Abstract
Background
Oral bisphosphonates are widely used in the treatment of bone resorptive diseases. There is an evidence that oral bisphosphonates can exert adverse effects on the oral mucosa independently of their effects on the jaw bones.
Objective
To systematically map the literature on adverse effects of oral bisphosphonates on the oral mucosa of adults with bone resorptive diseases.
Design
Scoping review of the literature, including different study designs.
Methods
Systematic searches of the PubMed, LILACS, Google Scholar and EMBASE databases were conducted. Two independent reviewers screened titles and abstracts according to predetermined criteria.
Results
The search retrieved 26 unique articles, comprising 22 case reports, one case series and three reviews describing a total of 56 cases of oral adverse events related to oral bisphosphonates. 88% of the reported cases were female suffering from comorbidities other than metabolic bone diseases. The improper use of the oral bisphosphonate was the most suspected cause of the adverse effect on the oral mucosa. Its management mainly involved withdrawal of the medication.
Conclusion
Adverse effects on the oral mucosa can develop from using oral bisphosphonates. Standardised registration of these adverse effects in university clinics and private practises could provide additional information about their occurrence and severity.
Keywords: bisphosphonates, erosive oral lesions, mouth ulcer, mucositis
1. INTRODUCTION
Bisphosphonates are anti‐resorptive agents highly efficient in binding bone hydroxyapatite. They can modulate the bone turnover and increase its mineral density. 1 , 2 Bisphosphonates are widely used to treat postmenopausal and corticosteroid‐induced osteoporosis. 3 The use of bisphosphonates in osteoporosis has been shown to improve bone strength and decreases the risk of fracture. 4 Moreover, bisphosphonates are used in Paget's disease and fibrous dysplasia, 5 to balance metastatic cancer (primarily breast and prostate) deposits in bone, 6 , 7 to cure severe hypercalcemia 8 and to treat the bone resorption defects of multiple myeloma. 8 Some examples of widely used oral bisphosphonates are alendronate, risedronate, etidronate, and minodronate. Although the most common oral formulation for these medications is tablets, a drinkable solution of alendronate and an alendronate oral jelly have been developed the last years. 9 , 10
The therapeutic boundaries of bisphosphonates have been explored for several years and several adverse effects have been reported. 11 , 12 Oral bisphosphonates, mainly used for the treatment of osteoporosis, have been linked to adverse events involving the upper gastrointestinal tract such as dysphagia, nausea, vomiting, dyspepsia, upper abdominal pain and discomfort. 13 , 14 , 15 Moreover, oral bisphosphonates may cause erosive esophagitis, gastritis, duodenitis, delayed healing and haemorrhage due to oesophageal, gastric or peptic ulcers. 14 , 16 , 17 Oral bisphosphonates may also cause musculoskeletal pain, ocular adverse events such as conjunctivitis and uveitis, hypocalcaemia and secondary hyperparathyroidism, atrial fibrillation and atypical fractures of the femoral diaphysis. 11 , 12
As with other anti‐resorptive agents, 18 bisphosphonate can cause medication‐related osteonecrosis of the jaws (MRONJ) is the most well‐known adverse effect in the field of oral and maxillofacial pathology. 19 Bisphosphonates can promote avascular necrosis of bone tissue, especially in the maxilla and mandible. 20 MRONJ appears either as exposed bone or as a non‐healing extraction socket. 2 The intravenous administration of nitrogen‐containing bisphosphonates remains the most common etiologic factor for MRONJ, 19 whereas the risk of MRONJ from oral bisphosphonates appears to increase when the therapy is continued for more than three years. 11
Bisphosphonate toxicity to gastrointestinal cells is well documented. 14 , 16 , 17 Recent in vitro studies provide evidence that high doses of oral bisphosphonates may also have a negative impact on the oral mucosa. 21 , 22 , 23 , 24 Doneti et al (2014) suggested that alendronic acid may have a negative effect on keratinised oral human mucosa of osteoporotic patients without clinical signs of MRONJ, 22 whilst recent experimental studies in animals have demonstrated that alendronic acid can induce an inflammatory process in the buccal and tongue mucosa. 23 , 24 Furthermore, adverse effects on the oral soft tissues have been described in several case reports and are accompanied by pain and great discomfort for the patient, whilst a life‐threatening situation has also been described. 25 Hence, it is essential for the dental practitioner to overcome the diagnostic challenges deriving from the abundance of the various oral mucosal lesions, identify the effects on the oral mucosa and provide an effective treatment. Nevertheless, the existing evidence is limited to scattered case reports and reviews focussed on a specific type of mucosal lesion. 26 , 27 The aim of the scoping review was to systematically map the literature to ascertain the adverse effects of oral bisphosphonates on the oral mucosa.
2. METHODS
2.1. Protocol
This scoping review was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses extension for Scoping Reviews (PRISMA‐ScR) guidelines. 28
2.2. Eligibility criteria
Peer‐reviewed journal articles were included if they involved adult human patients who used oral bisphosphonates as a therapeutic agent and had suffered from an adverse effect reported on their oral mucosa. Clinical trials, cohort studies, case series, case reports and systematic reviews reporting cases were included. Papers were excluded when they reported only MRONJ, mucosal adverse effects related with osteonecrosis of the jaws or did not report any cases. Surveys, comment letters, conference abstracts, ex‐vivo and in‐vitro studies and animal studies were also excluded (Table 1). There were no limitations concerning the language or publication status (accepted, in press, published).
TABLE 1.
Eligibility criteria for studies
| Eligibility criteria | |
|---|---|
| Inclusion | Exclusion |
|
Clinical trials, cohort studies, case series, case reports, reviews reporting cases. Adult patients taking oral bisphosphonates for bone resorptive diseases. Adverse effect of the bisphosphonates on the oral mucosa. |
Studies reporting on MRONJ only. Studies where the mucosal adverse effect is associated with pathology of the jaw structures. Reviews not reporting any original cases, surveys, comment letters, conference abstracts, letters to the editor. In vitro and ex vivo studies. Animal studies. |
2.3. Information sources and search strategy
To identify potentially relevant articles, the following online databases were searched from 1969 to June 2020: PubMed, LILACS, Google Scholar and EMBASE (from 1969 onwards). The search was contacted in a period from March until May 2020. The whole search process was repeated in December 2020.
Additionally, all issues of four dental journals, that were deemed relevant to the topic of this review, were hand‐searched for further potentially relevant publications: Journal of Oral Maxillofacial Surgery, Head and Neck Pathology, Journal of Oral Pathology and Medicine and Gerodontology. Grey literature was searched through Grey Literature report (http://www.greylit.org) and OpenGrey (htttp://www.opengrey.eu). Because of the challenges in finding papers with data on adverse oral effects not related to MRONJ, additional search strategies were also used. The reference list of all the full‐text articles selected after the screening and the list of articles citing these articles were hand‐searched for titles not identified with the previous methods.
2.4. Study selection process
Abstracts were obtained for all the studies identified during the electronic and hand‐searches. In cases where an abstract was not available, full‐text copies were obtained. Two reviewers (CP and ZP) screened titles and abstracts or full‐text copies independently to eliminate articles that clearly failed to meet the eligibility criteria (Table 1). Any disagreements were settled through discussion. Full‐text copies were obtained for all the selected studies. Articles in languages other than English were translated.
2.5. Data charting process and synthesis
Predetermined data (Table 2) were extracted from each study independently by two reviewers (CP and ZP) and arranged into data tables. The data abstraction table was pilot tested, by the two reviewers, during a preliminary extraction of the data from ten articles. Disagreements were solved through discussion between the two reviewers. When an article described multiple cases, only those that met the inclusion criteria were included in the review. Efforts were made to contact the author(s) and obtain additional information in cases when required data were not described in the articles. If this information could not be obtained, the data were coded as “Not reported” (NR). Cases described in more than one studies were identified (predominantly by using the extracted data) and excluded. Cases reported by individuals or organisations other than health care professionals (patients, marketing authorisation holder) were excluded.
TABLE 2.
Predetermined data items extracted from individual studies
|
Patient information and historyGender Age Primary Diagnosis and comorbid conditions Medications and allergies Type of bisphosphonate used and dosage Duration of bisphosphonate therapy History of current adverse event Major symptoms described from the patient Onset, location, duration of the symptoms Previous history of mucosal adverse events (before treatment with bisphosphonate) Clinical findings Health screening assessment (blood pressure, body temperature) Extraoral examination Intraoral examination Inspection and documentation of the mucosal adverse event (location, distribution and definition, size, shape, colour, consistency, texture) Nikolsky's sign examination Adjunctive visual tools (such as toluidine blue, fluorescence visualisation) Coexistence on other mucosae Differential Diagnosis Diagnostic tests Haematological and serological assays Saliva analysis Histopathological examination Suggested management Medical intervention/Drug holiday/Alteration of the medication Time until resolution of symptoms/Healing period |
2.6. Critical appraisal
No quality assessment of the included articles took place, which was in accordance with available guidelines on scoping review. 29
3. RESULTS
The electronic search yielded 104 potential articles. No relevant articles were recovered from the grey literature sources. After removing duplicates 60 unique articles were identified. Title and abstract screening resulted in the exclusion of 16 articles, so every possible effort was made to retrieve 44 articles in full text. Unfortunately, that was not possible for two studies. 30 , 31 After reading the full text, another 16 articles were excluded. Finally, 26 studies were included in this scoping review 26 , 27 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 (Figure 1).
FIGURE 1.
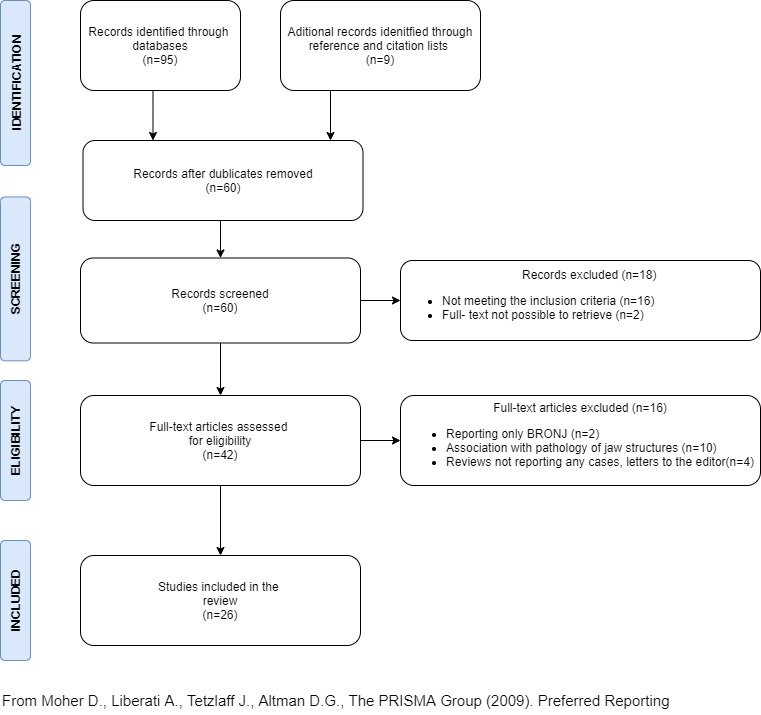
Flow chart of the literature search and the selection process
The PRISMA flow chart was adapted for use in this scoping review. The list of the excluded full‐text articles is presented as supplementary file in Table S1.
The publication years ranged from 1999 to 2020. Seventeen articles were written in English, three in French, 34 , 36 , 38 three in Japanese, 46 , 56 , 58 two in Spanish, 39 , 57 one in Turkish 40 and one in Dutch. 45 Two articles written from the same author 34 , 36 was describing the same patient and were included as one case. In one article, 27 two cases were reported by non‐health care professionals and were, therefore, excluded from this study, the included articles were twenty‐two case reports, one case series 55 and three reviews 26 , 27 , 48 describing in total 56 cases.
3.1. Mapping of the evidence
Most of the cases were female (n = 49). The age of the patients ranged from 48 to 96 years (median age 72 years, based on 53 patients). In three cases, the age of the patient was not mentioned. 27 , 51 Ten cases involved patients who were residents of a nursing facility or were receiving 24‐hour help at home. 43 , 44 , 50 , 54 , 55 , 56 , 59
The major indication for prescribing bisphosphonates was osteoporosis (51/56). In one case, the bisphosphonate was administrated because of osteopenia, a preosteoporotic condition. 50 Osteoarthritis was mentioned in one case. 55 In another case, the bisphosphonate was administered to reduce the risk of osteoporosis secondary to polymyalgia rheumatica. 55 In one case alendronate was prescribed after a knee replacement surgery. 41 In one case alendronate was administered as a treatment to vertebral fracture. 42
The chief complaint of the patient was reported in 37 cases and mostly involved pain (24 cases) and difficulty in eating (13 cases). Almost half of the cases (25/56) involved patients who were suffering from diseases other than the metabolic bone diseases. In 22 cases, the medical history is not mentioned.
Dementia (11/56), 45 , 50 , 52 , 54 , 55 , 56 , 58 , 59 Parkinson's disease 38 , 44 (2/56) and cerebrovascular incidents 44 , 46 , 56 (3/56) was reported in individual cases. One case reported a patient with Down Syndrome, 41 whilst another case involved a legally blind patient suffering from schizophrenia. 43 One case involved a patient with congenital deafness 52 and another case was a non‐verbal patient with learning difficulties. 59 Cerebral palsy, 43 hemiplegia, 43 severe postural instability 52 and a thoracic compression fracture 42 , 56 were described in five individual cases.
Polypharmacy (the concurrent use of five or more medications) was reported in eleven cases 27 , 43 , 44 , 45 , 46 , 52 , 55 , 56 (Table S3).
A previous history of adverse effects on the oral mucosa is not reported in any of the cases.
Nine patients were using a complete or partial denture. 33 , 35 , 37 , 39 , 44 , 50 , 56 , 58 One case was edentulous. 56 Dental status could not be verified for 46 cases.
Most of the cases involved patients who used oral bisphosphonates in the form of tablets, except for one case where the alendronate tablets had been recently replaced by a drinkable solution of alendronate. 59
Forty‐six patients used only alendronate (46/56), and one patient used alendronate in combination with etidronate and calcium. 43 Three patients used etidronate with calcium, 27 and two patients used only etidronate. 48 Two patients used minodronate, 54 , 56 and two patients used risedronate. 27 , 48
Of the 47 patients using alendronate, 21 patients were taking the medication on a weekly basis (44,7%), 16 patients were taking it daily (34%), whereas the dosage of alendronate was not specified for 10 cases. Etidronate was administrated daily in four cases, 27 , 48 whilst in one case there was no information available concerning the dosage of etidronate. 48 Minodronate was administered monthly. 54 , 56 Risedronate was administered in one case weekly 27 and in the other case daily. 48
The duration of the bisphosphonate administration was specified for twenty‐nine patients (51,8%) and ranged from 4 days 32 to 10 years. 58 Twelve patients used the bisphosphonate for one month or less; 11 patients had been using the medication for less than a year, and six patients had used the medication for a year or more.
Incorrect use of the bisphosphonate had been identified as the cause of the mucosal adverse effects in 30 of the cases (53,6%). In these cases, the bisphosphonate tablet was sucked, chewed, or allowed to dissolve on the oral mucosa before swallowing. In 10 cases incorrect use was attributed to physical or mental disability, whilst in other cases incorrect use was related with difficulty to swallow tablets (8/30)), distraction 32 , 42 (2/30), habit 33 , 35 (2/30), wrong administration from the care giver 43 , 59 (2/30), or wrong instruction given by the general practitioner 53 (1/30). The difficulty to swallow tablets was related in two cases to cerebral palsy, 43 or previous surgery for the removal of a thyroid tumour. 47 No explanation for the improper use was proposed for six cases.
The diagnostic process was reported for 34 cases. Intraoral and extraoral examination took place in all these cases. A physical examination was performed in 14 cases, whilst in 13 cases, other mucosae were also inspected. Adjunctive visual tools were used in eight cases, whilst Nikolsky's sign was used in three other cases. Histological examination of the mucosal lesions was used for 11 cases. Microbiological tests were used for 22 cases, whilst cytodiagnostic tests were performed in 10 cases. Finally, a CT scan was obtained in one case. A summary of the studies reporting a diagnostic process is presented in Table S2. In all of these cases, the diagnostic process was complex, involving a variety of the described tools.
The mucosal adverse effects were documented with respect to their location, size, shape and colour. Severe ulcerations of the oral mucosa, also described with the terms stomatitis and mucositis, were reported in most of the cases (80,3%). Erythema multiform minor 52 and oral lichenoid reaction 49 were described in two individual cases. Blisters in and around the mouth 48 (4/56), swelling 48 (2/56), vesicles 48 in the mouth (1/56) and petechiae 48 (1/56) were also reported. In one case, the term mucosal alteration 48 was used to describe the adverse effect on the oral mucosa, but no further explanation was provided (1/56). The most frequent location was the tongue and the lower lip, followed by the palate and the buccal mucosa.
The period from the onset of the mucosal adverse effect up to the day that the patient was reported by the health professional ranged from one day 32 , 48 to three years, 55 whilst it remains unknown for 16 cases. Twenty patients had suffered from the mucosal adverse effects for more than a month (20/40). In five of these cases, the patients had suffered from the adverse effects for more than a year 35 , 39 , 50 , 55 , 57 (Table S4).
The differential diagnosis was reported for 34 cases and included other mucosal disorders, traumatic ulcerations and lesions caused by bacterial or viral infections, aphthous, thyroid abnormalities, syphilis, blood deficiencies, immunobullous diseases (so as pemphigus and pemphigoid), oral lichen planus, erosive oral candidiasis, auto‐immune diseases and malignancy.
In most of the cases the management of the adverse effect included withdrawal of the oral bisphosphonate (85,7%); in three cases, this was followed by the administration of an alternative form of bisphosphonate. 27 , 56 , 59 Adjunctive regimens included the local application of corticosteroid 33 , 40 , 59 (4/48), or bicarbonate crème 40 (1/48) and the use of benzydamine oromucosal spray and chlorhexidine mouthwash 59 (1/48). In two cases, the dose of the bisphosphonate was reduced. 27 In one case, there is no change reported concerning the bisphosphonate. 27 In the rest of the cases, the administration of the bisphosphonate was corrected, and the patients were monitored ever since whilst taking the medication. The healing period ranged from 7 days 32 , 34 to 13 months. 44 The period required to achieve complete healing remains unknown for nine cases.
Previous unsuccessful treatment with analgesic and antiviral agents, topical and systemic corticosteroids, hyaluronic acid, topical and oral anti‐mycotic agents, antibiotic agents and chlorhexidine (without modification of the oral bisphosphonate administration) was reported for 12 cases.
4. DISCUSSION
The purpose of this scoping review was to collate the available information on the adverse effects for the oral mucosa by adults using oral bisphosphonates. The nature and the rare occurrence of these adverse effects preclude observational prospective or retrospective studies. As a result, the available evidence is limited to the uncontrolled information presented in case reports. Case reports are a low level of evidence, and they are prone to various types of bias. 60 Another limitation of case reports is that they cannot ascertain the cause of the adverse effects on the oral mucosa. The low prevalence of these adverse effects is the main reason why cases described in pharmaceutical databases 27 , 48 were included in our study despite their limited documentation.
The pathophysiology underlying the adverse effects on the oral mucosa remains unknown at the present time. Alendronic acid has been shown to initiate inhibition of oral keratinocyte proliferation, alteration of the progress of epithelial terminal differentiation and changes in molecular composition and desmosomal morphology in the human oral mucosa of osteoporotic women without clinical signs of medication‐related osteonecrosis of the jaws. 22 There is also evidence that alendronic acid has a cytotoxic effect on direct contact with periodontal ligament fibroblasts. 61 According to another study bisphosphonates have a strong negative effect on human oral keratinocytes by reducing cell viability, migration ability and accelerating the apoptosis rate of the oral mucosa. 21 In a recent in vitro study has been suggested that the decrease in the number of hemidesmosomes noticed after administration of alendronic acid could indicate the formation of ulcerous lesions in murine buccal mucosa. 23 Consequently, bisphosphonates may interfere in regenerative, apoptotic and ultrastructural cellular processes, leading to the development of mucosal adverse effects.
The majority of the patients in this study were older females, reflecting the higher prevalence of osteoporosis in older women. Two‐thirds of the patients (66%) expressed pain and great discomfort in eating. These symptoms are of great significance for health care professionals, especially when treating older adults. In ageing population, dysphagia (swallowing difficulty), is a growing health concern, contributing to a variety of negative health status changes; such as, increased risk of malnutrition, dehydration, weight loss and aspiration pneumonia. 62 Obviously, initiating a communication between the health care professionals can higher the awareness for the mucosal adverse effects and attribute to their early identification and management.
With respect to the type of oral bisphosphonate, alendronate was used in 84% of the cases, which reflects the wide use of this medication in patients with osteoporosis. Amongst the mucosal adverse effects of interest in this review, the most common one (80%) was oral ulceration. Erythema multiforme minor was also described in one case. 52 Nevertheless, we made no attempt to differentiate the erythema multiforme minor from oral ulcerations as a toxic reaction to the oral bisphosphonate. A lichenoid reaction following treatment with oral bisphosphonates was also described in one case. 49 A case of lichenoid dermatosis initialised by alendronate has been reported in the past. 63 In 16% of the cases, the diagnosis remains descriptive and vague. The limited awareness of these oral adverse effects and the diversity of the clinicians’ background, (dental surgeons, dermatologists), explain the diagnostic difficulties and justify the wide variations in the diagnostic process.
The incorrect administration of the bisphosphonates seems to be associated with the adverse effects on the oral mucosa (53,6%). This highlights the crucial role of proper administration in preventing the adverse effects. Currently, it is recommended that the bisphosphonate tablet is swallowed immediately along with plenty of water and the patient remains upright for 30 minutes afterwards. 64 However, this protocol requires that the patient has the cognitive ability to understand the recommended administration method, the physical ability to maintain a sitting position and a sufficient swallowing function. These requirements may not have been met in several of the cases included in this review, according to the reported medical histories.
Twenty‐three per cent of the patients suffered from mental disorders, such as dementia, Down syndrome or schizophrenia. An additional three per cent were coping with sensory impairments (congenital deafness, non‐verbal patient). Thus, just over one quarter of the patients experienced difficulties with medicine‐related tasks. Moreover, people with impaired cognitive ability are less able to indicate pain 65 so it is likely that adverse effects resulting from improper administration of oral bisphosphonates remained undiagnosed in some of these cases. Further, polypharmacy, which is associated with a greater likelihood of medication errors 66 was reported in 19 per cent of the patients.
Twelve per cent of the patients might have experienced difficulty to maintain an upright position, due to Parkinson's disease, cerebral palsy, hemiplegia, postural instability or compression fracture of the vertebra, whilst five per cent suffered from comorbidities (cerebrovascular incidents) that may impair the postural balance. Finally, twenty‐five per cent of the patients experienced difficulty in swallowing, which is a common problem in older adults and may be attributed to neurologic disorders, such as transient ischaemic attacks and strokes, and neurodegenerative diseases, such as Parkinson's disease. 67 All of these conditions are associated with a greater likelihood of dosing errors and the subsequent complications, so a detailed medical history prior to the prescription of oral bisphosphonates is indispensable. It is noteworthy that the current European guidelines for the treatment of osteoporosis 68 do not consider possible cognitive, postural, or swallowing impairment of the patient. Because incorrect administration can cause adverse effects on the oral mucosa, clear instructions on how to take oral bisphosphonates should be provided to all bisphosphonate users and, when applicable, their caregivers. When the administration of bisphosphonate tablets is contra‐indicated other types of bisphosphonate formulation can be considered, such as intravenous bisphosphonates and denosumab. 68 Intravenous bisphosphonates in the treatment of osteoporosis have a proven efficacy, a better bioavailability than oral bisphosphonates and a good safety profile. Moreover, the long dosing intervals improve patient convenience and help to overcome adherence to drug therapy issues, 69 whilst the risk of developing MRONJ in osteoporotic patients after exposure to intravenous bisphosphonates or denosumab remains very low. 18 With regard to denosumab, there are no published clinical data suggesting that this medication can cause adverse effects on the oral mucosa that do not relate to MRONJ. In addition, a drinkable form of alendronate could also be used. 15 Furthermore, an alendronate jelly administered once per week per os may be an effective alternative to tablets for older adults with swallowing difficulties. 10 Some limitations of this review are apparent, with regard to the use of the alendronate oral drinkable solution and the alendronate sodium hydrate (oral jelly). Firstly, there are no published clinical data concerning the occurrence of adverse effects on the oral mucosa. Secondly, alendronate sodium hydrate is approved and used only in Japan. 10 Regarding intravenous bisphosphonates and denosumab, there is limited data comparing their use with oral bisphosphonates in postmenopausal women with osteoporosis. These comparative studies include a small number of patients and have a relatively short term follow‐up. 69 , 70 , 71 As mentioned in the summary of the European guidance for the diagnosis and management for osteoporosis in postmenopausal women, oral bisphosphonates, when tolerated, remain the initial pharmacological intervention in the majority of the cases. 68
Oral mucosal lesions can develop as a result of a local factor as trauma or mechanical irritation or malignancy. They can be caused by a systematic disease or an auto‐immune disorder, a viral, bacterial or mycotic infection, a toxic reaction on medication or a vitamin deficiency. Hence, identifying an oral mucosal lesion can be a challenging process. The diagnosis should be based on a thorough investigation of the patient including extraoral, intraoral and mucosal lesion assessments combined with a comprehensive medical history. Identifying the side‐effects of the used medication can often provide valuable information. Additionally, diagnostic tests, including histological and microbiological samples, are necessary to establish a proper diagnosis.
An important limitation of the current review was the incomplete reporting of the cases in the included articles. Missing information about the medical history (39%), the exact number and type of medications taken (34%), the dental history (82%), the dosage of the bisphosphonates (19%), the administration period (48%), the onset and duration of the adverse effects (28,5%) and the healing period (16%) hindered mapping of the evidence. Evidently, incomplete reporting would be an obstacle for any systematic review on this topic. Hence, future case reports should adhere to more strict reporting standards and provide a more detailed description of the cases, since other types of clinical studies are not feasible. Standardised registration of oral mucosal adverse effects in university clinics and private practises could also provide additional information on these adverse effects.
Bisphosphonates are highly effective for the prevention of subsequent fractures in patients with osteoporosis or osteoporosis related fragility fractures. After discovering bisphosphonates in the nineties, 31 million prescriptions were dispensed in the United States only in 2008. Although the number of prescriptions is declining every year due to adverse effects, in 2012 still over 15 million prescriptions were dispensed. 72 From that perspective, the oral mucosa problems are very rare, but caution is strongly advised for the individual patient who suffers from comorbidities that can impair the correct administration of the medication.
5. CONCLUSION
Adverse effects on the oral mucosa can arise from using oral bisphosphonates. The most common adverse effect is oral ulceration. Half of the cases were related to improper administration of the medication. Health care professionals and oral health professionals in particular, should consider the possibility of adverse effects in older adults that are prescribed oral bisphosphonates, especially when complications in administration can be expected due to mental or physical difficulties. Standardised registration of these adverse effects in university clinics and private practises, could provide additional information about their prevalence and severity.
CONFLICT OF INTEREST
There are no conflicts of interests in connection with this article.
AUTHOR CONTRIBUTIONS
CP conceived and designed the study. CP and ZP conducted the study with the support of HCW, WJK and CDvdMW. HCW offered her expertise in geriatrics and osteoporosis. HCW, WJK and CDvdMW provided critical feedback and CP wrote the manuscript with input of all the authors. CDvdMW had the final planning and supervision of the study. All authors read and approved the final manuscript.
Supporting information
Table S1
Table S2
Table S3
Table S4
Psimma C, Psimma Z, Willems HC, Klüter WJ, van der Maarel‐Wierink CD. Oral bisphosphonates: Adverse effects on the oral mucosa not related to the jaw bones. A scoping review. Gerodontology. 2022;39:330–338. 10.1111/ger.12590
REFERENCES
- 1. De Ponte FS. Bisphosphonates and osteonecrosis of the jaw: a multidisciplinary approach. Springer; 2012:13‐21. [Google Scholar]
- 2. Yuan A, Woo SB. Adverse drug events in the oral cavity. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119(1):35‐47. 10.1016/j.oooo.2014.09.009 [DOI] [PubMed] [Google Scholar]
- 3. Bagger YZ, Tankó LB, Alexandersen P, Ravn P, Christiansen C. Alendronate has a residual effect on bone mass in postmenopausal Danish women up to 7 years after treatment withdrawal. Bone. 2003;33(3):301‐307. 10.1016/s8756-3282(03)00112-1 [DOI] [PubMed] [Google Scholar]
- 4. Fatoye F, Smith P, Gebrye T, Yeowell G. Real‐world persistence and adherence with oral bisphosphonates for osteoporosis: a systematic review. BMJ Open. 2019;9(4):e027049. 10.1136/bmjopen-2018-027049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kos M, Brusco D, Kuebler J, Engelke W. Clinical comparison of patients with osteonecrosis of the jaws, with and without a history of bisphosphonates administration. Int J Oral Maxillofac Surg. 2010;39(11):1097‐1102. 10.1016/j.ijom.2010.04.054 [DOI] [PubMed] [Google Scholar]
- 6. Marx RE, Sawatari Y, Fortin M, Broumand V. Bisphosphonate‐induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg. 2005;63(11):1567‐1575. 10.1016/j.joms.2005.07.010 [DOI] [PubMed] [Google Scholar]
- 7. Ruggiero SL. Bisphosphonate‐related osteonecrosis of the jaw (BRONJ): initial discovery and subsequent development. J Oral Maxillofac Surg. 2009;67(5 Suppl):13‐18. 10.1016/j.joms.2008.10.005 [DOI] [PubMed] [Google Scholar]
- 8. Ponte Fernández N, Estefania Fresco R, Aguirre Urizar JM. Bisphosphonates and oral pathology I. General and preventive aspects. Med Oral Patol Oral Cir Bucal. 2006;11(5):E396‐E400. [PubMed] [Google Scholar]
- 9. Giampà E, Di Bonito M, Ferretti V, et al. Effects of alendronate and calcifediol compared to alendronate and cholecalciferol in osteoporotic patients. Minerva Endocrinol. 2019;44(4):344‐350. 10.23736/S0391-1977.19.03052-9 [DOI] [PubMed] [Google Scholar]
- 10. Imai K. Alendronate sodium hydrate (oral jelly) for the treatment of osteoporosis: Review of a novel, easy to swallow formulation. Clin Interv Aging. 2013;8:681‐688. 10.2147/CIA.S37199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Papapetrou PD. Bisphosphonate‐associated adverse events. Hormones (Athens). 2009;8(2):96‐110. 10.14310/horm.2002.1226 [DOI] [PubMed] [Google Scholar]
- 12. Pazianas M, Abrahamsen B. Safety of bisphosphonates. Bone. 2011;49(1):103‐110. 10.1016/j.bone.2011.01.003 [DOI] [PubMed] [Google Scholar]
- 13. de Groen PC, Lubbe DF, Hirsch LJ, et al. Esophagitis associated with the use of alendronate. N Engl J Med. 1996;335(14):1016‐1021. 10.1056/NEJM199610033351403 [DOI] [PubMed] [Google Scholar]
- 14. Parfitt JR, Driman DK. Pathological effects of drugs on the gastrointestinal tract: a review. Hum Pathol. 2007;38(4):527‐536. 10.1016/j.humpath.2007.01.014 [DOI] [PubMed] [Google Scholar]
- 15. Brandi ML, Black D. A drinkable formulation of alendronate: Potential to increase compliance and decrease upper gi irritation. Clin Cases Miner Bone Metab. 2013;10(3):187‐190. [PMC free article] [PubMed] [Google Scholar]
- 16. Peng YL, Hu HY, Luo JC, Huo MC, Lin HC, Lee FY. Alendronate, a bisphosphonate, increased upper and lower gastrointestinal bleeding: risk factor analysis from a nationwide population‐based study. Osteoporos Int. 2014;25(5):1617‐1623. 10.1007/s00198-014-2647-z [DOI] [PubMed] [Google Scholar]
- 17. Modi A, Fan CS, Tang J, Weaver JP, Sajjan S. Association of gastrointestinal events with osteoporosis treatment initiation and treatment compliance in Germany: An observational study. Bone Rep. 2016;5:208‐213. 10.1016/j.bonr.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ruggiero SL, Dodson TB, Fantasia J, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication‐related osteonecrosis of the jaw‐‐2014 update. J Oral Maxillofac Surg. 2014;72(10):1938‐1956. [published correction appears in J Oral Maxillofac Surg. 2015 Jul;73(7):1440] [published correction appears in J Oral Maxillofac Surg. 2015 Sep;73(9):1879]. doi: 10.1016/j.joms.2014.04.031 [DOI] [PubMed] [Google Scholar]
- 19. Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: A review of 63 cases. J Oral Maxillofac Surg. 2004;62(5):527‐534. 10.1016/j.joms.2004.02.004 [DOI] [PubMed] [Google Scholar]
- 20. Bixofis RB, Sassi LM, Patussi C, da Silva WPP, Zanicotti RTS, Schuss JL. Implications of the use of bisphosphonates in dental treatment – Experience of the service of oral and maxillofacial surgery, RSBO. 2013;10(4):335‐342 [Google Scholar]
- 21. Pabst AM, Ziebart T, Koch FP, Taylor KY, Al‐Nawas B, Walter C. The influence of bisphosphonates on viability, migration, and apoptosis of human oral keratinocytes‐in vitro study. Clin Oral Investig. 2012;16(1):87‐93. 10.1007/s00784-010-0507-6 [DOI] [PubMed] [Google Scholar]
- 22. Donetti E, Gualerzi A, Sardella A, Lodi G, Carrassi A, Sforza C. Alendronate impairs epithelial adhesion, differentiation and proliferation in human oral mucosa. Oral Dis. 2014;20(5):466‐472. 10.1111/odi.12154 [DOI] [PubMed] [Google Scholar]
- 23. Papamitsou T, Fotiadou S, Papachristodoulou A, et al. Effect of alendronic acid on buccal mucosa. J. Dent. Oral Health. 2018;4(3):1‐6. [Google Scholar]
- 24. Papamitsou T, Morsi‐Yeroyannis A, Papanastasiou A, et al. Bisphosphonate’s effect on tongue mucosa: An experimental electron microscopy study. Med. 2020;56(2):1‐9. 10.3390/medicina56020051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Casiano V, Kalish VB, Unwin B. An unusual adverse event from a common medication in an individual with dementia. J Am Geriatr Soc. 2014;62(11):2223‐2224. 10.1111/jgs.13078 [DOI] [PubMed] [Google Scholar]
- 26. Kharazmi M, Sjöqvist K, Warfvinge G. Oral ulcers, a little known adverse effect of alendronate: Review of the literature. J Oral Maxillofac Surg. 2012;70(4):830‐836. 10.1016/j.joms.2011.03.046 [DOI] [PubMed] [Google Scholar]
- 27. Landelijke registratie evaluatie bijwerkingen (LAREB) . Bisphosphonates and stomatitis. https://databankws.lareb.nl/Downloads/kwb_2009_2_bisph.pdf
- 28. Tricco AC, Lillie E, Zarin W, et al. PRISMA Extension for Scoping Reviews (PRISMA‐ScR): Checklist and Explanation. Ann Intern Med. 2018;169(7):467‐473. 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 29. Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13(3):141‐146. 10.1097/XEB.0000000000000050 [DOI] [PubMed] [Google Scholar]
- 30. Chevallier JM. Oral ulcerations induced by monosodium alendronate. "Ulcérations buccales à l'alendronate monosodique". Nouvelles Dermatologiques. 2003;22;(9):612. [Google Scholar]
- 31. Spreux A, Amples M, Bolla G, Baldin B. Ulceration muqueuses topographique atypiques avec l'alendronate. Therapie. 1998;53:182‐192. [Google Scholar]
- 32. Demerjian N, Bolla G, Spreux A. Severe oral ulcerations induced by alendronate. Clin Rheumatol. 1999;18(4):349‐350. 10.1007/s100670050116 [DOI] [PubMed] [Google Scholar]
- 33. Gonzalez‐Moles MA, Bagan‐Sebastian JV. Alendronate‐related oral mucosa ulcerations. J Oral Pathol Med. 2000;29(10):514‐518. 10.1034/j.1600-0714.2000.291006.x [DOI] [PubMed] [Google Scholar]
- 34. Schmutz J, Barbaud A, Tréchot P. Alendronate monosodique (Fosamax®). (Esophagites et ulcerations orales). Ann Dermatol Venereol. 2000;127:1138. [PubMed] [Google Scholar]
- 35. Krasagakis K, Krüger‐Krasagakis S, Ioannidou D, Tosca A. Chronic erosive and ulcerative oral lesions caused by incorrect administration of alendronate [7]. J Am Acad Dermatol. 2004;50(4):651‐652. 10.1016/j.jaad.2003.07.030 [DOI] [PubMed] [Google Scholar]
- 36. Schmutz J‐L, Barbaud A, Tréchot P. Alendronate et ulcérations buccales: d’abord bien interroger le patient. Ann Dermatol Venereol. 2005;132(11):930. 10.1016/s0151-9638(05)79521-1 [DOI] [PubMed] [Google Scholar]
- 37. Rubegni P, Fimiani M. Bisphosphonate‐Associated Contact Stomatitis. N Engl J Med. 2006;355(22):e25. 10.1056/nejmicm054413 [DOI] [PubMed] [Google Scholar]
- 38. Dicko A, Toulemonde A, Hoareau F, et al. Ulcérations buccales. Ann Dermatol Venereol. 2007;134:389‐390. [DOI] [PubMed] [Google Scholar]
- 39. Cardona Tortajada F, Sainz Gómez E, Figuerido Garmendia J, de Robles L, Adsuar A. Ulcera oral crónica por ingestión de alendronato [Chronic mouth ulcer due to inadequate ingestion of alendronate]. Aten Primaria. 2008;40(8):430‐431. 10.1157/13125415140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cinemre H, Bilir C, Gökosmanoğlu F, Aytuğ NÖ. Oral Ulcers Caused by Alendronate use. Rheumatism. 2008;23:35‐37. [Google Scholar]
- 41. Aleid W, Sidebottom A. Oral mucosal irritation with incorrect use of alendronate. Br J Oral Maxillofac Surg. 2009;47(2):170‐171. 10.1016/j.bjoms.2008.08.005 [DOI] [PubMed] [Google Scholar]
- 42. García‐Font M, Curcó N, Prat C, Vives P. Mouth sores caused by alendronate. Actas Dermo‐Sifiliográficas. 2009;100(1):77‐78. 10.1016/s1578-2190(09)70016-2 [DOI] [PubMed] [Google Scholar]
- 43. Ng S, Chow L, Zed C. Oral erosive mucositis associated with improper administration of a drug. J can Dent Assoc. 2010;76:a156. [PubMed] [Google Scholar]
- 44. Kharazmi M, Sjöqvist K, Rizk M, Warfvinge G. Oral ulcer associated with alendronate: A case report. Oral Surgery, Oral Med Oral Pathol Oral Radiol Endodontology. 2010;110(6):1997‐1999. 10.1016/j.tripleo.2010.04.035 [DOI] [PubMed] [Google Scholar]
- 45. Dik EA, van Es RJ, Bergsma JE. A toxic reaction of the oral mucosa to alendronate (Fosamax). Ned Tijdschr Tandheelkd. 2010;117(7–8):387‐390. 10.5177/ntvt.2010.08.09189 [DOI] [PubMed] [Google Scholar]
- 46. Jinbu Y, Ioka Y, Kawashima R, Kato R, Kusama M. Oral ulcerations induced by sucking the tablets of bisphosphonate (alendronate) in the oral cavity. J Jpn Oral Muco Membr. 2010;16:24‐27. [Google Scholar]
- 47. Inui M, Asakura N, Nakamura S, Okumura K, Takeoka T, Tagawa T. Chemical burn of the tongue resulting from improper use of oral bisphosphonate. Asian J Oral Maxillofac Surg. 2011;23(4):204‐206. 10.1016/j.ajoms.2011.06.001 [DOI] [Google Scholar]
- 48. Kharazmi M, Persson U, Warfvinge G. Pharmacovigilance of oral bisphosphonates: Adverse effects manifesting in the soft tissue of the oral cavity. J Oral Maxillofac Surg. 2012;70(12):2793‐2797. 10.1016/j.joms.2012.01.025 [DOI] [PubMed] [Google Scholar]
- 49. Vučićević‐Boras V, Brailo V, Vidovic Juras D, Pandurić DG. An Alendronate‐Induced Oral Lichenoid Reaction. J Dent Med Sci. 2014;4(2):18‐21. http://www.interesjournals.org/full‐articles/an‐alendronate‐induced‐oral‐lichenoid‐reaction.pdf?view=inline%5Cnhttp://bib.irb.hr/prikazi‐rad?lang=en&rad=707962 [Google Scholar]
- 50. Lengfeld J, Buder‐Bakhaya K, Goebeler M, Wobser M. Bisphosphonate‐mediated oral ulcers: A rare differential diagnosis of erosive oral lesions. Dermatology. 2016;232(1):117‐121. 10.1159/000439347 [DOI] [PubMed] [Google Scholar]
- 51. Stanimirov P, Petkova M. Bisphosphonate ‐ Related Mucositis (Brm): a Case Report. J IMAB ‐ Annu Proceeding (Scientific Papers). 2017;23(1):1487‐1489. 10.5272/jimab.2017231.1487 [DOI] [Google Scholar]
- 52. de Arruda JA, Silva P, Amaral MB, Cotta F, Avendanho R, Mesquita R. Erythema multiforme induced by alendronate sodium in a geriatric patient: A case report and review of the literature. J Clin Exp Dent. 2017;9(7):e929‐e933. 10.4317/jced.53653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mascitti M, Santarelli A. Tongue, “heart” ulcer from bisphosphonate. J Dent Sci. 2018;13(3):182‐183. 10.1016/j.jds.2018.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Eguchi T, Basugi A, Kanai I, Miyata Y, Hamada Y. Multiple oral ulcers caused by incorrect use of oral bisphosphonate in a patient with dementia: A case report. Gerodontology. 2019;36(1):82‐84. 10.1111/ger.12378 [DOI] [PubMed] [Google Scholar]
- 55. Finn D, Field A, Rajlawat B, Randall C. Oral mucosal ulceration induced by alendronic acid: A case series. Dent Update. 2018;45(1):38‐42. 10.12968/denu.2018.45.1.38 [DOI] [Google Scholar]
- 56. Nitta T, Sakamoto R, Hirahara N, Matsumura Y, Nakamura K, Goto Y. Oral ulcers suspected to be caused by improper administration of bisphosphonate. Japanese Journal of Oral and Maxillofacial Surgery. 2019;65(9):599‐604. [Google Scholar]
- 57. Riera‐Monroig J, Riquelme‐Mc Loughlin C, Iranzo P. Úlceras y erosiones orales simulando penfigoide de mucosas: la importancia de asegurar la toma adecuada de alendronato [An uncommon cause of oral ulcers: Inappropriate intake of altendronate]. Aten Primaria. 2020;52(8):570‐571. 10.1016/j.aprim.2019.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ueda N, Yamamoto S, Takashima M, Takahashi Y, Kurokawa S, Kirita T. A case of bisphosphonate‐related oral mucosa ulcerations. Japanese Journal of Oral and Maxillofacial Surgery. 2020;66(1):25‐30. [Google Scholar]
- 59. McLean S, Kelly R, Rajlawat B, Field A. Alendronic acid‐induced oral mucosal ulceration: two case reports. Oral Surg. 2021;14(3):255‐258. 10.1111/ors.12540 [DOI] [Google Scholar]
- 60. Levine M, Ioannidis JPA, Haines AT, Guyatt G. Harm (observational studies). In: Guyatt G, Rennie D, Meade MO, Cook DJ, eds. Users’ guide to the medical literature. A manual for evidence‐based clinical practice, 3rd ed. McGrow‐Hill Education; 2015:301‐314. [Google Scholar]
- 61. Correia V, Caldeira C, Marques M. Cytotoxicity of sodium alendronate on cultured human periodontal ligament fibroblasts. Dent Traumatol. 2006;22:312‐317. [DOI] [PubMed] [Google Scholar]
- 62. Sura L, Madhavan A, Carnaby G, Crary MA. Dysphagia in the elderly: management and nutritional considerations. Clin Interv Aging. 2012;7:287‐298. 10.2147/CIA.S23404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Husein‐ElAhmed H, Aneiros‐Fernández J, Arias‐Santiago S, Anerios‐Cachaza J, Naranjo‐Sintes R. Lichenoid dermatosis induced by alendronate: An unusual skin drug reaction. Acta Derm Venereol. 2010;90(3):309‐310. 10.2340/00015555-0859 [DOI] [PubMed] [Google Scholar]
- 64. Graham DY. Excess gastric ulcers are associated with alendronate therapy. Am J Gastroenterol. 1998;93(8):1395‐1396. 10.1111/j.1572-0241.1998.01395.x [DOI] [PubMed] [Google Scholar]
- 65. Jones J, Sim TF, Hughes J. Pain assessment of elderly patients with cognitive impairment in the emergency department: Implications for pain management‐a narrative review of current practices. Pharmacy. 2017;5(2):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pérez‐Jover V, Mira JJ, Carratala‐Munuera C, et al. Inappropriate Use of Medication by Elderly, Polymedicated, or Multipathological Patients with Chronic Diseases. Int J Environ Res Public Health. 2018;15(2):310. 10.3390/ijerph15020310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Khan A, Carmona R, Traube M. Dysphagia in the Elderly. Clin Geriatr Med. 2014;30(1):43‐53. 10.1016/j.cger.2013.10.009 [DOI] [PubMed] [Google Scholar]
- 68. Kanis JA, Cooper C, Rizzoli R, Reginster JY. Executive summary of the European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Calcif Tissue Int. 2019;104(3):235‐238. 10.1007/s00223-018-00512-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Horikawa A, Miyakoshi N, Shimada Y, Sugimura Y, Kodama H. A comparative study between intravenous and oral alendronate administration for the treatment of osteoporosis. Springerplus. 2015;4:675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Miller PD, Pannacciulli N, Brown JP, et al. Denosumab or zoledronic acid in postmenopausal women with osteoporosis previously treated with oral bisphosphonates. J Clin Endocrinol Metab. 2016;101(8):3163‐3170. 10.1210/jc.2016-1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Horikawa A, Miyakoshi N, Hongo M, Kasukawa Y, Kodama H, Shimada Y. A prospective comparative study of intravenous alendronate and ibandronate for the treatment of osteoporosis. Medicine. 2019;98(6):e14340. 10.1097/MD.0000000000014340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wysowski DK, Greene P. Trends in osteoporosis treatment with oral and intravenous bisphosphonates in the United States, 2002–2012. Bone. 2013;57(2):423‐428. 10.1016/j.bone.2013.09.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Table S4


