Abstract
Redox equilibria and the modulation of redox signalling play crucial roles in physiological processes. Overproduction of reactive oxygen species (ROS) disrupts the body’s antioxidant defence, compromising redox homeostasis and increasing oxidative stress, leading to the development of several diseases. Manganese superoxide dismutase (MnSOD) is a principal antioxidant enzyme that protects cells from oxidative damage by converting superoxide anion radicals to hydrogen peroxide and oxygen in mitochondria. Systematic studies have demonstrated that MnSOD plays an indispensable role in multiple diseases. This review focuses on preclinical evidence that describes the mechanisms of MnSOD in diseases accompanied with an imbalanced redox status, including fibrotic diseases, inflammation, diabetes, vascular diseases, neurodegenerative diseases, and cancer. The potential therapeutic effects of MnSOD activators and MnSOD mimetics are also discussed. Targeting this specific superoxide anion radical scavenger may be a clinically beneficial strategy, and understanding the therapeutic role of MnSOD may provide a positive insight into preventing and treating related diseases.
Keywords: MnSOD, oxidative stress, ROS, human diseases, MnSOD mimetics
1. Introduction
Reactive oxygen species (ROS) are a series of molecular oxygen derivatives that regulate numerous physiological and pathological processes [1]. As a group of chemical species, ROS can be categorized into non-radical and free radical species. Non-radical ROS include hydrogen peroxide (H2O2), organic hydroperoxides (ROOH), singlet molecular oxygen (1O2), ozone (O3), hypochlorous acid (HOCl), and hypobromous acid (HOBr), whereas free radical ROS include superoxide anion radical (O2•−), hydroxyl radical (·OH), peroxyl radical (ROO·) and alkoxyl radical (RO·) [2]. Cellular ROS are generated endogenously through mitochondrial oxidative phosphorylation, or exogenously stimulated by xenobiotics, cytokines, and bacterial infection [3,4]. O2•− and H2O2 are acknowledged as the most physiologically relevant ROS. O2•− is mainly generated by nicotinamide adenine dinucleotide phosphate oxidases (NOXs), or the complexes I and III of the mitochondrial electron transport chain (ETC). H2O2 is generated by superoxide dismutases (SOD), NOX4, monoamine oxidases, and xanthine oxidases within a few different organelles (endoplasmic reticulum, mitochondria, and peroxisomes). More importantly, ROS are required for numerous cellular processes including cell growth, differentiation, and death by acting as signalling molecules [5]. Multiple signalling pathways are involved in mediating ROS, including nuclear factor kappa-B (NF-κB), mitogen-activated protein kinase (MAPK) cascade, Kelch-like ECH-associated protein 1-nuclear factor erythroid 2-related factor 2-antioxidant response elements (Keap1-Nrf2-ARE) signalling, adenosine 5′-monophosphate-activated protein kinase (AMPK), phosphoinositide-3 kinase-(PI3K-) Akt pathway, etc. [6,7].
The dynamic balance between ROS production and antioxidant capacity responsibly maintains the cellular redox homeostasis [8]. However, oxidative stress occurs when elevated ROS overwhelms the cellular antioxidant defence, damaging nucleic acids, proteins, and lipids [4,5,9]. To protect cells from oxidative damage as well as to maintain physiological ROS levels, organisms have developed antioxidant defence systems that comprise small-molecular-weight antioxidants and antioxidant enzymes. Small-molecular-weight antioxidants include glutathione (GSH), cysteine, ascorbic acid, and α-tocopherol, while antioxidant enzymes include SOD, catalase (CAT), glutathione peroxidase (GPX), glutaredoxin (GRX), thioredoxin (TXN), and peroxiredoxin (PRX) [10,11,12]. From a pharmacokinetic point of view in scavenging intracellular ROS, targeting antioxidant enzymes is more effective than small-molecule antioxidants, and ultimately maintains the antioxidant defence [13]. A series of antioxidant enzymes such as CAT, GPX, and PRX eliminate H2O2 by converting H2O2 into H2O [14,15,16]. On the other hand, SOD is the most powerful O2•− scavenger, which catalyses the dismutation of O2•− into H2O2 and O2 [17]. Currently, three isoforms of SOD have been discovered in mammalian cells, namely, copper–zinc superoxide dismutase (Cu/ZnSOD, SOD1), manganese superoxide dismutase (MnSOD, SOD2), and the extracellular superoxide dismutase (EcSOD, SOD3) [5]. Of these isoforms, MnSOD garners widespread interest as it potently scavenges mitochondrial O2•−, which is the major source of cellular ROS [18]. The indispensable role of MnSOD is further highlighted by the evidence of significant neonatal mortality in MnSOD-deficient mice, compared to the considerable neonatal survival in the SOD1- or SOD3-deficient mice [19,20,21,22].
MnSOD is a nuclear-encoded enzyme that translocates into the mitochondrial matrix [12]. It is constituted by a homotetramer containing an active site with manganese as a cofactor. Despite its enzymatic antioxidant function, MnSOD readily binds with iron, and the produced iron-substituted enzyme attains peroxidase activity that generates highly reactive hydroxyl radicals [23,24]. Therefore, in protecting against oxidative stress, manganese seems like the perfect metal, while the quaternary structure of MnSOD responsibly maintains the catalytic and dismutase activity [25]. The expression and activity of MnSOD is regulatable at multiple levels, from transcription and translation to posttranslational modifications [26]. NF-κB, specificity protein 1 (Sp1), activating protein-1 (Ap1), p53, and CCAAT binding protein (C/EBP) are the major transcription factors that regulate MnSOD gene expression by directly binding to specific DNA elements or interacting with its partners [27,28]. Furthermore, posttranslational modifications regulate MnSOD protein expression and activity through nitration, phosphorylation, and acetylation. Peroxynitrite (ONOO-) inactivates MnSOD by nitrating the tyrosine-34 amino acid residue of MnSOD protein. Acetylation at lysine-68, lysine-122, lysine-53, and lysine-89 amino acid residues, also inactivate MnSOD. In addition, sirtuin 3 (SIRT3) reactivates MnSOD by deacetylating the above lysine residues in response to irradiation, nutritional deprivation, and oxidative stress [25,27]. Moreover, MnSOD activity and stability are enhanced through phosphorylation at Ser106 by the mitochondrial cell-cycle kinase 1 (Cdk1) [29].
Considering that MnSOD engages in detoxifying ROS through O2•− dismutation, which possibly becomes a contributing factor or consequence in multiple diseases, it is vital to understand the physiological and pathological role of MnSOD. Recent evidence has revealed the pathological involvement of MnSOD in a number of diseases (Figure 1). For example, the enzymatic activity or expression of MnSOD is frequently downregulated in numerous diseases, such as diabetes and neurodegenerative diseases [30,31,32,33], whereas overexpression of MnSOD protects against pro-oxidant insults resulting from inflammatory cytokines, irradiation, hyperoxic injury, and ischaemia/reperfusion [34,35,36,37,38,39,40]. In addition, selective MnSOD mimetics have therapeutic potential in treating rheumatoid arthritis (RA), ischaemic stroke, and kidney diseases by mimicking the activity of MnSOD [41,42,43,44]. Nevertheless, a comprehensive summary of MnSOD regulatory roles in a range of diseases is lacking. In this review, we focus on the mechanisms of MnSOD in regulating various diseases that are associated with imbalanced redox status, including inflammation, fibrotic diseases, diabetes, neurodegenerative diseases, vascular diseases, and cancer (Figure 2). Advances in the therapeutic application of MnSOD activators and MnSOD mimetics are also discussed (Table 1). Moreover, limitations and critical issues in these MnSOD-related studies are addressed. Further understanding of how MnSOD can be regulated in the clinic may provide strategies and prospects for antioxidant therapy. Additionally, the comprehensive information and scientific advances presented in this review may facilitate the understanding of the physiological and pathological roles of MnSOD.
Figure 1.
An overview of MnSOD expression in various pathological states of human diseases. Abnormal expression levels of MnSOD have been detected in multiple diseases and have a wide impact on different organs, followed by various pathological alterations such as inflammation, fibrosis, sclerosis, and neuron degeneration. MnSOD, manganese superoxide dismutase; ROS, reactive oxygen species; AD, Alzheimer’s disease; Aβ, amyloid β-protein; HD, Huntington’s disease; HTT, huntingtin; PD, Parkinson’s disease; DA, dopaminergic; ALS, amyotrophic lateral sclerosis; IPF, idiopathic pulmonary fibrosis; ILD, interstitial lung disease; TNF, tumour necrosis factor; IL, interleukin; NASH, non-alcoholic steatohepatitis; HSCs, hepatic stellate cells; T2DM, type 2 diabetes mellitus; RA, rheumatoid arthritis; FLSs, fibroblast-like synoviocytes; MKD, mevalonate kinase deficiency; MI, myocardial infarction; AS, atherosclerosis; IBD, inflammatory bowel disease; UC, ulcerative colitis. ‘↑’ represents increased or upregulated, while ‘↓’ represents decreased or downregulated (created with BioRender.com (accessed on 4 November 2022)).
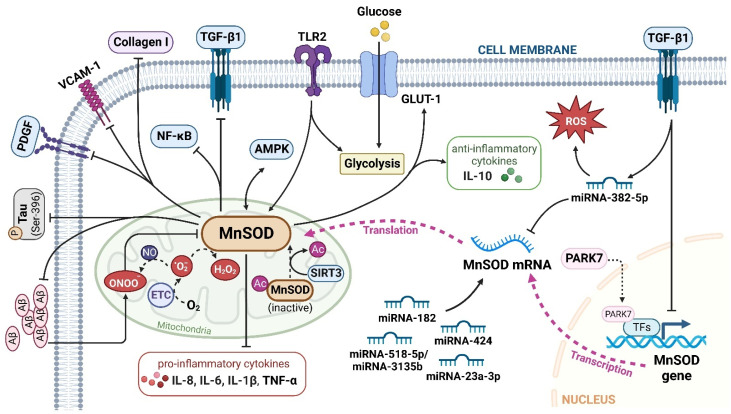
Figure 2.
The major signalling pathways involved in MnSOD regulation. MnSOD is localized in the mitochondrial matrix to catalyse the dismutation of O2•− to H2O2, and regulate cellular redox homeostasis. Multiple factors, cytokines, proteins, and miRNAs have been involved in the transcriptional and translational modulation of MnSOD. TGF-β1, transforming growth factor-β1; TLR2, toll-like receptor 2; Aβ, amyloid, β-protein; GLUT-1, glucose transporter member 1; IL, interleukin; VCAM-1, vascular cell adhesion molecule-1; AMPK, adenosine 5′-monophosphate-activated protein kinase; P, phospho; PDGF, platelet-derived growth factor; NF-κB, nuclear factor kappa-B; ETC, electron transport chain; PARK7, Parkinson disease protein 7; TFs, transcriptional factors; Ac, acetyl; SIRT3, sirtuin 3. ‘↑’ represents increased or upregulated, while ‘⊥’ represents suppressed or downregulated (created with BioRender.com (accessed on 4 November 2022)).
Table 1.
MnSOD-based therapeutic strategies.
| Type | Treatment | Chemical Structures | Disease | Mechanism | Therapeutic Effect | Ref |
|---|---|---|---|---|---|---|
| Clinical agents | Rebamipide |

|
Bowel inflammation | ↑MnSOD protein levels ↓superoxide anion leakage |
↓NSAID-induced mucosal injury | [45] |
| Melatonin |

|
Cardiac fibrosis | ↑MnSOD activity ↑SIRT3-mediated MnSOD deacetylation |
↓cardiac dysfunction and fibrosis | [46] | |
| Vitamin C |

|
Diabetes | ↑MnSOD mRNA levels | protected diabetic tissues | [47] | |
| NAC |

|
HD | ↑MnSOD activity ↓mitochondrial dysfunction |
↓neurobehavioural deficits | [48] | |
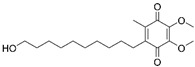
| Idebenone |
|
AS | ↑SIRT3-MnSOD-mtROS pathway | stabilized atherosclerotic plaques | [49] | |
| Asprosin | N/A | Ischaemic heart disease | ↑ERK1/2-MnSOD signalling ↓mesenchymal stromal cell apoptosis |
↑function of cardiac ejection ↓MI-induced myocardial remodelling |
[50] | |
| Nestorone |

|
Ischaemic stroke | ↑MnSOD protein levels | ↓behavioural and histological stroke outcomes | [51] | |
| Sevoflurane |

|
Myocardial I/R injury | ↑MnSOD activity and protein levels | ↓myocardial I/R injury | [36,37,38] | |
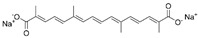
| Trans sodium crocetinate |
|
|||||
| Irisin | N/A | |||||
| MnSOD mimetics |
ZMTP | N/A | RA | ↑transition of M1 macrophages to M2 phenotype ↓IL-6, IL-1β and TNF-α ↑arginase-1 and IL-10 |
↑antiarthritic efficacy | [41] |
| MnTBAP |

|
Kidney disease | ↓angiotensin converting enzyme/angiotensin II signalling ↓mitochondria-derived oxidative stress |
↓albumin-overload-induced abnormalities | [42,44] | |
| AS | reversed the proatherosclerotic macrophage phenotype | ↓AS progression | [52] | |||
| MnTMPyP |

|
AD | ↓ROS during neurodegeneration | protected hippocampal neurons | [53] | |
| PD | ↑MnSOD protein levels ↓DA neurons loss |
↓nigrostriatal DA neurodegeneration | [54] | |||
| Ischaemic stroke | ↓intracellular superoxide radical levels | ↓cerebral infarct volume ↑neurological function |
[43] | |||
| mitoTEMPO |

|
Hypertension | ↑reendothelialization capability of endothelial progenitor cells | ↓blood pressure ↑vasorelaxation |
[55,56] | |
| AS | ↓intimal smooth muscle cell apoptosis and matrix degradation | ↓features of atherosclerotic plaque vulnerability | [57] | |||
| Natural compounds | Curcumin |

|
Hepatic fibrosis | ↑MnSOD activity and mRNA levels ↓HSC activation and ROS |
↓fibrogenesis | [58,59] |
| Alpha mangostin |

|
|||||
| Hawthorn fruit extract | N/A | AS | ↑MnSOD mRNA levels | ↓atherogenesis | [49] | |
| Proteins | rhMnSOD | N/A | RA | ↑plasma concentration of MnSOD | ↓paw swelling and bone destruction | [60] |
| Hepatic fibrosis | ↓HSC activation | ↓hepatic fibrosis | [61] | |||
| rhMnSOD-Hirudin fusion protein | N/A | IPF | ↓fibroblasts proliferation ↓profibrotic genes (Ctgf, Fn, Col1a1) |
↓lung inflammation and fibrosis | [62] | |
| Oncolytic vaccinia virus | OVV-MnSOD | N/A | Lymphoma | ↑lymphocyte infiltration ↑tumour sensitivity to anti-PD-L1 treatment |
↓tumour progression | [63] |
Note: mtROS, mitochondrial reactive oxygen species; NSAID, nonsteroidal anti-inflammatory drug; SIRT3, sirtuin 3; NAC, N-acetyl-L-cysteine; HD, Huntington’s disease; PD, Parkinson disease; AD, Alzheimer’s disease; PD-L1, programmed death ligand 1; ERK1/2, extracellular signal-regulated kinase 1/2; MI, myocardial infarction; I/R, ischaemia/reperfusion; IL, interleukin; TNF, tumour necrosis factor; DA, dopaminergic; HSC, hepatic stellate cell; OVV, oncolytic vaccinia virus; AS, atherosclerosis; RA, rheumatoid arthritis; IPF, idiopathic pulmonary fibrosis; ZMTP, Zn-MnIII meso-tetrakis (4-carboxyphenyl) porphyrin-polyvinylpyrrolidone; MnTBAP, MnIII tetrakis (4-benzoic acid) porphyrin chloride; MnTMPyP, MnIII tetrakis (1-methyl-4-pyridyl) porphyrin. ‘↑’ represents increased, upregulated, induced, enhanced, or activated, while ‘↓’ represents decreased, downregulated, or inhibited, and ‘N/A’ represents not applicable.
2. MnSOD in Diseases
2.1. Inflammatory Diseases
Inflammation acts as a defensive mechanism to confront harmful stimuli such as infection and tissue damage [64,65]. Oxidative stress in activated infiltrating immune cells and tissue-resident immune cells, such as macrophages and T cells, is responsible for inflammation-related diseases [66,67]. Numerous studies have demonstrated that MnSOD plays a vital role in protecting organismal integrity and homeostasis by regulating inflammatory chemokines and cytokines [68,69] (Figure 2).
2.1.1. Pulmonary Inflammation
MnSOD was found to attenuate pulmonary inflammation in different models of lung damage [34,39,70,71]. Exposure to high oxygen concentrations can cause hyperoxic lung injury, characterized by the accumulation of toxic ROS and the production of proinflammatory cytokines in the lung, such as interleukin (IL)-6, IL-8, and IL-1β [16,71,72]. MnSOD elevation weakened hyperoxia-induced A549 cell growth inhibition, at least in part by suppressing ROS-induced IL-8 production [34]. Likewise, MnSOD overexpression in the lung epithelium of transgenic mice prevented hyperoxic lung injury and improved the survival rate after exposure to 95% O2 [39]. Targeting MnSOD is also a promising strategy for treating radiation-induced lung damage. Chen et al. revealed that the tail vein injection of MnSOD-overexpressing mesenchymal stem cells (MnSOD-MSCs) alleviated radiation-induced lung histopathological injury and improved the mouse survival rate. Mechanistically, MnSOD-MSCs significantly mitigated lung inflammation by impeding proinflammatory cytokine activation (tumour necrosis factor (TNF)-α, IL-1β, and IL-6) and strengthening the anti-inflammatory cytokine (IL-10) effect in treated mice [35].
2.1.2. Bowel Inflammation
Oxidative stress increases damage to the mucosal layer in the gastrointestinal tract and stimulates an uncontrolled immune response, contributing to the initiation and development of inflammatory bowel disease (IBD) [73,74]. Ikumoto et al. found that MnSOD expression was elevated in the neutrophils, macrophages, and polymorphonuclear leukocytes in mucosal biopsy specimens of ulcerative colitis (UC) patients and characterized it as a potent diagnostic biomarker indicating a poor prognosis [75,76]. The engineered probiotic Bifidobacterium longum with a constructed expression system successfully secreted a biologically active penetratin-hMn-SOD fusion protein, which significantly inhibited ROS levels and cytokine release (TNF-α, IL-6, IL-1β, and IL-8), thus attenuating UC damage both in vitro and in vivo [77]. Consistently, Lactobacillus gasseri secreting MnSOD significantly inhibited inflammation in colonic tissues and alleviated IL-10-deficiency-induced colitis [78]. Moreover, rebamipide, an antiulcer agent, relieved mucosal injury and nonsteroidal anti-inflammatory drug (NSAID)-induced gastrointestinal complications such as gastric ulcers and erosions, by increasing MnSOD protein levels and suppressing superoxide anion leakage [45].
2.1.3. Renal Inflammation
MnSOD has been demonstrated to play a protective role in maintaining kidney function. MnSOD-deficient mice exhibited increased oxidative stress, renal interstitial T cells, macrophage infiltration, tubular damage, and glomerular sclerosis compared with wild-type mice [79]. Additionally, albumin-overloaded mice exhibited decreased MnSOD protein levels and increased mitochondrial oxidative stress and inflammation in the kidney. Applying the MnSOD mimic MnIII tetrakis (4-benzoic acid) porphyrin chloride (MnTBAP) rescued albumin overload-induced abnormalities by inhibiting angiotensin converting enzyme/angiotensin II signalling and renal sodium transporter expression [42,44].
2.1.4. Rheumatic and Musculoskeletal Diseases
Rheumatic and musculoskeletal diseases (RMDs) can be divided into autoinflammatory diseases and autoimmune diseases [80]. Both complex inflammatory processes and activated innate and adaptive immunity contribute to the pathogenesis of RMDs [81,82].
Autoinflammatory diseases are a group of hereditary disorders that are characterized by seemingly unprovoked episodes of periodic fever and inflammation at certain body parts, and may even involve multiple organ systems [83]. Mevalonate kinase deficiency (MKD) is a rare paediatric disease caused by mutations in the mevalonate kinase (MVK) gene and the accumulation of mevalonolactone (MEV) metabolites [84]. Gratton et al. suggested that excess MEV disrupted mitochondrial functions and induced MnSOD gene expression in response to augmented ROS production in U-87 MG human glioblastoma cells [85]. Another inflammatory disorder of unknown aetiology, Behcet’s disease, is characterized by recurrent aphthous ulcers [86,87]. The Val/Val genotype of the MnSOD gene was significantly associated with Behcet’s disease in a group of Turkish patients [88]. These findings indicate that MnSOD is correlated with autoinflammatory diseases, but the detailed mechanisms remain largely unknown.
Autoimmune diseases, such as psoriasis and RA, involve errors in self-discrimination by adaptive immune mechanisms [89,90]. MnSOD was found to be overexpressed in the skin tissue of psoriasis patients, which protected against skin damage caused by elevated levels of the proinflammatory factors IL-1β and TNF-α [91,92]. RA is a chronic autoimmune disease characterized by the abnormal proliferation of fibroblast-like synoviocytes (FLSs), leading to synovial joint inflammation and joint deformities [93,94]. A comparative proteomics study revealed that MnSOD was significantly downregulated in FLSs from patients with RA [95]. Recombinant human MnSOD (rhMnSOD) administration ameliorated paw swelling and bone destruction in an adjuvant-induced arthritis rat model [60]. Bowen et al. constructed a nanosheet, Zn-MnIII meso-tetrakis (4-carboxyphenyl) porphyrin-polyvinylpyrrolidone (ZMTP), to mimic MnSOD activity for RA treatment. They found that ZMTP mitigated oxidative stress in RA mice, decreased M1 macrophage markers (IL-6, IL-1β, and TNF-α), and promoted anti-inflammatory M2 phenotype markers (arginase-1 and IL-10) [41]. Seemingly contradictory, MnSOD was suppressed when RA-FLSs underwent mitochondrial-dependent apoptosis induced by resveratrol, indicating that the diverse roles of MnSOD in RA should be taken into consideration in future investigations [93].
2.2. Fibrotic Diseases
Fibrosis is defined by the excessive accumulation of extracellular matrix (ECM) components such as collagen and fibronectin in damaged tissues, which ultimately induce tissue sclerosis and organ failure [96]. This process is initiated by the continuous activation and transdifferentiation of local fibroblasts in response to chronic injury [97]. To date, the regulatory role of MnSOD in the fibrosis of multiple organs has been widely reported and summarized (Figure 1).
2.2.1. Hepatic Fibrosis
Hepatic fibrosis is the result of a sustained wound healing response to continuous liver injury, which is initiated by the activation and transdifferentiation of hepatic stellate cells (HSCs) [98,99,100]. Platelet-derived growth factor (PDGF) is the most potent HSC mitogen and is responsible for intracellular ROS production. MnSOD upregulation impedes PDGF-induced proliferation and ROS generation in HSCs [101]. Certain natural compounds, such as curcumin [58] and alpha mangostin [59], exert promising inhibitory effects against the fibrogenesis process by increasing MnSOD expression and ROS scavenging to hinder HSC activation (Table 1). In addition, Guillaume et al. reported that rhMnSOD treatment significantly reduced hepatic oxidative stress and deactivated HSCs in CCl4-induced cirrhotic rats [61]. Moreover, hepatic fibrosis progression is commonly accompanied by non-alcoholic fatty liver disease (NAFLD) [102]. Several studies have examined the relationship between NAFLD and a functional polymorphism in the targeting sequence of MnSOD. In brief, carriage of the ‘T’ allele in the SOD2 gene leads to less transport of MnSOD to the mitochondria, which is frequently found in non-alcoholic steatohepatitis (NASH) patients and is associated with the severity of liver fibrosis [103,104].
2.2.2. Cardiac Fibrosis
Evidence has shown that MnSOD plays an essential role in cardiac fibrosis progression. MnSOD-deficient mice developed heart hypertrophy with enhanced fibrosis [105], while MnSOD overexpression inhibited ageing-induced cardiac fibrosis in transgenic mice [106]. These studies indicate that MnSOD expression negatively regulates the severity of cardiac fibrosis but the underlying mechanisms vary. For example, Ma et al. reported that the expression of mouse myocardial MnSOD was upregulated by AMPK after swimming exercises, thus attenuating isoproterenol-induced cardiac fibrosis via ROS clearance [107]. Vivar et al. reported that MnSOD mRNA and protein expression levels were reduced by transforming growth factor (TGF)-β1 in cardiac fibroblasts (CFs), resulting in elevated ROS levels in CFs [108]. Consistently, MnSOD-overexpressing transgenic aged mice exhibited a significant decrease in cardiac oxidative stress and fibrotic marker (TGF-β1, collagen I) expression in the heart [106]. These findings indicate a potential regulatory loop between MnSOD and TGF-β1 in the activation of CFs and subsequent cardiac fibrosis (Figure 2). Interestingly, melatonin increased MnSOD activity by promoting SIRT3-mediated MnSOD deacetylation to attenuate mitochondrial oxidative injury, ultimately alleviating fine particulate matter (PM2.5) exposure-induced cardiac dysfunction and fibrosis [46].
2.2.3. Pulmonary Fibrosis
Pulmonary fibrosis is a common terminal syndrome in multiple lung diseases [109]. Since the lung is exposed to a hyperoxic environment and vulnerable to various oxidative pathogenic factors, MnSOD is especially vital for maintaining pulmonary redox balance to impede pulmonary fibrosis [110]. MnSOD activity was found to be significantly decreased in the fibrotic lung areas of patients with idiopathic pulmonary fibrosis (IPF). Further investigation showed that SIRT3 deficiency induced acetylation and the inactivation of MnSOD at lysine residue 68 exacerbated lung fibrosis in mice [111]. Moreover, a microarray indicated decreased MnSOD gene expression in systemic sclerosis interstitial lung disease (ILD) [112], and silencing MnSOD in fibroblasts derived from a non-ILD human resulted in a profibrotic phenotype [113]. Moreover, Zhang et al. reported that cigarette smoke extract induced lung fibrosis by downregulating MnSOD expression and enhancing collagen I expression in mice [114]. MnSOD is also recognized as a promising target for treating pulmonary fibrosis. Treatment with an rhMnSOD-Hirudin fusion protein effectively inhibited the proliferation of fibroblasts, suppressed profibrotic protein genes (Ctgf, Fn, Col1a1), and attenuated pulmonary fibrosis in vitro and in vivo [62].
2.2.4. Fibrosis in Other Organs
A reduction in MnSOD expression was found in the kidneys of unilateral ureteric obstruction (UUO)-induced renal fibrosis mice [115,116], while Parkinson disease protein 7 (PARK7) protected against chronic kidney injury and fibrosis by stimulating MnSOD expression, thus reducing oxidative stress in both tubular cells and kidney tissues of UUO mice [117]. Rossi et al. discovered that TGF-β1/miRNA-382-5p/MnSOD axis deregulation is vital in the pathogenesis of myelofibrosis by enhancing oxidative stress and inflammation [118]. In addition, intra-arterial injection of lentiviral MnSOD (LVMnSOD) in rats protected skin tissues from fibrosis by reducing the fibrotic burden and skin contracture after irradiation [40].
2.3. Diabetes
Diabetes mellitus occurs whereby an abnormally high blood glucose level (hyperglycaemia) results from insulin insufficiency, and/or impaired tissue response to insulin (i.e., insulin resistance) [119]. It is a heterogeneous disorder with two major forms, type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM), both of which are exacerbated by free radicals produced via glucose autoxidation [120,121,122]. Emerging evidence has indicated that the CT genotype of the MnSOD 47C/T gene is a significant risk factor for T1DM susceptibility (OR = 2.37; CI 95% = 1.03 to 5.46; p = 0.040) [123]. Here, we summarize the different roles of MnSOD in regulating diabetes and its complications.
2.3.1. Type 1 and Type 2 Diabetes Mellitus
T1DM is an autoimmune disease triggered by the immune system attacking β-cells in the pancreatic islets, leading to β-cell dysfunction and hyperglycaemia [124]. T2DM, accounting for 85% of the diabetes burden, is caused by insulin resistance and inadequate compensatory insulin secretion, which is associated with several lifestyle factors, such as age, obesity, and pregnancy [125,126]. Smriti et al. found that MnSOD mRNA levels in the serum of newly diagnosed T2DM patients were obviously decreased (p = 0.02), indicating a correlation between MnSOD and T2DM [31]. In an STZ-induced T1DM mouse model, MnSOD-overexpressing pancreatic β-cells improved blood glucose by inhibiting NF-κB activation and scavenging ROS [127]. Sadi et al. demonstrated that treatment with vitamin C enhanced the mRNA level but not the enzyme activity of MnSOD, thus protecting diabetic tissues against oxidative stress [47]. Islet transplantation is a promising therapy for diabetes; however, the islet isolation and transplantation processes generate oxygen radicals and cause oxidative stress damage, which is a major obstacle to islet replacement therapy [128]. To prevent oxidative damage, Suzanne et al. isolated MnSOD-overexpressing islets from young prediabetic NOD mice and transplanted them into STZ-induced T1DM NOD scid mice. The survival rate of islet graft mice was increased, and MnSOD overexpression prolonged islet function after transplantation [129].
2.3.2. Complications of Diabetes
Long-term hyperglycaemia causes diabetes complications that lead to damage throughout the body, which can be classified into microvascular and macrovascular damage [121,130,131]. Recent evidence has established that oxidative stress-regulated pathways in β-cell dysfunction and insulin resistance, collectively contribute to the development of diabetic complications, such as nephropathy, neuropathy, and retinopathy [132,133,134,135]. Oxidative stress has long been implicated in the progression of diabetes complications, leading to an increased risk of diabetic macrovascular and microvascular system lesions, such as diabetic cardiomyopathy and renal failure [133]. Increased MnSOD activity was observed as a self-defensive mechanism in the hearts of STZ-induced diabetic rats, indicating a protective role of MnSOD against oxidative stress-related damage in diabetic cardiomyopathy [136]. In addition, overexpression of MnSOD improved mitochondrial respiration and normalized contractility in diabetic cardiomyocytes by elevating cardiac catalase activity and preventing oxidative assaults [137]. Diabetic bladder dysfunction is another common diabetic consequence and has a profound impact on patient quality of life [138]. Excessive oxidative stress was observed in STZ-induced smooth muscle-specific MnSOD knockout mice, causing bladder dysfunction and apoptosis induction in detrusor smooth muscle, indicating a pivotal role of MnSOD in diabetic bladder dysfunction [139]. Moreover, MnSOD overexpression inhibited superoxide radicals and ameliorated the development of diabetic retinopathy by reducing glucose-induced mitochondrial DNA damage [140].
Taken together, these data highlight the involvement of MnSOD in regulating diabetes and its complications via oxidative stress inhibition.
2.4. Neurodegenerative Diseases
Neurodegenerative diseases are indicated by progressive degeneration and the selective loss of neuronal systems, leading to cognitive impairment, dementia, motor dysfunction, and even death [141]. The majority of neurodegenerative diseases are characterized by the accumulation of misfolded proteins in the central nerve system, e.g., amyloid-β (Aβ) and tau aggregates in Alzheimer disease (AD), SOD1 pathology in amyotrophic lateral sclerosis (ALS), and mutated huntingtin (HTT) in Huntington disease (HD), and accompanied by a progressive loss of neurons, e.g., the loss of dopaminergic (DA) neurons in PD (Parkinson’s disease) in the affected regions [142]. A number of studies have highlighted the potential therapeutic role of MnSOD in detoxifying cerebral ROS to inhibit the aggregation of misfolded proteins and protect against neurodegenerative disorders (Figure 1).
2.4.1. Alzheimer Disease
AD is a progressive neurodegenerative disorder characterized by impaired cognitive function and neuropsychiatric disorders. AD gradually destroys memory and thinking skills, and ultimately, disables the elderly from performing the simplest tasks [143,144,145]. Excessive accumulation of Aβ and phosphorylated tau are the main defining characteristics of AD [146,147]. Aβ peptides are generated from amyloid precursor protein (APP), which, together with presenilin 1 (PS-1) and PS-2, is suggested to be associated with the pathogenesis of early-onset AD [148]. It is well recognized that mitochondrial redox imbalance contributes to the neurodegeneration that occurs in AD; thus, MnSOD is involved in regulating AD progression, minimizing oxidative damage to the brain, and protecting cognitive function [149].
A decreased MnSOD level and an increased Aβ level were found in hippocampal neurons from autopsy-confirmed AD patients. This observation was also supported by spherical aggregation of Aβ in cultured human primary hippocampal neurons, which could be rescued by the MnSOD mimetic metalloporphyrin MnIII tetrakis (1-methyl-4-pyridyl) porphyrin (MnTMPyP) [53,150]. Moreover, deletion of one copy of the MnSOD allele exaggerated cerebrovascular amyloidosis, amyloid burden, and plaque-independent neuritic dystrophy. Mechanistically, the reduction in MnSOD exacerbated endogenous mitochondrial oxidative stress and increased the level of Ser-396 phosphorylated tau in a mutant human APP transgenic AD mouse model [151,152,153]. Interestingly, overexpression of MnSOD significantly reduced plaque deposition and ameliorated learning and spatial memory deficits by scavenging mitochondrial ROS in a Tg2576/MnSOD double transgenic mouse model [154]. Another study suggested that 12 weeks of treadmill exercise could have beneficial effects on modulating Aβ deposition and cognitive function in the early stage of AD. The level of MnSOD enzyme activity in the hippocampus was significantly increased after exercise and attenuated microglia-mediated neuroinflammation in an APP/PS-1 mouse model [155].
However, MnSOD plays a paradoxical role in AD. Using an MnSOD-deficient Saccharomyces cerevisiae strain, Franca et al. verified that oxidative stress is a consequence of amyloid induction, suggesting that MnSOD may not be directly associated with the amyloid process [156]. Furthermore, Aβ-induced oxidative stress results in highly nitrated MnSOD in the peripheral mononuclear blood cells of AD patients and APP/PS-1 mouse models, which likely contributes to the neurodegeneration in AD pathogenesis [157,158].
In conclusion, MnSOD plays an important role in regulating oxidative stress and protecting the neural system and thus has attracted increasing attention as a therapeutic target and drug candidate for AD.
2.4.2. Parkinson’s Disease
PD is a multifactorial neurodegenerative disease characterized by the loss of DA neurons in the substantia nigra, resulting in a significant reduction in dopamine levels in the striatum [159]. Numerous studies have revealed a link between oxidative stress and DA cell function in PD [160]. As an antioxidant enzyme, MnSOD enzyme activity was reduced by dopamine-quinone (DAQ), which is generated during DA neuron degeneration and causes oxidative stress in PD. This reduction further exacerbated oxidative damage and subsequent neuronal dysfunction and eventually led to cell death [30]. Decreased MnSOD expression was also observed in PD-causing neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated animal models, with severe loss of abnormal dopamine transporter (DAT)-immunoreactive DA neurons in the striatum and the substantia nigra [161,162]. This evidence indicates that MnSOD agonists may relieve PD by protecting DA neurons in the striatum and substantia nigra from oxidative stress. This has been confirmed by Dixit et al., who found that MnTMPyP could reverse MPTP-decreased MnSOD expression, thereby eliminating DA neuron loss in PD animals [54]. Moreover, increased MnSOD levels were found in the substantia nigra or frontal and motor cortex of PD patients, which could serve as an early diagnostic indicator [163,164,165]. Likewise, in two independent clinical trials, an increase in MnSOD mRNA levels was detected in 192 whole-blood samples from PD patients compared to healthy controls. A subsequent study suggested that the MnSOD mRNA level could be a potential blood biomarker for the diagnosis of PD patients [166]. A meta-analysis further revealed that the CC genotype of the MnSOD Val16Ala polymorphism indicated a higher susceptibility to PD in Han Chinese populations (OR = 1.77, 95% CI: 1.15–2.71, p = 0.01) [167].
2.4.3. Huntington Disease
HD is an autosomal dominant, neurodegenerative disease manifesting as progressive chorea, rigidity, dementia, and psychiatric disturbance caused by accumulation of the mutant HTT gene in neural and somatic cells [168]. It has been widely reported that oxidative stress and mitochondrial dysfunction are closely linked to HD, suggesting MnSOD as a potent therapeutic target to ameliorate neurodegeneration [169,170,171]. Astrocytes, the most abundant cell type in the brain, are implicated in neurodegenerative disorders and may contribute to striatal neuron loss or dysfunction in HD. HD astrocytes with the accumulation of mutant HTT showed a marked decrease in the expression of MnSOD, which is consistent with previous reports on brain samples from HD patients [32,33]. Similarly, MnSOD activity was suppressed in the mitochondria of a 3-nitropropionic acid (3-NP)-induced HD rat model, while ROS production and lipid peroxidation were enhanced, accompanied by increased neural space, neurodegeneration, and gliosis. N-acetyl-L-cysteine (NAC) administration significantly attenuated neurobehavioural deficits and mitochondrial dysfunction by increasing MnSOD activity [48]. Phenotypic alterations in skin fibroblasts from HD patients are another feature associated with HD pathogenesis. For instance, significantly increased expression of the MnSOD gene was observed in primary porcine skin fibroblasts from a transgenic (TgHD) minipig HD model. Moreover, MnSOD enzyme activity was markedly elevated in skin fibroblasts of HD patients and may serve as a defender against antioxidant damage and a potential biomarker of HD [172,173,174].
2.4.4. Amyotrophic Lateral Sclerosis
ALS is a rare and progressive neurodegenerative disease that is characterized by the degeneration of motor neurons in the brainstem and spinal cord, resulting in the loss of motor function and eventual death [175]. Although clinical evidence has confirmed that there is no significant correlation between MnSOD mutation and ALS, MnSOD can prevent SOD1 mutation-induced ALS [176]. For instance, MnSOD overexpression attenuated the neuronal cell death triggered by mutant SOD1G37R in neuroblastoma cells [177]. Partial deficiency of MnSOD in ALS mice, induced by SOD1G93A mutation, exacerbated motor neuron loss, motor deficits, and the death rate [178]. Increased MnSOD activity accompanied by excess superoxide production and lipid peroxidation was observed in the brain tissue homogenates of ALS rats [179]. Additionally, elevated MnSOD levels were observed in the motor neurons and glia of the brain stem in ALS patients, further supporting the proposed protective role of MnSOD in ALS [180,181].
2.5. Vascular Diseases
Redox homeostasis plays a vital role in vascular function. Dysregulated redox signalling can induce endothelial dysfunction and vascular abnormalities, contributing to different types of vascular diseases, such as hypertension and atherosclerosis (AS) and the occurrence of infarction [182,183,184]. Herein, we summarize the mechanisms of MnSOD-mediated protective effects through vascular remodelling in vascular diseases.
2.5.1. Hypertension
Hypertension is a disorder of circulatory regulation, with a persistent blood pressure elevation, and is listed as one of the major contributors to mortality [185]. In hypertension, abnormal oxidative stress increases both vascular stiffness and vascular smooth muscle cell adhesion, which further accelerates the development of endothelial dysfunction [186,187]. It has been reported that MnSOD in particular brain regions exerts antihypertensive effects by eradicating mitochondrial superoxide anions. For instance, MnSOD depletion in the brain subfornical organ (SFO) rather than in peripheral tissue significantly increased systemic mean arterial pressure and sensitized the pressor response, thus potentiating angiotensin II (AngII)-mediated hypertension in mice [188]. The gene expression and enzymatic activity of MnSOD were also found to be decreased in the brain stem rostral ventrolateral medulla (RVLM) of spontaneously hypertensive rats accompanied with elevated H2O2 and O2•− level. Microinjections of adenovirus encoding MnSOD into the RVLM elicited a long-lasting reduction in arterial pressure [189]. In addition, the elevation of blood pressure and early marker of endothelial dysfunction (vascular cell adhesion molecule-1, VCAM-1) was markedly suppressed in MnSOD-transduced carotid and femoral arteries in a hypertensive rat model [190]. Moreover, dysfunction of endothelial progenitor cells leads to impaired endothelial integrity in hypertension patients. Treatment with mitoTEMPO, a MnSOD mimetic, reduced blood pressure and oxidative stress and improved the reendothelialization capability of endothelial progenitor cells in AngII-induced hypertension mice, providing a potential vascular repair therapeutic target for hypertension [55,56].
Recent studies have reported that mitochondrial oxidative stress induced by MnSOD deficiency contributes to the development of persistent pulmonary hypertension of the newborn (PPHN). Afolayan et al. found that silencing the MnSOD gene reproduced PPHN phenotypes, such as right ventricular systolic pressure elevation and pulmonary artery endothelial cell (PAEC) apoptosis [191]. Furthermore, exogenous transduction of PAECs with MnSOD markedly improved the function of endothelial nitric oxide synthase and the relaxation response of pulmonary arteries in PPHN lambs, supporting the use of MnSOD as a therapeutic target in PPHN treatments [191,192].
2.5.2. Atherosclerosis
AS, a chronic disease defined as the formation of fibrofatty lesions in the artery wall, mainly results from pathologic processes characterized by vascular endothelial dysfunction and the formation of fibrous caps and plaques [193,194]. Emerging evidence has shown that MnSOD deficiency impairs vasomotor function and accelerates atherogenesis at arterial branch points [195,196]. Moreover, depletion of MnSOD increased the necrotic core, inflammatory cell infiltration, and the formation of atherosclerotic lesions in the arteries of an apolipoprotein E-deficient (ApoE-/-) spontaneous AS mouse model [57,197]. In addition, chronic inflammatory skin diseases, such as psoriasis, have been reported to accelerate AS. A significant reduction in MnSOD expression was found in psoriatic macrophages, while restoring MnSOD activity with MnTBAP reversed the proatherosclerotic phenotype in a mouse model [52]. Accordingly, elevating MnSOD activity could be a therapeutic approach to retard AS progression. For instance, treatment with the MnSOD mimetic mitoTEMPO attenuated atherosclerotic plaque vulnerability by preventing intimal smooth muscle cell apoptosis and matrix degradation in a mouse model [57]. The mitochondrial protective agent idebenone significantly stabilized atherosclerotic plaques in ApoE-/- mice by activating the SIRT3-MnSOD-mtROS pathway [49]. Treatment with hawthorn fruit extract significantly upregulates MnSOD expression, thus reducing trimethylamine-N-oxide-exacerbated atherogenesis in ApoE−/− mice [49].
2.5.3. Myocardial/Cerebral Infarction and Reperfusion Injury
Myocardial/cerebral infarction usually occurs when an atherosclerotic plaque hinders the blood supply in the heart or brain [194]. As a common therapy in the clinic, revascularization causes secondary damage to ischaemic tissues when blood flow is restored, which is called ischaemia/reperfusion (I/R) injury. MnSOD expression was elevated by an endogenous cardioprotective enzyme, dimethylarginine dimethylaminohydrolase 1 (DDAH1), to attenuate acute myocardial infarction (MI) by suppressing apoptosis and oxidative stress in cardiomyocytes [198]. Asprosin, a novel MI therapeutic agent, inhibited mesenchymal stromal cell apoptosis by activating extracellular signal-regulated kinase 1/2 (ERK1/2)-MnSOD signalling [50]. Furthermore, MnSOD is a key enzyme that plays a protective role after myocardial I/R injury. Various therapeutic agents used to treat I/R injury (Table 1), such as sevoflurane, trans sodium crocetinate, and irisin, were found to elevate MnSOD levels to protect cardiomyocytes from oxidative stress and apoptosis [36,37,38]. Moreover, MnSOD overexpression was verified to reduce the infarct heart size in a left coronary artery ligation I/R model in MnSOD transgenic mice [199].
Cerebral infarction (ischaemic stroke), defined as the sudden loss of focal neurological function due to cerebral ischaemia, is pathologically induced by hypertension, carotid stenosis, AS, or atrial fibrillation [200]. Zhao et al. reported that MnSOD was significantly elevated by miRNA-518-5p/miRNA-3135b signalling based on single-cell and bulk RNA-sequencing analyses, and this result was further validated in the ischaemic cerebral infarction group in a clinical cohort study [201]. An in vitro study demonstrated that a contraceptive drug, Nestorone, could ameliorate behavioral and histological stroke outcomes by activating MnSOD in neural stem cells [51]. Pretreatment with MnTMPyP significantly eliminated intracellular superoxide radical levels, and an in vivo study showed that it reduced cerebral infarct size and improved neurological function [43]. Moreover, elevated MnSOD expression in the cortex was demonstrated to ameliorate cerebral I/R-induced oxidative stress injury via several miRNAs (miRNA-424, miRNA-23a-3p, and miRNA-182) in a middle cerebral artery occlusion (MCAO) and reperfusion mouse model [202,203,204]. In addition, electroacupuncture pretreatment upregulated MnSOD expression to inhibit cellular apoptosis in the ischaemic penumbra in MCAO mice. Interestingly, the beneficial effects of electroacupuncture were reversed by MnSOD knockdown, indicating the unique role of MnSOD in protecting against I/R injury [205].
2.6. Cancer
Various aspects of the extensive role of MnSOD in cancer have been explored. MnSOD can directly mediate multiple cancer cell death signalling pathways, including apoptosis [206], pyroptosis [207], and autophagy [208]. High MnSOD expression contributes to chemoresistance [209,210,211] and radioresistance [212,213] in different cancer types. Recently, posttranslational modification of MnSOD, with particular emphasis on acetylation at lysine residue 68, was proposed and explored, which addressed its vital roles in promoting cancer progression [214,215,216]. These functional roles of MnSOD in cancer have been well-reviewed elsewhere [12,27,217,218]. Herein, we summarize some emerging research hotspots in MnSOD-regulated cancer progression, with an emphasis on metabolic reprogramming and the tumour immune microenvironment.
Metabolic reprogramming has been regarded as a hallmark of cancer for centuries. The Warburg effect primarily demonstrates that malignant cells preferentially rely on glycolysis rather than oxidative phosphorylation (OXPHOS) for energy supply [27,219,220]. Moreover, pathways involved in redox control are commonly reprogrammed due to tumorigenic mutations [221]. As MnSOD is one of the major redox metabolism regulators, the effect of MnSOD on cancer metabolic reprogramming has attracted broad attention. For instance, Liu et al. reported that toll-like receptor 2 (TLR 2) induced glycolysis and metabolic shift by markedly upregulating MnSOD expression in human gastric cancer cells. Furthermore, patient-derived tissue microarrays demonstrated a significant correlation between TLR2 and MnSOD expression in gastric tumours, indicating that the TLR2-MnSOD axis could serve as a potential biomarker for therapy decisions and prognosis determination in gastric cancer [222]. Zhou et al. observed that enhanced MnSOD expression activated AMPK signalling and shifted cell energy metabolism to glycolysis, thus facilitating tumorigenesis and progression in colorectal cancer cells [223]. Similarly, upregulation of MnSOD in cancer cells engaged AMPK to perform and sustain the Warburg effect, therefore supporting cancer cell survival [224]. Cells with a high level of MnSOD were more resistant to glucose-deprivation-induced cell death because MnSOD increased glucose uptake, glucose transporter member 1 (GLUT-1) availability, and OXPHOS electron transfer [225]. These results indicate a vital role of MnSOD in cancer glycolytic metabolism reprogramming, which deserves more in-depth mechanistic studies.
As the role of the immune system in cancer development has attracted increasing attention recently, the involvement of MnSOD in the tumour immune microenvironment has also been highlighted. Accumulating evidence has suggested that tumour-infiltrating immune cells participate in cancer progression, which is highly associated with MnSOD expression in different cancer types. For example, Su et al. analysed public datasets and found that MnSOD expression is positively correlated with CXCL8 and neutrophil infiltration, indicating an involvement of the ‘MnSOD-CXCL8-neutrophil recruitment’ axis in cancer progression [226]. MnSOD was also found to be positively correlated with CD68+ macrophage infiltration and may indicate poor outcomes in inflammation-driven lung adenocarcinoma [227]. High MnSOD expression was observed in aggressive triple-negative breast cancer (TNBC) patients and was regarded as a poor prognostic marker. MnSOD promotes the immunosuppressive tumour microenvironment by promoting M2 macrophage invasiveness and infiltration, supporting TNBC progression [228]. In addition, Lou et al. demonstrated that MnSOD-overexpressing oncolytic vaccinia virus (OVV-MnSOD) enhanced lymphocyte infiltration and tumour sensitivity to anti-PD-L1 treatment in lymphomatous mice, suggesting a promising therapeutic potential of MnSOD in cancer immunotherapy [63].
In addition, we recently explored whether MnSOD overexpression could prevent cancer relapse by inhibiting reactivation of quiescent cancer cells (data not published). This novel function of MnSOD further increases the potential benefits of targeting MnSOD in cancer treatment. Although the pleiotropic role of MnSOD in cancer progression has aroused extensive interest, the underlying mechanisms remain controversial and unclear. Breakthroughs in finding key players in MnSOD-cancer regulation will be a promising research area, and will provide positive insights into the development of new drugs based on MnSOD.
3. Discussion and Future Prospects
In this review, we described preclinical and clinical evidence on the involvement of MnSOD in inflammatory, fibrotic, neurodegenerative, and vascular diseases, as well as diabetes and cancers. Mechanistically, MnSOD mainly protects the host from oxidative stress by maintaining the redox homeostasis when exposed to stressors such as radiation, ultraviolet, chemotherapy, hypoxia, and hyperglycaemia [9,229,230,231]. Therefore, targeting MnSOD provides a promising strategy for preventing and treating redox-imbalance-related diseases.
However, several limitations and critical issues in these MnSOD-related studies remain to be elucidated. Though the above studies revealed that MnSOD is abnormally expressed in different diseases, whether these aberrant expressions are the cause or consequence of diseases remains undiscovered. Such a cause–effect relationship between MnSOD and diseases may largely determine the appropriateness of an MnSOD-targeting therapeutic approach. This point extends to another crucial question, whether oxidative stress is the principal pathogenesis in the above diseases? However, to date, the effectiveness of antioxidant defence is limited because oxidative stress is more often the secondary cause, rather than becoming the primary cause of disease [13]. Such an uncertain situation challenges the MnSOD-targeted strategy in effectively ameliorating disease. Exploring which oxygen-derived free radicals and/or molecule that principally trigger pathological consequences deserves serious consideration in stepping forward in this field. To this regard, applying the recent multi-omics and imaging technologies may provide comprehensive understanding on the above issue, to facilitate the identifying of impaired steps in redox metabolism, and ultimately, to evaluate the therapeutic effects of MnSOD-targeting agents.
Although few limitations in understanding the pathological role of MnSOD remain, emerging evidence has supported its broad clinical applicability and potential as a prognostic biomarker. For example, melatonin treatment (NCT02463318) attenuated oxidative stress by directly increasing the gene expression and enzyme activity of MnSOD in peripheral blood mononuclear cells (PBMCs) of relapsing–remitting multiple sclerosis (RRMS) patients [232]. A phase I-II study (NCT00618917) demonstrated that a swallowed MnSOD-plasmid/liposome (PL) transgene treatment alleviated radiation/chemotherapy-induced oesophagitis in advanced stage III non-small cell lung cancer patients. Moreover, it was found that MnSOD activity in the PBMCs of patients was linked to reduced diabetes- and cardiovascular-related complications [233,234]. After overall high-intensity training, T1DM patients (NCT02939768) showed increased MnSOD activity in mononuclear cells. A case–control study found that the MnSOD level in the PBMCs of patients could well reflect the therapeutic effect of resveratrol against coronary artery disease (NCT02137421).
MnSOD mimetics have recently gained increased attention, and a series of synthetic molecules were found to exhibit better uptake and ROS clearance efficiency [235,236]. For instance, M40403, a manganese poly-aza-macrocycle, synergistically enhanced the analgesic effects of morphine in postoperative dental pain [235]. Unfortunately, clinical trials investigating M40403 for cancer treatment have either been suspended (NCT00033956) or terminated (NCT00101621). MnBuOE, a type of manganese porphyrin, was used as a radioprotector of brain tissues in glioma patients (NCT02655601). In addition, MnBuOE was also studied in another phase II clinical trial to examine its protective effect against radiation-induced mucositis and xerostomia (NCT04607642). Furthermore, M40403 (NCT00033956), Tempol (NCT01324141), and calmangafodipir (NCT01619423) were studied in clinical trials as adjuvant agents to treat melanoma, renal cell carcinoma, anal cancer and advanced metastatic colorectal cancer. A preclinical study demonstrated that GC4419, a cyclic polyamine type of MnSOD mimetic, ameliorated the inflammatory response in chronic obstructive pulmonary disease [237]. GC4419 was studied in a phase II trial in patients with critical illness due to COVID-19 infection (NCT04555096) and might be a promising treatment for patients at risk of developing life-threatening SARS-CoV-2 [238].
Moreover, recent emphasis has been placed on reforming MnSOD or its mimetics to increase the efficiency and accuracy of tissue-specific delivery. An engineered recombinant MnSOD (rMnSOD) protein containing a leader peptide (rMnSOD-Lp) exhibited better cancer cell penetration ability [229,239]. This advantage also helps rMnSOD-Lp serve as a molecular carrier that can be conjugated with chemotherapeutic agents, such as cisplatin, and selectively delivered to cancer cells [240,241]. On the other hand, coupling MnSOD or its mimetics with nanoassemblies has shown success in potentiating therapeutic efficacies. A constructed two-dimensional nanosheet catalyst with an MnSOD mimetic (manganese porphyrin MnTCPP+), ZMTP, provided a much better and longer-lasting anti-inflammatory effect in RA than MnTCPP+ treatment alone. Further mechanistic studies revealed that ZMTP nanosheets significantly elevated catalytic activities accompanied by electron and proton transfers [41]. The incorporation of rMnSOD with a polymer multilayer nanocapsule significantly delayed rMnSOD denaturation, enhanced cytotoxicity, increased recognition of cluster of differentiation 44 (CD44), and prolonged the residence time in cancerous lesions [242]. Recently, facilitating MnSOD mimetics to enter mitochondria is a promising approach for better therapeutic efficiency. By conjugating MnSOD mimetics with lipophilic cations, the large negative inner mitochondrial membrane potential navigates these antioxidant mimics to mitochondria [238]. Nevertheless, these mitochondria-specific agents might be cytotoxic as they interfere the production of adenosine triphosphate (ATP), which should be taken into consideration in future investigations.
In conclusion, the expanding biological functions and therapeutic potentials of MnSOD have already been highlighted in numerous diseases. Broad discussion of the multiple aspects of MnSOD will shed light on the unknown mechanisms that govern redox-related diseases and provide optimized strategies for therapeutic intervention.
Acknowledgments
The authors acknowledge ‘BioRender.com (accessed on 4 November 2022)’. that was used to create schematic Figure 1 and Figure 2. All authors appreciate Wan Najbah Nik Nabil for her kind support in revising the manuscript.
Author Contributions
Conceptualization, M.L. and B.C.; validation, Z.X. and H.X.; investigation, M.L. and X.S.; resources, M.L. and X.S.; writing—original draft preparation, X.S. and B.C.; writing—review and editing, M.L., R.D. and Z.X.; visualization, M.L. and X.S.; supervision, Z.X. and H.X.; funding acquisition, Z.X. and H.X. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by National Natural Science Foundation of China (No. 82204437); Shanghai science and technology innovation action plan (No. 22YF1445100); Key-Area Research and Development Program of Guangdong Province (No. 2020B1111110003).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yang B., Chen Y., Shi J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem. Rev. 2019;119:4881–4985. doi: 10.1021/acs.chemrev.8b00626. [DOI] [PubMed] [Google Scholar]
- 2.Sies H., Jones D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020;21:363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 3.Bae Y.S., Oh H., Rhee S.G., Yoo Y.D. Regulation of reactive oxygen species generation in cell signaling. Mol. Cells. 2011;32:491–509. doi: 10.1007/s10059-011-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ray P.D., Huang B.W., Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014;24:R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J., Wang X., Vikash V., Ye Q., Wu D., Liu Y., Dong W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016;2016:4350965. doi: 10.1155/2016/4350965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Y., Hu X., Liu Y., Dong S., Wen Z., He W., Zhang S., Huang Q., Shi M. ROS signaling under metabolic stress: Cross-talk between AMPK and AKT pathway. Mol. Cancer. 2017;16:79. doi: 10.1186/s12943-017-0648-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Demir Y., Balcı N., Gürbüz M. Differential effects of selective serotonin reuptake inhibitors on paraoxonase-1 enzyme activity: An in vitro study. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019;226:108608. doi: 10.1016/j.cbpc.2019.108608. [DOI] [PubMed] [Google Scholar]
- 10.Sarsour E., Kalen A., Goswami P. Manganese Superoxide Dismutase Regulates a Redox Cycle Within the Cell Cycle. Antioxid. Redox Signal. 2013;20:1618–1627. doi: 10.1089/ars.2013.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sena L.A., Chandel N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell. 2012;48:158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim Y.S., Gupta Vallur P., Phaëton R., Mythreye K., Hempel N. Insights into the Dichotomous Regulation of SOD2 in Cancer. Antioxidants. 2017;6:86. doi: 10.3390/antiox6040086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forman H.J., Zhang H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021;20:689–709. doi: 10.1038/s41573-021-00233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lennicke C., Cochemé H.M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell. 2021;81:3691–3707. doi: 10.1016/j.molcel.2021.08.018. [DOI] [PubMed] [Google Scholar]
- 15.Holley A.K., Bakthavatchalu V., Velez-Roman J.M., St Clair D.K. Manganese superoxide dismutase: Guardian of the powerhouse. Int. J. Mol. Sci. 2011;12:7114–7162. doi: 10.3390/ijms12107114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demir Y. Naphthoquinones, benzoquinones, and anthraquinones: Molecular docking, ADME and inhibition studies on human serum paraoxonase-1 associated with cardiovascular diseases. Drug Dev. Res. 2020;81:628–636. doi: 10.1002/ddr.21667. [DOI] [PubMed] [Google Scholar]
- 17.Angelova P.R., Abramov A.Y. Functional role of mitochondrial reactive oxygen species in physiology. Free Radic. Biol. Med. 2016;100:81–85. doi: 10.1016/j.freeradbiomed.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Bresciani G., da Cruz I.B., Gonzalez-Gallego J. Manganese superoxide dismutase and oxidative stress modulation. Adv. Clin. Chem. 2015;68:87–130. doi: 10.1016/bs.acc.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Li Y., Huang T.T., Carlson E.J., Melov S., Ursell P.C., Olson J.L., Noble L.J., Yoshimura M.P., Berger C., Chan P.H., et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat. Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 20.Lebovitz R.M., Zhang H., Vogel H., Cartwright J., Jr., Dionne L., Lu N., Huang S., Matzuk M.M. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc. Natl. Acad. Sci. USA. 1996;93:9782–9787. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reaume A.G., Elliott J.L., Hoffman E.K., Kowall N.W., Ferrante R.J., Siwek D.F., Wilcox H.M., Flood D.G., Beal M.F., Brown R.H., Jr., et al. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat. Genet. 1996;13:43–47. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- 22.Carlsson L.M., Jonsson J., Edlund T., Marklund S.L. Mice lacking extracellular superoxide dismutase are more sensitive to hyperoxia. Proc. Natl. Acad. Sci. USA. 1995;92:6264–6268. doi: 10.1073/pnas.92.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aguirre J.D., Culotta V.C. Battles with iron: Manganese in oxidative stress protection. J. Biol. Chem. 2012;287:13541–13548. doi: 10.1074/jbc.R111.312181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamakura F., Kobayashi K., Furukawa S., Suzuki Y. In vitro preparation of iron-substituted human manganese superoxide dismutase: Possible toxic properties for mitochondria. Free Radic. Biol. Med. 2007;43:423–430. doi: 10.1016/j.freeradbiomed.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 25.Bonetta Valentino R. The structure-function relationships and physiological roles of MnSOD mutants. Biosci. Rep. 2022;42:1618–1627. doi: 10.1042/BSR20220202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zelko I.N., Mariani T.J., Folz R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002;33:337–349. doi: 10.1016/S0891-5849(02)00905-X. [DOI] [PubMed] [Google Scholar]
- 27.Dhar S.K., St Clair D.K. Manganese superoxide dismutase regulation and cancer. Free Radic. Biol. Med. 2012;52:2209–2222. doi: 10.1016/j.freeradbiomed.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Miao L., St Clair D.K. Regulation of superoxide dismutase genes: Implications in disease. Free Radic. Biol. Med. 2009;47:344–356. doi: 10.1016/j.freeradbiomed.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Candas D., Fan M., Nantajit D., Vaughan A.T., Murley J.S., Woloschak G.E., Grdina D.J., Li J.J. CyclinB1/Cdk1 phosphorylates mitochondrial antioxidant MnSOD in cell adaptive response to radiation stress. J. Mol. Cell Biol. 2013;5:166–175. doi: 10.1093/jmcb/mjs062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belluzzi E., Bisaglia M., Lazzarini E., Tabares L.C., Beltramini M., Bubacco L. Human SOD2 modification by dopamine quinones affects enzymatic activity by promoting its aggregation: Possible implications for Parkinson’s disease. PLoS ONE. 2012;7:e38026. doi: 10.1371/journal.pone.0038026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suri S., Mitra P., Bankul A., Saxena I., Garg M.K., Bohra G.K., Sharma P. Altered expression of specific antioxidant (SOD1 and SOD2) and DNA repair (XRCC1 and OGG1) genes in patients with newly diagnosed type-2 diabetes mellitus. Minerva Endocrinol. 2021;46 doi: 10.23736/S2724-6507.21.03417-5. [DOI] [PubMed] [Google Scholar]
- 32.Cho I.K., Yang B., Forest C., Qian L., Chan A.W.S. Amelioration of Huntington’s disease phenotype in astrocytes derived from iPSC-derived neural progenitor cells of Huntington’s disease monkeys. PLoS ONE. 2019;14:e0214156. doi: 10.1371/journal.pone.0214156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diaz-Castro B., Gangwani M.R., Yu X., Coppola G., Khakh B.S. Astrocyte molecular signatures in Huntington’s disease. Sci. Transl. Med. 2019;11:eaaw8546. doi: 10.1126/scitranslmed.aaw8546. [DOI] [PubMed] [Google Scholar]
- 34.Joseph A., Li Y., Koo H.C., Davis J.M., Pollack S., Kazzaz J.A. Superoxide dismutase attenuates hyperoxia-induced interleukin-8 induction via AP-1. Free Radic. Biol. Med. 2008;45:1143–1149. doi: 10.1016/j.freeradbiomed.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 35.Chen H.X., Xiang H., Xu W.H., Li M., Yuan J., Liu J., Sun W.J., Zhang R., Li J., Ren Z.Q., et al. Manganese Superoxide Dismutase Gene-Modified Mesenchymal Stem Cells Attenuate Acute Radiation-Induced Lung Injury. Hum. Gene Ther. 2017;28:523–532. doi: 10.1089/hum.2016.106. [DOI] [PubMed] [Google Scholar]
- 36.Dong J.W., Xu M.J., Zhang W.Y., Che X.M. Effects of Sevoflurane Pretreatment on Myocardial Ischemia-Reperfusion Injury Through the Akt/Hypoxia-Inducible Factor 1-alpha (HIF-1 alpha)/Vascular Endothelial Growth Factor (VEGF) Signaling Pathway. Med. Sci. Monit. 2019;25:3100–3107. doi: 10.12659/MSM.914265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z., Chen K., Han Y., Zhu H., Zhou X.Y., Tan T., Zeng J., Zhang J., Liu Y.K., Li Y., et al. Irisin Protects Heart Against Ischemia-Reperfusion Injury Through a SOD2-Dependent Mitochondria Mechanism. J. Cardiovasc. Pharmacol. 2018;72:259–269. doi: 10.1097/FJC.0000000000000608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang G.D., Chen Y.W., Zhang H.W., Zhou W. Trans sodium crocetinate alleviates ischemia/reperfusion-induced myocardial oxidative stress and apoptosis via the SIRT3/FOXO3a/SOD2 signaling pathway. Int. Immunopharmacol. 2019;71:361–371. doi: 10.1016/j.intimp.2019.03.056. [DOI] [PubMed] [Google Scholar]
- 39.Koo H.C., Davis J.M., Li Y., Hatzis D., Opsimos H., Pollack S., Strayer M.S., Ballard P.L., Kazzaz J.A. Effects of transgene expression of superoxide dismutase and glutathione peroxidase on pulmonary epithelial cell growth in hyperoxia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;288:L718–L726. doi: 10.1152/ajplung.00456.2003. [DOI] [PubMed] [Google Scholar]
- 40.Khan A.A., Paget J.T., McLaughlin M., Kyula J.N., Wilkinson M.J., Pencavel T., Mansfield D., Roulstone V., Seth R., Halle M., et al. Genetically modified lentiviruses that preserve microvascular function protect against late radiation damage in normal tissues. Sci. Transl. Med. 2018;10:eaar2041. doi: 10.1126/scitranslmed.aar2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang B., Yao H., Yang J., Chen C., Shi J. Construction of a two-dimensional artificial antioxidase for nanocatalytic rheumatoid arthritis treatment. Nat. Commun. 2022;13:1988. doi: 10.1038/s41467-022-29735-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jia Z., Zhuang Y., Hu C., Zhang X., Ding G., Zhang Y., Rohatgi R., Hua H., Huang S., He J.C., et al. Albuminuria enhances NHE3 and NCC via stimulation of mitochondrial oxidative stress/angiotensin II axis. Oncotarget. 2016;7:47134–47144. doi: 10.18632/oncotarget.9972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang H.-F., Guo F., Cao Y.-Z., Shi W., Xia Q. Neuroprotection by manganese superoxide dismutase (MnSOD) mimics: Antioxidant effect and oxidative stress regulation in acute experimental stroke. CNS Neurosci. Ther. 2012;18:811–818. doi: 10.1111/j.1755-5949.2012.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhuang Y., Yasinta M., Hu C., Zhao M., Ding G., Bai M., Yang L., Ni J., Wang R., Jia Z., et al. Mitochondrial dysfunction confers albumin-induced NLRP3 inflammasome activation and renal tubular injury. Am. J. Physiol. Ren. Physiol. 2015;308:F857–F866. doi: 10.1152/ajprenal.00203.2014. [DOI] [PubMed] [Google Scholar]
- 45.Nagano Y., Matsui H., Shimokawa O., Hirayama A., Tamura M., Nakamura Y., Kaneko T., Rai K., Indo H.P., Majima H.J., et al. Rebamipide attenuates nonsteroidal anti-inflammatory drugs (NSAID) induced lipid peroxidation by the manganese superoxide dismutase (MnSOD) overexpression in gastrointestinal epithelial cells. J. Physiol. Pharm. 2012;63:137–142. [PubMed] [Google Scholar]
- 46.Jiang J., Liang S., Zhang J., Du Z., Xu Q., Duan J., Sun Z. Melatonin ameliorates PM(2.5)-induced cardiac perivascular fibrosis through regulating mitochondrial redox homeostasis. J. Pineal Res. 2021;70:e12686. doi: 10.1111/jpi.12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sadi G., Güray T. Gene expressions of Mn-SOD and GPx-1 in streptozotocin-induced diabetes: Effect of antioxidants. Mol. Cell. Biochem. 2009;327:127–134. doi: 10.1007/s11010-009-0050-4. [DOI] [PubMed] [Google Scholar]
- 48.Sandhir R., Sood A., Mehrotra A., Kamboj S.S. N-Acetylcysteine reverses mitochondrial dysfunctions and behavioral abnormalities in 3-nitropropionic acid-induced Huntington’s disease. Neurodegener. Dis. 2012;9:145–157. doi: 10.1159/000334273. [DOI] [PubMed] [Google Scholar]
- 49.Jiang W., Geng H., Lv X., Ma J., Liu F., Lin P., Yan C. Idebenone Protects against Atherosclerosis in Apolipoprotein E-Deficient Mice Via Activation of the SIRT3-SOD2-mtROS Pathway. Cardiovasc. Drugs Ther. 2021;35:1129–1145. doi: 10.1007/s10557-020-07018-5. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Z.B., Tan Y.Z., Zhu L.W., Zhang B., Feng P., Gao E.H., Xu C.N., Wang X.M., Yi W., Sun Y. Asprosin improves the survival of mesenchymal stromal cells in myocardial infarction by inhibiting apoptosis via the activated ERK1/2-SOD2 pathway. Life Sci. 2019;231:116554. doi: 10.1016/j.lfs.2019.116554. [DOI] [PubMed] [Google Scholar]
- 51.Lee J.Y., Castelli V., Kumar N., Sitruk-Ware R., Borlongan C.V. Contraceptive drug, Nestorone, enhances stem cell-mediated remodeling of the stroke brain by dampening inflammation and rescuing mitochondria. Free Radic. Biol. Med. 2022;183:138–145. doi: 10.1016/j.freeradbiomed.2022.03.020. [DOI] [PubMed] [Google Scholar]
- 52.Baumer Y., Ng Q.M., Sanda G.E., Dey A.K., Teague H.L., Sorokin A.V., Dagur P.K., Silverman J.I., Harrington C.L., Rodante J.A., et al. Chronic skin inflammation accelerates macrophage cholesterol crystal formation and atherosclerosis. JCI Insight. 2018;3:e97179. doi: 10.1172/jci.insight.97179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sen A., Nelson T.J., Alkon D.L., Hongpaisan J. Loss in PKC Epsilon Causes Downregulation of MnSOD and BDNF Expression in Neurons of Alzheimer’s Disease Hippocampus. J. Alzheimer’s Dis. 2018;63:1173–1189. doi: 10.3233/JAD-171008. [DOI] [PubMed] [Google Scholar]
- 54.Dixit A., Srivastava G., Verma D., Mishra M., Singh P.K., Prakash O., Singh M.P. Minocycline, levodopa and MnTMPyP induced changes in the mitochondrial proteome profile of MPTP and maneb and paraquat mice models of Parkinson’s disease. Biochim. Biophys. Acta. 2013;1832:1227–1240. doi: 10.1016/j.bbadis.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 55.Dikalova A.E., Itani H.A., Nazarewicz R.R., McMaster W.G., Flynn C.R., Uzhachenko R., Fessel J.P., Gamboa J.L., Harrison D.G., Dikalov S.I. Sirt3 Impairment and SOD2 Hyperacetylation in Vascular Oxidative Stress and Hypertension. Circ. Res. 2017;121:564–574. doi: 10.1161/CIRCRESAHA.117.310933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He J., Liu X., Su C., Wu F., Sun J., Zhang J., Yang X., Zhang C., Zhou Z., Zhang X., et al. Inhibition of Mitochondrial Oxidative Damage Improves Reendothelialization Capacity of Endothelial Progenitor Cells via SIRT3 (Sirtuin 3)-Enhanced SOD2 (Superoxide Dismutase 2) Deacetylation in Hypertension. Arter. Thromb. Vasc. Biol. 2019;39:1682–1698. doi: 10.1161/ATVBAHA.119.312613. [DOI] [PubMed] [Google Scholar]
- 57.Vendrov A.E., Stevenson M.D., Alahari S., Pan H., Wickline S.A., Madamanchi N.R., Runge M.S. Attenuated Superoxide Dismutase 2 Activity Induces Atherosclerotic Plaque Instability During Aging in Hyperlipidemic Mice. J. Am. Heart Assoc. 2017;6:e006775. doi: 10.1161/JAHA.117.006775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhai X.G., Qiao H.W., Guan W., Li Z.Q., Cheng Y.Y., Jia X., Zhou Y.J. Curcumin regulates peroxisome proliferator-activated receptor-gamma coactivator-1 alpha expression by AMPK pathway in hepatic stellate cells in vitro. Eur. J. Pharmacol. 2015;746:56–62. doi: 10.1016/j.ejphar.2014.10.055. [DOI] [PubMed] [Google Scholar]
- 59.Lestari N., Louisa M., Soetikno V., Suwana A.G., Ramadhan P.A., Akmal T., Arozal W. Alpha Mangostin Inhibits the Proliferation and Activation of Acetaldehyde Induced Hepatic Stellate Cells through TGF-beta and ERK 1/2 Pathways. J. Toxicol. 2018;2018:5360496. doi: 10.1155/2018/5360496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shingu M., Takahashi S., Ito M., Hamamatu N., Suenaga Y., Ichibangase Y., Nobunaga M. Anti-inflammatory effects of recombinant human manganese superoxide dismutase on adjuvant arthritis in rats. Rheumatol. Int. 1994;14:77–81. doi: 10.1007/BF00300251. [DOI] [PubMed] [Google Scholar]
- 61.Guillaume M., Rodriguez-Vilarrupla A., Gracia-Sancho J., Rosado E., Mancini A., Bosch J., Garcia-Pagán J.C. Recombinant human manganese superoxide dismutase reduces liver fibrosis and portal pressure in CCl4-cirrhotic rats. J. Hepatol. 2013;58:240–246. doi: 10.1016/j.jhep.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 62.Shen L.H., Lei S.J., Huang L.Y., Li S.G., Yi S.Z., Breitzig M., Huang M.Y., Mo X.M., Sun H.X., Zheng Q., et al. Therapeutic effects of the rhSOD2-Hirudin fusion protein on bleomycin-induced pulmonary fibrosis in mice. Eur. J. Pharmacol. 2019;852:77–89. doi: 10.1016/j.ejphar.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 63.Lou J., Dong J., Xu R., Zeng H., Fang L., Wu Y., Liu Y., Wang S. Remodeling of the tumor microenvironment using an engineered oncolytic vaccinia virus improves PD-L1 inhibition outcomes. Biosci. Rep. 2021;41:BSR20204186. doi: 10.1042/BSR20204186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 65.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 66.Gray J.I., Farber D.L. Tissue-Resident Immune Cells in Humans. Annu. Rev. Immunol. 2022;40:195–220. doi: 10.1146/annurev-immunol-093019-112809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caiado F., Pietras E.M., Manz M.G. Inflammation as a regulator of hematopoietic stem cell function in disease, aging, and clonal selection. J. Exp. Med. 2021;218:e20201541. doi: 10.1084/jem.20201541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Biswas S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxid. Med. Cell. Longev. 2016;2016:5698931. doi: 10.1155/2016/5698931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McGarry T., Biniecka M., Veale D.J., Fearon U. Hypoxia, oxidative stress and inflammation. Free Radic. Biol. Med. 2018;125:15–24. doi: 10.1016/j.freeradbiomed.2018.03.042. [DOI] [PubMed] [Google Scholar]
- 70.Li C., Zhou H.M. The role of manganese superoxide dismutase in inflammation defense. Enzym. Res. 2011;2011:387176. doi: 10.4061/2011/387176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bhandari V. Developmental differences in the role of interleukins in hyperoxic lung injury in animal models. Front. Biosci. 2002;7:1624–1633. doi: 10.2741/bhan. [DOI] [PubMed] [Google Scholar]
- 72.Harijith A., Basa P., Ha A., Thomas J., Jafri A., Fu P., MacFarlane P.M., Raffay T.M., Natarajan V., Sudhadevi T. NOX4 Mediates Epithelial Cell Death in Hyperoxic Acute Lung Injury Through Mitochondrial Reactive Oxygen Species. Front. Pharmacol. 2022;13:880878. doi: 10.3389/fphar.2022.880878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mittal M., Siddiqui M.R., Tran K., Reddy S.P., Malik A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014;20:1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bourgonje A.R., Feelisch M., Faber K.N., Pasch A., Dijkstra G., van Goor H. Oxidative Stress and Redox-Modulating Therapeutics in Inflammatory Bowel Disease. Trends Mol. Med. 2020;26:1034–1046. doi: 10.1016/j.molmed.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 75.Kruidenier L., Kuiper I., van Duijn W., Marklund S.L., van Hogezand R.A., Lamers C.B., Verspaget H.W. Differential mucosal expression of three superoxide dismutase isoforms in inflammatory bowel disease. J. Pathol. 2003;201:7–16. doi: 10.1002/path.1407. [DOI] [PubMed] [Google Scholar]
- 76.Ikumoto T., Hayashi S., Tomita S., Miwa S., Mitomi H., Fujimori T., Imura J. Manganese superoxide dismutase plays an important role in the inflammatory process and predicts disease severity and activity in patients with ulcerative colitis. APMIS. 2014;122:512–517. doi: 10.1111/apm.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xie Y., Guo Q., Li S., Liu M., Zhang Q., Xu Z., Sun H. Anti-inflammatory properties of Bifidobacterium longum expressing human manganese superoxide dismutase using the TNBS-induced rats model of colitis. J. Microbiol. Biotechnol. 2017;27:1703–1708. doi: 10.4014/jmb.1703.03044. [DOI] [PubMed] [Google Scholar]
- 78.Carroll I.M., Andrus J.M., Bruno-Barcena J.M., Klaenhammer T.R., Hassan H.M., Threadgill D.S. Anti-inflammatory properties of Lactobacillus gasseri expressing manganese superoxide dismutase using the interleukin 10-deficient mouse model of colitis. Am. J. Physiol. Gastrointest Liver Physiol. 2007;293:G729–G738. doi: 10.1152/ajpgi.00132.2007. [DOI] [PubMed] [Google Scholar]
- 79.Rodriguez-Iturbe B., Sepassi L., Quiroz Y., Ni Z., Wallace D.C., Vaziri N.D. Association of mitochondrial SOD deficiency with salt-sensitive hypertension and accelerated renal senescence. J. Appl. Physiol. 2007;102:255–260. doi: 10.1152/japplphysiol.00513.2006. [DOI] [PubMed] [Google Scholar]
- 80.Szekanecz Z., McInnes I.B., Schett G., Szamosi S., Benko S., Szucs G. Autoinflammation and autoimmunity across rheumatic and musculoskeletal diseases. Nat. Rev. Rheumatol. 2021;17:585–595. doi: 10.1038/s41584-021-00652-9. [DOI] [PubMed] [Google Scholar]
- 81.Burmester G.R., Bijlsma J.W.J., Cutolo M., McInnes I.B. Managing rheumatic and musculoskeletal diseases—Past, present and future. Nat. Rev. Rheumatol. 2017;13:443–448. doi: 10.1038/nrrheum.2017.95. [DOI] [PubMed] [Google Scholar]
- 82.Badii M., Gaal O., Popp R.A., Crisan T.O., Joosten L.A.B. Trained immunity and inflammation in rheumatic diseases. Jt. Bone Spine. 2022;89:105364. doi: 10.1016/j.jbspin.2022.105364. [DOI] [PubMed] [Google Scholar]
- 83.Betrains A., Staels F., Schrijvers R., Meyts I., Humblet-Baron S., De Langhe E., Wouters C., Blockmans D., Vanderschueren S. Systemic autoinflammatory disease in adults. Autoimmun. Rev. 2021;20:102774. doi: 10.1016/j.autrev.2021.102774. [DOI] [PubMed] [Google Scholar]
- 84.Politiek F.A., Waterham H.R. Compromised Protein Prenylation as Pathogenic Mechanism in Mevalonate Kinase Deficiency. Front. Immunol. 2021;12:724991. doi: 10.3389/fimmu.2021.724991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gratton R., Tricarico P.M., Celsi F., Crovella S. Prolonged treatment with mevalonolactone induces oxidative stress response with reactive oxygen species production, mitochondrial depolarization and inflammation in human glioblastoma U-87 MG cells. Neurochem. Int. 2018;120:233–237. doi: 10.1016/j.neuint.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 86.Gul A. Behcet’s disease as an autoinflammatory disorder. Curr. Drug Targets Inflamm. Allergy. 2005;4:81–83. doi: 10.2174/1568010053622894. [DOI] [PubMed] [Google Scholar]
- 87.Bulur I., Onder M. Behcet disease: New aspects. Clin. Derm. 2017;35:421–434. doi: 10.1016/j.clindermatol.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 88.Uz E., Yilmaz H.R., Yağci R., Akyol İ., Ersoy T., Sungur G., Yiğit A., Duman S., Akyol Ö. Genetic Polymorphism of Manganese Superoxide Dismutase in Behçet’s Disease. Arch. Rheumatol. 2016;31:48–54. doi: 10.5606/ArchRheumatol.2016.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Surace A.E.A., Hedrich C.M. The Role of Epigenetics in Autoimmune/Inflammatory Disease. Front. Immunol. 2019;10:1525. doi: 10.3389/fimmu.2019.01525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang L., Wang F.S., Gershwin M.E. Human autoimmune diseases: A comprehensive update. J. Intern. Med. 2015;278:369–395. doi: 10.1111/joim.12395. [DOI] [PubMed] [Google Scholar]
- 91.Lontz W., Sirsjo A., Liu W., Lindberg M., Rollman O., Torma H. Increased mRNA expression of manganese superoxide dismutase in psoriasis skin lesions and in cultured human keratinocytes exposed to IL-1 beta and TNF-alpha. Free Radic. Biol. Med. 1995;18:349–355. doi: 10.1016/0891-5849(94)E0124-2. [DOI] [PubMed] [Google Scholar]
- 92.Gerbaud P., Petzold L., Therond P., Anderson W.B., Evain-Brion D., Raynaud F. Differential regulation of Cu, Zn- and Mn-superoxide dismutases by retinoic acid in normal and psoriatic human fibroblasts. J. Autoimmun. 2005;24:69–78. doi: 10.1016/j.jaut.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 93.Zhang J., Song X., Cao W., Lu J., Wang X., Wang G., Wang Z., Chen X. Autophagy and mitochondrial dysfunction in adjuvant-arthritis rats treatment with resveratrol. Sci. Rep. 2016;6:32928. doi: 10.1038/srep32928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang T., Wang G., Zhang Y., Zhang J., Cao W., Chen X. Effect of lentivirus-mediated overexpression or silencing of MnSOD on apoptosis of resveratrol-treated fibroblast-like synoviocytes in rheumatoid arthritis. Eur. J. Pharm. 2019;844:65–72. doi: 10.1016/j.ejphar.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 95.Wang J.G., Xu W.D., Zhai W.T., Li Y., Hu J.W., Hu B., Li M., Zhang L., Guo W., Zhang J.P., et al. Disorders in angiogenesis and redox pathways are main factors contributing to the progression of rheumatoid arthritis: A comparative proteomics study. Arthritis Rheum. 2012;64:993–1004. doi: 10.1002/art.33425. [DOI] [PubMed] [Google Scholar]
- 96.Wynn T.A., Ramalingam T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Henderson N.C., Rieder F., Wynn T.A. Fibrosis: From mechanisms to medicines. Nature. 2020;587:555–566. doi: 10.1038/s41586-020-2938-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Iredale J.P. Models of liver fibrosis: Exploring the dynamic nature of inflammation and repair in a solid organ. J. Clin. Investig. 2007;117:539–548. doi: 10.1172/JCI30542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Elpek G.O. Cellular and molecular mechanisms in the pathogenesis of liver fibrosis: An update. World J. Gastroenterol. 2014;20:7260–7276. doi: 10.3748/wjg.v20.i23.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schuppan D., Surabattula R., Wang X.Y. Determinants of fibrosis progression and regression in NASH. J. Hepatol. 2018;68:238–250. doi: 10.1016/j.jhep.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 101.Adachi M., Osawa Y., Uchinami H., Kitamura T., Accili D., Brenner D.A. The forkhead transcription factor FoxO1 regulates proliferation and transdifferentiation of hepatic stellate cells. Gastroenterology. 2007;132:1434–1446. doi: 10.1053/j.gastro.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 102.Lackner C., Tiniakos D. Fibrosis and alcohol-related liver disease. J. Hepatol. 2019;70:294–304. doi: 10.1016/j.jhep.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 103.Namikawa C., Shu-Ping Z., Vyselaar J.R., Nozaki Y., Nemoto Y., Ono M., Akisawa N., Saibara T., Hiroi M., Enzan H., et al. Polymorphisms of microsomal triglyceride transfer protein gene and manganese superoxide dismutase gene in non-alcoholic steatohepatitis. J. Hepatol. 2004;40:781–786. doi: 10.1016/j.jhep.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 104.Al-Serri A., Anstee Q.M., Valenti L., Nobili V., Leathart J.B., Dongiovanni P., Patch J., Fracanzani A., Fargion S., Day C.P., et al. The SOD2 C47T polymorphism influences NAFLD fibrosis severity: Evidence from case-control and intra-familial allele association studies. J. Hepatol. 2012;56:448–454. doi: 10.1016/j.jhep.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 105.Loch T., Vakhrusheva O., Piotrowska I., Ziolkowski W., Ebelt H., Braun T., Bober E. Different extent of cardiac malfunction and resistance to oxidative stress in heterozygous and homozygous manganese-dependent superoxide dismutase-mutant mice. Cardiovasc. Res. 2009;82:448–457. doi: 10.1093/cvr/cvp092. [DOI] [PubMed] [Google Scholar]
- 106.Kwak H.-B., Lee Y., Kim J.-H., Van Remmen H., Richardson A.G., Lawler J.M. MnSOD overexpression reduces fibrosis and pro-apoptotic signaling in the aging mouse heart. J. Gerontol. A Biol. Sci. Med. Sci. 2015;70:533–544. doi: 10.1093/gerona/glu090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ma X., Fu Y., Xiao H., Song Y., Chen R., Shen J., An X., Shen Q., Li Z., Zhang Y. Cardiac Fibrosis Alleviated by Exercise Training Is AMPK-Dependent. PLoS ONE. 2015;10:e0129971. doi: 10.1371/journal.pone.0129971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vivar R., Humeres C., Anfossi R., Bolivar S., Catalán M., Hill J., Lavandero S., Diaz-Araya G. Role of FoxO3a as a negative regulator of the cardiac myofibroblast conversion induced by TGF-β1. Biochim. Biophys. Acta Mol. Cell Res. 2020;1867:118695. doi: 10.1016/j.bbamcr.2020.118695. [DOI] [PubMed] [Google Scholar]
- 109.Otoupalova E., Smith S., Cheng G., Thannickal V.J. Oxidative Stress in Pulmonary Fibrosis. Compr. Physiol. 2020;10:509–547. doi: 10.1002/cphy.c190017. [DOI] [PubMed] [Google Scholar]
- 110.Nanthakumar C.B., Hatley R.J.D., Lemma S., Gauldie J., Marshall R.P., Macdonald S.J.F. Dissecting fibrosis: Therapeutic insights from the small-molecule toolbox. Nat. Rev. Drug Discov. 2015;14:693–720. doi: 10.1038/nrd4592. [DOI] [PubMed] [Google Scholar]
- 111.Jablonski R.P., Kim S.-J., Cheresh P., Williams D.B., Morales-Nebreda L., Cheng Y., Yeldandi A., Bhorade S., Pardo A., Selman M., et al. SIRT3 deficiency promotes lung fibrosis by augmenting alveolar epithelial cell mitochondrial DNA damage and apoptosis. FASEB J. 2017;31:2520–2532. doi: 10.1096/fj.201601077R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lindahl G.E., Stock C.J., Shi-Wen X., Leoni P., Sestini P., Howat S.L., Bou-Gharios G., Nicholson A.G., Denton C.P., Grutters J.C., et al. Microarray profiling reveals suppressed interferon stimulated gene program in fibroblasts from scleroderma-associated interstitial lung disease. Respir. Res. 2013;14:80. doi: 10.1186/1465-9921-14-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stock C.J.W., Michaeloudes C., Leoni P., Durham A.L., Mumby S., Wells A.U., Chung K.F., Adcock I.M., Renzoni E.A., Lindahl G.E. Bromodomain and Extraterminal (BET) Protein Inhibition Restores Redox Balance and Inhibits Myofibroblast Activation. BioMed. Res. Int. 2019;2019:1484736. doi: 10.1155/2019/1484736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang Y., Li T., Pan M.X., Wang W., Huang W.H., Yuan Y.F., Xie Z.Z., Chen Y.X., Peng J., Li X., et al. SIRT1 prevents cigarette smoking-induced lung fibroblasts activation by regulating mitochondrial oxidative stress and lipid metabolism. J. Transl. Med. 2022;20:222. doi: 10.1186/s12967-022-03408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu M., Sun Y., Xu M., Yu X., Zhang Y., Huang S., Ding G., Zhang A., Jia Z. Role of mitochondrial oxidative stress in modulating the expressions of aquaporins in obstructive kidney disease. Am. J. Physiol. Ren. Physiol. 2018;314:F658–F666. doi: 10.1152/ajprenal.00234.2017. [DOI] [PubMed] [Google Scholar]
- 116.Chevalier R.L., Forbes M.S., Thornhill B.A. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 2009;75:1145–1152. doi: 10.1038/ki.2009.86. [DOI] [PubMed] [Google Scholar]
- 117.Yin L., Li H., Liu Z., Wu W., Cai J., Tang C., Dong Z. PARK7 Protects Against Chronic Kidney Injury and Renal Fibrosis by Inducing SOD2 to Reduce Oxidative Stress. Front. Immunol. 2021;12:690697. doi: 10.3389/fimmu.2021.690697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rossi C., Zini R., Rontauroli S., Ruberti S., Prudente Z., Barbieri G., Bianchi E., Salati S., Genovese E., Bartalucci N., et al. Role of TGF-β1/miR-382-5p/SOD2 axis in the induction of oxidative stress in CD34+ cells from primary myelofibrosis. Mol. Oncol. 2018;12:2102–2123. doi: 10.1002/1878-0261.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sever B., Altıntop M.D., Demir Y., Türkeş C., Özbaş K., Çiftçi G.A., Beydemir Ş., Özdemir A. A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity. Open Chem. 2021;19:347–357. doi: 10.1515/chem-2021-0032. [DOI] [Google Scholar]
- 120.Ashcroft F.M., Rorsman P. Diabetes mellitus and the β cell: The last ten years. Cell. 2012;148:1160–1171. doi: 10.1016/j.cell.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rains J.L., Jain S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011;50:567–575. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wu J., Zhou Y., Hu H., Yang D., Yang F. Effects of β-carotene on glucose metabolism dysfunction in humans and type 2 diabetic rats. Acta Mater. Med. 2022;1:138–153. doi: 10.15212/AMM-2021-0009. [DOI] [Google Scholar]
- 123.Eddaikra A., Amroun H., Raache R., Galleze A., Abdallah-Elhadj N., Azzouz M., Meçabih F., Mechti B., Abbadi M.C., Touil-Boukoffa C., et al. Clinical variables and ethnicity may influenced by polymorphism of CAT-262C/T and MnSOD 47C/T antioxidant enzymes in Algerian type1 diabetes without complications. Gene. 2018;670:182–192. doi: 10.1016/j.gene.2018.05.105. [DOI] [PubMed] [Google Scholar]
- 124.Van Belle T.L., Coppieters K.T., von Herrath M.G. Type 1 diabetes: Etiology, immunology, and therapeutic strategies. Physiol. Rev. 2011;91:79–118. doi: 10.1152/physrev.00003.2010. [DOI] [PubMed] [Google Scholar]
- 125.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36((Suppl. 1)):S67–S74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Forbes J.M., Cooper M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013;93:137–188. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 127.Chen H., Li X., Epstein P.N. MnSOD and catalase transgenes demonstrate that protection of islets from oxidative stress does not alter cytokine toxicity. Diabetes. 2005;54:1437–1446. doi: 10.2337/diabetes.54.5.1437. [DOI] [PubMed] [Google Scholar]
- 128.Kim G., Lee H.S., Oh B.J., Kwon Y., Kim H., Ha S., Jin S.-M., Kim J.H. Protective effect of a novel clinical-grade small molecule necrosis inhibitor against oxidative stress and inflammation during islet transplantation. Am. J. Transpl. 2021;21:1440–1452. doi: 10.1111/ajt.16323. [DOI] [PubMed] [Google Scholar]
- 129.Bertera S., Crawford M.L., Alexander A.M., Papworth G.D., Watkins S.C., Robbins P.D., Trucco M. Gene transfer of manganese superoxide dismutase extends islet graft function in a mouse model of autoimmune diabetes. Diabetes. 2003;52:387–393. doi: 10.2337/diabetes.52.2.387. [DOI] [PubMed] [Google Scholar]
- 130.Cole J.B., Florez J.C. Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 2020;16:377–390. doi: 10.1038/s41581-020-0278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Akdağ M., Özçelik A.B., Demir Y., Beydemir Ş. Design, synthesis, and aldose reductase inhibitory effect of some novel carboxylic acid derivatives bearing 2-substituted-6-aryloxo-pyridazinone moiety. J. Mol. Struct. 2022;1258:132675. doi: 10.1016/j.molstruc.2022.132675. [DOI] [Google Scholar]
- 132.Sever B., Altıntop M.D., Demir Y., Yılmaz N., Akalın Çiftçi G., Beydemir Ş., Özdemir A. Identification of a new class of potent aldose reductase inhibitors: Design, microwave-assisted synthesis, in vitro and in silico evaluation of 2-pyrazolines. Chem. Biol. Interact. 2021;345:109576. doi: 10.1016/j.cbi.2021.109576. [DOI] [PubMed] [Google Scholar]
- 133.He X., Kan H., Cai L., Ma Q. Nrf2 is critical in defense against high glucose-induced oxidative damage in cardiomyocytes. J. Mol. Cell. Cardiol. 2009;46:47–58. doi: 10.1016/j.yjmcc.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 134.Zhang P., Li T., Wu X., Nice E.C., Huang C., Zhang Y. Oxidative stress and diabetes: Antioxidative strategies. Front. Med. 2020;14:583–600. doi: 10.1007/s11684-019-0729-1. [DOI] [PubMed] [Google Scholar]
- 135.Türkeş C., Arslan M., Demir Y., Çoçaj L., Nixha A.R., Beydemir Ş. N-substituted phthalazine sulfonamide derivatives as non-classical aldose reductase inhibitors. J. Mol. Recognit. JMR. 2022;35:e2991. doi: 10.1002/jmr.2991. [DOI] [PubMed] [Google Scholar]
- 136.Ivanović-Matić S., Mihailović M., Dinić S., Martinović V., Bogojević D., Grigorov I., Poznanović G. The absence of cardiomyopathy is accompanied by increased activities of CAT, MnSOD and GST in long-term diabetes in rats. J. Physiol. Sci. 2010;60:259–266. doi: 10.1007/s12576-010-0093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shen X., Zheng S., Metreveli N.S., Epstein P.N. Protection of cardiac mitochondria by overexpression of MnSOD reduces diabetic cardiomyopathy. Diabetes. 2006;55:798–805. doi: 10.2337/diabetes.55.03.06.db05-1039. [DOI] [PubMed] [Google Scholar]
- 138.Liu G., Daneshgari F. Diabetic bladder dysfunction. Chin. Med. J. 2014;127:1357–1364. [PMC free article] [PubMed] [Google Scholar]
- 139.Elrashidy R.A., Kavran M., Asker M.E., Mohamed H.E., Daneshgari F., Liu G. Smooth muscle-specific deletion of MnSOD exacerbates diabetes-induced bladder dysfunction in mice. Am. J. Physiol. Ren. Physiol. 2019;317:F906–F912. doi: 10.1152/ajprenal.00221.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Madsen-Bouterse S.A., Zhong Q., Mohammad G., Ho Y.-S., Kowluru R.A. Oxidative damage of mitochondrial DNA in diabetes and its protection by manganese superoxide dismutase. Free Radic. Res. 2010;44:313–321. doi: 10.3109/10715760903494168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lin M.T., Beal M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 142.Peng C., Trojanowski J.Q., Lee V.M. Protein transmission in neurodegenerative disease. Nat. Rev. Neurol. 2020;16:199–212. doi: 10.1038/s41582-020-0333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mahmudov I., Demir Y., Sert Y., Abdullayev Y., Sujayev A., Alwasel S.H., Gulcin I. Synthesis and inhibition profiles of N-benzyl- and N-allyl aniline derivatives against carbonic anhydrase and acetylcholinesterase—A molecular docking study. Arab. J. Chem. 2022;15:103645. doi: 10.1016/j.arabjc.2021.103645. [DOI] [Google Scholar]
- 144.Inuzuka H., Liu J., Wei W., Rezaeian A.H. PROTACs technology for treatment of Alzheimer’s disease: Advances and perspectives. Acta Mater. Med. 2022;1:24–41. doi: 10.15212/AMM-2021-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Güleç Ö., Türkeş C., Arslan M., Demir Y., Yeni Y., Hacımüftüoğlu A., Ereminsoy E., Küfrevioğlu Ö.İ., Beydemir Ş. Cytotoxic effect, enzyme inhibition, and in silico studies of some novel N-substituted sulfonyl amides incorporating 1,3,4-oxadiazol structural motif. Mol. Divers. 2022;26:2825–2845. doi: 10.1007/s11030-022-10422-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dujardin S., Commins C., Lathuiliere A., Beerepoot P., Fernandes A.R., Kamath T.V., De Los Santos M.B., Klickstein N., Corjuc D.L., Corjuc B.T., et al. Tau molecular diversity contributes to clinical heterogeneity in Alzheimer’s disease. Nat. Med. 2020;26:1256–1263. doi: 10.1038/s41591-020-0938-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Martini A.C., Gross T.J., Head E., Mapstone M. Beyond amyloid: Immune, cerebrovascular, and metabolic contributions to Alzheimer disease in people with Down syndrome. Neuron. 2022;110:2063–2079. doi: 10.1016/j.neuron.2022.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hutton M., Pérez-Tur J., Hardy J. Genetics of Alzheimer’s disease. Essays Biochem. 1998;33:117–131. doi: 10.1042/bse0330117. [DOI] [PubMed] [Google Scholar]
- 149.Flynn J.M., Melov S. SOD2 in mitochondrial dysfunction and neurodegeneration. Free Radic. Biol. Med. 2013;62:4–12. doi: 10.1016/j.freeradbiomed.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Furuta A., Price D.L., Pardo C.A., Troncoso J.C., Xu Z.S., Taniguchi N., Martin L.J. Localization of superoxide dismutases in Alzheimer’s disease and Down’s syndrome neocortex and hippocampus. Am. J. Pathol. 1995;146:357–367. [PMC free article] [PubMed] [Google Scholar]
- 151.Esposito L., Raber J., Kekonius L., Yan F., Yu G.-Q., Bien-Ly N., Puoliväli J., Scearce-Levie K., Masliah E., Mucke L. Reduction in mitochondrial superoxide dismutase modulates Alzheimer’s disease-like pathology and accelerates the onset of behavioral changes in human amyloid precursor protein transgenic mice. J. Neurosci. 2006;26:5167–5179. doi: 10.1523/JNEUROSCI.0482-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lee H.-P., Pancholi N., Esposito L., Previll L.A., Wang X., Zhu X., Smith M.A., Lee H.-G. Early induction of oxidative stress in mouse model of Alzheimer disease with reduced mitochondrial superoxide dismutase activity. PLoS ONE. 2012;7:e28033. doi: 10.1371/journal.pone.0028033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Melov S., Adlard P.A., Morten K., Johnson F., Golden T.R., Hinerfeld D., Schilling B., Mavros C., Masters C.L., Volitakis I., et al. Mitochondrial oxidative stress causes hyperphosphorylation of tau. PLoS ONE. 2007;2:e536. doi: 10.1371/journal.pone.0000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Massaad C.A., Washington T.M., Pautler R.G., Klann E. Overexpression of SOD-2 reduces hippocampal superoxide and prevents memory deficits in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2009;106:13576–13581. doi: 10.1073/pnas.0902714106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang X., He Q., Huang T., Zhao N., Liang F., Xu B., Chen X., Li T., Bi J. Treadmill Exercise Decreases Abeta Deposition and Counteracts Cognitive Decline in APP/PS1 Mice, Possibly via Hippocampal Microglia Modifications. Front. Aging Neurosci. 2019;11:78. doi: 10.3389/fnagi.2019.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.França M.B., Lima K.C., Eleutherio E.C.A. Oxidative Stress and Amyloid Toxicity: Insights from Yeast. J. Cell. Biochem. 2017;118:1442–1452. doi: 10.1002/jcb.25803. [DOI] [PubMed] [Google Scholar]
- 157.Delbarba A., Abate G., Prandelli C., Marziano M., Buizza L., Arce Varas N., Novelli A., Cuetos F., Martinez C., Lanni C., et al. Mitochondrial Alterations in Peripheral Mononuclear Blood Cells from Alzheimer’s Disease and Mild Cognitive Impairment Patients. Oxid. Med. Cell. Longev. 2016;2016:5923938. doi: 10.1155/2016/5923938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Anantharaman M., Tangpong J., Keller J.N., Murphy M.P., Markesbery W.R., Kiningham K.K., St Clair D.K. Beta-amyloid mediated nitration of manganese superoxide dismutase: Implication for oxidative stress in a APPNLH/NLH X PS-1P264L/P264L double knock-in mouse model of Alzheimer’s disease. Am. J. Pathol. 2006;168:1608–1618. doi: 10.2353/ajpath.2006.051223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bloem B.R., Okun M.S., Klein C. Parkinson’s disease. Lancet. 2021;397:2284–2303. doi: 10.1016/S0140-6736(21)00218-X. [DOI] [PubMed] [Google Scholar]
- 160.Jankovic J., Tan E.K. Parkinson’s disease: Etiopathogenesis and treatment. J. Neurol. Neurosurg. Psychiatry. 2020;91:795–808. doi: 10.1136/jnnp-2019-322338. [DOI] [PubMed] [Google Scholar]
- 161.Dionísio P.A., Amaral J.D., Rodrigues C.M.P. Oxidative stress and regulated cell death in Parkinson’s disease. Ageing Res. Rev. 2021;67:101263. doi: 10.1016/j.arr.2021.101263. [DOI] [PubMed] [Google Scholar]
- 162.Kurosaki R., Muramatsu Y., Kato H., Araki T. Biochemical, behavioral and immunohistochemical alterations in MPTP-treated mouse model of Parkinson’s disease. Pharm. Biochem. Behav. 2004;78:143–153. doi: 10.1016/j.pbb.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 163.Yoritaka A., Hattori N., Mori H., Kato K., Mizuno Y. An immunohistochemical study on manganese superoxide dismutase in Parkinson’s disease. J. Neurol. Sci. 1997;148:181–186. doi: 10.1016/S0022-510X(96)05339-7. [DOI] [PubMed] [Google Scholar]
- 164.Radunovic A., Porto W.G., Zeman S., Leigh P.N. Increased mitochondrial superoxide dismutase activity in Parkinson’s disease but not amyotrophic lateral sclerosis motor cortex. Neurosci. Lett. 1997;239:105–108. doi: 10.1016/S0304-3940(97)00905-1. [DOI] [PubMed] [Google Scholar]
- 165.Ferrer I., Perez E., Dalfo E., Barrachina M. Abnormal levels of prohibitin and ATP synthase in the substantia nigra and frontal cortex in Parkinson’s disease. Neurosci. Lett. 2007;415:205–209. doi: 10.1016/j.neulet.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 166.Santiago J.A., Scherzer C.R., Potashkin J.A. Network analysis identifies SOD2 mRNA as a potential biomarker for Parkinson’s disease. PLoS ONE. 2014;9:e109042. doi: 10.1371/journal.pone.0109042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wu Y.-R., Chang K.-H., Chao C.-Y., Lin C.-H., Chen Y.-C., Liu T.-W., Lee-Chen G.-J., Chen C.-M. Association of SOD2 p.V16A polymorphism with Parkinson’s disease: A meta-analysis in Han Chinese. J. Formos. Med. Assoc. 2021;120:501–507. doi: 10.1016/j.jfma.2020.06.023. [DOI] [PubMed] [Google Scholar]
- 168.Pan L., Feigin A. Huntington’s Disease: New Frontiers in Therapeutics. Curr. Neurol. Neurosci. Rep. 2021;21:10. doi: 10.1007/s11910-021-01093-3. [DOI] [PubMed] [Google Scholar]
- 169.Oh S.S., Sullivan K.A., Wilkinson J.E., Backus C., Hayes J.M., Sakowski S.A., Feldman E.L. Neurodegeneration and early lethality in superoxide dismutase 2-deficient mice: A comprehensive analysis of the central and peripheral nervous systems. Neuroscience. 2012;212:201–213. doi: 10.1016/j.neuroscience.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Naoi M., Maruyama W., Yi H., Inaba K., Akao Y., Shamoto-Nagai M. Mitochondria in neurodegenerative disorders: Regulation of the redox state and death signaling leading to neuronal death and survival. J. Neural Transm. 2009;116:1371–1381. doi: 10.1007/s00702-009-0309-7. [DOI] [PubMed] [Google Scholar]
- 171.Mena N.P., Urrutia P.J., Lourido F., Carrasco C.M., Nunez M.T. Mitochondrial iron homeostasis and its dysfunctions in neurodegenerative disorders. Mitochondrion. 2015;21:92–105. doi: 10.1016/j.mito.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 172.Jedrak P., Krygier M., Tonska K., Drozd M., Kaliszewska M., Bartnik E., Soltan W., Sitek E.J., Stanislawska-Sachadyn A., Limon J., et al. Mitochondrial DNA levels in Huntington disease leukocytes and dermal fibroblasts. Metab. Brain Dis. 2017;32:1237–1247. doi: 10.1007/s11011-017-0026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jędrak P., Mozolewski P., Węgrzyn G., Więckowski M.R. Mitochondrial alterations accompanied by oxidative stress conditions in skin fibroblasts of Huntington’s disease patients. Metab. Brain Dis. 2018;33:2005–2017. doi: 10.1007/s11011-018-0308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Smatlikova P., Askeland G., Vaskovicova M., Klima J., Motlik J., Eide L., Ellederová Z. Age-Related Oxidative Changes in Primary Porcine Fibroblasts Expressing Mutated Huntingtin. Neurodegener. Dis. 2019;19:22–34. doi: 10.1159/000500091. [DOI] [PubMed] [Google Scholar]
- 175.Hardiman O., Al-Chalabi A., Chio A., Corr E.M., Logroscino G., Robberecht W., Shaw P.J., Simmons Z., van den Berg L.H. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Prim. 2017;3:17071. doi: 10.1038/nrdp.2017.71. [DOI] [PubMed] [Google Scholar]
- 176.Tomkins J., Banner S.J., McDermott C.J., Shaw P.J. Mutation screening of manganese superoxide dismutase in amyotrophic lateral sclerosis. Neuroreport. 2001;12:2319–2322. doi: 10.1097/00001756-200108080-00008. [DOI] [PubMed] [Google Scholar]
- 177.Flanagan S.W., Anderson R.D., Ross M.A., Oberley L.W. Overexpression of manganese superoxide dismutase attenuates neuronal death in human cells expressing mutant (G37R) Cu/Zn-superoxide dismutase. J. Neurochem. 2002;81:170–177. doi: 10.1046/j.1471-4159.2002.00812.x. [DOI] [PubMed] [Google Scholar]
- 178.Andreassen O.A., Ferrante R.J., Klivenyi P., Klein A.M., Shinobu L.A., Epstein C.J., Beal M.F. Partial deficiency of manganese superoxide dismutase exacerbates a transgenic mouse model of amyotrophic lateral sclerosis. Ann. Neurol. 2000;47:447–455. doi: 10.1002/1531-8249(200004)47:4<447::AID-ANA7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 179.Stamenković S., Pavićević A., Mojović M., Popović-Bijelić A., Selaković V., Andjus P., Bačić G. In vivo EPR pharmacokinetic evaluation of the redox status and the blood brain barrier permeability in the SOD1 ALS rat model. Free Radic. Biol. Med. 2017;108:258–269. doi: 10.1016/j.freeradbiomed.2017.03.034. [DOI] [PubMed] [Google Scholar]
- 180.Wakai M., Mokuno K., Hashizume Y., Kato K. An immunohistochemical study of the neuronal expression of manganese superoxide dismutase in sporadic amyotrophic lateral sclerosis. Acta Neuropathol. 1994;88:151–158. doi: 10.1007/BF00294508. [DOI] [PubMed] [Google Scholar]
- 181.Liu Y., Brooks B.R., Taniguchi N., Hartmann H.A. CuZnSOD and MnSOD immunoreactivity in brain stem motor neurons from amyotrophic lateral sclerosis patients. Acta Neuropathol. 1998;95:63–70. doi: 10.1007/s004010050766. [DOI] [PubMed] [Google Scholar]
- 182.Fukai T., Ushio-Fukai M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011;15:1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Siti H.N., Kamisah Y., Kamsiah J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review) Vasc. Pharm. 2015;71:40–56. doi: 10.1016/j.vph.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 184.Perumareddi P. Prevention of Hypertension Related to Cardiovascular Disease. Prim. Care. 2019;46:27–39. doi: 10.1016/j.pop.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 185.Elliott W.J. Systemic hypertension. Curr. Probl. Cardiol. 2007;32:201–259. doi: 10.1016/j.cpcardiol.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 186.Guzik T.J., Touyz R.M. Oxidative Stress, Inflammation, and Vascular Aging in Hypertension. Hypertension. 2017;70:660–667. doi: 10.1161/HYPERTENSIONAHA.117.07802. [DOI] [PubMed] [Google Scholar]
- 187.Schulz E., Gori T., Münzel T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens. Res. 2011;34:665–673. doi: 10.1038/hr.2011.39. [DOI] [PubMed] [Google Scholar]
- 188.Case A.J., Tian J., Zimmerman M.C. Increased mitochondrial superoxide in the brain, but not periphery, sensitizes mice to angiotensin II-mediated hypertension. Redox Biol. 2017;11:82–90. doi: 10.1016/j.redox.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chan S.H.H., Tai M.-H., Li C.-Y., Chan J.Y.H. Reduction in molecular synthesis or enzyme activity of superoxide dismutases and catalase contributes to oxidative stress and neurogenic hypertension in spontaneously hypertensive rats. Free Radic. Biol. Med. 2006;40:2028–2039. doi: 10.1016/j.freeradbiomed.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 190.Li L., Crockett E., Wang D.H., Galligan J.J., Fink G.D., Chen A.F. Gene transfer of endothelial NO synthase and manganese superoxide dismutase on arterial vascular cell adhesion molecule-1 expression and superoxide production in deoxycorticosterone acetate-salt hypertension. Arter. Thromb. Vasc. Biol. 2002;22:249–255. doi: 10.1161/hq0202.104124. [DOI] [PubMed] [Google Scholar]
- 191.Afolayan A.J., Eis A., Teng R.-J., Bakhutashvili I., Kaul S., Davis J.M., Konduri G.G. Decreases in manganese superoxide dismutase expression and activity contribute to oxidative stress in persistent pulmonary hypertension of the newborn. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012;303:L870–L879. doi: 10.1152/ajplung.00098.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Schultz A., Olorundami O.A., Teng R.-J., Jarzembowski J., Shi Z.-Z., Kumar S.N., Pritchard K., Konduri G.G., Afolayan A.J. Decreased OLA1 (Obg-Like ATPase-1) Expression Drives Ubiquitin-Proteasome Pathways to Downregulate Mitochondrial SOD2 (Superoxide Dismutase) in Persistent Pulmonary Hypertension of the Newborn. Hypertension. 2019;74:957–966. doi: 10.1161/HYPERTENSIONAHA.119.13430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Libby P., Ridker P.M., Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 194.Libby P., Buring J.E., Badimon L., Hansson G.K., Deanfield J., Bittencourt M.S., Tokgozoglu L., Lewis E.F. Atherosclerosis. Nat. Rev. Dis. Prim. 2019;5:56. doi: 10.1038/s41572-019-0106-z. [DOI] [PubMed] [Google Scholar]
- 195.Ohashi M., Runge M.S., Faraci F.M., Heistad D.D. MnSOD Deficiency Increases Endothelial Dysfunction in ApoE-Deficient Mice. Arter. Thromb. Vasc. Biol. 2006;26:2331–2336. doi: 10.1161/01.ATV.0000238347.77590.c9. [DOI] [PubMed] [Google Scholar]
- 196.Ballinger S.W., Patterson C., Knight-Lozano C.A., Burow D.L., Conklin C.A., Hu Z., Reuf J., Horaist C., Lebovitz R., Hunter G.C., et al. Mitochondrial integrity and function in atherogenesis. Circulation. 2002;106:544–549. doi: 10.1161/01.CIR.0000023921.93743.89. [DOI] [PubMed] [Google Scholar]
- 197.Harrison C.M., Pompilius M., Pinkerton K.E., Ballinger S.W. Mitochondrial Oxidative Stress Significantly Influences Atherogenic Risk and Cytokine-Induced Oxidant Production. Environ. Health Perspect. 2011;119:676–681. doi: 10.1289/ehp.1002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hou L., Guo J.J., Xu F., Weng X.Y., Yue W.H., Ge J.B. Cardiomyocyte dimethylarginine dimethylaminohydrolase1 attenuates left-ventricular remodeling after acute myocardial infarction: Involvement in oxidative stress and apoptosis. Basic Res. Cardiol. 2018;113:28. doi: 10.1007/s00395-018-0685-y. [DOI] [PubMed] [Google Scholar]
- 199.Chen Z., Siu B., Ho Y.S., Vincent R., Chua C.C., Hamdy R.C., Chua B.H. Overexpression of MnSOD protects against myocardial ischemia/reperfusion injury in transgenic mice. J. Mol. Cell. Cardiol. 1998;30:2281–2289. doi: 10.1006/jmcc.1998.0789. [DOI] [PubMed] [Google Scholar]
- 200.Hankey G.J. Stroke. Lancet. 2017;389:641–654. doi: 10.1016/S0140-6736(16)30962-X. [DOI] [PubMed] [Google Scholar]
- 201.Zhao B.Y., Jiang X.F. hsa-miR-518-5p/hsa-miR-3135b Regulates the REL/SOD2 Pathway in Ischemic Cerebral Infarction. Front. Neurol. 2022;13:852013. doi: 10.3389/fneur.2022.852013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Liu P., Zhao H., Wang R., Wang P., Tao Z., Gao L., Yan F., Liu X., Yu S., Ji X., et al. MicroRNA-424 protects against focal cerebral ischemia and reperfusion injury in mice by suppressing oxidative stress. Stroke. 2015;46:513–519. doi: 10.1161/STROKEAHA.114.007482. [DOI] [PubMed] [Google Scholar]
- 203.Zhao H., Tao Z., Wang R., Liu P., Yan F., Li J., Zhang C., Ji X., Luo Y. MicroRNA-23a-3p attenuates oxidative stress injury in a mouse model of focal cerebral ischemia-reperfusion. Brain Res. 2014;1592:65–72. doi: 10.1016/j.brainres.2014.09.055. [DOI] [PubMed] [Google Scholar]
- 204.Yi H., Huang Y., Yang F., Liu W., He S., Hu X. MicroRNA-182 aggravates cerebral ischemia injury by targeting inhibitory member of the ASPP family (iASPP) Arch. Biochem. Biophys. 2017;620:52–58. doi: 10.1016/j.abb.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 205.Sun S., Chen X., Gao Y., Liu Z., Zhai Q., Xiong L., Cai M., Wang Q. Mn-SOD Upregulation by Electroacupuncture Attenuates Ischemic Oxidative Damage via CB1R-Mediated STAT3 Phosphorylation. Mol. Neurobiol. 2016;53:331–343. doi: 10.1007/s12035-014-8971-7. [DOI] [PubMed] [Google Scholar]
- 206.Li M., Wu C., Muhammad J.S., Yan D., Tsuneyama K., Hatta H., Cui Z.G., Inadera H. Melatonin sensitises shikonin-induced cancer cell death mediated by oxidative stress via inhibition of the SIRT3/SOD2-AKT pathway. Redox Biol. 2020;36:101632. doi: 10.1016/j.redox.2020.101632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Liu J., Yao L., Zhang M., Jiang J., Yang M., Wang Y. Downregulation of LncRNA-XIST inhibited development of non-small cell lung cancer by activating miR-335/SOD2/ROS signal pathway mediated pyroptotic cell death. Aging. 2019;11:7830–7846. doi: 10.18632/aging.102291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Jeong S., Kim B.G., Kim D.Y., Kim B.R., Kim J.L., Park S.H., Na Y.J., Jo M.J., Yun H.K., Jeong Y.A., et al. Cannabidiol Overcomes Oxaliplatin Resistance by Enhancing NOS3- and SOD2-Induced Autophagy in Human Colorectal Cancer Cells. Cancers. 2019;11:781. doi: 10.3390/cancers11060781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ma C.S., Lv Q.M., Zhang K.R., Tang Y.B., Zhang Y.F., Shen Y., Lei H.M., Zhu L. NRF2-GPX4/SOD2 axis imparts resistance to EGFR-tyrosine kinase inhibitors in non-small-cell lung cancer cells. Acta Pharmacol. Sin. 2021;42:613–623. doi: 10.1038/s41401-020-0443-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Paku M., Haraguchi N., Takeda M., Fujino S., Ogino T., Takahashi H., Miyoshi N., Uemura M., Mizushima T., Yamamoto H., et al. SIRT3-Mediated SOD2 and PGC-1alpha Contribute to Chemoresistance in Colorectal Cancer Cells. Ann. Surg. Oncol. 2021;28:4720–4732. doi: 10.1245/s10434-020-09373-x. [DOI] [PubMed] [Google Scholar]
- 211.Hsueh W.T., Chen S.H., Chien C.H., Chou S.W., Chi P.I., Chu J.M., Chang K.Y. SOD2 Enhancement by Long-Term Inhibition of the PI3K Pathway Confers Multi-Drug Resistance and Enhanced Tumor-Initiating Features in Head and Neck Cancer. Int. J. Mol. Sci. 2021;22:11260. doi: 10.3390/ijms222011260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhang Y., Xu Z., Ding J., Tan C., Hu W., Li Y., Huang W., Xu Y. HZ08 suppresses RelB-activated MnSOD expression and enhances Radiosensitivity of prostate Cancer cells. J. Exp. Clin. Cancer Res. CR. 2018;37:174. doi: 10.1186/s13046-018-0849-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Noh J.K., Woo S.R., Yun M., Lee M.K., Kong M., Min S., Kim S.I., Lee Y.C., Eun Y.G., Ko S.G. SOD2- and NRF2-associated Gene Signature to Predict Radioresistance in Head and Neck Cancer. Cancer Genom. Proteom. 2021;18:675–684. doi: 10.21873/cgp.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.He C., Danes J.M., Hart P.C., Zhu Y., Huang Y., de Abreu A.L., O’Brien J., Mathison A.J., Tang B., Frasor J.M., et al. SOD2 acetylation on lysine 68 promotes stem cell reprogramming in breast cancer. Proc. Natl. Acad. Sci. USA. 2019;116:23534–23541. doi: 10.1073/pnas.1902308116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hjelmeland A.B., Patel R.P. SOD2 acetylation and deacetylation: Another tale of Jekyll and Hyde in cancer. Proc. Natl. Acad. Sci. USA. 2019;116:23376–23378. doi: 10.1073/pnas.1916214116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zhu Y., Zou X., Dean A.E., Brien J.O., Gao Y., Tran E.L., Park S.-H., Liu G., Kieffer M.B., Jiang H., et al. Lysine 68 acetylation directs MnSOD as a tetrameric detoxification complex versus a monomeric tumor promoter. Nat. Commun. 2019;10:2399. doi: 10.1038/s41467-019-10352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kim A. Modulation of MnSOD in Cancer:Epidemiological and Experimental Evidence. Toxicol. Res. 2010;26:83–93. doi: 10.5487/TR.2010.26.2.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Konzack A., Kietzmann T. Manganese superoxide dismutase in carcinogenesis: Friend or foe? Biochem. Soc. Trans. 2014;42:1012–1016. doi: 10.1042/BST20140076. [DOI] [PubMed] [Google Scholar]
- 219.Gatenby R.A., Gillies R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 220.Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 221.Faubert B., Solmonson A., DeBerardinis R.J. Metabolic reprogramming and cancer progression. Science. 2020;368:eaaw5473. doi: 10.1126/science.aaw5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Liu Y.D., Yu L., Ying L., Balic J., Gao H., Deng N.T., West A., Yan F., Ji C.B., Gough D., et al. Toll-like receptor 2 regulates metabolic reprogramming in gastric cancer via superoxide dismutase 2. Int. J. Cancer. 2019;144:3056–3069. doi: 10.1002/ijc.32060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zhou C., Lyu L.-H., Miao H.-K., Bahr T., Zhang Q.-Y., Liang T., Zhou H.-B., Chen G.-R., Bai Y. Redox regulation by SOD2 modulates colorectal cancer tumorigenesis through AMPK-mediated energy metabolism. Mol. Carcinog. 2020;59:545–556. doi: 10.1002/mc.23178. [DOI] [PubMed] [Google Scholar]
- 224.Hart P.C., Mao M., de Abreu A.L.P., Ansenberger-Fricano K., Ekoue D.N., Ganini D., Kajdacsy-Balla A., Diamond A.M., Minshall R.D., Consolaro M.E.L., et al. MnSOD upregulation sustains the Warburg effect via mitochondrial ROS and AMPK-dependent signalling in cancer. Nat. Commun. 2015;6:6053. doi: 10.1038/ncomms7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Quiros-Gonzalez I., Gonzalez-Menendez P., Mayo J.C., Hevia D., Artime-Naveda F., Fernandez-Vega S., Fernandez-Fernandez M., Rodriguez-Gonzalez P., Garcia-Alonso J.I., Sainz R.M. Androgen-Dependent Prostate Cancer Cells Reprogram Their Metabolic Signature upon GLUT1 Upregulation by Manganese Superoxide Dismutase. Antioxidants. 2022;11:313. doi: 10.3390/antiox11020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Su H., Cai T., Zhang S., Yan X., Zhou L., He Z., Xue P., Li J., Zheng M., Yang X., et al. Identification of hub genes associated with neutrophils infiltration in colorectal cancer. J. Cell. Mol. Med. 2021;25:3371–3380. doi: 10.1111/jcmm.16414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Han X., Liu X., Wang X., Guo W., Wen Y., Meng W., Peng D., Lv P., Zhang X., Shen H. TNF-alpha-dependent lung inflammation upregulates superoxide dismutase-2 to promote tumor cell proliferation in lung adenocarcinoma. Mol. Carcinog. 2020;59:1088–1099. doi: 10.1002/mc.23239. [DOI] [PubMed] [Google Scholar]
- 228.Al Haq A.T., Tseng H.-Y., Chen L.-M., Wang C.-C., Hsu H.-L. Targeting prooxidant MnSOD effect inhibits triple-negative breast cancer (TNBC) progression and M2 macrophage functions under the oncogenic stress. Cell Death Dis. 2022;13:49. doi: 10.1038/s41419-021-04486-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Borrelli A., Schiattarella A., Bonelli P., Tuccillo F.M., Buonaguro F.M., Mancini A. The functional role of MnSOD as a biomarker of human diseases and therapeutic potential of a new isoform of a human recombinant MnSOD. Biomed. Res. Int. 2014;2014:476789. doi: 10.1155/2014/476789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wang Y., Branicky R., Noe A., Hekimi S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018;217:1915–1928. doi: 10.1083/jcb.201708007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Demir Y., Köksal Z. The inhibition effects of some sulfonamides on human serum paraoxonase-1 (hPON1) Pharmacol. Rep. 2019;71:545–549. doi: 10.1016/j.pharep.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 232.Emamgholipour S., Hossein-Nezhad A., Sahraian M.A., Askarisadr F., Ansari M. Evidence for possible role of melatonin in reducing oxidative stress in multiple sclerosis through its effect on SIRT1 and antioxidant enzymes. Life Sci. 2016;145:34–41. doi: 10.1016/j.lfs.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 233.Farinha J.B., De Carvalho N.R., Steckling F.M., De Vargas Lda S., Courtes A.A., Stefanello S.T., Martins C.C., Bresciani G., Dos Santos D.L., Soares F.A. An active lifestyle induces positive antioxidant enzyme modulation in peripheral blood mononuclear cells of overweight/obese postmenopausal women. Life Sci. 2015;121:152–157. doi: 10.1016/j.lfs.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 234.Farinha J.B., Ramis T.R., Vieira A.F., Macedo R.C.O., Rodrigues-Krause J., Boeno F.P., Schroeder H.T., Muller C.H., Boff W., Krause M., et al. Glycemic, inflammatory and oxidative stress responses to different high-intensity training protocols in type 1 diabetes: A randomized clinical trial. J. Diabetes Complicat. 2018;32:1124–1132. doi: 10.1016/j.jdiacomp.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 235.Bonetta R. Potential Therapeutic Applications of MnSODs and SOD-Mimetics. Chemistry. 2018;24:5032–5041. doi: 10.1002/chem.201704561. [DOI] [PubMed] [Google Scholar]
- 236.Miriyala S., Spasojevic I., Tovmasyan A., Salvemini D., Vujaskovic Z., St Clair D., Batinic-Haberle I. Manganese superoxide dismutase, MnSOD and its mimics. Biochim. Biophys. Acta. 2012;1822:794–814. doi: 10.1016/j.bbadis.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Rahman I., Adcock I.M. Oxidative stress and redox regulation of lung inflammation in COPD. Eur. Respir. J. 2006;28:219–242. doi: 10.1183/09031936.06.00053805. [DOI] [PubMed] [Google Scholar]
- 238.Karlsson J.O.G., Jynge P., Ignarro L.J. May Mangafodipir or Other SOD Mimetics Contribute to Better Care in COVID-19 Patients? Antioxidants. 2020;9:971. doi: 10.3390/antiox9100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Greenberger J.S., Mukherjee A., Epperly M.W. Gene Therapy for Systemic or Organ Specific Delivery of Manganese Superoxide Dismutase. Antioxidants. 2021;10:1057. doi: 10.3390/antiox10071057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Borrelli A., Schiattarella A., Mancini R., Morelli F., Capasso C., De Luca V., Gori E., Mancini A. The leader peptide of a human rec. MnSOD as molecular carrier which delivers high amounts of Cisplatin into tumor cells inducing a fast apoptosis in vitro. Int. J. Cancer. 2011;128:453–459. doi: 10.1002/ijc.25334. [DOI] [PubMed] [Google Scholar]
- 241.Borrelli A., Schiattarella A., Musella A., Mancini R., Capasso C., De Luca V., Carginale V., Sanseverino M., Tornesello A.L., Gori E., et al. A molecular carrier to transport and deliver cisplatin into endometrial cancer cells. Chem. Biol. Drug Des. 2012;80:9–16. doi: 10.1111/j.1747-0285.2012.01337.x. [DOI] [PubMed] [Google Scholar]
- 242.Russo G., Iaccarino G., Piccolo M., Ferraro M.G., Vecchione R., Grumetto L., Netti P.A., Santamaria R. Prolonged activity of a recombinant manganese superoxide dismutase through a formulation of polymeric multi-layer nanoassemblies targeting cancer cells. Eur. J. Pharm. Sci. 2021;162:105825. doi: 10.1016/j.ejps.2021.105825. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.