Abstract
Objective
Eribulin treatment improved overall survival with predictable toxicities in phase 3 trials of patients with previously treated, locally advanced/metastatic breast cancer. This study (NCT02443428) prospectively observed eribulin‐treated patients in real‐world clinical practice.
Methods
This observational multicentre registry study enrolled 76 patients with locally advanced/metastatic breast cancer who had ≤2 prior chemotherapeutic regimens for advanced disease. Eribulin was administered at a 1.23 mg/m2 dose (days 1 and 8 of every 21‐day cycle). Adverse events (AEs) were monitored and effectiveness was assessed per local practice.
Results
AEs occurred in 98.7% of patients; 88.2% had eribulin‐related AEs. The most common AEs were fatigue (64.5%), alopecia (36.8%), nausea (35.5%) and constipation (30.3%). Serious AEs occurred in 42.1% of patients. The most common grade 3/4 AEs were neutropenia (9.2%), febrile neutropenia (9.2%), dyspnoea (5.3%) and pleural effusion (5.3%). No fatal AEs occurred. Dose reductions occurred in 31.6% of patients, 42.1% experienced dose delays and 9.2% discontinued due to worsening condition. There were complete responses in 2.6% and partial responses in 15.8% of patients. Median time to progression and overall survival were 4.0 and 8.3 months, respectively.
Conclusion
Eribulin was well tolerated in real‐world clinical practice, comparable to safety and effectiveness reported in other clinical trials.
Keywords: adverse events, effectiveness, eribulin, metastatic, real‐world study
1. INTRODUCTION
Despite advances in early detection and treatment, metastatic breast cancer remains incurable, with a median overall survival of approximately 2–3 years (Cardoso et al., 2011, 2017; Saji, 2013). The use of cytotoxic regimens (e.g. anthracyclines and taxanes) in early disease has increased the number of pretreated and/or treatment‐resistant cases of metastatic disease, limiting therapeutic options after failure of first‐line treatment (Roché & Vahdat, 2011; Saji, 2013).
Eribulin is a nontaxane microtubule dynamic inhibitor, with a distinct mode of action compared with taxanes and vinca alkaloids, binding predominantly to a small number of high‐affinity sites on the growing (plus) ends of microtubules (Dybdal‐Hargreaves et al., 2015; Jordan et al., 2005; Smith et al., 2010). Eribulin also has nonmitotic effects, including increased vascular perfusion and reversal of the epithelial‐to‐mesenchymal transition (Dybdal‐Hargreaves et al., 2015; Funahashi et al., 2014; Ito et al., 2017; Kawano et al., 2016; Ueda et al., 2016; Yoshida et al., 2014).
Eribulin (Halaven; Eisai Ltd, Hatfield, Hertfordshire, UK) is licenced in the United Kingdom and European Union for the treatment of patients with locally advanced or metastatic breast cancer who have received at least one chemotherapeutic regimen for advanced disease (Eisai Europe Limited, 2021). Previous therapy should have included an anthracycline and a taxane in either the adjuvant or metastatic setting, unless the patient was not suitable for these treatments (Eisai Europe Limited, 2021).
In phase 3 clinical studies, eribulin was active in patients with pretreated advanced breast cancer (Cortes et al., 2011; Kaufman et al., 2015). In the phase 3 Eisai Metastatic Breast Cancer Study Assessing Physician's Choice Versus Eribulin (EMBRACE)/Study 305, eribulin significantly improved overall survival, compared with treatment of physician's choice, after at least two previous regimens for advanced breast cancer (median 13.1 vs. 10.6 months, respectively; hazard ratio 0.81; 95% confidence interval [CI]: 0.66–0.99; p = 0.041) (Cortes et al., 2011). In addition, a significantly greater objective response rate was observed with eribulin versus control treatments (12% vs. 5%, p = 0.002). A subsequent study in metastatic breast cancer compared eribulin with capecitabine in patients who had received up to two prior therapies, which included a taxane and an anthracycline (Kaufman et al., 2015). Although eribulin did not demonstrate an increase in overall survival in the overall population (hazard ratio 0.88; 95% CI: 0.77–1.00; p = 0.056), a prespecified subgroup analysis showed a survival benefit in certain subgroups. In these phase 3 studies, eribulin had a manageable toxicity profile similar to other chemotherapeutic agents in this setting (Cortes et al., 2011; Kaufman et al., 2015).
However, clinical trials utilise stringent inclusion and exclusion criteria, efficacy evaluations, side‐effect monitoring and patient management approaches, which may not reflect the actual use of eribulin in current clinical practice. There is, therefore, a need to evaluate the safety profile of eribulin in routine clinical practice. This need may take on even greater importance given emerging evidence of the utility of eribulin in combination with immunotherapy in triple‐negative breast cancer (Tolaney et al., 2021). The aim of this prospective, observational registry study was to monitor the safety and effectiveness of eribulin in routine clinical practice.
2. MATERIALS AND METHODS
2.1. Study design
This was a multicentre, prospective, observational registry study of patients with locally advanced or metastatic breast cancer treated with eribulin in routine clinical practice settings. Patients were enrolled from 13 centres in the United Kingdom, Ireland and Denmark. Data collection was from 25 July 2013 to 31 October 2015.
The recommended dose of eribulin was 1.23 mg/m2 (equivalent to 1.4 mg/m2 eribulin mesilate) administered intravenously on days 1 and 8 of every 21‐day cycle (except for patients with baseline characteristics for which the eribulin label recommends using a lower starting dose) (Eisai Europe Limited, 2021). Safety and clinical evaluations were recorded at each visit and radiological evaluation of tumour status was recorded as per local clinical practice until eribulin discontinuation. After eribulin discontinuation, patients were followed up for survival analysis for up to 1 year or until death.
As an observational study, all treatment decisions were at the discretion of the treating physician and were not mandated by study design or protocol. In all cases, the decision to treat was made prior to the decision to include the patient in this registry study.
2.2. Patients
The study included adults older than 18 years with locally advanced or metastatic breast cancer and with progression after up to two previous chemotherapy regimens for advanced disease, including an anthracycline and a taxane in either the adjuvant or advanced setting.
Additional inclusion criteria were adequate renal, liver and bone marrow function. Patients previously treated with eribulin or with more than two prior chemotherapeutic regimens for advanced disease were excluded.
Informed consent was obtained from all individual participants included in the study in accordance with the Principles of Good Clinical Practice and the Declaration of Helsinki. The study was approved by the following ethics committees: NRES Committee South Central, Berkshire, Bristol, UK, and the Research Ethics Committee of Mater Misericordiae, Dublin, Ireland.
2.3. Study assessments and source documents
All assessments were made according to the usual clinical practice of the treating investigator; assessments were not protocol driven. Parameters on the case report form (CRF) that were not normal practice of the investigator were not collected by that investigator.
The primary endpoint was safety, assessed by monitoring adverse events (AEs), including serious AEs (SAEs) and laboratory safety parameters. AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Secondary endpoints included tumour response, overall survival, duration of treatment, incidence of dose delays and reductions, and relative dose intensity of eribulin. Best overall response and time to progression were also assessed. All endpoints are defined in Appendix A. Tumour response was assessed according to the physician's usual clinical practice, occurred at each patient visit, and radiological status (complete response, partial response, stable disease or progressive disease) was recorded as per local clinical practice.
A CRF was completed for each patient and any data derived from source documents had to be consistent with the source documents (i.e. patient's medical notes) or the discrepancies had to be explained. The CRFs were monitored and collected regularly; ≥2 monitoring visits per centre were required during recruitment and a third at the end of the study.
2.4. Statistical analysis
Patient demographics were described by frequency distributions and/or basic summary statistics for continuous data. Kaplan–Meier curves were estimated for overall survival and time to progression together with median and two‐sided 95% CI. No adjustments were made for multiple comparisons. The safety population and analysis population were both defined as all patients who had received at least one dose of eribulin. Statistical analyses were performed using SAS version 9.2 or 9.4 (SAS, Cary, NC, USA).
3. RESULTS
3.1. Patient characteristics
This study enrolled 76 female patients, with a median age of 57.5 (range, 34.3–77.9) years. The majority (89.5% [68/76]) were white (Table 1). Eribulin represented third‐line treatment in 77.6% (59/76). Most patients had baseline lesions (73/76; 96.1%). Visceral disease was reported in 59 (80.1%) patients; the most common sites of metastases were the liver (50.0% [38/76]), lung (43.4% [33/76]), lymph nodes (43.4% [33/76]) and bone (35.5% [27/76]) (Table 1). Only one (1.3%) patient had bone‐only disease, and three (3.9%) had brain lesions. Although mainly a hormone‐receptor–positive population (57/76; 75.0% of patients), only 50.0% (38/76) received prior hormone therapy for advanced disease (includes eight [10.5%] patients with unknown prior therapies).
TABLE 1.
Patient baseline and primary diagnosis data
| Parameter | Safety population (N = 76) |
|---|---|
| Age, years | |
| Median | 57.5 |
| (minimum, maximum) | (34.3, 77.9) |
| Female, n (%) | 76 (100.0) |
| Race, n (%) | |
| White | 68 (89.5) |
| Black | 2 (2.6) |
| Asian | 4 (5.3) |
| Other a | 2 (2.6) |
| Hormone‐receptor status, n (%) | |
| Positive | 57 (75.0) |
| Negative | 18 (23.7) |
| Missing | 1 (1.3) |
| HER2 status, n (%) | |
| Positive | 10 (13.2) |
| Negative | 66 (86.8) |
| Triple‐negative disease, n (%) | |
| Yes | 17 (22.4) |
| No | 58 (76.3) |
| Missing | 1 (1.3) |
| Number of prior hormone therapies, n (%) | |
| 0 | 38 (50.0) |
| 1 | 14 (18.4) |
| 2 | 16 (21.1) |
| Unknown | 8 (10.5) |
| Number of prior chemotherapy treatments for advanced disease, n (%) | |
| 0 | 2 (2.6) |
| 1 | 15 (19.7) |
| 2 | 59 (77.6) |
| Any taxane, n (%) | |
| Yes | 71 (93.4) |
| No | 5 (6.6) |
| Any anthracycline, n (%) | |
| Yes | 61 (80.3) |
| No | 15 (19.7) |
| Number of sites of disease, n (%) | |
| Mean (SD) | 2.3 (1.19) |
| (minimum, maximum) | (1, 6) |
| Sites of disease, n (%) | |
| Liver | 38 (50.0) |
| Lung | 33 (43.4) |
| Lymph nodes | 33 (43.4) |
| Bone | 27 (35.5) |
| Chest wall | 10 (13.2) |
| Other | 29 (38.2) |
Abbreviations: HER2, human epidermal growth factor receptor 2; SD, standard deviation.
Includes mixed race or missing.
[Correction added on 12 November 2022, after first online publication: Data corresponding to “Triple‐negative disease, n (%)” in Table 1 was corrected in this version.]
3.2. Safety
Most patients experienced at least one AE (98.7% [75/76]). The most common all‐grade AEs were fatigue (64.5% [49/76] of patients), alopecia (36.8% [28/76]), nausea (35.5% [27/76]), constipation (30.3% [23/76]) and peripheral neuropathy (27.6% [21/76]) (Table 2). At least one SAE was reported in 32 (42.1%) patients, and 18 (23.7%) had at least one eribulin‐related SAE as assessed by the physician.
TABLE 2.
Adverse events reported in >10% of patients (all grades; safety population; N = 76)
| Adverse event, n (%) | All grades | Treatment related | Grade 3/4 |
|---|---|---|---|
| Fatigue | 49 (64.5) | 43 (56.6) | 3 (3.9) |
| Alopecia | 28 (36.8) | 26 (34.2) | 0 |
| Nausea | 27 (35.5) | 24 (31.6) | 2 (2.6) |
| Constipation | 23 (30.3) | 14 (18.4) | 0 |
| Peripheral neuropathy | 21 (27.6) | 16 (21.1) | 1 (1.3) |
| Decreased appetite | 17 (22.4) | 13 (17.1) | 1 (1.3) |
| Diarrhoea | 16 (21.1) | 8 (10.5) | 1 (1.3) |
| Headache | 15 (19.7) | 5 (6.6) | 0 |
| Dyspnoea | 15 (19.7) | 7 (9.2) | 4 (5.3) |
| Neutropenia | 14 (18.4) | 13 (17.1) | 7 (9.2) |
| Vomiting | 13 (17.1) | 10 (13.2) | 3 (3.9) |
| Pyrexia | 10 (13.2) | 7 (9.2) | 0 |
| Mucosal inflammation | 10 (13.2) | 8 (10.5) | 1 (1.3) |
| Arthralgia | 9 (11.8) | 3 (3.9) | 1 (1.3) |
| Cough | 9 (11.8) | 3 (3.9) | 0 |
| Stomatitis | 9 (11.8) | 8 (10.5) | 0 |
| Palmar‐plantar erythrodysaesthesia syndrome | 8 (10.5) | 7 (9.2) | 1 (1.3) |
Note: An adverse event with the same preferred term for the same patient was counted only once. If a patient had the same event more than once, the worst National Cancer Institute Common Terminology Criteria grade was selected within a same group of system/organ/class and preferred term per Medical Dictionary for Regulatory Activities version 17.0.
Grade 3 and 4 AEs occurred in 35 patients (46.1%) and 7 patients (9.2%), respectively. There were no fatal AEs. The most common grade 3/4 AEs were febrile neutropenia (9.2% [7/76]; all grade 3), neutropenia (9.2% [7/76]), dyspnoea (5.3% [4/76]) and pleural effusion (5.3% [4/76]). Treatment‐related AEs (all grades) were reported in 67 patients (88.2%); the most common being fatigue (56.6% [43/76]), alopecia (34.2% [26/76]) and nausea (31.6% [24/76]) (Table 2).
The time course of relevant AEs (i.e. any kind of neuropathy, febrile neutropenia, alopecia, nausea, or vomiting) demonstrated that most occurred during the first cycle of therapy (on day 1 or 8). During cycle 1, these included febrile neutropenia (57.1%; 4/7), nausea (44.4%; 12/27), alopecia (42.9%; 12/28), vomiting (38.5%; 5/13) and neuropathy (32.0%; 8/25).
Granulocyte‐colony stimulating factor (G‐CSF) was given to 15 (19.7%) patients—mainly pegfilgrastim (n = 9) or filgrastim (n = 7)—and one patient received both. Commonly, G‐CSF was initiated before treatment in a single treatment centre (5.3%) or in early treatment cycles (cycle 1, 3.9%; cycle 2, 2.6%; cycle 3, 2.6%). G‐CSF was administered to one patient each in cycles 4, 7, 8 and 9.
During eribulin treatment, 32 (42.1%) patients required dose delays and 24 (31.6%) required dose reductions. The main reason for dose delay was haematological AEs in 13 (17.1%) patients. The main reasons for dose reductions were nonhaematological AEs in 12 (15.8%) patients and haematological AEs in 10 (13.2%) patients. Seven (9.2%) patients had at least one AE that led to discontinuation due to worsening condition including low neutrophil count. The median (interquartile range: Q1, Q3) number of cycles of eribulin was 5.0 (2.0, 6.5) and the median (range) duration of eribulin treatment was 14.1 (5.2, 23.4) weeks. Median relative dose intensity (interquartile range: Q1, Q3) was 75.9% (65.1, 88.0).
3.3. Effectiveness
In the overall safety population, the best overall responses to eribulin treatment (assessed according to the physician's usual practice) were complete response and partial response in 2.6% (2/76) and 15.8% (12/76) of patients, respectively (Table 3). In patients without triple‐negative disease, a partial response was observed in 17.2% (10/58) of patients; none had a complete response. In triple‐negative disease, a complete response was observed in 11.8% (2/17) of patients and a partial response in another 11.8% (2/17) (Table 3). Treatment‐response data were missing or not available for 17 patients (Table 3). In the evaluable population of 59 patients with response data, a complete response was observed in 3.4%, a partial response in 20.3%, stable disease in 35.6% and progressive disease in 40.7% of patients.
TABLE 3.
Best overall response a in the overall safety population and by triple‐negative disease status
| Best overall response, n (%) | Safety population (N = 76) | Not TNBC (n = 58) | TNBC (n = 17) |
|---|---|---|---|
| Complete response | 2 (2.6) | 0 | 2 (11.8) |
| Partial response | 12 (15.8) | 10 (17.2) | 2 (11.8) |
| Stable disease | 21 (27.6) | 17 (29.3) | 4 (23.5) |
| Progressive disease b | 24 (31.6) | 17 (29.3) | 6 (35.3) |
| Not available/missing c | 17 (22.4) | 14 (24.1) | 3 (17.6) |
Abbreviation: TNBC, triple‐negative breast cancer.
Tumour response was assessed according to physician's usual clinical practice.
One patient with a best overall response of progressive disease had unknown TNBC status.
Scans were performed at the investigator's discretion and only after a patient had received at least three cycles of eribulin.
During the study period, 59 patients experienced disease progression and 57 patients died. The median time to progression was 4.0 (95% CI: 2.7–5.0) months (Figure 1). The median overall survival was 8.3 (95% CI: 6.2–11.1) months (Figure 2).
FIGURE 1.
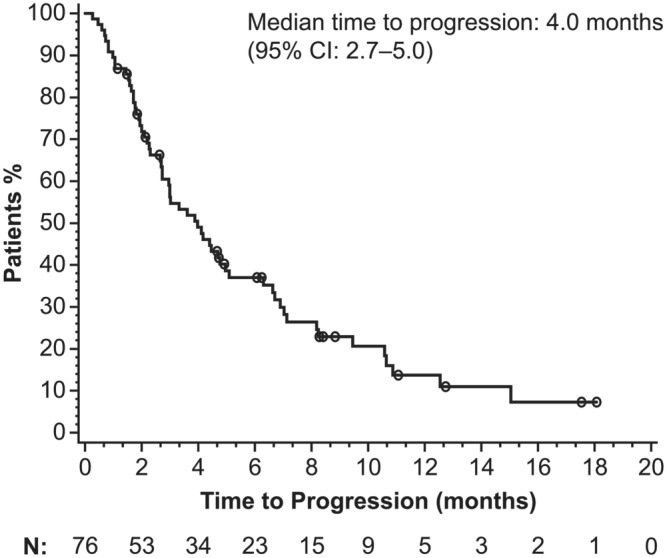
Kaplan–Meier estimation of time to progression. Tumour response was assessed according to the physician's usual practice rather than being protocol driven. Data shown are for the overall safety population (N = 76). CI, confidence interval
FIGURE 2.
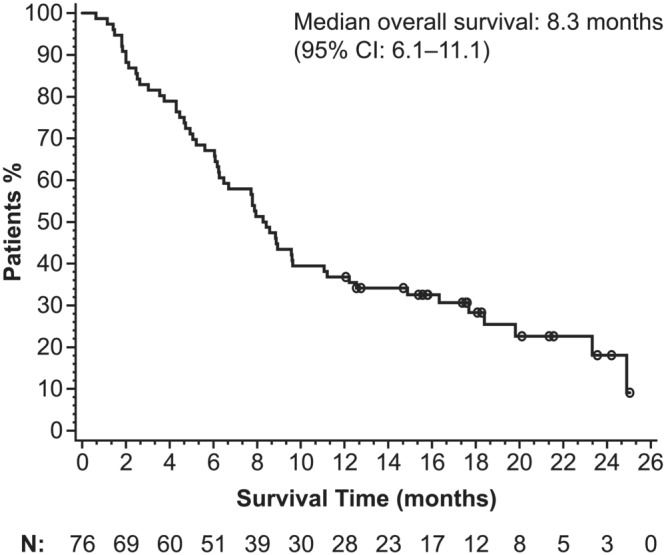
Kaplan–Meier estimation of overall survival. Data shown are for the overall safety population (N = 76). CI, confidence interval
After discontinuation of eribulin treatment, 34/76 (44.7%) patients had further chemotherapy and 25/76 (32.9%) received supportive or end‐of‐life care. Subsequent chemotherapy included capecitabine (17.6% [6/34]) and carboplatin with gemcitabine, paclitaxel or gemcitabine (14.7% [5/34] each).
4. DISCUSSION
The primary objective of this registry study was to monitor the safety of eribulin; it was found to be well tolerated in routine clinical use with a safety profile similar to that reported in randomised phase 3 trials (Cortes et al., 2011; Kaufman et al., 2015). The most common AEs (fatigue, alopecia, nausea and peripheral neuropathy) were consistent with the eribulin summary of product characteristics (Eisai Europe Limited, 2021). The majority of relevant AEs were reported during the first cycle of treatment, and the most common grade 3/4 AEs were febrile neutropenia, neutropenia and dyspnoea.
Effectiveness outcomes in this registry study were consistent with the efficacy previously observed in EMBRACE (Cortes et al., 2011), despite this being a more difficult‐to‐treat population. Time to progression in this registry study was similar to that reported in the eribulin arm of EMBRACE (4.0 months vs. a progression‐free survival of 3.7 months, respectively) as were overall response rates (18% vs. 12%). However, overall survival was lower than what was found in EMBRACE (8.3 vs. 13.1 months). Notably, a survival benefit has previously been demonstrated for eribulin in patients with triple‐negative breast cancer (Twelves et al., 2014), and this registry study confirmed a higher response rate with eribulin in this population. The effectiveness outcomes in this registry study are further bolstered by a prospective observational study of eribulin mesilate for use as a third‐line therapy in 118 patients with metastatic breast cancer (VESPRY) (Adamo et al., 2019). In VESPRY, eribulin had a notable antitumor effect as a third‐line therapy with median progression‐free survival of 5.5 months (95% CI: 4.2–6.6) and median overall survival of 31.8 months (95% CI: 27.9–34.4) (Adamo et al., 2019).
Disease characteristics of the patients in our registry study may have affected overall survival. The low rates of prior hormone therapies (50% of patients had received no prior hormone therapy) in a hormone‐receptor–positive population (75% of patients had hormone‐receptor–positive disease) may indicate a patient group with aggressive visceral disease; only one patient had bone‐only disease.
In this registry study, the rates of subsequent treatment following eribulin were low, with 45% of patients going on to receive further chemotherapy and 33% of patients going on to receive supportive or end‐of‐life care. A potential selection bias should be noted because patients with more than two prior lines of therapy were excluded. In contrast, in the EMBRACE study (Cortes et al., 2011), patients had a median of four prior lines of chemotherapy (range, 1–7 lines) and, therefore, this registry study may have selected for patients with more indolent and chemoresponsive tumours.
Any comparisons of outcomes between this registry study and randomised controlled clinical trials of eribulin should be made with caution because tumour responses in this study were assessed according to local guidelines whereas Response Evaluation Criteria in Solid Tumours is used in clinical studies. Further, time‐to‐progression assessment was not performed via centralised independent radiological review (Cortes et al., 2011; Kaufman et al., 2015).
While observational registry studies may provide valuable insights into safety and effectiveness in real‐world clinical practice, a limitation is an inability to establish causal relationships between variables. Other important limitations of this registry study should be noted: a small number of patients were enrolled and some selection criteria (e.g. the exclusion of patients who had received more than two prior chemotherapeutic regimens) do not allow the analysis of the complete population of patients who may receive eribulin in routine practice. As nearly 90% of the study population were white, it is also difficult to make any generalisations to a more ethnically diverse population.
5. CONCLUSION
The results of this prospective, observational, multicentre registry study show that the safety and effectiveness profiles of eribulin observed in clinical practice are consistent with the findings reported in clinical trials. These data further support the utility of eribulin as a valuable treatment option for patients with advanced and metastatic breast cancer, which was initially demonstrated in phase 3 clinical trials.
CONFLICTS OF INTEREST
Laura Kenny has served on advisory boards for Celgene and Novartis and given educational presentations sponsored by Lilly and Pfizer.
Mark Beresford has received honoraria and lecture fees from Eisai, Roche, Lilly, Pfizer and Novartis.
Ιan Brown is an employee of Eisai, Ltd.
Vivek Misra and Hartmut Kristeleit have received honoraria and consultancy fees from Eisai, Ltd, for educational lectures, presentation of audit findings and for attendance at Advisory Boards.
ACKNOWLEDGEMENTS
The authors would like to thank all patients, investigators and staff at the study sites. The authors also thank Dr Vanessa Christou and Dr Natasha Mithal.
Editorial support was provided Oxford PharmaGenesis Inc., Newtown, PA, USA, and was funded by Eisai Inc., Nutley, NJ, USA.
APPENDIX A. DEFINITION OF ENDPOINTS
Best overall response was the best status based on tumour assessment (i.e. lesions and overall lesions), with complete response being the best response and progressive disease the worst. Overall survival was measured from the first day of therapy to the date of death from any cause. Time to progression was the time between the first dose of eribulin to the date of the progression event (i.e. progressive disease response in overall lesions or death due to progression). Duration of treatment was from the first day of eribulin therapy to the date of last administration. Relative dose intensity of eribulin was the ratio of the delivered dose intensity (dose/surface area/time) to the standard intended dose intensity.
Kenny, L. , Beresford, M. , Brown, I. , Misra, V. , & Kristeleit, H. (2022). Eribulin for the treatment of advanced breast cancer: A prospective observational registry study. European Journal of Cancer Care, 31(6), e13747. 10.1111/ecc.13747
Funding information This work was supported by Eisai, Ltd, Hatfield, UK. Eisai was involved in study design, data collection and manuscript preparation. Dr Kenny was funded by an NIHR (National Institute for Health Research) CS09/009 clinician scientist fellowship; the study was supported by the National Cancer Research Network (NCRN).
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analysed during the current study are not publicly available because the data are commercially confidential. However, the data are available from the corresponding author on reasonable request.
REFERENCES
- Adamo, V. , Ricciardi, G. R. R. , Giuffrida, D. , Scandurra, G. , Russo, A. , Blasi, L. , Spadaro, P. , Iacono, C. , Soto Parra, H. J. , Savarino, A. , Ferraú, F. , Zerilli, F. , Verderame, F. , Butera, A. , Santangelo, C. , Franchina, V. , & Caruso, M. (2019). Eribulin mesylate use as third‐line therapy in patients with metastatic breast cancer (VESPRY): A prospective, multicentre, observational study. Therapeutic Advances in Medical Oncology, 11, 1758835919895755. 10.1177/1758835919895755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso, F. , Costa, A. , Senkus, E. , Aapro, M. , André, F. , Barrios, C. H. , Bergh, J. , Bhattacharyya, G. , Biganzoli, L. , Cardoso, M. J. , Carey, L. , Corneliussen‐James, D. , Curigliano, G. , Dieras, V. , el Saghir, N. , Eniu, A. , Fallowfield, L. , Fenech, D. , Francis, P. , … Winer, E. (2017). 3rd ESO‐ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 3). Annals of Oncology, 28, 16–33. 10.1093/annonc/mdw544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso, F. , Fallowfield, L. , Costa, A. , Castiglione, M. , Senkus, E. , & ESMO Guidelines Working Group . (2011). Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Annals of Oncology, 22, vi25–vi30. 10.1093/annonc/mdr372 [DOI] [PubMed] [Google Scholar]
- Cortes, J. , O'Shaughnessy, J. , Loesch, D. , Blum, J. L. , Vahdat, L. T. , Petrakova, K. , Chollet, P. , Manikas, A. , Diéras, V. , Delozier, T. , Vladimirov, V. , Cardoso, F. , Koh, H. , Bougnoux, P. , Dutcus, C. E. , Seegobin, S. , Mir, D. , Meneses, N. , Wanders, J. , & Twelves, C. (2011). Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): A phase 3 open‐label randomised study. Lancet, 377, 914–923. 10.1016/S0140-6736(11)60070-6 [DOI] [PubMed] [Google Scholar]
- Dybdal‐Hargreaves, N. F. , Risinger, A. L. , & Mooberry, S. L. (2015). Eribulin mesylate: Mechanism of action of a unique microtubule‐targeting agent. Clinical Cancer Research, 21, 2445–2452. 10.1158/1078-0432.CCR-14-3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisai Europe Limited . (2021). Halaven 0.44 mg/ml solution for injection [summary of product characteristics]. Hertfordshire, UK.
- Funahashi, Y. , Okamoto, K. , Adachi, Y. , Semba, T. , Uesugi, M. , Ozawa, Y. , Tohyama, O. , Uehara, T. , Kimura, T. , Watanabe, H. , Asano, M. , Kawano, S. , Tizon, X. , McCracken, P. J. , Matsui, J. , Aoshima, K. , Nomoto, K. , & Oda, Y. (2014). Eribulin mesylate reduces tumor microenvironment abnormality by vascular remodeling in preclinical human breast cancer models. Cancer Science, 105, 1334–1342. 10.1111/cas.12488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, K. , Hamamichi, S. , Abe, T. , Akagi, T. , Shirota, H. , Kawano, S. , Asano, M. , Asano, O. , Yokoi, A. , Matsui, J. , Umeda, I. O. , & Fujii, H. (2017). Antitumor effects of eribulin depend on modulation of the tumor microenvironment by vascular remodeling in mouse models. Cancer Science, 108, 2273–2280. 10.1111/cas.13392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan, M. A. , Kamath, K. , Manna, T. , Okouneva, T. , Miller, H. P. , Davis, C. , Littlefield, B. A. , & Wilson, L. (2005). The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Molecular Cancer Therapeutics, 4, 1086–1095. 10.1158/1535-7163.MCT-04-0345 [DOI] [PubMed] [Google Scholar]
- Kaufman, P. A. , Awada, A. , Twelves, C. , Yelle, L. , Perez, E. A. , Velikova, G. , Olivo, M. S. , He, Y. , Dutcus, C. E. , & Cortes, J. (2015). Phase III open‐label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. Journal of Clinical Oncology, 33, 594–601. 10.1200/JCO.2013.52.4892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano, S. , Asano, M. , Adachi, Y. , & Matsui, J. (2016). Antimitotic and non‐mitotic effects of eribulin mesilate in soft tissue sarcoma. Anticancer Research, 36, 1553–1561. [PubMed] [Google Scholar]
- Roché, H. , & Vahdat, L. T. (2011). Treatment of metastatic breast cancer: Second line and beyond. Annals of Oncology, 22, 1000–1010. 10.1093/annonc/mdq429 [DOI] [PubMed] [Google Scholar]
- Saji, S. (2013). Evolving approaches to metastatic breast cancer patients pre‐treated with anthracycline and taxane. BioDrugs, 27, 469–478. 10.1007/s40259-013-0038-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, J. A. , Wilson, L. , Azarenko, O. , Zhu, X. , Lewis, B. M. , Littlefield, B. A. , & Jordan, M. A. (2010). Eribulin binds at microtubule ends to a single site on tubulin to suppress dynamic instability. Biochemistry, 49, 1331–1337. 10.1021/bi901810u [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolaney, S. M. , Kalinsky, K. , Kaklamani, V. G. , D'Adamo, D. R. , Aktan, G. , Tsai, M. L. , O'Regan, R. M. , Kaufman, P. A. , Wilks, S. T. , Andreopoulou, E. , Patt, D. A. , Yuan, Y. , Wang, G. , Savulsky, C. , Xing, D. , Kleynerman, E. , Karantza, V. , & Diab, S. (2021). Eribulin plus pembrolizumab in patients with metastatic triple‐negative breast cancer (ENHANCE 1): A phase Ib/II study. Clinical Cancer Research, 27, 3061–3068. 10.1158/1078-0432.CCR-20-4726 [DOI] [PubMed] [Google Scholar]
- Twelves, C. , Cortes, J. , Vahdat, L. , Olivo, M. , He, Y. , Kaufman, P. A. , & Awada, A. (2014). Efficacy of eribulin in women with metastatic breast cancer: A pooled analysis of two phase 3 studies. Breast Cancer Research and Treatment, 148, 553–561. 10.1007/s10549-014-3144-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda, S. , Saeki, T. , Takeuchi, H. , Shigekawa, T. , Yamane, T. , Kuji, I. , & Osaki, A. (2016). In vivo imaging of eribulin‐induced reoxygenation in advanced breast cancer patients: A comparison to bevacizumab. British Journal of Cancer, 114, 1212–1218. 10.1038/bjc.2016.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida, T. , Ozawa, Y. , Kimura, T. , Sato, Y. , Kuznetsov, G. , Xu, S. , Uesugi, M. , Agoulnik, S. , Taylor, N. , Funahashi, Y. , & Matsui, J. (2014). Eribulin mesilate suppresses experimental metastasis of breast cancer cells by reversing phenotype from epithelial‐mesenchymal transition (EMT) to mesenchymal‐epithelial transition (MET) states. British Journal of Cancer, 110, 1497–1505. 10.1038/bjc.2014.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are not publicly available because the data are commercially confidential. However, the data are available from the corresponding author on reasonable request.


