Abstract
Chimeric antigen receptor T‐cell (CAR‐T) treatment has revolutionized the landscape of cancer therapy with significant efficacy on hematologic malignancy, especially in relapsed and refractory B cell malignancies. However, unexpected serious toxicities such as cytokine release syndrome (CRS) and immune effector cell‐associated neurotoxicity syndrome (ICANS) still hamper its broad application. Clinical trials using CAR‐T cells targeting specific antigens on tumor cell surface have provided valuable information about the characteristics of ICANS. With unclear mechanism of ICANS after CAR‐T treatment, unremitting efforts have been devoted to further exploration. Clinical findings from patients with ICANS strongly indicated existence of overactivated peripheral immune response followed by endothelial activation‐induced blood–brain barrier (BBB) dysfunction, which triggers subsequent central nervous system (CNS) inflammation and neurotoxicity. Several animal models have been built but failed to fully replicate the whole spectrum of ICANS in human. Hopefully, novel and powerful technologies like single‐cell analysis may help decipher the precise cellular response within CNS from a different perspective when ICANS happens. Moreover, multidisciplinary cooperation among the subjects of immunology, hematology, and neurology will facilitate better understanding about the complex immune interaction between the peripheral, protective barriers, and CNS in ICANS. This review elaborates recent findings about ICANS after CAR‐T treatment from bed to bench, and discusses the potential cellular and molecular mechanisms that may promote effective management in the future.
This article is categorized under:
Cancer > Biomedical Engineering
Immune System Diseases > Molecular and Cellular Physiology
Neurological Diseases > Molecular and Cellular Physiology
Keywords: chimeric antigen receptor T‐cell (CAR‐T), immune effector cell‐associated neurotoxicity syndrome (ICANS), management, mechanism
Concise mechanism of immune effector cell‐associated neurotoxicity syndrome (ICANS) after chimeric antigen receptor T‐cell (CAR‐T) treatment involving peripheral immune overactivation, endothelial activation‐induced blood–brain barrier dysfunction, and central nervous system inflammation.
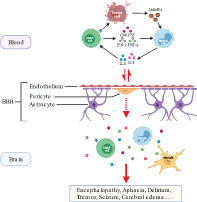
1. INTRODUCTION
Chimeric antigen receptor T‐cell (CAR‐T) treatment as a major success among adoptive cellular immunotherapy has revolutionized the landscape of cancer therapy, especially in hematological malignancies (Ding et al., 2021; Grupp et al., 2013; Locke et al., 2017; Maude et al., 2014; Mohty et al., 2019; Neelapu et al., 2017). By utilizing synthetic antigen recognition receptor implanted into T cells to facilitate targeting specific tumor‐associated antigens (TAAs) on cell surface, such as CD19, CD20, CD22 and BCMA (CD269), CAR‐T cells exert dramatic cytotoxicity on tumor cells independent of major histocompatibility complex‐restricted T‐cell receptor signaling (Ali et al., 2016; Larson & Maus, 2021; Maude et al., 2015; Ying et al., 2019). Among various antigen targets, CD19‐directed CAR‐T cell therapy is most widely used, with paramount efficacy of a nearly 70% overall response rate and a 52% complete response rate from real‐world post‐marketing data (Greenbaum et al., 2021). Despite making exciting progress in maintaining long‐term durable remission, concomitant adverse reactions have been reported as well, raising challenges to safe and broad application of CAR‐T treatment (Larson & Maus, 2021; Lynn et al., 2019; O'Rourke et al., 2017). Among those, cytokine release syndrome (CRS) and immune effector cell‐associated neurotoxicity syndrome (ICANS) are severe and life‐threatening (Miao et al., 2021; Morris et al., 2021; Shao et al., 2021; Zhu et al., 2021). Yet a detailed understanding about the mechanism and management of ICANS after CAR‐T treatment is extremely lacking.
ICANS manifests mainly as toxic encephalopathy with receptive aphasia, dysregulated motor function, and altered consciousness (Herr et al., 2020; Lee et al., 2019). Severe cases presented with seizures, coma, and cerebral edema that caused high mortality (Torre et al., 2018). Peripheral blood and cerebrospinal fluid (CSF) analysis revealed dramatically elevated cytokines with similar patterns in patients with ICANS (Hunter & Jacobson, 2019; Taraseviciute et al., 2018). Electroencephalogram (EEG) monitoring and imaging examination indicated extensive neurologic abnormality along with signs of interstitial or cytotoxic edema (Danish & Santomasso, 2021; Gust et al., 2021). FDG PET‐CT has a diagnostic value for predicting neurotoxicity after CAR‐T treatment (Wang et al., 2019). Postmortem pathologic biopsy also discovered perivascular lymphocyte and monocyte exudation with microhemorrhage (Gust et al., 2017; Hunter & Jacobson, 2019). Existing clinical findings and animal models all suggested peripheral immune overactivation and dysfunctional blood–brain barrier (BBB) with increased permeability in the development of ICANS (Giavridis et al., 2018; Larson & Maus, 2021; Pennell et al., 2018; Staedtke et al., 2018; Taraseviciute et al., 2018). However, the cellular and molecular mechanisms still remain vague due to lack of available experimental models and analytic methods to decipher complex interactions in both the thperipheral blood and the central nervous system (CNS).
Building on continuously updated discoveries, we focus on detailed elaboration of potential mechanisms of ICANS after CAR‐T treatment particularly in hematologic malignancies. Furthermore, we summarize novel management strategies to improve the safety without impairing efficacy according to related mechanisms.
2. CLINICAL CHARACTERISTICS OF ICANS
2.1. Incidence and risk factors
The reported incidence of ICANS varied, ranging from 15% to 70% (Table 1). Various factors including patient characteristics and specific features of CAR products could account for the relative high incidence (Gust et al., 2017). Current research illuminated that younger age, higher tumor burden, preexisting neurological symptoms, and factors related to CAR‐T cell therapy, such as preadministrated lymphodepletion agents, severe CRS, and high CSF protein level after treatment, were all risk factors of ICANS (Hunter & Jacobson, 2019). Particularly, the incidences of ICANS in pediatric and young adult B‐ALL range from 28.6% to 49%, which seems to be higher than that in grown adults (DiNofia & Maude, 2019; Gardner et al., 2017; Gofshteyn et al., 2018). Studies also found evidence of compromise of the neurovascular unit (NVU) and astrocyte injury in pediatrics (Gust et al., 2019). Indeed, in rats with the age comparable to human, astrocyte gradually becomes mature after birth, which is slower than other components like endothelial cell (EC) and pericyte, reflecting more susceptibility and less regulatory capacity of BBB in younger individuals (Bors et al., 2018; Delaney & Campbell, 2017). On the other hand, CAR structure like hinge, costimulatory domains, and transmembrane regions might also contribute (Brudno et al., 2020). Higher rates of ICANS after receiving CAR‐T cell infusion were observed in hematological malignancies, compared to that almost none was reported in solid tumor (Ahmed et al., 2015; Brown et al., 2016; Johnson et al., 2015; Kershaw et al., 2006). The recent clinical data of ICANS during CAR‐T cell therapy in hematological malignancies are listed below (Table 1).
TABLE 1.
Incidences of cytokine release syndrome (CRS) and immune effector cell‐associated neurotoxicity syndrome (ICANS) of chimeric antigen receptor T‐cell (CAR‐T) cell therapy overviewing 5 years' researches
| Trial registration | Disease | Target | CRS% | sCRS% | ICANS% | sICANS% | Time | Journal | |
|---|---|---|---|---|---|---|---|---|---|
| Cilta‐cel | NCT03548207 | MM | BCMA | 95 | 4 | 21 | 9 | 2021 | The Lancet |
| Relma‐cel | NCT04089215 | LBCL | CD19 | 47.50 | 5.10 | 20.30 | 5.10 | 2021 | Cancer Medicine |
| 4SCAR19 | ChiCTR‐OOC‐16007779 | NHL | CD19 | 14 | 0 | 4.76 | 4.76 | 2020 | Frontiers in Immunology |
| UCART19 | NCT02808442 and NCT02746952 | B‐ALL | CD19 | 91 | 14 | 38 | 0 | 2020 | The Lancet |
| LV20.19 | NCT03019055 | B cell malignancy | CD19/CD20 | 64 | 5 | 32 | 14 | 2020 | Nature Medicine |
| NCT04689659 | T‐ALL | CD7 | 90 | 10 | 15 | 0 | 2021 | Journal of Clinical Oncology | |
| HuCART19 | NCT02374333 | B‐ALL | CD19 | 84 | 6.80 | 39 | 4 | 2021 | Journal of Clinical Oncology |
| KTE‐X19 | NCT02614066 | B‐ALL | CD19 | 89 | 24 | 33 | 25 | 2021 | The Lancet |
| NCT01593696 | B‐ALL | CD19 | 100 | 30 | 20 | 8 | 2021 | Journal of Clinical Oncology | |
| Ide‐cel | NCT03361748 | MM | BCMA | 84 | 5 | 18 | 3 | 2021 | The New England Journal of Medicine |
| Liso‐cel | NCT02631044 | LBCL | CD19 | 42 | 2 | 30 | 10 | 2020 | The Lancet |
| Axi‐cel | non‐trial | B‐NHL | CD19 | 93 | 16 | 70 | 35 | 2020 | Journal of Clinical Oncology |
| Axi‐cel | NCT03153462 | LBCL | CD19 | 91.20 | 7 | 68.70 | 31 | 2020 | Journal of Clinical Oncology |
| NCT01747486 | CLL | CD19 | 63 | 24 | 40.63 | 9.38 | 2020 | Journal of Clinical Oncology | |
| NCT0231561 | B‐ALL | CD22 | 86.20 | 20.30 | 32.80 | 1.70 | 2020 | Journal of Clinical Oncology | |
| KTE‐X19 | NCT02601313 | MCL | CD19 | 91 | 15 | 63 | 31 | 2020 | The New England Journal of Medicine |
| Tisagenlecleucel | B‐ALL | CD19 | 94 | 18 | 40 | 6 | 2020 | Journal of Clinical Oncology | |
| Bb2121 | NCT02658929 | MM | BCMA | 76 | 6 | 42 | 3 | 2019 | The New England Journal of Medicine |
| Axi‐cel | NCT02348216 | LBCL | CD19 | 91.74 | 11 | 66.01 | 32 | 2019 | The Lancet Oncology |
| Tisagenlecleucel | NCT02445248 | DLBCL | CD19 | 58 | 22 | 21 | 12 | 2019 | The New England Journal of Medicine |
| NCT01044069 | B‐ALL | CD19 | 85 | 26 | 44 | 42 | 2018 | The New England Journal of Medicine | |
| Axi‐cel | NCT02435849 | B‐ALL | CD19 | 77 | 47 | 40 | 13 | 2018 | The New England Journal of Medicine |
| Axi‐cel | NCT02348216 | LBCL | CD19 | 93 | 13 | 64 | 28 | 2017 | The New England Journal of Medicine |
| CTL019 | NCT02030834 | B cell lymphoma | CD19 | 57 | 18 | 39 | 11 | 2017 | The New England Journal of Medicine |
| NCT01865617 | CLL | CD19 | 83 | 8 | 33 | 25 | 2017 | Journal of Clinical Oncology |
2.2. Clinical manifestation
The symptoms of ICANS resembled that of toxic encephalopathy, as receptive aphasia, dysregulation of fine motor function, and reduced level of attention (Herr et al., 2020; Lee et al., 2019). In mild cases, symptoms started early and patients tolerated well. While in severe cases, elevated intracranial pressure (ICP), seizures, and cerebral edema occurred with high mortality (Greenbaum et al., 2021; Rice et al., 2019). Though in general, ICANS is self‐limited and reversible, very few patients still suffer from long‐term sequelae (Maillet et al., 2021; Pensato et al., 2021). ICANS presents in a biphasic pattern. In one phase, ICANS concurrently happens with CRS (Brudno & Kochenderfer, 2019; Neelapu et al., 2018). In the other, ICANS presents after CRS remission but with high severity and long persistence. Extremely few cases with seizure or confusion took place 3–4 weeks after CAR‐T cells infusion as “delayed neurotoxicity” (Danish & Santomasso, 2021). With rapid onset and gradual remission, ICANS usually happens at 4–6 days after CAR‐T cell infusion, peaks at 7–9 days, and lasts for 5–13 days (Brown et al., 2021; Schuster et al., 2017).
2.3. Clinical examination
Laboratory examination, imaging, and EEG analysis are necessary for diagnosis. The CAR‐T cell kinetics and serum cytokines were connected with ICANS in a sequential time‐dependent manner (Morris et al., 2021), which also showed tightly correlation with CRS (Gust et al., 2020; Hong et al., 2021). In consideration of CNS symptoms as the main character of ICANS, the CSF analysis is thought to be a better diagnostic tool. The presence of CNS inflammation was indicated by cerebral infiltration CAR‐T cell and myeloid cell with elevated CSF level of interleukin (IL)‐1, IL‐6, IL‐10, Interferon‐γ (IFN‐γ), tumor necrosis factor‐α (TNF‐α), CCL2, CXCL10, and granzyme B (Brown et al., 2021; Hu et al., 2016; Miao et al., 2021). In addition, both GFAP (marker of astrocyte injury) and S100b (marker of astrocyte activation) significantly increased in the CSF compared to patients without ICANS, which strongly suggested local inflammatory response in the CNS (Gust et al., 2019). Furthermore, endothelium‐related factors showed a correlation with ICANS. For instance, decreased level of angiopoietin 1 (Ang1), increased level of Ang2, vWF, and CXCL8, were presented as unique markers in patients with severe ICANS (Mackall & Miklos, 2017; Miao et al., 2021).
Imaging results often exhibit no abnormal signs for patients with mild ICANS (Strati et al., 2020). Nevertheless, interstitial or cytotoxic edema with a particular susceptibility for certain brain regions like bilateral thalami, supratentorial white matter, and brainstem were often seen in severe cases (Gust et al., 2020; Rubin et al., 2019). Serving as a real‐time measure for brain function, EEG could help detect brain dysfunction especially for delirium and seizure, and provide evidence for diagnostic criteria and evaluation of therapeutic response. A study indicated that EEG background abnormalities were detected in all patients with ICANS, of which 8/19 developed clinical seizures (Gust et al., 2017; Santomasso et al., 2018). Additionally, abnormal EEG patterns graded by Synek scale were positively correlated with ICANS severity (Maziarz et al., 2020; Riegler et al., 2019).
2.4. Clinical diagnosis and grading
Early published grading systems for assessing encephalopathy after CAR‐T cell therapy include Common Terminology Criteria for Adverse Events (CTCAE), CAR‐T cell‐therapy‐associated TOXicity (CARTOX), and immune effector cell‐associated encephalopathy (ICE) score (Neelapu et al., 2018; Schmidts et al., 2021). In 2018, consensus was reached by the American Society for Blood and Marrow Transplantation (ASBMT), elucidating that neurotoxicity caused by CAR‐T cell therapy was re‐defined as ICANS and re‐graded into five levels mainly based on clinical symptoms (Lee et al., 2019).
3. POTENTIAL MECHANISMS OF ICANS
So far, diverse theories have been proposed to decipher the mechanisms of ICANS, but the precise pathophysiology still lacks an integral interpretation. Currently, CRS is considered as a crucial “initiating event” or cofactor for subsequent ICANS, with numerous overlapping aspects including timescale, cytokine profile, and immunocyte involvement (Miao et al., 2021; Morris et al., 2021; Teachey et al., 2016). Massive pro‐inflammatory cytokines are released from both activated CAR‐T cells and other host immune cells after engagement with tumor cells (Norelli et al., 2018), which induce endothelial activation with increased permeability and BBB dysfunction (Gust et al., 2017). Further infiltration of cytokines and immunocytes into the CNS triggers noninfectious neuroinflammation and corresponding symptoms (Galic et al., 2012; Turtle et al., 2016; Wendeln et al., 2018). The schematic illustration of ICANS is presented below (Figure 1).
FIGURE 1.
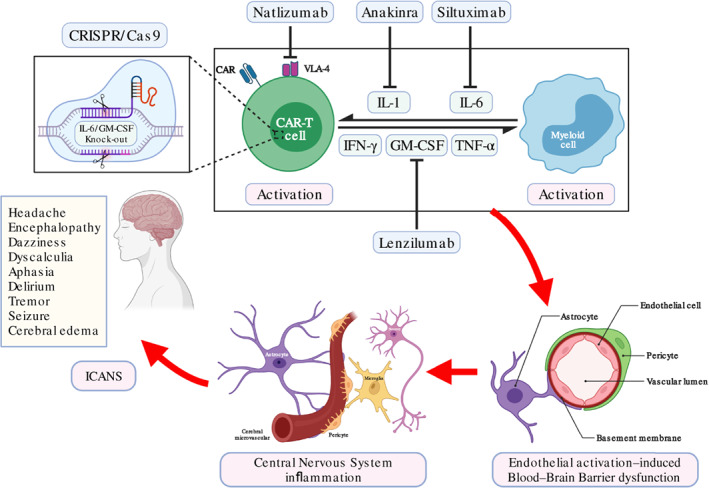
Schematic illustration of immune effector cell‐associated neurotoxicity syndrome (ICANS) pathogenesis after chimeric antigen receptor T‐cell (CAR‐T) treatment and potentially relevant management. After infusion of CAR‐T cell product into patients, CAR‐T cell is activated by engaging with tumor cell (not shown) and induces host myeloid cell activation. The interaction between CAR‐T cell and myeloid cell creates excessive circulating cytokines that contribute to endothelial activation‐induced blood–brain barrier (BBB) dysfunction. Increased permeability of BBB facilitates infiltration of peripheral immunocytes and cytokines into the central nervous system (CNS), which induces further CNS inflammation and abnormal neuronal function, together presenting typical symptoms of ICANS. Potentially relevant managements include key cytokines targeting, adhesion molecules blocking, and CAR‐T cell modulation to reduce immune effector cell‐associated neurotoxicity syndrome (ICANS) severity or prevent its occurrence.
3.1. CAR‐T cell‐induced peripheral immune overactivation
Significantly elevated circulating cytokines including IL‐1, IL‐6, IFN‐γ, TNF‐α, and GM‐CSF have been observed in patients with ICANS (Gust et al., 2020), which indicated overactivated peripheral immune system. Due to prior lymphodepletion, those excessive pro‐inflammatory mediators may lead to great damage on blood vessel and other normal organs in absence of normal immunosuppressive compartments (Kang & Kishimoto, 2021).
3.1.1. CAR‐T cell‐induced monocyte/macrophage activation
With significant CAR‐T cell proliferation in patients responsive to the therapy (Deng et al., 2020; Maude et al., 2014), CAR‐T cell itself is considered as a causative factor associated with occurrence and progression of ICANS. Clinical observations have demonstrated higher tumor burden, prior lymphodepletion, larger CAR‐T cell infusion doses, and faster CAR‐T cell expansion in vivo to be related to the onset and severity of ICANS (Brown et al., 2021; Gust et al., 2017; Holtzman et al., 2021; Karschnia et al., 2019). Activated CAR‐T cells release IFN‐γ, TNF‐α, and granulocyte‐macrophage colony stimulating factor (GM‐CSF) (Spear et al., 2012). Those factors not only exert cytotoxicity on tumor cells directly, but also activate monocyte/macrophage in a manner of cascade amplification.
Monocyte and macrophage are important innate immune cells in defending foreign pathogens and removing aging cells to maintain homeostasis (Mosser & Edwards, 2008). “Classically activated macrophages” respond to elevated CAR‐T cell‐derived cytokines including IFN‐γ, TNF‐α, and GM‐CSF (Norelli et al., 2018; Patel et al., 2017). Macrophages could also be activated by damage associated molecular patterns (DAMPs) released from lysed tumor cells, such as ATP, HMGB1, and histone H3, through binding of Toll‐like receptor (TLR) (Harada‐Shirado et al., 2020; Mosser & Edwards, 2008). In response to above mediators, activated macrophages enhance functional capacity and secrete IL‐1, IL‐6, and TNF‐α that act as both products and effectors throughout the whole immune activity (Hao et al., 2020).
3.1.2. Peripheral cytokines
Activated CAR‐T cell and macrophage secrete large amounts of cytokines into peripheral blood. IFN‐γ is a broad‐spectrum effector that exerts fundamental anti‐tumor and pro‐inflammatory functions by mediating activation of macrophage (Elinav et al., 2013). TNF‐α, a key mediator and regulator of immune activity (Bradley, 2008; Teachey et al., 2016), exerts different signal transduction pathways depending on expression patterns of TNF receptors on effector cells (Hopkins, 2013). In specific, TNF‐α activates monocyte/macrophage to promote proliferation, migration, and cytokine production depending on MAPK/NF‐κB pathway (Bradley, 2008). GM‐CSF predominantly promotes macrophage activation by enhancing its responsiveness to CSF‐1, a regulator of myeloid progenitor differentiation that enhances macrophage migration, proliferation, function, and survival (Becher et al., 2016; Hao et al., 2020; Xue et al., 2017).
IL‐1, known as human leukocytic pyrogen, is released from activated innate immune cells through ligand stimulation, and further promotes monocyte activation, neutrophil infiltration, and macrophage recruitment (Gottschlich et al., 2021). Emerging evidences also suggest that IL‐6 plays a central role in promoting cellular functions between CAR‐T cell and macrophage (Chen et al., 2020; Jiang et al., 2021; Norelli et al., 2018; Tanaka et al., 2016). IL‐6 is a multifunctional factor that regulates immune response, inflammation, and hematopoiesis (Hunter & Jones, 2015). IL‐6 initiates downstream signaling in effector cells in two alternative patterns, the cis‐activating pathway and the trans‐activating pathway (Rose‐John et al., 2006). In cis‐pathway, IL‐6 binds to membrane‐bound IL‐6 receptor (mIL‐6R), transduces signaling by dimerization of the membrane protein gp130, and activates JAK1/JAK2‐STAT3 and MAPK pathways (Zegeye et al., 2018). In trans‐pathway, soluble IL‐6 receptor (sIL‐6R) is produced from mIL‐6R by catalyzation of cellular protease, and then binds IL‐6. The IL‐6/sIL‐6R complex further interacts with gp130 on target cell surface and induces similar signalings. To determine the origin, researchers used T cells derived from HPSC‐humanized SGM3 mice to generate CAR‐T cell‐mediated CRS and ICANS mouse model (Norelli et al., 2018). They found that IL‐1 and IL‐6 primarily originated from monocytes by single‐cell RNA‐sequencing (scRNA‐seq) analysis.
Following CAR‐T cell infusion, IL‐1 elevation precedes IL‐6 up to 24 h in both the serum and CSF from patients with CRS and/or ICANS, indicating that IL‐1 might serve as a potential initiative factor (Gottschlich et al., 2021; Norelli et al., 2018). Intravenous application of anakinra, an IL‐1 blockade monoclonal antibody that can cross BBB, significantly relieved symptoms of CRS and ICANS at the same time (Giavridis et al., 2018). In addition, multifocal brain meningeal thickening accompanied by macrophage infiltration in lethal neurotoxicity could be effectively prevented by anakinra but not the IL‐6 blocker tocilizumab. However, the exact relationship between CAR‐T cell and IL‐6 is still controversial. Some indicated that IL‐6 from monocyte lineage had no effect on CAR‐T cell function in vitro (Singh et al., 2017), while others confirmed IL‐6 overexpression in CAR‐T cell could enhance expansion and antitumor activity via trans‐signaling pathway in xenograft models (Jiang et al., 2021). Another group discovered that IL‐6 knock‐down in CAR‐T cell significantly reduced IL‐6 secretion in monocytes, while maintained comparable antitumor efficacy (L. Kang et al., 2020). Further investigations should be conducted to determine the effective roles and exact source of IL‐6 in ICANS.
In ZUMA‐1 clinic trial, GM‐CSF along with IL‐2 and ferritin were proposed as biomarkers for CAR‐T cell‐mediated neurotoxicity by correlative analysis of pro‐inflammatory mediators (Locke et al., 2017), indicating GM‐CSF as a valuable therapeutic target. As for TNF‐α, although diverse TNF‐blocking agents showed great efficacy in inflammatory diseases, such as rheumatoid arthritis, psoriasis, and Crohn's disease (Adegbola et al., 2018; Bek et al., 2017; Tokuyama & Mabuchi, 2020), application of such strategies in ICANS have not been conducted yet due to concerns about impairing CAR‐T cell function.
3.1.3. Adrenal system activation
Elevated level of catecholamines were detected in patients with ICANS (Staedtke et al., 2018). Targeting adrenal signaling loop might attenuate ICANS toxicity. Catecholamines is a major group of effectors in adrenal system, including norepinephrine (NE), epinephrine (E), and dopamine (DA), with overall effect on immune activities among CAR‐T cells and other host immunocytes (Riddell, 2018). When challenged by stimulus, macrophages and neutrophils in host body produce catecholamines in a self‐amplifying loop, along with elevated levels of α1/α2‐adrenergic receptor on T cells, leading to immune dysregulation and injury (Bao et al., 2007; Bergquist et al., 1994). However, how immunocyte activation drives catecholamines production and how catecholamines boost cytokine production are currently unknown. The metyrosine is a tyrosine hydroxylase inhibitor that specifically inhibits catecholamines production (Staedtke et al., 2018). The atrial natriuretic peptide (ANP), a regulator of water–electrocyte balance, also possesses anti‐inflammatory function by inhibiting cytokine release. As application of ANP and metyrosine in patients with CRS significantly reduced cytokine secretion and improved survival, future exploration about the interactions among adrenal system, CAR‐T cell, and other components in host immune system will facilitate our understanding about catecholamines and improve ICANS management.
In conclusion, activated CAR‐T cell interacting with host monocyte/macrophage contributes to the peripheral immune overactivation. Adrenal system and tumor lysates, such as catecholamines and DAMPs, respectively, also exacerbate toxicity by cascade amplification (Figure 2).
FIGURE 2.
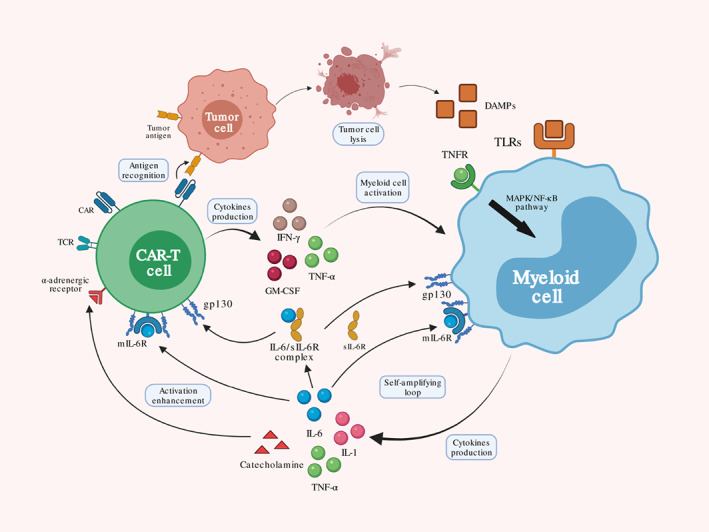
Peripheral immune overactivation. After tumor antigen recognition by CAR, activated chimeric antigen receptor T‐cell (CAR‐T) cell releases several cytokines including IFN‐γ, TNF‐α, and GM‐CSF. These cytokines further activate myeloid cell, such as monocyte and macrophage, and produce additional IL‐1, IL‐6, and TNF‐α. Damage associated molecular patterns (DAMPs) from tumor cell lysis may also contribute to myeloid cell activation through TCR signaling. Excessive IL‐6 directly binds to mIL‐6 or forms a complex with sIL‐6 binding to gp130 on CAR‐T cell and myeloid cell, establishing a self‐amplifying loop. Catecholamines from activated myeloid cell promote CAR‐T cell function though α‐adrenergic receptor that wield overall enhancement to immune effector cell‐associated neurotoxicity syndrome (ICANS).
3.2. Endothelial activation‐induced BBB dysfunction
Excessive circulating cytokines and inflammatory mediators establish positive feedbacks through interactions with ECs, and further induce BBB dysfunction (Jiang et al., 2021; Norelli et al., 2018).
3.2.1. EC function and homeostasis
BBB function largely depends on the functional integrity of EC. EC lines the inner layer of blood vessel as a permeable barrier, and supports vascular integrity together with smooth muscle cell and pericyte in outer layer to regulate substances exchange (Sturtzel, 2017). EC maintains immune system homeostasis but also mediates pathological change when under uncontrolled stimulation. Particularly, EC regulates cytokine secretion, immunocytes migration, and other inflammatory mediators during immune responses.
Sophisticated pathways regulate endothelial function and permeability through specific ligand‐receptor interaction, of which vascular endothelial growth factor (VEGF)/VEGF receptor axis and Ang/Tie axis contribute profoundly (Apte et al., 2019; Leligdowicz et al., 2018) (Figure 3). VEGF regulates vasculature formation and function, while the balance between Ang1 and Ang2 is crucial to the vascular homeostasis.
FIGURE 3.
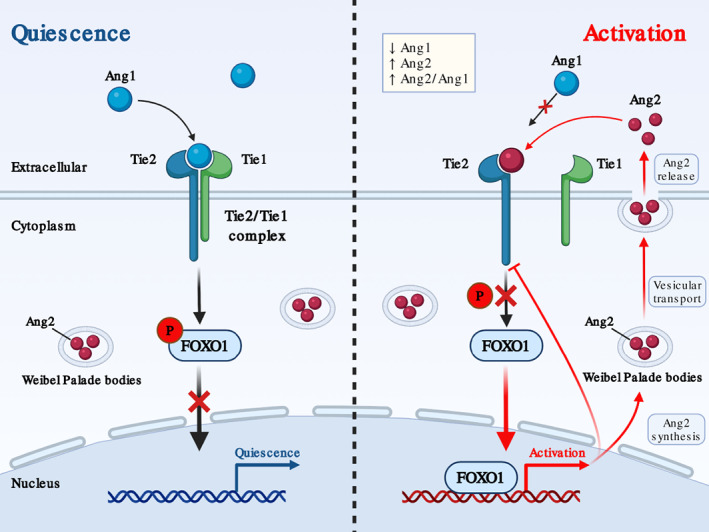
Endothelial homeostasis regulated by Ang/Tie axis. Under physiological status, Ang1 dominates and binds to Tie2 receptor on endothelial cell (EC) surface with Tie1 to form a stable complex. Ang1/Tie2 downstream signaling pathways phosphorylate FOXO1 to inhibit its nuclear translocation and maintain endothelium quiescence. After endothelial activation, Ang2 stored at Weibel–Palade bodies in EC is released into extracellular space and competitively binds to Tie2 with antagonism effect. FOXO1 nuclear translocation is therefore restored to promote endothelial activation by gene transcriptional regulation.
Ang1 predominates in vascular quiescence by forming a Tie1/Tie2 complex in a β1‐integrin‐dependent manner (Brindle et al., 2006). The Tie1/Tie2 heterodimer contains high affinity to fibronectin and collagen, which anchor EC to the extracellular matrix. Tie2 activation also induces downstream signaling pathways to prevent phosphorylate Forkhead box protein O1 (FOXO1) translocating into the nucleus (Daly et al., 2004). Upon inflammatory stimulation, Ang2, which is originally synthesized and stored within the Weibel–Palade bodies (WPB) in EC, is significantly released in an autocrine regulatory pattern (Fiedler et al., 2004). Large amount of Ang2 competes with Ang1 binding to Tie2 receptor, and exerts antagonistic effect on Tie2 signaling (Mandriota & Pepper, 1998). Interestingly, reduced Tie2 expression in EC under inflammation is mediated by NF‐κB‐dependent transcriptional modulation and proteolytic cleavage‐induced post‐translational regulation (Kurniati et al., 2013). Inactivation of Tie2 signaling restores FOXO1 activity, promotes subsequent nuclear translocation, and drives Ang2 expression in a positive feedback loop (Ghosh et al., 2012).
Clinical observations have discovered that low serum level of Ang1, high serum concentration of Ang2, high ratio of Ang2 to Ang1 along with significantly elevated levels of cytokines in both serum and CSF were all associated with severe CRS and ICANS (Gust et al., 2017). From another perspective, Ang1 overexpression in mouse model showed preserved endothelial nitric oxide synthase (eNOS), decreased nitric oxide synthesis, reduced microvascular permeability, and decreased leukocyte infiltration into interstitial spaces (Witzenbichler et al., 2005).
3.2.2. Cytokine‐mediated endothelial overactivation and injury
Elevated peripheral cytokines significantly alter the endothelium homeostasis in ICANS. IL‐6 and GM‐CSF are both considered as biomarkers in CRS and ICANS after CAR‐T treatment. TNF‐α, IL‐6, and other pro‐inflammatory mediators contribute to endothelial activation and increased permeability (Blecharz‐Lang et al., 2018; Gust et al., 2017; Kang & Kishimoto, 2021), participating in cerebrovascular and neurotoxic disease like cerebral ischemia, hemorrhage, and edema (S. Kang et al., 2020; Marcos‐Ramiro et al., 2014; Figure 4).
FIGURE 4.
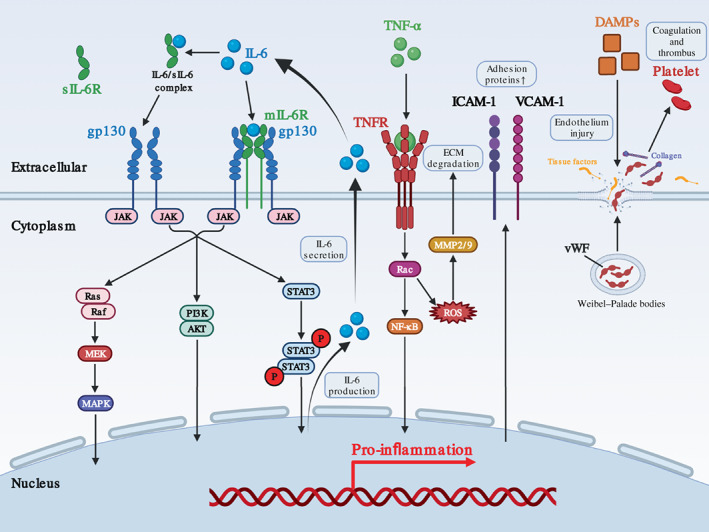
Molecular mechanism of endothelial cell dysregulation during immune effector cell‐associated neurotoxicity syndrome (ICANS) development. During ICANS after chimeric antigen receptor T‐cell (CAR‐T) treatment, excessive circulating IL‐6 along with IL‐6/sIL‐6 complex has pro‐inflammation effect on EC through Ras/MEK/MAPK pathway, PI3K/AKT pathway, and JAK/STAT3 pathway to produce more IL‐6 that establish positive feedback. TNF‐α binds to TNFR and transduces signaling through NF‐κB pathway to promote inflammation in EC. In addition, TNF increases ROS accumulation to upregulate MMP2/9‐mediated ECM degradation that contributes to endothelium disintegrity. Damage associated molecular patterns (DAMPs) may also lead to endothelium injury with exposure of tissue factor and collagen, and induce vWF release from Weibel–Palade bodies into extracellular space, which promote coagulation and thrombus.
TNF‐α influences endothelium homeostasis in a dose‐dependent manner by stimulating matrix metalloproteinase (MMP) production in ECs (Hosomi et al., 2005), which degrades extracellular matrix (Rosenberg & Navratil, 1997). Although low level of TNF‐α is seemed to be neuroprotective in certain conditions, excessive TNF‐α induces MMP‐2 and MMP‐9 production from ECs, which disrupt normal cell‐matrix adhesion and induce cell swelling and death, compromising microvascular integrity and permeability (Hosomi et al., 2005). TNF‐α also promotes leukocyte adhesion, diapedesis, and vascular leak through additional TFNR2‐mediated PI3K/Akt pathway (Bradley, 2008). TNF‐α neutralization by monoclonal antibody significantly reduced cerebral edema, supporting its essential participation.
Serum IL‐6 maintains at a very low level under physiological conditions but increases rapidly upon inflammation (Hunter & Jones, 2015). Evidences have shown that EC additionally secretes cascade‐amplified IL‐6 in response to existing soluble IL‐6 (Dalal et al., 2020; Garbuzova‐Davis et al., 2018; Kang & Kishimoto, 2021; Marin et al., 2001). IL‐6R and trans‐signaling pathway were both induced in peripheral and cerebral EC under inflammation (Kang & Kishimoto, 2021; Nishimoto et al., 2008). Endogenous inhibition of IL‐6 or GM‐CSF in CAR‐T cell reduced serum IL‐6 level and restored endothelium permeability via downregulating interactions with myeloid cells (Sachdeva et al., 2019; Sterner et al., 2019). Further investigation is encouraged to explore their contribution to cerebral EC injury and dysfunction.
3.2.3. Thrombotic microangiopathy
Elevated serum level of vWF and D‐dimer, and decreased fibrinogen and thrombocyte were accompanied by cerebral multifocal hemorrhage in patients with CRS and/or ICANS, indicating an involvement of thrombotic microangiopathy that compromised BBB function (Gust et al., 2017; Hunter & Jacobson, 2019; Schuster et al., 2017). Tumor lysates like HMGB1, histone H3, and ATP continuously activate and injure EC, exposing TF and collagen fibers that trigger extrinsic and intrinsic coagulation pathways, which threaten endothelium integrity and induce thrombotic microangiopathy (Miao et al., 2021; Figure 4). vWF, a marker of endothelium injury, is originally stored within WPB in ECs and released upon stimulation, participating in coagulation process by connecting platelet to collagen (Schwameis et al., 2015). The unbalance of these factors increases the risk of disseminated intravascular coagulation (Gust et al., 2017; Schuster et al., 2017).
3.2.4. BBB dysfunction
Post mortem examination discovered local inflammation with cellulosic exudation and even microhemorrhage around cerebral capillaries, indicating a participation of BBB dysfunction in ICANS (Hunter & Jacobson, 2019). BBB is a selective border formed by EC, pericyte, and astrocyte endfoot process that regulates immunocyte trafficking, transports necessary materials, and prevents entrance of noxious substances to protect the brain (Banks, 2015; Munji et al., 2019; Profaci et al., 2020). Pericyte predominantly regulates BBB permeability by stabilizing EC via direct contact or paracrine signaling (Birbrair, 2018). Due to endothelium dysfunction, exposed pericyte produces IL‐6 and VEGF in response to excessive IFN‐γ (Dalal et al., 2020; Siegler & Kenderian, 2020), which exacerbate BBB disruption by disarraying tight junctions (TJs) between brain microvascular ECs (Capaldo & Nusrat, 2009). Fibrous glia is a specific type of astrocyte that surrounds cerebral microvascular wall and regulates fluid exchange with endfoot process (Abbott et al., 2006). Astrocyte swelling and clasmatodendrosis restrains the ability of fluid control, leading to further BBB dysfunction and cerebral edema (Hulse et al., 2001; Rama Rao et al., 2010). Immunocyte migration and infiltration into the CNS is commonly observed in the CNS inflammation due to compromised BBB permeability. T cell trafficking through vessel is mediated by interaction of surface adhesion molecules between T cell and EC (Redondo‐Munoz et al., 2019). Under inflammation, expressions of VCAM‐1 and ICAM‐1 are elevated in cerebral EC to facilitate leukocyte infiltration (Savarin et al., 2015). Enrichment of peripheral immunocytes and their effector cytokines increase the risk of cerebral thrombosis, and intensify local immune response in the CNS by activating neuroglial cells.
In conclusion, cerebral EC overactivation and injury due to excessive pro‐inflammatory cytokines and mediators ultimately lead to increased permeability of cerebral capillary and BBB dysfunction, which facilitate the CNS infiltration of peripheral cytokines and immunocytes (Figure 5).
FIGURE 5.
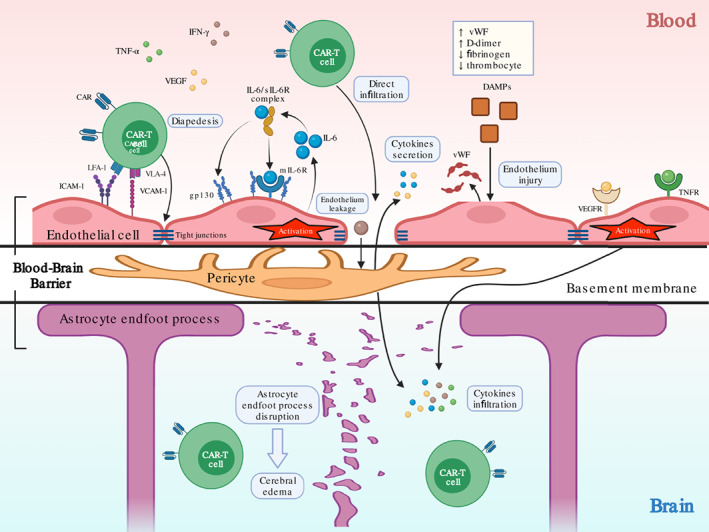
Endothelial activation‐induced blood–brain barrier dysfunction. Excessive circulating cytokines induces endothelial activation through receptors on cell surface including mIL‐6R, gp130, TNFR, and vascular endothelial growth factor receptor (VEGFR). Activated endothelial cell (EC) produces additional IL‐6 that forms positive feedback. Endothelial activation causes leakage with open tight junctions, which exposes pericyte at basement membrane to cytokines such as IFN‐γ to produces more IL‐6 and VEGF that exacerbate this process. EC may also be injured by damage associated molecular patterns (DAMPs) from tumor cell lysis. Endothelial leakage facilitates infiltration of peripheral chimeric antigen receptor T‐cell (CAR‐T) cell and cytokines into the central nervous system (CNS), and causes astrocyte endfoot process disruption with decreased fluid control and induces further cerebral edema.
3.3. Central nervous system inflammation
Normal CNS function relies on the BBB integrity to be free from peripheral stimulus. Endothelial activation‐induced BBB disruption with increased permeability initiates subsequent CNS noninfectious inflammation in ICANS after CAR‐T treatment. CNS infiltration of immunocytes with functional plasticity along with excessive cytokines may convert local immune landscape and induce subsequent astrocyte injury, microglia activation, and neuron dysfunction with corresponding symptoms.
3.3.1. CNS infiltration
CAR‐T cells and CD14+ myeloid cells were detected in CSF from patients with ICANS (Locke et al., 2017; Siegler & Kenderian, 2020). Infiltrative CAR‐T cells contained Th1, Th17, and Treg subsets (Norelli et al., 2018). scRNA‐seq discovered diverse features in infiltrative CAR‐T cells, including TCR, cytokine receptor, immune activation, trafficking, and differentiation (Li et al., 2021; Sterner et al., 2019; Xue et al., 2017). Interestingly, an unusual subgroup with monocyte‐like transcriptional characters correlated with severe ICANS (Martin‐Antonio, 2021). Substantial white matter infiltration and expansion of macrophages in perivascular space were found in brain tissues from lethal cases (Danish & Santomasso, 2021; Torre et al., 2018). A rhesus macaque model using autologous CD20‐specific CAR‐T cell demonstrated comparable results (Taraseviciute et al., 2018). Pathologic biopsy discovered CAR‐T cell, host T cell, and monocyte/macrophage in the brain parenchyma, with multifocal perivascular infiltration in the basal ganglia and parietal lobe. Upregulated VCAM‐1 and ICAM‐1 were found on the cerebral endothelium, together with significantly increased VLA‐4 on CAR‐T cell, indicating a potential adhesion‐mediated CAR‐T cell migration into the CNS. CSF cytokine analysis showed elevation of IFN‐γ, TNF‐α, GM‐CSF, IL‐1, IL‐6, and IL‐10, similarly in serum (Hunter & Jacobson, 2019; Taraseviciute et al., 2018). GM‐CSF neutralization reduced CNS infiltration of CAR‐T cells and myeloid cells in xenograft mouse models (Sterner et al., 2019).
3.3.2. Astrocyte activation, injury, and dysfunction
GFAP and S100b, markers of astrocyte activation and injury, increased along with the elevated cytokines in the CSF (Brown et al., 2021; Hunter & Jacobson, 2019). MRI analysis presented bilateral thalami swelling showing an interstitial or vasogenic edema (Gust et al., 2019, 2020; Santomasso et al., 2018). Post mortem examination found perivascular astrocyte clasmatodendrosis that indicated cytoplasmic swelling and vacuolation with extensive astrocyte loss (Torre et al., 2018). In severe cases, astrocyte clasmatodendrosis was presented throughout the cortex and white matter.
Astrocyte shares unique structural and functional properties in regulating substances exchange with endfoot process, and participates in neuron excitation by producing excitatory neurotransmitter glutamate (Hosomi et al., 2005). Astrocyte activation and injury present in inflammatory or degenerative neurological diseases, including multiple sclerosis (MS), Alzheimer disease (AD), and Parkinson's disease (PD) (Wolf et al., 2017). IL‐6 and GM‐CSF secreted by activated astrocytes contribute to disease progression in MS and PD (Sofroniew, 2020). Under physiological state, low level of IL‐6 promotes differentiation of oligodendrocytes to facilitate myelin repair, and maintains neuronal survival and regeneration. BBB dysfunction causes IL‐6 penetration into CNS from both peripheral blood and EC secretion. GM‐CSF is predominantly secreted by activated astrocytes (Linnerbauer et al., 2020). GM‐CSF receptors are generally expressed on brain macrophages, astrocytes, and microglia, suggesting broad downstream effects upon astrocyte activation. Intracranial ejection of IFN‐γ induced direct toxicity on astrocytes and exacerbated disease progression with CNS inflammation and immunocyte infiltration in a stage‐specific manner (Ottum et al., 2015; Savarin et al., 2015). Astrocyte injury and swelling further compromised the function in regulating fluid exchange, and resulted in subsequent cerebral edema (Sepehrinezhad et al., 2020).
3.3.3. Microglia activation
Microglia activation showed diverse patterns of location in fetal ICANS. One patient with CAR‐T cell‐related cerebral edema showed extensive distribution, while another presented perivascular localization (Torre et al., 2018). These differences might be attributed to individual heterogeneity with differential susceptibility to inflammatory stimulus in certain brain areas.
Microglia are CNS resident macrophages with highly heterogenic features including complement responsiveness, phagocytic ability, and neurodegeneration involvement (Han et al., 2020; Wendeln et al., 2018; Wolf et al., 2017). Microglia show distinct plasticity in activating diverse metabolic and immune pathways to modulate normal neuron functions (Zheng et al., 2021). Microglia express OX‐6 and OX‐42 as markers of activation (Jiang et al., 2009). During vasogenic edema, activated microglia secrete IL‐1 and TNF‐α that caused increased BBB permeability and neuron injury (Clark & Vissel, 2016; Conroy et al., 2004; Holmin & Mathiesen, 2000). IL‐1 induces temporary and reversible increased permeability by opening endothelial TJs, while TNF‐α causes long‐term effect due to endothelial cytotoxicity (Capaldo & Nusrat, 2009; Marcos‐Ramiro et al., 2014). IL‐6 also induces microglia activation and additional IL‐6 production, leading to neuron damage and astrogliosis (Sofroniew, 2015). IL‐6 neutralization upregulated expression of galectin‐1 on astrocytes and dramatically reduced microglia activation (Sirko et al., 2015). Another special microglia subset, the IFN‐M, was identified in AD models with multiple IFN‐stimulated transcriptional profiles, indicating its potential regulation of cognitive function (Olah et al., 2020). Microglia were also involved in cerebral malaria‐induced neurotoxicity with BBB disruption and cerebral edema (Dorovini‐Zis et al., 2011).
3.3.4. Abnormal neuronal function
Abnormal neuron function is related to the dysregulation of neuroglial cells and neurotransmitters by overwhelmed CNS inflammation. Significantly, high level of glutamate and quinolinic acid in the CSF may explain EEG background abnormalities and symptoms in patients with varying degrees of ICANS (Gust et al., 2021; Santomasso et al., 2018; Tallantyre et al., 2021). Glutamate regulates memory and learning process. Astrocytes exposed to IL‐1β showed decreased glutamate transporters with impaired function of glutamate re‐uptake (Rama Rao et al., 2010). Physiologic TNF‐α maintains normal neuron function, while excessive amount causes extracellular accumulation of glutamate by both increased release and inhibited re‐uptake (Clark & Vissel, 2016). Moreover, TNF‐α induces long‐term excitability alteration by glutamate receptor upregulation and GABA receptor endocytosis. Quinolinic acid, a stimulator for extracellular glutamate accumulation, induces synaptosome depolarization through exogenous Ca2+ influx (Hosomi et al., 2005). High level of IL‐6 altered neuronal excitability in Ca2+‐dependent manner with lower firing rate, oscillatory firing patterns, and prolonged inhibitory phase (Conroy et al., 2004), enhanced neurotoxicity, and blocked neurogenesis (Gruol & Nelson, 2005).
In conclusion, increased BBB permeability leads to neuroinflammation with astrocyte dysfunction, microglia activation, and abnormal neuronal function during CNS inflammation (Figure 6).
FIGURE 6.
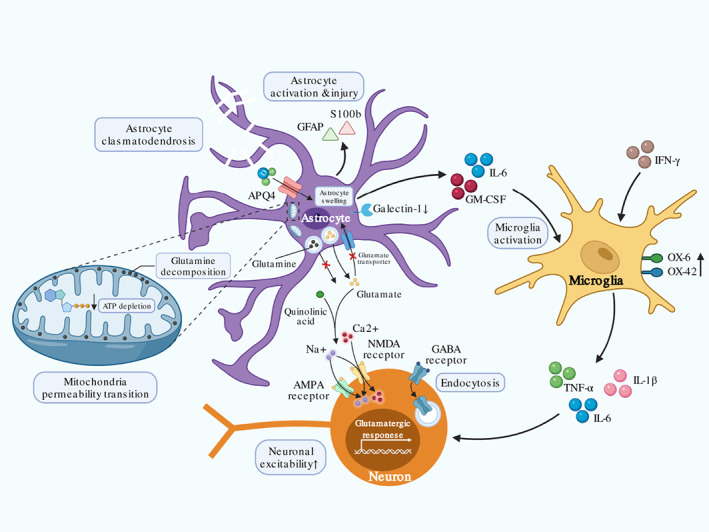
The Central nervous system (CNS) inflammation. Peripheral immunocytes and cytokines infiltration into the CNS contribute to the CNS inflammation. Activated astrocyte releases IL‐6 and GM‐CSF that induce microglia activation to produce additional IL‐1β, IL‐6, and TNF‐α, which further influence neuron function. Increased GFAP and S100b suggest astrocyte activation and injury, while downregulation of galectin‐1 indicates reduced control of microglia activation by astrocyte. Upregulated OX‐6 and OX‐42 on microglia indicate activated status. Astrocyte activation and injury induce glutamine accumulation and decomposition on mitochondria membrane, which lead to ATP depletion and mitochondria permeability transition. Energy insufficiency induces compromised water control by APQ4 and leads to astrocyte swelling. Dysfunctional astrocyte promotes extracellular glutamate accumulation by increased released and inhibited re‐uptake. Accumulative glutamate along with neuron GABA receptor endocytosis caused by TNF‐α increase neuronal excitability through NMDA and AMPA receptor in Ca2+ dependent manner. Abnormal neuron functions present with corresponding symptoms of immune effector cell‐associated neurotoxicity syndrome (ICANS) after chimeric antigen receptor T‐cell (CAR‐T) treatment.
4. OTHER POTENTIALLY RELEVANT FACTORS
4.1. CNS targeting by CAR‐T cell
4.1.1. CNS involvement
The CNS involvement is a severe situation in tumor progression with dismal prognosis in pediatrics and adults (del Principe et al., 2018). The existence of tumor cells in the CNS may contribute to a secondary neurotoxicity after CAR‐T cell targeting (Wang et al., 2021). Tumor cell may infiltrate into the CNS independent of BBB, followed by CAR‐T cell in the same routes (Yao et al., 2018). Patients with CNS leukemia experienced varying degrees of ICANS with detectable CAR‐T cell in the CSF (Santomasso et al., 2018). However, it is hard to distinguish whether increased CAR‐T cell in the CSF is due to local expansion in the CNS or massive infiltration from peripheral blood.
4.1.2. On‐target off‐tumor effect
On‐target off‐tumor effect is a common adverse reaction mostly seen in CAR‐T cell therapy and other antibody‐based target therapy. Normal tissues expressing the same TAAs may suffer additional cytotoxicity (Ahmed et al., 2015; Beatty et al., 2014; Brown et al., 2016; Johnson et al., 2015; Kershaw et al., 2006).
CD19 is an appropriate TAA candidate with confined expression on B cells, while B cell dysplasia usually happens due to normal B cells targeted by CD19 CAR‐T cells (Cordeiro et al., 2020). To our surprise, recent studies discovered that human mural cells also expressed CD19 (Parker et al., 2020; Figure 7). scRNA‐seq analysis of human prefrontal cortex cells found CD19 epitope on mural cells that could be recognized by clinical CAR‐T cells, which was further confirmed by perivascular staining at protein level. Mural cells are classified into vascular smooth muscle cell (vSMC) and pericyte in regard of its different localization along larger vessels or capillaries, respectively. The absence of pericyte impairs normal BBB development. Infusion of murine CD19‐directed CAR‐T cells into NSG mice induced BBB disruption with increased permeability. However, human brain pericytes are more susceptible to clinical CD19‐directed CAR‐T cells due to relative higher abundance of CD19 expression than mouse brain pericytes (Han et al., 2018).
FIGURE 7.
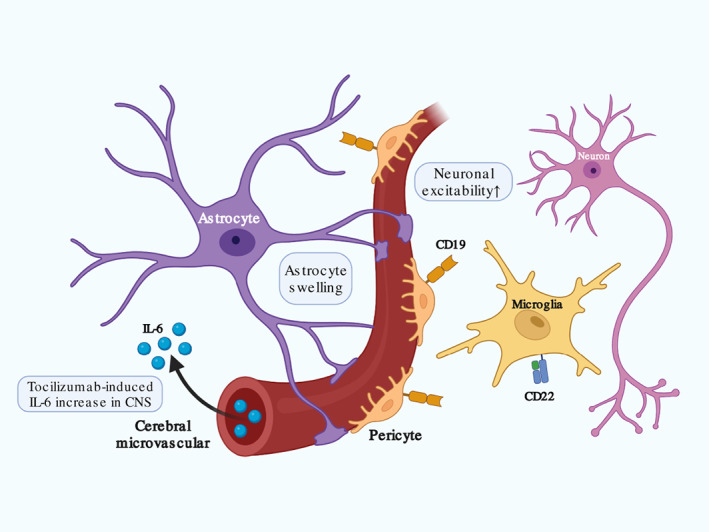
Other potentially relevant factors in immune effector cell‐associated neurotoxicity syndrome (ICANS) after chimeric antigen receptor T‐cell (CAR‐T) treatment. Previous cerebral disease may potentiate neuronal response to stimulus with increased neuronal excitability. CD19 expression on pericyte and CD22 expression on microglia may induce on‐target off‐tumor effect by CAR‐T cell that contributes to blood–brain barrier (BBB) dysfunction and CNS inflammation. Tocilizumab application in patients with cytokine release syndrome (CRS) after CAR‐T treatment may promote peripheral free IL‐6 accumulation and infiltration into the central nervous system (CNS) in absence of tocilizumab penetrating through BBB. Astrocyte swelling with endfoot process disruption and clasmatodendrosis compromises its fluid exchange between the peripheral blood and CNS.
CD22 substitutes for CD19 as a second target after tumor relapse (Fry et al., 2018). Increased incidences of inflammatory toxicities were reported in patients receiving CD22 CAR‐T product (Baird et al., 2021; Shah et al., 2020). scRNA‐seq data confirmed CD22 expression in human microglia, whose upregulation inhibited phagocytosis and impaired clearance of toxic metabolites like myelin debris (Olah et al., 2020) (Figure 7). Interestingly, infusing CD22 CAR‐T cells did not induce more frequent or severer ICANS than CD19 CAR‐T cells. The correlation between CD22 and ICANS remains to be elucidated.
4.2. Tocilizumab application
Tocilizumab is effective to manage patients with severe CRS after CAR‐T treatment by inhibiting both downstream cis and trans‐signaling in effector cells via competitively binding to membrane‐bound and soluble IL‐6R (Kotch et al., 2019; Le et al., 2018; Nishimoto et al., 2008). However, tocilizumab has an opposite effect on ICANS. Studies observed secondary neurotoxicity with serum IL‐6 elevation after CRS symptoms remission in patients receiving tocilizumab (Chen et al., 2016; Karschnia et al., 2019). Massive dissociated IL‐6 penetrates disrupted BBB into the CNS and enhances IL‐6 signaling cascades (Tanaka et al., 2016) (Figure 7). However, tocilizumab with large molecular size fails to reach into the CNS to exert inhibitory effect on microglia and neurons. Delayed ICANS occurred in very few patients with undetermined mechanism (Neelapu et al., 2018; Schuster et al., 2017). One explanation is that cerebral capillary remained integral until later disrupted by tocilizumab‐induced serum IL‐6 accumulation. Others include individual heterogeneity, previous medicine administration, and pharmacokinetics. Correlation between the use of tocilizumab and the onset of ICANS should be elucidated concretely for its safety to manage concurrent CRS.
4.3. Previous cerebral disease
Previous poor cerebral conditions such as epilepsy history and other pathological changes in focal lesions may potentiate ICANS occurrence after CAR‐T treatment. Epileptic seizure is a paroxysmal depolarizing shift caused by abnormal, excessive, and synchronized neuronal activities (Gavvala & Schuele, 2016). BBB disruption participates in epileptogenesis (Oby & Janigro, 2006). Dysfunctional ion channels and imbalanced excitatory or inhibitory circus result in abnormal neuron activation (Tian et al., 2012). Prolonged influx of Ca2+ and Na+ lead to long‐term depolarization and repetitive action potentials. Loss of inhibitory neuron and abnormal GABAergic signaling also induce neuron hyper‐excitability (Figure 7). Previous epilepsy may create persistent sublethal injury in neurons with potentiated responsiveness to extracellular ionic and other neurotransmitters such as GABA, quinolinic acid, and glutamate (Galic et al., 2012). Other pathological changes in focal lesions also influence neuronal electrophysiologic conditions (O'Rourke et al., 2017; Sepehrinezhad et al., 2020).
5. MANAGEMENT OF ICANS
According to the consensus from ASTCT, proper management should be based on ICANS grades (Lee et al., 2019). Supportive treatment with intravenous fluid infusion, aspiration support, and prophylactic anti‐seizure agents are recommended in mild ICANS. High‐dose corticosteroids or biological agent like slituximab and anakinra should be considered in severe ICANS (Davis et al., 2015; Giavridis et al., 2018). Potential biological agents with relevant mechanisms (Table 2) and CAR‐T cell modulation are promising strategies to manage or prevent ICANS (Figure 1).
TABLE 2.
Potential biological agents with relevant mechanisms in immune effector cell‐associated neurotoxicity syndrome (ICANS)
| Drug | Mechanism | Stage | Approved applications | Research related to CAR‐T |
|---|---|---|---|---|
| Corticosteroids | Anti‐inflammatory effect in nonspecific way | FDA approved and widely used for ICANS | Abundant applications concerning inflammation | Widely used to treat CRS and ICANS |
| Siltuximab | IL‐6 anatagonist | FDA approved | Castleman disease | Clinical trials in treating CRS and ICANS |
| Anakinra | IL‐1 receptor antagonist | FDA approved | RA/CAPS/deficiency of interleukin‐1 receptor antagonist | Clinical trials in treating CRS and ICANS |
| Lenzilumab | Anti‐GM‐CSF antibody | Under clinical investigation | Clinical trials for combination of lenzilumab and CART19 cell therapy | |
| Ruxolitinib | JAK inhibitor | FDA approved | MF/PV/aGVHD | Several cases reported for CRS treatment |
| Itacitinib | JAK inhibitor | Under clinical investigation | Clinical trials in treating CRS | |
| Ibrutinib | BTK inhibitor | FDA approved | MCL/CLL/SLL/WM/MZL/cGVHD | Clinical trials for combination of ibrutinib and CART cell therapy |
| Natalizumab | Anti‐VLA4 antibody | FDA approved | MS/Crohn's disease | No; possibly related to progressive multifocal leukoencephalopathy |
| Etanercept | TNF‐α inhibitor | FDA approved | RA/polyarticular JIA/PsA/AS/PsO | Preclinical study for CRS |
Abbreviations: aGVHD, acute graft‐versus‐host disease; AS, ankylosing spondylitis; cGVHD, chronic graft versus host disease; CAPS, cryopyrin‐associated periodic syndromes; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; JIA, juvenile idiopathic arthritis; MCL, mantle cell lymphoma; MF, myelofibrosis; MS, multiple sclerosis; MZL, marginal zone lymphoma; PsA, psoriatic arthriti; PsO, plaque psoriasis; PV, polycythemia vera; RA, rheumatoid arthritis; WM, Waldenstrom's macroglobulinemia.
5.1. Corticosteroids
Corticosteroids exert profound anti‐inflammatory effects on immune cells through rapid nongenomic alteration and gene transcriptive regulation to rebalance pro and anti‐inflammatory mediator production, block cytokine signaling or adhesion molecules interaction, and induce apoptosis (Timmermans et al., 2019). The application of high‐dose corticosteroids achieved excellent remission in severe CRS and ICANS (Brudno & Kochenderfer, 2019). However, corticosteroids may influence CAR‐T cell function and efficacy. Pioneer studies have observed CAR‐T cell functional deficiency after applying high‐dose or long‐period corticosteroids in patients with severe ICANS (Brentjens et al., 2013; Davila et al., 2014). Recently, investigators found that higher dose of corticosteroids, and earlier or longer‐period use was associated with significantly shorter progression‐free survival and overall survival in a retrospective study evaluating the efficacy of CAR‐T cell therapy among 100 patients with large B‐cell lymphoma (Strati et al., 2021). These results highlighted a cautious consideration before applying corticosteroids to manage ICANS.
5.2. Biological agents
5.2.1. IL‐6 signaling blockade
Siltuximab is an IL‐6 neutralization antibody different from tocilizumab (Chen et al., 2016; Davis et al., 2015). Intravenous administration of siltuximab did not increase the concentration of IL‐6 in the CSF (Neelapu et al., 2018), therefore to be suitable for managing CRS and ICANS simultaneously.
5.2.2. IL‐1 signaling blockade
Anakinra is an IL‐1 receptor antagonist with ability to penetrate BBB into the CNS for the treatment of both systemic and cerebral autoinflammatory disease, including Still's disease, rheumatoid arthritis, and macrophage activation syndrome (MAS) (Ramirez & Canete, 2018; Sonmez et al., 2018; Vastert et al., 2019). As discussed above, IL‐1 positively regulates both the peripheral and CNS inflammation. Therefore, targeting IL‐1 might prevent ICANS progression and avoid severe outcomes. Recent preclinical studies demonstrated that anakinra could alleviate severe neurotoxicity better than tocilizumab without impairing CAR‐T cell function in mice (Gottschlich et al., 2021; Norelli et al., 2018). Several clinical trials are currently evaluating the combinational strategy with CAR‐T cell and IL‐1 blockade (NCT04432506, NCT04359784, NCT04150913, NCT04148430, NCT04205838, and NCT03430011).
5.2.3. Targeting other cytokines
In preclinical study, GM‐CSF neutralization in xenograft mouse models by lenzilumab, an anti‐GM‐CSF antibody, showed reduced CNS infiltration of CAR‐T cells and myeloid cells, relieved signs of neurotoxicity, and improved overall survival without impairing CAR‐T cell function (Sterner et al., 2019). Inhibition of IFN‐γ production and signaling by using JAK inhibitors such as ruxolitinib or itacitinib (Elli et al., 2019; Huarte et al., 2020; Pan et al., 2021), and BTK inhibitor ibrutinib all successfully achieved ICANS remission, but doing great damage on CAR‐T cell function and efficacy (Gauthier et al., 2020; Ruella et al., 2017).
5.2.4. Adhesion molecules blockade
Alternative strategy to target specific surface molecules on CAR‐T cells and lymphocytes is also being developed recently. VLA‐4 plays a key role in lymphocytes trafficking into extravascular tissues through interactions with VCAM‐1 and fibronectin on EC (Redondo‐Munoz et al., 2019). As CAR‐T cells expressed more VLA‐4 than non‐CAR T cells, blocking VLA‐4 by natlizumab may prevent the CNS infiltration of CAR‐T cells and reduce CNS inflammation (Khoy et al., 2020; Taraseviciute et al., 2018).
5.3. CAR‐T modulation
CAR‐T cell modulation by redesigning CAR construct has drawn much attention into improving efficacy and reducing toxicities (Feucht et al., 2019; Hu et al., 2021; Wang et al., 2020; Ying et al., 2019). Specific tag or ON/OFF switch could be embedded into CAR construct in order to control CAR‐T cell biological functions in space and time (Feucht et al., 2019; Larson & Maus, 2021). Additional cell surface proteins such as EGFR or CD20 that are artificially introduced into CAR‐T cell could be targeted by exogenous monoclonal antibodies to induce specific elimination, such as cetuximab or rituximab, respectively (Paszkiewicz et al., 2016). However, such strategy remains risks of on‐target off‐tumor effect. Incorporation of inducible apoptosis signaling combined with specific receptor serves as a safety switch in CAR‐T cell. In a pioneer study, modified human caspase‐9 and FK506 binding protein (FKBP) were embedded into CD19‐targeted CAR construct to prevent severe ICANS progression via inducing CAR‐T cell apoptosis upon specific stimulation by chemical inducer of dimerization (CID) (Diaconu et al., 2017). Similarly, split CAR design facilitates selective and reversible modulation of CAR‐T cell function under certain circumstances (Mestermann et al., 2019). CAR‐T cells with further endogenous knock‐out of key cytokines like GM‐CSF by gene‐editing technology sufficiently reduced host monocyte/macrophage activation and cytokine release in mouse models, while preserving CAR‐T cell efficacy (Sachdeva et al., 2019; Sterner et al., 2019).
6. CONCLUSION
CAR‐T cell therapy provides with a promising strategy in cancer treatment, while specific toxic adverse events like CRS and ICANS still hinder its broad clinical application, with the precise mechanisms remaining largely unknown.
In our conclusion, CAR‐T cell‐induced overactivated peripheral immune activity with excessive systemic cytokines and pro‐inflammatory mediators contribute to endothelial activation and injury during the early stage of ICANS. Endothelial activation‐induced BBB dysfunction with increased cerebral microvascular permeability facilitates the CNS infiltration of peripheral cytokines and immunocytes, which result in subsequent CNS inflammation and neuron dysfunction. On‐target off‐tumor effect promotes pericyte‐mediate BBB disruption and induces microglia activation. Previous cerebral diseases and tocilizumab application might also increase CNS sensitivity to inflammatory stimulus and accelerate ICANS progression. Targeting key cytokines involved in ICANS induces rapid remission without impairing CAR‐T cell efficacy. Much effort should also be devoted to CAR‐T modulation and optimization with safety control to avoid rather than treat ICANS.
Challenges still remain despite existing progress. First, ICANS presents with diverse CNS symptoms with lack of universally adopted comprehensive description and category. Next, the absence of ideal experimental animal models and appropriate analytic technologies hinder our deeper understanding about ICANS. Single‐cell RNA sequencing might facilitate to decipher complex cellular and molecular interactions in the brain. Finally, discovering specific cytokines or adhesion molecules involved in the pathogenesis of ICANS might contribute to developing novel therapeutic target. Digging into the depth of mechanisms of ICANS will shed more light on the safe application of CAR‐T cell therapy in the future.
AUTHOR CONTRIBUTIONS
Tianning Gu: Conceptualization (lead); writing – original draft (lead); writing – review and editing (lead). Kejia Hu: Conceptualization (supporting); writing – original draft (supporting); writing – review and editing (supporting). Xiaohui Si: Conceptualization (supporting); writing – original draft (supporting); writing – review and editing (supporting). Yongxian Hu: Conceptualization (lead); supervision (lead). He Huang: Conceptualization (lead); supervision (lead).
CONFLICT OF INTEREST
The authors have declared no conflict of interest for this article.
RELATED WIREs ARTICLES
Applications of molecular engineering in T‐cell‐based immunotherapies
ACKNOWLEDGMENT
Thanks to Xia Li for useful discussion.
Gu, T. , Hu, K. , Si, X. , Hu, Y. , & Huang, H. (2022). Mechanisms of immune effector cell‐associated neurotoxicity syndrome after CAR‐T treatment. WIREs Mechanisms of Disease, 14(6), e1576. 10.1002/wsbm.1576
Edited by: Jessica Lawler, Executive Editor
Funding information National Natural Science Foundation of China, Grant/Award Number: 81730008
Contributor Information
Yongxian Hu, Email: 1313016@zju.edu.cn.
He Huang, Email: huanghe@zju.edu.cn.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
FURTHER READING
- McBride, D. A. , Kerr, M. D. , Wai, S. L. , & Shah, N. J. (2019). Applications of molecular engineering in T‐cell‐based immunotherapies. Wiley Interdisciplinary Reviews. Nanomedicine and Nanobiotechnology, 11(5), e1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
REFERENCES
- Abbott, N. J. , Ronnback, L. , & Hansson, E. (2006). Astrocyte‐endothelial interactions at the blood‐brain barrier. Nature Reviews. Neuroscience, 7(1), 41–53. 10.1038/nrn1824 [DOI] [PubMed] [Google Scholar]
- Adegbola, S. O. , Sahnan, K. , Warusavitarne, J. , Hart, A. , & Tozer, P. (2018). Anti‐TNF therapy in Crohn's disease. International Journal of Molecular Sciences, 19(8), 2244. 10.3390/ijms19082244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed, N. , Brawley, V. S. , Hegde, M. , Robertson, C. , Ghazi, A. , Gerken, C. , Liu, E. , Dakhova, O. , Ashoori, A. , Corder, A. , Gray, T. , Wu, M. F. , Liu, H. , Hicks, J. , Rainusso, N. , Dotti, G. , Mei, Z. , Grilley, B. , Gee, A. , … Gottschalk, S. (2015). Human epidermal growth factor receptor 2 (HER2)‐specific chimeric antigen receptor‐modified T cells for the immunotherapy of HER2‐positive sarcoma. JCO, 33(15), 1688–1696. 10.1200/JCO.2014.58.0225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali, S. A. , Shi, V. , Maric, I. , Wang, M. , Stroncek, D. F. , Rose, J. J. , Brudno, J. N. , Stetler‐Stevenson, M. , Feldman, S. A. , Hansen, B. G. , Fellowes, V. S. , Hakim, F. T. , Gress, R. E. , & Kochenderfer, J. N. (2016). T cells expressing an anti‐B‐cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood, 128(13), 1688–1700. 10.1182/blood-2016-04-711903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte, R. S. , Chen, D. S. , & Ferrara, N. (2019). VEGF in signaling and disease: Beyond discovery and development. Cell, 176(6), 1248–1264. 10.1016/j.cell.2019.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird, J. H. , Frank, M. J. , Craig, J. , Patel, S. , Spiegel, J. Y. , Sahaf, B. , Oak, J. S. , Younes, S. F. , Ozawa, M. G. , Yang, E. , Natkunam, Y. , Tamaresis, J. , Ehlinger, Z. , Reynolds, W. D. , Arai, S. , Johnston, L. , Lowsky, R. , Meyer, E. , Negrin, R. S. , … Muffly, L. (2021). CD22‐directed CAR T‐cell therapy induces complete remissions in CD19‐directed CAR‐refractory large B‐cell lymphoma. Blood, 137(17), 2321–2325. 10.1182/blood.2020009432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks, W. A. (2015). The blood‐brain barrier in neuroimmunology: Tales of separation and assimilation. Brain, Behavior, and Immunity, 44, 1–8. 10.1016/j.bbi.2014.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao, J. Y. , Huang, Y. , Wang, F. , Peng, Y. P. , & Qiu, Y. H. (2007). Expression of alpha‐AR subtypes in T lymphocytes and role of the alpha‐ARs in mediating modulation of T cell function. Neuroimmunomodulation, 14(6), 344–353. 10.1159/000129670 [DOI] [PubMed] [Google Scholar]
- Beatty, G. L. , Haas, A. R. , Maus, M. V. , Torigian, D. A. , Soulen, M. C. , Plesa, G. , Chew, A. , Zhao, Y. , Levine, B. L. , Albelda, S. M. , Kalos, M. , & June, C. H. (2014). Mesothelin‐specific chimeric antigen receptor mRNA‐engineered T cells induce anti‐tumor activity in solid malignancies. Cancer Immunology Research, 2(2), 112–120. 10.1158/2326-6066.CIR-13-0170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher, B. , Tugues, S. , & Greter, M. (2016). GM‐CSF: From growth factor to central mediator of tissue inflammation. Immunity, 45(5), 963–973. 10.1016/j.immuni.2016.10.026 [DOI] [PubMed] [Google Scholar]
- Bek, S. , Bojesen, A. B. , Nielsen, J. V. , Sode, J. , Bank, S. , Vogel, U. , & Andersen, V. (2017). Systematic review and meta‐analysis: Pharmacogenetics of anti‐TNF treatment response in rheumatoid arthritis. The Pharmacogenomics Journal, 17(5), 403–411. 10.1038/tpj.2017.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergquist, J. , Tarkowski, A. , Ekman, R. , & Ewing, A. (1994). Discovery of endogenous catecholamines in lymphocytes and evidence for catecholamine regulation of lymphocyte function via an autocrine loop. Proceedings of the National Academy of Sciences of the United States of America, 91(26), 12912–12916. 10.1073/pnas.91.26.12912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair, A. (2018). Pericyte biology: Development, homeostasis, and disease. Advances in Experimental Medicine and Biology, 1109, 1–3. 10.1007/978-3-030-02601-1_1 [DOI] [PubMed] [Google Scholar]
- Blecharz‐Lang, K. G. , Wagner, J. , Fries, A. , Nieminen‐Kelha, M. , Rosner, J. , Schneider, U. C. , & Vajkoczy, P. (2018). Interleukin 6‐mediated endothelial barrier disturbances can be attenuated by blockade of the IL6 receptor expressed in brain microvascular endothelial cells. Translational Stroke Research, 9(6), 631–642. 10.1007/s12975-018-0614-2 [DOI] [PubMed] [Google Scholar]
- Bors, L. , Tóth, K. , Tóth, E. Z. , Bajza, A. , Csorba, A. , Szigeti, K. , Máthé, D. , Perlaki, G. , Orsi, G. , Tóth, G. K. , & Erdő, F. (2018). Age‐dependent changes at the blood‐brain barrier. A comparative structural and functional study in young adult and middle aged rats. Brain Research Bulletin, 139, 269–277. 10.1016/j.brainresbull.2018.03.001 [DOI] [PubMed] [Google Scholar]
- Bradley, J. R. (2008). TNF‐mediated inflammatory disease. The Journal of Pathology, 214(2), 149–160. 10.1002/path.2287 [DOI] [PubMed] [Google Scholar]
- Brentjens, R. J. , Davila, M. L. , Riviere, I. , Park, J. , Wang, X. , Cowell, L. G. , Bartido, S. , Stefanski, J. , Taylor, C. , Olszewska, M. , Borquez‐Ojeda, O. , Qu, J. , Wasielewska, T. , He, Q. , Bernal, Y. , Rijo, I. V. , Hedvat, C. , Kobos, R. , Curran, K. , … Sadelain, M. (2013). CD19‐targeted T cells rapidly induce molecular remissions in adults with chemotherapy‐refractory acute lymphoblastic leukemia. Sci Transl Med, 5(177), 177ra138. 10.1126/scitranslmed.3005930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindle, N. P. , Saharinen, P. , & Alitalo, K. (2006). Signaling and functions of angiopoietin‐1 in vascular protection. Circulation Research, 98(8), 1014–1023. 10.1161/01.RES.0000218275.54089.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, B. D. , Tambaro, F. P. , Kohorst, M. , Chi, L. , Mahadeo, K. M. , Tewari, P. , Petropoulos, D. , Slopis, J. M. , Sadighi, Z. , & Khazal, S. (2021). Immune effector cell associated neurotoxicity (ICANS) in pediatric and young adult patients following chimeric antigen receptor (CAR) T‐cell therapy: Can we optimize early diagnosis? Front Oncol, 11, 634445. 10.3389/fonc.2021.634445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, C. E. , Alizadeh, D. , Starr, R. , Weng, L. , Wagner, J. R. , Naranjo, A. , Ostberg, J. R. , Blanchard, M. S. , Kilpatrick, J. , Simpson, J. , Kurien, A. , Priceman, S. J. , Wang, X. , Harshbarger, T. L. , D'Apuzzo, M. , Ressler, J. A. , Jensen, M. C. , Barish, M. E. , Chen, M. , … Badie, B. (2016). Regression of glioblastoma after chimeric antigen receptor T‐cell therapy. N Engl J Med, 375(26), 2561–2569. 10.1056/NEJMoa1610497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudno, J. N. , & Kochenderfer, J. N. (2019). Recent advances in CAR T‐cell toxicity: Mechanisms, manifestations and management. Blood Reviews, 34, 45–55. 10.1016/j.blre.2018.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudno, J. N. , Lam, N. , Vanasse, D. , Shen, Y. W. , Rose, J. J. , Rossi, J. , Xue, A. , Bot, A. , Scholler, N. , Mikkilineni, L. , Roschewski, M. , Dean, R. , Cachau, R. , Youkharibache, P. , Patel, R. , Hansen, B. , Stroncek, D. F. , Rosenberg, S. A. , Gress, R. E. , & Kochenderfer, J. N. (2020). Safety and feasibility of anti‐CD19 CAR T cells with fully human binding domains in patients with B‐cell lymphoma. Nat Med, 26(2), 270–280. 10.1038/s41591-019-0737-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldo, C. T. , & Nusrat, A. (2009). Cytokine regulation of tight junctions. Biochimica et Biophysica Acta, 1788(4), 864–871. 10.1016/j.bbamem.2008.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, F. , Teachey, D. T. , Pequignot, E. , Frey, N. , Porter, D. , Maude, S. L. , Grupp, S. A. , June, C. H. , Melenhorst, J. J. , & Lacey, S. F. (2016). Measuring IL‐6 and sIL‐6R in serum from patients treated with tocilizumab and/or siltuximab following CAR T cell therapy. Journal of Immunological Methods, 434, 1–8. 10.1016/j.jim.2016.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L. Y. C. , Hoiland, R. L. , Stukas, S. , Wellington, C. L. , & Sekhon, M. S. (2020). Confronting the controversy: Interleukin‐6 and the COVID‐19 cytokine storm syndrome. The European Respiratory Journal, 56(4), 2003006. 10.1183/13993003.03006-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, I. A. , & Vissel, B. (2016). Excess cerebral TNF causing glutamate excitotoxicity rationalizes treatment of neurodegenerative diseases and neurogenic pain by anti‐TNF agents. Journal of Neuroinflammation, 13(1), 236. 10.1186/s12974-016-0708-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy, S. M. , Nguyen, V. , Quina, L. A. , Blakely‐Gonzales, P. , Ur, C. , Netzeband, J. G. , Prieto, A. L. , & Gruol, D. L. (2004). Interleukin‐6 produces neuronal loss in developing cerebellar granule neuron cultures. Journal of Neuroimmunology, 155(1–2), 43–54. 10.1016/j.jneuroim.2004.06.014 [DOI] [PubMed] [Google Scholar]
- Cordeiro, A. , Bezerra, E. D. , Hirayama, A. V. , Hill, J. A. , Wu, Q. V. , Voutsinas, J. , Sorror, M. L. , Turtle, C. J. , Maloney, D. G. , & Bar, M. (2020). Late events after treatment with CD19‐targeted chimeric antigen receptor modified T cells. Biology of Blood and Marrow Transplantation, 26(1), 26–33. 10.1016/j.bbmt.2019.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal, P. J. , Muller, W. A. , & Sullivan, D. P. (2020). Endothelial cell calcium signaling during barrier function and inflammation. The American Journal of Pathology, 190(3), 535–542. 10.1016/j.ajpath.2019.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly, C. , Wong, V. , Burova, E. , Wei, Y. , Zabski, S. , Griffiths, J. , Lai, K. M. , Lin, H. C. , Ioffe, E. , Yancopoulos, G. D. , & Rudge, J. S. (2004). Angiopoietin‐1 modulates endothelial cell function and gene expression via the transcription factor FKHR (FOXO1). Genes Dev, 18(9), 1060–1071. 10.1101/gad.1189704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danish, H. , & Santomasso, B. D. (2021). Neurotoxicity biology and management. The Cancer Journal, 27(2), 126–133. 10.1097/ppo.0000000000000507 [DOI] [PubMed] [Google Scholar]
- Davila, M. L. , Riviere, I. , Wang, X. , Bartido, S. , Park, J. , Curran, K. , Chung, S. S. , Stefanski, J. , Borquez‐Ojeda, O. , Olszewska, M. , Qu, J. , Wasielewska, T. , He, Q. , Fink, M. , Shinglot, H. , Youssif, M. , Satter, M. , Wang, Y. , Hosey, J. , … Brentjens, R. (2014). Efficacy and toxicity management of 19‐28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med, 6(224), 224ra225. 10.1126/scitranslmed.3008226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, C. C. , Shah, K. S. , & Lechowicz, M. J. (2015). Clinical development of siltuximab. Current Oncology Reports, 17(7), 29. 10.1007/s11912-015-0453-1 [DOI] [PubMed] [Google Scholar]
- del Principe, M. I. , Buccisano, F. , Soddu, S. , Maurillo, L. , Cefalo, M. , Piciocchi, A. , Consalvo, M. I. , Paterno, G. , Sarlo, C. , de Bellis, E. , Zizzari, A. , de Angelis, G. , Fraboni, D. , Divona, M. , Voso, M. T. , Sconocchia, G. , del Poeta, G. , Lo‐Coco, F. , Arcese, W. , … Venditti, A. (2018). Involvement of central nervous system in adult patients with acute myeloid leukemia: Incidence and impact on outcome. Seminars in Hematology, 55(4), 209–214. 10.1053/j.seminhematol.2018.02.006 [DOI] [PubMed] [Google Scholar]
- Delaney, C. , & Campbell, M. (2017). The blood brain barrier: Insights from development and ageing. Tissue Barriers, 5(4), e1373897. 10.1080/21688370.2017.1373897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, Q. , Han, G. , Puebla‐Osorio, N. , Ma, M. C. J. , Strati, P. , Chasen, B. , Dai, E. , Dang, M. , Jain, N. , Yang, H. , Wang, Y. , Zhang, S. , Wang, R. , Chen, R. , Showell, J. , Ghosh, S. , Patchva, S. , Zhang, Q. , Sun, R. , … Green, M. R. (2020). Characteristics of anti‐CD19 CAR T cell infusion products associated with efficacy and toxicity in patients with large B cell lymphomas. Nat Med, 26(12), 1878–1887. 10.1038/s41591-020-1061-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaconu, I. , Ballard, B. , Zhang, M. , Chen, Y. , West, J. , Dotti, G. , & Savoldo, B. (2017). Inducible Caspase‐9 selectively modulates the toxicities of CD19‐specific chimeric antigen receptor‐modified T cells. Molecular Therapy, 25(3), 580–592. 10.1016/j.ymthe.2017.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, L. , Hu, Y. , & Huang, H. (2021). Novel progresses of chimeric antigen receptor (CAR) T cell therapy in multiple myeloma. Stem Cell Investigation, 8, 1. 10.21037/sci-2020-029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNofia, A. M. , & Maude, S. L. (2019). Chimeric antigen receptor T‐cell therapy clinical results in pediatric and young adult B‐ALL. Hema, 3(4), e279. 10.1097/HS9.0000000000000279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorovini‐Zis, K. , Schmidt, K. , Huynh, H. , Fu, W. , Whitten, R. O. , Milner, D. , Kamiza, S. , Molyneux, M. , & Taylor, T. E. (2011). The neuropathology of fatal cerebral malaria in Malawian children. The American Journal of Pathology, 178(5), 2146–2158. 10.1016/j.ajpath.2011.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinav, E. , Nowarski, R. , Thaiss, C. A. , Hu, B. , Jin, C. , & Flavell, R. A. (2013). Inflammation‐induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nature Reviews. Cancer, 13(11), 759–771. 10.1038/nrc3611 [DOI] [PubMed] [Google Scholar]
- Elli, E. M. , Barate, C. , Mendicino, F. , Palandri, F. , & Palumbo, G. A. (2019). Mechanisms underlying the anti‐inflammatory and immunosuppressive activity of Ruxolitinib. Frontiers in Oncology, 9, 1186. 10.3389/fonc.2019.01186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feucht, J. , Sun, J. , Eyquem, J. , Ho, Y. J. , Zhao, Z. , Leibold, J. , Dobrin, A. , Cabriolu, A. , Hamieh, M. , & Sadelain, M. (2019). Calibration of CAR activation potential directs alternative T cell fates and therapeutic potency. Nat Med, 25(1), 82–88. 10.1038/s41591-018-0290-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler, U. , Scharpfenecker, M. , Koidl, S. , Hegen, A. , Grunow, V. , Schmidt, J. M. , Kriz, W. , Thurston, G. , & Augustin, H. G. (2004). The Tie‐2 ligand angiopoietin‐2 is stored in and rapidly released upon stimulation from endothelial cell Weibel‐Palade bodies. Blood, 103(11), 4150–4156. 10.1182/blood-2003-10-3685 [DOI] [PubMed] [Google Scholar]
- Fry, T. J. , Shah, N. N. , Orentas, R. J. , Stetler‐Stevenson, M. , Yuan, C. M. , Ramakrishna, S. , Wolters, P. , Martin, S. , Delbrook, C. , Yates, B. , Shalabi, H. , Fountaine, T. J. , Shern, J. F. , Majzner, R. G. , Stroncek, D. F. , Sabatino, M. , Feng, Y. , Dimitrov, D. S. , Zhang, L. , … Mackall, C. L. (2018). CD22‐targeted CAR T cells induce remission in B‐ALL that is naive or resistant to CD19‐targeted CAR immunotherapy. Nat Med, 24(1), 20–28. 10.1038/nm.4441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galic, M. A. , Riazi, K. , & Pittman, Q. J. (2012). Cytokines and brain excitability. Frontiers in Neuroendocrinology, 33(1), 116–125. 10.1016/j.yfrne.2011.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbuzova‐Davis, S. , Ehrhart, J. , Sanberg, P. R. , & Borlongan, C. V. (2018). Potential role of humoral IL‐6 cytokine in mediating pro‐inflammatory endothelial cell response in amyotrophic lateral sclerosis. International Journal of Molecular Sciences, 19(2), 423. 10.3390/ijms19020423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner, R. A. , Finney, O. , Annesley, C. , Brakke, H. , Summers, C. , Leger, K. , Bleakley, M. , Brown, C. , Mgebroff, S. , Kelly‐Spratt, K. S. , Hoglund, V. , Lindgren, C. , Oron, A. P. , Li, D. , Riddell, S. R. , Park, J. R. , & Jensen, M. C. (2017). Intent‐to‐treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood, 129(25), 3322–3331. 10.1182/blood-2017-02-769208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier, J. , Hirayama, A. V. , Purushe, J. , Hay, K. A. , Lymp, J. , Li, D. H. , Yeung, C. C. S. , Sheih, A. , Pender, B. S. , Hawkins, R. M. , Vakil, A. , Phi, T. D. , Steinmetz, R. N. , Shadman, M. , Riddell, S. R. , Maloney, D. G. , & Turtle, C. J. (2020). Feasibility and efficacy of CD19‐targeted CAR T cells with concurrent ibrutinib for CLL after ibrutinib failure. Blood, 135(19), 1650–1660. 10.1182/blood.2019002936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavvala, J. R. , & Schuele, S. U. (2016). New‐onset seizure in adults and adolescents: A review. JAMA, 316(24), 2657–2668. 10.1001/jama.2016.18625 [DOI] [PubMed] [Google Scholar]
- Ghosh, C. C. , Mukherjee, A. , David, S. , Knaus, U. G. , Stearns‐Kurosawa, D. J. , Kurosawa, S. , & Parikh, S. M. (2012). Impaired function of the Tie‐2 receptor contributes to vascular leakage and lethality in anthrax. Proceedings of the National Academy of Sciences of the United States of America, 109(25), 10024–10029. 10.1073/pnas.1120755109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giavridis, T. , van der Stegen, S. J. C. , Eyquem, J. , Hamieh, M. , Piersigilli, A. , & Sadelain, M. (2018). CAR T cell‐induced cytokine release syndrome is mediated by macrophages and abated by IL‐1 blockade. Nature Medicine, 24(6), 731–738. 10.1038/s41591-018-0041-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gofshteyn, J. S. , Shaw, P. A. , Teachey, D. T. , Grupp, S. A. , Maude, S. , Banwell, B. , Chen, F. , Lacey, S. F. , Melenhorst, J. J. , Edmonson, M. K. J. , Panzer, J. , Barrett, D. M. , & McGuire, J. L. (2018). Neurotoxicity after CTL019 in a pediatric and young adult cohort. Ann Neurol, 84(4), 537–546. 10.1002/ana.25315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschlich, A. , Endres, S. , & Kobold, S. (2021). Therapeutic strategies for targeting IL‐1 in cancer. Cancers, 13(3), 477. 10.3390/cancers13030477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum, U. , Kebriaei, P. , Srour, S. A. , Olson, A. , Bashir, Q. , Neelapu, S. S. , Rezvani, K. , & Shpall, E. J. (2021). Chimeric antigen receptor T‐cell therapy toxicities. Br J Clin Pharmacol, 87(6), 2414–2424. 10.1111/bcp.14403 [DOI] [PubMed] [Google Scholar]
- Gruol, D. L. , & Nelson, T. E. (2005). Purkinje neuron physiology is altered by the inflammatory factor interleukin‐6. Cerebellum, 4(3), 198–205. 10.1080/14734220500199987 [DOI] [PubMed] [Google Scholar]
- Grupp, S. A. , Kalos, M. , Barrett, D. , Aplenc, R. , Porter, D. L. , Rheingold, S. R. , Teachey, D. T. , Chew, A. , Hauck, B. , Wright, J. F. , Milone, M. C. , Levine, B. L. , & June, C. H. (2013). Chimeric antigen receptor‐modified T cells for acute lymphoid leukemia. N Engl J Med, 368(16), 1509–1518. 10.1056/NEJMoa1215134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gust, J. , Annesley, C. E. , Gardner, R. A. , & Bozarth, X. (2021). EEG correlates of delirium in children and young adults with CD19‐directed CAR T cell treatment‐related neurotoxicity. Journal of Clinical Neurophysiology, 38(2), 135–142. 10.1097/WNP.0000000000000669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gust, J. , Finney, O. C. , Li, D. , Brakke, H. M. , Hicks, R. M. , Futrell, R. B. , Gamble, D. N. , Rawlings‐Rhea, S. D. , Khalatbari, H. K. , Ishak, G. E. , Duncan, V. E. , Hevner, R. F. , Jensen, M. C. , Park, J. R. , & Gardner, R. A. (2019). Glial injury in neurotoxicity after pediatric CD19‐directed chimeric antigen receptor T cell therapy. Ann Neurol, 86(1), 42–54. 10.1002/ana.25502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gust, J. , Hay, K. A. , Hanafi, L. A. , Li, D. , Myerson, D. , Gonzalez‐Cuyar, L. F. , Yeung, C. , Liles, W. C. , Wurfel, M. , Lopez, J. A. , Chen, J. , Chung, D. , Harju‐Baker, S. , Özpolat, T. , Fink, K. R. , Riddell, S. R. , Maloney, D. G. , & Turtle, C. J. (2017). Endothelial activation and blood‐brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR‐T cells. Cancer Discovery, 7(12), 1404–1419. 10.1158/2159-8290.CD-17-0698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gust, J. , Ponce, R. , Liles, W. C. , Garden, G. A. , & Turtle, C. J. (2020). Cytokines in CAR T cell‐associated neurotoxicity. Frontiers in Immunology, 11, 577027. 10.3389/fimmu.2020.577027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, X. , Wang, R. , Zhou, Y. , Fei, L. , Sun, H. , Lai, S. , Saadatpour, A. , Zhou, Z. , Chen, H. , Ye, F. , Huang, D. , Xu, Y. , Huang, W. , Jiang, M. , Jiang, X. , Mao, J. , Chen, Y. , Lu, C. , Xie, J. , … Guo, G. (2018). Mapping the mouse cell atlas by microwell‐Seq. Cell, 172(5), 1091–1107.e17. 10.1016/j.cell.2018.02.001 [DOI] [PubMed] [Google Scholar]
- Han, X. , Zhou, Z. , Fei, L. , Sun, H. , Wang, R. , Chen, Y. , Chen, H. , Wang, J. , Tang, H. , Ge, W. , Zhou, Y. , Ye, F. , Jiang, M. , Wu, J. , Xiao, Y. , Jia, X. , Zhang, T. , Ma, X. , Zhang, Q. , … Guo, G. (2020). Construction of a human cell landscape at single‐cell level. Nature, 581(7808), 303–309. 10.1038/s41586-020-2157-4 [DOI] [PubMed] [Google Scholar]
- Hao, Z. , Li, R. , Meng, L. , Han, Z. , & Hong, Z. (2020). Macrophage, the potential key mediator in CAR‐T related CRS. Experimental Hematology & Oncology, 9, 15. 10.1186/s40164-020-00171-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada‐Shirado, K. , Wang, X. , Mori, H. , Fukatsu, M. , Takahashi, H. , Shichishima‐Nakamura, A. , Kimura, S. , Ohkawara, H. , Yamada, S. , Ito, T. , & Ikezoe, T. (2020). Circulating intranuclear proteins may play a role in development of disseminated intravascular coagulation in individuals with acute leukemia. Int J Hematol, 111(3), 378–387. 10.1007/s12185-019-02798-5 [DOI] [PubMed] [Google Scholar]
- Herr, M. M. , Chen, G. L. , Ross, M. , Jacobson, H. , McKenzie, R. , Markel, L. , Balderman, S. R. , Ho, C. M. , Hahn, T. , & McCarthy, P. L. (2020). Identification of neurotoxicity after chimeric antigen receptor (CAR) T cell infusion without deterioration in the immune effector cell encephalopathy (ICE) score. Biology of Blood and Marrow Transplantation, 26(11), e271–e274. 10.1016/j.bbmt.2020.07.031 [DOI] [PubMed] [Google Scholar]
- Holmin, S. , & Mathiesen, T. (2000). Intracerebral administration of interleukin‐1beta and induction of inflammation, apoptosis, and vasogenic edema. Journal of Neurosurgery, 92(1), 108–120. 10.3171/jns.2000.92.1.0108 [DOI] [PubMed] [Google Scholar]
- Holtzman, N. G. , Xie, H. , Bentzen, S. , Kesari, V. , Bukhari, A. , el Chaer, F. , Lutfi, F. , Siglin, J. , Hutnick, E. , Gahres, N. , Ruehle, K. , Ahmad, H. , Shanholtz, C. , Kocoglu, M. H. , Badros, A. Z. , Yared, J. A. , Hardy, N. M. , Rapoport, A. P. , & Dahiya, S. (2021). Immune effector cell‐associated neurotoxicity syndrome after chimeric antigen receptor T‐cell therapy for lymphoma: Predictive biomarkers and clinical outcomes. Neuro‐Oncology, 23(1), 112–121. 10.1093/neuonc/noaa183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, R. , Hu, Y. , & Huang, H. (2021). Biomarkers for chimeric antigen receptor T cell therapy in acute lymphoblastic leukemia: Prospects for personalized management and prognostic prediction. Frontiers in Immunology, 12, 627764. 10.3389/fimmu.2021.627764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins, P. N. (2013). Molecular biology of atherosclerosis. Physiological Reviews, 93(3), 1317–1542. 10.1152/physrev.00004.2012 [DOI] [PubMed] [Google Scholar]
- Hosomi, N. , Ban, C. R. , Naya, T. , Takahashi, T. , Guo, P. , Song, X. Y. , & Kohno, M. (2005). Tumor necrosis factor‐alpha neutralization reduced cerebral edema through inhibition of matrix metalloproteinase production after transient focal cerebral ischemia. Journal of Cerebral Blood Flow and Metabolism, 25(8), 959–967. 10.1038/sj.jcbfm.9600086 [DOI] [PubMed] [Google Scholar]
- Hu, K. J. , Yin, E. T. S. , Hu, Y. X. , & Huang, H. (2021). Combination of CRISPR/Cas9 system and CAR‐T cell therapy: A new era for refractory and relapsed hematological malignancies. Curr Med Sci, 41(3), 420–430. 10.1007/s11596-021-2391-5 [DOI] [PubMed] [Google Scholar]
- Hu, Y. , Sun, J. , Wu, Z. , Yu, J. , Cui, Q. , Pu, C. , Liang, B. , Luo, Y. , Shi, J. , Jin, A. , Xiao, L. , & Huang, H. (2016). Predominant cerebral cytokine release syndrome in CD19‐directed chimeric antigen receptor‐modified T cell therapy. J Hematol Oncol, 9(1), 70. 10.1186/s13045-016-0299-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huarte, E. , O'Connor, R. S. , Peel, M. T. , Nunez‐Cruz, S. , Leferovich, J. , Juvekar, A. , Yang, Y. O. , Truong, L. , Huang, T. , Naim, A. , Milone, M. C. , & Smith, P. A. (2020). Itacitinib (INCB039110), a JAK1 inhibitor, reduces cytokines associated with cytokine release syndrome induced by CAR T‐cell therapy. Clinical Cancer Research, 26(23), 6299–6309. 10.1158/1078-0432.CCR-20-1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulse, R. E. , Winterfield, J. , Kunkler, P. E. , & Kraig, R. P. (2001). Astrocytic clasmatodendrosis in hippocampal organ culture. Glia, 33(2), 169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter, B. D. , & Jacobson, C. A. (2019). CAR T‐cell associated neurotoxicity: Mechanisms, clinicopathologic correlates, and future directions. Journal of the National Cancer Institute, 111(7), 646–654. 10.1093/jnci/djz017 [DOI] [PubMed] [Google Scholar]
- Hunter, C. A. , & Jones, S. A. (2015). IL‐6 as a keystone cytokine in health and disease. Nature Immunology, 16(5), 448–457. 10.1038/ni.3153 [DOI] [PubMed] [Google Scholar]
- Jiang, W. , Desjardins, P. , & Butterworth, R. F. (2009). Cerebral inflammation contributes to encephalopathy and brain edema in acute liver failure: Protective effect of minocycline. Journal of Neurochemistry, 109(2), 485–493. 10.1111/j.1471-4159.2009.05981.x [DOI] [PubMed] [Google Scholar]
- Jiang, Z. , Liao, R. , Lv, J. , Li, S. , Zheng, D. , Qin, L. , Wu, D. , Chen, S. , Long, Y. , Wu, Q. , Wang, S. , Lin, S. , Huang, X. , Tang, Z. , Shi, P. , Zhou, H. , Liu, Q. , Zhao, R. , Li, Y. , … Li, P. (2021). IL‐6 trans‐signaling promotes the expansion and anti‐tumor activity of CAR T cells. Leukemia, 35(5), 1380–1391. 10.1038/s41375-020-01085-1 [DOI] [PubMed] [Google Scholar]
- Johnson, L. A. , Scholler, J. , Ohkuri, T. , Kosaka, A. , Patel, P. R. , McGettigan, S. E. , Nace, A. K. , Dentchev, T. , Thekkat, P. , Loew, A. , Boesteanu, A. C. , Cogdill, A. P. , Chen, T. , Fraietta, J. A. , Kloss, C. C. , Posey, A. D., Jr. , Engels, B. , Singh, R. , Ezell, T. , … Maus, M. V. (2015). Rational development and characterization of humanized anti‐EGFR variant III chimeric antigen receptor T cells for glioblastoma. Sci Transl Med, 7(275), 275ra222. 10.1126/scitranslmed.aaa4963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, L. , Tang, X. , Zhang, J. , Li, M. , Xu, N. , Qi, W. , Tan, J. , Lou, X. , Yu, Z. , Sun, J. , Wang, Z. , Dai, H. , Chen, J. , Lin, G. , Wu, D. , & Yu, L. (2020). Interleukin‐6‐knockdown of chimeric antigen receptor‐modified T cells significantly reduces IL‐6 release from monocytes. Exp Hematol Oncol, 9, 11. 10.1186/s40164-020-00166-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, S. , & Kishimoto, T. (2021). Interplay between interleukin‐6 signaling and the vascular endothelium in cytokine storms. Experimental & Molecular Medicine, 53(7), 1116–1123. 10.1038/s12276-021-00649-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, S. , Tanaka, T. , Inoue, H. , Ono, C. , Hashimoto, S. , Kioi, Y. , Matsumoto, H. , Matsuura, H. , Matsubara, T. , Shimizu, K. , Ogura, H. , Matsuura, Y. , & Kishimoto, T. (2020). IL‐6 trans‐signaling induces plasminogen activator inhibitor‐1 from vascular endothelial cells in cytokine release syndrome. Proceedings of the National Academy of Sciences, 117(36), 22351–22356. 10.1073/pnas.2010229117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karschnia, P. , Jordan, J. T. , Forst, D. A. , Arrillaga‐Romany, I. C. , Batchelor, T. T. , Baehring, J. M. , Clement, N. F. , Gonzalez Castro, L. N. , Herlopian, A. , Maus, M. V. , Schwaiblmair, M. H. , Soumerai, J. D. , Takvorian, R. W. , Hochberg, E. P. , Barnes, J. A. , Abramson, J. S. , Frigault, M. J. , & Dietrich, J. (2019). Clinical presentation, management, and biomarkers of neurotoxicity after adoptive immunotherapy with CAR T cells. Blood, 133(20), 2212–2221. 10.1182/blood-2018-12-893396 [DOI] [PubMed] [Google Scholar]
- Kershaw, M. H. , Westwood, J. A. , Parker, L. L. , Wang, G. , Eshhar, Z. , Mavroukakis, S. A. , White, D. E. , Wunderlich, J. R. , Canevari, S. , Rogers‐Freezer, L. , Chen, C. C. , Yang, J. C. , Rosenberg, S. A. , & Hwu, P. (2006). A phase I study on adoptive immunotherapy using gene‐modified T cells for ovarian cancer. Clinical Cancer Research, 12(20 Pt 1), 6106–6115. 10.1158/1078-0432.CCR-06-1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoy, K. , Mariotte, D. , Defer, G. , Petit, G. , Toutirais, O. , & Le Mauff, B. (2020). Natalizumab in multiple sclerosis treatment: From biological effects to immune monitoring. Frontiers in Immunology, 11, 549842. 10.3389/fimmu.2020.549842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotch, C. , Barrett, D. , & Teachey, D. T. (2019). Tocilizumab for the treatment of chimeric antigen receptor T cell‐induced cytokine release syndrome. Expert Review of Clinical Immunology, 15(8), 813–822. 10.1080/1744666X.2019.1629904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurniati, N. F. , Jongman, R. M. , vom Hagen, F. , Spokes, K. C. , Moser, J. , Regan, E. R. , Krenning, G. , Moonen, J. R. A. J. , Harmsen, M. C. , Struys, M. M. R. F. , Hammes, H. P. , Zijlstra, J. G. , Aird, W. C. , Heeringa, P. , Molema, G. , & van Meurs, M. (2013). The flow dependency of Tie2 expression in endotoxemia. Intensive Care Med, 39(7), 1262–1271. 10.1007/s00134-013-2899-7 [DOI] [PubMed] [Google Scholar]
- Larson, R. C. , & Maus, M. V. (2021). Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nature Reviews. Cancer, 21(3), 145–161. 10.1038/s41568-020-00323-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- le, R. Q. , Li, L. , Yuan, W. , Shord, S. S. , Nie, L. , Habtemariam, B. A. , Przepiorka, D. , Farrell, A. T. , & Pazdur, R. (2018). FDA approval summary: Tocilizumab for treatment of chimeric antigen receptor T cell‐induced severe or life‐threatening cytokine release syndrome. The Oncologist, 23(8), 943–947. 10.1634/theoncologist.2018-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, D. W. , Santomasso, B. D. , Locke, F. L. , Ghobadi, A. , Turtle, C. J. , Brudno, J. N. , Maus, M. V. , Park, J. H. , Mead, E. , Pavletic, S. , Go, W. Y. , Eldjerou, L. , Gardner, R. A. , Frey, N. , Curran, K. J. , Peggs, K. , Pasquini, M. , DiPersio, J. F. , van den Brink, M. R. M. , … Neelapu, S. S. (2019). ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biology of Blood and Marrow Transplantation, 25(4), 625–638. 10.1016/j.bbmt.2018.12.758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leligdowicz, A. , Richard‐Greenblatt, M. , Wright, J. , Crowley, V. M. , & Kain, K. C. (2018). Endothelial activation: The Ang/Tie axis in sepsis. Frontiers in Immunology, 9, 838. 10.3389/fimmu.2018.00838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Guo, X. , Zhu, Y. , Wei, G. , Zhang, Y. , Li, X. , Xu, H. , Cui, J. , Wu, W. , He, J. , Ritchie, M. E. , Weiskittel, T. M. , Li, H. , Yu, H. , Ding, L. , Shao, M. , Luo, Q. , Xu, X. , Teng, X. , … Hu, Y. (2021). Single‐cell transcriptomic analysis reveals BCMA CAR‐T cell dynamics in a patient with refractory primary plasma cell leukemia. Molecular Therapy, 29(2), 645–657. 10.1016/j.ymthe.2020.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnerbauer, M. , Wheeler, M. A. , & Quintana, F. J. (2020). Astrocyte crosstalk in CNS inflammation. Neuron, 108(4), 608–622. 10.1016/j.neuron.2020.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke, F. L. , Neelapu, S. S. , Bartlett, N. L. , Siddiqi, T. , Chavez, J. C. , Hosing, C. M. , Ghobadi, A. , Budde, L. E. , Bot, A. , Rossi, J. M. , Jiang, Y. , Xue, A. X. , Elias, M. , Aycock, J. , Wiezorek, J. , & Go, W. Y. (2017). Phase 1 results of ZUMA‐1: A multicenter study of KTE‐C19 anti‐CD19 CAR T cell therapy in refractory aggressive lymphoma. Molecular Therapy, 25(1), 285–295. 10.1016/j.ymthe.2016.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn, R. C. , Weber, E. W. , Sotillo, E. , Gennert, D. , Xu, P. , Good, Z. , Anbunathan, H. , Lattin, J. , Jones, R. , Tieu, V. , Nagaraja, S. , Granja, J. , de Bourcy, C. F. A. , Majzner, R. , Satpathy, A. T. , Quake, S. R. , Monje, M. , Chang, H. Y. , & Mackall, C. L. (2019). c‐Jun overexpression in CAR T cells induces exhaustion resistance. Nature, 576(7786), 293–300. 10.1038/s41586-019-1805-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackall, C. L. , & Miklos, D. B. (2017). CNS endothelial cell activation emerges as a driver of CAR T cell‐associated neurotoxicity. Cancer Discovery, 7(12), 1371–1373. 10.1158/2159-8290.CD-17-1084 [DOI] [PubMed] [Google Scholar]
- Maillet, D. , Belin, C. , Moroni, C. , Cuzzubbo, S. , Ursu, R. , Sirven‐Villaros, L. , di Blasi, R. , Thieblemont, C. , & Carpentier, A. F. (2021). Evaluation of mid‐term (6‐12 months) neurotoxicity in B‐cell lymphoma patients treated with CAR T cells: A prospective cohort study. Neuro‐Oncology, 23(9), 1569–1575. 10.1093/neuonc/noab077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandriota, S. J. , & Pepper, M. S. (1998). Regulation of angiopoietin‐2 mRNA levels in bovine microvascular endothelial cells by cytokines and hypoxia. Circulation Research, 83(8), 852–859. 10.1161/01.res.83.8.852 [DOI] [PubMed] [Google Scholar]
- Marcos‐Ramiro, B. , Garcia‐Weber, D. , & Millan, J. (2014). TNF‐induced endothelial barrier disruption: Beyond Actin and rho. Thrombosis and Haemostasis, 112(6), 1088–1102. 10.1160/TH14-04-0299 [DOI] [PubMed] [Google Scholar]
- Marin, V. , Montero‐Julian, F. A. , Gres, S. , Boulay, V. , Bongrand, P. , Farnarier, C. , & Kaplanski, G. (2001). The IL‐6‐soluble IL‐6Ralpha autocrine loop of endothelial activation as an intermediate between acute and chronic inflammation: An experimental model involving thrombin. Journal of Immunology, 167(6), 3435–3442. 10.4049/jimmunol.167.6.3435 [DOI] [PubMed] [Google Scholar]
- Martin‐Antonio, B. (2021). Editorial: Understanding the cytokine release syndrome: Toward improving cancer immunotherapy. Frontiers in Immunology, 12, 666703. 10.3389/fimmu.2021.666703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude, S. L. , Frey, N. , Shaw, P. A. , Aplenc, R. , Barrett, D. M. , Bunin, N. J. , Chew, A. , Gonzalez, V. E. , Zheng, Z. , Lacey, S. F. , Mahnke, Y. D. , Melenhorst, J. J. , Rheingold, S. R. , Shen, A. , Teachey, D. T. , Levine, B. L. , June, C. H. , Porter, D. L. , & Grupp, S. A. (2014). Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med, 371(16), 1507–1517. 10.1056/NEJMoa1407222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude, S. L. , Teachey, D. T. , Porter, D. L. , & Grupp, S. A. (2015). CD19‐targeted chimeric antigen receptor T‐cell therapy for acute lymphoblastic leukemia. Blood, 125(26), 4017–4023. 10.1182/blood-2014-12-580068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maziarz, R. T. , Schuster, S. J. , Romanov, V. V. , Rusch, E. S. , Li, J. , Signorovitch, J. E. , Maloney, D. G. , & Locke, F. L. (2020). Grading of neurological toxicity in patients treated with tisagenlecleucel in the JULIET trial. Blood Advances, 4(7), 1440–1447. 10.1182/bloodadvances.2019001305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestermann, K. , Giavridis, T. , Weber, J. , Rydzek, J. , Frenz, S. , Nerreter, T. , Mades, A. , Sadelain, M. , Einsele, H. , & Hudecek, M. (2019). The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells. Sci Transl Med, 11(499), eaau5907. 10.1126/scitranslmed.aau5907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao, L. , Zhang, Z. , Ren, Z. , & Li, Y. (2021). Reactions related to CAR‐T cell therapy. Frontiers in Immunology, 12, 663201. 10.3389/fimmu.2021.663201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohty, M. , Gautier, J. , Malard, F. , Aljurf, M. , Bazarbachi, A. , Chabannon, C. , Kharfan‐Dabaja, M. A. , Savani, B. N. , Huang, H. , Kenderian, S. , Nagler, A. , & Perales, M. A. (2019). CD19 chimeric antigen receptor‐T cells in B‐cell leukemia and lymphoma: Current status and perspectives. Leukemia, 33(12), 2767–2778. 10.1038/s41375-019-0615-5 [DOI] [PubMed] [Google Scholar]
- Morris, E. C. , Neelapu, S. S. , Giavridis, T. , & Sadelain, M. (2021). Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nature Reviews. Immunology, 22, 85–96. 10.1038/s41577-021-00547-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser, D. M. , & Edwards, J. P. (2008). Exploring the full spectrum of macrophage activation. Nature Reviews. Immunology, 8(12), 958–969. 10.1038/nri2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munji, R. N. , Soung, A. L. , Weiner, G. A. , Sohet, F. , Semple, B. D. , Trivedi, A. , Gimlin, K. , Kotoda, M. , Korai, M. , Aydin, S. , Batugal, A. , Cabangcala, A. C. , Schupp, P. G. , Oldham, M. C. , Hashimoto, T. , Noble‐Haeusslein, L. J. , & Daneman, R. (2019). Profiling the mouse brain endothelial transcriptome in health and disease models reveals a core blood‐brain barrier dysfunction module. Nat Neurosci, 22(11), 1892–1902. 10.1038/s41593-019-0497-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelapu, S. S. , Locke, F. L. , Bartlett, N. L. , Lekakis, L. J. , Miklos, D. B. , Jacobson, C. A. , Braunschweig, I. , Oluwole, O. O. , Siddiqi, T. , Lin, Y. , Timmerman, J. M. , Stiff, P. J. , Friedberg, J. W. , Flinn, I. W. , Goy, A. , Hill, B. T. , Smith, M. R. , Deol, A. , Farooq, U. , … Go, W. Y. (2017). Axicabtagene ciloleucel CAR T‐cell therapy in refractory large B‐cell lymphoma. N Engl J Med, 377(26), 2531–2544. 10.1056/NEJMoa1707447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelapu, S. S. , Tummala, S. , Kebriaei, P. , Wierda, W. , Gutierrez, C. , Locke, F. L. , Komanduri, K. V. , Lin, Y. , Jain, N. , Daver, N. , Westin, J. , Gulbis, A. M. , Loghin, M. E. , de Groot, J. F. , Adkins, S. , Davis, S. E. , Rezvani, K. , Hwu, P. , & Shpall, E. J. (2018). Chimeric antigen receptor T‐cell therapy—Assessment and management of toxicities. Nat Rev Clin Oncol, 15(1), 47–62. 10.1038/nrclinonc.2017.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto, N. , Terao, K. , Mima, T. , Nakahara, H. , Takagi, N. , & Kakehi, T. (2008). Mechanisms and pathologic significances in increase in serum interleukin‐6 (IL‐6) and soluble IL‐6 receptor after administration of an anti‐IL‐6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood, 112(10), 3959–3964. 10.1182/blood-2008-05-155846 [DOI] [PubMed] [Google Scholar]
- Norelli, M. , Camisa, B. , Barbiera, G. , Falcone, L. , Purevdorj, A. , Genua, M. , Sanvito, F. , Ponzoni, M. , Doglioni, C. , Cristofori, P. , Traversari, C. , Bordignon, C. , Ciceri, F. , Ostuni, R. , Bonini, C. , Casucci, M. , & Bondanza, A. (2018). Monocyte‐derived IL‐1 and IL‐6 are differentially required for cytokine‐release syndrome and neurotoxicity due to CAR T cells. Nat Med, 24(6), 739–748. 10.1038/s41591-018-0036-4 [DOI] [PubMed] [Google Scholar]
- Oby, E. , & Janigro, D. (2006). The blood‐brain barrier and epilepsy. Epilepsia, 47(11), 1761–1774. 10.1111/j.1528-1167.2006.00817.x [DOI] [PubMed] [Google Scholar]
- Olah, M. , Menon, V. , Habib, N. , Taga, M. F. , Ma, Y. , Yung, C. J. , Cimpean, M. , Khairallah, A. , Coronas‐Samano, G. , Sankowski, R. , Grün, D. , Kroshilina, A. A. , Dionne, D. , Sarkis, R. A. , Cosgrove, G. R. , Helgager, J. , Golden, J. A. , Pennell, P. B. , Prinz, M. , … de Jager, P. L. (2020). Single cell RNA sequencing of human microglia uncovers a subset associated with Alzheimer's disease. Nat Commun, 11(1), 6129. 10.1038/s41467-020-19737-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke, D. M. , Nasrallah, M. P. , Desai, A. , Melenhorst, J. J. , Mansfield, K. , Morrissette, J. J. D. , Martinez‐Lage, M. , Brem, S. , Maloney, E. , Shen, A. , Isaacs, R. , Mohan, S. , Plesa, G. , Lacey, S. F. , Navenot, J. M. , Zheng, Z. , Levine, B. L. , Okada, H. , June, C. H. , … Maus, M. V. (2017). A single dose of peripherally infused EGFRvIII‐directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med, 9(399), eaaa0984. 10.1126/scitranslmed.aaa0984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottum, P. A. , Arellano, G. , Reyes, L. I. , Iruretagoyena, M. , & Naves, R. (2015). Opposing roles of interferon‐gamma on cells of the central nervous system in autoimmune neuroinflammation. Frontiers in Immunology, 6, 539. 10.3389/fimmu.2015.00539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, J. , Deng, B. , Ling, Z. , Song, W. , Xu, J. , Duan, J. , Wang, Z. , Chang, A. H. , Feng, X. , & Tan, Y. (2021). Ruxolitinib mitigates steroid‐refractory CRS during CAR T therapy. J Cell Mol Med, 25(2), 1089–1099. 10.1111/jcmm.16176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, K. R. , Migliorini, D. , Perkey, E. , Yost, K. E. , Bhaduri, A. , Bagga, P. , Haris, M. , Wilson, N. E. , Liu, F. , Gabunia, K. , Scholler, J. , Montine, T. J. , Bhoj, V. G. , Reddy, R. , Mohan, S. , Maillard, I. , Kriegstein, A. R. , June, C. H. , Chang, H. Y. , … Satpathy, A. T. (2020). Single‐cell analyses identify brain mural cells expressing CD19 as potential off‐tumor targets for CAR‐T immunotherapies. Cell, 183(1), 126–142.e17. 10.1016/j.cell.2020.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszkiewicz, P. J. , Fräßle, S. P. , Srivastava, S. , Sommermeyer, D. , Hudecek, M. , Drexler, I. , Sadelain, M. , Liu, L. , Jensen, M. C. , Riddell, S. R. , & Busch, D. H. (2016). Targeted antibody‐mediated depletion of murine CD19 CAR T cells permanently reverses B cell aplasia. Journal of Clinical Investigation, 126(11), 4262–4272. 10.1172/JCI84813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, A. A. , Zhang, Y. , Fullerton, J. N. , Boelen, L. , Rongvaux, A. , Maini, A. A. , Bigley, V. , Flavell, R. A. , Gilroy, D. W. , Asquith, B. , Macallan, D. , & Yona, S. (2017). The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. Journal of Experimental Medicine, 214(7), 1913–1923. 10.1084/jem.20170355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell, C. A. , Barnum, J. L. , McDonald‐Hyman, C. S. , Panoskaltsis‐Mortari, A. , Riddle, M. J. , Xiong, Z. , Loschi, M. , Thangavelu, G. , Campbell, H. M. , Storlie, M. D. , Refaeli, Y. , Furlan, S. N. , Jensen, M. C. , Kean, L. S. , Miller, J. S. , Tolar, J. , Osborn, M. J. , & Blazar, B. R. (2018). Human CD19‐targeted mouse T cells induce B cell aplasia and toxicity in human CD19 transgenic mice. Molecular Therapy, 26(6), 1423–1434. 10.1016/j.ymthe.2018.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pensato, U. , Muccioli, L. , Cani, I. , Janigro, D. , Zinzani, P. L. , Guarino, M. , Cortelli, P. , & Bisulli, F. (2021). Brain dysfunction in COVID‐19 and CAR‐T therapy: Cytokine storm‐associated encephalopathy. Ann Clin Transl Neurol, 8(4), 968–979. 10.1002/acn3.51348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Profaci, C. P. , Munji, R. N. , Pulido, R. S. , & Daneman, R. (2020). The blood‐brain barrier in health and disease: Important unanswered questions. The Journal of Experimental Medicine, 217(4), e20190062. 10.1084/jem.20190062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rama Rao, K. V. , Jayakumar, A. R. , Tong, X. , Alvarez, V. M. , & Norenberg, M. D. (2010). Marked potentiation of cell swelling by cytokines in ammonia‐sensitized cultured astrocytes. Journal of Neuroinflammation, 7, 66. 10.1186/1742-2094-7-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez, J. , & Canete, J. D. (2018). Anakinra for the treatment of rheumatoid arthritis: A safety evaluation. Expert Opinion on Drug Safety, 17(7), 727–732. 10.1080/14740338.2018.1486819 [DOI] [PubMed] [Google Scholar]
- Redondo‐Munoz, J. , Garcia‐Pardo, A. , & Teixido, J. (2019). Molecular players in hematologic tumor cell trafficking. Frontiers in Immunology, 10, 156. 10.3389/fimmu.2019.00156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice, J. , Nagle, S. , Randall, J. , & Hinson, H. E. (2019). Chimeric antigen receptor T cell‐related neurotoxicity: Mechanisms, clinical presentation, and approach to treatment. Current Treatment Options in Neurology, 21(8), 40. 10.1007/s11940-019-0580-3 [DOI] [PubMed] [Google Scholar]
- Riddell, S. R. (2018). Adrenaline fuels a cytokine storm during immunotherapy. Nature, 564(7735), 194–196. 10.1038/d41586-018-07581-w [DOI] [PubMed] [Google Scholar]
- Riegler, L. L. , Jones, G. P. , & Lee, D. W. (2019). Current approaches in the grading and management of cytokine release syndrome after chimeric antigen receptor T‐cell therapy. Therapeutics and Clinical Risk Management, 15, 323–335. 10.2147/TCRM.S150524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose‐John, S. , Scheller, J. , Elson, G. , & Jones, S. A. (2006). Interleukin‐6 biology is coordinated by membrane‐bound and soluble receptors: Role in inflammation and cancer. Journal of Leukocyte Biology, 80(2), 227–236. 10.1189/jlb.1105674 [DOI] [PubMed] [Google Scholar]
- Rosenberg, G. A. , & Navratil, M. (1997). Metalloproteinase inhibition blocks edema in intracerebral hemorrhage in the rat. Neurology, 48(4), 921–926. 10.1212/wnl.48.4.921 [DOI] [PubMed] [Google Scholar]
- Rubin, D. B. , Danish, H. H. , Ali, A. B. , Li, K. , LaRose, S. , Monk, A. D. , Cote, D. J. , Spendley, L. , Kim, A. H. , Robertson, M. S. , Torre, M. , Smith, T. R. , Izzy, S. , Jacobson, C. A. , Lee, J. W. , & Vaitkevicius, H. (2019). Neurological toxicities associated with chimeric antigen receptor T‐cell therapy. Brain, 142(5), 1334–1348. 10.1093/brain/awz053 [DOI] [PubMed] [Google Scholar]
- Ruella, M. , Kenderian, S. S. , Shestova, O. , Klichinsky, M. , Melenhorst, J. J. , Wasik, M. A. , Lacey, S. F. , June, C. H. , & Gill, S. (2017). Kinase inhibitor ibrutinib to prevent cytokine‐release syndrome after anti‐CD19 chimeric antigen receptor T cells for B‐cell neoplasms. Leukemia, 31(1), 246–248. 10.1038/leu.2016.262 [DOI] [PubMed] [Google Scholar]
- Sachdeva, M. , Duchateau, P. , Depil, S. , Poirot, L. , & Valton, J. (2019). Granulocyte‐macrophage colony‐stimulating factor inactivation in CAR T‐cells prevents monocyte‐dependent release of key cytokine release syndrome mediators. The Journal of Biological Chemistry, 294(14), 5430–5437. 10.1074/jbc.AC119.007558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santomasso, B. D. , Park, J. H. , Salloum, D. , Riviere, I. , Flynn, J. , Mead, E. , Halton, E. , Wang, X. , Senechal, B. , Purdon, T. , Cross, J. R. , Liu, H. , Vachha, B. , Chen, X. , DeAngelis, L. M. , Li, D. , Bernal, Y. , Gonen, M. , Wendel, H. G. , … Brentjens, R. J. (2018). Clinical and biological correlates of neurotoxicity associated with CAR T‐cell therapy in patients with B‐cell acute lymphoblastic leukemia. Cancer Discovery, 8(8), 958–971. 10.1158/2159-8290.CD-17-1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarin, C. , Hinton, D. R. , Valentin‐Torres, A. , Chen, Z. , Trapp, B. D. , Bergmann, C. C. , & Stohlman, S. A. (2015). Astrocyte response to IFN‐gamma limits IL‐6‐mediated microglia activation and progressive autoimmune encephalomyelitis. Journal of Neuroinflammation, 12, 79. 10.1186/s12974-015-0293-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidts, A. , Wehrli, M. , & Maus, M. V. (2021). Toward better understanding and management of CAR‐T cell‐associated toxicity. Annual Review of Medicine, 72, 365–382. 10.1146/annurev-med-061119-015600 [DOI] [PubMed] [Google Scholar]
- Schuster, S. J. , Svoboda, J. , Chong, E. A. , Nasta, S. D. , Mato, A. R. , Anak, O. , Brogdon, J. L. , Pruteanu‐Malinici, I. , Bhoj, V. , Landsburg, D. , Wasik, M. , Levine, B. L. , Lacey, S. F. , Melenhorst, J. J. , Porter, D. L. , & June, C. H. (2017). Chimeric antigen receptor T cells in refractory B‐cell lymphomas. N Engl J Med, 377(26), 2545–2554. 10.1056/NEJMoa1708566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwameis, M. , Schorgenhofer, C. , Assinger, A. , Steiner, M. M. , & Jilma, B. (2015). VWF excess and ADAMTS13 deficiency: A unifying pathomechanism linking inflammation to thrombosis in DIC, malaria, and TTP. Thrombosis and Haemostasis, 113(4), 708–718. 10.1160/TH14-09-0731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepehrinezhad, A. , Zarifkar, A. , Namvar, G. , Shahbazi, A. , & Williams, R. (2020). Astrocyte swelling in hepatic encephalopathy: Molecular perspective of cytotoxic edema. Metabolic Brain Disease, 35(4), 559–578. 10.1007/s11011-020-00549-8 [DOI] [PubMed] [Google Scholar]
- Shah, N. N. , Highfill, S. L. , Shalabi, H. , Yates, B. , Jin, J. , Wolters, P. L. , Ombrello, A. , Steinberg, S. M. , Martin, S. , Delbrook, C. , Hoffman, L. , Little, L. , Ponduri, A. , Qin, H. , Qureshi, H. , Dulau‐Florea, A. , Salem, D. , Wang, H. W. , Yuan, C. , … Fry, T. J. (2020). CD4/CD8 T‐cell selection affects chimeric antigen receptor (CAR) T‐cell potency and toxicity: Updated results from a phase I anti‐CD22 CAR T‐cell trial. JCO, 38(17), 1938–1950. 10.1200/JCO.19.03279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao, M. , Yu, Q. , Teng, X. , Guo, X. , Wei, G. , Xu, H. , Cui, J. , Chang, A. H. , Hu, Y. , & Huang, H. (2021). CRS‐related coagulopathy in BCMA targeted CAR‐T therapy: A retrospective analysis in a phase I/II clinical trial. Bone Marrow Transplant, 56(7), 1642–1650. 10.1038/s41409-021-01226-9 [DOI] [PubMed] [Google Scholar]
- Siegler, E. L. , & Kenderian, S. S. (2020). Neurotoxicity and cytokine release syndrome after chimeric antigen receptor T cell therapy: Insights into mechanisms and novel therapies. Frontiers in Immunology, 11, 1973. 10.3389/fimmu.2020.01973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, N. , Hofmann, T. J. , Gershenson, Z. , Levine, B. L. , Grupp, S. A. , Teachey, D. T. , & Barrett, D. M. (2017). Monocyte lineage‐derived IL‐6 does not affect chimeric antigen receptor T‐cell function. Cytotherapy, 19(7), 867–880. 10.1016/j.jcyt.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirko, S. , Irmler, M. , Gascón, S. , Bek, S. , Schneider, S. , Dimou, L. , Obermann, J. , de Souza Paiva, D. , Poirier, F. , Beckers, J. , Hauck, S. M. , Barde, Y. A. , & Götz, M. (2015). Astrocyte reactivity after brain injury: The role of galectins 1 and 3. Glia, 63(12), 2340–2361. 10.1002/glia.22898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew, M. V. (2015). Astrocyte barriers to neurotoxic inflammation. Nature Reviews. Neuroscience, 16(5), 249–263. 10.1038/nrn3898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew, M. V. (2020). Astrocyte reactivity: Subtypes, states, and functions in CNS innate immunity. Trends in Immunology, 41(9), 758–770. 10.1016/j.it.2020.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonmez, H. E. , Demir, S. , Bilginer, Y. , & Ozen, S. (2018). Anakinra treatment in macrophage activation syndrome: A single center experience and systemic review of literature. Clinical Rheumatology, 37(12), 3329–3335. 10.1007/s10067-018-4095-1 [DOI] [PubMed] [Google Scholar]
- Spear, P. , Barber, A. , Rynda‐Apple, A. , & Sentman, C. L. (2012). Chimeric antigen receptor T cells shape myeloid cell function within the tumor microenvironment through IFN‐gamma and GM‐CSF. Journal of Immunology, 188(12), 6389–6398. 10.4049/jimmunol.1103019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staedtke, V. , Bai, R. Y. , Kim, K. , Darvas, M. , Davila, M. L. , Riggins, G. J. , Rothman, P. B. , Papadopoulos, N. , Kinzler, K. W. , Vogelstein, B. , & Zhou, S. (2018). Disruption of a self‐amplifying catecholamine loop reduces cytokine release syndrome. Nature, 564(7735), 273–277. 10.1038/s41586-018-0774-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner, R. M. , Sakemura, R. , Cox, M. J. , Yang, N. , Khadka, R. H. , Forsman, C. L. , Hansen, M. J. , Jin, F. , Ayasoufi, K. , Hefazi, M. , Schick, K. J. , Walters, D. K. , Ahmed, O. , Chappell, D. , Sahmoud, T. , Durrant, C. , Nevala, W. K. , Patnaik, M. M. , Pease, L. R. , … Kenderian, S. S. (2019). GM‐CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR‐T cell function in xenografts. Blood, 133(7), 697–709. 10.1182/blood-2018-10-881722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strati, P. , Ahmed, S. , Furqan, F. , Fayad, L. E. , Lee, H. J. , Iyer, S. P. , Nair, R. , Nastoupil, L. J. , Parmar, S. , Rodriguez, M. A. , Samaniego, F. , Steiner, R. E. , Wang, M. , Pinnix, C. C. , Horowitz, S. B. , Feng, L. , Sun, R. , Claussen, C. M. , Hawkins, M. C. , … Neelapu, S. S. (2021). Prognostic impact of corticosteroids on efficacy of chimeric antigen receptor T‐cell therapy in large B‐cell lymphoma. Blood, 137(23), 3272–3276. 10.1182/blood.2020008865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strati, P. , Nastoupil, L. J. , Westin, J. , Fayad, L. E. , Ahmed, S. , Fowler, N. H. , Hagemeister, F. B. , Lee, H. J. , Iyer, S. P. , Nair, R. , Parmar, S. , Rodriguez, M. A. , Samaniego, F. , Steiner, R. E. , Wang, M. , Pinnix, C. C. , Adkins, S. , Claussen, C. M. , Martinez, C. S. , … Chi, T. L. (2020). Clinical and radiologic correlates of neurotoxicity after axicabtagene ciloleucel in large B‐cell lymphoma. Blood Advances, 4(16), 3943–3951. 10.1182/bloodadvances.2020002228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtzel, C. (2017). Endothelial cells. Advances in Experimental Medicine and Biology, 1003, 71–91. 10.1007/978-3-319-57613-8_4 [DOI] [PubMed] [Google Scholar]
- Tallantyre, E. C. , Evans, N. A. , Parry‐Jones, J. , Morgan, M. P. G. , Jones, C. H. , & Ingram, W. (2021). Neurological updates: Neurological complications of CAR‐T therapy. Journal of Neurology, 268(4), 1544–1554. 10.1007/s00415-020-10237-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, T. , Narazaki, M. , & Kishimoto, T. (2016). Immunotherapeutic implications of IL‐6 blockade for cytokine storm. Immunotherapy, 8(8), 959–970. 10.2217/imt-2016-0020 [DOI] [PubMed] [Google Scholar]
- Taraseviciute, A. , Tkachev, V. , Ponce, R. , Turtle, C. J. , Snyder, J. M. , Liggitt, H. D. , Myerson, D. , Gonzalez‐Cuyar, L. , Baldessari, A. , English, C. , Yu, A. , Zheng, H. , Furlan, S. N. , Hunt, D. J. , Hoglund, V. , Finney, O. , Brakke, H. , Blazar, B. R. , Berger, C. , … Jensen, M. C. (2018). Chimeric antigen receptor T cell‐mediated neurotoxicity in nonhuman primates. Cancer Discovery, 8(6), 750–763. 10.1158/2159-8290.CD-17-1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teachey, D. T. , Lacey, S. F. , Shaw, P. A. , Melenhorst, J. J. , Maude, S. L. , Frey, N. , Pequignot, E. , Gonzalez, V. E. , Chen, F. , Finklestein, J. , Barrett, D. M. , Weiss, S. L. , Fitzgerald, J. C. , Berg, R. A. , Aplenc, R. , Callahan, C. , Rheingold, S. R. , Zheng, Z. , Rose‐John, S. , … Grupp, S. A. (2016). Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T‐cell therapy for acute lymphoblastic leukemia. Cancer Discovery, 6(6), 664–679. 10.1158/2159-8290.CD-16-0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, L. , Ma, L. , Kaarela, T. , & Li, Z. (2012). Neuroimmune crosstalk in the central nervous system and its significance for neurological diseases. Journal of Neuroinflammation, 9, 155. 10.1186/1742-2094-9-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermans, S. , Souffriau, J. , & Libert, C. (2019). A general introduction to glucocorticoid biology. Frontiers in Immunology, 10, 1545. 10.3389/fimmu.2019.01545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyama, M. , & Mabuchi, T. (2020). New treatment addressing the pathogenesis of psoriasis. International Journal of Molecular Sciences, 21(20), 7488. 10.3390/ijms21207488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre, M. , Solomon, I. H. , Sutherland, C. L. , Nikiforow, S. , DeAngelo, D. J. , Stone, R. M. , Vaitkevicius, H. , Galinsky, I. A. , Padera, R. F. , Trede, N. , & Santagata, S. (2018). Neuropathology of a case with fatal CAR T‐cell‐associated cerebral edema. Journal of Neuropathology & Experimental Neurology, 77(10), 877–882. 10.1093/jnen/nly064 [DOI] [PubMed] [Google Scholar]
- Turtle, C. J. , Hanafi, L. A. , Berger, C. , Gooley, T. A. , Cherian, S. , Hudecek, M. , Sommermeyer, D. , Melville, K. , Pender, B. , Budiarto, T. M. , Robinson, E. , Steevens, N. N. , Chaney, C. , Soma, L. , Chen, X. , Yeung, C. , Wood, B. , Li, D. , Cao, J. , … Maloney, D. G. (2016). CD19 CAR‐T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. Journal of Clinical Investigation, 126(6), 2123–2138. 10.1172/JCI85309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vastert, S. J. , Jamilloux, Y. , Quartier, P. , Ohlman, S. , Osterling Koskinen, L. , Kullenberg, T. , Franck‐Larsson, K. , Fautrel, B. , & de Benedetti, F. (2019). Anakinra in children and adults with Still's disease. Rheumatology, 58(Supplement_6), vi9–vi22. 10.1093/rheumatology/kez350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Hu, Y. , Yang, S. , Wei, G. , Zhao, X. , Wu, W. , Zhang, Y. , Zhang, Y. , Chen, D. , Wu, Z. , Xiao, L. , Chang, A. H. , Huang, H. , & Zhao, K. (2019). Role of fluorodeoxyglucose positron emission tomography/computed tomography in predicting the adverse effects of chimeric antigen receptor T cell therapy in patients with non‐Hodgkin lymphoma. Biology of Blood and Marrow Transplantation, 25(6), 1092–1098. 10.1016/j.bbmt.2019.02.008 [DOI] [PubMed] [Google Scholar]
- Wang, X. , Huynh, C. , Urak, R. , Weng, L. , Walter, M. , Lim, L. , Vyas, V. , Chang, W. C. , Aguilar, B. , Brito, A. , Sarkissian, A. , Bandara, N. A. , Yang, L. , Wang, J. , Wu, X. , Zhang, J. , Priceman, S. J. , Qin, H. , Kwak, L. W. , … Forman, S. J. (2021). The cerebroventricular environment modifies CAR T cells for potent activity against both central nervous system and systemic lymphoma. Cancer Immunology Research, 9(1), 75–88. 10.1158/2326-6066.CIR-20-0236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Yang, Y. , Hong, R. , Zhao, H. , Wei, G. , Wu, W. , Xu, H. , Cui, J. , Zhang, Y. , Chang, A. H. , Hu, Y. , & Huang, H. (2020). A retrospective comparison of CD19 single and CD19/CD22 bispecific targeted chimeric antigen receptor T cell therapy in patients with relapsed/refractory acute lymphoblastic leukemia. Blood Cancer J, 10(10), 105. 10.1038/s41408-020-00371-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendeln, A. C. , Degenhardt, K. , Kaurani, L. , Gertig, M. , Ulas, T. , Jain, G. , Wagner, J. , Häsler, L. M. , Wild, K. , Skodras, A. , Blank, T. , Staszewski, O. , Datta, M. , Centeno, T. P. , Capece, V. , Islam, M. R. , Kerimoglu, C. , Staufenbiel, M. , Schultze, J. L. , … Neher, J. J. (2018). Innate immune memory in the brain shapes neurological disease hallmarks. Nature, 556(7701), 332–338. 10.1038/s41586-018-0023-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzenbichler, B. , Westermann, D. , Knueppel, S. , Schultheiss, H. P. , & Tschope, C. (2005). Protective role of angiopoietin‐1 in endotoxic shock. Circulation, 111(1), 97–105. 10.1161/01.CIR.0000151287.08202.8E [DOI] [PubMed] [Google Scholar]
- Wolf, S. A. , Boddeke, H. W. , & Kettenmann, H. (2017). Microglia in physiology and disease. Annual Review of Physiology, 79, 619–643. 10.1146/annurev-physiol-022516-034406 [DOI] [PubMed] [Google Scholar]
- Xue, Q. , Bettini, E. , Paczkowski, P. , Ng, C. , Kaiser, A. , McConnell, T. , Kodrasi, O. , Quigley, M. F. , Heath, J. , Fan, R. , Mackay, S. , Dudley, M. E. , Kassim, S. H. , & Zhou, J. (2017). Single‐cell multiplexed cytokine profiling of CD19 CAR‐T cells reveals a diverse landscape of polyfunctional antigen‐specific response. j immunotherapy cancer, 5(1), 85. 10.1186/s40425-017-0293-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, H. , Price, T. T. , Cantelli, G. , Ngo, B. , Warner, M. J. , Olivere, L. , Ridge, S. M. , Jablonski, E. M. , Therrien, J. , Tannheimer, S. , McCall, C. M. , Chenn, A. , & Sipkins, D. A. (2018). Leukaemia hijacks a neural mechanism to invade the central nervous system. Nature, 560(7716), 55–60. 10.1038/s41586-018-0342-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying, Z. , Huang, X. F. , Xiang, X. , Liu, Y. , Kang, X. , Song, Y. , Guo, X. , Liu, H. , Ding, N. , Zhang, T. , Duan, P. , Lin, Y. , Zheng, W. , Wang, X. , Lin, N. , Tu, M. , Xie, Y. , Zhang, C. , Liu, W. , … Chen, S. Y. (2019). A safe and potent anti‐CD19 CAR T cell therapy. Nat Med, 25(6), 947–953. 10.1038/s41591-019-0421-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegeye, M. M. , Lindkvist, M. , Fälker, K. , Kumawat, A. K. , Paramel, G. , Grenegård, M. , Sirsjö, A. , & Ljungberg, L. U. (2018). Activation of the JAK/STAT3 and PI3K/AKT pathways are crucial for IL‐6 trans‐signaling‐mediated pro‐inflammatory response in human vascular endothelial cells. Cell Commun Signal, 16(1), 55. 10.1186/s12964-018-0268-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, J. , Ru, W. , Adolacion, J. R. , Spurgat, M. S. , Liu, X. , Yuan, S. , Liang, R. X. , Dong, J. , Potter, A. S. , Potter, S. S. , Chen, K. , Chen, R. , Varadarajan, N. , & Tang, S. J. (2021). Single‐cell RNA‐seq analysis reveals compartment‐specific heterogeneity and plasticity of microglia. iScience, 24(3), 102186. 10.1016/j.isci.2021.102186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, F. , Wei, G. , Liu, Y. , Zhou, H. , Wu, W. , Yang, L. , Huang H., Hu, Y. (2021). Incidence and risk factors associated with infection after chimeric antigen receptor T cell therapy for relapsed/refractory B‐cell malignancies. Cell Transplant, 30, 09636897211025503. 10.1177/09636897211025503 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


