Abstract
Introduction
Adverse Events (AE) are one of the main problems in healthcare. Therefore, many policies have been developed worldwide to mitigate their impact. The Patient Safety Incident Study in Hospitals in the Community of Madrid (ESHMAD) measures the results of them in the region.
Methods
Cross‐sectional study, conducted in May 2019, in hospitalised patients in 34 public hospitals using the Harvard Medical Practice Study methodology. A logistic regression model was carried out to study the association of the variables with the presence of AE, calibrated and adjusted by patient.
Results
A total of 9975 patients were included, estimating a prevalence of AE of 11.9%. A higher risk of AE was observed in patients with surgical procedures (OR[CI95%]: 2.15[1.79 to 2.57], vs. absence), in Intensive Care Units (OR[CI95%]: 1.60[1.17 to 2.17], vs. Medical) and in hospitals of medium complexity (OR[CI95%]: 1.45[1.12 to 1.87], vs. low complexity). A 62.6% of AE increased the length of the stay or it was the cause of admission, and 46.9% of AE were considered preventable. In 11.5% of patients with AE, they had contributed to their death.
Conclusions
The prevalence of AE remains similar to the previously estimated one in studies developed with the same methodology. AE keep leading to longer hospital stays, contributing to patient's death, showing that it is necessary to put focus on patient safety again. A detailed analysis of these events has enabled the detection of specific areas for improvement according to the type of care, centre and patient.
1. INTRODUCTION
Adverse events (AE) related to healthcare include all the Patient Safety Incidents that produce harm to patients. 1 AE are a significant health problem, since they reduce the quality level of care and can lead to excess morbidity and mortality. 2 , 3 Likewise, the AE as a whole are the third cause of death in the United States (around 250,000 per year; 98,000 in hospital settings), 4 and have a significant economic impact (losses of over €194 million were estimated in Ireland in 2017). 5
Since the publication of the report To Err is Human 6, in 2000, the detection and epidemiological tracking of AE in the hospital setting has led to the implementation of actions to improve patient safety, on both at care and organisational level. Although AE have been studied in different care settings, AE are particularly prevalent in the hospital setting, making it an ideal environment to implement preventive barriers. 6
Several methods have been developed for the identification and surveillance of AE, ranging from the Global Trigger Tool 7 to the instrument previously developed by the Harvard Medical Practice Study, 8 which provides more complete information on AE, their nature and impact, and is the most widely used in the hospital setting. 5 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19
In Spain, concern for patient safety has grown in parallel with that in other European countries, allowing the performance of the Spanish National Study on Hospitalisation‐Related Adverse Events (ENEAS), 13 a pioneer quantifying the national incidence of AE in the hospital setting and which found an incidence of 9.3%. Studies were also conducted in the out‐of‐hospital setting, particularly the ‘Patient Safety in Primary Care Study’ in 2007 which found a point prevalence of 0.8%, 20 and the ‘Adverse effects at assisted patient care centres and living facilities’ in 2011, which showed that complications in care have the biggest impact at that level. 21
All this led to the promotion of public policies in Spain. ‘Patient Safety Strategy of the National Health System’ 22 and ‘Patient Safety Strategy of the Community of Madrid’, 23 both for the period 2015–2020, are the most recently developed strategic plans, at the national and local level, respectively. The fundamental pillars of both were to promote a safety culture at all levels of care, the development of safety incident notification systems and the promotion of safety practices.
Within the Community of Madrid, one of the strategic objectives was related to making a pioneering measurement of AE in all the hospitals in the region and the scientific dissemination of the results. In this context, the Patient Safety Incident Study of Hospitals in the Community of Madrid (ESHMAD) arises, whose main objective is to identify the prevalence, characteristics and impact of AE in hospitals in the region, as well as to analyse the factors associated with the development of AE.
2. MATERIALS AND METHODS
2.1. Design
An observational descriptive cross‐sectional study was carried out in all public hospitals in the Community of Madrid (34 hospitals in total), comprising 6 low complexity hospitals (with <5 clinical specialities), 13 medium complexity hospitals (5 to 10 clinical specialities), 8 high complexity hospitals (highly specialised, >10 clinical specialities), 2 support hospitals (caring for patients in the sub‐acute phase of reversible diseases), 3 long‐term care hospitals (caring for patients requiring chronic care) and 2 psychiatric hospitals. To construct the sample, each centre carried out a single cross‐sectional cut of hospitalised patients at the start of the care activity on a day in May 2019. Patients needed to have a hospital admission at the time of the cut and the only patients excluded where those in emergency units. A patient who was included in one hospital could not be included in another centre.
There was a scientific directorate, based at the Health Department of the Community of Madrid and the Hospital Universitario Ramón y Cajal, responsible for designing, planning, training, providing scientific technical support and analysing the results. A train‐the‐trainer structure was implemented, with one coordinator per centre for each phase of the study. 24 Each hospital was responsible for assigning the study to a team of reviewers, who received specific training for the study. The coordinator of each phase was in charge of carrying out a validation of the data, agreeing with the reviewer when there were discrepancies when categorising an event.
The study was conducted in two phases by clinical records review. It was based on the methodology of Brennan et al. and the Harvard Practice Medical Study 8 and the previous experience of the ENEAS 13 and IBEAS 25 study:
Screening for AE. After including all patients with a minimum stay of 24 hours following the methodology of the EPINE study, 26 the ESHMAD screening tool was used. It is an expanded questionnaire that includes two validated tools: the EPINE form, 27 which allows collecting all epidemiological variables, and the AE Screening Guide, 8 designed to identify warning signs of possible AE. Variables related to the healthcare provided and the diagnostic‐therapeutic procedure performed were also collected. According to the Harvard Medical Practice Study methodology, this phase could be performed by any healthcare worker recruited.
Review, confirmation and characterisation of the possible AE that obtained a positive result in the previous phase of screening. An adaptation of the Modular Review Form 2 (MRF2), 28 translated after the experience with the ENEAS 13 and IBEAS 25 studies, was used to gather structured information on the characteristics, consequences, impact and avoidability of each potential AE. It was conducted on discharge or, if still in hospital, 30 days after screening. This phase was carried out by a specifically trained physician.
The data were collected using a paper version of both tools. After that, the data were uploaded to a specific informatic software designed for the study, with data protection and safeguard mechanisms.
More information about the study design can be found in the publication of Valencia‐Martín et al. in 2021. 24 Reporting of the study conforms to broad EQUATOR guidelines. 29
2.2. Variables collected
As established by the Conceptual Framework of the International Classification for Patient Safety, published by the World Health Organisation in 2009, AE were classified as every incident during healthcare that causes physical harm, psychological damage or death to the patient; their degree of avoidability was also measured. 1 , 30
Subsequently, an analysis of the relationship between AE and healthcare was conducted on a 6‐point scale, requiring 4 or more to be considered linked. The same scale was used to assess their avoidability.
It was also collected intrinsic risk factors (IRF) of the patient at the moment of admission, (renal failure, cardiovascular disease, neoplasia, chronic obstructive pulmonary disease [EPOC], immunodeficiency, neutropenia, liver cirrhosis, hypoalbuminaemia, pressure ulcers, impaired mobility, sensory deficits, obesity and active smoking) and extrinsic risk factors (ERF) related to the healthcare delivered to the patient at the moment of screening, (previous surgery, peripheral vascular catheter, central vascular catheter, urinary catheterisation, intubation and number of drugs prescribed at the time of review). Age was classified into ‘<65 years’ and ‘≥ 65 years’ and IRF and ERF according to the number of factors present, allowing comparability with previous studies. 5 , 13 , 25 More information about these factors can be consulted at the Supplementary Material (Table S1).
The type of AE (according to the MRF2 classification: ‘Hospital acquired infection (HAI)’, ‘Complications in care’, ‘Complications of a procedure’, ‘Adverse effects of medication’ and ‘Other consequences’), 28 its causes and impacts in the form of consequential procedures, and severity (according to the World Health Organisation: ‘mild’, if it did not modify hospital stay; ‘moderate’, if it resulted in readmission or increased hospital days; and ‘severe’, if it lead to a surgical intervention or contributed to the patient's death 30 ) was also collected.
The disability of the AE was measured according to the clinical records with the Rosser Scale, 31 which allows to evaluate the grade of physical deterioration (from ‘mild or no disability’, ‘severe disability’ to ‘absolute disability’), the grade of pain (from ‘no pain’, ‘mild pain’, ‘moderate pain’ to ‘severe pain’) and the grade of emotional trauma (from ‘absent’, ‘minimum’, ‘moderate’, ‘acute’ to ‘severe’). If it was not possible to assess the disability caused by AE, reviewers were instructed to assign ‘unknown’.
2.3. Statistical analysis
Proportions were estimated for qualitative variables; and for quantitative variables, central and dispersion measures were estimated. A descriptive analysis of the patients included and the records with AE was performed, inspired by previous works. 18 , 25
The prevalence of the main events related to patient safety was estimated by patient (patients with an event, with respect to the total number of patients studied). Periodic control of the database was performed, and coordinators were contacted to reduce the number of missing data to guarantee the quality of the data collected.
Bivariate analysis was performed using Chi2 tests (if parametric test conditions were met) and Fisher's exact test (non‐parametric, if otherwise). The association of quantitative variables was estimated with the Student's t‐test or Mann–Whitney U test, depending on whether or not the normality criteria were met.
A multivariate logistic regression model was carried out to study the association of the variables with the presence of AE, calibrated and adjusted by patient. The model was started with all significant variables in the univariate analysis and followed a backward modelling strategy (output value p < .100). Length of stay was not considered in the multivariate model as it was considered an intermediate variable. To assess the goodness‐of‐fit and calibration of the model, the Hosmer–Lemeshow (H‐L) test was performed. 32 , 33
p‐values of less than .050 were considered significant.
IBM SPSS Statistics software was used. 34
2.4. Ethics committee
The study was approved on 19 March 2019 by the Ethics Committee of the Hospital Universitario Ramón y Cajal (reference 057/19), guaranteeing the anonymity and custody of the data gathered, which were transcribed to an anonymised online database, with security mechanisms and safeguarding of personal data.
The study was commissioned by the Ministry of Health of the Community of Madrid. All centres accepted their enrolment in the study.
After the approval of the Ethics Committee, informed consent was not necessary because no intervention was assessed on the participants.
3. RESULTS
3.1. Sample characteristics
A total of 9975 patients met the inclusion criteria, accounting for 81.4% of the 12,247 beds potentially available in the region's public hospitals. The mean and median ages were 63.5 (standard deviation [SD]: 25.5) and 68 (Interquartile range [IR]: 50 to 81), respectively. No relevant differences were found in relation to sex. The most frequent characteristics of the hospitalisation of the patients included were urgent admission (70%), a high complexity hospital (49.8%), or care in a Medical Care Unit (52%). At the time of screening, the median hospital stay was 6 days (IR: 2 to 15). A 82.3% of patients had ≥1 IRF and 47.6% had ≥3. A 74.1% of patients had ≥1 ERF, a figure that decreased to 25.1% if the peripheral venous catheter was excluded (Table 1).
TABLE 1.
Prevalence of adverse events according to patient and hospital admission characteristics
| Patient or hospital stay characteristics | Total | Prevalence of patients with AE | |
|---|---|---|---|
| n (%) | n (%) | p | |
| Age | |||
| < 65 years | 4559 (46%) | 456 (10.0%) | p < .001** |
| ≥ 65 years | 5344 (54%) | 717 (13.4%) | |
| Sex | |||
| Woman | 4986 (50%) | 544 (10.9%) | p = .002* |
| Man | 4.989 (50%) | 643 (12.9%) | |
| Type of hospital | |||
| Low complexity | 785 (7.9%) | 88 (11.2%) | p < .001** |
| Medium complexity | 3165 (31.7%) | 467 (14.8%) | |
| High complexity | 4972 (49.8%) | 578 (11.6%) | |
| Psychiatric | 461 (4.6%) | 11 (2.4%) | |
| Long‐term care hospital | 404 (4.1%) | 27 (6.7%) | |
| Support | 188 (1.9%) | 16 (8.5%) | |
| Type of Admitting Service | |||
| Medical specialities | 5184 (52%) | 591 (11.4%) | p < .001** |
| Surgical specialities | 3123 (31.3%) | 414 (13.3%) | |
| Psychiatric | 864 (8.7%) | 22 (2.6%) | |
| Other services | 424 (4.2%) | 47 (11.1%) | |
| Intensive medicine | 380 (3.8%) | 113 (29.7%) | |
| Hospital stay before screening | |||
| 0–2 days | 2615 (26.2%) | 106 (4.1%) | p < .001** |
| 2–6 days | 2552 (25.6%) | 213 (8.4%) | |
| 6–15 days | 2266 (22.7%) | 324 (14.3%) | |
| 15–30 days | 1060 (10.6%) | 216 (20.4%) | |
| ≥ 30 days | 1439 (14.4%) | 322 (22.4%) | |
| Unknown | 43 (0.4%) | 6 (14%) | |
| Type of admission | |||
| Scheduled | 2939 (29.5%) | 303 (10.3%) | p = .002* |
| Urgent | 6993 (70.1%) | 878 (12.6%) | |
| Unknown | 43 (0.4%) | 6 (14%) | |
| Surgical intervention | |||
| No intervention | 7309 (73.7%) | 703 (9.6%) | p < .001** |
| Intervention | 2666 (26.7%) | 484 (18.2%) | |
| Number Intrinsic Risk Factors | |||
| Absence | 1761 (17.6%) | 87 (4.9%) | p < .001** |
| 1 | 1729 (17.3%) | 169 (9.8%) | |
| 2 | 1743 (17.5%) | 203 (11.7%) | |
| ≥ 3 | 4742 (47.6%) | 728 (15.4%) | |
| Number Extrinsic Risk Factors | |||
| Absence | 2579 (25.7%) | 136 (5.3%) | p < .001** |
| 1 | 5408 (54.2%) | 613 (11.4%) | |
| 2 | 1505 (15.1%) | 299 (19.9%) | |
| ≥ 3 | 483 (4.8%) | 139 (28.8%) | |
| Total | 9975 | 1187 (11.9%) | |
Abbreviations: AE, Adverse events.
p: Ratio comparison (Chi2 or Fisher's exact test).
*p < .050; **p < .001.
3.2. Results by phase of the study
Of the initial 9975 patients, 36.6% (3649) had at least one positive screening criterion, comprising a total of 4660 records of possible AE. At the end of this first phase, 55 records were lost.
In the second phase, of the 4.660 records reviewed, 1967 had an AE where their relationship with healthcare was unknown (42.2%). A total of 2378 records were false positives (51%), 311 were non‐harm incidents (6.7%), and 4 records could not be classified. Of the 1967 AE, it was determined that 74% (1455) were really healthcare related. From the initial sample, this gave a prevalence of patients with AE of 11.9% (1187 patients with AE; 95%CI: 11.3 to 12.5). Of all the patients with AE, 34.9% (414) had more than one concurrent AE (Figure 1).
FIGURE 1.
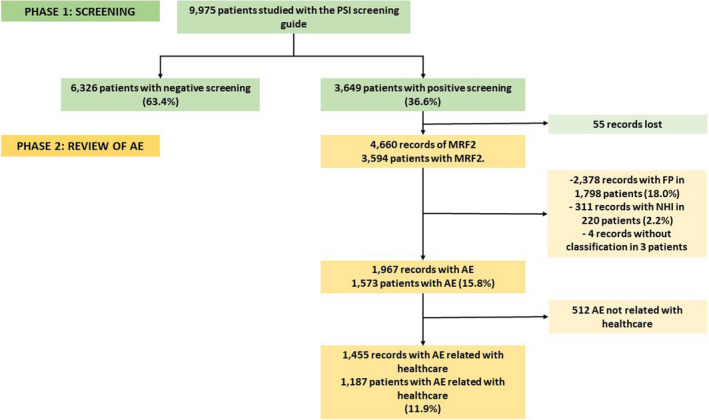
Study results tree. AE, Adverse Events; FP, False Positives; NHI, no‐harm incidents; PSI, Patient Safety Incidents.
3.3. Prevalence of AE and associated factors
Medium complexity hospitals had a higher prevalence of patients with AE (14.8%), followed by high complexity (11.6%), low complexity (11.2%), support (8.5%), long‐term care (6.7%) and psychiatric hospitals (2.4%). There was a higher prevalence of patients with AE in males (12.9% vs. 10.9% in females; p = .002), in patients aged ≥65 years (13.4% vs. 10% in patients aged <65 years; p < .001) and as the number of IRF (4.9% for patients without IRF; 15.6% for ≥3 IRF; p < .001) and ERF increased (5.3% for patients without ERF; 28.8% for ≥3 ERF; p < .001). Similarly, a higher prevalence of patients with AE was detected in Intensive Care Units (ICU) (29.7% vs. 13.3% in surgical and 11.4% in medical specialities; p < .001), in emergency admissions (12.6% vs. 10.3% in scheduled; p = .002) and with the increase in length of hospital stay, being 22.4% in ≥30 days (compared to 20.4% in 15–30 days, or 4.1% in 0–2 days; p < .001) (Table 1).
The gradual increase in ERF and IRF increased the risk of AE, being more than three times higher in patients ≥3 IRF (OR: 3.24; p < .001) and more than twice as high in patients ≥3 ERF (2.54; p < .001), compared to those without these characteristics. Urgent admission (1.43; p < .001), surgical interventions (2.15; p < .001) and ICU stay (1.60; p = .001) also increased the risk of AE. The prevalence of AE was lower in other services (0.15; p = .002) and in psychiatric services compared to medical specialities (0.47; p = .029) (Table 2).
TABLE 2.
Risk of developing an adverse event by hospitalised patients, according to the characteristics of the patient or the hospital stay
| Characteristics of the patient or the hospital stay | Total, n (%) | Prevalence AE, n (%) | Odds Ratio | CI 95% | p |
|---|---|---|---|---|---|
| Age | |||||
| < 65 years | 4559 (46%) | 456 (10%) | 1.00 | ‐ | ‐ |
| ≥ 65 years | 5344 (54%) | 717 (13.4%) | 0.92 | 0.80 to 1.06 | .289 |
| Sex | |||||
| Woman | 4986 (50%) | 544 (10.9%) | 1.00 | ‐ | ‐ |
| Male | 4989 (50%) | 643 (12.9%) | 1.10 | 0.97 to 1.25 | .178 |
| Type of hospital | |||||
| Low complexity | 785 (7.9%) | 88 (11.2%) | 1.00 | ‐ | ‐ |
| Medium complexity | 3165 (31.7%) | 467 (14.8%) | 1.45 | 1.12 to 1.87 | <.001** |
| High complexity | 4972 (49.8%) | 578 (11.6%) | 1.00 | 0.78 to 1.28 | .965 |
| Psychiatric | 461 (4.6%) | 11 (2.4%) | 0.62 | 0.38 to 1.03 | .070 |
| Long‐term care hospital | 404 (4.1%) | 27 (6.7%) | 1.01 | 0.42 to 2.46 | .963 |
| Support | 188 (1.9%) | 16 (8.5%) | 0.89 | 0.50 to 1.58 | .691 |
| Type of admission | |||||
| Scheduled | 2939 (29.5%) | 303 (10.3%) | 1.00 | ‐ | ‐ |
| Urgent | 6993 (70.1%) | 878 (12.6%) | 1.43 | 1.20 to 1.70 | <.001** |
| Surgical intervention | |||||
| No intervention | 7309 (73.7%) | 703 (9.6%) | 1.00 | ‐ | ‐ |
| Intervention | 2666 (26.7%) | 484 (18.2%) | 2.15 | 1.79 to 2.57 | <.001** |
| Type of service | |||||
| Medical speciality | 5184 (52%) | 591 (11.4%) | 1.00 | ‐ | ‐ |
| Surgical speciality | 3123 (31.3%) | 414 (13.3%) | 1.01 | 0.84 to 1.21 | .991 |
| Psychiatric | 864 (8.7%) | 22 (2.6%) | 0.47 | 0.25 to 0.89 | .029* |
| Other services | 424 (4.2%) | 47 (11.1%) | 0.16 | 1.12 to 2.29 | .002* |
| Intensive medicine | 380 (3.8%) | 113 (29.7%) | 1.60 | 1.17 to 2.17 | .001* |
| Number of Intrinsic Risk Factors | |||||
| Absence | 1761 (17.6%) | 87 (4.9%) | 1.00 | ‐ | ‐ |
| 1 | 1729 (17.3%) | 169 (9.8%) | 1.99 | 1.50 to 2.64 | <.001** |
| 2 | 1743 (17.5%) | 203 (11.7%) | 2.38 | 1.80 to 3.14 | <.001** |
| ≥ 3 | 4742 (47.6%) | 728 (15.4%) | 3.24 | 2.50 to 4.19 | <.001** |
| Number of Extrinsic Risk Factors | |||||
| Absence | 2579 (25.7%) | 136 (5.3%) | 1.00 | ‐ | ‐ |
| 1 | 5408 (54.2%) | 613 (11.4%) | 1.37 | 1.10 to 1.70 | .020* |
| 2 | 1505 (15.1%) | 299 (19.9%) | 2.32 | 1.82 to 2.96 | <.001** |
| ≥ 3 | 483 (4.8%) | 139 (28.8%) | 2.54 | 1.82 to 3.54 | <.001** |
| Constant | ‐ | ‐ | 0.02 | 0.01 to 0.03 | <.001** |
Notes: AE: Adverse event; CI 95%: 95% confidence interval; p: estimated p‐value in logistic regression.
Multivariate logistic regression analysis, adjusted for: sex, age, type of hospital, type of admission, type of surgery, type of service and number of intrinsic and extrinsic risk factors.
*p < .050; **p < .001.
Adjusted for surgical intervention, no differences were found in the association between being hospitalised in a surgical and a medical service. No significant differences were detected by sex and age when adjusting for the other main variables.
The Hosmer–Lemeshow test was performed to assess the goodness of fit of the model, obtaining a p = .389; there were no differences between the observed and expected prevalence.
3.4. Characteristics and consequences of the AE
The most frequent type of AE was hospital‐acquired infection (HAI) (553; 38%), followed by complications of care (336; 23.1%), complications of a procedure (309; 21.2%) and related to medication (199; 13.7%).
The most common HAI were surgical site infection (196, 13.5%), followed by device‐associated bacteraemia (80, 5.5%), urinary tract infection (78, 5.4%) and pneumonias (73, 5%). The most frequent healthcare complications were pressure ulcers (132; 9.1%) and phlebitis (127; 8.7%). The care process during which AE appeared was mainly in the hospitalisation ward (722; 49.8%), during the administration of medical treatment (190; 13.1%).
Of all 1455 AE, 55.2% required medical treatment and 18% required additional surgery to the patients affected by them. A 44.2% of AE increased part of the hospital stay and in 18.4% the AE itself was the cause of readmission (Table 3). Overall, they added a total of 16,227 days of hospitalisation (mean: 9 days/AE; median: 1 day) and 2666 days ICU stay (20 days/AE; 0 days). Patients with severe AE were in hospital for mean of 23 additional days.
TABLE 3.
Types and consequences of AE
| Characteristics of the AE | Total AE | |
|---|---|---|
| n | % | |
| Type of AE | ||
| Hospital acquired infections | 553 | 38% |
| Complications in care | 336 | 23.1% |
| Complications of a procedure | 309 | 21.2% |
| Adverse effects of medication | 199 | 13.7% |
| Other consequences | 58 | 4% |
| Additional assistance as a result of the AE | ||
| Medical treatment or rehabilitation (antibiotics, dressings, etc.) | 797 | 55.2% |
| Additional surgical intervention | 260 | 18% |
| No additional treatment or measures were required | 109 | 7.5% |
| Life support intervention or treatment (orotracheal intubation, etc.) | 106 | 7.3% |
| An additional test was required (x‐ray, culture, etc.) or other procedure | 104 | 7.2% |
| It required a higher level of observation and monitoring | 69 | 4.8% |
| Modification of hospitalisation by the AE | ||
| Unknown | 20 | 1.4% |
| It did not lengthen the stay | 507 | 35.1% |
| Extended part of the stay | 638 | 44.2% |
| Cause of readmission | 280 | 19.4% |
| Total | 1445 | 100% |
Abbreviation: AE, Adverse event.
A 62.8% of AE had a moderate or severe impact, while 35.5% were mild (for 1.4% of unknown records). On the Rosser scale, 33.3% of the AE identified resulted in severe or absolute physical disability, 25% of AE caused moderate or severe pain and 10% led to emotional trauma of more than a month.
In 11.5% (136) of patients with AE, it had contributed to their death, but no direct causation could be established. Of the 1455 AE related to healthcare, 46.9% (678) were avoidable (Table 4).
TABLE 4.
Patient impact and avoidability of AE
| Impact and avoidability of the AE | Total AE | |
|---|---|---|
| n | % | |
| Severity a | ||
| Unknown | 28 | 1.9% |
| Mild | 514 | 35.3% |
| Moderate | 612 | 42.1% |
| Severe | 301 | 20.7% |
| Physical deterioration | ||
| Unknown | 247 | 17% |
| Mild or no disability | 738 | 50.7% |
| Severe disability | 254 | 17.5% |
| Absolute disability | 216 | 14.8% |
| Pain | ||
| Unknown | 571 | 39.5% |
| No pain | 216 | 14.9% |
| Mild pain | 302 | 20.9% |
| Moderate pain | 273 | 18.9% |
| Severe pain | 83 | 5.7% |
| Emotional trauma b | ||
| Unknown | 985 | 68.2% |
| Absent | 206 | 14.3% |
| Minimum | 102 | 7.1% |
| Moderate | 95 | 6.6% |
| Acute | 30 | 2.1% |
| Severe | 27 | 1.9% |
| Death | ||
| No | 1278 | 87.8% |
| Yes | 177 | 12.2% |
| Avoidability | ||
| Unknown | 361 | 24.9% |
| No | 406 | 28.2% |
| Yes | 678 | 46.9% |
| Total | 1455 | 100% |
Abbreviation: AE, Adverse event.
Severity: Mild: it did not modify hospital stay; Moderate: it resulted in readmission or lengthened the hospital stay; Severe: if it led to a surgical intervention or contributed to the patient's death.
Minimal emotional trauma: with recovery in less than 1 month; Moderate: with recovery between 1 and 6 months; Acute: with recovery between 6 and 12 months; Severe: longer than 12 months.
4. DISCUSSION
A total of 9975 patients were studied in 34 public hospitals in the Community of Madrid, estimating a prevalence of AE of 11.9%. A higher risk of AE was observed in patients with surgical procedures, an Intensive Care Unit stay and in hospitals of medium complexity. A profile of a patient who suffers AE is an older adult, male, with urgent nature of admission, ICU or surgical stay, with prolonged hospital days, with a previous surgical intervention and with the presence of IRF and ERF. More than 9 out of 10 AE required additional assistance, and more than a half had a moderate or severe impact and increased the length of the stay or was the cause of admission.
The Harvard Medical Practice School methodology has been replicated on multiple occasions, although with significant differences, depending on the type of retrospective review carried out. Initially, this technique was used in the form of longitudinal reviews that estimated the incidence, providing an adequate approximation to the epidemiological variables related to AE and enabling the study of causality relations. 35 Using this methodology, in the United States, Brennan et al., in 1991, obtained an incidence of AE resulting from medical negligence of 3.7% 8 (sample [n] = 31,429); and later, in 1992, it was obtained similar results of 2.9% in Utah and Colorado (n = 14,732). 10 Later, other longitudinal studies wanted to identify all kind of AE and obtained higher incidence values: in Australia, a study in 1995 detected an incidence of 16.6% AE (n = 14,179) 9 ; and, in Spain, the ENEAS (hospital‐specific) in 2005, detected incidence of AE of 9.3% (n = 5624). 13
However, these longitudinal studies are often long and costly, requiring a detailed analysis of the entire patient episode. 36 ESHMAD therefore opted for a cross‐sectional design, previously validated and considered appropriate for the estimation and characterisation of AE. 28 , 37 , 38 Thus, compared with other cross‐sectional studies, the prevalence of ESHMAD AE (11.9%) is similar to that of IBEAS (10.5%; conducted in 2009 in hospitals in Colombia, Argentina, Costa Rica, Mexico and Peru) 25 ; but higher than the one found in two studies that analysed the period between 2005 and 2008 on hospitals in the Valencian Community, Spain (6% and 5.8%, respectively). 12 , 39
These and other results from the Harvard Medical Practice School methodology have been compiled in systematic reviews such as those carried out by De Vries et al. in 2008, 40 by Schwendimann et al. in 2018 41 and by Panagioti et al. in 2019. 42 The latter includes a meta‐analysis that determined a prevalence of patients with AE of 12%, this being a result entirely consistent with that obtained by ESHMAD.
The prevalence of patients with AE was higher in middle‐complexity hospitals (14.8%) than in high‐ and low‐complexity hospitals (11.6% and 11.2%). This differs from the results obtained by some studies, in which the increase in hospital complexity was associated with a higher frequency of AE, especially from certain interventions performed on patients with numerous comorbidities. 13 , 14 , 25 However, in other studies, no differences in complexity were detected, therefore, this would be an aspect that could benefit from complementary studies. 5 , 17
The prevalence of AE was higher in ICU (29.7%) and surgical services (15.2%), although these are lower than the figures obtained in the Panagioti et al.'s review (34% and 20%, respectively). 42 However, this is a common finding for most studies that use the Harvard Medical Practice School methodology, regardless of their cross‐sectional or longitudinal design. 11 , 12 , 13 , 14 , 16 , 19 , 25
In psychiatric services, in both specialised and acute hospitals, a prevalence of 2.6% has been found. This datum is slightly lower than studies carried out specifically in psychiatric hospitals, which put it between 5.4% 43 and 13.4%. 44 Our lower prevalence may be due to two reasons. First, most of the AEs identified in these studies are adverse drug effects, which are underestimated in a cross‐sectional design. The second one is that those studies used specific screening tools for AE in psychiatry and used more recent definitions of AE adapted to the particularities of the psychiatric patient. 45 In this study, we have attempted to use the same tool regardless of the service to be able to compare the type of hospital and service with the same measurement instrument. Future articles should adapt the approach to the psychiatric reality to give a more accurate measure of the problems in this area.
The avoidability of AE was 47%, although 25% of the sample did not record this data, so it may be slightly underestimated. However, this is consistent with the results of the meta‐analysis of Panagioti et al., which estimates it at 50%. 42
The main type of AE identified was HAI (38%), followed by complications in care (23%) and problems related to a procedure (21%). In the meta‐analysis by Schwendimann et al.41, the most frequent AE were those related to a procedure (40%), followed by those related to medication (19.3%) and HAI (17.7%). This discrepancy may be due to the fact that the same criteria were not used to define HAI (although ESHMAD adopted the valid and specific EPINE criteria for this objective 26 ); In the IBEAS, the most frequent were HAI and procedure‐related problems. 25
In the ESHMAD, only 13% of AE were related to medication. In this respect, another study, carried out in Spain between 2005 and 2013, estimated a frequency of 16.3% of this type of EA on a sample of 35,103 patients, noting a direct relationship between avoidability and severity. 46
Moderate severity AE were the most frequent (42%), followed by mild and severe AE. This is consistent with that obtained in other cross‐sectional studies, 25 , 35 but differs from that found in longitudinal studies, in which mild AE are usually more frequent (50%). 40 , 42
Cross‐sectional and longitudinal studies have their own particularities, which could explain some of the differences between the results derived from both types of studies: the longitudinal ones make it possible to estimate the incidence of AE and establish hypothesis of causality, 35 but they are also usually less operational because they present a greater duration and need of resources. 36
A common limitation of cross‐sectional studies, such as ESHMAD, is that long‐term findings (such as prolonged hospital stay, moderate–severe HAI or consequences) may be overrepresented, to the detriment of those that prolong it to a lesser extent or even reduce it (such as mild AE or premature discharge). 35 , 36 However, this should not affect the main objective of the study, which was to evaluate the condition of hospitals in the region, prioritising the AE that have the greatest impact on health management in the hospital setting. In addition, cross‐sectional studies, by making a more specific selection of the sample, allow to study the prevalence of AE efficiently and with a high ability to identify them. 28 , 37 , 38
Another limitation of ESHMAD, derived from the Harvard Medical Practice School methodology, is the reviewer subjectivity when establishing the possible relationship of AE with health care and its avoidability. However, this design was previously validated for the identification of AE and has optimal levels of consistency between evaluators. However, it would be interesting to develop more objective analysis tools to improve the validity of results. 28 , 37 , 38
That said, ESHMAD is a pioneer, being carried out simultaneously in all the hospitals of the Community of Madrid to ascertain the current situation regarding patients safety in the region. Its results identify areas for improvement, enabling the development of policies and strategies to mitigate the impact of the AE. The conducting of the study required the recruitment of a large number of health professionals from different fields and competencies, training them in aspects essential to the identification and systematic analysis of AE by means of cascade training. In addition to the positive impact on the institutional culture itself, an efficient methodology has been used, creating synergies between surveillance systems and making use of complementary approaches from different professional areas. All this has been done by applying a validated methodology, such as the screening questionnaires and MRF2, 28 but which has also been widely used in different regions and health systems before. 5 , 8 , 9 , 10 , 11 , 12 , 14 , 15 , 16 , 17 , 18 , 19 Therefore, in addition to studying the epidemiology of AE, ESHMAD is also designed to be a reproducible, practical and reliable AE surveillance and monitoring system. 35
5. CONCLUSIONS
Hospitals in the Community of Madrid have a prevalence of AE consistent with that obtained in other epidemiological studies conducted with a similar methodology. These AE produce avoidable and potentially improvable harm. In addition, prolonged hospital stay and resource consumption – derived from additional medical and surgical treatment – are clear examples of lost opportunities.
Patients with several comorbidities, who are often in a worse clinical condition and require more invasive procedures, develop AE more often. Likewise, HAI is the most common type of AE, so the development of specific measures to prevent nosocomial infections would be a beneficial strategy for all hospitals in the region.
ESHMAD has made it possible to estimate the frequency and characteristics of AE in all public hospitals in the Community of Madrid. This will identify areas for improvement and promote patient safety strategies that will improve the quality of care provided by healthcare providers.
The lack of changes in the patient safety results compared with that obtained globally in the last 20 years allows us to conclude that it is necessary to put the focus again on AE.
CONTRIBUTORS
ESHMAD Director Group and external advisers: Asunción Colomer Rosas, Inmaculada Mediavilla Herrera, Mª José Esteban Niveiro, Nieves López Fresneña, Cristina Díaz‐Agero Pérez, Pedro Ruiz Lopez, Isabel Carrasco Gonzalez, Cristina Navarro Royo, Carmen Albéniz Lizarraga, Yuri Fabiola Villan Villan, Ana Isabel Alguacil Pau, Alicia Díaz Redondo, Rosa Plá Mestre, Dolores Martín Ríos, Angels Figuerola Tejerina, Carlos Aibar Remón, José Joaquín Mira Solves, Juana Requena Puche, Idelfonso González Solana, Montserrat Salcero Guijarro, Delia Fernández Redondo, Esteban del Pozo García, Cornelia Bischofberger Valdés, Libertad Martín Prieto, Marta Grande Arnesto, Beatriz Nieto Pereda, Ana Herranz Alonso, Alicia Díaz Redondo, Laura Rubio Cirilo, Rafael Martos Martínez, María Teresa Ledo Varela, María Vicenta García Rosado, Jesús Minaya Saíz, María Jesús Labrador Domínguez, María José Pita López, Elia Mayoral Peccis, Marco Antonio Espinel Ruíz, Ana Polo Parada, Emely García Carrasco, Carlos Aranda Cosgaya, Carmen Gutiérrez Bezón, María de Sebastián Rueda, Miguel Ruíz Álvarez, Mercedes Vinuesa Sebastián, María Dolors Montserrat Capella, Carolina Ruíz Entrecanales, Sonia de Miguel Fernández, María Pilar González Sánchez, Felisa Jaén Herreros, María José Durá Jiménez, Carmen de Burgos Lunar, Anabel Alguacil Pau, María Ángel Valcárcel de la Iglesia, Laura Moratilla Monzó, Mercedes Ortiz Otero, Margarita Mosquera González, Susana Lorenzo Martínez, María Dolores Martín Ríos, Carolina Lucas Molina, María Teresa Sayalero Martín, María Dolores Calles Gato, Juan José Granizo Martínez, Juan Vega Barea, Eva Jiménez González de Buitrago, Inés Fernández Jiménez, Cristina García Fernández, Inmaculada López Carrillo, Ana Robustillo Rodela, Elena Ramírez García, Romina Sánchez Gómez, Nieves Franco Garrobo, Nieves Plana Farrá, Marta Macías Maroto, Marta Soler Vigil, Gonzalo de las Casas Cámara, Nuria Gálvez Carranza, Ana Belén Jiménez Muñoz, Belén Martínez Mondéjar, Beatriz Isidoro Fernández, Lourdes Sainz de los Terreros Soler, Carolina del Valle Giráldez García, Ruth González Ferrer, Guillermo Ordóñez León, Miguel Miró Murillo, Rosalía Hernández Holgado, Pilar Paloma Blanco Hernández, José Manuel Carrascosa Bernaldez, Sonia Fraile Gil, Beatriz Fidalgo Hermida, Francisco López Rodríguez Arias, Verónica Aranaz Ostáriz, María Pardo Ortiz.
AUTHOR CONTRIBUTIONS
Conception and design: Jesús María Aranaz‐Andrés, José L. Valencia‐Martín, Alberto Pardo‐Hernández.Acquistion of data: Diego San José‐Saras, Paloma Moreno‐Nunez, Jorge de Vicente‐Guijarro, ESHMAD Director Group and external advisers.Analysis and interpretation of data: Jose L. Valencia‐Martin, Jorge de Vicente‐Guijarro, Diego San José‐Saras. Drafting the manuscript: Jorge de Vicente‐Guijarro, Diego San José‐Saras.Critical revision of the manuscript for important intellectual content: José L. Valencia‐Martín, Jesús María Aranaz‐Andrés, Paloma Moreno‐Nunez, Alberto Pardo‐Hernandez. Supervision: Jesús María Aranaz‐Andrés, José L.Valencia‐Martín, Alberto Pardo‐Hernández.
Conflict of interests
None.
Supporting information
Table S1 Definitions of intrinsic and extrinsic risk factors
Valencia‐Martín JL, Vicente‐Guijarro J, San Jose‐Saras D, et al. Prevalence, characteristics, and impact of adverse events in 34 Madrid hospitals. The ESHMAD study. Eur J Clin Invest. 2022;52:e13851. doi: 10.1111/eci.13851
ESHMAD Director Group and external advisers Members of the research group present in Contributors section.
José L. Valencia‐Martín and Jorge Vicente‐Guijarro contributed equally to this work.
REFERENCES
- 1. Mitchell PH. Defining patient safety and quality care. In: Hughes RG, ed. Patient Safety and Quality: An Evidence‐Based Handbook for Nurses. Advances in Patient Safety. Agency for Healthcare Research and Quality (US); 2008. Accessed April 1, 2021. http://www.ncbi.nlm.nih.gov/books/NBK2681/ [Google Scholar]
- 2. Patient safety assessment manual 2nd edition [Internet]. : World Health Organization; 2016. [cited 2022 Jan 11]. Available from: https://www.who.int/publications‐detail‐redirect/9789290221203 [Google Scholar]
- 3. Kruk ME, Gage AD, Joseph NT, Danaei G, García‐Saisó S, Salomon JA. Mortality due to low‐quality health systems in the universal health coverage era: a systematic analysis of amenable deaths in 137 countries. Lancet. 2018;392(10160):2203‐2212. doi: 10.1016/S0140-6736(18)31668-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Makary MA, Daniel M. Medical error‐the third leading cause of death in the US. BMJ. 2016;353:i2139. doi: 10.1136/bmj.i2139 [DOI] [PubMed] [Google Scholar]
- 5. Rafter N, Hickey A, Conroy RM, et al. The Irish National Adverse Events Study (INAES): the frequency and nature of adverse events in Irish hospitals‐a retrospective record review study. BMJ Qual Saf. 2017;26(2):111‐119. doi: 10.1136/bmjqs-2015-004828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Institute of Medicine (US) Committee on Quality of Health Care in America . In: Kohn LT, Corrigan JM, Donaldson MS, eds. To Err Is Human: Building a Safer Health System. National Academies Press (US); 2000. Accessed March 27, 2021. http://www.ncbi.nlm.nih.gov/books/NBK225182/ [PubMed] [Google Scholar]
- 7. Institute for Healthcare Improvement . IHI Global Trigger Tool Guide. 2006. [Internet]. [cited 2021 Sep 30]. Available from: https://somuca.es/wp‐content/uploads/2011/11/GlobalTriggerToolGuide.pdf
- 8. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370‐376. doi: 10.1056/NEJM199102073240604 [DOI] [PubMed] [Google Scholar]
- 9. Wilson RM, Runciman WB, Gibberd RW, Harrison BT, Newby L, Hamilton JD. The Quality in Australian Health Care Study. Medical Journal of Australia. 1995;163(9):458‐471. doi: 10.5694/j.1326-5377.1995.tb124691.x [DOI] [PubMed] [Google Scholar]
- 10. Thomas EJ, Studdert DM, Burstin HR, et al. Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care. 2000;38(3):261‐271. doi: 10.1097/00005650-200003000-00003 [DOI] [PubMed] [Google Scholar]
- 11. Vincent C, Neale G, Woloshynowych M. Adverse events in British hospitals: preliminary retrospective record review. BMJ. 2001;322(7285):517‐519. doi: 10.1136/bmj.322.7285.517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Requena J, Aranaz JM, Gea MT, et al. Evolution of the adverse effects prevalence related to healthcare in hospitals of the Valencia community. Rev Calid Asist. 2010;25(5):244‐249. doi: 10.1016/j.cali.2010.03.007 [DOI] [PubMed] [Google Scholar]
- 13. Aranaz‐Andrés JM, Aibar‐Remón C, Vitaller‐Murillo J, Ruiz P. Estudio nacional sobre los efectos adversos ligados a la hospitalización: ENEAS 2005. Ministerio de Sanidad y Consumo; 2006. [Google Scholar]
- 14. Sousa P, Uva AS, Serranheira F, Uva MS, Nunes C. Patient and hospital characteristics that influence incidence of adverse events in acute public hospitals in Portugal: a retrospective cohort study. International J Qual Health Care. 2018;30(2):132‐137. doi: 10.1093/intqhc/mzx190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mostaza JL, Muinelo I, Teijo C, Pérez S. Prevalence and severity of adverse effects in hospitalized patients. Med Clin (Barc). 2005;124(2):77‐78. doi: 10.1157/13070462 [DOI] [PubMed] [Google Scholar]
- 16. Mendes W, Pavão ALB, Martins M, Travassos C. The application of Iberoamerican study of adverse events (IBEAS) methodology in Brazilian hospitals. International J Qual Health Care. 2018;30(6):480‐485. doi: 10.1093/intqhc/mzy055 [DOI] [PubMed] [Google Scholar]
- 17. Baker GR, Norton PG, Flintoft V, et al. The Canadian Adverse Events Study: the incidence of adverse events among hospital patients in Canada. CMAJ. 2004;170(11):1678‐1686. doi: 10.1503/cmaj.1040498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sommella L, de Waure C, Ferriero AM, et al. The incidence of adverse events in an Italian acute care hospital: findings of a two‐stage method in a retrospective cohort study. BMC Health Serv Res. 2014;14:358. doi: 10.1186/1472-6963-14-358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davis P, Lay‐Yee R, Briant R, Ali W, Scott A, Schug S. Adverse events in New Zealand public hospitals I: occurrence and impact. N Z Med J. 2002;115(1167):U271. [PubMed] [Google Scholar]
- 20. Aranaz‐Andrés JM, Aibar C, Limón R, et al. A study of the prevalence of adverse events in primary healthcare in Spain. Eur J Public Health. 2012;22(6):921‐925. doi: 10.1093/eurpub/ckr168 [DOI] [PubMed] [Google Scholar]
- 21. Estudio EARCAS . Eventos adversos en residencias y centros asistenciales sociosanitarios. Ministerio de Sanidad, Política Social e Igualdad; 2011. [Google Scholar]
- 22. Estrategia Seguridad del Paciente del Sistema Nacional de Salud 2015–2020 . Ministerio de Sanidad, Política Social e Igualdad. 2016 [Internet]. [cited 2022 Jul 9]. Available from: https://seguridaddelpaciente.es/resources/documentos/2015/Estrategia%20Seguridad%20del%20Paciente%202015‐2020.pdf
- 23. Estrategia de Seguridad del Paciente 2015‐2020 . Consejería de Sanidad de la Comunidad de Madrid. 2015; [Internet]. [cited 2022 Jul 9]. Available from: https://www.comunidad.madrid/sites/default/files/estrategia_de_seguridad_del_paciente_2015‐2020_sermas_rev.pdf
- 24. Valencia‐Martín JL, Martin‐Delgado J, Pardo‐Hernández A, et al. The Study on Safety in Hospitals in the Region of Madrid (ESHMAD) design: Screening and analysis of incidents and adverse events. J Healthc Qual Res. Published online May 6, 2021:S2603‐6479(21)00035‐X. doi: 10.1016/j.jhqr.2021.03.007 [DOI] [PubMed]
- 25. Aranaz‐Andrés JM, Aibar‐Remón C, Limón‐Ramírez R, et al. Prevalence of adverse events in the hospitals of five Latin American countries: results of the “Iberoamerican Study of Adverse Events” (IBEAS). BMJ Qual Saf. 2011;20(12):1043‐1051. doi: 10.1136/bmjqs.2011.051284 [DOI] [PubMed] [Google Scholar]
- 26. Estudio de Prevalencia de Infecciones Nosocomiales. Informe España . 2019. Sociedad Española de Medicina Preventiva, Salud Pública e Higiene. [cited 2022 Mar 27]. Available from: https://epine.es/api/documento‐publico/2019%20EPINE%20Informe%20Espa%C3%B1a%2027112019.pdf/reports‐esp
- 27. Protocolo Estudio EPINE‐EPPS . 2019. Sociedad Española de Medicina Preventiva, Salud Pública e Higiene. [cited 2022 Jul 22]. Available from: https://sagunto.san.gva.es/documents/7967159/8195564/Protocolo+Epine+2019.pdf
- 28. Woloshynowych M, Neale G, Vincent C. Case record review of adverse events: a new approach. Qual Saf Health Care. 2003;12(6):411‐415. doi: 10.1136/qhc.12.6.411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Simera I, Moher D, Hoey J, Schulz KF, Altman DG. A catalogue of reporting guidelines for health research. Eur J Clin Invest. 2010;40(1):35‐53. doi: 10.1111/j.1365-2362.2009.02234.x [DOI] [PubMed] [Google Scholar]
- 30. Conceptual framework for the International Classification for Patient Safety. World Health Organization. 2009. Available from: http://www.who.int/patientsafety/taxonomy/icps_full_report.pdf [Google Scholar]
- 31. Rosser R, Kind P. A scale of valuations of states of illness: is there a social consensus? Int J Epidemiol. 1978;7(4):347‐358. doi: 10.1093/ije/7.4.347 [DOI] [PubMed] [Google Scholar]
- 32. Nick TG, Campbell KM. Logistic regression. Methods Mol Biol. 2007;404:273‐301. doi: 10.1007/978-1-59745-530-5_14 [DOI] [PubMed] [Google Scholar]
- 33. Steyerberg EW, Vergouwe Y. Towards better clinical prediction models: seven steps for development and an ABCD for validation. Eur Heart J. 2014;35(29):1925‐1931. doi: 10.1093/eurheartj/ehu207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Corp IBM. IBM SPSS Statistics for Windows, Version 22.0. IBM Corp; 2013. [Google Scholar]
- 35. Aranaz Andrés JM, Limón Ramírez R, Aibar Remón C, et al. Comparison of two methods to estimate adverse events in the IBEAS Study (Ibero‐American study of adverse events): cross‐sectional versus retrospective cohort design. BMJ Open. 2017;7(10):e016546. doi: 10.1136/bmjopen-2017-016546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Michel P, Quenon JL, de Sarasqueta AM, Scemama O. Comparison of three methods for estimating rates of adverse events and rates of preventable adverse events in acute care hospitals. BMJ. 2004;328(7433):199‐190. doi: 10.1136/bmj.328.7433.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Walshe K. The reliability and validity of adverse‐event measures of the quality of health care:417.
- 38. Neale G, Woloshynowych M. Retrospective case record review: a blunt instrument that needs sharpening. Qual Saf Health Care. 2003;12(1):2‐3. doi: 10.1136/qhc.12.1.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Aranaz‐Andres JM, Agra Y. Medicina Clínica. Vol 135. Elsevier; 2010. [Google Scholar]
- 40. de Vries EN, Ramrattan MA, Smorenburg SM, Gouma DJ, Boermeester MA. The incidence and nature of in‐hospital adverse events: a systematic review. Qual Saf Health Care. 2008;17(3):216‐223. doi: 10.1136/qshc.2007.023622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schwendimann R, Blatter C, Dhaini S, Simon M, Ausserhofer D. The occurrence, types, consequences and preventability of in‐hospital adverse events ‐ a scoping review. BMC Health Serv Res. 2018;18(1):521. doi: 10.1186/s12913-018-3335-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Panagioti M, Khan K, Keers RN, et al. Prevalence, severity, and nature of preventable patient harm across medical care settings: systematic review and meta‐analysis. BMJ. Published online July 17. 2019;366:l4185. doi: 10.1136/bmj.l4185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Marcus SC, Hermann RC, Frankel MR, Cullen SW. Safety of Psychiatric Inpatients at the Veterans Health Administration. Psychiatr Serv. 2018;69(2):204‐210. doi: 10.1176/appi.ps.201700224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cullen SW, Xie M, Vermeulen JM, Marcus SC. Comparing Rates of Adverse Events and Medical Errors on Inpatient Psychiatric Units at Veterans Health Administration and Community‐based General Hospitals. Med Care. 2019;57(11):913‐920. doi: 10.1097/MLR.0000000000001215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Marcus SC, Hermann RC, Cullen SW. Defining Patient Safety Events in Inpatient Psychiatry. J Patient Saf. 2021;17(8):e1452‐e1457. doi: 10.1097/PTS.0000000000000520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mollar‐Maseres JB, Aranaz‐Andrés JM, Martin‐Moreno JM, et al. Adverse events related to medication in hospitals from the Valencian Community. EPIDEA Study 2005‐2013. Rev Esp Quimioter. 2017;30(5):319‐326. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Definitions of intrinsic and extrinsic risk factors


