Abstract
Previous studies have demonstrated a role for B cells, not associated with antibody production, in protection against lethal secondary infection of mice with Francisella tularensis live vaccine strain (LVS). However, the mechanism by which B cells contribute to this protection is not known. To study the specific role of B cells during secondary LVS infection, we developed an in vitro culture system that mimics many of the same characteristics of in vivo infection. Using this culture system, we showed that B cells do not directly control LVS infection but that control of LVS growth is mediated primarily by LVS-primed T cells. Importantly, B cells were not required for the generation of effective memory T cells since LVS-primed, B-cell-deficient (BKO) mice generated CD4+ and CD8+ T cells that controlled LVS infection similarly to LVS-primed CD4+ and CD8+ T cells from wild-type mice. The control of LVS growth appeared to depend primarily on gamma interferon and nitric oxide and was similar in wild-type and BKO mice. Rather, the inability of BKO mice to survive secondary LVS infection was associated with marked neutrophil influx into the spleen very early after challenge. The neutrophilia was directly associated with B cells, since BKO mice reconstituted with naive B cells prior to a secondary challenge with LVS had decreased bacterial loads and neutrophils in the spleen and survived.
Francisella tularensis, a gram-negative coccobacillus, is a facultative intracellular bacterium that causes a lethal disease in humans when delivered by the intravenous, intradermal, or aerosol route (35). Although natural infection in developed countries is rare, there has been renewed interest in F. tularensis as a human pathogen due to its potential as a biowarfare weapon (39). F. tularensis live vaccine strain (LVS) is an attenuated strain of F. tularensis that produces a lethal disease in mice quite similar to the disease in humans infected with fully virulent F. tularensis (3, 13, 16, 35). Further, LVS has been used as a human vaccine but the extent of protection afforded by LVS in humans is not well characterized (28, 35, 37); thus, it is important to understand the basis of protective immunity to this bacterium. Immunity to F. tularensis also has much in common with immunity to other intracellular bacteria, such as Listeria monocytogenes and Mycobacterium tuberculosis (3, 36), and the study of F. tularensis is considered a model for this important class of pathogens.
To date, it has been demonstrated that immunity against LVS is predominately cell mediated and dependent on the generation of tumor necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ) (2, 11, 12, 21). In vivo studies have demonstrated that either CD4+ or CD8+ T cells are sufficient for mediating control and resolution of LVS infection (41), while antibodies appear to have little, if any, role during protective immune responses to LVS infection (7, 9, 25, 41). For example, optimal protection is observed in CD4+ knockout mice that do not have specific immunoglobulin G (IgG) anti-LVS antibodies (41), and transfer of specific antibodies provides very limited protection (25). However, recent studies in our laboratory revealed a surprising role for B cells, that was not attributable to antibody production, in early and secondary immune responses against LVS (7, 9, 10). B-cell knockout (BKO) mice were only marginally defective in the primary response to an intradermal infection but were significantly compromised in the ability to survive a maximal secondary challenge (9). Reconstitution of BKO mice with B cells prior to a secondary challenge resulted in their survival despite the absence of circulating LVS-specific antibodies in their serum. This indicated that survival of a secondary lethal LVS infection was due to a non-antibody-related function provided by B cells themselves (9).
There have been several other reports in the literature suggesting a non-antibody-mediated role for B cells in protective immune responses against other bacterial pathogens. For example, following a pulmonary infection with Chlamydia trachomatis, B cells were implicated in the development of effective T-cell priming and secondary immunity (40). In that study, BKO mice exhibited suboptimal delayed-type hypersensitivity responses and reduced C. trachomatis-specific IFN-γ responses. A non-antibody-mediated role for B cells during primary (22) and secondary (20) respiratory Bordetella pertussis infections of mice has also been described. However, in these examples the specific mechanism of the dependence on B cells was also unclear.
To study the mechanism by which B cells contribute to immunity against LVS, we developed a new in vitro culture system to directly examine the role of B cells, T cells, and soluble mediators during secondary infection. Here we show that this in vitro system clearly reproduces known in vivo features of control of LVS infection, including control of intracellular LVS growth by either CD4+ or CD8+ T cells and thus is informative in examining questions that cannot be addressed readily in vivo. Using this system, we examined the contribution of B cells to the direct killing of intracellular bacteria, to antigen presentation, and to the generation of T effector cells for macrophage activation. We found that B cells are not required for these functions but, instead, appear to be intimately involved in regulating the appropriate in vivo trafficking of neutrophils.
MATERIALS AND METHODS
Animals.
Six- to 8-week-old, male, specific-pathogen-free C57BL6/J or B-cell-deficient mice (Igh6−; 18) on a C57BL6/J background were purchased from the Jackson Laboratory (Bar Harbor, Maine). Animals were housed in sterile microisolator cages in a barrier environment at the Center for Biologics Evaluation and Research. Mice were fed autoclaved food and water ad libitum. All experiments were performed under Animal Care and Use Committee guidelines.
Culture and infection of BMMφ with bacteria.
Since macrophages are the primary target for LVS in vivo (14, 15), we selected bone marrow macrophages (BMMφ) as the target cells for our in vitro system. BMMφ were cultured as previously described (26). Briefly, bone marrow was flushed from femurs of healthy C57BL6/J mice with Dulbecco minimum essential medium (DMEM; Life Technologies, Grand Island, N.Y.) supplemented with 10% heat-inactivated fetal calf serum (FCS; Hy-Clone, Logan, Utah), 10% L-929 conditioned medium, 0.2 mM l-glutamine (Life Technologies), 1 mM HEPES buffer (Life Technologies), and 0.1 mM nonessential amino acids (Life Technologies) (complete DMEM [cDMEM]). Cells were washed, a single-cell suspension was prepared by gentle pipetting, and cells were plated at 2 × 106/ml in 24-well plates (Costar, Corning, N.Y.) in cDMEM supplemented with gentamicin (Life Technologies) at 50 μg/ml and incubated at 37°C in 5% CO2. After 1 day of incubation, the medium was replaced with antibiotic-free cDMEM and the cells were incubated for an additional 6 days at 37°C in 5% CO2. The medium was replaced with fresh, gentamicin-free cDMEM every 2 days during the 7-day incubation period.
Following the 7-day incubation period, the concentration of BMMφ was estimated as 107 cells/well (estimated by both scraping off macrophages and removing macrophages by cold shock and then counting the cells) for both wild-type and BKO BMMφ. BMMφ were infected with LVS as previously described (26), with the following modifications. Briefly, F. tularensis LVS (American Type Culture Collection, Manassas, Va.) were diluted from frozen stocks in cDMEM and added at a multiplicity of infection (MOI) of 1:10 (bacterium- to -BMMφ ratio). As previously described (1, 15), an MOI of 1:10 was selected following the study of various MOIs in separate experiments, as this permitted a controlled infection of the macrophage monolayer that spread slowly and was sustained for 3 to 5 days before most of the macrophages died; these infection conditions therefore permitted time for lymphocyte-macrophage interaction. The infection inoculum was confirmed by plating serial dilutions of stock LVS on Mueller-Hinton agar plates immediately prior to addition to BMMφ cultures. LVS was coincubated with BMMφ at 37°C in 5% CO2 for 2 h and then replaced with 1 ml of cDMEM plus gentamicin (Life Technologies) at 50 μg/ml to eliminate extracellular bacteria. Cultures were incubated for an additional 45 min and washed five times with phosphate-buffered saline (PBS; Life Technologies). No bacteria were detected in supernatants of washed cells, confirming the elimination of extracellular bacteria. Following the last wash, PBS was replaced with cDMEM and the cells were incubated at 37°C in 5% CO2 for the remainder of the experiment. To determine bacterial uptake, some BMMφ were lysed with water for 5 min immediately after washing with PBS. Culture lysates were serially diluted and plated onto Mueller-Hinton agar plates and incubated at 37°C in 5% CO2 for approximately 48 h, and individual colonies of LVS were counted. BMMφ uptake of LVS was routinely between 102 and 103 bacteria/ml. Growth of LVS in BMMφ was monitored by lysing cultures at predetermined time points, plating lysates, and counting LVS colonies as described above. In some experiments, L. monocytogenes was used as the infecting bacterium at an MOI of 1:1,000 and cultures were treated as described above. Since L. monocytogenes replicates faster than LVS in vitro, a lower MOI was required to generate a growth curve similar to that of BMMφ infected with LVS.
Infection of mice with LVS.
Wild-type and BKO mice were infected intradermally with 8 × 104 LVS bacteria. This concentration of LVS has been previously determined to establish a sublethal infection in both wild-type and BKO mice that is eventually cleared from organs by 2 to 3 weeks and engenders long-term protective immunity to a lethal secondary LVS infection (9, 11, 41); such mice were therefore used as a source of LVS-primed splenocytes.
In experiments designed to study secondary immunity in vivo, mice were primed as described above. Thirty-five days after priming, mice were infected intraperitoneally (i.p.) with 5 × 105 LVS bacteria in 0.5 ml. This dose of LVS has been previously shown to be lethal to primed BKO mice, while reconstituted BKO mice and primed wild-type mice survive this dose and clear the bacteria (9). All in vivo experiments were done with three mice per group.
Harvesting and enrichment of splenocytes.
Four weeks following intradermal infection, or as indicated otherwise, spleens were aseptically removed from selected mice and disrupted with a 3-ml syringe plunger. A single-cell suspension was prepared, and erythrocytes were lysed with ammonium chloride. Cells were washed, viability was assessed by exclusion of trypan blue, and cells were resuspended in Dulbecco PBS–2% FCS at appropriate concentrations for flow cytometric analysis and cytospin centrifugation. Splenocytes were added to BMMφ cultures at various concentrations as indicated. Unless otherwise stated, 5 × 106 splenocytes were added to each well (approximately 1 splenocyte to 2 BMMφ). Since BKO mice had half as many splenocytes as wild-type mice, the number of whole BKO splenocytes added to the culture was half the number of wild-type splenocytes added, to mimic the number available in vivo. Numbers of B cells, whole T cells, and T-cell subpopulations added to BMMφ were proportional to their percentages (as determined by flow cytometry [see below]) in normal C57BL/6J spleens. T, CD4+, and CD8+ cells were enriched using T, CD4+, and CD8+ cell enrichment columns in accordance with the manufacturer's (R&D Systems, Minneapolis, Minn.) instructions. Purity of enriched cells was determined by flow cytometry as described below. Cell populations were greater than 90% of the desired population using this method of enrichment. B cells were enriched by negative selection as previously described (7, 9). Briefly, single-cell splenocytes suspensions were prepared from naive C57BL/6J mice and treated with ammonium chloride to deplete erythrocytes. Viable cells were enumerated by exclusion of trypan blue. Cells were treated with each of the following at 10 μg/ml in PBS–2% FCS; anti-CD3 (145-2C11), anti-CD4 (RM4-5), anti-CD8 (53-6.7), and anti-γδ T-cell receptor (GL3) (all purchased from PharMingen, San Diego, Calif.). Following incubation for 30 min at 4°C, cells were washed and treated with rabbit complement (Pel-Freeze Biologics, Browender, Wis.) diluted 1:10. Following incubation for 30 min at 37°C, cells were washed and viable cells were enumerated by exclusion of trypan blue. In all cases, starting and enriched splenocyte populations were analyzed by flow cytometry using a fluorescence-activated cell sorter scan as described below. Flow cytometry revealed that this method of enrichment for B cells resulted in less than 5% contaminating T cells in all B-cell preparations (data not shown). In some experiments, IFN-γ was neutralized in culture supernatants following addition of azide-free, low-endotoxin anti-mouse IFN-γ antibodies (XMG1.2; PharMingen) at a concentration of 10 μg/ml at the same time as splenocytes.
Quantitation of cytokines and NO in BMMφ culture supernatants.
Culture supernatants were assayed for IFN-γ, interleukin-12 (IL-12), TNF-α, IL-4, and IL-10 by standard sandwich enzyme-linked immunosorbent assays (ELISAs). All antibody pairs and standards were purchased from PharMingen. All ELISAs were performed in accordance with the manufacturer's instructions. Samples were read at 405 nm on a Versamax tunable microplate reader with a reference wavelength of 630 nm (Molecular Devices, Sunnyvale, Calif.). Cytokines were quantified by comparison to recombinant standards (all purchased from PharMingen) using four-parameter fit regression in the SoftMax Pro ELISA analysis software (Molecular Devices).
Nitric oxide (NO) was detected in culture supernatants by the Griess reaction (17). Briefly, 100-μl aliquots of culture supernatants were incubated with an equal volume of commercial Griess reagent (Sigma, St. Louis, Mo.) for 5 min at room temperature, and the absorbance of each sample at 490 nm was measured. NO2 was quantified by comparison to serially diluted NaNO2 as a standard using four-parameter fit regression in the SoftMax Pro ELISA analysis software (Molecular Devices).
Analysis of splenocyte populations by differential staining and flow cytometry.
To assess the morphology of different cell types present in the spleens, 5 × 105 cells per cytocentrifuge chamber (Shandon, Sewickley, Pa.) were spun through FCS onto slides and stained with modified Wright-Giemsa stain (Hema 3 stain set; Fisher Scientific, Pittsburgh, Pa.). Approximately 100 to 200 cells were counted from each slide to assess cell populations. Classification of cells was based on the following morphological characteristics: neutrophils, multilobed nuclei; lymphocytes, rounded nuclei with little cytoplasm; monocytes-macrophages, larger cells with kidney-shaped nuclei and an abundance of “foamy” cytoplasm.
Spleen cells were also analyzed by flow cytometry. Cells were prepared as described above and stained for B220+, CD4+, CD8+, and γδ+ surface markers. Single-cell suspensions were mixed with anti-CD16 (FcBlock; PharMingen) for 10 min on ice. Fluorescein isothiocyanate-conjugated rat IgG2a (R35-95), phycoerythrin (PE)-conjugated rat IgG2b (A95-1) (isotype controls), fluorescein isothiocyanate-conjugated anti-CD45/B220 (RA3-6B2), PE-conjugated anti-CD4 (RM4-4), PE-conjugated anti-CD8a (53-6.7), or PE-conjugated anti-γδ T-cell receptor (GL3) monoclonal antibody was added, and cells were incubated for an additional 30 min on ice. All antibodies were obtained from PharMingen, and optimal concentrations were determined in separate experiments. Cells were washed three times in PBS–2% FCS, fixed in 0.5% buffered paraformaldehyde, and analyzed using a Becton-Dickinson (San Jose, Calif.) FACScan flow cytometer with gates set for viable lymphocytes and monocytes according to forward- and side-scatter profiles. Among the total population of splenocytes, C57BL6/J wild-type mice had approximately 25% CD4+ T cells, 20% CD8+ T cells, and 46% B220+ cells. BKO mice had approximately 45% CD4+ and 35% CD8+ T cells. As expected, no B220+ cells were detected in BKO mice at any time. Thus, in order to mimic the in vivo relationships, in cultures containing B-cell populations, approximately 2.3 × 106 (45% of 5 × 106) wild-type cells were added. In cultures containing whole T-cell populations, approximately 2.3 × 106 (45% of 5 × 106) wild-type T cells were added while 2.0 × 106 (80% of 2.5 × 106) whole BKO splenocytes were added to cultures. In cultures containing T-cell subpopulations, approximately 1.3 × 106 (25% of 5.0 × 106) CD4+ or 1 × 106 (20% of 5.0 × 106) CD8+ wild-type cells were added while 1.1 × 106 (45% of 2.5 × 106) CD4+ or 8.8 × 105 (35% of 2.5 × 106) CD8+ BKO T cells were added to cultures.
RESULTS
Growth of LVS in wild-type and BKO murine BMMφ.
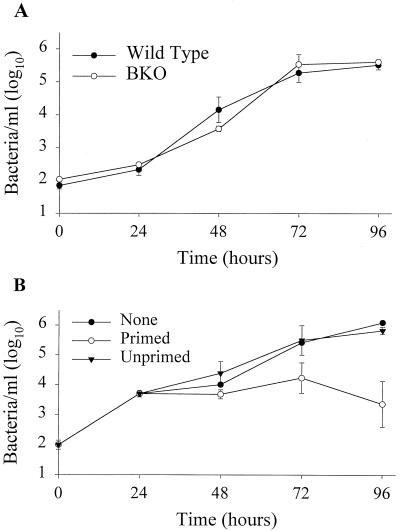
The primary host cell for LVS in mice is the macrophage (1, 14, 15, 35). To determine whether the uptake or growth of LVS differs in macrophages from BKO and wild-type mice, we first compared the growth of LVS in wild-type and BKO BMMφ in vitro. BKO and wild-type macrophages were similar in LVS uptake (Fig. 1A), and LVS grew logarithmically in both wild-type and BKO macrophages over time. The number of bacteria peaked by 72 h after infection in both wild-type and BKO macrophages and was never significantly different (Fig. 1A). Also of note is the fact that all of the LVS replication was attributed to growth in association with macrophages, as LVS did not replicate in tissue culture medium alone (15; data not shown).
FIG. 1.
Growth and control of LVS in BMMφ. (A) Growth of LVS in wild-type and BKO BMMφ. BMMφ from wild-type and BKO mice were infected with LVS at an MOI of 1:10 (bacterium-to-macrophage ratio). At the specified time points after infection, BMMφ were washed, lysed, and plated. Data points show the mean numbers ± the standard error of the mean (SEM) of viable bacteria (triplicate samples) recovered from wild-type (filled circles) and BKO (open circles) BMMφ at the indicated time points. (B) Control of LVS growth by LVS-primed splenocytes. Uninfected mice (Unprimed) or mice primed intradermally with LVS 4 weeks prior to the onset of the experiment (Primed) served as the source of LVS-primed splenocytes. Immediately following LVS infection of BMMφ, 5 × 106 splenocytes of each population were added to designated wells. At the indicated time points, cultures were assessed for intracellular bacteria. Data points show the mean numbers ± the SEM of viable bacteria (triplicate samples) recovered from macrophages alone (None, filled circles), cultures containing primed splenocytes (open circles), or cultures containing unprimed splenocytes (filled triangles) at the indicated time points; the SEM was too small to be visualized for some of the data points on this graph. The data in panel A are representative of three experiments similar in design, and those in panel B are representative of five experiments similar in design.
Control of LVS growth in wild-type and BKO murine BMMφ by LVS-primed splenocytes.
We next tested the ability of LVS-primed splenocytes to restrict the growth of LVS in vitro. Splenocytes from wild-type mice primed 4 weeks earlier with LVS were added at various time points after infection of BMMφ with LVS in vitro. In initial experiments, splenocytes were added on the day BMMφ were infected. Following addition of primed, but not unprimed, splenocytes, growth of LVS in BMMφ was controlled (Fig. 1B). Control of LVS growth was observed as early as 24 h after infection and was maximal by 72 h after the addition of primed splenocytes (Fig. 1B). Control of LVS growth 96 h after the addition of primed splenocytes was similar to that observed 72 h after their addition.
To determine if the time of splenocyte addition to infected BMMφ affects the control of LVS growth, splenocytes were added either on the day of infection or 24 h after infection of BMMφ (Fig. 2A). Splenocytes added at the time of infection or 24 h after infection exhibited maximal and comparable control of LVS growth in vitro 72 h after infection of BMMφ (Fig. 2A). Since LVS-primed splenocytes would be present in the spleen during a secondary infection with LVS in vivo, splenocytes were added on the day of BMMφ infection in all subsequent experiments. To determine if the control of LVS growth observed in our culture system was specific for LVS-infected macrophages, we tested the ability of LVS-primed splenocytes to control the growth of another intracellular pathogen, L. monocytogenes. Splenocytes from LVS-primed mice controlled the growth of LVS at 72 h as described above (Fig. 2B). However, neither unprimed nor LVS-primed splenocytes affected the growth of L. monocytogenes in BMMφ.
FIG. 2.
Determination of specificity, concentration, and time of splenocyte addition to cultures for optimal control of LVS growth. In each graph, “None” represents LVS bacterial growth in BMMφ alone. (A) BMMφ were infected with LVS. Immediately or 24 h after infection, primed (black bars) or unprimed (gray bars) splenocytes (5 × 106/well) were added to cultures. Seventy-two hours after infection, cultures were assessed for intracellular bacteria as described in the legend to Fig. 1. Error bars represent the SEM. (B) BMMφ were infected with LVS (gray bars) or L. monocytogenes (black bars); BMMφ were infected with L. monocytogenes at an MOI of 1:1,000 (bacterium-to-macrophage ratio). Twenty-four hours after infection of BMMφ splenocytes of each population (5 × 106/well) were added to designated wells. Seventy-two hours after addition of splenocytes, cultures were assessed for intracellular bacteria as described in the legend to Fig. 1. (C) BMMφ were infected with LVS. Twenty-four hours after infection, the indicated concentrations of primed (black bars) or unprimed (gray bars) splenocytes were added to cultures. Seventy-two hours after infection, cultures were assessed for intracellular bacteria. These data are representative of two experiments similar in design.
We further tested the ability of different concentrations of LVS-primed splenocytes to control LVS growth. As shown in Fig. 2C, as few as 5 × 105 LVS-primed splenocytes were capable of controlling LVS growth. Similar control of LVS growth was observed with all of the concentrations of splenocytes added to infected BMMφ (Fig. 2C), while splenocytes from unprimed mice were unable to control LVS growth at any of the numbers tested (Fig. 2C). Since the spleen has far greater numbers of lymphocytes compared to potentially infected macrophages, we used the highest concentration of splenocytes that was practical (5 × 106/ml) in subsequent experiments. Although splenocytes controlled the growth of LVS as early as 24 h after addition to infected BMMφ (Fig. 1A), subsequent experiments focused on events 72 h after infection, when control of LVS growth was maximal.
Contribution of lymphocyte subpopulations to the control of LVS infection in vitro.
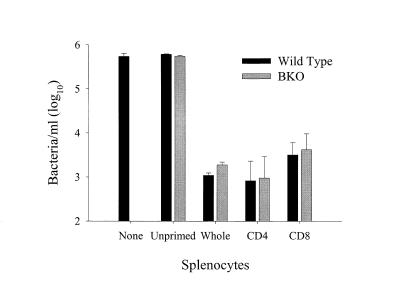
To examine the cellular basis of the control of LVS growth in infected BMMφ, T cells and B cells enriched from wild-type mice were compared for the ability to control the growth of LVS in vitro. LVS-primed T cells (2.3 × 106), but not B cells (2.3 × 106), controlled the growth of LVS in vitro (Fig. 3); neither unprimed whole splenocytes (Fig. 3) nor unprimed separated T or B cells from naive spleens (data not shown) were effective in controlling the growth of LVS. The control of LVS growth in cultures with LVS-primed T cells was not significantly different from that observed in cultures with whole splenocyte populations (Fig. 3).
FIG. 3.
Control of LVS growth by lymphocyte subpopulations. Uninfected mice served as a source of unprimed splenocytes. Mice primed intradermally with LVS 4 weeks prior to the onset of the experiment (Primed) served as the source of LVS-primed splenocytes. T and B lymphocytes were enriched from spleens of primed mice as described in Materials and Methods; efficacy of enrichment was determined by flow cytometry. Immediately following infection of BMMφ, unprimed splenocytes (5 × 106/well), primed (Whole) splenocytes (5 × 106/well), or primed T cells or B cells (2.3 × 106/well) were added. Seventy-two hours after infection, cultures were assessed for intracellular bacteria. These results are representative of three experiments similar in design.
To determine whether immune cells from BKO mice control LVS growth in vitro, we compared the ability of LVS-primed splenocytes from wild-type mice and BKO mice to control the growth of LVS in vitro. LVS-primed splenocytes from wild-type and BKO mice were not significantly different in the ability to control LVS growth in either BKO (Fig. 4B) or wild-type BMMφ (Fig. 4A). At each time point after infection, growth of LVS in either type of BMMφ was similarly controlled by both wild-type and BKO LVS-primed splenocytes (Fig. 4A and B). Since there were no significant differences in the abilities of wild-type and BKO macrophages to support LVS growth in vitro, we used wild-type BMMφ in subsequent experiments.
FIG. 4.
Growth and control of LVS in BMMφ by wild-type and BKO splenocytes. BMMφ from wild-type (A) or BKO (B) mice were infected with LVS. Immediately following infection, splenocytes from wild-type (5 × 106/well) or BKO (2.5 × 106/well) mice primed intradermally with LVS 4 weeks prior to the onset of the experiment were added to cultures. At the indicated time points after infection, cultures were assessed for intracellular bacteria. These results are representative of three experiments similar in design.
It has been previously shown that either CD4+ or CD8+ T cells in normal mice are capable of contributing to the survival of LVS infection in vivo (41). To determine if BKO mice generate T-cell subpopulations capable of controlling LVS growth similarly to wild-type T-cell subpopulations, CD4+ and CD8+ T cells enriched from spleens of both wild-type and BKO LVS-primed mice were compared. In agreement with in vivo data, both CD4+ and CD8+ T cells from wild-type mice were capable of controlling LVS growth in vitro (Fig. 5). Further, both CD4+ and CD8+ T cells from BKO mice were capable of controlling LVS growth in vitro (Fig. 5). There were no significant differences in the control of LVS growth by CD4+ and CD8+ T cells between wild-type and BKO mice. Neither unprimed splenocytes from either wild-type or BKO mice (Fig. 5) nor purified CD4+ or CD8+ T cells from naive wild-type or BKO mice (data not shown) were able to control LVS growth in vitro.
FIG. 5.
Control of LVS growth by T-lymphocyte subpopulations. Uninfected mice served as a source of unprimed splenocytes. Wild-type (black bars) and BKO (gray bars) mice primed intradermally with LVS 4 weeks prior to the onset of the experiment served as the sources of LVS-primed splenocytes. CD4+ and CD8+ T lymphocytes were enriched from the spleens of primed mice as described in Materials and Methods; efficacy of enrichment was determined by flow cytometry. Immediately following infection of BMMφ, the following were added to cultures: 5 × 106 unprimed splenocytes/well; 5 × 106 wild-type or 2.5 × 106 BKO (Whole) primed splenocytes/well; and 1.3 × 106 wild-type CD4+, 1 × 106 wild-type CD8+, 1.1 × 106 BKO CD4+, or 8.8 × 105 BKO CD8+ T cells/well. Seventy-two hours after infection, cultures were assessed for intracellular bacteria. These results are representative of three experiments similar in design.
Role of cytokines and NO in the control of LVS infection in vitro.
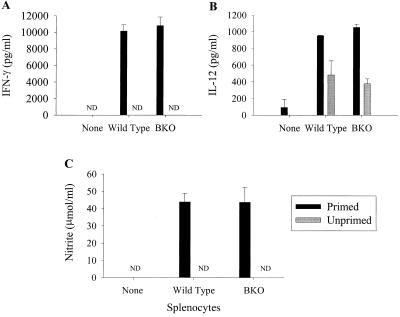
It has previously been demonstrated that TNF-α and IFN-γ play central roles in controlling LVS infection in vivo (2, 11, 12, 21). Further, one role of IFN-γ during LVS infection was the stimulation of macrophages to secrete NO, thus resulting in intracellular killing of LVS in vitro (15). To study the mechanism by which splenocytes from wild-type and BKO mice control LVS growth in vitro, secretion of cytokines and nitrite (as an indicator of NO production) into culture supernatants was examined. IFN-γ was secreted from both wild-type and BKO LVS-primed splenocytes, but not unprimed splenocytes, in response to LVS-infected macrophages (Fig. 6A). Supernatants from cultures containing primed wild-type splenocytes had concentrations of IFN-γ similar to those of supernatants from cultures containing primed BKO splenocytes (Fig. 6A). IFN-γ was not detected in the absence of splenocytes (Fig. 6A). Small amounts of IL-12 were detected in supernatants from LVS-infected BMMφ alone; these amounts increased upon addition of unprimed splenocytes and were largest upon addition of primed splenocytes (Fig. 6B). The concentration of IL-12 was similar in cultures containing either wild-type or BKO primed splenocytes (Fig. 6B). IL-4, IL-10, and TNF-α were not detected in any of the supernatants (<156 pg/ml; data not shown).
FIG. 6.
Secretion of cytokines and NO into culture supernatants following LVS infection. Splenocytes from either unprimed wild-type or BKO mice (gray bars) or mice primed with 8 × 104 LVS 4 weeks prior to the onset of the experiment (black bars) were added to cultures of LVS-infected BMMφ. Culture supernatants (triplicate samples) were tested for IFN-γ (A), IL-12 (B), or NO (C) 72 h after addition of splenocytes. “None” represents LVS-infected BMMφ alone. ND, not detected. Each bar represents the mean ± the SEM cytokine or NO concentration of a group. These results are representative of three experiments similar in design.
The secretion of NO by activated macrophages into the supernatant was also examined. NO (nitrite) was detected in supernatants of cultures containing either splenocytes from either wild-type or BKO LVS-primed mice (Fig. 6C). In the absence of LVS-primed splenocytes, NO was not detected (Fig. 6C). Cultures containing LVS-primed BKO splenocytes had concentrations of NO in their supernatants similar to those of cultures containing wild-type splenocytes (Fig. 6C).
Time course of development of cell-mediated immune responses that control LVS growth in vitro.
To examine events during the early phase and the development of the immune response, LVS-primed splenocytes from BKO and wild-type mice were tested for the ability to control LVS infection in vitro 1 to 4 weeks after priming. Previous experiments demonstrated no control of LVS growth by unprimed splenocytes (Fig. 1 to 3, 5, and 6); therefore, unprimed splenocytes were not included here. Mice were primed with LVS intradermally 1, 2, 3, or 4 weeks before splenocytes were harvested and were added simultaneously to LVS-infected BMMφ. Control of LVS growth was assessed 72 h after LVS infection of BMMφ (Fig. 7), and supernatants were evaluated for cytokines and NO (Fig. 8). Alternatively, groups of mice were primed at the same time and splenocytes from selected mice were harvested each week after priming for addition to LVS-infected BMMφ. Similar results for control of LVS growth and secretion of cytokines and NO were observed in experiments using either experimental design (data not shown).
FIG. 7.
Control of LVS in BMMφ by wild-type and BKO mouse splenocytes at various time points after priming. BMMφ were infected with LVS. Immediately following infection, splenocytes from wild-type or BKO mice primed intradermally with LVS 1, 2, 3, and 4 weeks prior to the onset of the experiment were added to cultures in cDMEM (A) or cDMEM containing anti-IFN-γ antibodies (10 μg/ml) (B) at 5 × 106 or 2.5 × 106 splenocytes/well, respectively. Seventy-two hours after infection, cultures were assessed for intracellular bacteria. The data shown are the mean numbers of viable bacteria ± the SEM recovered from macrophages alone (hatched bar) and cultures containing wild-type (black bars) or BKO (gray bars) splenocytes at the indicated time points. These results are representative of three experiments similar in design.
FIG. 8.
Secretion of cytokines and NO into culture supernatants following LVS infection. Splenocytes from wild-type (unfilled bars) or BKO (diagonal bars) mice primed intradermally with LVS 1, 2, 3, and 4 weeks prior to the onset of the experiment were added to cultures of LVS-infected BMMφ at 5 × 106 or 2.5 × 106 splenocytes/well, respectively. Culture supernatants (triplicate samples) were tested for IFN-γ (A), IL-12 (B), or NO (C) 72 h after addition of splenocytes. “None” represents LVS-infected BMMφ alone (black bars). ND, not detected. Each bar represents the mean concentration of cytokine or NO ± the SEM in a group. These results are representative of three experiments similar in design.
Both BKO and wild-type splenocytes were capable of controlling the growth of LVS as early as 1 week following priming (Fig. 7A). Splenocytes from both wild-type and BKO mice were equally capable of controlling LVS infection 3 and 4 weeks after priming (Fig. 7A). However, cultures containing BKO splenocytes 1 and 2 weeks after priming controlled growth significantly better than cultures containing wild-type splenocytes at these time points (Fig. 7A). As seen above, secretion of IFN-γ and nitrite, but not IL-4, was associated with control of LVS growth at each time point after LVS priming (Fig. 8A). Three weeks after priming, cultures containing wild-type or BKO splenocytes had similar concentrations of IFN-γ but greater concentrations of IFN-γ were detected in cultures with BKO splenocytes 1 and 2 weeks after priming (Fig. 8A), corresponding to greater control of LVS growth (Fig. 7A). To directly test the importance of IFN-γ in the control of LVS infection, we neutralized IFN-γ via inclusion of anti-IFN-γ antibodies at the outset of the in vitro culture. Neutralization of IFN-γ in cultures prevented detection of IFN-γ in culture supernatants (all supernatants contained less than 156 pg/ml) and inhibited the control of LVS growth in cultures containing either wild-type or BKO splenocytes at each time point after infection (Fig. 7B), with one exception. Anti-IFN-γ had no significant effect on the control of LVS growth by wild-type splenocytes obtained 1 week after LVS priming; further, neutralization of IFN-γ appeared to have a greater impact on the ability of BKO splenocytes to control growth 1 and 2 weeks after priming compared to wild-type splenocytes (Fig. 7B). As described above, small amounts of IL-12 were detected in supernatants from LVS-infected BMMφ (Fig. 8B). Secretion of IL-12 was greatest in cultures containing BKO splenocytes 1 week after priming and was similar in cultures containing BKO splenocytes 2, 3, and 4 weeks after priming (Fig. 8B). In cultures containing wild-type splenocytes, secretion of IL-12 peaked 3 and 4 weeks after priming (Fig. 8B). Although the concentration of IL-12 was greater in cultures containing BKO splenocytes than in those with wild-type splenocytes 1 week after priming, concentrations of IL-12 were similar in cultures containing either BKO or wild-type splenocytes thereafter (Fig. 8B). Both wild-type and BKO supernatants in these experiments were also tested for IL-4, IL-10, and TNF-α, none of which were detected at any time (<156 pg/ml; data not shown). Secretion of NO was similar between the two groups of mice throughout the course of the experiment (Fig. 8C), although the greatest amounts of NO were detected in supernatants from cultures containing LVS-primed splenocytes 1 week after priming. NO was not detected (less than 0.312 μmol/ml) at any time in cultures in which IFN-γ was neutralized.
Marked neutrophilia in BKO spleens compared to wild-type spleens after priming and during secondary infection with LVS.
Since no deficiencies in cytokine profiles or the capacity of T cells from wild-type and BKO mice to control LVS infection in vitro were detected, the in vivo trafficking of cells to the spleen, a major site of infection, was examined. The cellular composition of wild-type and BKO spleens was examined 1 to 4 weeks after primary infection (priming), as well as 1 to 3 days following a secondary lethal infection. Prior to priming, the primary cell type in both wild-type and BKO spleens was the lymphocyte (Fig. 9). Following intradermal priming with LVS, both wild-type and BKO mice had noticeable increases in all cell types (Fig. 9). However, BKO mice had significantly greater numbers of neutrophils during the first 2 weeks after priming compared to wild-type mice (P < 0.05) (Fig. 9). This marked neutrophil influx in BKO mice was threefold greater than in wild-type mice and represented as much as 50% of the total splenocyte population 1 week after priming. Two weeks after priming, the number of neutrophils had decreased in BKO mice but was still twofold greater compared to the number of neutrophils found in spleens of wild-type mice (Fig. 9). BKO mice also had a large increase in the number of lymphocytes during the first week after priming, such that the number of lymphocytes became similar to that found in wild-type mice at this time point (Fig. 9). The ratio of CD4+ to CD8+ T cells in wild-type mice rose slightly with time following priming but remained similar in BKO mice throughout the course of the experiment (data not shown).
FIG. 9.
Splenocyte populations in wild-type and BKO mice 1, 2, 3, and 4 weeks following priming with LVS. Splenocytes from mice (three per group) were harvested, adhered to slides by cytocentrifugation, and stained for examination of cell morphology using a modified Wright-Giemsa stain. Cells categorized as lymphocytes, macrophages, and neutrophils are represented. Splenocytes from uninfected (Unprimed) mice are also shown. BKO mice had a significantly greater numbers of neutrophils 1, 2, and 3 weeks after priming compared to wild-type mice (P < 0.05). Wild-type mice had significantly more lymphocytes at each time point before and after priming compared to BKO mice (all P < 0.05). These results are representative of two experiments similar in design.
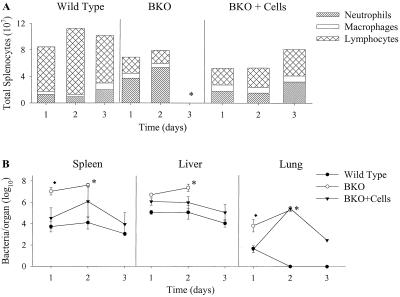
Following a secondary lethal LVS challenge, a similar pattern of neutrophilia in BKO mice was observed (Fig. 10A). Although an influx of neutrophils was observed in both wild-type and BKO spleens after challenge, BKO mice had three- to fivefold greater numbers of neutrophils compared to wild-type mice up to 2 days after challenge (Fig. 10A). Remarkably, neutrophils comprised as much as 65% of the total cellular population in BKO mice 1 and 2 days after challenge. Minimal changes were seen in lymphocyte or macrophage populations. The mean time to death in BKO mice was 3 days; thus, further analyses of cell populations in BKO spleens at later time points were not possible. However, as early as 1 day after challenge, BKO mice had significantly greater numbers of bacteria in all organs compared to wild-type mice (P < 0.05) (Fig. 10B). To determine if the presence of B cells was associated with control of bacterial growth and regulation of neutrophil accumulation in the spleen, BKO mice primed with LVS were reconstituted with naive B cells 7 days prior to a secondary challenge. As previously described (9), reconstitution of LVS-primed BKO mice with naive B cells restored the ability to survive a secondary LVS challenge; six of six mice survived through day 90 after challenge. Furthermore, reconstituted BKO mice had fewer bacteria in their spleens, livers, and lungs following a secondary challenge compared to nonreconstituted BKO mice (Fig. 10B). These bacterial burden differences were most dramatic in the spleen, where reconstituted BKO mice had nearly 1,000-fold fewer bacteria compared to nonreconstituted BKO mice 1 day after the challenge (Fig. 10B). When splenocytes were analyzed for cellular populations, reconstituted BKO mice had similar numbers of neutrophils in their spleens compared to wild-type mice and significantly fewer neutrophils in their spleens at each time point after infection, compared to nonreconstituted BKO mice (P < 0.05; Fig. 10A). Therefore, survival of a secondary LVS challenge was dependent on the presence of B cells and death was associated with very large numbers of neutrophils in the spleen very early after a lethal challenge.
FIG. 10.
Growth of LVS and analysis of splenocyte populations in wild-type C57BL/6J mice; BKO mice, and reconstituted BKO mice following i.p. infection. Wild-type mice, BKO mice, and BKO mice that received 2.3 × 107 naive B cells prior to infection were studied. All mice were primed intradermally with 8 × 104 LVS bacteria 4 weeks prior to i.p. infection. (A) Splenocyte populations in wild-type, BKO, and reconstituted BKO mice following i.p. challenge with 5 × 105 LVS bacteria. Splenocytes from mice were harvested, adhered to slides by cytocentrifugation, and stained for examination of cell morphology using a modified Wright-Giemsa stain. Cells categorized as lymphocytes, macrophages, and neutrophils are represented. BKO mice had significantly greater numbers of neutrophils 1 and 2 days after infection compared to wild-type and reconstituted BKO mice (P < 0.05). These results are representative of two experiments similar in design. (B) Growth of LVS in organs following i.p. challenge. The data shown are the mean numbers ± the SEM of viable bacteria recovered from the spleens, lungs, and livers at the indicated time points. ⧫, statistically significant difference (P < 0.05) compared to wild-type mice; ∗, all remaining mice dead by day 3 after infection. Although not represented here, BKO mice had statistically significantly greater numbers of bacteria in the spleens and lungs compared to reconstituted mice 1 day after infection. Both wild-type and reconstituted mice survived for more than 90 days following infection.
DISCUSSION
Previously, we demonstrated that B-cell-deficient mice have a defect in optimal protection against a secondary lethal F. tularensis LVS challenge that is due to a function of B cells not associated with antibody production. In this study, we directly examined three other possible B-cell functions that might explain this defect. Using a new in vitro culture system that clearly replicates in vivo features of LVS infection, we first demonstrated that B cells were not directly involved in controlling intracellular LVS growth (Fig. 3). Second, we demonstrated directly that B cells are not required for the development of maximal secondary CD4+ and CD8+ T-cell responses against LVS (Fig. 4, 5, and 7); thus, B cells do not appear to play a major role in antigen presentation and/or T-cell priming in LVS infection. Third, we demonstrated that the mechanisms by which BKO and wild-type mice control LVS growth in vitro appear to be similar: control of LVS growth was primarily, but not completely, dependent on IFN-γ (Fig. 7) and NO generation (Fig. 6 and 8). Instead, the inability of BKO mice to survive a lethal secondary challenge with LVS was associated with uncontrolled bacterial growth as early as 1 day after challenge (Fig. 9) and marked neutrophilia following challenge (Fig. 10). These increased bacterial burdens resulted in a massive influx of neutrophils into target organs, likely leading to neutrophil stimulation, degranulation, and subsequent development of shock. The neutrophilia and lack of control of bacterial growth were directly associated with the absence of B cells: reconstitution of BKO mice with naive B cells prior to a secondary lethal challenge resulted in control of bacterial growth (Fig. 10), largely reversed the neutrophilia observed in BKO mice (Fig. 10), and resulted in the survival of BKO mice (9; see Results).
There are several explanations as to why BKO mice may be unable to control the growth of LVS during the first 24 h of infection. First, BKO macrophages may be more permissive to LVS uptake or may kill intracellular LVS less efficiently. However, BMMφ from BKO and wild-type mice were similar in the ability to both support the growth of LVS and participate in the control of LVS growth (Fig. 1 and 5). Despite these similarities, it is possible that the mechanism of uptake and activation of peritoneal macrophages (the resident macrophages at the site of a secondary in vivo challenge) may be different from that of cultured, resting BMMφ. We believe this is unlikely, since previous reports have shown that thioglycolate-elicited peritoneal macrophages are similar to our BMMφ in the ability to take up, support the growth of, and participate in the killing of LVS (1, 6, 15). Additionally, to date, physiological differences between macrophages from BKO and wild-type mice have not been described.
Another possible explanation for the lack of control of bacterial growth in BKO was that the mechanism for the control of LVS growth is different or less efficient in BKO mice compared to wild-type mice. Previous studies have demonstrated an essential role for IFN-γ in the optimal control of LVS growth in vivo (2, 11, 12, 21, 32). Using either IFN-γ knockout mice or mice in which IFN-γ was neutralized following administration of anti-IFN-γ monoclonal antibodies, it was observed that IFN-γ was required very early following a challenge with LVS (1 to 3 days after challenge) to ensure survival of the host (10, 11, 12, 21). Therefore, we examined both the secretion of IFN-γ into culture supernatants containing BKO or wild-type splenocytes primed for various lengths of time and the effect that neutralizing IFN-γ has on the growth of LVS in vitro. In agreement with in vivo data, IFN-γ appeared to be central in the control of LVS growth in vitro for two reasons. First, IFN-γ was the predominant cytokine detected in culture supernatants; in fact, greater concentrations were detected in cultures containing BKO splenocytes compared to wild-type splenocytes during the first 2 weeks following priming (Fig. 8). Second, and importantly, neutralization of IFN-γ resulted in significant reversal of the control of LVS growth in cultures containing either wild-type or BKO splenocytes (Fig. 7). In addition to IFN-γ, a role for NO in the control of LVS growth in vitro has also been proposed (1, 6, 14, 15). Our data support those observations, since NO was detected in all of the cultures where LVS growth was controlled (Fig. 7 and 8). Additionally, with the exception of 1 week after priming, cultures containing BKO splenocytes had concentrations of NO similar to those of cultures with wild-type splenocytes (Fig. 6 and 8). Taken together, these findings indicate that the mechanisms of control of LVS growth by IFN-γ and NO appear to be similar in wild-type and BKO mice. Since blockade of IFN-γ did not completely reverse the control of LVS growth, it is likely that non-IFN-γ-mediated mechanisms play a role as well (24). Studies of the impact of inhibition of NO on the control of bacterial growth in these cultures are ongoing, and the results suggest that non-NO-mediated mechanisms are also involved in the response to LVS (data not shown; see reference 24).
We also examined the secretion of other cytokines following in vitro infection with LVS. IL-4 and IL-10 were not detected in culture supernatants, suggesting that their contribution to the control of LVS growth is minimal; this is consistent with previous in vivo studies indicating that IL-4 is not required for resolution of a primary LVS infection (21). IL-12 was readily detected in supernatants containing either primed of unprimed splenocytes, although the largest concentrations of IL-12 were detected in cultures containing primed splenocytes that included IFN-γ (38). Importantly, supernatants from cultures containing either wild-type or BKO splenocytes had similar concentrations of IL-12. In an effort to identify the role of IL-12 during LVS infection, we neutralized IL-12 in culture supernatants at the outset of infection. Surprisingly, although the same effect was observed in cultures containing either wild-type or BKO splenocytes, blockade of IL-12 had little, if any, effect on the ability of LVS-primed splenocytes to control the growth of LVS in vitro (data not shown). These observations are the subject of further studies by our laboratory (K. L. Elkins, A. C. Cooper, S. Colombini, and T. L. Kieffer, unpublished data).
Analysis of bacterial loads in target organs of BKO mice following a secondary challenge revealed unrestricted growth of LVS as early as 1 day (Fig. 9) and even 4 h after the challenge (data not shown). Thus, survival of LVS infection of BKO mice appeared to depend on events that occur within hours of a secondary infection. Thus, we examined cellular changes in wild-type and BKO mice that were given a secondary challenge (Fig. 10A). Remarkably, the largest difference between wild-type mice destined to survive the challenge and BKO mice destined to die was enormous neutrophilia in the spleen.
Although previous studies have shown that neutrophils are required for survival of LVS infection (5, 11, 31), our data suggested that excessive neutrophilia during an in vivo LVS infection is detrimental. Future studies will therefore examine the impact of intermediate levels of in vivo depletion of neutrophils. Preliminary results from our laboratory suggest that neutrophils do not directly participate in the control of LVS growth in BMMφ. In those studies, neither peritoneal neutrophils elicited by proteose peptone nor neutrophils enriched from spleens of BKO mice primed with LVS 1 week prior to their harvest controlled the growth of LVS in BMMφ (C. M. Bosio and K. L. Elkins, unpublished data). Other studies indicate that neutrophils contribute to the survival of primary and secondary LVS infections by killing infected hepatocytes (4, 5, 31). However, examination of liver sections from BKO mice stained with hematoxylin and eosin did not reveal a large neutrophil response in this organ following a lethal challenge compared to wild-type or reconstituted BKO mice, despite the presence larger numbers of bacteria in the BKO mouse livers (data not shown). Taken together, the data suggest that the defect in BKO mice is associated primarily with neutrophilia in the spleen.
Our observations of neutrophilia in BKO mice following a challenge with LVS are consistent with a recent report demonstrating persistent neutrophilia of both the liver and spleen in BKO mice following infection with Leishmania donovani. In that study, greater numbers of neutrophils were associated with marked tissue pathology following infection with L. donovani in BKO mice and depletion of neutrophils resulted in elevated parasite burdens in both BKO and wild-type mice (33). Similar to those of our studies, those results suggested that both B cells and neutrophils participate in establishing the fine line between effective control of pathogen growth and development of compromising pathology.
Other models of infectious disease have shown that early inflammatory events during the innate immune response, while not necessarily affecting the generation of specific cell-mediated immunity, can have profound effects on the outcome of infection. For example, mice treated with anti-IL-1 antibodies cannot survive infection with doses of L. monocytogenes that are sublethal in untreated mice (27), suggesting that the presence of adequate concentrations of IL-1 are important for optimal survival of a primary infection. On the other hand, overproduction of proinflammatory cytokines such as IL-1 has also been associated with poor outcome in a number of models for other disease states (19, 23) and with multiple organ failure and death (reviewed in references 8 and 30). IL-1 appears to play a key role in sustaining inflammatory responses associated with these conditions through recruitment of neutrophils and macrophages (8, 29, 34). To date, the role of IL-1 in LVS infection has not been studied. It is possible that BKO mice are unable to appropriately regulate IL-1, IL-1 receptor α, or IL-6. This, in turn, would result in their inability to control bacterial growth very early after infection, increased migration of neutrophils to the site of infection, and subsequent generation of even more proinflammatory cytokines; death from shock would ensue before T-cell-mediated immunity had an opportunity to intervene. Thus, future studies will examine immediate innate events in more detail, including the contribution by B cells to the regulation of proinflammatory cytokines and the accumulation and activation of neutrophils at the site of infection.
ACKNOWLEDGMENTS
We thank our CBER colleagues Suzanne Epstein and Steven Kozlowski for thoughtful critical review of the manuscript.
This research was supported in part by an appointment to the Aftergraduate Research Program at the Centers for Biologics, Evaluation, and Research administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. Food and Drug Administration.
REFERENCES
- 1.Anthony L S D, Burke R D, Nano F E. Growth of Francisella spp. in rodent macrophages. Infect Immun. 1991;59:3291–3296. doi: 10.1128/iai.59.9.3291-3296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anthony L S D, Ghadirian E, Nestel F P, Kongshavn P A L. The requirement for gamma interferon in resistance of mice to experimental tularemia. Microb Pathog. 1989;7:421–428. doi: 10.1016/0882-4010(89)90022-3. [DOI] [PubMed] [Google Scholar]
- 3.Anthony L S D, Kongshavn P A L. Experimental murine tularemia caused by Francisella tularensis, live vaccine strain: a model of acquired cellular resistance. Microb Pathog. 1987;2:3–14. doi: 10.1016/0882-4010(87)90110-0. [DOI] [PubMed] [Google Scholar]
- 4.Conlan J W, North R J. Neutrophil-mediated dissolution of infected host cells as a defense strategy against a facultative intracellular bacterium. J Exp Med. 1991;174:741–744. doi: 10.1084/jem.174.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conlan J W, North R J. Early pathogenesis of infection in the liver with the facultative intracellular bacteria Listeria monocytogenes, Francisella tularensis, and Salmonella typhimurium involves lysis of infected hepatocytes by leukocytes. Infect Immun. 1992;60:5164–5171. doi: 10.1128/iai.60.12.5164-5171.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cowley S, Myltseva S, Nano F E. Phase variation in Francisella tularensis affecting intracellular growth, lipopolysaccharide antigenicity, and nitric oxide production. Mol Microbiol. 1996;20:867–874. doi: 10.1111/j.1365-2958.1996.tb02524.x. [DOI] [PubMed] [Google Scholar]
- 7.Culkin S J, Rhinehart-Jones T, Elkins K L. A novel role for B cells in early protective immunity to an intracellular pathogen, Francisella tularensis strain LVS. J Immunol. 1997;158:3277–3284. [PubMed] [Google Scholar]
- 8.Dinarello C A. Proinflammatory and anti-inflammatory cytokines as mediators in the pathogenesis of septic shock. Chest. 1997;112(Suppl. 6):321S–329S. doi: 10.1378/chest.112.6_supplement.321s. [DOI] [PubMed] [Google Scholar]
- 9.Elkins K L, Bosio C M, Rhinehart-Jones T R. Importance of B cells, but not specific antibodies, in primary and secondary protective immunity to the intracellular bacterium Francisella tularensis live vaccine strain. Infect Immun. 1999;67:6002–6007. doi: 10.1128/iai.67.11.6002-6007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elkins K L, Leiby D A, Winegar R K, Nacy C A, Fortier A H. Rapid generation of specific protective immunity to Francisella tularensis. Infect Immun. 1992;60:4571–4577. doi: 10.1128/iai.60.11.4571-4577.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elkins K L, Rhinehart-Jones T R, Culkin S J, Yee D, Winegar R K. Minimal requirements for murine resistance to infection with Francisella tularensis LVS. Infect Immun. 1996;64:3288–3293. doi: 10.1128/iai.64.8.3288-3293.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elkins K L, Rhinehart-Jones T, Nacy C A, Winegar R K, Fortier A H. T-cell-independent resistance to infection and generation of immunity to Francisella tularensis. Infect Immun. 1993;61:823–829. doi: 10.1128/iai.61.3.823-829.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elkins K L, Winegar R K, Nacy C A, Fortier A H. Introduction of Francisella tularensis at skin sites induces resistance to infection and generation of protective immunity. Microb Pathog. 1992;13:417–421. doi: 10.1016/0882-4010(92)90085-3. [DOI] [PubMed] [Google Scholar]
- 14.Fortier A H, Green S J, Polsinelli T, Jones T R, Crawford R M, Leiby D A, Elkins K L, Meltzer M S, Nacy C A. Life and death of an intracellular pathogen: Francisella tularensis and the macrophage. Immunol Ser. 1994;60:349–361. [PubMed] [Google Scholar]
- 15.Fortier A H, Polsinelli T, Green S J, Nacy C A. Activation of macrophages for destruction of Francisella tularensis: identification of cytokines, effector cells, and effector molecules. Infect Immun. 1992;60:817–825. doi: 10.1128/iai.60.3.817-825.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fortier A H, Slayter M V, Ziemba R, Meltzer M S, Nacy C A. Live vaccine strain of Francisella tularensis: infection and immunity in mice. Infect Immun. 1991;59:2922–2928. doi: 10.1128/iai.59.9.2922-2928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green L C, Wagner A, Glogowski J, Skipper P L, Wishnok J S, Tannenbaum S R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 18.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 19.Kwak D J, Augustine N H, Borges W G, Joyner J L, Green W F, Hill H R. Intracellular and extracellular cytokine production by human mixed mononuclear cells in response to group B streptococci. Infect Immun. 2000;68:320–327. doi: 10.1128/iai.68.1.320-327.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leef M, Elkins K L, Barbic J, Shahin R D. Protective immunity to Bordetella pertussis requires both B cells and CD4+ T cells for key functions other than specific antibody production. J Exp Med. 2000;191:1841–1852. doi: 10.1084/jem.191.11.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leiby D A, Fortier A H, Crawford R M, Schreiber R D, Nacy C A. In vivo modulation of the murine immune response to Francisella tularensis LVS by administration of anticytokine antibodies. Infect Immun. 1992;60:84–89. doi: 10.1128/iai.60.1.84-89.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahon B P, Sheahan B J, Griffin F, Murphy G, Mills K H. Atypical disease after Bordetella pertussis respiratory infection of mice with targeted disruptions of interferon-γ receptor or immunoglobulin μ chain genes. J Exp Med. 1997;186:1843–1851. doi: 10.1084/jem.186.11.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orozco A S, Zhou X, Filler S G. Mechanisms of the proinflammatory response of endothelial cells to Candida albicans infection. Infect Immun. 2000;68:1134–1141. doi: 10.1128/iai.68.3.1134-1141.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polsinelli T, Meltzer M S, Fortier A H. Nitric oxide-independent killing of Francisella tularensis by IFN-γ-stimulated murine alveolar macrophages. J Immunol. 1994;153:1238–1245. [PubMed] [Google Scholar]
- 25.Rhinehart-Jones T R, Fortier A H, Elkins K L. Transfer of immunity against lethal murine Francisella infection by specific antibody depends on host gamma interferon and T cells. Infect Immun. 1994;62:3129–3137. doi: 10.1128/iai.62.8.3129-3137.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhoades E R, Orme I M. Susceptibility of a panel of virulent strains of Mycobacterium tuberculosis to reactive nitrogen intermediates. Infect Immun. 1997;65:1189–1195. doi: 10.1128/iai.65.4.1189-1195.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogers H W, Sheehan K C F, Brunt L M, Dower S K, Unuanue E R, Schreiber R D. Interleukin 1 participates in the development of anti-Listeria responses in normal and SCID mice. Proc Natl Acad Sci USA. 1992;89:1011–1015. doi: 10.1073/pnas.89.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandström G. The tularemia vaccine. J Chem Tech Biotechnol. 1994;59:315–320. doi: 10.1002/jctb.280590402. [DOI] [PubMed] [Google Scholar]
- 29.Sauder D N, Mounessa N L, Katz S I, Dinarello C A, Gallin J I. Chemotactic cytokines: the role of leukocytic pyrogen and epidermal cell thymocyte-activating factor in neutrophil chemotaxis. J Immunol. 1984;132:828–832. [PubMed] [Google Scholar]
- 30.Shanley T P, Warner R L, Ward P A. The role of cytokines and adhesion molecules in the development of inflammatory injury. Mol Med Today. 1995;1:40–45. doi: 10.1016/1357-4310(95)80019-0. [DOI] [PubMed] [Google Scholar]
- 31.Sjöstedt A, Conlan J W, North R J. Neutrophils are critical for host defense against primary infection with the facultative intracellular bacterium Francisella tularensis in mice and participate in defense against reinfection. Infect Immun. 1994;62:2779–2783. doi: 10.1128/iai.62.7.2779-2783.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sjöstedt A, North R J, Conlan J W. The requirement of tumour necrosis factor-alpha and interferon-gamma for the expression of protective immunity to secondary murine tularaemia depends on the size of the challenge inoculum. Microbiology. 1996;142:1369–1374. doi: 10.1099/13500872-142-6-1369. [DOI] [PubMed] [Google Scholar]
- 33.Smelt S C, Cotterell S E, Engwerda C R, Kaye P M. B cell-deficient mice are highly resistant to Leishmania donovani infection, but develop neutrophil-mediated tissue pathology. J Immunol. 2000;164:3681–3688. doi: 10.4049/jimmunol.164.7.3681. [DOI] [PubMed] [Google Scholar]
- 34.Sutton E T, Norman J, Rao P S, Graham L B, Newton C A, Richards I S. Pulmonary endothelial and epithelial integrity and neutrophil infiltration after endotoxin in interleukin-1 receptor knockout mice. Shock. 2000;13:117–125. doi: 10.1097/00024382-200013020-00005. [DOI] [PubMed] [Google Scholar]
- 35.Tärnvik A. Nature of protective immunity to Francisella tularensis. Rev Infect Dis. 1989;11:440–451. [PubMed] [Google Scholar]
- 36.Tärnvik A, Eriksson M, Sandström G, Sjöstedt A. Francisella tularensis—a model for studies of the immune response to intracellular bacteria in man. Immunology. 1992;76:349–354. [PMC free article] [PubMed] [Google Scholar]
- 37.Tigertt W D. Soviet viable Pasteurella tularensis vaccines. A review of selected articles. Bacteriol Rev. 1962;26:354–373. doi: 10.1128/br.26.3.354-373.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trinchieri G. Proinflammatory and immunoregulatory functions of interleukin-12. Int Rev Immunol. 1998;16:365–396. doi: 10.3109/08830189809043002. [DOI] [PubMed] [Google Scholar]
- 39.Weiner S L. Strategies for the prevention of a successful biological warfare aerosol attack. Mil Med. 1996;161:251–256. [PubMed] [Google Scholar]
- 40.Yang X, Brunham R C. Gene knockout B cell-deficient mice demonstrate that B cells play an important role in the initiation of T cell responses to Chlamydia trachomatis (mouse pneumonitis) lung infection. J Immunol. 1998;161:1439–1446. [PubMed] [Google Scholar]
- 41.Yee D, Rhinehart-Jones T R, Elkins K L. Loss of either CD4+ or CD8+ T cells does not affect the magnitude of protective immunity to an intracellular pathogen, Francisella tularensis strain LVS. J Immunol. 1996;157:5042–5048. [PubMed] [Google Scholar]