Abstract
Recently, the potential use of phytocannabinoids (pCBs) to treat different pathological conditions has attracted great attention in the scientific community. Among the different pCBs, cannabidiol (CBD) has showed interesting biological properties, making it a promising molecule with a high security profile that has been approved for treatment as an add-on therapy in patients afflicted by severe pharmaco-resistant epilepsy, including Dravet syndrome (DS), Lennox–Gastaut syndrome (LGS) and tuberous sclerosis complex (TSC). CBD is pharmacologically considered a “dirty drug”, since it has the capacity to bind different targets and to activate several cellular pathways. GABAergic impairment is one of the key processes during the epileptogenesis period able to induce a generalized hyperexcitability of the central nervous system (CNS), leading to epileptic seizures. Here, by using the microtransplantation of human brain membranes approach in Xenopus oocytes and electrophysiological recordings, we confirm the ability of CBD to modulate GABAergic neurotransmission in human cerebral tissues obtained from patients afflicted by different forms of pharmaco-resistant epilepsies, such as DS, TSC, focal cortical dysplasia (FCD) type IIb and temporal lobe epilepsy (TLE). Furthermore, using cDNAs encoding for human GABAA receptor subunits, we found that α1β2 receptors are still affected by CBD, while classical benzodiazepine lost its efficacy as expected.
Keywords: GABAA receptor, neurophysiology, epilepsy
1. Introduction
Cannabis sativa has been used for centuries for the treatment of different pathological conditions [1]. Among the hundreds of compounds present in cannabis flowers, over 100 phytocannabinoids (pCBs) have been discovered [1]. pCBs are lipidic molecules synthetized as acid compounds and then decarboxylated when dried or exposed to heat [1]. Among pCBs, CBD and Δ9-tetrahydrocannabinol (Δ9-THC) are the most studied molecules. They share a common precursor, namely, cannabigerol (CBG) [2]. After the discovery of Δ9-THC [3], new receptors were cloned, namely, cannabinoid receptors type 1 (CB1Rs) and type 2 (CB2Rs) [4,5]. In detail, CB1Rs are G-coupled metabotropic receptors that are highly expressed in the central nervous system (CNS), and are particularly abundant in the hippocampus, the basal ganglia and the cerebellum. They are located pre-synaptically on both the excitatory and inhibitory terminals, and they are activated mainly by Δ9-THC, responsible for the psychoactive effect of this compound. On the other hand, CB2Rs are mainly expressed on immune cells and in the peripheral nervous system [1].
However, up to now, other receptor targets for pCBs have been discovered, such as the transient receptor potential vanilloid type 1 (TRPV1) [6]. Indeed, it has been demonstrated that CBD can directly modulate these receptors and is able to inhibit the enzymatic inactivation of the main endocannabinoid anandamide (AEA). CBD is also able to modulate opioid receptors binding to an allosteric site of the µ and δ opioid receptors, adding further support to its anti-nociceptive action [7]. Furthermore, pCBs can interact with the G protein-coupled receptor GPR55 involved in glutamate release regulation [8,9] and voltage-gated calcium channels (VGCCs), thus regulating CNS excitability [10,11]. Moreover, other studies have highlighted the capability of pCBs to modulate glycine receptors [12,13], serotonin receptors [14,15] and acetylcholine receptors [16], opening new perspectives for their potential use in different pathological conditions.
Lately, it was demonstrated that pCBs, and CBD in particular, were able to significantly affect CNS excitability by acting on GABAergic neurotransmission at both the pre- and post-synaptic level [17]. In addition, CBD was recently approved by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) as an anti-seizure medication for three different neuropathologies characterized by pharmaco-resistant spontaneous recurrent seizures (SRSs), namely, Dravet syndrome (DS), Lennox-Gastaut syndrome (LGS) and tuberous sclerosis complex (TSC) [18,19,20].
However, studies showing CBD’s effects on GABAA receptors (GABAARs) from human epileptic tissues are not fully elucidated for two different reasons: the difficulty to obtain a proper amount of human material to perform electrophysiological recordings and, even more importantly, the lack of non-epileptic human tissues to compare the effects of CBD in normal versus pathological conditions.
In this study, taking advantage of the technique of voltage clamp recordings in Xenopus oocytes microinjected with human tissues [21], we bypass most of these problems, since we are able to record GABAergic currents from surgical and post-mortem tissues of human pharmaco-resistant epilepsies: DS, TSC, focal cortical dysplasia (FCD) type IIb and temporal lobe epilepsy (TLE). In addition, this approach is very powerful when human tissue is too scarce because of the rarity of these epileptic diseases, since it is possible to record neurotransmitter-evoked currents from just a few milligrams of brain tissue. Moreover, with this technique, we were able, as mentioned, to compare the CBD-mediated effect on evoked GABA currents (IGABA) in control healthy brain samples [22] versus epileptic brain tissues.
2. Materials and Methods
2.1. Patients
All the patients’ tissues (Table 1) used to perform the experiments reported here have been selected from the databases of the Department of Neuropathology, Amsterdam University Medical Center, University of Amsterdam (Amsterdam, The Netherlands); the Department of Neuropathology, University Medical Center Utrecht (Utrecht, The Netherlands); and the National Institute of Child Health and Human Development Brain and Tissue Bank for Developmental Disorders (Baltimore, MD, USA).
Table 1.
Patients’ clinical data.
| Patient | Age | Gender | Duration of Epilepsy | Brain Region | Type of Seizures | Diagnosis/Mut/Cause of Death | ASMs |
|---|---|---|---|---|---|---|---|
| #1 | 49 | M | 48 | T | FIAS/GS | DS/SCN1A mut/heart failure | CLB, STP, VPA |
| #2 | 46 | F | 44 | T | FIAS/GS | DS/SCN1A mut/ bronchopneumonia |
CLB, STP, VPA |
| #3 | 47 | M | 35 | T | FAS | TSC/TSC2 mut/Myocardial infarction | PHB, VPA, CBZ, CLB |
| #4 | 42 | F | 41 | T | FIAS | TSC/TSC2 mut/Myocardial infarction | PHB, VPA, CBZ |
| #5 | 41 | M | 21 | T | FIAS/GS | TLE-HS | CBZ, TPM |
| #6 | 54 | F | 42 | T | FIAS/GS | TLE-HS | CBZ, LMT, PHB |
| #7 | 52 | M | 42 | T | FIAS/GS | TLE-HS | CBZ, PHB, VGB |
| #8 | 18 | M | 16 | T | FAS | FCDIIb/mTOR mut | CBZ, VPA, LMT, LCM |
| #9 | 45 | M | 34 | T | FAS | FCDIIb | LEV, OCZ |
Patients #1, 2: tissues from autopsies of patients affected by Dravet syndrome (DS) with SCN1A mutation; Patients #3 and 4: tissues from tuberous sclerosis complex (TSC) patients (TSC2 mutation). All autopsies were performed within 24 h of death. Patients #5–7: temporal lobe epilepsy (TLE) patients with hippocampal sclerosis (HS). Patients #8 and 9: tissues from focal cortical dysplasia type IIb (FCDIIb). ASMs—anti-seizure medications; CBZ—carbamazepine; CLB—clobazam; F—female; FAS—focal aware seizures; FIAS—focal impaired awareness seizures; GS—generalized seizures; LCM—lacosamide; LEV—levetiracetam; LMT—lamotrigine; M—male; mut—mutation; OCZ—oxcarbazepine; PHB—phenobarbital; STP—stiripentol; T—temporal; TPM—topiramate; VGB—vigabatrin; VPA—valproic acid.
The patients in Table 1 underwent neurosurgical intervention for the treatment of pharmaco-resistant epilepsy due to TLE or FCDIIb. After resection, the tissue samples were instantaneously snap-frozen in liquid nitrogen, processed and subsequently used to perform our electrophysiology experiments. All the autopsies from which we obtained the DS and TSC samples were performed no more than 24 h after death, upon obtainment of specific written consent for the subsequent use for research purposes. Control cases (two samples: 61 and 38 years old, males) did not have a prior history of epilepsy, possessed a normal cortical histology that matched their age and did not have any relevant neuropathology. Both controls died of myocardial infarction. As previously reported, we already analyzed the immunoreactivity profiles of cortical tissues from autopsies and surgeries and found only slight differences between these [23,24]. All the procedures which required the use of human material were carried out following the guidelines from the Declaration of Helsinki and the Amsterdam UMC Research Code provided by the Medical Ethics Committee.
2.2. Membrane Preparation
The tissues were shipped in dry ice and either directly prepared for the electrophysiological recordings or conserved at −80 °C. The protocols for human membrane extraction and their microinjection in Xenopus oocytes have already been published and these procedures were carried out as previously described [25]. Concisely, tissues were homogenized in a membrane buffer solution (in mM: glycine 200, NaCl 150, EGTA 50, EDTA 50 and sucrose 300; plus 20 µL of protease inhibitors (P2714, Sigma); pH 9, adjusted with NaOH). Then, the samples were centrifuged for 15 min at 9500× g. Subsequently, the supernatant was centrifuged for 2 h at 100,000× g with an ultra-centrifuge (Beckman-Coulter). The pellet was rinsed with sterilized water and re-suspended in assay buffer (glycine 5 mM) for immediate use or stored at −80°. The use of laboratory animals (Xenopus laevis) and all the related procedures (surgery, oocytes extraction and their utilization) were validated by the Italian Ministry of Health and followed its guidelines (authorization no 427/2020-PR).
2.3. Xenopus oocytes Electrophysiology
The electrophysiological recordings on Xenopus oocytes were performed 24–48 h after cytoplasmic injection [25] using the technical approach of two-electrode voltage clamp. We recorded GABA-evoked currents (IGABA) [26] after placing the oocytes in a 0.1 mL recording chamber and continuously perfusing them with oocyte Ringer solution (OR, in mM: NaCl 82.5; KCl 2.5; CaCl2 2.5; MgCl2 1; Hepes 5, adjusted to pH 7.4 with NaOH) at room temperature (20–22 °C). The perfusion system was operated by a computer connected to a gravity-driven multi-valve device (9–10 mL/min; Biologique RSC-200; Claix, France), which granted precise control of the duration of GABA applications. With this setup, the whole volume of the recording chamber was entirely replaced in 0.5 to 1 s. For all the microtransplanted oocytes, we tested the stability of IGABA by assessing two consecutive GABA pulses, separated by a 4 min wash-out. In order to evaluate the acute effect of CBD (2 μM for 10 s), we used only the cells characterized by a <5% IGABA modification. We defined IGABA variation as a percent increase or decrease in the mean current elicited by two GABA applications before and after exposure to CBD. The GABA current run-down was defined as the decrease in the peak current amplitude after six consecutive GABA applications (10 s) spaced out by 40 s of wash-out and expressed as a percentage (I6th peak/I1st peak × 100). In another set of experiments, we used human GABAAR subunits encoding cDNAs (pCDM8 vector α1β2γ2 ratio 1:1:2; α1β2 ratio 1:1; cDNAs were a kind gift from Dr. K. Wafford). In this case, we performed an intranuclear injection in Xenopus oocytes with a pressure microinjector (PLI-100, Warner Instruments, Holliston, MA, USA).
2.4. Statistics
We assessed normal distribution with the Shapiro–Wilk test in order to choose parametric (Student’s t-test) or non-parametric (Wilcoxon signed rank test, Mann–Whitney rank sum test) tests before starting the data analysis process. Data were statistically analyzed using Sigmaplot 12 software, and differences between two data sets were considered significant when p < 0.05. Oocytes used in each experiment are indicated as (n).
3. Results
CBD modulation on GABA-evoked currents in Xenopus oocytes micro-transplanted with DS human tissues
DS is an epileptic disorder characterized by pharmaco-resistant recurrent seizures. CBD showed promising results both in animal models and in clinical trials [27,28,29], and was, thus, approved to treat this condition both in the USA and Europe [18,19]. In this set of experiments, we were able to test the effect of an acute application of CBD in two brain samples obtained from adult DS patients (Table 1). After the injection of oocytes with this membrane preparation, we elicited GABA-evoked currents (IGABA) with applications of 4 s of GABA (50 μM) (mean −65.0 ± 7.1 nA; Figure 1A; n = 20; #1, 2, Table 1).
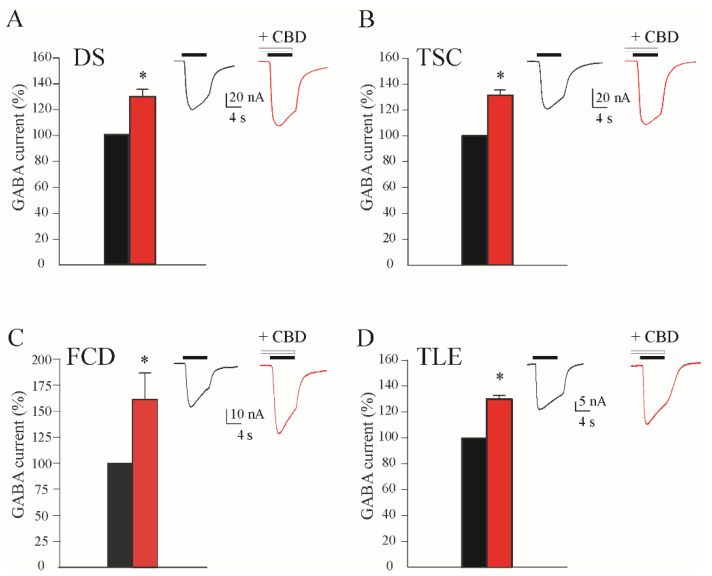
Figure 1.
CBD effect on GABA currents (IGABA) amplitude in oocytes transplanted with tissues from (A) Dravet syndrome (DS, IGABA range: from 11.2 to 122.4), (B) tuberous sclerosis complex (TSC, IGABA range: from 7.3 to 85.1), (C) focal cortical dysplasia type IIb (FCD, IGABA range: from 6.8 to 72.3) and (D) temporal lobe epilepsy (TLE, IGABA range: from 6.2 to 89.3) patients. All the bar-graphs show the % variation in the mean current amplitude after incubation with CBD 2 μM (black, % current before incubation; red, % current after incubation), * p < 0.05. In each panel, the inset represents sample currents with or without CBD. Black horizontal bars represent GABA 50 μM application; white horizontal bars represent CBD 2 μM application.
In order to confirm the recordings of genuine IGABA, we completely blocked these currents with high concentrations of bicuculline (100 μM; not shown). For comparison, we recorded, with the same approach, IGABA from oocytes injected with membranes obtained from two control patients without any neurological disease (see Methods). We found a comparable IGABA amplitude (mean −63.3 ± 3.5 nA; n = 12). When we recorded IGABA after the acute co-application of GABA and CBD, we found a significant increase in the elicited IGABA (+27.4 ± 4.8%; GABA 50 μM; CBD 2 μM; Figure 1A; n = 16; p < 0.05; #1–2; Table 1). This CBD effect on IGABA was fast and reversible, since after 5 min of washing with the oocyte Ringer solution (OR), the IGABA amplitude recovered to pre-treatment values (−2.7% ± 5.7%; GABA 50 μM; n = 8; p > 0.05 compared to the control current; #1–2; Table 1). Afterwards, with the same approach, we tested the effect of CBD on the oocytes injected with the control cortical membranes obtained from the autopsies of healthy patients. Interestingly, we found that CBD was able to increase the amplitudes of IGABA in the control tissues (+25.5 ± 2.4%; GABA 50 μM, n = 10; p < 0.05, CBD 2 μM), similarly to what was showed in DS.
CBD modulation on GABA-evoked currents in Xenopus oocytes micro-transplanted with TSC human tissues
Since the use of CBD was approved by both the FDA and the EMA to treat TSC patients as an add-on therapy [20], we obtained surgical brain samples from these patients in order to evaluate if CBD was able to modulate IGABA in this genetic epileptic condition. Indeed, TSC is a genetic, rare and multi-systemic disease characterized by different neurological alterations, including strong pharmaco-resistant seizures [30]. Different studies have highlighted an altered neurotransmission in this pathology, engaging both an inhibitory and excitatory transmission [31]. CBD was able to significantly increase evoked IGABA when applied together with GABA (CBD = +29.4 ± 3.0%; GABA 50 μM; CBD 2 μM; n = 18; p < 0.05; #3 and #4; Table 1; Figure 1B). Again, also in this case, the effect was reversible, since 5 min of wash-out with OR was able to recover the IGABA amplitudes to pre-CBD levels (−4.0% ± 2.5%; GABA 50 μM; n = 10; p > 0.05 compared to the control current; #1–2; Table 1).
CBD modulation on GABA-evoked currents in Xenopus oocytes micro-transplanted with FCDIIb human tissues
FCDs are a group of pathological conditions characterized by an altered cortical development often associated with pharmaco-resistant epilepsy [32,33,34]. Among them, FCD type IIb represents the most common malformation of cortical development [34]. The histopathological hallmark of FCDIIb is represented by an altered cortical lamination and the presence of cellular abnormalities (e.g., balloon cells and dysmorphic neurons) linked to an altered inhibitory neurotransmission, leading thus to SRSs [35,36]. We investigated the possible effect of CBD on GABAARs using brain tissues from FCD patients that underwent epilepsy surgery. We used the same approach used above for DS and TSC, recording bicuculline-sensitive IGABA (not shown) in order to test the CBD effect on these human samples. We obtained a statistically significant increase in IGABA amplitudes (CBD = +60.5 ± 25.5%; GABA 50 μM; CBD 2 μM; n = 11; p < 0.05; #8 and #9; Table 1; Figure 1C) that was, as in the case of DS and TSC, completely washable after 5 min of wash-out with OR solution (not shown).
CBD modulation on GABA-evoked currents in Xenopus oocytes micro-transplanted with TLE human tissues
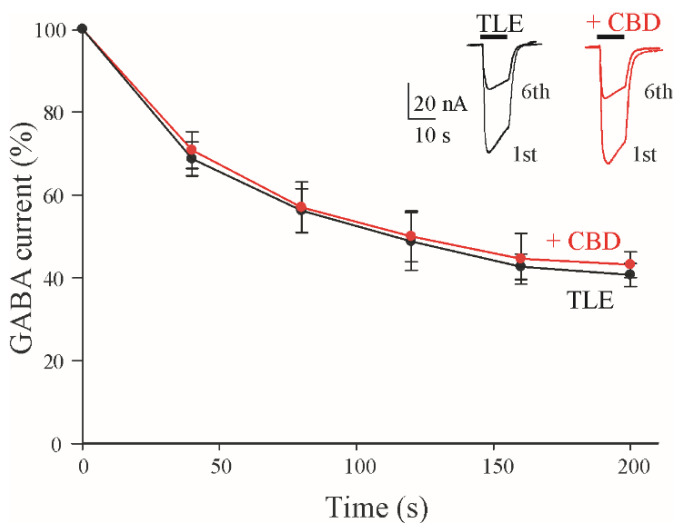
TLE represents the most common type of focal epilepsy in adulthood [37], and based on seizure semiology, it can fall into two different categories: the most common mesial form, which is characterized by mesial temporal lobe (mTLE) symptoms, and a rarer form with lateral temporal lobe symptoms (lTLE) [38]. Usually, after an epileptogenic insult (i.e., head trauma, stroke, brain tumor, brain infection), several pathological and physio-pathological alterations occur, and after a latent period that can last from hours to years, the brain becomes epileptic [37]. One of the main alterations that occurs during epileptogenesis, responsible for the recurrent seizures, is an imbalance between the excitatory and inhibitory neurotransmissions [39]. In particular, an increased use-dependent desensitization (i.e., GABA current run-down) of the GABAARs was described as a pathological hallmark of pharmaco-resistant mTLE [40,41,42]. Briefly, GABAARs from mTLE tissue become less responsive to repeated activation than those from healthy control tissue [41]. This GABA current run-down may imply hyper-excitability. In our experiments, we found that CBD application (2 μM) was able to induce a washable increase in IGABA (CBD = +30.4 ± 2.2%; GABA 50 μM; n = 20; #5–7; Table 1; Figure 1D). In another set of experiments, we tested whether CBD was able to improve the GABAA current run-down in order to restore a more physiological GABAergic neurotransmission. As previously reported [42,43], the application of 500 μM GABA in the oocytes injected with the membranes from the cortex of mTLE patients exhibited a statistically significant current run-down (the IGABA elicited by the sixth GABA application fell to 40.7 ± 2.8 %; n = 12; #5–7; Table 1; Figure 2).
Figure 2.
GABA current run-down in oocytes microtransplanted with mTLE tissues. Time course of GABA current run-down relative in mTLE without (●) and with (CBD) (●) application. IGABA were normalized to those elicited by the first GABA application. Data are expressed as mean ± SEM. Inset represents superimposed first and sixth GABA applications (500 μM) without (black traces) or with CBD (red traces). Black horizontal bars represent GABA application; CBD 2 μM was perfused during the run-down protocol (in red).
Surprisingly, an acute application of CBD 2 μM did not significantly change the rate of run-down (42.9 ± 2.8%; GABA+ CBD 2 μM; n = 16; #5–7; Table 1), while the increase in IGABA currents still persisted, even if to a lesser extent. This last evidence suggests that the CBD effect on GABA current amplitudes is not linked to the desensitization processes.
CBD modulation of GABAergic neurotransmission of α1β2 GABAA receptors
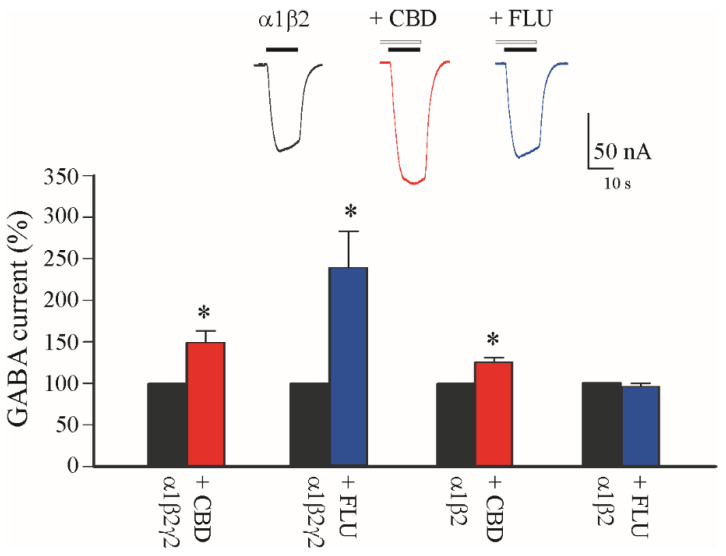
To better understand the CBD effect on IGABA amplitudes, we performed another set of experiments on oocytes expressing single GABAARs without ancillary or associated proteins. Thus, we intranuclearly injected cDNAs encoding for the most common GABAAR subunits in CNS (α1β2γ2) with or without the γ GABAAR subunit that is involved in the benzodiazepine (BDZ) binding site [44]. In these conditions, CBD was able to significantly increase the IGABA amplitudes (α1β2γ2: +48.0 ± 13.0%; GABA 5 μM; Figure 3; n = 6). For comparison, we also tested BDZ flunitrazepam (FLU, 2 μM), and we found, confirming previous reports [45], a strong potentiation of the elicited IGABA (+140 ± 43%; GABA 5 μM; Figure 3; n = 6). Subsequently, we expressed α1β2-containing GABAARs, and we found that, while the application with FLU was completely ineffective in increasing IGABA (−4 ± 3.5%; GABA 5 μM; Figure 3; n = 6), when we applied CBD, we still found a significant increase in IGABA (α1β2: +24.5 ± 3.8%; GABA 5 μM; Figure 3; n = 6).
Figure 3.
CBD effect in oocytes expressing α1β2γ2 and α1β2 GABAA receptor subunits. Bar graphs show the GABA current increase (as %) induced by CBD 2 μM (red bars) and flunitrazepam (FLU, blue bars) 6 μM in oocytes intranuclearly injected with cDNAs encoding human α1β2γ2 and α1β2 GABAAR subunits, * p < 0.05. In the inset, representative current traces of the experiment with α1β2-containing GABAARs in control condition (black trace), and with CBD (red trace) and FLU (blue trace) as indicated; note the ineffectiveness of FLU in this condition. Black horizontal bars represent GABA 5 μM application, white horizontal bars represent CBD 2 μM application.
4. Discussion
In this paper, using human epileptic brain tissues, we were able to highlight a modulation of CBD on the function of GABAARs. GABA impairment is a well-established mechanism involved in brain hyperexcitability [46]. Indeed, several studies have demonstrated how GABAAR subunits’ composition and functional properties change in epileptic disorders [42,47]. Furthermore, some of the most used ASMs, such as BDZ and barbiturates, act specifically on this class of ionotropic receptors, since GABAAR represents one of the main pharmacological targets in epileptic disorders, and the recovery of its physiological function could lead to a correct inhibitory neurotransmission [46]. In addition to BDZ and other GABAergic ASMs, pCBs represent a new class of pharmacological tools that show promising results in pre-clinical and clinical studies. Specifically, CBD obtained approval for use in some strongly pharmaco-resistant epilepsies such as DS, LG syndrome and, lately, TSC, as an add-on therapy [18,19,29]. The mechanism by which CBD and other pCBs exert their therapeutic effects still remains partially unclear, as multiple different targets have been identified [6,7,8,9,10,12,13,14,15,16,48]. Here, taking advantage of the micro-transplantation of human brain membranes in Xenopus laevis oocytes, we confirm that CBD can affect GABAergic neurotransmission by acting on human GABAARs. We tested CBD on four different epileptic conditions (DS, TSC, FCDIIb and mTLE), all characterized by a strong pharmaco-resistance to canonical ASMs, showing that CBD can significantly and reversibly potentiate GABAergic-evoked currents in all these diseases. Interestingly, in FCDIIb, the CBD effect seems more significant, thus suggesting that this potentiation may also be related to different cellular subtypes and/or different GABAAR arrangements. Further studies using human slices from FCDIIb patients could better elucidate this specific point. However, we can hypothesize that CBD’s ability to reduce the frequency and severity of epileptic seizures [18,19] is, at least in part, due to an interaction with GABAARs. In addition, we demonstrated that CBD also carries out its action on control tissues, indicating that this compound can also modulate the normal function of GABAARs. Interestingly, the aforementioned CBD effect is quite fast and rapidly washable, suggesting that its action is not mediated by the activation of intracellular pathways, as previously shown for other ASMs [46,49]. In line with this evidence, we did not observe any CBD effect on GABA current run-down in mTLE, an impairment that is prevented by acting on the phosphorylation mechanisms of GABAARs and/or their associated proteins [23,40,42]. To further exclude the possibility that CBD could interact with endogenous proteins and/or the activation of host cell intracellular signaling, we expressed, via the intranuclear injection of cDNAs, human GABAARs formed by α1β2γ2, the most common subunit composition, showing again a clear increase of IGABA. Altogether, these results strengthen our hypothesis that CBD can directly bind to GABAARs, increasing its efficacy, and that this effect is not mediated by specific CBRs [2,50]. Moreover, in different forms of epilepsy, GABAAR undergoes different rearrangements of its subunit composition, such as in DS [45] or mTLE [42,47]. These subunit changes can modify GABAAR’s function, often determining a reduced inhibitory tone and altering the effectiveness of ASMs on the original molecular target [51]. This hypothesis is strengthened by several studies that clearly showed CBD’s ability to modulate different GABAAR subunit compositions [17,45], such as α2, α3 and α6, indicating, thus, that its action is not linked to the different α subunit expressions. In the aforementioned conditions, drugs targeting new and alternative modulatory sites on GABAARs are likely to yield better results compared to classical ASMs. Noteworthily, we showed that CBD’s effect on IGABA still persists in α1β2—GABAARs, while a classical BDZ [44] did not show any significant effect. This last finding suggests that CBD may act also on defective GABAARs, especially in those conditions where BDZs are partially or completely ineffective [52]. Interestingly, as demonstrated in other studies [17], CBD is also able to modulate δ-containing GABAARs, making it an interesting pharmacological tool to potentially treat other epileptic pharmaco-resistant conditions characterized by a dysfunction of “tonic” GABAAR neurotransmission [53]. Further studies are required in order to better characterize CBD’s modulation of GABAAR’s function and identify the exact site of CBD binding on human GABAARs. In conclusion, we can hypothesize that CBD, being devoid of relevant psychotropic effects [11,54], can open new perspectives for its use, not only in epileptic diseases, but also in the treatment of other neurological and psychiatric conditions where GABAergic transmission is impaired, such as attention-deficit hyperactivity disorders (ADHD) [55] and neurodegenerative diseases [56,57,58].
Acknowledgments
The work was supported by grants from Ateneo Project (Sapienza University)”, grant n° RM11916B84D24429 (EP), RG12117A8697DCF1 (EP, GR) and AICE-FIRE 2022 (GR). GR was supported by the Italian Ministry of Health’s “Ricerca corrente”. This research was also supported by the intramural “DISCAB” GRANT 2022 code 07_DG_2022_05 to P.C.
Author Contributions
Conceptualization: E.P., E.A., G.R. and P.C.; Methodology: E.P., G.R. and P.C.; Investigation: A.G., B.C., C.P., G.R. and P.C.; Data Curation: A.G., A.M., B.C., C.P., G.R. and P.C.; Writing—Original Draft Preparation: E.P., E.A., G.R. and P.C.; Writing—Review and Editing: A.M., E.P., E.A., G.R. and P.C.; Supervision: E.P. and E.A.; Funding Acquisition: E.P. and E.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Human tissue was obtained and used in accordance with the Declaration of Helsinki and the Amsterdam UMC Research Code provided by the Medical Ethics Committee for studies involving humans. The use of Xenopus laevis frogs, the surgical procedures for oocytes extraction and their use conformed to the Italian Ministry of Health guidelines and were approved by the same institution (authorization no 427/2020-PR).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This project has received funding from the European Union’s Horizon 2020 Research and Innovation Program under grant agreement No. 952455.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Amin M.R., Ali D.W. Pharmacology of Medical Cannabis. Adv. Exp. Med. Biol. 2019;1162:151–165. doi: 10.1007/978-3-030-21737-2_8. [DOI] [PubMed] [Google Scholar]
- 2.Hill A.J., Williams C.M., Whalley B.J., Stephens G.J. Phytocannabinoids as Novel Therapeutic Agents in CNS Disorders. Pharmacol. Ther. 2012;133:79–97. doi: 10.1016/j.pharmthera.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Mechoulam R., Gaoni Y. A Total Synthesis of Dl-delta-1-tetrahydrocannabinol, the Active Constituent of Hashish. J. Am. Chem. Soc. 1965;87:3273–3275. doi: 10.1021/ja01092a065. [DOI] [PubMed] [Google Scholar]
- 4.Matsuda K. Synapse Organization and Modulation via C1q Family Proteins and Their Receptors in the Central Nervous System. Neurosci. Res. 2017;116:46–53. doi: 10.1016/j.neures.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Munro S., Thomas K.L., Abu-Shaar M. Molecular Characterization of a Peripheral Receptor for Cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 6.Bisogno T., Hanus L., De Petrocellis L., Tchilibon S., Ponde D.E., Brandi I., Moriello A.S., Davis J.B., Mechoulam R., Di Marzo V. Molecular Targets for Cannabidiol and Its Synthetic Analogues: Effect on Vanilloid VR1 Receptors and on the Cellular Uptake and Enzymatic Hydrolysis of Anandamide. Br. J. Pharmacol. 2001;134:845–852. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franco V., Perucca E. Pharmacological and Therapeutic Properties of Cannabidiol for Epilepsy. Drugs. 2019;79:1435–1454. doi: 10.1007/s40265-019-01171-4. [DOI] [PubMed] [Google Scholar]
- 8.Ryberg E., Larsson N., Sjögren S., Hjorth S., Hermansson N.-O., Leonova J., Elebring T., Nilsson K., Drmota T., Greasley P.J. The Orphan Receptor GPR55 Is a Novel Cannabinoid Receptor. Br. J. Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ibeas Bih C., Chen T., Nunn A.V.W., Bazelot M., Dallas M., Whalley B.J. Molecular Targets of Cannabidiol in Neurological Disorders. Neurotherapeutics. 2015;12:699–730. doi: 10.1007/s13311-015-0377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross H.R., Napier I., Connor M. Inhibition of Recombinant Human T-Type Calcium Channels by Delta9-Tetrahydrocannabinol and Cannabidiol. J. Biol. Chem. 2008;283:16124–16134. doi: 10.1074/jbc.M707104200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morano A., Fanella M., Albini M., Cifelli P., Palma E., Giallonardo A.T., Di Bonaventura C. Cannabinoids in the Treatment of Epilepsy: Current Status and Future Prospects. Neuropsychiatr. Dis. Treat. 2020;16:381–396. doi: 10.2147/NDT.S203782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiong W., Cui T., Cheng K., Yang F., Chen S.-R., Willenbring D., Guan Y., Pan H.-L., Ren K., Xu Y., et al. Cannabinoids Suppress Inflammatory and Neuropathic Pain by Targeting A3 Glycine Receptors. J. Exp. Med. 2012;209:1121–1134. doi: 10.1084/jem.20120242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahrens J., Demir R., Leuwer M., de la Roche J., Krampfl K., Foadi N., Karst M., Haeseler G. The Nonpsychotropic Cannabinoid Cannabidiol Modulates and Directly Activates Alpha-1 and Alpha-1-Beta Glycine Receptor Function. Pharmacology. 2009;83:217–222. doi: 10.1159/000201556. [DOI] [PubMed] [Google Scholar]
- 14.Russo E.B., Burnett A., Hall B., Parker K.K. Agonistic Properties of Cannabidiol at 5-HT1a Receptors. Neurochem. Res. 2005;30:1037–1043. doi: 10.1007/s11064-005-6978-1. [DOI] [PubMed] [Google Scholar]
- 15.Resstel L.B.M., Tavares R.F., Lisboa S.F.S., Joca S.R.L., Corrêa F.M.A., Guimarães F.S. 5-HT1A Receptors Are Involved in the Cannabidiol-Induced Attenuation of Behavioural and Cardiovascular Responses to Acute Restraint Stress in Rats. Br. J. Pharmacol. 2009;156:181–188. doi: 10.1111/j.1476-5381.2008.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahgoub M., Keun-Hang S.Y., Sydorenko V., Ashoor A., Kabbani N., Al Kury L., Sadek B., Howarth C.F., Isaev D., Galadari S., et al. Effects of Cannabidiol on the Function of A7-Nicotinic Acetylcholine Receptors. Eur. J. Pharmacol. 2013;720:310–319. doi: 10.1016/j.ejphar.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Bakas T., van Nieuwenhuijzen P.S., Devenish S.O., McGregor I.S., Arnold J.C., Chebib M. The Direct Actions of Cannabidiol and 2-Arachidonoyl Glycerol at GABAA Receptors. Pharmacol. Res. 2017;119:358–370. doi: 10.1016/j.phrs.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 18.U.S. Food and Drug Administration FDA Approves First Drug Comprised of an Active Ingredient Derived from Marijuana to Treat Rare, Severe Forms of Epilepsy. [(accessed on 5 November 2022)]; Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-comprised-active-ingredient-derived-marijuana-treat-rare-severe-forms.
- 19.GW Pharmaceuticals Receives European Commission Approval for Epidyolex. [(accessed on 5 November 2022)]. Available online: https://www.thepharmaletter.com/article/gw-pharmaceuticals-receives-european-commission-approval-for-epidyolex-for-seizures.
- 20.Thiele E.A., Bebin E.M., Filloux F., Kwan P., Loftus R., Sahebkar F., Sparagana S., Wheless J. Long-Term Cannabidiol Treatment for Seizures in Patients with Tuberous Sclerosis Complex: An Open-Label Extension Trial. Epilepsia. 2022;63:426–439. doi: 10.1111/epi.17150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miledi R., Palma E., Eusebi F. Microtransplantation of Neurotransmitter Receptors from Cells to Xenopus Oocyte Membranes: New Procedure for Ion Channel Studies. Methods Mol. Biol. 2006;322:347–355. doi: 10.1007/978-1-59745-000-3_24. [DOI] [PubMed] [Google Scholar]
- 22.Eusebi F., Palma E., Amici M., Miledi R. Microtransplantation of Ligand-Gated Receptor-Channels from Fresh or Frozen Nervous Tissue into Xenopus Oocytes: A Potent Tool for Expanding Functional Information. Prog. Neurobiol. 2009;88:32–40. doi: 10.1016/j.pneurobio.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Roseti C., Fucile S., Lauro C., Martinello K., Bertollini C., Esposito V., Mascia A., Catalano M., Aronica E., Limatola C., et al. Fractalkine/CX3CL1 Modulates GABAA Currents in Human Temporal Lobe Epilepsy. Epilepsia. 2013;54:1834–1844. doi: 10.1111/epi.12354. [DOI] [PubMed] [Google Scholar]
- 24.Roseti C., van Vliet E.A., Cifelli P., Ruffolo G., Baayen J.C., Di Castro M.A., Bertollini C., Limatola C., Aronica E., Vezzani A., et al. GABAA Currents Are Decreased by IL-1β in Epileptogenic Tissue of Patients with Temporal Lobe Epilepsy: Implications for Ictogenesis. Neurobiol. Dis. 2015;82:311–320. doi: 10.1016/j.nbd.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Palma E., Esposito V., Mileo A.M., Di Gennaro G., Quarato P., Giangaspero F., Scoppetta C., Onorati P., Trettel F., Miledi R., et al. Expression of Human Epileptic Temporal Lobe Neurotransmitter Receptors in Xenopus Oocytes: An Innovative Approach to Study Epilepsy. Proc. Natl. Acad. Sci. USA. 2002;99:15078–15083. doi: 10.1073/pnas.232574499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miledi R., Eusebi F., Martínez-Torres A., Palma E., Trettel F. Expression of Functional Neurotransmitter Receptors in Xenopus Oocytes after Injection of Human Brain Membranes. Proc. Natl. Acad. Sci. USA. 2002;99:13238–13242. doi: 10.1073/pnas.192445299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaplan J.S., Stella N., Catterall W.A., Westenbroek R.E. Cannabidiol Attenuates Seizures and Social Deficits in a Mouse Model of Dravet Syndrome. Proc. Natl. Acad. Sci. USA. 2017;114:11229–11234. doi: 10.1073/pnas.1711351114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang R., Fang F. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N. Engl. J. Med. 2017;377:699. doi: 10.1056/NEJMc1708349. [DOI] [PubMed] [Google Scholar]
- 29.Devinsky O., Cross J.H., Laux L., Marsh E., Miller I., Nabbout R., Scheffer I.E., Thiele E.A., Wright S. Cannabidiol in Dravet Syndrome Study Group Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N. Engl. J. Med. 2017;376:2011–2020. doi: 10.1056/NEJMoa1611618. [DOI] [PubMed] [Google Scholar]
- 30.Curatolo P., Specchio N., Aronica E. Advances in the Genetics and Neuropathology of Tuberous Sclerosis Complex: Edging Closer to Targeted Therapy. Lancet Neurol. 2022;21:843–856. doi: 10.1016/S1474-4422(22)00213-7. [DOI] [PubMed] [Google Scholar]
- 31.Ruffolo G., Iyer A., Cifelli P., Roseti C., Mühlebner A., van Scheppingen J., Scholl T., Hainfellner J.A., Feucht M., Krsek P., et al. Functional Aspects of Early Brain Development Are Preserved in Tuberous Sclerosis Complex (TSC) Epileptogenic Lesions. Neurobiol. Dis. 2016;95:93–101. doi: 10.1016/j.nbd.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 32.Iffland P.H., Crino P.B. Focal Cortical Dysplasia: Gene Mutations, Cell Signaling, and Therapeutic Implications. Annu. Rev. Pathol. 2017;12:547–571. doi: 10.1146/annurev-pathol-052016-100138. [DOI] [PubMed] [Google Scholar]
- 33.Najm I., Lal D., Alonso Vanegas M., Cendes F., Lopes-Cendes I., Palmini A., Paglioli E., Sarnat H.B., Walsh C.A., Wiebe S., et al. The ILAE Consensus Classification of Focal Cortical Dysplasia: An Update Proposed by an Ad Hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia. 2022;63:1899–1919. doi: 10.1111/epi.17301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blumcke I., Spreafico R., Haaker G., Coras R., Kobow K., Bien C.G., Pfäfflin M., Elger C., Widman G., Schramm J., et al. Histopathological Findings in Brain Tissue Obtained during Epilepsy Surgery. N. Engl. J. Med. 2017;377:1648–1656. doi: 10.1056/NEJMoa1703784. [DOI] [PubMed] [Google Scholar]
- 35.Blauwblomme T., Dossi E., Pellegrino C., Goubert E., Iglesias B.G., Sainte-Rose C., Rouach N., Nabbout R., Huberfeld G. Gamma-Aminobutyric Acidergic Transmission Underlies Interictal Epileptogenicity in Pediatric Focal Cortical Dysplasia. Ann. Neurol. 2019;85:204–217. doi: 10.1002/ana.25403. [DOI] [PubMed] [Google Scholar]
- 36.Cepeda C., Chen J.Y., Wu J.Y., Fisher R.S., Vinters H.V., Mathern G.W., Levine M.S. Pacemaker GABA Synaptic Activity May Contribute to Network Synchronization in Pediatric Cortical Dysplasia. Neurobiol. Dis. 2014;62:208–217. doi: 10.1016/j.nbd.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pitkänen A., Lukasiuk K. Mechanisms of Epileptogenesis and Potential Treatment Targets. Lancet Neurol. 2011;10:173–186. doi: 10.1016/S1474-4422(10)70310-0. [DOI] [PubMed] [Google Scholar]
- 38.Boluda S., Seilhean D., Bielle F. Neuropathology of the Temporal Lobe. Handb. Clin. Neurol. 2022;187:407–427. doi: 10.1016/B978-0-12-823493-8.00027-4. [DOI] [PubMed] [Google Scholar]
- 39.Gambardella A., Labate A., Cifelli P., Ruffolo G., Mumoli L., Aronica E., Palma E. Pharmacological Modulation in Mesial Temporal Lobe Epilepsy: Current Status and Future Perspectives. Pharmacol. Res. 2016;113:421–425. doi: 10.1016/j.phrs.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 40.Palma E., Ragozzino D.A., Di Angelantonio S., Spinelli G., Trettel F., Martinez-Torres A., Torchia G., Arcella A., Di Gennaro G., Quarato P.P., et al. Phosphatase Inhibitors Remove the Run-down of Gamma-Aminobutyric Acid Type A Receptors in the Human Epileptic Brain. Proc. Natl. Acad. Sci. USA. 2004;101:10183–10188. doi: 10.1073/pnas.0403683101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ragozzino D., Palma E., Di Angelantonio S., Amici M., Mascia A., Arcella A., Giangaspero F., Cantore G., Di Gennaro G., Manfredi M., et al. Rundown of GABA Type A Receptors Is a Dysfunction Associated with Human Drug-Resistant Mesial Temporal Lobe Epilepsy. Proc. Natl. Acad. Sci. USA. 2005;102:15219–15223. doi: 10.1073/pnas.0507339102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mazzuferi M., Palma E., Martinello K., Maiolino F., Roseti C., Fucile S., Fabene P.F., Schio F., Pellitteri M., Sperk G., et al. Enhancement of GABA(A)-Current Run-down in the Hippocampus Occurs at the First Spontaneous Seizure in a Model of Temporal Lobe Epilepsy. Proc. Natl. Acad. Sci. USA. 2010;107:3180–3185. doi: 10.1073/pnas.0914710107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palma E., Roseti C., Maiolino F., Fucile S., Martinello K., Mazzuferi M., Aronica E., Manfredi M., Esposito V., Cantore G., et al. GABA(A)-Current Rundown of Temporal Lobe Epilepsy Is Associated with Repetitive Activation of GABA(A) “Phasic” Receptors. Proc. Natl. Acad. Sci. USA. 2007;104:20944–20948. doi: 10.1073/pnas.0710522105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rudolph U., Knoflach F. Beyond Classical Benzodiazepines: Novel Therapeutic Potential of GABAA Receptor Subtypes. Nat Rev. Drug Discov. 2011;10:685–697. doi: 10.1038/nrd3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruffolo G., Cifelli P., Roseti C., Thom M., van Vliet E.A., Limatola C., Aronica E., Palma E. A Novel GABAergic Dysfunction in Human Dravet Syndrome. Epilepsia. 2018;59:2106–2117. doi: 10.1111/epi.14574. [DOI] [PubMed] [Google Scholar]
- 46.Palma E., Ruffolo G., Cifelli P., Roseti C., van Vliet E.A., Aronica E. Modulation of GABAA Receptors in the Treatment of Epilepsy. Curr. Pharm. Des. 2017;23:5563–5568. doi: 10.2174/1381612823666170809100230. [DOI] [PubMed] [Google Scholar]
- 47.Brooks-Kayal A.R., Russek S.J. Regulation of GABAA Receptor Gene Expression and Epilepsy. In: Noebels J.L., Avoli M., Rogawski M.A., Olsen R.W., Delgado-Escueta A.V., editors. Jasper’s Basic Mechanisms of the Epilepsies. National Center for Biotechnology Information (US); Bethesda, MD, USA: 2012. [PubMed] [Google Scholar]
- 48.Iannotti F.A., Hill C.L., Leo A., Alhusaini A., Soubrane C., Mazzarella E., Russo E., Whalley B.J., Di Marzo V., Stephens G.J. Nonpsychotropic Plant Cannabinoids, Cannabidivarin (CBDV) and Cannabidiol (CBD), Activate and Desensitize Transient Receptor Potential Vanilloid 1 (TRPV1) Channels in Vitro: Potential for the Treatment of Neuronal Hyperexcitability. ACS Chem. Neurosci. 2014;5:1131–1141. doi: 10.1021/cn5000524. [DOI] [PubMed] [Google Scholar]
- 49.Palma E., Ragozzino D., Di Angelantonio S., Mascia A., Maiolino F., Manfredi M., Cantore G., Esposito V., Di Gennaro G., Quarato P., et al. The Antiepileptic Drug Levetiracetam Stabilizes the Human Epileptic GABAA Receptors upon Repetitive Activation. Epilepsia. 2007;48:1842–1849. doi: 10.1111/j.1528-1167.2007.01131.x. [DOI] [PubMed] [Google Scholar]
- 50.Cifelli P., Ruffolo G., De Felice E., Alfano V., van Vliet E.A., Aronica E., Palma E. Phytocannabinoids in Neurological Diseases: Could They Restore a Physiological GABAergic Transmission? Int. J. Mol. Sci. 2020;21:723. doi: 10.3390/ijms21030723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Czornyj L., Auzmendi J., Lazarowski A. Transporter Hypothesis in Pharmacoresistant Epilepsies. Is It at the Central or Peripheral Level? Epilepsia Open. 2022;7((Suppl. S1)):S34–S46. doi: 10.1002/epi4.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burman R.J., Rosch R.E., Wilmshurst J.M., Sen A., Ramantani G., Akerman C.J., Raimondo J.V. Why Won’t It Stop? The Dynamics of Benzodiazepine Resistance in Status Epilepticus. Nat. Rev. Neurol. 2022;18:428–441. doi: 10.1038/s41582-022-00664-3. [DOI] [PubMed] [Google Scholar]
- 53.Cope D.W., Di Giovanni G., Fyson S.J., Orbán G., Errington A.C., Lorincz M.L., Gould T.M., Carter D.A., Crunelli V. Enhanced Tonic GABAA Inhibition in Typical Absence Epilepsy. Nat. Med. 2009;15:1392–1398. doi: 10.1038/nm.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scheffer I.E., Halford J.J., Miller I., Nabbout R., Sanchez-Carpintero R., Shiloh-Malawsky Y., Wong M., Zolnowska M., Checketts D., Dunayevich E., et al. Add-on Cannabidiol in Patients with Dravet Syndrome: Results of a Long-Term Open-Label Extension Trial. Epilepsia. 2021;62:2505–2517. doi: 10.1111/epi.17036. [DOI] [PubMed] [Google Scholar]
- 55.Poleg S., Golubchik P., Offen D., Weizman A. Cannabidiol as a Suggested Candidate for Treatment of Autism Spectrum Disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2019;89:90–96. doi: 10.1016/j.pnpbp.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 56.Cheng D., Spiro A.S., Jenner A.M., Garner B., Karl T. Long-Term Cannabidiol Treatment Prevents the Development of Social Recognition Memory Deficits in Alzheimer’s Disease Transgenic Mice. J. Alzheimers Dis. 2014;42:1383–1396. doi: 10.3233/JAD-140921. [DOI] [PubMed] [Google Scholar]
- 57.Stampanoni Bassi M., Sancesario A., Morace R., Centonze D., Iezzi E. Cannabinoids in Parkinson’s Disease. Cannabis Cannabinoid Res. 2017;2:21–29. doi: 10.1089/can.2017.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sieradzan K.A., Fox S.H., Hill M., Dick J.P., Crossman A.R., Brotchie J.M. Cannabinoids Reduce Levodopa-Induced Dyskinesia in Parkinson’s Disease: A Pilot Study. Neurology. 2001;57:2108–2111. doi: 10.1212/WNL.57.11.2108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.