Abstract
Chronic graft-versus-host disease (cGVHD) is a serious complication after allogenic hematopoietic stem cell transplantation (allo-HSCT), negatively affecting the morbidity and mortality of recipients. Skin involvement is the most common cGVHD manifestation with a wide range of pleomorphic features, from scleroderma to ulcerations and microangiopathic changes. Despite the access to many immunosuppressive drugs, therapy for cGVHD is challenging. Systemic steroids are recommended as the first-line treatment; but, in steroid-resistant patients, extracorporeal photopheresis (ECP) remains one of the subsequent therapeutic options. Here, we present a case report of a 31-year patient suffering from advanced steroid-refractory skin and oral mucosa cGVHD who was spectacularly treated with ECP. It was the first time we observed such “overnight” resolution of the graft-versus-host disease syndrome. The present report proves the important role of ECP in the treatment of steroid-resistant cGVHD, especially when other immunosuppressive therapies have failed.
Keywords: allogeneic hematopoietic stem cell transplantation, extracorporeal photopheresis, graft versus host disease, immunosuppressive drugs
1. Introduction
Graft-versus-host disease (GVHD) is one of the leading causes of morbidity and mortality after allogeneic hematopoietic stem cell transplantation (allo-HSCT) [1]. Its pathogenesis is associated with inflammation, immune-mediated organ injury, and subsequent fibrosis [2]. Chronic GVHD (cGVHD) usually develops months after allo-HSCT, but it sometimes may occur as a prolongation of prior acute manifestation. Chronic GVHD may affect every organ, but skin involvement remains the most frequent manifestation with a wide range of pleomorphic features, from scleroderma to lichen planus-like eruption, depigmentation, microangiopathic changes, and poikiloderma [3].
Despite the wide access to many immunosuppressive drugs, the treatment of cGVHD remains a challenge. Steroids are still the first-line treatment; but, in steroid refractory patients, extracorporeal photopheresis (ECP) remains one of the subsequent therapeutic choices [4]. It is a leukapheresis-based immunomodulatory therapy in which collected lymphocytes are exposed to ultraviolet-A radiation in the presence of the photosensitizing agent, 8-methylpsoralen, and then reinfused to the patient [5]. The efficacy of ECP in cGVHD is well-documented with an overall response rate varying from 31% to 93%, and the highest efficacy is noted for those with skin involvement [6]. Of note is that prolonged ECP is usually needed for evident visual effect [2,6].
Here, we present the case of a 31-year-old patient suffering from steroid-refractory skin and oral mucosa cGVHD who promptly and spectacularly responded to ECP.
2. Case Report
A 31-year-old male patient underwent allo-HSCT from a 9/10 HLA-matched male unrelated donor for acute myeloid leukemia (AML) in June 2021. He was diagnosed with AML and translocation t(9;11) six months earlier. The patient received induction chemotherapy consisted of daunorubicin and cytarabine. As a consequence, he achieved complete remission (CR) with negative measurable residual disease (MRD). The remission was consolidated by two cycles of high-doses of cytarabine. As a myeloablative conditioning, busulfan and cyclophosphamide were provided. For GVHD prophylaxis, he received cyclosporine with methotrexate as well as anti-thymocyte globulin (Thymoglobulin).The transplantation procedure as well as the early post-transplant period were unremarkable, and the patient was found to have full donor chimerism at discharge. CR was confirmed using repeated bone marrow examination, and cyclosporin was gradually reduced till complete withdrawal five months after allo-HSCT.
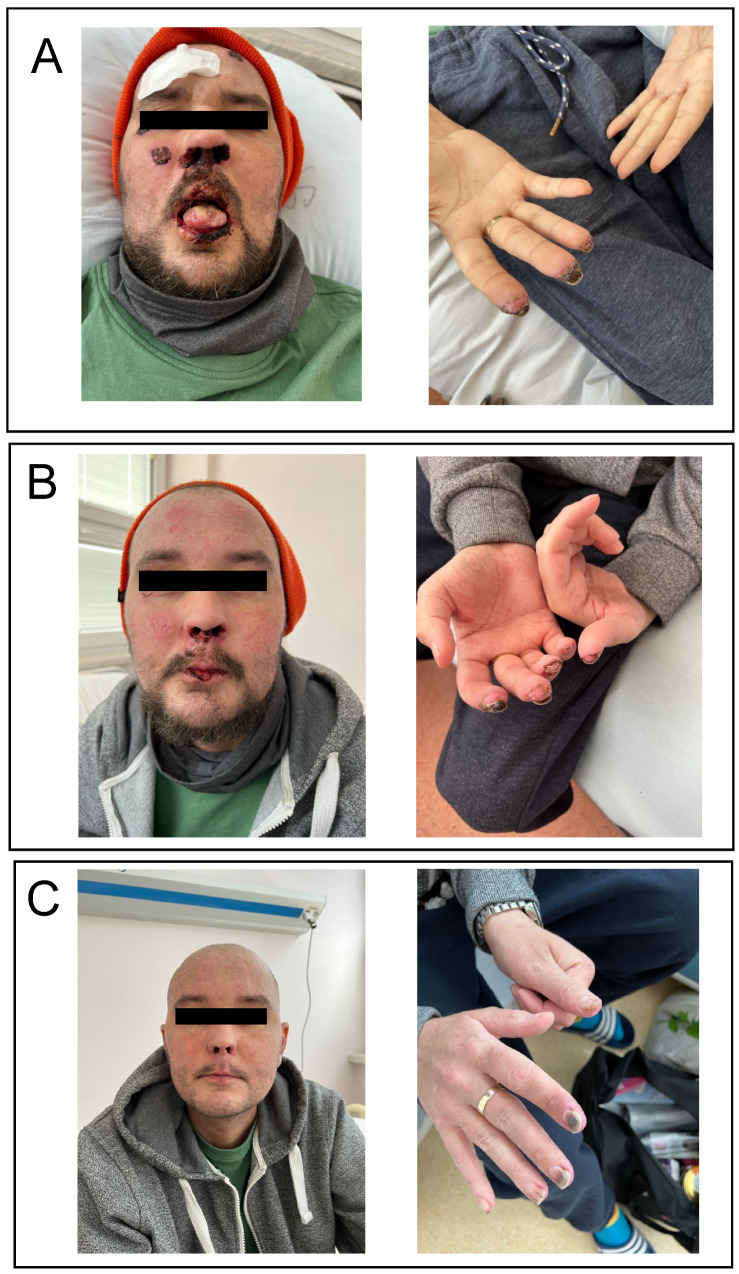
On day +212 after transplantation, while being off immunosuppressants, the patient was urgently admitted for progressive weakness, watery diarrhea (up to 3000 mL of stool daily), severe abdominal pain, and nausea. Blood count was within the normal limits. In biochemistry, hyperbilirubinemia (60 µmol/L; normal range 3.4–20.5) with elevated levels of transaminases and alkaline phosphatase was detected. Clostridium difficile as well as other bacterial, viral, and fungal infections were excluded. Finally, he was diagnosed with late onset acute GVHD grade 4 according to the MAGIC classification [7]. The patient received methylprednisolone (MP) at 2 mg/kg daily in combination with mycophenolate mofetil (MMF) 500 mg twice a day, but the symptoms deteriorated. The doses of MP were gradually diminished, MMF was discontinued, and ruxolitinib (RUX) was added at 5 mg twice a day. Due to the lack of response, Thymoglobulin at a total dose of 6.5 mg/kg was given on day +230 after allo-HSCT. As a result, normalization of serum bilirubin concentration and complete regression of gastrointestinal symptoms were observed. At the same time, symptoms of cGVHD affecting the skin as well as nasal and oral mucosa gradually developed. The patient was discharged on RUX, low doses of MP, and oral methotrexate once weekly. Despite the triple immunosuppressive therapy, cutaneous and oral manifestations progressed. Ten months after transplantation, the patient developed ulcerations with the presence of multiple necrotic foci as well as microangiopathic changes on face, oral mucosa, and distal phalanges (Figure 1A). Painful lesions in the mouth made it impossible to eat. Differential diagnosis excluded transplant-associated thrombotic microangiopathy and other possible causes of the present changes. He was diagnosed with severe cGvHD [8]. Due to pancytopenia, methotrexate was stopped and the patient started ECP while on low doses of MP and RUX. First, two procedures were performed on day +308 and +309 after allo-HSCT. Surprisingly, the visual effect of treatment was observed immediately on the next day (as presented on Figure 1B). The patient also reported an improvement in general condition and a reduction of pain. The continuation of ECP resulted in further resolution of skin and mucosa lesions (Figure 1C). Due to the reported favourable effects of RUX + ECP combination [9], the treatment was continued for six months. In total, the patient received eight ECP procedures which led to a complete deterioration of cGVHD symptoms. The dosage of RUX was diminished to 5 mg per day due to the occurrence of grade 3 thrombocytopenia. There were no significant infectious complications after the initiation of ECP treatment. At the time of observation, the patient remained in complete hematologic remission with negative MRD and full donor chimerism.
Figure 1.
Favourable effects of therapy with extracorporeal photopheresis (ECP) to treat skin and mucosa advanced chronic graft-versus-host disease (cGVHD): (A) presentation of symptoms before treatment (+304 day post allo-HSCT); (B) partial regression after two ECP procedures (+310 day post allo-HSCT); and (C) almost complete response after four ECP procedures (+326 day post allo-HSCT).
3. Discussion
The efficacy of ECP in cGVHD is well-established and reaches up to 70% in skin manifestation and 63% in oral mucosa, but the efficacy differs between studies [10]. Effective immunosuppressive treatment with ECP enables a decrease in steroid dose of more than 50%, which affects overall survival [11]. The procedures should be performed on two consecutive days every week and continued for at least eight cycles until there is a noticeable response [12]. The time till initial response to ECP differs between reports, but none of them describes an immediate improvement as presented in our case. The median time to response was 26 days in a paper by Couriel et al. [13]; however, it might be shorter [11].
Of note is that the differential diagnosis of microangiopathic changes presented in our patient includes TA-TMA, although GVHD may also play a role in the pathogenesis of this complication [14]. There is significant diversity among the diagnostic criteria of TA-TMA, but those presented by Jodele et al. [15] incorporate the recent findings in the field: (1) presence of schistocytes on blood smear; (2) elevated serum LDH; (3) proteinuria ≥ 30 mg/dL or hypertension; (4) de novo thrombocytopenia; (5) de novo anemia; and (6) elevation of terminal complement- sC5b-9 [14]. Although the measurement of terminal complement activation was not available in our center, our patient did not present any other abovementioned diagnostic criteria. It had been suggested that levels of sC5b-9, extracellular deoxyribonucleic acid-myeloperoxidase (DNA-MPO), and thrombin–antithrombin complex should be determined [16]. Higher levels of those parameters were detected in patients with TA-TMA when compared to GVHD patients [17].
One should remember that some drugs may have an impact on the development of microangiopathic changes. Calcineurin inhibitors, including cyclosporin A, tacrolimus, and sirolimus have been proven to increase the risk of TA-TMA, while steroids and MMF seem to be relatively safe in this aspect [18].
The exact mechanism of ECP in cGVHD is not well-understood. Some data suggest that ECP-induced T-cell tolerance depends on T-cell apoptosis and induction of regulatory T-cells [19]. The role of B-cells is also taken under consideration, but it requires further studies [20].
4. Conclusions
To the best of our knowledge, an “overnight” improvement of cGVHD after ECP has never been reported before. ECP may remain a valuable therapeutic option, especially for patients with skin and oral manifestation. The treatment with ECP should be initiated as soon as possible in those with steroid refractoriness.
Author Contributions
A.S.—conceptualization, investigation and writing—original draft preparation; I.G.-W.—investigation and writing—review and editing; M.P.-K.—investigation and visualization; K.G.—investigation and visualization; G.H.—supervision, conceptualization and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent was obtained from the patient to publish this paper.
Data Availability Statement
The data used in the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mohty B., Mohty M. Long-term complications and side effects after allogenic hematopoietic stem cell transplantation: An update. Blood Cancer J. 2011;1:e16. doi: 10.1038/bcj.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flowers M.E., Martin P.J. How we treat chronic graft-versus-host disease. Blood. 2015;125:606–615. doi: 10.1182/blood-2014-08-551994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strong Rodrigues K., Oliveira-Ribeiro C., de Abreu Fiuza Gomes S., Knobler R. Cutaneous graft-versus-host disease: Diagnosis and treatment. Am. J. Clin. Dermatol. 2018;19:33–50. doi: 10.1007/s40257-017-0306-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Penack O., Marchetti M., Ruutu T., Aljurf M., Bacigalupo A., Bonifazi F., Ciceri F., Cornelissen J., Malladi R., Duarte R.F., et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: Updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020;7:e157–e167. doi: 10.1016/S2352-3026(19)30256-X. [DOI] [PubMed] [Google Scholar]
- 5.Cho A., Jantschitsch C., Knobler R. Extracorporeal photopheresis-an overview. Front. Med. 2018;5:236. doi: 10.3389/fmed.2018.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drexler B., Buser A., Infanti L., Stehle G., Halter J., Holbro A. Extracorporeal photopheresis in graft-versus-host-disease. Transfus. Med. Hemother. 2020;47:214–224. doi: 10.1159/000508169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris A.C., Young R., Devine S., Hogan W.J., Ayuk F., Bunworasate U., Chanswangphuwana C., Efebera Y.A., Holler E., Litzow M., et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: A report from the Mount Sinai Acute GVHD International Consortium. Biol. Blood Marrow Transplant. 2016;22:4–10. doi: 10.1016/j.bbmt.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jagasia M.H., Greinix H.T., Flowers M.E. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging working group report. Biol. Blood Marrow Transplant. 2015;21:389–401. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maas-Bauer K., Kiote-Schmidt C., Bertz H., Apostolova P., Wäsch R., Ihorst G., Finke J., Zeiser R. Ruxolitinib-ECP combination treatment for refractory severe chronic graft-versus-host disease. Bone Marrow Transplant. 2021;56:909–916. doi: 10.1038/s41409-020-01122-8. [DOI] [PubMed] [Google Scholar]
- 10.Abu-Dalle I., Reljic T., Nishihori T., Antar A., Bazarbachi A., Djulbegovic B., Kumar A., Kharfan-Dabaja M.A. Extracorporeal photopheresis in steroid-refractory acute or chronic graft-versus-host disease: Results of a systemic review of prospective studies. Biol. Blood Marrow Transplant. 2014;20:1677–1686. doi: 10.1016/j.bbmt.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Oarbeascoa G., Lozano M.L., Guerra M.L., Amunarriz C., Saavedra C.A., Garcia-Gala J.M., Viejo A., Revilla N., Acosta Fleitas C., Arroyo J.L., et al. Grupo Español de Aféresis—Spanish Apheresis Group. Retrospective multicenter study of extracorporeal photopheresis in steroid-refractory acute and chronic graft-versus-host disease. Biol. Blood Marrow Transplant. 2020;26:651–658. doi: 10.1016/j.bbmt.2019.12.769. [DOI] [PubMed] [Google Scholar]
- 12.Mohammadi S., Malek Mohammadi A., Norooznezhad A.H., Heshmati F., Alimoghaddam K., Ghavamzadeh A. Extra corporeal photochemotherapy in steroid refractory graft versus host disease: A review of guidelines and recommendations. Transfus. Apher. Sci. 2017;56:376–384. doi: 10.1016/j.transci.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Couriel D.R., Hosing C., Saliba R., Shpall E.J., Anderlini P., Rhodes B., Smith V., Khouri I., Giralt S., de Lima M., et al. Extracorporeal photochemotherapy for the treatment of steroid-resistant chronic GVHD. Blood. 2006;107:3074–3080. doi: 10.1182/blood-2005-09-3907. [DOI] [PubMed] [Google Scholar]
- 14.Changsirikulchai S., Myerson D., Guthrie K.A., McDonald G.B., Alpers C.E., Hingorani S.R. Renal thrombotic microangiopathy after hematopoietic cell transplant: Role of GVHD in pathogenesis. Clin. J. Am. Soc. Nephrol. 2009;4:345–353. doi: 10.2215/CJN.02070508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jodele S., Laskin B.L., Dandoy C.E., Myers K.C., El-Bietar J., Davies S.M., Goebel J., Dixon B.P. A new paradigm: Diagnosis and management of HSCT-associated thrombotic microangiopathy as multi-system endothelial injury. Blood Rev. 2015;29:191–204. doi: 10.1016/j.blre.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lämmle B. Hematopoietic stem cell transplantation-associated thrombotic microangiopathy: Pathophysiology and differentiation from graft versus host disease. Thromb. Haemost. 2019;119:1382. doi: 10.1055/s-0039-1695731. [DOI] [PubMed] [Google Scholar]
- 17.Gavriilaki E., Chrysanthopoulou A., Sakellari I., Batsis I., Mallouri D., Touloumenidou T., Papalexandri A., Mitsios A., Arampatzioglou A., Ritis K., et al. Linking complement activation, coagulation and neutrophils in transplant-associated thrombotic microangiopathy. Thromb. Haemost. 2019;119:1433–1440. doi: 10.1055/s-0039-1692721. [DOI] [PubMed] [Google Scholar]
- 18.Rosenthal J. Hematopoietic cell transplantation-associated thrombotic microangiopathy: A review of pathophysiology, diagnosis and treatment. J. Blood Med. 2016;7:181–186. doi: 10.2147/JBM.S102235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aubin F., Mousson C. Ultraviolet light-induced regulatory (suppressor) T-cells: An approach for promoting induction of operational allograft tolerance? Transplantation. 2004;77:S29–S31. doi: 10.1097/01.TP.0000112969.24120.64. [DOI] [PubMed] [Google Scholar]
- 20.Whittle R., Taylor P.C. Circulating B-cell activating factor level predicts clinical response of chronic graft-versus-host-disease to extracorporeal photopheresis. Blood. 2011;118:6446–6449. doi: 10.1182/blood-2011-05-354019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in the current study are available from the corresponding author upon reasonable request.