Abstract
Introduction
Peptide‐C ampoules (PC) contain peptides, 10% of vitamin C, hyaluronic acid, and Vichy volcanic mineralizing water.
Aims
To assess the effectiveness and tolerability of PC.
Patients and Methods
An observational study conducted in 9 countries in women ≥30 years old with signs of facial skin aging (grade >0 for forehead and/or crow's feet wrinkles and bothered by skin quality). Investigator assessments and subject questionnaires were performed at initial visit and Day 30 after application of PC twice daily for 28 days. Tolerance was assessed throughout the study.
Results
Effectiveness and safety were analyzed in 1382 and 1742 subjects, respectively. Most subjects (mean age 48.5 ± 8.6 years) had skin phototype II or III (91.7%) and dry or combination skin (63.9%). PC was used as a standalone care or prior to a planned procedure (70%), or after a procedure (30%).
Between baseline and Day 30, 63% and 64% of all subjects (N = 1360) had an improvement in forehead wrinkles and crow's feet wrinkles, respectively. Skin hydration improved in 67.3% of subjects. According to investigator and subject assessments, skin quality, skin radiance, skin aging signs, wrinkles, complexion, and skin pores significantly improved by Day 30. Similar results were observed for subgroup analyses when PC was used as standalone skin care or after a procedure. Tolerance of PC was rated as good to very good by 97.7% of subjects.
Conclusions
Peptide‐C ampoules is effective in reducing visible signs of skin aging, and well tolerated, when used alone or as an adjunct to anti‐aging procedures.
Keywords: adjunct to procedures, anti‐aging product, observational study, peptides, topical formulation, vitamin C
1. INTRODUCTION
The anti‐aging formulation evaluated here contains pure vitamin C (10%), peptides (rice and lupin), hyaluronic acid (HA), and Vichy volcanic mineralizing water (Liftactiv Peptide‐C Ampoules, Laboratoires Vichy, France; PC). Vitamin C and peptides are widely used in dermocosmetics for their anti‐aging properties. 1 , 2 , 3 Topical L‐ascorbic acid, the most biologically active form of vitamin C, has antioxidant, photo‐protective, anti‐aging, and anti‐pigmentary benefits. 4 When vitamin C was added in culture medium of reconstructed human epidermis, significant protection against pollution and UVA1 was observed. 5 Previous in vitro and in vivo studies have reported that di‐ and tripeptides extracted from rice stimulate the proliferation of fibroblasts, the expression of pro‐collagen, collagen VII and fibrillin‐1 and have an anti‐wrinkle effect. 6 , 7 Similarly, peptides extracted from lupin stimulated the proliferation of fibroblasts. 6 Hyaluronic acid has viscoelastic and hygroscopic properties with a capacity to retain skin moisture and help prevent skin dryness, atrophy, and loss of elasticity. 8 Finally, Vichy volcanic mineralizing water is a highly mineralized water (containing 15 different minerals) used as a dermocosmetic ingredient for its anti‐inflammatory and antioxidant properties with potential benefits to strengthen the skin against exposome aggressions. 9
A daily dose format of PC is available in amber glass ampoules without preservatives in a minimalist formulation with low pH. Previous in vitro studies have shown that this formulation has antioxidant properties and protective effect against UVA and pollution. 10 Furthermore, three clinical studies with PC showed significant results on improving facial wrinkles and radiance. 11 However, there is a lack of studies demonstrating effectiveness in a real‐world setting. The objective of this observational study was to assess the effectiveness and tolerability of PC when applied as a standalone dermocosmetic, before a procedure, or after a procedure.
2. METHODS
2.1. Subjects
An international, observational study in 1756 women aged ≥30 years old, with signs of skin aging (grade >0 for at least one [forehead and/or crow's feet] wrinkle grade and bothered by skin quality), and recommended to apply PC on their face twice daily for 28 days.
2.2. Assessments
Investigator assessments by dermatologists at initial visit and Day 30 included skin improvement (not at all/slightly/moderately vs clearly/very clearly improved); global assessment (satisfactory/highly satisfactory vs rather unsatisfactory/not at all satisfactory); severity of forehead and crow's feet wrinkles (on a 6‐point scale except for the Russian Federation (RF) which used a 7‐point scale); and impact on skin quality (skin hydration, skin shininess, other skin aspects [quality, radiance]).
Subject questionnaire included self‐assessments on skin aspect, wrinkles, fine lines, skin complexion, skin tone, firmness, and skin pores; how much they were bothered by these signs on a scale from 0 to 10, cosmeticity and product satisfaction.
Tolerance was assessed throughout the study.
2.3. Statistical analysis
Quantitative variables were described as mean, standard deviation, median, minimum, and maximum. Change in skin hydration or skin shininess between visits were analyzed using the generalized mixed model for multinomial data. A student test for matched samples, or a Wilcoxon test for matched samples if the normality hypothesis of distributions was rejected by the Shapiro‐Wilk test at the threshold of 1%, was used to analyze changes in wrinkle grade, changes in skin quality; a separate analysis was performed for wrinkles in Russian women as a 7‐point scale was used. All statistical analyses were performed at a 5% significance level using 2‐sided tests, except a threshold of 1% was used for the normality Shapiro‐Wilk test. All summaries and statistical analyses were generated using SAS version 9.4.
2.4. Ethical statement
This cosmetic, open‐label, observational, international real‐world study was conducted according to the guidelines of the International Epidemiological Association for proper conduct in epidemiological research and in accordance with applicable regulatory requirements in Argentina, Czech Republic, Germany, Greece, Hungary, Portugal, Russia, Slovakia, and Turkey, between January 2020 and January 2021. Ethics committee approval was not required for this cosmetic product, observational study; local ethics committee approval was, however, obtained in Argentina.
For participating European countries, subjects were informed of their rights with regard to the processing of their personal data through an information leaflet translated into their native language, in accordance with the Regulation (EU) 2016/679 of the European Parliament and of the Council of April 27, 2016, on the protection of natural persons. For countries outside the EU, the study was conducted in accordance with all local legal requirements for this type of observational real‐world survey.
3. RESULTS
3.1. Baseline characteristics
Between January 2020 and January 2021, 1756 subjects were enrolled from 9 countries, mainly Russia and Greece (see Table 1). The effectiveness analysis was performed on 1382 subjects without any protocol deviations; 374 subjects had at least one deviation including incomplete observation (N = 9), age <30 years old (N = 36), off‐protocol wrinkle grades (N = 20), no indication for use (N = 13), <28 days of treatment (N = 295), and male subjects (N = 43). The safety analysis included 1742 subjects.
TABLE 1.
Baseline subject demographic and clinical characteristics
| Subjects, N (%) | |
|---|---|
| Age groups (years), N = 1382 | |
| [30–40] | 134 (9.7) |
| [40–50] | 676 (48.9) |
| [50–60] | 409 (29.6) |
| [60–70] | 136 (9.8) |
| ≥ 70 | 27 (2) |
| Country, N = 1382 | |
| Russia | 590 (42.7) |
| Greece | 342 (24.7) |
| Czech Rep | 113 (8.2) |
| Argentina | 105 (7.6) |
| Slovakia | 84 (6.1) |
| Germany | 64 (4.6) |
| Portugal | 36 (2.6) |
| Turkey | 30 (2.2) |
| Hungary | 18 (1.3) |
| Skin phototype, N = 1371 | |
| I | 75 (5.5) |
| II | 684 (49.9) |
| III | 573 (41.8) |
| IV | 39 (2.8) |
| Skin type, N = 1375 | |
| Combination | 488 (35.5) |
| Normal | 431 (31.3) |
| Dry | 391 (28.4) |
| Oily | 65 (4.7) |
| Previous use of anti‐aging treatment, N = 1380 | 1140 (82.5) |
| Dermocosmetic products | 570 (41.3) |
| Procedure | 933 (67.6) |
| Currently using a skin care product, N = 1380 | 1003 (72.7) |
| Currently using anti‐aging skin care cosmetic, N = 1376 | 798 (58) |
| Currently using daily photoprotection, N = 1381 | 791 (57.3) |
| Indication for Peptide‐C ampoules N = 1382 | |
| Standalone treatment | 627 (45.4) |
| Before a planned procedure | 340 (24.6) |
| After a procedure | 415 (30) |
At baseline, the mean age was 48.5 ± 8.6 years old (range 30–89 years) and most women were aged between 40 and 60 years old. Most subjects had skin phototype II or III, and 63.9% had dry or combination skin (Table 1).
Of 82.5% of subjects who had used previous anti‐aging treatments, 14.9% had used only dermocosmetic products, 41.2% had previously received only an anti‐aging a procedure, and 26.4% had used both. The last procedure performed was most commonly botulinum toxin injections, fillers or a peeling, as shown in Figure 1.
FIGURE 1.
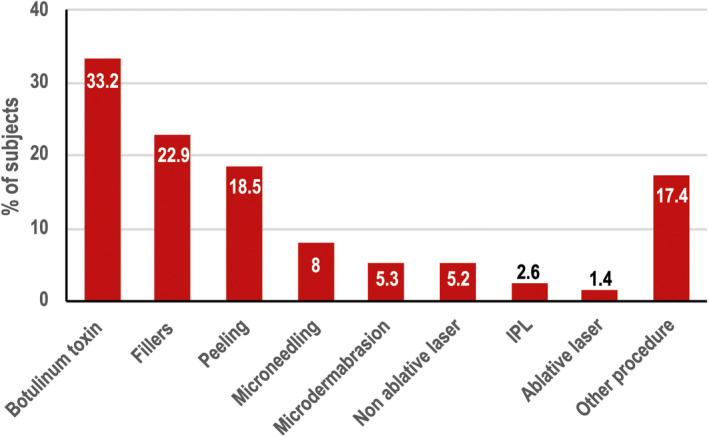
Previous anti‐aging procedures, N = 933. IPL, intense pulsed light therapy
At the start of the study, 57.3% of subjects indicated that they generally used daily photoprotection and 58% used anti‐aging skin care cosmetics. Of 1681 women who completed the exposome questionnaire, 59.9% indicated they were stressed, 67.2% lacked sleep, and 42.8% had irregular menstrual cycles. Concerning external environmental factors, 65.8% indicated they were exposed to environmental pollution, while 40.9% frequently exposed their face to the sun and 61.1% had done so in the past.
3.2. Effectiveness
Compliance data showed PC was applied twice daily (45%) or once daily (55%) for a mean duration of 30.6 ± 4.9 days.
After 30‐day application of PC, the global (N = 785) investigator‐assessed transversal forehead wrinkle score improved by 21% (2.94 ± 1.11 at baseline vs 2.27 ± 1.05 at Day 30 on a 0 [none]‐5 [deep wrinkles] scale; p < 0.0001). For the Russian Federation (N = 587), this score improved by 26% (3.35 ± 1.08 vs 2.45 ± 1.1 on a 0 [none] −6 [very deep wrinkles] scale; p < 0.0001).
The global (N = 785) investigator‐assessed crow's feet wrinkle score improved by 19.6% (3.18 ± 1.18 baseline vs 2.52 ± 1.16 Day 30 on a 0–6 scale; p < 0.0001). For the Russian Federation (N = 587), this score improved by 28% (3.31 ± 1.15 vs 2.36 ± 1.14 on a 0–7 scale; p < 0.0001). Overall, between baseline and Day 30, improvements in forehead wrinkles (N = 1360) and crow's feet wrinkles (N = 1372) were observed in 63% and 64% of subjects, respectively (Figure 2).
FIGURE 2.
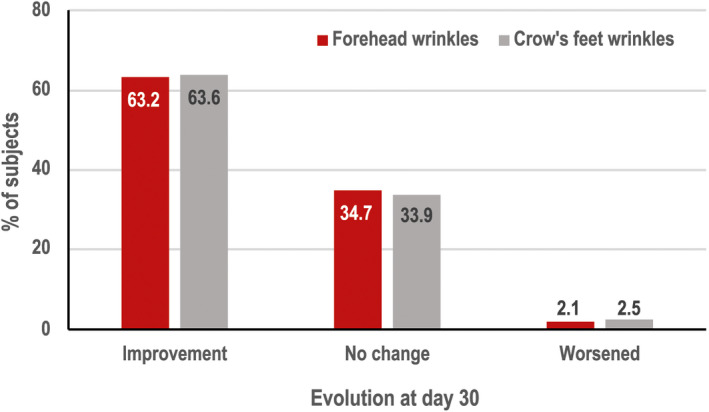
Percentage of subjects with improvement in investigator‐assessed forehead wrinkles (N = 1360) and crow's feet wrinkles (N = 1372) between baseline and Day 30
The percentage of women (N = 1357) with dry skin decreased from 28.2% at baseline to 16.9% on Day 30. After 30‐day application of PC, hydration was improved in 67.3% of subjects. According to investigator assessment (N = 1353), 66.9% had insufficiently hydrated skin (53.3% not enough and 13.6% not at all hydrated) at baseline, whereas this decreased to 14.3% (13.7% and 0.6% not enough and not at all, respectively) at Day 30 (p < .0001).
According to investigator and subject assessments, skin quality, skin radiance, skin aging signs, wrinkles, complexion, and skin pores had significantly improved (Table 2).
TABLE 2.
Improvements in skin according to investigator and subject assessments
| Dermatologist assessed skin quality | Very clearly or clearly improved | Moderately improved | Slightly or not at all improved | p‐value a |
|---|---|---|---|---|
| Overall quality, N = 1377 | 54.2 | 26.9 | 18.9 | 0.0016 |
| Skin radiance, N = 1376 | 54.9 | 23.5 | 21.5 | 0.0002 |
| Skin aging signs, N = 1373 | 46.5 | 31.4 | 22.1 | 0.0088 |
| Subject‐assessed improvement | Yes or yes, a great deal | Not at all or not much | p‐value b |
|---|---|---|---|
| Facial wrinkles, N = 1377 | 67.3 | 32.7 | <0.0001 |
| Skin complexion, N = 1378 | 82.6 | 17.4 | <0.0001 |
| Skin pores, N = 1304 | 57.1 | 42.9 | <0.0001 |
For not improved at all/slightly improved/moderately improved vs clearly improved/very clearly improved.
For yes vs not at all or not much.
The percentage of women (N = 1379) indicating their skin was very or extremely shiny decreased from 14.1% at baseline to 8.1% on Day 30. Of subjects with shiny skin at baseline (N = 830), 46.9% saw an improvement between baseline and Day 30, including 32% for whom the shininess disappeared.
Between baseline and after 30‐day application of PC, the decreases in subject‐assessed mean bother scores for skin aspect, wrinkles, fine lines, skin complexion, skin tone, skin firmness, and skin pores were 31%, 30%, 31%, 35%, 32%, 30%, and 31%, respectively (Figure 3).
FIGURE 3.
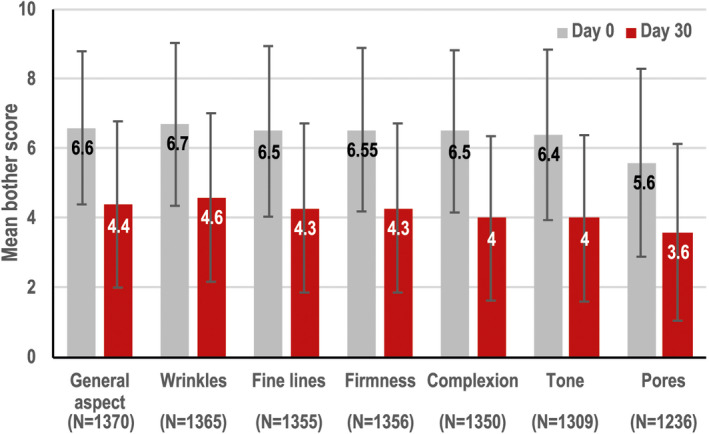
Subject‐assessed improvements in bother score between baseline and Day 30 for subjects who had a bother score above 0 at baseline (all p < 0.0001)
During the study, around half the subjects (45.4%) applied PC as a standalone treatment, while 24.6% applied PC before a planned procedure and 30% after a procedure. After the end of PC application, the mean time to the planned procedure was 17.9 ± 11.9 days. When PC was used to complement the treatment after a procedure, the mean time since the procedure was 21.1 ± 12.5 days.
Similar results were observed for the subgroup analyses of subjects having used PC as standalone or pre‐procedure skin care compared with the subgroup of subjects who applied PC after a procedure.
3.3. Tolerance
After 30‐day application of PC, 97.7% of subjects indicated the tolerance was very good (70%) or quite good (27.7%). Similar tolerability was observed when used as a standalone procedure or before a procedure (98.4% very/quite good tolerance) and when used as an adjunct after a procedure (97.8% very/quite good).
3.4. Satisfaction with the product
Investigators were overall satisfied with skin aspect, wrinkles, and radiance for 92%, 86%, and 88% of patients, respectively (Figure 4A).
FIGURE 4.
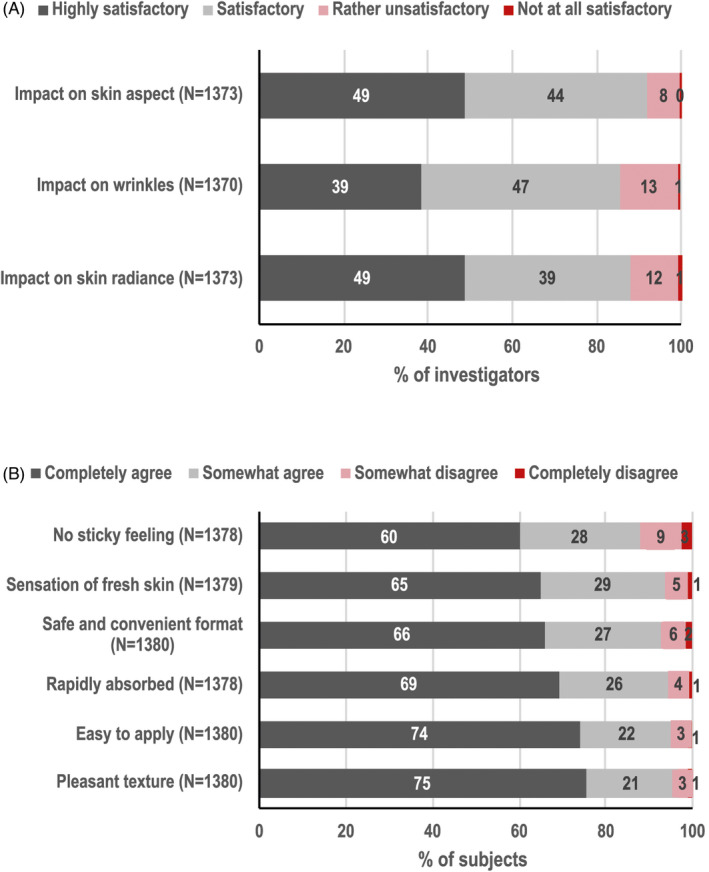
Overall dermatologist evaluations of the impact of PC on subject's skin (A) and subject‐assessed cosmetic acceptability (B)
Concerning patients who used PC before a planned procedure (N = 308), investigators agreed/totally agreed that PC prepared the skin well before a procedure in 93.5% of cases and were neutral/disagreed, or totally disagreed in 6.5% of cases. When PC was used after a procedure (N = 272), investigators agreed that application of PC improved or prolonged the effect of the procedure for 91.9% of patient cases.
The mean overall subject satisfaction was 8.6 ± 1.7 (on a scale from 0 not at all to 10 completely satisfied), and subject‐assessed cosmetic acceptability is summarized in Figure 4B. In the subgroup analyses, the mean overall subject satisfaction for subjects having used PC as a standalone or a pre‐procedure skin care (N = 963) was 8.5 ± 1.7 and was 8.7 ± 1.6 when it was used as a complement after a procedure (N = 415).
4. DISCUSSION
In this population of women consulting a dermatologist for anti‐aging solutions, the mean age was 48.5 years (range 30–89 years) and around two‐thirds of them had already undergone an anti‐aging procedure in the past, most frequently botulinum toxin or fillers. These real‐world data confirm high effectiveness, as well as high subject and investigator satisfaction, after only 30‐day application of PC and very similar results were obtained whether the ampoules were used as a standalone dermocosmetic, before a procedure, or as an adjunct post‐procedure. Limitations of the study include the short duration, as well as inherent limitations of observational studies, including selection bias or unmeasured confounding. However, the large sample size including subjects from 9 different countries provided real‐world information on a broad range of topics and in different situations, either before or after a procedure or as a standalone treatment.
Interestingly, data collected at inclusion showed that despite actively seeking anti‐aging solutions, only 57% of subjects indicated they apply daily photoprotection, suggesting that almost a half are missing the potential benefit of avoiding photodamage. An increasing body of evidence exists showing that daily use of broad‐spectrum sunscreen containing antioxidant and anti‐aging active ingredients is important for preventing or reducing extrinsic aging due to solar radiation and other exposome factors, such as pollution. 12 The exposome questionnaire responses revealed that around 6 out of 10 were exposed to various stressors, for example, internal exposome factors (stress, lack of sleep, and hormonal variations) and external exposome factors (pollution, solar radiation past exposure), while around 4 out of 10 indicated they currently frequently exposed their face to the sun. In addition to genetic factors, the sum of lifelong exposures (internal biological factors and external environmental factors), known as the exposome, have an impact on the pathophysiology of skin aging and oxidation plays a major role. 13 , 14
5. CONCLUSION
Whether PC is used alone or as an adjunct to procedures, PC is effective in reducing visible signs of skin aging and is well tolerated.
CONFLICT OF INTEREST
Delphine Kerob is a full‐time employee of Laboratoires Vichy, and Audrey Valois is a full‐time employee of L’Oréal. Monica Maiolino has received honoraria from Vichy. All other authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION
DK designed the study. CD, DK, and AV analyzed the data. IA, IS, EP, MM, SH, MS, BM, HR, and OT performed the study. IA, IS, EP, CD, DK, and AV contributed to writing the paper. All authors have reviewed and approved the final manuscript.
ETHICAL APPROVAL
This cosmetic, open‐label, observational, international real‐world study was conducted according to the guidelines of the International Epidemiological Association for proper conduct in epidemiological research and in accordance with applicable regulatory requirements in Argentina, Czech Republic, Germany, Greece, Hungary, Portugal, Russia, Slovakia, and Turkey, between January 2020 and January 2021. Ethics committee approval was not required for this cosmetic product, observational study; local ethics committee approval was, however, obtained in Argentina. For participating European countries, subjects were informed of their rights with regard to the processing of their personal data through an information leaflet translated into their native language, in accordance with the Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons. For countries outside the EU, the study was conducted in accordance with all local legal requirements for this type of observational real‐world survey.
ACKNOWLEDGMENTS
Writing assistance was provided by Helen Simpson PhD of My Word Medical Writing.
Akulinina I, Stefanaki I, Pavlíčková E, et al. Topical formulation containing peptides and vitamin C in ampoules improves skin aging signs: Results of a large, international, observational study. J Cosmet Dermatol. 2022;21:3908–3914. doi: 10.1111/jocd.14733
Funding information
The study and medical writing assistance were funded by Laboratoires Vichy, France.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Pullar JM, Carr AC, Vissers MCM. The roles of vitamin C in skin health. Nutrients. 2017;9(8):866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gorouhi F, Maibach HI. Role of topical peptides in preventing or treating aged skin. Int J Cosmet Sci. 2009;31(5):327‐345. [DOI] [PubMed] [Google Scholar]
- 3. Schagen SK . Topical peptide treatments with effective anti‐aging results. Cosmetics. 2017;4:1‐14. [Google Scholar]
- 4. Al‐Niaimi F, Chiang NYZ. Topical vitamin C and the skin: mechanisms of action and clinical applications. J Clin Aesthet Dermatol. 2017;10(7):14‐17. [PMC free article] [PubMed] [Google Scholar]
- 5. Dimitrov A, Zanini M, Zucchi H, et al. Vitamin C prevents epidermal damage induced by PM‐associated pollutants and UVA1 combined exposure. Exp Dermatol. 2021;30(11):1693‐1698. [DOI] [PubMed] [Google Scholar]
- 6. Jouandeaud M. Di‐ and tripeptides: a new approach to skin nutrition. Personal Care Mag. 2002;2002:1‐4. [Google Scholar]
- 7. Guzman A‐I, Boudier D, Guichard N, Gofflo S, Closs B. Natural peptides: “active”nutrients for deficient skins. Peptides naturels: des nutriments « actifs » pour les peaux carencées. Expr Cosmét Guide Cosmet Ingredients. 2015;2015:266‐270. [Google Scholar]
- 8. Papakonstantinou E, Roth M, Karakiulakis G. Hyaluronic acid: a key molecule in skin aging. Dermatoendocrinol. 2012;4(3):253‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tacheau C, Weisgerber F, Fagot D, et al. Vichy thermal spring water (VTSW), a cosmetic ingredient of potential interest in the frame of skin ageing exposome: an in vitro study. Int J Cosmet Sci. 2018;40(4):377‐387. [DOI] [PubMed] [Google Scholar]
- 10. Martín‐Martínez AS‐MN, Martínez‐Casanova D, Abarquero‐Cerezo M, Herranz‐López M, Barrajón‐Catalán E, Matabuena‐Yzaguirre M. High global anti‐oxidant protection (RMS) and stimulation of the collagen synthesis of new anti‐age product containing an optimized active‐mix. IMCAS World Congress poster presentation; Paris, France; 2020 30 January – 1 February. [Google Scholar]
- 11. Escobar S, Valois A, Nielsen M, Closs B, Kerob D. Effectiveness of a formulation containing peptides and vitamin C in treating signs of facial ageing: three clinical studies. Int J Cosmet Sci. 2021;43(2):131‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krutmann J, Schalka S, Watson REB, Wei L, Morita A. Daily photoprotection to prevent photoaging. Photodermatol Photoimmunol Photomed. 2021;37(6):482‐489. [DOI] [PubMed] [Google Scholar]
- 13. Wild CP. Complementing the genome with an "exposome": the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1847‐1850. [DOI] [PubMed] [Google Scholar]
- 14. Krutmann J, Bouloc A, Sore G, Bernard BA, Passeron T. The skin aging exposome. J Dermatol Sci. 2017;85(3):152‐161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


