Abstract
Identification of predictors of long COVID-19 is essential for managing healthcare plans of patients. This systematic literature review and meta-analysis aimed to identify risk factors not associated with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection, but rather potentially predictive of the development of long COVID-19. MEDLINE, CINAHL, PubMed, EMBASE, and Web of Science databases, as well as medRxiv and bioRxiv preprint servers were screened through 15 September 2022. Peer-reviewed studies or preprints evaluating potential pre-SARS-CoV-2 infection risk factors for the development of long-lasting symptoms were included. The methodological quality was assessed using the Quality in Prognosis Studies (QUIPSs) tool. Random-effects meta-analyses with calculation of odds ratio (OR) were performed in those risk factors where a homogenous long COVID-19 definition was used. From 1978 studies identified, 37 peer-reviewed studies and one preprint were included. Eighteen articles evaluated age, sixteen articles evaluated sex, and twelve evaluated medical comorbidities as risk factors of long COVID-19. Overall, single studies reported that old age seems to be associated with long COVID-19 symptoms (n = 18); however, the meta-analysis did not reveal an association between old age and long COVID-19 (n = 3; OR 0.86, 95% CI 0.73 to 1.03, p = 0.17). Similarly, single studies revealed that female sex was associated with long COVID-19 symptoms (n = 16); which was confirmed in the meta-analysis (n = 7; OR 1.48, 95% CI 1.17 to 1.86, p = 0.01). Finally, medical comorbidities such as pulmonary disease (n = 4), diabetes (n = 1), obesity (n = 6), and organ transplantation (n = 1) were also identified as potential risk factors for long COVID-19. The risk of bias of most studies (71%, n = 27/38) was moderate or high. In conclusion, pooled evidence did not support an association between advancing age and long COVID-19 but supported that female sex is a risk factor for long COVID-19. Long COVID-19 was also associated with some previous medical comorbidities.
Keywords: post-COVID-19 condition, long COVID-19 symptoms, risk factors, sex, age, co-morbidity
1. Introduction
Long COVID-19 is a term used for defining the persistence of signs and symptoms in people who recovered from an acute Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection [1]. Long COVID-19 is defined by the World Health Organization (WHO) as: “post-COVID-19 condition, occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually 3 months from the onset, with symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis [2].” Several meta-analyses investigating the prevalence of post-COVID-19 symptoms have been published, concluding that around 30–50% of subjects who recover from a SARS-CoV-2 infection develop persistent symptoms lasting up to one year [3,4]. A recent meta-analysis concluded that two years after the initial spread of coronavirus disease 2019 (COVID-19), up to 42% of infected patients experienced long COVID-19 symptoms [5].
Different narrative reviews have mentioned prognostic aspects, but no comprehensive synthesis has been provided so far [6,7,8,9]. Identification of potential risk factors associated with post-COVID-19 syndrome is important since identifying individuals at higher risk can guide management healthcare plans for these patients and reorganize healthcare accordingly. Iqbal et al. tried to pool data, but these authors were only able to pool prevalence data of post-COVID-19 symptomatology, but not risk factors [10]. All these narrative reviews have suggested that female sex, old age, higher number of comorbidities, higher viral load, and greater number of COVID-19 onset symptoms can be potential risk factors for long COVID-19 [6,7,8,9,10]. However, no systematic search or assessment of methodological quality was conducted in these reviews [6,7,8,9,10]. Two meta-analyses have recently focused on risk factors of long COVID-19. Maglietta et al. identified that female sex was a risk factor for long COVID-19 symptoms, whereas a more severe condition at the acute phase was associated just with long COVID-19 respiratory symptoms [11]. Thompson et al. found that old age, female sex, white ethnicity, poor pre-pandemic health, obesity, and asthma can predict long COVID-19 symptoms [12]. However, this analysis included just studies from the United Kingdom, and used the definition of long COVID-19 proposed by the National Institute for Health Care and Excellence (NICE) [13].
Accordingly, current evidence on risk factors associated with post-COVID-19 condition is heterogeneous. Risk factors can be classified as pre-infection (e.g., age, sex, previous comorbidities, and previous health status) and infection-associated (e.g., disease severity, symptoms at onset, viral load, hospitalization stay, and intensive care unit admission) factors. The current systematic review and meta-analysis aimed to identify factors not directly associated with acute SARS-CoV-2 infection (i.e., pre-infection factors) such as age, sex, and previous medical comorbidities, which may predict the development of long COVID-19 symptomatology.
2. Methods
This systematic literature review and meta-analysis aiming to identify the association of age, sex, and comorbidities as predictive factors for development of long COVID-19 was conducted following the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement of 2020 [14]. We also followed specific criteria recommended by Riley et al. to systematic reviews and the meta-analysis of prognostic factor studies [15]. The review study was prospectively registered in the Open Science Framework (OSF) database (https://osf.io/79pdg).
2.1. Search Strategy and Selection Criteria
Two different authors performed an electronic search for articles published up to 15 September 2022 MEDLINE, CINAHL, PubMed, EMBASE, and Web of Science databases, as well as on preprint servers medRxiv and bioRxiv, using the following search terms: “long COVID-19” OR “post-acute COVID” OR “post-COVID-19 condition” OR “long hauler” AND “age” OR “sex” OR “medical comorbidities” OR “transplant” OR “obesity” OR “diabetes” OR “hypertension” OR “pulmonary disease” OR “asthma” OR “chronic obstructive pulmonary disease”. The search was focused on the medical comorbidities likely associated with a more severe COVID-19 condition. Combinations of these search terms using Boolean operators are outlined in Table 1.
Table 1.
Database formulas during literature search.
| PubMed Search Formula |
| #1 “post-acute COVID-19 syndrome” [MeSH Terms] OR “long COVID-19” [All Fields] OR “long COVID-19 symptoms” [All Fields] OR “long hauler” [All Fields] OR “post-COVID-19” [All Fields] OR “post-acute COVID-19 symptoms” [All Fields] OR “COVID-19 sequelae” [All Fields] #2 “age” [All Fields] #3 “sex” [MeSH Terms] OR “sex” [All Fields] #4 “comorbidity” [MeSH Terms] OR (“transplants” [MeSH Terms] OR “transplantation” [MeSH Terms] OR transplant [All Fields]) OR (“obesity” [MeSH Terms] OR obesity [All Fields]) OR (“diabetes mellitus” [MeSH Terms] OR “diabetes insipidus” [MeSH Terms] OR diabetes [All Fields]) OR (“hypertension” [MeSH Terms] OR hypertension [All Fields]) OR (“lung diseases” [MeSH Terms] OR pulmonary disease [All Fields]) OR (“asthma” [MeSH Terms] OR asthma [All Fields]) OR (“pulmonary disease, chronic obstructive” [MeSH Terms] OR COPD [All Fields]) #5 #1 AND #2 #6 #1 AND #3 #7 #1 AND #4 |
| MEDLINE/CINAHL (via EBSCO) Search Formula |
| #1 “post-acute COVID-19 syndrome” OR “long COVID-19” OR “long COVID-19 symptoms” OR “long hauler” OR “post-COVID-19” OR “post-acute COVID-19 symptoms” OR “COVID-19 sequelae” #2 “age” #3 “sex” #4 “comorbidity” OR “transplants” OR “transplantation” OR “obesity” OR “diabetes mellitus” OR “diabetes” OR “hypertension” OR “pulmonary disease” OR “asthma” OR “chronic obstructive pulmonary disease” #5 #1 AND #2 #6 #1 AND #3 #7 #1 AND #4 |
| WOS (EMBASE)/Web of Science Search Formula |
| (“post-acute COVID-19 syndrome” OR “long COVID-19” OR “long COVID-19 symptoms” OR “long hauler” OR “post-COVID-19” OR “post-acute COVID-19 symptoms” OR “COVID-19 sequelae” AND ((“age”) OR (“sex”) OR (“comorbidity” OR “transplants” OR “transplantation” OR “obesity” OR “diabetes mellitus” OR “diabetes” OR “hypertension” OR “pulmonary disease” OR “asthma” OR “chronic obstructive pulmonary disease”)) |
The Population, Intervention, Comparison. and Outcome (PICO) principle was used to describe the inclusion and exclusion criteria:
Population: Adults (>18 years) infected by SARS-CoV-2 and diagnosed with real-time reverse transcription-polymerase chain reaction (RT-PCR) assay. Subjects could have been hospitalized or not by SARS-CoV-2 acute infection.
Intervention: Not applicable.
Comparison: People infected by SARS-CoV-2 who did not develop long COVID-19 symptoms.
Outcome: Collection of long COVID-19 symptoms developed after an acute SARS-CoV-2 infection by personal, telephone, or electronic interview. We defined post-COVID-19 condition according to Soriano et al. [2], where “post-COVID-19 condition occurs in individuals with positive history of probable or confirmed SARS-CoV-2 infection, usually 3 months from onset of COVID-19, with symptoms that last for at least 2 months and cannot be explained by alternative diagnosis.” We considered any long COVID-19 symptom appearing after the infection, e.g., fatigue, dyspnea, pain, brain fog, memory loss, skin rashes, palpitations, cough, and sleep problems. Results should be reported as odds ratio (OR), hazards ratio (HR), or mean incidence of the symptoms.
2.2. Screening Process, Study Selection, and Data Extraction
This review included observational cohort, cross-sectional, and case-control studies whether presence of risk factors for development of symptoms appearing after an acute SARS-CoV-2 infection were investigated in COVID-19 survivors, either hospitalized or non-hospitalized. The current review was limited to human studies and English language papers. Editorials, opinion, and correspondence articles were excluded.
Two authors screened title and abstract of publications obtained from database search and removed duplicates. Full text of eligible articles was retrieved and analyzed. The following data were extracted from each study: authors, country, design, sample size, age range, assessment of symptoms, long COVID-19 symptoms, and effect (measure) of risk factor studied. Discrepancies between reviewers in any part of the screening and data extraction process were resolved by a third author.
2.3. Risk of Bias
The Quality in Prognosis Studies (QUIPSs) tool was used to determine the risk of bias (RoB) of the studies [16]. The QUIPS consists of six domains such as study participation, study attrition, prognostic factor measurement, outcome measurement, adjustment for other prognostic factors, and statistical analysis. RoB was initially evaluated by two authors. If there is disagreement, a third researcher arbitrated a consensus decision. Risk of bias was scored as low, moderate, or high as follows: 1 if all domains are classified as having low RoB, or just one as moderate RoB, the paper was classified as low RoB (green); 2 if one or more domains are classified as having high RoB; or ≥3 if all domains have moderate RoB, the paper was classified as high RoB (red). All papers in between were classified as having moderate RoB (yellow) [17].
2.4. Data Synthesis
We conducted a qualitative synthesis of data for those risk factors where the heterogeneity of results did not permit to perform a meta-analysis. We only included articles in the meta-analysis that followed the Soriano et al. definition of post-COVID-19 condition [2], hence meta-analysis was possible for age and sex.
To synthesize the association between age and sex with post-COVID-19 condition, random-effects meta-analyses were performed using MetaXL software ( https://www.epigear.com/index_files/metaxl.html) to estimate weighted mean differences (for age) and pooled odds ratios (ORs) with 95% confidence intervals (CIs) for sex and age above 60 years (old adults). A p-value < 0.05 was considered statistically significant. Given the heterogeneity expected, a random-effects model was employed. Measures of heterogeneity such as the I square statistics and Cochran’s Q test statistic and p-value are also reported. When each age group was reported using median and interquartile range values, the method described by Wan was used for transformation in mean and standard deviation.
3. Results
3.1. Study Selection
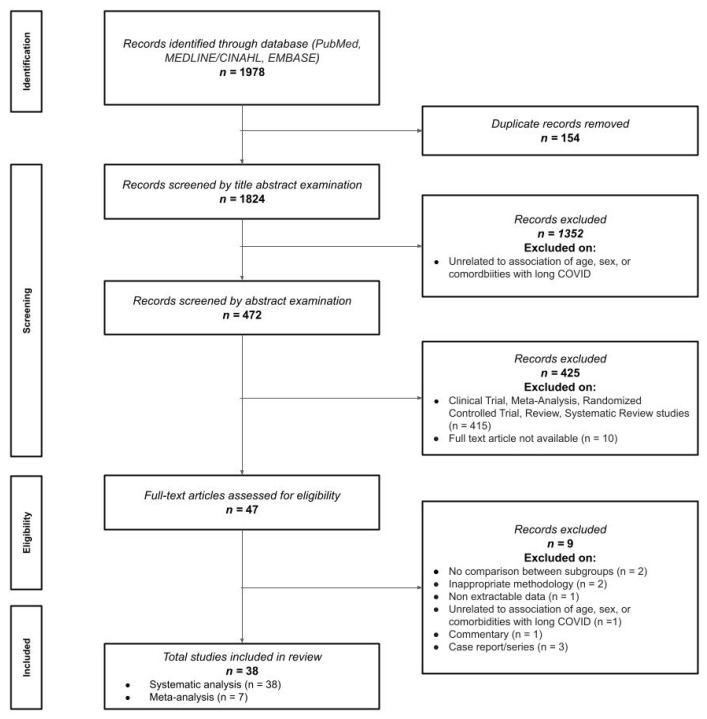
The electronic search allowed to initially identify 1978 titles for screening. After removing duplicates (n = 154) and papers not directly related to risk factors (n = 1352), 472 studies remained for abstract examination. Four hundred and twenty-five (n = 425) were excluded after reading the abstract, thus leading to a total of 47 text articles for eligibility (Figure 1). Nine articles were excluded because there were no comparisons between subgroups (n = 2) [18,19], inappropriate methodology (n = 2) [12,20], data not extractable (n = 1) [21], unrelated to association of risk factors (n = 1) [22], and type of literature commentary, case reports, and case series (n = 3) [23,24,25]. A total number of 37 peer-reviewed studies and one pre-print study were finally included [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63]. All papers could be included in qualitative analysis, whereas seven of these could also be pooled in the meta-analysis.
Figure 1.
Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) flow diagram.
3.2. Age and Post-COVID-19 Condition
A total of 18 articles, including 819,884 COVID-19 survivors analyzed age as a risk factor for developing long COVID-19 symptoms (Table 2) [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43]. Four articles used percentages [27,33,34,38], five used means [26,31,40,41,43], seven OR [28,29,30,32,36,37,39], one adjusted OR (aOR) [35], and one adjusted hazard ratio (aHR) [42]. Eight articles included population samples aged ≥50 years old [27,28,32,35,37,38,40,41], eight individuals aged between 40 and 49 years [26,29,31,33,34,39,42,43], and one a population between 18 and 64 years [27]. Two studies included children aged 10–12 years [30,36], but data from these age groups were not considered in the main analyses.
Table 2.
Studies investigating the effect of age in long COVID-19 symptomatology [10,26,27,28,29,30,31,32,33,35,36,37,38,39,40,41,42,43].
| Author | Country Study Period | Study Design Sample Size |
Age | Symptoms Assessment |
Post-COVID-19 Symptoms | Main Findings |
|---|---|---|---|---|---|---|
| Buonsenso et al., 2022 | Italy 1 April 2020–31 April 2021 |
Prospective cohort n = 507 |
Adults, 44 y | ISARIC Global COVID-19 protocol EQ-5D-5L |
Headache, Malaise, Fatigue | Probability of being fully recovered: 1–3 months Adults 0.83 (0.38), p = 0.001 6–9 months Adults 0.83 (0.38), p = 0.016 |
| Yellumahanthi et al., 2022 | USA 13 March 2020–12 March 2021 |
Prospective cohort n = 53 |
18–64 y (n = 38) ≥65 y (n = 15) |
Self-reported questionnaire three months after |
Fatigue, Brain fog Shortness of breath, Joint pain, Loss taste/smell, Anxiety/Depression, Hair loss, Sleep disturbances, Cough |
18–64 y Symptoms present n = 20 Symptoms absent n = 18 >65 y Symptoms present n = 7 Symptoms absent n = 8 p = 0.696 |
| Huang et al., 2021 | China 16 June 2020–13 September 2020 |
Cohort study n = 1733 |
mean 57 y | EQ-5D-5L | Fatigue, Sleep difficulties, Anxiety/Depression |
Per 10-year increase im age—Risk of Fatigue OR 1.17, 95% CI 1.07–1.27 * |
| Sudre et al., 2021 | UK, USA, Sweden March 2020–December 2020 |
Prospective cohort n = 8364 |
Positive for SARS-CoV-2 42 y (IQR 32–53) Negative for SARS-CoV-2 42 y (IQR 32–53) |
COVID-19 Symptoms Study app | Abdominal pain, Chest pain, Sore throat, Shortness of breath, Fatigue, Hoarse voice, Diarrhea, skipped meals, Cough, Muscle pain, Loss of smell, Headache | OR (95% CI)—18–30 y 30–40 y—OR from 2.11 to 4.12 * 40–50 y—OR from 2.24 to 4.35 * 50–60 y—OR from 6.65 to 11.49 * 60–70 y—OR from 6.53 to 14.0 * ≥70 y—OR from 5.46 to 18.56 * |
| Asadi-Pooya et al., 2021 # | Iran February 2020 –February 2021 |
Cross-Sectional n = 58 |
Mean age 12.3 y (SD 3.3) | Telephone interview | Fatigue, Shortness of breath, Exercise intolerance, Walking intolerance, Cough, Sputum, Sleep difficulty, Muscle/Joint pain, Headache, Chest pain, Palpitation, Loss of smell, Sore throat, Dizziness | OR 1.314 (95% CI 1.043–1.656), p = 0.002 * |
| Taquet et al., 2021 | USA January 2020–December 2020 |
Retrospective cohort n = 388,067 |
COVID-19 (unmatched) Mean age 46.3 y (SD 9.8) COVID-19 (matched) Mean age 39.4 y (SD 18.4) Influenza (matched) Mean age 38.3 y (SD 19.7) |
Electronic health records | Breathlessness, Fatigue/malaise, Chest pain, Throat pain, Headache, Abdominal pain, Myalgia, Cognitive symptoms, Anxiety/depression |
6-month incidence of long COVID-19 symptoms % (95% CI) 10–21 y—55.06 (54.34–55.77) 45–64 y—58.92 (58.24–59.59) ≥65 y—61.05 (60.29–61.81) |
| Peghin et al., 2021 | Italy March 2020–November 2020 |
Cohort n = 599 |
Mean age 53 y (SD 15.8) | Questionnaire via telephone interview | Dyspnea, Cough, Fatigue, Chest pain, Anosmia/Dysgeusia, Headache, Sleep disorders, Neurological Disorders, Brain Fog, Anxiety/Depression, Skin lesion, Gastrointestinal Dis., Hair loss, Ocular involvement |
41–60 vs.18–40 y OR 1.0 (95% CI 0.6–11.6), p = 0.9 >60 vs. 18–40 y OR 1.03 (95% CI 0.6–1.7), p = 0.9 >60 vs. 41–60 y OR 1.04 (95% CI 0.67–1.6), p = 0.8 |
| Carvalho-Schneider et al., 2021 | France March 2020–August 2020 |
Cohort n = 150 |
Mean age 49 y (IQR 34–64) | Telephone interviews | Dyspnea Chest pain Palpitations Anosmia/Ageusia Headache Cutaneous signs Arthralgia/Myalgia Digestive disorders Fever Sick leave |
One or more long COVID-19 symptom n (%) D30 (n = 150) * <30 y—7 (6.8) 30–39 y—21 (20.4) 40–49 y—24 (23.3) 50–59 y—28 (27.2) 60–69 y—11 (10.7) p = 0.06 D60 (n = 130) * <30 y—4 (4.7) 30–39 y—19 (22.1) 40–49 y—23 (26.7) 50–59 y—21 (24.4) 60–69 y—10 (11.6) p = 0.026 |
| Iqbal et al., 2021 | Pakistan September 2020–December 2020 |
Cross Sectional n = 158 |
Mean age 40.1 y (SD 12.42) | Questionnaire | Fatigue, Sleep quality, Anxiety/Depression Dyspnea, Joint pain, Loss of smell/taste, Cough, Loss Hair, Headache, Chest pain, Brain fog, Blurred vision, Tinnitus |
Relation of age with post-COVID-19 Dyspnea (p = 0.007) * Cough (p < 0.001) * Joint pain (p < 0.001) * Chest pain (p < 0.001) * |
| Tleyjeh et al., 2021 | Saudi Arabia May 2020–January 2021 |
Prospective Cohort n = 222 |
Mean age 52.5 y (IQR 38.52–66.42) | Structured interview via phone call | Insomnia, Fever, Fatigue, Joint pain, Muscle pain, Memory loss, Headaches, Loss of taste, Abdominal pain, Nausea/Vomiting, Diarrhea, Loss of smell, Sore throat, Runny nose, Chest pain, Cough, Shortness of breath |
Hazard model of new or persistent symptoms at follow-up (n = 222) Adjusted HR (95% CI) 0.99 (0.98–1.01), p = 0.38 |
| Osmanov et al., 2022 # | Russia April 2020–February 2021 |
Prospective Cohort n = 518 |
Mean age 10.4 y (IQR 3–15.2) | Telephone Interview— 1.0 ISARIC COVID-19 Health and Wellbeing Follow-Up Survey for Children |
Respiratory symptoms, Neurological symptoms, Sleep problems, Gastrointestinal Dermatological Cardiovascular Fatigue Musculoskeletal |
Presence of any persistent symptom at time of follow-up (n = 127) 2–5 y—OR 0.93 (95% CI 0.38–2.22) 6–11 y—OR 2.57 (95% CI 1.29–5.36) * 12–18 y—OR 2.52 (95% CI 1.34–5.01) * |
| Righi et al., 2022 | Italy February 2020 –February 2021 |
Prospective Cohort n = 465 |
Mean age 56 y (IQR 45–66) | Questionnaire | Cough, Diarrhea, Fatigue, Myalgia, Anosmia, Dysgeusia, Breathlessness |
Persistence of symptoms at 9-month follow-up >50 y—OR 2.5 (95% CI 1.28–4.88), p = 0.007 * Persistence of fatigue at 9-month follow-up >50 y—HR 0.98 (95% CI 0.97–0.99) |
| de Miranda et al., 2022 | Brazil March 2020–November 2021 |
Longitudinal study n = 646 |
Mean age 50.3 y (SD 15.8) | In person or virtual interview | Sore throat, Runny nose, Sputum, Skin lesion, Tachycardia, Vertigo, Chest pain, Joint pain, Diarrhea, Anxiety, Insomnia, Myalgia, Headache, Loss of smell/taste, Dyspnea, Fatigue | Mild COVID-19: 59.3% of 329 patients developed symptoms—<60 y: n = 162 (83.1%) Severe COVID-19: 33.1% of 260 patients developed symptoms ≤60 y old: n = 48 (55.8%) >60 y old: n = 38 (44.2%) |
| Loosen et al., 2022 | Germany 1 March 2020–31 March 2021 |
Cross-sectional n = 50,402 |
Mean age 48.8 y (SD 19.3) | Medical record data from the Disease Analyzer database | Fatigue, Abnormalities of breathing, Disturbances of smell/taste, Disturbances in attention | ≤30 years/COVID-19 patients: n = 10,443 Patients developing long COVID-19: n = 213 31–45 years/COVID-19 patients: n = 12,963 Patients developing long COVID-19: n = 379 46–60 years/COVID-19 patients: n = 14,424 Patients developing long COVID-19: n = 664 >60 years/COVID-19 patients: n = 12,572 Patients developing long COVID-19: n = 452 |
| Messin et al., 2021 | France March 2020–October 2020 |
Retrospective observational n = 74 With persistent symptoms: n = 53 Without persistent symptoms: n = 21 |
Mean age: 54.7 y (SD 16.9) | Telephone interview | Asthenia, Dyspnea, Anxiety, Anosmia, Ageusia, Nasal obstruction, Rhinorrhea, Sneezing, Odynophagia, Dysphonia, Chest pain, Palpitations, Headache, Dizziness, Drowsiness, Neuropathic pain, Depressive syndrome, Memory impairment, Attention deficit, Hair loss Diarrhea, Cough, Pain, Erectile dysfunction |
18–30 years—number (%) Symptoms: 5 (9.4)/No symptoms: 4 (19.1) 31–40 years—number (%) Symptoms: 8 (15.1)/No symptoms: 7 (33.3) 41–50 years—number (%) Symptoms: 8 (15.1)/No symptoms: 6 (28.6) 51–60 years—number (%) Symptoms: 9 (17)/No symptoms 0 61–70 years—number (%) Symptoms: 14 (26.4)/No symptoms: 0 >71 years—number (%) Symptoms: 9 (17)/No symptoms: 4 (19.1) |
| Kim et al., 2022 | Korea 31 August 2020–2 March 2021 |
Prospective cohort n = 170 With persistent symptoms: n = 129 Without persistent symptoms: n = 41 |
Median age: 51 y (IQR 37–61) | Individualized questionnaire | Fever, Myalgia, Cough, Arthralgia, Fatigue, Sore throat, Rhinorrhea, Chest pain, Dyspnea, Palpitation, Arrhythmia, Headache, Cognitive dysfunction, Dizziness, Insomnia, Depression/Anxiety, Vomiting, Diarrhea Anosmia, Ageusia, Tinnitus, Alopecia, Skin rash, Paresthesia |
20–29 years—number (%) Symptoms: 19 (14.7)/No symptoms: 10 (24.4) 30–39 years—number (%) Symptoms: 18 (14)/No symptoms: 6 (14.6) 40–49 years—number (%) Symptoms: 17 (13.2)/No symptoms: 9 (22) 50–59 years—number (%) Symptoms: 35 (27.1)/No symptoms: 9 (22) 60–70 years—number (%) Symptoms: 40 (31)/No symptoms: 7 (17.1) |
| Subramanian et al., 2022 | United Kingdom 31 January 2020–15 April 2021 |
Retrospective matched cohort study Non-hospitalized COVID-19 survivors n = 486,149 Matched patients with no evidence of COVID-19 n = 1,944,580 |
Patients infected with SARS-CoV-2 Mean age 44.1 y (SD 17.0) Comparator cohort Mean age 43.8 y (SD 16.9) |
Interviews and questionnaires | A total of 62 symptoms were significantly associated with SARS-CoV-2 infection after 12 weeks: Anosmia, Hair loss, Sneezing, Ejaculation difficulty, Reduced libido, Shortness of breath at rest, Fatigue, Chest pain, Hoarse voice, Fever |
18–29 years (n = 95,969) With symptoms: n (%) 6932 (7.2) 30–39 years (n = 78,302) With symptoms: n (%) 5805 (7.4) 40–49 years (n = 75,349) With symptoms: n (%) 5784 (7.7) 50–59 years (n = 73,262) With symptoms: n (%) 5485 (7.5) 60–69 years (n = 35,932) With symptoms: n (%) 2790 (7.8) ≥70 years (n = 25,323) With symptoms: n (%) 3073 (12.1) |
| Helmsdal et al., 2022 | Faroe Islands March 2020–January 2022 |
Cohort n = 180 |
Mean age 40 y (SD 19.4) | Standardized questionnaire via telephone interview | Fatigue, loss taste, loss smell, Headache, Skin rashes, Arthralgia, Dyspnea, Myalgia, Rhinorrhea, Chest tightness, Cough, Diarrhea, Nausea, Anorexia, Chills, Fever, Sore throat |
Prevalence (%) of long COVID-19 (n = 170) at 23-months Mean (SD) Age at symptom onset * Symptoms (n = 65) age: 45.1 (18.5) No symptoms (n = 105) age: 36.9 (19.3) p = 0.03 Persistent symptoms vs. No symptoms—n (%) 0–17 y—4 (6.2) vs. 17 (16.2) 18–34 y—16 (24.6) vs. 34 (32.4) 35–49 y—17 (26.2) vs. 22 (21.0) 50–67 y—18 (27.7) vs. 25 (23.8) >67 y—10 (15.4) vs. 7 (6.7) p = 0.1 |
* Statistically significant (p < 0.05); # Data from children were not considered in the analyses. y: years; SD: standard deviation.
Overall, most articles observed that old age was associated with long COVID-19 symptoms [26,28,29,31,33,34,35,37,38,39,40,41,43]. Contrastingly, Peghin et al. did not find an association between age and long COVID-19 symptoms [32]. Subramanian et al. stated that adults aged >70 years displayed lower risk of developing long COVID-19 symptoms than those aged 30–39 years [42].
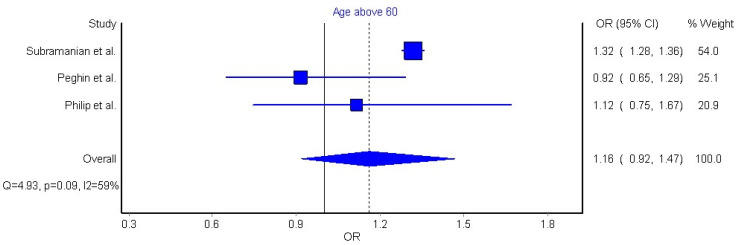
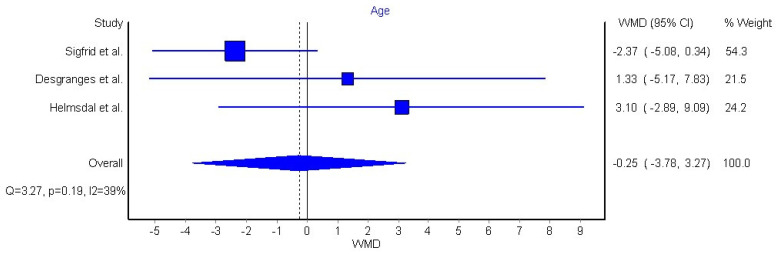
Three articles (n = 30,371 patients) were included in the meta-analysis based on their similar study design, study outcomes, and long COVID-19 definition [32,42,44]. We grouped individuals aged over 60 years old, since this age group is considered to be at higher risk of severe COVID-19. The meta-analysis did not reveal a significant association between old age and long COVID-19 symptomatology (OR 0.86, 95% CI 0.73 to 1.03, Q = 3.54, p = 0.17, I2: 44%, Figure 2). Another three articles reporting data as mean (with their standard deviation) or median (interquartile range) were also pooled [42,45,46]. We pooled these data through a random effects model, resulting in a non-significant weighted mean difference (WMD) of −0.25 (95% CI −3.78 to 3.27, Q = 3.27, p = 0.19, I2: 39%, Figure 3).
Figure 2.
Pooled analysis of odds ratio (OR) for the association between age older than 60 years and the presence of long COVID-19 symptoms [32,42,44].
Figure 3.
Pooled weighted mean difference (WMD) for the association between age as continuous variable and the presence of long COVID-19 symptoms [43,45,46].
3.3. Sex and Post-COVID-19 Condition
A total of 16 articles [32,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56] including 504,044 COVID-19 survivors were used in the analysis of sex as a risk factor for developing long COVID-19 symptomatology (Table 3). Data were presented as OR, aOR, HR, aHR, and percentage. Seven articles used OR [32,47,48,49,50,51,52], two used aOR [45,53], another two used both OR and aOR [46,54], while three articles used percentage [43,44,55], one article used both percentage and OR [56], and one article used both HR and aHR [42].
Table 3.
Studies investigating the effect of sex in long COVID-19 symptomatology [32,42,43,44,45,46,47,48,49,50,51,52,53,54,55].
| Author | Country Study Period |
Study Design Sample Size |
Age | Symptoms Assessment |
Post-COVID-19 Symptoms | Main Findings |
|---|---|---|---|---|---|---|
| Bai et al., 2021 | Italy 15 April 2020–15 December 2020 |
Prospective Cohort n = 377 Female 137 |
Median age 57 y (IQR 49–68) |
Interview and physical examination Impact of Event Scale-Revised (IES-R) |
Anosmia, Dysgeusia, Gastrointestinal symptoms, Fever, Joint pain, Myalgia, Dyspnea at rest, Exertional dyspnea, Fatigue, Brain fog, PTSD, Depression, Anxiety | Female Sex Risk Long COVID-19 OR 2.78 (95% CI 1.68–4.62) * Long COVID-19 AOR3.32 (95% CI 1.78–6.17) * |
| Pela et al., 2022 | Italy Follow-up: May 2020–March 2021 |
Cohort n = 223 Female 89 |
Mean age 59 y (SD 13) |
Retrospective Medical records Prospective Long COVID-19-19 reevaluation |
Dyspnea, Cough, Fatigue, Chest pain, Palpitations, Myalgia, Sleep disturbance | Female Sex Risk Dyspnea OR 2.35 (95% CI 1.12–4.94) * Fatigue OR6.72 (95% CI 2.34–19.26) * Chest pain OR 2.04 (95% CI 1.00–4.15) * Palpitation OR 2.30 (95% CI 1.14–4.65) * |
| Sigfrid et al., 2021 | UK NR |
Prospective Cohort n = 327 Female 135 |
Media age 60 y (IQR 51.7–67.7) | Washington group short scale MRC Dyspnea Scale EQ5D-5L |
Fatigue, Breathlessness, Sleep problems, Headache, Limb weakness, Muscle pain, Joint pain, Dizziness, Palpitations, Ocular problems, Stomach pain, Diarrhea, Cough, Chest pain, Loss of smell, Fever, Loss of taste, Nausea, Vomiting, Skin rashes |
Female Sex < 50 years Risk Long COVID-19 (AOR 5.09, 95% CI 1.64–15.74) * Fatigue (AOR 2.06, 95% CI 0.81–3.31) Breathlessness (AOR 7.15, 95% CI 2.24–22.83) * |
| Fernandez-de-las-Peñas et al., 2022 | Spain 10 March 2020–31 May 2020 |
Cross-sectional n = 1969 Female 915 |
Mean age 61 y (SD 16) |
Telephone interview | Fatigue, Dyspnea at rest, Dyspnea at exertion, Pain, Memory loss, Brain fog, Concentration loss, Hair loss, Palpitations, Skin rashes, Diarrhea, Voice problems, Gastrointestinal problems, Ageusia, Anosmia, Ocular Problems, Throat pain, Anxiety/Depression, Sleep quality | Female Sex Risk Symptoms (AOR 2.54, 95% CI 1.67–3.86) * Fatigue (AOR 1.51, 95% CI 1.04–2.20) * Dyspnea rest (AOR 1.42, 95% CI 1.08–1.88) * Dyspnea exertion (AOR 1.4, 95% CI 1.10–1.79) * Pain (AOR 1.34, 95% CI 1.05–1.72) * Hair loss (AOR 4.52, 95% CI 2.78–7.36) * Ocular problems (AOR 1.98, 95% CI 1.18–3.31) * Depression (AOR 1.60, 95% CI 1.00–2.57) * Sleep quality (AOR 1.63, 95% CI 1.09–2.43) * |
| Gebhard et al., 2022 | Switzerland February 2020–December 2020 |
Prospective cohort n = 2927 Female 1346 |
NR | Self-reported questionnaires | Dyspnea, Reduced exercise performance, Changes in smell and taste | Females reported at least one persistent symptom than males (43.5% vs. 32.0%, p < 0.001) The higher prevalence of PASC in females was observed in both outpatients (40.5% in females vs. 25.4% in males, p < 0.001) and hospitalized patients (63.1% in females vs. 55.2% in males, p < 0.001) |
| Tleyjeh et al., 2022 | Saudi Arabia May 2020–July 2020 |
Cohort n = 222 Female 51 |
Range > 18 y | Medical research council (MRC) dyspnea scale Metabolic equivalent of task (MET) score Chronic fatigability syndrome questionnaire | Breathlessness, Exercise intolerance, Chronic fatigue, Poor mental well-being |
Female Sex Risk Exertional Dyspnea OR4.36 (95% CI 2.25–8.46) * Lower MET exercise tolerance score OR0.19 (95% CI 0.09–0.42) * Chronic Fatigability Syndrome OR3.97 (95% CI 1.85–8.49) * |
| Desgranges et al., 2022 | Switzerland 26 February–27 April 2020 |
Prospective cohort n = 418 Female 261 |
Median age 41 y (IQR 31–54) | Structured and standardized phone survey | Fatigue, Smell or taste disorder, Dyspnea, Headache, Memory impairment, Hair loss, Sleep disorders | Female Sex Risk Symptoms AOR 1.67 (95% CI 1.09–2.56) * Dyspnea AOR1.71 (95% CI 0.93–3.16) Smell/taste disorder AOR 1.9 (95% CI 1.09–3.22) * Fatigue AOR1.61 (95% CI 1.00–2.59) * |
| García-Abellán at al., 2021 | Spain 10 March–30 June 2020 |
Prospective longitudinal study n = 146 Female 58 |
Median age 65 y (IQR 55–75) | Self-rated COVID-19 symptom questionnaire (CSQ) | Fatigue, Myalgia, Sweating, Headache, Cough, Difficulty breathing, Congestion, Sore throat, Anosmia, Diarrhea, Vomiting, Abdominal pain | Female Sex Risk Highest COVID-19 symptom questionnaire (CSQ) scores OR 2.41 (95% CI 1.20–4.82) * |
| Asadi-Pooya et al., 2021 | Iran 19 February 2020–20 November 2020 |
Retrospective observational study n = 4681 Female 2203 |
Mean age 52 y (SD 15) | Telephone interview | Weakness, Muscle pain, Fatigue, Sleep difficulty, Palpitations, Cough, Brain fog, Walking intolerance | Female Sex Risk Long COVID-19 Symptoms OR1.26 (95% CI1.12–1.43) * |
| Munblit et al., 2021 | Russia 8 April 2020–10 July 2020 |
Longitudinal cohort study n = 2649 Female 1353 |
Median age 56 y (IQR 46–66) | Study case report form (CRF) British Medical Research Council (MRC) dyspnoea scale EQ-5D-5L WHODAS 2.0 |
Fatigue, Breathlessness, Forgetfulness, Muscle weakness, Ocular problems, Hair loss, Sleeping problem | Female Sex Risk Symptoms OR1.83 (95% CI 1.55–2.17) * Fatigue OR1.67 (95% CI 1.39–2.02) * Neurological OR2.03 (95% CI 1.60–2.58) * Mood OR1.83 (95% CI 1.41–2.40) * Dermatological OR3.26 (95% CI 2.36–4.57) * Gastrointestinal OR2.50 (95% CI 1.64–3.89) * Sensory OR1.73 (95% CI 2.06–2.89) * Respiratory OR1.31 (95% CI 1.06–1.62) * |
| Chudzik et al., 2022 | Poland 1 September 2020–30 September 2021 |
Retrospective cohort n = 2218 Female 1410 |
Mean age 54 y (SD 13.5) | Health questionnaire | Fatigue, Headache, Cough Brain fog, Dyspnoea, Hair loss, Olfactory dysfunction, Osteoarticular pain |
Female Sex Risk Symptoms OR 1.44 (95% CI 1.20–1.72) * Brain fog OR1.15 (95% CI0.88–1.51) Fatigue OR1.06 (95% CI0.89–1.28) |
| Peghin et al., 2021 | Italy March 2020–November 2020 |
Cohort n = 599 Female 320 |
Mean age 53 y (SD 15.8) | Questionnaire via telephone interview | Dyspnea, Cough, Fatigue, Myalgia, Chest Pain, Anosmia/Dysgeusia, Headache, Arthralgia, Neurological Disorders Anxiety/Depression, Sleep Disorders, Brain Fog, Skin Lesions, Gastrointestinal Disorders, Hair Loss, Nose Cold, Sneezing, Odynophagia, Ocular Problems |
Female Sex Risk Long COVID-19 Symptoms OR 1.55 (95% CI 1.05–2.27) * |
| Philip et al., 2022 | UK October 2020 |
Retrospective cohort n = 4500 |
Range age 50–59 y |
Asthma UK and British Lung Foundation survey | Fatigue, Breathlessness, Pain (chest or whole body) | No association between female sex and long COVID-19 symptoms |
| Helmsdal et al., 2022 | Faroe Islands March 2020–January 2022 |
Cohort n= 180 Female 93 |
Mean age 40 y (SD 19.4) | Standardized questionnaire via telephone interview | Fatigue, affected taste, affected smell, Headache, Arthralgia, Dyspnea, Myalgia, Skin rashes, Rhinorrhea, Chest tightness, Cough, Nausea, Diarrhea, Fever, Sore throat | No association between female sex and long COVID-19 symptoms |
| Subramanian et al., 2022 | United Kingdom 31 January 2020–15 April 2021 |
Retrospective matched cohort study Non-hospitalized COVID-19 survivors n = 486,149 Matched patients with no evidence of COVID-19 n = 1,944,580 |
Patients infected with SARS-CoV-2 Mean age 44.1 y (SD 17.0) Comparator cohort Mean age 43.8 y (SD 16.9) |
Interviews and questionnaires | Anosmia, Hair loss, Sneezing, Ejaculation difficulty, Reduced libido, Shortness of breath at rest, Fatigue, Chest pain, Hoarse voice, Fever |
Female Sex Risk Long COVID-19 Symptoms HR 1.86 (95% CI 1.81–1.90) * aHR 1.52 (95% CI 1.48–1.56) * |
WHODAS: Washington disability score and World Health Organization Disability Assessment Schedule. * Statistically significant (p < 0.05). NR: not reported; y: years; SD: standard deviation.
Fourteen articles observed that female sex (n = 276,953) was associated with higher risk of long COVID-19 [32,42,45,46,47,48,49,50,51,52,53,54,55,56], whilst two articles (n = 475) reported that female sex was not associated with higher risk of long COVID-19 [43,44].
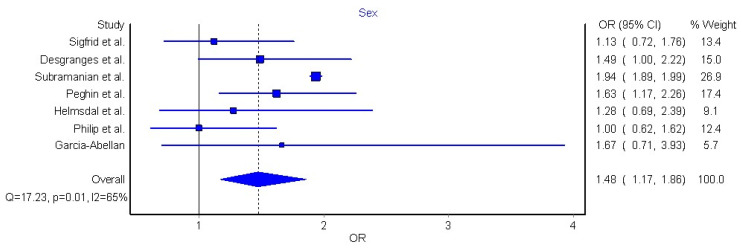
Seven articles (n = 386,234 COVID-19 patients who recovered from acute SARS-CoV-2 infection) were included in the meta-analysis due to their similarities in study design, definition of long COVID-19, as well as similarities in data presentation [32,42,43,44,45,46,50]. The meta-analysis revealed that female sex was significantly associated with nearly 50% higher risk (OR 1.48, 95% CI 1.17 to 1.86, Q = 17.2, p = 0.01, I2: 65%, Figure 4) of long COVID-19 symptomatology.
Figure 4.
Pooled analysis of odds ratio (OR) for the association between sex and the presence of long COVID-19 [32,42,43,44,45,46,50].
3.4. Medical Comorbidities and Post-COVID-19 Condition
A total of 12 articles with 677,045 COVID-19 survivors were analyzed for association between long COVID-19 and comorbidities (Table 4) [29,39,44,52,56,57,58,59,60,61,62,63]. Four comorbidities were included: pulmonary disease (n = 4), diabetes (n = 1), obesity (n = 6), and organ transplantation (n = 1). Data were presented as means, medians, percentages, odds ratio (OR), and incident rate ratio (IRR). One study used mean [61], one used both median and percentage [59], three used percentage only [44], five used OR [29,39,52,55,60], and two used both OR and IRR [57,59].
Table 4.
Studies investigating the effect of previous medical comorbidities in long COVID-19 symptomatology [29,39,44,52,53,56,58,59,60,61,62,63].
| Author | Country Study Period | Study Design Sample Size |
Age | Symptoms Assessment |
Post-COVID-19 Symptoms | Main Findings |
|---|---|---|---|---|---|---|
| Diabetes | ||||||
| Fernandez-de-las-Peñas et al., 2021 | Spain 1 March–31 May 2020 |
Case-control n = 435 Patients n = 145 Control n = 290 |
Patients Mean age 70.2 y (SD 13.2) Controls) Mean age 70.4 y (SD 13.4) |
Hospital medical records Telephonic interview | Fatigue, Dyspnea on exertion and at rest, Pain, Memory loss, Skin rashes, Gastrointestinal dis., Brain fog, Concentration loss, Ageusia, Ocular disorder, Anosmia, Tachycardia, Cough, Headache, Sleep, Depression/Anxiety | Number of post-COVID-19 symptoms (IRR 1.06, 95% CI 0.92–1.24) Fatigue (OR 1.45, 95% CI 0.93–2.25) Dyspnea (OR 0.97, 95% CI 0.64–1.47) Pain (OR 0.951, 95% CI 0.76–1.18) Anxiety (OR 1.30, 95% CI 0.77–2.20) Depression (OR 1.31, 95% CI 0.79–2.17) Poor sleep (OR 1.34, 95% CI 0.89–2.03) |
| Obesity | ||||||
| Lacavalerie et al., 2022 | France October 2020–June 2021 |
Retrospective observational n = 80 Patients n = 33 Controls n = 18 n = 29 |
Patients Mean age 60 y (SD 11) Controls Mean age 50 y (SD 13) |
Clinical evaluation with spirometry, cardiopulmonary and exercise testing | Fatigue, Dyspnea, Chest pain, Pulmonary function test, Cardiopulmonary exercise testing | Non-obese vs. obese, p value Pulmonary function test Predicted FEV1 (%) 87 ± 13/75 ± 13 p = 0.002 * Predicted FVC (%) 82 ± 16/74 ± 14 p = 0.04 * TLC (%) 79 ± 9/69 ± 12 p = 0.003 * RV (%) 71 ± 25/86 ± 24 p = 0.04 * KCO (%)100 ± 11/108 ± 12 p = 0.03 * Cardiopulmonary exercise testing Peak VE/VO2 35 ± 5/39 ± 7 p = 0.011 * Ventilatory reserve (%) 40 ± 14/25 ± 21 p = 0.011 * VE VCO2 slope 34 ± 6/31 ± 4 p = 0.045 * Peak SpO2 (%) 98 ± 2/96 ± 3 p = 0.036 * |
| Fernandez-de-las-Peñas et al., 2022 | Spain 1 March 2020–31 March 2021 |
Case-control n = 264 Patients n = 88 Control n = 176 |
Patients Mean age 52 y (SD 14.5) Controls Mean age 52.2 y (SD 14.2) |
Hospital medical records Telephonic interview | Fatigue, Dyspnea, Memory loss, Skin rashes, Brain Fog, Gastrointestinal disorders, Concentration loss, Ageusia, Ocular disorders, Tachycardia, Pain, Anosmia, Headache, Sleep, Depression/Anxiety | Number of post-COVID-19 symptoms (IRR 1.51, 95% CI 1.24–1.84) * Sleep quality (OR 2.27, 95% CI 1.34–3.86) * Fatigue (OR 1.39; 95% CI 0.79–2.43) Dyspnea (OR 1.41; 95% CI 0.79–2.53) Anxiety (OR 1.75, 95% CI 0.82–3.72) Depression (OR 0.83, 95% CI 0.40–1.73) |
| Loosen et al., 2022 | Germany 1 March 2020–31 March 2021 |
Retrospective Observational n = 50,402 |
Mean age 48.8 y (SD 19.3) |
Medical record | Fatigue, Abnormalities of breathing, Loss of smell and taste, disturbances in attention | Obesity (OR 1.25 95% CI 1.08–1.44) * Hypertension (OR 1.31, 95% CI 1.15–1.48) * |
| Shang et al., 2021 | Wuhan, China 20 February–20 March 2020 |
Cohort Study n = 118 Patients n = 53 Controls n = 65 |
Patients Mean age 51 y (IQR 41–58) Controls Mean age 57 y (IQR 48–62) |
Interview, Physical exam, Blood sample, Lung function test, CT scan |
Shortness of breath, Fatigue, Sleep problems, Joint pain, Smell disorder, Diarrhea, Constipation | No differences in the prevalence of long COVID-19 Symptoms existed between obese and non-obese patients. |
| Whitaker et al., 2022 | UK 15–28 September 2020 27 October–10 November 2020 25 January–8 February 2021 12–25 May 2021 |
Cohort n = 606,434 |
Age >18 | Online/telephone survey | Tiredness, Tight chest, Sore throat, Sore eyes, Sneezing, Shortness of breath, Fatigue, Runny nose, Skin lesions, Cough, Pain symptoms, Nausea, Vomiting, Loss of taste or smell, Hoarse voice, Headache, Dizziness, Difficulty sleeping, Diarrhoea, Chest pain, Abdominal pain | Persistence of one or more symptoms for 12 weeks or more Obesity (OR1.39, 95% CI 1.32–1.48) * |
| Chudzik et al., 2022 | Poland 1 September 2020–30 September 2021 |
Retrospective observational n = 2218 | Mean age = 53.8 ± 13.5 years | Health questionnaire | Cough, Dyspnea, Fatigue, Hair loss, Olfactory disturbances, Headache, Pain, Brain fog | Presence of overall persistent symptoms Obesity (OR1.16, 95% CI 0.96–1.41) Fatigue (OR 1.49, 95% CI 1.24–1.80) * |
| Pulmonary Disease | ||||||
| Sudre et al., 2021 | UK 25 March–30 June 2020 |
Prospective cohort n = 8364 COVID-19 n = 4182 No COVID-19 n = 4182 |
Mean age 46 y | COVID-19 Symptom Study app1 | Abdominal pain, Chest pain, Sore throat, Fatigue, Shortness of breath, Hoarse voice, Delirium, Diarrhea, Fever, Cough, Muscle pain, Anosmia, Headache | Presence of long COVID-19 symptoms Asthma (OR 2.14, 95% CI 1.55–2.96) * |
| Munblit et al., 2021 | Russia 8 April–10 July 2020 |
Prospective cohort n = 2649 |
Median age 56 y | ISARIC Long-term Follow-up Study questionnaire | Fatigue, Shortness of breath, Forgetfulness | Asthma and chronic pulmonary disease were not associated with persistent symptoms overall, but asthma was associated with neurological (OR1.95, 95% CI 1.25–2.98) * and chronic pulmonary disease was associated with fatigue (OR 1.68, 95% CI 1.21–2.32) * |
| Philip et al., 2022 | UK October 2020 |
Retrospective cohort n = 4500 COVID-19 n = 471 No COVID-19 n = 3036 COVID-19 n = 972 |
Range age 50–59 y |
Asthma UK and British Lung Foundation survey | Fatigue, Breathlessness, Pain (chest or whole body) | For many people with asthma, COVID-19 is associated with prolonged symptoms and worsening asthma control |
| Jia et al., 2022 | USA March 2020–February 2021 |
Prospective cohort n = 637 Patients n = 617 Controls n = 20 |
Patients Mean age 51 y Controls Mean age 54 y |
Survey | Cough, Shortness of breath, Fever, Nausea, Vomiting | Comorbid lung disease, asthma and lower levels of initial IgG response to SARS-CoV-2 nucleocapsid antigen were associated with longer symptom duration (mean days: 55 versus 44 days; p = 0.04) * |
| Transplant | ||||||
| Oto et al., 2022 | Turkey 15 March 2021–11 June 2021 |
Retrospective cohort n = 944 Patients n = 523 Control n = 421 |
Mean age 46 y | Survey | Respiratory symptoms | Persistence of respiratory symptoms without increased risk of acute rejection, BK and CMV infection, thromboembolic event or urinary tract infection |
* Statistically significant (p < 0.05). y: years; SD: standard deviation.
Three articles on pulmonary disease revealed an association between asthma and longer symptom duration among patients recovering from COVID-19 [29,44,60]. However, both asthma and chronic pulmonary disease were not associated with long COVID-19 in one study [52]. For diabetes, no difference was found in the number of long COVID-19 symptoms among diabetic and non-diabetic patients [57]. For obesity, all six articles noted that this metabolic disease was associated with worse health due to increased number of long COVID-19 symptoms [39,59], longer persistence of symptoms [56,63], more presence of pathological pulmonary limitations [61], and metabolic abnormalities [58]. Meanwhile, one study on kidney transplant patients revealed that patients have higher susceptibility to developing long COVID-19 symptoms, although this did not affect mortality rate [62].
3.5. Risk of Bias
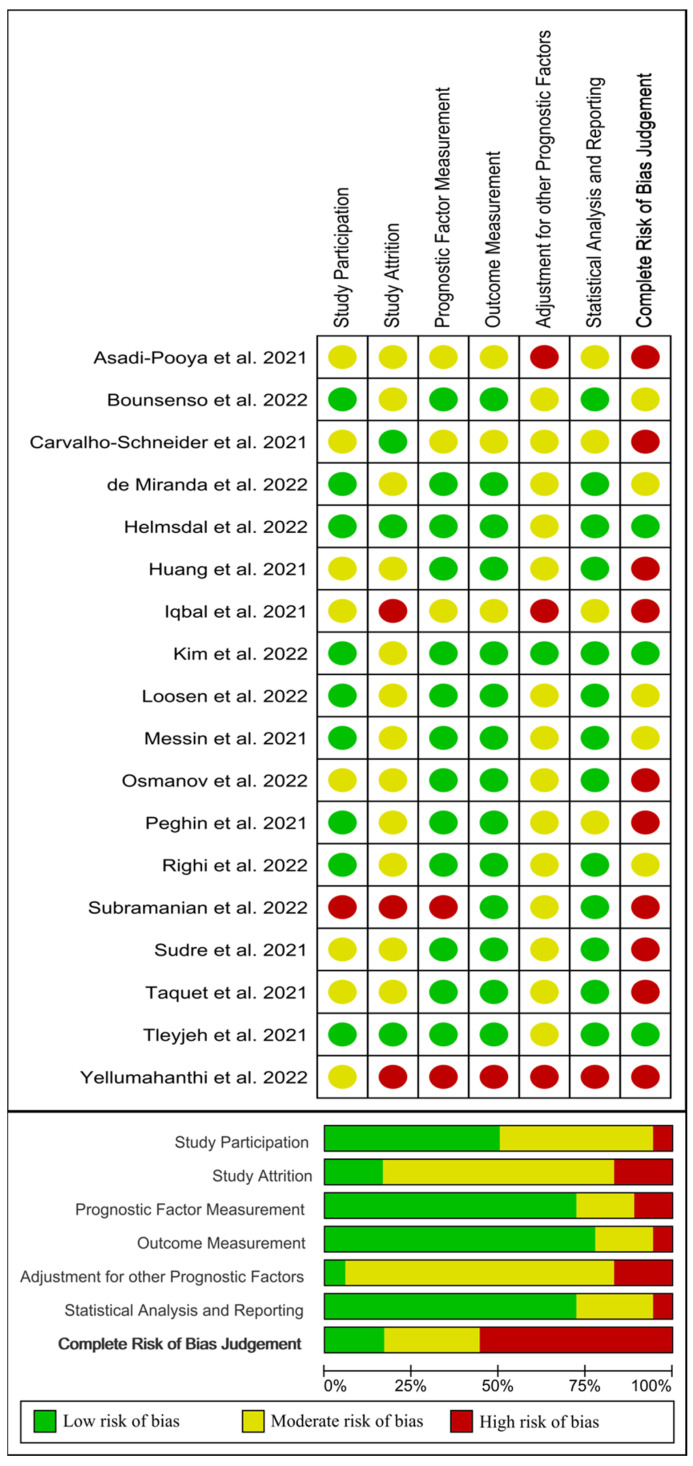
From out of 18 papers evaluating age as risk factor [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43], three [35,41,43] were classified as low risk of bias (green), five [26,37,38,39,40] as moderate risk of bias (yellow), and the remaining ten [27,28,29,31,32,33,34,36,42,51] as high risk of bias (red). Figure 5 visually graphs that the most frequent risk of bias was adjustment for other prognostic factor (i.e., if important potential confounding factors were appropriately accounted for), which was properly performed in just one study [41].
Figure 5.
Plot of the risk of bias of those studies investigating age as a risk factor of long COVID-19 [10,26,27,28,29,30,31,32,33,35,36,37,38,39,40,41,42,43].
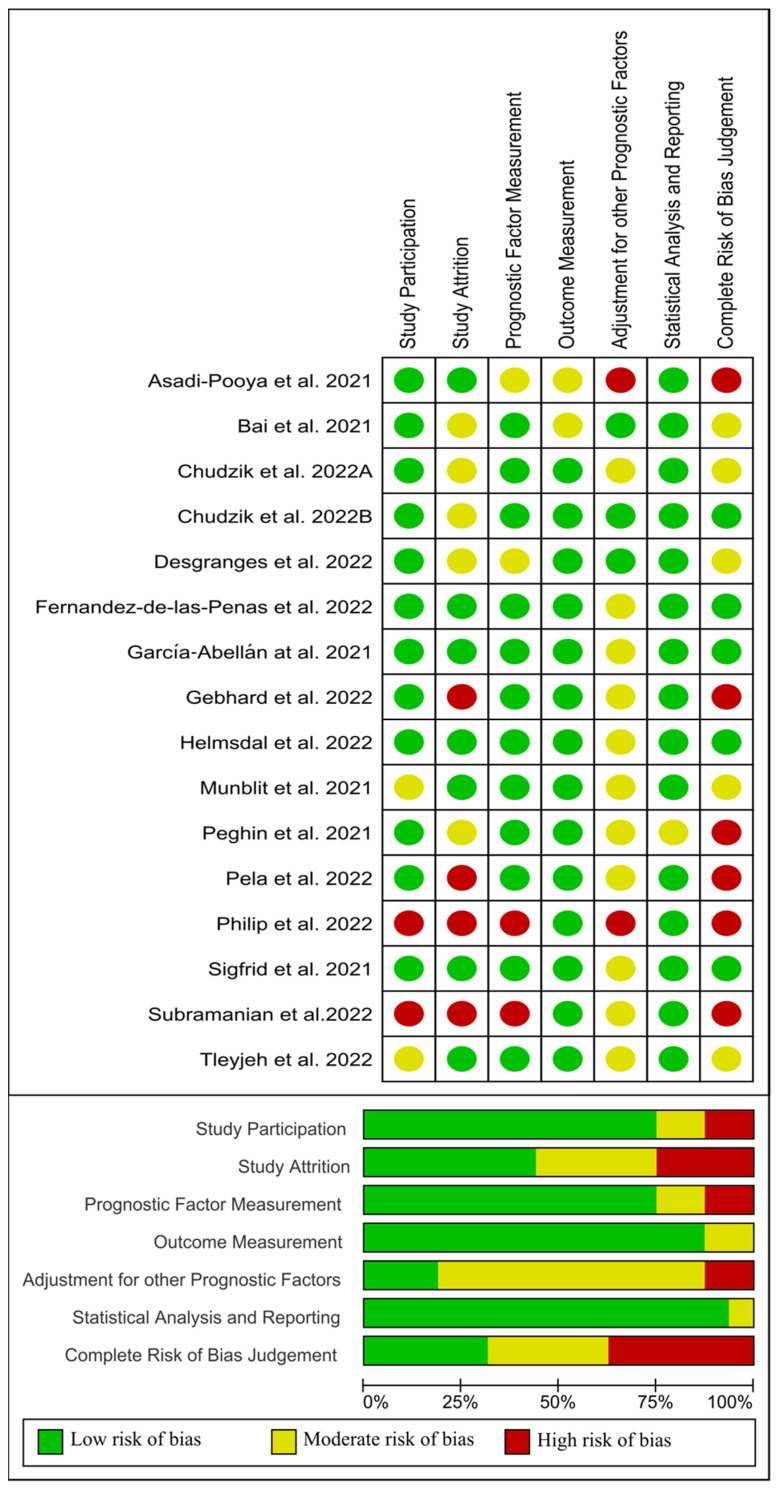
On the other hand, from 16 papers evaluating sex as a risk factor [32,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56], four studies [45,50,53,55] were classified as low risk of bias (green), five [46,49,52,54,56] as moderate risk of bias (yellow), and the remaining seven [32,42,43,44,47,48,51] as high risk of bias (red). Figure 6 visually graphs that the most frequent risk of bias in this group of studies were adjustment for other prognostic factors and study attrition (i.e., the representativeness of the participants with follow-up data with respect to those originally enrolled in the study, selection bias).
Figure 6.
Plot of the risk of bias of those studies investigating sex as a risk factor of long COVID-19 [1,32,42,43,44,45,46,47,48,49,50,51,52,54,55,56].
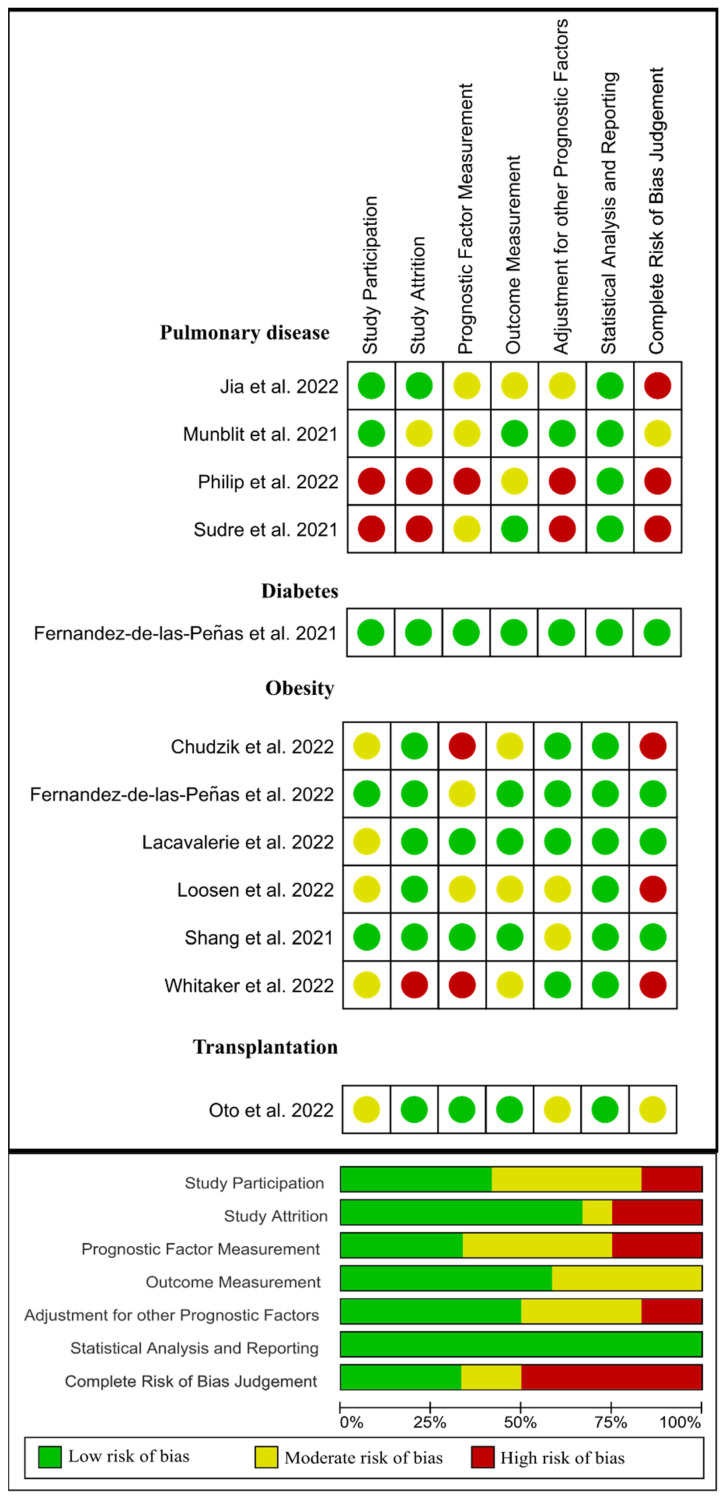
Lastly, from 12 papers evaluating previous medical comorbidities as a risk factor [29,39,44,52,56,57,58,59,60,61,62,63], four [57,58,59,61] were classified as low risk of bias (green), two [52,62] as moderate risk of bias (yellow), and the remaining six [29,39,44,56,60,63] as high risk of bias (red). Figure 7 visually graphs that the most frequent risk of bias in this group of studies was concerned prognostic factor measurement (i.e., if the prognostic factors were measured in a similar way for all the participants).
Figure 7.
Plot of the risk of bias of those studies investigating medical comorbidities as a risk factor of long COVID-19 [4,29,39,44,52,53,56,58,60,61,62,63].
4. Discussion
This systematic review and meta-analysis explored the association of long COVID-19 with risk factors not directly related to an acute SARS-CoV-2 infection (i.e., pre-infection factors), including age, sex, or previous comorbidities. The results support that female sex may be a predictor of long COVID-19 while old age was reported to be associated with long COVID-19 in single studies; however, the pooled evidence was not significant. Finally, prior medical comorbidities can also be potential predictors of long COVID-19 symptoms. These results should be considered with caution because most studies exhibited moderate to high risk of bias.
4.1. Old Age and Long COVID-19
Old age is an important risk factor of poor outcomes in COVID-19 hospitalization [64]; however, the impact of age on long COVID-19 is controversial. Old age is associated with higher risk of long COVID-19 symptomatology in single studies and in two previous reviews [10,12], but not in the meta-analysis by Maglietta et al. [11]. Results from our qualitative analysis suggest that older adults can develop more long COVID-19 symptoms than younger adults; however, this assumption was not supported when pooling data into a meta-analysis. We conducted two meta-analyses, the first one categorizing those adults older than 60 years (Figure 2), and a second one considering age as a continuous variable (Figure 3); neither analysis revealed an association between old age and risk of developing long COVID-19. Nevertheless, the number of studies pooled in our analyses of age was notably limited (n = 3). Our data are consistent with the meta-analysis of Maglietta et al. [11] but disagree with Thompson et al. [12]. Several differences can explain the discrepancy with Thompson et al. [12]. It is possible that the use of a different definition of long COVID-19 by these authors [12] can lead to inconclusive comparisons of results. In addition, Thompson et al. [12] did not pool data of age and long COVID-19 into a meta-analysis, but only calculated regression of proportions of subjects at each age group developing long COVID-19 symptoms. The significance of old age as a risk factor for long COVID-19 development requires further investigation. In fact, just three out of eighteen papers (16%) analyzing age as prognostic factor showed low risk of bias. The most significant bias of these studies was the proper control of other cofounding factors observed in older people, i.e., higher presence of medical comorbidities, or longer hospitalization stay, which can also be associated with long COVID-19.
4.2. Female Sex and Long COVID-19
Sex is another important risk factor which has been studied in relation to COVID-19 and long COVID-19. Evidence supports that men and women exhibit the same probability of being infected by SARS-CoV-2; however, males are at a higher risk of worse outcomes and death than females during the acute phase of infection [65]. Results from our systematic review and meta-analysis support that female sex may be associated with higher risk of developing long COVID-19 (OR 1.48, 95% CI 1.17 to 1.86). Our results are similar to those previously observed by Maglietta et al. [11], who also reported that female sex was associated with long COVID-19 symptoms (OR1.52, 95% CI 1.27–1.82), and with results (OR1.60, 95% CI 1.23–2.07) previously reported by Thompson et al. [12]. Based on available data, females are more vulnerable to develop long COVID-19 than males. Hence, considering sex differences in diagnosis, prevention and treatment are necessary, and fundamental steps towards precision medicine in COVID-19 [66]. Biological (i.e., hormones and immune responses), and sociocultural (i.e., sanitary-related behaviors, psychological stress, and inactivity) aspects play a significant role in creating sex-differences in long COVID-19 symptoms [48], although mechanisms behind increased risk of long COVID-19 in females remain unknown and warrant investigation.
4.3. Medical Comorbidities and Long COVID-19
Such as with old age, the presence of prior medical comorbidities (e.g., hypertension, obesity, diabetes, chronic kidney disease, cerebrovascular disease, chronic obstructive pulmonary disease, or cardiovascular disease) is known to induce a more severe COVID-19 disease progression [67,68]. A potential reason is that such comorbidities can contribute to degradation of angiotensin-converting enzyme 2 (ACE2). Since the SARS-CoV-2 virus uses this receptor as entry pathway in host cells, higher degradation of ACE2 could lead to a long-lasting inflammatory cytokine storm, oxidative stress, and hemostasis activation, which are all hallmarks of severe/critical COVID-19 illness [69]. Nevertheless, this hypothesis is not yet supported by the literature.
The current qualitative analysis suggests that prior comorbidities may contribute the risk of developing long COVID-19. Among different comorbidities, obesity seems to be associated; however, this assumption should be considered with caution at this stage, since potential cofounding factors, particularly those related to hospitalization (obese patients have more severe COVID-19 disease and higher hospitalization rates than non-obese patients), were not properly controlled in these studies. Moreover, the association of long COVID-19 with other medical comorbidities such as diabetes or transplants was only investigated in one prior study.
4.4. Strengths and Limitations
The results of this systematic review and meta-analysis should be considered according to potential strengths and limitations. Among the strengths, we conducted a systematic search of all the currently available evidence on factor not related to an acute SARS-CoV-2 infection but associated with higher risk of developing long COVID-19. This led to identification of thirty-eight studies. Second, this is the first time that several medical comorbidities have been systematically investigated as risk factors of long COVID-19.
One of the limitations is the lack of a consistent definition of long COVID-19 in available literatures. We included all identified studies within the qualitative analysis, but only those using the definition by Soriano et al. [2] of long COVID-19 were included in the meta-analyses. This assumption led to a small number of studies in the meta-analyses. Future studies using a more consistent definition of long COVID-19 are needed for improved quantification of the results. Another limitation is the lack of differentiation of risk factors between hospitalized and non-hospitalized patients. Similarly, no study investigating risk factors considered the SARS-CoV-2 variants of concern. Therefore, studies identifying long COVID-19 risk factors not directly associated with SARS-CoV-2 infection differentiating between hospitalized and non-hospitalized patients, and among different SARS-CoV-2 variants of concern are now needed. Finally, it should be considered that this systematic review and meta-analysis only investigated risk factors not associated with an acute SARS-CoV-2 infection. Other potential SARS-CoV-2-associated factors, such as severity of disease during the acute phase of infection or the number of COVID-19-associated onset symptoms have also been preliminarily identified as risk factors associated with long COVID-19 symptoms, particularly with respiratory symptoms [11]. Similarly, it is possible that some long COVID-19 symptoms can also be related to hospitalization factors which were also not investigated in this review.
5. Conclusions
The current review demonstrates that female sex and previous medical comorbidities may be predisposing factors for the development of long COVID-19 symptomatology. The current literature does not conclusively confirm that old age would significantly influence long COVID-19 risk. These results should be considered with caution due to moderate to high risk of bias in most published studies. These findings highlight the need for further research with improved control of confounding factors and use of a consistent and validated definition of long COVID-19.
Author Contributions
All the authors cited in the manuscript had substantial contributions to the concept and design, the execution of the work, or the analysis and interpretation of data; drafting or revising the manuscript and have read and approved the final version of the paper. K.I.N.: conceptualization, visualization, methodology, validation, formal analysis, data curation, writing—original draft, writing—review and editing. conceptualization, formal analysis, data curation, writing—review and editing. M.H.S.d.O.: methodology, validation, formal analysis, data curation, writing—original draft, writing—review and editing. P.J.P.: methodology, validation, formal analysis, data curation, writing—original draft, writing—review and editing. J.V.V.: methodology, validation, formal analysis, data curation, writing—original draft, writing—review and editing. I.M.: methodology, validation, formal analysis, data curation, writing—original draft, writing—review and editing. A.T.V.: methodology, validation, formal analysis, data curation, writing—original draft, writing—review and editing. F.C.P.: methodology, validation, formal analysis, data curation, writing—original draft, writing—review and editing. A.P.: methodology, validation, formal analysis, data curation, writing—original draft, writing—review and editing. N.G.: writing—review and editing. D.K.: writing—review and editing. M.M.L.G.: writing—review and editing. J.L.: writing—review and editing. G.L.: writing—review and editing. B.M.H.: writing—review and editing. C.F.-d.-l.-P.: conceptualization, visualization, validation, formal analysis, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
All data derived from the study are included in the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The project was supported by a grant associated to the Fondo Europeo De Desarrollo Regional—Recursos REACT-UE del Programa Operativo de Madrid 2014–2020, en la línea de actuación de proyectos de I+D+i en materia de respuesta a COVID-19 (LONG COVID-19-EXP-CM). The sponsor had no role in the design, collection, management, analysis, or interpretation of the data, draft, review, or approval of the manuscript or its content. The authors were responsible for the decision to submit the manuscript for publication, and the sponsor did not participate in this decision.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fernández-de-Las-Peñas C. Long COVID: Current Definition. Infection. 2022;50:285–286. doi: 10.1007/s15010-021-01696-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soriano J.B., Murthy S., Marshall J.C., Relan P., Diaz J.V. A Clinical Case Definition of Post-COVID-19 Condition by a Delphi Consensus. Lancet Infect. Dis. 2022;22:e102–e107. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han Q., Zheng B., Daines L., Sheikh A. Long-Term Sequelae of COVID-19: A Systematic Review and Meta-Analysis of One-Year Follow-Up Studies on Post-COVID Symptoms. Pathogens. 2022;11:269. doi: 10.3390/pathogens11020269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernández-de-Las-Peñas C., Palacios-Ceña D., Gómez-Mayordomo V., Florencio L.L., Cuadrado M.L., Plaza-Manzano G., Navarro-Santana M. Prevalence of Post-COVID-19 Symptoms in Hospitalized and Non-Hospitalized COVID-19 Survivors: A Systematic Review and Meta-Analysis. Eur. J. Intern. Med. 2021;92:55–70. doi: 10.1016/j.ejim.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen C., Haupert S.R., Zimmermann L., Shi X., Fritsche L.G., Mukherjee B. Global Prevalence of Post COVID-19 Condition or Long COVID: A Meta-Analysis and Systematic Review. J. Infect. Dis. 2022;226:1593–1607. doi: 10.1093/infdis/jiac136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yong S.J. Long COVID or Post-COVID-19 Syndrome: Putative Pathophysiology, Risk Factors, and Treatments. Infect. Dis. 2021;53:737–754. doi: 10.1080/23744235.2021.1924397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crook H., Raza S., Nowell J., Young M., Edison P. Long Covid-Mechanisms, Risk Factors, and Management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- 8.Akbarialiabad H., Taghrir M.H., Abdollahi A., Ghahramani N., Kumar M., Paydar S., Razani B., Mwangi J., Asadi-Pooya A.A., Malekmakan L., et al. Long COVID, a Comprehensive Systematic Scoping Review. Infection. 2021;49:1163–1186. doi: 10.1007/s15010-021-01666-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., Cook J.R., Nordvig A.S., Shalev D., Sehrawat T.S., et al. Post-Acute COVID-19 Syndrome. Nat. Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iqbal F.M., Lam K., Sounderajah V., Clarke J.M., Ashrafian H., Darzi A. Characteristics and Predictors of Acute and Chronic Post-COVID Syndrome: A Systematic Review and Meta-Analysis. eClinicalMedicine. 2021;36:100899. doi: 10.1016/j.eclinm.2021.100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maglietta G., Diodati F., Puntoni M., Lazzarelli S., Marcomini B., Patrizi L., Caminiti C. Prognostic Factors for Post-COVID-19 Syndrome: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022;11:1541. doi: 10.3390/jcm11061541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson E.J., Williams D.M., Walker A.J., Mitchell R.E., Niedzwiedz C.L., Yang T.C., Huggins C.F., Kwong A.S.F., Silverwood R.J., Di Gessa G., et al. Long COVID Burden and Risk Factors in 10 UK Longitudinal Studies and Electronic Health Records. Nat. Commun. 2022;13:3528. doi: 10.1038/s41467-022-30836-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Overview|COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19|Guidance|NICE. [(accessed on 17 October 2022)]. Available online: https://www.nice.org.uk/guidance/ng188.
- 14.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riley R.D., Moons K.G.M., Snell K.I.E., Ensor J., Hooft L., Altman D.G., Hayden J., Collins G.S., Debray T.P.A. A Guide to Systematic Review and Meta-Analysis of Prognostic Factor Studies. BMJ. 2019;364:k4597. doi: 10.1136/bmj.k4597. [DOI] [PubMed] [Google Scholar]
- 16.Hayden J.A., van der Windt D.A., Cartwright J.L., Côté P., Bombardier C. Assessing Bias in Studies of Prognostic Factors. Ann. Intern. Med. 2013;158:280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 17.Grooten W.J.A., Tseli E., Äng B.O., Boersma K., Stålnacke B.-M., Gerdle B., Enthoven P. Elaborating on the Assessment of the Risk of Bias in Prognostic Studies in Pain Rehabilitation Using QUIPS-Aspects of Interrater Agreement. Diagn. Progn. Res. 2019;3:5. doi: 10.1186/s41512-019-0050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tosato M., Carfì A., Martis I., Pais C., Ciciarello F., Rota E., Tritto M., Salerno A., Zazzara M.B., Martone A.M., et al. Prevalence and Predictors of Persistence of COVID-19 Symptoms in Older Adults: A Single-Center Study. J. Am. Med. Dir. Assoc. 2021;22:1840–1844. doi: 10.1016/j.jamda.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vanichkachorn G., Newcomb R., Cowl C.T., Murad M.H., Breeher L., Miller S., Trenary M., Neveau D., Higgins S. Post-COVID-19 Syndrome (Long Haul Syndrome): Description of a Multidisciplinary Clinic at Mayo Clinic and Characteristics of the Initial Patient Cohort. Mayo Clin. Proc. 2021;96:1782–1791. doi: 10.1016/j.mayocp.2021.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadat Larijani M., Ashrafian F., Bagheri Amiri F., Banifazl M., Bavand A., Karami A., Asgari Shokooh F., Ramezani A. Characterization of Long COVID-19 Manifestations and Its Associated Factors: A Prospective Cohort Study from Iran. Microb. Pathog. 2022;169:105618. doi: 10.1016/j.micpath.2022.105618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie Y., Bowe B., Al-Aly Z. Burdens of Post-Acute Sequelae of COVID-19 by Severity of Acute Infection, Demographics and Health Status. Nat. Commun. 2021;12:6571. doi: 10.1038/s41467-021-26513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ocsovszky Z., Otohal J., Berényi B., Juhász V., Skoda R., Bokor L., Dohy Z., Szabó L., Nagy G., Becker D., et al. The Associations of Long-COVID Symptoms, Clinical Characteristics and Affective Psychological Constructs in a Non-Hospitalized Cohort. Physiol. Int. 2022;109:230–245. doi: 10.1556/2060.2022.00030. [DOI] [PubMed] [Google Scholar]
- 23.Elhadedy M.A., Marie Y., Halawa A. COVID-19 in Renal Transplant Recipients: Case Series and a Brief Review of Current Evidence. NEF. 2021;145:192–198. doi: 10.1159/000512329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qui L., Zhang J., Huang Y., Cheng G., Chen Z., Ming C., Lu X., Gong N. Long-Term Clinical and Immunological Impact of Severe COVID-19 on a Living Kidney Transplant Recipient—A Case Report. Front. Immunol. 2021;12:3687. doi: 10.3389/fimmu.2021.741765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhoori S., Rossi R.E., Citterio D., Mazzaferro V. COVID-19 in Long-Term Liver Transplant Patients: Preliminary Experience from an Italian Transplant Centre in Lombardy. Lancet Gastroenterol. Hepatol. 2020;5:532–533. doi: 10.1016/S2468-1253(20)30116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buonsenso D., Munblit D., Pazukhina E., Ricchiuto A., Sinatti D., Zona M., De Matteis A., D’Ilario F., Gentili C., Lanni R., et al. Post-COVID Condition in Adults and Children Living in the Same Household in Italy: A Prospective Cohort Study Using the ISARIC Global Follow-Up Protocol. Front. Pediatr. 2022;10:447. doi: 10.3389/fped.2022.834875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yellumahanthi D.K., Barnett B., Barnett S., Yellumahanthi S. COVID-19 Infection: Its Lingering Symptoms in Adults. Cureus. 2022;14:e24736. doi: 10.7759/cureus.24736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., Kang L., Guo L., Liu M., Zhou X., et al. 6-Month Consequences of COVID-19 in Patients Discharged from Hospital: A Cohort Study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sudre C.H., Murray B., Varsavsky T., Graham M.S., Penfold R.S., Bowyer R.C., Pujol J.C., Klaser K., Antonelli M., Canas L.S., et al. Attributes and Predictors of Long COVID. Nat. Med. 2021;27:626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asadi-Pooya A.A., Nemati H., Shahisavandi M., Akbari A., Emami A., Lotfi M., Rostamihosseinkhani M., Barzegar Z., Kabiri M., Zeraatpisheh Z., et al. Long COVID in Children and Adolescents. World J. Pediatr. 2021;17:495–499. doi: 10.1007/s12519-021-00457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taquet M., Dercon Q., Luciano S., Geddes J.R., Husain M., Harrison P.J. Incidence, Co-Occurrence, and Evolution of Long-COVID Features: A 6-Month Retrospective Cohort Study of 273,618 Survivors of COVID-19. PLoS Med. 2021;18:e1003773. doi: 10.1371/journal.pmed.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peghin M., Palese A., Venturini M., De Martino M., Gerussi V., Graziano E., Bontempo G., Marrella F., Tommasini A., Fabris M., et al. Post-COVID-19 Symptoms 6 Months after Acute Infection among Hospitalized and Non-Hospitalized Patients. Clin. Microbiol. Infect. 2021;27:1507–1513. doi: 10.1016/j.cmi.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carvalho-Schneider C., Laurent E., Lemaignen A., Beaufils E., Bourbao-Tournois C., Laribi S., Flament T., Ferreira-Maldent N., Bruyère F., Stefic K., et al. Follow-up of Adults with Noncritical COVID-19 Two Months after Symptom Onset. Clin. Microbiol. Infect. 2021;27:258–263. doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iqbal A., Iqbal K., Arshad Ali S., Azim D., Farid E., Baig M.D., Bin Arif T., Raza M. The COVID-19 Sequelae: A Cross-Sectional Evaluation of Post-Recovery Symptoms and the Need for Rehabilitation of COVID-19 Survivors. Cureus. 2021;13:e13080. doi: 10.7759/cureus.13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tleyjeh I.M., Saddik B., AlSwaidan N., AlAnazi A., Ramakrishnan R.K., Alhazmi D., Aloufi A., AlSumait F., Berbari E., Halwani R. Prevalence and Predictors of Post-Acute COVID-19 Syndrome (PACS) after Hospital Discharge: A Cohort Study with 4 Months Median Follow-Up. PLoS ONE. 2021;16:e0260568. doi: 10.1371/journal.pone.0260568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osmanov I.M., Spiridonova E., Bobkova P., Gamirova A., Shikhaleva A., Andreeva M., Blyuss O., El-Taravi Y., DunnGalvin A., Comberiati P., et al. Risk Factors for Post-COVID-19 Condition in Previously Hospitalised Children Using the ISARIC Global Follow-up Protocol: A Prospective Cohort Study. Eur. Respir. J. 2022;59:2101341. doi: 10.1183/13993003.01341-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Righi E., Mirandola M., Mazzaferri F., Dossi G., Razzaboni E., Zaffagnini A., Ivaldi F., Visentin A., Lambertenghi L., Arena C., et al. Determinants of Persistence of Symptoms and Impact on Physical and Mental Wellbeing in Long COVID: A Prospective Cohort Study. J. Infect. 2022;84:566–572. doi: 10.1016/j.jinf.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Miranda D.A.P., Gomes S.V.C., Filgueiras P.S., Corsini C.A., Almeida N.B.F., Silva R.A., Medeiros M.I.V.A.R.C., Vilela R.V.R., Fernandes G.R., Grenfell R.F.Q. Long COVID-19 Syndrome: A 14-Months Longitudinal Study during the Two First Epidemic Peaks in Southeast Brazil. Trans. R. Soc. Trop. Med. Hyg. 2022;116:1007–1014. doi: 10.1093/trstmh/trac030. [DOI] [PubMed] [Google Scholar]
- 39.Loosen S.H., Jensen B.-E.O., Tanislav C., Luedde T., Roderburg C., Kostev K. Obesity and Lipid Metabolism Disorders Determine the Risk for Development of Long COVID Syndrome: A Cross-Sectional Study from 50,402 COVID-19 Patients. Infection. 2022;50:1165–1170. doi: 10.1007/s15010-022-01784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Messin L., Puyraveau M., Benabdallah Y., Lepiller Q., Gendrin V., Zayet S., Klopfenstein T., Toko L., Pierron A., Royer P.-Y. COVEVOL: Natural Evolution at 6 Months of COVID-19. Viruses. 2021;13:2151. doi: 10.3390/v13112151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim Y., Kim S.-W., Chang H.-H., Kwon K.T., Hwang S., Bae S. One Year Follow-Up of COVID-19 Related Symptoms and Patient Quality of Life: A Prospective Cohort Study. Yonsei Med. J. 2022;63:499–510. doi: 10.3349/ymj.2022.63.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Subramanian A., Nirantharakumar K., Hughes S., Myles P., Williams T., Gokhale K.M., Taverner T., Chandan J.S., Brown K., Simms-Williams N., et al. Symptoms and Risk Factors for Long COVID in Non-Hospitalized Adults. Nat. Med. 2022;28:1706–1714. doi: 10.1038/s41591-022-01909-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Helmsdal G., Hanusson K.D., Kristiansen M.F., Foldbo B.M., Danielsen M.E., Steig B.Á., Gaini S., Strøm M., Weihe P., Petersen M.S. Long COVID in the Long Run—23-Month Follow-up Study of Persistent Symptoms. Open Forum. Infect. Dis. 2022;9:ofac270. doi: 10.1093/ofid/ofac270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Philip K.E.J., Buttery S., Williams P., Vijayakumar B., Tonkin J., Cumella A., Renwick L., Ogden L., Quint J.K., Johnston S.L., et al. Impact of COVID-19 on People with Asthma: A Mixed Methods Analysis from a UK Wide Survey. BMJ Open Respir. Res. 2022;9:e001056. doi: 10.1136/bmjresp-2021-001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sigfrid L., Drake T.M., Pauley E., Jesudason E.C., Olliaro P., Lim W.S., Gillesen A., Berry C., Lowe D.J., McPeake J., et al. Long Covid in Adults Discharged from UK Hospitals after Covid-19: A Prospective, Multicentre Cohort Study Using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg. Health Eur. 2021;8:100186. doi: 10.1016/j.lanepe.2021.100186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Desgranges F., Tadini E., Munting A., Regina J., Filippidis P., Viala B., Karachalias E., Suttels V., Haefliger D., Kampouri E., et al. Post-COVID-19 Syndrome in Outpatients: A Cohort Study. J. Gen. Intern. Med. 2022;37:1943–1952. doi: 10.1007/s11606-021-07242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pelà G., Goldoni M., Solinas E., Cavalli C., Tagliaferri S., Ranzieri S., Frizzelli A., Marchi L., Mori P.A., Majori M., et al. Sex-Related Differences in Long-COVID-19 Syndrome. J. Women’s Health. 2022;31:620–630. doi: 10.1089/jwh.2021.0411. [DOI] [PubMed] [Google Scholar]
- 48.Gebhard C.E., Sütsch C., Bengs S., Todorov A., Deforth M., Buehler K.P., Meisel A., Schuepbach R.A., Zinkernagel A.S., Brugger S.D., et al. Understanding the Impact of Sociocultural Gender on Post-Acute Sequelae of COVID-19: A Bayesian Approach. medRxiv. 2021 doi: 10.1101/2021.06.30.21259757. [DOI] [Google Scholar]
- 49.Tleyjeh I.M., Saddik B., Ramakrishnan R.K., AlSwaidan N., AlAnazi A., Alhazmi D., Aloufi A., AlSumait F., Berbari E.F., Halwani R. Long Term Predictors of Breathlessness, Exercise Intolerance, Chronic Fatigue and Well-Being in Hospitalized Patients with COVID-19: A Cohort Study with 4 Months Median Follow-Up. J. Infect. Public Health. 2022;15:21–28. doi: 10.1016/j.jiph.2021.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.García-Abellán J., Padilla S., Fernández-González M., García J.A., Agulló V., Andreo M., Ruiz S., Galiana A., Gutiérrez F., Masiá M. Antibody Response to SARS-CoV-2 Is Associated with Long-Term Clinical Outcome in Patients with COVID-19: A Longitudinal Study. J. Clin. Immunol. 2021;41:1490–1501. doi: 10.1007/s10875-021-01083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asadi-Pooya A.A., Akbari A., Emami A., Lotfi M., Rostamihosseinkhani M., Nemati H., Barzegar Z., Kabiri M., Zeraatpisheh Z., Farjoud-Kouhanjani M., et al. Risk Factors Associated with Long COVID Syndrome: A Retrospective Study. Iran J. Med. Sci. 2021;46:428–436. doi: 10.30476/ijms.2021.92080.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Munblit D., Bobkova P., Spiridonova E., Shikhaleva A., Gamirova A., Blyuss O., Nekliudov N., Bugaeva P., Andreeva M., DunnGalvin A., et al. Incidence and Risk Factors for Persistent Symptoms in Adults Previously Hospitalized for COVID-19. Clin. Exp. Allergy. 2021;51:1107–1120. doi: 10.1111/cea.13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fernández-de-las-Peñas C., Martín-Guerrero J.D., Pellicer-Valero Ó.J., Navarro-Pardo E., Gómez-Mayordomo V., Cuadrado M.L., Arias-Navalón J.A., Cigarán-Méndez M., Hernández-Barrera V., Arendt-Nielsen L. Female Sex Is a Risk Factor Associated with Long-Term Post-COVID Related-Symptoms but Not with COVID-19 Symptoms: The LONG-COVID-EXP-CM Multicenter Study. JCM. 2022;11:413. doi: 10.3390/jcm11020413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bai F., Tomasoni D., Falcinella C., Barbanotti D., Castoldi R., Mulè G., Augello M., Mondatore D., Allegrini M., Cona A., et al. Female Gender Is Associated with Long COVID Syndrome: A Prospective Cohort Study. Clin. Microbiol. Infect. 2022;28:e9–e611. doi: 10.1016/j.cmi.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chudzik M., Lewek J., Kapusta J., Banach M., Jankowski P., Bielecka-Dabrowa A. Predictors of Long COVID in Patients without Comorbidities: Data from the Polish Long-COVID Cardiovascular (PoLoCOV-CVD) Study. JCM. 2022;11:4980. doi: 10.3390/jcm11174980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chudzik M., Babicki M., Kapusta J., Kałuzińska-Kołat Ż., Kołat D., Jankowski P., Mastalerz-Migas A. Long-COVID Clinical Features and Risk Factors: A Retrospective Analysis of Patients from the STOP-COVID Registry of the PoLoCOV Study. Viruses. 2022;14:1755. doi: 10.3390/v14081755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fernández-de-las-Peñas C., Guijarro C., Torres-Macho J., Velasco-Arribas M., Plaza-Canteli S., Hernández-Barrera V., Arias-Navalón J.A. Diabetes and the Risk of Long-Term Post-COVID Symptoms. Diabetes. 2021;70:2917–2921. doi: 10.2337/db21-0329. [DOI] [PubMed] [Google Scholar]
- 58.Shang L., Wang L., Zhou F., Li J., Liu Y., Yang S. Long-term Effects of Obesity on COVID-19 Patients Discharged from Hospital. Immun. Inflamm. Dis. 2021;9:1678–1685. doi: 10.1002/iid3.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fernández-de-las-Peñas C., Torres-Macho J., Elvira-Martínez C.M., Molina-Trigueros L.J., Sebastián-Viana T., Hernández-Barrera V. Obesity Is Associated with a Greater Number of Long-term Post-COVID Symptoms and Poor Sleep Quality: A Multicentre Case-control Study. Int. J. Clin. Pract. 2021;75:e14917. doi: 10.1111/ijcp.14917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jia X., Cao S., Lee A.S., Manohar M., Sindher S.B., Ahuja N., Artandi M., Blish C.A., Blomkalns A.L., Chang I., et al. Anti-Nucleocapsid Antibody Levels and Pulmonary Comorbid Conditions Are Linked to Post–COVID-19 Syndrome. JCI Insight. 2022;7:e156713. doi: 10.1172/jci.insight.156713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lacavalerie M.R., Pierre-Francois S., Agossou M., Inamo J., Cabie A., Barnay J.L., Neviere R. Obese Patients with Long COVID-19 Display Abnormal Hyperventilatory Response and Impaired Gas Exchange at Peak Exercise. Future Cardiol. 2022;18:577–584. doi: 10.2217/fca-2022-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oto O.A., Ozturk S., Arici M., Velioğlu A., Dursun B., Guller N., Şahin İ., Eser Z.E., Paydaş S., Trabulus S., et al. Middle-Term Outcomes in Renal Transplant Recipients with COVID-19: A National, Multicenter, Controlled Study. Clin. Kidney J. 2022;15:999–1006. doi: 10.1093/ckj/sfac045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Whitaker M., Elliott J., Chadeau-Hyam M., Riley S., Darzi A., Cooke G., Ward H., Elliott P. Persistent COVID-19 Symptoms in a Community Study of 606,434 People in England. Nat. Commun. 2022;13:1957. doi: 10.1038/s41467-022-29521-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Y., Ashcroft T., Chung A., Dighero I., Dozier M., Horne M., McSwiggan E., Shamsuddin A., Nair H. Risk Factors for Poor Outcomes in Hospitalised COVID-19 Patients: A Systematic Review and Meta-Analysis. J. Glob. Health. 2021;11:10001. doi: 10.7189/jogh.11.10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sahu A.K., Mathew R., Aggarwal P., Nayer J., Bhoi S., Satapathy S., Ekka M. Clinical Determinants of Severe COVID-19 Disease—A Systematic Review and Meta-Analysis. J. Glob. Infect. Dis. 2021;13:13–19. doi: 10.4103/jgid.jgid_136_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mauvais-Jarvis F., Bairey Merz N., Barnes P.J., Brinton R.D., Carrero J.-J., DeMeo D.L., De Vries G.J., Epperson C.N., Govindan R., Klein S.L., et al. Sex and Gender: Modifiers of Health, Disease, and Medicine. Lancet. 2020;396:565–582. doi: 10.1016/S0140-6736(20)31561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abumweis S., Alrefai W., Alzoughool F. Association of Obesity with COVID-19 Diseases Severity and Mortality: A Meta-Analysis of Studies. Obes. Med. 2022;33:100431. doi: 10.1016/j.obmed.2022.100431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen Z., Peng Y., Wu X., Pang B., Yang F., Zheng W., Liu C., Zhang J. Comorbidities and Complications of COVID-19 Associated with Disease Severity, Progression, and Mortality in China with Centralized Isolation and Hospitalization: A Systematic Review and Meta-Analysis. Front. Public Health. 2022;10:923485. doi: 10.3389/fpubh.2022.923485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu D., Yuan X., Gao F., Zhao B., Ding L., Huan M., Liu C., Jiang L. High Number and Specific Comorbidities Could Impact the Immune Response in COVID-19 Patients. Front. Immunol. 2022;13:899930. doi: 10.3389/fimmu.2022.899930. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data derived from the study are included in the paper.