Summary
Until now, no study has directly network meta‐analysed the impact of nasal masks, nasal pillows and oronasal masks on continuous positive airway pressure therapy in patients with obstructive sleep apnea. This study aimed to meta‐analyse the impact of three kinds of nasal interfaces with both network meta‐analysis and pairwise comparison. PubMed, EMBASE, CENTRAL and ClinicalTrials.gov were systematically searched from inception to December 2020 for studies that compared the three types of nasal interfaces for treating obstructive sleep apnea with continuous positive airway pressure. The outcomes were residual apnea–hypopnea index, continuous positive airway pressure, and nightly average usage. The network meta‐analysis was conducted using multivariate random‐effects in a frequentist framework where three interfaces were ranked with the surface under the cumulative ranking probabilities. The pairwise comparison was conducted using random‐effects meta‐analysis. Twenty‐nine articles comprising 6378 participants were included. The pairwise comparison showed both nasal masks and nasal pillows were associated with lower residual apnea–hypopnea index, lower continuous positive airway pressure, and higher continuous positive airway pressure adherence compared with oronasal masks. The surface under the cumulative ranking confirmed that nasal masks were associated with the lowest residual apnea–hypopnea index and highest adherence, while pillows were associated with the lowest continuous positive airway pressure. The meta‐regression identified that lower pretreatment apnea–hypopnea index and continuous positive airway pressure determined during continuous positive airway pressure titration (versus determined during continuous positive airway pressure therapy) was associated with lower continuous positive airway pressure with nasal masks and nasal pillows. In conclusion, compared with oronasal masks, nasal masks and nasal pillows are better interfaces, especially in patients with lower pretreatment apnea–hypopnea index and those with the therapeutic pressure determined during continuous positive airway pressure titration.
Keywords: compliance, positive airway pressure, randomized controlled trial, sleep‐disordered breathing, titration
1. INTRODUCTION
Continuous positive airway pressure (CPAP) is the standard treatment for obstructive sleep apnea (OSA), and has been shown to efficaciously reverse apnea–hypopnea, and improve daytime sleepiness, blood pressure, dyslipidaemia and quality of life (Patil et al., 2019). However, its impact on cardiovascular outcome was only noticed in the observational trials (Lin et al., 2018; Marin, Carrizo, Vicente, & Agusti, 2005) but not in the large‐scale randomized controlled trials (RCTs; Barbe et al., 2012; McEvoy et al., 2016; Peker et al., 2016). Two RCTs showed a significant cardiovascular risk reduction in patients who used CPAP ≥ 4 hr per night than those used < 4 hr per night (Barbe et al., 2012; Peker et al., 2016), while the association was not observed in other RCTs (McEvoy et al., 2016). The effectiveness of CPAP treatment is determined by both the therapeutic efficacy measured by the reduction of apnea–hypopnea index (AHI) and the CPAP adherence (Sutherland, Phillips, & Cistulli, 2015). Studies suggested that CPAP usage ≥ 4 hr per night and ≥ 6 hr per night are required for lowering blood pressure and improving daytime function, respectively (Fava et al., 2014; Weaver et al., 2007). Initial nasal interface fitting and CPAP adherence monitoring are crucial in improving CPAP adherence (R. G. Andrade et al., 2014; Bachour, Vitikainen, Virkkula, & Maasilta, 2013; Borel et al., 2013; Chai, Pathinathan, & Smith, 2006).
Nasal masks, nasal pillows and oronasal masks are the three commonly used nasal interfaces. Nasal masks and nasal pillows allow delivery of positive airway pressure through the nose, while oronasal masks allow airflow through both the nasal and oral routes. Overall, nasal masks work for the majority of patients. Alternatively, oronasal masks are options for patients with nasal obstruction or substantial oral leaks (Beecroft, Zanon, Lukic, & Hanly, 2003; Lebret et al., 2015; Lebret et al., 2018; Prosise & Berry, 1994). For those who have claustrophobia, mask‐induced nose bridge pressure sores, thick facial hairs, or frequent mask dislodgement due to tossing and turning in sleep, nasal pillows provide another suitable option. Previous studies suggested that nasal pillows have equal efficacy and objective adherence as nasal masks (Bachour et al., 2013; Borel et al., 2013; Ebben, Oyegbile, & Pollak, 2012; Lanza et al., 2018; Massie & Hart, 2003; Ryan, Garvey, Swan, Behan, & McNicholas, 2011; Zhu, Wimms, & Benjafield, 2013).
Several studies have compared the effects of different interfaces on outcomes including residual AHI, CPAP pressure, and adherence. Yet, the results remain inconsistent (Bachour et al., 2013; Bakker, Neill, & Campbell, 2012; Beecroft et al., 2003; Bettinzoli et al., 2014; Blanco, Ernst, Salvado, & Borsini, 2019; Borel et al., 2013; Casanova et al., 2013; Deshpande et al., 2016; Duarte, Mendes, Oliveira, Magalhaes‐da‐Silveira, & Gozal, 2020; Ebben, Milrad, Dyke, Phillips, & Krieger, 2016; Ebben, Narizhnaya, Segal, Barone, & Krieger, 2014; Ebben et al., 2012; Foellner et al., 2020; Goh et al., 2019; Kaminska et al., 2014; Lanza et al., 2018; Lebret et al., 2015; Lebret et al., 2018; Massie & Hart, 2003; Mortimore, Whittle, & Douglas, 1998; Prosise & Berry, 1994; Rowland et al., 2018; Ryan et al., 2011; Schell & Soose, 2017; Shirlaw, Duce, Milosavljevic, Hanssen, & Hukins, 2019; Teo et al., 2011; Westhoff & Litterst, 2015; Zampogna et al., 2019; Zhu et al., 2013) as RCTs are commonly underpowered by small sample sizes, while large‐scale observational studies may be biased due to various confounders derived from participant characteristics and study design. A Cochrane systematic review (Chai et al., 2006) previously concluded that the optimal form of CPAP delivery interface remained unclear. A more recent random‐effects meta‐analysis comparing the nasal masks and oronasal masks (R. G. S. Andrade et al., 2018) suggested that nasal masks were associated with better efficacy in lowering AHI, better adherence, and lower CPAP setting than oronasal masks. Since this meta‐analysis, more studies have compared the effects of different interfaces (Blanco et al., 2019; Duarte et al., 2020; Foellner et al., 2020; Goh et al., 2019; Lanza et al., 2018; Lebret et al., 2018; Rowland et al., 2018; Schell & Soose, 2017; Shirlaw et al., 2019; Zampogna et al., 2019). A recent systemic review for positive airway pressure treatment of adult OSA suggested that although the residual AHI was higher in oronasal interfaces than nasal interfaces, this difference may not be clinically significant (Patil et al., 2019).
Network meta‐analysis (NMA) has the capability to synthesize and compare both direct and indirect evidence from multiple clinical trials as randomized trials can rarely compare all available therapeutic options. NMA is especially useful when direct evidence is scarce or unavailable. In the case of CPAP mask comparison, oronasal masks and nasal pillows are often compared with nasal masks, but are seldom compared with each other. This study aimed to perform both NMA and pairwise meta‐analyses to compare the impact of nasal pillows, nasal masks and oronasal masks on residual AHI, CPAP pressure, and adherence in patients with OSA. We also performed subgroup analysis and meta‐regression to identify if the mask performance may be associated with patient characteristics such as pretreatment AHI level, CPAP‐naïvety (versus CPAP‐experienced), or outcomes determined during CPAP titration (versus determined during home CPAP therapy).
2. METHODS
2.1. Data source and search strategy
A systematic search was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement (Hutton et al., 2015). The present meta‐analysis was registered with the International Prospective Register for Systematic Reviews (CRD42018114447). The literature search, eligibility assessment, quality and risk of bias assessment, and data extraction were conducted by SWH, LYC and YHC independently, while any discrepancies were resolved through PLL. A systematic search of PubMed, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL) and ClinicalTrials.gov from their inception to 31 December 2020, using relevant text words and medical subject heading (MeSH) terms related to sleep apnea, CPAP and mask (Table S1), was performed. There were no time frame or language restrictions. The ClinicalTrial.gov website was also searched for trials registered but not yet published. The references listed in the included reports were manually scanned for relevant reports.
2.2. Screening of articles and inclusion criteria
After removing duplicates, the titles and abstracts of the retrieved studies were reviewed to select those eligible for full‐text review. Studies comparing the interfaces in patients with OSA, defined with AHI, respiratory disturbance index or oxygen desaturation index, were considered eligible. We excluded studies on paediatric patients (age < 18 years), without CPAP interventions, and studies without the comparison of interfaces. We also sequentially excluded non‐original studies (including case reports, case series, reviews, meta‐analyses or conference abstracts without further publication) and studies without the outcomes of interest.
2.3. Quality assessment
The risk of bias was assessed using Cochrane Collaboration's tool for RCTs (J. P. Higgins et al., 2011), with each being classified as low, high or unclear. For observational studies, the risk of bias was evaluated according to Risk Of Bias In Non‐randomized Studies of Interventions (ROBINS‐I; J. A. Sterne et al., 2016), with each being classified as mild, moderate, serious or critical (Supplementary Methods).
2.4. Data extraction
The data including the name of the author, year of publication, sample size, study design, mask type and manufacturer, participant characteristics (age, gender, body mass index), pretreatment AHI, CPAP treatment before participant enrollment, the timing of outcome determination, follow‐up duration, residual AHI, CPAP pressure, and adherence were extracted. For studies without the required information, we made attempts to contact the authors for additional details.
2.5. Pairwise comparison
The outcomes were residual AHI, CPAP pressure, and adherence measured according to the nightly use of CPAP in hours. Pairwise comparisons of mean and standard deviation (SD) were analysed. If the data were reported as the median and interquartile range (IQR), the median was used to estimate the mean difference, while the IQR was divided by 1.35 to estimate the standard deviation (J. P. T. Higgins & Deeks, 2011). The pooled effect of differences in outcomes was calculated using random‐effect generic inverse variance (J. P. Higgins, Thompson, Deeks, & Altman, 2003). Results were presented using mean difference (MD) with 95% confidence interval (CI), and were illustrated with the Forest plot.
Publication bias was assessed using Egger's test and visualized with funnel plots using MD with a standard error. Outcomes reported in fewer than 10 studies were not tested with Egger's test due to the lack of power to detect real asymmetry (J. A. C. Sterne, Egger, & Moher, 2011).
Heterogeneity across studies was assessed using the χ2‐test and I 2 statistics. A Cochran Q with a p‐value < 0.1 was considered to indicate heterogeneity, while the I 2 statistic was used to indicate low (0%–25%), moderate (26%–75%) and significant (76%–100%) heterogeneity. To explore heterogeneity, subgroup analyses were performed by grouping reports including trial design (RCT versus non‐RCT), CPAP experience before enrollment (CPAP‐naïve versus CPAP‐experienced), and the timing of CPAP pressure determination (during titration versus during CPAP therapy at home). The direct pairwise meta‐analyses were conducted using Review Manager 5.3 (The Cochrane Collaboration, Copenhagen, Denmark) and R 3.5.3 (R Development Core Team) with the metaphor package.
2.6. Network meta‐analysis
Frequentist NMA was implemented using the suite “network” for the statistical software STATA (version 17.0). Random‐effects models were utilized for all the analyses to account for the expected clinical and methodological heterogeneity. In the network diagram, each interface is represented by a node, and the comparisons between interfaces are represented by the edges between the nodes. The size of the nodes is proportional to the number of subjects receiving the intervention, and the width of the edge is proportional to the number of trials that compare the two interventions. The ranking of treatments was presented by the surface under the cumulative ranking curve (SUCRA), ranging from 0 to 1. SUCRA is interpreted as the performance of an intervention compared with a hypothetical perfect intervention. The greater the SUCRA value, the better the performance of an intervention is. We applied the design‐by‐treatment interaction model to assess the overall inconsistency within the NMA, and used the loop inconsistency model and side‐splitting model to evaluate the consistency between the direct and indirect evidence for any treatment comparison. Additionally, we assessed the quality of evidence contributing to each network estimate using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework (Salanti, Del Giovane, Chaimani, Caldwell, & Higgins, 2014).
Meta‐regression was also conducted in both pairwise standard meta‐analysis and NMA to investigate the associations between parameters (pretreatment AHI, CPAP‐naïve versus CPAP‐experienced patients, and pressure determined during CPAP titration versus home CPAP therapy) and outcomes to identify sources of heterogeneity.
3. RESULTS
3.1. Search results and included studies
The systematic literature search identified 7136 articles, of which 44 articles were reviewed for the full text. Among them, 29 articles (28 were in English and one was in French) comprising 6378 participants were included for the meta‐analysis (Figure 1; Table 1; Supplementary Results; Bachour et al., 2013; Bakker et al., 2012; Beecroft et al., 2003; Bettinzoli et al., 2014; Blanco et al., 2019; Borel et al., 2013; Casanova et al., 2013; Deshpande et al., 2016; Duarte et al., 2020; Ebben et al., 2016; Ebben et al., 2014; Ebben et al., 2012; Foellner et al., 2020; Goh et al., 2019; Kaminska et al., 2014; Lanza et al., 2018; Lebret et al., 2015; Lebret et al., 2018; Massie & Hart, 2003; Mortimore et al., 1998; Prosise & Berry, 1994; Rowland et al., 2018; Ryan et al., 2011; Schell & Soose, 2017; Shirlaw et al., 2019; Teo et al., 2011; Westhoff & Litterst, 2015; Zampogna et al., 2019; Zhu et al., 2013). The characteristics of these studies are listed in Table 1. The article by Mortimore et al. (1998) included both an RCT and an observational portion, and we reported them separately in Table 1. Among 18 observational studies, 14 included two or three groups of participants each for one specific interface (Bachour et al., 2013; Beecroft et al., 2003; Bettinzoli et al., 2014; Blanco et al., 2019; Borel et al., 2013; Casanova et al., 2013; Deshpande et al., 2016; Duarte et al., 2020; Ebben et al., 2016; Lanza et al., 2018; Lebret et al., 2015; Lebret et al., 2018; Schell & Soose, 2017; Zampogna et al., 2019), and four studied different interfaces in one group of subjects (Kaminska et al., 2014; Mortimore et al., 1998; Prosise & Berry, 1994; Westhoff & Litterst, 2015). The number of studies and participants comparing all interfaces on three primary outcomes are illustrated in Figure 2.
FIGURE 1.

Flow diagram of our literature search strategy
TABLE 1.
Study characteristics (a) and outcome (b) of 30 studies included
| (a) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author (year) | Design | CPAP‐naïve | Total no. | Nasal mask no. | Oronasal mask no. | Pillow no. | Age, mean (SD), years | Male, no. (%) | BMI, mean (SD), kg m–2 | Pretreatment AHI, mean (SD), events per hr | Outcome measured |
| Prosise (1994) | O | No | 5 | 5 | 5 | 0 | 60.0 (14.6) | NA | NA | 62.3 (28.2) | Titration |
| Mortimore (1998–1) | R | Yes | 20 | 20 | 20 | 0 | 52.0 (13.4) | NA | 32.0 (4.5) | 34.0 (23.3) | Therapy |
| Mortimore (1998–2) | O | No | 10 | 10 | 10 | 0 | 50.0 (9.5) | NA | 31.0 (12.6) | 46.0 (31.6) | Therapy |
| Beecroft (2003) | O | Yes | 72 | 65 | 7 | 0 | 50.6 (11.7) | 50 (70) | 32.5 (9.0) | 40.6 (25.8) | Titration, therapy |
| Massie (2003) | R | Yes | 39 | 39 | 0 | 39 | 48.7 (8.5) | 32 (82) | 35.9 (6.4) | 47.1 (35.1) | Titration, therapy |
| Ryan (2011) | R | Yes | 21 | 21 | 0 | 21 | 49.0 (10.0) | 19 (90) | 35.4 (7.6) | 52.4 (21.6) | Therapy |
| Teo (2011) | R | Yes | 24 | 24 | 24 | 0 | 51.3 (13.3) | 18 (75) | 33.8 (9.4) | 47.0 (15.2) | Titration |
| Bakker (2012) | R | No | 12 | 24 | 24 | 0 | 48.8 (NA) | 11 (92) | 37.7 (5.0) | 59.8 (28.6) | Therapy |
| Ebben (2012) | R | Yes | 55 | 21 | 16 | 18 | 55.6 (14.4) | 33 (60) | 33.2 (7.3) | 36.3 (22.0) | Titration |
| Bachour (2013) | O | No | 709 | 577 | 66 | 66 | 58.0 (12.0) | 569 (78) | NA | NA | Therapy |
| Borel (2013) | O | Yes | 2311 | 1443 | 605 | 263 | 57.3 (12.2) | 1641 (71) | 32.2 (6.6) | 40.7 (20.6) | Therapy |
| Casanova (2013) | O | No | 605 | 309 | 296 | 0 | 60.5 (12.5) | 445 (74) | NA | 41.7 (19.4) | Therapy |
| Zhu (2013) | R | No | 20 | 20 | 0 | 20 | 64.6 (9.5) | 15 (75) | NA | NA | Therapy |
| Bettinzoli (2014) | O | No | 109 | 67 | 42 | 0 | 58.3 (12.9) | 170 (78) | 33.9 (6.3) | 41.1 (20.5) | Titration |
| Ebben (2014) | R | Yes | 14 | 14 | 14 | 0 | 62.0 (15.0) | 11 (79) | 29.6 (6.0) | 36.5 (14.5) | Therapy |
| Kaminska (2014) | O | No | 6 | 6 | 6 | 0 | 53.5 (11.9) | 5 (83) | 28.1 (4.9) | 56.8 (40.1) | Titration |
| Lebret (2015) | O | No | 34 | 27 | 7 | 0 | 57.5 (6.7) e | 22 (65) | 32.6 (4.5) e | 45 (8.9) e | Therapy |
| Westhoff (2015) | O | No | 54 | 54 | 54 | 0 | 64.4 (12.8) | 61 (94) | 32.2 (8.1) | 31.8 (16.3) | Titration |
| Deshpande (2016) | O | Yes | 358 | 124 | 165 | 69 | 57.7 (14.1) | 226 (63) | 34.1 (7.9) | 41.9 (31.1) | Titration |
| Ebben (2016) | O | No | 10 a | 5 | 4 | 0 | 59.1 (11.1) | 8 (89) | 30.4 (6.4) | 46.0 (27.8) | Therapy |
| Schell (2017) | O | Mixed | 218 | 88 | 75 | 55 | 53.0 (12.6) | 163 (75) | 34.1 (7.3) | 32.4 (28.7) | Therapy |
| Goh (2018) | R | Yes | 85 | 85 | 85 | 85 | 46.0 (12.0) | 71 (84) | 29.9 (5.6) | 53.6 (24.0) | Therapy |
| Lanza (2018) | O | Yes | 144 | 42 | 0 | 102 | 58.1 (12.9) | 110 (76) | 33.9 (7.6) | 44.5 (NA) | Titration, therapy |
| Lebret (2018) | O | Yes | 74 b | 58 | 14 | 0 | 55.8 (13.0) e | 51 (65) | 32.9 (6.7) e | 44.3 (26.6) e | Therapy |
| Rowland (2018) | R | Yes | 48 c | 37 | 39 | 0 | 56.6 (1.8) | 32 (68) | 35.3 (1.1) | 57.6 (3.6) | Therapy |
| Shirlaw (2018) | R | No | 60 | 60 | 60 | 0 | 64.0 (10.0) | 45 (75) | 34.5 (6.7) e | 32.0 (18.8) e | Therapy |
| (a) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author (year) | Design | CPAP‐naïve | Total no. | Nasal mask no. | Oronasal mask no. | Pillow no. | Age, mean (SD), years | Male, no. (%) | BMI, mean (SD), kg m–2 | Pretreatment AHI, mean (SD), events per hr | Outcome measured |
| Blanco (2019) | O | Yes | 707 | 532 | 71 | 104 | 59.1 (12.2) | 513 (73) | 34.1 (6.7) | 33.0 | Titration |
| Zampogna (2019) | O | Yes | 147 d | 68 | 45 | 0 | 59.0 (4.7) e | 125 (85) | 31 (2.3) e | 40.0 (9.7) e | Therapy |
| Duarte (2020) | O | Yes | 436 | 283 | 153 | 0 | 56.0 (14.8) e | 287 (66) | 29.5 (4.9) e | NA | Titration |
| Foellner (2020) | R | No | 29 | 29 | 29 | 0 | 59.5 (NA) | 17 (59) | 33.1 (NA) | NA | Titration |
| (b) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Author (year) | Residual AHI, mean (SD), events per hr | CPAP pressure, mean (SD), cmH2O | Adherence, mean (SD), hr per night | ||||||
| Nasal | Oronasal | Pillow | Nasal | Oronasal | Pillow | Nasal | Oronasal | Pillow | |
| Prosise (1994) | 7.2 (3.5) | 7.6 (4.9) | NA | NA | NA | NA | NA | NA | NA |
| Mortimore (1998–1) | NA | NA | NA | NA | NA | NA | 5.3 (1.8) | 4.3 (2.2) | NA |
| Mortimore (1998–2) | NA | NA | NA | NA | NA | NA | 5.1 (2.2) | 4.0 (2.5) | NA |
| Beecroft (2003) | 6.7 (13.3) | 9.8 (12.8) | NA | 7.7 (2.1) | 9.7 (3.2) | NA | 5.0 (2.1) | 4.5 (1.9) | NA |
| Massie (2003) | 7.0 (7.7) | NA | 10.2 (9.8) | 8.4 (2.0) | NA | 8.9 (2.4) | 5.4 (1.6) | NA | 5.6 (1.3) |
| Ryan (2011) | 2.6 (2.7) | NA | 3.0 (2.9) | NA | NA | NA | 5.1 (1.9) | NA | 5.0 (1.7) |
| Teo (2011) | 5.3 (3.4) | 11.0 (10.4) | NA | 12.2 (2.2) | 11.9 (1.4) | NA | NA | NA | NA |
| Bakker (2012) f | 0.6 (1.2) | 2.2 (2.6) | NA | 11.0 (2.1) | 10.8 (1.7) | NA | 6.7 (1.0) | 6.4 (1.9) | NA |
| Ebben (2012) | 2.7 (2.3) | 3.6 (3.2) | 2.8 (2.1) | 7.6 (1.5) | 10.4 (3.6) | 8.8 (3.1) | NA | NA | NA |
| Bachour (2013) | NA | NA | NA | NA | NA | NA | 5.8 (2.8) | 4.7 (2.8) | 4.7 (3.2) |
| Borel (2013) | NA | NA | NA | 8.9 (2.7) | 9.6 (2.5) | 8.2 (2.4) | 5.5 (3.4) | 5.0 (2.7) | 5.3 (2.3) |
| Casanova (2013) | NA | NA | NA | NA | NA | NA | 5.6 (2.0) | 5.0 (2.2) | NA |
| Zhu (2013) | 1.7 (1.1) | NA | 1.9 (1.3) | 13.9 (1.9) | NA | 13.5 (1.4) | 7.2 (1.4) | NA | 7.4 (1.4) |
| Bettinzoli (2014) | 2.6 (2.5) | 4.5 (4.0) | NA | 10.0 (2.0) | 11.2 (2.1) | NA | NA | NA | NA |
| Ebben (2014) | 2.9 (2.3) | 7.9 (7.0) | NA | 7.4 (2.4) | 9.3 (4.2) | NA | 5.2 (1.7) | 4.6 (2.2) | NA |
| Kaminska (2014) | NA | NA | NA | 10.4 (3.0) | 16.3 (5.4) | NA | NA | NA | NA |
| Lebret (2015) | NA | NA | NA | NA | NA | NA | 6.4 (2.2) e | 6.2 (3.1) e | NA |
| Westhoff (2015) | 6.0 (3.6) | 31.8 (16.3) | NA | 7.2 (1.9) | 9.5 (2.3) | NA | NA | NA | NA |
| Deshpande (2016) | 6.4 (6.7) | 11.3 (11.7) | 6.7 (8.5) | 10 (3.0) | 12.0 (4.1) | 11.0 (3.3) | NA | NA | NA |
| Ebben (2016) | 2.5 (2.2) | 0.8 (0.9) | NA | 9.4 (4.0) | 6.8 (1.5) | NA | NA | NA | NA |
| Schell (2017) | NA | NA | NA | 12.4 (4.2) | 15.2 (4.1) | 12.3 (4.1) | NA | NA | NA |
| Goh (2018) | 4.0 (4.2) | 7.2 (5.2) | 4.1 (3.3) | 8.2 (2.2) | 7.7 (2.6) | 7.5 (2.0) | 4.0 (2.3) | 3.3 (2.2) | 3.5 (2.2) |
| Lanza (2018) | 1.1 (7.0) | NA | 0.7 (6.7) | 11.6 (1.6) | NA | 11.4 (1.5) | 5.3 (1.6) | NA | 5.5 (1.8) |
| Lebret (2018) | 10.3 (9.0) e | 17.5 (10.0) e | NA | 8.5 (2.6) | 8.9 (2.8) | NA | NA | NA | NA |
| Rowland (2018) | 4.0 (3.1) | 7.1 (7.7) | NA | NA | NA | NA | 5.7 (2.6) | 5.5 (2.4) | NA |
| Shirlaw (2018) | 4.9 (4.1) e | 5.3 (3.3) e | NA | 11.5 (1.3) e | 11.7 (1.6) e | NA | 7.3 (1.6) e | 7.3 (1.7) e | NA |
| Blanco (2019) | 4.6 (4.1) | 7.6 (5.2) | 3.7 (2.9) | 8.3 (2.1) | 9.3 (2.6) | 7.1 (1.9) | 6.3 (1.2) | 6.1 (1.1) | 6.2 (1.1) |
| Zampogna (2019) | 6.0 (4.4) | 6.0 (4.8) | NA | 10.7 (1.6) | 10.6 (1.6) | NA | 4.3 (2.7) | 4.3 (2.7) | NA |
| Duarte (2020) | 2.2 (2.5) e | 4.9 (5.8) e | NA | 10.7 (2.3) e | 12.9 (3.3) e | NA | NA | NA | NA |
| Foellner (2020) | 2.6 (2.3) | 8.5 (6.7) | NA | NA | NA | NA | NA | NA | NA |
AHI, apnea–hypopnea index; BMI, body mass index; CPAP, continuous positive airway pressure; NA, not available; O, observational; R, randomized controlled trial.
One patient with two unspecified pressure levels was excluded.
Mask type from two patients was missing.
Forty‐eight patients were randomized into the study. The characteristics at baseline were obtained from 34 completed data.
Data on the use of masks were available in 113 out of 147 patients.
Mean and SD were estimated using median and IQR/1.35.
Both APAP and CPAP data were grouped together.
FIGURE 2.

Network graph of interface comparison for residual apnea–hypopnea index (AHI), continuous positive airway pressure (CPAP) pressure and CPAP adherence. The number next to each node indicates the number of participants, and the size of each node reflects the proportion of participants. The number next to each edge indicates the number of studies and the edge thickness is proportional to the number of studies
Most of the enrolled participants were obese, male, and had a high pretreatment AHI (Table S2a). The timing of outcomes measured was listed in Table S2b. The titration duration ranged from 1 to 5 days, and the CPAP therapy duration ranged from 4 to a mean of 696 days. The information on products and manufacturers of masks is provided in the 20 studies (Table S2c), of which ResMed, Philips‐Respironics, and Fisher & Paykel are the top three most common. In nine studies, masks used were from more than one manufacturer, while masks were from the same manufacturer in 11 studies.
3.2. Bias assessment
Twelve RCTs were examined, and five of these studies properly described the procedure of randomization and allocation concealment (Bakker et al., 2012; Ebben et al., 2014; Goh et al., 2019; Rowland et al., 2018; Shirlaw et al., 2019). The data analysts were blinded in four studies (Bakker et al., 2012; Ebben et al., 2014; Goh et al., 2019; Rowland et al., 2018; Figure S1).
The 18 non‐RCTs were assessed using ROBINS‐I, and all were qualified for meta‐analysis (Table S3). Two studies (Kaminska et al., 2014; Westhoff & Litterst, 2015) had a critical bias in the selection of the participants as both studies recruited participants whose respiratory events were inadequately controlled with oronasal masks and were subsequently treated with nasal masks. The funnel plots and Egger's tests for pairwise comparisons were presented in Figure 3. There was no publication bias except for one study comparing nasal masks and nasal pillows on CPAP pressure showing positive Egger's test (p = 0.022). According to GRADE (Table S4), the quality of evidence was low to very low for all the comparison results in three outcomes as non‐RCTs formed most of the evidence.
FIGURE 3.

Funnel plot of included studies
3.3. Pairwise comparison outcomes
For residual AHI (Figure 4), nasal masks (−3.58 per hr, 95% CI –5.03 to −2.14, high heterogeneity) and nasal pillows (−3.03 per hr, 95% CI –4.46 to −1.59, moderate heterogeneity) were associated with a lower residual AHI compared with oronasal masks, while there was no difference between nasal masks and nasal pillows.
FIGURE 4.

Pairwise comparison of different interfaces for residual apnea–hypopnea index (AHI)
For CPAP pressure (Figure 5), nasal masks (−1.02 cmH2O, 95% CI –1.51 to −0.53, high heterogeneity) and nasal pillows (−1.45 cmH2O, 95% CI –2.15 to −0.76, high heterogeneity) were associated with a lower CPAP pressure compared with oronasal masks. There was no difference between nasal masks and nasal pillows.
FIGURE 5.

Pairwise comparison of different interfaces for continuous positive airway pressure (CPAP) pressure
For CPAP adherence (Figure 6), nasal masks (0.43 hr per night, 95% CI 0.29–0.58, low heterogeneity) and nasal pillows (0.18 hr per night, 95% CI –0.04 to 0.41, low heterogeneity) were associated with a higher adherence compared with oronasal masks. Nasal masks and nasal pillows had similar adherence.
FIGURE 6.

Pairwise comparison of different interfaces for CPAP adherence
3.4. NMA outcomes
Nasal masks were associated with the lower residual AHI (−3.88 per hr, 95% CI –5.98 to −1.78), lower CPAP pressure (−1.04 cmH2O, 95% CI –1.54 to −0.55) and higher adherence (0.43 hr per night, 95% CI 0.25–0.60) compared with oronasal masks. Nasal pillows were associated with lower residual AHI (−3.35 per hr, 95% CI –6.57 to −0.14), lower CPAP pressure (−1.29 cmH2O, 95% CI –1.97 to −0.60) and higher CPAP adherence (0.26 hr per night, 95% CI 0.04–0.49) compared with oronasal masks. There were no differences between nasal masks and nasal pillows. Nasal masks were best ranked in terms of low residual AHI and better CPAP adherence based on SUCRA (81.4% and 97.3%, respectively), while nasal pillows were ranked best in terms of low CPAP pressure (SUCRA 88.9%; Table 2). We found no evidence of inconsistency between direct and indirect evidence within our NMAs.
TABLE 2.
Cumulative ranking probabilities and SUCRA from NMA
| Nasal mask | Oronasal mask | Nasal pillow | ||
|---|---|---|---|---|
| Residual AHI | Ranking | 1 | 3 | 2 |
| SUCRA | 81.4 | 1.2 | 67.4 | |
| CPAP pressure | Ranking | 2 | 3 | 1 |
| SUCRA | 61.0 | 0.1 | 88.9 | |
| Adherence | Ranking | 1 | 3 | 2 |
| SUCRA | 97.3 | 0.2 | 52.4 | |
AHI, apnea–hypopnea index; CPAP, continuous positive airway pressure; SUCRA, surface under the cumulative ranking.
3.5. Subgroup analysis
In the subgroup analysis of RCTs and non‐RCTs (Figures S2–S4), the association of nasal masks and nasal pillows with lower pressure compared with oronasal masks was only observed in non‐RCTs (Figures S2b and S3b). The nasal pillows were also associated with lower AHI compared with nasal masks in non‐RCTs (Figure S4a).
In the subgroup analysis for CPAP‐naïve versus CPAP‐experienced participants, none of the three primary outcomes was associated with prior CPAP treatment (Figures S5, S6 and S7).
The subgroup analysis of outcomes measured during CPAP titration versus during CPAP therapy at home was performed except the outcome of CPAP adherence (Figures S8–S10). There was only one study (Blanco et al., 2019) that collected the data of CPAP adherence from the titration protocol, and its mean duration was only 3.2 days. It was inappropriate to evaluate the adherence in such a short duration. The subgroup effects were found on the residual AHI and the CPAP pressure. Nasal masks were associated with lower residual AHI and lower pressure measured during CPAP titration compared with oronasal masks (Figure S8a and b).
3.6. Meta‐regression
Mixed‐effects meta‐regression was performed for one‐tenth pretreatment AHI (AHI/10), CPAP treatment before enrollment, and timing of the outcome determined on both pairwise comparisons (Table S5) or NMA (Table S6). In the comparison of nasal masks versus oronasal masks, nasal masks were associated with lower CPAP pressure in the subgroup of CPAP pressure determined at CPAP titration (pairwise: coefficient − 1.255, 95% CI –2.393 to −0.016; Table S5a; NMA: coefficient − 1.239, 95% CI –2.123 to −0.354; Table S6). The pretreatment AHI/10 was associated with differences in CPAP pressure between nasal pillows and oronasal masks (pairwise: coefficient 1.015, 95% CI 0.662–1.368; Table S5b; NMA: coefficient 0.009, 95% CI 0.001–0.018; Table S6), which indicates at a lower pretreatment AHI and nasal pillows were associated with much lower CPAP pressure than oronasal masks (Figure 7).
FIGURE 7.
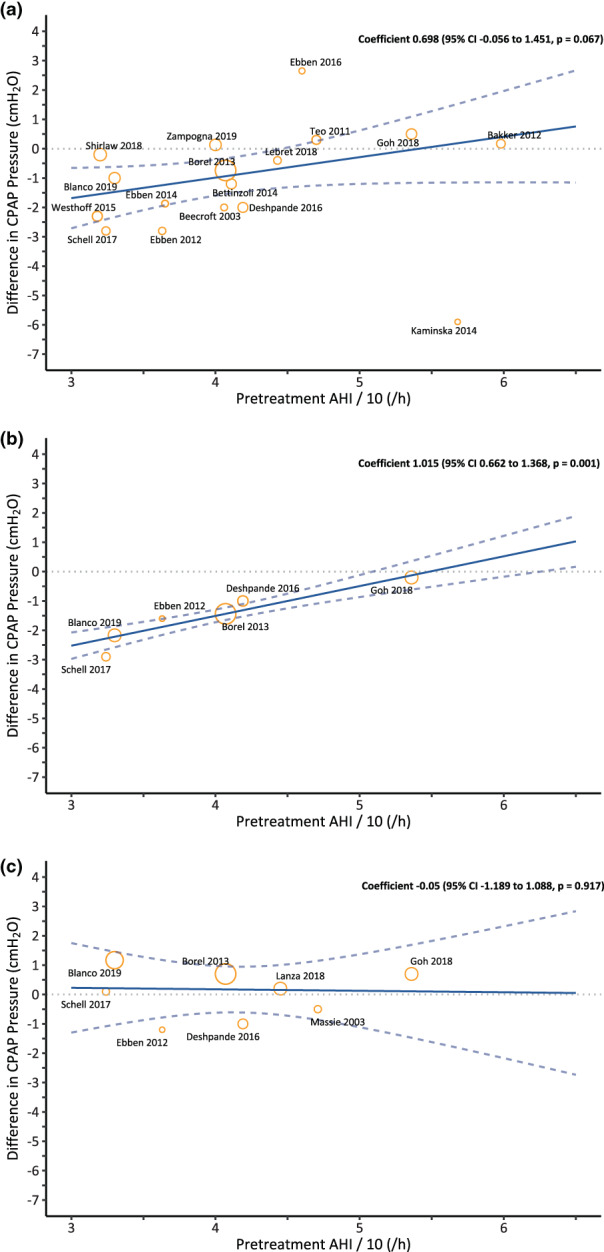
Pairwise meta‐regression to analyse the association between pretreatment apnea–hypopnea index (AHI) and differences in continuous positive airway pressure (CPAP) pressure among masks: (a) nasal mask versus oronasal mask; (b) nasal pillow versus oronasal mask; and (c) nasal mask versus nasal pillow
4. DISCUSSION
In this NMA study, we updated the literature search and compared three interfaces using both NMA and pairwise comparisons. We showed that oronasal masks were associated with higher residual AHI, CPAP pressure and lower adherence compared with the nasal masks and pillows, while nasal masks had no significant difference compared with nasal pillows. Subgroup effects were observed when the residual AHI and CPAP pressure were measured during titration in the comparison of nasal masks versus oronasal masks. The meta‐regression identified the lower pretreatment AHI, and the outcomes measured during titration were associated with lower CPAP pressure in nasal masks and nasal pillows compared with oronasal masks.
Our results showed nasal masks and nasal pillows offer modestly improved CPAP effectiveness by providing both better therapeutic efficacy (i.e. reduction of AHI), lower CPAP pressure and CPAP adherence compared with oronasal masks. The present study showed similar pairwise comparison results compared with Andrade's or Patil's meta‐analyses (Table S7; R. G. S. Andrade et al., 2018; Patil et al., 2019), but we demonstrated a smaller but explicit improvement on adherence with nasal masks in both RCTs and non‐RCTs. The difference may be due to more studies included and low heterogeneity (Figure S2c). Although a statistically significant difference exists, it remains debatable if these differences in CPAP adherence are of clinical significance (Patil et al., 2019).
Regarding the subgroup analysis and meta‐regression, the differences in CPAP pressure were significant only in the non‐RCTs subgroup with high heterogeneity, which might have resulted from a small number of participants in RCTs and the heterogeneity between the trials of each subgroup. This finding is similar to what R. G. S. Andrade et al. (2018) have reported. Another possibility was that in a couple of non‐RCT studies, the pretreatment AHI for oronasal masks was a bit higher than nasal masks and nasal pillows, which may contribute to the higher residual AHI of oronasal masks (Table S2a).
We identified in this analysis that the pressure difference between nasal masks and oronasal masks measured during titration was greater than that measured during home CPAP therapy, which has not been previously reported. One possible explanation for the larger pressure difference during CPAP titration is that patients who received oronasal masks due to mouth breathing may convert to nasal breathing during CPAP therapy and thus required lower therapeutic pressure to keep upper airway patent (Bachour & Maasilta, 2004). Another contributing factor could be the wide range of home CPAP therapy duration (Table S2b), which may have dampened the mask‐related difference in pressure.
Several possible mechanisms may lead to the poorer performance of oronasal masks than the nasal interfaces. First, oronasal masks have been shown to posteriorly displace the mandible, tongue and soft palate, and thus compromise the upper airway (R. G. Andrade et al., 2014; Bachour & Maasilta, 2004; Madeiro et al., 2019; Westhoff & Litterst, 2015). Second, positive airway pressure transmitted through the oral route may neutralize the upper airway splinting brought by positive airway pressure delivered through the nose and increase the upper airway surface tension (R. G. Andrade et al., 2014; Schorr, Genta, Gregorio, Danzi‐Soares, & Lorenzi‐Filho, 2012).
We did not meta‐analyse the leaks as mask leaks are reported in different manners by the CPAP manufacturers, which makes the outcome assessment less robust. Multiple studies have shown that oronasal masks may be associated with higher unintentional leaks, thus leading to poor adherence (R. G. S. Andrade et al., 2018; Bachour & Maasilta, 2004; Schorr et al., 2012). In contrast, a recent study demonstrated oronasal masks can effectively reduce unintentional oral air leaks (Lebret et al., 2018). In clinical practice, meticulous mask fitting and refitting are crucial, and mask selection must be individualized.
The strengths of the present study included the application of NMA analysis as well as the use of subgroup analysis and meta‐regression to clarify the factors contributing to the differences among nasal interfaces. NMA allows both direct and indirect comparisons across multiple studies with more than two different interventions, and has advantages over pairwise meta‐analysis in resolving inconsistent outcomes from multiple studies. Also, NMA can increase statistical power and cross‐validate the observed treatment effect of weak connections with reasonable network connectivity and sufficient sample sizes. There are a couple of limitations to this study. First, there was high heterogeneity among studies for outcomes including residual AHI and CPAP pressure, which made the quality of evidence low in those outcomes. Second, the outcomes in all of the included studies were measured during a short‐term CPAP treatment (Table S2b), and the results may not reflect the long‐term differences among masks. Third, we did not analyse the association between the mask manufacturers and outcomes, so it is not clear if the differences in manufacturers across the studies would be one of the sources of heterogeneity.
5. CONCLUSION
Both NMA and pairwise comparison showed that nasal masks and pillows are overall better nasal interfaces than oronasal masks, especially in patients with lower pretreatment AHI and those with the therapeutic CPAP pressure determined during CPAP titration.
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Conceptualization, PLL; methodology, PLL and MTL; software, YKT, SWH and LYC; formal analysis, YKT, SWH, LYC and YHC; writing—original draft preparation, SWH and LYC; writing—review and editing, PLL and AC; visualization, SWH, LYC and YHC; supervision, project administration and funding acquisition, PLL.
Supporting information
Appendix S1 Supporting Information
ACKNOWLEDGEMENT
The authors thank all authors who shared additional information from their published cohorts necessary for us to conduct the current study.
The study was supported by grants from the Ministry of Science and Technology, Taiwan (MOST 103‐2314‐B‐002‐139‐MY3;109‐2314‐B‐002‐252); National Scientific Council (NSC 100‐2314‐B‐002‐140‐; 101‐2314‐B‐002‐195‐; 102‐2314‐B002‐099); National Taiwan University (NTU‐ERP‐104R8951‐1; 105R8951‐1; 106R880301); National Taiwan University Hospital (NTUH 109‐042, 108‐S4331, 110‐N5164, 107‐19, 111‐S0298, 111‐X0033); and “Center for electronics technology integration (NTU‐107L900502; 108L900502; 109L900502)” from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan.
Chen, L.‐Y. , Chen, Y.‐H. , Hu, S.‐W. , Lin, M.‐T. , Lee, P.‐L. , Chiang, A. A. , & Tu, Y.‐K. (2022). In search of a better CPAP interface: A network meta‐analysis comparing nasal masks, nasal pillows and oronasal masks. Journal of Sleep Research, 31(6), e13686. 10.1111/jsr.13686
Funding information Ministry of Education (MOE) in Taiwan, Grant/Award Number: NTU‐107L900502; 108L900502; 109L900502); National Scientific Council, Grant/Award Numbers: NSC 100‐2314‐B‐002‐140‐, 101‐2314‐B‐002‐195‐, 102‐2314‐B002‐099; National Taiwan University Hospital, Grant/Award Numbers: NTUH 109‐042, 108‐S4331, 110‐N5164, 107‐19, 111‐S0298, 111‐X0033; National Taiwan University, Grant/Award Numbers: NTU‐ERP‐104R8951‐1, 105R8951‐1, 106R880301; Ministry of Science and Technology, Taiwan, Grant/Award Number: MOST 103‐2314‐B‐002‐139‐MY3;109‐2314‐B‐002‐252
DATA AVAILABILITY STATEMENT
Data available in article supplementary material
REFERENCES
- Andrade, R. G. , Piccin, V. S. , Nascimento, J. A. , Viana, F. M. , Genta, P. R. , & Lorenzi‐Filho, G. (2014). Impact of the type of mask on the effectiveness of and adherence to continuous positive airway pressure treatment for obstructive sleep apnea. Jornal Brasileiro de Pneumologia, 40(6), 658–668. 10.1590/S1806-37132014000600010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade, R. G. S. , Viana, F. M. , Nascimento, J. A. , Drager, L. F. , Moffa, A. , Brunoni, A. R. , … Lorenzi‐Filho, G. (2018). Nasal vs Oronasal CPAP for OSA treatment: A meta‐analysis. Chest, 153(3), 665–674. 10.1016/j.chest.2017.10.044 [DOI] [PubMed] [Google Scholar]
- Bachour, A. , & Maasilta, P. (2004). Mouth breathing compromises adherence to nasal continuous positive airway pressure therapy. Chest, 126(4), 1248–1254. 10.1378/chest.126.4.1248 [DOI] [PubMed] [Google Scholar]
- Bachour, A. , Vitikainen, P. , Virkkula, P. , & Maasilta, P. (2013). CPAP interface: Satisfaction and side effects. Sleep & Breathing, 17(2), 667–672. 10.1007/s11325-012-0740-0 [DOI] [PubMed] [Google Scholar]
- Bakker, J. P. , Neill, A. M. , & Campbell, A. J. (2012). Nasal versus oronasal continuous positive airway pressure masks for obstructive sleep apnea: A pilot investigation of pressure requirement, residual disease, and leak. Sleep & Breathing, 16(3), 709–716. 10.1007/s11325-011-0564-3 [DOI] [PubMed] [Google Scholar]
- Barbe, F. , Duran‐Cantolla, J. , Sanchez‐de‐la‐Torre, M. , Martinez‐Alonso, M. , Carmona, C. , Barcelo, A. , … Breathing, N. (2012). Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: A randomized controlled trial. JAMA, 307(20), 2161–2168. 10.1001/jama.2012.4366 [DOI] [PubMed] [Google Scholar]
- Beecroft, J. , Zanon, S. , Lukic, D. , & Hanly, P. (2003). Oral continuous positive airway pressure for sleep apnea: Effectiveness, patient preference, and adherence. Chest, 124(6), 2200–2208. 10.1378/chest.124.6.2200 [DOI] [PubMed] [Google Scholar]
- Bettinzoli, M. , Taranto‐Montemurro, L. , Messineo, L. , Corda, L. , Redolfi, S. , Ferliga, M. , & Tantucci, C. (2014). Oronasal masks require higher levels of positive airway pressure than nasal masks to treat obstructive sleep apnea. Sleep & Breathing, 18(4), 845–849. 10.1007/s11325-014-0954-4 [DOI] [PubMed] [Google Scholar]
- Blanco, M. , Ernst, G. , Salvado, A. , & Borsini, E. (2019). Impact of mask type on the effectiveness of and adherence to unattended home‐based CPAP titration. Sleep Disord, 2019, 4592462–4592467. 10.1155/2019/4592462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel, J. C. , Tamisier, R. , Dias‐Domingos, S. , Sapene, M. , Martin, F. , Stach, B. , … Scientific Council of The Sleep Registry of the French Federation of, P . (2013). Type of mask may impact on continuous positive airway pressure adherence in apneic patients. PLoS One, 8(5), e64382. 10.1371/journal.pone.0064382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova, F. , Leseux, L. , Fraysse, J. L. , Leophonte, P. , Muir, J. F. , & Didier, A. (2013). Impact of facial versus nose mask on the length of use of CPAP in patients with sleep apnea syndrome? Revue des Maladies Respiratoires, 30(5), 441–442. 10.1016/j.rmr.2013.01.006 [DOI] [PubMed] [Google Scholar]
- Chai, C. L. , Pathinathan, A. , & Smith, B. (2006). Continuous positive airway pressure delivery interfaces for obstructive sleep apnoea. Cochrane Database Syst, Rev(4), CD005308. 10.1002/14651858.CD005308.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande, S. , Joosten, S. , Turton, A. , Edwards, B. A. , Landry, S. , Mansfield, D. R. , & Hamilton, G. S. (2016). Oronasal masks require a higher pressure than nasal and nasal pillow masks for the treatment of obstructive sleep apnea. Journal of Clinical Sleep Medicine, 12(9), 1263–1268. 10.5664/jcsm.6128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte, R. L. M. , Mendes, B. A. , Oliveira, E. S. T. S. , Magalhaes‐da‐Silveira, F. J. , & Gozal, D. (2020). Nasal versus oronasal mask in patients under auto‐adjusting continuous positive airway pressure titration: A real‐life study. European Archives of Oto‐Rhino‐Laryngology, 277(12), 3507–3512. 10.1007/s00405-020-06242-x [DOI] [PubMed] [Google Scholar]
- Ebben, M. R. , Milrad, S. , Dyke, J. P. , Phillips, C. D. , & Krieger, A. C. (2016). Comparison of the upper airway dynamics of oronasal and nasal masks with positive airway pressure treatment using cine magnetic resonance imaging. Sleep & Breathing, 20(1), 79–85. 10.1007/s11325-015-1187-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebben, M. R. , Narizhnaya, M. , Segal, A. Z. , Barone, D. , & Krieger, A. C. (2014). A randomised controlled trial on the effect of mask choice on residual respiratory events with continuous positive airway pressure treatment. Sleep Medicine, 15(6), 619–624. 10.1016/j.sleep.2014.01.011 [DOI] [PubMed] [Google Scholar]
- Ebben, M. R. , Oyegbile, T. , & Pollak, C. P. (2012). The efficacy of three different mask styles on a PAP titration night. Sleep Medicine, 13(6), 645–649. 10.1016/j.sleep.2012.02.004 [DOI] [PubMed] [Google Scholar]
- Fava, C. , Dorigoni, S. , Dalle Vedove, F. , Danese, E. , Montagnana, M. , Guidi, G. C. , … Minuz, P. (2014). Effect of CPAP on blood pressure in patients with OSA/hypopnea a systematic review and meta‐analysis. Chest, 145(4), 762–771. 10.1378/chest.13-1115 [DOI] [PubMed] [Google Scholar]
- Foellner, S. , Guth, P. , Jorde, I. , Lucke, E. , Ganzert, C. , Stegemann‐Koniszewski, S. , & Schreiber, J. (2020). Prevention of leakage due to mouth opening through applying an oral shield device (Sominpax) during nasal CPAP therapy of patients with obstructive sleep apnea. Sleep Medicine, 66, 168–173. 10.1016/j.sleep.2019.06.023 [DOI] [PubMed] [Google Scholar]
- Goh, K. J. , Soh, R. Y. , Leow, L. C. , Toh, S. T. , Song, P. R. , Hao, Y. , … Ong, T. H. (2019). Choosing the right mask for your Asian patient with sleep apnoea: A randomized, crossover trial of CPAP interfaces. Respirology, 24(3), 278–285. 10.1111/resp.13396 [DOI] [PubMed] [Google Scholar]
- Higgins, J. P. , Altman, D. G. , Gotzsche, P. C. , Juni, P. , Moher, D. , Oxman, A. D. , … Cochrane Statistical Methods, G . (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ, 343, d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, J. P. , Thompson, S. G. , Deeks, J. J. , & Altman, D. G. (2003). Measuring inconsistency in meta‐analyses. BMJ, 327(7414), 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, J. P. T. , & Deeks, J. J. (2011). Chapter 7: Selecting studies and collecting data. In Cochrane handbook for systematic reviews of interventions version 5.1.0 (updated march 2011). The Cochrane Collaboration, 2011. Retrieved from https://handbook-5-1.cochrane.org/chapter_7/7_7_3_5_mediansand_interquartile_ranges.htm [Google Scholar]
- Hutton, B. , Salanti, G. , Caldwell, D. M. , Chaimani, A. , Schmid, C. H. , Cameron, C. , … Moher, D. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta‐analyses of health care interventions: Checklist and explanations. Annals of Internal Medicine, 162(11), 777–784. 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- Kaminska, M. , Montpetit, A. , Mathieu, A. , Jobin, V. , Morisson, F. , & Mayer, P. (2014). Higher effective oronasal versus nasal continuous positive airway pressure in obstructive sleep apnea: Effect of mandibular stabilization. Canadian Respiratory Journal, 21(4), 234–238. 10.1155/2014/408073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza, A. , Mariani, S. , Sommariva, M. , Campana, C. , Rubino, A. , Proserpio, P. , & Nobili, L. (2018). Continuous positive airway pressure treatment with nasal pillows in obstructive sleep apnea: Long‐term effectiveness and adherence. Sleep Medicine, 41, 94–99. 10.1016/j.sleep.2017.08.024 [DOI] [PubMed] [Google Scholar]
- Lebret, M. , Arnol, N. , Contal, O. , Martinot, J. B. , Tamisier, R. , Pepin, J. L. , & Borel, J. C. (2015). Nasal obstruction and male gender contribute to the persistence of mouth opening during sleep in CPAP‐treated obstructive sleep apnoea. Respirology, 20(7), 1123–1130. 10.1111/resp.12584 [DOI] [PubMed] [Google Scholar]
- Lebret, M. , Arnol, N. , Martinot, J. B. , Lambert, L. , Tamisier, R. , Pepin, J. L. , & Borel, J. C. (2018). Determinants of unintentional leaks during CPAP treatment in OSA. Chest, 153(4), 834–842. 10.1016/j.chest.2017.08.017 [DOI] [PubMed] [Google Scholar]
- Lin, M. T. , Lai, C. L. , Lee, P. L. , Shen, M. H. , Yu, C. J. , Fang, C. T. , & Chen, C. L. (2018). Timely diagnosis and treatment of sleep apnea reduce cardiovascular sequelae in patients with myocardial infarction. PLoS One, 13(7), e0201493. 10.1371/journal.pone.0201493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeiro, F. , Andrade, R. G. S. , Piccin, V. S. , Pinheiro, G. D. L. , Moriya, H. T. , Genta, P. R. , & Lorenzi‐Filho, G. (2019). Transmission of Oral pressure compromises Oronasal CPAP efficacy in the treatment of OSA. Chest, 156(6), 1187–1194. 10.1016/j.chest.2019.05.024 [DOI] [PubMed] [Google Scholar]
- Marin, J. M. , Carrizo, S. J. , Vicente, E. , & Agusti, A. G. (2005). Long‐term cardiovascular outcomes in men with obstructive sleep apnoea‐hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet, 365(9464), 1046–1053. 10.1016/S0140-6736(05)71141-7 [DOI] [PubMed] [Google Scholar]
- Massie, C. A. , & Hart, R. W. (2003). Clinical outcomes related to interface type in patients with obstructive sleep apnea/hypopnea syndrome who are using continuous positive airway pressure. Chest, 123(4), 1112–1118. 10.1378/chest.123.4.1112 [DOI] [PubMed] [Google Scholar]
- McEvoy, R. D. , Antic, N. A. , Heeley, E. , Luo, Y. , Ou, Q. , Zhang, X. , … Coordinators . (2016). CPAP for prevention of cardiovascular events in obstructive sleep apnea. The New England Journal of Medicine, 375(10), 919–931. 10.1056/NEJMoa1606599 [DOI] [PubMed] [Google Scholar]
- Mortimore, I. L. , Whittle, A. T. , & Douglas, N. J. (1998). Comparison of nose and face mask CPAP therapy for sleep apnoea. Thorax, 53(4), 290–292. 10.1136/thx.53.4.290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil, S. P. , Ayappa, I. A. , Caples, S. M. , Kimoff, R. J. , Patel, S. R. , & Harrod, C. G. (2019). Treatment of adult obstructive sleep apnea with positive airway pressure: An American Academy of sleep medicine systematic review, meta‐analysis, and GRADE assessment. Journal of Clinical Sleep Medicine, 15(2), 301–334. 10.5664/jcsm.7638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peker, Y. , Glantz, H. , Eulenburg, C. , Wegscheider, K. , Herlitz, J. , & Thunstrom, E. (2016). Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA randomized controlled trial. American Journal of Respiratory and Critical Care Medicine, 194(5), 613–620. 10.1164/rccm.201601-0088OC [DOI] [PubMed] [Google Scholar]
- Prosise, G. L. , & Berry, R. B. (1994). Oral‐nasal continuous positive airway pressure as a treatment for obstructive sleep apnea. Chest, 106(1), 180–186. 10.1378/chest.106.1.180 [DOI] [PubMed] [Google Scholar]
- Rowland, S. , Aiyappan, V. , Hennessy, C. , Catcheside, P. , Chai‐Coezter, C. L. , McEvoy, R. D. , & Antic, N. A. (2018). Comparing the efficacy, mask leak, patient adherence, and patient preference of three different CPAP interfaces to treat moderate‐severe obstructive sleep apnea. Journal of Clinical Sleep Medicine, 14(1), 101–108. 10.5664/jcsm.6892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan, S. , Garvey, J. F. , Swan, V. , Behan, R. , & McNicholas, W. T. (2011). Nasal pillows as an alternative interface in patients with obstructive sleep apnoea syndrome initiating continuous positive airway pressure therapy. Journal of Sleep Research, 20(2), 367–373. 10.1111/j.1365-2869.2010.00873.x [DOI] [PubMed] [Google Scholar]
- Salanti, G. , Del Giovane, C. , Chaimani, A. , Caldwell, D. M. , & Higgins, J. P. (2014). Evaluating the quality of evidence from a network meta‐analysis. PLoS One, 9(7), e99682. 10.1371/journal.pone.0099682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell, A. E. , & Soose, R. J. (2017). Positive airway pressure adherence and mask interface in the setting of sinonasal symptoms. Laryngoscope, 127(10), 2418–2422. 10.1002/lary.26486 [DOI] [PubMed] [Google Scholar]
- Schorr, F. , Genta, P. R. , Gregorio, M. G. , Danzi‐Soares, N. J. , & Lorenzi‐Filho, G. (2012). Continuous positive airway pressure delivered by oronasal mask may not be effective for obstructive sleep apnoea. The European Respiratory Journal, 40(2), 503–505. 10.1183/09031936.00145111 [DOI] [PubMed] [Google Scholar]
- Shirlaw, T. , Duce, B. , Milosavljevic, J. , Hanssen, K. , & Hukins, C. (2019). A randomised crossover trial comparing nasal masks with oronasal masks: No differences in therapeutic pressures or residual apnea‐hypopnea indices. Journal of Sleep Research, 28(5), e12760. 10.1111/jsr.12760 [DOI] [PubMed] [Google Scholar]
- Sterne, J. A. , Hernan, M. A. , Reeves, B. C. , Savovic, J. , Berkman, N. D. , Viswanathan, M. , … Higgins, J. P. (2016). ROBINS‐I: A tool for assessing risk of bias in non‐randomised studies of interventions. BMJ, 355, i4919. 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne, J. A. C. , Egger, M. , & Moher, D. (2011). Chapter 10: Addressing reporting biases. In: Cochrane handbook for systematic reviews of intervention. Version 5.1.0 (updated march 2011). The Cochrane collaboration, 2011. Cochrane handbook for systematic reviews of interventions: Online version . Retrieved from https://handbook-5-1.cochrane.org/chapter_10/10_4_3_1_recommendations_on_testing_for_funnel_plot_asymmetry.htm
- Sutherland, K. , Phillips, C. L. , & Cistulli, P. A. (2015). Efficacy versus effectiveness in the treatment of obstructive sleep apnea: CPAP and Oral appliances. Journal of Dental Sleep Medicine, 02(04), 175–181. 10.15331/jdsm.5120 [DOI] [Google Scholar]
- Teo, M. , Amis, T. , Lee, S. , Falland, K. , Lambert, S. , & Wheatley, J. (2011). Equivalence of nasal and oronasal masks during initial CPAP titration for obstructive sleep apnea syndrome. Sleep, 34(7), 951–955. 10.5665/SLEEP.1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver, T. E. , Maislin, G. , Dinges, D. F. , Bloxham, T. , George, C. F. , Greenberg, H. , … Pack, A. I. (2007). Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep, 30(6), 711–719. 10.1093/sleep/30.6.711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhoff, M. , & Litterst, P. (2015). Obstructive sleep apnoea and non‐restorative sleep induced by the interface. Sleep & Breathing, 19(4), 1317–1325. 10.1007/s11325-015-1173-3 [DOI] [PubMed] [Google Scholar]
- Zampogna, E. , Spanevello, A. , Lucioni, A. M. , Facchetti, C. , Sotgiu, G. , Saderi, L. , … Visca, D. (2019). Adherence to continuous positive airway pressure in patients with obstructive sleep Apnoea. A ten year real life study. Respiratory Medicine, 150, 95–100. 10.1016/j.rmed.2019.02.017 [DOI] [PubMed] [Google Scholar]
- Zhu, X. , Wimms, A. J. , & Benjafield, A. V. (2013). Assessment of the performance of nasal pillows at high CPAP pressures. Journal of Clinical Sleep Medicine, 9(9), 873–877. 10.5664/jcsm.2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information
Data Availability Statement
Data available in article supplementary material


