Abstract
Background
Post heart‐transplant survival has increased, but information is lacking on specific causes of death and life expectancy. We aimed to assess cause‐specific loss of life‐years compared to the general population, evaluate classification for cause of death after heart transplantation, and assess validity of cause of death data from the International Society of Heart and Lung Transplant (ISHLT) registry.
Methods
In this single center study, we included 239 heart recipients transplanted between 1988 and 2019 in Lund, Sweden (n = 239, 50% of the transplanted population where the cause of death was available). Two cardiologists retrospectively assigned causes of death according to a published classification (CLASS) in the 91 recipients who died during follow‐up. Life expectancy was compared to data from the general population.
Results
Compared to the average Swedish population, life expectancy for heart transplant recipients was 20 years shorter (IQR 12.9–27.2). The largest number of life‐years lost were for deaths due to acute (49 years) and chronic rejection (27 years). Primary graft dysfunction (24 years) accounted for 24% of deaths, followed by malignancy (20 years) and infection (17 years), each accounting for ∼20% of deaths. Use of CLASS revealed moderate inter‐rater agreement (56%) and moderate agreement with the ISHLT registry (62%).
Conclusions
Survival after heart transplantation was 20 years lower than in the general population. In the young, more life‐years were lost due to acute graft rejection, whereas chronic graft rejection and primary graft failure were more important causes of death in older patients. Agreement was moderate between CLASS and the ISHLT registry classifications.
Keywords: cause of death, heart transplant, ISHLT register, life‐years‐lost, Scandiatransplant, validation
1. INTRODUCTION
Survival of heart transplant recipients has increased steadily over the last decades, paralleling improvements in surgical technique, organ preservation, immunosuppression, transplantation logistics, infection control, and long‐term graft surveillance. 1 According to the International Society for Heart and Lung Transplantation (ISHLT) registry, the largest register in the world of heart and lung transplant recipients, which also includes information on time and cause of death from follow‐up, median survival has been reported to be 12.5 years. 2 Data on Scandinavian heart transplant recipients are entered into a common register (Scandiatransplant registry), from which data are exported to the ISHLT registry. This includes information on cause of death as coded according to the ISHLT. In Scandinavian heart transplant recipients, median survival was 13.2 years in 2009 3 and more recently published results from one of the two heart transplant centres in Sweden (Sahlgrenska University Hospital in Gothenburg) showed a median survival of 14.1 years at their centre. 4 Whereas the length of survival is well documented, information on the specific cause of death and life expectancy compared to the healthy population is limited.
Classification of cause of death is notoriously complicated and often displays substantial inter‐rater variability, with low validity in registries representing diagnoses from routine clinical practice. 5 , 6 , 7 For transplant recipients, it may be particularly difficult to identify a single cause of death. To our knowledge, no study has validated death causes in the ISHLT or Scandiatransplant registries.
Recently, a new method, the Classification of death causes after transplantation (CLASS) was developed in a broad transplantation context, and shown to display high reliability by Wareham et al. 8 Substantial discordance was observed between the CLASS classification and death causes reported from clinical routine in the Danish National Death Cause Registry. 8
The importance of individual causes of death can be described with several metrics. One highly relevant measure is life expectancy and life‐years lost, calculated from comparison with a normal population.
In this retrospective single center study from the heart transplant center at Skane University Hospital in Lund, Sweden, we aimed to validate the death causes reported in the ISHLT registry by application of the CLASS classification to heart transplant recipients, and additionally to evaluate the inter‐rater agreement for the CLASS system in this context. Further, we compared the transplant recipients to a matched non‐transplanted general population sample to determine cause‐specific life years lost in a transplanted population.
2. METHODS
2.1. Study sample
In this single center study, patients that had undergone heart transplantation in the period 1998–2019 and were followed at Skåne University Hospital, Lund Sweden, were included (Figure 1). The first heart transplant in Lund was performed in 1988. Since 2011 Lund has been one of two national centers for heart transplants in Sweden, performing an average of 28 heart transplants a year. Patients local to other Swedish university hospitals undergo transplant in Lund, but receive post‐transplant follow‐up at their home clinic. Subjects with unavailable paper journals or follow‐up at other hospitals were excluded due to insufficient data. Data extraction from patient journals and from the Scandiatransplant registry was approved by the local ethics committee (diary number 2016/987, 2017/538, 2018/77), and was conducted in accordance with the ISHLT Ethics statement.
FIGURE 1.

Patient selection
2.2. Cause of death classification
Two independent reviewers separately investigated the hospital records of deceased patients. The death cause was determined according to the CLASS death cause classification. 8 Agreement was defined as the same death cause (e.g., infection) including sub group (e.g., bacterial). In the cases where the selected death cause differed between the two reviewers, a board consisting of the two reviewers and a consultant heart failure cardiologist repeated the journal review and agreed on an adjudicated death cause. The validated death cause (i.e., reached through initial agreement between reviewers and after adjudication) was compared to the registered cause of death in the Scandiatransplant registry. For cause of death, the Scandiatransplant registry uses the ISHLT definition. For further comparison, the death causes from the CLASS system were translated into analogue death causes in the ISHLT classification, that is, “grouped death cause” (Table S2, Supplements).
2.3. Analysis of life‐years lost
The pathophysiologically relevant aggregated death cause was used for analysis of life‐years lost. Average life expectancy in Sweden between the years 1988–2019 for male and female residents of ages 0–73 was extracted from Statistics Sweden. 9 Statistics Sweden is a governmental agency responsible for official and governmental statistics in Sweden. The database is comprehensive, containing data on all registered inhabitants in Sweden. Data from the year 2020 was not available at the time, but life expectancy was considered the same as in 2019.
To calculate lost years of life, the life length of the transplanted patients was compared to the average life expectancy of the whole Swedish population matched by age and sex at the year of transplant. Patient length of survival after transplant was subtracted from the expected average years of life, and the median loss of life years was calculated overall and according to grouped death causes.
2.4. Statistical analysis
Continuous variables were presented as medians with first to third interquartile range (IQR). Categorical variables were presented as numbers and percentages. Inter‐reviewer agreement of CLASS cause of death and concordance between the CLASS and ISHLT death codes were assessed by Cohen's kappa test. Strength of agreement was considered slight (0.00–0.20), fair (0.21–0.40), moderate (0.41–0.60), substantial (0.61–0.80), or almost perfect (0.81–1.00). Cumulative incidence curves were created using a competing‐risks regression models (Fine and Gray) along with pointwise confidence intervals based on ln(−ln) transformation (“cmprsk” package in R, version 4.1.2). 10 , 11 Comparison of the number of life years lost was performed using quantile regression.
3. RESULTS
3.1. Deceased patients and clinical characteristics
Between 1988 and 2019, 472 patients underwent heart transplantation at Skåne University Hospital Lund. Two hundred and forty‐six patients received their follow‐up locally. Of these patients, 148 patients were still alive on December 31, 2019, while 98 patients were deceased. In the case of seven patients, we were unable to locate their old paper journal records. They were excluded from further analysis. The electronic journals of the remaining 91 patients were reviewed for cause of death analysis (Figure 1). Analyses were based on a sample size (n = 239. Fifty percent of whole population).
The baseline characteristics of the cohort are reported in Table 1.
TABLE 1.
Baseline characteristics
|
Baseline characteristics of transplanted patients 1988–2019 followed in Lund | |
|---|---|
| Number of transplants | 239 |
| Median age | 51 years |
| Average age | 45 years |
| Age range | 1–74 years |
| Female sex | 66 (28%) |
| Male sex | 173 (72%) |
| Alive at 31.12.2019 | 148 (62%) |
| Dead at 31.12.2019 | 91 (38%) |
| Females dead at 31.12.2019 | 25 (38%) |
| Males dead at 31.12.2019 | 66 (39%) |
| Retransplant | 2 (0.8%) |
| Mean survival | 16.3 years |
| Aetiology of heart failure | |
|---|---|
| Dilated cardiomyopathy, idiopathic | 110 (46%) |
| Coronary heart disease | 50 (21%) |
| Other aetiologies | 79 (33%) |
In brief, the major etiologies of heart failure prior to transplantation were idiopathic dilated cardiomyopathy (in 46%) and coronary artery disease (21%). The median age at transplantation was 51.2 years (IQR 37.0–58.4), and 72% were male. Two patients underwent re‐transplantation during the period. The median time of follow‐up was 8.1 years (IQR 3.0–14.0). Mortality was similar among men and women (39% vs. 38%). Survival after 1, 5, 10 and 20 years was 91.6%, 81.8%, 72.4%, and 37.8%, respectively. Median survival in the group was 16.3 years.
3.2. Cause of death classification and comparison with ISHLT
Using the CLASS methodology, there was initial agreement between the two reviewers in 51 cases (56%) and disagreement in 40 cases (44%) leading to a moderate inter‐reviewer agreement with a Cohen's Kappa Coefficient of 0.51. For these latter patients the specialist board determined the validated death cause. However, using the “grouped death causes” the inter‐reviewer agreement was substantial at 67% with a Cohen's Kappa coefficient of 0.63.
Cumulative incidence of the “grouped death causes” are presented in Figure 2. A summary of registered and grouped ISHLT death cause versus validated grouped CLASS death cause are presented in Table S3 (Supplements).
FIGURE 2.

Survival of heart transplant recipients according to grouped death cause
The validated CLASS death cause was compared with the registered ISHLT death cause and led to moderate agreement at 62%, with a Cohen's Kappa coefficient of 0.57.
Next, the “grouped death causes” in CLASS and validated CLASS codes were compared for each reviewer separately, and led to a substantial to almost perfect agreement rate of 79% and 85% with Cohen's Kappa coefficients of 0.76 and 0.83, respectively. However, agreement with the validated grouped CLASS codes and the ISHLT codes remained moderate with inter‐agreement of 62% and Cohen's Kappa of 0.57.
3.3. Life expectancy compared to the matched general population
Compared to an age and gender matched general Swedish population the heart transplant recipients lost a median of 20.0 years (IQR 12.9–27.2). Acute graft rejection resulted in most life years lost per deceased patient (median 49.7, IQR 23.3–61.2), followed by chronic graft rejection, primary graft failure, multi organ‐failure, malignancy, infection, cerebrovascular event, myocardial ischemia and major bleeding (Figure 3, Table S4, Supplements). Compared to acute graft rejection, all death causes resulted in less life years lost (p < 0.05). For patients who died from acute graft rejection, the median age at transplantation was 23 years, and median survival after transplantation was 5 years. Although patients who died of primary graft failure, chronic graft rejection, malignancy, infection and cerebrovascular events had similar post‐transplant survival, the resulting life‐years lost compared to the general population was less than in those who died from acute graft rejection, as they were transplanted at an older age.
FIGURE 3.
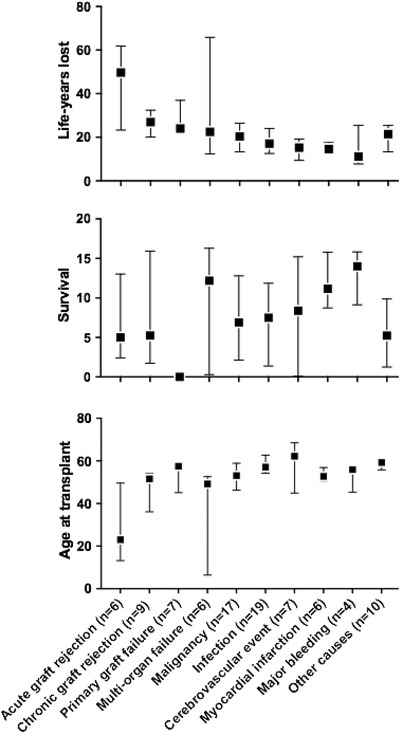
Average life‐years lost compared to the general population, years of survival and age at transplant according to aggregated validated death cause (Box = median. Bars = Inter quartile range (IQR))
4. DISCUSSION
To our knowledge this is the first study to systematically assess the validity of ISHLT data on cause of death. We found that specific causes of death in heart transplant recipients are difficult to determine, leading to moderate agreement between the heart specialists and compared with the registered ISHLT death causes. However, collapsing death causes in larger, pathophysiological relevant groups, improved agreement both between the specialists and when compared to the registered ISHLT death causes.
Furthermore, we compared our transplant population to a matched population sample and found a difference in life expectancy of 20 years.
Finally, we validated a new classification system of death causes, and applied it to assess the life‐years lost due to individual causes, identifying graft rejection together with primary graft failure as the two factors with the largest number of life‐years lost. Together, these death causes accounted for nearly one quarter of deaths, followed by infections and malignancy, each accounting for one fifth.
The validity of cause of death is notoriously poor in the general population. 12 , 13 In our study, we found a moderate validity on the cause of death registered in the Scandiatransplant/ISHLT registries, comparable to that found in previous studies in non‐transplanted populations. 5 , 14 Most clinicians are likely to have experienced that defining an individual underlying cause of death for patients with complex disease can be demanding. For example, patients who die with sepsis may also have been diagnosed with cancer, and kidney function may very well also be affected. It can be difficult to determine if these patients die from sepsis, cancer, electrolyte disturbances due to kidney failure or multi organ failure. Further, one could argue that in some cases it may be the combination of these conditions rather than one specific cause that leads to death in this vulnerable patient population. The complexity is reflected in the present study by the relatively high number of cases that required validation by a specialist board due to reviewers’ initial disagreement on the cause of death.
Further, the validated death causes differed significantly from the registered ISHLT death causes. This difference may be due to issues specific to our transplant center. However, more likely this is due to the complex nature of the courses leading to death in this patient population making it difficult to determine on a specific cause of death.
The CLASS as well as the ISHLT death cause classification system are detailed, but as demonstrated by this study, the possible advantage of richness in detail may be overshadowed by inaccuracies resulting from the complex procedure of deciding an individual death cause. An adjudication process involving two reviewers and a follow‐up procedure by a specialist board in those disagreed on as outlined in CLASS may lead to more accurate death causes, but this may not be feasible in most transplant centers due to the resource demanding nature of the process. A comparable validation from other centers contributing to the registry would contribute to further knowledge. We have shown that detailed data on death cause in the Scandiatransplant and ISHLT registries should be interpreted with caution. Grouping ISHLT death causes according to our suggestion could result in more robust data.
Grouping death causes into a larger and hence, less meticulous classification system would decrease the specificity of information, but potentially increase validity. In the present study, using the grouped death causes, the two reviewers had an almost perfect agreement with the validated CLASS methodology. However, agreement with the ISHLT causes of death remained only moderate even when using the larger pathophysiological relevant death codes.
In the second part of the study, we analyzed the grouped death causes according to the age at transplantation, survival and life years lost in comparison to a matched cohort. As shown in Figure 3, death as a result of acute graft rejection is most common in patients with a younger age at the time of transplantation. As previously known acute graft rejections often occur shortly after the transplant, and more often affect younger patients who have a more active immune system, 15 resulting in a high number of years lost. Although acute rejection is becoming less frequent with modern immunosuppressive therapy, 16 , 17 our findings underscore that it remains an important issue.
Chronic graft rejection including coronary allograft vasculopathy (CAV) is not uncommon, but probably underdiagnosed as it may be difficult to distinguish from graft failure from other causes. Separation from non‐rejection classical atherosclerosis, secondary effects of hypertension and possibly missed repeated acute rejections could also be demanding. Modern post‐transplant surveillance with diagnostic techniques such as improved biomarkers, computed tomography angiography, perfusion magnetic resonance imaging and conventional angiography supplemented by intravascular ultrasound or optical coherence tomography may improve the accuracy of diagnosing chronic graft rejections.
In comparison, death causes such as myocardial ischemia, infection, malignancy, cerebrovascular events and bleeding affect more, but relatively older patients and following several years of immunosuppression, resulting in a lower, although still high number of years lost compared to the non‐transplanted population. Multi‐organ failure affects patients of all ages, demonstrating that it often is the final consequence of other disease rather than an entity in itself, as well as a demonstration of the complexity of disease in transplanted patients.
In our material, the group major bleeding contains some patients with cardio‐ or cerebrovascular bleeding and some patients with other forms of bleeding (e.g., gastrointestinal or traumatic). One could argue the need to separate this groups, which we have chosen not to do in this material due to the small numbers.
The main limitation of the statistical analysis regarding death cause, is that the data are derived from a single center with a limited number of patients and small number in each stratification group. On the other hand, data was reviewed in detail. As approximately half of the patients transplanted at our center receive their follow‐up on other sites, and patient journals and hence death cause information on these patients were unavailable for this study, there is a risk of selection bias. The study demonstrates the problems with deciding on a single death cause for complex patients and defining the death cause even from such detailed data.
5. CONCLUSION
In this single center study, we have shown that the registered death causes in the Scandiatransplant registry according to the ISHLT death cause classification displayed moderate concordance with the validated death causes achieved by using the CLASS methodology.
After introducing a simplified version of the cause of death classification system, agreement between the two methods was markedly improved although at the cost of lower detail.
Survival after heart transplantation remains 20 years lower than in the general population, with the largest number of life‐years lost due to acute graft rejection, chronic graft rejection and primary graft failure. Although modern immunosuppressive treatment has improved during the last decades, rejection remains a considerable issue and remains an important factor.
CONFLICT OF INTEREST
No relevant financial conflicts of interest.
AUTHOR CONTRIBUTIONS
Conceptualization: Oscar Ö. Braun and J. Gustav Smith. Methodology: Oscar Ö. Braun, J. Gustav Smith, Johan Nilsson, Jakob Lundgren, Tomasz Czuba, and Grunde Gjesdal. Validation of death causes: Grunde Gjesdal, Jakob Lundgren and Oscar Ö. Braun. Analysis: Tomasz Czuba, Oscar Ö. Braun, J. Gustav Smith and Grunde Gjesdal. Jakob Lundgren contributed with analysis of death cause as the second adjudicator and member of the validation board. Writing – original draft: Grunde Gjesdal. Writing – review and editing: Oscar Ö. Braun, J. Gustav Smith, Johan Nilsson, Jakob Lundgren, Tomasz Czuba, Neval Ete Wareham, Finn Gustafsson and Grunde Gjesdal.
Supporting information
Supporting Information
ACKNOWLEDGEMENTS
We would like to express our gratitude to the heart transplant recipients who contributed to this study. J. Gustav Smith was supported by grants from the Swedish Heart‐Lung Foundation (2019‐0526), the Swedish Research Council (2017‐02554), the European Research Council (ERC‐STG‐2015‐679242), Skåne University Hospital, governmental funding of clinical research within the Swedish National Health Service, a generous donation from the Knut and Alice Wallenberg foundation to the Wallenberg Center for Molecular Medicine in Lund, and funding from the Swedish Research Council (Linnaeus grant Dnr 349‐2006‐237, Strategic Research Area Exodiab Dnr 2009‐1039) and Swedish Foundation for Strategic Research (Dnr IRC15‐0067) to the Lund University Diabetes Center. N. E. Wareham was supported by Danish National Research Foundation [Grant number 126]. J Nilsson was supported by the Swedish Research Council (2019‐00487), Swedish Heart‐Lung Foundation (20190623), a government grant for clinical research, region Skane research funds, donation funds from Skane University Hospital, and the Anna‐Lisa and Sven Eric Lundgrens Foundation.
Gjesdal G, Lundgren J, Czuba T, et al. Validation of cause of death classification after heart transplantation and cause‐specific life expectancy compared to the general population. Clin Transplant. 2022;36:e14756. 10.1111/ctr.14756
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Stehlik J, Kobashigawa J, Hunt SA, Reichenspurner H, Kirklin JK. Honoring 50 years of clinical heart transplantation in circulation: in‐depth state‐of‐the‐art review. Circulation. 2018;137:71–87. [DOI] [PubMed] [Google Scholar]
- 2. Khush KK, Cherikh WS, Chambers DC, et al. The international thoracic organ transplant registry of the international society for heart and lung transplantation: thirty‐sixth adult heart transplantation report – 2019; focus theme: donor and recipient size match. J Heart Lung Transplant. 2019;38:1056–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dellgren G, Geiran O, Lemstrom K, et al. Three decades of heart transplantation in Scandinavia: long‐term follow‐up. Eur J Heart Fail. 2013;15:308–315. [DOI] [PubMed] [Google Scholar]
- 4. Dellgren G, Westerlind A, Liden H, et al. Continuous improvement in outcome after heart transplantation – long‐term follow‐up after three decades of experience. Int J Cardiol. 2017;231:188–194. [DOI] [PubMed] [Google Scholar]
- 5. Alperovitch A, Bertrand M, Jougla E, et al. Do we really know the cause of death of the very old? Comparison between official mortality statistics and cohort study classification. Eur J Epidemiol. 2009;24:669–675. [DOI] [PubMed] [Google Scholar]
- 6. Burger EH, Groenewald P, Bradshaw D, Ward AM, Yudkin PL, Volmink J. Validation study of cause of death statistics in Cape Town, South Africa, found poor agreement. J Clin Epidemiol. 2012;65:309–316. [DOI] [PubMed] [Google Scholar]
- 7. Johansson LA, Bjorkenstam C, Westerling R. Unexplained differences between hospital and mortality data indicated mistakes in death certification: an investigation of 1,094 deaths in Sweden during 1995. J Clin Epidemiol. 2009;62:1202–1209. [DOI] [PubMed] [Google Scholar]
- 8. Wareham NE, Da Cunha‐Bang C, Borges AH, et al. Classification of death causes after transplantation (CLASS): evaluation of methodology and initial results. Medicine (Baltimore). 2018;97:e11564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. www.scb.se (Accessed 2021‐12‐10)
- 10. Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007;40:381–387. 10.1038/sj.bmt.1705727 [DOI] [PubMed] [Google Scholar]
- 11. Choudhury JB. Non‐parametric confidence interval estimation for competing risks analysis: application to contraceptive data. Statist Med. 2002;21:1129–1144. 10.1002/sim.1070 [DOI] [PubMed] [Google Scholar]
- 12. Mckee M. What do people die from? The challenges of measuring disease burden posed by multi‐morbidity. Israel J Health Policy Res. 2015;4. 10.1186/s13584-015-0047-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kircher T, Anderson RE. Cause of death. Proper completion of the death certificate. JAMA. 1987;258:349–352. [PubMed] [Google Scholar]
- 14. Rao C, Yang G, Hu J, Ma J, Xia W, Lopez AD. Validation of cause‐of‐death statistics in urban China. Int J Epidemiol. 2007;36:642–651. [DOI] [PubMed] [Google Scholar]
- 15. Kobashigawa JA, Kirklin JK, Naftel DC, et al. Pretransplantation risk factors for acute rejection after heart transplantation: a multiinstitutional study. The Transplant Cardiologists Research Database Group. J Heart Lung Transplant. 1993;12:355–366. [PubMed] [Google Scholar]
- 16. Patel JK, Kittleson M, Kobashigawa JA. Cardiac allograft rejection. Surgeon. 2011;9:160–167. [DOI] [PubMed] [Google Scholar]
- 17. Wever‐Pinzon O, Edwards LB, Taylor DO, et al. Association of recipient age and causes of heart transplant mortality: implications for personalization of post‐transplant management‐An analysis of the International Society for Heart and Lung Transplantation Registry. J Heart Lung Transplant. 2017;36:407–417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


