Abstract
Purpose
To analyse the development of neovascular age‐related macular degeneration (nAMD) in the fellow eye in patients initially presenting with unilateral nAMD, using data from the Swedish Macula Register.
Methods
This observational study included data on treatment‐naïve patients who initially underwent unilateral treatment for nAMD, and then required bilateral treatment, between 2010 and 2018, according to the Swedish Macula Register (SMR). The data were also stratified according into three time periods (2010–2013; 2014–2016; 2017–2018). Treatment duration, best‐corrected visual acuity (BCVA) in the first and second eye, number of injections in the first eye before falling ill in the second, and the time between the last injection in the first eye and the start of treatment of the fellow eye were analysed.
Results
5216 out of 28 670 (18%) patients treated for nAMD subsequently required bilateral treatment. The mean age was 77.7 ± 7.3 years, and 69% were female. The mean duration of treatment of the first eye before nAMD was diagnosed in the fellow eye was 1.58 years, and the mean number of injections in the first eye was 8.9 ± 8.6. Best‐corrected visual acuity, according to the ETDRS chart, was higher in the second eye at the time when treatment started in that eye compared to treatment start in the first eye: 62.8 (14.7) versus 57.6 (15.5); p < 0.001, and was higher in the 66% whose first eye was still undergoing treatment: 63.6 ± 14.5 versus 61.0 ± 14.8; p = 0.001.
Conclusions
The mean duration of treatment of the first eye before treatment started in the fellow eye was 19 months, and treatment of the second eye had started within 2 years in 61% of the patients. Best‐corrected visual acuity was higher in the second eye than in the first eye at the start of treatment of that eye and was higher in the second eye at the start of treatment of that eye when the first eye was still being treated.
Keywords: anti‐VEGF, fellow eye, neovascular macular degeneration, Swedish Macula Register
Introduction
Neovascular age‐related macular degeneration (nAMD) has been treated with anti‐VEGF injections for 14 years worldwide, but choroidal neovascularization (CNV) is still a major cause of visual impairment in the elderly (Colijn et al. 2017). Anti‐VEGF treatment is quite challenging due to the need for frequent injections, and treatment must be initiated as early as possible to prevent deterioration in visual acuity. Age‐related macular degeneration is a bilateral disease that progresses into nAMD in about 15% of the affected eyes. In most cases, nAMD disease is initially found in one eye, but several studies have shown that about 30% of patients will develop nAMD in the fellow eye after 2–6 years (Wong et al. 2008; Joachim et al. 2017; Bek & Klug 2018; Fasler et al. 2020). There is thus a risk of developing nAMD in the fellow eye.
We have previously shown that a higher baseline best‐corrected visual acuity (BCVA) before treatment for nAMD commences results in a better BCVA outcome (Lövestam Adrian et al. 2019). Studies on the treatment of fellow eyes with a better baseline BCVA than the first‐treated eye have also demonstrated a better BCVA outcome for the second‐treated eye (Zarranz‐Ventura et al. 2014; Chew et al. 2017). It is therefore of the greatest importance to detect nAMD in the second eye and treat it as early as possible, especially if this is the patient's best‐seeing eye.
The detection and treatment of nAMD in the second eye is dependent on the treatment regimen for the first eye, and how often the fellow eye is examined in connection with treatment of the first eye. Many studies have reported the general undertreatment of eyes with nAMD (Martin et al. 2012; Schmidt‐Erfurth et al. 2014; Maguire et al. 2016; Qin et al. 2018), which will affect the detection and treatment of nAMD in the second eye. New anti‐VEGF treatment regimens have been developed in an attempt to individualize and facilitate treatment and, when possible, reduce the number of injections and simplify the injection process. The first of these was pro re nata (PRN), that is injection when necessary, and recently, the treat and extend (T&E) regimen has been shown to be efficient and robust (Hatz & Prünte 2016; Hatz & Prünte 2017; Kvannli & Krohn 2017; Augsburger et al. 2019; Volkmann et al. 2020). However, examination and monitoring of the fellow eye may be missed or delayed when using these regimens.
The purpose of this study was to analyse data from the Swedish Macula Register on patients who initially underwent unilateral treatment for nAMD and who later underwent bilateral treatment. We investigated the time before the fellow eye was affected, the treatment regimen used and BCVA.
Methods
The study was conducted in accordance with the tenets of the Declaration of Helsinki and was approved by the Ethics Board of Lund University. The Swedish Macula Register (SMR) was established in 2007 and today includes data on about 86% of all eyes treated for nAMD in Sweden (Svenska Makula Registret 2019). Data were obtained from the SMR after approval from its Steering Committee.
Data collection and study population
The SMR has been described previously (Westborg et al. 2017). In 2015, data from about 1.5% of the Swedish population (aged ≥85 years) were included in the registry. By 2020, this had increased to 2.4%, and all 44 eye clinics in Sweden were contributing to the SMR. Comparison with the National Patient Diagnosis Register, where patients diagnosed with nAMD with an indication for intravitreal injection are included, confirmed that data from approximately 86% of all patients in Sweden treated for nAMD are included in the SMR. This study includes treatment‐naïve patients who initially received unilateral treatment, and later bilateral treatment, for nAMD during the period 2010–2018, and who were followed up until December 2020, at the latest.
Study variables and follow‐up
Data were extracted from the patients' electronic records in the SMR. Baseline data were collected from 1 January 2010 until 31 December 2018. The baseline visit was first defined as the first visit at which nAMD was diagnosed and treatment was prescribed for the first eye, and when treatment was started in the fellow eye, it was defined as the baseline for the fellow eye. Data at baseline were obtained on patients' age, sex, duration of symptoms (self‐reported symptoms associated with nAMD that caused the patient to consult an ophthalmologist, that is metamorphopsia, reduced visual acuity, problems with reading) and BCVA of the treated and fellow eye measured with the Snellen and ETDRS charts. At the time when treatment of the fellow eye was initiated, the following data were obtained for the first eye: duration of treatment, total number of injections given, treatment regimen (PRN or T&E), treatment intervals and BCVA. Data obtained for the fellow eye on the first occasion of treatment were the duration of symptoms and the BCVA using the Snellen and ETDRS charts. In cases where the BCVA had been measured using only the Snellen chart, the results were converted into ETDRS letters as described by Gregori et al. (2010). Results had been recorded using the ETDRS chart for about 80% of the patients at baseline and at 87% at the 1‐year follow‐up. Visual acuity determined using hand movements and amaurosis was expressed as ETDRS = 0.001.
Difference in BCVA at treatment start of the second eye, whether the first eye was still under treatment or not when initiating treatment of the second eye, was analysed. The data were also divided into different time periods: 2010–2013, 2014–2016 and 2017–2018, and analysed.
The primary aim was to study characteristics and the duration of treatment of the first eye before treatment of the second eye was initiated. The secondary aim was to compare the BCVA in the first and second eye at the start of treatment of each eye, and whether the VA in the second eye depended on whether the first eye was still being treated or not.
Statistical analysis
The data are the values obtained from the SMR, and the results are presented as the mean, median, standard deviation and range for continuous variables, and frequency counts and percentages for categorical variables. Comparisons of VA between two groups were based on student's t‐test. The chi‐squared test was used to compare frequencies. The Wilcoxon rank‐sum test was used when analysing nonparametric values. Statistical significance was defined as p < 0.05. SAS version 9.4 was used for the statistical calculations.
Results
Baseline characteristics
Of the 28 670 treatment‐naïve patients registered in the SMR between 2010 and 2018, 5216 (18%) were initially treated for nAMD unilaterally and then bilaterally. The mean age was 77.7 ± 7.3 years when treatment was initiated in the first eye, and 69% were female. The baseline characteristics are given in Table 1.
Table 1.
Baseline characteristics.
| N = 5216 | First eye | Second eye |
|---|---|---|
| Age | 77.7 ± 7.3 | 79.3 ± 7.9 |
| Sex, Female | 69% | 69% |
| CNV type | ||
| Not classified | 20% | 25% |
| Type 1 | 34% | 32% |
| Type 2 | 30% | 27% |
| RAP | 14% | 14% |
| PCV | 2% | 2% |
| Symptom duration | ||
| <0–2 month | 40% | 39% |
| 2 ≤ 4 month | 25% | 24% |
| 4–6 month | 15% | 14% |
| >6 month | 20% | 18% |
| Visual Acuity | ||
| ETDRS letters | 57.6 ± 15.4 | 62.8 ± 14.7*** |
| Snellen | 0.35 ± 0.20 | 0.43 ± 0.23 |
At treatment start of first/second eye.
p < 0.0001.
No difference was found in the reported duration of symptoms in the first‐ and second‐treated eyes. Best‐corrected visual acuity (ETDRS chart) before the start of treatment was higher in the second‐treated eye than in the first‐treated eye: 62.8 ± 14.7 versus 57.6 ± 15.4; p < 0.0001. However, the BCVA of the second‐treated eye decreased after the initiation of treatment in the first eye: from 73.1 ± 13.4 to 62.8 ± 14.7; p < 0.001 when treatment was initiated in the second eye. Best‐corrected visual acuity in the first eye was slightly improved when treatment began in the second eye: from 57.6 ± 15.5 to 58.2 ± 16.7.
Status of the first eye at time of initiating treatment of the fellow eye
The mean time between the initiation of treatment of the first eye and the second eye was 1.58 ± 1.54 years (19 months), median 1.11 (Q1 0.41; Q3 2.30) years for the whole group. The results obtained for the different time periods studied are shown in Fig. 1. Sixty‐one per cent of the patients had developed nAMD in the fellow eye within 2 years. The shorter treatment duration before the second eye was diagnosed with nAMD in the last time period reflects the shorter follow‐up time.
Fig. 1.
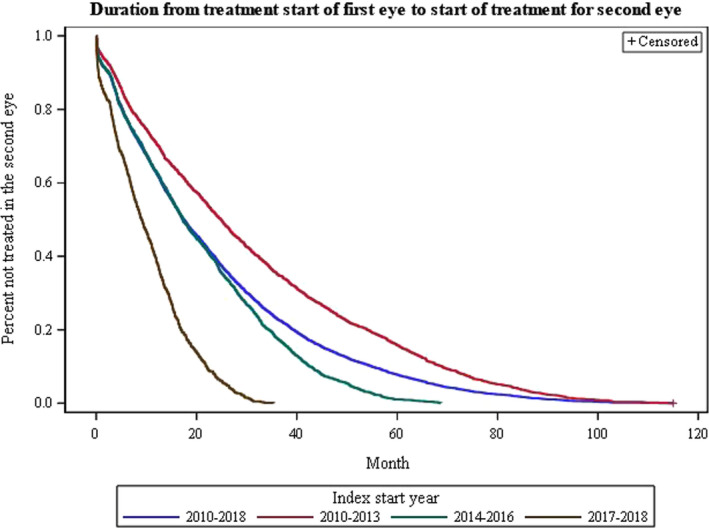
Kaplan‐Meier curve showing duration of treatment of the first eye before treatment start of the second eye for the different time periods. The number of patients; 2010–2018, n = 5218; 2010–2013, n = 2428; 2014–2016, n = 1946 and 2017–2018 n = 844. [Colour figure can be viewed at wileyonlinelibrary.com]
The mean number of injections in the first eye when treatment was initiated in the second eye was 8.9. The mean number of days for the latest treatment interval for the first eye, before initiating treatment in the second, was 60 days, and the number of days between the latest treatment in the first eye before starting treatment in the second was 67 days.
When dividing the data into three periods, the latest treatment interval for the first eye before initiating treatment in the fellow eye was 54 days in 2017–2018; 61 days in 2014–2016 and 61 days in 2010–2013. The number of days between the latest injection in the first eye and the start of treatment in the second was 44 days in 2017–2018; 62 days in 2014–2016 and 82 days in 2010–2013.
Over the whole period, 2010–2018, 68% (n = 3544) of the first‐treated eyes were still receiving treatment when treatment was initiated in the second eye. When the data were divided into different time periods, 78% were still receiving treatment in the first eye in 2017–2018; 72% in 2014–2016 but only 61% in 2010–2013; p < 0.0001, when treatment started in the second eye. The treatment regimen used for the first eye was not recorded during the first period of 2010–2013. In the period 2014–2016, the regimen was recorded as 21% T&E and 6% PRN; the corresponding values for the last period, 2017–2018, being 78% T&E and 12% PRN.
Visual acuity
Visual acuity during different time periods is shown in Fig. 2A. Over the whole study period, the baseline BCVA (ETDRS chart) was higher at the time treatment started in the second eye, than when treatment was started in the first eye: 62.8 (14.7) versus 57.6 (15.5); p < 0.0001. When analysing the data in different time periods, the BCVA at start of treatment of the second eye was higher in 2014–2016 than in 2010–2013; p = 0.0001 and higher in 2017–2018 versus 2010–2013; p = 0.0001. There was a decrease in BCVA in the second eye between the first measurement when treatment was started in the first eye and the measurement when treatment was started in the second eye. This decrease in BCVA in the second eye became smaller over time.
Fig. 2.
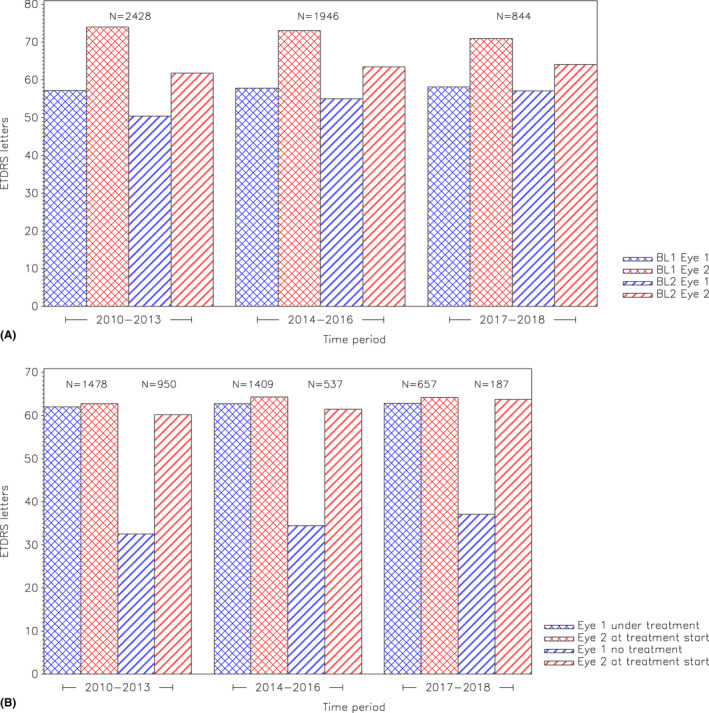
(A) Demonstrating different time periods and the number of patients within each time period. BL1 = Baseline 1; visual acuity for the first and second eye at treatment start of the first eye. BL2 = Baseline 2; visual acuity for the first and second eye at treatment start of the second eye. (B) Demonstrating different time periods and the number of patients at treatment start of the second eye divided by the first eye still treated or not. Visual acuity for the first eye, Eye 1, and second eye, Eye 2, at treatment start of the second eye divided by the first eye treated or not. [Colour figure can be viewed at wileyonlinelibrary.com]
At the start of treatment of the fellow eye, BCVA was higher when the first eye was still being treated than when the first eye was no longer being treated; 63.6 ± 14.5 versus 61.0 ± 14.8; p = 0.0001. This was also true for the time periods 2010–2013; p < 0.001 and 2014–2015; p = 0.0019, but not for the last time period; p = 0.4 (Fig. 2B).
A difference was found in the VA of the first eye when treatment was started in the second eye, between patients whose first eye was still being treated and those whose first eye was no longer being treated; p < 0.001. In patients still receiving treatment of the first eye, the first eye showed an increase in VA compared with the baseline, at the time when treatment was initiated in the second eye, while a decrease in VA was seen in the first eye in patients no longer receiving treatment in the first eye (Fig. 2A and B).
Discussion
In the present study, we found that the incidence of bilateral treatment in treatment‐naïve patients with nAMD who started with unilateral treatment was about 11% within 2 years. It is well known that CNV in one eye is a strong predictor of developing CNV in the second eye, and our findings are in parity with those of the CATT study (2013) (Comparison of Age‐Related Macular Degeneration Treatments Trials), where the incidence of CNV in the fellow eye within 2 years was found to be between 16% and 20%. However, other studies have reported widely varying results. For example, in a retrospective study, Barbazetto et al. (2010) reported the development of CNV in 24% to 39% of patients within 2 years, while Ueta et al. (2008) found involvement of the fellow eye in 3.4% after 1 year and in 9% after 3 years. Furthermore, the analysis of pooled results from several prospective studies has shown that 20%–25% of unilaterally treated patients required bilateral treatment within 5 years (Joachim et al. 2017).
In the present study, we found that nAMD was diagnosed in the fellow eye in 60% of the patients within 2 years and in about 20% within 6 months. The risk of developing nAMD in the second eye increased with time, and thus, the risk of nAMD developing in the second eye was greater in the group with the longer follow‐up period. However, in the two earlier periods, 50%–60% of the patients had started treatment in the second eye within 2 years.
Symptoms such as metamorphopsia, reading difficulties and impaired VA cause the patient to seek medical attention. We found no differences in the duration of symptoms between the first‐ and second‐treated eye. One reason for this could be the fact that the shortest symptom interval that can be chosen in the SMR is 0–2 months. This is quite a long time and does not discriminate between early symptom duration and longer. In the SMR, around 40% of the symptom durations are ticked at this interval. Another reason could be that many nAMD lesions may be asymptomatic for a long time (Chew et al. 2017) and that patients already receiving treatment in one eye may wait until their next visit to report symptoms in the other eye. With a mean interval for the last treatment interval of the first eye of 60 days, the symptom duration might well be 1–2 months for the second eye. In line with this, some membranes also might have been detected in connection with a clinical visit for the first eye before giving any obvious symptoms and therefore the clinician might have ticked a longer symptom duration. In order to include a new eye/patient in the register, it is obligatory to decide for a symptom duration. It is not possible to avoid it or to choose ‘unknown’. The longer symptom durations are on the whole more uncertain, since the patient group is quite old and it might be difficult to remember when the symptoms started. If there are uncertainties, it is challenging for the physician to decide and often a longer symptom duration is chosen.
Overall, many patients find it difficult to interpret their own visual changes, especially subtle, monocular or varying changes, even with the support of additional home tests as the Amsler test or when already receiving treatment in the other eye. Maybe it is part of the ordinary patient, calm culture and trust in the healthcare system to plan a follow‐up appointment at an appropriate time point that many patients have in Sweden.
To preserve a good visual outcome, it is crucial to detect and treat nAMD as early as possible, as demonstrated in a Danish study (Rasmussen et al. 2015). We have previously shown in the INSIGHT study, in which data on patients treated with aflibercept taken from the SMR were analysed, that a better baseline visual acuity is a strong predictor of good visual outcome after 2 and 3 years (Lövestam Adrian et al. 2019). This has also been shown in other large‐scale studies, for example, CATT and UK neovascular AMD EMR (Ying et al. 2013; Lee et al. 2015). In the present study, we found that the second eye had a higher BCVA when treatment was initiated than in the case of the first eye, although there was a decrease from the first registered BCVA. We also found that the BCVA of the second eye was higher at the start of treatment during the two later time periods, than in the earliest time period. However, this may be an effect of the shorter follow‐up time for the later time periods as more of the first eyes were still being treated, which we could demonstrate rendered a higher BCVA at the start of treatment in the second eye. During the latest time period, there was a trend towards higher BCVA in the second eye when the first eye was still being treated, although this was not statistically significant. This is probably due to the shorter follow‐up time, when more first eyes were still being treated.
Various treatment regimens have been developed since the start of the anti‐VEGF era. According to the data in the SMR, about 21% were treated with the T&E regime in 2014–2016 and up to 78% in more recent years, with the last treatment interval in the first eye before the second eye was diagnosed ranging from 54 to 60 days. The shorter interval for the latest time period, 2017–2018, reflects the short treatment duration of 10 months of the first eye in this group, given there had been less time to extend the intervals. In addition, the time between the last injection in the first eye and the first injection in the second eye differed between the time periods; being only around 40 days in the later time period, but more than 80 days for the first period, 2010–2013. The T&E regimen had not been introduced in the earliest period, and patients were treated more irregular in time. However, the T&E regimen does not necessarily mean that the second eye is examined on a regular basis. In an attempt to simplify the procedure, attention is mostly focussed on the eye being treated, and the clinical visit thus includes optical coherence tomography (OCT) imaging of the treated eye and an anti‐VEGF injection. This may lead to the risk of missing the development of a macular lesion in the second eye. We found that the BCVA in the second eye at the start of treatment of that eye was higher when the first eye was still being treated. This indicates a greater probability of early diagnosis of the second eye if the patient's first eye is still being treated and thus regularly examined. The importance of examining both eyes and regular monitoring of the second eye has been pointed out previously to ensure timely treatment and thus improved visual outcome (Wolf et al. 2016; Chew et al. 2017; Wong et al. 2020).
After the registration of baseline data for both eyes, registration of data on the second eye is optional in the SMR and is often done in connection with a more detailed clinical examination. We found that the baseline BCVA of the second eye at the start of treatment was higher in the later time periods, and the decrease in VA from the baseline when treatment was started in the first eye was less pronounced. One reason for this could be that patients are seen on a more regular basis in the T&E regimen. Another reason could be that 78% of the patients were still receiving treatment on their first eye when AMD was diagnosed in the second eye in the period 2017–2018, compared to 61% in the earlier period, 2010–2013.
In conclusion, the mean duration of treatment of the first eye before treatment was started in the fellow eye was 19 months, and treatment had been started in the second eye of 61% of the patients within 2 years. The visual acuity was higher in the second eye than in the first eye, at the time when treatment was initiated in that eye, and it was higher in the second eye at the start of treatment when the first eye was still being treated.
This study was supported by Skåne University Hospital (SUS) Research Grants and the Foundation for the Visually Impaired in the County of Malmöhus. We would also like to thank all the ophthalmology clinics in Sweden that register data in the Swedish Macula Register enabling studies such as this.
References
- Augsburger M, Sarra GM & Imesch P (2019): Treat and extend versus pro re nata regimens of ranibizumab and aflibercept in neovascular age‐related macular degeneration: a comparative study. Graefes Arch Clin Exp Ophthalmol 257: 1889–1895. [DOI] [PubMed] [Google Scholar]
- Barbazetto IA, Saroj N, Shapiro H, Wong P, Ho AC & Freund KB (2010): Incidence of new choroidal neovascularization in fellow eyes of patients treated in the MARINA and ANCHOR trials. Am J Ophthalmol 149: 939–946. [DOI] [PubMed] [Google Scholar]
- Bek T & Klug SE (2018): Incidence and risk factors for neovascular age‐related macular degeneration in the fellow eye. Graefes Arch Clin Exp Ophthalmol 256: 2061–2068. [DOI] [PubMed] [Google Scholar]
- Chew JK, Zhu M, Broadhead GK, Luo K, Hong T & Chang AA (2017): Bilateral neovascular age‐related macular degeneration: comparisons between first and second eyes. Ophthalmologica 238: 23–30. [DOI] [PubMed] [Google Scholar]
- Colijn JM, Buitendijk GH, Prokofyeva E et al. (2017): EYE‐RISK consortium; European Eye Epidemiology (E3) consortium. Prevalence of age‐related macular degeneration in Europe: the past and the future. Ophthalmology 124: 1753–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasler K, Fu DJ Moares G et al. (2020): Moorfields AMD database report 2: fellow eye involvement with neovascular age‐related macular degeneration. Br J Ophthalmol 104: 684–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregori NZ, Feuer W & Rosenfeld PJ (2010): Novel method for analyzing Snellen visual acuity measurements. Retina 30: 1046–1050. [DOI] [PubMed] [Google Scholar]
- Hatz K & Prünte C (2016): Changing from a pro re nata treatment regimen to a treat and extend regimen with ranibizumab in neovascular age‐related macular degeneration. Br J Ophthalmol 100: 1341–1345. [DOI] [PubMed] [Google Scholar]
- Hatz K & Prünte C (2017): Treat and Extend versus Pro Re Nata regimens of ranibizumab in neovascular age‐related macular degeneration: a comparative 12 Month study. Acta Ophthalmol 95: e67–e72. [DOI] [PubMed] [Google Scholar]
- Joachim N, Colijn JM, Kifley A et al. (2017): Five‐year progression of unilateral age‐related macular degeneration to bilateral involvement: the Three Continent AMD Consortium report. Br J Ophthalmol 101: 1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvannli L & Krohn J (2017): Switching from pro re nata to treat‐and‐extend regimen improves visual acuity in patients with neovascular age‐related macular degeneration. Acta Ophthalmol 95: 678–682. [DOI] [PubMed] [Google Scholar]
- Lee AY, Lee CS, Butt T et al. (2015): UKAMD EMR USERS GROUP REPORT V: benefits of initiating ranibizumab therapy for neovascular AMD in eyes with vision better than 6/12. Br J Ophthalmol 99: 1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lövestam Adrian M, Vassilev ZP & Westborg I (2019): Baseline visual acuity as a prognostic factor for visual outcomes in patients treated with aflibercept for wet age‐related macular degeneration: data from the INSIGHT study using the Swedish Macula Register. Acta Ophthalmol 97: 91–98. [DOI] [PubMed] [Google Scholar]
- Maguire MG, Martin DF, Ying GS et al. (2016): Five‐year outcomes with anti‐vascular endothelial growth factor treatment of neovascular age‐related macular degeneration: the comparison of age‐related macular degeneration treatments trials. Ophthalmology 123: 1751–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DF, Maguire MG, Fine SL et al. (2012): Ranibizumab and bevacizumab for treatment of neovascular age‐related macular degeneration: two‐year results. Ophthalmology 119: 1388–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin VL, Young J, Silva FQ, Conti FF & Singh RP (2018): Outcomes of patients with exudative age‐related macular degeneration treated with anti‐vascular endothelial growth factor therapy for three or more years: a review of current outcomes. Retina 38: 1500–1508. [DOI] [PubMed] [Google Scholar]
- Rasmussen A, Brandi S, Fuchs J, Hansen LH, Lund‐Andersen H, Sander B & Larsen M (2015): Visual outcomes in relation to time to treatment in neovascular age‐related macular degeneration. Acta Ophthalmol 93: 616–620. [DOI] [PubMed] [Google Scholar]
- Schmidt‐Erfurth U, Kaiser PK, Korobelnik JF et al. (2014): Intravitreal aflibercept injection for neovascular age‐related macular degeneration: ninety‐six‐ week results of the VIEW studies. Ophthalmology 121: 193–201. [DOI] [PubMed] [Google Scholar]
- Svenska Makula Registret (2019): Årsrapport 2019. Available at: https://rcsyd.se/makulareg/wp‐content/uploads/sites/2/2020/09/Årsrapport2019SMR.pdf. (Accessed on 10 May 2021).
- Ueta T, Iriyama A, Francis J et al. (2008): Development of typical age‐related macular degeneration and polypoidal choroidal vasculopathy in fellow eyes of Japanese patients with exudative age‐related macular degeneration. Am J Ophthalmol 146: 96–101. [DOI] [PubMed] [Google Scholar]
- Volkmann I, Knoll K, Wiezorrek M, Greb O & Framme C (2020): Individualized treat‐and‐extend regime for optimization of real‐world vision outcome and improved patients' persistence. BMC Ophthalmol 20: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westborg I, Granstam E, Rosso A, Albrecht S, Karlsson N & Lövestam‐Adrian M (2017): Treatment for neovascular age‐related macular degeneration in Sweden: outcomes at seven years in the Swedish Macula Register. Acta Ophthalmol 95: 787–795. [DOI] [PubMed] [Google Scholar]
- Wolf S, Bandello F, Loewenstein A, Slakter J, Katz T, Sowade O & Korobelnik JF (2016): Baseline characteristics of the fellow eye in patients with neovascular age‐related macular degeneration: post hoc analysis of the VIEW studies. Ophthalmologica 236: 95–99. [DOI] [PubMed] [Google Scholar]
- Wong TY, Chakravarthy U, Klein R et al. (2008): The natural history and prognosis of neovascular age‐related macular degeneration: a systematic review of the literature and meta‐analysis. Ophthalmology 115: 116–126. [DOI] [PubMed] [Google Scholar]
- Wong TY, Lanzetta P, Bandello F, Eldem B, Navarro R, Lövestam‐Adrian M & Loewenstein A (2020): Current concepts and modalities for monitoring the fellow eye in neovascular age‐related macular degeneration: an Expert Panel Consensus. Retina 40: 599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying GS, Huang J, Maguire MG et al. (2013): Baseline predictors for one‐year visual outcomes with ranibizumab or bevacizumab for neovascular age‐related macular degeneration. Ophthalmology 120: 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarranz‐Ventura J, Liew G, Johnston RL et al. (2014): The neovascular age‐related macular degeneration database: report 2: incidence, management, and visual outcomes of second treated eyes. Ophthalmology 121: 1966–1975. [DOI] [PubMed] [Google Scholar]


