Abstract
Objective
The contractile behavior of collecting lymphatic vessels occurs in essential hypertension in response to homeostasis, suggesting a possible role for microcirculation. We aimed to clarify the nature of the lymphatic microcirculation profile in spontaneously hypertensive rats (SHRs) and normotensive controls.
Methods
The vasomotion of collecting lymphatic vessels in eight‐ and thirteen‐week‐old SHRs and age‐matched Wistar‐Kyoto rats (WKYs, n = 4 per group) was visualized by intravital video and VasTrack. The lymphatic vasomotion profile (frequency and amplitude) and contractile parameters (contraction fraction and total contractility activity index) were compared. Plasma nitrite/nitrate levels were assessed by the Griess reaction, and plasma endothelin‐1 was measured by enzyme‐linked immunosorbent assay.
Results
WKYs and SHRs differed in the vasomotion of collecting lymphatic vessels. Both eight‐ and thirteen‐week‐old WKYs revealed a high‐amplitude pumping pattern, whereas a low‐amplitude pattern was observed in SHRs. Moreover, compared with age‐matched WKYs, SHRs exhibited deteriorated output and reflux capability and lost the ability to regulate collecting lymphatic vasomotion. Additionally, the chemistry complements the microcirculatory lymphatic profile as demonstrated by an increase in plasma nitrite, nitrate, and endothelin‐1 in SHRs. ET‐1 inhibitor meliorated the lymphatic contractile capability in SHRs partially through regulating frequency of lymphatic vasomotion.
Conclusions
We used an intravital lymphatic imaging system to observe that SHRs exhibit an impaired collecting lymphatic vasomotion profile and deteriorated contractility and reflux.
Keywords: collecting lymphatic vessels, endothelin‐1, hypertension, microcirculation, nitrate, nitrite, vasomotion
Abbreviations
- CF
contraction fraction
- DBP
diastolic blood pressure
- EDD
end‐diastolic diameter
- eNOS
endothelial nitric oxide synthase
- ESD
end‐systolic diameter
- ET‐1
endothelin‐1
- HR
heart rate
- KH
Krebs‐Henseleit
- MAP
mean arterial pressure
- SBP
systolic blood pressure
- SHRs
spontaneously hypertensive rats
- TCAI
total contraction activity index
- WKYs
Wistar‐Kyoto rats
1. INTRODUCTION
Essential hypertension is the most common type of high blood pressure and has no identifiable cause. The prevalence of essential hypertension is 23.2% (approximately 245 million), and another 41.3% (approximately 435 million) of the population is prehypertensive according to the data from China Hypertension Survey Groups. 1 Essential hypertension is generally recognized as a multifactorial disease of the cardiovascular system that is associated with genetic predisposition, epigenetic factors, and environmental determinants. Although the pathophysiological basis of essential hypertension remains unclear, recent findings have indicated that the disturbance of microcirculation might contribute to its pathogenesis and development. 2 , 3
Microcirculation refers to the terminal vascular and lymphatic network of the systemic circulation, which consists of arterioles, capillaries, venules, and lymphatic vessels, and plays a vital role in maintaining the physiological function of tissues. Accumulating data, including ours, have demonstrated that decreased microvascular blood perfusion contributes to the development of essential hypertension through a loss of endothelial nitric oxide synthase (eNOS) activity or reduction in bioavailable NO. 4 , 5 In lymphatic endothelial cells, eNOS is required for robust lymphatic contractions under physiological conditions, 6 and eNOS can stimulate lymph‐angiogenesis and modulate the activity of afferent and efferent collecting lymphatics, which therefore enable blood pressure to be maintained within normal ranges. Nevertheless, even though it is a comprehensive aspect of integrated microcirculation profile, the relationship between lymphatic microcirculation and essential hypertension is not well studied.
The lymphatic microcirculation is distributed throughout the body for the transport of protein and macromolecules through a process called vasomotion, 7 which refers to the rhythmic spontaneous constriction and dilation present in the lymphatics. In addition to passive forces, the active driving force derived from lymphatic vasomotion is required for the transport of lymph fluid. 8 The vasomotion of collecting lymphatic vessels exhibits a great variety of patterns from regular to disarranged oscillation. However, limited data are available regarding the dynamic changes in functional status of collecting lymphatic vessel vasomotion under hypertensive conditions. The major impediment to advances in understanding the link between lymphatic microcirculation and essential hypertension has been the inability to see changes in lymphatic vasomotion. Our group established an automated method for tracking the vasomotion of intravital collecting lymphatic vessels. 9 Additionally, lymphatic vessel contraction is sensitive to the length‐tension relationship. Hence, we hypothesize a pathological disorder of lymphatic microcirculation profile in the progression of hypertension. The present study aimed to determine the dynamic changes in functional status of lymphatic microcirculation in spontaneously hypertensive rats (SHRs) and age‐matched normotensive controls.
2. MATERIALS AND METHODS
2.1. Animals
This study was approved by the Institutional Animal Care and Use Committee (IACUC) at the Chinese Academy of Medical Sciences (CAMS), and all experimental procedures were performed according to the Guidelines for the Care and Use of Laboratory Animals. In vivo studies were performed in specific‐pathogen‐free male Wistar‐Kyoto rats (WKYs; eight‐ and thirteen‐week‐old) and age‐matched SHRs, all weighing 180–200 g. Rats (n = 4 per group) were purchased from the Institute of Laboratory Animal Sciences (CAMS, Beijing, China) and housed at a temperature of 22 ± 1℃ with 55%–65% humidity and a 12 h light: 12 h dark cycle. Rats had free access to water and food.
2.2. Measurement of blood pressure
A BIOPAC MP 150 system and AcqKnowledge software (BIOPAC) were employed to measure the blood pressure of rats. After acclimatization, rats were anesthetized by intraperitoneal injection of sodium pentobarbital (80 mg/kg). Following carotid artery intubation, a pressure transducer and transmitter were advanced into the carotid artery in a retrograde manner. The microcatheter was rotated and secured with a cephalad‐oriented tip. The MP 150 data acquisition analysis system and AcqKnowledge software (BIOPAC System, Goleta, CA, USA) were configured according to the manufacturer's instructions. The Systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), and heart rate (HR) of WKYs and SHRs were recorded.
2.3. Mesentery exteriorization preparation
To evaluate the functional status of lymphatic vasomotion, collecting lymphatic vessels located in the intestinal mesentery that were amenable to intravital microscopic observation were used as described previously. 10 , 11 , 12 Anesthetized rats were placed in the supine position and given a laparotomy by a midline incision. It has been reported the pathological changes of mesenteric microcirculation first and mainly affect the terminal ileum. 13 Based on this, the terminal ileum is thought to be a reasonable area for mesentery lymphatic microcirculatory observation. Following exteriorization from the peritoneal cavity, the terminal ileum mesentery was selected for microcirculatory lymphatic observation. Briefly, the mesentery was spread over an optical nonfluorescent glass pedestal that was placed in a transparent organic bath chamber with Krebs‐Henseleit (KH) buffer (pH 7.4) continually flowing at a rate of 6 ml/min. The KH solution contained 118.0 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.25 mM CaCl2, 1.2 mM KH2PO4, 25.0 mM NaHCO3, and 11.0 mM glucose, and was equilibrated with a 95% N2 and 5% CO2 mixture and warmed to 37 ± 0.5℃ by a thermostatic water bath. To prevent tissue dehydration, the exteriorized loop of the intestine adjacent to the mesenteric window was covered with medical gauze soaked in KH buffer (Figure 1A).
FIGURE 1.
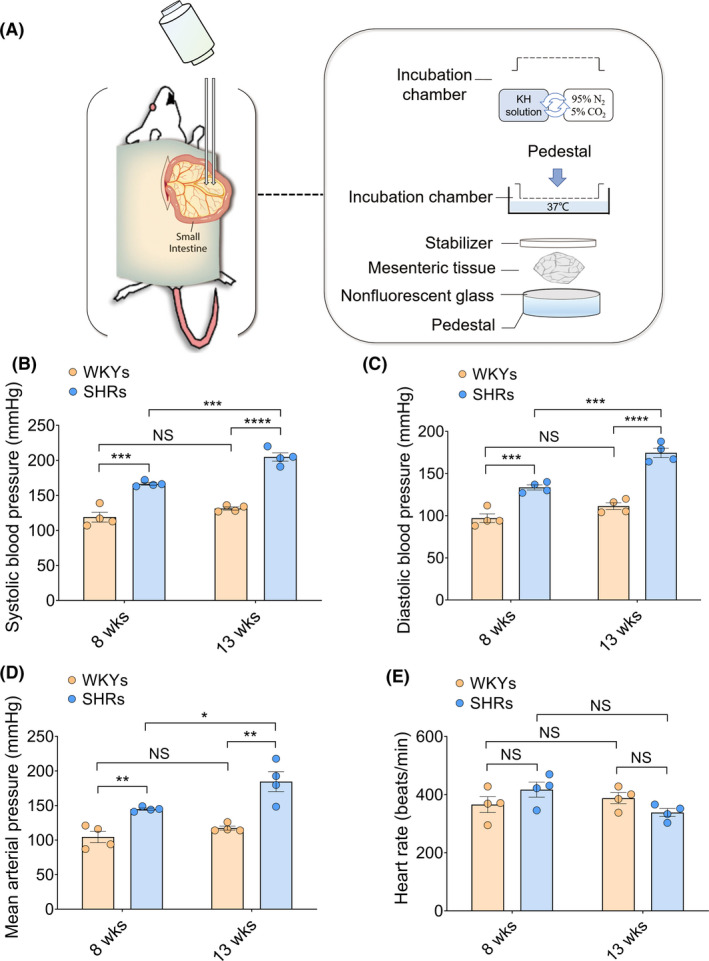
Comparisons of blood pressure in WKYs and SHRs at different ages. (A) Schematics of intravital mesenteric lymphatic imaging for lymphatic microcirculation visualization. In a transparent organic bath chamber, the rat mesentery was spread over an optical nonfluorescent glass pedestal and was continually superfused with Krebs‐Henseleit (KH) buffer (pH 7.4) at a rate of 6 mL/min. KH buffer was equilibrated with a 95% N2 and 5% CO2 mixture and warmed to 37 ± 0.5℃ by a thermostatic water bath. Mesenteric lymphatic contractile/diastolic oscillation was observed and recorded by Leitz intravital microscopy and a high‐resolution video camera. (B–E) Blood pressure, including systolic blood pressure (B), diastolic blood pressure (C), and mean arterial pressure (D), and heart rate (E) of eight‐week‐old and thirteen‐week‐old WKYs and SHRs were measured and recorded by a BIOPAC MP 150 system. Blood pressure values are expressed as means ± SEM. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. SHRs, spontaneously hypertensive rats; WKYs, Wistar‐Kyoto rats. n = 4 per group [Colour figure can be viewed at wileyonlinelibrary.com]
2.4. Intravital video recording
After equilibration, the mesentery was observed under a Leitz intravital microscope (ACM, ZEISS, Germany) equipped with a high‐resolution video camera (KY‐100, JVC, Japan) and a high‐pressure xenon light source. The lymphatic contractile/diastolic oscillation was continuously monitored and captured by TVHome Media3 software (Ver 1.0.2). All recorded videos were encoded by an XVID encoder and saved as MPEG4 format files for measurement and subsequent analysis. Video frames were set as 24‐bit true color with a resolution of 720 × 576 pixels. Red‐blue‐green (RBG) was set as 3 channels with 256 gray levels in each channel and 25 frames per second (PAL standard).
2.5. Characterization of collecting lymphatic vessels
To reduce interference derived from anatomical and regional variations in the lymphatic vessels, collecting lymphatic vessels were identified by the presence of a single smooth muscle layer and one‐way bicuspid valve with continuous quasiperiodic constriction‐dilation cycles. 14 Moreover, the data collection location was fixed downstream of the lymphatic valve of the collecting lymphatic vessels. Using VasTrack software, 9 the dynamic changes in diameter were tracked and captured automatically. Fifteen continuous and integrated systolic‐diastolic cycles of spontaneous lymphatic vasomotion were selected for analysis. Moreover, based on the end‐diastolic diameter (EDD) and end‐systolic diameter (ESD), the contractile activity of collecting lymphatic vessels was evaluated as previously reported. 10 , 15 The frequency of collecting lymphatic vasomotion was defined as the number of contraction/relaxation cycles per minute. Moreover, the relative amplitude was calculated by the following equation:
where A2 and A1 represent the EDD and ESD of collecting lymphatic vessels, respectively, and the B value is the diameter in the resting phase during the contraction/relaxation cycle.
2.6. Contraction fraction and total contraction activity index
The contraction fraction was developed to reveal the role of lymphatic autonomous contraction. 16 Moreover, the total contraction activity index (TCAI) represents lymphatic pumping function. 17 , 18 In the current study, we employed the contraction fraction and total contraction activity index to evaluate lymphatic contractile function. Based on the captured and measured dynamic changes in diameters, contraction fraction and total contraction activity index were calculated by the following equations:
where E is the contraction frequency, C is the maximum diastolic diameter and D is the maximum systolic diameter.
2.7. Enzyme‐linked immunosorbent assays (ELISAs)
A Griess reaction assay kit (R&D Systems, MN, USA) was used to measure the plasma nitrite/nitrate concentrations of WKYs and SHRs. Plasma endothelin‐1 (ET‐1) (R&D Systems) levels in WKYs and SHRs in both age groups were measured using ELISA kits in accordance with the manufacturer's protocols. A microplate reader (Thermo Scientific™ Multiskan™ GO, MA, USA) was used to record the optical density. All plasma samples were measured in duplicates.
2.8. Evaluation of an ET‐1 inhibitor in lymphatic microcirculation profile
To investigate the role of ET‐1 plays in pathological hypertension, additional groups of eight‐ and thirteen‐week‐old SHRs (n = 4 in each group) were treated with an ET‐1 receptor antagonist. Briefly, the mesentery was incubated with 10 μM bosentan, an ET‐1 receptor antagonist (Med Chem Express, Monmouth Junction, NJ, USA) for 30 min. As mentioned above, lymphatic microcirculatory data were collected before and after 10 μM bosentan incubation were collected and analyzed. The lymphatic vasomotion profile, contractility and reflux were compared.
2.9. Statistical analyses
Statistical analyses were performed using SPSS software (version 21.0; IBM, Armonk, NY). All collected lymphatic data are expressed as the mean ± standard error of the mean (mean ± SEM). For multivariate analysis, two‐way analysis of variance (ANOVA) was performed between genotype and age to determine which variables were independently associated with mesenteric lymphatic microcirculation. Data sets were subjected to Student's t‐tests after two‐way ANOVA for pairwise comparisons between two groups. A p value less than 0.05 was considered indicative of statistical significance. Furthermore, the correlations between mesenteric collecting lymphatic vasomotion profile and contractile parameters were analyzed with Pearson's correlation coefficients (r) and were considered relevant with associated p < 0.05 and values of r > 0.4 or r < −0.4.
3. RESULTS
3.1. Blood pressure
To investigate the changes in mesenteric lymphatic vasomotion under hypertensive conditions, rats at two different stages of hypertension and age‐matched normotension were enrolled in the analysis. SBP, DBP, MAP and HR in both WKYs and SHRs at eight and thirteen weeks are shown in Figure 1. As expected, SHRs had significantly higher SBP (167 ± 2 mmHg at eight weeks, 205 ± 6 mmHg at thirteen weeks), DBP (134 ± 3 mmHg at eight weeks, 175 ± 6 mmHg at thirteen weeks), and MAP (145 ± 2 mmHg at eight weeks, 185 ± 14 mmHg at thirteen weeks) compared with age‐matched WKYs at eight weeks (SBP, 119 ± 7 mmHg; DBP, 97 ± 5 mmHg; MAP,105 ± 8 mmHg) and thirteen weeks (SBP, 131 ± 2 mmHg; DBP, 111 ± 4 mmHg; MAP, 117 ± 3 mmHg). These values gradually increased with the progression of hypertension (Figure 1B–1D). However, no significant difference was observed in HR among the four groups (Figure 1E).
3.2. Abnormalities of mesenteric collecting lymphatic vasomotion in SHRs
The profiles of mesenteric collecting lymphatic vasomotion in the mesentery were revealed by intravital videos (Videos [Link], [Link], [Link], [Link]). Notably, the collecting lymphatic vasomotion in both WKYs and SHRs oscillated. Spontaneous phasic contractions/relaxation and valve opening/closing could be identified for most of the collective lymphatic vessels in the mesentery of both WKYs and SHRs. Moreover, the rats with and without hypertension differed in mesenteric collecting lymphatic vasomotion, as revealed by representative snapshots in the end‐diastolic and end‐systolic stages and by dynamic changes in collecting lymphatic vessel diameters (Figure 2). Notably, eight‐ and thirteen‐week‐old WKYs displayed a high‐amplitude collecting lymphatic vessel pumping pattern (Figure 2A,2C). Conversely, a low‐amplitude collecting lymphatic pumping pattern was observed in age‐matched SHRs (Figure 2B,2D). Due to systemic compensation for lymphatic contraction, 19 it is necessary to integrate the lymphatic diameter, amplitude, and frequency to globally evaluate the functional status of collecting lymphatic vasomotion.
FIGURE 2.
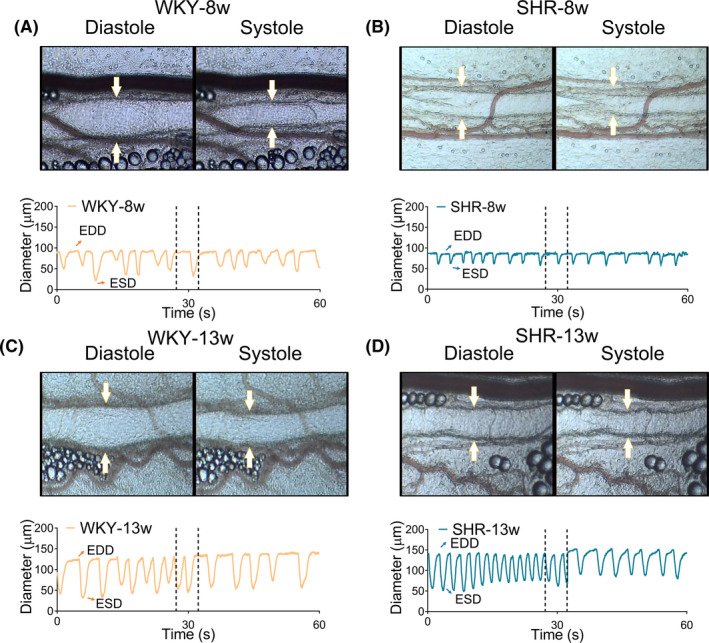
Mesenteric collecting lymphatic vasomotion of WKYs and SHRs. Representative collecting lymphatic contraction and dilation curves were generated from recordings of collecting lymphatic vessels using intravital microscopy and custom VasTrack software (n = 4). (A,B) Representative mesentery collecting lymphatic vasomotion of eight‐week‐old WKYs and SHRs. (C,D) Representative mesenteric collecting lymphatic vasomotion of thirteen‐week‐old WKYs and SHRs. White arrows indicate the lymphatic vessels. EDD, end‐diastolic diameter. ESD, end‐systolic diameter. SHRs, spontaneously hypertensive rats; WKYs, Wistar‐Kyoto rats. Source data are provided as Supplementary Videos [Colour figure can be viewed at wileyonlinelibrary.com]
3.3. Quantitative analysis of the mesenteric collecting lymphatic vasomotion profile
To unravel the effect of hypertension induction on lymphatic contractile function and to investigate the differences in collecting lymphatic vasomotion between SHRs and WKYs, four parameters of collecting lymphatic contractility (EDD, ESD, relative amplitude, and frequency) were subsequently quantitatively analyzed (Figure 3). No significant difference in the EDD was found between eight‐week‐old WKYs (105.30 ± 10.05 μm) and eight‐week‐old SHRs (104.50 ± 11.42 μm). Moreover, the EDD in thirteen‐week‐old SHRs (141.00 ± 21.69 μm) was also comparable to that in age‐matched WKYs (121.10 ± 10.21 μm) (Figure 3A). Although there was a trend toward a slight increase in the ESD of lymphatic vasomotion in eight‐ and thirteen‐week‐old SHRs compared with normotensive age‐matched WKYs, no significant differences were found (Figure 3B), indicating that lymphatic contractile capability may be impaired in the development of hypertension.
FIGURE 3.
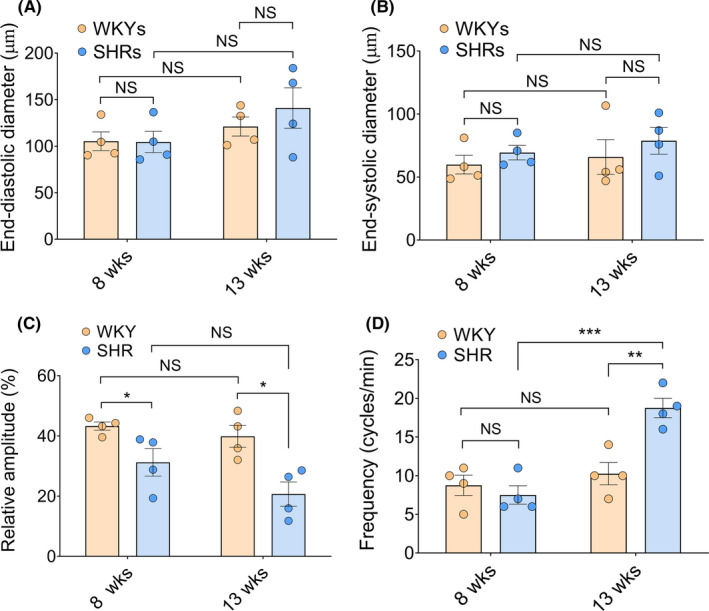
Parameters of collecting lymphatic contractility: end‐diastolic diameter, end‐systolic diameter, relative amplitude, and frequency of WKYs and SHRs at different ages (n = 4). (A,B) The end‐diastolic diameter (EDD) and end‐systolic diameter (ESD) are lymphatic vasomotion metrics that represent the diameters of lymph vessels per rhythmic constriction and relaxation, respectively. (C) The relative amplitude of collecting lymphatic vasomotion in eight‐week‐old and thirteen‐week‐old WKYs and SHRs (D) Frequency (cycle/min) of WKYs and SHRs at different ages. Values are expressed as means ± SEM. * p < 0.05, ** p < 0.01, ** p < 0.01, *** p < 0.001. SHRs, spontaneously hypertensive rats; WKYs, Wistar‐Kyoto rats. EDD, end‐diastolic diameter; ESD, end‐systolic diameter. n = 4 per group [Colour figure can be viewed at wileyonlinelibrary.com]
Frequency fluctuation of vasomotion is a passive phenomenon caused by natural variation in shear stresses. 20 Eight‐week‐old SHRs exhibited a low‐amplitude contraction and relaxation of collecting lymphatic vasomotion compared with age‐matched WKYs (31.20% ± 4.56% vs 43.26% ± 1.37%, p < 0.05), although the frequency of collecting lymphatic vasomotion was comparable between the groups of eight‐week‐old rat in the prehypertensive stage (8.75 ± 1.32 cycles/min vs 7.50 ± 1.19 cycles/min, p > 0.05). With the development of hypertension, thirteen‐week‐old SHRs revealed a low‐amplitude lymphatic vasomotion with a significantly increase in frequency (18.75 ± 1.25 cycles/min) (Figure 3C,3D), suggesting that SHRs lose the ability to regulate the amplitude and frequency of collecting lymphatic vasomotion.
3.4. Mesentery collecting lymphatic reflux in WKYs and SHRs
To further investigate the functional status of collecting lymphatic oscillations, the microcirculatory kinetics of collecting lymphatic vessels were analyzed. The CF and TCAI are lymphatic pumping metrics that represent the reflux of lymph fluid and output capability of lymph fluid per minute during a single contraction/relaxation cycle. As shown in Figure 4A, CF did not differ between eight‐week‐old SHRs (0.69 ± 0.10) and age‐matched WKYs (0.80 ± 0.09). The CF was greater in the thirteen‐week‐old normotensive WKYs than in the thirteen‐week‐old SHRs (0.78 ± 0.04 vs 0.36 ± 0.03, p < 0.001). Moreover, CF progressively and significantly decreased (eight‐week‐old SHRs vs thirteen‐week‐old SHRs) with the development of hypertension. Figure 4B displays the TCAI at different stages of hypertension. The TCAI tended to be slightly decreased in hypertensive rats (p > 0.05). The gradual deterioration of the output capability of lymphatic fluid in SHRs suggests a functional deficit in the development of hypertension.
FIGURE 4.
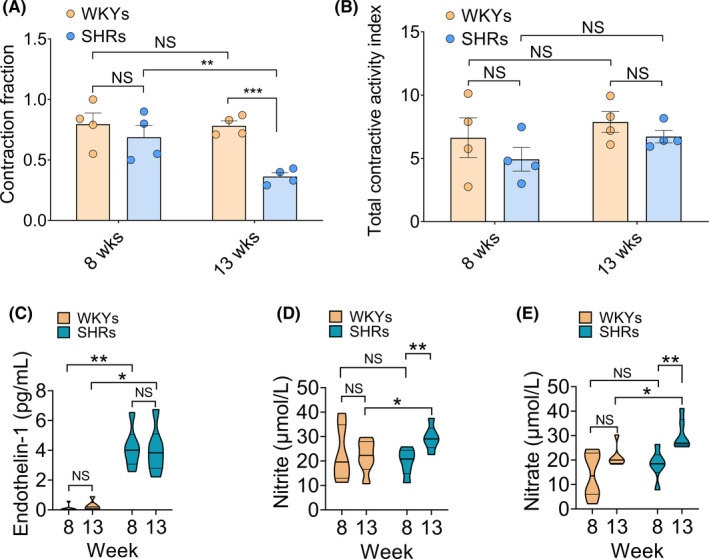
Mesenteric collecting lymphatic reflux and plasma chemical components in the progression of hypertension. The contraction fraction (CF) and total contraction activity index (TCAI) in mesenteric collecting lymphatic vessels of WKYs and SHRs were compared (n = 4). (A) CF of eight‐week‐old and thirteen‐week‐old WKYs and SHRs. (B) TCAI of eight‐week‐old and thirteen‐week‐old WKYs and SHRs. Data are expressed as means ± SEM. * p < 0.05, ** p < 0.01, *** p < 0.001. Collecting lymphatic vasomotion‐related plasma chemical components including concentrations of nitrite/nitrate and endothelin‐1, were determined by ELISAs. (C) The levels of plasma ET‐1 in eight‐week‐old and thirteen‐week‐old WKYs and SHRs. (D,E) The levels of plasma nitrite/nitrate in WKYs and SHRs at different ages. SHRs, spontaneously hypertensive rats; WKYs, Wistar‐Kyoto rats. n = 4 per group [Colour figure can be viewed at wileyonlinelibrary.com]
Previous reports have suggested that chemical components are crucial for endothelium‐dependent relaxation in lymphatic vessels. 21 To further assess the lymphatic microcirculatory function in WKYs and SHRs, the nitrite/nitrate and ET‐1 levels in plasma were evaluated. As illustrated in Figure 4D and 4E, plasma nitrite/nitrate levels were comparable in the prehypertensive stage (WKY: nitrite 20.87 ± 3.61 μmol/L, nitrate 17.71 ± 5.57 μmol/L, SHR: nitrite 21.33 ± 1.78 μmol/L, nitrate 16.60 ± 2.22 μmol/L, p > 0.05). In contrast, plasma nitrite/nitrate levels were significantly increased in thirteen‐week‐old SHRs (nitrite 31.26 ± 2.37 μmol/L, nitrate 31.64 ± 4.74 μmol/L) compared with age‐matched WKYs (nitrite 21.84 ± 2.80 μmol/L, nitrate 19.60 ± 0.63 μmol/L). Additionally, as a vasoconstrictor, the ET‐1 levels was significantly increased in SHRs from eight to thirteen weeks compared with normotensive controls (eight‐week‐old WKYs 0.14 ± 0.11 pg/mL, eight‐week‐old SHRs 4.12 ± 1.23 pg/mL, p < 0.01; thirteen‐week‐old WKYs 0.33 ± 0.15 pg/mL, thirteen‐week‐old SHRs 3.98 ± 1.40 pg/mL, p < 0.05), indicating an imbalance between lymphatic vasodilators and constrictors.
3.5. Effect of bosentan on mesenteric collecting lymphatic vasomotion
To assess the effect of ET‐1 on lymphatic vasomotion, we used 10 μM bosentan during measurements in a separate group of eight‐ and thirteen‐week‐old SHRs (Figure 5A, Videos [Link], [Link], [Link], [Link]). After recording spontaneous resting lymphatic vasomotion, oscillation was assessed in the same lymphatic vessel by adding 10 μM bosentan. Although bosentan acts as an ET‐1 inhibitor, both the EDD and ESD were comparable in eight‐ and thirteen‐week‐old SHRs before and after bosentan. Continuous recordings revealed spontaneous lymphatic vessel dilations and constrictions after bosentan administration (with a 3.59% diameter change in eight‐week‐old SHRs and a 12.10% diameter change in thirteen‐week‐old SHRs, Figure 5A and 5B). Furthermore, no significant differences were observed between the relative amplitude of lymphatic vasomotion in eight‐ and thirteen‐week‐old SHRs (Figure 5C). Notably, lymphatic vasomotion could be driven at different frequencies depending on the presence of bosentan (Figure 5D) without changes of CF and TCAI (Figure 5E, 5F), suggesting that administration of bosentan partially meliorated the lymphatic contractile capability in SHRs regulating frequency.
FIGURE 5.
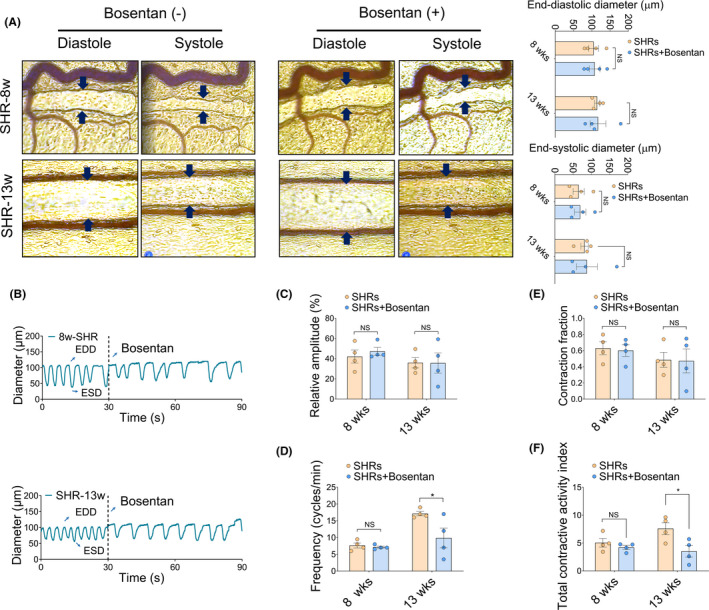
Effects of an ET‐1 inhibition on mesentery collecting lymphatic contractility and reflux. (A) Representative EDD and ESD of SHRs at different ages extracted from the collecting lymphatic vessels using intravital microscopy and custom VasTrack software. Black arrows indicate the collecting lymphatic vessels. The end‐diastolic diameter (EDD) and end‐systolic diameter (ESD) were compared in the right panel. (B) The representative collecting lymphatic contraction and dilation curves before and after 10 μM bosentan administration. (C‐F) Parameters of collecting lymphatic vasomotion. (C) Relative amplitude, (D) Frequency (cycle/min), (E) CF, and (F) TCAI. * p < 0.05 versus SHRs without bosentan. n = 4 per group. Source data are provided as Supplementary Videos [Colour figure can be viewed at wileyonlinelibrary.com]
3.6. Correlation analysis between the mesenteric lymphatic vasomotion profile and contractile parameters
The differences derived from genotype and age in mesenteric lymphatic vasomotion were summarized by two‐way ANOVA Table S1). Regarding the frequency of lymphatic vasomotion, two‐way ANOVA revealed main effects of genotype (p < 0.05) and age (p < 0.001), as well as an interaction (p < 0.05). Moreover, CF was significantly associated with genotype (p < 0.001) and age (p < 0.05). In addition, to further investigate whether there was an uncoupling association of the hypertensive phenotype with lymphatic microcirculation, we next analyzed the correlations between mesenteric lymphatic vasomotion profile and contractile parameters. As demonstrated in Figure 6, a significant positive correlation was revealed between the frequency of lymphatic vasomotion and TCAI in eight‐week‐old (r = 0.950, p < 0.05) and thirteen‐week‐old WKYs (r = 0.962, p < 0.05) (Figure 6A,6C). However, no significant correlations between frequency and TCAI were found in prehypertensive and hypertensive stages SHRs (Figure 6B,6D). Furthermore, no significant differences were found between the amplitude of lymphatic vasomotion and contractile parameters (CF and TCAI) in eight‐week‐old and thirteen‐week‐old WKYs and SHRs. Collectively, these lines of evidence suggest that under hypertensive conditions, functional status of mesenteric collecting lymphatic vasomotion deteriorates.
FIGURE 6.
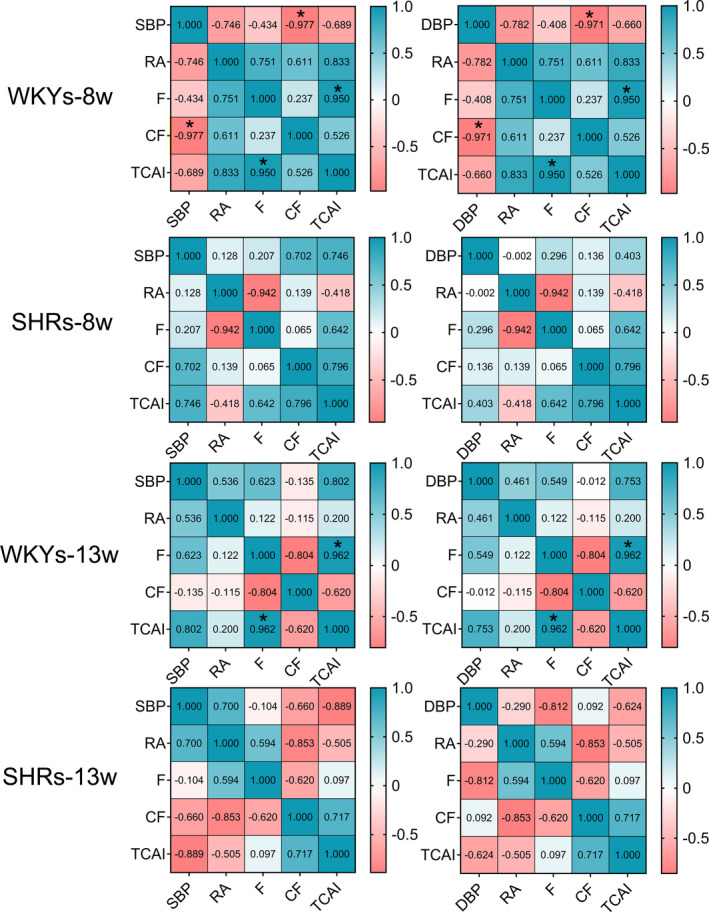
Correlation analysis between mesenteric lymphatic vasomotion profile and contractile parameters. Correlation coefficients in eight‐week‐old and thirteen‐week‐old WKYs and SHRs are shown. Numbers in the figure represent correlation coefficients r value. * p < 0.05. SHRs, spontaneously hypertensive rats; WKYs, Wistar‐Kyoto rats. SBP, systolic blood pressure; DBP, diastolic blood pressure; RA, relative amplitude; F, frequency; CF, contraction fraction; TCAI, total contraction activity index [Colour figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
Microcirculatory lymphatic vessels, an important component of microcirculation, exhibit a rhythmic constriction‐dilation cycle, which is responsible for both the propulsion of lymph and the modulation of vascular resistance and homeostasis. Dysfunction of microcirculatory lymphatic vessels leads to cardiovascular diseases, including essential hypertension. 22 Essential hypertension is closely related to both functional and structural alterations of the microcirculation. Although a previous study showed that contractile activity of the thoracic duct was functionally impaired with the development of hypertension in vitro, 23 there is little direct evidence regarding whether the development of hypertension is associated with dysfunctional lymphatic vasomotion. In the current study, we provide the first evidence to indicate that impaired functional status of lymphatic microcirculation in SHRs and disordered collecting lymphatic vasomotion might be involved in the pathogenesis and development of essential hypertension.
The lymphatic endothelial cells lining lymphatic vessels develop specialized cell‐cell junctions that are crucial for integrity and vascular functions of lymphatic vessels. 24 In addition to microhemodynamics, lymphatic microcirculation encompasses lymphatic capillaries and collecting lymphatic vessels, 25 , 26 , 27 , 28 and it drives and distributes microcirculatory fluid balance in a non‐linear way. 29 Starling forces (hydrostatic and colloid osmotic pressure) and their relevance to interstitial fluid and pressure homeostasis have revealed that the lymphatic vasculature plays a functional role in hypertension. 30 Physiological and metabolic abnormalities including elevated blood pressure, can result in collecting lymphatic remodeling, thereby reducing the potential load capability and impairing intrinsic contractility. In the present study, we showed the biological oscillation of collecting lymphatic vessels, providing evidence for the adaptability and autoregulation in normotensive WKYs. In contrast, SHRs at different ages (in the both prehypertensive and hypertensive stages) exhibited abnormal mesenteric lymphatic vasomotion with decreased relative amplitude and increased frequency, indicating two regulatory determinants of lymphatic microcirculation to balance fluid hemostasis.
Through the oscillating amplitude and frequency of lymphatic capillaries and collecting vessels, the lymphatic system absorbs excess interstitial fluid and transports it back into the blood microcirculation. 31 The accumulation of interstitial fluid results from insufficient lymphatic transportation caused by the dysfunctional status of collecting lymphatic vasomotion, supporting the view that lymphatic microcirculation is involved in the development of hypertension. The observed low‐amplitude high‐frequency structural pattern of collecting lymphatic vasomotion in the context of the hypertensive condition may be attributed to a compensatory mechanism for the elevated blood pressure to maintain hemostasis of the lymphatic microcirculation. Another interesting finding in the present study was that no correlations between functional collecting lymphatic parameters and blood pressure were found for WKYs. Conversely, CF was negatively correlated with DBP at both the prehypertensive and hypertensive stages of SHRs. We reported that microcirculatory blood perfusion showed a positive correlation with blood pressure. Thus, our data favor the view that microvascular blood perfusion might be a greater determinant than collecting lymphatic vasomotion in hypertension.
Lymphatic contractile function is regulated by multiple factors, including neuronal and humoral factors and shear stress. Immune cell trafficking through the lymphatic microcirculation is involved in the development of hypertension. 32 It is possible that the presence of M1 proinflammatory peritoneal macrophages leading to the abnormal lymphatic contraction in SHRs. Furthermore, under physiological conditions, eNOS produces nitric oxide (NO) in lymphatic endothelial cells during contraction cycles. The role of the endothelium‐derived relaxation factor NO in flow‐mediated dilation was investigated and demonstrated to be correlated with the shear stress applied to the vessels. NO is a predominant regulator of lymphatic vessel contraction and relaxation via its vasodilatory signal 33 , 34 and is also a regulator of lymphatic contraction through pacemaker‐like activity in mesentery lymphatic vessels. 35 , 36
Experiments have demonstrated that cross‐talk exists between the nitrate‐nitrite‐NO signaling pathway and the NOS‐dependent system in the regulation of NO homeostasis. Although there is still controversy regarding nitrate/nitrite levels in hypertensive conditions, decreased NO bioavailability has been viewed to be a major pathological phenomenon related to the impaired contractile activity of lymphatic vessels in hypertension. Our findings reveal that SHRs exhibit a higher total plasma nitrate/nitrite than the WKYs controls, which is in line with a previous observation. 37 A possible explanation for this discrepancy is the presence of a NOS‐independent pathway and increased iNOS activity that releases NO from protein‐bound dinitrosyl nonheme iron complexes. 38 , 39 These pathological chemical components are consistent to some extent with our recent finding that SHRs exhibit progressively disordered microcirculatory function.
Moreover, ET‐1 is a potent vasoconstrictor of lymphatic vessels and a regulator of microcirculatory blood perfusion that plays a critical role in blood pressure elevation in a model of experimental hypertension, 40 which contributes to microvascular remodeling and endothelial dysfunction. In agreement with studies where the plasma ET‐1 concentration is elevated in untreated patients with hypertension, 41 we found a higher level of ET‐1 during the development of hypertension. On the other hand, from prehypertensive stage to the hypertensive stage, the beneficial effect of bosentan in SHRs is associated with a decreased frequency of lymphatic vasomotion and an increased tendency of CF and relative amplitude of lymphatic vasomotion. Together, the elevated levels of plasma nitrite/nitrate and ET‐1 over their physiological ranges in hypertension are considered a compensatory response to imbalanced eNOS activity, proinflammatory cytokines, and oxidative stress which are indicative of the pathogenesis of hypertension. Our data further strengthen the importance of the lymphatic microcirculatory profile in hypertension.
5. CONCLUSION
Our study provides evidence that SHRs exhibit abnormal lymphatic microcirculatory profiles, including disarranged collecting lymphatic vessel vasomotion with reduced relative amplitude and increased oscillating frequency during the progression of hypertension. We believe that focusing on the nature of pathological changes in the lymphatic microcirculatory profile will help enhance the understanding of the microcirculation in hypertension.
6. PERSPECTIVES
We provided evidence of the occurrence of a gradually impaired lymphatic microcirculatory profile from the normotensive to the prehypertensive stage in SHRs. As it is an interconnected and integrated system, characteristics of lymphatic microcirculation provide crucial information for microcirculatory profile. The current finding suggests a need to establish “lymphatic microcirculatory observatories” to pave the way for basic and preclinical research of the microcirculation.
ACKNOWLEDGMENT
We gratefully acknowledge Xueting Liu at the Institute of Microcirculation, CAMS & PUMC, for the assistance of animal health and welfare.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTION
Mingming Liu and Honggang Zhang designed the research and obtained the research funding. Bing Wang, Youming Sheng, Bingwei Li performed the experiments and drafted the manuscript with the help of Yuan Li, Jian Zhang, and Ailing Li. Bing Wang, Youming Sheng, and Mingming Liu analyzed and interpreted the experimental data. Bing Wang, Ailing Li, Mingming Liu, and Ruijuan Xiu edited the manuscript.
Supporting information
Table S1
Video S1
Video S2
Video S3
Video S4
Video S5
Video S6
Video S7
Video S8
Wang B, Sheng Y, Li Y, et al. Lymphatic microcirculation profile in the progression of hypertension in spontaneously hypertensive rats. Microcirculation. 2022;29:e12724. 10.1111/micc.12724
Funding information
This study was financially supported by grants from the National Natural Science Foundation of China (No. 81900747), the Beijing Municipal Natural Science Foundation (No. 7212068), and the CAMS Initiative for Innovative Medicine (CAMS‐I2M) (No. 2016‐I2M‐3‐006)
Contributor Information
Mingming Liu, Email: mingmingliu@imc.pumc.edu.cn.
Honggang Zhang, Email: zhanghg1966126@163.com.
DATA AVAILABILITY STATEMENT
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Wang Z, Chen Z, Zhang L, et al. Status of hypertension in China: results from the china hypertension survey, 2012–2015. Circulation. 2018;137:2344‐2356. [DOI] [PubMed] [Google Scholar]
- 2. Junqueira CLC, Magalhaes MEC, Brandao AA, et al. Microcirculation and biomarkers in patients with resistant or mild‐to‐moderate hypertension: a cross‐sectional study. Hypertens Res. 2018;41:515‐523. [DOI] [PubMed] [Google Scholar]
- 3. Tsioufis C, Dimitriadis K, Katsiki N, et al. Microcirculation in hypertension: an update on clinical significance and therapy. Curr Vasc Pharmacol. 2015;13:413‐417. [DOI] [PubMed] [Google Scholar]
- 4. Liu M, Song X, Wang B, et al. Pancreatic microcirculation profiles in the progression of hypertension in spontaneously hypertensive rats. Am J Hypertens. 2021;34:100‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Song X, Li Y, Wang B, et al. Comparison of pancreatic microcirculation profiles in spontaneously hypertensive rats and Wistar‐kyoto rats by laser doppler and wavelet transform analysis. Physiol Res. 2020;69:1039‐1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liao S, Cheng G, Conner DA, et al. Impaired lymphatic contraction associated with immunosuppression (vol 108, pg 18784, 2011). Proc Natl Acad Sci USA. 2016;113:E5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zawieja DC, Davis KL, Schuster R, et al. Distribution, propagation, and coordination of contractile activity in lymphatics. Am J Physiol. 1993;264:H1283‐H1291. [DOI] [PubMed] [Google Scholar]
- 8. Aukland K, Reed RK. Interstitial‐lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev. 1993;73:1‐78. [DOI] [PubMed] [Google Scholar]
- 9. Sheng YM, Xiu RJ. Automated method for tracking vasomotion of intravital microvascular and microlymphatic vessels. Clin Hemorheol Microcirc. 2012;52:37‐48. [DOI] [PubMed] [Google Scholar]
- 10. Zhang J, Xiu R. The characteristics of spontaneous rhythmical microlymphomotion. Zhonghua Yi Xue Za Zhi. 1995;75(262–265):318. [PubMed] [Google Scholar]
- 11. Baluk P, Tammela T, Ator E, et al. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005;115:247‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Azimi MS, Myers L, Lacey M, et al. An ex vivo model for anti‐angiogenic drug testing on intact microvascular networks. PLoS One. 2015;10:e0119227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koike Y, Li B, Ganji N, et al. Remote ischemic conditioning counteracts the intestinal damage of necrotizing enterocolitis by improving intestinal microcirculation. Nat Commun. 2020;11:4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Breslin JW, Yang Y, Scallan JP, et al. Lymphatic vessel network structure and physiology. Compr Physiol. 2018;9:207‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Benoit JN, Zawieja DC. Effects of f‐Met‐Leu‐Phe‐induced inflammation on intestinal lymph flow and lymphatic pump behavior. Am J Physiol. 1992;262:G199‐202. [DOI] [PubMed] [Google Scholar]
- 16. Liao S, Jones D, Cheng G, et al. Method for the quantitative measurement of collecting lymphatic vessel contraction in mice. J Biol Methods. 2014;1:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zawieja SD, Wang W, Wu X, et al. Impairments in the intrinsic contractility of mesenteric collecting lymphatics in a rat model of metabolic syndrome. Am J Physiol Heart Circ Physiol. 2012;302:H643‐653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang J, Xiu R. The characteristics of spontaneous rhythmical microlymphomotion. Zhonghua Yi Xue Za Zhi. 1995;75:262‐265. [PubMed] [Google Scholar]
- 19. Liao S, Cheng G, Conner DA, et al. Impaired lymphatic contraction associated with immunosuppression. Proc Natl Acad Sci USA. 2011;108:18784‐18789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buerk DG, Riva CE. Vasomotion and spontaneous low‐frequency oscillations in blood flow and nitric oxide in cat optic nerve head. Microvasc Res. 1998;55:103‐112. [DOI] [PubMed] [Google Scholar]
- 21. Kesler CT, Liao S, Munn LL, et al. Lymphatic vessels in health and disease. Wiley Interdiscip Rev Syst Biol Med. 2013;5:111‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brakenhielm E, Alitalo K. Cardiac lymphatics in health and disease. Nat Rev Cardiol. 2019;16:56‐68. [DOI] [PubMed] [Google Scholar]
- 23. Mukohda M, Mizuno R, Ozaki H. Increased blood pressure causes lymphatic endothelial dysfunction via oxidative stress in spontaneously hypertensive rats. Hypertension. 2020;76:598‐606. [DOI] [PubMed] [Google Scholar]
- 24. Zhang F, Zarkada G, Yi S, et al. Lymphatic endothelial cell junctions: molecular regulation in physiology and diseases. Front Physiol. 2020;11:509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. von der Weid PY, Zawieja DC. Lymphatic smooth muscle: the motor unit of lymph drainage. Int J Biochem Cell Biol. 2004;36:1147‐1153. [DOI] [PubMed] [Google Scholar]
- 26. Betterman KL, Harvey NL. The lymphatic vasculature: development and role in shaping immunity. Immunol Rev. 2016;271:276‐292. [DOI] [PubMed] [Google Scholar]
- 27. Oliver G. Lymphatic vasculature development. Nat Rev Immunol. 2004;4:35‐45. [DOI] [PubMed] [Google Scholar]
- 28. Schulte‐Merker S, Sabine A, Petrova TV. Lymphatic vascular morphogenesis in development, physiology, and disease. J Cell Biol. 2011;193:607‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Possenti L, Casagrande G, Di Gregorio S, et al. Numerical simulations of the microvascular fluid balance with a non‐linear model of the lymphatic system. Microvasc Res. 2019;122:101‐110. [DOI] [PubMed] [Google Scholar]
- 30. Machnik A, Neuhofer W, Jantsch J, et al. Macrophages regulate salt‐dependent volume and blood pressure by a vascular endothelial growth factor‐C‐dependent buffering mechanism. Nat Med. 2009;15:545‐552. [DOI] [PubMed] [Google Scholar]
- 31. Mukherjee A, Hooks J, Nepiyushchikh Z, et al. Entrainment of lymphatic contraction to oscillatory flow. Sci Rep. 2019;9:5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Solak Y, Afsar B, Vaziri ND, et al. Hypertension as an autoimmune and inflammatory disease. Hypertens Res. 2016;39:567‐573. [DOI] [PubMed] [Google Scholar]
- 33. Schmid‐Schonbein GW. Nitric oxide (NO) side of lymphatic flow and immune surveillance. Proc Natl Acad Sci USA. 2012;109:3‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bohlen HG, Gasheva OY, Zawieja DC. Nitric oxide formation by lymphatic bulb and valves is a major regulatory component of lymphatic pumping. Am J Physiol Heart Circ Physiol. 2011;301:H1897‐1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bohlen HG, Wang W, Gashev A, et al. Phasic contractions of rat mesenteric lymphatics increase basal and phasic nitric oxide generation in vivo. Am J Physiol Heart Circ Physiol. 2009;297:H1319‐1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. von der Weid PY, Zhao J, Van Helden DF. Nitric oxide decreases pacemaker activity in lymphatic vessels of guinea pig mesentery. Am J Physiol Heart Circ Physiol. 2001;280:H2707‐2716. [DOI] [PubMed] [Google Scholar]
- 37. Ling WC, Murugan DD, Lau YS, et al. Sodium nitrite exerts an antihypertensive effect and improves endothelial function through activation of eNOS in the SHR. Sci Rep. 2016;6:33048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao Y, Vanhoutte PM, Leung SW. Endothelial nitric oxide synthase‐independent release of nitric oxide in the aorta of the spontaneously hypertensive rat. J Pharmacol Exp Ther. 2013;344:15‐22. [DOI] [PubMed] [Google Scholar]
- 39. Hong HJ, Loh SH, Yen MH. Suppression of the development of hypertension by the inhibitor of inducible nitric oxide synthase. Br J Pharmacol. 2000;131:631‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schiffrin EL. Role of endothelin‐1 in hypertension and vascular disease. Am J Hypertens. 2001;14:83S‐89S. [DOI] [PubMed] [Google Scholar]
- 41. Xu M, Lu YP, Hasan AA, et al. Plasma ET‐1 concentrations are elevated in patients with hypertension ‐ meta‐analysis of clinical studies. Kidney Blood Press Res. 2017;42:304‐313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Video S1
Video S2
Video S3
Video S4
Video S5
Video S6
Video S7
Video S8
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.


