Abstract
Molecularly-targeted agents have improved outcomes for a subset of patients with BRAF-mutated melanoma, but treatment of resistant and BRAF wild-type tumors remains a challenge. The MERTK receptor tyrosine kinase is aberrantly expressed in melanoma and can contribute to oncogenic phenotypes. Here we report the effect of treatment with a MERTK-selective small molecule inhibitor, UNC2025, in preclinical models of melanoma. In melanoma cell lines, treatment with UNC2025 potently inhibited phosphorylation of MERTK and downstream signaling, induced cell death, and decreased colony formation. In patient-derived melanoma xenograft models, treatment with UNC2025 blocked or significantly reduced tumor growth. Importantly, UNC2025 had similar biochemical and functional effects in both BRAF-mutated and BRAF wild-type models and irrespective of NRAS mutational status, implicating MERTK inhibition as a potential therapeutic strategy in tumors that are not amenable to BRAF-targeting and for which there are limited treatment options. In BRAF-mutated cell lines, combined treatment with UNC2025 and the BRAF inhibitor vemurafenib provided effective inhibition of oncogenic signaling through ERK, AKT, and STAT6, increased induction of cell death, and decreased colony forming potential. Similarly, in NRAS-mutated cell lines, addition of UNC2025 to cobimetinib therapy increased cell death and decreased colony-forming potential. In a BRAF-mutated patient-derived xenograft, treatment with combined UNC2025 and vemurafenib was well-tolerated and significantly decreased tumor growth compared to vemurafenib alone. These data support the use of UNC2025 for treatment of melanoma, irrespective of BRAF or NRAS mutational status, and suggest a role for MERTK and targeted combination therapy in BRAF and NRAS-mutated melanoma.
Keywords: MERTK, Melanoma, Small Molecule Inhibitor, Targeted Therapy, BRAF, Receptor Tyrosine Kinase
INTRODUCTION
Metastatic melanoma remains one of the most aggressive forms of cutaneous cancer, accounting for the majority of deaths from skin cancers. Current treatments for advanced disease have significant side effects, are effective in only a subset of individuals, and can lead to resistance. Poor effectiveness of melanoma treatments is likely due to the complex and heterogeneous etiology of melanoma, which can be attributed to activating mutations, such as those in BRAF and NRAS (50% and 20% of melanomas, respectively (1, 2)), or to differential expression of molecules affecting oncogenic intracellular signaling pathways, such as receptor tyrosine kinases (RTKs). Molecularly targeted agents, such as ipilimumab (anti-CTLA-4 antibody) and vemurafenib (mutant BRAF inhibitor) have been FDA-approved for treatment of metastatic melanoma for several years (3–5). However, these therapies are not effective in all patients. In the case of vemurafenib, 20% of BRAF-mutated melanomas do not respond to treatment (6) and even those that initially respond often develop resistance (7–9). Mutated NRAS is the second most common oncogenic driver in melanoma and the MEK inhibitor binimetinib has had clinical efficacy in this setting, but improvements in progression-free survival over standard chemotherapy have been limited to only 1–2 months (10). Combined treatment with BRAF and MEK inhibitors, such as vemurafenib and cobimetinib, delayed onset of resistance compared to BRAF inhibition alone (11), leading to improved clinical responses and prolonged survival (12). Newer agents include the immune checkpoint inhibitors, nivolumab and pembrolizumab (anti-PD-1 monoclonal antibodies), which are FDA-approved for up-front treatment of unresectable or metastatic melanoma. However, despite the superiority of these agents over previous regimens, median progression-free survival remains 6 months or less and approximately 10% of patients suffer grade 3 or higher immune-related adverse effects (13), underscoring the need for development of new therapeutic targets.
Expression and activation of TAM (TYRO3, AXL and MERTK) family receptor tyrosine kinases (RTKs) has been reported in many human cancers where they contribute to chemoresistance, cell survival, migration and invasion, and angiogenesis (14, 15), making them attractive targets for cancer therapies. MERTK is abnormally expressed in hematopoietic malignancies and numerous solid tumors (16–20) where it regulates intracellular pro-survival and anti-apoptotic signaling pathways that are aberrantly activated in cancer cells, including JAK/STAT, ERK, and PI3K/AKT. MERTK also mediates an anti-inflammatory response in the tumor microenvironment (21) and enhances tumor cell migration and invasion (14, 15, 19, 22).
TAM kinases are overexpressed in melanoma (23–28). In a phosphoproteomic screen, TAM family members were the most consistently activated RTKs in early passage melanoma cultures, and shRNA-mediated knockdown of AXL led to decreased proliferation and migration (23). Similarly, TYRO3 was identified as a regulator of the critical melanocyte transcription factor MITF and TYRO3 knock-down inhibited tumor formation in murine melanoma models (26). Our laboratory and others have previously demonstrated an oncogenic role for MERTK in melanoma. MERTK was overexpressed in 58% of primary melanoma cell cultures (28) and approximately 50% of melanoma cell lines (25). Moreover, its expression correlated with disease progression, implicating MERTK in development of metastatic disease (25, 28). While the mechanisms underlying MERTK activation in melanoma have not been fully elucidated, melanoma cell lines express and secrete the MERTK ligand GAS6 (24), suggesting autocrine stimulation of MERTK. In both BRAF-mutated and wild-type melanoma cell lines, MERTK inhibition by shRNA decreased signaling through pro-survival pathways STAT6, AKT, and ERK, and decreased colony forming potential greater than two-fold (25). Similarly, expression of MERTK was associated with increased cell expansion in early passage patient-derived melanoma cell cultures and shRNA-mediated MERTK inhibition reduced signaling through AKT, mTOR, and P70S6 kinase, decreased cell expansion, induced apoptosis and decreased clonogenic colony-formation (28). In these studies, expression of constitutively active AKT was sufficient to reverse the apoptotic and proliferative phenotypes associated with MERTK inhibition, implicating AKT as a critical downstream mediator of MERTK signaling in this context. Additionally, in a BRAF wild-type melanoma xenograft model, shRNA-mediated MERTK inhibition delayed tumor progression (25). Importantly, MERTK expression did not correlate with oncogenic mutations in BRAF and NRAS (25, 28) indicating its potential as a target across biologic subsets. Furthermore, MERTK inhibition could be an effective therapeutic option for patients whose tumors have no known oncogenic driver and who currently have limited treatment options.
Since two of the signaling pathways downstream of MERTK, MAPK/ERK and PI3K/AKT, are frequently dysregulated in melanoma (2) and are involved in primary and secondary resistance to BRAF inhibitors (7–9), we hypothesize that targeting MERTK could provide a novel therapeutic advantage since it would disrupt multiple downstream effectors that mark a site of RTK pathway convergence and are involved in tumorigenesis (29, 30). Indeed, recent data demonstrated upregulation of both MERTK and its ligand, PROS1, in melanoma cells after treatment with an early generation BRAF inhibitor (31). In addition, tumor progression was more effectively inhibited in response to treatment with vemurafenib in melanoma xenografts with shRNA-mediated MERTK knockdown compared to controls, implicating MERTK as an attractive target for combination therapy. We previously reported UNC2025, a novel tyrosine kinase inhibitor (TKI) that is highly potent against MERTK, with an IC50 of 2.7 nmol/L in cell based assays, and has >15-fold selectivity for MERTK over the other TAM family members (32). UNC2025 is orally-bioavailable and has efficacy in murine models of non-small cell lung cancer (16), acute lymphoblastic leukemia and acute myeloid leukemia (33), making it an ideal candidate for translational studies.
Here, we report that inhibition of MERTK activity via UNC2025 downregulates survivin and other oncogenic signaling pathways, induces cell death, and suppresses colony formation in wild-type and BRAF or NRAS mutant melanoma cell lines models, and delays tumor progression in melanoma patient-derived xenografts (PDX). In addition, MERTK inhibition enhanced the efficacy of vemurafenib and cobimetinib therapy in BRAF and NRAS mutant models, respectively. Together these data validate MERTK as a versatile and important target in melanomas with and without activating BRAF or NRAS mutations, particularly in combination with other therapies currently in clinical use.
MATERIALS AND METHODS
Cell culture and reagents
HMCB, MB2141, MB2204, and MB2724 were propagated in RPMI medium and G361, A101D, SK-MEL-2, SK-MEL-5, SK-MEL-119, and MeWo in DMEM. Media were supplemented with 10% or 20% (SK-MEL-5 and SK-MEL-119) fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 ug/ml). Cell lines were verified by short tandem repeat analysis and BRAF and NRAS mutation status was determined using the COSMIC database (34, 35). MB2141, MB2204, and MB2724 were derived as previously described (36). Cell lines were tested for Mycoplasma contamination using a PCR-based kit (ATCC) and confirmed negative within 20 passages or 3 months before use. UNC2025 was synthesized as previously described (32). Vemurafenib was from LC Labs (Woburn, MA) and cobimetinib from MedChem Express (Monmouth Junction, NJ). For in vitro studies, UNC2025 (3 mM), cobimetinib (3 mM) and vemurafenib (10 mM) stock solutions were prepared in DMSO. Adherent cells were collected using trypsin-EDTA (Invitrogen) or 0.02% EDTA in PBS and/or mechanical dissociation with a cell scraper.
Signaling assay
Sub-confluent cultures were incubated in serum-free medium for two hours and in the presence of UNC2025, vemurafenib, or DMSO vehicle for an additional 90 minutes prior to stimulation with 200 nM recombinant human GAS6 (R&D Systems) for 10 minutes. Alternatively, cultures were treated with inhibitors or vehicle control for 72 hours. For detection of phosphorylated MERTK, cultures were treated with freshly prepared pervanadate (0.12 mmol/L Na3VO4 in 0.002% H2O2 in PBS) for 3 minutes as previously described (16).
Immunoblotting Analysis
Lysates were prepared in 50 nM HEPES pH 7.5, 150 mM NaCl, 10 mM EDTA, 10% glycerol, and 1% Triton X-100, supplemented with phosphatase inhibitors (1 mM Na3VO4, 0.1 mM Na2MoO4) and protease inhibitors (Roche Complete Mini). Immunoblotting was performed as previously described (19) using the following primary antibodies: pAKT (S473, Cat# 9271), AKT (Cat# 9272), pERK1/2 (T202/Y204, Cat# 9106), ERK1/2 (Cat# 9102), SURVIVIN (Cat# 2808), and TYRO3 (Cat# 5585) from Cell Signaling; pMERTK (Y749, Y753, Y754, PhosphoSolutions), MERTK (Abcam ab52968), AXL (R&D Systems, AF154), and ACTIN (Santa Cruz sc-1616). Proteins were detected by enhanced chemiluminescence using horseradish peroxidase (HRP)-conjugated goat anti-rabbit (Bio-Rad 170–6515), goat anti-mouse (Bio-Rad 170–6516), and donkey anti-goat (Santa Cruz sc-2020) secondary antibodies and enhanced chemiluminescence.
Apoptosis assay
Cell lines were treated with UNC2025, vemurafenib, cobimetinib and/or DMSO vehicle control for 48 hours and then stained with PO-PRO™−1 iodide (Invitrogen) and 7-AAD (7-amino-actinomycin D, eBioscience) dyes per manufacturer’s instructions. Uptake of dyes was assessed by flow cytometry using a CyAn or Cytoflex analyzer (Beckman Coulter, Brea, CA) and FlowJo software (FlowJo, LLC, Ashland, OR).
Clonogenic colony formation assay
Cells (500–700 per well) were cultured in medium with inhibitors or DMSO vehicle control for 10 days. Media and inhibitors were replaced on day 5. Cells were stained with 0.5% crystal violet in 25% methanol (Fisher) and colonies were enumerated with a GelCount colony counter (Oxford Optronix, Abington, UK).
MTT assay
Cells (1×104/well) were cultured in triplicate in medium with UNC2025 or DMSO vehicle for 44 hours, then stained with 1/10 volume thiazolyl blue tetrazolium bromide (MTT) for an additional 4 hours before addition of an equal volume of 10% sodium dodecyl sulfate in 0.01 M hydrochloric acid. Absorbance at 590nM was detected the next day using a Synergy 2 plate reader (BioTek, Winooski, VT) and normalized to the mean intensity in vehicle-treated samples.
Patient-derived xenograft models
PDX models were generated as previously described (37, 38). Mice with established tumors were randomized to groups and treated with UNC2025 twice daily, vemurafenib once daily, UNC2025 and vemurafinib combined, or 10 mL/kg vehicle. UNC2025 was administered in saline and vemurafenib and combination therapy were administered in 0.04% DMSO in saline. All treatments were administered by oral gavage. Experiments involving animals were approved by the University of Colorado Institutional Animal Care and Use Committee.
Statistical analysis
Statistical analyses were performed using Prism 5 software (GraphPad Software, Inc.). One-way ANOVA with Bonferroni’s correction was used to assess differences in cell culture models and two-way ANOVA with Bonferroni’s correction was used to compare tumor volumes in xenograft studies. Mean values and standard errors are shown.
RESULTS
UNC2025 inhibits activation of MERTK in BRAF and NRAS mutated melanoma cell lines
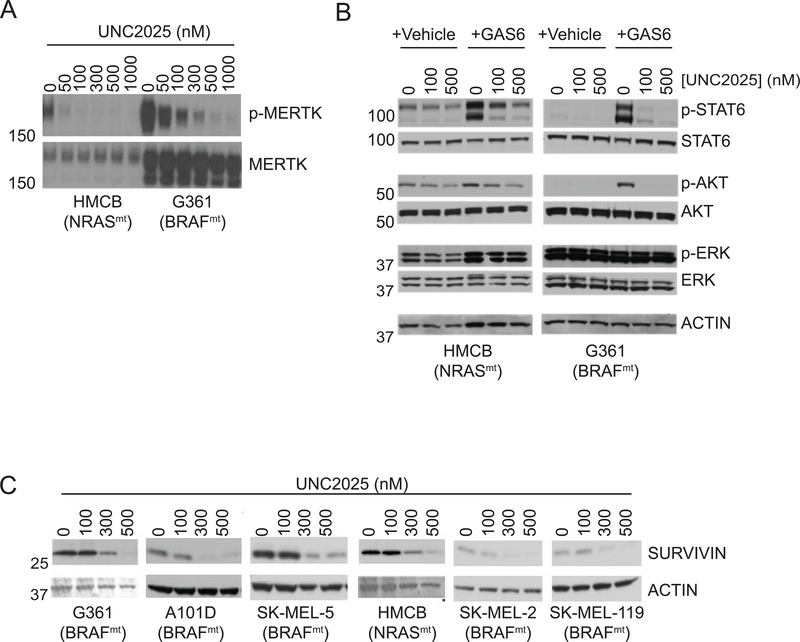
Phosphorylated MERTK levels were determined in melanoma cell lines as a marker of MERTK activation. Treatment with UNC2025 resulted in a dose-dependent decrease in phosphorylated MERTK in both BRAF wild-type, NRAS-mutated (HMCB - BRAFwt, NRASmt) and BRAF-mutated, NRAS wild-type (G361 - BRAFmt, NRASwt) cell lines (Fig. 1A). In the HMCB cell line, near-complete inhibition of MERTK activation was observed in response to treatment with 300 nM UNC2025. The G361 cell line expressed higher levels of both total and phosphorylated MERTK, but inhibition of MERTK activation was still nearly complete in cultures treated with 1000 nM UNC2025. These data demonstrate potent inhibition of MERTK activity mediated by UNC2025 in both BRAFmt and NRASmt cell lines.
Figure 1: UNC2025 inhibits MERTK activation and downstream signaling in melanoma cell lines.
A) Cultures of the indicated cell lines were treated with UNC2025 for 90 minutes, and then with pervanadate phosphatase inhibitor for 3 minutes. MERTK was immunoprecipitated from cell lysates, and phosphorylated and total MERTK proteins were detected by immunoblot. B) Serum starved cultures of the indicated cell lines were treated with UNC2025 for 90 min and then stimulated with 200 nM GAS6 ligand or an equivalent volume of vehicle for an additional 10 min. Cell lysates were prepared and the indicated phosphorylated (denoted by p-) and total proteins were detected by immunoblot. ACTIN is shown as a loading control. C) The indicated cell lines were treated with UNC2025 for 72 h. Cell lysates were prepared and SURVIVIN was detected by immunoblot. Data are representative of at least 3 independent experiments.
Treatment with UNC2025 inhibits oncogenic signaling downstream of MERTK
MERTK signals through many pathways known to play roles in oncogenesis, including the PI3K/AKT, MAPK, and STAT pathways (39). To assess the effects of UNC2025 on signaling pathways downstream of MERTK in melanoma cells, levels of phosphorylated and total STAT6, AKT, and ERK were determined. Serum starved cells were stimulated with GAS6, a TAM kinase ligand, to allow assessment of protein phosphorylation downstream of MERTK (Fig. 1B). Alternatively, cells cultured in normal growth media were treated with UNC2025 in the absence of GAS6 stimulation (Supplemental Fig. 1). Phosphorylation of STAT6 and AKT was induced in response to GAS6 stimulation in both the HMCB and G361 cells and phosphorylated ERK levels were also increased in the HMCB cell line. Conversely, treatment with UNC2025 decreased both basal and GAS6-depedendent phosphorylation of STAT6, AKT and ERK at doses consistent with those required for MERTK inhibition. As expected, total protein levels were not affected by treatment with UNC2025. Of note, MERTK inhibition did not change ERK phosphorylation in the G361 cell line, likely due to constitutive activation downstream of activated BRAF. Previous studies also implicated the anti-apoptotic protein survivin downstream of MERTK (16, 19). Consistent with these data, survivin expression was decreased in all melanoma cell lines evaluated and was almost completely inhibited in response to higher doses of 500 nM (Fig. 1C). Taken together, these data demonstrate effective inhibition of MERTK and downstream oncogenic signaling in melanoma cells irrespective of oncogenic mutation status.
UNC2025 decreases colony formation and cell density in BRAFmt and NRASmt melanoma cell lines
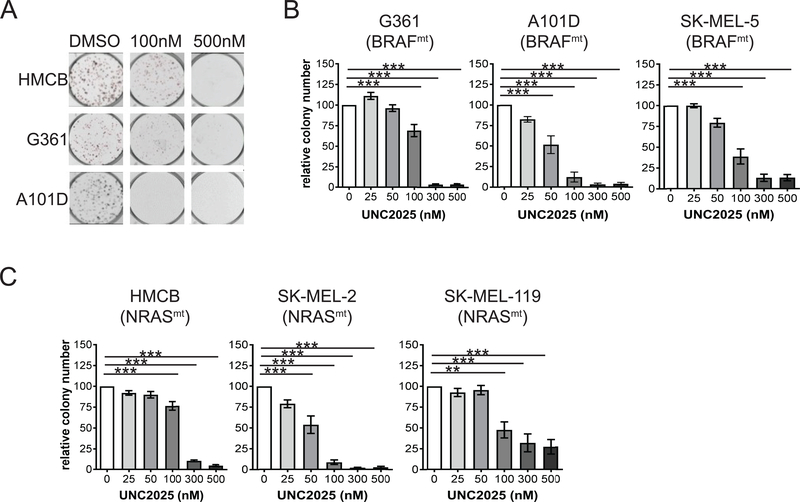
To determine the functional effects of UNC2025-mediated MERTK inhibition, colony-forming potential was assessed in a panel of melanoma cell lines with different oncogenic mutations, including BRAFmt NRASwt (G361, A101D, SK-MEL5) and BRAFwt NRASmt (HMCB, SK-MEL-2, SK-MEL-119). In all cell lines there was a statistically significant decrease in colony formation in response to treatment with 100 nM UNC2025 (Fig 2. and Supplemental Fig. 2), consistent with the doses that mediated robust biochemical changes (Fig. 1). Inhibition of colony-formation was dose-dependent, with higher doses of 300–500 nM mediating near complete inhibition in most cell lines. The A101D (BRAFmt, NRASwt) cell line was particularly sensitive, and exhibited a significant decrease in colony number in response to treatment with UNC2025 at concentrations as low as 50 nM (Fig. 2B). When cultured at higher density, expansion of BRAFmt (G361) and NRASmt (HMCB) cell lines was significantly inhibited by treatment with 100–200nM UNC2025; however, melanoma cells persisted even after treatment with very high doses of UNC2025 (Supplemental Fig. 3). All of the cell lines in the panel expressed MERTK protein to varying degrees (Supplemental Fig. 4). The level of MERTK expression did not predict sensitivity to MERTK inhibition.
Figure 2: UNC2025 reduces colony-forming potential in BRAFmt and NRASmt melanoma cell lines.
Melanoma cell lines were cultured at low density and treated with UNC2025 for 10 days, then colonies were stained with crystal violet and enumerated. A) Images from a representative experiment. B) Colony number in BRAFmt G361, A101D, and SK-MEL-5 cell lines relative to cultures treated with vehicle (0 = DMSO), n = 4–7 independent experiments. C) Relative colony number in NRASmt cell lines HMCB, SK-MEL-2, and SK-MEL-119, n = 3–5 independent experiments. Mean values and standard errors are shown. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, one-way ANOVA.
UNC2025 induces melanoma cell death
To determine whether treatment with UNC2025 led to changes in melanoma cell survival, melanoma cell lines were treated with UNC2025 then stained with PO-PRO®−1-iodide (PO-PRO) and 7-AAD and early apoptotic (PO-PRO+, 7-AAD negative) and dead (7-AAD+) cells were detected by flow cytometry. In the BRAFmt G361 cell line treatment with UNC2025 resulted in a dose-dependent and statistically significant increase in the fraction of apoptotic and dead cells (Supplemental Fig. 5). In the BRAFwt HMCB cell line treatment with UNC2025 also induced cell death, however this effect was not statistically significant. In both cell lines, induction of cell death was observed in response to treatment with UNC2025 at doses of 300 nM or greater, consistent with the doses required to impact survivin levels (Fig. 1C).
UNC2025 inhibits oncogenic signaling and colony formation in BRAF/NRAS wild-type melanoma cell lines
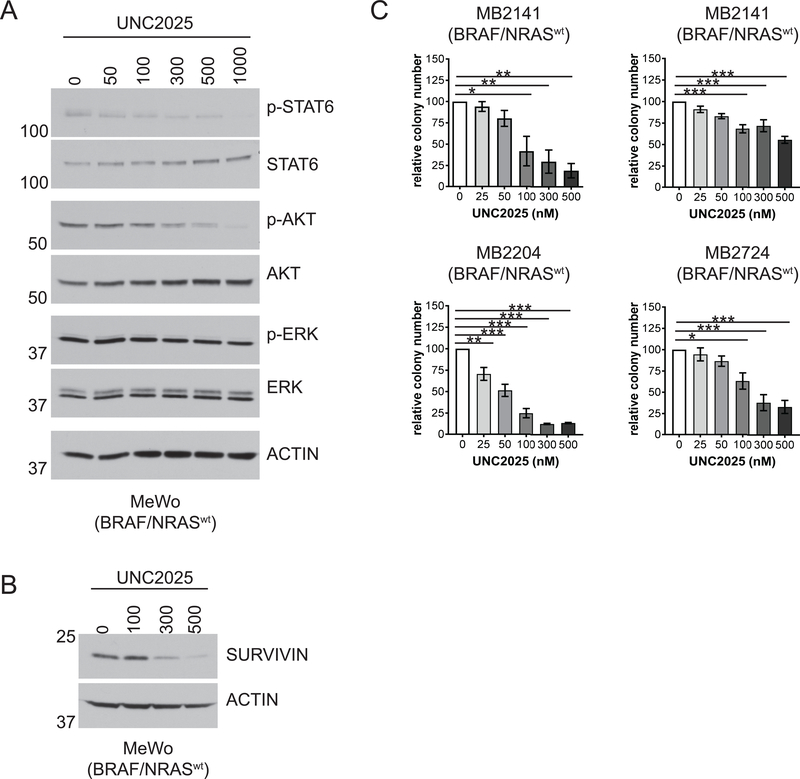
To determine whether pharmacologic MERTK inhibition would decrease activation of oncogenic signaling pathways in melanomas without BRAF or NRAS mutations, the BRAFwt/NRASwt MeWo cell line was treated with UNC2025 and levels of phosphorylated and total STAT6, AKT, and ERK were assessed. Activation of both STAT6 and AKT was inhibited in cells treated with 100–300nM UNC2025, with near complete abrogation of phosphorylation by 1000nM (Fig. 3A). Interestingly, treatment with UNC2025 had no effect on ERK phosphorylation, similar to findings in the BRAFmt cell line G361 (Fig. 1B). Survivin levels were also reduced in MeWo cells after 72 hours of treatment with 100 nM UNC2025 (Fig. 3B), consistent with our findings in BRAF or NRAS mutated cells. The impact of treatment with UNC2025 on colony-formation was assessed in a panel of wild-type cell lines, including MeWo and three patient-derived cell lines, MB2141, MB2204, and MB2724. Colony-forming potential was significantly reduced in three of the four cell lines in response to treatment with 50–300nM UNC2025 (Fig. 3C), similar to the doses that inhibited colony-formation in BRAF or NRAS mutated cell lines (Fig. 2). Although there was significant inhibition of colony-forming potential in the MB2141 cell line, a higher dose of 500nM was required indicating relatively less sensitivity to MERTK inhibition. Melanoma cell expansion was also inhibited in higher density cultures of three BRAF/NRAS wild-type cell lines evaluated, although again the impact of treatment with UNC2025 was less substantial than observed in lower density cultures (Supplemental Fig. 3).
Figure 3: UNC2025 inhibits oncogenic signaling and reduces colony-forming potential in BRAFwt/NRASwt melanoma cell lines.
A-B) MeWo BRAFwt/NRASwt cell cultures were treated with the indicated concentrations of UNC2025 for 90 minutes (A) or 72 hours (B). Cell lysates were prepared and the indicated phosphorylated (denoted by p-) and total proteins were detected by immunoblot. C) BRAFwt/NRASwt cell lines MeWo, MB2141, MB2204, and MB2724 were cultured in triplicate at low density and treated with the indicated concentrations of UNC2025 for 10 days. Colonies were stained with crystal violet and enumerated. Colony numbers relative to vehicle-treated (DMSO) cultures are shown. n=3–4 independent experiments, *p<0.05, **p<0.01, ***p<0.001, one-way ANOVA.
Combined treatment of BRAFmt or NRASmt melanoma cells with UNC2025 and BRAF or MEK inhibitors enhances inhibition of pro-survival signaling, cell survival, and colony formation compared to single agents
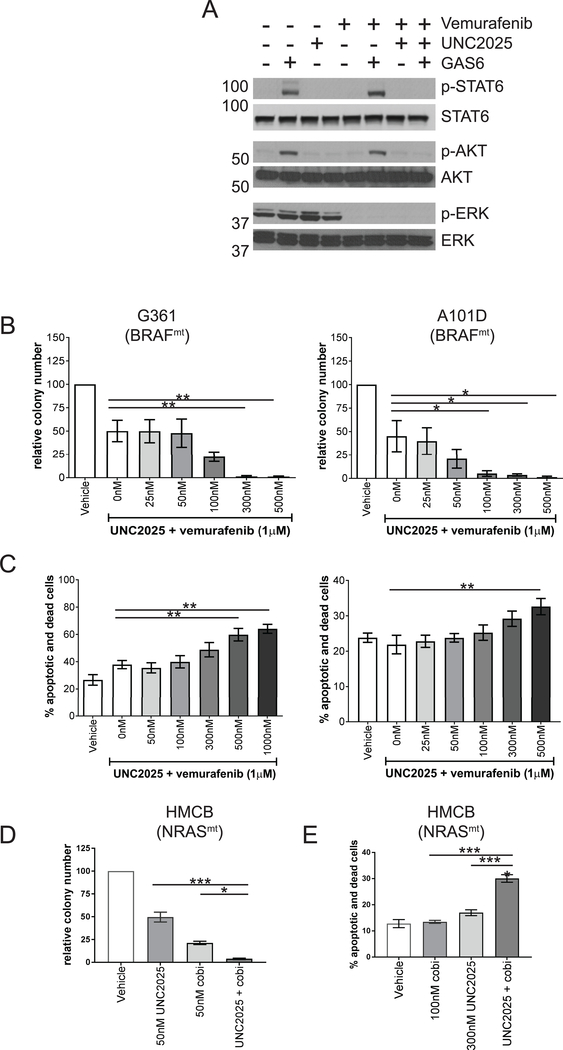
As combined BRAF and MEK inhibition is the current standard of care for patients with BRAFmt melanoma, additional studies were conducted to determine the impact of treatment with UNC2025 in combination with the BRAF inhibitor vemurafenib in BRAFmt cell lines. In the G361 cell line, treatment with UNC2025 alone inhibited GAS6-depedendent phosphorylation of STAT6 and AKT, but ERK was constitutively phosphorylated and was not impacted by treatment with the MERTK inhibitor (Fig. 4A). Conversely, treatment with vemurafenib monotherapy inhibited only ERK activation, but did not affect GAS6-dependent activation of STAT6 or AKT. However, in cells treated with a combination of UNC2025 and vemurafenib, all three oncogenic signaling pathways were effectively blocked. Furthermore, treatment with a combination of UNC2025 and vemurafenib mediated more effective inhibition of colony formation than treatment with vemurafenib alone in the BRAFmt G361 and A101D cell lines (Fig. 4B) and cell death was induced in a greater fraction of cells in response to treatment with the combination therapy compared to vemurafenib alone (Fig. 4C). Similarly, combined treatment with UNC2025 and the MEK inhibitor cobimetinib significantly increased induction of apoptosis and decreased colony-forming potential in cultures of the NRAS-mutant HMCB cell line compared to either drug alone (Fig. 4D and E).
Figure 4: Combined treatment with UNC2025 and a BRAF or MEK inhibitor enhances functional anti-tumor effects in BRAFmt and NRASmt melanoma cells.
A) Serum starved BRAFmt G361 cell cultures were treated with 300 nM UNC2025 and/or 675 nM vemurafenib or DMSO vehicle for 90 min and then stimulated with 200 nM GAS6 ligand or an equivalent volume of vehicle for an additional 10 min. Cell lysates were prepared and the indicated phosphorylated (denoted by p-) and total proteins were detected by immunoblot. B) G361 and A101D cell lines were cultured at low density and treated with 1μM vemurafenib alone or in combination with the indicated concentrations of UNC2025, or with vehicle (DMSO) only for 10 days. Colonies were stained with crystal violet and enumerated. Colony numbers relative to vehicle treated cultures are shown. C) G361 and A101D cell cultures were treated with 1μM vemurafenib alone or in combination with the indicated concentrations of UNC2025 or with vehicle (DMSO) only for 48 h and then stained with PO-PRO™−1 iodide (PO-PRO) and 7-AAD. Apoptotic (PO-PRO+, 7-AAD negative) and dead (7-AAD+) cells were detected by flow cytometry. Dead and dying cells were enumerated via flow cytometric analysis based on PO-PRO™-1 and 7-AAD uptake. D&E) HMCB cell cultures were treated with the indicated concentrations of cobimetinib (cobi), UNC2025, the combination, or vehicle and colony formation (D) and induction of cell death (E) were assessed as above. n=3–4 independent experiments, *p < 0.05, **p < 0.01, ***p<0.001, one-way ANOVA.
Treatment with UNC2025 reduces tumor growth in patient-derived melanoma xenograft models alone and in combination with vemurafenib
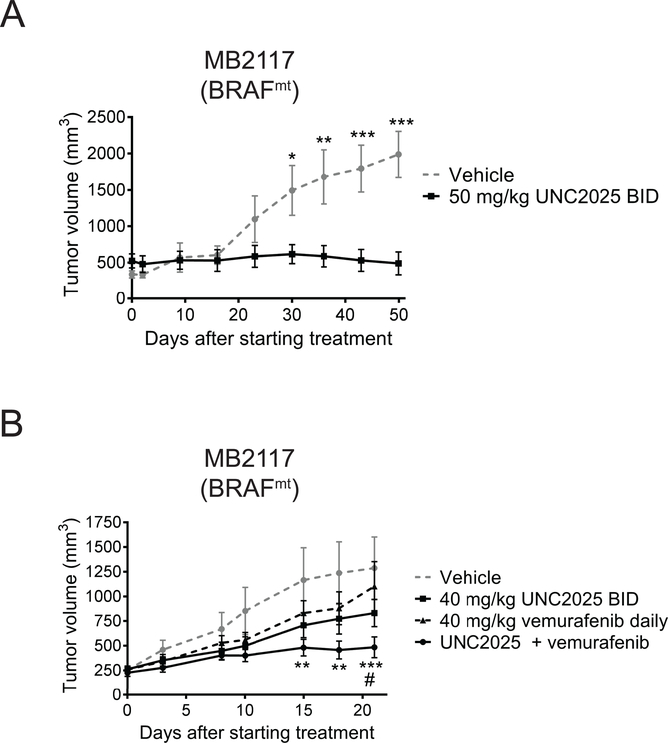
To determine whether UNC2025 has therapeutic activity against melanoma, patient-derived xenograft models were generated by transplanting biologically stable tumor samples into immune-compromised athymic nude mice (37) and the impact of treatment with UNC2025 on tumor growth was monitored. Mice bearing tumors with a BRAF mutation were treated with 50 mg/kg UNC2025 or vehicle control twice daily by oral gavage. The mean trough plasma concentration in mice treated with UNC2025 using this dosing strategy is of 161 nM (16), which corresponds with the doses that mediate effective anti-tumor activity in cell culture assays (Fig. 2). Tumor growth was significantly decreased in mice treated with UNC2025 compared to vehicle-treated control mice (Fig. 5A). In addition, mean tumor volume remained stable in mice treated with UNC2025 and was not significantly different after 50 days of treatment (489.9±157.3 mm3) compared to mean tumor volume at initiation of treatment (522.9±97.1 mm3, p = 0.4307). Tumor volume was also significantly decreased in response to treatment with UNC2025 in a PDX model of BRAFwt melanoma (Supplemental Fig. 6).
Figure 5: Treatment with UNC2025 inhibits tumor growth in a BRAFmt patient-derived melanoma xenograft model alone and in combination with vemurafenib.
A xenograft-passaged BRAFmt melanoma patient sample (MB2117) was transplanted into nude mice, and mice with established tumors were treated with 40 or 50 mg/kg UNC2025 twice daily (BID), 40 mg/kg vemurafenib once daily, or equivalent doses of UNC2025 and vemurafenib in combination, or vehicle. Tumor volumes were measures at intervals. A) n=6, * p < 0.05, ** p < 0.01, *** p < 0.001, two-way ANOVA B) n=10–13, ** p <0.01 versus vehicle, *** p < 0.001 versus vehicle, # p <0.05 versus vemurafenib monotherapy, two-way ANOVA. Asterisks denote comparison between vehicle and combination treatment. Data shown are representative of two independent experiments.
To determine whether the enhanced anti-tumor activity mediated by combined treatment with UNC2025 and vemurafenib in cell culture assays could be translated to an in vivo model, mice bearing patient-derived BRAFmt tumors were generated and then treated with vehicle control, 40 mg/kg UNC2025 twice daily, 40 mg/kg vemurafenib once daily, or equivalent doses of UNC2025 and vemurafenib. Sub-optimal doses of both UNC2025 and vemurafenib were used to allow assessment of combinatorial therapeutic effects. The combination therapy was well-tolerated and significant weight loss or other overt evidence of toxicity was not observed in mice treated with the combination therapy (Supplemental Fig. 7). Although there was a trend toward decreased tumor volume in mice treated with UNC2025 or vemurafenib alone, tumor volumes were not significantly different than in mice treated with vehicle (Fig. 5B). In contrast, tumor growth was significantly inhibited in mice treated with UNC2025 and vemurafenib compared to mice treated with vehicle. Differences were evident after 15 days of treatment and continued through the end of the study. Additionally, at the end of the study, tumors were significantly smaller in mice treated with the combination therapy compared to mice treated with vemurafenib monotherapy.
DISCUSSION
The complex and heterogeneous etiology of melanoma presents a challenge for development of effective therapies. Furthermore, the roles for active RTKs in this disease have not been completely characterized and this has limited the use of RTK-directed therapies. We and others have shown that members of the TAM family of RTKs are aberrantly expressed in melanoma (23, 25, 31). Of these, MERTK has been validated as a potential therapeutic target, conferring a survival advantage for melanoma cells and possibly contributing to metastasis (25). The data presented here demonstrate decreased oncogenic signaling mediated by UNC2025, a MERTK-selective small molecule inhibitor, resulting in functional anti-tumor activity, both in vitro and in vivo. Our finding that treatment with UNC2025 halted or dramatically suppressed tumor growth in mice with patient-derived xenografts is of particular significance as these models more closely represent the biology of de novo melanomas in patients and may more accurately predict therapeutic effects that will translate to clinical application (37). Importantly, the therapeutic effects mediated by UNC2025 in both cell culture and animal models were independent of oncogenic driver status (BRAF and NRAS), implicating MERTK as a promising therapeutic target for patients whose tumors have acquired resistance to other targeted therapies and for patients whose tumors do not have known mutations (BRAFwt, NRASwt). This finding is notable as, while BRAF-targeted agents provide therapeutic benefit for patients whose tumors have activating BRAF mutations, these mutations are only present in approximately half of the patient population and therapeutic options for treatment of BRAFwt melanoma are much more limited. In our studies, treatment with UNC2025 inhibited oncogenic signaling pathways, decreased colony formation, and reduced viable cell numbers in BRAF and NRAS wild-type melanoma cell line cultures, implicating MERTK inhibition as an attractive strategy for treatment of melanomas without BRAF or NRAS mutations using a molecularly-targeted approach. In addition, previous data demonstrating enhanced chemosensitivity upon MERTK inhibition (17, 19) suggest that MERTK therapy could also be used in combination with standard chemotherapeutic agents in wild-type melanoma, permitting dose reduction and reduced toxicity.
Treatment with UNC2025 inhibited MERTK activation and activation of downstream signaling pathways known to be important in oncogenesis, including STAT6 and AKT (40), suggesting a biochemical mechanism for UNC2025-mediated therapeutic activity. Although in other tumor models pharmacologic inhibition of MERTK inhibits activation of ERK (16, 33), there was not a significant decrease in ERK phosphorylation in response to UNC2025, consistent with previous data demonstrating minimal regulation of ERK signaling in response to shRNA-mediated MERTK inhibition in melanoma cell lines (25, 28). In these studies, expression of constitutively active AKT overcame the shMERTK-mediated decreases in cell survival and tumor cell expansion in culture, implicating inhibition of AKT as a critical mechanism underlying the anti-tumor effects of UNC2025 (28).
Survivin protein levels were also decreased in response to treatment with UNC2025, consistent with previously published data demonstrating inhibition of survivin expression in response to shRNA-mediated MERTK inactivation (19). Survivin is expressed in most human cancers, where it plays roles in apoptosis, cell cycle control and immune evasion (41). Inhibition of survivin is of particular interest in melanoma, where it functions both as an anti-apoptotic protein and an enhancer of cell migration and invasion (42). In melanoma survivin expression has been associated with more aggressive disease and poor prognosis (43, 44) and is not normally expressed in terminally differentiated adult tissues (41). Thus, survivin protein levels could be particularly useful as a pharmacodynamic biomarker of MERTK inhibition in tumor cells and may also predict therapeutic response. All melanoma cell lines in the current study demonstrated sensitivity to UNC2025 treatment in a dose range consistent with that required to inhibit survivin protein levels, but further studies are needed to identify and validate potential biomarker candidates.
In BRAFmt melanoma cell lines, treatment with a combination of UNC2025 and vemurafenib mediated more effective induction of cell death and inhibition of colony formation than either single agent. Furthermore, while treatment with vemurafinib alone was sufficient to inhibit ERK activity in BRAFmt cells and UNC2025 inhibited AKT and STAT6, only the combination therapy effectively inhibited all three pathways. These data suggest a biochemical mechanism by which MERTK inhibitors can cooperate with BRAF or MEK inhibitors. Importantly, combined treatment with UNC2025 and vemurafenib demonstrated superior therapeutic activity in vivo compared to either drug alone, consistent with published data using shMERTK and vemurafenib (31). This strategy of combining BRAF inhibitors with other molecularly-targeted agents in order to inhibit common pathways has already shown promise in clinical studies. Specifically, given the survival benefit seen in patients treated with concurrent BRAF and MEK inhibitors (12), BRAF/MEK inhibitor combination therapy has become the current standard of care for patients with BRAFmt tumors. However, median overall survival remains approximately 2 years with up to one-third of patients experiencing grade III/IV adverse events. The efficacy and tolerability of combined MERTK and BRAF inhibition in our preclinical models suggest MERTK as an alternative target for BRAF inhibitor combination therapy. Furthermore, in an NRASmt cell line, treatment with a combination of UNC2025 and the MEK inhibitor cobimetinib mediated enhanced anti-tumor effects compared to either agent alone, implicating combined MERTK and MEK inhibition as a potential therapeutic option for this class of tumors. Targeted treatment options for this large subset of melanoma have been lacking to date and single-agent MEK inhibitors have had limited clinical activity (10), making these findings particularly intriguing. Further studies to elucidate the effectiveness of MERTK inhibition in vivo in combination with BRAF or MEK inhibitors and other targeted therapies are warranted.
While the studies described here clearly demonstrate induction of cell death as a mechanism of anti-tumor activity mediated by UNC2025 in melanoma cell cultures, it is likely that other mechanisms contribute. Studies using genetic knockdown models implicated MERTK in cell cycle regulation, autophagy and induction of cellular senescence (17, 31, 45). However, it is not known whether MERTK kinase activity is involved and additional studies are needed to determine what affect, if any, pharmacologic MERTK inhibition has on these processes.
Additional studies to assess the effect of MERTK inhibition in combination with immunotherapy would also be of interest, given the known roles for MERTK in the immune system. Genetic deletion of MERTK reprograms macrophages to express a pro-inflammatory phenotype, leading to increased infiltration and activation of CD8 T cells in the tumor microenvironment and enhanced anti-tumor immunity in both breast carcinoma and melanoma models (21). In this context, MERTK inhibition was sufficient to reduce primary tumor incidence and volume as well as tumor metastasis. More recent studies have implicated MERTK in the regulation of PD-L1 and PD-L2 immune checkpoint ligands (46). Ectopic expression of MERTK induced expression of PD-L1 and PD-L2 in 293TN cells and shRNA-mediated MERTK inhibition suppressed PD-L1 expression in a breast cancer cell line. Immune-mediated tumor rejection and inhibition of PD-1 signaling may be particularly important therapeutic mechanisms in melanoma, which is an immunotherapy-responsive disease. Monoclonal antibodies against the immune regulatory molecules PD-1 and CTLA-4 are the current mainstay of treatment for advanced melanoma in the absence of targetable driver mutations and in the recent KEYNOTE-002 trial treatment with the anti-PD-1 antibody pembrolizumab resulted in greater than 2-fold increase in progression-free survival compared to cytotoxic chemotherapies (47). These data underscore the importance of immuno-therapeutic approaches for treatment of melanoma and suggest that MERTK-targeted therapies such as UNC2025, which are expected to mediate both direct anti-tumor effects and immunomodulatory effects through PD-1 and other mechanisms, may be particularly effective in this context.
Although UNC2025 is a MERTK-selective TKI, it is possible that some of the effects of treatment with UNC2025 described here are mediated by off-target inhibition of other kinases; however, several lines of evidence argue against this possibility. The kinome inhibitory profile of UNC2025 against 305 kinases revealed FLT3 as the only other kinase that is targeted by UNC2025 with similar potency to MERTK (IC50 = 0.35 nM and 0.46 nM in enzymatic assays, respectively) (32). Although FLT3 expression has been well-characterized in hematologic malignancies, it is not expressed in melanoma (48) and therefore the effects of UNC2025 in melanoma cells are likely independent of FLT3. The next most potently inhibited kinases are AXL and TRK-A (32). In cell-based assays, treatment with 300 nM UNC2025 had minimal or no effects on phosphorylation of endogenous AXL in non-small cell lung cancer models (16). While the concentrations used for the cell culture assays described here may target AXL and TRK-A to some extent, the degree of MERTK inhibition correlated with the changes in downstream signaling and functional anti-tumor activity. In addition, the biochemical and functional effects of treatment with UNC2025, both as a monotherapy and in combination with vemurafenib, recapitulated the phenotypic effects of shRNA mediated MERTK inhibition (25, 31). In aggregate, these findings suggest that the anti-tumor effects of treatment with UNC2025 described here are mediated by MERTK inhibition. However, AXL and TRK-A can also contribute to oncogenic phenotypes in melanoma. AXL promotes melanoma cell migration and invasion, and is commonly expressed in NRASmt melanomas (23, 24, 28). The TRKA locus is amplified in 50% of primary melanomas and TRKA amplification and activation have both been associated with poorer prognosis (49, 50). Given that both AXL and TRK-A play roles in melanoma, off-target inhibition of these proteins may be a desirable approach with potential to improve therapeutic benefits.
In conclusion, the data presented here demonstrate therapeutic activity mediated by UNC2025 in all subsets of melanoma, regardless of the presence or absence of BRAF or NRAS mutations, and reveal an added benefit in BRAFmt or NRASmt melanoma when UNC2025 is combined with vemurafenib or cobimetinib, respectively. While many strides have been made in recent years to improve treatment of melanoma, including combined BRAF/MEK inhibition and advancement of immunotherapies such as the PD-1 inhibitors, further improvements are still needed and identification of novel targets to improve outcomes for patients with advanced melanoma is a critical goal. The promising effects mediated by UNC2025 in preclinical studies and the versatile role that MERTK plays in tumors and the tumor microenvironment support further investigation toward clinical development of MERTK-targeted TKIs for treatment of melanoma.
Supplementary Material
ACKNOWLEDGEMENTS:
The authors wish to thank Christine Childs and the University of Colorado Cancer Center Flow Cytometry Core Facility (NIH P30CA046934) for expert technical assistance,the University of Colorado Diabetes and Endocrinology Research Center Molecular Biology Core Facility (NIH P30DK57516) for cell line authentication services, the Children’s Healthcare of Atlanta and Emory University Pediatric Flow Cytometry Core for use of core facilities, and Eleana Vasileaidi for technical assistance.
FINANCIAL SUPPORT: This work was supported by a sponsored research agreement with Merck Inc. to D.K. Graham, grant support from the Melanoma Research Alliance (Saban Family Foundation-MRA Team Science Award to R. Kami, T. Burstyn-Cohen, S.G. Earnhardt, S.V. Frye, H.S. Earp, and D.K. Graham), and a generous contribution from Dr. Bill Robinson (University of Colorado). K.A. Minson is supported by the Atlanta Pediatric Scholars Program sponsored by the NIH National Institute of Child Health and Human Development Child Health Research Career Development Award (K12HD072245). Medicinal chemistry efforts (X. Wang and S.V. Frye) were supported by the University Cancer Research Fund and Federal Funds from the National Cancer Institute, National Institute of Health, under Contract No. HHSN261200800001E.
Footnotes
CONFLICT OF INTEREST: D.K.G., D.D. and H.S.E. have filed patents on targeting of MERTK tyrosine kinase as cancer therapy. X.W., and S.F. have a patent on UNC2025. D.D., X.W., S.F., H.S.E., and D.K.G. have stock in Meryx, Inc (a company developing novel therapeutics against MERTK). The remaining authors have declared that no conflict of interest exists.
REFERENCES
- 1.Wellbrock C, Ogilvie L, Hedley D, Karasarides M, Martin J, Niculescu-Duvaz D, et al. V599EB-RAF is an oncogene in melanocytes. Cancer Res. 2004;64(7):2338–42. [DOI] [PubMed] [Google Scholar]
- 2.Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, et al. A landscape of driver mutations in melanoma. Cell. 2012;150(2):251–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. The New England journal of medicine. 2011;364(26):2507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010;363(8):711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JH, Choi JW, Kim YS. Frequencies of BRAF and NRAS mutations are different in histological types and sites of origin of cutaneous melanoma: a meta-analysis. The British journal of dermatology. 2011;164(4):776–84. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez-Laorden B, Viros A, Girotti MR, Pedersen M, Saturno G, Zambon A, et al. BRAF inhibitors induce metastasis in RAS mutant or inhibitor-resistant melanoma cells by reactivating MEK and ERK signaling. Science signaling. 2014;7(318): ra30. [DOI] [PubMed] [Google Scholar]
- 7.Atefi M, von Euw E, Attar N, Ng C, Chu C, Guo D, et al. Reversing melanoma cross-resistance to BRAF and MEK inhibitors by co-targeting the AKT/mTOR pathway. PloS one. 2011;6(12):e28973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468(7326):973–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poulikakos PI, Persaud Y, Janakiraman M, Kong X, Ng C, Moriceau G, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E). Nature. 2011;480(7377):387–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dummer R, Schadendorf D, Ascierto PA, Arance A, Dutriaux C, Di Giacomo AM, et al. Binimetinib versus dacarbazine in patients with advanced NRAS-mutant melanoma (NEMO): a multicentre, open-label, randomised, phase 3 trial. The Lancet Oncology. 2017;18(4):435–45. [DOI] [PubMed] [Google Scholar]
- 11.Paraiso KH, Fedorenko IV, Cantini LP, Munko AC, Hall M, Sondak VK, et al. Recovery of phospho-ERK activity allows melanoma cells to escape from BRAF inhibitor therapy. British journal of cancer. 2010;102(12):1724–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. The Lancet. 2015;386(9992):444–51. [DOI] [PubMed] [Google Scholar]
- 13.Marconcini R, Spagnolo F, Stucci LS, Ribero S, Marra E, Rosa F, et al. Current status and perspectives in immunotherapy for metastatic melanoma. Oncotarget. 2018;9(15):12452–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linger RM, Keating AK, Earp HS, Graham DK. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Advances in cancer research. 2008;100:35–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verma A, Warner SL, Vankayalapati H, Bearss DJ, Sharma S. Targeting Axl and Mer kinases in cancer. Molecular cancer therapeutics. 2011;10(10):1763–73. [DOI] [PubMed] [Google Scholar]
- 16.Cummings CT, Zhang W, Davies KD, Kirkpatrick GD, Zhang D, DeRyckere D, et al. Small Molecule Inhibition of MERTK Is Efficacious in Non-Small Cell Lung Cancer Models Independent of Driver Oncogene Status. Molecular cancer therapeutics. 2015;14(9):2014–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keating AK, Kim GK, Jones AE, Donson AM, Ware K, Mulcahy JM, et al. Inhibition of Mer and Axl receptor tyrosine kinases in astrocytoma cells leads to increased apoptosis and improved chemosensitivity. Molecular cancer therapeutics. 2010;9(5):1298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham DK, Salzberg DB, Kurtzberg J, Sather S, Matsushima GK, Keating AK, et al. Ectopic expression of the proto-oncogene Mer in pediatric T-cell acute lymphoblastic leukemia. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12(9):2662–9. [DOI] [PubMed] [Google Scholar]
- 19.Linger RM, Cohen RA, Cummings CT, Sather S, Migdall-Wilson J, Middleton DH, et al. Mer or Axl receptor tyrosine kinase inhibition promotes apoptosis, blocks growth and enhances chemosensitivity of human non-small cell lung cancer. Oncogene. 2013;32(29):3420–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu YM, Robinson DR, Kung HJ. Signal pathways in up-regulation of chemokines by tyrosine kinase MER/NYK in prostate cancer cells. Cancer Res. 2004;64(20):7311–20. [DOI] [PubMed] [Google Scholar]
- 21.Cook RS, Jacobsen KM, Wofford AM, DeRyckere D, Stanford J, Prieto AL, et al. MerTK inhibition in tumor leukocytes decreases tumor growth and metastasis. The Journal of clinical investigation. 2013;123(8):3231–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogers AE, Le JP, Sather S, Pernu BM, Graham DK, Pierce AM, et al. Mer receptor tyrosine kinase inhibition impedes glioblastoma multiforme migration and alters cellular morphology. Oncogene. 2012;31(38):4171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tworkoski K, Singhal G, Szpakowski S, Zito CI, Bacchiocchi A, Muthusamy V, et al. Phosphoproteomic screen identifies potential therapeutic targets in melanoma. Molecular cancer research : MCR. 2011;9(6):801–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sensi M, Catani M, Castellano G, Nicolini G, Alciato F, Tragni G, et al. Human cutaneous melanomas lacking MITF and melanocyte differentiation antigens express a functional Axl receptor kinase. The Journal of investigative dermatology. 2011;131(12):2448–57. [DOI] [PubMed] [Google Scholar]
- 25.Schlegel J, Sambade MJ, Sather S, Moschos SJ, Tan AC, Winges A, et al. MERTK receptor tyrosine kinase is a therapeutic target in melanoma. The Journal of clinical investigation. 2013;123(5):2257–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu S, Wurdak H, Wang Y, Galkin A, Tao H, Li J, et al. A genomic screen identifies TYRO3 as a MITF regulator in melanoma. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(40):17025–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demarest SJ, Gardner J, Vendel MC, Ailor E, Szak S, Huang F, et al. Evaluation of Tyro3 expression, Gas6-mediated Akt phosphorylation, and the impact of anti-Tyro3 antibodies in melanoma cell lines. Biochemistry. 2013;52(18):3102–18. [DOI] [PubMed] [Google Scholar]
- 28.Tworkoski KA, Platt JT, Bacchiocchi A, Bosenberg M, Boggon TJ, Stern DF. MERTK controls melanoma cell migration and survival and differentially regulates cell behavior relative to AXL. Pigment cell & melanoma research. 2013;26(4):527–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knight ZA, Lin H, Shokat KM. Targeting the cancer kinome through polypharmacology. Nature reviews Cancer. 2010;10(2):130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu AM, Huang PH. Receptor tyrosine kinase coactivation networks in cancer. Cancer Res. 2010;70(10):3857–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xue G, Kohler R, Tang F, Hynx D, Wang Y, Orso F, et al. mTORC1/autophagy-regulated MerTK in mutant BRAFV600 melanoma with acquired resistance to BRAF inhibition. Oncotarget. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang W, DeRyckere D, Hunter D, Liu J, Stashko MA, Minson KA, et al. UNC2025, a potent and orally bioavailable MER/FLT3 dual inhibitor. Journal of medicinal chemistry. 2014;57(16):7031–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeRyckere D, Lee Sherick AB, Huey MG, Hill AA, Tyner JW, Jacobsen KM, et al. UNC2025, a MerTK small molecule inhibitor, is therapeutically effective alone and in combination with methotrexate in leukemia models. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forbes SA, Beare D, Boutselakis H, Bamford S, Bindal N, Tate J, et al. COSMIC: somatic cancer genetics at high-resolution. Nucleic acids research. 2017;45(D1):D777–d83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.COSMIC, Catalogue of Somatic Mutations in Cancer 13-Feb-18. [cited 2018 4/24]. Available from: https://cancer.sanger.ac.uk/cosmic.
- 36.Couts KL, Bemis J, Turner JA, Bagby SM, Murphy D, Christiansen J, et al. ALK Inhibitor Response in Melanomas Expressing EML4-ALK Fusions and Alternate ALK Isoforms. Molecular cancer therapeutics. 2018;17(1):222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tentler JJ, Tan AC, Weekes CD, Jimeno A, Leong S, Pitts TM, et al. Patient-derived tumour xenografts as models for oncology drug development. Nature reviews Clinical oncology. 2012;9(6):338–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bagby S, Messersmith WA, Pitts TM, Capasso A, Varella-Garcia M, Klauck PJ, et al. Development and Maintenance of a Preclinical Patient Derived Tumor Xenograft Model for the Investigation of Novel Anti-Cancer Therapies. Journal of visualized experiments : JoVE. 2016(115). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graham DK, DeRyckere D, Davies KD, Earp HS. The TAM family: phosphatidylserine sensing receptor tyrosine kinases gone awry in cancer. Nature reviews Cancer. 2014;14(12):769–85. [DOI] [PubMed] [Google Scholar]
- 40.Meier F, Schittek B, Busch S, Garbe C, Smalley K, Satyamoorthy K, et al. The RAS/RAF/MEK/ERK and PI3K/AKT signaling pathways present molecular targets for the effective treatment of advanced melanoma. Frontiers in bioscience : a journal and virtual library. 2005;10:2986–3001. [DOI] [PubMed] [Google Scholar]
- 41.Altieri DC. Validating survivin as a cancer therapeutic target. Nature reviews Cancer. 2003;3(1):46–54. [DOI] [PubMed] [Google Scholar]
- 42.McKenzie JA, Grossman D. Role of the apoptotic and mitotic regulator survivin in melanoma. Anticancer research. 2012;32(2):397–404. [PubMed] [Google Scholar]
- 43.Takeuchi H, Morton DL, Elashoff D, Hoon DS. Survivin expression by metastatic melanoma predicts poor disease outcome in patients receiving adjuvant polyvalent vaccine. International journal of cancer. 2005;117(6):1032–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gradilone A, Gazzaniga P, Ribuffo D, Scarpa S, Cigna E, Vasaturo F, et al. Survivin, bcl-2, bax, and bcl-X gene expression in sentinel lymph nodes from melanoma patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21(2):306–12. [DOI] [PubMed] [Google Scholar]
- 45.Sufit A, Lee-Sherick AB, DeRyckere D, Rupji M, Dwivedi B, Varella-Garcia M, et al. MERTK Inhibition Induces Polyploidy and Promotes Cell Death and Cellular Senescence in Glioblastoma Multiforme. PloS one. 2016;11(10):e0165107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen KQ, Tsou WI, Calarese DA, Kimani SG, Singh S, Hsieh S, et al. Overexpression of MERTK receptor tyrosine kinase in epithelial cancer cells drives efferocytosis in a gain-of-function capacity. The Journal of biological chemistry. 2014;289(37):25737–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. The Lancet Oncology. 2015;16(8):908–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serve H, Flesch K, Serve S, Fenski R, Berdel WE. Expression and function of Flt3/flk2 in human tumor cell lines. International journal of oncology. 1999;14(4):765–70. [DOI] [PubMed] [Google Scholar]
- 49.Florenes VA, Maelandsmo GM, Holm R, Reich R, Lazarovici P, Davidson B. Expression of activated TrkA protein in melanocytic tumors: relationship to cell proliferation and clinical outcome. American journal of clinical pathology. 2004;122(3):412–20. [DOI] [PubMed] [Google Scholar]
- 50.Pasini L, Re A, Tebaldi T, Ricci G, Boi S, Adami V, et al. TrkA is amplified in malignant melanoma patients and induces an anti-proliferative response in cell lines. BMC cancer. 2015;15:777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.