Abstract
Introduction
The evidence for characteristics of persons with subjective cognitive decline (SCD) associated with amyloid positivity is limited.
Methods
In 1640 persons with SCD from 20 Amyloid Biomarker Study cohort, we investigated the associations of SCD‐specific characteristics (informant confirmation, domain‐specific complaints, concerns, feelings of worse performance) demographics, setting, apolipoprotein E gene (APOE) ε4 carriership, and neuropsychiatric symptoms with amyloid positivity.
Results
Between cohorts, amyloid positivity in 70‐year‐olds varied from 10% to 76%. Only older age, clinical setting, and APOE ε4 carriership showed univariate associations with increased amyloid positivity. After adjusting for these, lower education was also associated with increased amyloid positivity. Only within a research setting, informant‐confirmed complaints, memory complaints, attention/concentration complaints, and no depressive symptoms were associated with increased amyloid positivity. Feelings of worse performance were associated with less amyloid positivity at younger ages and more at older ages.
Discussion
Next to age, setting, and APOE ε4 carriership, SCD‐specific characteristics may facilitate the identification of amyloid‐positive individuals.
Keywords: Alzheimer's disease, amyloid, cerebrospinal fluid, positron emission tomography, subjective cognitive decline
1. INTRODUCTION
Cognitively normal persons with evidence of cerebral amyloidosis, as measured with positron emission tomography (PET) or cerebrospinal fluid (CSF), are considered an early stage of Alzheimer's disease (AD), that is, preclinical AD. 1 , 2 These persons are at an increased risk of developing AD‐type dementia. 3 , 4 Identification of preclinical AD is important for the development of treatments that aim to preserve cognitive function. Cognitively unimpaired persons experiencing subjective cognitive decline (SCD) are also at an increased risk of developing dementia. 5 , 6 SCD is characterized by perceived cognitive decline in the absence of cognitive impairment. 7 It has been hypothesized that SCD may be an early manifestation of amyloidosis, and practical, low‐cost characteristics of SCD could aid in the identification of potential at‐risk participants. However, previous studies have shown conflicting results on whether SCD is a specific indicator of amyloidosis, and improved understanding of the association of SCD‐specific characteristics with amyloid positivity is needed.
Some studies reported an association between memory complaints or concerns and presence of amyloid pathology, 8 , 9 , 10 , 11 whereas others reported similar levels of amyloid pathology in cognitively normal individuals with and without SCD. 3 , 12 These inconsistent findings may be due to heterogeneity in the definition and assessment of SCD and specific complaints 13 and in the underlying causes of SCD. In an attempt to reduce part of this heterogeneity between studies, Jessen et al. 13 , 14 suggested characteristics to be collected in studies on SCD that are assumed to increase the likelihood of the presence of amyloid pathology in persons with SCD, such as memory complaints rather than attention/concentration complaints, concerns associated with SCD, and informant confirmation of complaints. 13 , 14 It remains unknown whether these SCD‐specific enrichment characteristics for preclinical AD are associated with amyloid positivity across studies. Furthermore, the characteristics suggested for studies on SCD also included more general risk factors for amyloidosis such as older age, 15 , 16 apolipoprotein E gene (APOE) ε4 carriership, 15 and the presence of neuropsychiatric symptoms, 17 which may further influence the potential associations between SCD characteristics and amyloid positivity, as well as study setting. 6 , 18 Previous studies examining the association of characteristics of persons with SCD with amyloid positivity were performed within a single cohort 19 , 20 or focused on age and APOE ε4 carriership as characteristics of persons with SCD. 10 , 11
RESEARCH IN CONTEXT
Systematic review: Previous inconsistent findings on subjective cognitive decline (SCD) as a specific indicator of amyloid positivity, with some studies reporting an association of SCD with amyloid and others reporting no association, may be due to heterogeneity in the definition of SCD and specific complaints. Improved understanding of associations of SCD‐specific characteristics with amyloid positivity is needed.
Interpretation: We observed variability in the frequency of amyloid positivity between cohorts ranging from 10% to 76% when estimated at age 70, which was partly attributable to setting and apolipoprotein E gene (APOE) ε4 carriership. In addition to age, setting, and APOE‐ε4 carriership, we found associations between amyloid positivity and informant confirmation of complaints, memory complaints, attention/concentration complaints, feelings of worse performance, and depressive symptoms. These associations were found mainly in a research setting.
Future directions: Future research areas are longitudinal relationships of SCD characteristics with amyloid and other AD biomarkers, and associations with clinical progression.
HIGHLIGHTS
We examined subjective cognitive decline characteristics associated with amyloid.
Large variability exists in amyloid positivity between cohorts of persons with subjective cognitive decline (SCD).
Older age, memory clinic setting, and apolipoprotein E gene (APOE) ε4 carriership were related to amyloid positivity.
A number of SCD‐specific characteristics were also related to amyloid positivity.
SCD‐specific characteristics may facilitate identifying amyloid‐positive persons.
This study investigates which general and SCD‐specific characteristics are associated with amyloid positivity using individual participant‐level data from 20 cohorts included in the Amyloid Biomarker Study. 3 , 21 In addition, sources of heterogeneity in amyloid positivity between cohorts are examined.
2. METHODS
2.1. Participants
Participants were selected from the Amyloid Biomarker Study, an ongoing worldwide data‐pooling initiative that collects data from cohorts using amyloid biomarkers on PET or CSF. 3 , 21 The aim of the Amyloid Biomarker Study is to obtain a better understanding of the pathophysiology of AD. Study selection and data collection are described in Jansen et al. 3 For the current analyses, we included 1640 participants with SCD from 20 cohorts (Table 1). All participants experienced cognitive decline in the absence of objective decline on neuropsychological assessment, and each participant was classified as SCD either based on presentation to a memory clinic or upon assessment. In research settings, participants were typically classified as SCD based on an interview or questionnaire. In clinical settings, SCD was classified based on a clinical interview, a questionnaire, or a multidisciplinary consensus meeting (Supplemental Table 3). Characteristics of the included cohorts are displayed in Supplemental Table 1. Written informed consent to participate was obtained from all participants and data were de‐identified by the respective cohorts. Study protocols were approved by the respective local ethics committees.
TABLE 1.
Data availability for the different measures
| Measure | N individuals | N cohorts |
|---|---|---|
| Age | 1640 | 20 |
| Sex | 1640 | 20 |
| Education | 1481 | 20 |
| APOE ε4 carriership | 1354 | 16 |
| Setting | 1639 | 20 |
| Memory‐specific complaints | 1133 | 18 |
| Attention/concentration‐specific complaints | 688 | 11 |
| Informant confirmation of complaints | 693 | 12 |
| Concerns or worries about the complaints | 684 | 9 |
| Feelings of worse performance compared to others of the same age group | 1144 | 13 |
| Symptoms of depression | 1257 | 19 |
| Symptoms of anxiety | 1015 | 17 |
Abbreviation: APOE, apolipoprotein E gene.
2.2. Measures
2.2.1. Amyloid assessment
Our primary outcome measure was amyloid beta (Aß) deposition measured by PET or CSF biomarkers, dichotomized as normal and abnormal using study‐specific cut‐offs or upon visual read for PET (Supplemental Table 2). Amyloid positivity was assessed using amyloid‐PET in 499 participants (six cohorts) and using amyloid ß1‐42 level in CSF in 1141 participants (15 cohorts). When both PET and CSF amyloid measures were available, we selected the modality that resulted in the greatest number of participants for each cohort for the primary analyses.
2.2.2. AD risk factors and SCD‐specific characteristics
We examined risk factors previously associated with AD and SCD‐specific characteristics. The AD risk factors included age, sex, education, setting, presence of APOE ε4 genotype, and symptoms of depression and anxiety. SCD‐specific characteristics included 13 memory‐specific complaints, attention/concentration‐specific complaints, informant confirmation of complaints, concerns about the complaints, and feelings of worse cognitive performance compared to others of the same age group. Not all characteristics were available for all cohorts, as shown in Table 1. Education was dichotomized as lower or higher than the total sample median of 14 years. Setting was defined as clinical if patients presented with cognitive complaints at a health care facility or as research if participants were asked to participate in research and recruited through advertisements or from other departments within the health care facility. All SCD‐specific characteristics were dichotomized as present or absent. Supplemental Tables 3 and 4 show the definition of SCD and measurement details for each of these characteristics for the different cohorts.
2.3. Statistical analyses
Differences in AD risk factors and SCD‐specific characteristics between amyloid‐positive and amyloid‐negative participants were analyzed using independent samples t tests for continuous variables and χ2 tests for categorical variables. Generalized estimating equations (GEEs) were used to examine the combined individual participant‐level data from different cohorts, and GEEs allow for modeling non‐independencies in the data such as clustering of participants within cohorts. 21 , 22 , 23 A logit link function for binary outcome with an exchangeable correlation structure was assumed to account for within‐cohort correlation. First, we examined heterogeneity in amyloid‐positivity frequencies between the different cohorts. Second, we examined the association of each AD risk factor and SCD‐specific characteristic with the frequency of amyloid positivity in separate, uncorrected GEE analyses. Third, we combined all significant characteristics in one GEE model and examined the effect of the remaining characteristics by adding them one‐by‐one separately to the GEE model and by testing two‐way interaction effects with the significant characteristics from analysis 2. For any interaction effect with one of the significant characteristics from analysis 2, we calculated stratified mean predicted values for various levels of the individual characteristics (eg, for different ages, for APOE ε4 carriers vs non‐carriers, or for research vs clinical settings). Nonsignificant terms were removed step by step for each model based on the highest P‐values. Terms were retained in each model equation when the Wald statistic was significant (P < .05). Significant terms in each model are described in Supplemental Table 5. Additional analyses included examining discrepancies in amyloid positivity between PET and CSF measures and examining discrepancies between self‐ and informant‐reported complaints. All analyses were performed using SPSS statistical software, version 26 (IBM Corp).
3. RESULTS
We included 1640 participants with SCD (mean age 66.8 (SD 7.95) years), of which 863 (53%) were women and 363 (21%) were amyloid positive. This is comparable to the observed prevalence of amyloid positivity in 697 participants with SCD included in our previous study (22% in CSF and 23% in PET). 23 Table 2 shows sample characteristics according to amyloid status. Compared with amyloid‐negative persons, amyloid‐positive persons were older, more often CSF phosphorylated tau (p‐tau) positive, more often APOE ε4 carriers, more often recruited from a memory clinic setting, more often had attention/concentration complaints, and informants more often confirmed their complaints.
TABLE 2.
Sample characteristics according to amyloid status
| All | Amyloid negative | Amyloid positive | |
|---|---|---|---|
| N individuals | 1640 | 1277 | 363 |
| Age, y (SD) | 66.8 (7.95) | 66.1 (7.91) | 69.2 (7.61)** |
| Education, N (%) low (< 14 years) | 810/1481 (55) | 618/1144 (54) | 192/337 (57) |
| MMSE, N, mean (SD) | 1141, 28.6 (1.64) | 849, 28.6 (1.62) | 292, 28.6 (1.69) |
| Sex, N (%) women | 863/1640 (53) | 677/1277 (53) | 186/363 (51) |
| p‐tau positive, N (%) | 357/1093 (33) | 237/839 (28) | 120/254 (47)** |
| APOE ε4 carrier, N (%) | 464/1354 (34) | 303/1074 (28) | 161/208 (58)** |
| Setting, N (%) memory clinic | 1108/1639 (68) | 822/1276 (64) | 286/363 (79)** |
| Memory‐specific complaints, N (%) yes | 702/1133 (62) | 534/884 (60) | 168/249 (68) |
| Attention/concentration‐specific complaints, N (%) yes | 261/952 (38) | 193/553 (35) | 68/135 (50)* |
| Informant confirmation of complaints, N (%) yes | 377/693 (54) | 292/559 (52) | 85/134 (63)* |
| Concerns or worries about the complaints, N (%) yes | 492/684 (72) | 399/558 (72) | 93/126 (74) |
| Feelings of worse performance compared to others of the same age group, N (%) yes | 501/1144 (44) | 349/901 (44) | 107/243 (44) |
| Symptoms of depression, N (%) yes | 227/1257 (18) | 185/980 (19) | 42/277 (15) |
| Symptoms of anxiety, N (%) yes | 166/1015 (16) | 132/791 (17) | 34/224 (15) |
Note: N is displayed as N/total available N for each characteristic, % is the percentage within the available N.
SD, standard deviation; APOE, apolipoprotein E; p‐tau, phosphorylated tau.
P < .05 or.
P < .001 for difference between amyloid positive persons and amyloid‐negative persons. Data availability for the different measures is shown in Table 1.
3.1. Heterogeneity in amyloid positivity
Figure 1 demonstrates heterogeneity between cohorts in age‐related frequencies of amyloid positivity for persons with SCD. There was considerable heterogeneity, as mean amyloid positivity estimates at age 70 ranged from 10% to 76% (Supplemental Table 6).
FIGURE 1.
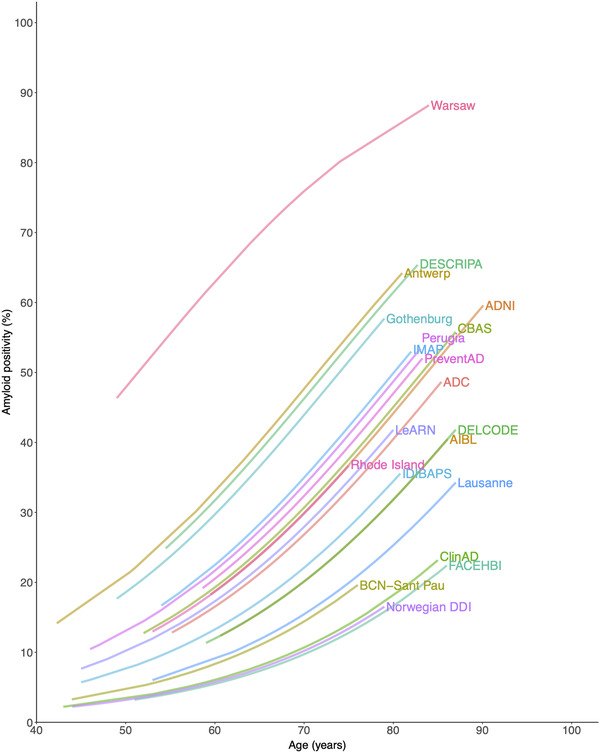
Heterogeneity in amyloid positivity between the included cohorts. Note: Prevalence of amyloid positivity is shown on the Y‐axis and age is shown on the X‐axis. Each line represents a different cohort. Characteristics of the different cohorts are described in Supplemental Table 1
3.2. Associations with amyloid positivity
In univariate analyses with AD risk factors, older age (18% at age 60, 27% at age 70, and 39% at age 80; P < .001), a memory clinic setting (28% vs 17% for research setting, P = .008), and APOE ε4 carriership (37% vs 16% for APOE ε4 non‐carriers, P < .001) were associated with higher frequencies of amyloid positivity, whereas sex, education, and symptoms of depression and anxiety were not (Figure 2, Supplemental Table 5, analysis 1 and analysis 2). None of the SCD‐specific characteristics were associated with amyloid positivity in these univariate analyses.
FIGURE 2.
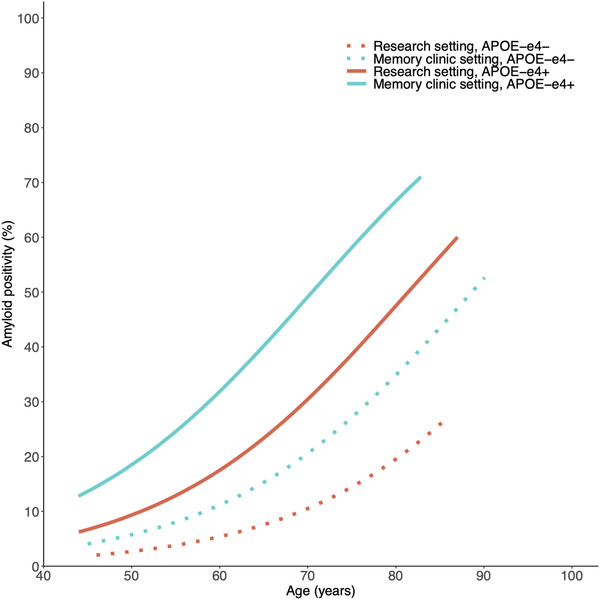
Amyloid positivity by age, setting, and APOE ε4 carriership. Note: Frequency of amyloid positivity for individuals with SCD is shown on the Y‐axis, and age is shown on the X‐axis. Each line represents a different combination of APOE ε4 carriership and setting. Number of participants included for each combination is shown in Supplemental Table 8
Because the AD risk factors age, setting, and APOE ε4 carriership were associated with amyloid positivity in the univariate analyses, we next performed multivariate analyses including age, setting, and APOE ε4 carriership, and we tested separately for each variable that was not significant in the univariate analyses whether they had an association with amyloid positivity or an interaction effect with one of these three factors. We found a higher frequency of amyloid positivity in persons with a lower education (28% at age 70, vs 23% for higher education, P < .001, Figure 3E and Supplemental Table 5, analysis 3).
FIGURE 3.
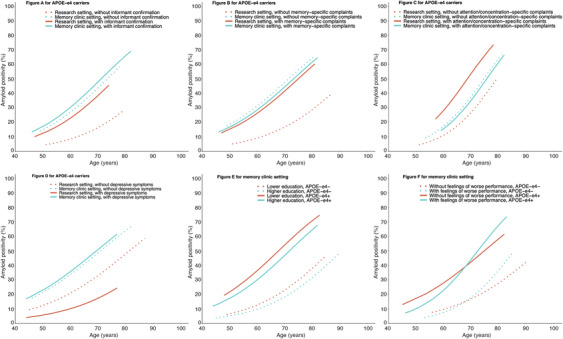
Amyloid positivity by age within APOE ε4 carriers (A‐D) according to different subgroups of setting and informant confirmation (3A), setting and memory complaints (3B), setting and attention/concentration complaints (3C), or setting and depression (3D); and amyloid positivity by age within a memory clinic setting according to different subgroups of APOE ε4 carriership and education (3E), or APOE ε4 and feelings of worse performance (3F). Note: (A–D) show interaction with setting, and (E and F) show interactions with APOE ε4 carriership or age. A was based on the model including age, setting, APOE ε4 carriership, and informant confirmation of complaints, and shows estimated amyloid positivity for APOE ε4 carriers only. (B) was based on the model, including age, setting, APOE ε4 carriership, and complaints specific to memory, and shows estimated amyloid positivity for APOE ε4 carriers only. (C) was based on the model, including age, setting, APOE ε4 carriership, and complaints specific to attention/concentration, and shows estimated amyloid positivity for APOE ε4 carriers only. (D) was based on the model including age, setting, APOE ε4 carriership, and depressive symptoms, and shows estimated amyloid positivity for APOE‐ε4 carriers only. (E) was based on the model including age, setting, APOE ε4 carriership, and education, and shows estimated amyloid positivity for a memory clinic setting only. (F) was based on the model including age, setting, APOE ε4 carriership, and feelings of worse performance, and shows estimated amyloid positivity for a memory clinic setting only. Results shown in (A–D) show a similar pattern for APOE ε4 non‐carriers, and results shown in (E and F) show a similar pattern for persons in a research setting (Supplemental Figure 1A‐1F). Data for each combination of characteristics are shown in Supplemental Table 7
In addition, the associations of informant confirmation of complaints, memory complaints, attention/concentration complaints, and depressive symptoms with amyloid positivity were dependent on setting (P < .001 for informant confirmation of complaints, P < .001 for memory complaints, P < .001 for attention/concentration complaints, and P = .035 for depressive symptoms, Supplemental Table 5, analysis 3). Within a research setting, amyloid positivity was higher if complaints were confirmed by an informant (21% at age 70) compared to those without confirmation (8%, Figure 3A and Supplemental Figure 1A), higher in persons with complaints specific to memory (25% at age 70) compared to persons without complaints specific to memory (9%, Figure 3B and Supplemental Figure 1B), higher in persons with complaints specific to attention/concentration (34% at age 70) compared to persons without complaints specific to attention/concentration (14%, Figure 3C and Supplemental Figure 1C), and higher in persons without depressive symptoms (17% at age 70) compared to persons with depressive symptoms (8%, Figure 3D and Supplemental Figure 1D). Within a memory clinic setting, informant confirmation of complaints, memory complaints, attention/concentration complaints, and depressive symptoms were not associated with amyloid positivity.
Furthermore, the influence of feelings of worse performance was dependent on age (P = .005). In younger persons, feelings of worse performance were associated with a lower frequency of amyloid positivity (at age of 50: 4% with feelings of worse performance, vs 7% without feelings of worse performance, P = .017), whereas in older persons, feelings of worse performance were associated with a higher frequency of amyloid positivity (at age 90: 68% with feelings of worse performance, vs 50% without feelings of worse performance, P = .021, Figure 3F and Supplemental Figure 1F). No associations with amyloid positivity were found of sex, anxiety, and concerns about the complaints.
The number of participants for which data were available for each combination of characteristics is shown in Supplemental Table 7. Figure 4 is a heat map showing the estimated frequency of amyloid positivity based on combinations of characteristics (associated confidence intervals are displayed in Supplemental Table 8). The heat map visualizing the frequency of amyloid positivity by age, setting, APOE ε4 carriership, and other SCD characteristics may assist interpretation of our findings. For example, in 70‐year‐olds, the frequency of amyloid positivity was lowest in APOE ε4 non‐carriers in a research setting without informant confirmation of complaints (3%) and highest in APOE ε4 carriers in a memory clinic setting with lower educational attainment (54%). Although differences were relatively small compared with the effects of age, setting, and APOE ε4 carriership, the estimates differed up to 31% for persons with versus without specific SCD characteristics with similar age, setting, and APOE ε4 carriership.
FIGURE 4.
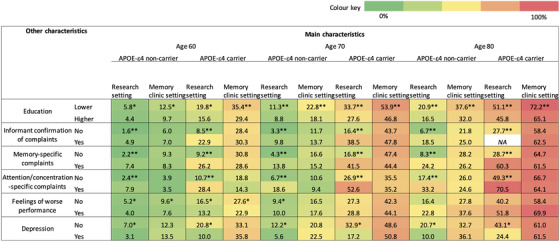
Heat map showing the mean predicted amyloid positivity by age, setting, APOE ε4 carriership, and significant characteristics from analysis 3. Note: Green colors indicate lower frequencies of amyloid positivity, and red colors indicate higher frequencies of amyloid positivity. Table columns contain the main characteristics age, setting, and APOE ε4 carriership, and rows contain additional characteristics that were associated with lower or higher frequency of amyloid positivity. Results are displayed for age 60, 70, and 80. Some of the combinations of characteristics were not available for persons age60, 70, or 80 and a slightly younger or older age was selected in 9% of the combinations (range from 3 years younger to 1 year older). *P < .05 or **P < .001 for difference between row characteristic (lower education vs higher education, without vs with informant confirmation of complaints, without vs with complaints specific to memory, without vs with complaints specific to attention/concentration, without vs with feelings of worse performance, without vs with depressive symptoms, respectively).
3.3. Sensitivity analyses
3.3.1. Heterogeneity when taking into account age, setting, and APOE ε4 carriership
Supplemental Figure 2 demonstrates heterogeneity in amyloid‐positivity frequencies between the included cohorts, when taking into account age, setting, and APOE ε4 carriership. Variability in amyloid positivity between cohorts was larger in cohorts from a memory clinic setting than from a research setting (estimates at age 70 ranged from 7% to 73% for APOE ε4 non‐carriers in a memory clinic setting [11 cohorts] vs 2% to 19% for APOE ε4 non‐carriers in a research setting [6 cohorts]) and ranged from 23% to 91% for APOE ε4 carriers in a memory clinic setting [11 cohorts] vs 6% to 45% for APOE ε4 carriers in a research setting [6 cohorts]).
3.3.2. Influence of amyloid biomarker modality
No difference was found in the in age‐related frequency of amyloid positivity by biomarker modality. The frequency of amyloid positivity at age 70 was 26% (20% to 33% confidence interval) based on PET, and 28% (22% to 35% confidence interval, P = .556) based on CSF. Sample sizes did not allow examining the association of each characteristic with amyloid positivity based on PET or CSF separately.
3.3.3. Discrepancy between self‐reported and informant‐reported complaints
Discrepancy between self‐reported memory complaints and informant confirmation of complaints occurred in 170 individuals (reported in 546 participants from nine cohorts), but was not associated with amyloid positivity in the model, including age, setting, and APOE ε4 carriership (16% for discrepancy vs 15% for no discrepancy, P = .443).
4. DISCUSSION
The present study examined the association of different AD risk factors and SCD‐specific characteristics with amyloid positivity in a large sample of 1640 participants with SCD from 20 cohorts. We found that in individuals with SCD, only older age, a memory clinic setting, and APOE ε4 carriership were univariately associated with amyloid positivity, and none of the SCD‐specific characteristics. When adjusting for these three factors, lower education was associated with increased amyloid positivity. The associations of the SCD‐specific characteristics informant confirmation of complaints, memory complaints, attention/concentration complaints, and depressive symptoms with amyloid positivity were dependent on setting and were found within a research setting only. The association of feelings of worse performance depended on age: there was a higher frequency of amyloid positivity at older ages and a lower frequency at lower ages.
We noted a large variability in the frequency of amyloid positivity between cohorts ranging from 10% to 76% when estimated at age 70. This indicates that SCD indeed is a heterogeneous concept and the concept itself does not seem to be a predictor of amyloid positivity. We found that part of this heterogeneity can be attributed to age, setting, and APOE ε4 carriership. Older age, a memory clinic setting, and APOE ε4 carriership were associated with a higher frequency of amyloid positivity, which is in line with previous studies. 6 , 15
When adjusting for age, setting, and APOE ε4 carriership, we found that higher education was associated with a lower frequency of amyloid positivity, which is in accordance with a lower risk for AD‐type dementia in persons with a higher educational attainment but conflicts with the general explanation that education lowers the risk of developing dementia by compensation of the effects of brain pathology. 24 , 25 In our previous study, 3 we reported an increased frequency of amyloid positivity with higher education in persons with normal cognition, but we did not separately examine associations of education with amyloid positivity in persons with SCD. Furthermore, higher educational attainment was more frequent in the group of persons without SCD (64% >14 years) compared to our present sample of persons with SCD (45% >14 years), and none of the participants with normal cognition were from a memory clinic setting.
Some SCD‐specific characteristics were associated with amyloid positivity only in a research setting. Memory complaints as opposed to complaints in non‐memory domains, attention/concentration‐specific complaints, and informant confirmation of complaints were indicative of amyloid positivity in a research setting only. Although previous studies that were conducted within a single research or clinical setting reported associations of memory complaints 8 , 20 , 26 and informant confirmation of complaints 20 , 27 with amyloid positivity, we did not observe such associations in our analyses. Possibly the lack of discriminative value of these characteristics in a memory clinic setting results from the fact that memory complaints and informant confirmation are common in this setting (Supplemental Table 7). Moreover, a previous single‐center study did not find an association of attention/concentration complaints with amyloid positivity, whereas we did observe an association. 28 Because the single‐center study corrected for age, sex, education, and depressive symptoms, direct comparisons are difficult. Furthermore, we found that depressive symptoms were associated with a lower frequency of amyloid positivity within a research setting only. Affective disorders are commonly associated with SCD, 13 but affective symptoms have also been associated with incident AD‐type dementia in at‐risk individuals. 29 The risk of underlying pathophysiology is apparently partly associated with setting and may be related to medical help‐seeking. 30
Of interest, the presence of feelings of worse performance was associated with a higher frequency of amyloid positivity in older persons only. In younger persons, feelings of worse performance might be due to functional cognitive disorders 31 rather than underlying AD pathology. Similar results were described in reviews by Jonker et al., 32 who found that in younger persons with memory complaints that these complaints generally refer to neuropsychiatric symptoms or personality factors, and Rabin et al., 33 who also found that underlying causes of complaints vary with age.
Overall, effect sizes of education, informant confirmation of complaints, memory complaints, attention/concentration complaints, depressive symptoms, and feelings of worse performance were relatively small and of limited added value in explaining heterogeneity in amyloid positivity in persons with SCD next to age, setting, and APOE ε4 carriership, which showed the strongest effects in our analyses. Most associations of SCD‐specific characteristics with amyloid positivity were only seen in a research setting, implicating potential value within research settings. The lack of associations with amyloid positivity of these SCD‐specific characteristics in clinical settings could mean that they are of less value within clinical settings.
In the present study, we combined data collected within a large number of cohorts in different settings to examine associations between AD risk factors and SCD characteristics and amyloid positivity. A limitation of our study is that most cohorts only collected data on a subset of characteristics of the subjective complaints. This may be explained by the fact that most cohorts started data collection before the introduction of the SCD research criteria and that collection of these characteristics has not been fully implemented in clinical and research practice. Therefore, a limitation inherent to our approach is that each analysis included a different sample and that statistical power was relatively small for a number of characteristics. A limitation inherent to combining data from multiple studies is the use of different assessment methods to define each characteristic. Although all assessment methods are transparently shown in Supplemental Table 3, caution is warranted in comparing studies. However, despite considerable heterogeneity, associations were found across the different cohorts. Examining the influence of assessment method (clinical interview vs scale) for the different SCD characteristics was not possible due to the used definitions for SCD characteristics: most studies defined the presence of specific characteristics based on one item from a questionnaire or based on a specific question within a structured interview. Furthermore, sample sizes did not allow analyses stratified by modality, and potential influence of amyloid modality on the associations reported in the present study cannot be excluded. However, no main effect of modality was found in univariate analyses. Finally, the cross‐sectional nature of the present study does not allow assessment of stability of the SCD characteristics over time.
5. CONCLUSIONS AND IMPLICATIONS
Our findings show that next to age, setting, and APOE ε4 carriership, other characteristics in the context of SCD related to amyloid pathology are education, informant confirmation of complaints, memory complaints, attention/concentration complaints, feelings of worse performance, and depression, but mostly within a research setting only. Our findings show that enrichment characteristics for preclinical AD proposed in the SCD research criteria 13 indeed are associated with amyloid pathology, but these associations often depend on age and setting. Our study also suggests that education is an additional characteristic important to consider in studies on SCD. Inclusion of practical, low‐cost AD risk factors, and SCD‐specific characteristics aids in the identification of individuals that will likely benefit from disease‐modifying treatment. However, the effect sizes of the SCD‐specific characteristics were relatively small and of limited added value next to age, APOE ε4 carriership, and across settings. Because it has been suggested previously that consistency over time of SCD characteristics is associated with a greater risk of future decline, 14 , 34 the longitudinal relationship between SCD characteristics with amyloid and other AD biomarkers, as well as associations with clinical progression are important areas for future research. Future research might also examine this longitudinal relationship in individuals with evidence of both amyloidosis and tauopathy, since these individuals might be at increased risk of clinical progression.
The Amyloid Biomarker Study was supported by Biogen, which provided financial support for the conduct of the research. Biogen was not involved in the collection, analysis, and interpretation of data, or in the writing of the report or the decision to submit the article for publication.
CONFLICT OF INTEREST
Willemijn Jansen reports receiving research support from Biogen.
Olin Janssen reports receiving research support from Biogen.
Stephanie Vos reports receiving a personal grant from ZonMw, and research support from Alzheimer Nederland and Stichting Rinsum Ponssen.
Merce Boada reports receiving research support from Grifols and the Instituto de Salud Carlos III (ISCIII) grant; receiving consulting fees; and participating on Data Safety Monitoring Board or Advisory Board from Biogen, Roche, Merck, Zambon, Cortexyme.
Lucilla Parnetti reports receiving funds for clinical trials from INDENA S.P.A., Biogen, EISAI Co., Ltd.; payments were made to University of Perugia, Department of Medicine and Surgery. ‐ From 2019: MIRIADE funded under Marie Skłodowska‐Curie actions by the European Union's Horizon 2020 research and innovation programme. Payments were made to University of Perugia. From 2019: bPRIDE project funded by the European Joint Programming on Neurodegenerative Diseases (JPND, European Union's Horizon 2020 research and innovation programme). Payments were made to University of Perugia; is member of the Editorial Board of the following journals (unpaid): • Neurology • Movement Disorders • Biomolecules • Scientific Reports • Frontiers in Aging Neuroscience • Cells • Journal of Alzheimer Disease • Biomarker Insights • Journal of Integrative Neuroscience Lucilla Parnetti is member of the following societies (unpaid): ‐ Since 2019: Member of the Fresco Network for Parkinson's disease. ‐ Since 2020: Member of the Research Council of Norway, Member of the Icelandic Centre for Research, Coordinator of a Center of Excellence of the Parkinson Foundation; and received Lumipulse kits from Fujirebio and ELISA kits from Euroimmun (2021)
Tormod Fladby reports receiving research support for the present manuscript from European Union (EU)/Joint Programming on Neurodegenerative Diseases (JPND) and The Norwegian Research Council; receiving consulting fees from Biogen and Novo Nordisk; having several planned, issued, and pending patents regarding innate immune amyloid beta clearance and regulation of inflammation; participating on Data Safety Monitoring Board or Advisory Board of Biogen, Novo, and Nordisk; and is leader of the board of Nansen Neuroscience (unpaid).
José Luis Molinuevo is currently a full‐time employee of Lundbeck and earlier has served as a consultant or at advisory boards for the following for‐profit companies, or has given lectures in symposia sponsored by the following for‐profit companies: Roche Diagnostics, Genentech, Novartis, Lundbeck, Oryzon, Biogen, Lilly, Janssen, Green Valley, MSD, Eisai, Alector, BioCross, GE Healthcare, ProMIS Neurosciences, NovoNordisk, Zambón, Cytox, and Nutricia.
Sylvia Villeneuve reports receiving research support from Canadian Institutes of Health Research (CIHR).
Jakub Hort reports research support from Czech Ministry of Health and honoraria for lectures by Schwabe and Lundbeck, as well as travel grant from Czech grant agency and participating in a Data Safety Monitoring Board or Advisory Board from Axon company and having stock options in Alzheon company.
Stéphane Epelbaum reports research support from Fondation Recherche Alzheimer and consulting fees from Biogen and Roche, honoraria for presentation from Eisai, and travel support from Edimark.
Alberto Lleó reports research support from Instituto de Carlos III: Fondo de Investigación Sanitario (PI17:01896; PI20/01330; AC19:00103); payment to the institution Instituto de Carlos III: CIBERNED (COEN); payment to the institution, patent royalties (personal and institutional payments); personal consulting fees from Biogen, Nutricia, Roche, and Fujirebio‐Europe; personal fees from Biogen and Nutricia for lectures or educational programs; and patent WO2019175379 A1 Markers of synaptopathy in neurodegenerative disease issued.
Sebastiaan Engelborghs reports participating in Data Safety Monitoring Boards or Advisory Boards of Biogen, Eisai, Icometrix, Novartis, Pfizer, and Roche.
Wiesje M van der Flier reports receiving research funding from ZonMW, NWO, EU‐FP7, EU‐JPND, Alzheimer Nederland, CardioVascular Onderzoek Nederland, Health∼Holland, Topsector Life Sciences & Health, stichting Dioraphte, Gieskes‐Strijbis fonds, stichting Equilibrio, Pasman stichting, Biogen MA Inc, Boehringer Ingelheim, Life‐MI, AVID, Roche BV, Fujifilm, and Combinostics. WF holds the Pasman chair. WF has performed contract research for Biogen MA Inc and Boehringer Ingelheim. WF has been an invited speaker at Boehringer Ingelheim, Biogen MA Inc, Danone, Eisai, WebMD Neurology (Medscape). All funding is paid to her institution; and she is consultant to Oxford Health Policy Forum CIC, Roche, and Biogen MA Inc, and is associate editor at Alzheimer's, Research & Therapy.
Susan Landau reports receiving support for the present manuscript from NIH U19 AG024904 (PI: Weiner), funds to the institution (UC Berkeley); honorarium for speaking at Hillblom Symposium UC San Francisco 2019; travel fees paid for attending meetings and conferences as member of Scientific Program Committee for the Alzheimer's Association International Conference 2017‐2019; and participating in Advisory Board for KeifeRx.
Julius Popp reports receiving support from Synapsis Foundation Alzheimer Swiss (to institution; Swiss National Research foundation (to institution); Om Pharma Switzerland (to institution), and receiving consulting fees from Om pharma Switzerland (to institution).
Anders Walling reports receiving payment for expert testimony from Lundbeck lecture.
Philip Scheltens reports receiving research support from ZonMW and Alzheimer Nederland and has received consultancy fees (paid to the institution) from AC Immune, Alkermes, Alnylam, Alzheon, Anavex, Biogen, Brainstorm Cell, Cortexyme, Denali, EIP, ImmunoBrain Checkpoint, GemVax, Genentech, Green Valley, Novartis, Novo Nordisk, PeopleBio, Renew LLC, and Roche. He is PI of studies with AC Immune, CogRx, FUJI‐film/Toyama, IONIS, UCB, and Vivoryon. He is a part‐time employee of Life Sciences Partners Amsterdam. He serves on the board of Brain Research Center and New Amsterdam Pharma, and participated on the Data Safety Monitoring Board of Genentech.
Peter J. Snyder is a consultant to AlzeCure Pharma (Sweden).
Chris Rowe reports Cerveau Technologies grant paid to institution Biogen grant paid to institution, Abbvie grant paid to institution, consulting fees from Cerveau Technologies, educational materials preparation payments from Biogen, participating in Cerveau Technologies Scientific Advisory Board, and receiving salary payment from Australian Dementia Network.
Gaël Chételat has received research support from the European Union's Horizon 2020 research and innovation program (grant agreement No. 667696), Inserm, Fondation d'entreprise MMA des Entrepreneurs du Futur, Fondation Alzheimer, Programme Hospitalier de Recherche Clinique, Région Normandie, Association France Alzheimer et maladies apparentées, and Fondation Vaincre Alzheimer (all to Inserm); and personal fees from Fondation d'entreprise MMA des Entrepreneurs du Futur. No stock options, patents, or royalties.
Agustin Ruíz receives research support from the Innovative Medicines Initiative 2 Joint Undertaking, which receives support from the European Union's Horizon 2020 research and innovation programme (ADAPTED Grant No. 115975). AR's research is also supported by Instituto de Salud Carlos III (ISCIII) grants PI16/01861 and PI19/01301. Acción Estratégica en Salud, integrated in the Spanish National R+D+I Plan and funded by ISCIII‐Subdirección General de Evaluación and the Fondo Europeo de Desarrollo Regional (FEDER‐“Una manera de Hacer Europa) and JPco‐fuND‐2 “Multinational research projects on Personalised Medicine for Neurodegerative Diseases” PREADAPT project, and reports consulting fees from Landsteiner Genmed, Grifols, Araclon biotech and lecture fees from Araclon Biotech; has EU Patent EP21382305.7; and participated on Data Safety Monitoring Board or Advisory Board of Grifols SA and Landsteiner Genmed.
Marta Marquié receives research support from the Instituto de Salud Carlos III (ISCIII) grant PI19/00335 Acción Estratégica en Salud, integrated in the Spanish National R+D+I Plan and financed by ISCIII‐Subdirección General de Evaluación and the Fondo Europeo de Desarrollo Regional (FEDER‐“Una manera de Hacer Europa).
PI17/01474 Acción Estratégica en Salud, integrated in the Spanish National R+D+I Plan and financed by ISCIII‐Subdirección General de Evaluación and the Fondo Europeo de Desarrollo Regional (FEDER‐Una manera de hacer Europa), and reports consulting fees from Grifols, Araclon, Biogen, Roche. Lilly, Schwabe, Cortexyme, Merck and Nutricia.
Henning Boecker received 2 x Promotionsstipendium, University Clinic Bonn, payments were made to institution, and receiving travel support from 2019: Sino‐German Center for research promotion, co‐funded by both DFG and National Science Foundation of China (NSFC) ‐ payments were made to institution (travel reimbursement).
Oliver Peters reports receiving institutional grants from Astra Zeneca, Novartis, Biogen, Genentech, Roche, Eisai, and Fujifilm; and received Consulting fees from Biogen, Roche, Eisai, Abbvie, and Griffols, and received payment or honoraria for lectures from Schwabe, MSD.
Lorena Rami reports research Grant from Spanish government to IDIBAPS (PI Lorena Rami) grant no. PI19/00745.
Alexa Pichet Binette reports Human Amyloid Imaging conference: payment made to me Alzheimer's Association: payments made directly to waive conference fees.
Judes Poirier reports support provided by the Fonds de la Recherche en Santé du Québec and the JL Levesque Foundation and has served at the scientific advisory board of the CIHR and Alzheimer Foundation of France.
Jiri Cerman reports receiving research support from Charles University, and consulting fees from Biogen, as well as participating on Data Safety Monitory Board or Advisory Board of Biogen.
Bruno Dubois received consulting fees from Biogen and research grants for his institution from Roche and Fondation Merck‐Avenir and received Equipment SIMOA paid by Lions Alzheimer.
Daniele Altomare received research support from Institute of Health Carlos III (ISCIII), Spain research grants PI18/00435 and INT19/00016 to DA Department of Health Generalitat de Catalunya PERIS research program SLT006/17/125 to DA; consulting fees for his participation in advisory boards from Fujirebio‐Europe and Roche Diagnostics; speaker honoraria from Fujirebio‐Europe, Roche Diagnostics, Nutricia, Krka Farmacéutica S.L., Zambon S.A.U. and Esteve Pharmaceuticals S.A.; and support for attending meetings from Nutricia.
Juan Fortea reports funding from Fondo de Investigaciones Sanitario (FIS), Instituto de Salud Carlos III, to my institution; National Institutes of Health, to institution; Fundació La Marató de TV3, to my institution; Generalitat de Catalunya, to institution; Fundació Catalana Síndrome de Down, to institution; and ‐Fundació Víctor Grífols i Lucas; and has served as a consultant for Novartis and Lundbeck, has received honoraria for lectures from Roche, NovoNordisk, Esteve, and Biogen; has a patent WO2019175379 A1 Markers of synaptopathy in neurodegenerative disease issued; and has served at advisory boards for AC Immune, Zambon, and Lundbeck, as well as institution received support from AC Immune to acquire Quanterix NfL Kits.
Marie Eckerström reports receiving consulting fees from Medavante‐prophase, payment for on‐demand independent reviewer assignments, and payment for lectures from Department of Psychology, University of Gothenburg.
Louisa Thompson reports receiving grant AACSF‐20‐685786 Alzheimer's Association 5/01/2020 – 4/31/2023 payments made to Brown University, and payment or honoraria for lectures from Sage Bionetworks payments.
Victor Villemagne reports receiving consulting fees and payment or honoraria for lectures from IXICO Hospicom ACE Alzheimer's Center.
Rachel Buckley reports research support from NIH‐NIA K99/R00 Pathway to Independence award Alzheimer's Association Research Fellowship and Alzheimer's Association International Conference travel fellowship (2019).
Samantha Burnham reports receiving research support from grants NHMRC GNT1161706, NHMRC GNT1156891, NHMRC GNT1191535, NHMRC GNT2001320, and NHMRC MRFF117 1338.
Marion Delarue reports wages for my work in Chetelat's lab (U1237‐PhIND) at Caen paid by INSERM or University of Caen Normandy (since July 2017); wages for my work in LPCN‐EA7452 at University of Caen Normandy paid by University from IReSP fundings (from January 2021); Punctual Sessions at IFRES (from 2015), paid by "Association Pierre Noal."
Inez Ramakers reports research support from Janssen Pharmaceuticals > to institution and EIT Health > to institution.
Hilkka Soininen reports grants from the Academy of Finland, European Union, and Danone which were paid to institution; as well as personal consultation fee from Novo Nordisk; personal payment for membership of a Data Safety Monitoring Board, of ACImmune. Se also reports having been the head of the board of Muistiliitto, which is the Finnish Alzheimer Association from 2018‐2020 for which no payment was received.
Harald Hampel is an employee of Eisai Inc. and serves as Senior Associate Editor for the journal Alzheimer's & Dementia and does not receive any fees or honoraria since May 2019. Before May 2019 he had received lecture fees from Servier, Biogen, and Roche; research grants from Pfizer, Avid, and MSD Avenir (paid to the institution); travel funding from Eisai, Functional Neuromodulation, Axovant, Eli Lilly and company, Takeda and Zinfandel, GE‐Healthcare, and Oryzon Genomics; consultancy fees from Qynapse, Jung Diagnostics, Cytox Ltd., Axovant, Anavex, Takeda and Zinfandel, GE Healthcare, Oryzon Genomics, and Functional Neuromodulation; and participated in scientific advisory boards of Functional Neuromodulation, Axovant, Eisai, Eli Lilly and company, Cytox Ltd., GE Healthcare, Takeda and Zinfandel, Oryzon Genomics, and Roche Diagnostics. He is co‐inventor in the following patents as a scientific expert and has received no royalties: In Vitro Multiparameter Determination Method for The Diagnosis and Early Diagnosis of Neurodegenerative Disorders Patent Number: 8916388; In Vitro Procedure for Diagnosis and Early Diagnosis of Neurodegenerative Diseases Patent Number: 8298784; Neurodegenerative Markers for Psychiatric Conditions Publication Number: 20120196300; In Vitro Multiparameter Determination Method for The Diagnosis and Early Diagnosis of Neurodegenerative Disorders Publication Number: 20100062463; In Vitro Method for The Diagnosis and Early Diagnosis of Neurodegenerative Disorders Publication Number: 20100035286; In Vitro Procedure for Diagnosis and Early Diagnosis of Neurodegenerative Diseases Publication Number: 20090263822; In Vitro Method for The Diagnosis of Neurodegenerative Diseases Patent Number: 7547553; CSF Diagnostic in Vitro Method for Diagnosis of Dementias and Neuroinflammatory Diseases Publication Number: 20080206797; In Vitro Method for The Diagnosis of Neurodegenerative Diseases Publication Number: 20080199966; Neurodegenerative Markers for Psychiatric Conditions Publication Number: 20080131921. This work has been performed during his previous position at Sorbonne University, Paris, France. At Sorbonne University he was supported by the AXA Research Fund, the “Fondation partenariale Sorbonne Université” and the “Fondation pour la Recherche sur Alzheimer,” Paris, France.
Betty M. Tijms is inventor on patent on biological subtypes in AD, No. PCT/NL2020/050216 (owner stichting VUmc).
Frans Verhey reports receiving research support from Profile, EMR Interreg DISTINCT H2020 All to our institution.
Frank Jessen reports receiving research support for the present manuscript from German Center for Neurodegenerative Disorders (DZNE); personal fees for lectures from Lilly and Roche; and personal fees for participating on Data Safety Monitoring Boards or Advisory Boards of Abbvie, AC Immune, Biogen, Böhringer, Danone, Eisai, GE Healthcare, Green Valley, hummingbird, Janssen, MSDOM Pharma, Roche, and Vifer; Executive committee German Psychiatric Association (DGPPN) Vice chair European Alzheimer's Disease Consortium (EADC).
Pieter Jelle Visser reports receiving research support from ZonMW (Redefining AD), from ZonMW (Netherlands Consortium of Dementia Cohorts (NCDC), from Innovative Medicine Initiatives (IMI) (Amypad), from IMI (RADAR‐AD), and from Biogen (Amyloid biomarker study group). All payment were made to institution. He also reports receiving payment for Workshop grant writing organized by Synapsis; patent PCT/NL2020/050216, and is Senior editor Alzheimer Research and Therapy and Biomed Central (BMC) Geriatrics and a Member of the Executive board of European Alzheimer's Disease Cohorts (EADC).
Tomasz Gabryelewicz, Marcel Olde Rikkert, Elena Chipi, Steffen Wolfsgruber, Michael Heneka, Jonas Jarholm, Adrià Tort‐Merino, Pedro Rosa‐Neto, Jiri Cerman, Marc Teichmann, M. Belén Sánchez‐Saudinós, Jarith Ebenau, Cornelia Pocnet, Yvonne Freund‐Levi, Åsa K. Wallin, Magda Tsolaki, Luiza Spiru, and Rik Ossenkoppele have nothing to disclose.
Supporting information
Supporting Information
ACKNOWLEDGEMENTS
*A proportion of the data used in preparation of this article was obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data, but they did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp‐content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
The ADNI was launched in 2003 as a public‐private partnership led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early Alzheimer's disease (AD).
Data collection and sharing for this project was funded by the ADNI (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH‐12‐2‐0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol‐Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann‐La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. A proportion of data used in preparation of this article were obtained from the Pre‐symptomatic Evaluation of Novel or Experimental Treatments for Alzheimer's Disease (PREVENT‐AD) program (htpp://douglas.research.mcgill.ca/stop‐ad‐centre), data release 5.0 (November 30, 2017). As such, the investigators of the PREVENT‐AD program contributed to the design and implementation of PREVENT‐AD and/or provided data but did not participate in analysis or writing of this report. A complete listing of PREVENT‐AD Research Group can be find in the PREVENT‐AD database: http://preventad.loris.ca/acknowledgements/acknowledgements.php?date = [2020 = 04‐01].
The present study was supported by European Medical Information Framework (EMIF)‐AD. The EMIF‐AD project has received support from the Innovative Medicines Initiative Joint Undertaking under EMIF grant agreement no. 115372, resources of which are composed of financial contribution from the European Union's Seventh Framework Program (FP7/2007‐2013) and EFPIA companies’ in kind contribution.
The Fundació ACE Healthy Brain Initaitive (FACEHBI) study was supported by funds from Fundació ACE Institut Català de Neurociències Aplicades, Grifols, Life Molecular Imaging, Araclon Biotech, Alkahest, Laboratorio de análisis Echevarne and IrsiCaixa.
Research of Alzheimer Centre Amsterdam is part of the neurodegeneration research program of Amsterdam Neuroscience. Alzheimer Centre Amsterdam is supported by Stichting Alzheimer Nederland and Stichting VUmc fonds. The SCIENCe project is supported by research grants from Gieskes‐Strijbis fonds and stichting Dioraphte. Wiesje van der Flier holds the Pasman chair.
The Sant Pau Memory Unit has received funding from Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas (CIBERNED); Instituto de Salud Carlos III; jointly funded by Fondo Europeo de Desarrollo Regional (FEDER), Unión Europea, “Una manera de hacer Europa”; Generalitat de Catalunya; Fundació "La Marató TV3” Fundació Bancària Obra Social La Caixa; Fundación BBVA; Fundación Española para el Fomento de la Investigación de la Esclerosis Lateral Amiotrófica (FUNDELA); Global Brain Health Institute; Fundació Catalana Síndrome de Down; and Fundació Víctor Grífols i Lucas.
The Imagerie Multimodale de la maladie d'Alzheimer a un stade Precoce (IMAP) study (G Chételat, Caen) was supported by the Programme Hospitalier de Recherche Clinique (PHRCN 2011‐A01493‐38 and PHRCN 2012 12‐006‐0347), the Agence Nationale de la Recherche (ANR LONGVIE 2007), Fondation Plan Alzheimer (Alzheimer Plan 2008–2012), Association France Alzheimer et maladies apparentées AAP 2013, the Région Basse Normandie and the Institut National de la Santé et de la Recherche Médicale (INSERM).
A proportion of data used in preparation of this article were obtained from the LeARN study and prepared by Ron Handels. The LeARN study was performed within the framework of CTMM, the Center for Translational Molecular Medicine, a Dutch public‐private partnership; project LeARN (grant 02 N‐101).
Janssen O, Jansen WJ, Vos SJB, et al. Characteristics of subjective cognitive decline associated with amyloid positivity. Alzheimer's Dement. 2022;18:1832–1845. 10.1002/alz.12512
REFERENCES
- 1. Morris JC. Early‐stage and preclinical Alzheimer disease. Alzheimer Dis Assoc Disord. 2005;19:163‐165. [DOI] [PubMed] [Google Scholar]
- 2. Vos SJ, Xiong C, Visser PJ, et al. Preclinical Alzheimer's disease and its outcome: a longitudinal cohort study. Lancet Neurol. 2013;12:957‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jansen WJ, Ossenkoppele R, Knol DL, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta‐analysis. JAMA. 2015;313:1924‐1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ebenau JL, Timmers T, Wesselman LMP, et al. ATN classification and clinical progression in subjective cognitive decline: the SCIENCe project. Neurology. 2020;95:e46‐e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta‐analysis. Acta Psychiatr Scand. 2014;130:439‐451. [DOI] [PubMed] [Google Scholar]
- 6. Slot RER, Sikkes SAM, Berkhof J, et al. Subjective cognitive decline and rates of incident Alzheimer's disease and non‐Alzheimer's disease dementia. Alzheimers Dement. 2019;15:465‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stewart R. Subjective cognitive impairment. Curr Opin Psychiatry. 2012;25:445‐450. [DOI] [PubMed] [Google Scholar]
- 8. Amariglio RE, Becker JA, Carmasin J, et al. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia. 2012;50:2880‐2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Perrotin A, Mormino EC, Madison CM, Hayenga AO, Jagust WJ. Subjective cognition and amyloid deposition imaging: a Pittsburgh Compound B positron emission tomography study in normal elderly individuals. Arch Neurol. 2012;69:223‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buckley RF, Sikkes S, Villemagne VL, et al. Using subjective cognitive decline to identify high global amyloid in community‐based samples: a cross‐cohort study. Alzheimers Dement (Amst). 2019;11:670‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mielke MM, Wiste HJ, Weigand SD, et al. Indicators of amyloid burden in a population‐based study of cognitively normal elderly. Neurology. 2012;79:1570‐1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chetelat G, Villemagne VL, Bourgeat P, et al. Relationship between atrophy and beta‐amyloid deposition in Alzheimer disease. Ann Neurol. 2010;67:317‐324. [DOI] [PubMed] [Google Scholar]
- 13. Jessen F, Amariglio RE, van Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10:844‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jessen F, Amariglio RE, Buckley RF, et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2020;19:271‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Risacher SL, Kim S, Nho K, et al. APOE effect on Alzheimer's disease biomarkers in older adults with significant memory concern. Alzheimers Dement. 2015;11:1417‐1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buckley RF, Mormino EC, Amariglio RE, et al. Sex, amyloid, and APOE epsilon4 and risk of cognitive decline in preclinical Alzheimer's disease: findings from three well‐characterized cohorts. Alzheimers Dement. 2018;14:1193‐1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jorm AF, Butterworth P, Anstey KJ, et al. Memory complaints in a community sample aged 60‐64 years: associations with cognitive functioning, psychiatric symptoms, medical conditions, APOE genotype, hippocampus and amygdala volumes, and white‐matter hyperintensities. Psychol Med. 2004;34:1495‐1506. [DOI] [PubMed] [Google Scholar]
- 18. Perrotin A, La Joie R, de La Sayette V, et al. Subjective cognitive decline in cognitively normal elders from the community or from a memory clinic: differential affective and imaging correlates. Alzheimers Dement. 2017;13:550‐560. [DOI] [PubMed] [Google Scholar]
- 19. Slot RER, Verfaillie SCJ, Overbeek JM, et al. Subjective Cognitive Impairment Cohort (SCIENCe): study design and first results. Alzheimers Res Ther. 2018;10:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miebach L, Wolfsgruber S, Polcher A, et al. Which features of subjective cognitive decline are related to amyloid pathology? Findings from the DELCODE study. Alzheimers Res Ther. 2019;11:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ossenkoppele R, Jansen WJ, Rabinovici GD, et al. Prevalence of amyloid PET positivity in dementia syndromes: a meta‐analysis. JAMA. 2015;313:1939‐1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hubbard AE, Ahern J, Fleischer NL, et al. To GEE or not to GEE: comparing population average and mixed models for estimating the associations between neighborhood risk factors and health. Epidemiology. 2010;21:467‐474. [DOI] [PubMed] [Google Scholar]
- 23. Jansen WJ, Ossenkoppele R, Tijms BM, et al. Association of cerebral amyloid‐beta aggregation with cognitive functioning in persons without dementia. JAMA Psychiatry. 2018;75:84‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Evans DA, Hebert LE, Beckett LA, et al. Education and other measures of socioeconomic status and risk of incident Alzheimer disease in a defined population of older persons. Arch Neurol. 1997;54:1399‐1405. [DOI] [PubMed] [Google Scholar]
- 25. Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:S69‐S74. [DOI] [PubMed] [Google Scholar]
- 26. Shokouhi S, Conley AC, Baker SL, et al. The relationship between domain‐specific subjective cognitive decline and Alzheimer's pathology in normal elderly adults. Neurobiol Aging. 2019;81:22‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Valech N, Mollica MA, Olives J, et al. Informants' perception of subjective cognitive decline helps to discriminate preclinical Alzheimer's disease from normal aging. J Alzheimers Dis. 2015;48(1):S87‐S98. [DOI] [PubMed] [Google Scholar]
- 28. Verfaillie SCJ, Timmers T, Slot RER, et al. Amyloid‐beta load is related to worries, but not to severity of cognitive complaints in individuals with subjective cognitive decline: the SCIENCe Project. Front Aging Neurosci. 2019;11:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rosenberg PB, Mielke MM, Appleby BS, Oh ES, Geda YE, Lyketsos CG. The association of neuropsychiatric symptoms in MCI with incident dementia and Alzheimer disease. Am J Geriatr Psychiatry. 2013;21:685‐695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Espenes R, Kirsebom BE, Eriksson C, et al. Amyloid plaques and symptoms of depression links to medical help‐seeking due to subjective cognitive decline. J Alzheimers Dis. 2020;75:879‐890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McWhirter L, Ritchie C, Stone J, Carson A. Functional cognitive disorders: a systematic review. Lancet Psychiatry. 2020;7:191‐207. [DOI] [PubMed] [Google Scholar]
- 32. Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population‐based studies. Int J Geriatr Psychiatry. 2000;15:983‐991. [DOI] [PubMed] [Google Scholar]
- 33. Rabin LA, Smart CM, Crane PK, et al. Subjective Cognitive decline in older adults: an overview of self‐report measures used across 19 international research studies. J Alzheimers Dis. 2015;48(1):S63‐S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wolfsgruber S, Kleineidam L, Wagner M, et al. Differential risk of incident Alzheimer's disease dementia in stable versus unstable patterns of subjective cognitive decline. J Alzheimers Dis. 2016;54:1135‐1146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


