Abstract
The clinical translation of messenger mRNA (mRNA)-based therapeutics requires safe and effective delivery systems. Although considerable progress has been made on the development of mRNA delivery systems, many challenges, such as the dose-limiting toxicity and specific delivery to extrahepatic tissues, still remain. Cell-derived vesicles, a type of endogenous membranous particle secreted from living cells, can be leveraged to load mRNA during or after their biogenesis. Currently, they have received increasing interest for mRNA delivery due to their natural origin, good biocompatibility, cell-specific tropism, and unique ability to cross physiological barriers. In this review, we provide an overview of recent advances in the naturally occurring mRNA delivery platforms and their biomedical applications. Furthermore, the future perspectives on clinical translation of cell-derived vesicles have been discussed.
Keywords: cell-derived vesicles, extracellular vesicles, exosome, microvesicles, mRNA delivery
1. Introduction
Messenger RNA (mRNA) was first discovered in the early 1960s as a critical intermediary between genes and proteins [1]. Since then, significant work has been done towards improving mRNA’s stability and reducing its immunogenicity [2,3,4]. Additionally, the creation of mRNA delivery devices has drawn the attention of numerous researchers [5,6,7,8]. Recently, two coronavirus disease 2019 (COVID-19) mRNA vaccines have just received approval to prevent severe acute respiratory coronavirus 2 (SARS-CoV-2) infection. These progresses have paved the road for mRNA as a new class of drug [2,5,6,7,9,10]. Nowadays, mRNA-based therapeutics have displayed broad applications in prophylactic vaccines, protein replacement therapy, cancer immunotherapy, and gene editing [9,10,11,12,13,14].
It is difficult to exert physiological effects for exogenous mRNA without the assistance of delivery systems, indicating the importance of carriers for mRNA-based therapeutics [5,6,7,8]. Currently, a variety of nanomaterials including lipid- and polymer-based nanoparticles have been developed for mRNA delivery [15,16,17,18]. Among them, lipid nanoparticles are commonly used in preclinical and clinical trials [19,20,21]. Despite the growing number of studies on delivery platforms, many challenges, such as the dose-limiting toxicity and specific delivery of mRNA to extrahepatic tissues, still remain [22,23,24]. In this context, cell-derived vesicles such as extracellular vesicles (EVs) have received increasing attention in the field of mRNA delivery due to their good biocompatibility, cell-specific tropism, and unique ability to cross physiological barriers, such as blood–brain barriers [25,26]. This review summarizes the recent advances in cell-derived vesicle-mediated mRNA delivery and their applications in biomedicine.
2. Cell-Derived Vesicles for mRNA Delivery
Cell-derived extracellular vesicles refer to a heterogeneous population of membranous particles that are secreted from living cells, including mammalian cells, bacteria, and fungi [27]. They can be roughly classed into exosomes, microvesicles (MVs), and apoptotic bodies, depending on their biogenesis pathway and size [28]. Exosomes (30–150 nm) are formed as intraluminal vesicles through inward budding of early endosomes and subsequently released into the extracellular space. The plasma membrane directly protrudes outward to generate microvesicles, which range in size from 100 to 1000 nm. When cells die, apoptotic bodies (100–5000 nm) leak out through blebbing plasma membranes. EVs secreted from the source cells (also known as producer cells) transport endogenously produced biomolecules to the nearby recipient cells, or the distant cells through biological fluids [27,29]. Upon entry into the recipient cells, EVs exert important physiological and pathophysiological activity such as modulation of the tumor microenvironment, immunostimulatory functions, and tissue regeneration [29,30,31,32,33,34,35]. Due to their unique biological functions, natural origin, and good biocompatibility, as well as the ability to cross physiological barriers, EVs have attracted great interest as drug delivery platforms for delivery of small molecules (Curcumin, Doxorubicin, Paclitaxel, etc.) and biomacromolecules (siRNA, miRNA, tumor antigen, etc.) [36,37,38,39,40,41,42]. Recently, they have also been leveraged in the field of mRNA delivery and shown promise in multiple therapeutic applications (Table 1) [25]. Other cell-derived vesicles are still relatively unexplored. Until recently, virus-mimicking cell membranes have been reported for coating mRNA-loaded poly (lactic-co-glycolic acid) (PLGA) nanoparticles for enhancing their delivery efficiency, both in vitro and in vivo [43].
Table 1.
Cell-derived vesicles for mRNA loading and delivery.
| Cell-Derived Nanocarriers | Surface Markers | Size | Source Cell | Loading Strategies |
mRNA Cargos |
Target Strategies |
Application | Ref. |
|---|---|---|---|---|---|---|---|---|
| Exosomes | TSG101 and CD9 | 50–200 nm | 293T | Passive loading | Luciferase or PGC1α | miRNA-dependent mRNA expression and ultrasound | Obesity | [44] |
| Exosomes | CD9, TSG101, and CD63 | 30–160 nm | 293T | Passive loading | Bmp7 | Ultrasound | Obesity | [45] |
| MVs | NA | Mostly 100–150 nm | HEK-293T | Passive loading | CD-UPRT-EGFP | Prodrug | Schwannoma tumor | [46] |
| Exosomes | TSG101 and CD9 | 50–200 nm | HEK293T | Passive loading | Il-10 | miRNA- dependent mRNA expression | Inflammation of atherosclerosis | [47] |
| Exosomes | Lamp2b, CD63, Alix, and Tsg101 | 20–500 nm | HEK293 | Passive loading | Nerve growth factor | RVG-Lamp2b | Cerebral ischemia | [48] |
| Exosomes | CD9, CD63, CD47, and Tsg101 | NA | MEFs and DCs | Passive loading based on a cellular nanoporation system | PTEN | Fuse glioma-targeting peptides to CD47 | Glioma tumor | [49] |
| Exosomes | CD9, TSG101 | 30–150 nm | AML12 | Passive loading | Ldlr | NA | Familial hypercholesterolemia | [50] |
| Exosomes | CD63, Lamp2b, CD9 and TSG101 | 50–200 nm | HEK-293T | Active loading based on L7Ae-CD63 fusion protein | nluc or catalase | RVG-Lamp2b | Parkinson’s disease | [51] |
| Exosomes | Lamp2b and CD63 | 50–200 nm | 293FT | TAMEL platform | DTomato or Cas9 | NA | NA | [52] |
| Exosomes | CD63, CD9, and TSG101 | 100–200 nm | 293T | Active loading based on CD9-HUR fusion protein | Cas9 | NA | NA | [53] |
| Exosomes | CD9 and Lamp2b | 100–300 nm | 293T | Active loading based on a specific DNA aptamer | GFP, PGC1α or Il-10 | ATS-Lamp2b | Obesity and intestinal inflammation |
[54] |
| Exosomes | CD63, Alix, and TSG101 | Average ~100 nm | HEK293T | Active loading based on L7Ae-CD63 fusion protein | ZPAMt | NA | HIV-1 infection | [55] |
| Exosomes | CD63, CD81, and lactadherin | 30–100 nm; | HEK293 | Active loading based on EV-loading zipcode sequence | HChrR6 | Prodrug | HER2 + ve human breast cancer | [56] |
| MVs | ARRDC1 | <100 nm | 293T | Active loading based on Tat-ARRDC1 fusion protein | GFP or p53 | NA | NA | [57] |
| Outer membrane vesicles | ClyA | average 28.1 nm | BL21 (DE3) Escherichia coli | Active loading based on ClyA-L7Ae fusion protein | EGFP, OVA or ADPGK | NA | Melanoma and colon cancer | [58] |
| Exosomes | Glycophorin A, ALIX, and TSG1–1 | 120–200 nm | Red blood cells | Post-loading based on REG1 loading reagent | Luciferase | An enzymatic method | NA | [59] |
| A mixture of exosomes and MVs | ALIX, TSG101, hemoglobin A, and Stomatin | 100–300 nm (average ~140 nm) | Red blood cells | Post-loading based on electroporation | Cas9 | NA | NA | [60] |
| Exosomes | CD63 | ~110 nm | HEK293T or lung spheroid cells | Post-loading based on electroporation | GFP | NA | NA | [61] |
| Exosomes | NA | ~200 nm | HEK293T or lung spheroid cells | Post-loading based on electroporation | GFP or SARS-CoV-2 spike protein | NA | COVID-19 | [62] |
| Cell membrane-coated PLGA NPs | NA | average 185 nm | B16F10 | Double emulsion method with the assistance of G0-C14 | EGFP or Cypridina luciferase | NA | NA | [43] |
MEFs, mouse embryonic fibroblasts; DCs, dendritic cells; HUR, human antigen R; ARRDC1, arrestin domain containing protein; Cas9, CRISPR-associated protein 9; GFP, green fluorescent protein; nluc, NanoLuc luciferase; EGFP, enhanced green fluorescent protein; OVA, ovalbumin; Ldlr, low density lipoprotein receptor; RVG, rabies viral glycoprotein. PTEN, a tumor suppressor phosphatase and tensin homolog deleted on chromosome 10; Lamp2b, lysosome-associated membrane protein 2; SARS-CoV-2, severe acute respiratory coronavirus 2; COVID-19, coronavirus disease 2019; NA, not applicable.
2.1. Preparation of Cell-Derived Vesicles
Commonly, EVs with or without mRNA cargos should be produced firstly from the producer cells for further isolation and purification. Several types of cells have been explored as source cells of EVs, such as red blood cells, human embryonic kidney 293T cells, bone marrow-derived dendritic cells, and Gram-negative bacteria [58,63,64,65,66]. For EV-based mRNA delivery, 293T were the mostly commonly used cells, accounting for about 80% in all source cells (Table 1). Following secretion, multiple techniques such as ultra-centrifugation, density gradient centrifugation, size exclusion chromatography, and filtration methods have been exploited to obtain purified EVs from source cells [67,68,69,70].
To boost the capability of exosomes production, potential production boosters including STEAP3, syndecan-4, and a fragment of L-aspartate oxidase were employed [51]. STEAP3 and L-aspartate oxidase-related fragment involve the biogenesis and cellular metabolism of exosomes, while syndecan-4 contributes to the inward budding of early endosomes membranes [51]. Co-overexpression of all three boosters in 293T cells significantly increased exosome production [51]. Apart from production boosters, cellular nanoporation (CNP) consisting of a nanochannel array (~500 nm in diameter) has also been proposed for increasing the production of mRNA-loaded exosomes via transient electrical pulses [49]. The yield of mRNA-bearing exosomes for CNP was more than 50-fold higher in comparison with the traditional electroporation method [49]. Furthermore, abundant EVs (1013–1014 EVs) were produced by treating red blood cells with calcium ionophore [60].
It is worth noting that the heterogeneity of EVs resulted from the process of production and separation has an important impact on their delivery efficiency. Thus, thorough characterization of EVs, including the particle size, zeta potential, morphologies, and surface markers, is necessary for subsequent quality control and biomedical applications [35,67,69,71].
2.2. Strategies for mRNA Loading into Cell-Derived Vesicles
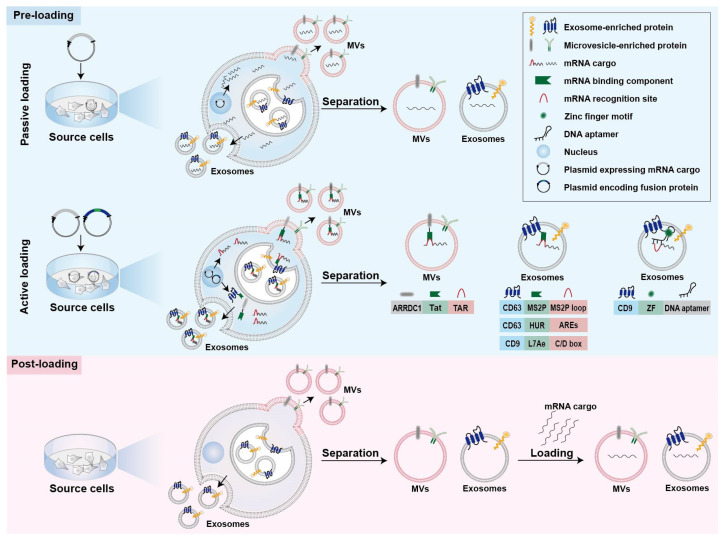
Strategies for mRNA loading into cell-derived vesicles could be simply classified into two main categories, namely pre-loading methods and post-loading methods (Figure 1) [25,72]. Pre-loading methods (also called pre-separation or endogenous loading methods) heavily rely on the producer cells to pack the mRNA cargos into EVs during their biogenesis (Figure 1) [72]. Sometimes, mRNA-encoded proteins are also simultaneously packaged into cell-derived vesicles during the endogenous loading process [46,48]. Pre-loading methods can be further divided into passive and active categories (Figure 1), as described below [52]. Post-loading methods are also called post-separation or exogenous loading methods (Figure 1) [72]. In this case, exogenous mRNA is loaded into isolated EVs via electroporation or chemical transfection reagents [59,60].
Figure 1.
Strategies for mRNA loading into EVs. Strategies are classified into pre-loading methods and post-loading methods. The former can be further divided into passive and active methods. ARRDC1, arrestin domain containing protein 1; Tat, transactivator of transcription protein; TAR, trans-activating response element; MS2P, MS2 bacteriophage coat protein; HUR, human antigen R; MVs, microvesicles; ARE, AU-rich elements; ZF, zinc finger motif.
2.2.1. Passive Pre-Loading Methods
The most common approach for passive pre-loading methods is to introduce plasmid into producer cells to obtain the transcribed mRNA of interest. Overexpression of target mRNA could facilitate its enrichment into EVs. Such strategy has been employed by several studies for loading various mRNA into EVs (Table 1) [47,48,50]. For example, low-density lipoprotein receptor (Ldlr) mRNA was encapsulated into exosomes via forced overexpression in the source cells [50]. After plasmid transfection, the level of Ldlr mRNA in source cells increased more than 100-fold compared with cells transfected with control plasmid, thus leading to a similar increase in mRNA cargos in the secreted exosomes [50].
Because small RNAs are the dominant modalities of RNAs within secreted exosomes, encapsulation of large mRNA into nano-sized exosomes is technically challenging for passive pre-loading method [25,73]. It is revealed that the aforementioned CNP technology not only increases the yield of exosomes but also improves mRNA content in the exosomes [49]. In comparison with exosomes produced endogenously without external stimulation, the mRNA loading efficiency of CNP-treated exosomes produced by the same source cells increased by three or four orders of magnitude (one mRNA within every 103 exosomes vs. two to ten mRNA per exosome) [49]. Additionally, this strategy also led to a 100-folded higher loading of mRNA into exosomes relative to conventional electroporation method [49].
2.2.2. Active Pre-Loading Methods
Another strategy to improve the loading efficiency of mRNA cargos is to transfect the producer cells with two types of plasmid. One type of the plasmid encodes fusion proteins comprised of mRNA binding components and EV-enriched proteins such as surface markers CD9, CD63, or cytosolic protein Hspa8 (Figure 1) [51,52,53]. Generally speaking, the mRNA of interest transcribed from plasmids contains intentionally engineered recognition sites, which can specifically bind with the mRNA binding components of fusion proteins. The remaining part of the fusion proteins, EV-enriched proteins, are then incorporated into EVs during their biogenesis to achieve active mRNA pre-loading.
Targeted and Modular EV loading (TAMEL) is an active loading platform developed for actively loading mRNA into exosomes via fusing a EV-enriched protein such as Lamp2b, CD63, and Hspa8 to the MS2 bacteriophage coat protein (Figure 1) [52]. The cognate MS2 stem loop sequence was then incorporated into the mRNA cargos to promote mRNA binding and loading into the EVs interior [52]. It has been found that the loading efficiency decreases with the increase in mRNA size [52].
Several other fusion proteins have also been designed for active loading of mRNA into EVs (Figure 1) [51,53]. Archaeal ribosomal protein L7Ae or human antigen R fused to surface marker of EVs are leveraged to bind to the introduced C/D box RNA structure and AU-rich elements in the mRNA cargos, respectively [51,53]. In addition, fusing the transactivator of the transcription protein to the C-terminus of arrestin domain containing protein 1, which mediated the budding of MVs, confers high affinity for binding the stem-loop-containing trans-activating response element introduced at the 5′ end of mRNA cargos [57]. In general, the high binding affinity between mRNA binding components and mRNA recognition site facilitates the active packaging of specific mRNA into EVs.
Apart from these mRNA binding components, DNA aptamer was also used to specifically recognize and actively load the mRNA of interest (Figure 1) [54]. In this case, a specific DNA aptamer consisting of two parts was designed as a bridge for connection between mRNA cargos and EVs [54]. The single strand part of the DNA aptamer could recognize the region surrounding start codon AUG of target mRNA, which was thought to be beneficial for the sorting of mRNA into EVs [54]. The double strand part of the DNA aptamer could be recognized by zinc finger motifs (ZF) that were tailored to specifically bind to the sequence of any double-stranded DNA [54]. To facilitate the recruit of DNA aptamer as well as sorting of the complexed mRNA into EVs, the ZF was fused with an exosomal surface marker, CD9 [54]. As a result, this designed DNA aptamer resulted in a ~2.5-fold increase in the enrichment effect of large PGC1α mRNA into EVs [54].
2.2.3. Post-Loading Methods
So far, post-loading mRNA into EVs largely depends on electroporation and commercial loading reagents (Table 1 and Figure 1). Electroporation is a commonly used method for loading of various molecules, including siRNA and miRNA, as well as mRNA, to purified EVs [60,61,62,74,75]. About one fifth of Cas9 mRNA can be loaded into red blood cell-derived EVs by electroporation [60]. Furthermore, a commercial loading reagent named REG1 has also been used for loading mRNA into EVs after their isolation [59].
2.3. Strategies for Tissue-Specific mRNA Expression
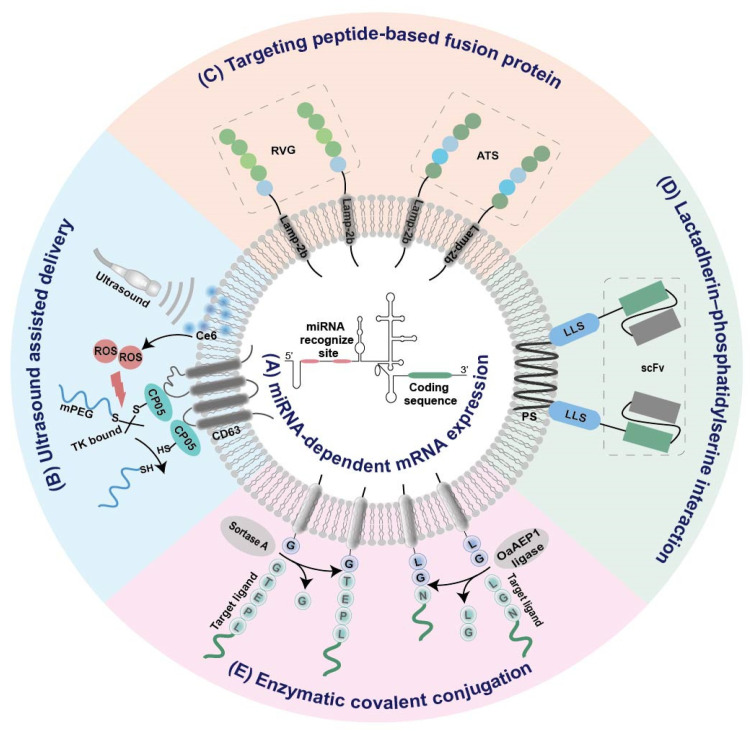
To enhance the tissue specificity of cell-derived biomimetic vesicles, several strategies have been proposed, as below (Figure 2).
Figure 2.
Summary of EV-based platforms for tissue-specific mRNA expression. (A) miRNA-dependent mRNA expression. (B) Ultrasound assisted delivery. (C) Targeting peptide-based fusion protein. (D) Lactadherin–phosphatidylserine interaction. (E) Enzymatic covalent conjugation. IRES, internal ribosome entry site; RVG, rabies viral glycoprotein; ATS, adipocyte-targeting sequence; scFv, single chain variable fragment; LLS, lactadherin leader sequence; Ce6, sonosensitizer chlorin e6; ROS, reactive oxygen species; PS, phosphatidylserine; TK, thioketal.
2.3.1. Tissue-Specific miRNA-Dependent mRNA Expression
It has been found that the internal ribosome entry site (IRES) at the 5′ end of the hepatitis C virus RNA can be specifically recognized by liver-specific miRNA-122, and thus initiates its tissue-specific mRNA translation [76]. Replacement of this miR-122 recognition site at the IRES with sequences recognized by other tissue-specific miRNA enables miRNA-specific activation of mRNA translation in specific tissues (Figure 2A). According to this principle, an adipose-specific translation system (miR-148a-IRES-PGC1α) was constructed by substituting miR-122 recognition sites at the IRES with sequences recognized by adipose-specific miR-148a at the upstream of the PGC1α mRNA coding sequence [44]. Injection of exosome loading with such a system resulted in a significant increase in PGC1α protein expression in the adipose tissue of mice, but a decrease in lung, spleen, and kidney [44]. Using a similar strategy, the same group also constructed inflammation-responsive Il-10 mRNA by replacing miR-122 with miR-155 enriched in the inflammatory sites of atherosclerosis [47]. The expression of Il-10 mRNA in exosomes was specifically activated by miR-155 in the inflamed macrophages, while its expression in other tissues without obvious inflammation was rare [47].
2.3.2. Ultrasound Assisted Tissue-Specific Delivery
To minimize the off-target effects, exosomal delivery strategies assisted by ultrasound have been explored [44,45]. Recently, two ultrasound-assisted exosomal platforms have been established for the specific delivery of mRNA to adipose tissue [44,45]. In one study, target uptake of EVs was achieved with the assistance of ultrasound-targeted microbubble destruction (UTMD) as well as the tissue-specific miRNA-dependent expression system mentioned above [44]. As the microbubble destruction at the ultrasound site may enhance the cell membrane permeability and cellular uptake of recipient cells, the delivery of exosomes into the adipose tissue should be increased by UTMD. Consistent with this assumption, the distribution of DiR-labeled exosomes in the adipose tissue significantly increased under the assistance of UTMD [44].
In the other study, a smart exosome-based delivery platform was designed for escaping from phagocytosis and locally delivering mRNA to the omental adipose tissue [45]. Firstly, CP05-thioketal (TK)-mPEG was anchored onto exosomes through interaction between the peptide CP05 and the CD63 marker of exosomes (Figure 2B) [45]. The mPEG chain could protect the carrier platform from aggregation, opsonization, and phagocytosis, thus prolonging the in vivo circulation time [45]. Then, reactive oxygen species produced by sonosensitizer chlorin e6 under ultrasound triggered the break of TK bonds between CP05 and mPEG (Figure 2B) [45]. Eventually, the carrier core was exposed by removing the PEG corona and specifically internalized by recipient cells at the ultrasound site, thus leading to a dramatic increase in the expression of exosome-encapsulated mRNA in adipose tissue under ultrasound [45].
2.3.3. Targeted Modification
Conjugation of targeting ligands to EVs via genetic engineering, enzymatic, or affinity-based methods was proven to be effective for exosome-based targeted delivery [77,78,79,80]. For example, when the central nervous system-specific rabies viral glycoprotein (RVG) was fused to an exosomal membrane protein Lamp2b, the fusion protein RVG-Lamp2b facilitated transport of EVs across the blood–brain barrier (Figure 2C) [48,51]. To fulfill the targeted delivery of EVs to adipose tissues, an adipocyte-targeting sequence (ATS, CKGGRAKDC) was also fused to the N-terminus of Lamp2b (Figure 2C) [54]. Moreover, an anti-HER2 single chain variable fragment was connected to a lactadherin leader sequence (Figure 2D) [56]. The former was capable of targeting HER2 overexpressing cells via antigen–antibody interactions, while the latter could bind to EVs based on affinity with their surface phosphatidylserine (Figure 2D) [56]. As a consequence, the modified EVs were selectively internalized by HER2-positive cells [56].
Despite its straightforwardness, the fusion protein-based targeting method always require genetic engineering. Therefore, covalently conjugating peptides or nanobodies onto EVs without any genetic modification of source cells provide an alternative method for the targeted modification of EVs [59]. The versatile targeting platform leverages protein-ligating enzymes (Sortase A and OaAEP1 ligase) to catalyze covalent-bonding reactions between the membrane proteins of EVs and targeting ligands, including targeting peptides, ‘self’ peptides, as well as nanobodies (Figure 2E) [59].
3. Application
EVs themselves, or when used as drug delivery systems, are currently being assessed in clinical trials for diagnostic or therapeutic purposes [37]. When functioning as mRNA delivery systems, EVs exhibit a great potential for biomedical applications, including tumor, central nervous system diseases, obesity, and anti-inflammation (Table 1).
3.1. Tumor
In 2013, Arda Mizrak et al. pioneered the use of MVs as mRNA carriers for the treatment of tumor [46]. In that study, mRNA encoding cytosine deaminase (CD)-uracil phosphoribosyltransferase (UPRT) fusion proteins were encapsulated into MVs and injected into tumors, in combination with intravenously injected prodrug 5-fluorocytosine (5-FC). The expression of CD and UPRT in tumor cells promoted the conversion of 5-FC to the cytotoxic drug 5-fluoro-deoxyuridine monophosphate, thus inhibiting DNA synthesis and inducing tumor cell apoptosis. The remarkable inhibition of schwannoma tumor growth and regression of tumor size in two animal models indicated the feasibility of MVs as mRNA carrier for tumor therapy [46].
Subsequently, the therapeutic potential of exosomes as mRNA carriers was also explored for tumor therapy [56]. HChrR6 is an optimized bacterial enzyme that could convert prodrug CNOB into cytotoxic drug MCHB. Sequential injection of anti-HER2 scFv antibody-modified HchrR6 mRNA-loaded exosomes and CNOB led to the specific activation of CNOB and subsequent MCHB generation at tumor site, causing near-complete inhibition of orthotopic HER2-positive BT474 xenografts [56]. Additionally, glioma-targeting peptide-modified exosomes carrying PTEN mRNA were applied to brain tumor therapy and increased survival time for U87 or GL261 glioma-bearing mice [49].
EVs derived from BL21 (DE3) Escherichia coli with intrinsic function of innate immunity stimulation have also been employed as an mRNA delivery platform for a personalized tumor vaccine [58]. This nanocarrier platform possessed a “Plug-and-Display” feature and allowed the rapid display of various tumor antigens, thus enabling rapid preparation of personalized cancer vaccines. After subcutaneous injection, ovalbumin or ADPGK mRNA delivered by this platform led to significantly strong inhibition of melanoma progression. Moreover, complete regression was observed for three out of eight mice bearing colon cancer [58].
3.2. Central Nervous System Diseases
The ability to cross blood–brain barriers makes EVs a promising carrier candidate for the treatment of various brain diseases. Ryosuke Kojima et al. constructed an EXOsomal Transfer Into Cells system (EXOtic) consisting of an exosome production booster, a specific mRNA packaging device, a cytosolic delivery helper, and a brain targeting module (RVG-Lamp2b) [51]. Implantation of exosome producer cells engineered by the EXOtic system into living mice was adopted for the in situ production and delivery of a therapeutic mRNA-containing exosome for treatment of Parkinson’s disease [51]. It has been found that catalase mRNA was delivered into brain tissue by the in situ produced exosomes, resulting in attenuation of neuroinflammation and area-specific rescue of neuronal cell death [51]. Recently, the utility of an EV-based delivery strategy for treatment of cerebral ischemia has also been reported [48]. RVG-modified exosomes loaded with nerve growth factor both in mRNA and protein format were able to reach the ischemic region of the brain following tail vein injection and alleviate ischemic injury by reduced inflammation, improved cell survival, and promoted neurogenesis in ischemia mice [48].
3.3. Obesity
Obesity is becoming a health burden worldwide that increases risks for various diseases. EV-mediated mRNA delivery holds promise for obesity therapy [44,45,54]. Two studies have revealed that ultrasound assisted exosome delivery platforms were capable of enhancing the efficacy of functional mRNA at the adipose tissue [44,45]. As both PGC1α (an essential transcription factor for fat browning) and the bone morphology protein 7 (Bmp7, an important inducer of brown adipocyte differentiation) play great roles in the induction of brown adipose tissue, delivery of Bmp7 mRNA or PGC1α mRNA-exosomes to the adipose tissues under ultrasound significantly induced the browning effect, decreased body weight, and reduced off-target effects [44,45]. Furthermore, PGC1α mRNA has been selectively delivered to the adipose via exosomes modified with adipocyte-targeting sequence, eventually leading to a significant decrease in body weight along with an increase in brown adipose tissue [54].
3.4. Anti-Inflammation
Il-10, a soluble anti-inflammatory cytokine, plays an important role in inflammation. Thus, Il-10 mRNA-loaded exosomes have been used for the treatment of inflammation related diseases, including atherosclerosis [47] and inflammatory bowel disease [54]. For atherosclerosis, the inflammation responsive Il-10 mRNA-loaded exosomes were efficiently delivered to inflammatory macrophages and precisely translationally activated in inflamed tissues after systemic administration, demonstrating on-demand anti-inflammatory effects with decreased expression of inflammation cytokines, including Il-1β, Tnf-α, and Il-6 [47]. Furthermore, the anti-inflammatory effects also alleviated the atherosclerosis in ApoE-/- mice, with lower atherosclerotic plaques and lesion size [47]. Recently, Il-10 mRNA delivered by exosomes has been reported for the treatment of inflammatory bowel disease [54]. Systemic injection of Il-10 mRNA-loaded exosomes not only reduced inflammatory responses, but also prevented body weight loss and colon length shortening in a mouse model [54].
3.5. Other Diseases
Most recently, new attempts to deliver mRNA with cell-derived vesicles have been made in treating other diseases, including familial hypercholesterolemia, acquired immunodeficiency syndrome, and COVID-19 [50,55,62]. Exosomes carrying Ldlr mRNA could restore Ldlr protein expression and further decreased the high serum cholesterol level in Ldlr-/- mice following intravenous injection. Systemic administration of exosomes loaded with ZPAMt mRNA targeting CPG methylation of the 5′ long terminal repeat achieved stably repression of HIV-1 [55]. Furthermore, such exosomes were found to be useful for crossing the blood–brain barrier and inhibiting HIV-1 expression in the brain [55]. More recently, mRNA vaccines against COVID-19 were prepared by electroporation of mRNA encoding SARS-CoV-2 spike into lung-derived exosomes. Such vaccines were able to elicit more potent immune responses than liposome-based counterpart in mice by dry powder inhalation [62].
4. Conclusions and Outlook
Cell-derived vesicles hold promise for delivering various small molecules, siRNA, and miRNA. In recent years, these naturally occurring vesicles have been adapted for mRNA delivery. They offer a promising opportunity to enhance the efficacy of mRNA in treating a variety of diseases, ranging from tumor to COVID-19. Nevertheless, some barriers have yet to be solved before translational applications. These include standardization of the critical parameters of inherently heterogeneous EVs, such as surface signatures and internal autologous contents. Furthermore, biodistribution in target organs merits further improvement. Future work on hybrid EVs containing synthetic nanocarriers or the rational design of engineered EVs would provide great value for the development of more effective and selective cell-derived vesicles for mRNA delivery, thus accelerating their clinic translation.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the National Natural Science Foundation of China, grant number 82072053, the Shenzhen Science and Technology Program, grant number RCYX20200714114539061, and the Shenzhen Fundamental Research Foundation, grant number JCYJ20190806150018265.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cobb M. Who discovered messenger RNA? Curr. Biol. 2015;25:R526–R532. doi: 10.1016/j.cub.2015.05.032. [DOI] [PubMed] [Google Scholar]
- 2.Dammes N., Peer D. Paving the Road for RNA Therapeutics. Trends Pharmacol. Sci. 2020;41:755–775. doi: 10.1016/j.tips.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boo S.H., Kim Y.K. The emerging role of RNA modifications in the regulation of mRNA stability. Exp. Mol. Med. 2020;52:400–408. doi: 10.1038/s12276-020-0407-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sahin U., Karikó K., Türeci Ö. mRNA-based therapeutics--developing a new class of drugs. Nat. Rev. Drug Discov. 2014;13:759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 5.Xiao Y., Tang Z., Huang X., Chen W., Zhou J., Liu H., Liu C., Kong N., Tao W. Emerging mRNA technologies: Delivery strategies and biomedical applications. Chem. Soc. Rev. 2022;51:3828–3845. doi: 10.1039/D1CS00617G. [DOI] [PubMed] [Google Scholar]
- 6.Paunovska K., Loughrey D., Dahlman J.E. Drug delivery systems for RNA therapeutics. Nat. Rev. Genet. 2022;23:265–280. doi: 10.1038/s41576-021-00439-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ibba M.L., Ciccone G., Esposito C.L., Catuogno S., Giangrande P.H. Advances in mRNA non-viral delivery approaches. Adv. Drug Deliv. Rev. 2021;177:113930. doi: 10.1016/j.addr.2021.113930. [DOI] [PubMed] [Google Scholar]
- 8.Li B., Zhang X., Dong Y. Nanoscale platforms for messenger RNA delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019;11:e1530. doi: 10.1002/wnan.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbier A.J., Jiang A.Y., Zhang P., Wooster R., Anderson D.G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 2022;40:840–854. doi: 10.1038/s41587-022-01294-2. [DOI] [PubMed] [Google Scholar]
- 10.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beck J.D., Reidenbach D., Salomon N., Sahin U., Türeci Ö., Vormehr M., Kranz L.M. mRNA therapeutics in cancer immunotherapy. Mol. Cancer. 2021;20:69. doi: 10.1186/s12943-021-01348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Z., Li Z., Li B. Nonviral Delivery of CRISPR/Cas Systems in mRNA Format. Adv. NanoBiomed Res. 2022;2:2200082. doi: 10.1002/anbr.202200082. [DOI] [Google Scholar]
- 13.Qiu M., Li Y., Bloomer H., Xu Q. Developing Biodegradable Lipid Nanoparticles for Intracellular mRNA Delivery and Genome Editing. Acc. Chem. Res. 2021;54:4001–4011. doi: 10.1021/acs.accounts.1c00500. [DOI] [PubMed] [Google Scholar]
- 14.Huang X., Kong N., Zhang X., Cao Y., Langer R., Tao W. The landscape of mRNA nanomedicine. Nat. Med. 2022;28:2273–2287. doi: 10.1038/s41591-022-02061-1. [DOI] [PubMed] [Google Scholar]
- 15.Patel S., Ashwanikumar N., Robinson E., Xia Y., Mihai C., Griffith J.P., 3rd, Hou S., Esposito A.A., Ketova T., Welsher K., et al. Naturally-occurring cholesterol analogues in lipid nanoparticles induce polymorphic shape and enhance intracellular delivery of mRNA. Nat. Commun. 2020;11:983. doi: 10.1038/s41467-020-14527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu S., Cheng Q., Wei T., Yu X., Johnson L.T., Farbiak L., Siegwart D.J. Membrane-destabilizing ionizable phospholipids for organ-selective mRNA delivery and CRISPR-Cas gene editing. Nat. Mater. 2021;20:701–710. doi: 10.1038/s41563-020-00886-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li M., Li S., Huang Y., Chen H., Zhang S., Zhang Z., Wu W., Zeng X., Zhou B., Li B. Secreted Expression of mRNA-Encoded Truncated ACE2 Variants for SARS-CoV-2 via Lipid-Like Nanoassemblies. Adv. Mater. 2021;33:e2101707. doi: 10.1002/adma.202101707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren J., Cao Y., Li L., Wang X., Lu H., Yang J., Wang S. Self-assembled polymeric micelle as a novel mRNA delivery carrier. J. Control. Release. 2021;338:537–547. doi: 10.1016/j.jconrel.2021.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou X., Zaks T., Langer R., Dong Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021;6:1078–1094. doi: 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eygeris Y., Gupta M., Kim J., Sahay G. Chemistry of Lipid Nanoparticles for RNA Delivery. Acc. Chem. Res. 2022;55:2–12. doi: 10.1021/acs.accounts.1c00544. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y., Sun C., Wang C., Jankovic K.E., Dong Y. Lipids and Lipid Derivatives for RNA Delivery. Chem. Rev. 2021;121:12181–12277. doi: 10.1021/acs.chemrev.1c00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dowdy S.F. Overcoming cellular barriers for RNA therapeutics. Nat. Biotechnol. 2017;35:222–229. doi: 10.1038/nbt.3802. [DOI] [PubMed] [Google Scholar]
- 23.Loughrey D., Dahlman J.E. Non-liver mRNA Delivery. Acc. Chem. Res. 2022;55:13–23. doi: 10.1021/acs.accounts.1c00601. [DOI] [PubMed] [Google Scholar]
- 24.Cheng Q., Wei T., Farbiak L., Johnson L.T., Dilliard S.A., Siegwart D.J. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat. Nanotechnol. 2020;15:313–320. doi: 10.1038/s41565-020-0669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Brien K., Breyne K., Ughetto S., Laurent L.C., Breakefield X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020;21:585–606. doi: 10.1038/s41580-020-0251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scully M.A., Sterin E.H., Day E.S. Membrane-wrapped nanoparticles for nucleic acid delivery. Biomater. Sci. 2022;10:4378–4391. doi: 10.1039/D2BM00447J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Niel G., D’Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 28.Cheng L., Hill A.F. Therapeutically harnessing extracellular vesicles. Nat. Rev. Drug Discov. 2022;21:379–399. doi: 10.1038/s41573-022-00410-w. [DOI] [PubMed] [Google Scholar]
- 29.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tkach M., Théry C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell. 2016;164:1226–1232. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 31.Marar C., Starich B., Wirtz D. Extracellular vesicles in immunomodulation and tumor progression. Nat. Immunol. 2021;22:560–570. doi: 10.1038/s41590-021-00899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagelkerke A., Ojansivu M., van der Koog L., Whittaker T.E., Cunnane E.M., Silva A.M., Dekker N., Stevens M.M. Extracellular vesicles for tissue repair and regeneration: Evidence, challenges and opportunities. Adv. Drug Deliv. Rev. 2021;175:113775. doi: 10.1016/j.addr.2021.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Mathieu M., Martin-Jaular L., Lavieu G., Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019;21:9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z.G., Buller B., Chopp M. Exosomes—beyond stem cells for restorative therapy in stroke and neurological injury. Nat. Rev. Neurol. 2019;15:193–203. doi: 10.1038/s41582-018-0126-4. [DOI] [PubMed] [Google Scholar]
- 35.Lai J.J., Chau Z.L., Chen S.Y., Hill J.J., Korpany K.V., Liang N.W., Lin L.H., Lin Y.H., Liu J.K., Liu Y.C., et al. Exosome Processing and Characterization Approaches for Research and Technology Development. Adv. Sci. 2022;9:e2103222. doi: 10.1002/advs.202103222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kooijmans S.A.A., de Jong O.G., Schiffelers R.M. Exploring interactions between extracellular vesicles and cells for innovative drug delivery system design. Adv. Drug Deliv. Rev. 2021;173:252–278. doi: 10.1016/j.addr.2021.03.017. [DOI] [PubMed] [Google Scholar]
- 37.Herrmann I.K., Wood M.J.A., Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021;16:748–759. doi: 10.1038/s41565-021-00931-2. [DOI] [PubMed] [Google Scholar]
- 38.Elsharkasy O.M., Nordin J.Z., Hagey D.W., de Jong O.G., Schiffelers R.M., Andaloussi S.E., Vader P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020;159:332–343. doi: 10.1016/j.addr.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 39.Fu P., Zhang J., Li H., Mak M., Xu W., Tao Z. Extracellular vesicles as delivery systems at nano-/micro-scale. Adv. Drug Deliv. Rev. 2021;179:113910. doi: 10.1016/j.addr.2021.113910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horodecka K., Düchler M. CRISPR/Cas9: Principle, Applications, and Delivery through Extracellular Vesicles. Int. J. Mol. Sci. 2021;22:6072. doi: 10.3390/ijms22116072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu M., Zhou X., Tang J. Non-Coding RNAs Delivery by Small Extracellular Vesicles and Their Applications in Ovarian Cancer. Front. Bioeng. Biotechnol. 2022;10:876151. doi: 10.3389/fbioe.2022.876151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bost J.P., Barriga H., Holme M.N., Gallud A., Maugeri M., Gupta D., Lehto T., Valadi H., Esbjörner E.K., Stevens M.M., et al. Delivery of Oligonucleotide Therapeutics: Chemical Modifications, Lipid Nanoparticles, and Extracellular Vesicles. ACS Nano. 2021;15:13993–14021. doi: 10.1021/acsnano.1c05099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park J.H., Mohapatra A., Zhou J., Holay M., Krishnan N., Gao W., Fang R.H., Zhang L. Virus-Mimicking Cell Membrane-Coated Nanoparticles for Cytosolic Delivery of mRNA. Angew. Chem. Int. Ed. Engl. 2022;61:e202113671. doi: 10.1002/anie.202113671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun W., Xing C., Zhao L., Zhao P., Yang G., Yuan L. Ultrasound Assisted Exosomal Delivery of Tissue Responsive mRNA for Enhanced Efficacy and Minimized Off-Target Effects. Mol. Nucleic Acids. 2020;20:558–567. doi: 10.1016/j.omtn.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo Y., Wan Z., Zhao P., Wei M., Liu Y., Bu T., Sun W., Li Z., Yuan L. Ultrasound triggered topical delivery of Bmp7 mRNA for white fat browning induction via engineered smart exosomes. J. Nanobiotechnology. 2021;19:402. doi: 10.1186/s12951-021-01145-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mizrak A., Bolukbasi M.F., Ozdener G.B., Brenner G.J., Madlener S., Erkan E.P., Ströbel T., Breakefield X.O., Saydam O. Genetically engineered microvesicles carrying suicide mRNA/protein inhibit schwannoma tumor growth. Mol. Ther. 2013;21:101–108. doi: 10.1038/mt.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bu T., Li Z., Hou Y., Sun W., Zhang R., Zhao L., Wei M., Yang G., Yuan L. Exosome-mediated delivery of inflammation-responsive Il-10 mRNA for controlled atherosclerosis treatment. Theranostics. 2021;11:9988–10000. doi: 10.7150/thno.64229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang J., Wu S., Hou L., Zhu D., Yin S., Yang G., Wang Y. Therapeutic Effects of Simultaneous Delivery of Nerve Growth Factor mRNA and Protein via Exosomes on Cerebral Ischemia. Mol. Ther. Nucleic Acids. 2020;21:512–522. doi: 10.1016/j.omtn.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Z., Shi J., Xie J., Wang Y., Sun J., Liu T., Zhao Y., Zhao X., Wang X., Ma Y., et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat. Biomed. Eng. 2020;4:69–83. doi: 10.1038/s41551-019-0485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Z., Zhao P., Zhang Y., Wang J., Wang C., Liu Y., Yang G., Yuan L. Exosome-based Ldlr gene therapy for familial hypercholesterolemia in a mouse model. Theranostics. 2021;11:2953–2965. doi: 10.7150/thno.49874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kojima R., Bojar D., Rizzi G., Hamri G.C., El-Baba M.D., Saxena P., Ausländer S., Tan K.R., Fussenegger M. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat. Commun. 2018;9:1305. doi: 10.1038/s41467-018-03733-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hung M.E., Leonard J.N. A platform for actively loading cargo RNA to elucidate limiting steps in EV-mediated delivery. J. Extracell. Vesicles. 2016;5:31027. doi: 10.3402/jev.v5.31027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Z., Zhou X., Wei M., Gao X., Zhao L., Shi R., Sun W., Duan Y., Yang G., Yuan L. In Vitro and in Vivo RNA Inhibition by CD9-HuR Functionalized Exosomes Encapsulated with miRNA or CRISPR/dCas9. Nano Lett. 2019;19:19–28. doi: 10.1021/acs.nanolett.8b02689. [DOI] [PubMed] [Google Scholar]
- 54.Zhang S., Dong Y., Wang Y., Sun W., Wei M., Yuan L., Yang G. Selective Encapsulation of Therapeutic mRNA in Engineered Extracellular Vesicles by DNA Aptamer. Nano Lett. 2021;21:8563–8570. doi: 10.1021/acs.nanolett.1c01817. [DOI] [PubMed] [Google Scholar]
- 55.Shrivastava S., Ray R.M., Holguin L., Echavarria L., Grepo N., Scott T.A., Burnett J., Morris K.V. Exosome-mediated stable epigenetic repression of HIV-1. Nat. Commun. 2021;12:5541. doi: 10.1038/s41467-021-25839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang J.H., Forterre A.V., Zhao J., Frimannsson D.O., Delcayre A., Antes T.J., Efron B., Jeffrey S.S., Pegram M.D., Matin A.C. Anti-HER2 scFv-Directed Extracellular Vesicle-Mediated mRNA-Based Gene Delivery Inhibits Growth of HER2-Positive Human Breast Tumor Xenografts by Prodrug Activation. Mol. Cancer Ther. 2018;17:1133–1142. doi: 10.1158/1535-7163.MCT-17-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Q., Yu J., Kadungure T., Beyene J., Zhang H., Lu Q. ARMMs as a versatile platform for intracellular delivery of macromolecules. Nat. Commun. 2018;9:960. doi: 10.1038/s41467-018-03390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Y., Ma X., Yue Y., Zhang K., Cheng K., Feng Q., Ma N., Liang J., Zhang T., Zhang L., et al. Nie, Rapid Surface Display of mRNA Antigens by Bacteria-Derived Outer Membrane Vesicles for a Personalized Tumor Vaccine. Adv. Mater. 2022;34:e2109984. doi: 10.1002/adma.202109984. [DOI] [PubMed] [Google Scholar]
- 59.Pham T.C., Jayasinghe M.K., Pham T.T., Yang Y., Wei L., Usman W.M., Chen H., Pirisinu M., Gong J., Kim S., et al. Covalent conjugation of extracellular vesicles with peptides and nanobodies for targeted therapeutic delivery. J. Extracell. Vesicles. 2021;10:e12057. doi: 10.1002/jev2.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Usman W.M., Pham T.C., Kwok Y.Y., Vu L.T., Ma V., Peng B., Chan Y.S., Wei L., Chin S.M., Azad A., et al. Efficient RNA drug delivery using red blood cell extracellular vesicles. Nat. Commun. 2018;9:2359. doi: 10.1038/s41467-018-04791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Popowski K.D., Abad B.L.d.J., George A., Silkstone D., Belcher E., Chung J., Ghodsi A., Lutz H., Davenport J., Flanagan M., et al. Inhalable exosomes outperform liposomes as mRNA and protein drug carriers to the lung. Extracell. Vesicle. 2022;1:100002. doi: 10.1016/j.vesic.2022.100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Popowski K.D., Moatti A., Scull G., Silkstone D., Lutz H., de Juan Abad B.L., George A., Belcher E., Zhu D., Mei X., et al. Inhalable dry powder mRNA vaccines based on extracellular vesicles. Matter. 2022;5:2960–2974. doi: 10.1016/j.matt.2022.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meng W., He C., Hao Y., Wang L., Li L., Zhu G. Prospects and challenges of extracellular vesicle-based drug delivery system: Considering cell source. Drug Deliv. 2020;27:585–598. doi: 10.1080/10717544.2020.1748758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grange C., Skovronova R., Marabese F., Bussolati B. Stem Cell-Derived Extracellular Vesicles and Kidney Regeneration. Cells. 2019;8:1240. doi: 10.3390/cells8101240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Veerman R.E., Akpinar G.G., Eldh M., Gabrielsson S. Immune Cell-Derived Extracellular Vesicles—Functions and Therapeutic Applications. Trends Mol. Med. 2019;25:382–394. doi: 10.1016/j.molmed.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 66.Hade M.D., Suire C.N., Suo Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells. 2021;10:1959. doi: 10.3390/cells10081959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Buschmann D., Mussack V., Byrd J.B. Separation, characterization, and standardization of extracellular vesicles for drug delivery applications. Adv. Drug Deliv. Rev. 2021;174:348–368. doi: 10.1016/j.addr.2021.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jia Y., Yu L., Ma T., Xu W., Qian H., Sun Y., Shi H. Small extracellular vesicles isolation and separation: Current techniques, pending questions and clinical applications. Theranostics. 2022;12:6548–6575. doi: 10.7150/thno.74305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alzhrani G.N., Alanazi S.T., Alsharif S.Y., Albalawi A.M., Alsharif A.A., Abdel-Maksoud M.S., Elsherbiny N. Exosomes: Isolation, characterization, and biomedical applications. Cell Biol. Int. 2021;45:1807–1831. doi: 10.1002/cbin.11620. [DOI] [PubMed] [Google Scholar]
- 70.Chen J., Li P., Zhang T., Xu Z., Huang X., Wang R., Du L. Review on Strategies and Technologies for Exosome Isolation and Purification. Front. Bioeng. Biotechnol. 2021;9:811971. doi: 10.3389/fbioe.2021.811971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coumans F.A.W., Brisson A.R., Buzas E.I., Dignat-George F., Drees E.E.E., El-Andaloussi S., Emanueli C., Gasecka A., Hendrix A., Hill A.F., et al. Methodological Guidelines to Study Extracellular Vesicles. Circ. Res. 2017;120:1632–1648. doi: 10.1161/CIRCRESAHA.117.309417. [DOI] [PubMed] [Google Scholar]
- 72.Schulz-Siegmund M., Aigner A. Nucleic acid delivery with extracellular vesicles. Adv. Drug Deliv. Rev. 2021;173:89–111. doi: 10.1016/j.addr.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 73.Tang T.T., Wang B., Lv L.L., Dong Z., Liu B.C. Extracellular vesicles for renal therapeutics: State of the art and future perspective. J. Control. Release. 2022;349:32–50. doi: 10.1016/j.jconrel.2022.06.049. [DOI] [PubMed] [Google Scholar]
- 74.Munir J., Yoon J.K., Ryu S. Therapeutic miRNA-Enriched Extracellular Vesicles: Current Approaches and Future Prospects. Cells. 2020;9:2271. doi: 10.3390/cells9102271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Izco M., Alvarez-Erviti L. siRNA Loaded-Exosomes. Methods Mol. Biol. 2021;2282:395–401. doi: 10.1007/978-1-0716-1298-9_21. [DOI] [PubMed] [Google Scholar]
- 76.Schult P., Roth H., Adams R.L., Mas C., Imbert L., Orlik C., Ruggieri A., Pyle A.M., Lohmann V. microRNA-122 amplifies hepatitis C virus translation by shaping the structure of the internal ribosomal entry site. Nat. Commun. 2018;9:2613. doi: 10.1038/s41467-018-05053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Salunkhe S., Dheeraj, Basak M., Chitkara D., Mittal A. Surface functionalization of exosomes for target-specific delivery and in vivo imaging & tracking: Strategies and significance. J. Control. Release. 2020;326:599–614. doi: 10.1016/j.jconrel.2020.07.042. [DOI] [PubMed] [Google Scholar]
- 78.Liang Y., Duan L., Lu J., Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183–3195. doi: 10.7150/thno.52570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bashyal S., Thapa C., Lee S. Recent progresses in exosome-based systems for targeted drug delivery to the brain. J. Control. Release. 2022;348:723–744. doi: 10.1016/j.jconrel.2022.06.011. [DOI] [PubMed] [Google Scholar]
- 80.Choi H., Yim H., Park C., Ahn S.H., Ahn Y., Lee A., Yang H., Choi C. Targeted Delivery of Exosomes Armed with Anti-Cancer Therapeutics. Membranes. 2022;12:85. doi: 10.3390/membranes12010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.