Abstract
Evolution in response to a change in ecology often coincides with various morphological, physiological, and behavioral traits. For most organisms little is known about the genetic and functional relationship between evolutionarily derived traits, representing a critical gap in our understanding of adaptation. The Mexican tetra, Astyanax mexicanus, consists of largely independent populations of fish that inhabit at least 30 caves in Northeast Mexico, and a surface fish population, that inhabit the rivers of Mexico and Southern Texas. The recent application of molecular genetic approaches combined with behavioral phenotyping have established A. mexicanus as a model for studying the evolution of complex traits. Cave populations of A. mexicanus are interfertile with surface populations and have evolved numerous traits including eye degeneration, insomnia, albinism, and enhanced mechanosensory function. The interfertility of different populations from the same species provides a unique opportunity to define the genetic relationship between evolved traits and assess the co‐evolution of behavioral and morphological traits with one another. To define the relationships between morphological and behavioral traits, we developed a pipeline to test individual fish for multiple traits. This pipeline confirmed differences in locomotor activity, prey capture, and startle reflex between surface and cavefish populations. To measure the relationship between traits, individual F2 hybrid fish were characterized for locomotor behavior, prey‐capture behavior, startle reflex, and morphological attributes. Analysis revealed an association between body length and slower escape reflex, suggesting a trade‐off between increased size and predator avoidance in cavefish. Overall, there were few associations between individual behavioral traits, or behavioral and morphological traits, suggesting independent genetic changes underlie the evolution of the measured behavioral and morphological traits. Taken together, this approach provides a novel system to identify genetic underpinnings of naturally occurring variation in morphological and behavioral traits.
Keywords: cavefish, development, behavior
Individual hybrid fish (top) were assayed for various behaviors (bottom) revealing genetic relationships between behaviors.
Research highlights
-
1)
We examined the relationship between evolved morphological traits in the cave‐dwelling, form and the behavioral changes the cave form exhibits.
-
2)
We find that shared genetic architecture regulates numerous traits associated with body and size.
-
3)
We identified behavioral and morphological traits are largely regulated by independent genetic architecture.
-
4)
These experiments provide a framework for future studies that apply genetic mapping to identify genetic architecture regulating complex traits.
1. INTRODUCTION
Environmental changes often drive the evolution of morphological, behavioral, and physiological traits (Rose, 2007). Often trait evolution involves complex changes in genetic architecture that include pleiotropic gene function, or traits that independently evolve in parallel (Stern, 2013), yet systematically testing the evolutionary relationship between traits has been challenging. Subterranean environments present a unique opportunity to investigate the relationship between environment and trait evolution because aspects of the environment, such as loss of light, are often well defined and present across independently evolved populations (Culver & Pipan, 2009). In addition, in many cases, a closely related species or population remains in the surface environment, allowing for a direct comparison between species that inhabit different environments (Borowsky, 2018; Elliott, 2016). Finally, many traits associated with subterranean evolution including albinism, reduced eye size, lower metabolic rate, and loss of circadian rhythms, have repeatedly evolved in distantly‐related species in cavefish and subterranean mammals (Jeffery, 2009; Poulson, 2001; Tian et al., 2017). Therefore, investigating trait evolution in subterranean species has potential to uncover whether seemingly distinct traits are genetically linked and may have co‐evolved.
The Mexican tetra Astyanax mexicanus is a leading model to study the evolution of complex traits (Jeffery, 2020; Keene et al., 2015). The repeated evolution of cavefish from surface‐like ancestors in geographically distinct cave environments produced two morphologically distinct forms of A. mexicanus. The first is a surface‐dwelling form with fully developed eyes found in above‐ground rivers and streams of northeast Mexico and parts of southern Texas, and the second includes at least 30 populations of cave‐dwelling forms, mostly found within the Sierra de El Abra region of northeast Mexico (Gross, 2012; Jeffery, 2001; Mitchell et al., 1977). Genomic and geological data suggest that multiple cavefish populations evolved independently (with some admixture) providing the opportunity to test the repeatability of evolution (Herman et al., 2018; Mitchell et al., 1977; Strecker et al., 2004). Cave‐dwelling forms have converged on distinct morphological traits, including albinism and eye loss (Jeffery, 2020). In addition, cavefish evolved numerous behavioral changes including different prey capture, startle response, and increased locomotor activity (Duboué et al., 2011; Lloyd et al., 2018; Paz et al., 2020). Overall many of these changes are thought to be critical for foraging in the absence of visual cues (Keene & Duboue, 2018; McGaugh et al., 2020; Yoshizawa, 2015). Therefore, the robust phenotypic differences between surface fish and cavefish provide an opportunity to examine the relationship between the evolution of behaviors and morphological traits.
Cavefish and surface fish are interfertile, allowing for the generation of hybrid fish that can be used to assess whether shared or independent genetic architecture regulates seemingly distinct cave‐like traits (O'Quin & McGaugh, 2016; Protas et al., 2006; Yoshizawa et al., 2012). Quantitative trait loci (QTL) analyses for multiple morphological and behavioral traits support the notion that genetic pleiotropy may contribute to the evolution of multiple traits (Kowalko et al., 2013; McGaugh et al., 2014; Yoshizawa et al., 2012). In addition, numerous functional interactions have been identified, including interactions between eye loss and the expansion of the jaw and hypothalamus (Atukorala & Franz‐Odendaal, 2018; Pottin et al., 2011; Yamamoto et al., 2009). Studies have also found genetic interactions between albinism, elevated catecholamines, and anesthesia resistance (Bilandzija et al., 2013; Bilandžija et al., 2018). A later study found that mutation of the oca2 gene causes sleep loss and increased locomotor activity (O'gorman et al., 2021). Therefore, investigating many different cave‐evolved traits in individual hybrids has potential to identify the degree to which evolved traits relate to one another.
Here, we generated a pipeline for analyzing behavior and morphology in individual fish. We applied this to phenotype F2 hybrids from surface and Pachón cavefish, a highly troglomorphic population, and measured the relationship between traits. We systematically investigated the relationships between individual behaviors as well as the relationships between these behaviors and morphological traits. These studies suggest that behavioral and morphological traits are largely regulated independently, suggesting independent evolution of many cave‐associated traits.
2. MATERIALS AND METHODS
2.1. Fish husbandry
Animal husbandry was carried out as previously described (Borowsky, 2008a) and all protocols were approved by the IACUC Florida Atlantic University. Fish were housed in the Florida Atlantic University core facilities at 23°C ± 1°C constant water temperature throughout rearing for behavior experiments (Borowsky, 2008a). Lights were kept on a 14:10 h light–dark cycle that remained constant throughout the animal's lifetime. Light intensity was kept between 25 and 40 lx for both rearing and behavior experiments. Adult fish were fed a diet of black worms to satiation twice daily at zeitgeber time (ZT) 2 and ZT12, (Aquatic Foods) and standard flake fish food during periods when fish were not being used for breeding (Tetramine Pro). All fry used for experiments were reared on live Artemia beginning at 4dfp and fed twice daily through the end of experiments at 7 dpf.
2.2. Behavioral analysis pipeline
All fish tested, including surface fish, Pachón cavefish, and surface × cave F2 hybrids followed the same behavioral analysis pipeline. At 6 dpf fish were removed from bowls, transferred to plates, and tested for startle reflex as described below between ZT0 and ZT4 in the light. Following completion of startle response assays, larvae were returned to their well plates and transferred to the locomotor assay. Locomotion behavior experiments were run as four trials a day, with a 60 min trial interval. Behavioral videos were captured between ZT4 and ZT8. Immediately following locomotor assays at ZT8, larvae were returned to their well plates and then transferred to prey capture arenas containing Artemia nauplii, where their behavior was recorded for a single period of 30 min. After this period, larvae were returned to their original well plates and incubated overnight. The following day, larvae were retrieved and photographed for measurement of morphological traits. The details of each part of the procedure are described below. At all stages care was taken to avoid mixing up individual fish throughout the process.
2.3. Prey capture
Prey capture behavior was recorded as previously described, with minor modifications, described below (Lloyd et al., 2018). Video was acquired using a USB 3.0 camera (LifeCam Studio, Microsoft) fitted with a zoom lens (75 mm DG Series Fixed Focal Length Lens, Edmund Optics Worldwide), and recorded with VirtualDub2 (v44282). All images were acquired at 30 frames per second. Recording chambers were illuminated with custom‐designed infrared LED source (Infrared (IR) 850 nm 5050 LED Strip Light, Environmental Lights). All recordings were performed in 6 dpf fry from zeitgeber (ZT) 0 to ZT3, shortly after the onset of lights on. For larval fish recordings, individual fish were placed in 24 well tissue culture plates (Cellvis) or custom‐made chambers, filled with approximately 3 mm of water to constrict the larvae to a single focal plane. Fish were allowed to acclimate for 2 min before the start of the experiment. To record feeding behavior, approximately 30 Artemia nauplii were added to each well and fish were imaged for 30 min.
Recordings were analyzed using ImageJ 1.52a (National Institutes of Health). Chamber diameter was set using ImageJ's native “Set Scale” function, and strike distance and angle were measured for all successful feeding events, using ImageJ's “Line” and “Angle” tools. Measurements of both strike distance and angle were taken in the frame before initiation of movement towards the prey. Strike distance was defined as the shortest distance between the edge of the fish's body and the prey. Strike angle was defined as the angle between a line extending down the fish's midline, terminating parallel with the pectoral fins, and a line extending from this point to the center of the prey. Measurements of each strike were averaged to calculate the mean strike distance and angle for that individual, and any recording with fewer than three feeding events was excluded from analysis.
2.4. Startle response
We assessed startle response probability and kinematics as previously described (Paz et al., 2020). Assays were conducted in a temperature‐controlled environment maintained at 24°C. Individual F2, surface, or Pachon larvae were placed in square wells on a custom 3D printed polyactic acid 16‐well plate, which was mounted onto a vertically‐oriented vibration Exciter controlled by a multi‐function I/O device and custom Labview 2018 v.18.0f2 (National Instruments) scripts. To optimize the quality of video recordings, only 8 wells were used at a time. Each assay consisted of an initial 10min acclimation period followed by six 500 Hz square wave stimuli of 50 ms duration with a 10 min interstimulus interval, resulting in a total duration of 1 h per assay. A total of 128 F2 larvae were assayed. An LED was connected directly to the signal driving the exciter so that its flashing could be used to identify the start and end of each stimulus in video recordings. C‐start responses were identified as accelerated, simultaneous flexion of the head and tail in the same direction. Response probability is reported as the total number of c‐starts performed by a larva divided by the total number of stimuli to which the larva was exposed (six). Beginning from the frame immediately preceding the stimulus start (as indicated by the LED turning on), the “angle” tool on ImageJ 1.52a (National Institutes of Health) was used to determine the change in orientation of the larvae over the course of the stimulus, and these measurements were used to determine response latency, angular speed, and peak angle. Response latency is defined as the time interval between stimulus onset and a change in orientation of at least 10°.
2.5. Locomotor behavior
We measured locomotion activity as previously described (Jaggard JB, 2019). Videos were captured using a Basler ace ac1300–200 μm USB 3.0 digital camera (Edmund Optics Inc., NJ; CAT#33978) with a 16 mm C series lens (Edmund Optics Inc., CAT#67714) and a UV‐VIS filter (Edmund Optics Inc., CAT#65716). Individual Astyanax mexicanus were placed in single wells of a 6‐well plate (34.8 mm diameter; #S3506, Corning Inc.), acclimated for 10min and recorded for 1 h. Distance, velocity, and time spent in the center and border were tracked and analyzed using the software EthoVision (Ethovision X14, Noldus Information Technology). Raw data was binned and transformed using custom made MATLAB scripts (available on request).
2.6. Morphological analysis
Pure surface and Pachón cavefish or F2 offspring were anesthetized in 0.1 M Tricaine at 7 days post‐fertilization and placed under a light microscope for imaging. Each fish was imaged both dorsally and laterally. Images were standardized against a 1 mm measurement and were uploaded to Fiji ImageJ (Schindelin et al., 2012). The 1 mm measurement was measured by the line variable in ImageJ and established as a global calibration for all future measurements. Each fish image was used to measure lengths for the following: standard length, head depth, eye size lateral, eye size dorsal, jaw width, head width, head length, fin length left and right (which were averaged to a single value). Standard length measurement connected the tip of the upper lip to the end of the tail at its greatest length. Head depth was measured as the length from the dorsal edge of the head to the ventral edge of the head. Eye size lateral was determined as the length between either side of the widest part of the right eye (under microscope when fish is placed laterally). The remaining measurements were done from a dorsal perspective. Eye size dorsal was measured from the outside of the widest part of the eye from the center edge to the lateral edge. Jaw width was measured as the length of the widest part of the head rostral to the eyes. Head width was measured as the widest length of the head caudal to the eyes. Head length was recorded as the length from the tip of the upper lip to the end of the head, established when it meets the swimmer bladder. Finally, fin lengths were measured as the greatest length where each fin attaches to the head to the tip of the end of each fin. Every measurement was completed by two different raters to determine inter‐rater reliability, and both measurements were averaged to find final values for analysis.
2.7. Statistical analysis
All morphological and behavioral traits are presented as violin plots; indicating the median, 25th, and 75th percentiles. All statistical analyses were performed using Instat software (Graphpad Prism 8.4.3). For each trait, normality was assessed visually from a QQ plot and then a parametric t‐test was performed. To visualize the relationship between two traits, a linear regression was performed. R2 heatmaps were generated using python's SciPy and Seaborn modules. R 2 values were obtained by performing an independent linear regression on each pair of variables.
3. RESULTS
In zebrafish and A. mexicanus, 6 days post‐fertilization (dpf) larvae are often studied because their transparency and small size is amenable to brain imaging and high‐throughput behavioral analysis (Halpern et al., 2008; Keene & Appelbaum, 2019). We designed our experiments to measure behavior in 6 dpf fish, followed by morphological analysis at 7 dpf. To define morphological differences between surface and cavefish populations, we compared multiple anatomical traits in surface and Pachón cavefish. At this timepoint, the overall developmental stage of surface and cavefish is largely similar, allowing for direct comparisons of anatomical features (Hinaux et al., 2011). We quantified eight traits related to overall body size, craniofacial development, and pigmentation (Figures 1a,b and S1). At 7 dpf, cavefish were on average larger than surface fish with increased body length, height, and head length, revealing an overall increase in the body and head size of Pachón cavefish (Figures S2 and 1e). Consistent with the previous reports, we found that jaw size is significantly increased in cavefish and eye size is significantly reduced, raising the possibility of a trade‐off between eye and jaw size (Figure 1c,d; Pottin et al., 2011; Yamamoto et al., 2009). Nearly all differences were maintained when individual traits were normalized to length body length (Figure S3), confirming that the observed differences are not due to overall changes in size. Together, these analyses confirm the presence of numerous morphological differences between surface and Pachón cavefish at 7 dpf, providing a platform to investigate the genetic relationship between these traits.
Figure 1.
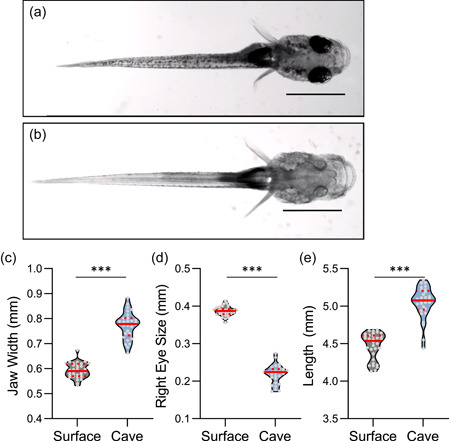
Morphological differences between purebred surface and Pachón cavefish populations. (a and b) Dorsal image of surface (a) and Pachón cavefish (Bb). Scale bars denote 1 mm. (c) Jaw width is significantly greater in cavefish compared to surface fish (t‐test: t 59 = 16.48, p < .0001). (d) Eye size is significantly greater in surface fish (t‐test: t 59 = 33.80, p < .0001). (e) Length is significantly greater in cavefish compared to surface fish (t‐test: t 59 = 11.55, p < .0001). For each trait, the median (center line) as well as 25th and 75th percentiles (top and bottom lines) are shown. Circles represent values from individual fish. ***denotes p < .001. [Color figure can be viewed at wileyonlinelibrary.com]
To define behavioral differences between surface fish and Pachón cavefish, we developed a behavioral analysis pipeline to quantify ecologically relevant behaviors in succession, assessing startle response kinematics, followed by prey capture, and finally locomotor activity in the same individual fish. We focused on behaviors related to foraging and predator evasion, as food scarcity and reduced predation are major changes associated with the cave environment (Elliott, 2016; McGaugh et al., 2020). To measure feeding behavior, we recorded the response of surface and cavefish during Artemia feeding and quantified the angle and distance of prey capture, two kinematic components that differ between visually and nonvisually related feeding (Figure 2a; Jaggard et al., 2020; Lloyd et al., 2018). Consistent with previous reports, strike angle was significantly greater in cavefish (Figure 2b; Lloyd et al., 2018). We also found the strike distance was reduced in Pachón cavefish compared to surface fish (Figure 2c). To assess escape response kinematics, plates containing fish in individual wells were fastened to a small vibration excitor and the response to escape‐inducing vibration was measured with a high‐speed camera (Figure 2d). The peak angle of cavefish was reduced, approaching significance (p = .06), while the angular speed was also reduced (Figure 2e,f) consistent with a previous report that the escape response is blunted in cavefish (Paz et al., 2020). Finally, we measured locomotor activity in cavefish because it is a critical for aspects of predator avoidance and foraging. Cavefish are more active, presumably to allow increased foraging activity and exhibit increased wall‐following behavior (Duboué et al., 2011). We quantified total locomotor activity and time spent in the center of the arena over a 1‐h assay (Figure 2g). Pachón cavefish spent more time in the center of the test arena compared to surface fish and exhibited a greater total amount of activity (Figure 2h,i). Together, these findings are consistent with previously published reports revealing robust differences in sensory and foraging behavior. The establishment of these phenotypes in 6 dpf fish tested in succession for each behavior provides an assay for examining interindividual variability.
Figure 2.
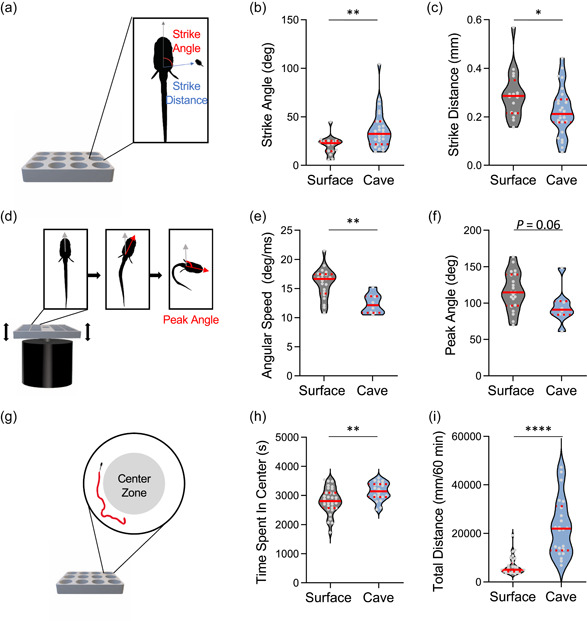
Behavioral variation in surface and cavefish (a) Diagram of prey capture apparatus. Videos were used to extract strike angle (red) and strike distance (blue) between Artemia and the head of the fish. (b) Strike angle is significantly greater in cavefish than surface fish (t‐test: t 41 = 3.006, p < .0045). (c) Strike distance in surface fish is significantly greater than in cavefish (t‐test: t 41 = 2.209, p < .0328). (a) Image of startle reflex set up. Plate sits on a mini‐shaker (black) to induce a startle. Videos were used to extract angular speed and peak angle (red). Grey arrow denotes head orientation at the initiation of the startle stimulus. (e) Angular speed in surface fish is significantly greater than in cavefish (t‐test: t 27 = 3.629, p < .0012). (f) Peak angle in surface fish is greater, approaching significance, than cavefish (t‐test: t 27 = 1.928, p < .0645). (g) Image of locomotor assay where fish were recorded in individual wells for 1‐h and to analyze total locomotor activity and time in the center of the well (grey area). (h) Time spent in the center in surface fish is significantly greater than cavefish (t‐test: t37 = 4.710, p < .0013) (i) Total distance in surface fish is significantly less than in cavefish. (t‐test: t 37 = 6.506, p < .0001). For each trait, the median (center line) as well as 25th and 75th percentiles (dotted lines) are shown. Circles represent values from individual fish. *denotes p < .05, **denotes p < .01; ***denotes p < .001. [Color figure can be viewed at wileyonlinelibrary.com]
To examine the relationship between traits in surface and Pachón cavefish, we first examined the correlations between morphological traits. For most morphological traits there was a strong correlation between eye size, head width, length, and jaw width, suggesting many differences are related to overall body size (Figure S4A,B). Conversely, there were far fewer significant correlations between individual components of behavior (Figure S4C,D). In cavefish, total locomotor activity was significantly correlated with peak angle, and angular speed, revealing a relationship between locomotor behavior and startle reflex (Figure S4C). In addition, in both surface fish and Pachón cavefish, angular speed was correlated with peak angle, suggesting a relationship between both metrics of startle reflex (Figure S4C,D). Finally, we examined the relationship between morphological and behavioral traits (Figure S4E,F). There were far fewer correlations between behavioral and morphological traits, than for morphology alone. In cavefish, total distance traveled was correlated with many aspects of size, yet this was not observed in surface fish (Figure S4E,F). Taken together, these findings reveal strong associations between morphological traits and fewer between individual behaviors in pure populations of surface and cavefish.
We sought to define whether any of the morphological differences identified between surface fish and cavefish genetically segregate, a result that would suggest they are governed by shared genetic architecture. To examine the relationship between different morphological traits, including body sizes, eye size, and pigmentation, we generated F2 surface‐cave hybrids by crossing F1 offspring of surface fish and Pachón cavefish (Figure 3a). We were not able to detect intermediate pigmentation levels. Therefore, this trait was scored in a binary fashion, with fish classified as albino (no melanin pigment present) or pigmented (any level of pigmentation present). Thus, pigmented fish are either heterozygous and homozygous for surface fish oca2 allele. We assessed individual fish for numerous traits including eye size, length, height, and jaw size (Figure 3c–e). First, we quantified whether there was an interaction between these traits and pigmentation. Across all morphological traits measured in F2 offspring, there were no significant differences between pigmented and albino fish (Table S1 and Figure S5A–G). We performed a Spearman's Rank Correlation Coefficient across all variables and found traits related to size had strong associations with one another (Figure 3b). These studies revealed that the majority of morphological traits were linked, including an association between jaw width and overall size. While the difference did not reach significance, albino fish trended towards having larger eyes (p = .06; Figure 3c). However, when eye‐size was corrected for body length, eye size was larger in pigmented hybrids than albino hybrids (p < .05; Figure S5H), raising the possibility that shared genes, or closely linked genes, contribute to both phenotypes. It has previously been suggested that jaw width is associated with eye‐size (Yamamoto et al., 2009) and we observed a significant correlation between these traits (Figure 3d). We also observed a significant interaction between jaw width and overall head width, suggesting jaw width is likely specified by the overall head size of the animal (Figure 3e). Together, these findings suggest the overall growth rate of cavefish is accelerated and the genes regulating different features of growth co‐segregate in surface‐cave hybrids.
Figure 3.
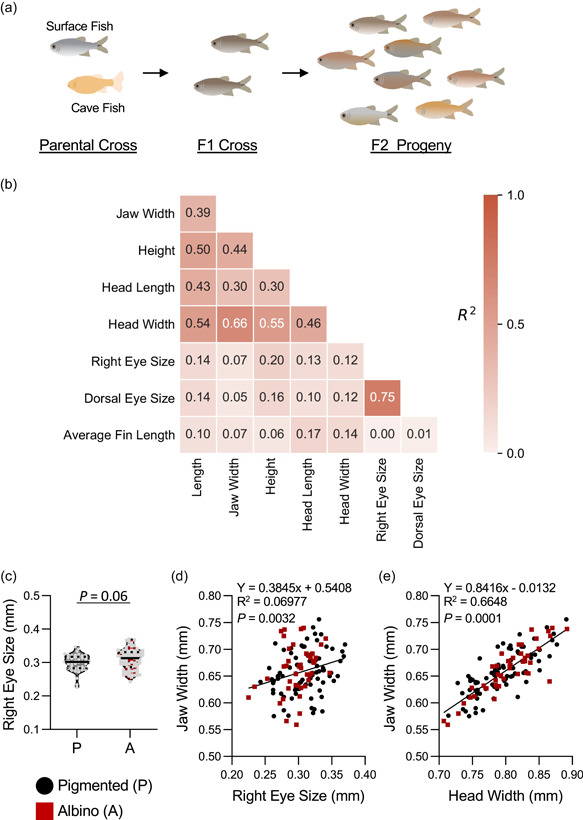
Morphological traits in F2 hybrid offspring. (a) Cross‐breeding process between purebred Surface (silver) and Pachón (albino) to produce F1 progeny, and F1 crosses to produce F2 hybrids used for study. (b) Heat map of the correlations between morphological traits in F2 offspring (R 2 values shown). (c) Eye size does not differ between pigmented and albino individuals (t‐test: t 121 = 1.856, p < .0659). (d) Linear regression between jaw width and eye size reveals a significant association (F 1,121 = 9.076, p < .0032). (e) Linear regression between head width and jaw width reveals a significant association (F 1,121 = 137.5, p < .0001). Albino individuals are depicted as red squares, while pigmented individuals are depicted as black circles. [Color figure can be viewed at wileyonlinelibrary.com]
It is possible that the many behavioral differences in cavefish evolved independently of one another, or that they are governed by shared genetic architecture. To examine the relationships between these traits, we measured the behavior of individual F2 hybrids for locomotor behavior, prey‐capture, and escape reflex reflex in individual F2 hybrids (Figure 4a). We then performed rank‐correlation analyses between all behavioral traits (Figure 4b). We identified a correlation between angular speed and peak angle in escape responses, but not with other behavioral variables tested (Figure 4c). Additionally, no significant associations were identified for variables of prey capture and escape reflexes, suggesting the evolved differences for each behavior in cavefish occurred through independent genetic mechanisms (Figure 4d). Together, these findings suggest there is little shared genetic or functional relationships between three behaviors that are thought to be critical to cave evolution.
Figure 4.
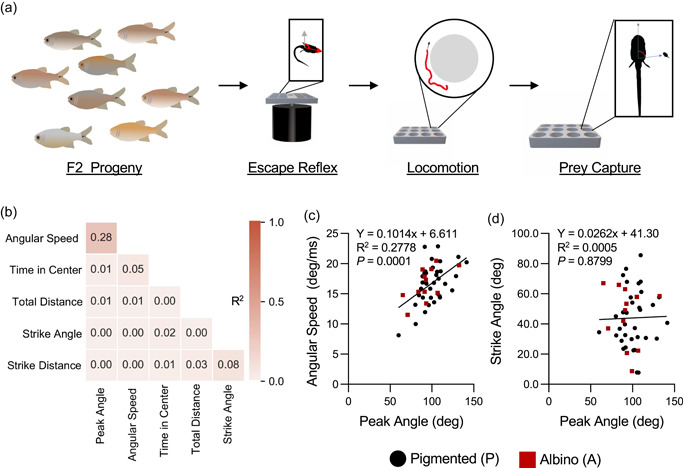
Relationship between behavioral traits within F2 hybrids. (a) Schematic for behavioral analysis where individual F2 fish were tested for locomotor activity, escape reflex, and then prey capture in succession. (b) Heat map of the correlations between behavioral traits in F2 offspring (R 2 values shown). (c) Peak angle of the escape reflex is associated with angular speed (F 1,45 = 17.31, p < .0001). (d) Peak angle during the escape reflex is not associated with feeding strike angle (F 1,44 = 0.0231, p < .8799). Albino individuals are depicted as red squares, while pigmented individuals are depicted as black circles. [Color figure can be viewed at wileyonlinelibrary.com]
Numerous studies have revealed associations between morphological and behavioral evolution (Kowalko, 2020). To examine the possibility that the behaviors studied in our analysis pipeline relate to anatomical changes, we compared the associations between anatomical and behavioral traits measured in F2 offspring (Figure 5a). We identified significant negative correlations between angular speed in escape response and body length as well as between peak angle in escape response and head length, suggesting a trade‐off between increased size and reduced escape response performance in cavefish (Figure 5d). We also identified an association between albinism and total swimming distance, consistent with the notion that mutations in oca2 confer sleep loss and altered locomotor activity (Figure 5b; O'Gorman et al., 2020). These differences are likely not reflective of general locomotor abnormalities in albino hybrids because the time in the center did not differ across each population (Figure 5c). Taken together, these findings suggest the evolution of behavioral and morphological phenotypes are largely governed by independent genetic architecture.
Figure 5.
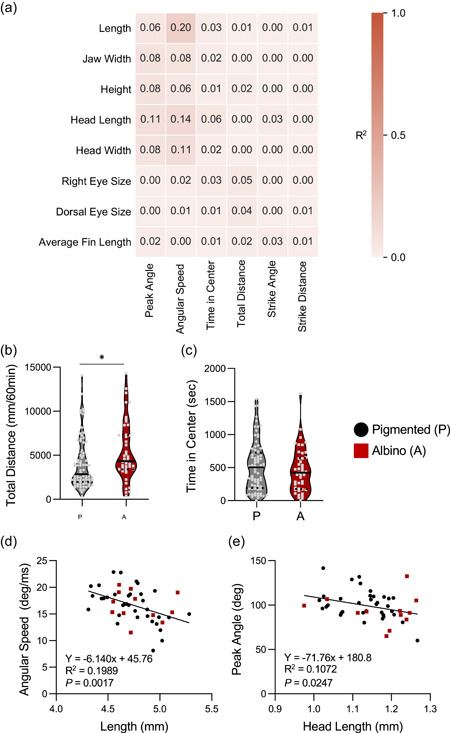
Comparison between morphological and behavioral traits within F2 hybrid offspring. (a) Heat map of the correlations between morphological and behavioral traits in F2 offspring (R 2 values shown). (b) Total distance is significantly greater in albino (A) than in pigmented (P) individuals (t‐test: t 121 = 2.452, p < .0157). (c) Time in the center does not differ between pigmented and albino individuals (t‐test: t121 = 0.8957, p < .3722). For each trait, the median (center line) as well as 25th and 75th percentiles (dotted lines) are shown. (d) Standard length and angular speed are significantly correlated in F2 hybrid individuals (R 2 = 0.1989). (e) There is a significant association between peak angle and head length in F2 individuals (R 2 = 0.1072). Albino individuals are depicted as red squares, while pigmented individuals are depicted as black circles. **denotes p < .01. [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
Across taxa, environmental perturbation leads to the evolution of many behavioral and morphological traits (Stern, 2013). Co‐segregation of traits can influence the rate and direction of phenotypic evolution as seen in the three‐spine stickleback populations, resulting in changes in body armor, aggression, and social behaviors (Leinonen et al., 2011; Peichel & Marques, 2017). Similarly, rapid evolution of species in East African cichlids has led to dramatic changes in many traits including coloration, craniofacial morphology, aggression, and locomotor behavior (Kocher, 2004; Powder & Albertson, 2016; Salzburger, 2018). Understanding how defined ecological factors impact the evolution of these traits, and uncovering their genetic bases are central questions in evolutionary biology. Examining numerous behavioral and morphological traits in hybrids with robust evolutionarily‐derived differences can be performed to examine the genetic basis of trait evolution, and whether traits are governed by shared genetic architecture.
To investigate the relationship between genetic architecture underlying the evolution of behavioral and morphological traits, we quantified these traits in surface‐Pachón cave F2 hybrids. The approach of examining numerous behavioral and morphological traits in hybrids with robust evolutionarily‐derived differences can be applied to a other species to examine the genetic basis of trait evolution, and whether traits are governed by shared genetic architecture.
We performed broad analyses of morphological and behavioral traits that suggest the genetic architecture underlying multiple aspects of increased body size including head size, head width and body length, and jaw width are all related. This suggests defined genetic changes have led to an overall increase in growth rate during early development in cavefish. We examined a correlation between eye size and albinism, which is supported by QTL analysis that eye size and albinism localize to overlapping QTL (Protas et al., 2008). Further, increased jaw size has been previously associated with a reduction in eye size(Yamamoto et al., 2009). Therefore, increasing the sample size or testing at different developmental stages may identify additional associations between traits including developmentally‐specified associations, with interactions at some stages, but not early development. Alternatively, the effect size of many traits may be small and were missed during this study. It is important to note that we only examined superficial morphological traits, and it is possible that a more detailed analysis would uncover additional traits that are related. For example, cavefish have differences in craniofacial morphology, tooth development, and an expansion of lateral line neuromasts (Atukorala & Franz‐Odendaal, 2018; Gross et al., 2014; Varatharasan et al., 2009; Yoshizawa et al., 2010). In addition, we did not test possible interactions between morphological traits and the differences in brain neuroanatomy that have previously been reported (Loomis et al., 2019; 2019). Therefore, it is possible that a more detailed analysis will reveal broader genetic interactions.
Most behavioral traits studied here are unlinked from the observed anatomical changes. We did, however, identify an association between increased length and reduced angular speed during the escape response, revealing a potential trade‐off between size and escape performance in fish. While limited information is available about the relationship between body size and escape response in larvae, in adult damsel fish size is correlated with increased escape velocity (McCormick et al., 2019). Given that cavefish appear to lack macroscopic predators (Jeffery, 2008; Kowalko, 2020), it is possible that rapid growth is advantageous to develop resistance to starvation, even at the expense of reduced escape abilities. Broadly, we found that pigmentation did not associate with nearly all traits tested (with the exception of eye size and locomotor activity), suggesting that loss of oca2 function is relatively specific to albinism and does not impact the behaviors tested. This is surprising given the role of oca2 as in monoamine function. In oca2 mutants, norepinephrine and dopamine levels are elevated, and these neurotransmitters are linked to many behaviors including foraging (Bilandzija et al., 2013, 2018). We did observe increased locomotor behavior in albino mutants, consistent with previous findings that sleep is reduced F2 surface x cave hybrids or surface fish with engineered mutations in oca2 (O'gorman et al., 2021). Therefore, our findings suggest that the differences in behavioral and morphological traits examined here largely evolved through independent genetic mechanisms, though there are likely to be trade‐offs between body size and escape behavior during early development.
A central question in the field relates to the ecological factors that drive many of the evolved differences in behavior and morphology in cavefish. Here, we confirm previous findings revealing sensory‐motor changes in prey capture and startle response, as well as changes in locomotion (Jaggard et al., 2020; Lloyd et al., 2018). It is unclear which aspects of cave ecology are likely to drive these differences. Caves and surface habitats differ in many ways, including constant darkness, which is proposed to underlie increased dependence on the lateral line during feeding behavior (Yoshizawa et al., 2010, 2012). There is also speculation that the caves are nutrient poor compared to surface environments, and this underlies the evolution of sleep loss, however this has not been investigated systematically in the cave environment (Krishnan & Rohner, 2017). It is possible that the increased jaw size in cavefish is related to the size of the prey consumed by juveniles, allowing for larger prey, greater suction during feeding, or improved success during lateral‐line dependent feeding that involves lateral movement during capture (Holzman et al., 2014; Lloyd et al., 2018; Yoshizawa et al., 2010). Conversely, it has previously been shown that enhanced prey capture abilities of larval cavefish are independent from eye loss (Espinasa et al., 2014). Little is known of the foraging behavior of fish in natural conditions, especially at the larval stage. The stomach contents of adult fish, identifying a diet of arthropods and there is speculation that cavefish consume bat guanos deposited from bat colonies that inhabit the majority of the caves (Espinasa et al., 2017). Further investigation of the abiotic and biotic ecology of the caves are likely to contribute to our understanding of evolution, and comparisons of surface and cavefish across different developmental stages should improve our understanding of how the studied traits have evolved.
We examined hybrids of the surface and Pachón cave populations. We chose this population because geological, genomic, and morphological evidence suggests the Pachón population is one of the most troglomorphic, and therefore the most commonly used in Mexican tetra studies. The largely independent evolution of at least 30 different cave populations offers a unique opportunity to study the evolution of various traits. Shared genetic changes underlie evolution in a number of populations. For example, different mutations in the pigmentation gene oca2 directly lead to albinism in the Molino and Pachón populations (Protas et al., 2008). In addition, complementation analysis between independently evolved cavefish populations suggest different genetic changes underlie eye loss in the Pachón and Molino populations (Borowsky, 2008b; Sifuentes‐Romero et al., 2020; Wilkens & Strecker, 2003). However, the presence of convergent evolution in these populations increases the likelihood of differences in traits among individual populations. For example, sleep loss in the Pachón population is dependent on enhanced lateral line function, while sleep loss in Tinaja and Molino fish is independent of the lateral line (Jaggard et al., 2017). Therefore, the systematic relationship between evolved traits across multiple independently evolved populations of cavefish has potential to uncover whether shared principles governed repeated evolution following similar ecological changes.
In this study we exclusively examined behavior at 6 days post fertilization. This is an age typically used in zebrafish for genetic manipulations including performing whole brain imaging, and a recently developed neuroanatomical atlas in A. mexicanus compared different populations at this age (Halpern et al., 2008; Jaggard et al., 2020; Keene & Appelbaum, 2019). Further, hybrid analysis studies often require large numbers of fish, and testing fish at 7dpf is much more accessible. While the differences between surface fish and cavefish behavior for sleep, foraging, and wall‐following (or reduced time in center) are similar in 7 dpf fish and adults, there may be developmentally‐specified effects. For example, at 30 dpf the prey capture distance (distance between prey and fish at the start of the attack) is greater in surface fish than cavefish (Lloyd et al., 2018), however, we report that here it is reduced at this timepoint at 6 dpf. Despite these differences, multiple studies now confirm that the attack angle is greater in cavefish as early as 7 dpf through 30 dpf. Therefore, the phenotypes observed may vary across time, and therefore any genetic relationships identified through the approach used here may not generalize across development. In addition to the behaviors examined, the behaviors of adults are thought to be more complex, and therefore may allow for more detailed analysis of the relationship between differentially‐evolved behaviors. Many behavioral differences have only been described in adults including schooling, aggression, vocalizations, and vibration attraction, and therefore could not be included in the analysis applied here. The approach of generating a pipeline for examining trait interactions could be applied to adult animals allowing for investigation of the interactions between a broader number of traits.
This investigation sought to understand the relationship between many different evolved traits in F2 surface x cave hybrid fish. While our analysis was limited to phenotyping, previous studies have performed mapping studies to localize genomic regions associated with numerous traits including albinism, locomotor behavior, eye size, social behavior, nonvisual sensory systems (O'Quin & McGaugh, 2016). Sequenced genomes for Pachón cave and surface populations of A. mexicanus are available and can be applied to identify candidate genes from mapping studies (McGaugh et al., 2014; Warren et al., 2021). The behavioral pipeline approach used in this study would be particularly powerful because it would allow for genomic mapping of many traits in a relatively small number of animals. In addition to genomic approaches, gene‐editing approaches have been applied to functionally validate data obtained from genomic mapping or transcriptional analysis, revealing potential for this approach to identify novel genetic regulators of many different cave evolved traits (Klaassen et al., 2018; Ma et al., 2015; Stahl et al., 2019). Finally, the approach used and its potential for mapping is not limited to A. mexicanus. Hybrid analysis and mapping is widely used in other fish species and applying a behavioral pipeline to identify genetic architecture associated with trait evolution has potential for identifying genes in many different models of evolution.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Supplementary information.
Supplementary information.
Supplementary information.
ACKNOWLEDGEMENTS
The authors are grateful to Julia Pasquale for assistance with behavioral analysis, as well as Peter Lewis and Arthur Lopatto for assistance with fish care. This study was supported by an NIGMS U‐RISE award (T34GM136486) to ACK an NIGMS R01 (1R01GM127872) to ACK and an NSF‐EDGE award to JEK and ERD (1923372).
Oliva, C. , Hinz, N. K. , Robinson, W. , Barrett Thompson, A. M. , Booth, J. , Crisostomo, L. M. , Zanineli, S. , Tanner, M. , Lloyd, E. , O'Gorman, M. , McDole, B. , Paz, A. , Kozol, R. , Brown, E. B. , Kowalko, J. E. , Fily, Y. , Duboue, E. R. , & Keene, A. C. (2022). Characterizing the genetic basis of trait evolution in the Mexican cavefish. Evolution & Development, 24, 131–144. 10.1111/ede.12412
DATA AVAILABILITY STATEMENT
All analyzed data have been made available in supplemental material. The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Atukorala, A. D. S. , & Franz‐Odendaal, T. A. (2018). Genetic linkage between altered tooth and eye development in lens‐ablated Astyanax mexicanus . Developmental Biology, 441, 235–241. [DOI] [PubMed] [Google Scholar]
- Bilandzija, H. , Ma, L. , Parkhurst, A. , & Jeffery, W. (2013). A potential benefit of albinism in Astyanax cavefish: Downregulation of the oca2 gene increases tyrosine and catecholamine levels as an alternative to melanin synthesis. PLoS One, 8, e80823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilandžija, H. , Abraham, L. , Ma, L. , Renner, K. J. , & Jeffery, W. R. (2018). Behavioural changes controlled by catecholaminergic systems explain recurrent loss of pigmentation in cavefish. Proceedings of the Royal Society B: Biological Sciences, 16, 1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowsky, R. (2008a). Handling Astyanax mexicanus eggs and fry. Cold Spring Harbor Protocols, 2008, 3. [DOI] [PubMed] [Google Scholar]
- Borowsky, R. (2008b). Restoring sight in blind cavefish. Current Biology, 18, R23–R24. [DOI] [PubMed] [Google Scholar]
- Borowsky, R. (2018). Cavefishes. Current Biology, 28, R60–R64. [DOI] [PubMed] [Google Scholar]
- Culver, D. C. , & Pipan, T. (2009). The Biology of Caves and Other Subterranean Habitats. Oxford University Press. [Google Scholar]
- Duboué, E. R. E. R. , Keene, A. C. C. A. C. , & Borowsky, R. L. R. L. R. L (2011). Evolutionary convergence on sleep loss in cavefish populations. Current Biology, 21, 671–676. [DOI] [PubMed] [Google Scholar]
- Elliott, W. R. , 2016. Cave Biodiversity and Ecology of the Sierra de El Abra Region, 59–76 in Biology and Evolution of the Mexican Cavefish.
- Espinasa, L. , Bibliowicz, J. , Jeffery, W. R. , & Rétaux, S. (2014). Enhanced prey capture skills in Astyanax cavefish larvae are independent from eye loss. EvoDevo, 5, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinasa, L. , Bonaroti, N. , Wong, J. , Pottin, K. , Queinnec, E. , et al. (2017). Contrasting feeding habits of post‐larval and adult Astyanax cavefish. subterranean. Biology, 21, 1–17. [Google Scholar]
- Gross, J. B. (2012). The complex origin of Astyanax cavefish. BMC Evolutionary Biology, 12, 105. 10.1186/1471-2148-12-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross, J. B. , Krutzler, A. J. , & Carlson, B. M. (2014). Complex craniofacial changes in blind cave‐dwelling fish are mediated by genetically symmetric and asymmetric loci. Genetics, 196, 1303–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern, M. E. , Rhee, J. , Goll, M. G. , Akitake, C. M. , Parsons, M. , & Leach, S. D. (2008). Gal4/UAS transgenic tools and their application to zebrafish. Zebrafish, 5, 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman, A. , Brandvain, Y. , Weagley, J. , Jeffery, W. R. , Keene, A. C. , Kono, T. , Bilandžija, H. , Borowsky, R. , Espinasa, L. , O'Quin, K. , Ornelas‐García, C. P. , Yoshizawa, M. , Carlson, B. , Maldonado, E. , Gross, J. B. , Cartwright, R. A. , Rohner, N. , Warren, W. C. , & McGaugh, S. E. (2018). The role of gene flow in rapid and repeated evolution of cave‐related traits in Mexican tetra, Astyanax mexicanus . Molecular Ecology, 22, 4397–4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinaux, H. , Pottin, K. , Chalhoub, H. , Père, S. , Elipot, Y. , Legendre, L. , & Rétaux, S. (2011). A developmental staging table for Astyanax mexicanus surface fish and Pacho 'n cavefis. Zebrafish, 8, 155–165. [DOI] [PubMed] [Google Scholar]
- Holzman, R. , Perkol‐Finkel, S. , & Zilman, G. (2014). Mexican blind cavefish use mouth suction to detect obstacles. The Journal of Experimental Biology, 217, 1955–1962. [DOI] [PubMed] [Google Scholar]
- Jaggard, J. , Robinson, B. G. , Stahl, B. A. , Oh, I. , Masek, P. , Yoshizawa, M. , & Keene, A. C. (2017). The lateral line confers evolutionarily derived sleep loss in the Mexican cavefish. Journal of Experimental Biology, 220, 284–293. [DOI] [PubMed] [Google Scholar]
- Jaggard, J. B. , Lloyd, E. , Yuiska, A. , Patch, A. , Fily, Y. , Kowalko, J. E. , Appelbaum, L. , Duboue, E. R. , & Keene, A. C. (2020). Cavefish brain atlases reveal functional and anatomical convergence across independently evolved populations. Science Advances, 6, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery, W. R. (2001). Cavefish as a model system in evolutionary developmental biology. Developmental Biology, 231, 1–12. [DOI] [PubMed] [Google Scholar]
- Jeffery, W. R. (2008). Emerging model systems in evo‐devo: cavefish and microevolution of development. Evolution & Development, 10, 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery, W. R. (2009). Regressive evolution in Astyanax cavefish. Annual Review of Genetics, 43, 25–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery, W. R. (2020). Astyanax surface and cave fsh morphs. EvoDevo . 11 [DOI] [PMC free article] [PubMed]
- Keene, A. C. , & Appelbaum, L. (2019). Sleep in Fish Models
- Keene, A. C. , & Duboue, E. R. (2018). The origins and evolution of sleep. The Journal of Experimental Biology , [DOI] [PMC free article] [PubMed]
- Keene, A. C. , Yoshizawa, M. , & McGaugh, S. E. (2015). Biology and Evolution of the Mexican Cavefish .
- Klaassen, H. , Wang, Y. , Adamski, K. , Rohner, N. , & Kowalko, J. E. (2018). CRISPR mutagenesis confirms the role of oca2 in melanin pigmentation in Astyanax mexicanus. Developmental Biology , 441, 313–318. [DOI] [PubMed] [Google Scholar]
- Kocher, T. D. (2004). Adaptive evolution and explosive speciation: The cichlid fish model. Nature Reviews Genetics, 5, 288–298. [DOI] [PubMed] [Google Scholar]
- Kowalko, J. (2020). Utilizing the blind cavefish Astyanax mexicanus to understand the genetic basis of behavioral evolution. The Journal of Experimental Biology, 223, 223. [DOI] [PubMed] [Google Scholar]
- Kowalko, J. E. , Rohner, N. , Rompani, S. B. , Peterson, B. K. , Linden, T. A. , Yoshizawa, M. , Kay, E. H. , Weber, J. , Hoekstra, H. E. , Jeffery, W. R. , Borowsky, R. , & Tabin, C. J. (2013). Loss of schooling behavior in cavefish through sight‐dependent and sight‐independent mechanisms. Current Biology, 23, 1874–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan, J. , & Rohner, N. (2017). Cavefish and the basis for eye loss. Philosophical Transactions of the Royal Society of London Series B, Biological sciences , 372. [DOI] [PMC free article] [PubMed]
- Leinonen, T. , Cano, J. M. , & Merilä, J. (2011). Genetics of body shape and armour variation in threespine sticklebacks. Journal of Evolutionary Biology , 24, 206–218. [DOI] [PubMed] [Google Scholar]
- Lloyd, E. , Olive, C. , Stahl, B. A. , Jaggard, J. B. , Amaral, P. , Duboué, E. R. , & Keene, A. C. (2018). Evolutionary shift towards lateral line dependent prey capture behavior in the blind Mexican cavefish. Developmental Biology, 441, 328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis, C. , Peuß, R. , Jaggard, J. B. , Wang, Y. , Mckinney, S. A. , Raftopoulos, S. C. , Raftopoulos, A. , Whu, D. , Green, M. , McGaugh, S. E. , Rohner, N. , Keene, A. C. , & Duboue, E. R. (2019). Cavefish brain atlases reveal functional and anatomical convergence across independently evolved populations. bioRxiv (in press at Science Advances), 13, 832543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis, C. , Peuß, R. , Jaggard, J. B. , Wang, Y. , Mckinney, S. A. , Raftopoulos, S. C. , Raftopoulos, A. , Whu, D. , Green, M. , McGaugh, S. E. , Rohner, N. , Keene, A. C. , & Duboue, E. R. (2019). An adult brain Atlas reveals broad neuroanatomical changes in independently evolved populations of Mexican cavefish. BioRxiv, 13, 0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, L. , Jeffery, W. R. , Essner, J. J. , & Kowalko, J. E. (2015). Genome editing using TALENs in blind Mexican cavefish, Astyanax mexicanus . PLoS One, 10, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick, M. I. , Fakan, E. P. , & Palacios, M. M. (2019). Habitat degradation and predators have independent trait‐mediated effects on prey. Scientific Reports, 9, 15705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh, S. E. , Gross, J. B. , Aken, B. , Blin, M. , Borowsky, R. , Chalopin, D. , Hinaux, H. , Jeffery, W. R. , Keene, A. , Ma, L. , Minx, P. , Murphy, D. , O'Quin, K. E. , Rétaux, S. , Rohner, N. , Searle, S. M. , Stahl, B. A. , Tabin, C. , Volff, J. N. , … Warren, W. C. (2014). The cavefish genome reveals candidate genes for eye loss. Nature Communications, 5, 5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh, S. E. , Kowalko, J. E. , Duboué, E. , Lewis, P. , Franz‐Odendaal, T. A. , Rohner, N. , Gross, J. B. , & Keene, A. C. (2020). Dark world rises: The emergence of cavefish as a model for the study of evolution, development, behavior, and disease. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution, 334, 397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, R. W. , Russell, W. H. , & Elliott, W. R. (1977). In Mackey C., & Barnett G. E. (Eds.), Mexican eyeless characin fishes, genus Astyanax: Environment, distribution, and evolution. Texas Tech Press. [Google Scholar]
- O'gorman, M. , Thakur, S. , Imrie, G. , Moran, R. L. , Choy, S. , Sifuentes‐Romero, I. , Bilandžija, H. , Renner, K. J. , Duboué, E. , Rohner, N. , McGaugh, S. E. , Keene, A. C. , & Kowalko, J. E. (2021). Pleiotropic function of the oca2 gene underlies the evolution of sleep loss and albinism in cavefish. Current biology: CB, 31, 3694–3701. [DOI] [PubMed] [Google Scholar]
- O'Gorman, M. , Thakur, S. , Imrie, G. , Moran, R. L. , Duboue, E. , Rohner, N. , McGaugh, S. E. , Keene, A. C. , & Kowalko, J. E. (2020). Pleiotropic function of the oca2 gene underlies the evolution of sleep loss and albinism in cavefish. BioRxiv , 10.1101/2020.09.27.314278 [DOI] [PubMed]
- O'Quin, K. , & McGaugh, S. E. (2016). Mapping the genetic basis of troglomorphy in astyanax: How far we have come and where do we go from here?, Biology and Evolution of the Mexican Cavefish (pp. 111–135). Elsevier Inc. [Google Scholar]
- Paz, A. , McDole, B. , Kowalko, J. , Duboue, E. , & Keene, A. (2020). Evolution of the acoustic startle response of Mexican cavefish. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution, 334, 474–485. [DOI] [PubMed] [Google Scholar]
- Peichel, C. L. , & Marques, D. A. (2017). The genetic and molecular architecture of phenotypic diversity in sticklebacks. Philosophical Transactions of the Royal Society, B: Biological Sciences, 372, 20150486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottin, K. , Hinaux, H. , & Rétaux, S. (2011). Restoring eye size in Astyanax mexicanus blind cavefish embryos through modulation of the Shh and Fgf8 forebrain organising centres. Development (Cambridge, England), 138, 2467–2476. [DOI] [PubMed] [Google Scholar]
- Poulson, T. L. (2001). Morphological and physiological correlates of evolutionary reduction of metabolic rate among amblyopsid cave fishes. Environmental Biology of Fishes, 62, 239–249. [Google Scholar]
- Powder, K. E. , & Albertson, R. C. (2016). Cichlid fishes as a model to understand normal and clinical craniofacial variation. Developmental Biology, 415, 338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protas, M. , Tabansky, I. , Conrad, M. , Gross, J. B. , Vidal, O. , Tabin, C. J. , & Borowsky, R. (2008). Multi‐trait evolution in a cave fish, Astyanax mexicanus . Evolution and Development, 10, 196–209. [DOI] [PubMed] [Google Scholar]
- Protas, M. E. , Hersey, C. , Kochanek, D. , Zhou, Y. , Wilkens, H. , Jeffery, W. R. , Zon, L. I. , Borowsky, R. , & Tabin, C. J. (2006). Genetic analysis of cavefish reveals molecular convergence in the evolution of albinism. Nature Genetics, 38, 107–111. [DOI] [PubMed] [Google Scholar]
- Rose, S. (2007). The Richness of Life: The Essential Stephen Jay Gould (1st ed.). W. W. Norton & Company. [Google Scholar]
- Salzburger, W. (2018). Understanding explosive diversification through cichlid fish genomics. Nature Reviews Genetics, 19, 705–717. [DOI] [PubMed] [Google Scholar]
- Schindelin, J. , Arganda‐Carreras, I. , Frise, E. , Kaynig, V. , Longair, M. , Pietzsch, T. , Preibisch, S. , Rueden, C. , Saalfeld, S. , Schmid, B. , Tinevez, J. Y. , White, D. J. , Hartenstein, V. , Eliceiri, K. , Tomancak, P. , & Cardona, A. (2012). Fiji: an open‐source platform for biological‐image analysis. Nature Methods, 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifuentes‐Romero, I. , Ferrufino, E. , Thakur, S. , Laboissonniere, L. A. , Solomon, M. , Smith, C. L. , Keene, A. C. , Trimarchi, J. M. , & Kowalko, J. E. (2020). Repeated evolution of eye loss in Mexican cavefish: Evidence of similar developmental mechanisms in independently evolved populations. Journal of Experimental Zoology. Part B, Molecular and Developmental Evolution, 334, 423–437. [DOI] [PubMed] [Google Scholar]
- Stahl, B. A. , Jaggard, J. B. , Chin, J. , Kowalko, J. E. , Keene, A. C. , & Duboué, E. R. (2019). Manipulation of gene function in Mexican cavefish. Journal of Visualized Experiments , 146. 10.3791/59093 [DOI] [PubMed]
- Stern, D. (2013). The genetic causes of convergent evolution. Nature Reviews Genetics, 14, 751–764. [DOI] [PubMed] [Google Scholar]
- Strecker, U. , Faúndez, V. H. , & Wilkens, H. (2004). Phylogeography of surface and cave Astyanax (teleostei) from Central and North America based on cytochrome b sequence data. Molecular Phylogenetics and Evolution, 33, 469–481. [DOI] [PubMed] [Google Scholar]
- Tian, R. , Yin, D. , Liu, Y. , Seim, I. , Xu, S. , & Yang, G. (2017). Adaptive evolution of energy metabolism‐related genes in hypoxia‐tolerant mammals. Frontiers in Genetics, 8, 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varatharasan, N. , Croll, R. P. , & Franz‐Odendaal, T. (2009). Taste bud development and patterning in sighted and blind morphs of Astyanax mexicanus . Developmental Dynamics, 238, 3056–3064. [DOI] [PubMed] [Google Scholar]
- Warren, W. C. , Boggs, T. E. , Borowsky, R. , Carlson, B. M. , Ferrufino, E. , Gross, J. B. , Hillier, L. , Hu, Z. , Keene, A. C. , Kenzior, A. , Kowalko, J. E. , Tomlinson, C. , Kremitzki, M. , Lemieux, M. E. , Graves‐Lindsay, T. , McGaugh, S. E. , Miller, J. T. , Mommersteeg, M. , Moran, R. L. , … Rohner, N. (2021). A chromosome‐level genome of Astyanax mexicanus surface fish for comparing population‐specific genetic differences contributing to trait evolution. Nature Communications, 12, 1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkens, H. , & Strecker, U. (2003). Convergent evolution of the cavefish Astyanax (characidae: teleostei): Genetic evidence from reduced eye‐size and pigmentation. Biological Journal of the Linnean Society, 80, 545–554. [Google Scholar]
- Yamamoto, Y. , Byerly, M. S. , Jackman, W. R. , & Jeffery, W. R. (2009). Pleiotropic functions of embryonic sonic hedgehog expression link jaw and taste bud amplification with eye loss during cavefish evolution. Developmental Biology, 330, 200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa, M. (2015). Behaviors of cavefish offer insight into developmental evolution. Molecular Reproduction and Development, 82, 268–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa, M. , Gorički, Š. , Soares, D. , & Jeffery, W. R. (2010). Evolution of a behavioral shift mediated by superficial neuromasts helps cavefish find food in darkness. Current Biology, 20, 1631–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa, M. , Yamamoto, Y. , O'Quin, K. E. , & Jeffery, W. R. (2012). Evolution of an adaptive behavior and its sensory receptors promotes eye regression in blind cavefish. BMC Biology, 10, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.
Supplementary information.
Supplementary information.
Data Availability Statement
All analyzed data have been made available in supplemental material. The data that support the findings of this study are available from the corresponding author upon reasonable request.


