Abstract
Purpose
The purpose of the study was to analyse the development of ocular complications and visual prognosis in juvenile idiopathic arthritis associated uveitis (JIA‐uveitis) compared to the previous decade in the light of new treatment guidelines.
Methods
In this retrospective cohort, 143 patients with JIA‐uveitis were stratified into two cohorts based on the year of diagnosis of uveitis, <2010 (n = 61) and ≥2010 (n = 82). Development of ocular complications and visual outcomes were analysed by univariate and multivariate methods. Treatment with systemic corticosteroids and immunomodifying medication (IMT) were documented.
Results
In total, 109 and 133 affected eyes, respectively, for cohort 1 (<2010) and cohort 2 (≥2010) were included for analysis. In the multivariate analysis with correction for paired eyes, patients in cohort 1 were at higher risk for cataract surgery (p = 0.03) and secondary glaucoma (p = 5.15 × 10−3). Also, the number of eyes that were legally blind and visually impaired at 5 years of follow‐up was significantly higher in cohort 1 (7% versus 2% and 8% versus 0%, p = 0.01 respectively). The number of patients that started IMT was significantly higher in cohort 2 (57% versus 98%, p = 2.17 × 10−6). In cohort 2, both methotrexate and anti‐TNF‐α therapy were prescribed earlier in the disease course (1.41 versus 0.05 years, p = 8.31 × 10−6 and 6.07 versus 1.84 years, p = 5.14 × 10−5 respectively).
Conclusions
The prognosis of JIA‐uveitis has improved during the last decade. There is a reduction in the number of cataract surgeries and secondary glaucoma and fewer patients lose their vision parallel with earlier access to tertiary care and earlier introduction of IMT.
Keywords: cataract, glaucoma, juvenile idiopathic arthritis, uveitis
Introduction
Juvenile idiopathic arthritis (JIA) is the most common rheumatic disease in children with some genetic predisposition but still unclarified triggers (Angeles‐Han et al. 2015a, 2015b, 2015c, Haasnoot et al. 2018). Depending on JIA subtype, approximately one‐third of JIA patients develops uveitis within 4 years after JIA onset (Heiligenhaus et al. 2007; Angeles‐Han et al. 2015a). Uveitis in JIA (JIA‐uveitis) is the leading cause of uveitis in children, with high risk of development of complications (e.g. cataract and glaucoma). (Tugal‐Tutkun 2011, Edelsten et al. 2002, Carvounis et al. 2006, Saurenmann et al. 2007, Thorne et al. 2007, Bolt et al. 2008, Skarin et al. 2009, Cann et al. 2018, Heiligenhaus et al. 2019, Sen & Ramanan 2020, Rypdal et al. 2021). Consequently, by adulthood, approximately 30% of the eyes with JIA‐uveitis become visually impaired which poses a significant burden on the quality of life (Angeles‐Han et al. 2015a, 2015c; Haasnoot et al. 2016). Male gender, short interval between arthritis and uveitis diagnosis, uveitis diagnosis prior to arthritis diagnosis, young age at uveitis onset, disease severity and presence of complications at first ophthalmological visit have been reported as risk factors for visual impairment and ocular complications (Edelsten et al. 2002; Zulian et al. 2002; Heiligenhaus et al. 2007; Thorne et al. 2007; Woreta et al. 2007; Holland et al. 2009; Kalinina Ayuso et al. 2010; Angeles‐Han et al. 2013; Heiligenhaus et al. 2019; Sen & Ramanan 2020; Rypdal et al. 2021). Therefore, the combination of established standardized uveitis screening for early detection, specialized care by a uveitis expert and the development of novel immunomodifying treatments are expected to improve outcomes. Formerly, topical and/or systemic corticosteroids were used for rapid control of intraocular inflammation (Simonini et al. 2010, Heiligenhaus et al. 2012a, Constantin et al. 2018). However, given the potentially severe side effects of both topical (cataract and glaucoma) and systemic corticosteroids (e.g. Cushing's syndrome), adding immunomodifying treatment (IMT) like methotrexate (MTX) earlier in the disease course is widely recommended nowadays so that topical and systemic corticosteroids can be tapered (Hoes et al. 2009, Simonini et al. 2010, 2013, Thorne et al. 2010, Gregory et al. 2013, Heiligenhaus et al., 2012a, Kothari et al. 2015, Blum‐Hareuveni et al. 2017, Constantin et al. 2018, Angeles‐Han et al. 2019). Further, the therapeutic potential of tumour necrosis factor alpha (TNF‐α) inhibitors, in addition to methotrexate, is significant (Ramanan et al. 2017). Treatment with biologics in JIA has been registered since 2010 and changed the clinical practices completely. Therefore, in this retrospective study, we compared the clinical outcomes in children with JIA‐uveitis diagnosed before 2010 to children diagnosed in 2010 and after.
Methods
Patient and data collection
The study was approved by the Medical Ethical Research Committee of the University Medical Center Utrecht in concordance with the Declaration of Helsinki. In this retrospective study, we compared the clinical outcomes of a previously reported cohort of children with JIA‐uveitis diagnosed <2010 to a new cohort of children diagnosed ≥2010 (Kalinina Ayuso et al. 2010). All patients were seen by a paediatric rheumatologist who diagnosed the patients with JIA based on the criteria of the International League of Associations for Rheumatology or by former criteria [e.g. European League Against Rheumatism (EULAR) (Wood 1978; Berntson et al. 2001; Petty et al. 2004]. All patients had an uveitis screening according to the guideline the of American Academy of Paediatrics (AAPS 1993). Uveitis was diagnosed by an ophthalmologist specialized in paediatric uveitis at the Department of Ophthalmology, University Medical Centre, Utrecht, the Netherlands, a tertiary referral centre. Patients with SUN ≥1+ cells or more in the anterior chamber were diagnosed with JIA‐associated anterior uveitis (Jabs et al. 2005).
Clinical data were retrospectively collected from the patient files. The following information from the medical data of the patients were recorded: gender, age of onset of uveitis, age of onset of JIA, laterality, laboratory results of antinuclear antibodies (ANA), ocular complications, best‐corrected visual acuities (BCVA) and systemic treatment. The following ocular complications were registered: ocular hypertension, posterior synechiae, secondary glaucoma, glaucoma requiring surgery, cataract requiring surgery, cystoid macula oedema (CME), papillitis and hypotony. Ocular hypertension was defined as three successive intraocular pressure measurements higher than 21 mmHg for which anti‐glaucomatous treatment had been started in the absence of pathologic optic disc cupping or visual field changes (Sijssens et al. 2006). Secondary glaucoma was classified as the presence of pathologic disc cupping and/or glaucomatous visual field, in combination with history of intraocular pressure higher than 21 mmHg. Preperimetric glaucoma was defined as glaucomatous changes on the optical coherence tomography (OCT) retinal nerve fibre layer (RNFL). Since the OCT was not available in our clinic until 2003, CME was defined as the presence of macular thickening with cyst formation observed by fundoscopy or by OCT, before and after 2003 respectively. Visual impairment was defined as BCVA between 20/50 and 20/200, and legal blindness as BCVA of 20/200 or worse or a visual field less than 10 degrees (Kalinina Ayuso et al. 2010).
The data regarding treatment with corticosteroid and IMT were collected. Because of missing values regarding the amount of drops and duration of treatment with topical corticosteroids was excluded from the analysis.
Statistical analyses
Statistical analyses of the data were performed using r software version 3.6.1. Baseline characteristics and administration of therapy were analysed per patient. Data are presented as medians with an interquartile range. The Pearson chi‐squared test or Fisher exact test was used for univariate analysis of categorical variables, and the Mann–Whitney U test was used to compare medians. Statistical analysis of ocular complications and BCVA were performed ‘by eye’, only including affected eyes with a 4‐year follow‐up with correction for paired eyes. Generalized estimating equations (GEE) was used for correction of paired eyes (Katz et al. 1994). A Cox proportion hazard regression was applied for multivariate analysis including all affected eyes with correction of standard error with clustering using robust method (Lin & Wei 1989). The model was adjusted for age of onset of uveitis (numeric variable), gender and uveitis diagnosis before arthritis onset. P‐values of <0.05 were considered statistically significant. All tests were two‐tailed.
Results
General characteristics of the study population
Sixty‐one patients diagnosed with JIA‐uveitis before 2010 were included in cohort 1. Cohort 2 included 82 patients diagnosed with JIA‐uveitis in 2010 and after (Table 1) (Kalinina Ayuso et al. 2010). Children in cohort 1 were younger at uveitis onset and had a longer interval between diagnosis of uveitis and initial visit at a tertiary referral centre than the children in cohort 2. Other comparisons of baseline characteristics can be seen in Table 1.
TABLE 1.
General characteristics of the study population according to cohort.
| Timing of uveitis | Cohort 1 | Cohort 2 | p‐value |
|---|---|---|---|
| <2010 | ≥2010 | ||
| Total number of patients | 61 | 82 | |
| Total number of eyes | 109 | 133 | |
| Male, n (%) | 15 (25) | 19 (23) | 1.00 |
| Bilateral, n (%) | 48 (78) | 51 (62) | 0.05 |
| ANA seropositive, n (%) | 54 (89) | 64 (78) | 0.26 |
| Age of onset JIA, median (IQR) | 3.3 (2.0–5.0) | 3.0 (3.3–7.4) | 0.82 |
| Age of onset uveitis, median (IQR) | 4.2 (3.1–6.2) | 5.2 (3.8–7.6) | 0.03 |
| Uveitis before JIA, n (%) | 15 (25) | 9 (11) | 0.05 |
| Time between diagnosis of arthritis and uveitis, median in years (IQR) | 1.3 (0.3–1.0) | 1.0 (0.4–2.0) | 0.27 |
| Time between diagnosis of uveitis and initial visit at the tertiary centre, median in years (IQR) | 2.2 (0.7–5.5) | 0.9 (0.1–2.2) | 8.08 × 10−4 |
| Follow‐up time, median in years (IQR) | 10 (9.1–10) | 5.1 (2.6–6.9) | 8.90 × 10−14 |
ANA = antinuclear antibodies, IQR = interquartile range, JIA = Juvenile idiopathic arthritis.
Complications
Cohort 1 and cohort 2
Cumulative incidences of ocular complications according to the two different cohorts are presented in Table 2. After adjusting for paired eyes, we found that in cohort 1, cataract surgeries were more common than in cohort 2 at 5 years of follow‐up (40% versus 20%, p = 0.02, GEE). No other differences were found after adjusting for paired eyes. Of the eyes that needed glaucoma surgery at 5 years of follow‐up, the indications were ocular hypertension (40% versus 50%), preperimetric glaucoma (32% versus 30%) and secondary glaucoma (28% versus 20%, p = 0.90).
Table 2.
Cumulative incidences of ocular complications of patients with juvenile idiopathic arthritis associated uveitis at diagnosis and at 5 years of follow‐up according to cohort.
| Timing of uveitis | Cohort 1 | Cohort 2 | p‐value * |
|---|---|---|---|
| <2010 | ≥2010 | ||
| At diagnosis | |||
| Number of eyes | 109 | 133 | |
| Posterior synechiae, n (%) | 24 (22) | 24 (18) | 0.44 |
| Cataract surgery, n (%) | 0 (0) | 0 (0) | NA |
| Ocular hypertension, n (%) | 6 (6) | 18 (14) | 0.04 |
| Secondary glaucoma, n (%) | 1 (1) | 0 (0) | 0.45 |
| Glaucoma surgery, n (%) | 0 (0) | 1 (1) | 1.00 |
| CME, (%) | 2 (2) | 6 (5) | 0.30 |
| Papillitis, n (%) | 5 (5) | 8 (6) | 0.62 |
| Hypotony, n (%) | 0 (0) | 0 (0) | NA |
| Follow‐up at 5 years | |||
| Number of eyes | 105 | 76 | |
| Posterior synechiae, n (%) | 47 (45) | 22 (29) | 0.03 |
| Cataract surgery, n (%) | 42 (40) | 15 (20) | 3.79 × 10−3 |
| Secondary glaucoma, n (%) | 22 (21) | 8 (10) | 0.06 |
| Glaucoma surgery, n (%) | 25 (24) | 10 (13) | 0.07 |
| CME, (%) | 20 (19) | 9 (12) | 0.19 |
| Papillitis, n (%) | 16 (15) | 15 (20) | 0.43 |
| Hypotony, n (%) | 3 (4) | 0 (0) | 1.00 |
Analysis performed by “eye”.
CME = cystoid macular oedema, NA = not applicable.
The Pearson χ 2 test or Fisher exact test.
Uveitis diagnosis before arthritis onset
After adjusting for paired eyes, the group with uveitis diagnosis before arthritis onset developed more frequently posterior synechiae (29% versus 74%, p = 6.00 × 10−6 ), CME (10% versus 39%, p = 1.94 × 10−4) and needed to undergo cataract surgery more frequently (24% versus 59%, p = 2.64 × 10−3) at 5 years of follow‐up.
Risk factors for ocular complications
Cox proportion hazard regression analysis with correction for age, gender and uveitis diagnosis before arthritis onset, revealed that patients in cohort 1 had an increased risk for cataract surgery (HR = 1.84, 95% CI: 1.06–3.20, p = 0.03; Fig. 1A). Furthermore, male gender and uveitis diagnosis before arthritis onset also appeared to be an independent risk factor for cataract surgery (HR = 1.68, 95% CI: 1.00–2.80, p = 4.79 × 10−2 and HR = 2.25, 95% CI: 1.33–3.82, p = 2.43 × 10−3 respectively). Cohort 1 was the only independent risk factor for secondary glaucoma (HR = 2.81, 95% CI: 1.36–5.79, p = 5.15 × 10−3; Fig. 1B). Independent risk factors for posterior synechiae were uveitis diagnosis before arthritis and younger age of uveitis onset (HR = 3.11, 95% CI: 1.95–4.98, p = 2.13 × 10−6 and HR = 0.90, 95% CI: 0.83–0.99, p = 0.03 respectively).
Fig. 1.
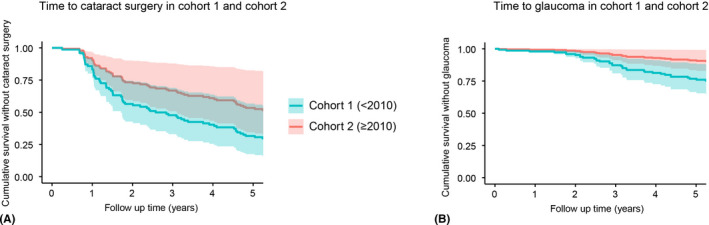
Time to the different complications. (A) Time to cataract surgery. HR = 1.84, 95% CI: 1.06–3.20, p = 0.03. (B) Time to secondary glaucoma. HR = 2.81, 95% CI: 1.36–5.79, p = 5.15 × 10−3. Analysis is adjusted for age of uveitis, uveitis diagnosis before arthritis onset, gender and paired eyes. CI = confidence interval; HR = hazard ratio.
Next, to identify the potential effect of early start with IMT we compared the interval between diagnosis of uveitis and treatment with methotrexate and anti‐TNF‐α between patients with and without ocular complications in the two different cohorts (Table 3). In cohort 1, patients with secondary glaucoma and children with cataract requiring surgery had a longer interval between diagnosis of uveitis and start with methotrexate and start with anti‐TNF‐α therapy compared to patients without these complications, although this was not significant (Table 3). These differences were not observed in cohort 2. Patients from cohort 2 with cataract requiring surgery had a significant shorter time between diagnosis of uveitis and initial visit at the tertiary centre (Table 3).
Table 3.
Time between diagnosis of uveitis and start with immunosuppressive therapy and time between diagnosis of uveitis and initial tertiary centre visit in patients with and without complications in the two different cohorts.
| Cohort 1 | p‐value | Cohort 2 | p‐value | |||
|---|---|---|---|---|---|---|
| Yes | No | Yes | No | |||
| Cataract surgery | ||||||
| Number of patients, n (%) | 36 (59) | 25 (41) | 17 (21) | 65 (79) | ||
| Time between diagnosis of uveitis and start methotrexate, median in years (IQR) | 2.5 (0.5–5.8) | 0.4 (0.0–3.3) | 0.10 | 0.0 (0.0–0.1) | 0.1 (0.0–0.7) | 0.22 |
| Time between diagnosis of uveitis and start anti‐TNFα therapy, median in years (IQR) | 7.0 (3.7–9.5) | 5.1 (3.2–7.0) | 0.45 | 1.8 (0.7–3.7) | 1.9 (0.7–4.5) | 0.85 |
| Time between diagnosis of uveitis and initial visit at tertiary centre, median in years (IQR) | 1.3 (0.7–5.5) | 3.0 (2.2–4.8) | 0.57 | 0.1 (0.0–0.9) | 0.9 (0.2–2.4) | 0.04 |
| Secondary glaucoma | ||||||
| Number of patients, n (%) | 28 (46) | 33 (54) | 9 (9) | 73 (91) | ||
| Time between diagnosis of uveitis and start methotrexate, median in years (IQR) | 2.5 (0.5–5.7) | 0.6 (0.0–3.9) | 0.16 | 0.0 (0.0–0.0) | 0.08 (0.0–0.6) | 0.02 |
| Time between diagnosis of uveitis and start anti‐TNF‐α therapy, median in years (IQR) | 8.0 (5.2–9.5) | 3.9 (2.8–5.7) | 0.19 | 1.6 (0.6–2.1) | 2.1 (0.7–4.5) | 0.54 |
| Time between diagnosis of uveitis and initial visit at tertiary centre, median in years (IQR) | 1.7 (0.9–3.3) | 4.4 (0.7–8.3) | 0.29 | 0.5 (0.1–1.3) | 0.9 (0.1–7.2) | 0.80 |
anti‐TNF‐α = anti‐tumour necrosis factor alpha, IQR = interquartile range.
Visual outcomes
Median BCVA's and categorized BCVA's at standard points of follow‐up, analysed according to cohort, are presented in Table 4. After adjusting for paired eyes, the total number of legally blind and visually impaired eyes was higher in cohort 1 (7% versus 2% and 8% versus 0%, p = 0.02, GEE). No other differences were identified (Table 4).
Table 4.
Median best‐corrected visual acuity and categorized visual outcomes in eyes affected by juvenile idiopathic arthritis associated uveitis, according to cohort.
| Timing of uveitis | Cohort 1 | Cohort 2 | p‐value * |
|---|---|---|---|
| >2010 | ≥2010 | ||
| At diagnosis | |||
| Number of eyes | 109 | 133 | |
| BCVA, median (range) | 1.0 (0.8–1.0) | 1.00 (0.8–1.0) | 0.22 |
| ≤20/200, n (%) | 3 (5) | 3 (5) | 0.98 |
| 20/100–20/50, n (%) | 4 (7) | 5 (8) | |
| ≥20/40, n (%) | 50 (88) | 58 (88) | |
| Follow‐up at 5 years | |||
| Number of eyes | 105 | 76 | |
| BCVA, median (range) | 1.0 (0.6–1.0) | 1.20 (1.0–1.2) | 1.48 × 10−9 |
| ≤20/200, n (%) | 6 (7) | 1 (2) | 0.01 |
| 20/100–20/50, n (%) | 8 (8) | 0 (0) | |
| ≥20/40, n (%) | 78 (85) | 63 (98) | |
Analysis performed “by eye”.
BCVA = best‐corrected visual acuity.
The Pearson χ 2 test or Fisher exact test.
Treatment
The differences in treatment with systemic corticosteroids and IMT at 5 years of follow‐up are presented in Table 5. There was no difference in the number of patients that were treated with systemic corticosteroids; however, in cohort 2, systemic corticosteroids were predominately prescribed between 2011 and 2014. All patients requiring systemic treatment were initiated with methotrexate. In cohort 2, both methotrexate and anti‐TNF‐α therapy were prescribed earlier in the disease course compared to cohort 1 (1.41 [0.07–4.72] years versus 0.05 [0.00–0.37] years, p = 8.31 × 10−6 and 6.07 [3.40–9.23] years versus 1.84 [0.66–4.28] years, p = 5.14 × 10−5 respectively; Fig. 2).
Table 5.
Treatment with systemic corticosteroids and immunomodifying treatment at 5 years of follow‐up according to cohort.
| Timing of uveitis | Cohort 1 | Cohort 2 | p‐value |
|---|---|---|---|
| <2010 | ≥2010 | ||
| Follow‐up at 5 years | |||
| Number of patients, n (%) | 58 (95) | 45 (55) | |
| Systemic corticosteroids, n (%)a | 13 (23) | 12 (27) | 0.65 |
| Methotrexate, n (%) | 33 (57) | 44 (98) | 2.17 × 10−6 |
| Anti‐TNF‐α therapy | 6 (10) | 22 (49) | 1.30 × 10−5 |
| Adalimumab, n (%) | 6 (100) | 20 (91) | |
| Infliximab, n (%) | 0 (0) | 2 (9) | |
anti‐TNF‐α = anti‐tumour necrosis factor alpha.
Perioperative use of systemic corticosteroids was not included.
Fig. 2.
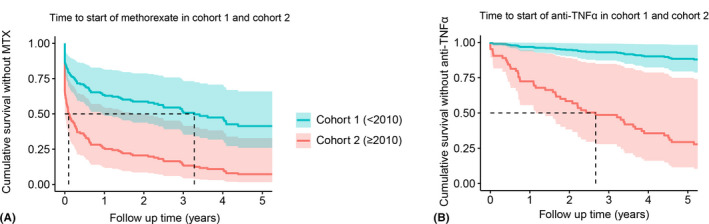
Time to immunomodifying treatment. (A) Time to treatment with methotrexate. HR = 0.34, 95% CI: 0.22–0.52, p = 1.04 × 10−6. (B) Time to treatment with anti‐TNF‐α therapy. HR = 0.10, 95% CI: 0.05–0.20, p = 1.98 × 10−10. Analysis is adjusted for age of uveitis, uveitis diagnosis before arthritis onset and gender. The dotted line represents the median survival. CI = confidence interval; HR, hazard ratio.
Discussion
Our retrospective cohort study shows a reduction in the rates of cataract surgeries, secondary glaucoma development, visual impairment and blindness among patients with JIA‐uveitis diagnosed ≥2010 compared with patients diagnosed <2010 along with more intensive therapy and earlier access to a tertiary centre. Since most of the prognostic studies in patients with JIA‐uveitis are from the previous decade, before the new treatment guidelines, a new prognostic study is of great value both for ophthalmologists and patients (Constantin et al. 2018).
In our study, the rate of visual impairment reduced substantially from 8% to 0%. A reduction in the number of eyes with visual impairment was also reported in an earlier study by Tappeiner (Tappeiner et al. 2015). According to studies from previous decades, cataract occurs in 15–46% and glaucoma in 7–27% of affected eyes of patients with JIA‐uveitis, depending on follow‐up (Kanski 1977; Wolf et al. 1987; Carvounis et al. 2006; Sijssens et al. 2007; Thorne et al. 2007; Bolt et al. 2008; Papadopoulou et al. 2017; Cann et al. 2018; Rypdal et al. 2021). A recent study by Tappeiner revealed a significant decrease in the prevalence of cataract (from 28% to 16%) and secondary glaucoma (from 10% to 5%) between 2009 and 2013 (Tappeiner et al. 2015). In our study, the rate of cataract surgery (20% in cohort 2) and secondary glaucoma (10% in cohort 2) were higher than the recent studies of Tappeiner et al. (2015) and Heiligenhaus et al. (2019). The difference in the number of complications between studies can be caused by a difference in corticosteroid treatment, follow‐up or definition of a complication which is not always reported in the studies (Kanski 1977; Wolf et al. 1987; Carvounis et al. 2006; Sijssens et al. 2007; Thorne et al. 2007; Bolt et al. 2008; Tappeiner et al. 2015; Papadopoulou et al. 2017; Cann et al. 2018; Heiligenhaus et al. 2019; Rypdal et al. 2021). This emphasizes the unmet need for a study that describes the prognostic changes in children with JIA‐uveitis using the recommended standardized outcomes (Heiligenhaus et al. 2012b; Foeldvari et al. 2019).
Male gender, short interval between arthritis and uveitis diagnosis, uveitis diagnosis prior to arthritis diagnosis, young age at uveitis onset and disease severity are associated with a poor prognosis (Edelsten et al. 2002; Zulian et al. 2002; Heiligenhaus et al. 2007; Thorne et al. 2007; Woreta et al. 2007; Holland et al. 2009; Kalinina Ayuso et al. 2010; Angeles‐Han et al. 2013; Heiligenhaus et al. 2019; Sen & Ramanan 2020; Rypdal et al. 2021). In our study, patients in cohort 1 were significantly younger of age at uveitis diagnosis. The difference in age at uveitis diagnosis might be explained by the fact that nowadays MTX is well‐established as first‐line treatment in the management of JIA (Ramanan et al. 2017) which might delay or even prevent the occurrence of uveitis.
General opinion is that early treatment with immunomodifying agents can reduce the rate of complications and this is in line with this study (Sijssens et al. 2007, Heiligenhaus et al., 2012a, Gregory 2nd et al. 2013, Constantin et al. 2018). However, due to the retrospective design of our study, a causal role of immunomodifying therapy to prevent ocular complications cannot be proven. Early treatment with immunomodifying agents is also recommended so that topical and systemic corticosteroids can be tapered to prevent long‐term treatment with corticosteroids. In our study, there was no difference in the number of patients that were treated with systemic corticosteroids between the cohorts. Besides earlier treatment with immunomodifying agents, we hypothesize that the clinical improvement is also influenced by better access to multidisciplinary specialized care between ophthalmologists and paediatric rheumatologists.
Unfortunately, due to the retrospective design of the study, we were unable to take into account the use of topical corticosteroids. The number of corticosteroid drops and duration of treatment is related to cataract and glaucoma development as has been shown in several studies (Thorne et al. 2010; Blum‐Hareuveni et al. 2017).
In conclusion, our study reports a significant decrease in the number of cataract surgeries, secondary glaucoma development and lower rates of visual impairment parallel with earlier access to a tertiary referral centre and more intensive IMT. These data on clinical outcome and visual prognosis are relevant for ophthalmologists who need to council the child and their parents for the need of intensive IMT for uveitis. Additionally, our study will contribute in creating awareness among ophthalmologists about the increased risks of complications in patients with JIA‐uveitis. Further studies are required to verify the causal association of early IMT on the course of uveitis in JIA.
This study was financially supported by the Dutch Ophthalmology Foundation ‘UitZicht’ and ‘ODAS stichting’. Funding entities had no role in the conducting or presentation of this study.
References
- AAPS (1993): American Academy of Pediatrics Section on Rheumatology and Section on Ophthalmology: Guidelines for ophthalmologic examinations in children with juvenile rheumatoid arthritis. Pediatrics 92: 295–296. [PubMed] [Google Scholar]
- Angeles‐Han ST, Yeh S & Vogler LB (2013): Updates on the risk markers and outcomes of severe juvenile idiopathic arthritis‐associated uveitis. Int J Clin Rheumatol 8. 10.2217/ijr.12.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeles‐Han ST, McCracken C, Yeh S et al. (2015a): HLA associations in a cohort of children with juvenile idiopathic arthritis with and without uveitis. Invest Ophthalmol Vis Sci 56: 6043–6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeles‐Han ST, McCracken C, Yeh S et al. (2015b): Characteristics of a cohort of children with juvenile idiopathic arthritis and JIA‐associated uveitis. Pediatr Rheumatol Online J 13: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeles‐Han ST, Yeh S, McCracken C et al. (2015c): Using the effects of youngsters' eyesight on quality of life questionnaire to measure visual outcomes in children with uveitis. Arthritis Care Res 67: 1513–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeles‐Han ST, Ringold S, Beukelman T et al. (2019): 2019 American College of Rheumatology/arthritis foundation guideline for the screening, monitoring, and treatment of juvenile idiopathic arthritis‐associated uveitis. Arthritis Care Res 71: 703–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum‐Hareuveni T, Seguin‐Greenstein S, Kramer M et al. (2017): Risk factors for the development of cataract in children with uveitis. Am J Ophthalmol 177: 139–143. [DOI] [PubMed] [Google Scholar]
- Berntson L, Fasth A, Andersson‐Gäre B et al. (2001): Construct validity of ILAR and EULAR criteria in juvenile idiopathic arthritis: a population based incidence study from the Nordic countries. International League of Associations for Rheumatology. European League Against Rheumatism. J Rheumatol 28: 2737–2743. [PubMed] [Google Scholar]
- Bolt IB, Cannizzaro E, Seger R & Saurenmann RK (2008): Risk factors and longterm outcome of juvenile idiopathic arthritis‐associated uveitis in Switzerland. J Rheumatol 35: 703–706. [PubMed] [Google Scholar]
- Cann M, Ramanan AV, Crawford A, Dick AD, Clarke SLN, Rashed F & Guly CM (2018): Outcomes of non‐infectious Paediatric uveitis in the era of biologic therapy. Pediatr Rheumatol Online J 16: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvounis PE, Herman DC, Cha S & Burke JP (2006): Incidence and outcomes of uveitis in juvenile rheumatoid arthritis, a synthesis of the literature. Graefes Arch Clin Exp Ophthalmol 244: 281–290. [DOI] [PubMed] [Google Scholar]
- Constantin T, Foeldvari I, Anton J et al. (2018): Consensus‐based recommendations for the management of uveitis associated with juvenile idiopathic arthritis: the SHARE initiative. Ann Rheum Dis 77: 1107–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelsten C, Lee V, Bentley CR, Kanski JJ & Graham EM (2002): An evaluation of baseline risk factors predicting severity in juvenile idiopathic arthritis associated uveitis and other chronic anterior uveitis in early childhood. Br J Ophthalmol 86: 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foeldvari I, Klotsche J, Simonini G et al. (2019): Proposal for a definition for response to treatment, inactive disease and damage for JIA associated uveitis based on the validation of a uveitis related JIA outcome measures from the Multinational Interdisciplinary Working Group for Uveitis in Childhood (MIWGUC). Pediatr Rheumatol Online J 17: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory AC 2nd, Kempen JH, Daniel E et al. (2013): Risk factors for loss of visual acuity among patients with uveitis associated with juvenile idiopathic arthritis: the Systemic Immunosuppressive Therapy for Eye Diseases Study. Ophthalmology 120: 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haasnoot AJ, Vernie LA, Rothova A, van der Does P, Los LI, Schalij‐Delfos NE & de Boer JH (2016): Impact of juvenile idiopathic arthritis associated uveitis in early adulthood. PloS one 11: e0164312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haasnoot A, Schilham MW, Kamphuis S et al. (2018): Identification of an amino acid motif in HLA‐DRβ1 that distinguishes uveitis in patients with juvenile idiopathic arthritis. Arthritis & rheumatology (Hoboken, NJ) 70: 1155–1165. [DOI] [PubMed] [Google Scholar]
- Heiligenhaus A, Niewerth M, Ganser G, Heinz C, Minden K & German Uveitis in Childhood Study Group (2007): Prevalence and complications of uveitis in juvenile idiopathic arthritis in a population‐based nation‐wide study in Germany: suggested modification of the current screening guidelines. Rheumatology (Oxford) 46: 1015–1019. [DOI] [PubMed] [Google Scholar]
- Heiligenhaus A, Michels H, Schumacher C et al. (2012a): Evidence‐based, interdisciplinary guidelines for anti‐inflammatory treatment of uveitis associated with juvenile idiopathic arthritis. Rheumatol Int 32: 1121–1133. [DOI] [PubMed] [Google Scholar]
- Heiligenhaus A, Foeldvari I, Edelsten C et al. (2012b): Proposed outcome measures for prospective clinical trials in juvenile idiopathic arthritis‐associated uveitis: a consensus effort from the multinational interdisciplinary working group for uveitis in childhood. Arthritis Care Res 64: 1365–1372. [DOI] [PubMed] [Google Scholar]
- Heiligenhaus A, Klotsche J, Tappeiner C et al. (2019): Predictive factors and biomarkers for the 2‐year outcome of uveitis in juvenile idiopathic arthritis: data from the Inception Cohort of Newly diagnosed patients with Juvenile Idiopathic Arthritis (ICON‐JIA) study. Rheumatology (Oxford) 58: 975–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoes JN, Jacobs JW, Verstappen SM, Bijlsma JW & Van der Heijden GJ (2009): Adverse events of low‐ to medium‐dose oral glucocorticoids in inflammatory diseases: a meta‐analysis. Ann Rheum Dis 68: 1833–1838. [DOI] [PubMed] [Google Scholar]
- Holland GN, Denove CS & Yu F (2009): Chronic anterior uveitis in children: clinical characteristics and complications. Am J Ophthalmol 147: 667–678.e5. [DOI] [PubMed] [Google Scholar]
- Jabs DA, Nussenblatt RB, Rosenbaum JT & Standardization of Uveitis Nomenclature (SUN) Working Group (2005): Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol 140: 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinina Ayuso V, Ten Cate HA, van der Does P, Rothova A & de Boer JH (2010): Male gender as a risk factor for complications in uveitis associated with juvenile idiopathic arthritis. Am J Ophthalmol 149: 994–999.e5. [DOI] [PubMed] [Google Scholar]
- Kanski JJ (1977): Anterior uveitis in juvenile rheumatoid arthritis. Arch Ophthalmol (Chicago, Ill. : 1960) 95: 1794–1797. [DOI] [PubMed] [Google Scholar]
- Katz J, Zeger S & Liang KY (1994): Appropriate statistical methods to account for similarities in binary outcomes between fellow eyes. Invest Ophthalmol Vis Sci 35: 2461–2465. [PubMed] [Google Scholar]
- Kothari S, Foster CS, Pistilli M et al. (2015): The Risk of Intraocular Pressure Elevation in Pediatric Noninfectious Uveitis. Ophthalmology 122: 1987–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DY & Wei LJ (1989): The robust inference for the cox proportional hazards model. J Am Sat Assoc 84: 1074–1078. [Google Scholar]
- Papadopoulou M, Zetterberg M, Oskarsdottir S & Andersson Grönlund M (2017): Assessment of the outcome of ophthalmological screening for uveitis in a cohort of Swedish children with juvenile idiopathic arthritis. Acta Ophthalmol 95: 741–747. [DOI] [PubMed] [Google Scholar]
- Petty RE, Southwood TR, Manners P et al. (2004): International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 31: 390–392. [PubMed] [Google Scholar]
- Ramanan AV, Dick AD, Jones AP et al. (2017): Adalimumab plus Methotrexate for Uveitis in Juvenile Idiopathic Arthritis. N Engl J Med 376: 1637–1646. [DOI] [PubMed] [Google Scholar]
- Rypdal V, Glerup M, Songstad NT et al. (2021): Uveitis in juvenile idiopathic arthritis: 18‐year outcome in the population‐based nordic cohort study. Ophthalmology 128: 598–608. [DOI] [PubMed] [Google Scholar]
- Saurenmann RK, Levin AV, Feldman BM, Rose JB, Laxer RM, Schneider R & Silverman ED (2007): Prevalence, risk factors, and outcome of uveitis in juvenile idiopathic arthritis: a long‐term followup study. Arthritis Rheum 56: 647–657. [DOI] [PubMed] [Google Scholar]
- Sen ES & Ramanan AV (2020): Juvenile idiopathic arthritis‐associated uveitis. Clin Immunol (Orlando, Fla) 211: 108322. [DOI] [PubMed] [Google Scholar]
- Sijssens KM, Rothova A, Berendschot TT & de Boer JH (2006): Ocular hypertension and secondary glaucoma in children with uveitis. Ophthalmology 113: 853–9.e2. [DOI] [PubMed] [Google Scholar]
- Sijssens KM, Rothova A, Van De Vijver DA, Stilma JS & De Boer JH (2007): Risk factors for the development of cataract requiring surgery in uveitis associated with juvenile idiopathic arthritis. Am J Ophthalmol 144: 574–579. [DOI] [PubMed] [Google Scholar]
- Simonini G, Cantarini L, Bresci C, Lorusso M, Galeazzi M & Cimaz R (2010): Current therapeutic approaches to autoimmune chronic uveitis in children. Autoimmun Rev 9: 674–683. [DOI] [PubMed] [Google Scholar]
- Simonini G, Paudyal P, Jones GT, Cimaz R & Macfarlane GJ (2013): Current evidence of methotrexate efficacy in childhood chronic uveitis: a systematic review and meta‐analysis approach. Rheumatology (Oxford) 52: 825–831. [DOI] [PubMed] [Google Scholar]
- Skarin A, Elborgh R, Edlund E & Bengtsson‐Stigmar E (2009): Long‐term follow‐up of patients with uveitis associated with juvenile idiopathic arthritis: a cohort study. Ocul Immunol Inflamm 17: 104–108. [DOI] [PubMed] [Google Scholar]
- Tappeiner C, Klotsche J, Schenck S, Niewerth M, Minden K & Heiligenhaus A (2015): Temporal change in prevalence and complications of uveitis associated with juvenile idiopathic arthritis:data from a cross‐sectional analysis of a prospective nationwide study. Clin Exp Rheumatol 33: 936–944. [PubMed] [Google Scholar]
- Thorne JE, Woreta F, Kedha SR, Dunn JP & Jabs DA (2007): Juvenile idiopathic arthritis‐associated uveitis: incidence of ocular complications and visual acuity loss. Am J Ophthalmol 143: 840–846. [DOI] [PubMed] [Google Scholar]
- Thorne JE, Woreta FA, Dunn JP & Jabs DA (2010): Risk of cataract development among children with juvenile idiopathic arthritis‐related uveitis treated with topical corticosteroids. Ophthalmology 117: 1436–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugal‐Tutkun I (2011): Pediatric uveitis. J Ophthalmic Vis Res 6: 259–269. [PMC free article] [PubMed] [Google Scholar]
- Wolf MD, Lichter PR & Ragsdale CG (1987): Prognostic factors in the uveitis of juvenile rheumatoid arthritis. Ophthalmology 94: 1242–1248. [DOI] [PubMed] [Google Scholar]
- Wood PHN (1978): Diagnosis criteria, nomenclature, classification. In: Munthe E (ed.). The care of rheumatic children. Basel: EULAR Publishers; 42–50. [Google Scholar]
- Woreta F, Thorne JE, Jabs DA, Kedhar SR & Dunn JP (2007): Risk factors for ocular complications and poor visual acuity at presentation among patients with uveitis associated with juvenile idiopathic arthritis. Am J Ophthalmol 143: 647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulian F, Martini G, Falcini F, Gerloni V, Zannin ME, Pinello L, Fantini F & Facchin P (2002): Early predictors of severe course of uveitis in oligoarticular juvenile idiopathic arthritis. J Rheumatol 29: 2446–2453. [PubMed] [Google Scholar]


