Abstract
Background and Aims
Availability of age‐ and sex‐specific reference values for sex steroids and sex steroid‐binding globulin (SHBG) levels allows for appropriate interpretation of research findings and their clinical applications. We report the sex‐specific distribution and reference levels of sex steroids, including total estradiol, total testosterone and (calculated) free androgen index (cFAI), SHBG and other androgens dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulphate (DHEAS) and androstenedione across age.
Methods
Using data from 3291 participants from the prospective population‐based Rotterdam Study (2006–2008), we visualised the distribution of sex steroids and SHBG levels by calculating and depicting the 5th, 25th, 50th, 75th and 95th percentiles per year and per age‐year across 5‐year age bands to provide reference value ranges in men and women. Total estradiol and SHBG were measured using automated immunoassay and androgens using liquid chromatography‐mass spectrometry (LC–MS/MS).
Result
Mean age was 56.8 (range 45.6–79.9) years in men and 56.9 (range 45.7–79.9) years in women. Amongst men, total estradiol and SHBG showed an increasing trend from 45 years onwards. In women, total estradiol and SHBG showed a decreasing trend from 45 years until the age of 60. From 60 years onwards, SHBG showed an increasing trend. For total testosterone, a clear declining trend was observed amongst men but not women. Other androgens showed a similar decreasing trend in both sexes from 45 years onwards.
Discussion and Conclusion
Our study underlines sex‐specific trends in sex steroids and SHBG levels with ageing. This warrants taking into account sex‐ and age‐specific reference values for sex steroids and SHBG when investigating their impact on health outcomes to prevent controversial results and allow for their appropriate clinical application.
Keywords: epidemiology, reference values, sex steroid‐binding globulin, sex steroids
1. INTRODUCTION
Increasing body of evidence has shown the pivotal role of sex steroids and its main binding protein the sex steroid‐binding globulin (SHBG) for overall health outcomes and well‐being, as well as comorbid conditions. 1 , 2 Sex steroids influence, amongst others, tissue and bone mass, 3 , 4 cognitive functioning, 5 brain health 6 , 7 , 8 and cardiometabolic health 9 via various pathways. Moreover, besides the impact on somatic disorders and symptoms, prior studies have suggested sex steroids may also play an important role in mental health and pathophysiology of mental disorders. 10 , 11
Despite the wide range of health effects of sex steroids, current results regarding the association of sex steroids with various outcomes, in particular with cardiometabolic outcomes and changes in cardiovascular risk, are not conclusive.
Previous studies have suggested age‐related variations in serum concentrations and health‐related effects of sex steroids. 12 , 13 , 14 The impact of sex steroids is dependent on free available steroid concentrations, and therefore, SHBG levels are also important to be taken into consideration when studying sex steroids. It is known that the interplay between sex steroids and SHBG is complex (a simplified overview of the sex steroidal pathway is provided in Figure 1). Failing to take into account such complexities might, at least partly, account for the discrepancies in the results of studies regarding the associations of sex steroids with various outcomes.
FIGURE 1.
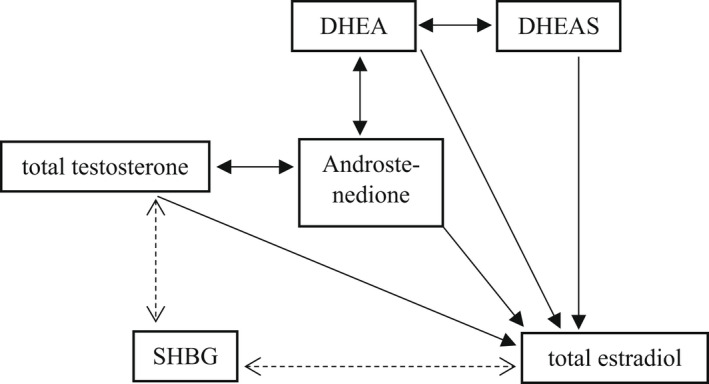
Simplified overview of the sex steroidal pathway. DHEA; dehydroepiandrosterone; DHEAS; dehydroepiandrosterone sulphfate; SHBG; sex steroid‐binding globulin.
The availability of age‐specific reference values for sex steroids and SHBG levels is crucial to allow for appropriate interpretation of the studies and their clinical applications. However, current literature on age‐specific reference values is scarce 15 , 16 , 17 , 18 and limited to investigation of only one specific hormone or focussing on only one sex. Therefore, studies reporting age‐specific reference values for a broad range of sex steroids and SHBG in a wide age range of both men and women are lacking. For this purpose, more robust epidemiological data, based on large population‐based cohort studies, comprehensively investigating oestrogens, androgens and SHBG in both sexes are required. We, therefore, report the distribution and concentrations of sex steroids, including total estradiol, total testosterone and (calculated) free androgen index (cFAI), SHBG and other androgens dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulphate (DHEAS) and androstenedione across age. Using data from the large prospective Rotterdam Study cohort, we provide age‐ and sex‐specific reference values for sex steroids and SHBG amongst middle‐aged and elderly men and women from general population.
2. MATERIALS AND METHODS
2.1. Study population
This study was embedded within the Rotterdam Study, an ongoing prospective, population‐based cohort study amongst individuals of 55 years and older living in the Ommoord district of Rotterdam, the Netherlands. The rationale and study design have been described in detail elsewhere. 19 The baseline examination of the Rotterdam Study included 7983 individuals between 1989 and 1993 (Rotterdam Study‐I). Rotterdam Study has been extended twice (Rotterdam Study‐II: 2000–2001, Rotterdam Study III: 2006–2008) to include participants who were 45 years or older or who had moved to the study research area. The overall response for all three Rotterdam Study cycles at baseline was 72.0% (14.926 of 20.744). This study included participants from the extended cohort Rotterdam Study III‐1 (2006–2008). Data for any sex steroids and SHBG were available in 3449 participants. We excluded 115 women and three men with current use of sex‐steroid medication, defined as use before 90 days of blood collection. Next, we also excluded 43 participants aged >80 years. This resulted in 3291 participants with available sex steroids and SHBG measurements. (Figure 2).
FIGURE 2.
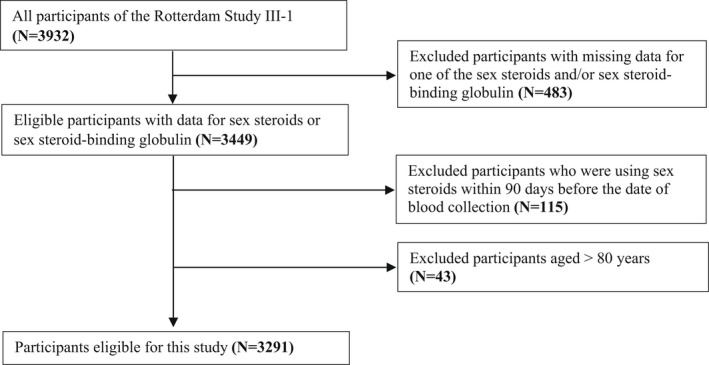
Flow chart of the study population. N, number.
2.2. Assessment of sex steroids and SHBG
All blood samples were drawn in the morning (≤11 am) and were fasting. Sex steroids and SHBG were measured using automated immunoassay or liquid chromatography‐mass spectrometry (LC–MS/MS).
The same method (per measure) was used throughout the entire Rotterdam Study to measure sex steroids and SHBG. The choice of the method was based on sensitivity, specifically in the lower ranges, given also the epidemiologic approach in this study. Total estradiol and SHBG were measured using automated immunoassay and androgens using liquid chromatography‐mass spectrometry. Total estradiol levels were measured with COBAS 8000 Modular Analyser (Roche Diagnostics GmbH), serum levels of total testosterone with liquid chromatography–tandem mass spectrometry (LC–MS/MS), and sex steroid‐binding globulin (SHBG) was measured as with the Immulite platform (Diagnostics Products Corporation Breda). The corresponding interassay coefficients of variations for total estradiol, total testosterone and SHBG were <7%, <5% and <5%. The minimum detection limit for estradiol was 18.35 pmol/L. Undetectable estradiol was scored as 18.35. The (calculated) free androgen index was calculated in women as (total testosterone/SHBG)*100. 20 DHEA, DHEAS and androstenedione were measured on a Waters XEVO‐TQ‐S system (Waters) using the CHS™ MSMS Steroids Kit (Perkin Elmer). The interassay coefficients of variation of DHEA, DHEAS and androstenedione were <6.5%. Serum was immediately frozen (within 2 h) at −150°C and afterwards stored at −80°C.
2.3. Other measurements (provided as Appendix S1)
An interview was performed to obtain information on current health status, medical history, medication use (including hormones), menopausal status and smoking. Blood pressure was measured in the sitting position on the right upper arm with a random‐zero sphygmomanometer. The retrospective data on self‐reported use of antihypertensive were collected by a questionnaire during the home interview. Smoking status was assessed by asking participants whether they were current smokers of cigarettes, cigars or pipe. Postmenopausal women were defined as women who reported the absence of menstrual periods for 12 months. The retrospective data on self‐reported use of antihypertensive were collected by a questionnaire during the home interview. Smoking status was assessed asking participants whether they were current smokers of cigarettes, cigars or pipe. Body mass index was calculated as weight (kg) divided by height squared (m2). Waist circumference was measured in standing position, without heavy outer garments, midway between the lower rib and iliac crest. Waist–hip ratio was calculated. All biochemical parameters were assessed in fasting serum. Total cholesterol, high‐density lipoprotein cholesterol (HDL) and C‐reactive protein were measured on the COBAS 8000 Modular Analyser (Roche Diagnostics GmbH). The corresponding interassay coefficients of variations are the following: lipids <2.1% and C‐reactive protein <16.9%. Prevalent CHD was defined as a history of myocardial infarction or coronary revascularization including PCI or CABG. Diabetes mellitus type 2 diagnosis was considered present if a participant used glucose‐lowering drugs, in case a nonfasting random serum glucose level was found to be ≥11 mmol/L or use of antidiabetic medication.
2.4. Statistical analysis
Characteristics of women and men were presented as mean (standard deviation; SD) or median (25th ‐75th quartile) for continuous variables and number (percentage) for categorical variables.
All analyses were performed separately for men and women. First, we visualised the distribution of sex steroids and SHBG across age by calculating and depicting the 25th, 50th and 75th percentiles per age‐year. Smoothed percentile lines were plotted to visualise the data. We also calculated the 5th, 25th, 50th, 50th and 95th percentiles for 5‐year age bands to provide reference value ranges. The following age categories were constructed: 45–49 years, 50–54 years, 55–59 years, 60–64 years, 65–69 years, 70–74 years and 75–79 years.
As a sensitivity analysis, we further assessed the trend of sex steroids and SHBG across age amongst overweight and nonoverweight participants separately and after restricting the population to postmenopausal women only.
Statistical analysis was performed with IBM SPSS statistics software version 24 and R version 3.4.4. R package ‘ggplot2’ was used to construct the plots. Generalised additive mode smoothing (GAM) method was used to visualise the data using smoothed lines.
3. RESULTS
3.1. Characteristics of the study population
Table 1 depicts the characteristics of the study population. The mean age in men was 56.8 (range 45.6–79.9 years) and in women 56.9 (range 45.7–79.9 years).
TABLE 1.
Characteristics of the study population
| Men (N = 1490) | Women (N = 1801) | |
|---|---|---|
| Age, years | 56.75 (6.18) | 56.87 (6.09) |
| Body mass index, kg/m2 | 27.88 (3.90) | 27.68 (5.09) |
| Waist–hip ratio | 0.93 (0.07) | 0.83 (0.07) |
| Current smoking, N (%) | 426 (31.2) | 401 (23.1) |
| Diastolic blood pressure, mmHg | 83.38 (10.67) | 82.09 (11.05) |
| Systolic blood pressure, mmHg | 135.44 (17.76) | 130.20 (19.37) |
| Antihypertensive therapy, N (%) | 420 (28.5) | 447 (25.0) |
| Total cholesterol, mg/dl | 5.43 (1.02) | 5.76 (1.06) |
| High‐density lipoprotein cholesterol, mmol/L | 1.24 (0.35) | 1.58 (0.44) |
| Lipid lowering medication, N (%) | 383 (25.9) | 357 (19.9) |
| C‐reactive protein, mg/L | 1.10 [0.50, 2.50] | 1.30 [0.60, 3.00] |
| Prevalent diabetes, N (%) | 194 (13.0) | 131 (7.3) |
| Prevalent coronary heart disease, N (%) | 100 (6.8) | 24 (1.3) |
| Sex steroids | ||
| Total estradiol, pmol/L | 94.07 [73.78, 118.60] | 30.96 [18.35, 66.54] |
| Total testosterone, nmol/L | 16.85 [13.38, 21.28] | 0.79 [0.57, 1.08] |
| Calculated free androgen index | 0.42 [0.35, 0.50] | 0.01 [0.01, 0.02] |
| Sex steroid‐binding globulin, nmol/L | 40.37 [30.83, 50.92] | 56.76 [40.88, 78.32] |
| Dehydroepiandrosterone, nmol/L | 13.10 [8.37, 19.20] | 13.36 [8.68, 19.90] |
| Dehydroepiandrosterone sulphate, nmol/L | 3619.85 [2352.41, 5082.61] | 2316.89 [1448.54, 3454.40] |
| Androstenedione, nmol/L | 3.32 [2.60, 4.40] | 2.65 [1.92, 3.70] |
| Female‐specific variables | ||
| Age at menopause, years | NA | 48.11 (6.30) |
| Time since menopause, years | NA | 10.53 (7.76) |
| Natural menopause, N (%) | NA | 1028 (75.9) |
Note: Values are reported as number (percentage) for categorical variables and mean (SD) or median [25th–75th quartile] for continuous variables.
Abbreviations: N; number, NA; not applicable.
Figure 3 depicts the relationship between age and concentrations of total estradiol, total testosterone and SHBG. Figure S1 depicts the relationship between age and levels of DHEA, DHEAS and androstenedione. Age‐ and sex‐specific percentile values for sex steroids and SHBG are provided in Table 2.
FIGURE 3.
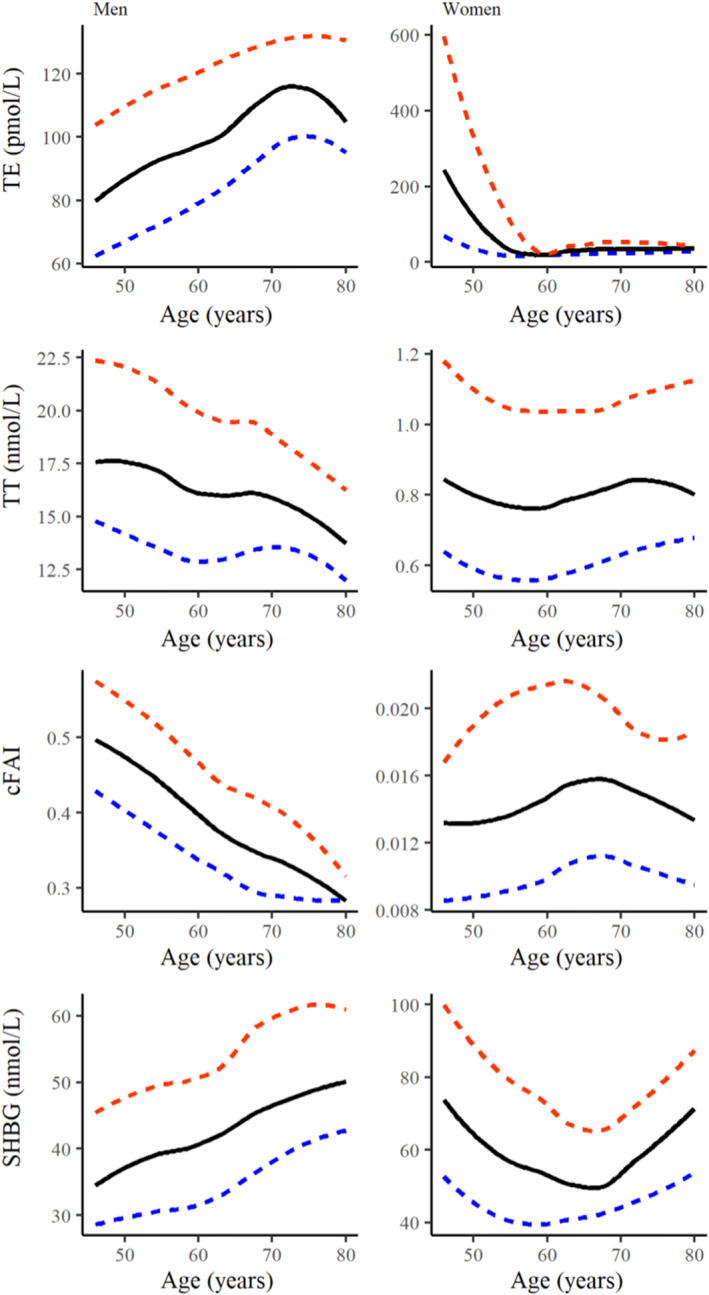
Levels of total estradiol, total testosterone, cFAI and SHBG across age. Smoothed percentile plots of total estradiol, total testosterone, cFAI, and SHBG for each age‐year in 1490 men (left) and 1801 women (right). The median is a solid line, 25th and 75th percentile are depicted as dashed lines. cFAI, calculated free androgen index; SHBG, sex steroid‐binding globulin; TE, total estradiol; TT, total testosterone.
TABLE 2.
Age‐ and sex‐specific percentile values for serum sex steroids and sex steroid‐binding globulin
| (A) Total estradiol (pmol/L) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | ||||||||||||
| Age‐groups | N | 5th | 25th | 50th | 75th | 95th | Age‐groups | N | 5th | 25th | 50th | 75th | 95th |
| 45–49 year | 212 | 41.93 | 63.70 | 86.92 | 109.43 | 155.55 | 45–49 year | 245 | 18.35 | 32.37 | 165.30 | 512.50 | 1719.00 |
| 50–54 year | 391 | 46.90 | 71.62 | 90.78 | 115.80 | 154.95 | 50–54 year | 456 | 18.35 | 18.35 | 40.75 | 129.98 | 800.20 |
| 55–59 year | 471 | 45.09 | 74.06 | 94.47 | 115.70 | 160.10 | 55–59 year | 582 | 18.35 | 18.35 | 18.49 | 43.60 | 85.84 |
| 60–64 year | 300 | 54.02 | 80.51 | 99.78 | 125.73 | 161.30 | 60–64 year | 387 | 18.35 | 18.35 | 27.80 | 45.30 | 79.32 |
| 65–69 year | 49 | 64.69 | 83.17 | 104.40 | 129.30 | 165.16 | 65–69 year | 51 | 18.35 | 18.35 | 32.59 | 50.32 | 110.60 |
| 70–74 year | 39 | 64.79 | 95.28 | 123.20 | 131.90 | 155.29 | 70–74 year | 41 | 18.35 | 24.28 | 38.43 | 51.62 | 138.20 |
| 75–79 year | 23 | 51.11 | 75.21 | 114.40 | 125.45 | 161.81 | 75–79 year | 29 | 18.35 | 18.96 | 34.40 | 44.02 | 68.68 |
| (B) Total testosterone (nmol/L) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | ||||||||||||
| Age‐groups | N | 5th | 25th | 50th | 75th | 95th | Age‐groups | N | 5th | 25th | 50th | 75th | 95th |
| 45–49 year | 212 | 10.05 | 14.43 | 17.98 | 22.97 | 28.00 | 45–49 year | 245 | 0.37 | 0.59 | 0.80 | 1.06 | 1.66 |
| 50–54 year | 391 | 9.97 | 13.97 | 17.27 | 21.79 | 27.63 | 50–54 year | 452 | 0.38 | 0.57 | 0.80 | 1.10 | 1.63 |
| 55–59 year | 471 | 9.60 | 13.05 | 16.67 | 20.82 | 27.70 | 55–59 year | 580 | 0.34 | 0.55 | 0.73 | 1.01 | 1.70 |
| 60–64 year | 298 | 9.25 | 12.78 | 16.31 | 20.43 | 26.95 | 60–64 year | 386 | 0.38 | 0.59 | 0.82 | 1.12 | 1.68 |
| 65–69 year | 49 | 9.55 | 12.46 | 17.26 | 20.30 | 27.82 | 65–69 year | 51 | 0.36 | 0.59 | 0.86 | 1.11 | 1.53 |
| 70–74 year | 39 | 9.61 | 12.15 | 15.77 | 19.43 | 24.08 | 70–74 year | 41 | 0.30 | 0.55 | 0.81 | 1.13 | 1.61 |
| 75–79 year | 23 | 10.34 | 12.01 | 13.46 | 18.00 | 22.84 | 75–79 year | 28 | 0.31 | 0.60 | 0.78 | 1.14 | 2.74 |
| (C) cFAI | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | ||||||||||||
| Age‐groups | N | 5th | 25th | 50th | 75th | 95th | Age‐groups | N | 5th | 25th | 50th | 75th | 95th |
| 45–49 year | 212 | 0.33 | 0.40 | 0.49 | 0.57 | 0.71 | 45–49 year | 245 | 0.004 | 0.009 | 0.013 | 0.018 | 0.028 |
| 50–54 year | 391 | 0.30 | 0.39 | 0.45 | 0.53 | 0.70 | 50–54 year | 452 | 0.004 | 0.009 | 0.013 | 0.021 | 0.034 |
| 55–59 year | 471 | 0.25 | 0.35 | 0.41 | 0.49 | 0.62 | 55–59 year | 580 | 0.005 | 0.009 | 0.014 | 0.020 | 0.039 |
| 60–64 year | 298 | 0.26 | 0.33 | 0.40 | 0.47 | 0.60 | 60–64 year | 386 | 0.006 | 0.011 | 0.016 | 0.023 | 0.037 |
| 65–69 year | 49 | 0.23 | 0.29 | 0.35 | 0.43 | 0.55 | 65–69 year | 51 | 0.006 | 0.011 | 0.014 | 0.022 | 0.032 |
| 70–74 year | 39 | 0.19 | 0.25 | 0.31 | 0.40 | 0.52 | 70–74 year | 41 | 0.005 | 0.009 | 0.017 | 0.021 | 0.038 |
| 75–79 year | 23 | 0.24 | 0.27 | 0.28 | 0.34 | 0.37 | 75–79 year | 28 | 0.005 | 0.007 | 0.012 | 0.018 | 0.035 |
| (D) SHBG (nmol/L) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | ||||||||||||
| Age‐groups | N | 5th | 25th | 50th | 75th | 95th | Age‐groups | N | 5th | 25th | 50th | 75th | 95th |
| 45–49 year | 212 | 18.92 | 28.57 | 37.85 | 47.68 | 66.01 | 45–49 year | 245 | 26.18 | 45.27 | 67.08 | 92.76 | 171.94 |
| 50–54 year | 391 | 19.21 | 29.53 | 38.61 | 50.11 | 65.40 | 50–54 year | 456 | 25.38 | 42.26 | 59.41 | 82.89 | 128.95 |
| 55–59 year | 471 | 21.61 | 31.68 | 41.12 | 50.75 | 70.68 | 55–59 year | 582 | 24.70 | 39.53 | 54.89 | 76.49 | 115.51 |
| 60–64 year | 300 | 20.40 | 31.76 | 41.73 | 52.78 | 76.27 | 60–64 year | 387 | 25.79 | 39.61 | 52.31 | 71.97 | 109.15 |
| 65–69 year | 49 | 25.36 | 35.48 | 43.48 | 58.55 | 90.36 | 65–69 year | 51 | 32.66 | 41.40 | 53.65 | 69.00 | 96.64 |
| 70–74 year | 39 | 24.00 | 38.45 | 47.32 | 63.30 | 91.73 | 70–74 year | 41 | 27.57 | 38.93 | 49.29 | 75.93 | 110.80 |
| 75–79 year | 23 | 32.03 | 40.48 | 49.14 | 64.64 | 89.16 | 75–79 year | 29 | 34.23 | 52.26 | 74.90 | 86.62 | 132.14 |
| (E) DHEA (nmol/L) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | ||||||||||||
| Age‐groups | N | 5th | 25th | 50th | 75th | 95th | Age‐groups | N | 5th | 25th | 50th | 75th | 95th |
| 45–49 year | 209 | 6.85 | 12.67 | 17.77 | 25.32 | 46.77 | 45–49 year | 241 | 5.12 | 10.14 | 14.71 | 23.24 | 45.23 |
| 50–54 year | 389 | 6.10 | 9.45 | 14.42 | 20.84 | 38.67 | 50–54 year | 450 | 4.83 | 10.17 | 14.94 | 22.03 | 38.70 |
| 55–59 year | 459 | 5.07 | 8.51 | 12.69 | 18.19 | 30.93 | 55–59 year | 573 | 4.54 | 8.67 | 13.24 | 19.18 | 32.92 |
| 60–64 year | 294 | 4.25 | 7.23 | 10.15 | 16.40 | 27.64 | 60–64 year | 384 | 4.28 | 8.52 | 12.92 | 19.34 | 34.77 |
| 65–69 year | 49 | 3.75 | 6.28 | 9.12 | 12.62 | 23.47 | 65–69 year | 51 | 3.08 | 7.34 | 9.19 | 13.22 | 23.78 |
| 70–74 year | 39 | 2.74 | 3.91 | 6.42 | 10.05 | 19.62 | 70–74 year | 41 | 2.63 | 4.13 | 7.28 | 11.98 | 22.34 |
| 75–79 year | 23 | 2.47 | 3.39 | 4.61 | 9.72 | 14.45 | 75–79 year | 27 | 2.20 | 3.15 | 5.60 | 8.88 | 16.88 |
| Men | Women | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age‐groups | N | 5th | 25th | 50th | 75th | 95th | Age‐groups | N | 5th | 25th | 50th | 75th | 95th |
| 45–49 year | 193 | 2027.79 | 3487.16 | 4835.29 | 6171.53 | 7458.33 | 45–49 year | 242 | 786.85 | 1878.14 | 3067.44 | 4197.49 | 6546.83 |
| 50–54 year | 360 | 1613.14 | 2839.77 | 4055.80 | 5367.02 | 7030.06 | 50–54 year | 447 | 866.30 | 1662.73 | 2545.95 | 3771.91 | 5684.64 |
| 55–59 year | 455 | 1175.83 | 2472.76 | 3612.80 | 5043.47 | 6815.23 | 55–59 year | 577 | 660.39 | 1408.54 | 2323.50 | 3317.62 | 5200.07 |
| 60–64 year | 286 | 946.81 | 1872.69 | 2913.95 | 4264.78 | 7004.31 | 60–64 year | 386 | 649.74 | 1313.61 | 2016.49 | 2923.86 | 5072.82 |
| 65–69 year | 49 | 788.18 | 1470.75 | 2393.82 | 3464.91 | 5564.23 | 65–69 year | 51 | 522.88 | 1034.04 | 1592.58 | 2745.01 | 4593.76 |
| 70–74 year | 39 | 668.93 | 1057.61 | 1508.26 | 2525.77 | 4943.30 | 70–74 year | 41 | 251.97 | 862.73 | 1494.59 | 2077.82 | 3038.15 |
| 75–79 year | 23 | 558.24 | 970.90 | 1491.19 | 2573.66 | 4170.95 | 75–79 year | 29 | 136.07 | 541.30 | 1065.05 | 1588.92 | 3108.19 |
| (G) Androstenedione (nmol/L) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | ||||||||||||
| Age‐groups | N | 5th | 25th | 50th | 75th | 95th | Age‐groups | N | 5th | 25th | 50th | 75th | 95th |
| 45–49 year | 211 | 2.00 | 3.04 | 3.86 | 4.75 | 8.01 | 45–49 year | 245 | 1.32 | 2.30 | 3.33 | 4.39 | 7.33 |
| 50–54 year | 391 | 2.02 | 2.81 | 3.48 | 4.49 | 6.55 | 50–54 year | 451 | 1.26 | 2.15 | 2.97 | 3.98 | 6.17 |
| 55–59 year | 469 | 1.79 | 2.55 | 3.19 | 4.30 | 6.18 | 55–59 year | 577 | 1.17 | 1.80 | 2.49 | 3.30 | 5.57 |
| 60–64 year | 298 | 1.73 | 2.43 | 3.10 | 4.14 | 5.87 | 60–64 year | 386 | 1.12 | 1.84 | 2.53 | 3.39 | 5.41 |
| 65–69 year | 49 | 1.47 | 2.07 | 2.73 | 3.67 | 5.85 | 65–69 year | 50 | 1.18 | 1.65 | 2.28 | 3.01 | 4.50 |
| 70–74 year | 39 | 1.25 | 1.76 | 2.60 | 3.56 | 4.74 | 70–74 year | 41 | 0.94 | 1.57 | 1.79 | 2.64 | 4.64 |
| 75–79 year | 23 | 1.28 | 1.93 | 2.18 | 3.03 | 4.20 | 75–79 year | 29 | 0.78 | 1.37 | 1.80 | 2.42 | 3.60 |
Abbreviations: N; number, cFAI; calculated free androgen index, SHBG; sex steroid‐binding globulin, DHEA; dehydroepiandrosterone, DHEAS; dehydroepiandrosterone sulfate.
3.2. Total estradiol
Amongst men, total estradiol levels increased from the age of 45 years onwards. A steady increase was observed until the age of 75 years and a decline in the eldest age group 75–79 years. In women, total estradiol levels declined starting from 45 years until approximately 60 years. Due to the lower assay detection limit of 18.35 pmol/L, further decline was not detectable in this study.
3.3. Total testosterone and cFAI
In men, both total testosterone and cFAI declined with increasing age. Whilst a more prominent decline was observed after the age of approximately 65 years for total testosterone, cFAI showed a steady constant decline from 45 years onwards.
Amongst women, after an initial decrease, a slight increase was observed for total testosterone after 60 years. However, cFAI in women showed first a slight increase from the age of 45 to approximately 65 years, reflecting the trend of SHBG with increasing age. Afterwards, a declining trend was observed.
3.4. SHBG
Serum SHBG levels decreased from the age of 45 years onwards in men. Amongst women, a U‐shaped trend was observed. SHBG levels decreased until the age of 60, followed by a steady and stronger increase until older ages.
3.5. DHEA, DHEAS and androstenedione
In both sexes, DHEA, DHEAS and androstenedione declined with a constant slope from 45 years onwards.
Whilst in men, the slope of decline for DHEA slowed down after the age of 60 years; in women, this occurred approximately after the age of 70 years. However, the slope of decline for DHEAS in men occurred approximately at the age of 70 years, Whilst in women, this occurred around the age of 65 years.
The pattern of decline for androstenedione was similar amongst men and women; the decline slowed down after the age of 60 years.
3.6. Sensitivity analysis
After stratifying for overweight and nonoverweight participants, similar trend of changes was observed in the two groups in both men and women. (Figure S2) However, in both sexes, SHBG levels were lower amongst overweight individuals. Amongst men, total testosterone levels were also lower in overweight individuals. In women, higher cFAI levels amongst overweight individuals were observed across all age categories.
After restricting the population to postmenopausal women, a similar trend, as in the total population of women, was observed for total estradiol and SHBG.(Figure S3) A different pattern was observed for total testosterone, and other androgens (DHEA, DHEAS and androstenedione), showing an increasing trend from 45 years onwards to approximately 55 years in postmenopausal women. However, after the age of 55 years, similar decreasing trends were observed.
4. DISCUSSION
In this large population‐based cohort study, we describe the distribution of sex steroids and SHBG and provide age‐ and sex‐specific reference values for middle‐aged and elderly men and women from general population. Our study underlines sex‐specific trends in the levels of sex steroids and SHBG with ageing and shows reference ranges differ by age category.
4.1. Total estradiol
We observed a sex‐specific trend for total estradiol levels with increasing age. Whilst estradiol levels declined in women with ageing, we observed an opposite trend in men. Although the age‐related decline of total estradiol in women is known to be related to the perimenopausal period, the underlying mechanisms for the observed age‐related increase in men remain unknown. Nevertheless, we speculate that these changes may reflect, partly, age‐related changes in body weight and/or increase in comorbid conditions. A prior study similarly reported an increasing trend of total estradiol levels in middle‐aged men. 21
4.2. SHBG
Evident sex differences in the trend of SHBG levels with ageing were observed. Whilst SHBG levels increased from the age of 45 years onwards amongst men, a U‐shaped trend was observed amongst women. However, the lowest and highest reference ranges were different for age categories in both men and women. Importantly, the U‐shaped trend in women was also reflected in the reference ranges. A previous study has also showed a similar U‐shaped trend for SHBG amongst women. 12 Another study showed similarly a decreasing trend from SHBG during the menopausal transition. 22
Whilst the decrease of SHBG in women after the age of 45 years mirrors the parallel decrease of estradiol, the observed increase from the age of 60 years onwards merits further investigations. Similarly, the underlying mechanisms for the observed trend in men also remain to be established. Previous studies have suggested estradiol levels may increase and androgens may decrease SHBG levels. 23 , 24 However, the exact role of sex steroids and their interplay with SHBG, which is known to be complex for the regulation of SHBG levels and may be bidirectional, remains also to be elucidated.
4.3. Total testosterone and cFAI
In line with previous reports, amongst Danish, 25 European, 26 Australian 13 and American 27 population, we observed a decrease in total testosterone levels with ageing in men. Although it was previously suggested that testosterone levels decrease in women from adolescence until menopause, 13 we observed increasing levels with ageing from 60 years onwards similar to other studies. 13 , 14
We observed similar decreasing trend for total testosterone and cFAI amongst men. However, total testosterone and cFAI showed different patterns with ageing amongst women. This could be a reflection of the differences in the trend of SHBG with increasing age in men and women (constant increasing trend in men vs. U‐shaped trend in women).
4.4. DHEA, DHEAS and androstenedione
Our study showed a declining trend for DHEA, DHEAS and androstenedione after the age of 45 years, which is consistent with previous studies in men 28 and in women. 14 The approximate age at which the slope of the trend changed was similar in both men and women for androstenedione, but differed by sex for DHEA and DHEAS.
An increasing trend for testosterone and other androgens was observed amongst the younger postmenopausal women aged 45 until 55 years, which is the average age of 5 years since menopause.
We speculate that this could be explained by the late perimenopausal and early menopausal androgen production in the ovaries in response to stimulation by gonadotropins (luteinizing hormone and follicle‐stimulating hormone) produced in the pituitary, which disappears later in the menopause. 29
4.5. Age‐specific reference ranges
Our results show that normal reference ranges (i.e., P5‐P95 or P25‐P75) are inconsistent for sex steroids and SHB across different age categories in both genders. This further highlights the clinical importance of taking into account age‐related changes. Our findings also, at least partly, explain the inconsistencies in proposed reference values by various studies. 30 , 31 , 32 The age‐specific reference values (for women, both pre‐ and postmenopausal) could also, in part, be responsible for discrepancies in the reported associations between sex steroids and SHBG with health outcomes in various studies. 9 , 33 , 34 , 35 , 36 Our findings warrant both clinicians and researchers to account for age‐related changes in sex steroids when diagnosing or investigating hypo‐ and hyperandrogenism. Consideration of age‐specific reference values for sex steroids and SHBG levels will allow for appropriate interpretation of the studies and their clinical applications.
4.6. Strength and limitations
The major strengths of this study include the large community‐dwelling study sample of men and women from a prospective cohort study. Moreover, a broad range of sex steroids and SHBG was simultaneously available in both women and men. Finally, total testosterone was measured using the golden standard method. Limitations of this study include the cross‐sectional design that does not allow for trajectories of longitudinal changes within the same individuals. In this study, we used only single sex steroid measurements, whilst sex steroids levels may fluctuate due to diurnal variation. However, in this study, blood was drawn in the morning in all participants to minimise influence of diurnal rhythm. Moreover, at older ages and in postmenopausal women, sex steroids levels are more stable over time. Finally, the immunoassay to measure estradiol had a minimum detection limit of 18.35 pmol/L. We did not exclude participants with comorbid conditions, including CHD, diabetes and obesity, whilst previous studies have suggested these conditions are associated with sex steroids and SHBG. Additionally, no separate subgroup analysis was performed for participants with endocrine disorders. However, this was not the aim of this study as we aimed to provide reference values for a general population, which can be used for future clinical purposes and settings. The use of different laboratory assays may lead to variation in measurements of sex steroids and SHBG values, and reference ranges may vary amongst different laboratories. As such, this should be taken into account when extrapolating the data for use of measurements performed by other assays than those used in this study. Although this may affect the absolute sex steroids or SHBG levels, it does not affect observed age‐ and sex‐specific trends. Additionally, the majority of the Rotterdam Study participants were of Euroancestry. Therefore, ethnicity‐specific reference values were not available.
In our study, serum albumin levels were not available. As sex steroids are partly bound to albumin, future studies should also investigate age‐specific reference values for serum albumin levels.
5. CONCLUSIONS
In this large population‐based cohort study, we describe the distribution of sex steroids and SHBG and provide age‐ and sex‐specific reference values for middle‐aged and elderly men and women from general population. Our study underlines sex‐specific trends in the levels of sex steroids and SHBG with ageing, which was also reflected in the reference ranges across different age categories. This warrants taking into account sex‐ and age‐specific reference values for sex steroids and SHBG when investigating their impact on health outcomes to prevent controversial results. Consideration of age‐specific reference values (for women both pre‐ and postmenopausal) for sex steroids and SHBG levels will allow for appropriate interpretation of the studies and their clinical applications. Future studies assessing longitudinal within‐person changes are warranted.
AUTHOR CONTRIBUTIONS
EA, JERL, YBR and MK contributed to the conception or design of the work. EA, JERL, YBR, JSEL, MAI, RPP and MK contributed to the acquisition, analysis or interpretation of data for the work. EA drafted the manuscript. All critically revised the manuscript and gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
FUNDING INFORMATION
The Rotterdam Study is funded by Erasmus Medical Center and Erasmus University Rotterdam, the Netherlands Organization for the Health Research and Development (ZonMw), the Research Institute for Diseases in the Elderly (RIDE), the Ministry of Education, Culture and Science, the Ministry for Health, Welfare and Sports, the European Commission (DGXII), and the Municipality of Rotterdam. Further support was obtained from the Netherlands Consortium for Healthy Aging and the Dutch Heart Foundation (2012 T008) and the Dutch Cancer Society (NKI‐20157737). This project is further supported by the Gender and Prevention grant (555003017) from ZonMw and Senior Scientist Grant from Dutch Heart Foundation.
CONFLICT OF INTEREST
EA, JERL, YBR, MAI, RPP and MK have nothing to disclose. JSEL reports grants from fAstellas (Tokyo, Japan), Dutch Heart Association (Utrecht, the Netherlands), ZonMw (Amsterdam, the Netherlands), personal fees from Titus Healthcare (Hoofddorp, the Netherlands) and grants and personal fees from Ferring (Hoofddorp, the Netherlands), Ansh Labs (Webster, Tx, USA), outside the submitted work.
INFORMED CONSENT
Informed consent was obtained from all individual participants included in the study.
Supporting information
Appendix S1
ACKNOWLEDGEMENT
We gratefully acknowledge the dedication, commitment and contribution of the inhabitants, general practitioners and pharmacists of the Ommoord district to the Rotterdam Study.
Aribas E, Roeters van Lennep JE, De Rijke YB, et al. Sex steroids and sex steroid‐binding globulin levels amongst middle‐aged and elderly men and women from general population. Eur J Clin Invest. 2022;52:e13866. doi: 10.1111/eci.13866
REFERENCES
- 1. Kelly DM, Jones TH. Testosterone: a vascular hormone in health and disease. J Endocrinol. 2013;217(3):R47‐R71. [DOI] [PubMed] [Google Scholar]
- 2. Goldstajn MS, Toljan K, Grgic F, Jurkovic I, Baldani DP. Sex hormone binding globulin (SHBG) as a marker of clinical disorders. Coll Antropol. 2016;40(3):211‐218. [PubMed] [Google Scholar]
- 3. Mohamad N‐V, Soelaiman I‐N, Chin K‐Y. A concise review of testosterone and bone health. Clin Interv Aging. 2016;11:1317‐1324. doi: 10.2147/cia.S115472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paller CJ, Shiels MS, Rohrmann S, et al. Relationship of sex steroid hormones with bone mineral density (BMD) in a nationally representative sample of men. Clin Endocrinol. 2009;70(1):26‐34. doi: 10.1111/j.1365-2265.2008.03300.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gurvich C, Hoy K, Thomas N, Kulkarni J. Sex differences and the influence of sex hormones on cognition through adulthood and the aging process. Brain Sci. 2018;8(9):163. doi: 10.3390/brainsci8090163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li R, Cui J, Shen Y. Brain sex matters: estrogen in cognition and Alzheimer's disease. Mol Cell Endocrinol. 2014;389(1–2):13‐21. doi: 10.1016/j.mce.2013.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pike CJ. Sex and the development of Alzheimer's disease. J Neurosci Res. 2017;95(1–2):671‐680. doi: 10.1002/jnr.23827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Glisic M, Mujaj B, Rueda‐Ochoa OL, et al. Associations of endogenous estradiol and testosterone levels with plaque composition and risk of stroke in subjects with carotid atherosclerosis. Circ Res. 2018;122(1):97‐105. [DOI] [PubMed] [Google Scholar]
- 9. Brand JS, van der Schouw YT. Testosterone, SHBG and cardiovascular health in postmenopausal women. Int J Impot Res. 2010;22:91‐104. doi: 10.1038/ijir.2009.64 [DOI] [PubMed] [Google Scholar]
- 10. Colangelo LA, Craft LL, Ouyang P, et al. Association of sex hormones and sex hormone‐binding globulin with depressive symptoms in postmenopausal women: the multiethnic study of atherosclerosis. Menopause. 2012;19(8):877‐885. doi: 10.1097/gme.0b013e3182432de6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Asselmann E, Kische H, Haring R, et al. Prospective associations of androgens and sex hormone‐binding globulin with 12‐month, lifetime and incident anxiety and depressive disorders in men and women from the general population. J Affect Disord. 2019;245:905‐911. [DOI] [PubMed] [Google Scholar]
- 12. Maggio M, Lauretani F, Basaria S, et al. Sex hormone binding globulin levels across the adult lifespan in women–The role of body mass index and fasting insulin. J Endocrinol Investig. 2008;31(7):597‐601. doi: 10.1007/bf03345608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Handelsman DJ, Sikaris K, Ly LP. Estimating age‐specific trends in circulating testosterone and sex hormone‐binding globulin in males and females across the lifespan. Ann Clin Biochem. 2016;53(Pt 3):377‐384. [DOI] [PubMed] [Google Scholar]
- 14. Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90(7):3847‐3853. [DOI] [PubMed] [Google Scholar]
- 15. Qin X, Lv H, Mo Z, et al. Reference intervals for serum sex hormones in Han Chinese adult men from the Fangchenggang area male health and examination survey. Clin Lab. 2012;58(3–4):281‐290. [PubMed] [Google Scholar]
- 16. Bhasin S, Pencina M, Jasuja GK, et al. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community‐based sample of healthy nonobese young men in the Framingham heart study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab. 2011;96(8):2430‐2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bjerner J, Biernat D, Fosså SD, Bjøro T. Reference intervals for serum testosterone, SHBG, LH and FSH in males from the NORIP project. Scand J Clin Lab Invest. 2009;69(8):873‐887. doi: 10.3109/00365510903380886 [DOI] [PubMed] [Google Scholar]
- 18. Shen X, Wang R, Yu N, et al. Reference ranges and association of age and lifestyle characteristics with testosterone, sex hormone binding globulin, and luteinizing hormone amongst 1166 Western Chinese men. PLoS One. 2016;11(10):e0164116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ikram MA, Brusselle GGO, Murad SD, et al. The Rotterdam study: 2018 update on objectives, design and main results. Eur J Epidemiol. 2017;32(9):807‐850. doi: 10.1007/s10654-017-0321-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Position statement: utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab. 2007;92(2):405‐413. [DOI] [PubMed] [Google Scholar]
- 21. Jasuja GK, Travison TG, Davda M, et al. Age trends in estradiol and estrone levels measured using liquid chromatography tandem mass spectrometry in community‐dwelling men of the Framingham heart study. J Gerontol A Biol Sci Med Sci. 2013;68(6):733‐740. doi: 10.1093/gerona/gls216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burger HG, Dudley EC, Cui J, Dennerstein L, Hopper JL. A prospective longitudinal study of serum testosterone, dehydroepiandrosterone sulfate, and sex hormone‐binding globulin levels through the menopause Transition1. J Clin Endocrinol Metab. 2000;85(8):2832‐2838. doi: 10.1210/jcem.85.8.6740 [DOI] [PubMed] [Google Scholar]
- 23. Kalme T, Loukovaara M, Koistinen R, et al. Estradiol increases the production of sex hormone‐binding globulin but not insulin‐like growth factor binding protein‐1 in cultured human hepatoma cells. Fertil Steril. 1999;72(2):325‐329. doi: 10.1016/s0015-0282(99)00229-0 [DOI] [PubMed] [Google Scholar]
- 24. Ruokonen A, Alén M, Bolton N, Vihko R. Response of serum testosterone and its precursor steroids, SHBG and CBG to anabolic steroid and testosterone self‐administration in man. J Steroid Biochem. 1985;23(1):33‐38. doi: 10.1016/0022-4731(85)90257-2 [DOI] [PubMed] [Google Scholar]
- 25. Andersson AM, Jensen TK, Juul A, Petersen JH, Jorgensen T, Skakkebaek NE. Secular decline in male testosterone and sex hormone binding globulin serum levels in Danish population surveys. J Clin Endocrinol Metab. 2007;92(12):4696‐4705. [DOI] [PubMed] [Google Scholar]
- 26. Wu FC, Tajar A, Pye SR, et al. Hypothalamic‐pituitary‐testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European male aging study. J Clin Endocrinol Metab. 2008;93(7):2737‐2745. [DOI] [PubMed] [Google Scholar]
- 27. Travison TG, Araujo AB, O'Donnell AB, Kupelian V, McKinlay JB. A population‐level decline in serum testosterone levels in American men. J Clin Endocrinol Metab. 2007;92(1):196‐202. [DOI] [PubMed] [Google Scholar]
- 28. Damgaard‐Olesen A, Johannsen TH, Holmboe SA, et al. Reference ranges of 17‐hydroxyprogesterone, DHEA, DHEAS, androstenedione, total and free testosterone determined by TurboFlow‐LC‐MS/MS and associations to health markers in 304 men. Clin Chim Acta. 2016;454:82‐88. [DOI] [PubMed] [Google Scholar]
- 29. Lasley BL, Crawford S, McConnell DS. Adrenal androgens and the menopausal transition. Obstet Gynecol Clin N Am. 2011;38(3):467‐475. doi: 10.1016/j.ogc.2011.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vesper HW, Botelho JC. Standardization of testosterone measurements in humans. J Steroid Biochem Mol Biol. 2010;121(3–5):513‐519. doi: 10.1016/j.jsbmb.2010.03.032 [DOI] [PubMed] [Google Scholar]
- 31. Demers LM, Hankinson SE, Haymond S, et al. Measuring estrogen exposure and metabolism: workshop recommendations on clinical issues. J Clin Endocrinol Metab. 2015;100(6):2165‐2170. doi: 10.1210/jc.2015-1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stanczyk FZ, Lee JS, Santen RJ. Standardization of steroid hormone assays: why, how, and when? Cancer Epidemiol Biomark Prev. 2007;16(9):1713‐1719. doi: 10.1158/1055-9965.epi-06-0765 [DOI] [PubMed] [Google Scholar]
- 33. Chen Y, Zeleniuch‐Jacquotte A, Arslan AA, et al. Endogenous hormones and coronary heart disease in postmenopausal women. Atherosclerosis. 2011;216(2):414‐419. doi: 10.1016/j.atherosclerosis.2011.01.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jaspers L, Dhana K, Muka T, et al. Sex steroids, sex hormone‐binding globulin and cardiovascular health in men and postmenopausal women: the Rotterdam study. J Clin Endocrinol Metab. 2016;101(7):2844‐2852. doi: 10.1210/jc.2016-1435 [DOI] [PubMed] [Google Scholar]
- 35. Ouyang P, Vaidya D, Dobs A, et al. Sex hormone levels and subclinical atherosclerosis in postmenopausal women: the multi‐ethnic study of atherosclerosis. Atherosclerosis. 2009;204(1):255‐261. doi: 10.1016/j.atherosclerosis.2008.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Khatibi A, Agardh CD, Shakir YA, et al. Could androgens protect middle‐aged women from cardiovascular events? A population‐based study of Swedish women: the Women's health in the Lund area (WHILA) study. Climacteric. 2007;10(5):386‐392. doi: 10.1080/13697130701377265 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


