Abstract
Introduction
The present review aimed to establish prevalence rates of anxiety and depression in adults with haematology cancer, with a focus on the differences between patients under treatment and patients under watchful waiting.
Method
Five databases (Scopus, Medline, PsycINFO, EThOS, CINAHL) were searched throughout June 2021. Key search terms included haematology cancer, anxiety, depression, in treatment and watchful waiting. Study and sample characteristics, prevalence rates and mean self‐reported scores of anxiety and depression data were extracted.
Results
A total of 18 eligible papers were included in the review. Quality appraisal indicated papers were of adequate standard. Depression data from 2720 participants (14.5% under watchful waiting) and anxiety data from 2520 participants (15.9% under watchful waiting) were analysed through subgroup meta‐analyses. The prevalence of anxiety was 34% amongst adults receiving treatment and 24.5% amongst those under watchful waiting. The prevalence of depression amongst adults receiving treatment was 31.3%, significantly higher than 16.1% of adults under watchful waiting.
Conclusion
Overall, adults with haematology cancer were at greater risk of experiencing anxiety and depression than the general population, with greatest risk in those under treatment. The findings indicate the need for future research to examine availability and effectiveness of targeted psychological interventions.
Keywords: anxiety, cancer, depression, haematology, meta‐analysis, prevalence
1. INTRODUCTION
Haematology cancer (HC) describes any cancer affecting the blood, bone or lymphatic system (NICE, 2016). The term describes over 90 diagnoses, each associated with different characteristics (Blood Cancer UK, 2019). HC accounts for an estimated 5% of cancer cases globally (Sung et al., 2021) and 9% of those diagnosed in high economically developed countries (Smith et al., 2011). Though HC affects individuals across the lifespan, there is a marked increase in incidence in individuals aged 50 years and over (Smith et al., 2011).
On average, 50% of people diagnosed with HC in the United Kingdom are expected to survive for 10 years or more, thanks to advances in treatment development (Foster et al., 2018). The move from viewing cancer as an acute life‐threatening illness to a chronic condition means focus has shifted to exploring how individuals can live well alongside their diagnosis (Pitman et al., 2018). Anxiety and depression are commonly diagnosed disorders that can significantly increase the psychosocial burden of living with cancer (Gold et al., 2020). It is estimated that depression affects approximately 20%, and anxiety approximately 10%, of people living with cancer, higher than prevalence rates seen in the general population (Pitman et al., 2018). Psycho‐oncology theories have aimed to explain mechanisms of increased risk of psychological distress in cancer (Barroilhet et al., 2005). The Folkman model (Holland, 2002), based on the stress and coping model (Lazarus & Folkman, 1984), proposes that a person's appraisal of the cancer diagnosis, and their coping style, influence the emotional response to having cancer. Appraisals of high threat and unhelpful coping styles have been linked to increased reports of experiencing psychological distress (Greer et al., 1989). Though such theories may require updating, recent research has found the model remains applicable in cancer settings (Admiraal, 2020; Kim et al., 2019).
The prevalence of anxiety in people living with HC is estimated to be 20%–37% (Clinton‐McHarg et al., 2014), compared to 7% in the general population (Steel et al., 2014). In addition to impacting patients' quality of life, anxiety in cancer has been linked to an increase in pain, vomiting and sleep disturbance (Baqutayan, 2012). Depression is estimated to affect between 17% and 51% (Clinton‐McHarg et al., 2014) of the HC population, substantially higher than the estimated 5% in the general population (Steel et al., 2014). A comorbid diagnosis of depression has been linked to poorer adherence to cancer treatment, consequent poorer health outcomes and increased mortality (Sherrill et al., 2017). It is important to note that prevalence of anxiety and depression is the most commonly assessed through self‐report measures (Mitchell et al., 2010), a method that has received criticism. In cancer patients, high base rates for adjustment disorders are reported, and generic mood questionnaires do not differentiate between these and anxiety and depression (Vodermaier & Millman, 2011). In addition, self‐reporting relies on the responder's understanding and openness and omits clinical judgement. Therefore, the way in which self‐report measures have been applied may hold implications for prevalence rates reported. However, research also benefits from such validated tools due to their ease of application and utility in allowing for comparisons across samples (Mitchell et al., 2010).
Comorbid psychological distress has implications for wider society (Mausbach et al., 2020). Cancer patients diagnosed with anxiety or depression were significantly more likely to attend an emergency department and to require hospitalisations and, on average, were hospitalised for 73% longer than cancer patients without anxiety or depression (Mausbach et al., 2020). In total, cancer patients presenting with anxiety and/or depression incurred healthcare costs that were over double cancer patients without anxiety and/or depression. Consequently, anxiety and depression place additional financial pressure on countries with national healthcare systems, such as the United Kingdom. For individuals who pay for their healthcare, psychological distress is likely to increase the financial burden of having cancer, which, in turn, has been linked to higher risk of anxiety and depression (Hall et al., 2016).
Some HC diagnoses are classed as acute and require immediate and often debilitating treatment, such as chemotherapy, radiotherapy or a bone marrow transplant (NICE, 2016). Side effects from such treatments can include fatigue, nausea, vomiting, neutropenia and an increased risk of infection (MacMillan Cancer Support, 2020). Aggressive treatments with such side effects have been associated with an increased risk of individuals presenting with anxiety and depression (Allart‐Vorelli et al., 2015). Other HC diagnoses are slow‐growing chronic diseases that may not require immediate treatment. Patients with such diagnoses can be placed under ‘watchful waiting’, a disease monitoring pathway whereby patients attend regular check‐ups with their care team (also known as active surveillance or active monitoring) (Blood Cancer UK, 2019). Whilst patients under watchful waiting do not experience debilitating treatment side effects, managing a chronic HC significantly impacts individuals' ability to work, plan for the future and maintain a family life (Evans et al., 2012).
In the wider cancer population, anxiety and depression are more commonly noted in the early stages of receiving a diagnosis, with symptoms reducing following commencement of treatment (Niedzwiedz et al., 2019). In contrast, in HC patients, anxiety and depression are reported to remain high over time (Oerlemans et al., 2014) and do not correlate with receiving treatment (Walker et al., 2014). Despite preliminary findings indicating HC patients under watchful waiting and under treatment experience significant levels of anxiety and depression, provision of psychological support differs. Leukaemia Care (2019) surveyed 1152 HC patients about support provision when under treatment and when under watchful waiting. Those under watchful waiting were significantly less likely to be offered information on emotional support (39% vs. 69%) or access to a clinical nurse specialist, despite this being identified as a key driver of improved patient experience by NHS England (Leukaemia Care, 2019).
Whilst there is extensive research examining the prevalence of anxiety and depression and associated effective support in the wider cancer population, there are comparatively few studies examining the prevalence in HC patients and even fewer in those under watchful waiting. Moreover, there are several key differences associated with HC that may make findings from the wider psycho‐oncology literature inapplicable. Such differences include lack of a solid tumour site, absence of physical signs of illness and differences in treatment regimens (Swash, 2015). There are also important differences in watchful waiting pathways across cancer diagnoses. For example, in the United Kingdom, prostate cancer patients typically have choice between watchful waiting and surgical intervention (NICE, 2019), whereas treatment is predominantly dictated by disease stage in HC (NICE, 2016).
Depression and anxiety can significantly impact the quality of life of people living with cancer (Pitman et al., 2018), as well as increase financial pressure on individuals or healthcare systems. Understanding the prevalence of depression and anxiety is essential for understanding the need for, and development of, evidence‐based psychological interventions to address distress. However, prevalence estimates vary across individual studies. Therefore, this paper aims to systematically review literature to answer the question: ‘What are the prevalence rates of anxiety and depression in adults living with HC in ongoing treatment and under watchful waiting?’ It is hoped that an indication of overall prevalence, as well as differences between treatment pathways, will inform policymakers and service providers in developing effective care pathways in supporting people living with HC.
2. METHOD
2.1. Protocol and registration
The present systematic review was registered with PROSPERO (registration number CRD42021265435). The Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines provided a framework for development of the review protocol (Page et al., 2021).
2.2. Search strategy
PsycINFO, Medline, CINAHL, Scopus and EThOS databases were systematically searched for literature examining anxiety and depression in people with HC. Searches were initiated and completed in June 2021. No date limit was set. On PsycINFO, Medline and CINAHL, both free text and index terms were used; on Scopus and EThOS, only free‐text search was available. Given the scarcity of research examining patients under watchful waiting and in treatment in a single study, two separate searches were conducted across all databases. Both searches contained terms relating to depression, anxiety and HC. In addition, one search included search terms relating to the watchful waiting pathway, whilst the other search included terms relating to ongoing treatment. Broad search terms and multiple alternatives to key words were used to increase opportunity to identify relevant literature. A published review was also used as a key terms guide (Watts et al., 2014). An example of a full search strategy can be viewed in Figure 1. Bibliographies of relevant reviews and included articles were examined to identify additional relevant literature.
FIGURE 1.
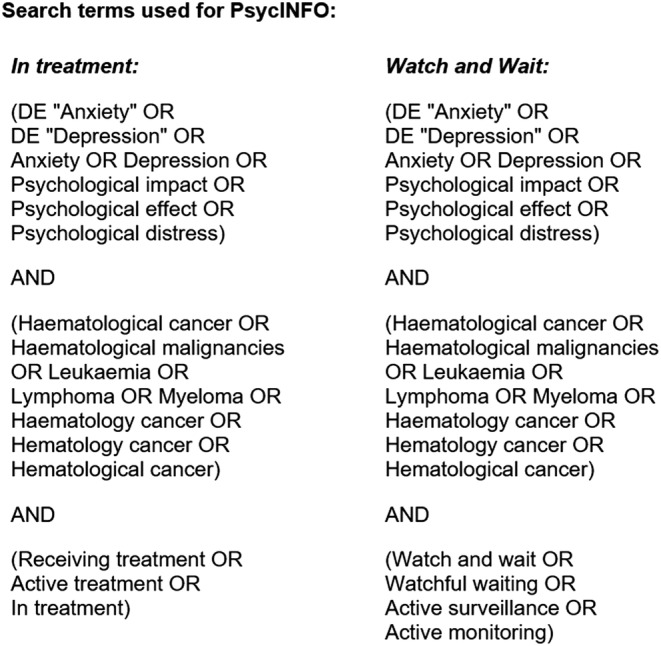
Full search strategies used in PsycINFO database via the EBSCO host platform
2.3. Study selection
Following identification of potentially relevant studies, duplicates were removed, and article titles and abstracts were screened by the main author. Full‐text papers were then obtained and screened for inclusion in the review against the eligibility criteria. Grey literature was included to minimise the impact of publication bias, given recruitment issues in this population are not uncommon (Swash, 2015).
2.4. Eligibility criteria
Articles that met the following criteria were considered for inclusion:
Sample comprised participants over the age of 18 and diagnosed with an HC at the time of study participation.
Participants were described as being under watchful waiting or in ongoing treatment.
Empirical study reporting original quantitative anxiety and/or depression data, determined using a validated measure.
Available in English.
Studies were excluded if:
Participants had completed treatment and awaiting further treatment. This excluded studies that examined the impact of discrete treatments such as a bone marrow transplant. Patients undergoing such treatments were viewed as being on a third treatment trajectory, beyond the scope of the present review.
Participants were identified as having anxiety or depression diagnoses prior to cancer diagnosis.
- Inappropriate data presentation meaning suitable data could not be extracted:
- Depression and anxiety scores were combined, and separate means or prevalence rates were not presented.
- Depression and anxiety scores for those under watchful waiting and those in treatment were combined, or the paper did not explicitly state the treatment trajectory of the sample the scores represented.
- Depression and anxiety scores included participants with non‐haematological cancers.
2.5. Data abstraction
The main author extracted and recorded key data from included papers, including study design, sample characteristics and outcome measures of anxiety and depression.
2.6. Quality appraisal
Each included article was given a quality rating by both authors. Due to lack of a ‘gold standard’ quality assessment tool, one tool was adapted to ensure the included reviews were thoroughly appraised (Quigley et al., 2019). It was anticipated that articles reporting various study designs would be included in the review. Therefore, an adapted version of the Standard Quality Assessment Criteria for Evaluating Quantitative Primary Research Papers from a Variety of Fields (QualSyst) (Kmet et al., 2004) was used. Adaptions included omitting three questions pertaining to randomisation of participants due to the review only considering baseline data (see Section 2.4), and therefore, randomisation to intervention group being irrelevant to the quality of the baseline data reported. One question from the Quality Assessment Tool for Quantitative Studies (Thomas et al., 2004) on sample representativeness was included to capture risk of selection bias. Each article was given a rating out of 2 (2 = criterion met; 1 = criterion partially met or cannot tell; 0 = criterion not met) on each question of the appraisal. A total score was provided for each question and for each article. The Oxford Levels of Evidence were also used to appraise the level of evidence in each study (OCEBM, 2011).
2.7. Data analysis
Depression and anxiety data were analysed separately through an identical meta‐analysis process. Descriptive data [means, standard deviations (SD), sample prevalence percentages and sample sizes] were extracted and entered into meta‐analysis software: OpenMeta[Analyst] (Wallace et al., 2012). Subgroup analyses were conducted for means data and prevalence data. Random effects modelling (DerSimonian & Laird, 1986) was chosen due to non‐randomisation of participants to each group and the heterogeneity between samples. Pooled estimates of prevalence of anxiety and depression in each subgroup were computed using 95% confidence intervals (CI). Pooled estimates of subgroup means and SDs were computed. Pooled estimates were compared to test for statistical significance through further random‐effects subgroup analysis (Borenstein et al., 2009).
3. RESULTS
3.1. Systematic search results
Systematic database searching returned 1856 articles. After removing duplicates, 1695 articles remained, and 1677 of these were excluded at title, abstract or full‐text level, depending on when ineligibility became evident. Manual searching of reference lists returned one previously unidentified article. Following screening, 18 articles met eligibility criteria. Figure 2 illustrates a detailed breakdown of article selection and exclusion.
FIGURE 2.
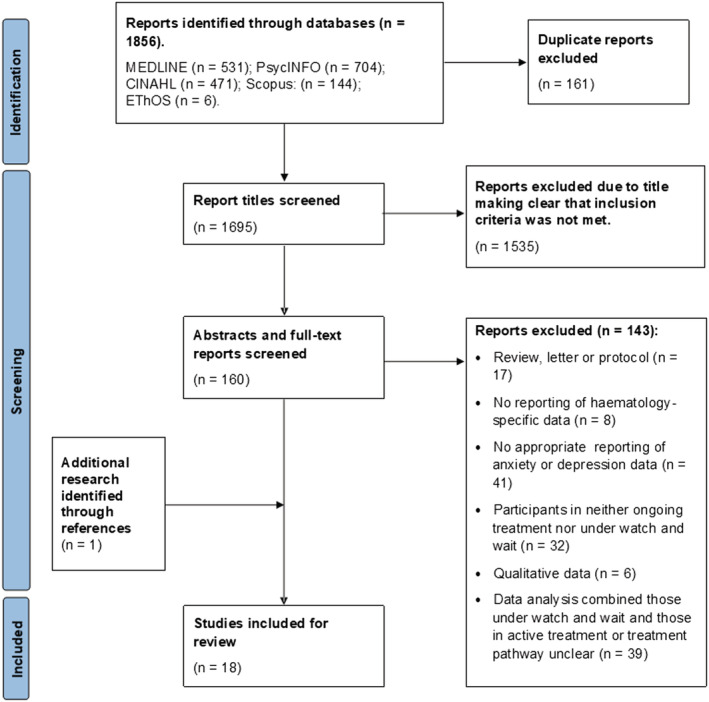
PRISMA diagram illustrating article selection and exclusion process (Page et al., 2021)
3.2. Study characteristics
Table 1 outlines study characteristics. Studies comprised cross‐sectional and longitudinal designs. All 18 reported depression data (17 reported means; 13 reported prevalence), whilst 17 reported anxiety data (16 reported means; 11 reported prevalence). In total, depression data were extracted from 2720 participants (14.5% under watchful waiting); anxiety data were extracted from 2520 (15.9% under watchful waiting). The difference resulted from one study (n = 200) only measuring depression (5). Sample sizes ranged from 17 to 489 participants. The average age of participants across studies was 53 years with 61% male. Though average age and gender ratios vary depending on diagnosis, generally HC is more commonly diagnosed in males aged over 50 years (Smith et al., 2011), as reflected in the overall characteristics. Only five studies reported ethnicity (2, 10, 13, 14, 15), each reporting a majority Caucasian sample (range 75%–100%). Studies were conducted in 11 countries, across five continents; however, all studies reporting ethnicity were based in the United Kingdom or the United States. Therefore, the overall ethnicity of the sample was unknown. Across studies, 11 types of HC diagnoses were reported, with multiple myeloma and chronic lymphocytic leukaemia mostly commonly described.
TABLE 1.
Study characteristics
| No. | Author and country | Study design (OCEBM) level of evidence | Sample size (% men); mean age (age range); sample ethnicity | Haematology diagnosis | Treatment received | Outcome measures | Key relevant findings | |
|---|---|---|---|---|---|---|---|---|
|
Anxiety Mean (SD) |
Depression Mean (SD) |
|||||||
| 1 |
Bellali et al. (2020) Greece |
Cross sectional (Level 3) | 76 (65.8%); 58.4 years (not given); not given |
Non‐Hodgkin's lymphoma, chronic lymphatic leukaemia, Hodgkin's lymphoma, multiple myeloma |
In treatment: chemotherapy |
Anxiety: HADS a Depression: HADS a |
12.7 (4.5) | 11.1 (4.0) |
| 2 |
Bryant et al. (2018) USA |
Randomised control trial (Level 3) | 17 (62.5%); 50 years (28–69); 76% White, 24% African American | Acute myeloid Leukaemia, acute lymphoblastic Leukaemia | In treatment: Chemotherapy |
Anxiety: PROMIS b Depression: PROMIS b |
Baseline for intervention group: 58.2 Baseline for control group: 59.4 Prevalence: 58% |
Baseline for intervention group: 58 Baseline for control group: 59.5 Prevalence: 58% |
| 3 |
Fukushima et al. (2018) Japan |
Cohort study (Level 3) | 44 (47.7%); 68 years (41–86); not given | Acute myeloid leukaemia, acute lymphoblastic leukaemia, multiple myeloma, chronic myeloid leukaemia | In treatment: Chemotherapy |
Anxiety: HADS a Depression: HADS a |
High exercise engagement: 5.8 (3.2) Low exercise engagement: 6.4 (4.8) |
High exercise engagement: 7.0 (3.0) Low exercise engagement: 5.9 (3.9) |
| 4 |
Furzer et al. (2016) Australia |
Randomised control trial (Level 3) | 37 (not given); 49 years (22–68); not given |
Non‐Hodgkin's lymphoma Hodgkin's lymphoma Multiple myeloma |
In treatment: chemotherapy (100%), radiotherapy (27%), stem cell transplant (13.5%) |
Anxiety: HADS a Depression: HADS a |
Baseline for intervention group: 5.3 (2.9) Baseline for control group: 6.8 (4.6) |
Baseline for intervention group: 4.7 (3.4) Baseline for control group: 5.1 (4.6) |
| 5 |
Kapoor et al. (2015) India |
Cross sectional (level 3) | 100 (63%); 41 years (18–70); not given | Chronic myeloid leukaemia | In treatment: Imatinib | Depression: PHQ‐9 c |
N/A |
Adherence group: 2.48 (2.88) Non‐adherence group: 7.16 (5.84) Prevalence: 23% |
| 6 |
Kiely et al. (2017) Ireland |
Cross sectional (Level 3) | 41 (59%); 64 years (46–86); not given | Multiple myeloma |
In treatment: Thalidomide Lenalidomide Bortezomib Chemotherapy Stem cell transplant |
Anxiety: HADS a Depression: HADS a |
Median (SD) 9.8 (3.76) Prevalence: 30% |
Median (SD) 10.1 (3.68) Prevalence: 37% |
| 7 |
Koizumi et al. (2018) Japan |
Cross sectional (level 3) | 27 (74%); 60 years (not given); not given |
Leukaemia, myelodysplastic syndrome Non‐Hodgkin's lymphoma, aplastic anaemia, multiple myeloma |
In treatment: Chemotherapy |
Anxiety: POMS d Depression: POMS d |
17.0 (7.1) Prevalence: 55% |
15.9 (9.5) Prevalence: 33% |
| 8 |
Mianmian et al. (2019) China |
Cross sectional (Level 3) | 180 (56.1%); 51 years (not given); not given | Acute myeloid leukaemia (AML) | In treatment: Chemotherapy |
Anxiety: HADS a Depression: HADS a |
Relapsed and remitting AML: 9.1 (4.0) De novo AML: 7.5 (3.4) Prevalence: 53.9% Prevalence: 40% |
Relapsed and remitting AML: 8.0 (3.7) De novo AML: 6.7 (3.0) Prevalence: 45.6% Prevalence: 29.4% |
| 9 |
Oerlemans et al. (2014) Netherlands |
Cohort study (Level 3) | 489 (61%); 57.5 years (not given); not given |
Hodgkin's lymphoma (HL) Diffuse large B‐cell lymphoma (DLBCL) |
In treatment: Chemotherapy |
Anxiety: HADS a Depression: HADS a |
HL—4.8 (4) DLBCL—4.1 (4) Prevalence: 24% (HL) and 17% (DLBCL) |
HL—3.7 (4) DLBCL–4.3 (4) Prevalence: 18% (HL) and 19% (DLBCL) |
| 10 |
Robbertz et al. (2020) USA |
Cohort study (Level 3) | 106 (67%); 60 years (24–82); 95.6% Caucasian, 2.30% African American | Chronic lymphatic leukaemia | In treatment: Chemotherapy |
Anxiety: GAD‐7 e Depression: PHQ‐9 c |
None‐mild anxiety: 2.09 (2.40) Moderate–severe: 13.00 (2.45) Prevalence: 10% |
None‐mild depression: 2.25 (2.00) Moderate–severe: 11.73 (3.43) Prevalence: 14% |
| 11 |
Wang et al. (2016) China |
Cross sectional (Level 3) | 227 (51.5%); 45 years (18–83); not given | Leukaemia, multiple myeloma, lymphoma | In treatment: Chemotherapy |
Anxiety: Zung self‐rating anxiety scale Depression: CES‐D f |
47.87 (11.21) Prevalence: 45.8% |
20.18 (9.65) Prevalence: 65.0% |
| 12 |
Zittoun et al. (1999) France |
Cohort study (Level 3) | 178 (55.6%); 44 years (16–83); not given | Acute leukaemia, chronic myeloid leukaemia, Hodgkin's lymphoma, non‐Hodgkin's lymphoma, multiple myeloma | In treatment: Chemotherapy, bone marrow transplant |
Anxiety: HADS a Depression: HADS a |
5.55 (4.14) Prevalence: 33% |
5.95 (4.53) Prevalence: 23% |
| 13 |
Levin et al. (2007) USA |
Cross sectional (Level 3) | 105 (63%); 59 years (not given); 95% White | Chronic lymphatic leukaemia |
In treatment (48%) Watchful waiting (57%) |
Anxiety: BAI g Depression: BDI‐II h |
Watchful waiting: <60 years: 6.18 (5.47) >60 years: 2.91 (3.04) Ongoing treatment: <60 years: 4.75 (4.01) >60 years: 5.38 (6.46) |
Watchful waiting: <60 years: 8.09 (6.07) >60 years: 4.75 (4.54) Ongoing treatment: <60 years: 8.62 (6.78) >60 years: 6.39 (4.54) |
| 14 |
Morrison et al. (2016) USA |
Cross sectional (Level 3) | 112 (55%); 61 year (37–76); 100% Caucasian | Chronic lymphocytic leukaemia | Watchful waiting |
Anxiety: GAD‐7 e Depression: CES‐D f |
3.8 (6.5) Prevalence: 20.5% |
10.2 (8.1) Prevalence: 20.6% |
| 15 |
O'Byrne (2018) UK |
Cohort (Level 3) | 33 (57%); 70 years (50–89); 96% White, 3% other | Chronic lymphatic lymphoma, low‐grade lymphoma | Watchful waiting |
Anxiety: HADS a Depression: HADS a |
Median: 5 Prevalence: 43% |
Median: 3 Prevalence: 12% |
| 16 |
Santos et al. (2006) Brazil |
Cross sectional (Level 3) | 107 (55%); 50 years (21–84); not given | Non‐Hodgkin's lymphoma, acute myeloid leukaemia, acute lymphocytic leukaemia, multiple myeloma |
In treatment (49%) Watchful waiting (51%) |
Anxiety: HADS a Depression: HADS a |
Watchful waiting: 4.63 Ongoing medication: 3.2 Radiotherapy: 3.0 Chemotherapy: 6.45 |
Watchful waiting: 3.47 Ongoing medication: 2.2 Radiotherapy: 1.8 Chemotherapy: 4.64 |
| 17 |
Swash (2015) UK |
Cross sectional (Level 3) | 71 (46.8%); 61 years (19–97); 97.4% White, 1.3% Black Caribbean, 1.3% Chinese | Non‐Hodgkin's lymphoma, chronic lymphocytic leukaemia, chronic myeloid leukaemia, Hodgkin's lymphoma |
In treatment (69%) Watchful waiting (31%) |
Anxiety: HADS a Depression: HADS a |
Watchful waiting: 6.00 (1.3) In treatment: 7.6 (0.62) |
Watchful waiting: 4.33 (1.05) In treatment: 5.42 (0.56) |
| 18 |
Van den Broek et al. (2014) Netherlands |
Cross sectional (Level 3) | 125 (67%); 61 years (not given); not given | Chronic lymphocytic leukaemia, small lymphocytic leukaemia |
In treatment (46%): Chemotherapy, radiotherapy Watchful waiting (54%) |
Anxiety: HADS a Depression: HADS a |
Watchful waiting: 4.5 (3.7) Ongoing treatment: Chlorambucil 6.0 (4.2) Chemo/immunotherapy 3.5 (3.7) Prevalence: 18% |
Watchful waiting: 3.6 (3.5) Ongoing treatment: Chlorambucil 4.9 (4.1) Chemo/immunotherapy 4.1 (4.1) Prevalence: 13% |
Source: Levels of Evidence based on the Oxford Centre for Evidence‐Based Medicine (OCEBM) Levels of Evidence (2011).
Hospital Anxiety and Depression scale (HADS).
Patient‐reported Outcome Measurement Information System (PROMIS).
Patient Health Questionnaire (PHQ‐9).
Profile of mood states (POMS) .
Generalised Anxiety Disorder Assessment (GAD‐7).
Centre for Epidemiologic Studies Depression Scale (CES‐D).
Beck's Anxiety Inventory (BAI).
Beck's Depression Inventory (BDI‐ll).
All studies used self‐report measures to assess anxiety and depression. Across studies, 10 outcome measures were used. The Hospital Anxiety and Depression Scale (Zigmond & Snaith, 1983) was most commonly reported (1, 3, 4, 6, 8, 9, 12, 15, 16, 17, 18). Four studies compared psychological distress in those under watchful waiting and those in treatment (13, 16, 17, 18). Two examined distress in those under watchful waiting only (14,15), and the remaining 12 examined only those in treatment (1–12). Ongoing treatments included chemotherapy, radiotherapy and other drug therapies.
3.3. Quality appraisal
All studies were deemed to be ‘Level 3’ evidence: ‘Non‐randomised controlled trials with low/moderate risk of bias or randomised controlled trials at high risk of bias’ (OCEBM, 2011). Table 2 outlines the quality ratings of each study. Each received a score out of 24, with a higher score indicating higher study quality. A score of 50% or less was deemed a liberal estimate of low quality by QualSyst developers (Kmet et al., 2004). The average study quality score was 21, equivalent to 88% (SD = 7.9%; range: 67%–92%). Thirteen scored above 75% (1, 3–6, 8–12, 14, 16, 18); no study scored 50% or below. Though the tool used in the present review was an adapted version, 11/12 questions were taken from QualSyst, and so the 50% mark has been deemed relevant as a general guide. Therefore, the overall study quality was considered good.
TABLE 2.
Quality ratings (based on quality assessment tools by Kmet et al., 2004 and Critical Appraisal Skills Programme [CASP], 2019)
| Question | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bellali et al. (2020) | Bryant et al. (2018) | Fukushima et al. (2018) | Furzer et al. (2016) | Kapoor et al. (2015) | Kiely et al. (2017) | Koizumi et al. (2018) | Mianmian et al. (2019) | Oerlemans et al. (2014) | Robbertz et al. (2020) | Wang et al. (2016) | Zittoun et al. (1999) | Levin et al. (2007) | Morrison et al. (2016) | O'Byrne (2018) | Santos et al. (2006) | Swash (2015) | Van den Broek et al. (2014) | ||
| 1. Was the research question or aims clearly stated? a | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 36 |
| 2. Were the individuals selected to participate in the study likely to represent the target population? b | 2 | 1 | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 2 | 2 | 2 | 30 |
| 3. Is the method of subject selection described and appropriate? a | 2 | 2 | 2 | 1 | 1 | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 2 | 26 |
| 4. Was the sample size appropriate? a | 2 | 0 | 1 | 1 | 2 | 1 | 0 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 0 | 1 | 0 | 2 | 22 |
| 5. Are the subject characteristics sufficiently described? a | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 33 |
| 6. Is the design evident and appropriate to answer study question? a | 1 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 33 |
| 7. Was the outcome measure well defined and robust to measurement/misclassification bias? a | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 35 |
| 8. Is the analysis described and appropriate? a | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 35 |
|
9. Controlled for confounding? a |
1 | 1 | 0 | 2 | 2 | 1 | 0 | 0 | 2 | 1 | 2 | 1 | 1 | 2 | 1 | 1 | 2 | 1 | 21 |
| 10. Is an estimate of variance for the main outcome given? a | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 2 | 19 |
| 11. Are results reported in sufficient detail? a | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 2 | 31 |
| 12. Do the results support the conclusions drawn? a | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 35 |
| Total raw score | 22 | 18 | 19 | 22 | 22 | 20 | 16 | 20 | 21 | 19 | 21 | 20 | 18 | 22 | 17 | 19 | 18 | 22 | Average: 21 |
| Total percentage (%) | 92 | 75 | 79 | 92 | 92 | 83 | 67 | 83 | 88 | 79 | 88 | 83 | 75 | 92 | 70 | 79 | 75 | 92 | Average: 88 |
All studies stated the research question or aims, and all samples were at least partially representative of the wider HC population in terms of gender, age or diversity in HC diagnoses. Most lacked detail on sample ethnicity. Studies generally scored poorly on appropriate sample size, which may hold implications for generalisability of individual study findings to the wider HC population. Generally, studies scored highly on analysis and reporting of results, with many offering an estimate of variance of their main findings or controlling for confounding factors.
3.4. Anxiety and depression
In some studies, an overall sample mean or prevalence was not reported and instead, samples were split by a factor relevant to the individual study, for example, Kapoor et al. (2015) presented their means for a medication‐adherence group and a non‐adherence group. In these cases, samples were treated independently and entered separately into OpenMeta. Further details on split samples within single studies can be viewed in Table 1.
Meta‐analyses included only studies reporting HADS mean and SD data. This decision was made on the basis that transforming raw scores from several measures into comparable data would weaken the clinical utility of the findings, as clinical cut‐offs would be inapplicable. The HADS is a 14‐item self‐report scale that has demonstrated acceptable internal consistency in a cancer population (Cronbach's α = 0.85) (Rodgers et al., 2005). Scoring gives separate scores of anxiety symptoms and depressive symptoms, each out of 21. A score below 8 is deemed ‘normal;’ between 8 and 10: ‘mild’; 11 and 14: ‘moderate’ and over 14: ‘severe’. Two studies only reported median HADS data (15, 16), and so it was assumed that sample distribution was not normal and transformation from median to mean scores was not possible. Therefore, the samples were excluded from the means meta‐analysis. Both studies were included in the prevalence analysis.
Table 3 outlines the key findings relating to anxiety and depression data in HC patients.
TABLE 3.
Prevalence and symptoms of anxiety and depression across groups
| Anxiety | Depression | |||
|---|---|---|---|---|
| Prevalence % (C.I.) | Mean (SD) | Prevalence % (C.I.) | Mean (SD) | |
| Watchful waiting | 24.5 (13.5–35.4) | 5.1 (1.1) | 16.1 (10.7–22.4) | 4.1 (0.7) |
| Under ongoing treatment | 34.0 (24.7–43.3) | 6.4 (1.0) | 31.3 (22.1–40.7) | 6.0 (0.9) |
| Overall | 32.3 (24.4–40.1) | 6.1 (0.7) | 28.2 (20.2–36.2) | 5.7 (0.8) |
3.4.1. Prevalence of anxiety
Across nine studies reporting anxiety prevalence in those in ongoing treatment (2, 6–12,18) (n = 1502), sample prevalence ranged between 9% and 59%. In the three studies examining those under watchful waiting (14, 15, 18), the prevalence range was 17.6%–42.4%. Subgroup analysis revealed the pooled estimated prevalence of anxiety in those undergoing treatment was 34.0% (CI 24.7%–43.3%; I 2 = 93.8%), higher than the estimated prevalence in the watchful waiting group (24.5%; CI 13.5%–35.4%; I 2 = 70.0%), though this difference was not statistically significant (z = 1.296, p = 0.195). The study reporting the highest prevalence in those under watchful waiting (15) (42.4%) was also deemed to be at highest risk of bias compared to the other studies. Similarly, the studies reporting the highest prevalence rates in those undergoing treatment (2, 7) received the lowest quality ratings of the included studies. Therefore, the upper limits of both estimated prevalence rates should be considered lightly. Subgroup and overall analyses revealed the prevalence (I 2) of anxiety in those undergoing treatment at more than 90%, indicating high heterogeneity existed amongst the reviewed articles. Figure 3 illustrates the prevalence of anxiety across studies.
FIGURE 3.
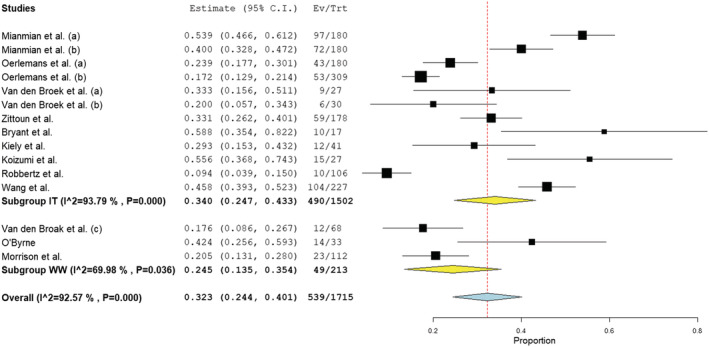
Forest plot of prevalence of anxiety based on random‐effects model by subgroup (IT, in treatment; WW, watchful waiting)
3.4.2. Anxiety means
Meta‐analysis of 10 studies reporting HADS data was conducted. Of the studies that examined patients under treatment (1, 3, 4, 8, 9,12,16–18), 14 samples were included (n = 1354). The remaining three samples (16–18) comprised patients under watchful waiting (n = 143). Reported means ranged from 3.5 to 12.7 for those under treatment and 4.5 to 6.0 in those under watchful waiting. The estimated overall means were 6.4 (SD = 1; I 2 = 89.2%) in those under treatment and 5.1 (SD = 1.1; I 2 = 79.8%) in those under watchful waiting. Both overall means were below clinical cut‐off and the difference between the groups was not significant (z = 1.650, p = 0.099). The overall average mean across treatment pathways was 6.1 (SD = 0.7; I 2 = 87.8%). Visually, one study appeared anomalous (1) (see Figure 4), with a mean two times greater than the pooled estimated mean for those in treatment. However, risk of bias in this study was deemed low. Of the nine studies measuring those in ongoing treatment that did not use the HADS, only two studies reported clinically significant means (2, 11). Despite these two studies varying in quality (75% and 88% respectively), both reported means marginally above the cut‐off point for clinical significance.
FIGURE 4.
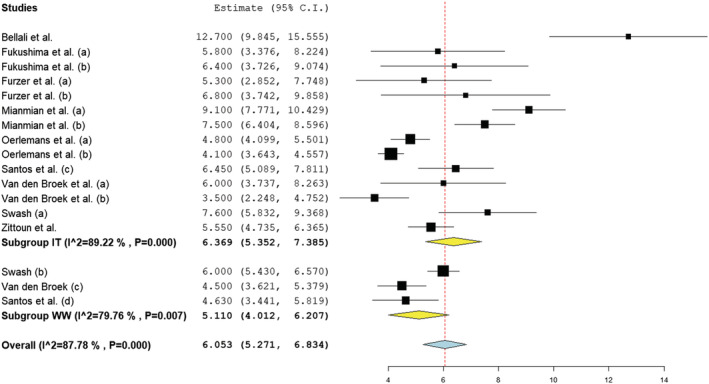
Forest plot of anxiety symptoms based on random‐effects model by subgroup (IT, in treatment; WW, watchful waiting)
3.4.3. Prevalence of depression
Prevalence of depression was examined in 13 samples of patients under treatment (2, 5–12,18) (n = 1702) and three samples of patients under watchful waiting (14, 15, 1) (n = 213). Reported prevalence ranged between 14.2% and 66.1% for those under treatment and 12.1% and 20.5% for those under watchful waiting. Subgroup analysis reported prevalence of depression in those under treatment (31.3%; CI 22.1%–40.7%; I 2 = 94.8%) was significantly higher than in those under watchful waiting (16.1%; CI 10.7%–22.4%; I 2 = 14.01%) (z = 2.800, p < 0.05). The overall prevalence across studies was 28.2% (CI 20.2%–36.2%; I 2 = 94.0%). Study 11 reported the highest prevalence in those under treatment, with a finding 2.1 times greater than the overall under treatment average. Quality appraisal suggested a strong study design and a comparatively large sample size, with good sample representation. Interestingly, Study 2 reported a similarly high prevalence (58.8%), but quality was comparatively low, with a small sample size (n = 17) and weak analysis. In Figures 5 displays subgroup and overall analyses with prevalence (I 2) of depression in those undergoing treatment at 94.77% and 94.04% respectively, indicating high heterogeneity existed amongst the reviewed articles. In contrast, heterogeneity amongst watchful waiting samples was low, despite variation in appraised quality, and all individually reported watchful wait prevalence rates fell below the overall prevalence rate, as illustrated in the forest plot (Figure 5).
FIGURE 5.
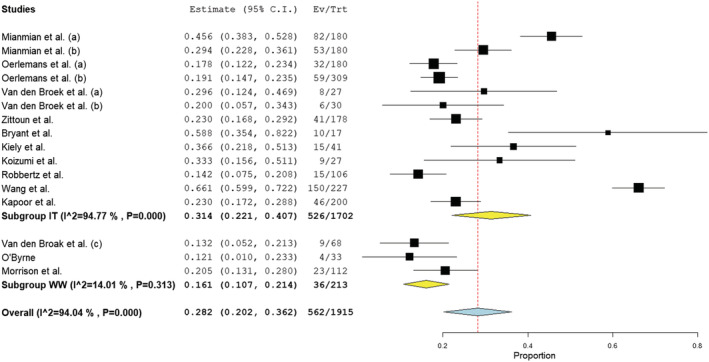
Forest plot of prevalence of depression based on random‐effects model by subgroup (IT, in treatment; WW, watchful waiting)
3.4.4. Depression means
Of the studies that examined patients under treatment (1, 3, 4, 8, 9, 12, 17, 18), 13 samples were included (n = 1312). The remaining two samples comprised patients under watchful waiting (17, 18) (n = 88). The pooled mean average of depressive symptoms in those under treatment (mean = 6.0; SD = 0.9; I 2 = 96.4%) was found to be significantly higher than those reported by patients under watchful waiting (mean = 4.1; SD = 0.7; I 2 = 55.85%) (z = 3.370, p < 0.001). The overall average mean was 5.7 (SD = 0.8; I 2 = 96.4%). All pooled means fell below clinical cut‐off. Similar to findings in Section 3.4.2, one finding appeared anomalous (1) (see Figure 6), despite quality appraisal indicating a low risk of bias. Heterogeneity across studies was found to be high and this can be observed visually in Figure 6. Of the studies reporting depression means via outcome measures other than the HADS, only one study (11), of high quality, reported a mean above the clinical cut‐off point. No study examining those under watchful waiting reported a clinically significant mean, consistent with studies included in the meta‐analysis (17, 18).
FIGURE 6.
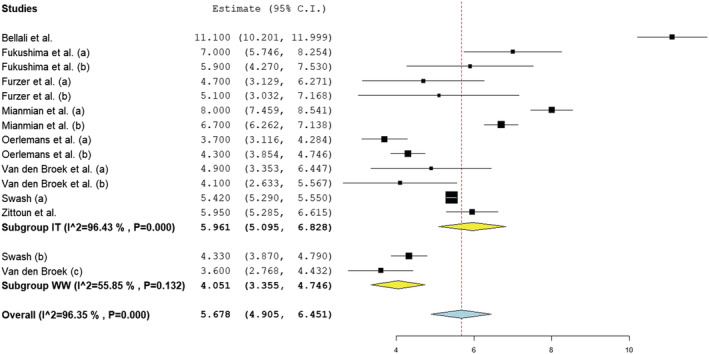
Forest plot of depression symptoms based on random‐effects model by subgroup (IT, in treatment; WW, watchful waiting)
4. DISCUSSION
4.1. Summary of main findings
The current review aimed to further understanding of anxiety and depression in HC patients, with a focus on differences between those in ongoing treatment and those under watchful waiting. A total of 18 studies involving 2720 adults with HC (14.5% under watchful waiting), carried out between 1999 and 2020, were reviewed.
Meta‐analysis reported no significant difference in anxiety prevalence between those under watchful waiting (24.5%) and those under treatment (34%), supporting findings that HC is associated with increased risk of anxiety, regardless of whether immediate treatment is offered (Holtzer‐Goor et al., 2015). Both estimated prevalence rates were in line with previous estimations in the HC population (Clinton‐Mcharg et al., 2014) and substantially higher than the estimated prevalence in the general population (7%) (Steel et al., 2014).
Prevalence of depression in HC patients under watchful waiting was also found to be greater than that in the general population (16% vs. 5%) (Steel et al., 2014) and greatest in those undergoing treatment (31.3%). In contrast with previous findings (Walker et al., 2014), a significantly higher proportion of patients under treatment reported depression, compared with those under watchful waiting. Psycho‐oncology theories, focusing on appraisal and coping as factors influencing emotional distress, can be used to hypothesise about the observed findings (Barroilhet et al., 2005). It is possible that patients receiving treatment appraised their situation as more severe and had a lower perception of their ability to cope with aggressive treatments, increasing the likelihood of developing depression, compared with patients under watchful waiting. In contrast, both groups may have experienced equal levels of anxiety because a cancer diagnosis may be appraised as threatening and uncertain, regardless of treatment pathway. Such hypotheses require testing and future research should focus on understanding underlying causes of distress in those under watchful waiting and those in treatment.
Across studies, prevalence rates of anxiety and depression in patients under watchful waiting were estimated to be higher than in the general population. This discovery contradicts findings in prostate cancer research where patients are reported to cope well under watchful waiting (Matheson et al., 2019). Psychological theories of needs, where psychological distress is thought to indicate unmet psychosocial needs, can be considered to understand why such a difference has been observed. Self‐determination theory (Deci & Ryan, 1985) posits that autonomy is one of three vital needs for psychological well‐being. A lack of autonomy over treatment pathway could contribute to the differences observed between HC patients and prostate cancer patients under watchful waiting. Future research could test such hypotheses to further understanding of the specific challenges associated with the HC watchful waiting pathway and enable development of effective interventions.
Exploring symptom prevalence revealed HC patients under treatment were more likely to report depressive symptoms than those under watchful waiting and equally likely to experience anxiety. However, all overall means were under the threshold for clinical significance. The disparity between the substantially increased prevalence rates and the within ‘normal’ mean scores suggest that a proportion of HC patients cope well with their diagnosis and resulting treatment pathway. Study quality did not appear to correlate with level of anxiety or depression recorded, with both high‐ and low‐quality studies reporting highest means.
4.2. Limitations and future research
The most significant limitation of the current review was the dearth of research examining depression and anxiety in those under watchful waiting. Of the six studies included, two constituted grey literature and were of lower quality than other included studies (15, 17), predominantly due to recruitment difficulties. The substantially smaller overall sample size of patients under watchful waiting somewhat limits the ability to comprehensively answer the review question. Future research should focus on increasing understanding of prevalence and symptoms of psychological distress in those under watchful waiting, and consequently, what effective support would comprise.
Other limitations relate to the heterogeneity of the included studies. Whilst breadth in haematology diagnosis was important for generalising findings to the HC population, most studies did not offer prevalence or symptom rate by diagnosis. HC diagnoses can vary in prognosis, treatment intensity and symptom severity (Blood Cancer UK, 2019), which may impact levels of anxiety or depression. In addition, few studies reported ethnicity. Given there is a relationship between psychological distress and ethnicity (Flaskerud, 2000), research should explore this further. The included studies were conducted across 11 countries, indicating potential for cultural variation. The impact of certain cultural factors, such as the quality and availability of psychological support (Niedzwiedz et al., 2019), cultural conceptualisations of anxiety and depression (Maters et al., 2013) or healthcare costs (Hall et al., 2016), could partially account for variance seen between study estimations. For example, significantly higher mean scores were reported in a Greek population (1), where access to psychological support is reported as low (Madianos, 2020). Data unavailability and small sample sizes meant that formal analysis of ethnicity, culture or HC diagnosis‐related factors was not possible, and this limits the extent to which prevalence estimations can be understood. Future research is required to explore moderating and mediating factors on levels of anxiety and depression in the HC population.
None of the included studies reported whether participants were receiving psychological support or taking mood medication at the time of, or prior to, study participation. Understanding proportions accessing treatment for psychological distress is vital to accurately assessing the risk of anxiety and depression in the HC population. Without accounting for these data, the present review may have underestimated prevalence, as those receiving treatment may have consequently scored out of clinical ranges. Future research should consider recording such information to develop a more comprehensive understanding of distress in HC.
Finally, all studies reported anxiety and depression data via self‐report measures, which has acknowledged limitations (Vodermaier & Millman, 2011). Preferably, prevalence estimations should be based on clinical assessment in addition to self‐report screening tools (Mitchell et al., 2010). Further, only data from the HADS were included in the means meta‐analyses. Pooling estimates from a range of different measures may have increased accuracy in estimations due to increasing the evidence base. Despite these limiting factors, research examining prevalence in HC patients through clinical interviews found that 26% of the sample met criteria for major depressive disorder and 31% for an anxiety disorder (Allart et al., 2013)—figures very similar to overall prevalence reported in the present review (28% and 32%, respectively). Therefore, the current prevalence estimations appear consistent with wider literature.
4.3. Strengths and implications
Whilst living with cancer is widely understood to be associated with increased risk of anxiety and depression (Pitman et al., 2018), the present review furthers understanding on the impact of treatment pathway. Reviewing the literature has highlighted the scarcity of research exploring psychological distress in HC patients under watchful waiting. To the best of the authors' knowledge, the present review is the first of its kind to evaluate and synthesise current available evidence with a focus on treatment pathway in HC. The use of meta‐analysis has the benefit of increasing the accuracy of estimated prevalence rates, compared to considering each study individually (Haidich, 2010). Evidence of estimated prevalence of anxiety and depression for patients on each pathway can inform policymakers and healthcare providers around the need for psychological support in haemo‐oncology services. Further, the findings can increase clinicians' awareness of the risk of patients presenting with anxiety or depression in outpatients' appointments and consequently signposting to appropriate support.
4.4. Conclusion
The present review examined prevalence and symptoms of anxiety and depression amongst adults with HC, both under watchful waiting and under treatment. Analysis indicated high prevalence of anxiety and depression in both groups, highlighting the need for evidence‐based psychological interventions. Those under treatment were at most risk of experiencing depression, and both groups were equally at risk of experiencing anxiety. Reviewing the literature demonstrated a paucity in studies involving HC patients under watchful waiting. The findings therefore emphasise the need for future high‐quality research exploring the experiences of those under watchful waiting and what effective psychological support would comprise.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
This review was funded as part of the Trent Doctorate in Clinical Psychology (DClinPsy) Programme by NHS Health Education England.
Russell, K. , Moghaddam, N. , & Tickle, A. (2022). Examining anxiety and depression in haematology cancer patients in ongoing treatment and under watchful waiting: A systematic review and meta‐analysis. European Journal of Cancer Care, 31(6), e13678. 10.1111/ecc.13678
Funding information This review was funded by Health Education England as part of a Doctorate of Clinical Psychology.
DATA AVAILABILITY STATEMENT
No original data are associated with this review article: All the necessary information required for a reader to access the reviewed studies by the same means as the authors are provided.
REFERENCES
- Admiraal, J. (2020). Supporting cancer patients in managing distress: New insights in the use of the distress htermometer & problem list and effects of web‐based support programs [Unpublished doctoral dissertation]. University of Groningen. 10.33612/diss.112109033 [DOI]
- Allart, P. , Soubeyran, P. , & Cousson‐Gélie, F. (2013). Are psychosocial factors associated with quality of life in patients with haematological cancer? A critical review of the literature. Psycho‐Oncology, 22(2), 241–249. 10.1002/pon.3026 [DOI] [PubMed] [Google Scholar]
- Allart‐Vorelli, P. , Porro, B. , Baguet, F. , Michel, A. , & Cousson‐Gélie, F. (2015). Haematological cancer and quality of life: A systematic literature review. Blood Cancer Journal, 5, 1–10. 10.1038/bcj.2015.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baqutayan, S. (2012). The effect of anxiety on breast cancer patients. Indian Journal of Psychological Medicine, 34(2), 119–123. 10.4103/0253-7176.101774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroilhet, S. , Forjaz, M. , & Landivar, G. (2005). Concepts, theories and psychosocial factors in cancer adaptation. Actas Españolas de Psiquiatría, 33(6), 390–397. [PubMed] [Google Scholar]
- Bellali, T. , Manomenidis, G. , Meramveliotaki, E. , Minasidou, E. , & Galanis, P. (2020). The impact of anxiety and depression in the quality of life and psychological well‐being of Greek hematological cancer patients on chemotherapy. Psychology, Health & Medicine, 25(2), 201–213. 10.1080/13548506.2019.1695864 [DOI] [PubMed] [Google Scholar]
- Blood Cancer UK . (2019). Facts and information about blood cancer. https://bloodcancer.org.uk/news/blood-cancer-facts/
- Borenstein, M. , Hedges, L. V. , Higgins, J. P. T. , & Rothstein, H. R. (2009). Introduction to meta‐analysis. John Wiley & Sons. 10.1002/9780470743386 [DOI] [Google Scholar]
- Bryant, A. , Deal, A. , Battaglini, C. , Phillips, B. , Pergolotti, M. , Coffman, E. , & Reeve, B. (2018). The effects of exercise on patient‐reported outcomes and performance‐based physical function in adults with acute leukaemia undergoing induction therapy: Exercise and quality of life in acute leukaemia (EQUAL). Integrative Cancer Therapies, 17(2), 263–270. 10.1177/1534735417699881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton‐Mcharg, T. , Carey, M. , Sanson‐Fisher, R. , Tzelepis, F. , Bryant, J. , & Williamson, A. (2014). Anxiety and depression among haematological cancer patients attending treatment centres: Prevalence and predictors. Journal of Affective Disorders, 165, 176–181. 10.1016/J.JAD.2014.04.072 [DOI] [PubMed] [Google Scholar]
- Critical Appraisal Skills Programme (CASP) . (2019). CASP systematic review checklist. https://casp-uk.net/casp-tools-checklists/
- Deci, E. L. , & Ryan, R. M. (1985). Intrinsic motivation and self‐determination in human behavior. Plenum. 10.1007/978-1-4899-2271-7 [DOI] [Google Scholar]
- DerSimonian, R. , & Laird, N. (1986). Meta‐analysis in clinical trials. Controlled Clinical Trials, 7, 177–188. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- Evans, J. , Ziebland, S. , & Pettitt, A. (2012). Incurable, invisible and inconclusive: Watchful waiting for chronic lymphocytic leukaemia and implications for doctor‐patient communication. European Journal of Cancer Care, 21(1), 67–77. 10.1111/j.1365-2354.2011.01278.x [DOI] [PubMed] [Google Scholar]
- Flaskerud, J. H. (2000). Ethnicity, culture, and neuropsychiatry. Issues in Mental Health Nursing, 21(1), 5–29. 10.1080/016128400248248 [DOI] [PubMed] [Google Scholar]
- Foster, C. , Calman, L. , Richardson, A. , Pimperton, H. , & Nash, R. (2018). Improving the lives of people living with and beyond cancer: Generating the evidence needed to inform policy and practice. Journal of Cancer Policy, 15(Part B), 92–95. 10.1016/J.JCPO.2018.02.004 [DOI] [Google Scholar]
- Fukushima, T. , Nakano, J. , Ishii, S. , Natsuzako, A. , Sakamoto, J. , & Okita, M. (2018). Low‐intensity exercise therapy with high frequency improves physical function and mental and physical symptoms in patients with haematological malignancies undergoing chemotherapy. European Journal of Cancer Care, 27(6), 1–10. 10.1111/ecc.12922 [DOI] [PubMed] [Google Scholar]
- Furzer, B. , Ackland, T. , Wallman, K. , Petterson, A. , Gordon, S. , Wright, K. , & Joske, D. (2016). A randomised controlled trial comparing the effects of a 12‐week supervised exercise versus usual care on outcomes in haematological cancer patients. Supportive Care in Cancer, 24(4), 1697–1707. 10.1007/s00520-015-2955-7 [DOI] [PubMed] [Google Scholar]
- Gold, S. , Köhler‐Forsberg, O. , Moss‐Morris, R. , Mehnert, A. , Miranda, J. , Bullinger, M. , & Otte, C. (2020). Comorbid depression in medical diseases. Nature Reviews. Disease Primers, 6(1), 1–22. 10.1038/s41572-020-0200-2 [DOI] [PubMed] [Google Scholar]
- Greer, S. , Morrey, S. , & Watson, M. (1989). Patient's adjustment to cancer: The mental adjustment to cancer (MAC) scale vs clinical ratings. Journal of Psychosomatic Research, 33(3), 373–377. 10.1016/0022-3999(89)90027-5 [DOI] [PubMed] [Google Scholar]
- Haidich, A. B. (2010). Meta‐analysis in medical research. Hippokratia, 14(Suppl 1), 29–37. [PMC free article] [PubMed] [Google Scholar]
- Hall, A. , Sanson‐Fisher, R. , Carey, M. , Paul, C. , Williamson, A. , Bradstock, K. , & Campbell, H. (2016). Prevalence and associates of psychological distress in haematological cancer survivors. Supportive Care in Cancer, 24(10), 4413–4422. 10.1007/S00520-016-3282-3 [DOI] [PubMed] [Google Scholar]
- Holland, J. (2002). History of psycho‐oncology: Overcoming attitudinal and conceptual barriers. Psychosomatic Medicine, 64(2), 206–221. 10.1097/00006842-200203000-00004 [DOI] [PubMed] [Google Scholar]
- Holtzer‐Goor, K. , Schaafsma, M. , Joosten, P. , Posthuma, E. , Wittebol, S. , Huijgens, P. , & Uyl‐de Groot, C. (2015). Quality of life of patients with chronic lymphocytic leukaemia in the Netherlands: Results of a longitudinal multicentre study. Quality of Life Research, 24(12), 2895–2906. 10.1007/S11136-015-1039-Y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor, J. , Agrawal, N. , Ahmed, R. , Sharma, S. , Gupta, A. , & Bhurani, D. (2015). Factors influencing adherence to imatinib in Indian chronic myeloid leukemia patients: A cross‐sectional study. Mediterranean Journal of Haematology and Infectious Diseases, 7(1), e2015013. 10.4084/MJHID.2015.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiely, F. , Cran, A. , Finnerty, D. , & O'Brien, T. (2017). Self‐reported quality of life and symptom burden in ambulatory patients with multiple myeloma on disease‐modifying treatment. American Journal of Hospice & Palliative Medicine, 34(7), 671–676. 10.1177/1049909116646337 [DOI] [PubMed] [Google Scholar]
- Kim, Y. , Mitchell, H. , & Ting, A. (2019). Application of psychological theories on the role of gender in caregiving to psycho‐oncology research. Psycho‐Oncology, 28(2), 228–254. 10.1002/PON.4953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmet, L. , Lee, R. , & Cook, L. (2004). Standard quality assessment criteria for evaluating primary research papers from a variety of fields. Alberta Heritage Foundation for Medical Research (AHFMR). [Google Scholar]
- Koizumi, K. , Tayama, J. , Ishioka, T. , Nakamura‐Thoma, H. , Suzuki, M. , Hara, M. , & Hamaguchi, T. (2018). Anxiety, fatigue, and attentional bias toward threat in patients with hematopoietic tumors. PLoS ONE, 13(2), 1–13. 10.1371/journal.pone.0192056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus, R. , & Folkman, S. (1984). Stress, appraisal, and coping. Springer. [Google Scholar]
- Leukaemia Care . (2019). The emotional impact of watch and wait for Chronic Lymphatic Leukaemia [Poster presentation]. European Hematology Association 24 Digital Congress, Amsterdam, Netherlands. https://media.leukaemiacare.org.uk/wp‐content/uploads/The‐Emotional‐Impact‐of‐Watch‐and‐Wait‐for‐CLL‐EHA‐2019‐Poster.pdf
- Levin, T. , Li, Y. , Riskind, J. , & Rai, K. (2007). Depression, anxiety and quality of life in a chronic lymphocytic leukaemia cohort. General Hospital Psychiatry, 29(3), 251–256. 10.1016/j.genhosppsych.2007.01.014 [DOI] [PubMed] [Google Scholar]
- MacMillan Cancer Support . (2020). Side effects of cancer treatment. https://cdn.macmillan.org.uk/dfsmedia/1a6f23537f7f4519bb0cf14c45b2a629/3718‐source/mac12921‐side‐fx‐e05‐pdf?_ga=2.119064961.966690433.1628268516‐360091843.1627289726
- Madianos, M. (2020). The adventures of psychiatric reform in Greece: 1999–2019. BJPsych International, 17(2), 26–28. 10.1192/BJI.2019.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maters, G. A. , Sanderman, R. , Kim, A. Y. , & Coyne, J. C. (2013). Problems in cross‐cultural use of the hospital anxiety and depression scale: “No butterflies in the desert”. PLoS ONE, 8(8), 1–11. 10.1371/JOURNAL.PONE.0070975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson, L. , Wilding, S. , Wagland, R. , Nayoan, J. , Rivas, C. , Downing, A. , & Watson, E. (2019). The psychological impact of being on a monitoring pathway for localised prostate cancer: A UK‐wide mixed methods study. Psycho‐Oncology, 28(7), 1567–1575. 10.1002/pon.5133 [DOI] [PubMed] [Google Scholar]
- Mausbach, B. , Decastro, G. , Schwab, R. , Tiamson‐Kassab, M. , & Irwin, S. (2020). Healthcare use and costs in adult cancer patients with anxiety and depression. Depression and Anxiety, 37(9), 908–915. 10.1002/DA.23059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mianmian, G. , Xiaohong, H. , Lin, C. , Jie, S. , Gu, M. , Hao, X. , & Sun, J. (2019). The prevalence, risk factors, and prognostic value of anxiety and depression in refractory or relapsed acute myeloid leukaemia patients of North China. Medicine, 98(50), 1–8. 10.1097/MD.0000000000018196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, A. J. , Meader, N. , & Symonds, P. (2010). Diagnostic validity of the hospital anxiety and depression scale (HADS) in cancer and palliative settings: A meta‐analysis. Journal of Affective Disorders, 126(3), 335–348. 10.1016/j.jad.2010.01.067 [DOI] [PubMed] [Google Scholar]
- Morrison, E. , Flynn, J. , Jones, J. , Byrd, J. , Andersen, B. , Morrison, E. , & Andersen, B. (2016). Individual differences in physical symptom burden and psychological responses in individuals with chronic lymphocytic leukaemia. Annals of Hematology, 95(12), 1989–1997. 10.1007/s00277-016-2790-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence . (2016) Haematological cancers: Improving outcomes. www.nice.org.uk/guidance/ng47 [PubMed]
- National Institute for Health and Care Excellence . (2019). Prostate cancer: diagnosis and management. www.nice.org.uk/guidance/ng131 [PubMed]
- Niedzwiedz, C. , Knifton, L. , Robb, K. , Katikireddi, S. , & Smith, D. (2019). Depression and anxiety among people living with and beyond cancer: A growing clinical and research priority. BMC Cancer, 19(1), 1–8. 10.1186/S12885-019-6181-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Byrne, S. (2018). “Watch and wait” – Examining the potential impact of uncertainty in illness on the mental health of individuals with chronic lymphocytic leukaemia and low grade Lymjmphomas. [Doctoral disseration]. University of Essex, UK.
- OCEBM Levels of Evidence Working Group . 2011. Levels of evidence. Oxford Centre for Evidence‐Based Medicine. http://www.cebm.net/index.aspx?o=5653
- Oerlemans, S. , Mols, F. , Nijziel, M. , Zijlstra, W. , Willem, J. , & Coebergh, W. (2014). The course of anxiety and depression for patients with Hodgkin's lymphoma or diffuse large B cell lymphoma: A longitudinal study of the PROFILES registry. Journal of Cancer Survivorship, 8, 555–564. 10.1007/s11764-014-0367-1 [DOI] [PubMed] [Google Scholar]
- Page, M. J. , McKenzie, J. E. , Bossuyt, P. M. , Boutron, I. , Hoffmann, T. C. , Mulrow, C. D. , & Moher, D. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. British Medical Journal, 372, 1–9. 10.1136/BMJ.N71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman, A. , Suleman, S. , Hyde, N. , & Hodgkiss, A. (2018). Depression and anxiety in patients with cancer. British Medical Journal, 361, 1–6. 10.1136/bmj.k1415 [DOI] [PubMed] [Google Scholar]
- Quigley, J. , Thompson, J. , Halfpenny, N. , & Scott, D. (2019). Critical appraisal of non‐randomized studies – A review of recommended and commonly used tools. Journal of Evaluation in Clinical Practice, 25(1), 44–52. 10.1111/JEP.12889 [DOI] [PubMed] [Google Scholar]
- Robbertz, A. , Weiss, D. , Awan, F. , Byrd, J. , Rogers, K. , & Woyach, J. A. (2020). Identifying risk factors for depression and anxiety symptoms in patients with chronic lymphocytic leukaemia. Supportive Care in Cancer, 28(4), 1799–1807. 10.1007/s00520-019-04991-y [DOI] [PubMed] [Google Scholar]
- Rodgers, J. , Martin, C. R. , Morse, R. C. , Kendell, K. , & Verrill, M. (2005). An investigation into the psychometric properties of the hospital anxiety and depression scale in patients with breast cancer. Health and Quality of Life Outcomes, 3(41), 1–6. 10.1186/1477-7525-3-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos, F. , Kozasa, E. , Chauffaille, M. , Colleoni, G. , & Leite, J. (2006). Psychosocial adaptation and quality of life among Brazilian patients with different hematological malignancies. Journal of Psychosomatic Research, 60(5), 505–511. 10.1016/j.jpsychores.2005.08.017 [DOI] [PubMed] [Google Scholar]
- Sherrill, C. , Smith, M. , Mascoe, C. , Bigus, E. , & Abbitt, D. (2017). Effect of treating depressive disorders on mortality of cancer patients. Cureus, 9(10), e1740. 10.7759/CUREUS.1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, A. , Howell, D. , Patmore, R. , Jack, A. , & Roman, E. (2011). Incidence of haematological malignancy by sub‐type: A report from the haematological malignancy research network. British Journal of Cancer, 105(11), 1684–1692. 10.1038/bjc.2011.450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel, Z. , Marnane, C. , Iranpour, C. , Chey, T. , Jackson, J. , Patel, V. , & Silove, D. (2014). The global prevalence of common mental disorders: A systematic review and meta‐analysis 1980‐2013. International Journal of Epidemiology, 43(2), 476–493. 10.1093/ije/dyu038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung, H. , Ferlay, J. , Siegel, R. , Laversanne, M. , Soerjomataram, I. , Jemal, A. , & Bray, F. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer Journal for Clinicians, 71(3), 209–249. 10.3322/CAAC.21660 [DOI] [PubMed] [Google Scholar]
- Swash, B. (2015). The unmet psychosocial needs of haematological cancer patients and their impact upon psychological wellbeing. [Doctoral dissertation]. University of Chester, United Kingdom. [Google Scholar]
- Thomas, B. , Ciliska, D. , Dobbins, M. , & Micucci, S. (2004). A process for systematically reviewing the literature: Providing the research evidence for public health nursing interventions. Worldviews on Evidence‐Based Nursing, 1(3), 176–184. 10.1111/J.1524-475X.2004.04006.X [DOI] [PubMed] [Google Scholar]
- Van den Broek, E. , Oerlemans, S. , Nijziel, M. , Posthuma, E. , Coebergh, J. , & Poll‐Franse, L. (2014). Impact of active surveillance, chlorambucil, and other therapy on health‐related quality of life in patients with CLL/SLL in the Netherlands. Annals of Hematology, 94, 45–56. 10.1007/s00277-014-2161-6 [DOI] [PubMed] [Google Scholar]
- Vodermaier, A. , & Millman, R. (2011). Accuracy of the hospital anxiety and depression scale as a screening tool in cancer patients: A systematic review and meta‐analysis. Supportive Care in Cancer, 19(12), 1899–1908. 10.1007/S00520-011-1251-4 [DOI] [PubMed] [Google Scholar]
- Walker, J. , Hansen, C. , Martin, P. , Symeonides, S. , Ramessur, R. , Murray, G. , & Sharpe, M. (2014). Prevalence, associations, and adequacy of treatment of major depression in patients with cancer: A cross‐sectional analysis of routinely collected clinical data. The Lancet Psychiatry, 1(5), 343–350. 10.1016/S2215-0366(14)70313-X [DOI] [PubMed] [Google Scholar]
- Wallace, B. , Dahabreh, I. , Trikalinos, T. , Lau, J. , Trow, P. , & Schmid, C. (2012). Closing the gap between methodologists and end‐users: R as a computational back‐end. Journal of Statistical Software, 49(5), 1–15. 10.18637/jss.v049.i05 [DOI] [Google Scholar]
- Wang, Z. , Liu, L. , Shi, M. , & Wang, L. (2016). Exploring correlations between positive psychological resources and symptoms of psychological distress among hematological cancer patients: A cross‐sectional study. Psychology, Health & Medicine, 21(5), 571–582. 10.1080/13548506.2015.1127396 [DOI] [PubMed] [Google Scholar]
- Watts, S. , Leydon, G. , Birch, B. , Prescott, P. , Lai, L. , Eardley, S. , & Lewith, G. (2014). Depression and anxiety in prostate cancer: A systematic review and meta‐analysis of prevalence rates. British Medical Journal Open, 4, 3901. 10.1136/bmjopen-2013-003901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond, A. S. , & Snaith, R. P. (1983). The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica, 67(6), 361–370. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- Zittoun, R. , Achard, S. , & Ruszniewski, M. (1999). Assessment of quality of life during intensive chemotherapy or bone marrow transplantation. Psycho‐Oncology, 8(1), 64–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No original data are associated with this review article: All the necessary information required for a reader to access the reviewed studies by the same means as the authors are provided.


