Abstract
Identifying relatively intact areas within ecosystems and determining the conditions favoring their existence is necessary for effective management in the context of widespread environmental degradation. In this study, we used 3766 surveys of randomly selected sites in the United States and U.S. Territories to identify the correlates of sites categorized as “oases” (defined as sites with relatively high total coral cover). We used occupancy models to evaluate the influence of 10 environmental predictors on the probability that an area (21.2‐km2 cell) would harbor coral oases defined at four spatial extents: cross‐basin, basin, region, and subregion. Across all four spatial extents, oases were more likely to occur in habitats with high light attenuation. The influence of the other environmental predictors on the probability of oasis occurrence were less consistent and varied with the scale of observation. Oases were most likely in areas of low human population density, but this effect was evident only at the cross‐basin and subregional extents. At the regional and subregional extents oases were more likely where sea‐surface temperature was more variable, whereas at the larger spatial extents the opposite was true. By identifying the correlates of oasis occurrence, the model can inform the prioritization of reef areas for management. Areas with biophysical conditions that confer corals with physiological resilience, as well as limited human impacts, likely support coral reef oases across spatial extents. Our approach is widely applicable to the development of conservation strategies to protect biodiversity and ecosystems in an era of magnified human disturbance.
Keywords: climate change, conservation, ecosystem, management, occupancy models, persistence, resilience
INTRODUCTION
Although all ecosystems have been impacted by human activities, they have not been affected equally (Bowler et al., 2020). There are examples of communities that are resilient (sensu O'Leary et al., 2017) to contemporary environmental conditions despite an increasing frequency and intensity of disturbances (Davis et al., 2013; Turner & Corlett, 1996), whereas other communities persist by escaping the stressors causing degradation in other areas (e.g., Kavousi & Keppel, 2018). Although the mechanisms contributing to community persistence in an anthropogenically disturbed world are not fully understood, there is growing interest in such “bright spots” (Cinner et al., 2016; O'Leary et al., 2017) or “oases” (Guest et al., 2018). There is an urgent need to identify these areas, and to determine the conditions favoring their occurrence in order to prioritize areas for human intervention, an approach known as “predict‐and‐prescribe” management (Webster et al., 2017).
Coral cover has declined globally in recent decades primarily as a result of the combined effects of climate warming and land‐use changes (Bruno & Selig, 2007; Gardner et al., 2003). These declines are predicted to continue into the future as global warming exacerbates coral mortality due to bleaching and disease (Donner et al., 2005; Randall & van Woesik, 2017). Despite past, current, and projected declines in coral cover, a few reef locations have maintained higher coral cover relative to neighboring locations, and these have been referred to as “oases” (Guest et al., 2018; Lirman et al., 2011). Reef oases in Guest et al. (2018) were defined by coral cover that was high relative to nearby sites, but oasis status was also associated with relatively high coral community calcium carbonate production, an emergent property of coral reefs with important functional implications (Courtney et al., 2020; Perry & Alvarez‐Filip, 2019). In most cases, it is unclear whether reef oases have maintained their high coral cover by escaping, resisting, or recovering from disturbances. Regardless of their mechanistic origins, oases may serve as sources of functionally important organisms to repopulate other locations while work is undertaken to mitigate the effects of environmental change and to restore degraded reefs (Darling et al., 2019; Guest et al., 2018; O'Leary et al., 2017).
Predictions of reef condition have been aided by advances in computing and the availability of remotely sensed environmental data. State variables in recent predictive studies of coral reef condition have included fish biomass (Cinner et al., 2016; Williams, Baum, et al., 2015), benthic cover (Darling et al., 2019; Robinson et al., 2018; Williams, Gove, et al., 2015), and the extent of coral bleaching (Barnes et al., 2015; Donovan et al., 2020; Safaie et al., 2018; Sully et al., 2019). Most studies to date have focused on biophysical predictors of reef condition (but see Cinner et al., 2016; Darling et al., 2019), but local human impacts may disrupt functionally important associations between the abundance of benthic taxa and their biophysical predictors (Williams, Gove, et al., 2015). Moreover, local human impacts tend to be weaker correlates of the abundance of benthic organisms than fish biomass, probably because fishing directly influences fish populations (Bruno et al., 2019). Changes in the human use of coastal land can directly impact benthic community structure in near‐shore habitats (Fabricius, 2005), but reefs with relatively high coral cover can be found in heavily disturbed environments (Guest et al., 2016; Morgan et al., 2016).
Despite the advances made in characterizing the conditions associated with relatively high coral cover (e.g., Darling et al., 2019; Robinson et al., 2018), most studies addressing this topic have favored biophysical and socioeconomic covariates of reef condition at large spatial scales (i.e., tens of thousands of kilometers). This focus has proved useful for understanding macroecological patterns on coral reefs but is less useful for the management of reefs at the scales typical of most management regimes (i.e., hundreds of kilometers). The essence of the oasis concept (sensu Guest et al., 2018) is to identify reefs that may be targeted for effective conservation and management based on their relatively high and stable coral cover, but this goal requires the capacity to predict the occurrence of oases at spatial scales relevant for management. Recognizing that a coordinated international effort to secure the future of all the world's reefs is needed (Hoegh‐Guldberg et al., 2018), here we focus on a gradient of spatial scales within the sociopolitical entity of the United States and U.S. Territories as a case study for identifying effective predictors of the occurrence of coral reef oases. We used occupancy models with imperfect detection (MacKenzie et al., 2002) to predict the probability of occurrence of a coral reef oasis for spatial units of typical remotely sensed data sets (i.e., 21.2 km2) rather than trying to predict coral cover at specific sites. We considered explicitly the effect of spatial scale when predicting the occurrence of coral reef oases by adjusting the spatial extent (sensu Wiens, 1989), i.e., the area over which we defined oases and standardized covariates. For example, we expected mean sea‐surface temperature to be associated positively with oasis occurrence at the cross‐basin extent, consistent with the global distribution of tropical coral reefs (Robinson et al., 2018; Williams, Gove, et al., 2015). However, the strength or direction of this association may not hold at regional or subregional extents depending on the degree of localized adaptation or acclimatization in corals (Oliver & Palumbi, 2011; Palumbi et al., 2014). The occupancy modeling framework presented here can be applied to any ecosystem in which oases of high organismal abundance (or diversity, ecosystem function, etc.) can be defined and in situations where identifying predictors of their occurrence is desirable.
METHODS
Previously, we developed a framework for identifying coral reef oases based on spatiotemporal variability in coral cover (Guest et al., 2018). An “oasis” was conceptualized as a reef site (10–100 m2) exhibiting consistently higher coral cover relative to other sites within the same region (~80–17,000 km2); “consistently” referred to the proportion of occasions over a decadal scale when a reef exhibited coral cover above the regional mean value. Here, we evaluated whether the locations of coral reef oases could be predicted from environmental conditions. Modeling the probability of occurrence of coral reef oases required data over a large spatial extent, which was obtained at the expense of temporal sampling at any one site. Therefore, if we wish to use “snapshot” data (i.e., from a single survey) instead of time‐series data, we must evaluate the assumption that snapshot data of coral cover can be used to identify reef oases with an effectiveness that is similar to that achievable using results of surveys over multiple years (as in Guest et al., 2018). This was done by calculating the sensitivity (true positive rate) and specificity (true negative rate) of oasis detection using simulations of the spatiotemporal data in Guest et al. (2018) (Appendix S1: Figure S1). The sensitivity of oasis detection was high (>95%) for three of four geographic regions included in Guest et al. (2018), even when only 1 year of the time series data was used (Appendix S1: Figure S1). The specificity of oasis detection was also very high (>95%) for all four regions. These results demonstrate that using snapshot data to designate oases may occasionally overlook exceptional sites but will rarely misidentify an average or below‐average site as an oasis. Given these results, we conceptualized a coral oasis as a reef site that exhibited higher coral cover relative to sites within a defined spatial extent based on a single survey during the study period.
In order to evaluate the occurrence of coral oases, we sought biotic data in the public domain that provided: broad spatial coverage (i.e., sampling across millions of square kilometers), large sample size (i.e., thousands of sites), and random selection of sites. Data from the United States National Oceanic and Atmospheric Administration's (NOAA) Coral Reef Conservation Program's (CRCP) National Coral Reef Monitoring Program met these criteria and were accessed for a 6‐year period (2012–2017). The data were structured according to the following spatial hierarchy: cross‐basin, basin, region, and subregion (Appendix S1: Section S1 and Appendix S2: Table S1). Regional and subregional scales are most relevant to reef management (NOAA Coral Program, 2014). The cross‐basin spatial extent included the entire data set, while the basin spatial extent was applied to the western Atlantic and Pacific (0.6–1.2 × 106 km2). Nested within these two basins were seven regions (Florida, Puerto Rico, U.S. Virgin Islands, main Hawaiian Islands, northwest Hawaiian Islands, Mariana Islands, American Samoa; 0.4–11 × 104 km2, median = 4.4 × 104 km2) and 32 subregions (e.g., Tortugas, lower Keys, middle Keys, upper Keys, and southeast Florida in the Florida region, as well as islands in most other regions; 8–12,000 km2, median = 572 km2). At each site (n = 3766), coral cover was assessed for a 30‐m transect using photographic images of 0.7‐m2 quadrats. Using these data, the total cover of all reef‐building corals (including the octocoral Heliopora coerulea and the hydrozoan Millepora spp.) was calculated for each reef. The period sampled for these data included worldwide bleaching events that occurred between 2014 and 2017 (Eakin et al., 2019). Therefore, we tested whether the year in which reefs were sampled influenced coral cover by comparing a linear mixed‐effects model (random intercepts of subregion) with a coefficient for year to a null model with only an intercept term. We found no effect of year on coral cover during our study (χ2 = 0.08, p = 0.78; Appendix S1: Figure S2).
We chose 10 environmental covariates hypothesized to be associated with oasis occurrence either at a broad, macroecological spatial extent over millions of square kilometers, or at smaller spatial extents over hundreds of square kilometers relevant for local management, or both (Appendix S1: Table S2). Covariates were resolved at the 2.5 arc‐minute scale (21.2 km2) at varying temporal resolutions. For each grid cell (n = 890), we calculated the mean and coefficient of variation of monthly sea‐surface temperature (SST; °C) and monthly light attenuation (Kd490, m−1) from 2003 to 2017 (Appendix S1: Section S1). Light attenuation, as measured by Kd490, results from suspended particles (which both absorb and scatter light) and dissolved organic matter (DOM; which only absorbs light). In contrast, turbidity describes only particles, and thus we do not use the term turbidity to describe Kd490. We also retrieved published estimates (Yeager et al., 2017) of primary productivity (mean of weekly means, 2003–2013; mg C m−2 day−1), wave energy flux (mean of weekly means, 1971–2009; kW m−1), land area ≤50 km from each site (km2), and human population density (in 2015) ≤50 km from each site. Finally, we calculated the total number of category 3, 4, and 5 storms (on the Saffir‐Simpson scale) passing ≤100 km from each site during the 30 years prior to the year of sampling (Knapp et al., 2010). We did not include seawater depth as a predictor in the model because depth varies considerably within a 2.5 arc‐minute grid cell, and moreover, the NOAA sampling protocol uses stratified random sampling by depth (e.g., 0–6, 6–18, 18–30 m depth in the Pacific; 0–10, >10 m depth in the Atlantic). In addition, a statistical analysis demonstrated no effect of depth on coral cover in our data set (Appendix S1: Figure S3).
Once we had assembled a database of coral cover and the environmental covariates, we proceeded with a workflow to generate a gridded database of coral oases (Figure 1). Coral oases exhibiting exceptional coral cover relative to nearby sites were defined at the four spatial extents. A site was considered exceptional if its coral cover was >2 standard deviations (SD) above the mean coral cover of sites (i.e., z score > 2) within the chosen spatial extent; a site could be considered an oasis at one spatial extent but not another. This threshold was set higher than the z score of >0 applied in Guest et al. (2018) because “snapshot” data were used in the current analysis. A higher threshold to detect coral oases was necessary to achieve the high sensitivity and specificity of oasis designation (Appendix S1: Section S1).
FIGURE 1.
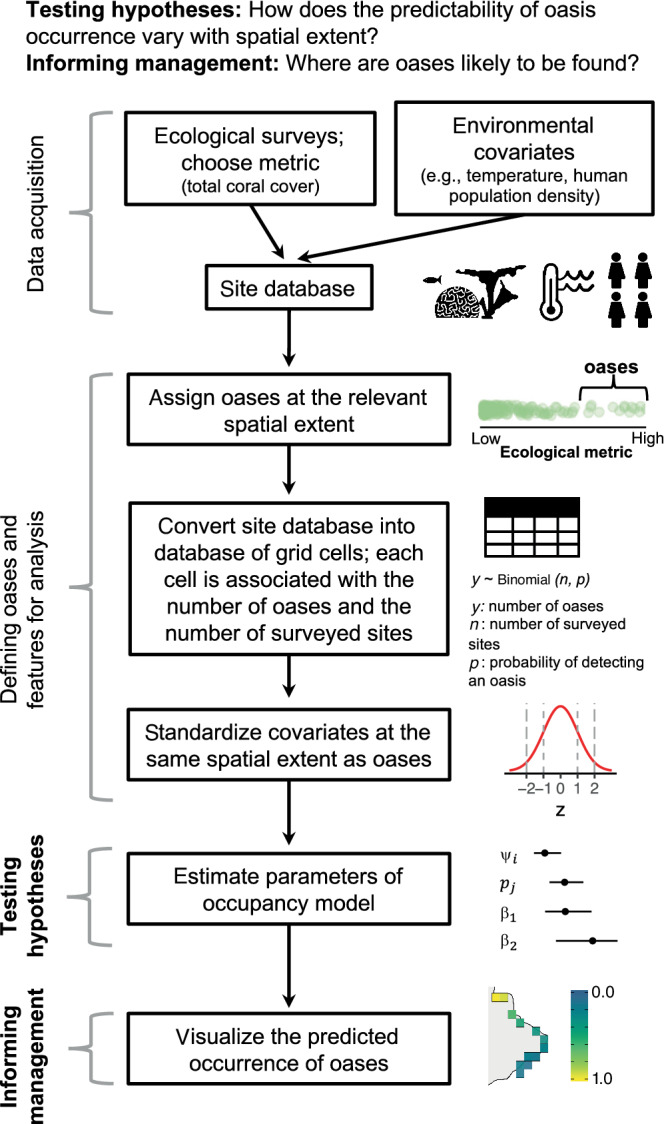
A schematic of the steps necessary to proceed from a database of ecological surveys and environmental covariates to predictions of oasis occurrence.
To evaluate how the environmental covariates of oasis designation varied spatially, we compared qualitatively the results of four statistical models, one for each spatial extent, in which the covariates were standardized at the same spatial extent at which we defined the oases (Appendix S1: Figures S4–S7). By standardizing predictors relative to each spatial extent, an increase of one unit will differ (in absolute terms) between each basin, region, or subregion, but the relative increase is consistent. This is different from most global‐scale syntheses of ecological data, where predictors are scaled relative to the entire data set (e.g., Cinner et al., 2016; Darling et al., 2019). To avoid multicollinearity, we removed predictors with the highest variance inflation factors (VIFs) and Pearson correlation coefficients (r) sequentially until all VIFs were <3 and correlation coefficients were <0.7 (Appendix S1: Table S3). Land area was correlated strongly with human population density at cross‐basin (r = 0.84), basin (r = 0.83), and regional extents (r = 0.74); land area was therefore removed from all analyses. Mean SST was correlated strongly with the CV of SST at the basin scale (r = −0.80) and had a high VIF (3.0) at the cross‐basin scale; mean SST was therefore removed from analysis at those two spatial extents.
Because coral oases are uncommon (by definition), any given grid cell is unlikely to harbor an oasis. Modeling the true oasis occurrence (presence/absence) in a grid cell is challenging because an absence of detected oases can arise from more than one process. Zeros can occur in a grid cell because that cell truly does not harbor an oasis or because we did not sample an oasis even though an oasis truly occurs in the cell. We considered these two processes independently with a site occupancy model (Appendix S2: Section S1). We assumed that the true state of oasis occupancy (i.e., occurrence) did not change over the course of the study because there was no evidence for trends in coral cover (Appendix S1: Figure S2). We modeled the true probability of oasis occurrence using a deterministic equation with the environmental covariates, and the detection probability using a binomial distribution with the number of trials (sampled sites) and successes (oases). We used group‐level intercepts for subregions to allow for the non‐independence of occurrence and detection. Our approach permitted inferences about oasis occurrence at spatial extents of tens to hundreds of square kilometers, rather than at individual sites (<100 m2). It also reflected the available resolution (21.2 km2) of many of our chosen socioenvironmental predictors.
Site occupancy models were conceived originally with respect to modeling the occurrence of species when the process of detection is imperfect (MacKenzie et al., 2002). When considering the detection of species, there are many reasons why a species may not be detected at a site (e.g., weather conditions, observer skill). Thus, detection probability in the species occupancy framework is estimated from repeated visits to the same site. In our case, however, detection probability was estimated from multiple sites (sampled only once) within a grid cell, and thus detection probability for a grid cell is a consequence of variation in space (and necessarily time, for logistical reasons). Therefore, detection in our modeling framework can be interpreted as the spatial consistency of detecting oases. For example, a high probability of oasis detection indicates that, within a particular grid cell, a high proportion of sites will exceed the chosen threshold of coral cover. In our models, we allowed detection probability to vary by subregion due to expected natural variability in the spatial consistency of oases.
Models were fit in a Bayesian framework. We discarded the first 40,000 iterations of each Markov chain as warm‐up, fit the models with 80,000 iterations per chain, and retained every 10th sample to reduce autocorrelation and file size; this resulted in a posterior sample of 8000 for each chain (24,000 iterations per parameter). We visually inspected the chains for convergence, confirmed that the upper confidence interval (97.5th percentile) of the scale‐reduction factor (R hat) was <1.05 for all parameters of each model, and ensured that the minimum effective sample size (n eff) was >1000 for all the parameters (Gelman et al., 2013). To assess model fit, we used graphical posterior predictive checks of observed and simulated data. We also calculated Bayesian p values for the discrepancy between the observed number of oases and simulated values using a Pearson χ2 metric and a Freeman‐Tukey metric, the latter being less sensitive to small expected values than the former (Kéry & Royle, 2016). Extremely small Bayesian p values (p < 0.05) indicate that the model predicted a smaller metric (e.g., the number of oases per grid cell) than observed in the data; extremely large Bayesian p values (p < 0.95) indicate that the model predicted a larger metric than observed in the data. Models were fit using a Gibbs sampler (Plummer, 2003) in R 4.0.3 (R Development Core Team, 2020). All data and code have been deposited in a permanent digital repository (Elahi et al., 2021).
The posterior predictive checks were useful to assess model fit but we sought to use the model in a more practical way relevant to reef managers. Specifically, we used the estimated probability of oasis occurrence (per grid cell) to inform the selection of grid cells for management and determined how this model‐based selection performed against a null model with respect to choosing cells with a detected oasis. For each Markov chain iteration, we selected the cells with the highest estimated probability of oasis occurrence and then calculated the total number of selected cells with an oasis detection. The total number of selected cells was equivalent to the observed number of cells with detected oases, at the appropriate spatial extent. That is, for the cross‐basin analysis, at least one oasis was detected in 116 of 890 cells, and thus we selected 116 cells with the highest probability of oasis occurrence at every iteration; the response of interest was the percentage of these cells with an oasis detection. This procedure resulted in a posterior distribution of the percentage of oasis detection in cells chosen by the statistical model, derived from the posterior probability distributions of the model parameters. We compared the performance of our statistical model to a null model, which selected cells randomly from each level (as appropriate) in the hierarchy. For example, at the basin scale, 66 cells were chosen from the western Atlantic and 59 cells were chosen from the Pacific, and similarly, for regions and subregions. In an extreme case, if a subregion did not harbor any oases, the null model did not sample any cells from that subregion. Thus, the null model used more information than choosing cells entirely at random.
RESULTS
Across the entire data set of sites in the United States and U.S. Territories, mean coral cover was 11% ± 14% (mean ± SD; n = 3766) from 2012 to 2017. Our designated threshold for a coral oasis at the cross‐basin scale was 39.3% coral cover (Figure 2a). In the western Atlantic (n = 1994) and Pacific (n = 1772) ocean basins, mean coral cover was 7% ± 8% and 16% ± 17%, respectively, and the corresponding oasis thresholds were 23.5% and 50.6% coral cover (Figure 2a). At the regional scale, mean coral cover ranged from 7% to 8% (SD 8%) in the western Atlantic, and 12%–26% (SD 13%–18%) in the Pacific. Corresponding oasis thresholds ranged from 23.1% to 23.9% in the western Atlantic, and 40.5% to 61.9% in the Pacific. At the subregional scale, mean coral cover ranged from 2% to 33% (SD, 3%–23%) and oasis thresholds at this scale ranged from 7% to 67% coral cover. Given the differences in mean coral cover between the ocean basins, the percentage of sites classified as oases depended on the spatial extent at which oases were defined. When oases were defined at the cross‐basin extent, the percentage of sites identified as oases in the Pacific was highest (5%–23% of sites), while oases were rare in the western Atlantic (0.1% of sites; Figure 2b). However, when coral oases were defined at the regional extent, the percentage of sites categorized as oases ranged from 4% to 8% and was similar between the western Atlantic and Pacific basins. At the subregional extent, the percentage of sites categorized as oases ranged from 0% to 10%.
FIGURE 2.
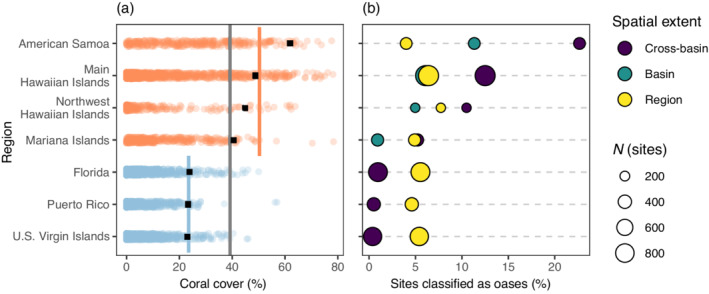
The proportion of sites classified as coral cover oases in a region depends on the spatial extent of comparison. In (a), coral cover (%) is plotted for each site (n = 3766), represented by small translucent points colored according to ocean basin (blue, western Atlantic; orange, Pacific). The gray line represents the threshold of coral cover (mean + 2 SD) above which a given site is designated an oasis for the entire data set (cross‐basin). Similarly, the colored lines represent the threshold for all regions within each basin. The black squares represent the threshold for each region. The raw data in (a) are summarized in (b), where the percentage of sites classified as oases is plotted for each region and spatial extent.
Similar to the results for oases, the spatial extent at which environmental correlates were standardized influenced patterns across regions. When standardized to the entire data set (i.e., the cross‐basin extent), environmental correlates displayed considerable variation within and between ocean basins (Appendix S1: Figure S4). Pacific sites displayed lower primary productivity, higher mean wave exposure, and fewer storms than sites in the western Atlantic. Sea‐surface temperature (SST) differed greatly among regions in both ocean basins, reflecting the latitudinal range of the sites included in our data set. However, when standardized at regional and subregional extents, these inter‐ocean differences were largely eliminated (Appendix S1: Figures S5–S7).
We used Bayesian hierarchical occupancy models to estimate the probability of occurrence of oases at different spatial extents. The models fit the data well, as indicated by graphical posterior predictive checks and Bayesian p values between 0.12 and 0.46 (Appendix S1: Table S4). Therefore, we proceeded with inference on the estimated parameters. To aid in the discussion of the environmental covariates of occurrence of oases, we describe a positive or negative association between a covariate and the probability of an oasis when the 80% Bayesian credible intervals (CI) did not include zero (Figure 3). When the 80% CI did overlap zero, we considered these trends to be less credible; we discuss these less credible trends in the context of the proportion of Markov chain iterations (p mc) that were greater or less than 0 (Appendix S1: Table S5).
FIGURE 3.
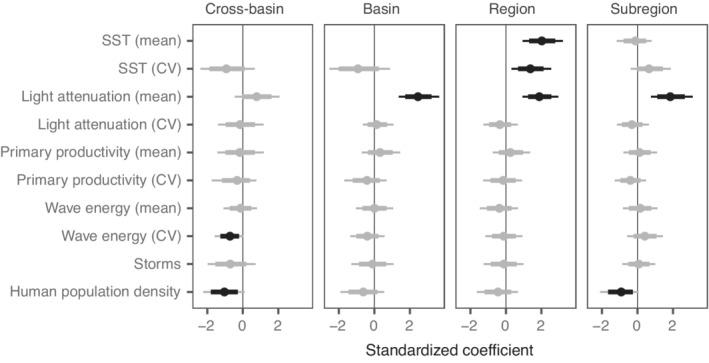
Relationships between covariates and the probability of oasis occurrence () vary with spatial extent. Standardized coefficients are Bayesian posterior median values with 95% and 80% credible intervals (thin and thick lines, respectively). Points and credible intervals are colored black when the intervals do not overlap 0. Each panel represents the spatial extent at which the covariates were standardized; from left (cross‐basin) to right (subregion), the relationships shift from a macroecological perspective to a local perspective. SST, sea‐surface temperature; CV, coefficient of variation.
The most consistent and credible predictors of oasis occurrence were mean light attenuation and human population density (Figure 3). Mean light attenuation was correlated positively with oasis occurrence at all spatial extents (0.89 < p mc ≤ 1), but this effect was less credible at the cross‐basin extent. Human population density was correlated negatively with oasis occurrence at all spatial extents (0.78 < p mc ≤ 0.97), but this effect was most credible at the cross‐basin and subregional extents. Variability in SST was correlated negatively with oasis occurrence at the cross‐basin and basin extents (0.85 < p mc ≤ 0.88) but was associated positively with oasis occurrence at regional and subregional extents (p mc > 0.89); the strongest effect was observed at the regional scale (Figure 3). Due to the negative correlation between the mean and CV of SST at large spatial extents, we included mean SST as a predictor in the model only for the two smallest spatial extents. At the regional scale, mean SST was associated positively (p mc = 1) with oasis occurrence, but there was no effect at the subregional scale. Variability in wave energy was correlated negatively with oasis occurrence at the cross‐basin scale (p mc > 0.96) but this negative relationship became weaker with smaller spatial extents and eventually positive at the subregional scale (p mc > 0.79). The remaining predictors had minimal influence on the probability of oasis occurrence (Figure 3).
Estimates of oasis occurrence (median ) varied between subregions at all spatial extents (Figure 4). There was also considerable variability in median ψ within subregions (i.e., interquartile ranges in Figure 4), reflecting variation in the environmental predictors among grid cells. For example, the Lower and Upper Florida Keys at the basin and regional scales displayed more variation in median ψ than the Middle Keys and the Tortugas subregion (Figure 4). At the subregional extent, median ψ was similar among and within subregions, because oases were defined in each subregion. In general, the estimated median ψ was higher than the observed proportion of oases (i.e., naïve occurrence; diamonds in Figure 4). However, in a few subregions at certain spatial extents (e.g., Molokai at cross‐basin, basin, and regional extents), naïve occurrence was comparable to estimated occurrence, owing to higher detection probabilities (p) in those subregions (Figure 5). That is, when detection probability is low, the naïve estimate of occurrence will necessarily be an underestimate of true occurrence (MacKenzie et al., 2002). An extreme case of this discrepancy was observed in the northwest Hawaiian Islands. In the French Frigate subregion, naïve occurrence was relatively high (and comparable to the lower bounds of median ψ) at cross‐basin, basin, and regional extents. However, naïve occurrence dropped to zero at the subregional extent because no sites exceeded the coral cover threshold at the subregional extent (67.7%), and thus no oases were detected (Figure 4).
FIGURE 4.
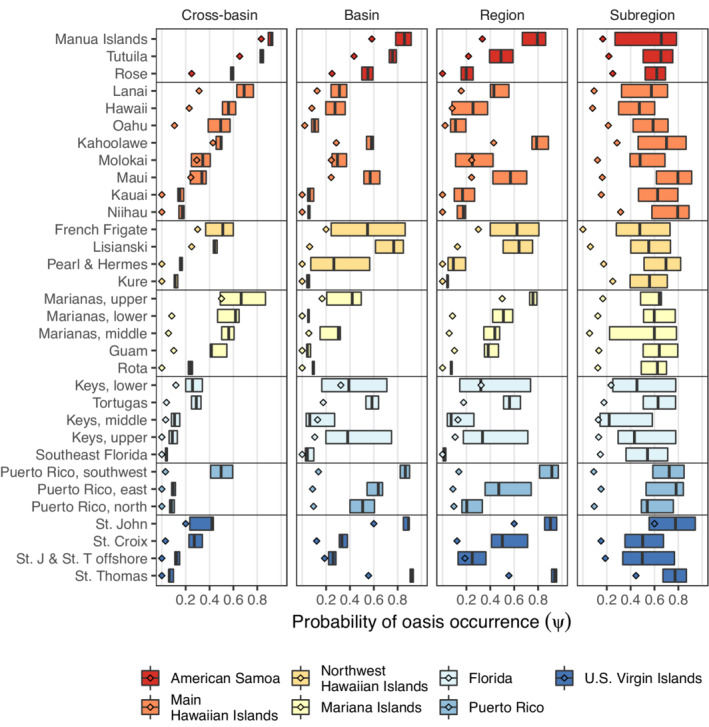
The probability of oasis occurrence () within each subregion and by spatial extent. Boxplots indicate the median and quartiles of posterior median probability of oasis occurrence for grid cells within each subregion. Diamonds indicate the observed proportion of grid cells with an oasis (i.e., naïve oasis occurrence).
FIGURE 5.
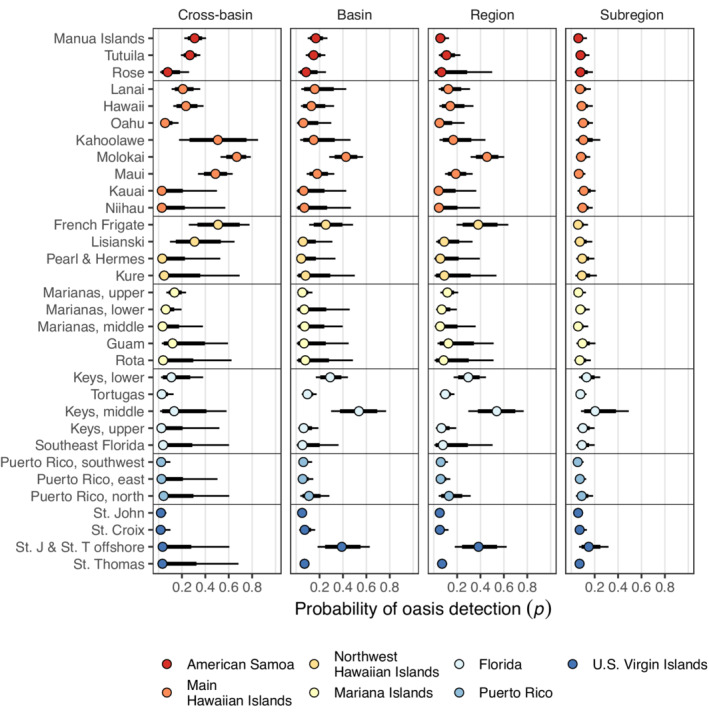
The probability of detecting an oasis (p) for each subregion and by spatial extent. Probabilities are Bayesian posterior median values with 95% and 80% credible intervals (thin and thick lines, respectively).
A high detection probability (p) indicates that within a grid cell, a high proportion of visited sites was defined as an oasis. Similar to the probability of occurrence, the probability of detection was permitted to vary by subregion in our hierarchical model. In general, detection probability was low (<0.1) and varied considerably within subregions at the cross‐basin extent, except for a few Pacific subregions (e.g., Molokai and Manua Islands). At the basin and regional spatial extents, detection probability tended to increase and become less variable, particularly for subregions with low detection probability at the cross‐basin extent (e.g., the western Atlantic). At the subregional extent, detection probability was the lowest and least variable, owing to the definition of oases at that extent.
The occupancy model performed consistently better than a null model when selecting a subset of cells based on predicted oasis occurrence (ψ; Appendix S1: Figure S8). The median percentage of selected cells with oasis detections ranged from 25% to 29% for the occupancy model, but 13%–20% for the null model. The performance of the null model improved as spatial extent declined, a consequence of how oases were defined. For example, if oases were defined within a subregion, the probability of randomly selecting a cell with a detected oasis in that subregion was higher due to a higher probability of oasis occurrence (by definition) and a smaller sample of grid cells.
Estimates of oasis occurrence and detection probability aggregated by subregion (Figures 4 and 5) offer a useful summary of the model output. However, reef managers make decisions about management in a spatial and environmental context. Across the entire data set, light attenuation and SST (mean and CV) were associated positively with oasis occurrence at the regional spatial extent (Figure 3). Therefore, we examined these predictors in a case study of four subregions in Florida at the regional extent (Figure 6), omitting one subregion (southeast Florida) due to nearly zero oasis occurrence (Figure 4). Nearly all the surveyed grid cells in these four subregions were within the boundaries of the Florida Keys National Marine Sanctuary, Florida State Parks, or U.S. National Parks (Figure 6a). The probability of oasis occurrence was highest in the Tortugas subregion (which includes the Dry Tortugas and the Marquesas Keys) and nearshore grid cells (i.e., containing Hawk Channel patch reefs) in the Florida Keys. Particularly in the Keys, the median probability of oasis occurrence was associated with high light attenuation (Figure 6b) and SST variability (Figure 6c). Notably, light attenuation was correlated positively with SST variability (Appendix S1: Figure S9), and the latter was correlated negatively with mean SST (Appendix S1: Figure S10). Together, these patterns of covariance resulted in a lower probability of oasis occurrence in warmer waters (Appendix S1: Figure S10), contrary to the overall model results at the regional extent (Figure 3). In conclusion, given the data and model, the Tortugas subregion and nearshore waters of the Florida Keys are most likely to harbor coral oases in Florida, and are within the boundaries of existing managed areas. Maps of the probability of oasis occurrence for all the subregions are available in the permanent digital repository (Elahi et al., 2021).
FIGURE 6.
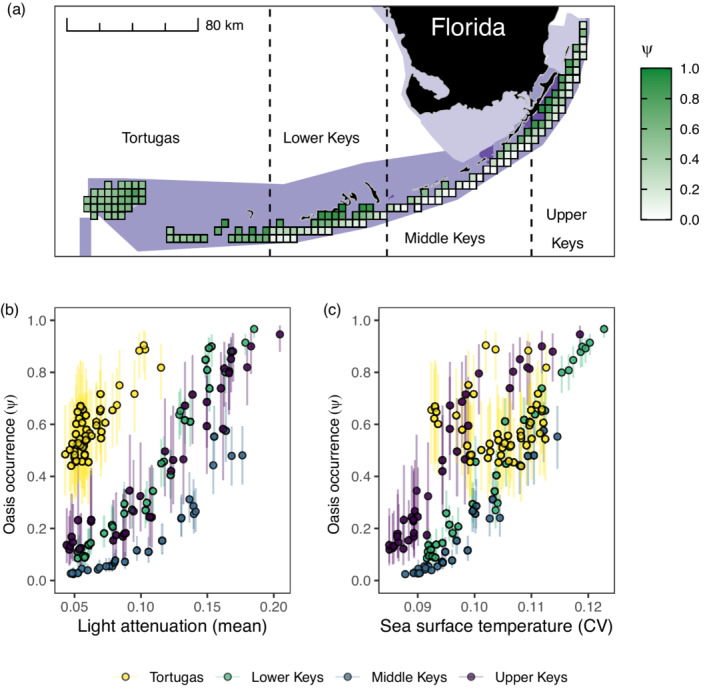
The median probability of oasis occurrence () in grid cells of four subregions (Tortugas, Lower Keys, Middle Keys, Upper Keys) in Florida, USA, plotted geographically (a) and as a function of mean light attenuation (Kd490, m−1; b) and the coefficient of variation (CV) in monthly sea surface temperature (SST in °C; c). Oases were defined at the regional spatial extent, i.e., across the five subregions in Florida (the southeast Florida subregion was not included in the map for figure clarity). In (a), grid cells represent 21.2 km2 areas that were surveyed for coral cover at least once. Light, medium, and dark purple polygons represent areas within United States National Parks, the Florida Keys National Marine Sanctuary, and Florida State Parks, respectively. In (b) and (c), error bars are 50% credible intervals.
DISCUSSION
The selection of a spatial or temporal scale at which to view ecological patterns affects the interpretation of their underlying causes, and consequently, has implications for ecosystem management. One aspect of spatial scale is the area encompassed by the study, referred to as spatial extent. In this study, we first showed that the choice of spatial extent (cross‐basin, basin, region, and subregion) determined whether sites were designated as coral oases. Next, we used occupancy models to test the effects of environmental covariates on the designation of oases across different spatial extents. Finally, we applied model predictions to a case study in Florida to demonstrate the utility of our approach to ecologists and managers.
The designation of sites as oases is a necessary first step in determining their association with environmental correlates. Central to this designation is identifying the benchmark for comparison. As a result of differences in average coral cover, the designation of a site as an oasis depended on the spatial extent. The 50% coral cover threshold required to classify a site as an oasis in the Pacific Ocean was far greater than the 23% required to classify a site as an oasis in the western Atlantic. The baseline levels of coral cover between these two ocean basins are striking and the difference is likely related to basin‐scale discrepancies in the ability of coral communities to recover from disturbances. These discrepancies have been hypothesized to stem from variation in species redundancy within functional groups (sensu Rosenfeld, 2002), the propensity of macroalgal blooms, and herbivory (Roff & Mumby, 2012). In our study, sites in the western Atlantic displayed higher levels of net primary productivity, more major storms, and lower wave energy than sites in the Pacific, highlighting the distinct environments of the sites in these basins (Appendix S1: Figure S4). Despite these stark differences, our models highlighted several useful environmental predictors of oasis occurrence across basins.
The strongest and most consistent predictor of oasis occurrence was light attenuation in seawater (Kd490). Higher values of Kd490 represented greater light attenuation and thus lower water clarity and vice versa. The remotely sensed measurement of light attenuation is influenced simultaneously by a number of physical and biological processes in the water column and can have both positive and negative effects on coral physiology. For example, solar irradiance is an important requirement for reef corals to sustain symbiont photosynthesis (Falkowski et al., 1990), but excessive light can exacerbate temperature‐related bleaching in corals (Lesser et al., 1990). Protection from high light, through cloud cover or productive nearshore waters, can ameliorate coral bleaching associated with exceptional warming events (Fitt & Warner, 1995; Mumby et al., 2001). Another possible explanation for the positive effect of light attenuation could be increased plankton availability for heterotrophic feeding by corals, which can help some species better resist or recover from heat stress (Grottoli et al., 2006). Turbidity, however, is typically considered a stressor to corals that requires specific mechanisms of adaptation (Anthony & Larcombe, 2000) due to the smothering effects of sedimentation and associated reductions in available light for photosynthesis (Kleypas, 1996). We found that mean light attenuation was associated positively with the occurrence of coral oases at all spatial extents, suggesting a net advantage of high light attenuation (i.e., low water clarity) for total coral cover.
The relationship between human population density and the occurrence of oases was consistently negative (78%–97% probability) and most evident at the largest and smallest spatial extents. From a causal perspective, human population size might be deleterious to coral cover because it is associated with activities that have negative effects on coral health (reviewed in Birkeland, 2015; Dubinsky & Stambler, 2010). Indeed, other studies carried out over spatial scales similar to our basins and regions have found that increased proximity to human populations has a deleterious effect on coral reef health (Sandin et al., 2008). An Indo‐Pacific‐wide study by Darling et al. (2019) found a negative association between human population size and coral cover, mediated primarily through declines in abundance of corals assigned to stress tolerant and generalist functional groups. However, in a global analysis, Bruno and Valdivia (2016) found only a weak relationship between human population size and coral cover, leading them to suggest that local‐scale reef management was unlikely to increase the resilience of coral cover to global‐scale disturbances. These previous studies, and our mixed results, support the notion that the relationship between human population size and coral abundance is complex. For example, reefs in marginal environments, close to urban centers, with relatively high rates of sedimentation can be resilient with high cover and diversity of corals (e.g., Singapore; Guest et al., 2016, and nearshore Great Barrier Reef; Morgan et al., 2016). Moreover, the estimate of human population density does not account for seasonal tourist populations, which may be considerable in certain regions and subregions. On balance, human population density may sometimes, but not always, serve as a proxy for the causal drivers of reef decline.
The effects of sea‐surface temperature (SST) on oasis occurrence were variable and depended on spatial extent. High SST variability was associated negatively with low oasis occurrence at cross‐basin and basin scales (>84% probability), but positively at the regional and subregional scales (>88% probability). At large spatial extents, SST variability was correlated negatively with mean SST, and thus the negative association between SST variability and oasis occurrence likely reflects the macroecological pattern of greater coral cover at lower latitudes (Robinson et al., 2018). At small spatial extents, our results agree with observations of lower bleaching rates on reefs exposed to more variable SST (Safaie et al., 2018; Sully et al., 2019), as well as evidence that corals have at least some capacity to acclimatize and adapt to thermal variation, thereby increasing their tolerance of high temperatures (Coles & Riegl, 2013; Oliver & Palumbi, 2011; Palumbi et al., 2014).
The other predictors we considered in our models were primary productivity, wave energy, and storms, all of which demonstrated weak and variable associations with oases. We found no association between primary productivity and the occurrence of coral oases, in contrast to the negative association between primary productivity and coral cover reported for the Indo‐Pacific (Darling et al., 2019), and the positive association between water‐column chlorophyll a concentration and coral cover in the central western Pacific (Robinson et al., 2018; Williams, Gove, et al., 2015). We also found no association between oasis occurrence and storms. This may be a consequence of the spatial resolution of this predictor (storms within a 100‐km radius of a site), because it is well known that storms can damage reefs at local spatial scales (Massel & Done, 1993; Woodley et al., 1981). Lower variability in wave energy was associated weakly with a higher probability of oasis occurrence, but only at the cross‐basin extent. This is likely due to unusually low levels of variability in wave energy in American Samoa (Appendix S1: Figure S4), which also had the greatest number of oases at the cross‐basin extent (Figure 2). In conclusion, the utility of primary productivity, wave energy, and storms as predictors of oasis occurrence appears minimal.
Oasis occurrence is best conveyed in a spatial context, and thus we used Florida as a case study to emphasize the model predictions at the regional extent. Many subregions in Florida are degraded and harbored very few oases at cross‐basin or basin extents, owing to widespread coral mortality caused by a multitude of disturbances (Palandro et al., 2008; Ruzicka et al., 2013). Notably, all the sites in the Florida Keys and Tortugas subregions were located in managed areas (State or National Parks; National Marine Sanctuary), but the specific levels of protection and enforcement are likely to vary between and within these managed areas, and only small areas of reef are completely protected at the level of “no‐take” (Keller & Causey, 2005; Kuffner et al., 2009). Somewhat paradoxically, the nearshore waters closest to the human settlements of the Keys, with relatively high light attenuation and thermal variability, were most likely to harbor coral oases. Indeed, in the Florida Keys (2003–2012), coral bleaching was associated with high light levels (Barnes et al., 2015), consistent with the positive effect of light attenuation on oasis occurrence in our study. In Florida, light attenuation covaried with the mean and variability of SST. Notably, due to the covariance between these three predictors at the regional extent in Florida (but not across the entire data set), the model inference regarding mean SST (positive effect on oasis occurrence; Figure 3) did not hold in Florida. That is, oases were less likely to be associated with higher mean SST (Appendix S1: Figure S10). This counterintuitive result cautions against relying on inferences drawn from the entire data set when focusing on specific areas. This warning extends beyond our study, because synthetic studies typically screen for covariance in predictors for the entire data set (as we did) but does not preclude the potential for covariance in smaller subsets of data. In summary, the occupancy model was able to identify promising areas in Florida for increased protection of potential source populations, but attributing oasis occurrence (or any measure of coral reef condition) to causal drivers remains problematic.
Our focus on oases is but one approach for the identification of potential candidates for marine spatial planning. For example, the “bright spots” approach identifies sites that are exceptional relative to model predictions (Cinner et al., 2016), as opposed to the simpler (model‐free) approach based on relative coral cover employed in our oasis approach. Sites identified as both oases and bright spots warrant particular attention, and any discrepancies between the two approaches would likely result in value‐based decision making. A simpler approach is a universal threshold of coral cover, such as 10%, to help inform decisions regarding protection or restoration (Darling et al., 2019). In the latter case, sites with at least 10% cover of framework‐building corals, also associated with low thermal stress (e.g., degree heating weeks), would be prioritized for protection whereas sites with higher thermal stress would be targeted for restoration efforts (Darling et al., 2019). Given our results, we support the inclusion of light attenuation as another key environmental feature for consideration by managers. A local proxy for this can be obtained through remote sensing of Kd490, as in the present study, but values in nearshore, shallow, and turbid environments are best measured by underwater sensors.
One advantage of the oasis approach in identifying reefs for enhanced protection is an explicit consideration of the detection vs. occurrence processes, permitting inference for ~5 × 5 km areas, rather than individual sites. The management of coral reef resources frequently relies on limited sampling, and the use of occupancy models offers an explicit way to cope with this limitation. Random sampling is of great importance in the selection of reefs for quantitative surveys used in the construction of occupancy models, both for inference and to permit the extrapolation (if desired) of predicted oasis occurrence to unvisited areas. Although random sampling is widely accepted as an important aspect of coral reef monitoring, syntheses of coral reef condition typically do not report how (or whether) randomization was employed for site selection. For this reason, and because the present analysis yielded different inferences depending on how the data were partitioned (by spatial extent), we caution against relying on a single analysis for the “best” predictors of reef condition. Instead, it is preferable to emphasize consistent results within a study (e.g., light attenuation for coral oases; this study) or across studies (e.g., thermal variability for coral bleaching; Safaie et al., 2018; Sully et al., 2019).
Under a predict‐and‐prescribe approach to reef management (sensu Webster et al., 2017), our framework could be used to identify and protect areas where sites are likely to maintain high coral cover despite ongoing anthropogenic disturbances. For example, at regional spatial extents, grid cells with high SST variability and strong light attenuation were more likely to harbor coral oases. For oases at subregional extents, areas with lower human population density may also be prioritized for protection. These correlations suggest that oases have escaped or resisted disturbances related to thermal bleaching and high human population densities. Implicit in our framework is that we prioritize the protection of such oases because they may persist through future disturbance, serve as a larval reservoir for nearby degraded reefs, and maintain genetic diversity of a broader meta‐population. Protecting the oases may be particularly relevant when the majority of sites in an area are in a degraded state and oases appear to have escaped or resisted disturbances (Game et al., 2008). The division of areas into regions or subregions can reflect ecological features (e.g., islands), sociopolitical entities (e.g., U.S. Virgin Islands), or other arbitrary divisions (e.g., sections of reef), but calls attention to the need for coordination across such boundaries when considering areas for targeted management. Although the mechanisms underpinning local reef resilience to global stressors are uncertain (Bruno et al., 2019), local conditions do mediate patterns of coral bleaching in response to widespread thermal stress (Donovan et al., 2020; Donovan et al., 2021). Thus, the impetus for management action at regional and subregional scales remains, despite the global scale of the climate crisis.
AUTHOR CONTRIBUTIONS
Robin Elahi, Peter J. Edmunds, Ruth D. Gates, and Ilsa B. Kuffner conceived the ideas and designed the research. Robin Elahi, Brian B. Barnes, Iliana Chollett, T. Shay Viehman, and Ivor D. Williams assembled the database. Robin Elahi developed the model, analyzed the data, and led the writing of the manuscript with essential feedback from Peter J. Edmunds and Ilsa B. Kuffner. All authors contributed critically to subsequent drafts.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Appendix S1
Appendix S2
ACKNOWLEDGMENTS
This manuscript is a product of a U.S. Geological Survey's John Wesley Powell Center for Analysis and Synthesis working group led by Peter J. Edmunds, Ruth D. Gates, and Ilsa B. Kuffner. We are indebted to the agencies that published their data in the public domain. We thank S. Zeigler, M. L. Baskett, and two anonymous reviewers for their comments on the manuscript. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. With this paper we celebrate the memory of Ruth D. Gates, whose passion and positivity continues to inspire current and future generations of marine scientists.
Elahi, Robin , Edmunds Peter J., Gates Ruth D., Kuffner Ilsa B., Barnes Brian B., Chollett Iliana, Courtney Travis A., et al. 2022. “Scale Dependence of Coral Reef Oases and Their Environmental Correlates.” Ecological Applications 32(7): e2651. 10.1002/eap.2651
Handling Editor: Marissa Leanne Baskett
Funding information US Geological Survey John Wesley Powell Center for Analysis and Synthesis
DATA AVAILABILITY STATEMENT
Data and code (Elahi et al., 2021) are available in Stanford Digital Repository: https://doi.org/10.25740/zb133qp4049.
REFERENCES
- Anthony, K. R. N. , and Larcombe P.. 2000. “Coral Reefs in Turbid Waters: Sediment‐Induced Stresses in Corals and Likely Mechanisms of Adaptation.” In Proceedings of the 9th International Coral Reef Symposium, Bali, Indonesia, edited by Moosa M. K., Soemodihardjo S., Romimohtarto K., Nontji A., and Soekarno S.. [Google Scholar]
- Barnes, B. B. , Hallock P., Chuanmin H., Muller‐Karger F., Palandro D., Walter C., and Zepp R.. 2015. “Prediction of Coral Bleaching in the Florida Keys Using Remotely Sensed Data.” Coral Reefs 34(2): 491–503. [Google Scholar]
- Birkeland, C. 2015. “Coral Reefs in the Anthropocene.” In Coral Reefs in the Anthropocene, edited by Birkeland C., 1–15. Cham: Springer. [Google Scholar]
- Bowler, D. E. , Bjorkman A. D., Dornelas M., Myers‐Smith I. H., Navarro L. M., Niamir A., Supp S. R., et al. 2020. “Mapping Human Pressures on Biodiversity across the Planet Uncovers Anthropogenic Threat Complexes.” People and Nature 2: 380–94. [Google Scholar]
- Bruno, J. F. , Côté I. M., and Toth L. T.. 2019. “Climate Change, Coral Loss, and the Curious Case of the Parrotfish Paradigm: Why don't Marine Protected Areas Improve Reef Resilience?” Annual Review of Marine Science 11: 307–34. [DOI] [PubMed] [Google Scholar]
- Bruno, J. F. , and Selig E. R.. 2007. “Regional Decline of Coral Cover in the Indo‐Pacific: Timing, Extent, and Subregional Comparisons.” PLoS One 2(8): e711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno, J. F. , and Valdivia A.. 2016. “Coral Reef Degradation Is Not Correlated with Local Human Population Density.” Scientific Reports 6: 29778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinner, J. E. , Cindy Huchery M., MacNeil A., Graham N. A. J., McClanahan T. R., Maina J., Maire E., Kittinger J. N., Hicks C. C., and Mora C.. 2016. “Bright Spots Among the world's Coral Reefs.” Nature 535(7612): 416–9. [DOI] [PubMed] [Google Scholar]
- Coles, S. L. , and Riegl B. M.. 2013. “Thermal Tolerances of Reef Corals in the Gulf: A Review of the Potential for Increasing Coral Survival and Adaptation to Climate Change through Assisted Translocation.” Marine Pollution Bulletin 72(2): 323–32. [DOI] [PubMed] [Google Scholar]
- Courtney, T. A. , Barnes B. B., Chollett I., Elahi R., Gross K., Guest J. R., Kuffner I. B., Lenz E. A., Nelson H. R., and Rogers C. S.. 2020. “Disturbances Drive Changes in Coral Community Assemblages and Coral Calcification Capacity.” Ecosphere 11(4): e03066. [Google Scholar]
- Darling, E. S. , McClanahan T. R., Maina J., Gurney G. G., Graham N. A. J., Januchowski‐Hartley F., Cinner J. E., Mora C., Hicks C. C., and Maire E.. 2019. “Social–Environmental Drivers Inform Strategic Management of Coral Reefs in the Anthropocene.” Nature Ecology & Evolution 3(9): 1341–50. [DOI] [PubMed] [Google Scholar]
- Davis, J. , Pavlova A., Thompson R., and Sunnucks P.. 2013. “Evolutionary Refugia and Ecological Refuges: Key Concepts for Conserving Australian Arid Zone Freshwater Biodiversity under Climate Change.” Global Change Biology 19(7): 1970–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner, S. D. , Skirving W. J., Little C. M., Oppenheimer M., and Hoegh‐Guldberg O. V. E.. 2005. “Global Assessment of Coral Bleaching and Required Rates of Adaptation under Climate Change.” Global Change Biology 11(12): 2251–65. [DOI] [PubMed] [Google Scholar]
- Donovan, M. K. , Adam T. C., Shantz A. A., Speare K. E., Munsterman K. S., Rice M. M., Schmitt R. J., Holbrook S. J., and Burkepile D. E.. 2020. “Nitrogen Pollution Interacts with Heat Stress to Increase Coral Bleaching across the Seascape.” Proceedings of the National Academy of Sciences USA 117(10): 5351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan, M. K. , Burkepile D. E., Kratochwill C., Shlesinger T., Sully S., Oliver T. A., Hodgson G., Freiwald J., and van Woesik R.. 2021. “Local Conditions Magnify Coral Loss after Marine Heatwaves.” Science 372(6545): 977–80. [DOI] [PubMed] [Google Scholar]
- Dubinsky, Z. , and Stambler N.. 2010. Coral Reefs: An Ecosystem in Transition. Berlin: Springer Science & Business Media. [Google Scholar]
- Eakin, C. M. , Sweatman H. P. A., and Brainard R. E.. 2019. “The 2014–2017 Global‐Scale Coral Bleaching Event: Insights and Impacts.” Coral Reefs 38(4): 539–45. [Google Scholar]
- Elahi, R. , Barnes B., Chollett I., Viehman S., and Williams I.. 2021. “Occupancy Models for Coral Reef Oases.” Stanford Digital Repository. 10.25740/zb133qp4049. [DOI]
- Fabricius, K. E. 2005. “Effects of Terrestrial Runoff on the Ecology of Corals and Coral Reefs: Review and Synthesis.” Marine Pollution Bulletin 50(2): 125–46. [DOI] [PubMed] [Google Scholar]
- Falkowski, P. G. , Jokiel P. L., and Kinzie R. A.. 1990. “Irradiance and Corals.” In Ecosystems of the World, edited by Dubinsky Z., 89–107. Amsterdam: Elsevier. [Google Scholar]
- Fitt, W. K. , and Warner M. E.. 1995. “Bleaching Patterns of Four Species of Caribbean Reef Corals.” The Biological Bulletin 189(3): 298–307. [DOI] [PubMed] [Google Scholar]
- Game, E. T. , McDonald‐Madden E., Puotinen M. L., and Possingham H. P.. 2008. “Should Ee Protect the Strong or the Weak? Risk, Resilience, and the Selection of Marine Protected Areas.” Conservation Biology 22(6): 1619–29. [DOI] [PubMed] [Google Scholar]
- Gardner, T. A. , Côté I. M., Gill J. A., Grant A., and Watkinson A. R.. 2003. “Long‐Term Region‐Wide Declines in Caribbean Corals.” Science 301: 958–60. [DOI] [PubMed] [Google Scholar]
- Gelman, A. , Carlin J. B., Stern H. S., Dunson D. B., Vehtari A., and Rubin D. B.. 2013. Bayesian Data Analysis. London: Chapman and Hall/CRC. [Google Scholar]
- Grottoli, A. G. , Rodrigues L. J., and Palardy J. E.. 2006. “Heterotrophic Plasticity and Resilience in Bleached Corals.” Nature 440(7088): 1186–9. [DOI] [PubMed] [Google Scholar]
- Guest, J. R. , Edmunds P. J., Gates R. D., Kuffner I. B., Andersson A. J., Barnes B. B., Chollett I., et al. 2018. “A Framework for Identifying and Characterising Coral Reef “Oases” against a Backdrop of Degradation.” Journal of Applied Ecology 55(6): 2865–75. [Google Scholar]
- Guest, J. R. , Tun K., Low J., Vergés A., Marzinelli E. M., Campbell A. H., Bauman A. G., Feary D. A., Chou L. M., and Steinberg P. D.. 2016. “27 Years of Benthic and Coral Community Dynamics on Turbid, Highly Urbanised Reefs off Singapore.” Scientific Reports 6: 36260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoegh‐Guldberg, O. , Kennedy E. V., Beyer H. L., McClennen C., and Possingham H. P.. 2018. “Securing a Long‐Term Future for Coral Reefs.” Trends in Ecology & Evolution 33(12): 936–44. [DOI] [PubMed] [Google Scholar]
- Kavousi, J. , and Keppel G.. 2018. “Clarifying the Concept of Climate Change Refugia for Coral Reefs.” ICES Journal of Marine Science 75(1): 43–9. [Google Scholar]
- Keller, B. D. , and Causey B. D.. 2005. “Linkages between the Florida Keys National Marine Sanctuary and the South Florida Ecosystem Restoration Initiative.” Ocean & Coastal Management 48(11–12): 869–900. [Google Scholar]
- Kéry, M. , and Royle J. A.. 2016. Applied Hierarchical Modelling in Ecology—Modeling Distribution, Abundance and Species Richness Using R and BUGS.Volume 1: Prelude and Static Models. Cambridge, MA: Elsevier/Academic Press. [Google Scholar]
- Kleypas, J. A. 1996. “Coral Reef Development under Naturally Turbid Conditions: Fringing Reefs near Broad Sound, Australia.” Coral Reefs 15(3): 153–67. [Google Scholar]
- Knapp, K. R. , Kruk M. C., Levinson D. H., Diamond H. J., and Neumann C. J.. 2010. “The International Best Track Archive for Climate Stewardship (IBTrACS) Unifying Tropical Cyclone Data.” Bulletin of the American Meteorological Society 91(3): 363–76. [Google Scholar]
- Kuffner, I. B. , Paul V. J., Ritson‐Williams R., Hickey T. D., and Walters L. J.. 2009. “Reef Communities in the Dry Tortugas (Florida, USA): Baseline Surveys for the New No‐Take Area.” In Proceedings of the 11th International Coral Reef Symposium, Ft. Lauderdale, Florida, 7–11 July 2008, edited by Riegl B. M., and Dodge R. E.. [Google Scholar]
- Lesser, M. P. , Stochaj W. R., Tapley D. W., and Shick J. M.. 1990. “Bleaching in Coral Reef Anthozoans: Effects of Irradiance, Ultraviolet Radiation, and Temperature on the Activities of Protective Enzymes against Active Oxygen.” Coral Reefs 8(4): 225–32. [Google Scholar]
- Lirman, D. , Schopmeyer S., Manzello D., Gramer L. J., Precht W. F., Muller‐Karger F., Banks K., Barnes B., Bartels E., and Bourque A.. 2011. “Severe 2010 Cold‐Water Event Caused Unprecedented Mortality to Corals of the Florida Reef Tract and Reversed Previous Survivorship Patterns.” PLoS One 6(8): e23047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie, D. I. , Nichols J. D., Lachman G. B., Sam Droege J., Royle A., and Langtimm C. A.. 2002. “Estimating Site Occupancy Rates when Detection Probabilities Are Less than One.” Ecology 83(8): 2248–55. [Google Scholar]
- Massel, S. R. , and Done T. J.. 1993. “Effects of Cyclone Waves on Massive Coral Assemblages on the Great Barrier Reef: Meteorology, Hydrodynamics and Demography.” Coral Reefs 12(3–4): 153–66. [Google Scholar]
- Morgan, K. M. , Perry C. T., Smithers S. G., Johnson J. A., and Daniell J. J.. 2016. “Evidence of Extensive Reef Development and High Coral Cover in Nearshore Environments: Implications for Understanding Coral Adaptation in Turbid Settings.” Scientific Reports 6(1): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby, P. J. , Chisholm J. R. M., Edwards A. J., Andrefouet S., and Jaubert J.. 2001. “Cloudy Weather May Have Saved Society Island Reef Corals during the 1998 ENSO Event.” Marine Ecology Progress Series 222: 209–16. [Google Scholar]
- NOAA Coral Program . 2014. “ NOAA Coral Reef Conservation Program: National Coral Reef Monitoring Plan ,” edited by Morgan Jessica Alicia. Silver Spring, MD: NOAA Coral Reef Conservation Program. [Google Scholar]
- O'Leary, J. K. , Micheli F., Airoldi L., Boch C., De Leo G., Elahi R., Ferretti F., Graham N. A. J., Litvin S. Y., and Low N. H.. 2017. “The Resilience of Marine Ecosystems to Climatic Disturbances.” Bioscience 67(3): 208–20. [Google Scholar]
- Oliver, T. A. , and Palumbi S. R.. 2011. “Do Fluctuating Temperature Environments Elevate Coral Thermal Tolerance?” Coral Reefs 30(2): 429–40. [Google Scholar]
- Palandro, D. A. , Andréfouët S., Chuanmin H., Hallock P., Müller‐Karger F. E., Dustan P., Callahan M. K., Kranenburg C., and Beaver C. R.. 2008. “Quantification of Two Decades of Shallow‐Water Coral Reef Habitat Decline in the Florida Keys National Marine Sanctuary Using Landsat Data (1984–2002).” Remote Sensing of Environment 112(8): 3388–99. [Google Scholar]
- Palumbi, S. R. , Barshis D. J., Traylor‐Knowles N., and Bay R. A.. 2014. “Mechanisms of Reef Coral Resistance to Future Climate Change.” Science 344(6186): 895–8. [DOI] [PubMed] [Google Scholar]
- Perry, C. T. , and Alvarez‐Filip L.. 2019. “Changing Geo‐Ecological Functions of Coral Reefs in the Anthropocene.” Functional Ecology 33(6): 976–88. [Google Scholar]
- Plummer, M. 2003. “JAGS: A Program for Analysis of Bayesian Graphical Models Using Gibbs Sampling.” In Proceedings of the 3rd International Workshop on Distributed Statistical Computing. Vienna, Austria.
- R Development Core Team . 2020. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Randall, C. J. , and van Woesik R.. 2017. “Some Coral Diseases Track Climate Oscillations in the Caribbean.” Scientific Reports 7(1): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, J. P. W. , Williams I. D., Yeager L. A., McPherson J. M., Clark J., Oliver T. A., and Baum J. K.. 2018. “Environmental Conditions and Herbivore Biomass Determine Coral Reef Benthic Community Composition: Implications for Quantitative Baselines.” Coral Reefs 37(4): 1157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roff, G. , and Mumby P. J.. 2012. “Global Disparity in the Resilience of Coral Reefs.” Trends in Ecology & Evolution 27(7): 404–13. [DOI] [PubMed] [Google Scholar]
- Rosenfeld, J. S. 2002. “Functional Redundancy in Ecology and Conservation.” Oikos 98(1): 156–62. [Google Scholar]
- Ruzicka, R. R. , Colella M. A., Porter J. W., Morrison J. M., Kidney J. A., Brinkhuis V., Lunz K. S., Macaulay K. A., Bartlett L. A., and Meyers M. K.. 2013. “Temporal Changes in Benthic Assemblages on Florida Keys Reefs 11 Years after the 1997/1998 El Niño.” Marine Ecology Progress Series 489: 125–41. [Google Scholar]
- Safaie, A. , Silbiger N. J., McClanahan T. R., Pawlak G., Barshis D. J., Hench J. L., Rogers J. S., Williams G. J., and Davis K. A.. 2018. “High Frequency Temperature Variability Reduces the Risk of Coral Bleaching.” Nature Communications 9(1): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandin, S. A. , Smith J. E., DeMartini E. E., Dinsdale E. A., Donner S. D., Friedlander A. M., Konotchick T., Malay M., Maragos J. E., and Obura D.. 2008. “Baselines and Degradation of Coral Reefs in the Northern Line Islands.” PLoS One 3(2): e1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sully, S. , Burkepile D. E., Donovan M. K., Hodgson G., and Van Woesik R.. 2019. “A Global Analysis of Coral Bleaching over the Past Two Decades.” Nature Communications 10(1): 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, I. M. , and Corlett R. T.. 1996. “The Conservation Value of Small, Isolated Fragments of Lowland Tropical Rain Forest.” Trends in Ecology & Evolution 11(8): 330–3. [DOI] [PubMed] [Google Scholar]
- Webster, M. S. , Colton M. A., Darling E. S., Armstrong J., Pinsky M. L., Knowlton N., and Schindler D. E.. 2017. “Who Should Pick the Winners of Climate Change?” Trends in Ecology & Evolution 32(3): 167–73. [DOI] [PubMed] [Google Scholar]
- Wiens, J. A. 1989. “Spatial Scaling in Ecology.” Functional Ecology 3(4): 385–97. [Google Scholar]
- Williams, G. J. , Gove J. M., Eynaud Y., Zgliczynski B. J., and Sandin S. A.. 2015. “Local Human Impacts Decouple Natural Biophysical Relationships on Pacific Coral Reefs.” Ecography 38(8): 751–61. [Google Scholar]
- Williams, I. D. , Baum J. K., Heenan A., Hanson K. M., Nadon M. O., and Brainard R. E.. 2015. “Human, Oceanographic and Habitat Drivers of Central and Western Pacific Coral Reef Fish Assemblages.” PLoS One 10(4): e0120516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodley, J. D. , Chornesky E. A., Clifford P. A., Jackson J. B. C., Kaufman L. S., Knowlton N., Lang J. C., Pearson M. P., Porter J. W., and Rooney M. C.. 1981. “Hurricane Allen's Impact on Jamaican Coral Reefs.” Science 214(4522): 749–55. [DOI] [PubMed] [Google Scholar]
- Yeager, L. A. , Marchand P., Gill D. A., Baum J. K., and McPherson J. M.. 2017. “Marine Socio‐Environmental Covariates: Queryable Global Layers of Environmental and Anthropogenic Variables for Marine Ecosystem Studies.” Ecology 98(7): 1976–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Data Availability Statement
Data and code (Elahi et al., 2021) are available in Stanford Digital Repository: https://doi.org/10.25740/zb133qp4049.


