Summary
Sleep inertia is the brief period of performance impairment and reduced alertness experienced after waking, especially from slow‐wave sleep. We assessed the efficacy of polychromatic short‐wavelength‐enriched light to improve vigilant attention, alertness and mood immediately after waking from slow‐wave sleep at night. Twelve participants (six female, 23.3 ± 4.2 years) maintained an actigraphy‐confirmed sleep schedule of 8.5 hr for 5 nights, and 5 hr for 1 night prior to an overnight laboratory visit. In the laboratory, participants were awakened from slow‐wave sleep, and immediately exposed to either dim, red ambient light (control) or polychromatic short‐wavelength‐enriched light (light) for 1 hr in a randomized crossover design. They completed a 5‐min Psychomotor Vigilance Task, the Karolinska Sleepiness Scale, and Visual Analogue Scales of mood at 2, 17, 32 and 47 min after waking. Following this testing period, lights were turned off and participants returned to sleep. They were awakened from their subsequent slow‐wave sleep period and received the opposite condition. Compared with the control condition, participants exposed to light had fewer Psychomotor Vigilance Task lapses (χ 2[1] = 5.285, p = 0.022), reported feeling more alert (Karolinska Sleepiness Scale: F 1,77 = 4.955, p = 0.029; Visual Analogue Scalealert: F 1,77 = 8.226, p = 0.005), and reported improved mood (Visual Analogue Scalecheerful: F 1,77 = 8.615, p = 0.004). There was no significant difference in sleep‐onset latency between conditions following the testing period (t 10 = 1.024, p = 0.330). Our results suggest that exposure to polychromatic short‐wavelength‐enriched light immediately after waking from slow‐wave sleep at night may help improve vigilant attention, subjective alertness, and mood. Future studies should explore the potential mechanisms of this countermeasure and its efficacy in real‐world environments.
Keywords: awakening, intervention, on‐call workers, reactive countermeasure, shiftwork
1. INTRODUCTION
Sleep inertia refers to the transient neurobehavioural impairments experienced immediately after waking from sleep. This period of reduced alertness and performance poses a significant safety risk to on‐call workers who may be required to perform a safety‐critical task immediately after waking (e.g. emergency services, health care and military). In these types of operations, the need for a rapid return to full alertness is critical to mission safety and success.
Previous research into the factors influencing sleep inertia has led to countermeasures that proactively minimize the severity and/or duration of sleep inertia. For example, the risks posed by sleep inertia can be reduced by avoiding known factors that exacerbate it, such as wake‐up times during the biological night (Scheer et al., 2008); prior sleep loss or periods of extended wakefulness (Dinges et al., 1985; McHill et al., 2019); and waking from deep sleep (i.e. slow‐wave sleep [SWS]; Dinges et al., 1985). These proactive strategies, however, are not guaranteed to eliminate sleep inertia after waking. Indeed, sleep inertia can occur even in the absence of these exacerbating factors (Achermann et al., 1995; Hofer‐Tinguely et al., 2005; Scheer et al., 2008; Wertz et al., 2006), suggesting that it is a ubiquitous phenomenon that, while dynamic in severity through interaction with the two‐processes model (Borbély, 1982), can potentially occur after any sleep–wake transition.
Proactive strategies are not always feasible to implement. A short recuperative nap break while driving long distances, for example, is often taken following extended wakefulness and/or to alleviate sleepiness at night, and therefore increases the risk of severe sleep inertia (Hilditch et al., 2016,2017). In fact, many of the conditions that are known to exacerbate sleep inertia effects are common, particularly in shift‐working and on‐call operations (Dawson et al., 2020; Ferguson et al., 2016; Vincent et al., 2018). Furthermore, although interventions administered pre‐sleep or requiring a pre‐determined wake time may be appropriate in some settings, they are of limited use in operational settings in which sleep opportunities are unpredictable (Figueiro et al., 2019; Hilditch et al., 2016; Scheuermaier et al., 2018). Hence, there is an obvious need for reactive countermeasures to sleep inertia (i.e. countermeasures that can be implemented after any awakening to rapidly improve alertness and performance).
A recent review of the literature on reactive countermeasures highlighted several research gaps and promising candidate interventions for further investigation (Hilditch, Dorrian, et al., 2016). One viable countermeasure identified was light exposure upon awakening. Bright, short‐wavelength‐enriched light has been shown to elicit acute alerting effects during wakefulness (Lok et al., 2018; Souman et al., 2018), but its effects on alertness and cognitive performance during the sleep inertia period have yet to be convincingly demonstrated. Light does not appear to be an effective countermeasure to sleep inertia experienced upon waking in the morning after a night of sleep (Santhi et al., 2013) or after a nap in the daytime (Hayashi et al., 2003). However, the acute alerting effects of light on alertness and performance are dampened during the day relative to the night, suggesting that using light as a countermeasure to sleep inertia during the night may be more effective than using it during the day (Lok et al., 2018; Souman et al., 2018).
Thus, we tested the use of polychromatic short‐wavelength‐enriched light as a countermeasure to sleep inertia following awakenings under conditions known to exacerbate sleep inertia symptoms (i.e. from SWS during the biological night, and following sleep restriction), in a within‐subject, randomized, crossover intervention study. We hypothesized that under these conditions reflecting real‐world awakenings with combined influences from the three‐process model of alertness (Folkard & Åkerstedt, 1992), the intervention would improve vigilant attention, alertness and mood during the awakening period relative to the control condition.
2. MATERIALS AND METHODS
2.1. Participants
Twelve healthy young adults were recruited to participate in the study. Participants were subjected to the following inclusion criteria: (1) normal sleepers (Pittsburgh Sleep Quality Index ≤ 5; no self‐reported sleep problems; habitual sleep 7–9 hr); (2) healthy (General Health Screening Questionnaire, personal physician's permission to participate, approval from onsite physician upon review of full blood work screening); (3) free of illicit substances and nicotine (urine toxicology screen at on‐boarding and each laboratory visit); (4) free of alcohol during the study period (breathalyser at each laboratory visit); and (5) no shiftwork or travel > 3 time zones in the past 3 months (self‐report).
Participants provided written informed consent prior to participation in the screening activities and the in‐laboratory protocol. The study protocol was approved by the NASA Ames Research Center Institutional Review Board.
2.2. Protocol
Participants completed a 2‐week within‐subject, randomized, crossover intervention study including two in‐laboratory overnight visits. The methods and results of 1 week of this study are presented here, which, for half of the sample, was their first week in the study. The sleep–wake protocol during the other week was identical to the one described here. The presentation order of the intervention or control at wake‐up within the experimental night was randomized by sex.
2.2.1. At‐home
Participants were required to follow a fixed sleep–wake schedule for the 6 nights leading up to the in‐laboratory visit. The at‐home sleep–wake schedule consisted of 5 nights of 8.5 hr of time‐in‐bed followed by 1 sleep‐restricted night of 5 hr of time‐in‐bed (Figure 1). Sleep timing was determined by each participant's self‐reported habitual sleep pattern. Sleep on the sixth night was restricted by delayed sleep onset, allowing for a fixed morning wake time across the protocol. The purpose of the sleep restriction was to increase sleep pressure (ÅKerstedt & Gillberg, 1986; Tassi et al., 2006). The following day, participants were provided with transport to the Fatigue Countermeasures Laboratory at NASA Ames Research Center for an overnight visit.
FIGURE 1.
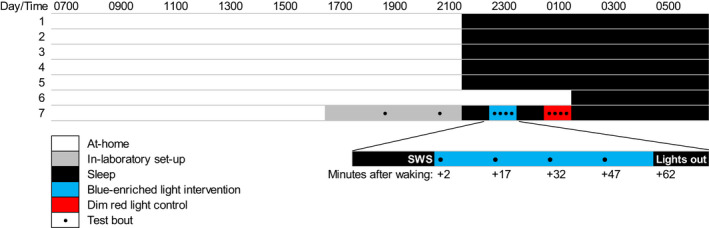
Protocol schematic. White shading indicates the awake at‐home portion of study. Grey shading indicates in‐laboratory activities including baseline testing. Black shading indicates sleep periods. Black circles represent test bouts. Order of polychromatic short‐wavelength‐enriched light intervention (blue shading) and dim red light control (red shading) were randomized. Times shown are approximate and varied depending on habitual sleep–wake times and slow‐wave sleep (SWS) periods
2.2.2. In‐laboratory
Participants arrived at the laboratory approximately 5 hr before their habitual bedtime. During the evening, participants were familiarized with the test battery and study procedures, performed two baseline test batteries, and were set‐up for sleep recording.
Participants slept and performed all study tasks in a light‐controlled, sound‐attenuated, temperature‐controlled room (22 ± 1°C). At the participant's habitual bedtime, all lights were turned off (< 0.3 lux) and the participant was allowed to sleep. Electroencephalography (EEG; BrainVision Recorder, Brain Products GmBH) was monitored during the sleep period to identify SWS stages (Stages 3 and 4; Rechtschaffen & Kales, 1968). Participants were awakened after a minimum of 10 consecutive live‐scored 30‐s epochs of SWS. Sleep was re‐scored offline to confirm sleep stage at awakening.
The awakening procedure involved an alarm (similar to a phone ring tone), immediately followed by a researcher knocking on and opening the bedroom door with the instructions, “NAME, it's time to wake up, please sit up carefully on the side of the bed ready for testing”. A dim, red ambient light (0.26 lux, 0.00 W m–2, 0.10 equivalent daylight [D65] illuminance [EDI] melanopic lux, and peak at 714 nm) was turned on as the researcher entered the room during each night awakening. The intervention and testing equipment were moved into position ready for the intervention (or control) to be delivered 1 min after the wake‐up call, and for testing to begin 2 min after the wake‐up call.
The test battery was repeated four times at 15‐min intervals (test bout 1, 2, 3, 4 at +2, +17, +32 and +47 min after the wake‐up call, respectively). At the end of the testing period, all lights were turned off and the participant was then allowed to fall back asleep. The participant's EEG was monitored again until the next period of 10 consecutive live‐scored 30‐s epochs of SWS occurred, at which time the participant was awakened again using the same procedure as described above. Following the second testing period, all lights were turned off and the participant was allowed to sleep undisturbed until their habitual wake time. Participants were allowed to leave the laboratory approximately 1 hr after their habitual wake time.
2.3. Intervention
A 12" x 24" canvas of polychromatic short‐wavelength‐enriched light‐emitting diodes (Circadian Positioning Systems) was positioned at 15 degrees to horizontal angle of gaze and approximately 56 cm away from the participant. Illuminance, irradiance, melanopic EDI and peak spectra were measured via a Spectroradiometer ILT950 (International Lighting Technologies) as 242.77 lux, 0.95 W m–2, 338.03 melanopic lux and 456 nm, respectively. Light readings were confirmed prior to each experimental night. The light canvas was turned on 1 min after the wake‐up call to allow time to set the lighting rig into the correct position and have the participant sit up on the side of the bed. The light was illuminated 1 min before testing began and remained on throughout the sleep inertia testing period (~1 hr), and was turned off immediately after the completion of the final task. The light was positioned identically in the control condition but remained off.
2.4. Measures
A 5‐min version of the Psychomotor Vigilance Task (PVT) was used to assess vigilant attention (PVT‐192; Ambulatory Monitoring; Loh et al., 2004; Roach et al., 2006). We assessed the mean of the reciprocal response times (1000/RT, response speed) and number of lapses (reaction time > 500 ms) at baseline and during the sleep inertia arousals. These outcome metrics were chosen based on their sensitivity to sleep loss as reported by Basner and Dinges (2011), and previous demonstrations of these metrics being sensitive to sleep inertia (Van Dongen et al., 2001; Hilditch, Centofanti, et al., 2016).
Subjective sleepiness was measured using a pen‐and‐paper version of the 9‐point Karolinska Sleepiness Scale (KSS; Miley et al., 2016) ranging from 1 (Very alert) to 9 (Very sleepy, great effort to keep awake). Participants rated their mood on a range of Visual Analogue Scales (VAS). Each 100‐point VAS was anchored by a mood descriptor at each end of the scale. Scales included: (1) alert–sleepy; (2) cheerful–miserable; (3) calm–tense; (4) depressed–elated; (5) stressed–relaxed; (6) peaceful–hostile; (7) greedy–generous; (8) aggressive–easygoing; and (9) lethargic–energetic. Participants marked along the line with a pen on paper to indicate their mood relative to the anchor terms. The ambient light in the control condition was sufficient to easily read and respond to these scales.
2.4.1. Polysomnography
Sleep was monitored and recorded using BrainVision 32‐channel caps (Brain Products GmbH, Munich, Germany) and BrainVision Recorder software (Brain Products GmbH). Recording was set to a sampling rate of 500 Hz, high cut‐off filter of 70 Hz and notch filter of 60 Hz. Electrode impedances were checked and confirmed as < 10 kOhms prior to recording. Sleep during the in‐laboratory night was monitored and live‐scored for a minimum of 10 consecutive 30‐s epochs of Stage 3 or 4 sleep (i.e. SWS), the observation of which would trigger the wake‐up protocol described above. Sleep was scored offline by a single blinded scorer (EEF‐E) following Rechtschaffen and Kales (1968) rules using BrainVision Analyzer (Brain Products GmbH).
2.5. Statistics
To determine the impact of light across the testing period, we used a linear mixed‐effects model with fixed effects of condition (light, control), test (1–4, at +2, +17, +32, +47 min post‐wake‐up call), condition × test interaction, and a random effect of participant (intercept). The average of the two pre‐sleep baseline tests was included as a covariate. Due to overdispersion, a negative binomial mixed‐effects model with the same factors was used to analyse the lapse data. Effect size was calculated using R 2 (marginal and conditional). Sleep metrics were compared between conditions using paired samples t‐tests with effect size calculated using Hedge's g. An α‐value of 0.05 was considered significant except when corrected for multiple comparisons within a construct using the Bonferroni technique.
3. RESULTS
Twelve participants completed the study and are described in Table 1. Due to an equipment failure, one participant was excluded from the PVT analyses (n = 11). Due to data file corruption, one participant was excluded from the sleep‐onset latency analysis in the control condition (n = 11).
TABLE 1.
Participant demographics (n = 12)
| Male | n (%) | ||
|---|---|---|---|
| 6 (50%) | |||
| Mean | SD | Range | |
|
Age (years) At‐home sleep duration (hr) |
23.3 | 4.2 | 19–35 |
| Nights 1–5 | 7.2 | 0.6 | 6.0–8.3 |
| Night 6 | 4.4 | 0.3 | 3.8–5.1 |
| Bedtime | |||
| Nights 1–5 | 23:53 | 00:49 | 21:59–0:15 |
| Night 6 | 02:28 | 00:56 | 1:27–3:36 |
| Wake time | |||
| Nights 1–5 | 07:24 | 00:49 | 6:25–8:44 |
| Night 6 | 07:28 | 00:55 | 6:29–8:32 |
| Questionnaires | |||
| PSQI | 2.0 | 1.2 | 0–4 |
| FSS | 27.1 | 6.7 | 16–39 |
| MEQ | 55.6 | 6.4 | 45–64 |
Sleep variables estimated by actigraphy: sleep duration = sleep period minus wake after sleep onset; bed time = start of attempted sleep period; wake time = end of attempted sleep period. Range and SD are based on all individual nights, not means by participant.
FSS, Fatigue Severity Scale; MEQ, Morningness–Eveningness Questionnaire; PSQI, Pittsburgh Sleep Quality Index; SD, standard deviation.
Compared with the control condition, participants exposed to polychromatic short‐wavelength‐enriched light had fewer lapses on the PVT (main effect of condition, χ 2 [1] = 5.285, p = 0.022; marginal R 2 (R 2 M) = 0.265, conditional R 2 (R 2 C) = 0.752; Figure 2). There was no significant effect of light on PVT speed (F 1,70 = 1.984, p = 0.163). A summary of statistics can be found in Table 2. While not our primary PVT metrics of interest, median RT and fastest 10% RT have previously been shown to improve with blue‐enriched light interventions (Chellappa et al., 2011), and are plotted in the supplemental material for reference (Figure S1).
FIGURE 2.
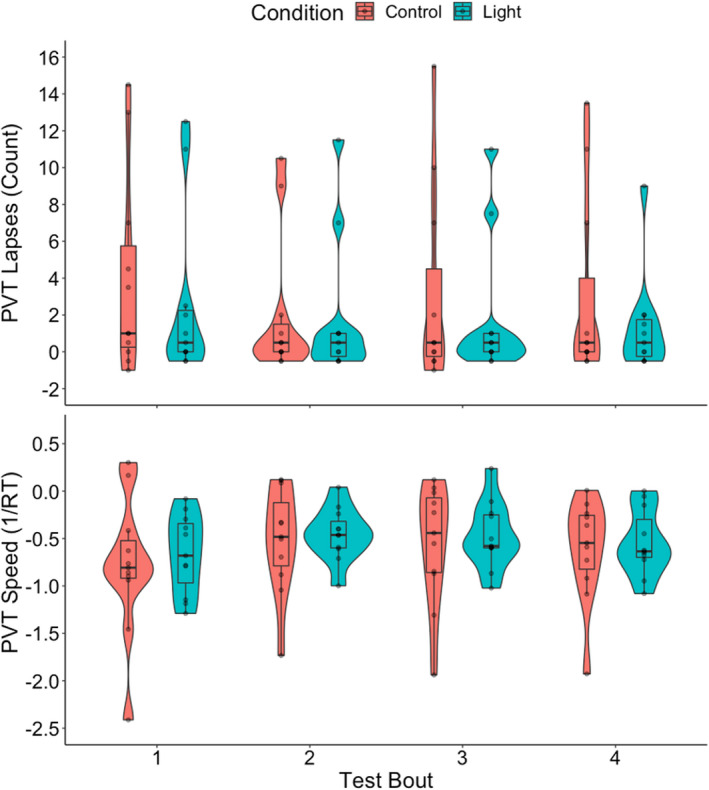
Violin plot overlaid with box plot and individual data points depicting change from baseline for Psychomotor Vigilance Task (PVT) performance across the sleep inertia testing period by condition (blue = light; red = control). Bold lines indicate median, box hinges represent first and third quartiles, whiskers extend to 1.5 times the interquartile range (IQR), individual data points displayed and visualized with density curves. RT = reaction time. Main effect (Bonferroni‐corrected) of condition for lapses (p = 0.022)
TABLE 2.
Summary of statistics
| Variable | R 2 | p‐Values | |||
|---|---|---|---|---|---|
| Marginal | Conditional | Condition | Test | Condition × Test | |
| PVT lapses | 0.265 | 0.752 | 0.022a,b | 0.261 | 0.944 |
| PVT speed | 0.572 | 0.859 | 0.163 | 0.043a | 0.984 |
| KSS | 0.107 | 0.623 | 0.029a,b | 0.359 | 0.753 |
| VAS alert | 0.086 | 0.651 | 0.005a,b | 0.109 | 0.875 |
| VAS cheerful | 0.216 | 0.782 | 0.004a,b | 0.504 | 0.822 |
| VAS calm | 0.064 | 0.595 | 0.640 | 0.800 | 0.761 |
| VAS depressed | 0.480 | 0.697 | 0.034a | 0.714 | 0.587 |
| VAS stressed | 0.087 | 0.600 | 0.810 | 0.677 | 0.895 |
| VAS peaceful | 0.046 | 0.629 | 0.224 | 0.768 | 0.883 |
| VAS greedy | 0.234 | 0.753 | 0.491 | 0.166 | 0.969 |
| VAS aggressive | 0.188 | 0.654 | 0.236 | 0.912 | 0.938 |
| VAS lethargic | 0.114 | 0.760 | 0.020a | 0.834 | 0.852 |
Trigamma R‐squared values are reported for PVT lapses. Alpha values were adjusted by construct using the Bonferroni correction: PVT (α = 0.025); KSS (α = 0.05); VAS (α = 0.006).
aUncorrected significant p‐values.
bAdjusted significant p‐values.
KSS, Karolinska Sleepiness Scale; PVT, Psychomotor Vigilance Task; VAS, Visual Analogue Scale.
Compared with the control condition, participants exposed to polychromatic short‐wavelength‐enriched light reported feeling more alert (main effect of condition, KSS: F 1,77 = 4.955, p = 0.029, R 2 M = 0.107, R 2 C = 0.623; VASalert: F 1,77 = 8.226, p = 0.005, R 2 M = 0.086, R 2 C = 0.651), more cheerful (VAScheerful: F 1,77 = 8.615, p = 0.004, R 2 M = 0.216, R 2 C = 0.782), less depressed (VASdepressed: F 1,77 = 4.649, p = 0.034, R 2 M = 0.480, R 2 C = 0.697) and less lethargic (VASlethargic: F 1,77 = 5.652, p = 0.020, R 2 M = 0.114, R 2 C = 0.760; Figure 3). The significant changes in depression and lethargy did not persist after corrections for multiple comparisons (α = 0.006). There was no significant effect of light on the other mood scales (p > 0.05).
FIGURE 3.
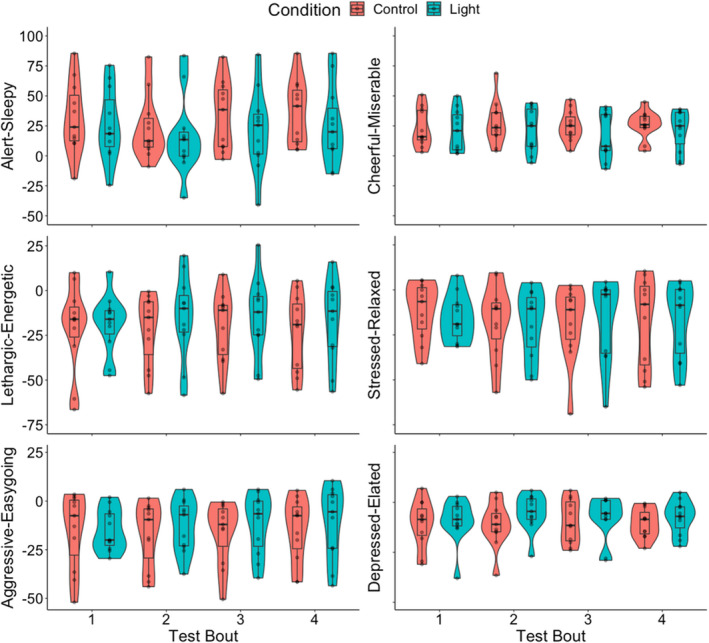
Violin plot overlaid with box plot and individual data points depicting change from baseline for subjective Visual Analogue Scales (VAS) of mood across the sleep inertia testing period by condition (blue = light; red = control). Bold lines indicate median, box hinges represent first and third quartiles, whiskers extend to 1.5 times the interquartile range (IQR), individual data points displayed and visualized with density curves. Main effect (Bonferroni‐corrected) of condition for alertness (p = 0.005) and cheerfulness (p = 0.004)
3.1. Sleep
Offline scoring confirmed that each wake‐up episode was immediately preceded by SWS. The average (± SD) consecutive SWS minutes prior to wake up was 7.1 (± 2.4) in the light condition and 7.3 (± 2.2) in the control condition. Average sleep‐onset latency following the testing period was 7.7 (± 4.5) min in the light condition and 5.3 (± 5.9) min in the control condition. Two‐tailed paired‐samples t‐tests revealed no significant differences between conditions with regard to amount of consecutive SWS before wake‐up (t 11 = −0.219, p = 0.831; Hedge's g = −0.061), or sleep‐onset latency after the testing period (t 10 = 1.024, p = 0.330; Hedge's g = 0.298). Table 3 displays the sleep stage statistics for the sleep episodes prior to each wake‐up by condition. There were no significant differences between the two conditions for any sleep metric (all p > 0.05).
TABLE 3.
Mean (SD) sleep statistics for the sleep episodes prior to each wake‐up condition
| Variable | Light | Control | p (df = 11) | Hedge's g |
|---|---|---|---|---|
| Wake (min) | 7.2 (5.4) | 8.3 (6.2) | 0.569 | −0.164 |
| Stage 1 (min) | 1.6 (1.5) | 2.6 (2.4) | 0.172 | −0.407 |
| Stage 2 (min) | 16.3 (12.6) | 15.0 (11.9) | 0.307 | 0.298 |
| Stage 3 (min) | 2.8 (1.2) | 3.5 (2.0) | 0.186 | −0.394 |
| Stage 4 (min) | 5.9 (3.5) | 5.8 (3.1) | 0.915 | 0.031 |
| REM (min) | 0.0 (0.0) | 0.4 (1.4) | 0.339 | −0.279 |
| TIB (min) | 34.1 (14.0) | 35.6 (16.6) | 0.464 | −0.212 |
| TST (min) | 26.5 (13.3) | 27.2 (11.6) | 0.710 | −0.106 |
| SE (%) | 77.7 (13.5) | 78.0 (10.3) | 0.945 | −0.019 |
Alpha values were adjusted using the Bonferroni correction: α = 0.006.
df, degrees of freedom; REM, rapid eye movement; SD, standard deviation; SE, sleep efficiency; TIB, time in bed; TST, total sleep time.
On average, the first wake‐up occurred at 23:46 ± 01:11 hours with a sleep‐onset latency of 7.1 ± 6.4 min; the second wake‐up occurred at 01:16 ± 00:52 hours with a sleep‐onset latency of 5.9 ± 3.6 min. It should be noted, however, that bedtimes varied based on habitual sleep patterns of participants. Therefore, variation in clock timing within a wake‐up does not necessarily reflect variation in circadian timing across individuals.
4. DISCUSSION
We found that participants had fewer lapses of attention upon awakening when exposed to polychromatic short‐wavelength‐enriched light compared with dim red light. In addition, participants reported feeling more alert, more cheerful, less depressed and less lethargic in the polychromatic short‐wavelength‐enriched light condition. Participants did not take longer to return to sleep after the light intervention compared with control. Given the need to mitigate the potential impact of sleep inertia on safety‐critical tasks in on‐call operations, our findings suggest that polychromatic short‐wavelength‐enriched light exposure upon awakening may help improve elements of cognitive performance, alertness and mood during the sleep inertia period following awakening from deep, nocturnal sleep.
Our study showed that polychromatic short‐wavelength‐enriched light was able to improve alertness, mood and vigilant attention following an awakening from SWS compared with dim light. We chose to focus our study on awakenings from SWS as previous studies have shown a positive association between waking from SWS, or the amount of slow‐wave activity in a sleep episode, and sleep inertia severity (Bruck & Pisani, 1999; Dinges et al., 1985; Stampi, 1992; Vallat et al., 2019). Furthermore, our study design incorporates several other known exacerbating factors of sleep inertia, namely, prior sleep loss (McHill et al., 2019; Miccoli et al., 2008) and awakening at night (Scheer et al., 2008). This finding suggests that light is a relatively effective countermeasure given that it is able to produce an effect during severe sleep inertia. Light could be useful as a countermeasure against the influence of sleep inertia when waking from other stages of sleep at night, although further research is needed to identify the mechanism(s) by which it exerts its influence. For example, on‐call awakenings could also occur towards the end of the night when rapid eye movement (REM) sleep is more prevalent (Czeisler et al., 1980). A complementary study addressing the efficacy of this countermeasure during early morning awakenings from REM sleep would be a valuable addition to this field of research.
Despite several papers referencing reduced mood during the sleep inertia period, closer inspection of these studies revealed that “mood” often refers to measures of sleepiness rather than traditional mood states (Asaoka et al., 2010; Kubo et al., 2010). To our knowledge, there are no prior reports of a mood assessment during the sleep inertia period in a non‐clinical population (see Trotti, 2017 for a review in clinical populations). Hayashi and colleagues (2003) did collect a single 5‐point scale of mood following a daytime nap but did not report the results relative to a baseline or control, thus limiting interpretation of the results. Thus, our observations of worsened mood during awakenings from SWS at night relative to pre‐sleep appear to be the first in this field. Future studies dedicated to the assessment of mood using more thorough mood scales such as Profile of Mood States or Positive and Negative Affect Scale are of interest to define the mood profile of the post‐awakening state under different conditions. Furthermore, our observation of improved mood in the light condition supports prior studies and is an important benefit when translated to occupational settings (Viola et al., 2008). Negative mood has the potential to impact teamwork dynamics and ultimately performance outcomes (Jordan et al., 2006).
We are the first to report a significant effect of post‐awakening, polychromatic short‐wavelength‐enriched light on a cognitive performance task during the first hour after waking. The acute alerting effects of short‐wavelength‐enriched light on cognitive performance have been reported during wakefulness, but prior attempts to demonstrate this effect when administered during the sleep inertia period have not been successful (Hayashi et al., 2003; Santhi et al., 2013). Our significant findings are unlikely to solely be due to the duration, intensity or spectrum of light exposure given that Santhi and colleagues’ (2013) study exposed participants to bright, short‐wavelength‐enriched light (750 lux; Melanopsin Weighted Photon Density [MWPD] 2.32 × 1018 photons m–2 s–1) for 6.5 hr. We theorize that our observations are, at least in part, due to the time of day that the light intervention was delivered. We woke participants during their habitual sleep time as opposed to during an afternoon nap (Hayashi et al., 2003) or at their habitual wake time (Santhi et al., 2013). Evidence from studies assessing the acute alerting effect of light during the daytime have had mixed results (Lok et al., 2018; Souman et al., 2018), with many failing to observe any improved outcomes (Segal et al., 2016), or reporting a reduced effect relative to exposure at night (Rahman et al., 2014). Although the phase–response curve of light with regard to circadian shifts is well established, a response profile for the acute effects of light has yet to be systematically determined, nor is the effect of light during the sleep inertia period well understood. Interestingly, Figueiro and colleagues (2019) demonstrated a beneficial effect of red light delivered through goggles after a nighttime awakening. In this study, sleep was not objectively measured, therefore the impacts of sleep depth and sleep stage at awakening on the results are unknown. While we observed better outcomes with the polychromatic short‐wavelength‐enriched light intervention compared with our red light control, a direct comparison of the light devices used in the respective studies, under controlled sleep stage conditions, is needed in order to determine the relative influence of light wavelength during the sleep inertia period.
A potential mechanism for the acute effects of light demonstrated in our study could be the suppression of melatonin. Lockley and colleagues (2006) showed a positive correlation between the degree of melatonin suppression, cognitive performance, subjective alertness and decreased power in the delta–theta range at night. We did not measure melatonin in our study, but prior studies have demonstrated that the composition of the light that we used is sufficient to suppress melatonin (West et al., 2011). The acute effects of light may also, or alternatively, be working through other physiological systems independent of melatonin such as distal skin temperature (Lok et al., 2019), which is a known biomarker of the sleep–wake transition (Kräuchi et al., 2004, 2005) and can be manipulated with light (Cajochen et al., 2005). It is possible that the effects we observed during the sleep inertia period were, at least partially, a result of light directly acting on the circadian and/or homeostatic system rather than on sleep inertia per se. In addition, the absence of a test or condition‐by‐test interaction effect may have been due to a commensurate elevation in homeostatic pressure as sleep inertia dissipated. Our study measured alertness and vigilant attention under a combination of pressures from the three‐process model, therefore we are unable to disentangle the relative actions of light on these individual systems. It is important to understand the mechanisms underlying the observed effects in order to further develop targeted countermeasures for different scenarios. Nevertheless, our demonstration of light improving alertness, mood and vigilant attention immediately after waking from SWS at night provides an important example of an effective countermeasure in a wake‐up scenario common to on‐call workers.
We assessed sleep‐onset latency following each awakening and observed no significant differences between the two conditions. This finding suggests that exposure to polychromatic short‐wavelength‐enriched light of moderate intensity for an hour during the first half of the night did not impair participants’ ability to return to sleep. The lack of differences between sleep‐onset latency in the two conditions is an important finding because countermeasures that disrupt subsequent sleep may not be appropriate in on‐call scenarios where an opportunity to return to sleep after a wake‐up is provided, or in the case of false alarms. Overall, these findings suggest that moderate‐intensity short‐wavelength‐enriched light exposure as a countermeasure to sleep inertia in the first half of the night may be a more suitable candidate in some scenarios compared with caffeine, which has a long half‐life and can impair subsequent sleep opportunities (Juliano & Griffiths, 2004; McHill et al., 2014). Further research is needed to determine whether light of different timing, spectra or intensity would yield the same results.
We evaluated an operationally viable countermeasure to sleep inertia to allow for translation and implementation of these results into strategies for promoting alertness in the workplace. Polychromatic short‐wavelength‐enriched light exposure after waking from deep sleep could improve the alertness, mood and vigilant attention of on‐call and on‐site napping workers such as first responders, doctors and military service members. The light canvas we used is a practical intervention that is easy to install. Furthermore, light is a passive intervention in that it allows personnel to perform other tasks during the intervention (unlike other potential interventions such as exercise). We used a dim red light as our control condition. Further research is needed to determine whether polychromatic short‐wavelength‐enriched light is effective relative to other occupational light settings.
Although our study employed a randomized, crossover, within‐subjects design to test the effect of light on sleep inertia following nocturnal awakenings from deep sleep, it is not without limitations. Firstly, we do not have a measure of circadian phase. To reduce circadian variability between participants, all participants were regular healthy sleepers, non‐extreme on the Morningness–Eveningness Questionnaire (MEQ), and were scheduled according to their habitual bedtime. However, within the study night, the intervention and control wake‐ups were conducted at slightly different circadian times and following differing sleep pressures. Our randomized order of intervention aimed to address this potential order effect. Furthermore, we do not know whether light exposure altered circadian phase within the night and how this might affect the use of light as a countermeasure to sleep inertia in repeated exposures. However, field studies of nightshift‐workers have shown that several consecutive nights of nocturnal light exposure only facilitate a modest phase shift at best (Ferguson et al., 2012; Stone et al., 2018). Given the degree of individual variation in circadian adaptation and response to light in real‐world settings (Stone et al., 2018), future studies should include a measure of circadian phase and trial repeated light exposures across several wake‐ups to test both the efficacy of repeated exposures and the potential influence on circadian rhythms. In addition, studies employing a larger sample size to investigate potential sex differences in the response to light during the sleep inertia period are warranted given the findings from Chellappa et al. (2017) showing males have a stronger response to blue‐enriched light. Finally, given the novelty of our study design and intervention, we chose to focus our assessment of cognitive performance using the well‐established PVT. It is difficult to assess the impact of sleep inertia on multiple cognitive domains in one study as the effects of sleep inertia are uniquely time‐critical. Future studies should trial other, more complex cognitive performance tasks that are also sensitive to sleep inertia (e.g. visual search task; Burke et al., 2015) to determine whether the effects of light on vigilant attention extend to other cognitive domains.
5. CONCLUSION
The risk of cognitive performance impairment during the sleep inertia period needs to be managed when safety critical tasks are required soon after waking. Our study showed that exposure to bright, polychromatic short‐wavelength‐enriched light following awakening from SWS improved vigilant attention, alertness and mood compared with a dim, red light. Further research is required in order to determine the mechanisms through which light exerted these effects and whether there are any potential circadian side‐effects. Our light intervention should also be tested in the workplace to confirm its efficacy under real‐world conditions. Further exploration into other reactive countermeasures, and potentially their combination, is needed in order to improve the performance and safety of those required to undertake safety‐critical tasks soon after waking.
CONFLICTS OF INTEREST
The authors report no conflicts of interest in this work.
AUTHOR CONTRIBUTIONS
CJH: conception, methodology, data collection, data analysis, manuscript writing, manuscript review; LRW: methodology, data collection, manuscript review; NGB: recruitment, data analysis, manuscript review; NHF: methodology, data collection, data analysis; SP: data analysis, manuscript review; AS: methodology, manuscript review; NS: methodology, manuscript review; EEF: conception, methodology, data collection, data analysis, manuscript review.
Supporting information
Fig S1
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in part by the Naval Medical Research Center's Naval Advanced Medical Development Program (MIPR N3239820WXHN007), and the NASA Airspace Operations and Safety Program, System‐Wide Safety. The authors thank all the participants who volunteered their time for this study.
Hilditch, C. J. , Wong, L. R. , Bathurst, N. G. , Feick, N. H. , Pradhan, S. , Santamaria, A. , Shattuck, N. L. , & Flynn‐Evans, E. E. (2022). Rise and shine: The use of polychromatic short‐wavelength‐enriched light to mitigate sleep inertia at night following awakening from slow‐wave sleep. Journal of Sleep Research, 31, e13558. 10.1111/jsr.13558
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Achermann, P. , Werth, E. , Dijk, D. J. , & Borbely, A. (1995). Time course of sleep inertia after nighttime and daytime sleep episodes. Archives Italiennes De Biologie, 134(1), 109–119. [PubMed] [Google Scholar]
- Åkerstedt, T. , & Gillberg, M. (1986). A dose‐response study of sleep loss and spontaneous sleep termination. Psychophysiology, 23(3), 293–297. 10.1111/j.1469-8986.1986.tb00635.x [DOI] [PubMed] [Google Scholar]
- Asaoka, S. , Masaki, H. , Ogawa, K. , Murphy, T. I. , Fukuda, K. , & Yamazaki, K. (2010). Performance monitoring during sleep inertia after a 1‐h daytime nap. Journal of Sleep Research, 19(3), 436–443. 10.1111/j.1365-2869.2009.00811.x [DOI] [PubMed] [Google Scholar]
- Basner, M. , & Dinges, D. F. (2011). Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep, 34(5), 581–591. 10.1093/sleep/34.5.581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbély, A. A. (1982). A two process model of sleep regulation. Human Neurobiology, 1(3), 195–204. [PubMed] [Google Scholar]
- Bruck, D. , & Pisani, D. L. (1999). The effects of sleep inertia on decision‐making performance. Journal of Sleep Research, 8(2), 95–103. 10.1046/j.1365-2869.1999.00150.x [DOI] [PubMed] [Google Scholar]
- Burke, T. M. , Scheer, F. A. , Ronda, J. M. , Czeisler, C. A. , & Wright, K. P. Jr (2015). Sleep inertia, sleep homeostatic and circadian influences on higher‐order cognitive functions. Journal of Sleep Research, 24(4), 364–371. 10.1111/jsr.12291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajochen, C. , Münch, M. , Kobialka, S. , Kräuchi, K. , Steiner, R. , Oelhafen, P. , Orgül, S. , & Wirz‐Justice, A. (2005). High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. The Journal of Clinical Endocrinology & Metabolism, 90(3), 1311–1316. 10.1210/jc.2004-0957 [DOI] [PubMed] [Google Scholar]
- Chellappa, S. L. , Steiner, R. , Blattner, P. , Oelhafen, P. , Götz, T. , & Cajochen, C. (2011). Non‐visual effects of light on melatonin, alertness and cognitive performance: Can blue‐enriched light keep us alert? PLoS One, 6(1), e16429. 10.1371/journal.pone.0016429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellappa, S. L. , Steiner, R. , Oelhafen, P. , & Cajochen, C. (2017). Sex differences in light sensitivity impact on brightness perception, vigilant attention and sleep in humans. Scientific Reports, 7(1), 1–9. 10.1038/s41598-017-13973-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeisler, C. A. , Zimmerman, J. C. , Ronda, J. M. , Moore‐Ede, M. C. , & Weitzman, E. D. (1980). Timing of REM sleep is coupled to the circadian rhythm of body temperature in man. Sleep, 2(3), 329–346. [PubMed] [Google Scholar]
- Dawson, D. , Ferguson, S. A. , & Vincent, G. E. (2020). Safety implications of fatigue and sleep inertia for emergency services personnel. Sleep Medicine Reviews, 55, 101386. [DOI] [PubMed] [Google Scholar]
- Dinges, D. F. , Orne, M. T. , & Orne, E. C. (1985). Assessing performance upon abrupt awakening from naps during quasi‐continuous operations. Behavior Research Methods, Instruments, & Computers, 17(1), 37–45. 10.3758/BF03200895 [DOI] [Google Scholar]
- Ferguson, S. A. , Kennaway, D. J. , Baker, A. , Lamond, N. , & Dawson, D. (2012). Sleep and circadian rhythms in mining operators: Limited evidence of adaptation to night shifts. Applied Ergonomics, 43(4), 695–701. 10.1016/j.apergo.2011.11.003 [DOI] [PubMed] [Google Scholar]
- Ferguson, S. A. , Paterson, J. L. , Hall, S. J. , Jay, S. M. , & Aisbett, B. (2016). On‐call work: To sleep or not to sleep? It Depends. Chronobiology International, 33(6), 678–684. 10.3109/07420528.2016.1167714 [DOI] [PubMed] [Google Scholar]
- Figueiro, M. G. , Sahin, L. , Roohan, C. , Kalsher, M. , Plitnick, B. , & Rea, M. S. (2019). Effects of red light on sleep inertia. Nature and Science of Sleep, 11, 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkard, S. , & Åkerstedt, T. (1992). A three‐process model of the regulation of alertness‐sleepiness. In Broughton R. J., & Ogilvie R. D. (Eds.)., Sleep, arousal, and performance (pp. 11–26). Birkhäuser. [Google Scholar]
- Hayashi, M. , Masuda, A. , & Hori, T. (2003). The alerting effects of caffeine, bright light and face washing after a short daytime nap. Clinical Neurophysiology, 114(12), 2268–2278. 10.1016/S1388-2457(03)00255-4 [DOI] [PubMed] [Google Scholar]
- Hilditch, C. J. , Centofanti, S. A. , Dorrian, J. , & Banks, S. (2016). A 30‐minute, but not a 10‐minute nighttime nap is associated with sleep inertia. Sleep, 39(3), 675–685. 10.5665/sleep.5550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilditch, C. J. , Dorrian, J. , & Banks, S. (2016). Time to wake up: Reactive countermeasures to sleep inertia. Industrial Health, 54, 528–541. 10.2486/indhealth.2015-0236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilditch, C. J. , Dorrian, J. , Centofanti, S. A. , Van Dongen, H. P. , & Banks, S. (2017). Sleep inertia associated with a 10‐min nap before the commute home following a night shift: A laboratory simulation study. Accident Analysis & Prevention, 99, 411–415. 10.1016/j.aap.2015.11.010 [DOI] [PubMed] [Google Scholar]
- Hofer‐Tinguely, G. , Achermann, P. , Landolt, H.‐P. , Regel, S. J. , Rétey, J. V. , Dürr, R. , Borbély, A. A. , & Gottselig, J. M. (2005). Sleep inertia: performance changes after sleep, rest and active waking. Cognitive Brain Research, 22(3), 323–331. 10.1016/j.cogbrainres.2004.09.013 [DOI] [PubMed] [Google Scholar]
- Jordan, P. J. , Lawrence, S. A. , & Troth, A. C. (2006). The impact of negative mood on team performance. Journal of Management & Organization, 12(2), 131–145. 10.5172/jmo.2006.12.2.131 [DOI] [Google Scholar]
- Juliano, L. M. , & Griffiths, R. R. (2004). A critical review of caffeine withdrawal: Empirical validation of symptoms and signs, incidence, severity, and associated features. Psychopharmacology (Berl), 176(1), 1–29. 10.1007/s00213-004-2000-x [DOI] [PubMed] [Google Scholar]
- Kräuchi, K. , Cajochen, C. , & Wirz‐Justice, A. (2004). Waking up properly: Is there a role of thermoregulation in sleep inertia? Journal of Sleep Research, 13(2), 121–127. 10.1111/j.1365-2869.2004.00398.x [DOI] [PubMed] [Google Scholar]
- Kräuchi, K. , Cajochen, C. , & Wirz‐Justice, A. (2005). Thermophysiologic aspects of the three‐process‐model of sleepiness regulation. Clinics in Sports Medicine, 24(2), 287–300. 10.1016/j.csm.2004.12.009 [DOI] [PubMed] [Google Scholar]
- Kubo, T. , Takahashi, M. , Takeyama, H. , Matsumoto, S. , Ebara, T. , Murata, K. , Tachi, N. , & Itani, T. (2010). How do the timing and length of a night‐shift nap affect sleep inertia? Chronobiology International, 27(5), 1031–1044. 10.3109/07420528.2010.489502 [DOI] [PubMed] [Google Scholar]
- Lockley, S. W. , Evans, E. E. , Scheer, F. A. , Brainard, G. C. , Czeisler, C. A. , & Aeschbach, D. (2006). Short‐wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep, 29(2), 161–168. [PubMed] [Google Scholar]
- Loh, S. , Lamond, N. , Dorrian, J. , Roach, G. , & Dawson, D. (2004). The validity of psychomotor vigilance tasks of less than 10‐minute duration. Behavior Research Methods, Instruments, & Computers, 36(2), 339–346. 10.3758/BF03195580 [DOI] [PubMed] [Google Scholar]
- Lok, R. , Smolders, K. C. , Beersma, D. G. , & de Kort, Y. A. (2018). Light, alertness, and alerting effects of white light: A literature overview. Journal of Biological Rhythms, 33(6), 589–601. 10.1177/0748730418796443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok, R. , van Koningsveld, M. J. , Gordijn, M. C. , Beersma, D. G. , & Hut, R. A. (2019). Daytime melatonin and light independently affect human alertness and body temperature. Journal of Pineal Research, 67(1), e12583. 10.1111/jpi.12583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHill, A. W. , Hull, J. T. , Cohen, D. A. , Wang, W. , Czeisler, C. A. , & Klerman, E. B. (2019). Chronic sleep restriction greatly magnifies performance decrements immediately after awakening. Sleep, 42(5), zsz032. 10.1093/sleep/zsz032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHill, A. W. , Smith, B. J. , & Wright, K. P. Jr (2014). Effects of caffeine on skin and core temperatures, alertness, and recovery sleep during circadian misalignment. Journal of Biological Rhythms, 29(2), 131–143. 10.1177/0748730414523078 [DOI] [PubMed] [Google Scholar]
- Miccoli, L. , Versace, F. , Koterle, S. , & Cavallero, C. (2008). Comparing sleep‐loss sleepiness and sleep inertia: Lapses make the difference. Chronobiology International, 25(5), 725–744. 10.1080/07420520802397228 [DOI] [PubMed] [Google Scholar]
- Miley, A. Å. , Kecklund, G. , & Åkerstedt, T. (2016). Comparing two versions of the karolinska sleepiness scale (KSS). Sleep and Biological Rhythms, 14(3), 257–260. 10.1007/s41105-016-0048-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman, S. A. , Flynn‐Evans, E. E. , Aeschbach, D. , Brainard, G. C. , Czeisler, C. A. , & Lockley, S. W. (2014). Diurnal spectral sensitivity of the acute alerting effects of light. Sleep, 37(2), 271–281. 10.5665/sleep.3396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechtschaffen, A. , & Kales, A. (1968). A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Brain Information Service/Brain Research Institute. [Google Scholar]
- Roach, G. D. , Dawson, D. , & Lamond, N. (2006). Can a shorter psychomotor vigilance task be usedas a reasonable substitute for the ten‐minute psychomotor vigilance task? Chronobiology International, 23(6), 1379–1387. 10.1080/07420520601067931 [DOI] [PubMed] [Google Scholar]
- Santhi, N. , Groeger, J. A. , Archer, S. N. , Gimenez, M. , Schlangen, L. J. , & Dijk, D. J. (2013). Morning sleep inertia in alertness and performance: Effect of cognitive domain and white light conditions. PLoS One, 8(11), e79688. 10.1371/journal.pone.0079688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer, F. A. , Shea, T. J. , Hilton, M. F. , & Shea, S. A. (2008). An endogenous circadian rhythm in sleep inertia results in greatest cognitive impairment upon awakening during the biological night. Journal of Biological Rhythms, 23(4), 353–361. 10.1177/0748730408318081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuermaier, K. , Münch, M. , Ronda, J. M. , & Duffy, J. F. (2018). Improved cognitive morning performance in healthy older adults following blue‐enriched light exposure on the previous evening. Behavioural Brain Research, 348, 267–275. 10.1016/j.bbr.2018.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal, A. Y. , Sletten, T. L. , Flynn‐Evans, E. E. , Lockley, S. W. , & Rajaratnam, S. M. (2016). Daytime exposure to short‐and medium‐wavelength light did not improve alertness and neurobehavioral performance. Journal of Biological Rhythms, 31(5), 470–482. 10.1177/0748730416659953 [DOI] [PubMed] [Google Scholar]
- Souman, J. L. , Tinga, A. M. , Te Pas, S. F. , Van Ee, R. , & Vlaskamp, B. N. (2018). Acute alerting effects of light: A systematic literature review. Behavioural Brain Research, 337, 228–239. 10.1016/j.bbr.2017.09.016 [DOI] [PubMed] [Google Scholar]
- Stampi, C. (1992). The effects of polyphasic and ultrashort sleep schedules. In Stampi C. (Ed.). Why we nap (pp. 137–179). Springer. [Google Scholar]
- Stone, J. E. , Sletten, T. L. , Magee, M. , Ganesan, S. , Mulhall, M. D. , Collins, A. , Howard, M. , Lockley, S. W. , & Rajaratnam, S. M. W. (2018). Temporal dynamics of circadian phase shifting response to consecutive night shifts in healthcare workers: Role of light–dark exposure. The Journal of Physiology, 596(12), 2381–2395. 10.1113/JP275589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassi, P. , Bonnefond, A. , Engasser, O. , Hoeft, A. , Eschenlauer, R. , & Muzet, A. (2006). EEG spectral power and cognitive performance during sleep inertia: The effect of normal sleep duration and partial sleep deprivation. Physiology & Behavior, 87(1), 177–184. 10.1016/j.physbeh.2005.09.017 [DOI] [PubMed] [Google Scholar]
- Trotti, L. M. (2017). Waking up is the hardest thing I do all day: Sleep inertia and sleep drunkenness. Sleep Medicine Reviews, 35, 76–84. 10.1016/j.smrv.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallat, R. , Meunier, D. , Nicolas, A. , & Ruby, P. (2019). Hard to wake up? The cerebral correlates of sleep inertia assessed using combined behavioral, EEG and fMRI measures. NeuroImage, 184, 266–278. 10.1016/j.neuroimage.2018.09.033 [DOI] [PubMed] [Google Scholar]
- Van Dongen, H. P. , Price, N. J. , Mullington, J. M. , Szuba, M. P. , Kapoor, S. C. , & Dinges, D. F. (2001). Caffeine eliminates psychomotor vigilance deficits from sleep inertia. Sleep, 24(7), 813–819. 10.1093/sleep/24.7.813 [DOI] [PubMed] [Google Scholar]
- Vincent, G. E. , Kinchin, I. , Ferguson, S. A. , & Jay, S. M. (2018). The cost of inadequate sleep among on‐call workers in Australia: A workplace perspective. International Journal of Environmental Research and Pblic Health, 15(3), 398. 10.3390/ijerph15030398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola, A. U. , James, L. M. , Schlangen, L. J. , & Dijk, D. J. (2008). Blue‐enriched white light in the workplace improves self‐reported alertness, performance and sleep quality. Scandinavian Journal of Work, Environment & Health, 297–306. 10.5271/sjweh.1268 [DOI] [PubMed] [Google Scholar]
- Wertz, A. T. , Ronda, J. M. , Czeisler, C. A. , & Wright, K. P. (2006). Effects of sleep inertia on cognition. JAMA, 295(2), 159–164. [DOI] [PubMed] [Google Scholar]
- West, K. E. , Jablonski, M. R. , Warfield, B. , Cecil, K. S. , James, M. , Ayers, M. A. , Maida, J. , Bowen, C. , Sliney, D. H. , Rollag, M. D. , Hanifin, J. P. , & Brainard, G. C. (2011). Blue light from light‐emitting diodes elicits a dose‐dependent suppression of melatonin in humans. Journal of Applied Physiology, 110(3), 619–626. 10.1152/japplphysiol.01413.2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


