Abstract
In the present study we observed that the Haemophilus influenzae type b (Hib) porin, among the different surface bacterial components, is involved in the pathophysiology of bacterial meningitis. This study demonstrates that inoculation of Hib porin into the fourth cerebral ventricle causes the simultaneous expression of interleukin-1α (IL-1α), tumor necrosis factor alpha (TNF-α), and macrophage inflammatory protein 2 (MIP-2) at 6 h after inoculation. At 24 h, the expression of MIP-2 decreases while the expression of IL-1α and TNF-α increases. The mRNA expression of IL-1α, TNF-α, and MIP-2 is correlated with injury to the blood-brain barrier as demonstrated by the appearance of serum proteins and leukocytes in cerebrospinal fluid and by the increase in brain water content.
Most diseases caused by Haemophilus influenzae type b (Hib) occur in young children (under the age of 5 years). This microorganism is a common cause of meningitis. The advent of vaccination programs has almost eradicated the disease from wealthy countries, but the infection is still present in less developed areas (1, 27, 30).
An understanding of the pathophysiology of bacterially induced brain injury is essential in identifying mediators which may be important in this process. It is generally accepted that the inflammatory response to gram-negative bacteria infection is induced by endotoxin. Hib lipopolysaccharide (LPS) plays a fundamental role in rats meningeal inflammation (39, 47). Although LPS has been clearly documented to play an important role in the pathogenesis of gram-negative infections (53), very little is known about the functions of other bacterial components. In fact, there is significant evidence that other components of gram-negative bacteria also play an important role in the pathology associated with these infections (12, 25, 46).
Hib porin (also known as P2 or protein BLC) is the most dominant band in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of outer membrane protein preparations (31, 35, 37); its molecular mass varies between 36 and 42 kDa (37). This protein exists as a trimer and functions as a porin (8). Hib porin has also been reported to be an important immunoprotection target (23).
Tumor necrosis factor alpha (TNF-α) and interleukin-1 (IL-1) play important roles in the host response to bacteria and their products. Astrocytes and microglial cells produce IL-1 and TNF-α within the central nervous system (CNS) (13, 15). The evolution of the inflammatory process in the CNS depends on the set of cytokines released during the first steps of the infection. Noxious stimuli that act directly on neuronal soma are highly effective in generating chemokine expression. IL-1 is a polypeptide that acts as a soluble mediator in immunological and inflammatory reactions. Woolpe et al. (52) characterized a novel cytokine termed macrophage inflammatory protein 2 (MIP-2) that exhibits a strong affinity for heparin. MIP-2 is secreted by endotoxin-stimulated macrophages. Sequence analysis indicates that MIP-2 is a member of the platelet factor 4 family. This mediator is an extremely potent chemotactic agent for human neutrophils but induces little chemokinetic activity in vitro (10, 48).
Within the first 24 h after a mechanical trauma to the CNS, reactive astrogliosis develops and injury sites are infiltrated by mononuclear phagocytes derived from blood-borne monocytes and endogenous microglia (2, 3). The chemokine expression in posttraumatic inflammation is generally restricted to the monocyte chemoattractant MCP-1 (44) and occurs before hematogenous cells penetrate neuronal tissues.
It is still unclear how leukocytes migrate through the blood-brain barrier. Two types of mechanisms are involved during the migration of blood cells across the tight endothelial cell barrier of the brain vessels: (i) cellular adhesion molecules of endothelial cells and their counterreceptors on leukocytes induce the attachment of circulating blood cells to the vessel wall, and (ii) chemokines activate and attract specific leukocyte subpopulations, leading to translocation and accumulation of these cells in the inflamed tissue. Among the other components of the surface of gram-negative organisms, the porins activate the adhesion molecules (11), induce leukocyte transmigration through human endothelial cells in vitro (22), and stimulate cytokine liberation (17, 20, 21, 26).
Furthermore, to establish the exact pathogenic mechanisms by which Hib contributes to signaling of the inflammatory cascade, it is important to characterize the pattern of cytokine release after stimulation with Hib cellular components.
In this work we investigate the potential role of Hib porin in the pathophysiology of bacterial brain inflammation.
MATERIALS AND METHODS
Bacteria and growth conditions.
Hib was obtained from the American Type Culture Collection (ATCC 9795) and grown in CY medium (9) for 18 to 24 h at 37°C; the cells were harvested at the end of the exponential growth phase.
Animals.
Male Sprague-Dawley rats (250 to 300 g each) with free access to food and water were housed at constant temperature (21 ± 1°C) and relative humidity (60%). The animals were maintained under a regular 12-h light/12-h dark schedule (light from 7.00 a.m. to 7.00 p.m.).
Surgical preparation and treatment.
The rats were placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, Calif.) under ketamine anesthesia (100 mg/kg intraperitoneally), and a craniotomy was performed by following the coordinates of the atlas of Paxinos and Watson (42) (measured in millimeters from the bregma; AP.−11.60; L. 2.3; V. 7.5) to permit microinjection into the fourth ventricle (4 V). The intracerebroventricular microinjections (time zero) were conducted with a Hamilton 10-μl syringe attached to the stereotaxic micromanipulator and inserted into the cerebral tissue. A volume of 7 μl was used either for control injections (0.2 M phosphate-buffered saline) (PBS) (pH 6.5) as well as for injection of drugs, over a period of 10 s. After this procedure the rats were allowed to recover until the brain was removed for in vitro assays.
After the completion of the experiment, at each time point considered the rats were killed with a high dose of pentobarbital (200 mg/kg intravenously) and the brains were removed. They were immersed into D solution (4 M guanidine thiocyanate, 25 mM sodium citrate [pH 7.0], 0.5% sarcosyl, 0.1 M β-mercaptoethanol) for RNA extraction.
A separate series of experiments were performed to verify the injection site. The rats subjected to the above surgical procedure were injected intracerebroventricularly 10 μl of 5 mM (0.2%) methylene blue solution before being sacrificed with a high dose of pentobarbital (200 mg/kg intravenously). The animals were immediately perfused intracardially with 50 ml of PBS followed by 50 ml of a 10% formalin solution in PBS. The brains were removed and immersed in saturated formalin for 24 h. The injection site was verified using two consecutive sections (40 μm), one stained with cresyl violet to identify the nuclei and the other unstained to determine the diffusion of the dye.
Preparation of the porin.
The porin was isolated and purified from cells of Hib ATCC 9795 using the modified method of Nurminen (41). Briefly, the bacterial envelopes were treated with Triton X-100 buffer for 2 h at 37°C with rotary shaking, dissolved in SDS buffer (4% [wt/vol] SDS, 0.1 M sodium phosphate [pH 7.2]), and applied to an Ultragel ACA34 column equilibrated with 0.25% SDS-sodium azide buffer. Elution flow through the column was 8 ml h−1, and 2 ml was collected. The fraction containing proteins, identified by measuring the absorption at 280 nm, was extensively dialyzed and checked by SDS-PAGE by the method of Laemmli (28). The protein content of the porin preparation was determined by the method of Lowry et al. (32). All possible traces of LPS were revealed on SDS-PAGE gels stained with silver nitrate as described by Tsai and Frasch (50) and by the Limulus amoebocyte lysate assay as described by Thye Yin et al. (49).
The pore-forming ability of our preparation was checked by a functional assay (liposome-swelling assay) after incorporation into proteoliposomes by the method of Nikaido and Rosenberg (40).
Preparation of Hib LPS.
Hib LPS was extracted by the phenol-chloroform-petroleum ether method of Galanos et al. (16).
N-terminal sequencing of Hib porin.
The Applied Biosystems model 477A automatic protein sequencer has been used to determine the N-terminal sequence of the Hib porin. The phenylthiohydantoin (PTH)-aa released during the degradation have been identified by high-performance liquid chromatography on a model 120A high-performance liquid chromatograph (Applied Biosystem) with a Brownlee C18 column. Separation was performed with an eluent gradient as suggested by the manufacturer. The column was set at 54°C, and the elution was monitored by measuring the absorbance at 269 nm. Data were elaborated by a computer Macintosh Ilsi.
Inocula.
Highly purified porin (5 μg in 7 μl) from Hib outer membranes were used. In some assays the LPS activity in the porins was neutralized by adding polymyxin B (PB) at room temperature for 1 h (4, 34). The porin-PB mixture was used in pyrogen-free PBS. The controls were inoculated with bovine serum albumin (5 μg in 7 μl) or PBS (7 μl) or porin-PB mixture (5 μg in 7 μl plus 5 ng) or PB (1 μg in 7 μl) or LPS (1 μg in 7 μl) or LPS-PB mixture (1 μg in 7 μl plus 1 μg).
Determination of the water and protein contents in the brain.
At 6 and 24 h after postintracerebroventricular inoculation, the rats were killed by an overdose of pentobarbital given intravenously. Immediately after death, a craniotomy was performed; the brain without the cerebellum and medulla was removed, weighed in aluminium boats, and dried in a stove for 16 h at 130°C to a stable weight. The brain water content, expressed as grams of water per 100 g of dry weight, was determined by using the formula [(wet weight − dry weight)/wet weight] × 100 and used as an estimation of brain edema. The cerebrospinal fluid (CSF) samples were centrifuged at 10,000 × g for 5 min and the supernatant fluid was assayed to establish the protein content by the Bradford method (5).
CSF pleocytosis and histologic assays.
The CSF was collected from rats by intra-4 V puncture at 6 and 24 h after inoculation. Leukocyte concentrations were measured with a hemocytometer and by microscopic evaluation after May-Grunwald coloration. Histologic assays were performed on transverse sections.
RNA isolation and cDNA preparation.
The brain samples were collected 6 and 24 h after stimulation, and total RNA was extracted by the method of Chomczynski and Sacchi (7). The RNA pellet was resuspended in 75% (vol/vol) ethanol, sedimented, vacuum dried, and dissolved in 15 μl of RNase-free water. 1 μg of oligo(dT) (Promega, Madison, Wis.) was added to the suspension, and the mixture was heated at 65°C for 5 min. After cooling on ice, the mixture was incubated for 2 h at 42°C with 14 μl of the following solution: 20 mM dithiothreitol (Sigma), 1 mM (each) dATP, dGTP, dCTP, and dTTP, 35 U of RNasin (Promega), and 525 U of Moloney murine leukemia virus reverse transcriptase (Promega) in reverse transcription buffer.
PCR procedure.
Murine cytokine primer pairs sequences were designed on the basis of published gene sequences as reported in Table 1. The primer sequences were complementary to sequences in the exons or spanned exon-exon junctions and thus were RNA specific. A 2-μl volume of cDNA prepared as described above was amplified in the presence of 500 nM (final concentration) 5′ and 3′ primers, 200 μM (each) dATP, dGTP, dCTP, dTTP, and 1.25 U of Taq DNA polymerase (Promega) in a final volume of 50 μl of 10× Taq DNA polymerase buffer (Promega). The PCR was performed in a Perkin-Elmer thermal cycler for 30 cycles of 1 min of denaturation at 94°C, 2 min of annealing at 60°C, and 3 min of extension at 72°C. The reaction product was visualized by electrophoresis using 25 μl of the reaction mixture at 100 V in a 1.5% agarose gel containing ethidium bromide (1 μg/ml). The gels were then examined on an UV light box and photographed. BglI- and HinfI-digested pBR328 DNA (Boehringer Mannheim) (1 μg) was run in parallel as a molecular size marker (providing bands at 2,176, 1,766, 1,230, 1,033, 653, 517, 453, 394, 298, 234, 220, and 154 bp).
TABLE 1.
Primer sequences used for PCR-assisted mRNA amplification
| Target primer | Oligonucleotide sequence |
|---|---|
| IL-1α | 5′AAGATGTCCAACTTCACCTTCAAGGAGAGCCG3′ |
| 5′AGGTCGGTCTCACTACCTGTGATGAGTTTTGG3′ | |
| TNF-α | 5′TTCTGTCTACTGAACTTCGGGGTGATCGGTCC3′ |
| 5′GTATGAGATAGCAAATCGGCTGACGGTGTGTGGG3′ | |
| IL-6 | 5′ATGAAGTTCCTCTCTGCAAGAGACT3′ |
| 5′CACTAGGTTTGCCGAGTAGATCTC3′ | |
| IFN-γ | 5′TGCATCTTGGCTTTGCAGCTCTTCCTCATGGC3′ |
| 5′TGGACCTGTGGGTTGTTGACCTCAAACTTGGC3′ | |
| IL-4 | 5′ATGGGTCTCAACCCCCAGCTAGT3′ |
| 5′GCTCTTTAGGCTTTCCAGGAAGTC3′ | |
| IL-10 | 5′CTGGGAAGACCAAGGTGTCTAC3′ |
| 5′GAGCTGCTGCAGGAATGATGA3′ | |
| MIP-2 | 5′CTGCGCTGTCAATGCCTG3′ |
| 5′AGCCTTGCCTTTGTTCAGTA3′ | |
| β-actin | 5′GTGGGCCGCTCTAGGCACCAA3′ |
| 5′CTCTTTGATGTCACGCACGATTTC3′ |
Statistics.
All experiments were carried out in triplicate; the results are expressed as the mean ± standard error. Comparisons between tests were done by Student's t test, with statistical significance considered to be indicated by P < 0.05.
RESULTS
Purity of Hib porin preparations.
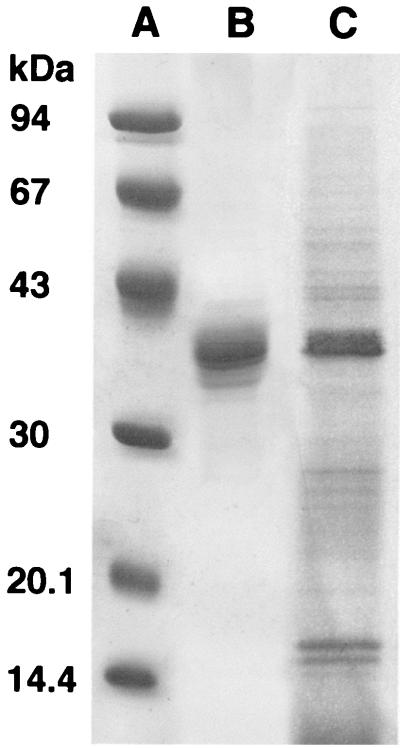
The purity of the porin preparation from Hib, as checked by SDS-PAGE, is shown in Fig. 1. SDS-PAGE revealed one band with a molecular mass of approximately 40 kDa, as previously reported by Coulton et al. (8). In the same gel, we found a Hib outer membrane preparation (Fig. 1, lane C), demonstrating that the 40-kDa band is the most dominant in the bacterial strain used. This protein showed porin activity as demonstrated by its ability to form transmembrane aqueous channels (data not shown). Moreover, the N-terminal sequence (Ala-Val-Val-Tyr-Asn-Asn-Glu-Gly-Thr-Asn-Val) of the isolated protein corresponds to the known sequence for the protein as reported in literature (24, 36). The purification and contamination by LPS in the preparations obtained have been amply addressed in previous works (18, 20). Using the Limulus test, the LPS contamination in the porin preparation was estimated to be about 0.001% (wt/wt) compared with a standard Hib LPS solution.
FIG. 1.
SDS-PAGE analysis of the outer membrane protein preparation and porin extract from Hib ATCC 9795. The gel was stained with Coomassie blue. Lanes: A, molecular mass standards (phosphorylase b, 94 kDa; albumin, 67 kDa; ovalbumin, 43 kDa; carbonic anydrase, 30 kDa; trypsin inhibitor, 20 kDa; α-lactalbumin, 14 kDa); B, Hib porin (10 μg); C, Hib OMP preparation (10 μg).
Water and protein contents in the brain.
Intracerebral inoculation of rats with Hib porin was performed with a dose of 5 μg, which is able to achieve a porin concentration in CSF approximately within the range of those that elicited a biological effect in vitro (19, 21, 22). The water content in the brain was significantly elevated in rats inoculated intracerebrally with porin compared to rats inoculated with PBS, BSA, LPS-PB, or PB alone. The results of the edema evaluation are reported in Table 2. The porin caused also an increase in protein concentration in CSF; 24 h after inoculation, the protein content was notably increased compared to the controls (Table 2).
TABLE 2.
Ability of Hib porin to provoke meningeal inflammation after 24 h of treatmenta
| Inoculant | Dose | Leukocyte countb | Protein contentc | Brain edemad |
|---|---|---|---|---|
| PBS | 7 μl | 40 ± 5 | 2.5 ± 0.5 | 75 ± 1.2 |
| Hib LPS | 1 μg | 350 ± 120* | 101 ± 7* | 77 ± 1.4** |
| Hib LPS plus PB | 1 μg + 1 μg | 36 ± 5 | 3.0 ± 0.5 | 75 ± 1.2 |
| Hib porin | 5 μg | 378 ± 130* | 98.0 ± 7.0* | 78 ± 1.5** |
| Hib porin plus PB | 5 μg + 5 ng | 395 ± 140* | 94.0 ± 7.0* | 78 ± 1.6** |
| PB | 1 μg | 45 ± 5 | 2.8 ± 0.5 | 75 ± 1.2 |
| BSA | 5 μg | 50 ± 5 | 2.7 ± 0.5 | 75 ± 1.1 |
All results are the means ± standard errors of three separate experiments with five animals each. ∗, P < 0.001; ∗∗, P < 0.5.
Leukocyte counts are expressed as cells per microliter of CSF.
Protein content is expressed as milligrams of protein per 100 ml of CSF.
Brain edema is expressed as grams of H2O/100 g of brain dry weight at 24 h.
CSF pleocytosis and histologic examinations.
Intra-4 V inoculation of Hib porin elicited a discrete pleocytosis compared to controls. Inoculation of 5 μg of Hib porin at 24 h increased the leukocyte concentration in CSF (mean, 378 ± 130/μl) (P < 0.001) much more than that found when using PBS or other controls (mean, 40 ± 5/μl). Also, Hib LPS (1 μg) at 24 h increased the leukocyte concentration in CSF (mean, 350 ± 120/μl) (P < 0.001). No significant differences were observed with respect to controls after 6 h. The results are reported in Table 2. Histologic assays of the transverse brain sections obtained 24 h after the treatment with porin showed a small infiltration of polymorphonuclear cells that was higher than that in controls (data not shown).
Cytokine mRNA expression in the brain.
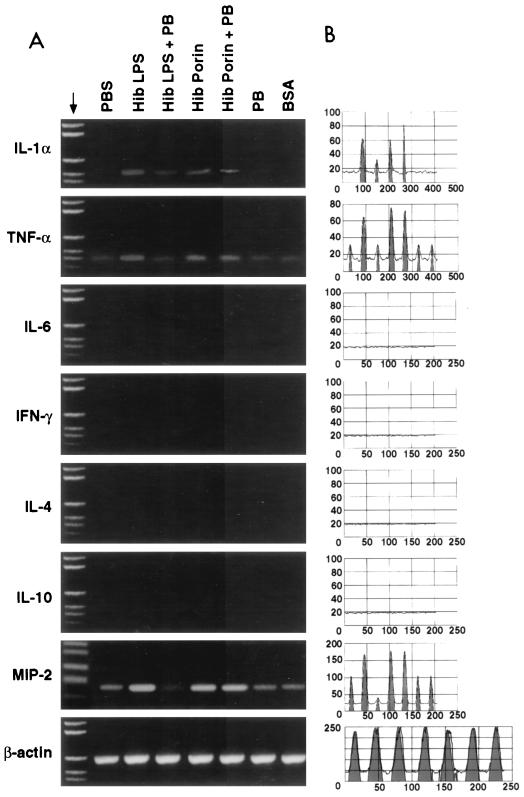
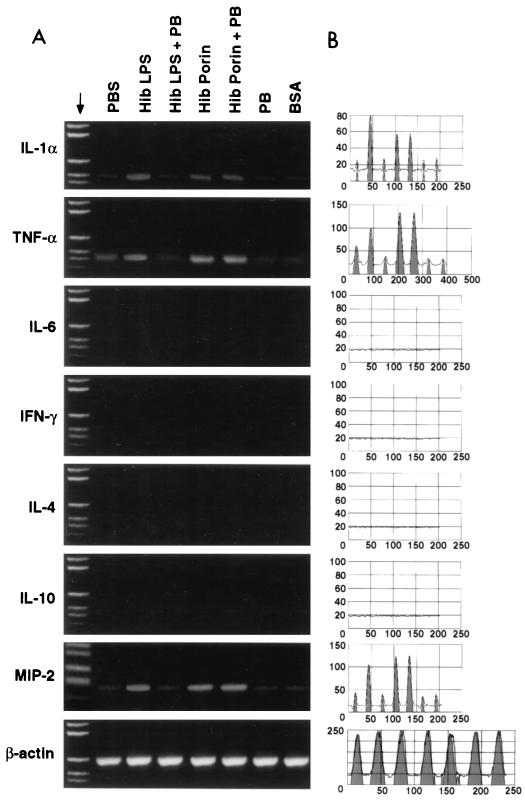
An analysis of cytokine mRNA in brain tissues from rats intracerebroventricularly inoculated with Hib porin and different controls was performed using reverse transcription-PCR (RT-PCR) and focused on tissues obtained from rats at 6 and 24 h after treatment. At our limits of detection, mRNAs for gamma interferon (IFN-γ), IL-6, IL-4, and IL-10 were not expressed in brain tissues from untreated rats. TNF-α and MIP-2 mRNA bands were weak in controls inoculated intracerebroventricularly with PBS, PB, LPS-PB, or BSA at 6 and 24 h, whereas IL-1α mRNA was detected in controls only after 24 h. The patterns of cytokine mRNA expression at 6 h postinoculation in brains from rats inoculated intra-4 V with Hib porin or LPS as a positive control are shown in Fig. 2A, while the equivalent results 24 h postinfection are shown in Fig. 3A. The results obtained were confirmed by quantitation of mRNA using Sigma Gel software (Fig. 2B and Fig. 3B); the percentages of integrated peak areas are reported in Table 3. As detected by RT-PCR analysis, cytokine mRNA bands were present for IL-1α, TNF-α, and MIP-2 at 6 and 24 h after treatment. No effect was detected for IFN-γ, IL-6, IL-4, and IL-10 mRNA under the same experimental conditions. TNF-α and IL-1α mRNA were found only after 6 h, and their levels increased after 24 h; in contrast, MIP-2 mRNA was found at 6 h and its level decreased at 24 h. The MIP-2 band intensity at 6 h after treatment with porin was higher than the TNF-α and IL-1α band intensities obtained at 24 h. As a control, we measured the levels of β-actin mRNA, which is a cell cycle-independent mRNA. The actin mRNA levels remained unchanged, indicating that the changes in cytokine mRNA levels were not caused by a general increase in all poly(A)+ RNA species. Similar results were obtained in independent experiments with brains from different rats.
FIG. 2.
A representative experiment (IL-1α, TNF-α, IL-6, IFN-γ, IL-4, IL-10, and MIP-2) showing mRNA expression in rat brain 6 h after Hib porin inoculation. (A) The brain samples were collected after stimulation with PBS (7 μl), LPS Hib (1 μg), LPS Hib plus PB (1 μg + 1 μg), Hib porin (5 μg), Hib porin plus PB (5 μg + 5 ng), PB (1 μg), or BSA (5 μg) and then subjected to RNA extraction and RT-PCR amplification. Reaction products were run on a 1.5% agarose gel in the presence of appropriate molecular mass markers; β-actin was the positive transcription control. (B) RT-PCR quantitation of the cytokine results by Sigma Gel software.
FIG. 3.
A representative experiment (IL-1α, TNF-α, IL-6, IFN-γ, IL-4, IL-10, and MIP-2) showing mRNA expression in rat brain 24 h after Hib porin inoculation. (A) The brain samples were collected after stimulation with PBS (7 μl), LPS Hib (1 μg), LPS Hib plus PB (1 μg + 1 μg), Hib porin (5 μg), Hib porin plus PB (5 μg + 5 ng), PB (1 μg), or BSA (5 μg) and then subjected to RNA extraction and RT-PCR amplification. Reaction products were run on a 1.5% agarose gel in the presence of appropriate molecular mass markers; β-actin was the positive transcription control. (B) RT-PCR quantitation of cytokine results by Sigma Gel software.
TABLE 3.
Quantitation (by Sigma Gel software) of cytokine mRNA signals at 6 and 24 h after treatment
| Inoculant | % Integrated areas of the peaksa for:
|
|||||
|---|---|---|---|---|---|---|
| IL-1α
|
TNF-α
|
MIP-2
|
||||
| 6 h | 24 h | 6 h | 24 h | 6 h | 24 h | |
| PBS | 0 | 4 | 14 | 19 | 22 | 11 |
| Hib LPS | 32 | 47 | 52 | 79 | 91 | 42 |
| Hib LPS plus PB | 8 | 5 | 14 | 13 | 12 | 10 |
| Hib porin | 18 | 33 | 63 | 74 | 97 | 63 |
| Hib porin plus PB | 19 | 29 | 57 | 73 | 84 | 62 |
| PB | 0 | 4 | 13 | 13 | 22 | 8 |
| BSA | 0 | 4 | 14 | 12 | 22 | 10 |
The results are the means of three separate experiments with five animals each.
DISCUSSION
Our results show that the Hib porin induces the early release of cytokines by CNS cells, amplifying the inflammatory response. The Hib porin, inoculated at 5 μg into the fourth ventricle of the brain, elicited the appearance of serum proteins in CSF and the development of brain edema, as demonstrated by an increase in the water content in the brain. These modifications were followed by a small increase in the number of neutrophils both in CSF and in the tissue sections around the inoculation site. IL-1α, TNF-α, and MIP-2 mRNA appeared quickly in the tissue near the inoculation site. No information concerning the possible role of porins in inducing the symptoms of bacterial meningitis has been published to date. It can be ruled out that the effects shown by Hib porin are attributable to contaminating traces of LPS, because the same results were obtained using Hib porin plus PB whereas LPS plus PB did not show any activity. The concentration of porins used contains a biologically inactive percentage of LPS (0.001%, wt/wt), which is unable to elicit the results obtained under our experimental conditions. An active concentration of both LPS and porin can frequently be reached at infection sites from outer membrane blebbing or bacterial lysis of gram-negative bacteria as a consequence of host defenses (53). Considering that the level of porins is about 105 molecules/cell and that of LPS is about 3.4 × 106 molecules/cell (6), 108 bacterial cells are enough to reach concentrations of about 5 μg of porins per ml (about 0.2 μM) and 1 μg of LPS per ml (about 0.5 μM).
Furthermore, the effects observed were not caused by the mechanical trauma following the intracerebral inoculation of extraneous substances; indeed, PBS, PBS plus PB, or PBS plus BSA did not show the same intensity of the signal from IL-1α and TNF-α mRNA. Moreover, the alterations in the parameters of the blood-brain barrier are significantly different in the rats treated with the Hib porin compared to controls. Within the first 24 h after mechanical trauma to the CNS, it was possible to observe increased levels of MCP-1 mRNA; astrocytes are the cellular source of MCP-1 mRNA at early times after mechanical brain injury (44). This is in accordance with previous studies showing that TNF-α and IL-1 have been detected in the CSF of patients with bacterial meningitis caused by gram-positive and gram-negative microorganisms (29, 33) and in the CSF of animals experimentally inoculated with Hib (38), Hib LPS (B. Wispelwey, W. J. Long, J. M. Castracane, and W. M. Scheld, Program Abstr. 28th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 873, 1988), Neisseria meningitidis (51) and pneumococcal cell wall fragment (I. Riesenfeld-Orn, J. Garcìa-Bustos, M. Hoffman, and E. Tuomanen, Program Abstr. 28th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 876, 1988). TNF-α and IL-1, produced from astrocytes and microglial cells in the early stages of the inflammatory process, play an important role in the host response to bacteria and their products (43). Indeed, monoclonal antibody to TNF-α reduced inflammation and was also protective against brain edema in a rabbit model of pneumococcal meningitis (45). Furthermore, injection of meningococcal LPS into the subarachnoid space of rabbits induced the subsequent appearance of TNF-α, IL-1, and IL-6 followed by the migration of leukocytes into the CSF compartment (51). Therefore, our results show that the Hib porin also initially induces the synthesis of TNF-α and IL-1α mRNA followed by brain edema and a slight neutrophil infiltration. In our experiments, we did not observe the expression of IL-6 mRNA by astrocytes and microglial cells. Its expression probably occurs later than that of other cytokines; in fact it was demonstrated that IL-6 mRNA appears in CNS viral infection only after 48 h (14).
Hib porin, even at an early stage, induces the appearance of leukocytes in CSF. For this reason, we looked for the presence of any chemotactic cytokines. MIP-2 is a chemoattractant (10) and an activating factor for neutrophils (48). MIP-2 is made by cytokine- or LPS-activated monocytes and also by endothelial cells. The release of MIP-2 into the tissue of the CNS may be caused by both in situ and infiltrated cells.
We could not observe any expression of IFN-γ, IL-4, and IL-10 mRNA in the CNS tissue at the times of incubation we used, probably because the lymphocyte infiltration happens later.
In conclusion, the interplay between bacterial components and host inflammatory cells is likely to be a major determinant of the experimental presentation and outcome of bacterial brain edema. The further study of regulation and coordination of chemokine release and production by bacterial components in brain tissue may lead to novel therapeutic approaches to bacterially induced brain inflammation.
REFERENCES
- 1.Adegbola R A, Usen S O, Weber M, Lloyd-Evans N, Jobe K, Mulholland K, McAdam K P W J, Greenwood B M, Milligan P J M. Haemophilus influenzae type b meningitis in the Gambia after introduction of a conjugate vaccine. Lancet. 1999;354:1091–1092. doi: 10.1016/s0140-6736(99)03010-x. [DOI] [PubMed] [Google Scholar]
- 2.Balasingam V, Dickson K, Brade A, Yong V W. Astrocyte reactivity in neonatal mice: apparent dependence on the presence of reactive microglia/macrophages. Glia. 1996;18:11–26. doi: 10.1002/(SICI)1098-1136(199609)18:1<11::AID-GLIA2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Balasingam V, Tejada-Berges T, Wright E, Bouckova R, Yong V W. Reactive astrogliosis in the neonatal mouse brain and its modulation by cytokines. J Neurosci. 1994;14:846–856. doi: 10.1523/JNEUROSCI.14-02-00846.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanchard D K, Djeun J Y, Klein T W, Friedman H, Stewart W E. Interferon-gamma induction by lipopolysaccharide: dependence on interleukin 2 and macrophages. J Immunol. 1986;136:963–970. [PubMed] [Google Scholar]
- 5.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Brass J M. The cell envelope of Gram-negative bacteria: new aspect of its function in transport and chemotaxis. Curr Top Microbiol Immunol. 1986;29:1–8. doi: 10.1007/978-3-642-71399-6_1. [DOI] [PubMed] [Google Scholar]
- 7.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenoi-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 8.Coulton J W, Chin A C, Vachon V. Recombinant porin of Haemophilus influenzae type b. J Infect Dis. 1992;165(Suppl. 1):S188–S191. doi: 10.1093/infdis/165-supplement_1-s188. [DOI] [PubMed] [Google Scholar]
- 9.Coulton J W, Wan I F. The outer membrane of Haemophilus influenzae type b: cell envelope associations of major proteins. Can J Microbiol. 1983;29:280–287. doi: 10.1139/m83-046. [DOI] [PubMed] [Google Scholar]
- 10.Diab A, Abdalla H, Li H L, Shi F D, Zhu J, Hojberg B, Lindquist L, Wretlind B, Bakhiet M, Link H. Neutralization of macrophage inflammatory protein 2 (MIP-2) and MIP-1 α attenuate neutrophils recruitment in the central nervous system during experimental bacterial meningitis. Infect Immun. 1999;67:2590–2601. doi: 10.1128/iai.67.5.2590-2601.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donnarumma G, Brancaccio F, Cipollaro de l'Ero G, Folgore A, Marcatili A, Galdiero M. Release of GM-CSF, sELAM-1 and sICAM-1 by human vascular endothelium stimulated with Gram-negative and Gram-positive components. Endothelium. 1996;4:11–22. [Google Scholar]
- 12.Evans T J, Strivens E, Carpenter A, Cohen J. Differences in cytokine response and induction of nitric oxide synthase in endotoxin-resistant and endotoxin-sensitive mice after intravenous Gram-negative infection. J Immunol. 1993;150:5033–5040. [PubMed] [Google Scholar]
- 13.Fontana A, Hengartner H, de Tribolet N, Weber E. Glioblastoma cells release interleukin-1 and factors inhibiting interleukin-2 mediated effects. J Immunol. 1984;132:1837–1844. [PubMed] [Google Scholar]
- 14.Frei K, Malipiero U V, Leist T P, Zinkernagel R M, Schwab M E, Fontana A. On the cellular source and function of interleukin 6 produced in the central nervous system in viral diseases. Eur J Immunol. 1989;19:689–694. doi: 10.1002/eji.1830190418. [DOI] [PubMed] [Google Scholar]
- 15.Frei K, Siepl C, Groscurth P, Bodmer S, Schwerdel C, Fontana A. Antigen presentation and tumor cytotoxicity by interferon-γ-treated microglial cells. Eur J Immunol. 1987;17:1271–1278. doi: 10.1002/eji.1830170909. [DOI] [PubMed] [Google Scholar]
- 16.Galanos C, Luderitz O, Westphal O. A new method for the extraction of R lipopolysaccharide. Eur J Biochem. 1969;9:245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 17.Galdiero F, Cipollaro de l'Ero G, Benedetto N, Galdiero M, Tufano M A. Release of cytokines induced by Salmonella typhimurium porins. Infect Immun. 1993;61:155–159. doi: 10.1128/iai.61.1.155-161.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galdiero F, Tufano M A, Galdiero M, Masiello S, Di Rosa M. Inflammatory effect of Salmonella typhimurium porins. Infect Immun. 1990;58:3183–3188. doi: 10.1128/iai.58.10.3183-3186.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galdiero F, Tufano M A, Sommese L, Folgore A, Tedesco F. Activation of the complement system by porins extracted from Salmonella typhimurium. Infect Immun. 1984;46:559–563. doi: 10.1128/iai.46.2.559-563.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galdiero M, Cipollaro de l'Ero G, Donnarumma G, Marcatili A, Galdiero F. Interleukin-1 and interleukin-6 gene expression in human monocytes stimulated with Salmonella typhimurium porins. Immunology. 1995;86:612–619. [PMC free article] [PubMed] [Google Scholar]
- 21.Galdiero M, De Martino L, Marcatili A, Nuzzo I, Vitiello M, Cipollaro de l'Ero G. Th1 and Th2 cell involvement in immune response to Salmonella typhimurium porins. Immunology. 1998;94:5–13. doi: 10.1046/j.1365-2567.1998.00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galdiero M, Folgore A, Molitierno M, Greco R. Porins and lipopolysaccharide (LPS) from Salmonella typhimurium induce leukocyte transmigration through human endothelial cells in vitro. Clin Exp Immunol. 1999;116:453–461. doi: 10.1046/j.1365-2249.1999.00904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groeneveld K, van Alphen L, Voorter C, Eijk P P, Jansen H M, Zanen H C. Antigenic drift of Haemophilus influenzae in patients with chronic obstructive pulmonary disease. Infect Immun. 1989;57:3038–3044. doi: 10.1128/iai.57.10.3038-3044.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen E J, Hasemann C, Clausell A, Capra J D, Orth K, Moomaw C R, Slaughter C A, Latimer J L, Miller E E. Primary structure of the porin of Haemophilus influenzae type b determined by nucleotide sequence analysis. Infect Immun. 1989;57:1100–1107. doi: 10.1128/iai.57.4.1100-1107.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hopkins W J, Gendron-Fitzpatrick A, McCarthy D O, Haine J E, Uehling D T. LPS-responder and nonresponder C3H mouse strains are equally susceptible to an induced Escherichia coli urinary tract infection. Infect Immun. 1996;64:1369–1374. doi: 10.1128/iai.64.4.1369-1372.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iovane G, Pagnini P, Galdiero M, Cipollaro de l'Ero G, Vitiello M, D'Isanto M, Marcatili A. Role of Pasteurella multocida porin on cytokine expression and release by murine splenocytes. Vet Immunol Immunopathol. 1998;66:391–404. doi: 10.1016/s0165-2427(98)00183-4. [DOI] [PubMed] [Google Scholar]
- 27.Kamiya H, Uehara S, Kato T, Shiraki K, Togashi T, Morishima T, Satoh O, Standaert S M. Childhood bacterial meningitis in Japan. Pediatr Infect Dis J. 1998;17:S183–S185. doi: 10.1097/00006454-199809001-00019. [DOI] [PubMed] [Google Scholar]
- 28.Laemmli U K. Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Leist T P, Frei K, Kam-Hansen S, Zinkernagel R M, Fontana A. Tumor necrosis factor-α in cerebrospinal fluid during bacterial, but not viral, meningitis. Evaluation in murine model infections and patients. J Exp Med. 1988;167:1743–1748. doi: 10.1084/jem.167.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levine O S, Schwartz B. The rationale for population-based surveillance for Haemophilus influenzae type b meningitis. Pediatr Infect Dis J. 1998;17:S195–S198. doi: 10.1097/00006454-199809001-00024. [DOI] [PubMed] [Google Scholar]
- 31.Loeb M R, Smith D H. Outer membrane protein composition in disease isolates of Haemophilus influenzae: pathogenic and epidemiological implications. Infect Immun. 1980;30:709–717. doi: 10.1128/iai.30.3.709-717.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with Folin phenol reagent. J Biol Chem. 1985;193:265–275. [PubMed] [Google Scholar]
- 33.McCracken G H, Jr, Mustafa M M, Ramilo O, Olsen K D, Risser R C. Cerebrospinal fluid interleukin-1β and tumor necrosis factor concentrations and outcome from neonatal gram-negative enteric bacillary meningitis. Pediatr Infect Dis J. 1989;8:155–159. [PubMed] [Google Scholar]
- 34.Morrison D C, Jacobs D M. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 1976;13:813–818. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- 35.Munson R S, Jr, Shenep J L, Barenkamp S J. Purification and comparison of outer membrane protein P2 from Haemophilus influenzae type b isolates. J Clin Investig. 1983;72:677–684. doi: 10.1172/JCI111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munson R S, Jr, Tolan R W., Jr Molecular cloning, expression, and primary sequence of outer membrane protein P2 gene of Haemophilus influenzae type b. Infect Immun. 1989;57:88–94. doi: 10.1128/iai.57.1.88-94.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy T F, Dudas K C, Mylotte J M, Apicella M A. A subtyping system for nontypable Haemophilus influenzae based on outer membrane proteins. J Infect Dis. 1983;147:838–846. doi: 10.1093/infdis/147.5.838. [DOI] [PubMed] [Google Scholar]
- 38.Mustafa M M, Ramilo O, Olsen K D, Franklin P S, Hansen E J, Beutler B, McCracken G H., Jr Tumor necrosis factor in mediating experimental Haemophilus influenzae type b meningitis. J Clin Investig. 1989;84:1253–1259. doi: 10.1172/JCI114292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mustafa M M, Ramilo O, Syrogiannopoulos G A, Olsen K D, McCracken G H, Jr, Hansen E J. Induction of meningeal inflammation by outer membrane vesicles of Haemophilus influenzae type b. J Infect Dis. 1989;159:917–922. doi: 10.1093/infdis/159.5.917. [DOI] [PubMed] [Google Scholar]
- 40.Nikaido H, Rosenberg E Y. Porin channels in Escherichia coli: studies with liposomes reconstituted from purified proteins. J Bacteriol. 1983;153:241–252. doi: 10.1128/jb.153.1.241-252.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nurminen M. Isolation of porin trimers. Enterobacterial surface antigen: methods for molecular characterization. FEMS Symp. 1985;25:294. [Google Scholar]
- 42.Paxinos G, Watson C. The rat brain in the stereotaxic coordinates. 2nd ed. Sydney, Australia: Academic Press; 1986. [Google Scholar]
- 43.Ramilo O, Sàez-Llorens X, Mertsola J, Jafari H, Olsen K D, Hansen E J, Yoshinaga M, Ohkawara S, Nariuchi H, McCracken G H., Jr Tumor necrosis factor α/cachectin and interleukin 1β initiate meningeal inflammation. J Exp Med. 1990;172:497–507. doi: 10.1084/jem.172.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ransohoff R M. Chemokines in neurological disease models: correlation between chemokine expression patterns and inflammatory pathology. J Leukoc Biol. 1997;62:645–652. doi: 10.1002/jlb.62.5.645. [DOI] [PubMed] [Google Scholar]
- 45.Saukkonen K, Sande S, Cioffe C, Wolpe S, Sherry B, Cerami A, Tuomanen E. The role of cytokines in the generation of inflammation and tissue damage in experimental gram-positive meningitis. J Exp Med. 1990;171:439–448. doi: 10.1084/jem.171.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shenep J L, Flynn P M, Barrett E F, Stidham G L, Westenkirchner D F. Serial quantitation of endotoxemia and bacteremia during therapy for gram negative bacterial sepsis. J Infect Dis. 1988;157:565–568. doi: 10.1093/infdis/157.3.565. [DOI] [PubMed] [Google Scholar]
- 47.Syrogiannopoulos G A, Hansen E J, Erwin A L, Munnford R S, Rutledge J, Reisch J S, McCracken G H., Jr Haemophilus influenzae type b lipooligosaccharide induces meningeal inflammation. J Infect Dis. 1988;157:237–244. doi: 10.1093/infdis/157.2.237. [DOI] [PubMed] [Google Scholar]
- 48.Tekamp-Olson P, Gallegos C, Bauer D, McClain J, Sherry R, Fabre M, van Deventer S, Cerami A. Cloning and characterization of cDNA for murine macrophage inflammatory protein and its human homologues. J Exp Med. 1990;172:911–919. doi: 10.1084/jem.172.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thye Yin E, Galanos C, Kinsky S, Bradshow R A, Wessler S, Luderitz O, Sarmiento M F. Picogram sensitive assay for endotoxin: gelatin of Limulus polyphemus blood cell lysate induced by purified lipopolysaccharide and lipid A from Gram-negative bacteria. Biochim Biophys Acta. 1972;261:245–249. doi: 10.1016/0304-4165(72)90340-6. [DOI] [PubMed] [Google Scholar]
- 50.Tsai C M, Frasch C E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 51.Waage A, Halstensen A, Shalaby R, Brandtzaeg P, Kierulf P, Espevik T. Local production of tumor necrosis factor α, interleukin 1, and interleukin 6 in meningococcal meningitis. J Exp Med. 1989;170:1859–1867. doi: 10.1084/jem.170.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woolpe S D, Sherry B, Jurres D, Davatelis G, Yurt R W, Cerami A. Identification and characterization of macrophage inflammatory protein 2. Proc Natl Acad Sci USA. 1989;86:612–617. doi: 10.1073/pnas.86.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang H, Nielsen D W, Peterson J W, Klimpel G R. Lipoprotein release by bacteria: potential factor in bacterial pathogenesis. Infect Immun. 1998;66:5196–5201. doi: 10.1128/iai.66.11.5196-5201.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]