Abstract
The recent COVID‐19 pandemic has demonstrated again the global threat posed by emerging zoonotic coronaviruses. During the past two decades alone, humans have experienced the emergence of several coronaviruses, such as SARS‐CoV in 2003, MERS‐CoV in 2012, and SARS‐CoV‐2 in 2019. To date, MERS‐CoV has been detected in 27 countries, with a case fatality ratio of approximately 34.5%. Similar to other coronaviruses, MERS‐CoV presumably originated from bats; however, the main reservoir and primary source of human infections are dromedary camels. Other species within the Camelidae family, such as Bactrian camels, alpacas, and llamas, seem to be susceptible to the infection as well, although to a lesser extent. In contrast, susceptibility studies on sheep, goats, cattle, pigs, chickens, and horses obtained divergent results. In the present study, we tested nasal swabs and/or sera from 55 sheep, 45 goats, and 52 cattle, collected at the largest livestock market in the United Arab Emirates, where dromedaries are also traded, for the presence of MERS‐CoV nucleic acid by RT‐qPCR, and for specific antibodies by immunofluorescence assay. All sera were negative for MERS‐CoV‐reactive antibodies, but the nasal swab of one sheep (1.8%) repeatedly tested positive for MERS‐CoV nucleic acid. Next generation sequencing (NGS) of the complete N gene of the sheep‐derived MERS‐CoV revealed >99% nucleotide identity to MERS‐CoV sequences of five dromedaries in nearby pens and to three reference sequences. The NGS sequence of the sheep‐derived MERS‐CoV was confirmed by conventional RT‐PCR of a part of the N gene and subsequent Sanger sequencing. All MERS‐CoV sequences clustered within clade B, lineage 5. In conclusion, our study shows that noncamelid livestock, such as sheep, goats, and cattle do not play a major role in MERS‐CoV epidemiology. The one sheep that tested positive most likely reflects an accidental viral spillover event from infected dromedaries in nearby pens.
Keywords: cattle, goat, MERS‐CoV, Middle East respiratory syndrome‐related coronavirus, Middle East respiratory syndrome, sheep
1. INTRODUCTION
Since the 1930s, members of the family Coronaviridae have been identified in humans and many different animal species such as cats, dogs, horses, pigs, cattle, mice, and fowl (Lai & Cavanagh, 1997). In the last 20 years, three zoonotic coronaviruses have emerged into the human population: Severe acute respiratory syndrome‐related coronavirus (SARS‐CoV) in 2003, Middle East respiratory syndrome‐related coronavirus (MERS‐CoV) in 2012, and most recently, SARS‐CoV‐2 in 2019, which led to the so far most devastating pandemic of the 21st century (Drosten et al., 2003; Wu et al., 2020; Zaki et al., 2012). Since 2012, MERS‐CoV has been detected in 27 countries, with 84.0% of cases found in Saudi Arabia, and a case fatality ratio of approximately 34.5% (WHO, 2019). Similar to other coronaviruses, MERS‐CoV presumably originated from bats; however, the main reservoir and primary source of human infections are dromedaries (Camelus dromedarius) (Azhar et al., 2014; Corman et al., 2014; Hemida et al., 2014; Muhairi et al., 2016; Nowotny & Kolodziejek, 2014; Reusken, Haagmans et al., 2013). Although dromedaries usually remain asymptomatic, or develop only mild symptoms due to MERS‐CoV infection, it has been shown that they can shed considerable amounts of virus (Adney et al., 2014; Farag et al., 2015). Studies on Bactrian camels (Camelus bactrianus), hybrid camels, alpacas (Vicugna pacos), llamas (Lama glama), and even pigs (Sus scrofa), have revealed that these animals are also susceptible to MERS‐CoV infection (Adney et al., 2019; Lau et al., 2020; Reusken et al., 2016; Vergara‐Alert et al., 2017). In contrast, studies on sheep, goats, cattle, horses, and chickens have indicated that, although sheep and goats may produce antibodies to MERS‐CoV, none of these species effectively shed the virus (Adney et al., 2016; Hemida et al., 2013; Reusken, Ababneh et al., 2013; Vergara‐Alert et al., 2017). A study about domestic mammals in contact with infected dromedaries found MERS‐CoV nucleic acid in nasal swabs not only from sheep and goats, but also from a cow and donkeys (Kandeil et al., 2019). To provide more information about the potential exposure and infection of other livestock, we performed a survey for MERS‐CoV at a livestock market in the United Arab Emirates (UAE), as live animal markets are known to be common sources of virus spillover.
2. MATERIALS AND METHODS
2.1. Sampling
Sampling was performed at the largest national livestock market in the UAE, the Al Ain central market in the emirate of Abu Dhabi, in October 2019. The market was opened in February 2009 and is situated on an 82‐acre site divided into three main areas, with mostly mixed sheep and goat pens located between camel and cattle stalls (Figure 1a). The distance between camel pens and the closest pens holding sheep and goats is approximately 20 m. The market is open daily from 6 a.m. to 7 p.m., and there are 300 animal pens, 2 slaughterhouses, 10 veterinary clinics, 10 veterinary pharmacies, 10 advanced livestock testing laboratories (e.g., for pre‐market entry Brucella testing), 20 feed shops, and 3500 parking spaces for animal pick‐up and drop‐off. On average, approximately 600‐1000 animals are received daily at the market, predominantly from all over the UAE, but also from neighbouring Oman and Saudi Arabia, doubling to approximately 1200‐2000 during weekends (Friday‐Saturday), and increasing to approximately 2000‐3000 animals during Ramadan and Eid. Daily sold animals are approximately half the number of those received. Camels usually stay between 1 and 21 days (until they are either sold to camel farms or taken to the abattoir for meat production), the average length of stay for goats is 7‐10 days, for sheep 10‐14 days, and between 14 and 30 days for cattle.
FIGURE 1.
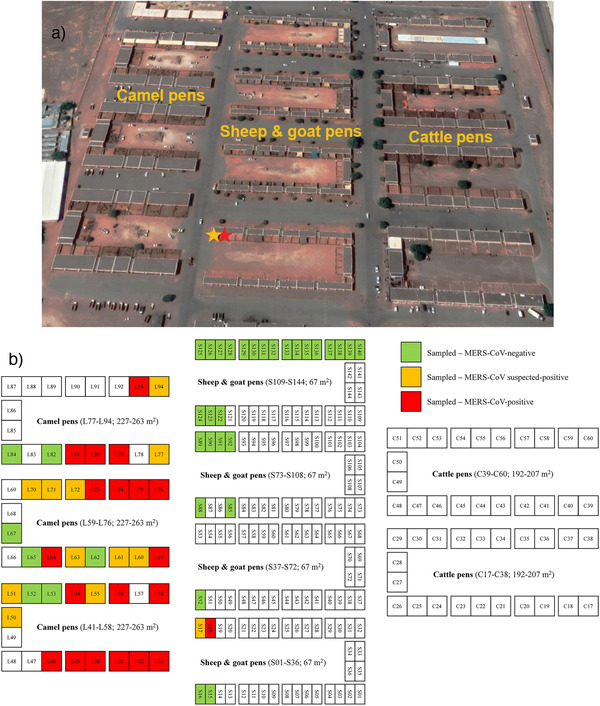
(a) Satellite image of the Al Ain central market in Abu Dhabi. Camel pens are located on the left side, cattle stalls on the right side, and sheep and goats are kept in between. The distance between camel pens and the closest pens holding sheep and goats is approximately 20 m (separated by a road). The red asterisk indicates sheep pen S18, in which the MERS‐CoV‐positive sheep was sampled, the orange asterisk indicates the mixed pen S17, in which the suspected positive goat was kept. Image retrieved from Google Maps. (b) Schematic diagram of the Al Ain central market in Abu Dhabi. Pens holding animals that tested negative for MERS‐CoV nucleic acid are indicated in green, pens with suspected positive animals are marked in orange, and pens with MERS‐CoV‐positive animals are highlighted in red. Pens S13‐S14, S19‐S20, S49‐S51, S86‐S87, and S121 were empty at the time of sampling. Unfortunately, the individual cattle samples could not be traced back to single pens anymore.
In total, we sampled 152 animals, which appeared healthy at the time of sampling. Nasal swabs and blood samples were taken from 55 sheep (41 females and 14 males) with an age range from 3 months to 4 years (mean of 19 months), and 45 goats (31 females and 14 males) from 3 to 40 months old (mean of 12 months). In addition, we collected blood samples from 52 cattle (43 females and 9 males) from 1 to 6 years (mean of 3.8 years). Nasal swabs were collected in tubes containing a virus inactivation solution (DNA/RNA Shield, ZymoResearch, Irvine, CA, USA). Serum was separated from each blood sample by centrifugation. All samples were then stored at −80°C at the laboratory of the College of Medicine, Mohammed Bin Rashid University of Medicine and Health Sciences, Dubai, UAE, before shipment on dry ice to the University of Veterinary Medicine, Vienna, Austria, for virological and serological investigations.
2.2. MERS‐CoV nucleic acid screening
Nasal swabs of sheep (n = 55) and goats (n = 45), as well as serum samples of all animals (n = 152) were screened for MERS‐CoV nucleic acid by reverse transcription real‐time (RT‐q)PCR. For this, 200 μL of each sample were subjected to automated nucleic acid extraction employing QIAamp Viral RNA Mini Kit on QIAcube HT (both Qiagen, Hilden, Germany). MERS‐CoV‐specific RT‐qPCR was performed using qScript XLT One Step RT‐qPCR ToughMix (QuantaBio, MA, USA) and previously described primers and probe in the open reading frame (ORF) 1a gene (Corman, Müller et al., 2012). RT‐qPCR‐positive samples were additionally tested by conventional RT‐PCRs (OneStep RT‐PCR Kit, Qiagen) employing two previously published primer pairs within the nucleocapsid (N) and ORF1b genomic regions of MERS‐CoV (Corman, Eckerle et al., 2012; Corman, Müller et al., 2012). Amplicons from the RT‐PCRs were subjected to Sanger sequencing (Eurofins Genomics, Ebersberg, Germany). In addition, next generation sequencing (NGS) was performed on suspected positive samples and five selected MERS‐CoV‐positive samples from dromedaries in nearby pens. For this, adapter sequences were trimmed using bcl2fastq2 conversion software version 2.20.0, and high‐quality sequencing reads were aligned to three reference MERS‐CoV genome sequences (GenBank acc. numbers KF192507, MG757604, and MK462253) using BWA version 0.7.17. Consensus sequences were generated for the N gene using GATK version 3.8–1–0 and BCFtools version 1.3.1. Multiple sequence alignment of the N gene of MERS‐CoV (1.2 kb) derived from one sheep and five dromedary camels was performed using MAFFT version 7.455.
2.3. MERS‐CoV antibody testing
Altogether 148 serum samples from sheep (n = 55), goats (n = 45), and cattle (n = 48) were available in sufficient volumes to perform MERS‐CoV antibody testing. MERS‐CoV spike protein‐based immunofluorescence assay (IFA) was done as described previously (Corman, Müller et al., 2012; Wölfel et al., 2020). Initial screening was performed with sample dilutions of 1:40, retesting of suspected positive (questionable) samples was done with a dilution series of 1:10‐1:320. Detection was facilitated using 1:200 dilutions of donkey‐anti sheep, donkey‐anti goat, or goat‐anti bovine secondary antibodies, all labelled with Alexa Fluor 488 (Dianova, Hamburg, Germany).
3. RESULTS
3.1. MERS‐CoV nucleic acid and antibody prevalence
The nasal swab of one sheep (1.8%) repeatedly tested positive for MERS‐CoV nucleic acid by RT‐qPCR (Ct values 32.5‐34.8; Table 1), and the nasal swab of one goat was suspected positive (Ct values 38.1‐39.4; Table 1). The positive sheep was a 2‐year‐old female, presumably locally raised Nuaimi breed. It was kept in pen S18 at the market, used for sheep only, which was the second closest pen to the camel area (approximately 25 m away; Figure 1, Supporting information Table S1). The suspected positive goat, a 1‐year‐old locally raised male Omani goat, was kept in mixed pen S17, directly adjacent to the camel pens, with only approximately 20 m between them (Figure 1, Supporting information Table S1).
TABLE 1.
Results of the MERS‐CoV screening in sheep, goats, and cattle
| Nucleic acid‐positive | |||||
|---|---|---|---|---|---|
| Species | n (females/males) | Age range (average) | Nasal swabs | Serum samples | Antibody‐positive |
| Sheep | 55 (41/14) | 3‐48 months (19 months) | 1/55 (1.8%) | 0/55 | 0/55 |
| Goat | 45 (31/14) | 3‐40 months (12 months) | 1(s) † /45 (2.2%) | 0/45 | 0/45 |
| Cattle | 52 (43/9) | 1‐6 years (3.8 years) | n.a ‡ | 0/52 | 0/48 |
| Total | 152 (115/37) | 3 months–6 years | 1 + 1(s)/100 (1‐2%) | 0/152 | 0/148 |
s, suspected.
n.a., not applicable.
Nasal swabs from sheep and goats, and sera from all animals were tested for MERS‐CoV nucleic acid by ORF1a RT‐qPCR. Serum samples from all but four animals (i.e., cattle sera with insufficient volumes) were investigated for MERS‐CoV‐specific antibodies by IFA.
The RT‐qPCR‐positive sheep sample was confirmed by two different RT‐PCRs. The obtained sequences (lengths without primer sequences: N–293 bp [GenBank acc. number: MZ558082], ORF1b–341 bp [GenBank acc. number: MZ558083]) were >99% identical to many MERS‐CoV sequences belonging to clade B, lineage 5 (according to the classification by Hemida et al., 2020) including human and camel strains from the UAE, Saudi Arabia, South Korea, Jordan, Qatar, and the United Kingdom. Unfortunately, we were not able to uncover the complete MERS‐CoV genomes using NGS; however, we succeeded to establish the complete N gene sequences of the virus‐positive sheep and five selected MERS‐CoV‐positive dromedaries (Figure 2). The sheep‐derived N gene sequences obtained by NGS and Sanger sequencing were identical. By NGS, the average sequencing coverage of the N gene from the sheep (S90_N) and five camel samples (C53_N, C5_N, C6_N, C28_N, and C50_N) was >6X across all samples (Figure 2a). The percentage of sequence identity of the N gene of the same samples along with three reference MERS‐CoV sequences (GenBank acc. numbers KF192507, MG757604, and MK462253) revealed that the sheep sample showed high nucleotide identity (>99%) with all other MERS‐CoV sequences (Figure 2b). In detail, multiple sequence alignment revealed five positions of nucleotide variations between the sheep‐derived MERS‐CoV sequence and the camel‐derived sequences and/or reference sequences (representative positions 533 and 637 of the N gene are shown in Figure 2c). The GenBank accession numbers of the complete MERS‐CoV N gene sequences are: sheep‐derived (S90_N): MZ558076; dromedary‐derived: C53_N ‐ MZ558080; C5_N ‐ MZ558077; C6_N ‐ MZ558078; C28_N ‐ MZ558079; and C50_N ‐ MZ558081. Unfortunately, the suspected positive result of the goat sample could not be confirmed by either RT‐PCR or NGS.
FIGURE 2.
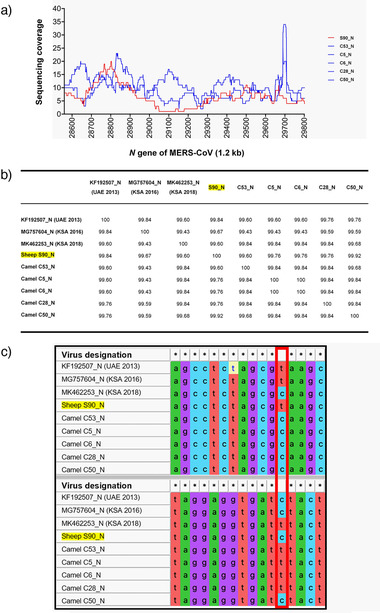
Next generation sequencing analysis of the MERS‐CoV N gene of sheep and camels. (a) Sequencing coverage of the MERS‐CoV N gene derived from one sheep (S90_N) and five camels (C53_N, C5_N, C6_N, C28_N, and C50_N). The average coverage of the N gene across all samples was >6X. X‐axis = N gene of MERS‐CoV (1.2 kb) and Y‐axis = sequencing coverage; red line = sheep‐derived MERS‐CoV, blue lines = dromedary‐derived MERS‐CoV. (b) Percentage of N gene sequence identity of the same samples along with three references MERS‐CoV sequences (GenBank acc. numbers KF192507, MG757604, and MK462253). The sample collected from the sheep showed high nucleotide identity (>99%) with all camel‐derived MERS‐CoV sequences as well as the reference sequences. (c) Multiple sequence alignment of the N gene of the same MERS‐CoV samples (1.2 kb). Two short representative regions with nucleotide sequence variations are shown: positions 533 and 637 of the N gene (highlighted by a red vertical box); conserved positions are indicated by asterisks (*). Multiple sequence alignment was performed using MAFFT version 7.455
The serological survey revealed that, although in the initial screening with 1:40 dilutions 15 serum samples (eight sheep, four goats, three cattle) appeared questionable, the presence of antibodies could not be confirmed in any sample by retesting using a dilution series of 1:10‐1:320 (Supporting information Tables S1 and S2).
4. DISCUSSION
Previous studies have demonstrated that, besides dromedaries, several other members of the Camelidae family are susceptible to MERS‐CoV infection, such as Bactrian camels, alpacas, and llamas (Adney et al., 2019; Lau et al., 2020; Reusken et al., 2016; Vergara‐Alert et al., 2017), although human contact infections have only been reported from dromedary camels (WHO, 2019). However, the existing data on the susceptibility of non‐camelids are conflicting. Our results are in line with a series of publications demonstrating that non‐camelids do not play a major role in the transmission cycle (Adney et al., 2016; Hemida et al., 2013; Meyer et al., 2015; Reusken, Ababneh et al., 2013). For instance, in a seroepidemiological study in Saudi Arabia (2010‐2013), no antibodies were detected in sheep, goats, cattle, or chickens, but a high prevalence was found in camels (Hemida et al., 2013). Similarly, after an experimental challenge of sheep, goats, and horses by intranasal MERS‐CoV inoculation, none of the animals effectively shed the virus, and neutralizing antibodies were only found in goats (Adney et al., 2016). Further regarding the family Equidae, including horses, donkeys, and mules, although viral replication has been observed in primary horse kidney cells, no antibodies have been found in any of 1053 equid sera collected in the UAE and Spain (Meyer et al., 2015). A study on livestock cell lines from the Arabian Peninsula showed that MERS‐CoV can replicate in goat, alpaca, and dromedary cells, in contrast to sheep, cattle, rodent, and insectivore cells (Eckerle et al., 2014). In a study experimentally challenging animals from France and Spain, MERS‐CoV nucleic acid in the respiratory tract as well as seroconversion were detected only in llamas and pigs, but not in sheep and horses (Vergara‐Alert et al., 2017). A serosurvey in a MERS‐CoV‐affected area in Jordan identified MERS‐CoV neutralising antibodies in the sera of all camels but none in goats and cattle, while 4.8% of sheep sera reacted with MERS‐CoV antigen (Reusken, Ababneh et al., 2013). In a monitoring study of several mammalian species in direct contact with infected dromedaries in Egypt, Tunisia, and Senegal (2015‐2017), antibodies were identified in an astonishing 55.6% of sheep from Senegal, 1.8% of sheep from Tunisia, and 0.9% of goats from Egypt. MERS‐CoV RNA was detected in nasal swabs from 1.2% sheep and 4.1% goats from Egypt and Senegal, as well as 1.9% cattle and 7.1% donkeys from Egypt (Kandeil et al., 2019). In conclusion, it seems that several non‐camelid species may become infected with MERS‐CoV during viral spillover events when they are in direct contact with virus‐shedding dromedaries. This susceptibility may be due to the close relatedness of the Betacoronaviruses (specifically their receptors) infecting camels and especially bovids, but also humans, porcines, and equids (Su et al., 2016; van Doremalen et al., 2014). However, whether these animals are implicated in the MERS‐CoV transmission cycle remains unknown, but seems to be highly unlikely.
The fact that none of the sheep, goats, and cattle in our survey tested positive for antibodies indicates that none of them had previously experienced a MERS‐CoV infection, although with an age range of 3 months to 6 years, all animals could have encountered the virus in the past. Moreover, as the average time of stay at the market for goats is usually 7‐10 days, 10‐14 days for sheep, and between 14 and 30 days for cattle, there is also a potential risk of infection at the market. However, the nasal swab of one sheep (1.8%) contained MERS‐CoV nucleic acid (Supporting information Table S1), suggesting either a current infection, or a spillover event from nearby dromedaries, which appears to be more likely since no antibodies were detected and the sheep was kept in a pen in close proximity to the camel pens (approximately 25 m away; Figure 1). Of note, we additionally sampled 90 dromedaries at the same market and at the same time as the non‐camelids, of which 35.6% were positive for MERS‐CoV nucleic acid, and another 24.4% were considered suspected positive (Ct values 38.6‐41.2). The locations of the camels sampled at the market can be seen in Figure 1b. Moreover, 91.1% of the 90 camel sera were MERS‐CoV antibody‐positive by ELISA, and 96.7% by IFA (data can be provided upon request), further supporting the high prevalence of MERS‐CoV infections in dromedaries. This hypothesis is also supported by our latest study, in which we screened 76 dromedaries during spring and autumn 2019 at the same market, and found MERS‐CoV nucleic acid in 57.9% of their nasal swabs (Lado et al., 2021).
The positive sheep from this study was a 2‐year‐old female, presumably locally raised Nuaimi breed. However, due to the fact that sex was not equally distributed across the animals we sampled (115 females, 75.7%, compared to 37 males, 24.3%), no conclusions can be drawn regarding the susceptibility of the different sexes. The same is true for the different breeds, with only one positive animal, we cannot infer any breed‐specific genetic predispositions to MERS‐CoV infection.
In conclusion, our results indicate that non‐camelids, such as sheep, goats, and cattle do not play a major role in MERS‐CoV epidemiology. The one sheep that tested RNA‐positive in our study most likely reflects a viral spillover event from infected dromedaries in nearby pens. Furthermore, serology revealed that the sheep did not develop an antibody response, suggesting that the infection was very recent, or the sheep only carried viral particles on its nasal mucosa without actually being infected.
ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. No ethical approval was required for sampling of the involved animals.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors thank the General Manager of the Al Ain City Municipality, H.E. Dr. Matar Mohammed Saif Al Nuaimi, and his team, including Dr. Babiker Mohammed Osman, for supporting the study, as well as Hassan Mohammed Alkaabi, Head of Veterinary Services, Hashim Ahmed Saeed and Md. Helal Ahmed for assistance with sampling at the livestock market. We are grateful for the help of Noushad Karuvantevida, Athiq Ahmed Wahab, and Abubakkar Babuhan, from the Mohammed Bin Rashid University of Medicine and Health Sciences, in facilitating the study.
This work was supported by research grants from the College of Medicine, Mohammed Bin Rashid University of Medicine and Health Sciences, Dubai, United Arab Emirates (Grant no. MBRU‐CM‐RG2018‐14 to N.N., and Grant no. MBRU‐CM‐RG2019‐13 to T.L.).
Weidinger, P. , Kolodziejek, J. , Camp, J. V. , Loney, T. , Kannan, D. O. , Ramaswamy, S. , Tayoun, A. A. , Corman, V. M. , & Nowotny, N. (2022). MERS‐CoV in sheep, goats, and cattle, United Arab Emirates, 2019: Virological and serological investigations reveal an accidental spillover from dromedaries. Transboundary and Emerging Diseases, 69, 3066–3072. 10.1111/tbed.14306
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Adney, D. R. , Brown, V. R. , Porter, S. M. , Bielefeldt‐Ohmann, H. , Hartwig, A. E. , & Bowen, R. A. (2016). Inoculation of goats, sheep, and horses with MERS‐CoV does not result in productive viral shedding. Viruses, 8(8): 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adney, D. R. , Letko, M. , Ragan, I. K. , Scott, D. , van Doremalen, N. , Bowen, R. A. , & Munster, V. J. (2019). Bactrian camels shed large quantities of Middle East respiratory syndrome coronavirus (MERS‐CoV) after experimental infection. Emerging Microbes & Infections, 8(1), 717–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adney, D. R. , van Doremalen, N. , Brown, V. R. , Bushmaker, T. , Scott, D. , Wit, E. , Bowen, R. A. , & Munster, V. J. (2014). Replication and shedding of MERS‐CoV in upper respiratory tract of inoculated dromedary camels. Emerging Infectious Diseases, 20(12), 1999–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhar, E. I. , El‐Kafrawy, S. A. , Farraj, S. A. , Hassan, A. M. , Al‐Saeed, M. S. , Hashem, A. M. , & Madani, T. A. (2014). Evidence for camel‐to‐human transmission of MERS coronavirus. New England Journal of Medicine, 370(26), 2499–2505. [DOI] [PubMed] [Google Scholar]
- Corman, V. M. , Müller, M. A. , Costabel, U. , Timm, J. , Binger, T. , Meyer, B. , Kreher, P. , Lattwein, E. , Eschbach‐Bludau, M. , Nitsche, A. , Bleicker, T. , Landt, O. , Schweiger, B. , Drexler, J. F. , Osterhaus, A. D. , Haagmans, B. L. , Dittmer, U. , Bonin, F. , Wolff, T. , & Drosten, C. (2012). Assays for laboratory confirmation of novel human coronavirus (hCoV‐EMC) infections. Eurosurveillance, 17(49), 20334. [DOI] [PubMed] [Google Scholar]
- Corman, V. M. , Eckerle, I. , Bleicker, T. , Zaki, A. , Landt, O. , Eschbach‐Bludau, M. , van Boheemen, S. , Gopal, R. , Ballhause, M. , Bestebroer, T. M. , Muth, D. , Müller, M. A. , Drexler, J. F. , Zambon, M. , Osterhaus, A. D. , Fouchier, R. M. , & Drosten, C. (2012). Detection of a novel human coronavirus by real‐time reverse‐transcription polymerase chain reaction. Euro Surveillance, 17(39), 20285. [DOI] [PubMed] [Google Scholar]
- Corman, V. M. , Ithete, N. L. , Richards, L. R. , Schoeman, M. C. , Preiser, W. , Drosten, C. , & Drexler, J. F. (2014). Rooting the phylogenetic tree of Middle East respiratory syndrome coronavirus by characterization of a conspecific virus from an African bat. Journal of Virology, 88(19), 11297–11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten, C. , Günther, S. , Preiser, W. , van der Werf, S. , Brodt, H.‐R. , Becker, S. , Rabenau, H. , Panning, M. L. , Kolesnikova, R. A. M. F , Berger, A. , Burguière, A.‐M. , Cinatl, J. , Eickmann, M. , Escriou, N. , Grywna, K. , Kramme, S. J.‐C. , Manuguerra, S. M , Rickerts, V. , Stürmer, M., S. , Vieth, H.‐D. K , Osterhaus, A. D. M. E. , Schmitz, H. , & Doerr, H. W. (2003). Identification of a novel coronavirus in patients with severe acute respiratory syndrome. New England Journal of Medicine, 348(20), 1967–1976. [DOI] [PubMed] [Google Scholar]
- Eckerle, I. , Corman, V. M. , Müller, M. A. , Lenk, M. , Ulrich, R. G. , & Drosten, C. (2014). Replicative capacity of MERS coronavirus in livestock cell lines. Emerging Infectious Diseases, 20(2), 276–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag, E. A. B. A. , Reusken, C. B. E. M. , Haagmans, B. L. , Mohran, K. A. , Raj, V. S. , Pas, S. D. , Voermans, J. , Smits, S. L. , Godeke, G.‐J. , Al‐Hajri, M. M. , Alhajri, F. H. , Al‐Romaihi, H. E. , Ghobashy, H. , El‐Maghraby, M. M. , El‐Sayed, A. M. , Al Thani, M. H. J. , Al‐Marri, S. , & Koopmans, M. P. G. (2015). High proportion of MERS‐CoV shedding dromedaries at slaughterhouse with a potential epidemiological link to human cases, Qatar 2014. Infection Ecology & Epidemiology, 5(1), 28305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemida, M. G. , Chu, D. K. W. , Chor, Y. Y. , Cheng, S. M. S. , Poon, L. L. M. , Alnaeem, A. , & Peiris, M. (2020). Phylogenetic analysis of MERS‐CoV in a camel abattoir, Saudi Arabia, 2016‐2018. Emerging Infectious Diseases, 26(12), 3089–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemida, M. G. , Chu, D. K. W. , Poon, L. L. M. , Perera, R. A. P. M. , Alhammadi, M. A. , Ng, H.‐Y. , Siu, L. Y. , Guan, Y. , Alnaeem, A. , Peiris, M. , Siu, L. Y. , & Guan, Y. (2014). MERS coronavirus in dromedary camel herd, Saudi Arabia. Emerging Infectious Diseases, 20(7), 1231–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemida, M. G. , Perera, R. A. , Wang, P. , Alhammadi, M. A. , Siu, L. Y. , Li, M. , Poon, L. L. , Saif, L. , Alnaeem, A. , & Peiris, M. (2013). Middle East Respiratory Syndrome (MERS) coronavirus seroprevalence in domestic livestock in Saudi Arabia, 2010 to 2013. Euro Surveillance: Bulletin Europeen Sur Les Maladies Transmissibles = European Communicable Disease Bulletin, 18(50), 20659. [DOI] [PubMed] [Google Scholar]
- Kandeil, A. , Gomaa, M. , Shehata, M. , El‐Taweel, A. , Kayed, A. E. , Abiadh, A. , Jrijer, J. , Moatasim, Y. , Kutkat, O. , Bagato, O. , Mahmoud, S. , Mostafa, A. , El‐Shesheny, R. , Perera, R. A. P. M. , Ko, R. L. W. , Hassan, N. , Elsokary, B. , Allal, L. , Saad, A. , Sobhy, H. , McKenzie, P. P. , Webby, R. J. , Peiris, M. , Ali, M. A. & Kayali, G. (2019). Middle East respiratory syndrome coronavirus infection in non‐camelid domestic mammals. Emerging Microbes & Infections, 8(1), 103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lado, S. , Elbers, J. P. , Plasil, M. , Loney, T. , Weidinger, P. , Camp, J. V. , Kolodziejek, J. , Futas, J. , Kannan, D. A. , Wengel, P. , Horin, P. , Nowotny, N. , & Burger, P. A. (2021). Innate and adaptive immune genes associated with MERS‐CoV infection in dromedaries. Cells, 10(6), 1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, M. M. C. , & Cavanagh, D. (1997). The molecular biology of coronaviruses. In Maramorosch K., Murphy F. A., & Shatkin A. J. (Eds.), Advances in virus research, 48, pp. 1–100. Academic Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau, S. K. P. , Li, K. S. M. , Luk, H. K. H. , He, Z. , Teng, J. L. L. , Yuen, K.‐Y. , Yuen, K.‐Y. , Wernery, U. , & Woo, P. C. Y. (2020). Middle East respiratory syndrome coronavirus antibodies in Bactrian and hybrid camels from Dubai. MSphere, 5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, B. , García‐Bocanegra, I. , Wernery, U. , Wernery, R. , Sieberg, A. , Müller, M. A. , Drexler, J. F. , Drosten, C. , & Eckerle, I. (2015). Serologic assessment of possibility for MERS‐CoV infection in equids. Emerging Infectious Diseases, 21(1), 181–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhairi, S. A. , Hosani, F. A. , Eltahir, Y. M. , Mulla, M. A. , Yusof, M. F. , Serhan, W. S. , Serhan, W. S. , Hashem, F. M. , Elsayed, E. A. , MarzougB, A. , & Abdelazim, A. S. (2016). Epidemiological investigation of Middle East respiratory syndrome coronavirus in dromedary camel farms linked with human infection in Abu Dhabi Emirate, United Arab Emirates. Virus Genes, 52(6), 848–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowotny, N. , & Kolodziejek, J. (2014). Middle East respiratory syndrome coronavirus (MERS‐CoV) in dromedary camels, Oman, 2013. Euro Surveillance: Bulletin Europeen Sur Les Maladies Transmissibles=European Communicable Disease Bulletin, 19(16), 20781. [DOI] [PubMed] [Google Scholar]
- Reusken, C. B. , Ababneh, M. , Raj, V. S. , Meyer, B. , Eljarah, A. , Abutarbush, S. , Godeke, G. J. , Bestebroer, T. M. , Zutt, I. , Muller, M. A. , Bosch, B. J. , Rottier, P. J. , Osterhaus, A. D. , Drosten, C. , Haagmans, B. L. , & Koopmans, M. P. (2013). Middle East respiratory syndrome coronavirus (MERS‐CoV) serology in major livestock species in an affected region in Jordan, June to September 2013. Advance online publication. 18, 20662. [DOI] [PubMed] [Google Scholar]
- Reusken, C. B. E. M. , Schilp, C. , Raj, V. S. , De Bruin, E. , Kohl, R. H. G. , Farag, E. A. B. A. , Haagmans, B. L. , Al‐Romaihi, H. , Le Grange, F. , Bosch, B.‐J. , & Koopmans, M. P. G. (2016). MERS‐CoV infection of alpaca in a region where MERS‐CoV is endemic. Emerging Infectious Disease, 22(6), 1129–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusken, C. B. , Haagmans, B. L. , Müller, M. A. , Gutierrez, C. , Godeke, G.‐J. , Meyer, B. , Muth, D. , Stalin Raj, V. , Smits‐De Vries, L. , Corman, V. M. , Drexler, J.‐F. , Smits, S. L. , El Tahir, Y. E. , De Sousa, R. , van Beek, J. , Nowotny, N. , van Maanen, K. , Hidalgo‐Hermoso, E. , Bosch, B.‐J. , Rottier, P. , Osterhaus, A. , Gortázar‐Schmidt, C. , Drosten, C. , & Koopmans, M. P. G. (2013). Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: a comparative serological study. Lancet Infectious Diseases, 13(10), 859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, S. , Wong, G. , Shi, W. , Liu, J. , Lai, A. C.K. , Zhou, J. , Liu, W. , Bi, Y. , & Gao, G. F. (2016). Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends in Microbiology, 24(6), 490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Doremalen, N. , Miazgowicz, K. L. , Milne‐Price, S. , Bushmaker, T. , Robertson, S. , Scott, D. , Kinne, J. , McLellan, J. S. , Zhu, J. , & Munster, V. J. (2014). Host species restriction of Middle East respiratory syndrome coronavirus through its receptor, dipeptidyl peptidase 4. Journal of Virology, 88(16), 9220–9232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara‐Alert, J. , van den Brand, J. M. A. , Widagdo, W. , Muñoz, M. , Raj, S. , Schipper, D. , Solanes, D. , Cordón, I. , Bensaid, A. , Haagmans, B L. , & Segalés, J. (2017). Livestock susceptibility to infection with Middle East respiratory syndrome coronavirus. Emerging Infectious Diseases, 23(2), 232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . (2019). Retrieved from World Health Organization website. MERS global summary and assessment of risk. https://apps.who.int/iris/bitstream/handle/10665/326126/WHO‐MERS‐RA‐19.1‐eng.pdf [Google Scholar]
- Wölfel, R. , Corman, V. M. , Guggemos, W. , Seilmaier, M. , Zange, S. , Müller, M. A. , Niemeyer, D. , Jones, T. C. , Vollmar, P. , Rothe, C. , Hoelscher, M. , Bleicker, T. , Brünink, S. , Schneider, J. , Ehmann, R. , Zwirglmaier, K. , Drosten, C. , & Wendtner, C. (2020). Virological assessment of hospitalized patients with COVID‐2019. Nature, 581(7809), 465–469. [DOI] [PubMed] [Google Scholar]
- Wu, A. , Peng, Y. , Huang, B. , Ding, X. , Wang, X. , Niu, P. , Meng, J. , Zhu, Z. , Zhang, Z. , Wang, J. , Sheng, J. , Quan, L. , Xia, Z. , Tan, W. , Cheng, G. , & Jiang, T. (2020). Genome composition and divergence of the novel coronavirus (2019‐nCoV) originating in China. Cell Host & Microbe, 27(3), 325–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki, A. M. , van Boheemen, S. , Bestebroer, T. M. , Osterhaus, A. D. M. E. , & Fouchier, R. A. M. (2012). Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. New England Journal of Medicine, 367(19), 1814–1820. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


