Abstract
Objectives
This study examined whether sociodemographic factors, including distance to hospital, were associated with differences in the diagnostic interval and the treatment interval for colorectal cancer in northern Sweden.
Methods
Data were retrieved from the Swedish cancer register on patients (n = 446) diagnosed in three northern regions during 2017–2018, then linked to data from Statistics Sweden and medical records. Also, Google maps was used to map the distance between patients' place of residence and nearest hospital. The different time intervals were analysed using Mann–Whitney U‐test and Cox regression.
Results
Differences in time to diagnosis were found between groups for income and distance to hospital, favouring those with higher income and shorter distance. The unadjusted regression analysis showed higher income to be associated with more rapid diagnosis (HR 1.004, CI 1.001–1.007). This association remained in the fully adjusted model for income (HR 1.004, CI 1.000–1.008), but not for distance. No differences between sociodemographic groups were found in the treatment interval.
Conclusion
Higher income and shorter distance to hospital were in the unadjusted models associated with shorter time to diagnosis for patients with CRC in northern Sweden. The association remained for income when adjusting for other variables even though the difference was small.
Keywords: colorectal cancer, distance to hospital, socio‐economic factors, time intervals, time to diagnosis
1. INTRODUCTION
Income and education are important determinants of health (Lindstrand, 2006); thus, lower socio‐economic position is associated with poorer health outcomes for many diseases, including cancer (Dalton et al., 2008; Kivimäki et al., 2020; Sommer et al., 2015). Patients with lower socio‐economic positions are more likely to be diagnosed with colorectal cancer (CRC) at a late stage, have poorer health outcomes and lower cancer survival (Dalton et al., 2019; Feller et al., 2018; Finke et al., 2021; Frederiksen et al., 2009). Longer distance to hospital is associated with increased time to diagnosis for cancer in general (Flytkjær Virgilsen et al., 2019), and worse health outcomes for patients with CRC (Beckmann et al., 2016).
CRC often presents with symptoms that are ambiguous and common among the general population, which can influence the time to diagnosis and make it difficult to both suspect and expediently diagnose affected individuals (Adelstein et al., 2011; Hamilton et al., 2009; Walter et al., 2016). In the literature, time to diagnosis contains various intervals between certain events within patients' care trajectories, which may be interrelated but also alone represent a specific actor's—patient, doctor or system—accountability for that specific part of the diagnostic process. The total interval (i.e., from first symptom appearance to treatment start) covers a broad period including several time‐intervals in which the patient interval, that is, time from first symptom to first healthcare visit is one of them, and the diagnostic interval (DI), that is, time from first symptom presentation in healthcare to diagnosis and treatment interval (TI), that is, time from diagnosis to start of treatment are others (Weller et al., 2012). When symptoms are ambiguous it may result in a prolonged DI, which in turn can potentially result in poorer health outcomes and higher mortality (Neal et al., 2015; Redaniel et al., 2015; Tørring et al., 2013; Tørring et al., 2017), possibly due to that longer DIs seems to be associated with more advanced CRC (Tørring et al., 2017). However, while socio‐economic positions seem to influence cancer health outcomes (Barclay et al., 2021; Feller et al., 2018), the association between socio‐economic positions and DI is not well established.
The setting of the present study in northern Sweden is heterogenous, characterised by abundant rurality with long distances to healthcare services, combined with urban areas where the availability of such services is greater, which makes the setting of this study particularly interesting since it captures the extremities. Additionally, since CRC is a common cancer disease, and since the incidence is quite similar among women and men (Ferlay et al., 2018), it is appropriate to examine the influence of socio‐economic disparities on time to diagnosis and treatment for this cancer disease, as one way to scrutinise healthcare's management. For these reasons, the aim of this study is to examine whether sociodemographic factors, including distance to hospital, are associated with differences in the DI and the TI for CRC in northern Sweden.
2. METHODS
2.1. Design and setting
This study is a retrospective cohort study that took place in three northern regions out of Sweden's 21 regions, namely, Region Jämtland Härjedalen (RJH), Region Västerbotten (RVB) and Region Västernorrland (RVN). The northern part of Sweden is geographically large but sparsely populated, encompassing challenges with accessing and delivering healthcare. The population in the tree northern regions included in this study was 647,893 during 2019, and the population density was 5.18/km2 the same year, compared with 25.4/km2 for Sweden as whole (statistikdatabasen SCB, n.d.).
When this study was conducted, there was no screening for CRC in the study regions. However, Sweden, among other countries, has during recent years invested in Standardized Cancer Patient Pathways (CPPs) in primary and secondary care, with the intention to shorten time to diagnosis and treatment and decrease regional differences in management of cancer (Wilkens et al., 2016). The CPP for CRC was introduced in Sweden during 2016 and includes allocated time frames for diagnostic events leading up to diagnosis and treatment. Primary care is most often patients first instance of care, as well as the main gateway for accessing secondary care. On referral, most patients are given an appointment in secondary care within 90 days based on the Swedish Healthcare Guarantee (Vårdgaranti in Swedish) (Health and Medical Act, 2017, p. 80). However, following the guidelines from the introduction of CPP, the numbers of days for a specialist appointment for a patient with well‐founded suspicion of CRC has been greatly reduced and is specified to 10 days (Regional Cancer Center, n.d.).
The Swedish healthcare system is decentralised, publicly funded and ensures healthcare for all residents. Besides the publicly financed healthcare system, some private healthcare services exist. Which primary healthcare centre (PHC) the residents want to receive care from is free of choice. In RJH there exists 26 PHCs and one hospital, in RVB: 38 PHCs and three hospitals and in RVN: 31 PHCs and three hospitals (numbers from 2021).
2.2. Data collection and study population
All patients who were reported in the Swedish Cancer Register (with 98% coverage) with incident CRC during the study period in the study regions were included in this study. Each study region has joint systems for Electronic Medical Records (EMRs) for primary and secondary care; however, four healthcare providers in RVB have a separate system; thus, patients receiving care from these healthcare providers were excluded. Also, patients diagnosed in other regions than their region of residence were excluded, which in previous projects have been shown to be few, <1%. Thus, approximately 97% of the total number of the eligible patients from the study regions were included in the study and had their data retrieved from the Swedish cancer register. No other exclusion criteria were undertaken. The sample size of the study was based on a power analysis including standard deviations (SD = 137) from our previous research where our primary outcome was the reduction of time interval from the first visit with the physician to diagnosis. To detect a difference of 10 days between two samples (significance level 5%, power 80%) where a smaller difference was judged not to be clinically relevant, a total of 320 patients was needed.
Firstly, cases were identified though the Swedish Cancer Register; thus, data were retrieved on all incident CRC patients with primary CRC tumours (n = 447) diagnosed during 1 year (2017‐07/2018‐06) in the three included regions. Secondly, reviews of these patients' EMRs were performed by one physician from each region, including data from primary and secondary care. Thirdly, Google maps was used to map the distance between the patients' place of residence and their nearest hospital. Lastly, the data set was combined with data from Statistics Sweden (SCB), which consist of data on socio‐economic and demographic parameters. Of the 447 eligible patients retrieved from the cancer register, one person was excluded from the data set due to having an unreasonable number of days (6230 days) from first contact with care to diagnosis.
2.3. Outcome variables
The study outcomes were defined as (i) time (in days) from first presentation/clinical appearance to diagnosis, that is, the DI (in our data: time from first appointment with physician to diagnosis), and (ii) the time (in days) from diagnosis to start of treatment, that is, the TI.
The dates of diagnosis were retrieved from the EMRs, that is, the first date when a clinician diagnosed the patient with CRC, that is, when it is clinically obvious that the patient suffers from CRC, and this is what clinicians report to the Swedish Cancer Register. A clinical diagnosis could be based on a clinical examination (e.g., colonoscopy), radiology, findings at an operation etc. The date when a treatment was initiated, that is, decided upon, which include palliative care treatments. However, 20 patients had no date for start of treatment registered. In those cases, we used the date of when it was decided that the patient should undergo a certain treatment, including palliative care treatments. The date for deciding upon treatment was missing for three patients (0.7%).
2.4. Exposure variables
The exposures of the study were socio‐economic and demographic parameters, such as income, education and distance from residence to closest hospital, as well as age and sex. The income variable was dichotomized across the median into low income (<166,200 SEK/year ≈ 16,500 €/year) versus high income. The education variable was dichotomized as short education (completed elementary school, or completed elementary school and high school), and long education (completed post‐high school education, e.g., university education). The variable distance to closest hospital was dichotomized across the 80th percentile, defined as long distance (>57 km, i.e., ≥P 80), and short distance (<P 80, i.e., all others). Presenting categorical results often facilitates the interpretation (Naggara et al., 2011); however, in the regression analyses the continuous variables for income and distance were used. Furthermore, the variable tumour localisation was dichotomized into right tumour (tumour in the right side of the colon), and left/rectum tumour (tumour in the transverse or left side of the colon including rectum or unspecified localisation).
2.5. Analyses
The majority of the data was skewed, which required us to use non‐parametric methods. Normality of the data were assessed using Skewness–Kurtosis test, and by visual inspection of the histograms. We applied the Mann–Whitney U‐test to examine differences between the groups. Since age was normally distributed, differences in mean age were analysed by one‐way ANOVA. Also, to examine the relationship between our categorical variables we applied Pearson's Chi‐squared Test. Furthermore, since our data were skewed, but all cases in our data had a diagnosis date, and the great majority had a date when treatment was initiated, we applied Cox‐regression to be able to analyse time to diagnosis and treatment while adjusting for other variables (Vach, 2013). By applying Cox‐regression, we could analyse the number of observed events (diagnoses and initiated treatments respectively) per observed time unit (DI, TI), which provides us with a measure of the number of events per time unit and can be interpreted as time to diagnosis. Also, we assessed the assumption of proportional hazards with regression of Schoenfeld residuals against time and found that no significant violations of the assumption were made. However, when we assessed the assumption of linearity of proposed continuous independent variables in the regression models, we found the association between continuous age and DI to show signs of non‐linearity, resulting in age being included as a categorical variable based on quartiles in the regression. Lastly, we performed sub‐group analyses for those who had primary care as their first healthcare contact.
Statistical significance for all analyses were defined at p < 0.05, with 95% confidence interval. Analyses were performed using IBM SPSS Statistics ver. 24, and the assumption of proportional hazards was checked with R ver. 4.0.5. Ethical approval was granted from the regional ethical review board (Dnr. 2018/305–31), and all analyses were performed at a secure platform provided by SCB.
3. RESULTS
A total of 446 patients fulfilled the inclusion criteria and are thus included in this study; Figure 1 specifies the included patients' first contact with healthcare.
FIGURE 1.
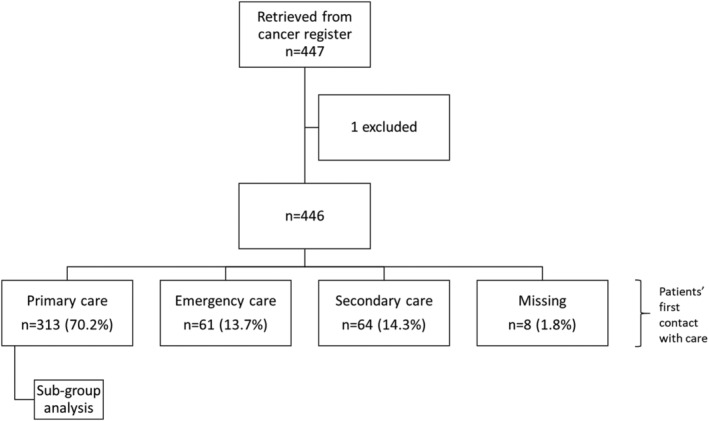
Flowchart with patients' first healthcare contact specified
3.1. Time to diagnosis and treatment—Differences between groups of sociodemographic and clinical parameters
Table 1 offers a description of the characteristics of the cohort, and Table 2 presents an overview of the cohort and differences between groups of socio‐economic and demographic parameters, that is, income, education and distance to hospital.
TABLE 1.
Characteristics of the study sample
| All | ||||
|---|---|---|---|---|
| n (%) | Mean/median | CI/IQI | Range | |
| n | 446 (100) | |||
| Age (year) a | 72.3 | 71.3–73.3 | 37–93 | |
| Sex n (%) | ||||
| Female | 194 (43.5) | |||
| Male | 252 (56.5) | |||
| Diagnostic interval (days) b | 436 (97.8) | 29.0 | 9.0–74.0 | 0–673 |
| Treatment interval (days) b | 443 (99.3) | 32.0 | 20.0–48.0 | 0–510 |
| Distance to nearest hospital (km) b | 446 (100) | 18.0 | 5–49 | 1–248 |
| Education n (%) | ||||
| Short | 325 (72.9) | |||
| Long | 104 (23.3) | |||
| Income n (%) | ||||
| Low | 216 (48.4) | |||
| High | 216 (48.4) | |||
| First care contact n (%) | ||||
| Primary care | 313 (70.2) | |||
| Secondary care (not emergency) | 64 (14.3) | |||
| Emergency care | 61 (13.7) | |||
| Investigation in emergency care (EC) n (%) | ||||
| No contact with EC | 263 (59.0) | |||
| Initially referred to EC | 75 (16.8) | |||
| Patient initially seeks EC | 65 (14.6) | |||
| EC after investigation has started in primary care | 38 (8.5) | |||
| Tumour stage n (%) | ||||
| Tx without metastasis | 11 (2.5) | |||
| T1‐T2 without metastasis | 76 (17.0) | |||
| T3‐T4 without metastasis | 126 (28.3) | |||
| Tx, T1‐T 4 with metastasis to lymfo‐node | 128 (28.7) | |||
| Tx, T1‐T4 with remote metastasis | 85 (19.1) | |||
| Tumour localisation n (%) | ||||
| Right colon | 143 (32.1) | |||
| Left colon | 126 (28.3) | |||
| Rectum | 141 (31.6) | |||
| Transversum | 34 (7.6) | |||
| Unspecified | 2 (0.4) | |||
Note: Low income defined as <median income (166,200 sek/year ≈ 16,500 €) and High income defined as >median income. Short education was defined as completed elementary school, or elementary school and high school, whereas Long education was defined as post‐high school education, for example, university education. Long distance was defined as the 20% with the longest distance to hospital (57–248 km), whereas Short distance was defined as having <57 km to nearest hospital. Tumour staging according to TNM stageing, Tx = unspecified stage.
Values presented are means with confidence intervals (CI).
Median values with Inter Quartile Interval (IQI).
TABLE 2.
Patients' characteristics and differences between sociodemographic groups
| Income | Education | Distance to hospital | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | CI/IQI | High | CI/IQI | p | Short | CI/IQI | Long | CI/IQI | p | Short | CI/IQI | Long | CI/IQI | p | |
| n | 216 | 216 | 325 | 104 | 357 | 89 | |||||||||
| Age (year) a | 75.9 | 74.8–77.1 | 68.4 | 66.8–69.9 | <0.001 | 72.6 | 71.4–73.7 | 70.5 | 68.3–72.7 | 0.09 | 71.8 | 70.7–72.9 | 74.3 | 72.2–76.5 | 0.043 |
| Sex n (%) | 0.002 d | 0.024 d | 0.020 d | ||||||||||||
| Female | 109 (50.5) | na | 77 (35.6) | na | 131 (40.3) | na | 55 (52.9) | na | 165 (46.2) | na | 29 (32.6) | na | |||
| Male | 107 (49.5) | na | 139 (64.4) | na | 194 (59.7) | na | 49 (47.1) | na | 192 (53.8) | na | 60 (67.4) | na | |||
| Diagnostic interval (days) b | 39.5 | 13.3–92.0 | 24.5 | 8.0–56.5 | 0.004 c | 30.0 | 10.3–73.8 | 24.0 | 7.0–85.0 | 0.380 c | 27.0 | 8.0–74.0 | 39.0 | 15.0–76.5 | 0.037 c |
| Treatment interval (days) b | 31.5 | 21.0–49.0 | 34.0 | 22.0–47.0 | 0.782 c | 33.5 | 21.0–47.8 | 31.5 | 19.8–52.3 | 0.883 c | 32.0 | 19.0–47.0 | 33.0 | 21.0–52.0 | 0.369 c |
| Distance to nearest hospital (km) b | 20.5 | 5.0–54.5 | 16.0 | 4.3–42.0 | 0.085 c | 21.0 | 5.0–51.5 | 9.0 | 4.0–31.8 | 0.007 c | na | na | |||
| Education n (%) | <0.001 d | na | 0.13 d | ||||||||||||
| Short | 187 (86.5) | na | 138 (63.9) | na | na | na | 256 (71.7) | na | 69 (77.5) | na | |||||
| Long | 26 (12.0) | na | 78 (36.1) | na | na | na | 89 (24.9) | na | 15 (16.9) | na | |||||
| Income n (%) | na | <0.001 d | 0.054 d | ||||||||||||
| Low | na | na | 187 (57.5) | 26 (25.0) | 165 (46.2) | 51 (57.3) | |||||||||
| High | na | na | 138 (42.5) | 78 (75.0) | 181 (50.7) | 35 (39.3) | |||||||||
| First care contact n (%) | 0.38 d | 0.59 d | 0.07 d | ||||||||||||
| Primary care | 157 (72.7) | 149 (69.0) | 233 (71.7) | 71 (68.3) | 246 (68.9) | 67 (75.3) | |||||||||
| Secondary care (not emergency) | 26 (12.0) | 35 (16.2) | 43 (13.2) | 18 (17.3) | 48 (13.4) | 16 (18.0) | |||||||||
| Emergency care | 31 (14.4) | 26 (12.0) | 42 (12.9) | 14 (13.5) | 55 (15.4) | 6 (6.7) | |||||||||
| Investigation in emergency care (EC) n (%) | 0.39 d | 0.44 d | 0.19 d | ||||||||||||
| No contact with EC | 125 (57.9) | 135 (62.5) | 193 (59.4) | 65 (62.5) | 203 (56.9) | 60 (67.4) | |||||||||
| Initially referred to EC | 43 (19.9) | 31 (14.4) | 61 (18.8) | 13 (12.5) | 61 (17.1) | 14 (15.7) | |||||||||
| Patient initially seeks EC | 29 (13.4) | 29 (13.4) | 41 (12.6) | 16 (15.4) | 58 (16.2) | 7 (7.9) | |||||||||
| EC after investigation has started in primary care | 15 (6.9) | 20 (9.3) | 25 (7.7) | 10 (9.6) | 30 (8.4) | 8 (9.0) | |||||||||
| Tumour stage n (%) | 0.37 d | 0.99 d | 0.64 d | ||||||||||||
| Tx without metastasis | 7 (3.2) | 4 (1.9) | 8 (2.5) | 2 (1.9) | 7 (2.0) | 4 (4.5) | |||||||||
| T1‐T2 without metastasis | 40 (18.5) | 35 (16.2) | 56 (17.2) | 19 (18.3) | 60 (16.8) | 16 (18.0) | |||||||||
| T3‐T4 without metastasis | 66 (30.6) | 58 (26.9) | 93 (28.6) | 30 (28.8) | 103 (28.9) | 23 (25.8) | |||||||||
| Tx, T1‐T 4 with metastasis to lymfo‐node | 55 (25.5) | 73 (33.8) | 95 (29.2) | 32 (30.8) | 104 (29.1) | 24 (27.0) | |||||||||
| Tx, T1‐T4 with remote metastasis | 37 (17.1) | 41 (19.0) | 60 (18.5) | 18 (17.3) | 66 (18.5) | 19 (21.3) | |||||||||
| Tumour localisation n (%) | 0.37 d | 0.96 d | 0.07 d | ||||||||||||
| Right colon | 72 (33.3) | 66 (30.6) | 103 (31.7) | 35 (33.7) | 116 (32.5) | 27 (30.3) | |||||||||
| Left colon | 63 (29.2) | 58 (26.9) | 92 (28.3) | 28 (26.9) | 99 (27.7) | 27 (30.3) | |||||||||
| Rectum | 61 (28.2) | 78 (36.1) | 103 (31.7) | 34 (32.7) | 108 (30.3) | 33 (37.1) | |||||||||
| Transversum | 19 (8.8) | 14 (6.5) | 26 (8.0) | 7 (6.7) | 33 (9.2) | 1 (1.1) | |||||||||
| Unspecified | 1 (0.5) | 0 (0.0) | 1 (0.3) | 0.0 | 1 (0.3) | 1 (1.1) | |||||||||
Note: Bold type indicates significant p values. Mean values in age are analysed using one‐way ANOVA. Low income defined as <median income (166,200 sek/year ≈ 16,500 €) and High income defined as >median income. Short education was defined as completed elementary school, or elementary school and high school, whereas Long education was defined as post‐high school education, for example, university education. Long distance was defined as the 20% with the longest distance to hospital (57–248 km), whereas Short distance was defined as having <57 km to nearest hospital. Tumour staging according to TNM stageing, Tx = unspecified stage.
Means with confidence intervals (CI).
Median values with Inter Quartile Interval (IQI).
Mann–Whitney U‐test (Exact Sig. two‐tailed).
Pearson Chi‐Square Test.
3.1.1. Diagnostic interval
Our analysis showed no differences in time intervals when we compared education groups, but we found differences in the DI when we compared income and distance to hospital. Patients in the high‐income group had a shorter DI compared with patients in the low‐income group, 24.5 days (IQI 8.0–56.5) compared with 39.5 days (IQI 13.3–92.0) (p 0.004). Similarly, patients with short distance to hospital had a shorter DI compared patients with long distance to hospital, 27 days (IQI 8.0–74.0) compared with 39 days (IQI 15.0–76.5) (p 0.037) (Table 2).
When performing the Cox regression analysis, the unadjusted analysis showed that having higher income is associated with being more rapidly diagnosed (HR 1.004, CI 1.001–1.007, p 0.020). This association remained when adjusting for age and sex, and in the fully adjusted model (HR 1.004, CI 1.000–1.008, p 0.034) (Table 3).
TABLE 3.
Hazard ratios of diagnoses per observed time unit for diagnostic interval
| Unadjusted | Model 1 | Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| Income a | 1.004 | 1.001–1.007 | 0.020 | 1.004 | 1.001–1.008 | 0.019 | 1.004 | 1.000–1.008 | 0.034 |
| Distance in km | 0.998 | 0.955–1.000 | 0.054 | 0.997 | 0.955–1.000 | 0.031 | 0.998 | 0.995–1.000 | 0.060 |
| Long education | 1.018 | 0.814–1.273 | 0.876 | 1.014 | 0.809–1.271 | 0.901 | 0.987 | 0.780–1.249 | 0.914 |
| Left colon/rectum tumour | 1.336 | 1.092–1.636 | 0.005 | 1.334 | 1.083–1.642 | 0.007 | 1.359 | 1.099–1.681 | 0.005 |
| Men | 1.124 | 0.929–1.360 | 0.229 | na | 1.158 | 0.949–1.412 | 0.149 | ||
| Age (ref > 81) | 0.096 | na | 0.036 | ||||||
| <66 | 1.222 | 0.934–1.598 | 0.144 | 1.093 | 0.823–1.452 | 0.538 | |||
| 67–73 | 1.394 | 1.068–1.820 | 0.014 | 1.470 | 1.120–1.930 | 0.005 | |||
| 74–80 | 1.128 | 0.860–1.479 | 0.383 | 1.171 | 0.884–1.552 | 0.271 | |||
Note: Hazard ratios (HRs) represent being diagnosed. HR = Hazard ratio; CI = 95% confidence interval; bold type indicates significant p values. Reference categories: Short education (completed elementary school, or completed elementary school and high school); right colon tumour; women; age > 81 years. Model 1: Adjusted for age and sex. Model 2: adjusted as model 1 plus for income, distance, education and tumour localisation.
Income per 10,000 SEK (≈1000 EUR).
Additionally, we found associations between age and tumour localisation and the DI in the adjusted analysis. Patients with tumours on the left side of their colon, or in rectum, were more rapidly diagnosed than those with tumours on the right side of their colon (HR 1.359, CI 1.099–1.681, p 0.005), and patients aged between 67 and 73 were the age group with the most rapid time to diagnosis (HR 1.470, CI 1.120–1.930, p 0.005) (Table 3).
3.1.2. Treatment interval
As seen in Table 2, no differences were found in the TI when specifically comparing groups with different income, education and distance to hospital, nor did we find any association in the regression analysis regarding income, education and distance to hospital (Table 4).
TABLE 4.
Hazard ratios of initiated treatments per observed time unit for treatment interval
| Unadjusted | Model 1 | Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| Income a | 1.002 | 0.998–1.005 | 0.440 | 1.001 | 0.997–1.005 | 0.626 | 1.001 | 0.997–1.005 | 0.571 |
| Distance in km | 1.000 | 0.997–1.002 | 0.750 | 1.000 | 0.997–1.002 | 0.790 | 1.000 | 0.997–1.002 | 0.893 |
| Long education | 0.968 | 0.774–1.210 | 0.773 | 0.936 | 0.745–1.176 | 0.570 | 0.919 | 0.727–1.162 | 0.482 |
| Left colon/rectum tumour | 0.725 | 0.592–0.888 | 0.002 | 0.711 | 0.577–0.877 | 0.001 | 0.707 | 0.572–0.875 | 0.001 |
| Men | 0.911 | 0.754 | 1.099 | na | 0.986 | 0.809–1.202 | 0.889 | ||
| Age (ref > 81) | 0.344 | na | 0.233 | ||||||
| <66 | 1.070 | 0.819–1.397 | 0.621 | 1.126 | 0.845–1.499 | 0.418 | |||
| 67–73 | 0.846 | 0.648–1.105 | 0.220 | 0.851 | 0.646–1.122 | 0.253 | |||
| 74–80 | 0.986 | 0.752–1.293 | 0.920 | 0.925 | 0.696–1.228 | 0.589 | |||
Note: Hazard ratios (HRs) represent initiated treatments. HR = Hazard ratio, CI = 95% confidence interval; bold type indicates significant p‐values. Reference categories: Short education (completed elementary school, or completed elementary school and high school); right colon tumour; women; age >81 years. Model 1: Adjusted for age and sex. Model 2: adjusted as model 1 plus for income, distance, education and tumour localisation.
Income per 10,000 SEK (≈1000 EUR).
However, we found an association between tumour localisation and the TI. Patients with tumours on the left side of their colon, or in rectum, had in the adjusted model longer time to start of treatment than with tumours on the right side of their colon (HR 0.707, CI 0.572–0.875, p 0.001) (Table 4).
3.2. Sub‐group analysis—Primary care as first healthcare contact
Of the 446 patients in the total sample, 313 patients (70.2%) had primary care as their first healthcare contact during their trajectory (Table 1). This unadjusted sub‐group analysis showed, as expected, that patients who seek primary care as first instance, have more days to diagnosis compared with those who have emergency care or secondary care as first healthcare contact. Patients who seek primary care as first instance have 33 days to diagnosis (IQI 14.0–83.5), compared with 19 days for the others (IQI 2.0–63.0) (p < 0.005) (further data not presented).
4. DISCUSSION
This study examined if sociodemographic factors, including distance to hospital, is associated with the DI and TI for patients diagnosed with CRC in northern Sweden. Our results showed minor differences in the DI based on income and distance to hospital; however, the differences for distance did not remain in the fully adjusted model.
Income is known to be an important determinant of health (Lindstrand, 2006). The results from the fully adjusted models in our study showed an association, yet small, between income and DI, but no association between income and TI. A Norwegian study, examining waiting time or access to treatment for patients with CRC in an emergency care context, found no association with income. However, the same study report that people with higher income are less likely to present with CRC in emergency care, insinuating that income do influence the CRC diagnostic process. (Nilssen et al., 2021).
In our study, education did not independently influence the DI and TI, which might be interpreted as being in contrast to results reported in other studies; nevertheless the other studies have admittedly analysed the association between socio‐economic factors and stage at diagnosis and CRC survival (Coughlin, 2020; Egeberg et al., 2008; Finke et al., 2021; McDaniel et al., 2017; Rosskamp et al., 2021), and factors related to survival might differ from those related to time to diagnosis and treatment. However, one study, even though performed in a socioeconomically disadvantaged area, reports no strong associations with socio‐economic factors (Barclay et al., 2015), which is similar to our findings. In general, education is widely known for being associated with better health outcomes (Hahn & Truman, 2015; Lindstrand, 2006; Luy et al., 2019; The Lancet Public, 2020), therefore, that our study did not find an association with education is somewhat surprising. Furthermore, our results showed that patients aged 67–73 have a short DI than the other age groups. This association is probably explained by that CRC is less prevalent in the younger age group and that older patients sometimes have a prolonged DI due to comorbidities, which possibly influence the awareness within the healthcare organisation.
While the association between DI and distance to hospital found in this study did not remain in the fully adjusted models, scholars report inconsistency of findings. Flytkjær Virgilsen et al. report that longer distance to hospital increase the DI for some cancers, especially for those cancers that are more difficult to diagnose (2019). Research from Scotland suggest that rural living does not lead to an increased DI for cancer in general (Murchie, Adam, et al., 2020), and rural primary care practitioner initiated diagnostic action within the system just as likely when comparing with their urban counterparts in a European setting (Murchie, Khor, et al., 2020). However, Bergin et al. (2018) report that rural living is associated with longer time to diagnosis and treatment for CRC in Australia (Bergin et al., 2018). Interestingly, another study from Scotland reports that patients with longer distance to healthcare have shorter DI and TI compared with those living closer to healthcare facilities (Turner et al., 2017). Additionally, a study comparing travel times to healthcare facilities in Denmark and Scotland for patients diagnosed with CRC report that travel times seems to influence health outcomes differently in the two countries (Murchie et al., 2021).
The underlying mechanisms of diagnosing cancer, measuring time intervals and analysing the contextual effect are multifaceted, influenced by patient, provider and organisational factors. Consequently, it is not suspiring that inconsistent findings have been reported when it comes to time to diagnosis for patients with CRC (Golding et al., 2020), stemming from different definitions of time intervals and biases related to time intervals (Neal, 2009; Weller et al., 2012), factors influencing time intervals (Golding et al., 2020), as well as the variety of contextual factors (Dobson et al., 2020). Furthermore, inter alia, patients' age (Esteva et al., 2014), interpretation of bodily changes, availability healthcare services, travel time and poor access to public transport are all factors that influence help‐seeking behaviours (Carriere et al., 2018; Emery et al., 2013), which in turn might influence patients' health outcomes (Carriere et al., 2018). Also, the impact of rurality may be difficult to address and interpret, perhaps since rurality differs immensely between countries, settings and populations (Dobson et al., 2020). In a study from the United Kingdom, long travel time was defined as >30 min (Murage et al., 2019), while in our data, patients travelled up to 250 km, which would take approximately 3 h, consequently, the preconditions across different settings varies vastly. Thus, comparing results across different settings and contexts is challenging, considering the variety of influencing factors and underlying mechanisms.
Since the present study was conducted only shortly (1–2 years) after the introduction of CPP, it is rather unlikely that this result is a consequence stemming from the new CPP reform. However, even though CPPs may theoretically counterbalance socio‐economic disparities, insights from other countries show that people with lower income, older age and female sex seem to be less likely referred into a standardised pathway (Nilssen et al., 2020; Zhou et al., 2018). Additionally, a recent study from another Swedish region conclude that CPP does not seem to improve the prognosis for patients diagnosed with CRC (Andersson et al., 2021).
4.1. Strengths and limitations
This study has strengths that we want to highlight. Firstly, the Swedish cancer register has almost a 100% coverage. Secondly, we argue that this study examined a geographic area that is quite unique since it encompasses areas of great rurality, but at the same time have larger urban cities. Thus, this study provides results from a very mixed milieu with very different kinds of challenges related to organising and providing healthcare services, as well as it includes the differences that people in these regions face when seeking and accessing healthcare. Lastly, that our results showed an expected association between tumour localisation and time to diagnosis reinforces the internal validity of our results.
However, there are some limitations that merit comments. First off, that we have used the distance from residence to closest hospital instead of closest PHC could be seen as a limitation. However, since Sweden's citizens can choose which PHC they want to be listed at and we do not know which PHC they have chosen; thus, we argue that using the distance to hospital was the most suitable option in the present study. Secondly, our results might be limited since only some variables of importance for socio‐economic inequities could be included in the analyses. Thus, we call for more extensive research that includes further variables and that also address intersectionality from a social epidemiological perspective. Thirdly, even though dichotomized variables facilitate interpretation, it might risk loss of power and information; we chose to present dichotomized variables in the initial analysis. However, in the regression analyses, the variables (except age) were used, and presented, as continuous. Forth, even if this is a study that consists of all incident CRC patients, during 1 year, it is possible that no statistically significant differences were found in the adjusted models due to lack of power. To gain power by, for example, extending the study period might generate different results. However, the sample size of this study was based on a power analysis from our previous research. Nevertheless, we call for more research in the study regions. Lastly, due to time intervals not being normally distributed, comparing mean time intervals is not a completely suitable method, and the use of Cox regression is unconventional in this setting, especially considering that we have no lost participants at follow‐up. Furthermore, Cox regression does not allow for a comparison across the whole distribution of length of the time interval. Nonetheless, since our data were skewed, and we wanted to adjust for multiple exposures and covariates we found Cox regression to be an appropriate statistical method to investigate our data. This because Cox regression closely resembles parametric models (Kleinbaum & Klein, 2005); furthermore, no violation of the proportional hazards was made.
5. CONCLUSION
Higher income and shorter distance to hospital were in the unadjusted models associated with shorter time to diagnosis for patients with CRC in northern Sweden. The association remained for income when adjusting for other variables even though the difference was small.
CONFLICT OF INTEREST
The authors declare no competing interests.
Hultstrand, C. , Hörnsten, C. , Lilja, M. , Coe, A.‐B. , Fjällström, P. , & Hajdarevic, S. (2022). The association between sociodemographic factors and time to diagnosis for colorectal cancer in northern Sweden. European Journal of Cancer Care, 31(6), e13687. 10.1111/ecc.13687
Funding information This study was founded by a regional agreement between Umeå University and Västerbotten County Council (ALF), grant numbers RV‐855211, RV‐731891 and RV‐931881; The Cancer Research Foundation in Northern Sweden, grant number LP‐18‐2193; VISARE NORR Fund, Northern country council Regional federation, grant number 838121 and 939897; Strategic Research Area Health Care Science (SFO‐V); and The JC Kempe's foundation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are not publicly available due to ethical restrictions.
REFERENCES
- Adelstein, B.‐A. , Macaskill, P. , Chan, S. F. , Katelaris, P. H. , & Irwig, L. (2011). Most bowel cancer symptoms do not indicate colorectal cancer and polyps: A systematic review. BMC Gastroenterology, 11, 65–65. 10.1186/1471-230X-11-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson, E. , Nyhlin, N. , & Van Nieuwenhoven, M. A. (2021). The effectiveness of the colorectal cancer referral pathway—Identification of colorectal cancer in a Swedish region. Scandinavian Journal of Gastroenterology, 56(5), 1–7. [DOI] [PubMed] [Google Scholar]
- Barclay, K. L. , Goh, P. J. , & Jackson, T. J. (2015). Socio‐economic disadvantage and demographics as factors in stage of colorectal cancer presentation and survival. ANZ Journal of Surgery, 85, 135–139. 10.1111/ans.12709 [DOI] [PubMed] [Google Scholar]
- Barclay, M. E. , Abel, G. A. , Greenberg, D. C. , Rous, B. , & Lyratzopoulos, G. (2021). Socio‐demographic variation in stage at diagnosis of breast, bladder, colon, endometrial, lung, melanoma, prostate, rectal, renal and ovarian cancer in England and its population impact. British Journal of Cancer, 124, 1320–1329. 10.1038/s41416-021-01279-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann, K. R. , Bennett, A. , Young, G. P. , Cole, S. R. , Joshi, R. , Adams, J. , Singhal, N. , Karapetis, C. , Wattchow, D. , & Roder, D. (2016). Sociodemographic disparities in survival from colorectal cancer in South Australia: A population‐wide data linkage study. BMC Health Services Research, 16, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergin, R. J. , Emery, J. , Bollard, R. C. , Falborg, A. Z. , Jensen, H. , Weller, D. , Menon, U. , Vedsted, P. , Thomas, R. J. , Whitfield, K. , & White, V. (2018). Rural‐urban disparities in time to diagnosis and treatment for colorectal and breast Cancer. Cancer Epidemiology, Biomarkers & Prevention : A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology, 27, 1036–1046. 10.1158/1055-9965.EPI-18-0210 [DOI] [PubMed] [Google Scholar]
- Carriere, R. , Adam, R. , Fielding, S. , Barlas, R. , Ong, Y. , & Murchie, P. (2018). Rural dwellers are less likely to survive cancer—An international review and meta‐analysis. Health & Place, 53, 219–227. 10.1016/j.healthplace.2018.08.010 [DOI] [PubMed] [Google Scholar]
- Coughlin, S. S. (2020). Social determinants of colorectal cancer risk, stage, and survival: A systematic review. International Journal of Colorectal Disease, 35, 985–995. 10.1007/s00384-020-03585-z [DOI] [PubMed] [Google Scholar]
- Dalton, S. O. , Olsen, M. H. , Johansen, C. , Olsen, J. H. , & Andersen, K. K. (2019). Socioeconomic inequality in cancer survival—Changes over time. A population‐based study, Denmark, 1987‐2013. Acta Oncologica, 58, 737–744. 10.1080/0284186X.2019.1566772 [DOI] [PubMed] [Google Scholar]
- Dalton, S. O. , Steding‐Jessen, M. , Gislum, M. , Frederiksen, K. , Engholm, G. , & Schüz, J. (2008). Social inequality and incidence of and survival from cancer in a population‐based study in Denmark, 1994–2003: Background, aims, material and methods. European Journal of Cancer, 44, 1938–1949. 10.1016/j.ejca.2008.06.010 [DOI] [PubMed] [Google Scholar]
- Dobson, C. , Rubin, G. , Murchie, P. , MacDonald, S. , & Sharp, L. (2020). Reconceptualising rural cancer inequalities: Time for a new research agenda. International Journal of Environmental Research and Public Health, 17, 1455. 10.3390/ijerph17041455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeberg, R. , Halkjær, J. , Rottmann, N. , Hansen, L. , & Holten, I. (2008). Social inequality and incidence of and survival from cancers of the colon and rectum in a population‐based study in Denmark, 1994–2003. European Journal of Cancer, 44, 1978–1988. 10.1016/j.ejca.2008.06.020 [DOI] [PubMed] [Google Scholar]
- Emery, J. D. , Walter, F. M. , Gray, V. , Sinclair, C. , Howting, D. , Bulsara, M. , Bulsara, C. , Webster, A. , Auret, K. , Saunders, C. , Nowak, A. , & Holman, C. D. A. (2013). Diagnosing cancer in the bush: A mixed‐methods study of symptom appraisal and help‐seeking behaviour in people with cancer from rural Western Australia. Family Practice, 30, 294–301. 10.1093/fampra/cms087 [DOI] [PubMed] [Google Scholar]
- Esteva, M. , Ruiz, A. , Ramos, M. , Casamitjana, M. , Sánchez‐Calavera, M. A. , González‐Luján, L. , Pita‐Fernández, S. , Leiva, A. , Pértega‐Díaz, S. , Costa‐Alcaraz, A. M. , Macià, F. , Espí, A. , Segura, J. M. , Lafita, S. , Novella, M. T. , Yus, C. , Oliván, B. , Cabeza, E. , Seoane‐Pillado, T. , … Llobera, J. (2014). Age differences in presentation, diagnosis pathway and management of colorectal cancer. Cancer Epidemiology, 38, 346–353. 10.1016/j.canep.2014.05.002 [DOI] [PubMed] [Google Scholar]
- Feller, A. , Schmidlin, K. , Bordoni, A. , Bouchardy, C. , Bulliard, J. L. , Camey, B. , Konzelmann, I. , Maspoli, M. , Wanner, M. , Zwahlen, M. , Clough‐Gorr, K. M. , Egger, M. , Spoerri, A. , Puhan, M. , Bopp, M. , Künzli, N. , Bochud, M. , & Oris, M. (2018). Socioeconomic and demographic inequalities in stage at diagnosis and survival among colorectal cancer patients: Evidence from a Swiss population‐based study. Cancer Medicine (Malden, MA), 7, 1498–1510. 10.1002/cam4.1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay, J. , Colombet, M. , Soerjomataram, I. , Dyba, T. , Randi, G. , Bettio, M. , Gavin, A. , Visser, O. , & Bray, F. (2018). Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. European Journal of Cancer, 103, 356–387. 10.1016/j.ejca.2018.07.005 [DOI] [PubMed] [Google Scholar]
- Finke, I. , Seppä, K. , Malila, N. , Jansen, L. , Brenner, H. , & Pitkäniemi, J. (2021). Educational inequalities and regional variation in colorectal cancer survival in Finland. Cancer Epidemiology, 70, 101858–101858. 10.1016/j.canep.2020.101858 [DOI] [PubMed] [Google Scholar]
- Flytkjær Virgilsen, L. , Møller, H. , & Vedsted, P. (2019). Cancer diagnostic delays and travel distance to health services: A nationwide cohort study in Denmark. Cancer Epidemiology, 59, 115–122. 10.1016/j.canep.2019.01.018 [DOI] [PubMed] [Google Scholar]
- Frederiksen, B. L. , Osler, M. , Harling, H. , Ladelund, S. , & Jørgensen, T. (2009). Do patient characteristics, disease, or treatment explain social inequality in survival from colorectal cancer? Social Science & Medicine, 1982(69), 1107–1115. [DOI] [PubMed] [Google Scholar]
- Golding, H. , Webber, C. E. , & Groome, P. A. (2020). Factors contributing to time to diagnosis in symptomatic colorectal cancer: A scoping review. European Journal of Cancer Care, 30, e13397. [DOI] [PubMed] [Google Scholar]
- Hahn, R. A. , & Truman, B. I. (2015). Education improves public health and promotes health equity. International Journal of Health Services, 45, 657–678. 10.1177/0020731415585986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, W. , Lancashire, R. , Sharp, D. , Peters, T. J. , Cheng, K. K. , & Marshall, T. (2009). The risk of colorectal cancer with symptoms at different ages and between the sexes: A case‐control study. BMC Medicine, 7, 17–17. 10.1186/1741-7015-7-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health and Medical Act . (2017). 80 https://www.riksdagen.se/sv/dokument-lagar/dokument/svensk-forfattningssamling/halso--och-sjukvardsforordning-201780_sfs-2017-80
- Kivimäki, M. , Batty, G. D. , Pentti, J. , Shipley, M. J. , Sipilä, P. N. , Nyberg, S. T. , Suominen, S. B. , Oksanen, T. , Stenholm, S. , Virtanen, M. , Marmot, M. G. , Singh‐Manoux, A. , Brunner, E. J. , Lindbohm, J. V. , Ferrie, J. E. , & Vahtera, J. (2020). Association between socioeconomic status and the development of mental and physical health conditions in adulthood: A multi‐cohort study. The Lancet. Public Health, 5, e140–e149. 10.1016/S2468-2667(19)30248-8 [DOI] [PubMed] [Google Scholar]
- Kleinbaum, D. G. , & Klein, M. (2005). Survival analysis: A self‐learning text. Springer New York. 10.1007/0-387-29150-4 [DOI] [Google Scholar]
- Lindstrand, A. (2006). Global health: An introductory textbook. Studentlitteratur. [Google Scholar]
- Luy, M. , Zannella, M. , Wegner‐Siegmundt, C. , Minagawa, Y. , Lutz, W. , & Caselli, G. (2019). The impact of increasing education levels on rising life expectancy: A decomposition analysis for Italy, Denmark, and the USA. Genus, 75, 1–21. 10.1186/s41118-019-0055-0 [DOI] [Google Scholar]
- McDaniel, J. T. , Nuhu, K. , Ruiz, J. , & Alorbi, G. (2017). Social determinants of cancer incidence and mortality around the world: An ecological study. Global Health Promotion, 26, 41–49. [DOI] [PubMed] [Google Scholar]
- Murage, P. , Bachmann, M. O. , Crawford, S. M. , McPhail, S. , & Jones, A. (2019). Geographical access to GPs and modes of cancer diagnosis in England: A cross‐sectional study. Family Practice, 36, 284–290. 10.1093/fampra/cmy077 [DOI] [PubMed] [Google Scholar]
- Murchie, P. , Adam, R. , Khor, W. L. , Smith, S. , McNair, E. , Swann, R. , Witt, J. , & Weller, D. (2020). Impact of geography on Scottish cancer diagnoses in primary care: Results from a national cancer diagnosis audit. Cancer Epidemiology, 66, 101720–101720. 10.1016/j.canep.2020.101720 [DOI] [PubMed] [Google Scholar]
- Murchie, P. , Falborg, A. Z. , Turner, M. , Vedsted, P. , & Virgilsen, L. F. (2021). Geographic variation in diagnostic and treatment interval, cancer stage and mortality among colorectal patients—An international comparison between Denmark and Scotland using data‐linked cohorts. Cancer Epidemiology, 74, 102004. 10.1016/j.canep.2021.102004 [DOI] [PubMed] [Google Scholar]
- Murchie, P. , Khor, W. L. , Adam, R. , Esteva, M. , Smyrnakis, E. , Petek, D. , Thulesius, H. , Vedsted, P. , Mclernon, D. , & Harris, M. (2020). Influences of rurality on action to diagnose cancer by primary care practitioners—Results from a Europe‐wide survey in 20 countries. Cancer Epidemiology, 65, 101698. 10.1016/j.canep.2020.101698 [DOI] [PubMed] [Google Scholar]
- Naggara, O. , Raymond, J. , Guilbert, F. , Roy, D. , Weill, A. , & Altman, D. G. (2011). Analysis by categorizing or dichotomizing continuous variables is inadvisable: An example from the natural history of Unruptured aneurysms. American Journal of Neuroradiology, 32, 437–440. 10.3174/ajnr.A2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal, R. D. (2009). Do diagnostic delays in cancer matter? British Journal of Cancer, 101, S9–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal, R. D. , Tharmanathan, P. , France, B. , Din, N. U. , Cotton, S. , Fallon‐Ferguson, J. , Hamilton, W. , Hendry, A. , Hendry, M. , Lewis, R. , Macleod, U. , Mitchell, E. D. , Pickett, M. , Rai, T. , Shaw, K. , Stuart, N. , Tørring, M. L. , Wilkinson, C. , Williams, B. , … Emery, J. (2015). Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. British Journal of Cancer, 112, S92–S107. 10.1038/bjc.2015.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilssen, Y. , Brustugun, O. T. , Eriksen, M. T. , Haug, E. S. , Naume, B. , & Møller, B. (2020). Patient and tumour characteristics associated with inclusion in Cancer patient pathways in Norway in 2015–2016. BMC Cancer, 20, 488–488. 10.1186/s12885-020-06979-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilssen, Y. , Eriksen, M. T. , Guren, M. G. , & Møller, B. (2021). Factors associated with emergency‐onset diagnosis, time to treatment and type of treatment in colorectal cancer patients in Norway. BMC Cancer, 21, 757. 10.1186/s12885-021-08415-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redaniel, M. T. , Martin, R. M. , Ridd, M. J. , Wade, J. , & Jeffreys, M. (2015). Diagnostic intervals and its association with breast, prostate, lung and colorectal cancer survival in England: Historical cohort study using the clinical practice research datalink. PLoS ONE, 10, e0126608–e0126608. 10.1371/journal.pone.0126608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- REGIONAL CANCER CENTER . (n.d.) Standardized cancer patient pathway colorectal cancer. https://kunskapsbanken.cancercentrum.se/diagnoser/tjock-och-andtarmscancer/vardforlopp/#-Utredning-och-beslut-om-behandling Accessed 2021‐07‐07
- Rosskamp, M. , Verbeeck, J. , Gadeyne, S. , Verdoodt, F. , & De Schutter, H. (2021). Socio‐economic position, Cancer incidence and stage at diagnosis: A Nationwide cohort study in Belgium. Cancers (Basel), 13, 933. 10.3390/cancers13050933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer, I. , Griebler, U. , Mahlknecht, P. , Thaler, K. , Bouskill, K. , Gartlehner, G. , & Mendis, S. (2015). Socioeconomic inequalities in non‐communicable diseases and their risk factors: An overview of systematic reviews. BMC Public Health, 15, 914–914. 10.1186/s12889-015-2227-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- STATISTIKDATABASEN. STATISTICS SWEDEN . (n.d.) http://www.statistikdatabasen.scb.se/pxweb/sv/ssd/START__BE__BE0101__BE0101C/BefArealTathetKon/?loadedQueryId=83180&timeType=from&timeValue=2000 Accessed 2021‐02‐11
- THE LANCET PUBLIC, H . (2020). Education: A neglected social determinant of health. The Lancet Public Health, 5, e361. 10.1016/S2468-2667(20)30144-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tørring, M. L. , Frydenberg, M. , Hansen, R. P. , Olesen, F. , & Vedsted, P. (2013). Evidence of increasing mortality with longer diagnostic intervals for five common cancers: A cohort study in primary care. European Journal of Cancer, 49, 2187–2198. 10.1016/j.ejca.2013.01.025 [DOI] [PubMed] [Google Scholar]
- Tørring, M. L. , Murchie, P. , Hamilton, W. , Vedsted, P. , Esteva, M. , Lautrup, M. , Winget, M. , & Rubin, G. (2017). Evidence of advanced stage colorectal cancer with longer diagnostic intervals: A pooled analysis of seven primary care cohorts comprising 11 720 patients in five countries. British Journal of Cancer, 117, 888–897. 10.1038/bjc.2017.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, M. , Fielding, S. , Ong, Y. , Dibben, C. , Feng, Z. , Brewster, D. H. , Black, C. , Lee, A. , & Murchie, P. (2017). A cancer geography paradox? Poorer cancer outcomes with longer travelling times to healthcare facilities despite prompter diagnosis and treatment: A data‐linkage study. British Journal of Cancer, 117, 439–449. 10.1038/bjc.2017.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vach, W. (2013). Regression models as a tool in medical research. CRC Press. 10.1201/b12925 [DOI] [Google Scholar]
- Walter, F. M. , Emery, J. D. , Mendonca, S. , Hall, N. , Morris, H. C. , Mills, K. , Dobson, C. , Bankhead, C. , Johnson, M. , Abel, G. A. , Rutter, M. D. , Hamilton, W. , & Rubin, G. P. (2016). Symptoms and patient factors associated with longer time to diagnosis for colorectal cancer: Results from a prospective cohort study. British Journal of Cancer, 115, 533–541. 10.1038/bjc.2016.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller, D. , Vedsted, P. , Rubin, G. , Walter, F. M. , Emery, J. , Scott, S. , Campbell, C. , Andersen, R. S. , Hamilton, W. , Olesen, F. , Rose, P. , Nafees, S. , Rijswijk, E. V. , Hiom, S. , Muth, C. , Beyer, M. , & Neal, R. D. (2012). The Aarhus statement: Improving design and reporting of studies on early cancer diagnosis. British Journal of Cancer, 106, 1262–1267. 10.1038/bjc.2012.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkens, J. , Thulesius, H. , Schmidt, I. , & Carlsson, C. (2016). The 2015 National Cancer Program in Sweden: Introducing standardized care pathways in a decentralized system. Health Policy, 120, 1378–1382. 10.1016/j.healthpol.2016.09.008 [DOI] [PubMed] [Google Scholar]
- Zhou, Y. , Mendonca, S. C. , Abel, G. A. , Hamilton, W. , Walter, F. M. , Johnson, S. , Shelton, J. , Elliss‐Brookes, L. , McPhail, S. , & Lyratzopoulos, G. (2018). Variation in 'fast‐track' referrals for suspected cancer by patient characteristic and cancer diagnosis: Evidence from 670 000 patients with cancers of 35 different sites. British Journal of Cancer, 118, 24–31. 10.1038/bjc.2017.381 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not publicly available due to ethical restrictions.


