Abstract
Objective
Chemoradiotherapy (CRT) for head and neck cancer (HNC) is associated with high toxicity that adversely affects physical functioning, body composition, fatigue, quality of life and treatment outcomes. Exercise interventions during treatment might counteract these negative effects. We therefore assessed the feasibility of an exercise programme for HNC patients during CRT.
Methods
Forty patients were offered a tailored 10‐week endurance and resistance training with supervised and home‐based sessions. Feasibility endpoints were (1) adherence (main outcome): ≥60% attendance; (2) recruitment: ≥30%; (3) retention rate: ≥85% and (4) compliance rate: ≥60%. Physical performance, muscle strength, body composition, quality of life and fatigue were assessed pre‐ and post‐intervention.
Results
Overall adherence was 54%. The recruitment rate was 36%, and the retention rate was 65%. Compliance to the supervised intervention protocol was 66%. Statistically significant decreases were found in mean grip strength, fat‐free mass and clinically relevant deteriorations on several domains of quality of life, and fatigue subscales were found.
Conclusion
We conclude that this exercise programme for HNC patients during CRT in its current form is feasible for only a minority of patients. We suggest adaptations to improve adherence and retention rates for a definitive multicentre trial.
Trial registration
This study is registered at the Netherlands Trial Register (NTR7305), 6 June 2018, retrospectively registered.
Keywords: chemoradiotherapy, endurance training, exercise intervention, feasibility study, head and neck cancer, resistance training
1. INTRODUCTION
Radiotherapy combined with concurrent chemotherapy (CRT) for locally advanced head and neck cancer (HNC) is associated with high toxicity with a negative impact on physical functioning, body composition, fatigue and quality of life (Bressan et al., 2016; Rogers et al., 2006; Silver et al., 2007; Taylor et al., 2004). Treatment toxicity contributes to unintentional weight loss, which is a key characteristic of malnutrition. Already at diagnosis prevalence of critical weight loss is substantial (19%) (Jager‐Wittenaar et al., 2007), and may increase up to 50%, despite intensive nutritional support (Beijer et al., 2013; Jager‐Wittenaar et al., 2011). Weight loss during HNC treatment is characterised by loss of lean body mass, including muscle mass (Hunter & Jolly, 2013; Jager‐Wittenaar et al., 2011). Loss of muscle mass is associated with a decreased health‐related quality of life (HR‐QoL), physical decline, increased risk of treatment toxicity, higher complication rates, and lower survival rates in patients with HNC (Grossberg et al., 2016; Jung et al., 2019; Rogers et al., 2006; Sealy et al., 2020; Silver et al., 2007; Wendrich et al., 2017). To maintain or restore muscle mass during and after treatment, an adequate nutritional intake combined with physical exercise are prerequisites (Fearon et al., 2013). Physical exercise interventions during and after anti‐cancer treatment in cancer populations positively affect fitness, fatigue, HR‐QoL, and treatment completion rates (Campbell et al., 2019; Scott et al., 2018; van Waart et al., 2015). Moreover, higher levels of physical activity and fitness are associated with prolonged survival in several cancer populations (Patel et al., 2019). Most of this evidence is based on studies in patients with breast or colon cancer. Patients with HNC, however, are generally less active compared to other cancer patients: only 30.5% meet physical activity public health guidelines before diagnosis, which further decreases to 8.5% after diagnosis (Rogers et al., 2006). This sedentary behaviour can exacerbate the loss of muscle mass due to decreased muscle activity. Therefore, interventions aiming at improving physical activity and preserving muscle mass are needed. On average, compared to other cancer patients, the HNC population is older, less educated and has a less healthy lifestyle, with higher tobacco and alcohol consumption (Hashibe et al., 2009). Moreover, there is an increase in HNC caused by human papillomavirus (HPV), with better prognosis and different patient characteristics leading to a more heterogeneous HNC group (Sabatini & Chiocca, 2020). Therefore, effects from exercise interventions in other cancer populations may not be generalizable to the HNC population. Pilot studies investigating physical exercise during (chemo)radiation in HNC are limited, have small sample sizes, and mainly focus on efficacy outcomes (e.g., physical functioning and HR‐QoL) instead of feasibility outcomes (Rogers et al., 2013; Samuel et al., 2013; Zhao et al., 2016). It therefore remains unclear whether patients with HNC will be able to complete an exercise intervention to a sufficient degree for the intended effects to occur during CRT.
In a previous study on exercise preferences, only 50% of the HNC patients indicated that they felt being able to participate in an exercise programme (Rogers et al., 2009). The majority preferred to exercise alone, unsupervised, and with flexible scheduling. We therefore developed an exercise programme during CRT adjusted to these preferences, incorporating strength and endurance training at moderate intensity, in a combined supervised and home‐based setting. All exercises were suitable for training at home and tailored to patients' individual capacity.
The primary aim of this study was to assess the feasibility of this tailored exercise programme for HNC patients during CRT. Our secondary aim was to assess changes from pre‐ to post‐intervention in physical performance, muscle strength, body composition, fatigue and health related quality of life (HR‐QoL).
2. METHODS
2.1. Participants and design
Consecutive patients with locally advanced HNC were recruited at the University Medical Center Utrecht and the Netherlands Cancer Institute, between January 2018 and January 2020. Study inclusion criteria were (1) scheduled to receive CRT; (2) age ≥18 years; (3) sufficient Dutch writing and reading skills; (4) Karnofsky Performance status >60; (5) able to walk ≥60 m without aid and (6) no contraindication for physical activity. Demographic and medical data were collected by a study‐specific baseline questionnaire and chart review. Weekly dietary consultations were scheduled as part of usual care. The study was approved by the Medical Ethics Committee of the University Medical Center Utrecht (17‐630) and by the Institutional Review Board of the Netherlands Cancer Institute. Written informed consent was obtained from all participants.
2.2. Exercise intervention
The exercise intervention consisted of a 10‐week combined endurance and resistance training with supervised sessions as well as home‐based sessions. The 10‐week intervention started, preferably, the week before the start of the 7‐week CRT, continued during treatment, and ended 2 to 5 weeks after CRT completion. Due to the short time frame between treatment decision and the start of treatment, the study protocol was adapted 6 weeks after start of the study, allowing baseline measurements also in the first or second week of CRT. Patients attended one session per week at the hospital, supervised by a physiotherapist (PT). Patients were instructed to perform home‐based endurance exercise for 6 days a week and resistance training three times a week.
The endurance training consisted of 30 min moderate‐intensity physical activity; 15 min brisk walking and another 15 min of physical activity of their own choice. Patients were instructed to use the Borg scale (6–20) to rate perceived exertion (RPE) to guide exercise intensity for the endurance training (Borg, 1982), aiming for an RPE between 12 and 15. An activity tracker, the Fitbit Zip (Fitbit LLC, San Francisco, CA), with daily step count was used to motivate patients and provide them with feedback during home‐based activities. Individual targets were based on the distance achieved during the 6MWT.
For the resistance training, patients were instructed to perform six exercises three times a week, targeting major muscle groups (arms, legs, shoulders and core) using body weight and elastic bands for resistance. One of the resistance training sessions per week was performed at the hospital. Exercise type and resistance was adjusted to the participants' capacity based on pragmatic 15‐RM testing and RPE range 12 to 15. Exercise intensity was increased in steps of 10% if patients exceeded the prescribed 15 repetitions. Likewise, intensity was decreased if patients were unable to complete 12 repetitions or reported worsening of symptoms due to the exercise.
2.3. Primary outcome: Feasibility
The primary outcome of this study was the feasibility of the exercise intervention. Feasibility endpoints and accompanying success criteria were based on previous studies (Singh et al., 2018): adherence (main outcome): ≥60% attendance to the supervised training sessions; recruitment: ≥30% of approached patients participating; retention rate: ≥ 85% completing the intervention, and compliance: ≥60% exercising according to the protocol. Adherence to the supervised sessions was defined as the number of attended sessions out of the 10 offered sessions and was recorded by the physiotherapist. Adherence, recruitment and retention rates were obtained by keeping a clinical research file. Compliance with supervised exercise sessions was registered by the physiotherapists, and home‐based sessions were recorded by patients in an exercise log.
2.4. Secondary outcomes
Secondary outcomes included physical performance, muscle strength, body composition, HR‐QoL and fatigue. Physical performance was measured with the 6‐Minute Walk Test (6‐MWT) (Schmidt et al., 2013). Hand grip strength was assessed using the JAMAR dynamometer (Patterson Medical, Warrenville, IL), upper leg and arm muscle strength was assessed by using the Microfet handheld dynamometer (Hoggan scientific, Salt Lake City, UT) according to standardised procedures using the best of three trials on each side for analysis (Trutschnigg et al., 2008). Functional lower body strength was measured by the 30‐Second Chair Stand Test (30‐SCST) (Jones et al., 1999) (Jones et al., 1999) (Jones et al., 1999). Body composition was assessed by Bioelectrical Impedance Analysis (BIA) using the Quadscan 4000 (Bodystat Ltd, Douglas, Isle of Man) according to the standard operating procedures (Zweers et al., 2018) in a fasted state for at least 2 h. The Kyle equation was used to calculate fat‐free mass (FFM) (Kyle et al., 2004). Fat‐free mass index (FFMI) was derived from FFM (kg) divided by height (m) squared (kg/m2). Baseline measurements of physical performance, muscle strength, and body composition were performed at the hospital and were re‐assessed post‐intervention (10 to12 weeks post‐baseline).
HR‐QoL was measured using the EORTC QLQ‐C30 and QLQ‐H&N35 questionnaires (Aaronson et al., 1993; Bjordal et al., 1999). Fatigue was measured using the Multidimensional Fatigue Inventory (MFI) (Smets et al., 1996). Clinically important differences were defined as a change in scores of at least 10 points on the EORTC subscales and two points on MFI subscales. Questionnaires were administered on paper at baseline, midway (5 weeks post‐baseline), and post‐intervention (10 to12 weeks post‐baseline). Participants who dropped out were asked to provide the main reason for dropout and to complete the post‐intervention assessments. Consecutive participants and non‐participants were approached for an interview (until data saturation was reached) to gain insight into exercise preferences, barriers and facilitators. These qualitative data will be reported in another paper. Data was captured and stored in Castor (Amsterdam, The Netherlands), an electronic data capture system.
2.5. Sample size calculation
The aim for our main outcome, i.e., adherence, was at least 60% with a minimal acceptable adherence of 45%. Therefore, a sample size of 37 patients (power of 80%) was needed. For compliance, the same precision applies. With 37 patients, a precision resulting in a one‐sided 95% lower limit confidence interval (CI) of 17.5% (80% power) was estimated.
2.6. Statistical analyses
Analyses were performed using IBM SPSS version 26.0 (IBM Corporation, Armonk, NY). Demographic and clinical data were reported as proportions, mean with standard deviation, or median with interquartile range. Feasibility outcomes were described in counts and frequencies with 95% confidence intervals. Paired t tests were used to examine within‐group changes in physical performance.
Within‐group mean changes for patient reported outcomes at baseline, midway and post intervention were evaluated using linear mixed modelling with a random intercept and time as fixed factor, adjusted for centre.
3. RESULTS
In total, 231 patients were screened for inclusion. One hundred and ten patients met the inclusion criteria and were approached for participation in the study. Of those, 40 patients (36%) signed informed consent. Five initially included patients cancelled their participation before the first session of the exercise intervention, due to treatment toxicity and/or emotional distress. Finally, 35 patients (of 110) started the intervention (Figure 1). One participant withdrew consent for using his data, leaving 34 participants for analysis. Due to the COVID‐19 pandemic, recruitment had to be terminated after Participant 35 started the intervention. Patients' baseline characteristics are listed in Table 1.
FIGURE 1.
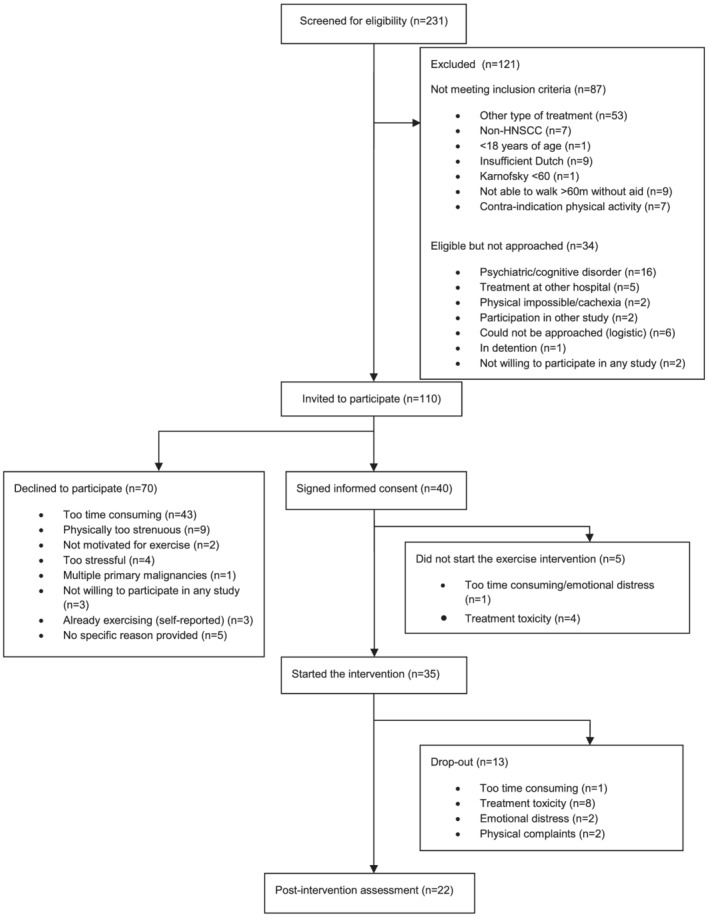
Flow chart participant recruitment and retention
TABLE 1.
Demographic and clinical baseline characteristics of the participants
| Variables | Overall study population (n = 34) |
|---|---|
| Sex; n (%) | |
| Male | 27 (79.4) |
| Female | 7 (20.6) |
| Age (years); median (sd) | 58 (35–70) |
| BMI (kg/m2); mean (sd) | 24.9 (5.4) |
| Educational level; n (%) | |
| Low | 11 (32.4) |
| Middle | 10 (29.4) |
| High | 10 (29.4) |
| Missing | 3 (8.8) |
| Marital status; n (%) | |
| Single/divorced/widowed | 11 (32.4) |
| Married/living together | 23 (67.6) |
| Employment; n (%) | |
| Paid employed | 16 (48.5) |
| Self‐employed | 7 (21.2) |
| Unemployed/household/retired | 5 (15.2) |
| Disabled for work/other | 5 (15.2) |
| Smoking status; n (%) | |
| Current | 2 (5.9) |
| Past | 22 (64.7) |
| Never | 8 (23.5) |
| Missing | 2 (5.9) |
| Alcohol consumption; n (%) | |
| Current user | 17 (50.0) |
| Stopped | 12 (35.3) |
| Never | 4 (11.8) |
| Missing | 1 (2.9) |
| Tumour location; n (%) | |
| Oral cavity | 6 (17.6) |
| Oropharynx | 17 (50.0) |
| Hypopharynx | 3 (8.8) |
| Larynx | 2 (5.9) |
| Nasopharynx | 3 (8.8) |
| Unknown primary tumour | 3 (8.8) |
|
TNM stage; n (%) |
|
| Stage III | 13 (38.2) |
| Stage IV | 21 (61.8) |
| HPV positive; n (%) | 15 (44.1) |
| Type of treatment | |
| CRT | 32 (94.1) |
| BRT | 2 (5.9) |
| Adjuvant CRT | 5 (14.7) |
| Comorbidities; n (%) | 11 (32.4) |
Abbreviations: BMI, body mass index; BRT, cetuximab‐based bioradiotherapy; CRT, cisplatin‐based chemoradiotherapy; HPV, human papilloma virus; TNM, tumour, node, metastasis classification according to the 8th edition.
3.1. Primary outcome: Feasibility
3.1.1. Adherence
Overall adherence to the supervised sessions for the 34 participants was 182 of 340 sessions (54%). Fifteen of the 34 participants (44%) attended at least 60% of the sessions (Figure 2). Patients who completed the intervention (n = 22; 63%) attended a median number of eight supervised sessions (IQR 4–9), while patients who dropped‐out during intervention (n = 13; 37%) attended a median number of two supervised sessions (IQR 2–3). Attendance during the sessions planned after completion of cancer treatment was lower as compared to during CRT, respectively 41% versus 58% (Figure 2). Reasons for not attending or cancelling the supervised session are shown in Table 2, in which treatment toxicity was most often mentioned.
FIGURE 2.
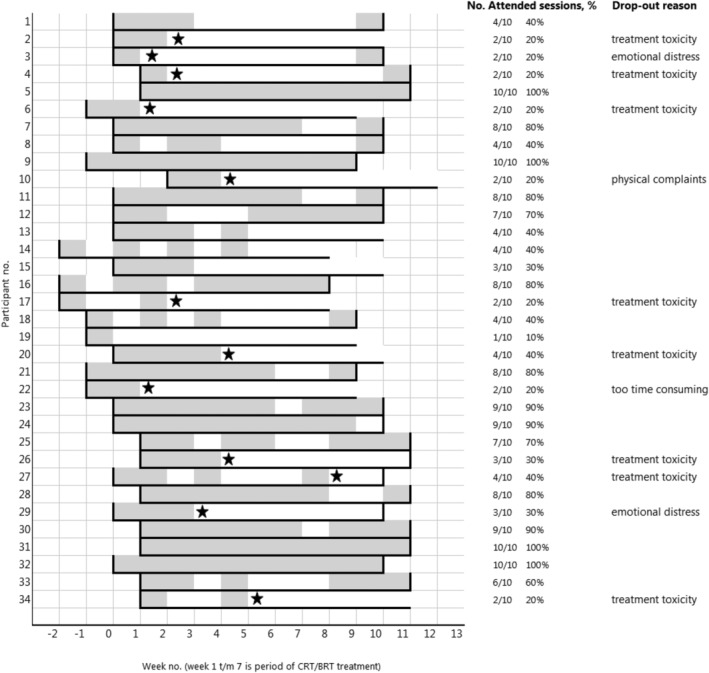
Number of attended training sessions per patient. Each grey box represents an attended session. Each white box represents a not attended session. Horizontal black lines represents the 10‐week intervention period. Vertical black lines at the start and end of the intervention period represent the baseline and post‐intervention measurements. Star symbols represent the timing of dropout.
TABLE 2.
Reasons for not attending a training session in the first 5 weeks and last 5 weeks of the exercise intervention of the participants who completed the 10‐week exercise intervention
| Main reason for absence training session | Missed training sessions Week 1–5 (n = 24, 100%) | Missed training sessions Week 6–10 (n = 44, 100%) |
|---|---|---|
|
Organisational (planning/conflicting appointments) |
4 (16.7%) | 0 (0.0%) |
|
Patient‐related (planning/lack of motivation) |
2 (8.3%) | 8 (18.2%) |
| Treatment toxicity | 8 (33.3%) | 20 (45.5%) |
|
Hospitalisation for chemotherapy |
2 (8.3%) | 3 (6.8%) |
|
Physical complaints (not related to treatment) |
3 (12.5%) | 0 (0.0%) |
|
Gastrostomy placement |
1 (4.2%) | 0 (0.0%) |
|
Missing |
4 (16.7%) | 13 (29.6%) |
Abbreviation: n, the number of missed training sessions.
3.1.2. Recruitment rate
Recruitment rate was 36% (95%CI: 27%–45%) and thus exceeded the feasibility criterion for recruitment (30%). The most common reason for declining participation in the study was the perception that it would be too time consuming (n = 43, 61%) (Figure 1). Due to a slow inclusion, the inclusion criteria were broadened in October 2018; from then also patients receiving a combination of cetuximab and radiotherapy were eligible to participate if meeting all other inclusion criteria.
3.1.3. Retention rate
Twenty‐two of the 34 (65%) participants completed the 10‐week intervention period, resulting in a dropout rate of 35%. The most important reason for dropping‐out was treatment toxicity (n = 8, 67%) (Figure 2).
3.1.4. Compliance
Compliance with the home‐based programme could not be assessed, as only three participants (9%) returned complete exercise logs. Compliance of the supervised strength exercises, defined as an RPE range of 12 to 15 combined with ≥15 RM‐testing, showed compliance to the protocol in 66% over the sessions attended.
3.1.5. Adverse events
Two serious adverse events occurred. One participant was admitted to the hospital for analysis of loss of arm strength and sensation. These symptoms seemed to be related to previous surgery and were already present prior to study entry, but intensified during the intervention. The participant was able to continue to participate in the intervention without arm strength exercises. The other participant collapsed during the first training session due to orthostatic hypotension, probably as a result of exercise in combination with chemo‐induced dehydration and antihypertensive medication. After stabilisation and monitoring in the emergency unit, the participant was dismissed the next day and discontinued study participation.
3.2. Secondary outcomes
3.2.1. Physical performance, muscle strength and body composition
Twenty‐four participants completed both the baseline and post‐intervention physical performance measurements. No significant differences in physical performance and knee extension strength were found between baseline and post‐intervention measurements. Mean handgrip strength and elbow flexion strength significantly decreased during the intervention period (grip strength: −2 kg [95% CI:−4; 0]; elbow flexion strength: −28 N [95%CI: −43; −12]). Mean body weight significantly decreased from baseline to post‐intervention: −5.7 kg (95%CI: −7.5; −3.8) of which 49% was loss of FFM: −2.8 kg (95%CI: −4.1; −1.6) (Table 3).
TABLE 3.
Physical performance, muscle strength and body composition at baseline and post‐intervention presented in means (sd) and mean differences (95% CI)
| Variables | Baseline (n = 24) | 12 weeks after baseline (post‐intervention) (n = 24) | Baseline to 12 weeks Differences | p value |
|---|---|---|---|---|
|
Physical performance |
||||
| 6‐min walking test [min] | 522 (109) | 517 (89) | −5 (−43; 33) | 0.79 |
| 30‐sec chair stand test [n] | 15 (5) | 15 (5) | 0 (−2; 2) | 0.73 |
|
Muscle strength |
||||
| Hand grip strength [kg] | 42 (11) | 40 (10) | −2 (−4; 0) | <0.01 |
| Knee extension [N] | 289 (97) | 298 (101) | 10 (−37; 56) | 0.67 |
| Elbow flexion [N] | 241 (89) | 213 (77) | −28 (−43; −12) | <0.01 |
|
Body composition |
||||
| Body weight [kg] | 78.3 (16.2) | 72.6 (15.3) | −5.7 (−7.5; −3.8) | <0.01 |
| Fat‐free mass [kg] | 57.1 (12.2) | 54.3 (11.6) | −2.8 (−4.1; −1.6) | <0.01 |
| Fat‐free mass [%] | 73.5 (6.5) | 75.5 (7.0) | 2.0 (0.7; 3.2) | <0.01 |
| Fat‐free mass index [kg/m2] | 17.9 (2.8) | 17.0 (2.7) | −0.9 (−1.3; −0.5) | <0.01 |
3.2.2. Health related quality of life and fatigue
Results for HR‐QoL are shown in Appendix S1 (Table S4). Overall, HR‐QoL deteriorated during CRT (week 5 post‐baseline). Some domains recovered at 12‐week post‐baseline, shortly after treatment, whereas scores on most symptom scales were still higher at that time, as compared to baseline.
Appendix S1 (Table S5) shows the results for fatigue. At week 5 post‐baseline, patients reported clinically relevant increases on all domains, except for mental fatigue. Scores on general fatigue, physical fatigue and reduced motivation in week 12 slightly improved as compared to week 5 post‐baseline.
4. DISCUSSION
The primary aim of our study was to assess the feasibility of a tailored exercise programme with combined home‐based and supervised endurance and strength sessions, for patients with HNC during CRT. To assess feasibility, we focused on adherence (main outcome), recruitment, retention and compliance rates. With an overall adherence of 54%, we did not achieve our goal of at least 60%. Recruitment rate was sufficient but the retention rate was lower than expected; 65% instead of 85%. Attendance to the supervised sessions declined after treatment completion, once the participants no longer visited the hospital for radiation treatment. Although the exercise intervention was adjusted to the participants' (changing) capacity during treatment, treatment toxicity was still the most common reason for not attending an exercise session and premature ending study participation. Protocol compliance during the supervised sessions was 66%.
In a recent review on exercise interventions in HNC patients during treatment adherence rates varied between 45% up to 94% (Bye et al., 2020). Our adherence rate of 54% was lower than our aim, and in the lower range compared to the other studies. Especially in the period shortly after treatment a high number of sessions were missed, and we hypothesise that on‐site training at the hospital does not seem to be feasible after HNC treatment completion. Probably this is due to the highest level of treatment toxicity at the end of CRT and the first weeks afterwards (Beijer et al., 2013; Driessen et al., 2016). Symptom burden of HNC treatment was also considered as a reason for non‐adherence in an exploratory trial (Capozzi et al., 2016). Also, long travelling distance to the hospital and planning difficulties (patients prefer to schedule training sessions combined with medical visits, which are less frequent after treatment) were reported in our study as reasons for not attending the training sessions after treatment.
The recruitment rate of 36% exceeded the 30% we aimed for and corresponds to previous studies (Singh et al., 2018). Yet the recruitment period was twice as long as expected, even after broadening our eligibility criteria to include patients receiving cetuximab and radiotherapy due to a lower number of eligible patients. Other studies reported even higher recruitment rates of approximately 60% (Capozzi et al., 2016; Lonkvist et al., 2017). In accordance with other studies, time constraints often due to travelling time was the main reason for not being willing to participate in our study, even only one session per week was hospital‐based (Sheill et al., 2019).
Almost two thirds of the 34 participants starting with the exercise intervention completed the intervention, resulting in a retention rate of 65%, which was fairly equal to the 60% reported previously (Capozzi et al., 2016). Other studies reported much higher retention rates varying between 83% and 100% (Grote et al., 2018; Rogers et al., 2013; Zhao et al., 2016). However, these studies were not completely comparable to ours. Some focused on training during radiotherapy with patients possible experiencing less toxicity as compared to CRT (Grote et al., 2018; Rogers et al., 2013). In other studies, interventions were delivered on‐site during treatment and post‐CRT at home with telephonic support (Rogers et al., 2013; Zhao et al., 2016). Participants preterm ending their study participation mostly stopped at or before week 5. Treatment toxicity, decreased motivation, and physical inability were the main reasons for dropout in our study.
While compliance to the home‐based intervention could not be assessed, compliance to the protocol of the supervised exercises was 66%. Thus, for those attending the supervised sessions, it seems that the resistance exercises were feasible and sufficiently tailored to their personal capacities.
Two serious adverse events were reported resulting in unplanned hospital admissions. We cannot rule out that the exercise intervention contributed to these events, which both occurred during CRT. Careful monitoring of patients before and during the exercise intervention is therefore advised.
The secondary aim of our study was to assess changes in physical performance, muscle strength, body composition, HR‐QoL and fatigue. We did not find significant changes in knee extension strength and physical performance. Significant decreases in grip strength and elbow flexion strength were found. Regardless of the exercise intervention and dietary treatment, body weight and FFM significantly declined during CRT. Previous research showed weight loss during CRT for HNC was particularly loss of fat‐free mass; i.e., 71% of weight loss was due to loss of FFM (Jager‐Wittenaar et al., 2011). In our study, 60% of weight loss could be attributed to a loss in fat mass and only 49% to loss of FFM. Our results suggest that an exercise intervention might help to counteract loss of FFM, but only a large randomised controlled trial would allow definitive conclusions. It is important to prevent FFM loss during CRT as loss of FFM has adverse effect on treatment toxicity, tolerance and survival (Willemsen et al., 2020). Therefore, exercise interventions during treatment should preferably be combined with intensive nutritional support and monitoring.
On average, a relevant decline in HR‐QoL during treatment was found, despite the exercise intervention. Fatigue scores increased from baseline to week 5 and remained stable until week 12. A randomised pilot study (Grote et al., 2018) showed a 9% increase in general fatigue during treatment for the intervention group and a 40% increase for the control group, suggesting a positive effect of exercise on cancer‐related fatigue for cachectic patients with HNC during radiotherapy, as was also shown for other cancer types (Cramp & Byron‐Daniel, 2012). Due to our small study sample and the lack of a control group, we cannot draw conclusions about whether our exercise intervention led to less deterioration of HR‐QoL and less increase in fatigue than would have been the case without the intervention.
4.1. Strengths and limitations
With 34 participants at two study sites, this is one of the largest pilot studies assessing the feasibility of a tailored exercise intervention in HNC during CRT with combined supervised and home‐based sessions. With this sample size, we were able to report feasibility outcomes with sufficient power. However, we also have to consider limitations of our study. Firstly, participants of our study are likely to already be more active than non‐participants, which might have resulted in selection bias. This can be inferred from the baseline results of the 6MWT, which show higher scores as compared to comparable HNC populations (Samuel et al., 2013; Zhao et al., 2016). Also, compared to data from the Dutch Head and Neck audit, participants in our study are younger, and the prevalence of HPV is high (44.1%) (van Overveld et al., 2018). Lastly, the lack of a control group makes it difficult to attribute changes in physical capacity, performance, HR‐QoL, and fatigue between baseline and post‐intervention to the exercise intervention. However, these preliminary data can be used for sample size calculations for future large‐scale interventions.
4.2. Recommendations for future exercise trials in HNC
This feasibility study revealed several barriers that could be addressed to increase inclusion and adherence. Lowering the study load for participants (e.g., less travel, improved logistics planning and fewer questionnaires), using activity trackers that automatically record and store data and give immediate feedback might increase adherence, recruitment and completeness of data collection. To offer a more tailored exercise intervention and to improve feasibility, understanding of patients' preferences to determine preferable timing, intensity and setting is needed.
Some recommend to engage patients with a training programme before treatment but start the actual training programme after treatment (Capozzi et al., 2016; Lonbro et al., 2013). This might result in higher retention and recruitment rates. We suggest to adapt the training schedule in weeks 6 to 10 by replacing on‐site supervised training by home‐based sessions with remote support, to account for the increasing treatment toxicity. After treatment, patients with HNC prefer training at a community location (Jackson et al., 2018). In other cancer patient populations home based training sessions combined with supervised sessions by a physiotherapist resulted in higher adherence rates and showed positive effects on fatigue, cardiorespiratory fitness and muscle strength (van Waart et al., 2015; Velthuis et al., 2010). Training at a community location will be more convenient for our patient population and diminish travel time. The benefits of training in group classes should also be further explored as one study showed that patients with HNC preferred exercise alone prior to participating in an exercise trial, but afterwards preferred group classes which increased motivation for some participants (Jackson et al., 2018). This also emphasises the need for tailored exercise interventions: participants should be able to choose between home‐based, on‐site, alone or group classes. Furthermore, careful focus on the personal goals and capacity of ‘hard to engage’ patients and addressing knowledge gaps about benefits of physical activity and their perceived barriers might increase recruitment and adherence rate (Chinn et al., 2006). Analysis of our qualitative data will give insight into exercise preferences, and possible barriers and facilitators from patients' perspective.
We conclude that this intensive exercise training during CRT for patients with HNC is feasible for a minority of patients in its current form. Adherence to the supervised exercise sessions was lower than expected, although the recruitment rate, retention rate and compliance rate during supervised sessions were reasonably good. We suggest adaptations to improve adherence and retention rates. A more personalised approach, including better motivators and immediate feedback by activity trackers, needs further investigation prior to conducting a definitive multicentre trial.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Supporting information
Table S4. Results of functional and symptom scales of the EORTC C30 and EORTC H&N35 quality of life questionnaires at baseline, week 5 (mid‐intervention) and week 12 (post‐intervention) in means (sd) and mean differences (95% CI).
Table S5. Results of the Multidimensional Fatigue Inventory at baseline, week 5 and week 12 post‐intervention in means (sd) and mean differences (95% CI).
ACKNOWLEDGEMENTS
We would like to thank all physiotherapists, i.e., Ad Kerst, Yvonne Huibers, Boukje van der Zee, Kim van Reysen, Sarah Kager and Danique Deen, for conducting the exercise intervention and dietitians, i.e., Sheena Tjon a Joe, Sara Verschure, Denise Homan, Anne van Dijk, Francis Hollander and Elles Steenhagen, for performing BIA and grip strength measurements. We thank Carina van Deemter for support in literature review, data entry and analysis. Also, special thanks to Chantal Westerink and José Klomp, oncological nurse specialists, for their involvement in preselecting eligible patients at the UMC Utrecht.
Kok, A. , Passchier, E. , May, A. M. , van den Brekel, M. W. M. , Jager‐Wittenaar, H. , Veenhof, C. , de Bree, R. , Stuiver, M. M. , & Speksnijder, C. M. (2022). Feasibility of a supervised and home‐based tailored exercise intervention in head and neck cancer patients during chemoradiotherapy. European Journal of Cancer Care, 31(6), e13662. 10.1111/ecc.13662
Annemieke Kok and Ellen Passchier contributed equally to this manuscript.
Martijn M. Stuiver and Caroline M. Speksnijder contributed equally to this manuscript.
Funding information
This study was supported by a grant from the World Cancer Research Fund International (2016/1632).
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Aaronson, N. K. , Ahmedzai, S. , Bergman, B. , Bullinger, M. , Cull, A. , Duez, N. J. , Filiberti, A. , Flechtner, H. , Fleishman, S. B. , de Haes, J. C. , & Kaasa, S. (1993). The European Organization for Research and Treatment of Cancer QLQ‐C30: A quality‐of‐life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute, 85(5), 365–376. 10.1093/jnci/85.5.365 [DOI] [PubMed] [Google Scholar]
- Beijer, Y. J. , Koopman, M. , Terhaard, C. H. , Braunius, W. W. , van Es, R. J. , & De, G. A. (2013). Outcome and toxicity of radiotherapy combined with chemotherapy or cetuximab for head and neck cancer: Our experience in one hundred and twenty‐five patients. Clinical Otolaryngol, 38(1), 69–74. 10.1111/coa.12002 [DOI] [PubMed] [Google Scholar]
- Bjordal, K. , Hammerlid, E. , Ahlner‐Elmqvist, M. , de Graeff, A. , Boysen, M. , Evensen, J. F. , Biorklund, A. , de Leeuw, J. R. , Fayers, P. M. , Jannert, M. , Westin, T. , & Kaasa, S. (1999). Quality of life in head and neck cancer patients: Validation of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire‐H&N35. Journal of Clinical Oncology, 17(3), 1008–1019. 10.1200/JCO.1999.17.3.1008 [DOI] [PubMed] [Google Scholar]
- Borg, G. A. (1982). Psychophysical bases of perceived exertion. Medicine and Science in Sports and Exercise, 14(5), 377–381. PMID: https://www.ncbi.nlm.nih.gov/pubmed/7154893 [PubMed] [Google Scholar]
- Bressan, V. , Stevanin, S. , Bianchi, M. , Aleo, G. , Bagnasco, A. , & Sasso, L. (2016). The effects of swallowing disorders, dysgeusia, oral mucositis and xerostomia on nutritional status, oral intake and weight loss in head and neck cancer patients: A systematic review. Cancer Treatment Reviews, 45, 105–119. 10.1016/j.ctrv.2016.03.006 [DOI] [PubMed] [Google Scholar]
- Bye, A. , Sandmael, J. A. , Stene, G. B. , Thorsen, L. , Balstad, T. R. , Solheim, T. S. , Pripp, A. H. , & Oldervoll, L. M. (2020). Exercise and nutrition interventions in patients with head and neck cancer during curative treatment: A systematic review and meta‐analysis. Nutrients, 12(11), 3233–3257. 10.3390/nu12113233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, K. L. , Winters‐Stone, K. M. , Wiskemann, J. , May, A. M. , Schwartz, A. L. , Courneya, K. S. , Zucker, D. S. , Matthews, C. E. , Ligibel, J. A. , Gerber, L. H. , Morris, G. S. , Patel, A. V. , Hue, T. F. , Perna, F. M. , & Schmitz, K. H. (2019). Exercise guidelines for cancer survivors: Consensus statement from international multidisciplinary roundtable. Medicine and Science in Sports and Exercise, 51(11), 2375–2390. 10.1249/MSS.0000000000002116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capozzi, L. C. , McNeely, M. L. , Lau, H. Y. , Reimer, R. A. , Giese‐Davis, J. , Fung, T. S. , & Culos‐Reed, S. N. (2016). Patient‐reported outcomes, body composition, and nutrition status in patients with head and neck cancer: Results from an exploratory randomized controlled exercise trial. Cancer, 122(8), 1185–1200. 10.1002/cncr.29863 [DOI] [PubMed] [Google Scholar]
- Chinn, D. J. , White, M. , Howel, D. , Harland, J. O. , & Drinkwater, C. K. (2006). Factors associated with non‐participation in a physical activity promotion trial. Public Health, 120(4), 309–319. 10.1016/j.puhe.2005.11.003 [DOI] [PubMed] [Google Scholar]
- Cramp, F. , & Byron‐Daniel, J. (2012). Exercise for the management of cancer‐related fatigue in adults. Cochrane Database of Systematic Reviews, 11, CD006145. 10.1002/14651858.CD006145.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen, C. M. , Janssens, G. O. , van der Graaf, W. T. , Takes, R. P. , Merkx, T. A. , Melchers, W. J. , Kaanders, H. A. , & van Herpen, C. M. (2016). Toxicity and efficacy of accelerated radiotherapy with concurrent weekly cisplatin for locally advanced head and neck carcinoma. Head & Neck, 38(Suppl 1), E559–E565. 10.1002/hed.24039 [DOI] [PubMed] [Google Scholar]
- Fearon, K. , Arends, J. , & Baracos, V. (2013). Understanding the mechanisms and treatment options in cancer cachexia. Nature Reviews. Clinical Oncology, 10(2), 90–99. 10.1038/nrclinonc.2012.209 [DOI] [PubMed] [Google Scholar]
- Grossberg, A. J. , Chamchod, S. , Fuller, C. D. , Mohamed, A. S. , Heukelom, J. , Eichelberger, H. , Kantor, M. E. , Hutcheson, K. A. , Gunn, G. B. , Garden, A. S. , Frank, S. , Phan, J. , Beadle, B. , Skinner, H. D. , Morrison, W. H. , & Rosenthal, D. I. (2016). Association of body composition with survival and locoregional control of radiotherapy‐treated head and neck squamous cell carcinoma. JAMA Oncologia, 2(6), 782–789. 10.1001/jamaoncol.2015.6339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote, M. , Maihofer, C. , Weigl, M. , Davies‐Knorr, P. , & Belka, C. (2018). Progressive resistance training in cachectic head and neck cancer patients undergoing radiotherapy: A randomized controlled pilot feasibility trial. Radiation Oncology, 13(1), 215–225. 10.1186/s13014-018-1157-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashibe, M. , Brennan, P. , Chuang, S. C. , Boccia, S. , Castellsague, X. , Chen, C. , Curado, M. P. , Dal Maso, L. , Daudt, A. W. , Fabianova, E. , Fernandez, L. , Wunsch‐Filho, V. , Franceschi, S. , Hayes, R. B. , Herrero, R. , Kelsey, K. , Koifman, S. , la Vecchia, C. , Lazarus, P. , … Boffetta, P. (2009). Interaction between tobacco and alcohol use and the risk of head and neck cancer: Pooled analysis in the international head and neck Cancer epidemiology consortium. Cancer Epidemiology, Biomarkers & Prevention, 18(2), 541–550. 10.1158/1055-9965.EPI-08-0347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter, K. U. , & Jolly, S. (2013). Clinical review of physical activity and functional considerations in head and neck cancer patients. Support Care Cancer, 21(5), 1475–1479. 10.1007/s00520-013-1736-4 [DOI] [PubMed] [Google Scholar]
- Jackson, C. , Dowd, A. J. , Capozzi, L. C. , Bridel, W. , Lau, H. Y. , & Culos‐Reed, S. N. (2018). A turning point: Head and neck cancer patients' exercise preferences and barriers before and after participation in an exercise intervention. European Journal of Cancer Care, 27(2), e12826. 10.1111/ecc.12826 [DOI] [PubMed] [Google Scholar]
- Jager‐Wittenaar, H. , Dijkstra, P. U. , Vissink, A. , Langendijk, J. A. , van der Laan, B. F. , Pruim, J. , & Roodenburg, J. L. (2011). Changes in nutritional status and dietary intake during and after head and neck cancer treatment. Head & Neck, 33(6), 863–870. 10.1002/hed.21546 [DOI] [PubMed] [Google Scholar]
- Jager‐Wittenaar, H. , Dijkstra, P. U. , Vissink, A. , van der Laan, B. F. , van Oort, R. P. , & Roodenburg, J. L. (2007). Critical weight loss in head and neck cancer‐‐prevalence and risk factors at diagnosis: An explorative study. Support Care Cancer, 15(9), 1045–1050. 10.1007/s00520-006-0212-9 [DOI] [PubMed] [Google Scholar]
- Jones, C. J. , Rikli, R. E. , & Beam, W. C. (1999). A 30‐s chair‐stand test as a measure of lower body strength in community‐residing older adults. Research Quarterly for Exercise and Sport, 70(2), 113–119. 10.1080/02701367.1999.10608028 [DOI] [PubMed] [Google Scholar]
- Jung, A. R. , Roh, J. L. , Kim, J. S. , Kim, S. B. , Choi, S. H. , Nam, S. Y. , & Kim, S. Y. (2019). Prognostic value of body composition on recurrence and survival of advanced‐stage head and neck cancer. European Journal of Cancer, 116, 98–106. 10.1016/j.ejca.2019.05.006 [DOI] [PubMed] [Google Scholar]
- Kyle, U. G. , Bosaeus, I. , de Lorenzo, A. D. , Deurenberg, P. , Elia, M. , Gomez, J. M. , Heitmann, B. L. , Kent‐Smith, L. , Melchior, J. C. , Pirlich, M. , Scharfetter, H. , Schols, A. M. , & Pichard, C. (2004). Bioelectrical impedance analysis—Part I: Review of principles and methods. Clinical Nutrition, 23(5), 1226–1243. 10.1016/j.clnu.2004.06.004 [DOI] [PubMed] [Google Scholar]
- Lonbro, S. , Dalgas, U. , Primdahl, H. , Johansen, J. , Nielsen, J. L. , Aagaard, P. , Hermann, A. P. , Overgaard, J. , & Overgaard, K. (2013). Progressive resistance training rebuilds lean body mass in head and neck cancer patients after radiotherapy‐‐results from the randomized DAHANCA 25B trial. Radiotherapy and Oncology, 108(2), 314–319. 10.1016/j.radonc.2013.07.002 [DOI] [PubMed] [Google Scholar]
- Lonkvist, C. K. , Vinther, A. , Zerahn, B. , Rosenbom, E. , Deshmukh, A. S. , Hojman, P. , & Gehl, J. (2017). Progressive resistance training in head and neck cancer patients undergoing concomitant chemoradiotherapy. Laryngoscope Investig Otolaryngol, 2(5), 295–306. 10.1002/lio2.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, A. V. , Friedenreich, C. M. , Moore, S. C. , Hayes, S. C. , Silver, J. K. , Campbell, K. L. , Winters‐Stone, K. , Gerber, L. H. , George, S. M. , Fulton, J. E. , Denlinger, C. , Morris, G. S. , Hue, T. , Schmitz, K. H. , & Matthews, C. E. (2019). American College of Sports Medicine roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Medicine and Science in Sports and Exercise, 51(11), 2391–2402. 10.1249/MSS.0000000000002117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers, L. Q. , Anton, P. M. , Fogleman, A. , Hopkins‐Price, P. , Verhulst, S. , Rao, K. , Malone, J. , Robbs, R. , Courneya, K. S. , Nanavati, P. , Mansfield, S. , & Robbins, K. T. (2013). Pilot, randomized trial of resistance exercise during radiation therapy for head and neck cancer. Head & Neck, 35(8), 1178–1188. 10.1002/hed.23118 [DOI] [PubMed] [Google Scholar]
- Rogers, L. Q. , Courneya, K. S. , Robbins, K. T. , Malone, J. , Seiz, A. , Koch, L. , Rao, K. , & Nagarkar, M. (2006). Physical activity and quality of life in head and neck cancer survivors. Support Care Cancer, 14(10), 1012–1019. 10.1007/s00520-006-0044-7 [DOI] [PubMed] [Google Scholar]
- Rogers, L. Q. , Malone, J. , Rao, K. , Courneya, K. S. , Fogleman, A. , Tippey, A. , Markwell, S. J. , & Robbins, K. T. (2009). Exercise preferences among patients with head and neck cancer: Prevalence and associations with quality of life, symptom severity, depression, and rural residence. Head & Neck, 31(8), 994–1005. 10.1002/hed.21053 [DOI] [PubMed] [Google Scholar]
- Sabatini, M. E. , & Chiocca, S. (2020). Human papillomavirus as a driver of head and neck cancers. British Journal of Cancer, 122(3), 306–314. 10.1038/s41416-019-0602-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel, S. R. , Maiya, G. A. , Babu, A. S. , & Vidyasagar, M. S. (2013). Effect of exercise training on functional capacity & quality of life in head & neck cancer patients receiving chemoradiotherapy. The Indian Journal of Medical Research, 137(3), 515–520. PMID: https://www.ncbi.nlm.nih.gov/pubmed/23640558 [PMC free article] [PubMed] [Google Scholar]
- Schmidt, K. , Vogt, L. , Thiel, C. , Jager, E. , & Banzer, W. (2013). Validity of the six‐minute walk test in cancer patients. International Journal of Sports Medicine, 34(7), 631–636. 10.1055/s-0032-1323746 [DOI] [PubMed] [Google Scholar]
- Scott, J. M. , Zabor, E. C. , Schwitzer, E. , Koelwyn, G. J. , Adams, S. C. , Nilsen, T. S. , Moskowitz, C. S. , Matsoukas, K. , Iyengar, N. M. , Dang, C. T. , & Jones, L. W. (2018). Efficacy of exercise therapy on cardiorespiratory fitness in patients with cancer: A systematic review and Meta‐analysis. Journal of Clinical Oncology, 36(22), 2297–2305. 10.1200/JCO.2017.77.5809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealy, M. J. , Dechaphunkul, T. , van der Schans, C. P. , Krijnen, W. P. , Roodenburg, J. L. N. , Walker, J. , Jager‐Wittenaar, H. , & Baracos, V. E. (2020). Low muscle mass is associated with early termination of chemotherapy related to toxicity in patients with head and neck cancer. Clinical Nutrition, 39(2), 501–509. 10.1016/j.clnu.2019.02.029 [DOI] [PubMed] [Google Scholar]
- Sheill, G. , Guinan, E. , Brady, L. , Hevey, D. , & Hussey, J. (2019). Exercise interventions for patients with advanced cancer: A systematic review of recruitment, attrition, and exercise adherence rates. Palliative & Supportive Care, 17(6), 686–696. 10.1017/S1478951519000312 [DOI] [PubMed] [Google Scholar]
- Silver, H. J. , Dietrich, M. S. , & Murphy, B. A. (2007). Changes in body mass, energy balance, physical function, and inflammatory state in patients with locally advanced head and neck cancer treated with concurrent chemoradiation after low‐dose induction chemotherapy. Head & Neck, 29(10), 893–900. 10.1002/hed.20607 [DOI] [PubMed] [Google Scholar]
- Singh, B. , Spence, R. R. , Steele, M. L. , Sandler, C. X. , Peake, J. M. , & Hayes, S. C. (2018). A systematic review and meta‐analysis of the safety, feasibility, and effect of exercise in women with stage II+ breast cancer. Archives of Physical Medicine and Rehabilitation, 99(12), 2621–2636. 10.1016/j.apmr.2018.03.026 [DOI] [PubMed] [Google Scholar]
- Smets, E. M. , Garssen, B. , Cull, A. , & de Haes, J. C. (1996). Application of the multidimensional fatigue inventory (MFI‐20) in cancer patients receiving radiotherapy. British Journal of Cancer, 73(2), 241–245. 10.1038/bjc.1996.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, J. C. , Terrell, J. E. , Ronis, D. L. , Fowler, K. E. , Bishop, C. , Lambert, M. T. , Myers, L. L. , Duffy, S. A. , Bradford, C. R. , Chepeha, D. B. , Hogikyan, N. D. , Prince, M. E. , Teknos, T. N. , & Wolf, G. T. (2004). Disability in patients with head and neck cancer. Archives of Otolaryngology – Head & Neck Surgery, 130(6), 764–769. 10.1001/archotol.130.6.764 [DOI] [PubMed] [Google Scholar]
- Trutschnigg, B. , Kilgour, R. D. , Reinglas, J. , Rosenthall, L. , Hornby, L. , Morais, J. A. , & Vigano, A. (2008). Precision and reliability of strength (Jamar vs. Biodex handgrip) and body composition (dual‐energy X‐ray absorptiometry vs. bioimpedance analysis) measurements in advanced cancer patients. Applied Physiology, Nutrition, and Metabolism, 33(6), 1232–1239. 10.1139/H08-122 [DOI] [PubMed] [Google Scholar]
- van Overveld, L. F. J. , Takes, R. P. , Braspenning, J. C. C. , Baatenburg de Jong, R. J. , de Boer, J. P. , Brouns, J. J. A. , Bun, R. J. , Dik, E. A. , van Dijk, B. A. C. , van Es, R. J. J. , Hoebers, F. J. P. , Kolenaar, B. , Kropveld, A. , Langeveld, T. P. M. , Verschuur, H. P. , de Visscher, J. , van Weert, S. , Witjes, M. J. H. , Smeele, L. E. , … Hermens, R. (2018). Variation in integrated head and neck cancer care: Impact of patient and hospital characteristics. Journal of the National Comprehensive Cancer Network, 16(12), 1491–1498. 10.6004/jnccn.2018.7061 [DOI] [PubMed] [Google Scholar]
- van Waart, H. , Stuiver, M. M. , van Harten, W. H. , Geleijn, E. , Kieffer, J. M. , Buffart, L. M. , de Maaker‐Berkhof, M. , Boven, E. , Schrama, J. , Geenen, M. M. , Meerum Terwogt, J. M. , van Bochove, A. , Lustig, V. , van den Heiligenberg, S. M. , Smorenburg, C. H. , Hellendoorn‐van Vreeswijk, J. A. , Sonke, G. S. , & Aaronson, N. K. (2015). Effect of low‐intensity physical activity and moderate‐ to high‐intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: Results of the PACES randomized clinical trial. Journal of Clinical Oncology, 33(17), 1918–1927. 10.1200/JCO.2014.59.1081 [DOI] [PubMed] [Google Scholar]
- Velthuis, M. J. , May, A. M. , Koppejan‐Rensenbrink, R. A. , Gijsen, B. C. , van Breda, E. , de Wit, G. A. , Schroder, C. D. , Monninkhof, E. M. , Lindeman, E. , van der Wall, E. , & Peeters, P. H. (2010). Physical activity during Cancer treatment (PACT) study: Design of a randomised clinical trial. BMC Cancer, 10, 272–281. 10.1186/1471-2407-10-272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendrich, A. W. , Swartz, J. E. , Bril, S. I. , Wegner, I. , de Graeff, A. , Smid, E. J. , de Bree, R. , & Pothen, A. J. (2017). Low skeletal muscle mass is a predictive factor for chemotherapy dose‐limiting toxicity in patients with locally advanced head and neck cancer. Oral Oncology, 71, 26–33. 10.1016/j.oraloncology.2017.05.012 [DOI] [PubMed] [Google Scholar]
- Willemsen, A. C. H. , Hoeben, A. , Lalisang, R. I. , van Helvoort, A. , Wesseling, F. W. R. , Hoebers, F. , Baijens, L. W. J. , & Schols, A. (2020). Disease‐induced and treatment‐induced alterations in body composition in locally advanced head and neck squamous cell carcinoma. Journal of Cachexia, Sarcopenia and Muscle, 11(1), 145–159. 10.1002/jcsm.12487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, S. G. , Alexander, N. B. , Djuric, Z. , Zhou, J. , Tao, Y. , Schipper, M. , Feng, F. Y. , Eisbruch, A. , Worden, F. P. , Strath, S. J. , & Jolly, S. (2016). Maintaining physical activity during head and neck cancer treatment: Results of a pilot controlled trial. Head & Neck, Suppl 1(Suppl 1), E1086–E1096. 10.1002/hed.24162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweers, H. , Kruizenga, H. , van den Berg, A. , Reijven, N. , & Hulshof, P. (2018). Standard operating procedures bio‐electrical impedance analysis. Nutrional Assessment Platform, 1.3(1‐13). https://nutritionalassessment.nl/wp-content/uploads/2019/02/NAP-BIA-SOP-english.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S4. Results of functional and symptom scales of the EORTC C30 and EORTC H&N35 quality of life questionnaires at baseline, week 5 (mid‐intervention) and week 12 (post‐intervention) in means (sd) and mean differences (95% CI).
Table S5. Results of the Multidimensional Fatigue Inventory at baseline, week 5 and week 12 post‐intervention in means (sd) and mean differences (95% CI).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


