Abstract
Legionella pneumophila is an intracellular pathogen of protozoa and alveolar macrophages. This bacterium contains a gene (pilD) that is involved in both type IV pilus biogenesis and type II protein secretion. We previously demonstrated that the PilD prepilin peptidase is crucial for intracellular infection by L. pneumophila and that the secreted pilD-dependent proteins include a metalloprotease, an acid phosphatase, an esterase/lipase, a phospholipase A, and a p-nitrophenyl phosphorylcholine hydrolase. Since mutants lacking type IV pili, the protease, or the phosphorylcholine hydrolase are not defective for intracellular infection, we sought to determine the significance of the secreted acid phosphatase activity. Three mutants defective in acid phosphatase activity were isolated from a population of mini-Tn10-mutagenized L. pneumophila. Supernatants as well as cell lysates from these mutants contained minimal acid phosphatase activity while possessing normal levels of other pilD-dependent exoproteins. Genetic studies indicated that the gene affected by the transposon insertions encoded a novel bacterial histidine acid phosphatase, which we designated Map for major acid phosphatase. Subsequent inhibitor studies indicated that Map, like its eukaryotic homologs, is a tartrate-sensitive acid phosphatase. The map mutants grew within macrophage-like U937 cells and Hartmannella amoebae to the same degree as did wild-type legionellae, indicating that this acid phosphatase is not essential for L. pneumophila intracellular infection. However, in the course of characterizing our new mutants, we gained evidence for a second pilD-dependent acid phosphatase activity that, unlike Map, is tartrate resistant.
Legionella pneumophila, the etiologic agent of Legionnaires' disease, is a gram-negative, facultative intracellular bacterium (10). Normally, L. pneumophila is an inhabitant of fresh water, where it lives as part of biofilms or as a parasite of protozoa (28). However, it can reach the human respiratory tract following inhalation or aspiration of contaminated water (20, 26, 60). In the lung, the legionellae replicate within alveolar macrophages, eventually causing much cell death and damage to lung tissue (24, 60).
The identification of an L. pneumophila pilD gene (31, 32) afforded new insight into the molecular pathogenesis of legionellosis. In a variety of gram-negative bacteria, PilD is an inner membrane peptidase that cleaves and methylates the pilin and pilin-like proteins that will form the type IV pili (2, 3, 21, 38, 49, 57). In addition, PilD processes a second set of pilin-like molecules which contribute to the formation of a type II protein secretion system (9, 21, 22, 33, 43, 48, 56). The two functions of PilD have been confirmed in L. pneumophila; i.e., a mutation in pilD renders bacteria nonpiliated and deficient for protein secretion (5, 31). The existence of the Legionella type II secretion system was later confirmed by the identification of some of the genes encoding components of the secretion apparatus (23). Importantly, the L. pneumophila pilD mutant is severely defective (i.e., ca. 1,000-fold) for replication within freshwater protozoa and human macrophages (31). Although type IV pili are modestly involved in the attachment of the bacteria to host cells, they are not critical for intracellular growth and survival (31, 55). Consequently, it became more significant to identify and characterize the secreted activities that are absent in the pilD mutant. Several Legionella enzymatic activities have recently been shown to be secreted in a pilD-dependent manner, including a zinc metalloprotease, phospholipase A, acid phosphatase, p-nitrophenyl phosphorylcholine (pNPPC) hydrolase, RNase, and esterase/lipase (5, 31). Since mutants specifically defective for the production of either the pNPPC hydrolase activity or the metalloprotease proved not to be impaired for intracellular infection (5, 58), the critical pilD-dependent factors remain to be identified.
For three reasons, we targeted the acid phosphatase activity for further analysis. First, phosphatase activity is diminished in type II secretion mutants that are defective for intracellular growth (O. Rossier and N. P. Cianciotto, submitted for publication). Second, our observations in Legionella represent the first connection between an acid phosphatase and PilD or type II secretion. Third, in other pathogens, acid phosphatases have been postulated to have a role in intracellular infection (7, 11, 30, 45, 46, 50, 51). In this work, we provide evidence that L. pneumophila possesses two acid phosphatase activities and report the identification and mutation of the major, pilD-dependent acid phosphatase. Although the major acid phosphatase proved to be unique among bacterial acid phosphatases in terms of its sequence and secretion, it was not essential for the intracellular replication of L. pneumophila in macrophages or amoebae.
MATERIALS AND METHODS
Bacteria and media.
L. pneumophila serogroup 1 strain 130b (ATCC BAA 74), a virulent clinical isolate, and NU243, a direct derivative of 130b that contains a stable mini-Tn10 insertion in the Legionella pilD gene, were described previously (19, 31). A population of 130b randomly mutagenized with mini-Tn10 was also reported before (41). Legionellae were routinely cultured on buffered charcoal yeast extract (BCYE) agar for 3 days at 37°C (17). In order to determine the levels of secreted enzymatic activities and compare the growth rates between strains, bacteria were cultured in buffered yeast extract (BYE) broth (5). The extent of bacterial growth was assessed by measuring the optical density of the cultures at 660 nm (OD660) (31). To ultimately screen for mutants deficient in acid phosphatase activity, colonies of randomly mutagenized 130b bacteria were taken from BCYE plates and inoculated into 100 μl of BYE contained in wells of 96-well plates. After overnight incubation at 37°C with shaking, 10-μl aliquots from each culture were assayed for acid phosphatase activity (see below). Escherichia coli DH5α was used as the host for recombinant plasmids (6). E. coli was grown in Luria-Bertani broth or agar, with ampicillin (100 μg/ml) added when needed.
Preparation of supernatants and cell lysates.
To test for secreted or cell-associated enzymes, supernatants and lysates from L. pneumophila cultures were prepared as before (5). Briefly, supernatants were obtained by centrifugation followed by filtration (0.22 μm pore size) and in some cases were concentrated 100-fold through a Millipore YM10 ultrafiltration cell. Lysates were prepared by treatment with Triton X-100 and lysozyme.
Phosphatase and other enzymatic assays.
Phosphatase activity was measured as the ability of the sample to release p-nitrophenol (pNP) from p-nitrophenyl phosphate (pNPP) (Sigma Chemical, St. Louis, Mo.) (5). Bacterial supernatant or cell lysates were incubated with 7.6 mM pNPP in 50 mM citric acid buffer (pH 5) or 0.2 M acetate buffer (pH 5.5) at 37°C. Since pNP is colorless at acid pH, concentrated NaOH was added to the reactions after the appropriate incubation at 37°C, and the production of pNP was monitored at 410 nm. In some experiments, sodium tartrate and sodium molybdate, compounds that usually inhibit acid phosphatases, were assayed throughout a concentration range from 0.001 to 10 mM (15, 29, 50, 54). To measure alkaline phosphatase activity, the pNPP hydrolysis reactions were performed in 100 mM Tris (pH 10) (5). One unit of acid or alkaline phosphatase activity was defined as that which releases 1 nmol of pNP in 1 min. Acid phosphatase activity was also determined in samples electrophoresed through polyacrylamide gels (47, 52). Proteins in 100-fold-concentrated supernatants were separated in a discontinuous system with a 10% polyacrylamide separating gel under nondenaturing conditions. After the electrophoresis, the gel was equilibrated in 0.2 M acetate buffer (pH 5.5) and incubated with 7.6 mM pNPP in the same buffer at room temperature. To then detect the release of pNP, the solution was made alkaline by the addition of concentrated NaOH. The reactive band was isolated from the gel by electroelution under nondenaturing conditions following the manufacturer's recommendations (Bio-Rad Laboratories, Hercules, Calif.), and the proteins contained within were examined for pNPP hydrolysis and for size by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (27).
Bacterial supernatants were assayed for their ability to release pNP from p-nitrophenyl caprylate or palmitate (indicative of a lipase/esterase activity) and from pNPPC as described previously (5).
PCR, sequencing, and clone analysis.
Genomic DNA from L. pneumophila was extracted as previously described (42). Based upon data from the L. pneumophila Philadelphia I genome project (http://genome3.cpmc.columbia.edu/∼legion/), three DNA primers were designed for the amplification of an acid phosphatase gene: 1, 5′-GCCATCTTCCAAGGTATAGC, corresponding to a site 1,020 bp upstream of the gene; 2, 5′-ACCAACGGTGGCAAGATACG, from a site 74 bp upstream; and 3, 5′-ATTCCGAGCACGACCACAAC, from a site 201 bp downstream of the gene. To determine the approximate position of the mini-Tn10 in several acid phosphatase mutants, a standard PCR was performed using primer 3 and a primer designed from the end of the transposon (5′-CCTTAACTTAATGATTTTTAC) (32). PCR products obtained with wild-type DNA and primers 1 plus 3 and 2 plus 3 were used for sequencing the 130b acid phosphatase gene. Sequencing reactions were performed using the BigDye terminator cycle sequencing mix from PE Applied Biosystems (Foster City, Calif.). Primer synthesis and automated sequence analysis on an ABI Prism 373 DNA sequencer (Applied Biosystems) were performed at the Biotech Facility at Northwestern University Medical School, Chicago, Ill. Sequence database searches were performed using programs based on the BLAST algorithm (4). The nucleotide sequence was analyzed for promoter prediction (44). The predicted protein was analyzed with the SignalP program (36) and Psort (35) for a signal sequence and for protein motifs with the PROSITE database (8). The PCR fragments that were used in sequencing experiments were also cloned into the pGem-T Easy vector (Promega, Madison, Wis.). The 2.3-kb fragment derived using primers 1 and 3 generated plasmid pVA13, whereas the 1.3-kb fragment obtained with primers 2 and 3 generated pVA12.
Intracellular infection of U937 cells and Hartmannella amoebae.
U937, a human cell line that differentiates into macrophage-like cells after treatment with phorbol esters, served as a host for in vitro infection by L. pneumophila (13). The cell line was infected as previously described (31), with some modifications. To quantitate intracellular growth, monolayers containing 106 macrophages were inoculated with approximately 105 CFU, incubated for 0, 6, 18, 24, 48, or 72 h, and then lysed. Serial dilutions of the lysates were plated on BCYE agar, supplemented with kanamycin for the mutants, to determine the number of bacteria per monolayer. To establish the cytopathic effect of L. pneumophila on U937 cells, the viability of infected monolayers was tested by their ability to reduce alamar blue, as recommended by the manufacturer (Biosource International, Vacaville, Calif.). Briefly, at 0, 24, 48, and 72 h, the wells were thoroughly washed to eliminate the extracellular bacteria. Medium (100 μl) with 10 μl of alamar blue was added to each well and incubated at 37°C for 4 h. After this time, the fluorescence (excitation, 540 nm; emission, 584 nm) was read in a Spectra Max Gemini fluorescence reader (Molecular Devices, Sunnyvale, Calif.). To examine the ability of legionellae to grow within a protozoan host, Hartmannella vermiformis was infected as previously indicated (14, 31). Approximately 105 CFU were added to wells containing 105 amoebae, and at 0, 24, 48, or 72 h postinoculation, the numbers of bacteria within the coculture were determined by serial dilution.
Statistical analysis.
To study the statistical significance of the differences observed between samples, Student's t test was used. The P values are given when appropriate.
Nucleotide accession number.
The L. pneumophila map locus sequence has been deposited in the GenBank database at the National Center for Biotechnology Information under accession number AF299349.
RESULTS
Isolation of L. pneumophila acid phosphatase mutants.
With the purpose of determining the role of the acid phosphatase(s) in L. pneumophila intracellular infection, we sought to isolate and characterize mutants specifically deficient in this enzymatic activity. Toward that end, 1,767 colonies from a randomly mutagenized population of L. pneumophila 130b were inoculated into 100 μl of BYE in microtiter plates. After overnight growth at 37°C, an aliquot from each culture was tested for phosphatase activity with pNPP at pH 5. Five mutants with reduced activity, which was visually apparent, were selected and grown in standard culture tubes for retesting. The three mutants that appeared to produce a reduced level of acid phosphatase upon reexamination were designated NU254, NU255, and NU256. These mutants replicated in BYE broth with a growth profile similar to that of wild-type 130b (data not shown), indicating that the reduction in acid phosphatase activity was not due to a general growth defect.
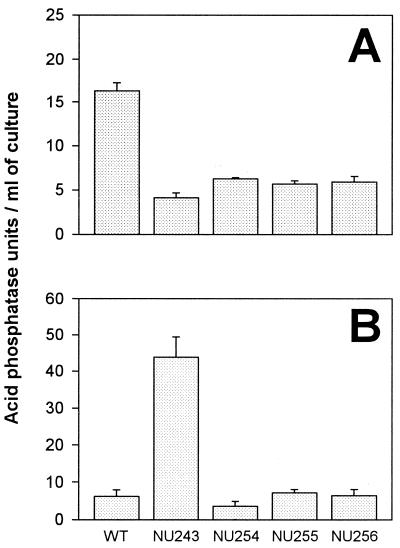
Previously, we had shown that the highest level of acid phosphatase activity in L. pneumophila supernatants occurs at late log phase (5). Therefore, late-log-phase BYE cultures of NU254, NU255, and NU256 were analyzed for phosphatase activity at pH 5 in order to confirm and quantitate their enzymatic defect. The supernatants from the mutants presented a level of acid phosphatase activity significantly reduced compared with the wild type (P < 0.001) (Fig. 1A). Indeed, the level of acid phosphatase activity found for NU254, NU255, and NU256 supernatants was similar to the level found for the secretion-defective pilD mutant (Fig. 1A). To determine whether the reduction in pNPP hydrolysis was due to the absence of one or more secreted proteins, concentrated late-log-phase supernatants were electrophoresed through polyacrylamide under nondenaturing conditions, and then the gel was incubated in the presence of pNPP. After 5 min of incubation, wild-type samples yielded a single reactive band, whereas the samples from the phosphatase mutants and the pilD mutant failed to exhibit any reactive band (data not shown). When the reactive band in wild-type samples was electroeluted from the gel, it showed hydrolytic activity against pNPP at pH 5 (5.6 U/ml) but not at pH 10 (−0.6 U/ml). These data confirm that NU254, NU255, and NU256 lack most of the secreted acid phosphatase activity.
FIG. 1.
Acid phosphatase activity produced by L. pneumophila strains. Late-log-phase (OD660 = 1.8 to 1.9) supernatants (A) and cell lysates (B) of wild-type strain 130b (WT), pilD mutant NU243, NU254, NU255, and NU256 were examined for their ability to release pNP from pNPP at pH 5. Bars represent the means ± standard deviation of the activity found in three cultures and are representative of the results seen in three independent experiments.
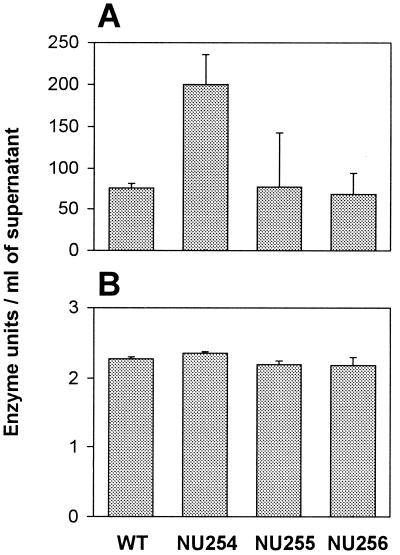
To begin to examine the reason for the observed losses of acid phosphatase activity, cell lysates from the mutants were tested for their ability to hydrolyze pNPP. Unlike the pilD mutant, NU254, NU255, and NU256 did not show accumulated acid phosphatase activity in their lysates, suggesting that they are not secretion mutants (Fig. 1B). The supernatants of the new mutants were next tested for esterase/lipase and pNPPC hydrolase activities, two functions known to be secreted in a pilD-dependent fashion (5). The levels of these activities were similar for the mutants and the wild type (Fig. 2), confirming that NU254, NU255, and NU256 do not have a general defect in secretion. Incidentally, the fact that these mutants had wild-type levels of pNPPC hydrolysis confirms our earlier conclusion that the L. pneumophila pNPPC hydrolase activity is not due to any major acid phosphatase activity (5). To address the possibility that the mutants had a regulatory defect, affecting the stage at which they produce the acid phosphatase, supernatants from log-phase (OD660 = 0.8 to 0.9), late-log-phase (OD660 = 1.8 to 1.9), and stationary-phase (OD660 = 2.1 to 2.2) cultures were examined for the presence of acid phosphatase. At log phase, the acid phosphatase level in wild-type cultures was barely detectable, with only 3.8 (±1.2) U/ml, but was already higher than that in the supernatant from the pilD mutant (1.6 ± 0.1; P < 0.05) or NU254 and NU255 (1.5 ± 0.2 and 1.6 ± 0.2, respectively; P < 0.05). The difference in the amount of activity present in wild-type and mutant supernatants was greater at late log and stationary phases, similar to the results presented in Fig. 1. Taken together, these results suggest that NU254, NU255, and NU256 contain a mutation in the gene encoding an L. pneumophila acid phosphatase.
FIG. 2.
Lipase/esterase and pNPPC hydrolase activity secreted by L. pneumophila strains. Late-log-phase (OD660 = 1.8 to 1.9) supernatants of wild-type 130b (WT), NU254, NU255, and NU256 were tested for their ability to release pNP from p-nitrophenyl palmitate (A) and pNPPC (B). Bars represent the means ± standard deviation of the activity found in three cultures and are representative of the results obtained in three independent experiments. Similar to the results in panel A, the three mutants were not defective in the hydrolysis of p-nitrophenyl caprylate (data not shown)
Nucleotide sequence of the major L. pneumophila acid phosphatase (map) gene.
To establish the genetic basis of the defect in NU254, NU255, and NU256, we sought to determine the sequences into which the transposon had inserted in each mutant. At the time we were starting this analysis using inverse PCR, as we have done in the past for other mutants (32), the sequence of an open reading frame (ORF) with homology to acid phosphatases became available in the developing L. pneumophila Philadelphia I genome database. To determine if this was the gene mutated in our 130b acid phosphatase mutants, PCR experiments were performed using a primer corresponding to sequences just 3′ of the ORF and a primer for the mini-Tn10. The PCR fragments amplified from DNA of the three mutants indicated that this was the gene interrupted; i.e., we amplified fragments of 430 bp for NU254, 260 bp for NU255, and 960 bp for NU256. The exact position of the transposon in NU255 was confirmed by sequencing of the PCR fragment (see below).
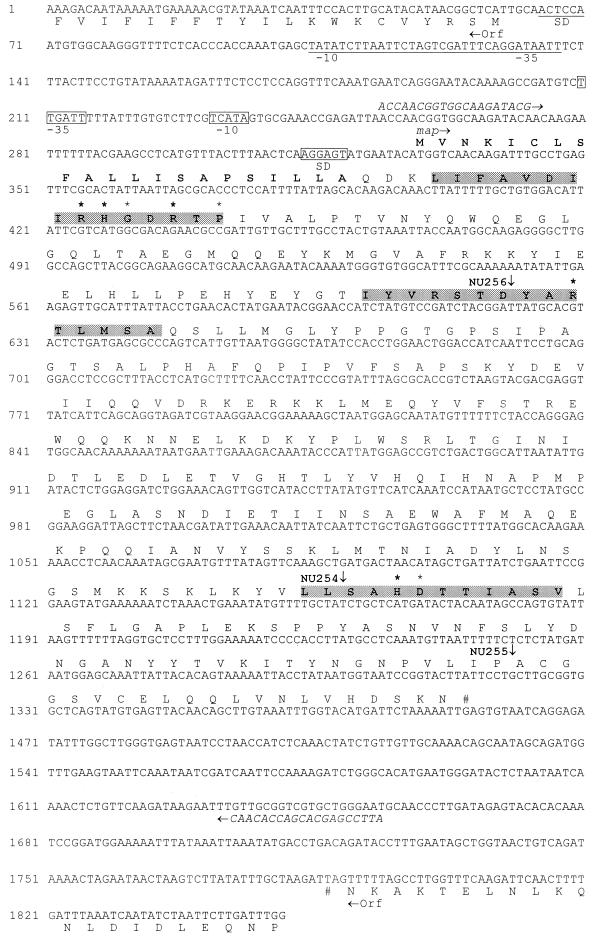
Using genomic DNA from strain 130b, the gene was sequenced in its entirety, along with up and downstream regions (Fig. 3). The ORF had 1,059 bp, encoding a predicted protein of 352 amino acids (39.4 kDa). This size was in agreement with the migration observed for the electroeluted acid phosphatase (see above) when it was analyzed by SDS-PAGE (39 kDa) (data not shown). Analysis of the protein sequence showed the presence of a signal peptide (Fig. 3), supporting the secreted nature of the L. pneumophila acid phosphatase activity. Importantly, the predicted protein contained the motif [LIVM]-X-X-[LIVMA]-X-X-[LIVM]-X-R-H-[GN]-X-R-X-[PAS] characteristic of histidine acid phosphatases (Fig. 3). An alignment with the amino acid sequence of the well-studied Escherichia coli periplasmic acid phosphatase indicated that the Legionella protein had all the residues that are essential for enzymatic activity (39) (bold asterisks in Fig. 3). Interestingly, the protein showed its greatest overall homology to eukaryotic acid phosphatases, such as a lysosomal acid phosphatase from mouse (protein accession number P24638), the human prostatic acid phosphatase (2HPA), the Drosophila acid phosphatase (S64682), and the Leishmania donovani acid phosphatases (AAC79513 and AAC47744), with identities ranging from 28 to 30% and similarities ranging from 45 to 46%. To confirm that map encodes a functional acid phosphatase, we examined two recombinant E. coli clones that contain the entire gene for pNPP hydrolysis. DH5α(pVA12) had 110.3 ± 0.58 U/ml of culture, and DH5α(pVA13) had 92 ± 1.9 U/ml, while DH5α containing the pGem-T Easy vector had just 1.6 ± 0.17 U/ml of culture. Given this result, the sequence data, and the behavior of the L. pneumophila mutants, we named this new L. pneumophila gene map for major acid phosphatase gene.
FIG. 3.
Nucleotide sequence of the L. pneumophila 130b map locus. The deduced amino acid sequences of the various ORFs and the termination codons (#) are indicated. For the predicted Map protein, the putative signal peptide is indicated in bold, and conserved protein domains are shadowed. Within the conserved domains, asterisks indicate conserved amino acids, and bold asterisks indicate amino acids that are essential for activity in the E. coli periplasmic acid phosphatase. Putative promoter sequences are indicated by the −10 and −35 designations, and the Shine-Dalgarno sequence is indicated (SD). The mini-Tn10 insertions are indicated by an arrow alongside the name of the corresponding mutant. The positions of the insertions are approximate for NU254 and NU256. Primers 2 and 3 (see Materials and Methods) are shown in italics at their binding regions.
Immediately upstream of map, a putative promotor was detected, as well as a ribosome-binding sequence (Fig. 3). Two hundred sixty-eight base pairs upstream of the map start codon, and oriented in the opposite direction, the beginning of an ORF with high homology to the d-stereospecific peptide hydrolase of Bacillus cereus (protein accession number AJ011526) was detected. Downstream of map, and also oriented in the opposite direction, the beginning of an ORF without clear homology to known proteins was detected. These data suggest that map is expressed as a monocistronic message. Therefore, the transposon insertions in NU254, NU255, and NU256 would not be expected to produce polar effects. The GC content in the map locus was 39%, similar to the %GC of the L. pneumophila genome (10).
Identification of a minor acid phosphatase activity produced by L. pneumophila.
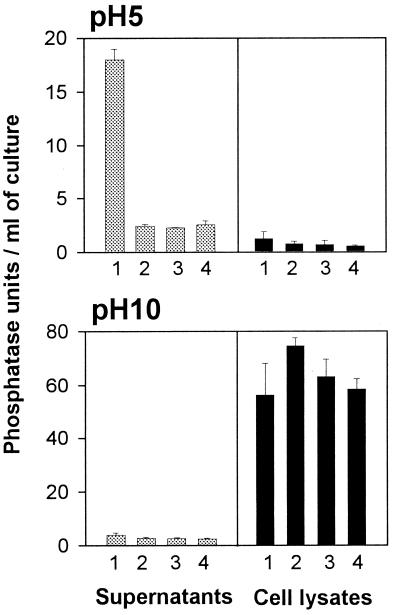
Although an acid phosphatase gene was interrupted in NU254, NU255, and NU256, the supernatants from the mutants always showed a low level of activity in the pNPP assay (Fig. 1A) which could be eliminated by 10 min of incubation at 100°C. To determine whether this activity was simply cell-associated alkaline phosphatase that was released by spontaneous lysis of some cells in the culture (5, 25), supernatants and cell lysates from the wild type and map mutants were assayed for hydrolysis of pNPP at both pH 5 and 10 (Fig. 4). The supernatants showed a small amount of phosphatase activity at pH 10. However, the cell lysates, which were very rich in alkaline phosphatase, showed a minimal pNPP reaction at pH 5, indicating that alkaline phosphatase cannot be responsible for the activity in the supernatants from the mutants. Since two of the mutants had transposon insertions in domains of the protein that are likely essential for activity (Fig. 3), and all of them have the same level of pNPP hydrolytic activity (Fig. 1A and 4), it is unlikely that the residual activity is due to low levels of truncated Map. Consequently, we suspected that the phosphatase activity remaining in the supernatants of the map mutants was due to another (minor) acid phosphatase activity, since bacteria can have multiple acid phosphatases (47, 50).
FIG. 4.
Acid and alkaline phosphatase activity in L. pneumophila cultures. Supernatants and cell lysates from the wild type (bar 1) and map mutants NU254 (bar 2), NU255 (bar 3), and NU256 (bar 4) were examined for their ability to hydrolyze pNPP at pH 5 and 10. Bars represent the means ± standard deviation of three cultures. The only significant difference between the wild type and mutants was observed with supernatants tested at pH 5 (P < 0.01).
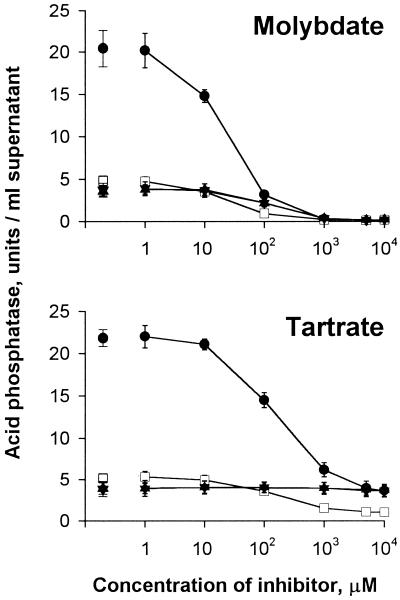
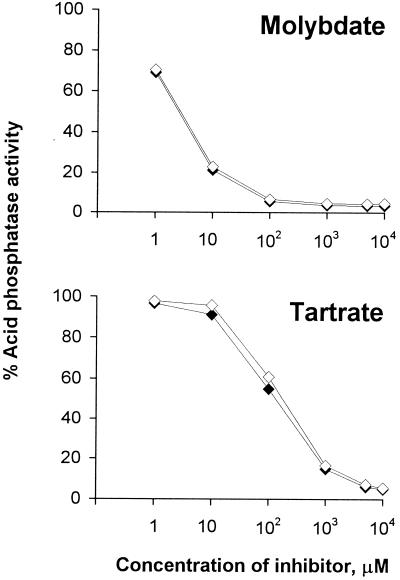
To explore this possibility, we examined the effect of molybdate and tartrate on the pNPP hydrolysis mediated by mutant and wild-type supernatants, since acid phosphatases are commonly molybdate sensitive but only some are sensitive to tartrate (15, 29, 50, 54). The activity in 130b supernatants was completely inhibited by molybdate (Fig. 5). In contrast, it was only partially inhibited by tartrate; even a concentration of 10 mM tartrate inhibited the activity only up to 75% (Fig. 5). The activity present in the supernatants of the map mutants was completely inhibitable by sodium molybdate but interestingly, was not affected by sodium tartrate (Fig. 5). The level of activity remaining in NU254 and NU255 corresponded to the level of tartrate-resistant activity found in the wild type, demonstrating that the mutants indeed lack a tartrate-sensitive acid phosphatase activity while retaining a tartrate-resistant one. In support of these data, the activity expressed by recombinant, map-containing E. coli (Fig. 6), as well as the electroeluted pNPP-reactive protein band (data not shown), was completely inhibited by either tartrate or molybdate. Altogether, these results indicate that L. pneumophila has two different acid phosphatase activities: one that is molybdate and tartrate sensitive and is completely missing in NU254, NU255, and NU256, and one that is molybdate sensitive but tartrate resistant and is still present in the supernatants of the mutants. Since the level of tartrate-resistant activity is reduced in the supernatant of NU243 (P < 0.01 at 1 to 10 mM tartrate; Fig. 5), this second acid phosphatase activity is probably pilD dependent.
FIG. 5.
L. pneumophila map mutants lack a tartrate-sensitive acid phosphatase. Supernatants from wild-type 130b (●), the pilD mutant NU243 (□), and the mutants NU254 (▴) and NU255 (▾) were examined for their ability to hydrolyze pNPP in the presence of different concentrations of molybdate or tartrate. The isolated symbols represent the level of activity without inhibitor. The results are the means ± standard deviation of the activity found in three cultures. Similar results were obtained in two independent experiments using concentrated supernatants (data not shown).
FIG. 6.
Effect of molybdate and tartrate on the cloned L. pneumophila Map activity. Whole cultures of E. coli DH5α(pVA12) (◊) and DH5α(pVA13) (⧫) were tested for acid phosphatase activity in acetate buffer (pH 5.5) in the presence of different concentrations of sodium molybdate (top panel) or sodium tartrate (bottom panel). The results are expressed as the percentage of refractive activity and are the means ± standard deviation of the activity found in three cultures and representative of two independent experiments. The error bars are too small to be seen.
Intracellular infection by L. pneumophila map mutants.
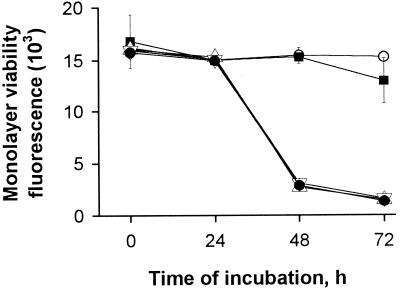
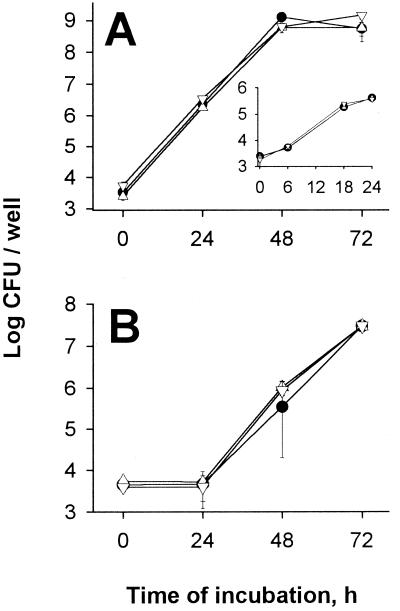
To determine the role of the major acid phosphatase in intracellular infection, we tested the map mutants for their relative ability to infect macrophages and protozoa. It is well documented that the intracellular growth of L. pneumophila results in the destruction of the macrophage host (1, 12, 40). Therefore, the acid phosphatase mutants, together with the wild type and the pilD mutant, were first inoculated into U937 cells and examined for cytopathic effects at different times after infection (Fig. 7). The macrophages infected with the wild type showed a substantial decrease in viability after 48 and 72 h of infection, only 19 and 9% viable macrophages, respectively (P < 0.001). Contrary to these results, the macrophages infected with the pilD mutant showed little, if any, reduction in viability after 72 h, as has been seen previously (5, 31). The map mutants showed a pattern of cytopathicity that was identical to that of the wild type (Fig. 7). To determine if this cytopathic effect correlated with a normal capacity to replicate inside macrophages, the mutants were inoculated into U937 monolayers, and the number of bacteria was recorded after various incubation intervals (Fig. 8A). Wild-type 130b showed the typical intracellular growth pattern, increasing 10,000- to 100,000-fold by 48 h after infection. Interestingly, the same results were obtained with the acid phosphatase mutants, indicating that these strains are not defective for intracellular replication in U937 cells. Moreover, the mutants did not seem to have a defect in entry, since the numbers recovered after the uptake period were the same as for the wild type (see t = 0 in Fig. 8A). To determine the role of the major acid phosphatase in amoeba infection, H. vermiformis cultures were inoculated with the wild type and map mutants, and the number of bacteria was recorded at different times. As in the macrophage infection, the same numbers of bacteria were recovered from cocultures with the wild type or with the mutants (Fig. 8B). Together, these results indicate that the major acid phosphatase is not essential for macrophage or amoeba infection by L. pneumophila.
FIG. 7.
Cytopathic effect of L. pneumophila on U937 cells. At different times after inoculation, the viability of the macrophage monolayer that had been infected at a multiplicity of infection of 0.1 with 130b (●), pilD mutant NU243 (■), NU254 (▵), or NU255 (▿) was measured by fluorescence associated with alamar blue reduction. As a control, an uninfected monolayer (○) was processed in parallel. Results represent the means ± standard deviation of triplicate wells and are representative of two independent experiments.
FIG. 8.
Intracellular infection by wild-type and mutant L. pneumophila. U937 cells (A) and H. vermiformis amoebae (B) were infected at multiplicities of infection of 0.1 and 1, respectively, with wild-type 130b (●), NU254 (▵), or NU255 (▿). The number of bacteria in each well was quantitated at 0, 24, 48, and 72 h by plating aliquots on BCYE agar. Results represent the means ± standard deviation of triplicate wells and are representative of two independent experiments. In a third U937 experiment, the numbers of bacteria were also determined at 6 and 18 h (inset).
DISCUSSION
An acid phosphatase activity was first described in 1981 for the Legionella genus (34). That activity was detected in all the strains tested, i.e., representatives of L. pneumophila serogroups 1 to 6 and Legionella species L. bozemanii, L. dumoffii, L. gormanii, L. longbeachae, and L. micdadei (34, 37, 59). Subsequently, two cell-associated acid phosphatases, ACP1 and ACP2, were purified from L. micdadei (50). Recently, we demonstrated that the acid phosphatase activity of L. pneumophila is actually secreted in a pilD-dependent fashion (5). Our current data indicate that the secreted acid phosphatase activity of L. pneumophila is the result of at least two different enzymes, which can be discriminated by their different sensitivity to tartrate. Mutant and sequence analyses indicated that the majority of activity is due to a tartrate-sensitive histidine acid phosphatase, which we have named Map. The Map protein is notable among bacterial acid phosphatases in two aspects. First, it is an extracellularly secreted enzyme, while the rest of the bacterial acid phosphatases characterized to date are periplasmic or membrane-bound proteins (47). Second, Map aligned with significant homology only with eukaryotic histidine acid phosphatases that are also secreted. Although several bacterial acid phosphatases have been sequenced (47), they did not show homology with L. pneumophila Map as determined by the NCBI BLAST programs. Thus, our data represent the first evidence for a bacterial extracellularly secreted histidine acid phosphatase.
L. pneumophila mutants containing insertions in map did not show a defect in infection of U937 cells or amoebae. Therefore, Map either has no role in L. pneumophila intracellular replication or has a dispensable one. As we have demonstrated, L. pneumophila has at least one other acid phosphatase, and this, or other enzymes, may compensate for the lack of Map. Also, even though Map is not essential for U937 cell infection, it may play a role in Legionella survival within other host cells, such as activated macrophages or neutrophils. Although there is no bacterial precedent for Map, two secretory histidine acid phosphatases, SAcP-1 and SAcP-2, have been identified in the macrophage parasite Leishmania donovani (53). It is believed that the parasitic phosphatases do have a role in infection, because they are produced during human infection (18). Since Map and the SAcP enzymes showed significant homology, they may have a common target or function during intracellular infection. On the other hand, it is entirely possible that Map and its parasite analog promote extracellular survival and spread in vivo.
Although the loss of Map secretion was not the reason for the intracellular infectivity defect of the pilD mutant, it is possible that the pilD-dependent tartrate-resistant acid phosphatase is important for U937 cell or amoeba infection. Indeed, in addition to L. micdadei ACP2 noted above, tartrate-resistant acid phosphatases from Francisella tulariensis, Coxiella burnetii, and Leishmania donovani have been implicated in the resistance of these intracellular pathogens to the oxidative burst of phagocytes; the purified enzymes inhibit superoxide production by fMet-Leu-Phe-activated cells (7, 30, 45, 46, 50). In the case of L. micdadei and Leishmania donovani, the enzymes interfere in phosphoinositide metabolism by degrading phosphatidylinositol diphosphate and inositol trisphosphate (16, 51). Although the tartrate-resistant activity is a minor one in L. pneumophila BYE culture supernatants, it may be a major secreted factor when the bacteria are exposed to host cells or tissue. Thus, the tartrate-resistant acid phosphatase activity that we have uncovered, as well as other pilD-dependent factors, may be quite relevant for the pathogenesis of L. pneumophila.
ACKNOWLEDGMENTS
We thank Ombeline Rossier for first noticing the acid phosphatase gene in the Legionella database and for helpful discussions.
This work was supported by NIH grant AI43987 awarded to N.P.C.
REFERENCES
- 1.Abu Kwaik Y. Fatal attraction of mammalian cells to Legionella pneumophila. Mol Microbiol. 1998;30:689–695. doi: 10.1046/j.1365-2958.1998.01092.x. [DOI] [PubMed] [Google Scholar]
- 2.Alm R A, Hallinan J P, Watson A A, Mattick J S. Fimbrial biogenesis genes of Pseudomonas aeruginosa: pilW and pilX increase the similarity of type 4 fimbriae to the GSP protein-secretion systems and pilY1encodes a gonococcal PilC homologue. Mol Microbiol. 1996;22:161–173. doi: 10.1111/j.1365-2958.1996.tb02665.x. [DOI] [PubMed] [Google Scholar]
- 3.Alm R A, Mattick J S. Identification of two genes with prepilin-like leader sequences involved in type 4 fimbrial biogenesis in Pseudomonas aeruginosa. J Bacteriol. 1996;178:3809–3817. doi: 10.1128/jb.178.13.3809-3817.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aragon V, Kurtz S, Flieger A, Neumeister B, Cianciotto N P. Secreted enzymatic activities of wild-type and pilD-deficient Legionella pneumophila. Infect Immun. 2000;68:1855–1863. doi: 10.1128/iai.68.4.1855-1863.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ausubel F M, Brent R B, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N. Y: John Wiley & Sons; 1987. [Google Scholar]
- 7.Baca O G, Roman M J, Glew R H, Christner R F, Buhler J E, Aragon A S. Acid phosphatase activity in Coxiella burnetii: a possible virulence factor. Infect Immun. 1993;61:4232–4239. doi: 10.1128/iai.61.10.4232-4239.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bairoch A, Bucher P, Hofmann K. The PROSITE database: its status in 1997. Nucleic Acids Res. 1997;25:217–221. doi: 10.1093/nar/25.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bally M, Wretlind B, Lazdunski A. Protein secretion in Pseudomonas aeruginosa: molecular cloning and characterization of the xcp-1gene. J Bacteriol. 1989;171:4342–4348. doi: 10.1128/jb.171.8.4342-4348.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brenner D J, Steigerwalt A G, Weaver R E, McDade J E, Feeley J C, Mandel M. Classification of the Legionnaires' disease bacterium: an interim report. Curr Microbiol. 1978;1:7–10. [Google Scholar]
- 11.Chhatwal G S, Walker M J, Yan H, Timmis K N, Guzman C A. Temperature dependent expression of an acid phosphatase by Bordetella bronchiseptica: role in intracellular survival. Microb Pathog. 1997;22:257–264. doi: 10.1006/mpat.1996.0118. [DOI] [PubMed] [Google Scholar]
- 12.Cianciotto N, Eisenstein B I, Engleberg N C, Shuman H. Genetics and molecular pathogenesis of Legionella pneumophila, an intracellular parasite of macrophages. Mol Biol Med. 1989;6:409–424. [PubMed] [Google Scholar]
- 13.Cianciotto N P, Eisenstein B I, Mody C H, Toews G B, Engleberg N C. A Legionella pneumophilagene encoding a species-specific surface protein potentiates initiation of intracellular infection. Infect Immun. 1989;57:1255–1262. doi: 10.1128/iai.57.4.1255-1262.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cianciotto N P, Fields B S. Legionella pneumophila mipgene potentiates intracellular infection of protozoa and human macrophages. Proc Natl Acad Sci USA. 1992;89:5188–5191. doi: 10.1073/pnas.89.11.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colon L S, Jimenez N M, Zlotnik H. Properties of a partially purified acid phosphatase from pathogenic Nocardia brasiliensis. Mycopathologia. 1992;118:85–93. doi: 10.1007/BF00442536. [DOI] [PubMed] [Google Scholar]
- 16.Das S, Saha A K, Remaley A T, Glew R H, Dowling J N, Kajiyoshi M, Gottlieb M. Hydrolysis of phosphoproteins and inositol phosphates by cell surface phosphatase of Leishmania donovani. Mol Biochem Parasitol. 1986;20:143–153. doi: 10.1016/0166-6851(86)90026-5. [DOI] [PubMed] [Google Scholar]
- 17.Edelstein P H. Improved semiselective medium for isolation of Legionella pneumophilafrom contaminated clinical and environmental specimens. J Clin Microbiol. 1981;14:298–303. doi: 10.1128/jcm.14.3.298-303.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellis S L, Shakarian A M, Dwyer D M. Leishmania: amastigotes synthesize conserved secretory acid phosphatases during human infection. Exp Parasitol. 1998;89:161–168. doi: 10.1006/expr.1998.4298. [DOI] [PubMed] [Google Scholar]
- 19.Engleberg N C, Drutz D J, Eisenstein B I. Cloning and expression of Legionella pneumophila antigens in Escherichia coli. Infect Immun. 1984;44:222–227. doi: 10.1128/iai.44.2.222-227.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fields B S. The molecular ecology of legionellae. Trends Microbiol. 1996;4:286–290. doi: 10.1016/0966-842x(96)10041-x. [DOI] [PubMed] [Google Scholar]
- 21.Filloux A, Michel G, Bally M. GSP-dependent protein secretion in Gram-negative bacteria: the Xcp system of Pseudomonas aeruginosa. FEMS Microbiol Rev. 1998;22:177–198. doi: 10.1111/j.1574-6976.1998.tb00366.x. [DOI] [PubMed] [Google Scholar]
- 22.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hales L M, Shuman H A. Legionella pneumophilacontains a type II general secretion pathway required for growth in amoebae as well as for secretion of the Msp protease. Infect Immun. 1999;67:3662–2666. doi: 10.1128/iai.67.7.3662-3666.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horwitz M A. Interactions between macrophages and Legionella pneumophila. Curr Top Microbiol Immunol. 1992;181:265–282. doi: 10.1007/978-3-642-77377-8_10. [DOI] [PubMed] [Google Scholar]
- 25.Kim M J, Rogers J E, Hurley M C, Engleberg N C. Phosphatase-negative mutants of Legionella pneumophila and their behavior in mammalian cell infection. Microb Pathog. 1994;17:51–62. doi: 10.1006/mpat.1994.1051. [DOI] [PubMed] [Google Scholar]
- 26.Kramer M H, Ford T E. Legionellosis: ecological factors of an environmentally ‘new‘ disease. Zentralbl Hyg. 1994;195:470–482. [PubMed] [Google Scholar]
- 27.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Lee J V, West A A. Survival and growth of Legionellaspecies in the environment. J Appl Bacteriol Symp Suppl. 1991;70:121S–129S. [PubMed] [Google Scholar]
- 29.Li H C, Chernoff J, Chen L B, Kirschonbaum A. A phosphotyrosyl-protein phosphatase activity associated with acid phosphatase from human prostate. Eur J Biochem. 1984;138:45–51. doi: 10.1111/j.1432-1033.1984.tb07879.x. [DOI] [PubMed] [Google Scholar]
- 30.Li Y P, Curley G, Lopez M, Chavez M, Glew R, Aragon A, Kumar H, Baca O G. Protein-tyrosine phosphatase activity of Coxiella burnetiithat inhibits human neutrophils. Acta Virol. 1996;40:263–272. [PubMed] [Google Scholar]
- 31.Liles M R, Edelstein P H, Cianciotto N P. The prepilin peptidase is required for protein secretion by and the virulence of the intracellular pathogen Legionella pneumophila. Mol Microbiol. 1999;31:959–970. doi: 10.1046/j.1365-2958.1999.01239.x. [DOI] [PubMed] [Google Scholar]
- 32.Liles M R, Viswanathan V K, Cianciotto N P. Identification and temperature regulation of Legionella pneumophilagenes involved in type IV pilus biogenesis and type II secretion. Infect Immun. 1998;66:1776–1782. doi: 10.1128/iai.66.4.1776-1782.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez A, Ostrovsky P, Nunn D N. Identification of an additional member of the secretin superfamily of proteins in Pseudomonas aeruginosathat is able to function in type II secretion. Mol Microbiol. 1998;28:1235–1246. doi: 10.1046/j.1365-2958.1998.00888.x. [DOI] [PubMed] [Google Scholar]
- 34.Muller H E. Enzymatic profile of Legionella pneumophila. J Clin Microbiol. 1981;13:423–426. doi: 10.1128/jcm.13.3.423-426.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakai K, Kanehisa M. Expert system for predicting protein localization in Gram-negative bacteria. Proteins Struct Funct Genet. 1991;11:95–110. doi: 10.1002/prot.340110203. [DOI] [PubMed] [Google Scholar]
- 36.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavages sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 37.Nolte F S, Hollick G E, Robertson R G. Enzymatic activities of Legionella pneumophila and Legionella-like organisms. J Clin Microbiol. 1982;15:175–177. doi: 10.1128/jcm.15.1.175-177.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nunn D N, Lory S. Product of the Pseudomonas aeruginosa gene pilDis a prepilin leader peptidase. Proc Natl Acad Sci USA. 1991;88:3281–3285. doi: 10.1073/pnas.88.8.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ostanin K, Harms E H, Stevis P E, Kuciel R, Zhou M-M, Etten R L V. Overexpression, site-directed mutagenesis, and mechanism of Escherichia coliacid phosphatase. J Biol Chem. 1992;267:22830–22836. [PubMed] [Google Scholar]
- 40.Pearlman E, Jiwa A H, Engleberg N C, Eisenstein B I. Growth of Legionella pneumophilain a human macrophage-like (U937) cell line. Microb Pathog. 1988;5:87–95. doi: 10.1016/0882-4010(88)90011-3. [DOI] [PubMed] [Google Scholar]
- 41.Pope C D, Dhand L, Cianciotto N P. Random mutagenesis of Legionella pneumophila with mini-Tn10. FEMS Microbiol Lett. 1994;124:107–111. doi: 10.1111/j.1574-6968.1994.tb07269.x. [DOI] [PubMed] [Google Scholar]
- 42.Pope C D, O’Connel W, Cianciotto N P. Legionella pneumophilamutants that are defective for iron acquisition and assimilation and intracellular infection. Infect Immun. 1996;64:629–636. doi: 10.1128/iai.64.2.629-636.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reese M G, Harris N L, Eeckman F H. Large scale sequencing specific neural networks for promoter and splice site recognition. In: Hunter L, Klein T E, editors. Biocomputing: proceedings of the 1996 Pacific symposium. Singapore, Singapore: World Scientific Publishing Co.; 1996. pp. 737–738. [Google Scholar]
- 45.Reilly T J, Baron G S, Nano F E, Kuhlenschmidt M S. Characterization and sequencing of a respiratory burst-inhibiting acid phosphatase from Francisella tularensis. J Biol Chem. 1996;271:10973–10983. doi: 10.1074/jbc.271.18.10973. [DOI] [PubMed] [Google Scholar]
- 46.Remaley A T, Kubus D B, Basford R H, Glew R H, Kaplan S S. Leishmanial phosphatase blocks neutrophil O2production. J Biol Chem. 1984;259:11173–11175. [PubMed] [Google Scholar]
- 47.Rossolini G M, Schippa S, Riccio M L, Berlutti F, Macaskie L E, Thaller M C. Bacterial nonspecific acid phosphohydrolases: physiology, evolution and use as tools in microbial biotechnology. Cell Mol Life Sci. 1998;54:833–850. doi: 10.1007/s000180050212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Russel M. Macromolecular assembly and secretion across the bacterial cell envelope: type II protein secretion systems. J Mol Biol. 1998;279:485–499. doi: 10.1006/jmbi.1998.1791. [DOI] [PubMed] [Google Scholar]
- 49.Russell M A, Darzins A. The pilE gene product of Pseudomonas aeruginosa, required for pilus biogenesis, shares amino acid sequence identity with the N-termini of type 4 prepilin proteins. Mol Microbiol. 1994;13:973–985. doi: 10.1111/j.1365-2958.1994.tb00489.x. [DOI] [PubMed] [Google Scholar]
- 50.Saha A K, Dowling J N, LaMarco K K, Das S, Remaley A T, Olomur N, Pope M T, Glew R H. Properties of an acid phosphatase from Legionella micdadeiwhich blocks superoxide anion production by human neutrophils. Arch Biochem Biophys. 1985;243:150–160. doi: 10.1016/0003-9861(85)90783-0. [DOI] [PubMed] [Google Scholar]
- 51.Saha A K, Dowling J N, Pasculle A W, Glew R H. Legionella micdadeiphosphatase catalyzes the hydrolysis of phosphatidylinositol 4,5-biphosphate in human neutrophils. Arch Biochem Biophys. 1988;265:94–104. doi: 10.1016/0003-9861(88)90375-x. [DOI] [PubMed] [Google Scholar]
- 52.Salvano M A, Domenech C E. Kinetic properties of purified Pseudomonas aeruginosaphosphorylcholine phosphatase indicated that this enzyme may be utilized by the bacteria to colonize in different environments. Curr Microbiol. 1999;39:1–8. doi: 10.1007/pl00006819. [DOI] [PubMed] [Google Scholar]
- 53.Shakarian A M, Ellis S L, Mallinson D J, Olafson R W, Dwyer D M. Two tandemly arrayed genes encode the (histidine) secretory acid phosphatases of Leishmania donovani. Gene. 1997;196:127–137. doi: 10.1016/s0378-1119(97)00218-7. [DOI] [PubMed] [Google Scholar]
- 54.Stankiewicz P J, Gresser M J. Inhibition of phosphatase and sulfatase by transition-state analogues. Biochemistry. 1988;27:206–12. doi: 10.1021/bi00401a031. [DOI] [PubMed] [Google Scholar]
- 55.Stone B J, Abu Kwaik Y. Expression of multiple pili by Legionella pneumophila: identification and characterization of a type IV pilin gene and its role in adherence to mammalian and protozoan cells. Infect Immun. 1998;66:1768–1775. doi: 10.1128/iai.66.4.1768-1775.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Strom M S, Nunn D, Lory S. Multiple roles of the pilus biogenesis protein PilD: involvement of PilD in excretion of enzymes from Pseudomonas aeruginosa. J Bacteriol. 1991;173:1175–1180. doi: 10.1128/jb.173.3.1175-1180.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strom M S, Nunn D N, Lory S. A single bifunctional enzyme, PilD, catalyzes cleavage and N-methylation of proteins belonging to the type IV pilin family. Proc Natl Acad Sci USA. 1993;90:2404–2408. doi: 10.1073/pnas.90.6.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Szeto L, Shuman H A. The Legionella pneumophilamajor secretory protein, a protease, is not required for intracellular growth or cell killing. Infect Immun. 1990;58:2585–2592. doi: 10.1128/iai.58.8.2585-2592.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thorpe T C, Miller D. Extracellular enzymes of Legionella pneumophila. Infect Immun. 1981;33:632–635. doi: 10.1128/iai.33.2.632-635.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Winn W C J. Legionnaires' disease: historical perspective. Clin Microbiol Rev. 1988;1:60–81. doi: 10.1128/cmr.1.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]