Abstract
The ADP-ribosylating enterotoxins, cholera toxin (CT) and the Escherichia coli heat-labile toxin (LT-IIa), have been shown to enhance mucosal and systemic antibody (Ab) responses to coadministered antigens. The purpose of the present study was to compare the ability of the nontoxic A2/B subunits of these toxins, which have distinct targeting properties, to augment the immunogenicity of a genetically coupled protein antigen. Structurally similar chimeric proteins were generated by genetically replacing the toxic A1 subunit of CT or LT-IIa with the saliva-binding region (SBR) from the streptococcal adhesin AgI/II. Intranasal immunization of BALB/c mice with either chimeric protein induced significantly higher plasma and mucosal anti-SBR immunoglobulin A (IgA) and IgG Ab responses than SBR alone. Moreover, compared to SBR–LT-IIaA2/B, SBR-CTA2/B elicited significantly higher levels of plasma IgG1 and salivary IgA anti-SBR Ab responses. Ex vivo and in vitro experiments revealed that SBR-CTA2/B selectively up-regulated B7-2 expression on murine B cells isolated from both the nasal associated lymphoid tissue, cervical lymph nodes, and spleen. In contrast, SBR–LT-IIaA2/B had little effect on B7-1 or B7-2 expression on B220+, CD11b+, or CD11c+ cells. Analysis of the functional costimulatory activity of SBR-CTA2/B-treated B cells revealed a significant enhancement in anti-CD3-stimulated CD4+ T-cell proliferative responses, and this proliferation was significantly reduced by treatment with anti-B7-2 but not with anti-B7-1 or isotype control Abs. Thus, SBR-CTA2/B and SBR–LT-IIaA2/B exhibit distinct patterns of antibody responses associated with differential effects on B7-2 expression and subsequent costimulatory effects on CD4+ T cells.
Cholera toxin (CT) produced by Vibrio cholerae and the labile toxins (LT) from Escherichia coli are structurally related heat-labile enterotoxins (HLE) that have been employed as adjuvants to augment both mucosal and systemic immune responses to coadministered antigens (Ag) (3, 12). These oligomeric toxins consist of an A subunit noncovalently coupled to five B polypeptides (42). After proteolytic cleavage and reduction of an intrachain disulfide bond, the A subunit is cleaved into a toxic A1 and a linking A2 polypeptide. Initial studies using HLE as mucosal adjuvants in animal models led to the conclusion that their adjuvanticity was due to the toxic ADP-ribosyltransferase activity of the A1 subunit (31). ADP ribosylation of the Gsα subunit of adenylate cyclase results in abnormally high levels of intracellular cyclic AMP (cAMP) (24, 42) and subsequent chloride ion efflux into the lumen of the gut, which is ultimately responsible for the characteristic watery diarrhea.
Due to the toxic nature of the holotoxins, many investigators have tried to dissociate the toxicity associated with the A1-subunit from the adjuvanticity of the AB5 complex and have attempted to address the immunostimulatory effects of B subunit receptor-mediated interactions. Earlier studies using commercial CTB preparations contaminated with intact CT made it impossible to distinguish between the adjuvanticity associated with ADP-ribosyltransferase activity and the binding properties of the AB5 complex. This issue was further complicated by the synergistic effect of holotoxin on the adjuvanticity of the B subunit (45, 48). However, with the aid of recombinant techniques, mutant constructs of CT and LT-I, which lacked ADP-ribosyltransferase activity, were shown to retain many of the adjuvant properties of the native toxin (11, 15, 52, 53). Studies comparing recombinantly produced wild type and a LT-I B subunit (LT-I B) mutant that lacks GM1 binding further demonstrated that both immunogenicity and adjuvanticity were dependent upon GM1 binding (35). Additional experiments have demonstrated that the up-regulation of various costimulatory molecules on Ag-presenting cells (APCs) by LT-I B or nontoxic derivatives of CT was abrogated when GM1 binding was blocked (34, 52). These studies demonstrate that the GM1 binding properties of the type I HLE appear to be necessary for their adjuvant properties.
Two types of HLE have been distinguished on the basis of distinct immunoreactivity (22, 37): type I HLE are represented by CT and LT-I (25, 37); type II HLE include E. coli LT-IIa and LT-IIb (16–18, 23). Although type I and type II HLE are structurally homologous and catalyze similar enzymatic reactions, comparison of the predicted amino acid sequences reveals considerable variability between type I and type II enterotoxin B subunits (22, 37–39). This extensive diversity imparts different ganglioside-binding properties to the respective B subunits. The cellular receptor for CT has been shown to be the monosialoganglioside GM1 (14). The B subunit of LT-IIa binds with high affinity to GD1b and less strongly to GT1b, GD2, GD1a, GM1, and GM2 (14). Gangliosides are glycosphingolipids in which the polar head groups consist of carbohydrate moieties with a lipophilic ceramide tail anchored in the lipid bilayer of membranes (33). Gangliosides are primarily components of the exoplasmic leaflet and have been shown to vary widely at the cell, tissue, and organ level, as well as between species (33). There is significant information demonstrating that various gangliosides play important roles in signal transduction pathways involving cellular immunomodulation, proliferation, differentiation, transformation, and suppression (20, 34, 35, 47, 49).
Our laboratory has recently demonstrated that compared to CT, the type II HLE exhibit potent and distinct adjuvant properties for stimulating mucosal and systemic immune responses to a noncoupled protein immunogen after parenteral or mucosal immunization (5, 32). Furthermore, a study comparing the adjuvanticity of CT and LT-I, which share >80% amino acid sequence homology but differ with respect to the receptor binding properties of their B subunits, showed that CT and LT-I induced different antibody (Ab) and cellular responses after mucosal immunization (44). However, it is not clear if the different response patterns observed from these studies were related to the more promiscuous binding activities of the B subunits of LT-I or LT-II compared to CT or possible differences associated with their ADP-ribosyltransferase activity.
An alternative approach to the use of HLE as mucosal adjuvants when coadministered with an Ag has been the development of potent mucosal immunogens by coupling protein Ag to the nontoxic B subunit of CT (9, 19, 51). Initially, proteins were coupled to CTB by chemical conjugation, but later a genetic strategy for fusing an antigenic polypeptide to the A2 subunit of CT and coexpressing this with CTB was devised, yielding a chimeric immunogen in the form of Ag-CTA2/B (19, 26). Given that LT-IIa binds a greater diversity of ganglioside receptors compared to only GM1 for CT, we have postulated that the targeting properties of a chimeric protein consisting of Ag-LT-IIaA2/B would have advantageous immunogenic qualities compared to those of a chimeric protein consisting of Ag-CTA2/B. Therefore, the purpose of the present study was to compare the mucosal immunogenicity of two nontoxic chimeric proteins composed of the same protein Ag genetically coupled in an identical manner to the A2 and B subunits of CT or LT-IIa, and to investigate the mechanistic basis for the observed differences.
MATERIALS AND METHODS
Genetic constructs.
The recombinant plasmid encoding the saliva-binding region (SBR)–LT-IIaA2/B hybrid protein was constructed in two steps. The first step involved engineering an intermediate plasmid designated pSBR/KS+. The plasmid pSBR-CTA2/B (19) was digested with XbaI and XhoI to release a 1.2-kbp fragment containing the SBR gene, a PelB leader sequence and a ribosomal binding site. The 1.2-kbp fragment, and all other fragments used in engineering the chimera, were isolated using agarose gel electrophoresis and a Gene Clean Kit (Bio 101, Inc., Vista, Calif.), ligated into pBluescriptKS(+) (Stratagene, La Jolla, Calif.) which had also been digested with XbaI and XhoI, and transformed into E. coli DH5αF′ tet cells (Life Technologies, Bethesda, Md.) using osmotic shock.
In the second step, PCR was used to amplify a fragment encoding the LT-IIa A2 domain and the B polypeptide from pTDC200, a plasmid which contained the genes encoding the type II HLT LT-IIa holotoxin (6, 7). The oligonucleotide primers Blue-619 (5′-GTAAAACGACGGCCAGTGAG-3′) and Xho-I (5′-GTAACCTCGAGGCCTGGAGAG-3′) and a Perkin-Elmer DNA thermal cycler (model 480) were used to amplify a 1,025-bp fragment from pTDC200 (PCR conditions: denaturation at 92°C for 30 s, annealing at 52°C for 60 s, extension at 72°C for 60 s; 30 cycles). The amplified fragment was digested with SmaI, which cleaved at a site located 3′ to the terminus of the B polypeptide gene, and with XhoI which was incorporated into the fragment using PCR primer Xho-I. This fragment was ligated into pSBR/KS+ which had been sequentially digested with KpnI, blunt ended using Klenow fragment, and digested with XhoI. Sites and oligonucleotides were chosen such that ligation of the fragments would place the SBR gene and the LT-IIa A2 sequences in the same reading frame. After transformation of the ligation mix into E. coli DH5αF′ tet, a clone containing a plasmid encoding the SBR–LT-IIaA2/B chimera was isolated and verified by genetic sequencing. This plasmid was designated pVAR9. Since the chimeric gene was oriented in the vector such that expression depended upon the T7 promoter, pVAR9 was subsequently transformed into E. coli BL21(DE3) which encodes an isopropyl-β-d-thiogalactoside (IPTG)-inducible T7 polymerase from a lysogenized prophage. A modified GD1b-ganglioside enzyme-linked immunosorbent assay (ELISA) (7) and anti-SBR antiserum were used to demonstrate not only that the plasmid expressed the SBR–LT-IIaA2 and SBR–LT-IIaB polypeptides but that the polypeptides also assembled into the expected chimeric holotoxin and retained ganglioside binding activity (19).
Growth and purification of recombinant proteins.
Growth of E. coli and purification of recombinant SBR-CTA2/B were performed as previously described (19). To isolate SBR–LT-IIaA2/B, the E. coli strain expressing plasmid pVAR9 was grown at 37°C with vigorous shaking (225 rpm) in Luria-Bertani broth supplemented with ampicillin (150 μg/ml) and tetracycline (10 μg/ml). Target gene expression was induced at mid-log phase (optical density [OD] ≈0.4) by the addition of IPTG to 1 mM. Growth was terminated 12 to 16 h after induction, and cells were harvested by centrifugation. The bacterial pellet was resuspended to 1/10 the original culture volume in ice-cold 100 mM Tris-HCl, pH 8.0, containing 20% sucrose, 5 mM EDTA, and lysozyme (0.5 mg/ml) to release the periplasm contents. After 30 min of incubation at 4°C, the supernatant was harvested by centrifugation and subjected to 50% ammonium sulfate saturation. The subsequent precipitate was obtained by centrifugation and resuspended in 10 mM Tris-HCl, pH 8.0, containing 0.3 M NaCl. In order to separate properly assembled SBR–LT-IIaA2/B from unassembled SBR-A2 and -B subunits, the dissolved precipitate was subjected to gel filtration using a Sephacryl-100 gel (Pharmacia). Eluted fractions possessing anti-SBR reactivity in a GD1b-ELISA were then pooled and loaded onto an anion-exchange Mono Q column (Pharmacia). The fractions containing purified SBR–LT-IIaA2/B were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), Western blotting, and a GD1b-ELISA (7). Recombinant proteins were analyzed for endotoxin content by means of a Quantitative Chromagenic Limulus Amebocyte Lysate assay kit (Bio Whittaker, Inc., Walkersville, Md.) using an E. coli K235 lipopolysaccharide standard. The endotoxin content for either recombinant protein was less than 0.5 ng of lipopolysaccharide per μg of purified chimeric protein.
Animals and immunizations.
Female BALB/c mice, 8 to 12 weeks of age, were immunized by the intranasal (i.n.) route. Groups of six to eight mice were immunized four times at 7-day intervals (i.e., days 0, 7, 14, and 21) with phosphate-buffered saline (PBS), 50 μg of SBR-CTA2/B, 50 μg of SBR–LT-IIaA2/B, or an equimolar amount (20 μg) of SBR alone. The vaccines were administered in a standardized volume of 20 μl and applied slowly to both external nares of nonanesthetized mice. All animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham.
Collection of mucosal secretions and plasma.
Samples of plasma, saliva, and vaginal washes were collected from individual mice 1 day before the first immunization (day 0), and on days 8, 18, 28, 42, and 70. Saliva was collected with a micropipetter after stimulation of salivary flow by injecting each mouse intraperitoneally with 5 μg of carbachol (Sigma Chemical Company, St. Louis, Mo.) in 0.1 ml PBS. Plasma samples were obtained following centrifugation of blood collected from the tail vein using a calibrated heparinized capillary tube. Vaginal washes were collected by flushing the vaginal vault two times with 75 μl of sterile PBS. Mucosal secretions and plasma samples were stored at −70 and −20°C, respectively, until assayed for antibody activity.
Antibody analysis.
The levels of isotype-specific antibodies in saliva, plasma, and vaginal washes were assayed by ELISA. Polystyrene microtiter plates (96-well; Nunc, Roskilde, Denmark) were coated overnight at 4°C with SBR (5 μg/ml), GD1b (2 μg/ml), or GM1 (1 μg/ml) (Matreya, Pleasant Gap, Pa.). GM1- and GD1b-treated plates were washed and incubated with CT (1 μg/ml) or LT-IIa (2 μg/ml), respectively, for 30 min at 37°C. Total immunoglobulin (Ig) isotype concentrations were determined by coating plates with goat anti-mouse Ig isotype-specific antibodies (Southern Biotechnology Associates, Birmingham, Ala.). Serial twofold dilutions of plasma or secretion samples were added in duplicate, and plates were incubated overnight at 4°C. Plates were then washed with PBS containing 0.1% Tween (PBS-Tw) and incubated at room temperature with the appropriate peroxidase-conjugated goat anti-mouse Ig isotype-specific reagent (Southern Biotechnology). Plates were washed and developed with o-phenylenediamine and hydrogen peroxide. The color reaction was stopped after 15 min, and OD was measured at 490 nm. The levels of antibodies and of total Ig in samples were calculated by interpolation on calibration curves generated by using a mouse Ig reference serum (ICN Biomedicals, Aurora, Ohio). In order to compensate for variations arising from salivary flow rate and dilutions of secretions, mucosal IgA responses are reported as the ratio of specific antibody IgA to total IgA.
Purification of B220+ B and CD4+ T cells.
Nasal associated lymphoid tissue (NALT), cervical lymph node (CLN), or splenic CD4+ T cells were purified by use of magnetized polystyrene beads (Dynabeads) coated with a rat monoclonal Ab (MAb) specific for the CD4 (L3T4) membrane Ag (Dynal A.S., Oslo, Norway). NALT was isolated as previously described (50). A single-cell suspension prepared from NALT, CLN, or spleen was incubated at 4°C for 20 min in the presence of mouse CD4 Dynabeads. CD4+ T cells were then positively selected with the aid of a Dynal magnetic particle concentrator. CD4+ T cells were then washed three times in PBS containing 1% fetal calf serum (FCS) and 2 mM EDTA. Isolated CD4+ T cells were then detached from L3T4 Dynabeads using a DETACHaBEAD Mouse CD4 Ag (Dynal A.S.). Cells were then washed three times and resuspended in complete medium (RPMI 1640) [Cellgro Mediatech, Washington, D.C.] containing 10% FCS, 40 μm of 2-mercaptoethanol, 1% l-glutamine, 10 mM HEPES, 10 U of penicillin per ml, 100 μg of streptomycin per ml, and 50 μg of gentamicin per ml) before use. This procedure routinely resulted in >99% pure CD4+ T cells as shown by flow cytometry.
B220+ B cells were isolated from the CD4+-depleted cell populations by use of a depletion column using CD43 MicroBeads (Miltenyi Biotec, Sunnyvale, Calif.). CD43 MicroBeads were incubated with the cell suspension for 15 min at 6°C in PBS containing 0.5% bovine serum albumin (BSA) and 2 mM EDTA. The cell suspension was then washed by adding a 20-fold excess of PBS containing 0.5% BSA and 2 mM EDTA. After centrifugation, cells were resuspended in 1 ml of PBS–0.5% BSA and added to the magnetic depletion column. CD43− B cells were eluted with 15 ml of PBS containing 0.5% BSA and 2 mM EDTA. Cells were then washed twice and resuspended in complete medium before use. This procedure routinely yielded >99% pure B220+ cells as shown by flow cytometry.
Cell staining.
Cell suspensions were stained with allophycocyanin-conjugated B220+, CD11b+, or CD11c+ and then costained with fluorescein isothiocyanate-conjugated anti-B7-2 and phycoerythrin-conjugated anti-B7- 1 (Caltag). Fluorochrome-labeled cells were analyzed by flow cytometry using a FACStar flow cytometer (Becton Dickinson, Mountain View, Calif.).
To examine the effects of SBR, SBR-CTA2/B, SBR–LT-IIaA2/B, recombinant LT-IIaB (rLT-IIaB), and recombinant CTB (rCTB) on costimulatory molecule up-regulation by B220+, CD11b+, or CD11c+ cells, single-cell suspensions or purified B220+ cells (0.15 to 2.0 × 106 cells/ml) were incubated with various concentrations of recombinant proteins. In order to assess the effects of ganglioside binding, SBR-CTA2/B or SBR–LT-IIaA2/B, were first blocked by incubating with a 20:1 molar ratio of GM1 or GD1b, respectively (Matreya), for 1 h at 37°C and then added to B220+ cell cultures. Following a 20-h incubation, cell suspensions or purified B cells were incubated for 15 min in PBS containing 0.1% sodium azide and 3% FCS and washed extensively.
Proliferation assay.
To assess the costimulatory function of B7-1 and B7-2 up-regulation, B cells were treated with SBR-CTA2/B, SBR–LT-IIaA2/B, or medium alone for 20 h, washed in ice-cold PBS, and fixed in 0.5% paraformaldehyde. CD4+ T cells (2 × 106 cells/ml) were cultured in complete medium containing 0.5% paraformaldehyde fixed B cells (1 × 106 cells/ml) in the presence of 100 ng of anti-CD3 Ab per ml for 5 days. Approximately 18 h before harvesting, the cells were pulsed with 0.5 μCi of [3H]thymidine, and [3H]thymidine uptake was determined by using a liquid scintillation counter.
Statistical analysis.
Analysis of variance with the Tukey-Kramer multiple test was used for multiple comparisons, and unpaired t tests were performed to analyze differences between two groups. Differences were considered significant at the P < 0.05 level.
RESULTS
Characterization of the expressed chimeric protein SBR–LT-IIaA2/B.
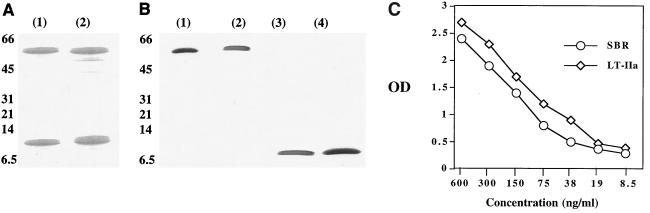
Periplasmic fractions of E. coli expressing SBR–LT-IIaA2/B were purified by gel filtration and anion-exchange chromatography. Eluted fractions were monitored by GD1b-ELISA and SDS-PAGE. As shown by SDS-PAGE and Western blotting, SBR–LT-IIaA2/B was purified to homogeneity and contained both antigenic determinants, SBR and LT-IIa (Fig. 1A and B). Furthermore, purified SBR–LT-IIaA2/B was shown to bind GD1b-coated plates and contained both SBR and LT-IIa epitopes, indicating successful assembly of an intact chimeric protein that possessed ganglioside binding (Fig. 1C). These results, along with the nature of the genetic construction of the chimeric protein, indicate that SBR–LT-IIaA2/B is structurally similar to the SBR-CTA2/B chimeric protein (19) in possessing equimolar ratios of SBR and A2/B5 subunits.
FIG. 1.
(A) SDS-PAGE of purified SBR-CTA2/B (lane 1) and SBR–LT-IIaA2/B (lane 2) dissociated into the SBR-A2 subunit (∼60 kDa) and B subunit (∼12.5 kDa) monomers. (B) Western blot of recombinant SBR-CTA2/B (lane 1) and SBR-LT-IIaA2/B (lane 2) probed with polyclonal anti-SBR Abs and of SBR-CTA2/B (lane 3) and SBR-LT-IIaA2/B (lane 4), using polyclonal Abs to CT or LT-IIa, respectively. The positions of the molecular mass markers (kilodaltons) are indicated. (C) Titration of purified SBR–LT-IIaA2/B in a GD1b-ELISA using polyclonal Abs to SBR and LT-IIa antigenic determinants. Each point represents the mean OD of triplicate values.
Ab responses to SBR in mucosal secretions.
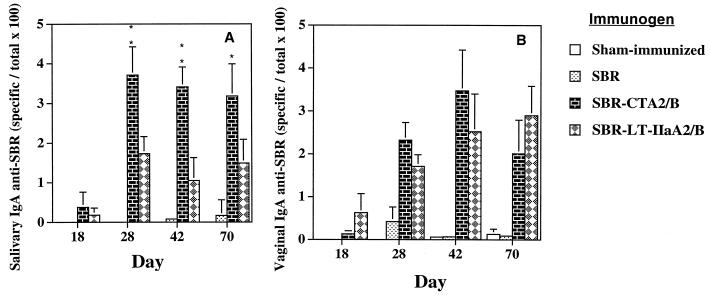
In order to compare the mucosal immunogenicity of SBR, SBR-CTA2/B, and SBR–LT-IIaA2/B, groups of mice were immunized by the i.n. route with equimolar amounts of each immunogen, and saliva and vaginal wash samples were analyzed for IgA anti-SBR activity. Both chimeric proteins induced SBR-specific IgA in saliva that persisted at greater than 1% of total salivary IgA through day 70 (Fig. 2A). SBR-CTA2/B and SBR–LT-IIaA2/B induced maximal levels of salivary IgA anti-SBR Abs on day 28, which reached almost 4 and 2% of total salivary IgA, respectively (Fig. 2A). On days 28 through 70, mice immunized with SBR-CTA2/B had significantly higher levels (P < 0.05) of SBR-specific IgA in the saliva than mice immunized with SBR–LT-IIaA2/B (Fig. 2A). Vaginal IgA anti-SBR Ab responses were detected on day 18 in mice immunized with SBR-CTA2/B or SBR–LT-IIaA2/B and persisted at high levels through day 70 (Fig. 2B). Peak vaginal anti-SBR IgA (3.6%) levels in SBR-CTA2/B mice occurred on day 42, while peak responses in mice immunized with SBR–LT-IIaA2/B (∼3%) occurred on day 70. Mice immunized with SBR alone had low to nondetectable levels of SBR-specific Abs in saliva (Fig. 2A) and vaginal wash (Fig. 2B). These results suggest that SBR-CTA2/B was more effective at inducing salivary IgA Abs to SBR than SBR–LT-IIaA2/B. However, this effect was not observed in vaginal wash samples.
FIG. 2.
Salivary (A) and vaginal (B) IgA Ab responses to SBR after i.n. immunization of mice with SBR alone, SBR-CTA2/B, or SBR-LT-IIaA2/B. Results are the arithmetic means + standard deviations (error bars) of eight mice per group. In panel A, ∗ and ∗∗ indicate statistically significant differences at P < 0.05 and P < 0.01, respectively, compared to SBR–LT-IIaA2/B.
Plasma Ab responses to SBR.
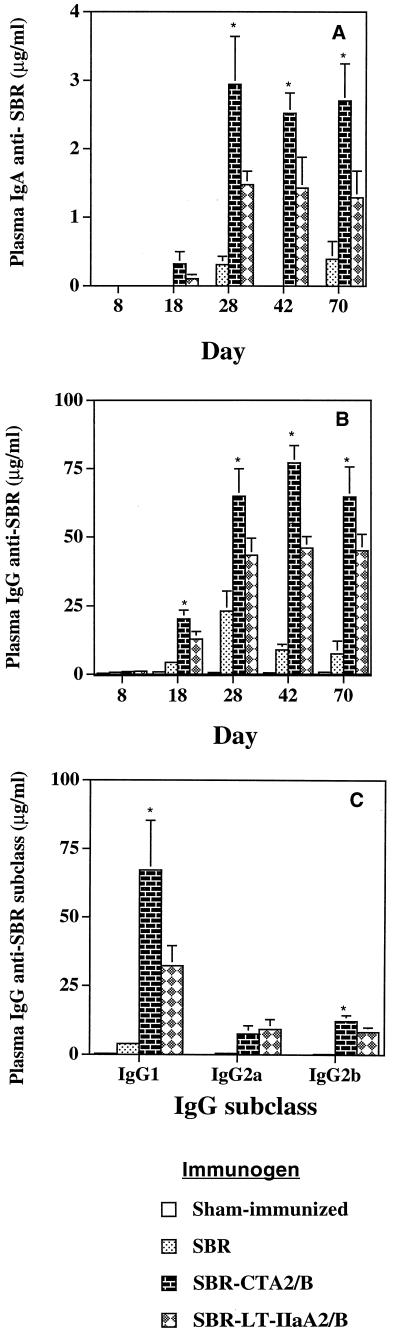
Mice immunized by the i.n. route with SBR-CTA2/B or SBR–LT-IIaA2/B had significantly (P < 0.05) higher (3- to 10-fold) levels of plasma IgA anti-SBR Abs than mice immunized with SBR alone (Fig. 3A). On day 28 through day 70, SBR-CTA2/B-immunized mice had significantly (P < 0.05) higher levels of SBR-specific plasma IgA than mice immunized with SBR–LT-IIaA2/B (Fig. 3A). Plasma IgG anti-SBR levels were also significantly elevated in mice immunized with SBR-CTA2/B or SBR–LT-IIaA2/B, compared to SBR alone (Fig. 3B). Furthermore, on days 18 to 70, plasma IgG anti-SBR Abs were significantly (P < 0.05) higher in mice immunized with SBR-CTA2/B than in mice receiving the SBR–LT-IIaA2/B chimera (Fig. 3B). Analysis of the IgG subclasses revealed that the major SBR-specific subclass was IgG1, followed by lower levels of IgG2a and IgG2b (Fig. 3C). SBR-specific IgG1 Ab in SBR-CTA2/B-immunized mice was significantly elevated compared to SBR- or SBR–LT-IIaA2/B-immunized mice (Fig. 3C). Furthermore, the ratios of SBR-specific IgG1 to IgG2a were markedly different between groups. Mice immunized with SBR-CTA2/B had an IgG1/IgG2a ratio of 10:1, whereas those immunized with SBR–LT-IIaA2/B had an IgG1/IgG2a ratio of 4:1 (Fig. 3C). Thus, it appears that the greater IgG Ab response induced by SBR-CTA2/B is largely due to a selective increase in IgG1 Abs.
FIG. 3.
Plasma IgA (A), IgG (B), and IgG subclass (day 42) (C) antibody responses to SBR from mice immunized by the i.n. route with SBR alone, SBR-CTA2/B, or SBR–LT-IIaA2/B. Data represent the arithmetic means + standard deviations (error bars) of eight mice per group. ∗, statistically significant differences at P < 0.05 compared to SBR–LT-IIaA2/B.
Ab responses to CT and LT-IIa.
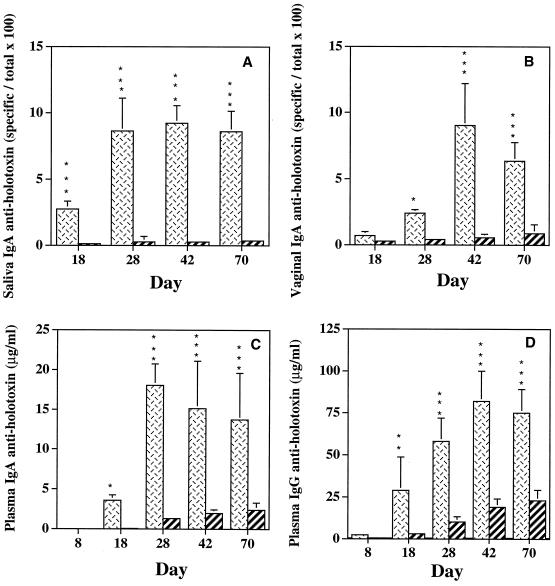
We also analyzed the immunogenicity of the CTA2/B and LT-IIaA2/B components of the two chimeras by employing a ganglioside-dependent ELISA (43). Optimal concentrations of GM1, GD1b, CT, and LT-IIa for coating the assay plates were first determined by titration curves generated with various concentrations of the ganglioside, CT, and LT-IIa. SBR-CTA2/B induced significantly higher (P < 0.05 to P < 0.001) salivary IgA and vaginal IgA anti-CT Ab responses compared to the anti-LT-IIa Ab responses induced in mice immunized with SBR–LT-IIaA2/B (Fig. 4A and B). Plasma IgA and IgG Ab responses to LT-IIa were likewise lower than the anti-CT responses (Fig. 4C and D). These data demonstrate that the CTA2/B subunits of CT were consistently more immunogenic than the LT-IIaA2/B subunits of LT-IIa in the respective chimeras.
FIG. 4.
Salivary IgA (A), vaginal IgA (B), plasma IgA (C), and plasma IgG (D) responses to CT or LT-IIa in mice immunized by the i.n. route with SBR-CTA2/B (cross-hatched bars) or SBR–LT-IIaA2/B (striped bars), respectively. Data represent the arithmetic means + standard deviations (error bars) of eight mice per group. ∗ and ∗∗∗, statistical significance at P < 0.05 and P < 0.001, respectively.
Analysis of B7-1 and B7-2 expression ex vivo after i.n. immunization.
Upon recognition of major histocompatibility complex (MHC) Ag by the cognate T-cell receptor, the engagement of B7 present on APCs with CD28 on T cells results in a second signal pathway for the activation of T cells. Since previous studies have demonstrated that the adjuvanticity of native CT is highly dependent upon B7 up-regulation, we wanted to determine if either chimeric protein was capable of up-regulating B7 in vivo. In order to investigate the effects of the CT- and LT-IIa-based chimeric proteins on B7-1 (CD80) and B7-2 (CD86) expression on APCs, we immunized BALB/c mice i.n. with PBS, 50 μg of either chimeric protein, or an equimolar amount of SBR in a standardized volume and isolated the NALT and CLN 24 h later (Table 1). Cell suspensions were examined by flow cytometry and gated on B220+ (B cells), CD11b+ (macrophage), or CD11c+ (dendritic cell) populations which were costained with B7-1 and B7-2. Analysis of CD11b+ and CD11c+ cells from the NALT (Table 1) and CLN (data not shown) revealed no significant up-regulation of B7-1 or B7-2 expression between the control and experimental groups. However, B220+ cells isolated from the NALT of SBR-CTA2/B-immunized mice exhibited a significant (P < 0.05) enhancement in B7-2 expression, while B7-1 expression was not up-regulated (Table 1). These results demonstrate that SBR-CTA2/B selectively up-regulates B7-2 expression on mucosal B cells isolated from the NALT after i.n. immunization.
TABLE 1.
Increase of B7-1 and B7-2 expression on various cells isolated from BALB/c mice immunized via the i.n. route
| Immunogenb | Frequency of expression (mean ± SD) on indicated cellsa
|
|||||
|---|---|---|---|---|---|---|
| B220+
|
CD11b+
|
CD11c+
|
||||
| B7-1 | B7-2 | B7-1 | B7-2 | B7-1 | B7-2 | |
| SBR | <1 | 2.11 ± 1.88 | <1 | <1 | <1 | <1 |
| SBR-CTA2/B | <1 | 19.52 ± 4.06c | <1 | 1.86 ± 0.97 | <1 | <1 |
| SBR–LT-IIaA2/B | <1 | 4.64 ± 3.09 | <1 | 1.09 ± 0.82 | <1 | <1 |
Increase in the frequency of B7-1 and B7-2 expression on NALT B220+, CD11b+, or CD11c+ cells were shown as the difference between PBS-immunized and immunogen-immunized mice compared to isotype-matched controls after gating on B220+, CD11b+, or CD11c+ cells. NALT cells were isolated 24 h after i.n. immunization. Data represent the arithmetic mean ± standard deviation of three separate experiments.
Mice were immunized i.n. with 50 μg of SBR-CTA2/B, 50 μg of SBR-LT-IIaA2/B, or an equimolar amount of SBR.
P < 0.05 compared to SBR- or SBR–LT-IIaA2/B-immunized mice.
Expression of B7-1 and B7-2 on B cells.
Several studies have demonstrated the importance of B7 molecules on APCs in providing costimulatory function for T-cell activation (27, 29). Furthermore, it has been shown that the adjuvanticity of CT is strongly dependent upon the up-regulation of B7 expression (4). However, it is not known if the immunogenicity of Ags genetically coupled to the B subunit depends on B7 up-regulation. Due to the selective up-regulation of B7-2 on NALT B220+ cells observed in SBR-CTA2/B-immunized mice, we wanted to determine whether the observed B7-2 up-regulation was due to the targeting properties of SBR-CTA2/B, and if so, whether the enhanced B7-2 expression induces a functional costimulatory signal for T-cell activation.
Incubation of SBR-CTA2/B with NALT B cells from naive mice resulted in a significant (P < 0.05) up-regulation of B7-2 expression, which was almost fivefold higher than that induced by SBR–LT-IIaA2/B (Table 2). In contrast, SBR-CTA2/B had no effect on the level of B7-1 expression on B220+ cells. SBR or rCTB alone caused no change in B7-1 expression and only a modest increase in B7-2 (Table 2). rLT-IIa B alone induced an increase in B7-1 expression similar to that seen with SBR–LT-IIaA2/B but did not affect B7-2 expression (Table 2). Similar results were obtained with splenic B220+ cells (data not shown).
TABLE 2.
Expression of B7-1 and B7-2 on treated NALT B220+ cellsa
| Stimulant | % Positive cells (mean ± SD)b
|
|
|---|---|---|
| B7-1 | B7-2 | |
| SBR-CTA2/B | <1 | 25.19 ± 4.29 |
| SBR-LT-IIaA2/B | 3.94 ± 1.12 | 5.11 ± 3.45 |
| SBR | <1 | 2.88 ± 1.37 |
| rCTB | <1 | 7.67 ± 4.43 |
| rLT-IIaB | 5.78 ± 2.22 | <1 |
| SBR-CTA2/B (GM1 treated) | <1 | 4.79 ± 1.82 |
| SBR–LT-IIaA2/B (GD1b treated) | <1 | 3.57 ± 3.71 |
NALT B220+ cells (1.5 × 105/ml) were incubated with chimeric protein (20 μg/ml) or equimolar concentrations of SBR, rCTB, or rLT-IIaB for 20 h. Cells were washed and costained with phycoerythrin-conjugated anti-B7-1 and fluorescein isothiocyanate-conjugated anti-B7-2.
Values are the increase in the expression of B7 molecules and are shown as the difference between experimental and control (untreated) from three separate experiments (mean ± standard deviation).
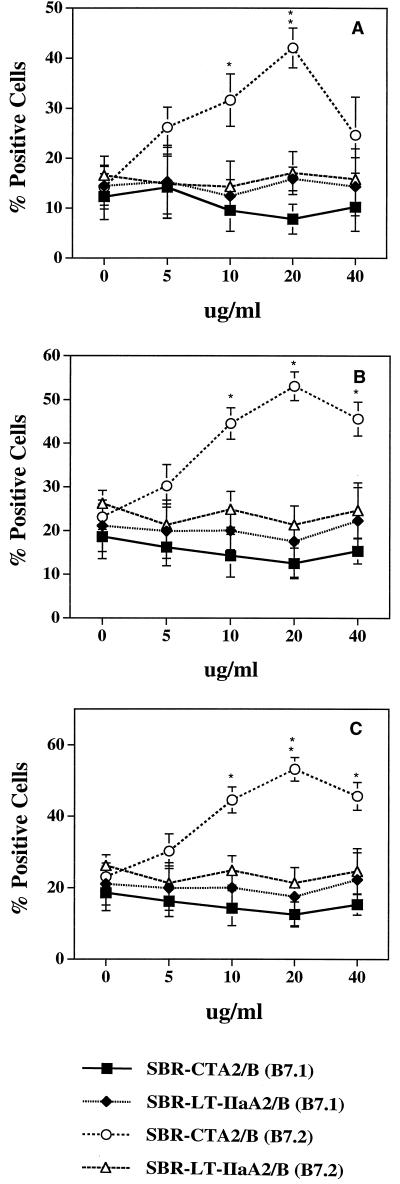
The up-regulation of B7-2 by SBR-CTA2/B on NALT, CLN, and splenic B cells was dose-dependent, reaching a maximum at 20 μg/ml (Fig. 5). In contrast, SBR–LT-IIaA2/B had only a minor effect on B7-1 and B7-2 expression at all concentrations tested (Fig. 5). To demonstrate that the up-regulation of B7 expression by the chimeric proteins was dependent upon ganglioside binding, SBR-CTA2/B and SBR–LT-IIaA2/B were incubated with GM1 or GD1b, respectively, before addition to B-cell cultures.
FIG. 5.
Dose-response effect of SBR-CTA2/B and SBR–LT-IIaA2/B on B7-1 and B7-2 expression by NALT (A) or splenic (B) or CLN (C) B220+ B cells. B220+ B cells were incubated for 20 h with various concentrations of chimeric protein and then costained with anti-B7-1 and anti-B7-2. Results shown are expressed as the arithmetic means ± standard deviations (error bars) of three experiments. ∗ and ∗∗, statistically significant differences at P < 0.05 and P < 0.01, respectively, compared to SBR-LT-IIaA2/B.
Pretreatment of either chimera with its receptor significantly reduced B7 expression by B cells to levels accountable for by the effect of SBR alone (Table 2). These results demonstrate that SBR-CTA2/B and SBR–LT-IIaA2/B differ in their ability to enhance B7 expression, and suggest that the up-regulation of B7-2 observed on SBR-CTA2/B-treated B cells depends on the recognition of its ganglioside receptor GM1.
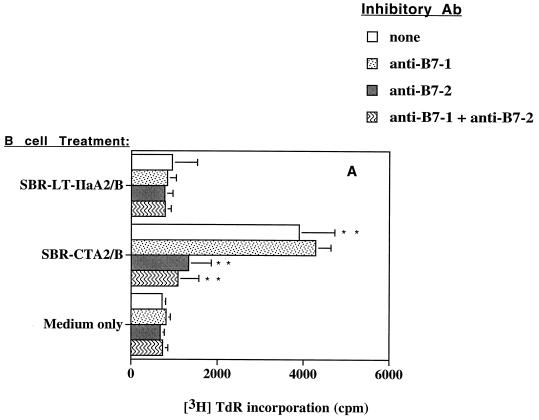
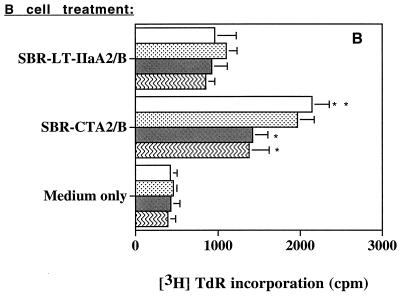
To determine if the observed increases in B7 expression on B cells promoted a functional costimulatory activity, we assessed CD4+ T-cell proliferative responses. NALT or splenic CD4+ T cells were stimulated with a suboptimal concentration of anti-CD3 in the presence of B cells that had previously been incubated with SBR-CTA2/B or SBR–LT-IIaA2/B (or medium alone) and then fixed with paraformaldehyde. CD4+ T cells cocultured with untreated B cells (medium only) displayed a low level of stimulation by anti-CD3 (Fig. 6). SBR–LT-IIaA2/B-treated NALT B cells did not significantly enhance T cell proliferation under anti-CD3 stimulation compared to untreated B cells (Fig. 6A). Spleen B cells treated with SBR–LT-IIaA2/B appeared to stimulate T cells more than NALT B cells (Fig. 6B), but this effect was not significant. In contrast, NALT or splenic B cells incubated with SBR-CTA2/B induced significantly (P < 0.05 to P < 0.01) higher T-cell proliferative responses compared to both the SBR–LT-IIaA2/B-treated and control groups (Fig. 6). To determine if the increased CD4+ T-cell proliferation in response to B cells was dependent on B7 up-regulation, MAbs to B7-1 or B7-2, or isotype control Abs were added to the cultures. The MAb anti-B7-1, anti-B7-2, or both had no effect on the B-cell costimulatory activity induced by SBR–LT-IIaA2/B (Fig. 6). The addition of MAb anti-B7-1 had no significant effect on T-cell proliferation induced by SBR-CTA2/B-treated B cells. In contrast, the addition of MAb anti-B7-2 significantly (P < 0.05) diminished the T-cell proliferative response induced by SBR-CTA2/B-treated B cells (Fig. 6). Isotype control Abs did not have any effect on proliferative responses (data not shown). These data suggest that SBR-CTA2/B-treated B cells selectively stimulated CD4+ T cells to proliferate, and this effect was associated with B7-2 but not B7-1 up-regulation.
FIG. 6.
Proliferative responses of NALT (A) or splenic (B) CD4+ T cells in the presence of anti-CD3 stimulation and B220+ cells that were unstimulated or treated with SBR-CTA2/B or SBR–LT-IIaA2/B in the absence or presence of inhibitory Abs to B7-1 or B7-2. Results shown are expressed as the arithmetic means + standard deviations (error bars) of three experiments. TdR, thymidine. ∗ and ∗∗, statistically significant differences at P < 0.05 and P < 0.01, respectively, compared to no inhibitory Ab or SBR–LT-IIaA2/B.
DISCUSSION
This study demonstrates the construction and expression of a recombinant chimeric protein in which the toxic ADP-ribosylating A1-subunit of LT-IIa was genetically replaced with SBR from the streptococcal adhesin AgI/II. This chimera, designated SBR–LT-IIaA2/B, was shown to act as a mucosal immunogen which induced significantly higher anti-SBR Ab responses in both plasma and mucosal secretions compared to an equivalent dose of SBR alone. However, the immunogenic properties of a previously constructed chimeric protein based on CT (19), designated SBR-CTA2/B, were significantly greater than those observed with SBR–LT-IIaA2/B. The greater immunogenicity of SBR-CTA2/B was revealed in higher salivary and serum IgA Abs to the SBR component and in a selective enhancement of IgG1 subclass Abs. These results suggest that the CT-based chimera had a greater capacity to induce responses governed by type 2 T helper cells (Th2). A similar bias was found in our previous studies on the comparative adjuvant properties of the intact holotoxins, CT and the type II HLE (32). A mechanism contributing to the differences in the immunogenic qualities of the two chimeras is associated with marked differences in B7 up-regulation. SBR-CTA2/B was shown to significantly enhance B7-2 expression on NALT B220+ cells after i.n. immunization. Moreover, incubation of NALT, CLN, or splenic B220+ cells with SBR-CTA2/B in vitro resulted in the selective up-regulation of B7-2 that was capable of exerting a functional costimulatory effect on CD4+ T cells.
The adjuvant properties of intact CT when coadministered with an Ag have been associated with the ADP-ribosyltransferase activity of the A1 subunit (1, 31). Moreover, elevated intracellular cAMP induced by CT, or the use of cAMP analogs, has been shown to enhance the expression of B7-2 on B cells and macrophages (4, 10). Studies addressing the ability of native CT to up-regulate B7 expression on Peyer's patch CD11b+ and CD11c+ cells demonstrated that CT selectively up-regulated B7-2 expression and that this effect could be mimicked by a cAMP analog but not rCTB (4). Alternatively, a study examining the immunomodulatory effects of rLTB demonstrated that cAMP was not necessary to up-regulate B7 expression on rLTB-treated B220+ cells (34). The data presented here are in agreement with these observations. SBR-CTA2/B, which lacks the ADP-ribosylating A1 subunit, significantly enhanced B7-2 expression on mucosal and systemic B cells; however, this effect was not observed on macrophages or dendritic cells as identified by the expression of CD11b or CD11c markers. Thus, it appears from these studies that cAMP may be necessary for the up-regulation of B7 expression on CD11b+ and CD11c+ cells but not required for the enhancement of B7 molecules on B cells. However, a recent study by others using a nontoxic mutant of CT, in which a point mutation was inserted into one of the ADP-ribosyltransferase sites and which thus lacks the ability to increase cAMP, demonstrated that the mutant CT enhanced both B7-1 and B7-2 expression on B220+ and CD11b+ cells isolated from Peyer's patches (52). Unlike rCTB, rLTB, or the chimeric protein SBR-CTA2/B, the inactive mutant contains the entire A1 subunit. Taken together, these data suggest that the A subunit may possess additional immunostimulatory qualities that are responsible for the observed differences between the native and nontoxic derivatives of CT or LT-I.
Earlier studies addressing the effects of B7-1 and B7-2 on T helper cells suggested that these two B7 molecules do not confer equivalent costimulatory signals. Unlike B7-1, B7-2 preferentially stimulates interleukin-4 production from human T cells and may be initially involved in establishing the Th2 phenotype (13, 27). Moreover, it has been reported that Th1 and Th2 cells differ in their requirements for CD28 ligation. The initial activation of Th2 cells is highly dependent upon B7-CD28 interactions, whereas Th1 cells initially appear to be less dependent on CD28 ligation but require B7-CD28 interactions for their maintenance (29, 46). The initial dependence of Th2 cells on B7-CD28 interactions may be the result of early B7-2–CD28 ligation that results in enhancing interleukin-4 receptor sensitivity and thus driving a Th2 phenotype (28). A study assessing the adjuvant properties of CT demonstrated that blocking B7-2–CD28 interactions resulted in a down-regulation of Ag-specific IgG1 but not IgG2a in vivo and that the adjuvant properties of CT were the result of preferentially up-regulating B7-2 (4). Our results with SBR-CTA2/B are in agreement with these observations. SBR-CTA2/B significantly enhanced B7-2 expression, which in turn provided a costimulatory signal via B7-2 to CD4+ T cells. Moreover, analysis of the IgG subclasses revealed that the enhanced IgG Abs observed in mice immunized with SBR-CTA2/B were due to the selective enhancement of IgG1 Abs, which, compared to responses in mice immunized with SBR–LT-IIaA2/B, exhibited more than a twofold increase in the ratio of SBR-specific IgG1 to IgG2a, indicative of a Th2-dominated immune response (36, 41). Thus, the selective enhancement of B7-2 may play a critical role in the IgG1-dominated Ab subclass response observed in SBR-CTA2/B-immunized mice.
The chimeric protein SBR–LT-IIaA2/B induced significantly lower plasma and mucosal Ab responses, as well as costimulatory B7 expression, than SBR-CTA2/B. However, SBR–LT-IIaA2/B did exhibit enhanced mucosal immunogenicity compared to an equimolar amount of SBR alone. One proposed mechanism responsible for the immunoenhancing effect of chimeric proteins based on HLE B subunits is their targeting of cell surface gangliosides resulting in enhanced immunogen uptake. It has recently been shown that the conjugation of rCTB to the surface of liposomes greatly enhanced their adjuvant properties compared to liposomes containing rCTB in the encapsulated aqueous phase (21). Subsequent studies using both surface-linked and encapsulated rCTB demonstrated enhanced uptake of rCTB-coated liposomes by murine Peyer's patches (E. Harokopakis and S. M. Michalek, unpublished results). Therefore, compared to SBR alone, the ability of SBR–LT-IIaA2/B to target cell-surface gangliosides could result in the enhanced uptake and increased delivery of Ag to immunocompetent cells.
The up-regulation of B7 molecules by SBR-CTA2/B was dependent upon recognition of its ganglioside GM1 receptor. Due to the genetic construction used in this study, the two chimeras are structurally similar and differ only at the amino acid level in their A2/B5 components, which confer different ganglioside-binding properties. Thus, the lower immunoenhancing effect of SBR–LT-IIaA2/B may be the result of its preferential binding to non-GM1 receptors (14). Previous studies addressing receptor-mediated immunomodulation by the B subunit of the type I HLE, LT-I, showed that targeting its high-affinity receptor, GM1, was largely responsible for its immunogenic and adjuvant properties (34, 35). Despite the ability of LT-I B to bind non-GM1 receptors, GM1 appears to be its dominant receptor (14). Moreover, a recent study has demonstrated that membrane lipid rafts selectively enriched in GM1, which are believed to play an important role in signal transduction and membrane trafficking, were selectively targeted to the MHC class II peptide-loading compartment after B-cell receptor cross-linking (2, 40). Therefore, the ability to preferentially target GM1 may also increase Ag delivery and subsequent MHC class II presentation by B cells.
Activation of CD4+ T cells requires, in addition to recognition by the T-cell receptor of antigenic peptide in the context of MHC class II, costimulation of CD28 by B7 molecules on the APC (27, 29). B cells normally serve as APCs in the secondary immune response, when adequate numbers of specific antibody-expressing cells are available from the clonal expansion occurring in the primary immune response. Previous studies addressing the effects of the HLE and their B subunits on B-lymphocyte functional activation (4, 34, 52), as well as data presented here, demonstrate that naive B cells can become activated prior to interaction with T cells and subsequently express high levels of B7 costimulatory molecules and thereby serve as APCs in a primary response. Our ex vivo data demonstrated that B cells with elevated B7-2 expression are present in the NALT after i.n. immunization with SBR-CTA2/B. The recruitment of activated B cells expressing these phenotypic markers has been shown to be important for the activation of naive T cells (8). Furthermore, experiments addressing germinal center formation by Ag-specific B lymphocytes during the initial immune response suggest that as few as two or three activated B cells are required for the induction of productive T cell-B cell interactions (30). Thus, the low frequency of Ag-specific B cells during a primary immune response may not be a limiting factor for the activation of naive T cells, when HLE-coupled immunogens are used. Thus, based on the data presented in this study, it appears that chimeric immunogens based on HLE exert their immunoenhancing effects by targeting and activating B cells as APCs.
ACKNOWLEDGMENTS
We thank Dana Stinson for her excellent technical assistance.
This work was supported in part by grants DE 06746, DE 08182, DE 09081, and T32-AI07051.
REFERENCES
- 1.Agren L C, Ekman B, Lowenadler B, Lycke N Y. Genetically engineered nontoxic vaccine adjuvant that combine B cell targeting with immunomodulation. J Immunol. 1997;158:3936–3946. [PubMed] [Google Scholar]
- 2.Cheng P C, Dykstra M L, Mitchell R N, Pierce S K. A role for lipid rafts in B cell antigen receptor signalling and antigen targeting. J Exp Med. 1999;190:1549–1560. doi: 10.1084/jem.190.11.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clements J D, Hartzog N M, Lyon F L. Adjuvant activity of Escherichia coli heat-labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens. Vaccine. 1988;6:269–277. doi: 10.1016/0264-410x(88)90223-x. [DOI] [PubMed] [Google Scholar]
- 4.Cong Y, Weaver C T, Elson C O. The mucosal adjuvanticity of cholera toxin involves enhancement of costimulatory activity by selective up-regulation of B7.2 expression. J Immunol. 1997;159:5301–5308. [PubMed] [Google Scholar]
- 5.Connell T D, Cornelia S, Metzger D, Evans R T. Immunostimulatory activity of LT-IIa, a type II heat-labile enterotoxin of Escherichia coli. Immunol Lett. 1998;62:117–120. doi: 10.1016/s0165-2478(98)00038-8. [DOI] [PubMed] [Google Scholar]
- 6.Connell T D, Holmes R K. Characterization of hybrid toxins produced in Escherichia coli by assembly of A and B polypeptides from type I and type II heat-labile enterotoxins. Infect Immun. 1992;60:1653–1661. doi: 10.1128/iai.60.4.1653-1661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connell T D, Holmes R K. Molecular genetic analysis of ganglioside GD1b-binding activity of Escherichia coli type IIa heat-labile enterotoxin by use of random and site-directed mutagenesis. Infect Immun. 1992;60:63–70. doi: 10.1128/iai.60.1.63-70.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Croft M. Activation of naive, memory and effector T cells. Curr Opin Immunol. 1994;6:431–437. doi: 10.1016/0952-7915(94)90123-6. [DOI] [PubMed] [Google Scholar]
- 9.Czerkinsky C, Russell M W, Lycke N, Lindblad M, Holmgren J. Oral administration of a streptococcal antigen coupled to cholera toxin B subunit evokes strong antibody responses in salivary glands and extramucosal tissues. Infect Immun. 1989;57:1072–1077. doi: 10.1128/iai.57.4.1072-1077.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeBenedette M A, Chu N R, Pollock K E, Hurtado J, Wade W F, Kwon B S, Watts T H. Role of 4-1BB ligand in costimulation of T lymphocyte growth and its upregulation on M12 B lymphomas by cAMP. J Exp Med. 1995;181:985–992. doi: 10.1084/jem.181.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickinson B L, Clements J D. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect Immun. 1995;63:1617–1623. doi: 10.1128/iai.63.5.1617-1623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elson C O. Cholera toxin as a mucosal adjuvant—the effect of H-2 genes. Fed Proc. 1987;46:1778. [Google Scholar]
- 13.Freeman G J, Boussiotis V A, Anumanthan A, Bernstein G M, Ke X Y, Rennert P D, Gray G S, Gribbon J G, Nadler L M. B7–1 and B7–2 do not deliver identical costimulatory signals, since B7–2 but not B7–1 preferentially costimulates the initial production of IL-4. Immunity. 1995;2:523–532. doi: 10.1016/1074-7613(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 14.Fukuta S, Magnani J L, Twiddy E M, Holmes R K, Ginsburg V. Comparison of the carbohydrate-binding specificities of cholera toxin and Escherichia coli heat-labile enterotoxins LTh-I, LT-IIa, and LT-IIb. Infect Immun. 1988;56:1748–1753. doi: 10.1128/iai.56.7.1748-1753.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giuliani M M, Del Giudice G, Giannelli V, Dougan G, Douce G, Rappuoli R, Pizza M. Mucosal adjuvanticity and immunogenicity of LTR72, a novel mutant of Escherichia coli heat-labile enterotoxin with partial knockout of ADP-ribosyltransferase activity. J Exp Med. 1998;187:1123–1132. doi: 10.1084/jem.187.7.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green B A, Neill R J, Ruyechan W T, Holmes R K. Evidence that a new enterotoxin of Escherichia coli which activates adenylate cyclase in eucaryotic target cells is not plasmid mediated. Infect Immun. 1983;41:383–390. doi: 10.1128/iai.41.1.383-390.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guth B E, Pickett C L, Twiddy E M, Holmes R K, Gomes T A, Lima A A, Guerrant R L, Franco B D, Trabulsi L R. Production of type II heat-labile enterotoxin by Escherichia coli isolated from food and human feces. Infect Immun. 1986;54:587–589. doi: 10.1128/iai.54.2.587-589.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guth B E, Twiddy E M, Trabulsi L R, Holmes R K. Variation in chemical properties and antigenic determinants among type II heat-labile enterotoxins of Escherichia coli. Infect Immun. 1986;54:529–536. doi: 10.1128/iai.54.2.529-536.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hajishengallis G, Hollingshead S K, Koga T, Russell M W. Mucosal immunization with a bacterial protein antigen genetically coupled to cholera toxin A2/B subunits. J Immunol. 1995;154:4322–4332. [PubMed] [Google Scholar]
- 20.Hannun Y A, Linardic C M. Sphingolipid breakdown products: anti-proliferative and tumor-suppressor lipids. Biochim Biophys Acta. 1993;1154:223–236. doi: 10.1016/0304-4157(93)90001-5. [DOI] [PubMed] [Google Scholar]
- 21.Harokopakis E, Hajishengallis G, Michalek S M. Effectiveness of liposomes possessing surface-linked recombinant B subunit of cholera toxin as an oral antigen delivery system. Infect Immun. 1998;66:4299–4304. doi: 10.1128/iai.66.9.4299-4304.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holmes R K, Jobling M G, Connell T D. Cholera toxin and related enterotoxins of gram negative bacteria. In: Moss J, Iglewski B, Vaughn M, Tu A T, editors. Handbook of natural toxins. Vol. 8. New York, N.Y: Marcel Dekker, Inc.; 1995. pp. 225–255. [Google Scholar]
- 23.Holmes R K, Twiddy E M, Pickett C L, Marcus H, Jobling M G, Pettijean F M J. The Escherichia coli/Vibrio cholerae family of enterotoxins. In: Pohland A E, Dowell V R, Richard J L, editors. Symposium on Molecular Mode of Action of Selected Microbial Toxins in Foods and Feeds. New York, N.Y: Plenum Press; 1990. pp. 91–102. [Google Scholar]
- 24.Holmgren J. Actions of cholera toxin and the prevention and treatment of cholera. Nature. 1981;292:413–417. doi: 10.1038/292413a0. [DOI] [PubMed] [Google Scholar]
- 25.Honda T, Tsuji T, Takeda Y, Miwatani T. Immunological nonidentity of heat-labile enterotoxins from human and porcine enterotoxigenic Escherichia coli. Infect Immun. 1981;34:337–340. doi: 10.1128/iai.34.2.337-340.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jobling M G, Holmes R K. Fusion proteins containing the A2 domain of cholera toxin assemble with B polypeptides of cholera toxin to form immunoreactive and functional holotoxin-like chimeras. Infect Immun. 1992;60:4915–4924. doi: 10.1128/iai.60.11.4915-4924.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.June C H, Bluestone J A, Nadler L M, Thompson C B. The B7 and CD28 receptor families. Immunol Today. 1994;15:321–331. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 28.Kubo M, Yamashita M, Abe R, Tada T, Okumura K, Ransom J T, Nakayama T. CD28 costimulation accelerates IL-4 receptor sensitivity and IL-4-mediated Th2 differentiation. J Immunol. 1999;163:2432–2442. [PubMed] [Google Scholar]
- 29.Lenschow D J, Walunas T L, Bluestone J A. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y J, Zhang J, Lane P J, Chan E Y T, MacLennan I C M. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur J Immunol. 1991;21:2951–2962. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- 31.Lycke N, Tsuji T, Holmgren J. The adjuvant effect of Vibrio cholerae and Escherichia coli heat-labile enterotoxins is linked to their ADP-ribosyltransferase activity. Eur J Immunol. 1992;22:2277–2281. doi: 10.1002/eji.1830220915. [DOI] [PubMed] [Google Scholar]
- 32.Martin M, Metzger D J, Michalek S M, Connell T D, Russell M W. Comparative analysis of the mucosal adjuvanticity of the type II heat-labile enterotoxins, LT-IIa and LT-IIb. Infect Immun. 2000;68:281–287. doi: 10.1128/iai.68.1.281-287.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagai Y, Iwamori M. Ganglioside distribution at different levels of organization and its biological implications. Adv Exp Med Biol. 1984;174:135–136. doi: 10.1007/978-1-4684-1200-0_12. [DOI] [PubMed] [Google Scholar]
- 34.Nashar T O, Hirst T R, Williams N A. Modulation of B-cell activation by the B subunit of Escherichia coli enterotoxin: receptor interaction up-regulates MHC class II, B7, CD40, CD25 and ICAM-1. Immunology. 1997;91:572–578. doi: 10.1046/j.1365-2567.1997.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nashar T O, Webb H M, Eaglestone S, Williams N A, Hirst T R. Potent immunogenicity of the B subunits of Escherichia coli heat-labile enterotoxin: receptor binding is essential and induces differential modulation of lymphocyte subsets. Proc Natl Acad Sci USA. 1996;93:226–230. doi: 10.1073/pnas.93.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paul W E. Interleukin 4/B cell stimulatory factor 1: one lymphokine, many functions. FASEB J. 1987;1:456–461. doi: 10.1096/fasebj.1.6.3315808. [DOI] [PubMed] [Google Scholar]
- 37.Pickett C L, Twiddy E M, Belisle B W, Holmes R K. Cloning of genes that encode a new heat-labile enterotoxin of Escherichia coli. J Bacteriol. 1986;165:348–352. doi: 10.1128/jb.165.2.348-352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pickett C L, Twiddy E M, Coker C, Holmes R K. Cloning, nucleotide sequence, and hybridization studies of the type IIb heat-labile enterotoxin gene of Escherichia coli. J Bacteriol. 1989;171:4945–4952. doi: 10.1128/jb.171.9.4945-4952.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pickett C L, Weinstein D L, Holmes R K. Genetics of type IIa heat-labile enterotoxin of Escherichia coli: operon fusions, nucleotide sequence, and hybridization studies. J Bacteriol. 1987;169:5180–5187. doi: 10.1128/jb.169.11.5180-5187.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 41.Snapper C M, Peschel C, Paul W E. IFN-gamma stimulates IgG2a secretion by murine B cells stimulated with bacterial lipopolysaccharide. J Immunol. 1988;140:2121–2127. [PubMed] [Google Scholar]
- 42.Spangler B D. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol Rev. 1992;56:622–647. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Svennerholm A M, Holmgren J. Identification of Escherichia coli heat-labile enterotoxin by means of a ganglioside immunosorbant assay (GM1-ELISA) procedure. Curr Microbiol. 1978;1:19. [Google Scholar]
- 44.Takahashi I, Marinaro M, Kiyono H, Jackson R J, Nakagawa I, Fujihashi K, Hamada S, Clements J D, Bost K L, McGhee J R. Mechanisms for mucosal immunogenicity and adjuvancy of Escherichia coli labile enterotoxin. J Infect Dis. 1996;173:627–635. doi: 10.1093/infdis/173.3.627. [DOI] [PubMed] [Google Scholar]
- 45.Tamura S, Shoji Y, Hasiguchi K, Aizawa C, Kurata T. Effects of cholera-toxin adjuvant on IgE antibody-response to orally. Vaccine. 1994;12:1238–1240. doi: 10.1016/0264-410x(94)90250-x. [DOI] [PubMed] [Google Scholar]
- 46.Thompson C B. Distinct roles for the costimulatory ligands B7–1 and B7–2 in T helper cell differentiation? Cell. 1995;81:979–982. doi: 10.1016/s0092-8674(05)80001-7. [DOI] [PubMed] [Google Scholar]
- 47.Truitt R L, Hanke C, Radke J, Mueller R, Barbieri J. Glycosphingolipids as novel targets for T-cell suppression by the B subunit of recombinant heat-labile enterotoxin. Infect Immun. 1998;66:1299–1308. doi: 10.1128/iai.66.4.1299-1308.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilson A D, Clarke C J, Stokes C R. Whole cholera toxin and B subunit act synergistically as an adjuvant for the mucosal immune system response of mice to keyhole limpet haemocyanin. Scand J Immunol. 1990;31:443–451. doi: 10.1111/j.1365-3083.1990.tb02791.x. [DOI] [PubMed] [Google Scholar]
- 49.Wolf A A, Jobling M G, Wimer-Mackin S, Ferguson-Maltzman M, Madara J L, Holmes R K, Lencer W I. Ganglioside structure dictates signal transduction by cholera toxin and association with caveolae-like membrane domains in polarized epithelia. J Cell Biol. 1998;141:917–927. doi: 10.1083/jcb.141.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu H-Y, Nguyen H H, Russell M W. Nasal lymphoid tissue (NALT) as a mucosal inductive site. Scand J Immunol. 1997;46:506–513. doi: 10.1046/j.1365-3083.1997.d01-159.x. [DOI] [PubMed] [Google Scholar]
- 51.Wu H-Y, Russell M W. Induction of mucosal and systemic immune responses by intranasal immunization using recombinant cholera toxin B subunit. Vaccine. 1998;16:286–292. doi: 10.1016/s0264-410x(97)00168-0. [DOI] [PubMed] [Google Scholar]
- 52.Yamamoto M, Kiyono H, Yamamoto S, Batanero E, Kweon M-N, Otake S, Azuma M, Takeda Y, McGhee J R. Direct effects on antigen-presenting cells and T lymphocytes explain the adjuvanticity of a nontoxic cholera toxin mutant. J Immunol. 1999;162:7015–7021. [PubMed] [Google Scholar]
- 53.Yamamoto S, Kiyono H, Yamamoto M, Imaoka K, Fujihashi K, Van Ginkel F W, Noda M, Takeda Y, McGhee J R. A nontoxic mutant of cholera toxin elicits TH2-type responses for enhanced mucosal immunity. Proc Natl Acad Sci USA. 1997;94:5267–5272. doi: 10.1073/pnas.94.10.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]