Abstract
Urolithins, metabolites produced by the gut microbiota from the polyphenols ellagitannins and ellagic acid, are discovered by the research group in humans almost 20 years ago. Pioneering research suggests urolithins as pleiotropic bioactive contributors to explain the health benefits after consuming ellagitannin‐rich sources (pomegranates, walnuts, strawberries, etc.). Here, this study comprehensively updates the knowledge on urolithins, emphasizing the review of the literature published during the last 5 years. To date, 13 urolithins and their corresponding conjugated metabolites (glucuronides, sulfates, etc.) have been described and, depending on the urolithin, detected in different human fluids and tissues (urine, blood, feces, breastmilk, prostate, colon, and breast tissues). There has been a substantial advance in the research on microorganisms involved in urolithin production, along with the compositional and functional characterization of the gut microbiota associated with urolithins metabolism that gives rise to the so‐called urolithin metabotypes (UM‐A, UM‐B, and UM‐0), relevant in human health. The design of in vitro studies using physiologically relevant assay conditions (molecular forms and concentrations) is still a pending subject, making some reported urolithin activities questionable. In contrast, remarkable progress has been made in the research on the safety, bioactivity, and associated mechanisms of urolithin A, including the first human interventions.
Keywords: ellagitannins, gut microbiota, metabotype, polyphenol, urolithins
About 20 years ago, urolithins are discovered as bioavailable metabolites produced by the human gut microbiota from ellagitannins and ellagic acid. Pioneering investigations suggested pleiotropic effects for urolithins that growing evidence confirms, especially for urolithin A. Here, we update the knowledge on urolithins metabolism, bioactivity, and associated gut microbiota, emphasizing the literature published in the last 5 years.
1. Introduction
Urolithins (Uros) are gut microbiota metabolites of ellagitannins (ETs) and ellagic acid (EA) that were first discovered as bioavailable metabolites from pomegranate ETs almost 20 years ago in animal models[ 1 , 2 ] and humans.[ 3 ] Since then, Uros have been studied for different health effects highlighted in a previous review published in 2017.[ 4 ] In the last few years, the number of research articles on Uros has increased exponentially, with significant advances in their biological effects and the production by gut microbes. Therefore, in the present review, we update the knowledge on Uros, emphasizing the review of the literature published during the last 5 years (2017–2021).
1.1. Advances in Bioavailability, Metabolism, and Tissue Distribution
ETs are one of the main groups of hydrolyzable tannins. They can occur in different plant foods, including pomegranates, berries (strawberries, raspberries, blackberries, etc.), walnuts, many tropical fruits, medicinal plants, and herbal teas, including green and black Camelia sinensis teas.[ 4 ] The bioavailability of ETs and EA is very low. They undergo extensive metabolism by the gut microbiota to produce 6H‐dibenzo[b,d]pyran‐6‐one derivatives, known as Uros. In some animal species, EA can be dehydroxylated to produce nasutins, in which the dilactone structure of EA is preserved. The gut microbiota can transform EA into Uros by lactone‐ring cleavage, decarboxylation, and dehydroxylation reactions, starting with pentahydroxy‐Uro (Uro‐M5) and following to tetrahydroxy‐Uros (Uro‐D, Uro‐E, and Uro‐M6) and trihydroxy‐Uros (Uro‐C and Uro‐M7) to finalize in dihydroxy‐Uros (Uro‐A and isoUro‐A) and monohydroxy‐Uro (Uro‐B), which is generally detected when isoUro‐A is also produced (Figure 1 ).[ 4 ] The pathway of Uros formation and the place in the intestinal tract where they are produced were elucidated using a gastrointestinal simulation model (TWIN–SHIME) with fecal samples from two individuals with distinct urolithin metabotypes (UMs). Differences in the Uro profile were observed between UMs and along the large intestine, showing predominant Uro production in the distal colon region.[ 5 ]
Figure 1.
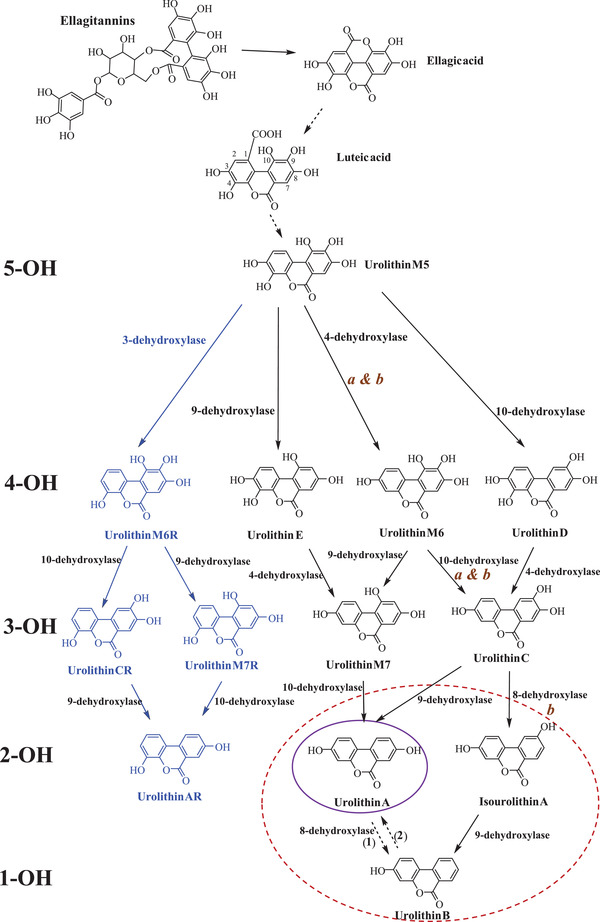
Catabolic pathway of ellagitannins and ellagic acid to urolithins, showing the gut microbial enzymatic activities involved*. 5‐OH, 4‐OH, 3‐OH, 2‐OH, and 1‐OH refer to the number of hydroxyl groups for each urolithin group (penta‐, tetra‐, tri‐, di‐ and monohydroxy urolithins, respectively. *Adapted from [ 4 ] and [ 52 ]. a) Gordonibacter urolithinfaciens, G. pamelaeae; (b) Ellagibacter isourolithinifaciens. 1) Suggested by Espín et al.[ 47 ] and described by Gasperotti et al.[ 49 ] in mice. 2) Described in vitro[ 100 ] and in vivo[ 132 ]) and tentatively attributed to a cytochrome P450‐mediated hydroxylation. The purple and red circles designate the final urolithins produced in UM‐A and UM‐B, respectively. Uro‐AR can be found in both metabotypes.[ 6 ]
In the last years, four new Uros have been identified in human feces after the intake of a pomegranate extract: 4,8,9,10‐tetrahydroxy‐Uro (Uro‐M6R), 4,8,10‐trihydroxy‐Uro (Uro‐M7R), 4,8,9‐trihydroxy‐Uro (Uro‐CR), and 4,8‐dihydroxy‐Uro (Uro‐AR). These new metabolites have in common that they need a bacterial 3‐dehydroxylase activity for their production (Figure 1). Phase‐II conjugates of the novel Uros were also detected in urine.[ 6 ]
An update of the Uro concentrations in biological fluids is shown in Table 1 . Concentrations in human plasma were in the range 0.003−5.2 µM and in urine up to 50 µM. The high variability observed was affected by the dose administered, the source of ETs, and the analytical methods used. The Uro profile was also characterized in the breast milk of mothers consuming walnuts as a dietary source of ETs. Total Uro concentration in milk ranged from 8.5 to 176.9 nM, being glucuronide and sulfate conjugates the predominant compounds. The type of Uros excreted in breast milk depended on the maternal UM.[ 7 ]
Table 1.
Bioavailability and metabolism of urolithins in different biological fluids.*
| Administration | Quantified metabolites | Reference | |
|---|---|---|---|
|
Wistar rats (5 groups, 8 rats) |
Blackberry fiber (BF) 6% Extracted blackberry fiber (EBF) 6% Blackberry polyphenols (BP) 1% Defatted blackberry seeds (DBS) 6% 4 weeks |
Cecal Digesta (µg g−1) Uro‐A: 26 (BF), 17 (BP), 13 (DBS) Nasutin A: 76 (BF), 29 (EBF), 87 (BP), 241 (DBS) EA: 3400 (BF), 1000 (EBF), 2800 (BP), 3400 (DBS) |
[ 10 ] |
| Male Wistar rats***(3 groups, 10 rats) |
Strawberry preparations rich in monomeric ellagitannins (EM) or dimeric ellagitannins (ED) and free EA (0.10−0.15% of the diet) 7 days |
Stomach digesta (µg g−1) EA: 3.8 (EM), 24.1 (ED), 355.7 (EA); Nasutin A: 5.5 (EM), 8 (ED) Small intestine digesta (µg g−1) EA: 9.4 (EA); Uro‐A: 0.2 (EM), 0.2 (ED), 53.5 (EA); Nasutin A: 1 (EM), 0.3 (ED); Uro A‐glur: 3.7 (EM), 95.7 (EA); Nasutin A glur: 0.5 (EM) Cecal digesta (µg g−1) EA: 1.6 (EA); Uro‐A: 8.3 (EM), 0.2 (ED), 224.3 (EA); Nasutin A: 44.8 (EM), 5.8 (ED) Feces (µg g−1) Uro‐A: 89.3(EA); Nasutin A: 46.9 (EM), 3.9 (ED) Urine 24 h (µM)c Uro‐A: 5.57 (EM), 3.9 (ED), 99.5 (EA); Nasutin A: 1.4 (EM), 0.3 (ED), 0.4 (EA); Uro‐A glur: 19.1 (EM), 0.02 (ED), 84.3 (EA); Nasutin A glur: 10.5 (EM), 1.1 (ED), 0.2 (EA); Isonasutin A glur: 0.4 (EM), 0.3 (ED), 1.2 (EA) Plasma (nM) Uro‐A glur: 495 (EA); Nasutin A glur: 45 (EM), 22.4 (ED); DMEAG: 891 (EA) Results are the mean of three days: 2, 4 and 7 |
[ 11 ] |
|
Wistar rats (6 groups, 8 rats) |
Monomeric ellagitannins (ME) 0.23% ME+ FOS 0.23% Dimeric ellagitannins (DE) 0.24% DE+FOS 0.24% 4 weeks |
Cecal digesta (µg g−1) Nasutin A: 62.7 (ME), 79.2 (ME+FOS), 6.3 (DE), 10.3 (DE+FOS) Isonasutin A glur: 5.1 (ME), 9.2 (ME+FOS), 6.7 (DE), 7.8 (DE+FOS) Uro‐A: 1.1 (ME+FOS) EA: 1900 (ME), 2400 (ME+FOS), 1600 (DE), 2000 (DE+FOS) Plasma (nM) Uro‐A: 1030 (ME+FOS), 596 (DE), 1188 (DE+FOS) Isonasutin A glur: 22.6 (ME+FOS), 22.6 (DE), 24.2 (DE+FOS) Nasutin A glur: 18.2 (ME), 6.1 (ME+FOS), 0.7 (DE) |
[ 12 ] |
|
Male wistar rats (6 groups, 8 rats) |
Strawberry phenolic fraction (20 mg kg−1 of BW) (57.3 g ETs/100g) 4 days Different post‐intake time and duration of ingestion |
F1‐4 (4 days of ingestion, 12 h after last administration) Cecal digesta (µg g−1): 0.9 EA, 45.1 Uro‐A, 122 Nasutin A Urine (µM): 0.5 Uro‐A, 0.3 Nasutin A, 2.2 Uro‐A glur, 1.3 Nasutin A glur Plasma (nM): 109 Uro A‐glur, 31.4 Nasutin A glur, 17.8 DMEAG |
[ 13 ] |
|
Male wistar rats (6 groups, 8 rats) |
Strawberry ET‐rich extract (0.24% or 0.72% of the diet) (57.3 g ETs/100g) 4 weeks Different diet type and ET dosage |
Standard diet and high dose Urine 24h (µM)c: 156 Uro‐A glur, 46 Nasutin A glur, 650 Uro‐A, 119 Nasutin Plasma (nM): 4.1 Nasutin A glur, 59.4 DMEAG, 66.3 Nasutin A |
[ 14 ] |
| CD1 male mice (n = 5‐7) | Epilobium angustifolium extract (0.6 mg mouse‐1)>15% oenothein 2 weeks |
Plasma (nM) (24 h/1week/2weeks): 10.9/28.5/21.9 Uro‐A, 35.8/37.3/32.5 Uro‐B Pellet (nM) (24 h/1week/2weeks): ‐/29.8/38.6 Uro‐A; ‐/38.2/45.3 Uro‐B |
[ 16 ] |
| Healthy women (n = 10) | Grumixama juice (10 mL kg−1 body weigh) 14.6 mg total EA/100 mL. Acute intake |
Plasma: no detection Urine 0–24 h Cmax (µmol mmol−1 creatinine): 144 Uro‐A glur, 19.4 Uro‐B glur, 25.3 Uro‐C methyl ether glur, 20.3 Uro‐D glur AUC(µmol h−1): 1376 Uro‐A glur, 96.8 Uro‐B glur, 1142 Uro‐C methyl ether glur, 198 Uro‐D glur. Concentrations in the group of high producers |
[ 17 ] |
| Healthy volunteers (n = 35) | Jabuticaba dessert (20 g, 1492 mg EA and ETs). Acute intake |
Urine (0‐48 h) (µM)c 24.9 Uro‐A 3‐glur, 0.4 Uro‐A sulfate, 1.4 Uro‐A, 5.3 isoUro‐A 3‐glur, 2.6 isoUro‐A 9‐glur, 0.6 isoUro‐A, 2 Uro‐B glur, 0.03 Uro‐B sulfate, 0.06 Uro‐B, 0.2 Uro‐C, 0.007 Uro‐M6, 0.01 Uro‐M7, 0.02 EA |
[ 18 ] |
| Healthy subjects (n = 16) | Cagaita (Eugenia dysenterica DC) juice (300 mL, 70 mg total EA) |
Urine (24 h) µM 2.3‐14.4 Uro‐A glur, 2.3‐22.1 isoUro‐A glur, 0.4‐29.1 Uro‐B glur |
[ 20 ] |
|
Healthy volunteers (n = 10) |
Tea (5 g in 250 mL of boiling water) 3 mg EAE d−1 for 2 days | Uro‐A glur, Uro‐A sulfate, Uro‐A, isoUro‐A glur, isoUro‐A, Uro‐B glur, Uro‐B sulfate, Uro‐B (no quantification) | [ 21 ] |
| Hysterectomy women (n = 33) | Oak wood extracts 300 mg d−1 (3.5 mg ETs). 4/8 weeks |
Serum (nM) 4/8 weeksb: 58/61.6 Uro‐A; 94.2/105.4 Uro‐B; 9.8/10.9 Uro‐C. Blood cells (ng g−1) 4/8 weeksb: 2.32/1.52 Uro‐A; |
[ 24 ] |
|
Barrett´s esophagus patients (n = 20) |
Powdered lyophilized berries (32 g females /45 g males,) 12/26 weeks | Urine (µM) 12weeks/26 weeks: 3.2/4.3 Uro‐A glur; 0.37/0.38 Uro‐A sulfate; 0.09/0.02 DMEAG | [ 25 ] |
|
Healthy older adults (n = 38) |
Free‐dried strawberry (24 g d−1) 90 days (Chronic intake) | Plasma (nM) 45/90 days: 1073/1964 Uro‐A glur, 2000/5200 isoUro‐A glur, 828/646 Uro‐B glur | [ 26 ] |
|
Men with prostate cancer (n = 24) |
Usual American diet (1568 mg day−1 polyphenols) | Urine 24 h (µM)b: 0−36.8 Uro‐A; 0−47.3 Uro‐B; 1.8−5.1 Uro‐D | [ 29 ] |
| Men with prostate cancer (n = 40) (5 groups, 8 subjects) | Confection (C) or nectar (N) (10 or 20 g day−1) (25 or 50 mg ETs + EA) 3 weeks |
Urine 24 h (nM)b Uro‐A: 1581 (N10), 4320 (N20), 3317 (C10), 4807 (C20) Uro‐B: 0.63 (N10), 29 (N20), n.d. (C10), 312 (C20) Uro‐C: 17.3 (N10), 52 (N20), 24 (C10), 59 (C20) Uro‐D: 3.3 (N10), 2 (N20), 5.3 (C10), 4.7 (C20) DMEA: 393 (N10), 755 (N20), 533 (C10), 1176 (C20) |
[ 30 ] |
|
Healthy (n = 10) and individuals with prediabetes and insulin resistance (PreDM‐IR) (n = 26) |
Red raspberry‐based test drink (125 mg ETs and EA) Acute intake |
Plasma (0‐24h) (AUC µmol h L−1)a Uro‐A derivatives: 0.1 (PreDM‐IR), 0.4 (healthy) Urine (0‐24h) (AUC nmol h µmol−1 creatinine)a Uro‐A derivatives: 9 (PreDM‐IR), 28 (healthy) |
[ 31 ] |
| Healthy (n = 10) and individuals with prediabetes and insulin resistance (PreDM‐IR) (n = 25) |
Red raspberry‐based test drink (125 mg ETs). 1 cup day−1 with or without FOS supplementation (8 g) for 4 weeks Pharmacokinetics after intake of 2 cups of RRB‐drink in week 0 (baseline (B)) and week 4 (W4) |
Plasma (0‐24 h) (AUC nmol hL−1) Total urolithins: 429 (B), 1044 (W4), 1110 (W4 + FOS) Uro‐A 3‐glur: 311 (B), 569 (W4), 887 (W4 + FOS) Uro‐A glur: 91 (B), 133 (W4), 261 (W4 + FOS) isoUro‐A glur: 129 (B), 112 (W4), 91 (W4 + FOS) Uro‐B glur: 130 (B), 1376 (W4), 305 (W4 + FOS) Urine (0‐24 h) (AUC)(nmol h µmol−1 creatinine) Total urolithins: 23 (B), 51 (W4), 66 (W4 + FOS) Uro‐A: 0.2 (B), 0 (W4), 2.4 (W4 + FOS) Uro‐A 3‐glur: 14.7 (B),26.7 (W4), 46.8 (W4 + FOS) Uro‐A glur: 7.7 (B), 12.6 (W4), 13.7 (W4 + FOS) isoUro‐A glur: 1.9 (B), 0.2 (W4), 1 (W4 + FOS) Uro‐A sulfglur: 2.3 (B), 4.6 (W4), 11.9 (W4 + FOS) Uro‐B glur: 5.3 (B), 44.4 (W4), 9 (W4 + FOS) Uro‐B sulfglur: 1 (B), 3.9 (W4), 3.4 (W4 + FOS) |
[ 32 ] |
| Healthy (n = 10) and individuals with prediabetes and insulin resistance (PreDM‐IR) (n = 21) | Frozen red raspberry (125 o 250 g/29 mg or 58 mg ETs and EA). Acute intake |
Plasma (0‐24 h) AUC (nmol h L−1) Uro‐A glur: 854 (PreDM‐IR 125), 1861 (PreDM‐IR 250), 823 (healthy 125), 704 (healthy 250) Uro‐B glur: 186 (PreDM‐IR 125), 493 (PreDM‐IR 250), 20 (healthy 125), 22 (healthy 250) Cmax (nM) Uro‐A glur: 92 (PreDM‐IR 125), 198 (PreDM‐IR 250), 75 (healthy 125), 63 (healthy 250) Uro‐B glur: 21 (PreDM‐IR 125), 62 (PreDM‐IR 250), 3 (healthy 125), 3 (healthy 250) |
[ 33 ] |
| Healthy volunteers (n = 100) | Food product containing Uro‐A (500 mg) or pomegranate juice (PJ) (240 mL) (71 mg punicalagins and 36 mg EA) |
Plasma (0‐24 h) iAUC (nmol mL−1) Uro‐A glur: 301 (PJ), 2069 (Uro‐A) Cmax (nM), (Tmax (h)): Uro A‐glur: 273 (24) (PJ), 1190 (6) (Uro‐A)Comparable absolute change for Uro‐A sulfate and Uro‐A |
[ 35 ] |
| Male SD rats (4 groups, 4 animals) |
Encapsulation of Uro‐A in nanoparticles: 25 mg P2Ns‐encapsulated Uro‐A (P2Ns‐UA) or 10 or 25 mg P2Ns‐GA‐UA. Oral gavage |
Plasma (0‐48 h) AUC (nmol h L−1): Uro‐A: 9951 (Uro‐A 50), 19078 (P2Ns UA 25), 32429 (P2Ns‐GA UA 25), 12728 (P2Ns‐GA UA 10) Cmax (nM): Uro‐A: 820 (Uro‐A 50), 894 (P2Ns UA 25), 2403 (P2Ns‐GA UA 25), 1157 (P2Ns‐GA UA 10) |
[ 36 ] |
| Healthy women (n = 27) | 30 g peeled walnuts for 3 days | Breastmilk (nM): Uro‐A glur: 27.6 (UM‐A), 31.1 (UM‐B); Uro‐A‐sulfate: 7.9 (UM‐A), 17.2 (UM‐B); isoUro‐A glur: 14.8 (UM‐B); Uro‐B glur: 19.8 (UM‐B); Uro‐A: 4.7 (UM‐A), 3.3 (UM‐B); isoUro‐A: 2.7 (UM‐B); Uro‐B: 4.3 (UM‐B) | [ 7 ] |
*Primarily focused on the last five years. Check the reference [ 4 ] for a systematic review up to 2017. All the results of plasma and urine were expressed in nM and µm, respectively. Some results had to be recalculated from original data to be expressed in these units; a) Quantitative mean values were estimated from graphics present in the original paper; b) quantification after enzymatic hydrolysis; c) Concentrations in urine were estimated in µM assuming an average urine excretion of 1.5 L 24 h−1 in humans and 10 mL 24 h−1 in rats; FOS, fructooligosaccharides: DMEAG: ellagic acid dimethyl ether glucuronide; EAE: ellagic acid equivalent; GA: gambogic acid; P2Ns: polymer nanosystem: UM‐A: metabotype A; UM‐B: Metabotype B; iAUC: incremental area under the curve.
The profile of metabolites produced by the gut microbiota depends on the host species' characteristics, probably linked to individual differences in the microbiota composition.[ 8 ] In previous investigations, nasutin was identified as one of the ET metabolites in rats.[ 9 ] In the last years, new studies with rats have corroborated the presence of nasutin and their conjugates as the primary ET metabolites in biological fluids. The supplementation of rat diets with different formulations of blackberry (poly)phenols, mostly rich in dimeric and monomeric ETs, has shown relatively high amounts of nasutin A in the cecal digesta (29−241 µg g−1).[ 10 ] In another study with rats, Milala et al.[ 11 ] demonstrated that the degree of polymerization (DP) of ETs affected metabolite formation. Uro‐A was the predominant metabolite in rats fed a diet supplemented with EA. At the same time, nasutin was the primary metabolite in rats fed diets rich in ETs, showing higher concentrations in the case of ETs with a lower DP.[ 11 ] Similarly, concentrations of nasutin A in the cecal digesta and its glucuronide in plasma of rats were higher after the consumption of monomeric ETs compared to dimeric ETs, suggesting that monomeric ETs are more easily degraded than those with a higher DP.[ 12 ] Besides, the absorption of these metabolites could be increased by co‐ingestion with dietary fructooligosaccharides (FOS). The effect of other experimental factors: post‐intake time, duration of the administration, diet type (standard and high‐fat), and ET dosage (without, low, and high ET intake) in the ETs metabolism was evaluated in the blood serum and urine of rats consuming strawberry phenolics.[ 13 , 14 ] The highest concentrations were obtained after 2–4 days of the administration, 12 h after the last administration, with a standard diet and the high ET dose.
The metabolism of ETs from herbal medicinal products and new food products has also been evaluated. In the case of Epilobium sp, rich in oenothein B, interspecies differences were observed in its gut microbiota metabolism. Conjugates of nasutin were observed after oral supplementation of rats with Epilobium angustifolium aqueous extract, whereas in human volunteers supplemented with Epilobium tea, only Uro conjugates were present.[ 15 ] In another study, Uro‐A and Uro‐B were quantified in plasma, pellet (blood cells), and whole blood samples in CD1 mice after the oral administration of an Epilobium angustifolium extract.[ 16 ] Concentrations of both metabolites increased with the duration of treatment, confirming that Uros are produced after prolonged ET administration.
The metabolism of ETs to Uros following consumption of different Brazilian fruits (grumixama juice, jabuticaba, and cagaita) has also been documented in a non‐European population.[ 17 , 18 , 19 ] Transformation of jabuticaba bioactive compounds during colonic fermentation was also evaluated in vitro.[ 20 ]
Uros detection in volunteers' urine after dietary tea intake has demonstrated that this could be used to determine the volunteer UM, despite the low amount of EA ingested (3 mg of EA d−1 for 2 days).[ 21 ] In another study, the presence of Uro‐A in human urine and plasma was related to the consumption of a diet supplemented with the Mankai plant, a (poly)phenol‐rich aquatic plant containing EA.[ 22 ]
ETs present in Lythrum salicaria (herb of purple loosestrife) were metabolized to Uros (Uro‐M6, Uro‐M7, Uro‐C, Uro‐A, and isoUro‐A) in ex vivo anaerobic cultures of piglets cecal and distal colon microbiota. A new metabolite originating from the flavogalloyl moiety of the C‐glucosidic ETs was detected. Based on the mass and UV spectra, this metabolite was assigned as a dehydroxy‐flavogallonic acid dilactone, although NMR analyses would be necessary to confirm the identity of this metabolite. This is the first time a different ET moiety than the hexahydroxydiphenoyl group is a substrate for gut microbiota metabolism.[ 23 ]
Several studies have also investigated the microbial capacity to catabolize (poly)phenols in disease and pre‐disease states in the last years. Uro‐A (61.6 nM), Uro‐B (105.4 nM), and Uro‐C (10.9 nM) were detected after enzymatic hydrolysis in serum samples of patients with hysterectomy after receiving 300 mg of an extract from French oak wood.[ 24 ] Uro‐A (2.32 ng g−1) was also quantified in blood cells for the first time. Blood cell/serum ratios were clearly below 1.0, indicating that after intake of the extract, the metabolite was not enriched in blood cells. The Uro levels were consistent after 4 and 8 weeks.
Levels of ET metabolites [Uro‐A glucuronide (glur) (4.3 µM), Uro‐A sulfate (0.38 µM), and dimethylellagic acid glur (DMEAG) (0.02 µM)] in urine following treatment with lyophilized black raspberries were reported in Barrett's esophagus patients. Similar concentrations were also obtained after 12 and 26 weeks of ingestion.[ 25 ] Higher concentrations, but not significantly different, were observed after 90 days of strawberry (poly)phenol intake compared with 45 days, suggesting that the continuous intake of ETs could enhance either the abundance of some bacterial species or the capacity of certain species to increase Uro production.[ 26 ] This study confirmed a previous study in which metabolic syndrome patients consumed ET‐rich berries for 3 months.[ 27 ]
Several studies have reported the presence of Uro conjugates in plasma and urine of men with localized prostate cancer after consuming a pomegranate fruit extract,[ 28 ] a usual American diet,[ 29 ] or standardized black raspberry products.[ 30 ]
Zhang et al.[ 31 ] evaluated the functional capacity to metabolize (poly)phenols from a red raspberry drink in individuals with prediabetes and insulin resistance (PreDM‐IR, n = 26) compared to a metabolically healthy reference group (n = 10).[ 31 ] PreDM‐IR group showed a lower concentration of Uro‐A conjugates than the reference group in plasma and urine, indicating that metabolic status was a factor in metabolite response. More recently, the same authors reported no significant differences between both groups, attributed to the high interindividual variability.[ 32 ] Higher Uro concentrations were observed in the same PreDM‐IR group, after supplementation for 4 weeks but only in the samples from 0 to 4 h, while their excretion remained unchanged at 24 h. Therefore, 4‐weeks supplementation contributed to the accumulation of Uros but did not produce higher concentrations of these metabolites. Besides, adding FOS did not affect the concentration of excreted metabolites.[ 33 ]
In a pioneer human study with direct oral Uro‐A supplementation in healthy elderly, Uro‐A underwent intensive phase‐II metabolism. The plasma concentrations of Uro‐A glur and Uro‐A sulfate showed a Tmax around 6 h, with a half‐life in circulation being approximately a day.[ 34 ] More recently, a human pharmacokinetic study demonstrated that Uro‐A, Uro‐A glur, and Uro‐A sulfate concentrations were significantly higher (>30‐fold at 6 h and >2‐fold higher at 24 h) with the direct supplementation with Uro‐A compared with pomegranate juice intake. Although direct Uro‐A supplementation can overcome microbiome and dietary variability that leads to Uro‐A heterogeneity in the population, another study design (e.g., another ETs source) could have yielded much lower differences.[ 35 ] Another study showed that nanoparticle encapsulation of Uro‐A with polymer nanosystems conjugated with gambogic acid significantly increased the bioavailability of Uro‐A.[ 36 ]
The knowledge of Uros tissue distribution (qualitatively and quantitatively) is essential to explain their health effects and design new strategies using ET‐rich foods to prevent several diseases. In the last years, tissue distribution of Uros has been reported in animal models[ 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 ] and humans[ 34 , 46 ] (Table 2 ). Most of the animal studies were based on Uro‐A administration,[ 34 , 37 , 38 , 39 , 40 , 41 ] although dietary interventions with ET‐rich extracts,[ 45 , 46 ] or ET‐rich products such as black raspberry[ 44 ] or pomegranate juice were also considered.[ 42 , 43 ]
Table 2.
Quantification of urolithins in tissues.*
| Animals | Administration | Tissue distribution | Reference |
|---|---|---|---|
| Male SD rats (n = 12) | Uro‐A (25 mg kg−1 day−1) 2 or 7 days. | Vastus lateralis muscle (ng g−1)a: 12 Uro‐A (2 days); 9 Uro‐A (7 days). | [ 37 ] |
|
Male C57BL/6 mice (n = 8) |
Uro‐A i.p. (20 µg) 12 weeks. | Trace amounts of Uro‐A metabolites were detected in the adipose tissue. | [ 38 ] |
| Male Wistar rats with streptozotocin‐induced type‐1 diabetes (n = 20) | Daily i.p. injection of Uro‐A or Uro‐B (2.5 mg kg−1day−1) 3 weeks. |
Liver (nmol g−1)a: 9 Uro‐A; 58 Uro‐A‐sulfate; 100 Uro‐B; 220 Uro‐B sulfate. Pancreas (nmol g−1)a: 10 Uro‐A; 80 Uro‐B; 1 Uro‐A sulfate; 160 Uro‐B sulfate. Heart (nmol g−1)a: 400 Uro‐A; 15 Uro‐A sulfate; 500 Uro‐B; 225 Uro‐B sulfate. |
[ 39 ] |
| Wistar rats (males n = 16; females n = 16) | 14C radiolabeled Uro‐A (1000 mg kg−1 bw). |
% of total radioactivity from the administered dose Liver: 0.009−0.018. Kidney: 0.005−0.021. Carcass: 0.180−0.896. GI tract: 87−121. Heart, lung, spleen, testes, prostate, pancreas, abdominal fat, brown fat, muscle adrenals, thymus, thyroid, bone marrow, brain, skin and bone <0.001%. |
[ 40 ] |
| Male SD rats (n = 14) | Uro‐A by oral gavage (26 mg kg−1 bw). Two groups: LPS i.p (n = 7) vs no LPS. |
LPS/NO LPS Stomach content (µmol)a: 0.25/0.15 Uro‐A; 0.02/0.01 Uro‐A sulfate; 0.08/0.04 Uro‐A glur. Small intestine content (µmol)a: 0.12/0.13 Uro‐A; 0.02/0.02 Uro‐A sulfate; 0.09/0.16 Uro‐A glur. Cecum content (µmol)a: 0.1/0.5 Uro‐A; 0.01/0.01 Uro‐A sulfate and Uro‐A glur. Colon content (µmol)a: 0.1/0.4 Uro‐A; 0.01/0.01 Uro‐A sulfate. Kidney (nmol g−1)a: 0.5/0.01 Uro‐A; 1/1 Uro‐A sulfate; 4/2.5 Uro‐A glur. Bladder (nmol g−1)a: 0.15/0.01 Uro‐A; 0.05/0.1 Uro‐A sulfate; 0.1/0.01 Uro‐A glur. Spleen (nmol g−1)a: 1.7/0.01 Uro‐A; 0.3/0.02 Uro‐A sulfate; 0.2/0.02 Uro‐A glur. Lung (nmol g−1)a: 1.4/0.1 Uro‐A; 1.2/0.2 Uro‐A sulfate; 1/0.4 Uro‐A glur. Liver (nmol g−1)a: 0.7/0.01 Uro‐A; 0.2/0.05 Uro‐A sulfate; 0.4/0.3 Uro‐A glur. |
[ 41 ] |
|
Male albino Wistar rats (n = 3) |
Pomegranate juice (500 mg kg−1 b.w.) per day for 10 days. | Brain (ng g−1)b: 1.68 Uro‐A. | [ 42 ] |
|
Male albino Wistar rats (n = 6) |
Pomegranate juice (500 mg kg−1 b.w per day (i.g.)) 45 days. | Brain (ng g−1)b: 2.07 Uro‐A. | [ 43 ] |
| Male C57BL/6 mice (n = 12) | 10% w/w freeze‐dried black raspberries powder, 6 weeks. | Liver (nmol g−1): 0.35 Uro‐A; 0.004 Uro‐C. Colon: 3.93 Uro‐A; 0.16 Uro‐C. Luminal content (nmol g−1): 29.14 Uro‐A; 0.067 Uro‐B; 4.28 Uro‐C; 0.03 Uro‐D. Prostate (nmol g−1):0.15 Uro‐A. | [ 44 ] |
| Female SD rats (n = 28) | One capsule by gavage (2.2 mg RSV, 6.7 mg pomegranate, 6.7 mg olive, 6.7 mg cocoa, 2.2 mg orange, 2.2 mg lemon, and 2.2 mg grapeseed). | Mammary tissues (pmol g−1)a: Cmax: 6.7 Uro‐A glur; 6 Uro‐A sulfate. | [ 45 ] |
| Humans | |||
| Breast cancer patients (n = 19) | Plant extract mixture (53.85 mg trans‐RSV, 161.5 mg pomegranate extract, 53.85 mg orange extract, 53.85 mg lemon extract, 161.5 mg olive extract, 161.5 mg cocoa extract and 53.85 mg grape seed extract) 3 capsules/day, 6 days. |
Normal mammary tissue (pmol g−1): 27.4 Uro‐A glur; 9.6 isoUro‐A glur; 5.4 Uro‐A sulfate; 14.4 Uro‐B glur; 1.3 Uro‐B sulfate. Malignant mammary tissue (pmol g−1): 29.4 Uro‐A glur; 4.8 Uro‐A sulfate; 11.3 Uro‐B glur; 1.0 Uro‐B sulfate. Trace amounts of Uro‐A glur were detected in the adipose mammary tissue. |
[ 46 ] |
| Healthy elderly volunteers (n = 6) | 2000 mg of Uro‐A delivered orally using softgels. | Skeletal muscle tissue (ng g−1)a: 0.9 Uro‐A (eight hours after oral dose). | [ 34 ] |
*Primarily focused on the last five years. Check the reference [ 4 ] for a systematic review up to 2017. aQuantitative mean values were estimated from graphics in the original paper; bQuantification after enzymatic hydrolysis; i.g., intragastric; i.p., intraperitoneal; RSV, resveratrol; SD: Sprague‐Dawley.
The oral administration of Uro‐A allowed the quantification of 0.9 ng g−1 of this compound in human skeletal muscle tissue[ 34 ] and up to 12 ng g−1 in the vastus muscle from rats.[ 37 ] So far, the presence of Uro‐A in skeletal muscle tissue had only been reported in Iberian pigs after oak acorn intake.[ 47 ] Trace amounts of Uro‐A metabolites were detected in the adipose tissue of mice after intraperitoneal injection of Uro‐A.[ 38 ] In another study, concentrations of Uro‐A and Uro‐B and their sulfate conjugates were quantified (nmol g−1) in the liver, pancreas, and heart after intraperitoneal injection of Uro‐A and Uro‐B in Wistar rats.[ 39 ] Furthermore, the oral administration of 14C radiolabeled Uro‐A to rats allowed to detect it in many rat tissues, showing a huge variability between males and females.[ 40 ] In another study, after gavage administration of Uro‐A in rats, this compound and its conjugated metabolites were quantified in the stomach, small intestine, cecum, colon, kidney, bladder, spleen, lung, and liver.[ 41 ] The amount of Uro‐A and derived metabolites in the tissues was higher in lipopolysaccharide (LPS)‐treated versus control rats. Tissue deconjugation of Uro‐A glur to produce Uro‐A after LPS‐induced inflammation was described for the first time.[ 41 ]
In previous studies, methyl‐Uro‐A[ 48 ] and Uro‐A[ 49 ] were quantified in the brain of rats after direct administration of Uro‐A, suggesting its ability to cross the blood‐brain barrier. However, the presence of metabolites in the irrigating brain blood cannot be ruled out since no perfusion was performed. Recently, the detection of Uro‐A in the brain after pomegranate juice intake has been reported,[ 42 , 43 ] with concentrations around 1.68 and 2.07 ng g−1 tissue. However, information about the brain‐occurring Uro metabolites after ET‐rich food intake is still missing as the available quantifications were done after enzymatic hydrolysis.
In another study, different unconjugated Uros (Uro‐A, Uro‐B, Uro‐C, and Uro‐D) were quantified in the liver, colon, luminal content, and prostate of mice after consuming a 10% black raspberries powder diet for 6 weeks. Uro‐A's highest concentration was observed in the luminal content (29.14 ± 9.42 µmol kg−1).[ 44 ]
Recently, the distribution of Uros has also been reported in mammary tissues from animals[ 45 ] and humans [ 46 ] after consuming a mixture of extracts containing pomegranate extract, among others. In rats, Uro‐A glur and Uro‐A sulfate were detected at 6.7 and 6 pmol g−1, respectively. In humans, glucuronide and sulfate conjugates of Uro‐A and Uro‐B were determined in normal and malignant mammary tissues with similar concentrations, from 1.0 to 27.4 pmol g−1 depending on the metabolite.[ 46 ]
Overall, Uros' bioavailability, metabolism, and tissue distribution are essential to understanding the Uros‐health binomial. Next, it is crucial to elucidate the current evidence on the biological activity of Uros based on in vitro and in vivo models.
1.2. Evidence for Health Properties of Urolithins Based on In Vitro and Animal Model Studies
After almost two decades of intense research since their identification, Uros have gained recognition as one of the main drivers for the health effects related to the consumption of ET rich foods both at the gastrointestinal and systemic levels.
Many in vitro studies describe the beneficial effects of Uros (especially Uro‐A), which include anti‐inflammatory, anticancer, antioxidant, cardioprotective, neuroprotective, and estrogenic effects, among others.[ 4 , 50 , 51 ] Nevertheless, despite the promising in vitro results, the in vivo direct evidence and their mechanisms of action supporting the health benefits are still under debate.[ 52 , 53 ] In this regard, a common concern within the (poly)phenols and health interaction research resides in the fact that many in vitro studies overlook the metabolism and tissue distribution of Uros, using both non‐physiological concentrations and inadequate metabolic forms.[ 54 , 55 ]
The design of physiologically relevant in vitro studies using appropriate metabolites (free forms or conjugates) and concentrations detected in vivo is essential to investigate the biological effects of Uros. The growing knowledge on their metabolism and tissue distribution and the higher availability of relevant phase‐II Uro conjugates in the market allow scientists to design proper in vitro approaches. However, as shown in Tables S1‐S5 (Supporting Information), many in vitro studies exploring the biological activity of Uros (free forms and phase‐II conjugates) use physiologically irrelevant assay conditions.
In the past few years, there has been a considerable increase of in vivo studies using animal models reporting biological activities after Uros administration, both orally and intraperitoneally, to unequivocally demonstrate their involvement in the beneficial effects attributed to the intake of ET‐rich foods. To the best of our knowledge, from a total of 35 studies performed so far, over 90% were published in the last five years (Table 3 ). Here, we summarize and discuss the main relevant in vitro and animal studies to show the current evidence of the biological properties attributed to Uros relevant to different chronic diseases.
Table 3.
Biological activities of urolithins in animal models
| Animal model | Assay conditions | Main outcomes | Reference |
|---|---|---|---|
| Anti‐inflammatory activity | |||
|
F344 rat |
Uro‐A (15 mg kg−1 d−1 p.o.; HED: ∼150 mg 70 kg−1 person) for 25 days prior to DSS‐induced colon inflammation (UC colitis model). |
Preservation of colonic architecture; ↓iNOS, COX‐2 and PTGES protein expression; ↓pro‐inflammatory IL‐1β and IL‐4 gene expression. |
[ 58 ] |
| ICR mice | Uro‐A (300 mg kg−1 d−1 p.o.; HED: ∼1.5 g 70 kg−1 person) for 1 or 6 h prior to inducing inflammation (carrageenan‐induced paw edema model). | ↓Volume of paw edema; ↑ORAC antioxidant activity in plasma. | [ 98 ] |
| Wistar rats | Uro‐A or Uro‐B (2.5 mg kg−1 d−1 i.p.) for 3 weeks in a streptozotocin‐induced type‐1 diabetes model. | ↓Fractalkine; Prevention of cardiac dysfunction; ↑maximal rate of ventricular pressure rise and parallel ↓reduction in the isovolumic contraction time; recovery of cardiomyocyte contractility and Ca+2 dynamics and ↑velocity of shortening (only for Uro‐B). | [ 39 ] |
| Sprague‐Dawley rats | Uro‐A (50 mg kg−1 d−1 p.o.) for 5 days in a cisplatin‐induced nephrotoxicity model. | ↓Cisplatin‐induced inflammatory cascade and inhibition of the proapoptotic pathway. Prevention of renal dysfunction and histopathological damage. | [ 162 ] |
|
C57BL/6J or Nrf2−/− mice |
1) Uro‐A (20 mg kg−1 d−1 p.o.) at 0, 6, 12, 18, and 24 h before LPS‐induced peritonitis in C57BL/6J mice. 2) Uro‐A (20 mg kg−1 d−1 p.o.) (4 or 20 mg kg−1 d−1 p.o.) after 12 h of TNBS‐induced colitis (C57BL/6 or Nrf2−/− mice) and every 12 h thereafter up to 72 h. 3) Uro‐A (20 mg kg−1 d−1 p.o.) on the 4th and 6th day of DSS‐induced colitis C57BL/6 model. |
1) ↓LPS‐induced increase in serum IL‐6 and TNF‐α levels.; 2) Protection of TNBS‐induced tissue damage (body weight loss, reduction of DAI score, intestinal permeability, colon shortening and weight to length ratio) and inflammation scores (reduction of neutrophil infiltration, MPO activity, and serum inflammatory markers such as IL‐6, TNF‐α, CXCL1, and IL‐1β); 3) Protection of DSS‐induced acute colitis (↓DAI scores, colon shortening, gut permeability and increase of colon weight/length ratio); ↓inflammation (serum IL‐6, IL‐1β, TNF‐α and colonic tissue MPO levels). | [ 59 ] |
| C57BL/6J mice | Uro‐A (nanoparticle encapsulated) (50 mg kg−1 d−1 p.o.) for 19 days in cisplatin‐induced acute kidney injury model. | Attenuation of the histopathological hallmarks of cisplatin‐induced acute kidney injury; ↓mortality by lower renal oxidative and apoptotic stress (Nrf2/antioxidant response element and P53 pathways). | [ 36 ] |
| C57BL/6 mice | Uro‐A (20 mg kg−1 d−1 i.g.) for 8 weeks in surgically osteoarthritis model. | Protective effect in osteoarthritis development by ↓OARSI score, ↓PI3K/AKT pathway activation and the nuclear p65 expression in chondrocytes. | [ 166 ] |
| C57BL/6 mice | Uro‐A (50 mg kg−1 d−1 p.o.) for 3 days and 30 min before surgery in a model of ischemia reperfusion injury. | ↓TNFα, IL1β, MIP1α and MIP2 mRNA expression; ↑autophagy; attenuation of associated kidney injury; protection against ischemia reperfusion injury. | [ 164 ] |
| C57BL/6 mice | Uro‐A (100 mg kg−1 d−1 i.p.) for 5 days in a cisplatin‐induced ischemic neuronal injury model. | ↓Histological damage in proximal tubular cells; ↓cisplatin‐induced pro‐inflammatory cytokines/chemokines (TNF‐α, IL‐23, IL‐18 and MIP2) and attenuation of renal oxidative/nitrative stress. | [ 99 ] |
| IL‐10−/− C57BL/6j mice |
Uro‐A (0.114 mg kg−1 d−1 p.o.) for 2 days in Campylobacter jejuni infected, microbiota‐depleted IL‐10−/− mice as preclinical inflammation model. |
Improve clinical outcome and less pronounced macroscopic (less colonic shrinkage) and microscopic (less colonic histopathology and apoptosis) inflammatory sequelae of infection; ↓intestinal pro‐inflammatory immune responses (IFN‐γ, TNF‐α, MCP‐1 and NO) and systemic markers (IFN‐γ, MCP‐1 and IL‐6); ↓abundance of macrophages, monocytes and T lymphocytes in the mucosa and lamina propria. | [ 168 ] |
| FUNDC1f/f mice and cardiomyocyte‐specific FUNDC1 knockout (FUNDC1CKO) mice | Uro‐A (30.0 mg kg−1 i.p.) prior to LPS treatment (48 h) to induce septic cardiomyopathy. | Attenuate inflammation‐mediated myocardial injury levels and normalization of cardiac function, including LVEF, LVDd, and FS in FUNDC1f/f mice, but not in FUNDC1CKO mice. | [176] |
| Neuroprotective effect and(or) improvement of cognitive function | |||
| Transgenic (express human amyloid β 1–42 in the muscle tissue after a heat shock) Caenorhabditis elegans (CL4176) | Exposure to Uro‐A (43.8 µM), Uro‐B (47.2 µM), methyl‐Uro‐A (41.3 µM), methyl‐Uro‐B (44.2 µM). |
Only methyl‐Uro‐B has a protective effect against amyloid β 1–42 induced neurotoxicity and worm paralysis. |
[ 83 ] |
| Alzheimer's disease APP/PS1 transgenic mice model | Uro‐A (300 mg kg−1 d−1 p.o.) for 14 days. | Amelioration of learning and memory deficits; prevention of neuronal apoptosis; ↑neurogenesis; ↓plaque Aβ deposition and peri‐plaque microgliosis and astrocytosis in the cortex and hippocampus; Anti‐(neuro)‐inflammatory activity: ↓pro‐inflammatory cytokine levels and activation of NF‐κB p65 subunit and p38 (MAPK). | [ 79 ] |
| ICR mice | Uro‐A (150, 100 or 50 mg kg−1 d−1 p.o.) for 8 weeks in a D‐gal‐induced brain aging model. | ↓D‐gal‐induced cognitive impairment; ↓brain aging by suppression of miR‐34a induced upregulation; ↓apoptosis induction; ↑autophagy by upregulating the SIRT1 signaling pathway and downregulating the mTOR signaling pathway. | [ 165 ] |
| C57BL/6 mice | Uro‐A (2.5 or 5.0 mg kg−1 d−1 i.p.) for 24 h and 1 h before surgery in an ischemic neuronal injury model. | ↓Infarction volume; reinforcement of ischemia‐induced autophagy by ↑LC3‐II and ↓p62 level; ↓ER stress by autophagy activation. | [ 80 ] |
|
ICR mice |
Uro‐A (1.5 or 2.0 mg kg−1 d−1 i.p.) at 1 and 24 h prior to surgery, and 1 h after surgery in an ischemic neuronal injury model (transient middle cerebral artery occlusion). | Ameliorate infarction, neurological deficit scores, and spatial memory deficits after cerebral ischemia; ↓neuron loss and ↑neurogenesis after ischemic stroke; Attenuate apoptosis by regulating apoptotic‐related proteins; ↓glial activation via affecting inflammatory signaling pathways (↑AMPK and IκBα activation, and ↓Akt, NFκB p65, ERK, JNK, and p38). | [ 170 ]*** |
|
ICR mice |
Uro‐A (2.5 mg kg−1 d−1 i.p.) for 8 weeks in an STZ‐induced diabetic mouse model. | Alleviate APP and BACE1 expressions, Tau phosphorylation, Aβ deposition, and cognitive impairment; ameliorate the high glucose‐induced TGM2 expression. | [ 82 ] |
| Cardioprotective activity | |||
| C57BL/6J mice | Uro‐A (1 mg kg−1 d−1 i.p.) at 24 and 1 h before ischemia induction in a myocardial ischemia reperfusion injury model. | Improvement of cardiac function by ↓myocardial infarct size, prevention of cardiomyocyte apoptosis and ↑serum CK and LDH activities after ischemia. | [ 161 ] |
| Wistar rat | Uro‐A (3 mg kg−1 d−1 p.o.) combined with a high cholesterol diet supplemented with Vit. D3 for 3 days prior to the balloon injury of the aorta and 12 weeks of treatment. | Improvement of aortic atherosclerotic lesions; ↓plasma lipid (total cholesterol, TGs, and LDL) and angiotensin II levels in aortic tissue. | [ 88 ] |
| ApoE−/− mice | Uro‐B (10 mg kg−1 d−1 p.o.; equal to 1.11 mg kg−1 to human) for 14 days. | ↓Lipid plaque deposition and oxidized‐LDL uptake. | [ 91 ] |
| C57BL/6 mice | Uro‐A (20 µg d−1 i.p.) accompanied with a high‐fat diet for 12 weeks. | Anti‐obesity activity by ↑systemic insulin sensitivity, ↓total and LDL cholesterol levels. In liver: ↓TGs accumulation, inflammation and elevation of mitochondrial biogenesis. In adipose tissue: ↓adipocyte hypertrophy and macrophage infiltration. | [ 38 ] |
| Sprague Dawley rats | Uro‐B (0.7 mg kg−1 d−1 i.p.) at 24 and 48 h before ischemia induction in a myocardial ischemia reperfusion injury model. | ↓Myocardial infarct size; ↓cardiac dysfunction after ischemia reperfusion; protection against myocardial ischemia/reperfusion injury via p62/Keap1/Nrf2 signaling pathway. | [ 167 ]*** |
| Wistar rats | Uro‐A or Uro‐B (2.5 mg kg−1 d−1 i.p.) four times a week for 4 weeks, in rats fed on a high‐fat diet | Anti‐obesity effect by ↓body weight and visceral adipose tissue mass; restore hepatic antioxidant capacity, serum lipid profile; ↓lipid accumulation; ↑fecal fat excretion. ↓LXRα and SREBP1c (lipogenesis) level; ↓PERK and IRE1α (hepatic endoplasmic reticulum stress) level; ↑PPARα expression (fatty acid oxidation). | [ 89 , 90 ] |
|
C57BL/6 mice and ob/ob mice |
Uro‐A (30 mg kg−1 d−1 i.g.) for 10 weeks, in mice fed on a high‐fat diet. | ↓HFD‐induced and genetic obesity; ↑in energy expenditure via ↑thermogenesis in brown adipose tissue and ↑browning of white adipose tissue. |
[ 172 ] |
|
DBA2J mice |
Uro‐A or Uro‐A and ellagic acid (0.1 % p.o.) for 8 weeks, in mice fed on a high fat/high sucrose diet (starting 8 weeks before to induce insulin resistance). | ↓Diet‐induced insulin resistance via ↓fasting glucose, serum free fatty acids and TGs levels and ↑adiponectin fasting. Differential expression of genes related to mitochondrial function in liver and skeletal muscle. |
[ 169 ] |
|
C57BL/6 mice |
Uro‐A (50 mg kg−1 d−1 i.p.) alone or in combination with chloroquine for 8 weeks in an induced by high fat and STZ‐induced type 2 diabetic model. | Improvement of diabetic symptoms:↓high water intake and urine volumes, ↓fasting blood glucose, glycated hemoglobin levels, plasma C‐peptide, MDA and IL‐1β level; ↑reduced glutathione, IL‐10 content, glucose tolerance, and pancreatic function indexes such as HOMA‐β; ↓mitochondrial swelling and myelin‐like cytoplasmic inclusions; ↑upregulate the LC3‐II and beclin1; ↓sequestosome 1 (p62) accompanied by ↓apoptotic protein cleaved caspase3 in pancreas via regulating autophagy and AKT/mTOR signaling pathway. | [ 174 ] |
| Other biological activities | |||
|
F344 rat |
Uro‐A (15 mg kg−1 d−1 p.o.; HED: ∼150 mg/70 kg person) for 25 days before inducing DSS‐induced colon inflammation (UC model). | Gut microbiota modulation: ↑bifidobacteria and lactobacilli. | [ 58 ] |
| C57BL/6J mice and Caenorhabditis elegans |
1) Uro‐A (25 or 50 mg kg−1 d−1 p.o.) for 6 weeks and 8 months, respectively, in age‐related muscle decline mice model. 2) Exposure to Uro‐A, Uro‐B, Uro‐C or Uro‐D (50 µM) in C. elegans for 50 days. |
1) Improvement of exercise capacity via ↑muscle function manifested by greater grip strength and level of spontaneous exercise. 2) Uro‐A, Uro‐B, Uro‐C or Uro‐D extended lifespan by 45.4, 36.6, 36.0 and 19.0%, respectively. |
[ 37 ] |
| Sprague–Dawley rats | Uro‐A (25 mg kg−1 d−1 p.o.) for one day after surgery, and for 4 weeks of treatment in intervertebral disc degeneration (needle‐punctured tail) model. | Amelioration of intervertebral disc degeneration mediated by ↓loss and destruction of disc height, and osteophyte formation. |
[ 163 ] |
| BALB/c athymic mice (nu/nu) | Uro‐A (50 mg kg−1, 5 days per week p.o.) for 4–5 weeks in xenograft with PC‐3 and C4‐2B cells model. | Anticancer activity: ↓tumor growth and Ki‐67 expression in both PC‐3 and C4‐2B xenografts; ↓AR/pAKT signaling in C4‐2B tumors. | [ 76 ] |
| Nude mice | Uro‐B (40 mg kg−1 i.p. and s.c.) every 2 days for 30 days in a subcutaneous xenograft with HEG2 cells model. | Anticancer activity: ↓average tumor volume, weight, and Ki‐67 levels. | [ 77 ] |
|
C57BL/6 mice (wild type, Nrf2−/− and AhR−/−) |
Uro‐A (20 mg kg−1 d−1 p.o.) for 7 days. |
Improvement of gut barrier function: activation of AhR‐Nrf2‐dependent pathways to upregulate epithelial tight junction proteins (Cldn4, NQO1, Ocln, ZO1, and TJP3). Cyp1A1 activity induction in colon and liver of wild type but not in AhR−/− mice. | [ 59 ] |
| C57BL/6 mice | Uro‐A (10 mg kg−1 d−1 i.g.) for 12–16 weeks. | Angiogenic effect: ↑angiogenic pathways and markers such as VEGFA and CDH5, which were blunted in skeletal muscles; ↑skeletal muscle vascularization via silent information regulator 1 and PGC‐1α pathway; ↑ATP and NAD+ levels in skeletal muscle. |
[ 171 ] |
|
ICR mice |
Uro‐A (80 or 240 mg kg−1 d−1 p.o.) for 1 or 3 days in a purine bodies‐induced hyperuricemic model. | Antihyperuricemic effect: Inhibit the increase in plasma uric acid levels and hepatic xanthine oxidase activity; ↓expression of genes associated with hepatic purine metabolism. | [ 173 ] |
| C57BL/6 mice | Uro‐A (10, 25, or 50 mg kg−1 d−1 p.o.) at 0, 11 and 17 day after immunization in an EAE model. | Effect against autoimmune diseases: Suppression of disease progression at prevention, induction, and effector phases of preclinical EAE at the highest dose; ↓number of inflammatory cells and demyelination; lower numbers of M1‐type microglia and activate dendritic cells; ↓infiltrating Th1/Th17 cells in the CNS | [ 175 ] |
|
mdx and mdx/Utr −/− (DKO) mice, and Caenorhabditis elegans dys‐1;hlh‐1 strain |
1) Uro‐A (mg kg−1 d−1 p.o.) for 10 weeks in DMD mice models. 2) Exposure to Uro‐A (25 µM) for 4 days in C. elegans dys‐1;hlh‐1 model (lacking the human DMD gene) |
1) Improvement of muscle function by ↑mitophagy in muscular dystrophy: ↑skeletal muscle respiratory capacity, and improved MuSCs' regenerative ability, resulting in the recovery of muscle function and ↑survival in DMD mouse models. 2) ↑Expression of pink‐1 and pdr‐1 mitophagy genes, with no impact on the expression of autophagy genes. Improvement in the mitochondrial network, mitochondrial respiration, citrate synthase activity, and the mitochondrial DNA over nuclear DNA (mtDNA/nDNA) ratio. Positive impact on muscle function and motility of the dystrophic worms. |
[ 103 ] |
|
Wistar rats |
Uro‐A or Uro‐B (2.5 mg kg−1 d−1 i.p.) four times a week for 4 weeks, in rats fed on a high‐fat diet | Gut microbiota modulation: modulate gut microbes related to body weight, dysfunctional lipid metabolism and inflammation. | [ 90 ] |
αKGDH, alpha‐ketoglutarate dehydrogenase; AhR, aryl hydrocarbon receptor; AMP, adenosine monophosphate; AMPK, AMP activated protein kinase; APP, amyloid precursor protein; AR, androgen receptor; ATP, adenosine triphosphate; BACE1, β‐secretase‐1; CDH5, cadherin 5; CK, creatine kinase; Cldn4, claudin 4; CNS, central nervous system; COX, cyclooxygenase; CXCL1, chemokine ligand 1; CYP, cytochrome P450; DAI, disease activity index; DHT, 5α‐dihydrotestosterone; DMBA, dimethylbenz[a]anthracene; DMD; Duchenne muscular dystrophy; DSS, dextran sulfate sodium; EAE, experimental autoimmune encephalomyelitis; ER, endoplasmic reticulum; ERα, estrogen receptor alpha; ERK, extracellular signal‐regulated kinase; FRAP, ferric‐reducing antioxidant power; FS, fractional shortening; GDX, gonadectomized; GnRH, gonadotropin releasing hormone; GR, glutathione reductase; GSH, reduced glutathione; GSSG, oxidized glutathione; HED, human equivalent dose; HOMA, homeostasis model assessment; ICR, Institute of Cancer Research; IFN, intereferon; IκBα, nuclear factor of kappa light polypeptide gene enhancer in B‐cells inhibitor alpha; IL, interleukin; iNOS, nitric oxid synthase; IRE1α, inositol‐requiring transmembrane kinase/endoribonuclease 1α; JNK, c‐Jun N‐terminal kinase; Keap1, Kelch like ECH associated protein 1; LC3‐II, protein levels of microtubule‐associated protein 1 light chain 3‐II; LDL, low‐density lipoprotein; LDH, lactate dehydrogenase; LH, luteinizing hormone; LPS, lipopolysaccharide; LVDd, left ventricular diastolic; LVEF, left ventricular ejection fraction; LXRα Liver X receptor α; MAPK, mitogen‐activated protein kinase; MCP‐1, monocyte chemoattractant protein 1; MDA, malondialdehyde; MIP, macrophage inflammatory protein; miR, microRNA; MPO, myeloperoxidase; mTOR, mammalian target of rapamycin; NAD, nicotinamide adenine dinucleotide; NF‐κB, nuclear factor kappa‐light‐chain‐enhancer of activated B cells; NO, nitric oxide; NQO1, NAD(P)H dehydrogenase [quinone] 1; Nrf2, nuclear factor erythroid 2‐related factor 2; OARSI, Osteoarthritis Research Society International; Ocln, occludin; ODMA, O‐desmethylangolensin; ORAC, oxygen radical absorbance capacity; OVX, ovariectomy; PDH, pyruvate dehydrogenase; PERK, protein kinase R‐like endoplasmic reticulum kinase; PGC‐1α, peroxisome proliferator‐activated receptor‐gamma coactivator‐1‐alpha; PI3K, phosphoinositide 3‐kinase; PPARα, peroxisome proliferator‐activated receptor α; PRL, prolactin; PTGES, prostaglandin E synthase; SIRT, sirtuin 1; SOD, superoxide dismutase; SREBP1, sterol regulatory element binding protein 1; STZ, streptozotocin; TBARS, thiobarbituric acid reactive substances; TC, total cholesterol; TERP, truncated estrogen receptor product; TG, triglycerides; TGM2, transglutaminase type 2; TJP3, tight junction protein 3; TNBS, 2,4,6‐Trinitrobenzenesulfonic acid; TNF‐α, tumor necrosis factor alpha; TP, testosterone propionate; UC, ulcerative colitis; Uro, urolithin; VEGFA, vascular endothelial growth factor A; ZO1, zonula occludens‐1.
1.3. Anti‐inflammatory Effects
To date, the evidence related to the anti‐inflammatory effects of Uros encompasses the highest number of studies, mainly within the colonic environment. Pioneer investigations reported the benefits associated with Uro‐A in relevant animal and cellular models of intestinal inflammation. These benefits included protection of the inflammation‐induced damage in colon tissues, the modulation of genes and proteins (such as iNOS, COX‐2, PTGES, and NF‐κB) as well as molecules (i.e., prostaglandins and cytokines) involved in the regulation of inflammation and processes associated like fibroblasts migration and monocyte adhesion.[ 56 , 57 , 58 ] Further evidence supporting these benefits comes from additional preclinical studies which described molecular mechanisms related to the anti‐inflammatory effects[ 4 ] (Table S1, Supporting Information). In this line, recent investigations with rodent and cellular models of inflammation further showed that Uro‐A protects against the inflammation‐induced intestinal barrier damage through the activation of the aryl hydrocarbon receptor (AhR)‐nuclear factor erythroid 2‐related factor 2 (Nrf2)‐dependent pathways, amelioration of cytokines biosynthesis (such as TNF‐α and IL‐6), and modulation of the tight junction proteins expression.[ 59 , 60 ]
Regarding the systemic environment, growing evidence on the anti‐inflammatory properties of Uro‐A is supported by seven animal studies. These studies describe that Uro‐A administration (orally or intraperitoneally) shows the capacity to protect or attenuate inflammation‐induced injury in different inflammation models, including ischemia/reperfusion and osteoarthritis, associated with decreasing systemic pro‐inflammatory cytokines and the signaling pathways increased by the inflammation status (Table 3). However, despite the pharmacological context, the underlying molecular mechanisms involved in the anti‐inflammatory effect of the circulating conjugated Uros remain puzzling.
In this context, since many anti‐inflammatory in vitro studies have been performed in immune, endothelial or neuronal cells, the physiological relevance of several investigations published in the last years is still questionable as only a few studies have taken into consideration the effect of the phase‐II urolithin conjugates at physiological concentrations attained at the systemic level[ 4 , 54 ] (Table S1, Supporting Information).
A common result in these studies, regardless of the inflammatory cell model, is the lower or lack of anti‐inflammatory effect of the circulating conjugated Uros compared with their aglycones.[ 61 , 62 , 63 , 64 ] Nonetheless, considering the positive impact observed after oral administration of Uro‐A in animal models (Table 3), we cannot discard that these conjugated metabolites could exert anti‐inflammatory effects involving alternative mechanisms of action and indirect effects, as well as possible in situ deconjugation processes that could release their more bioactive free forms. However, this hypothesis should be considered with caution as only one in vivo study has explored and reported the tissue deconjugation of Uro‐A glur to give Uro‐A under specific LPS‐induced systemic inflammation conditions in rats.[ 41 ] Considering this, further in vitro studies are needed with the phase‐II Uro metabolites and more in vivo studies.
1.4. Anticancer Effects
In 2009, a pioneer in vitro study reported the antiproliferative effect of colonic Uros, mainly Uro‐A, against different colon cancer cells.[ 65 ] Since then, the anticancer properties of Uros, including antiproliferation, apoptosis promotion, and anti‐angiogenesis, have attracted significant attention against a wide diversity of cellular models of cancer such as colon, prostate, breast, kidney, liver, and brain[ 4 , 66 ] (Table S2, Supporting Information). In this line, accumulating in vitro evidence indicate that Uros exert different anticancer mechanisms of action through the modulation of the expression of genes and proteins of key cancer markers (such as p21) and signaling pathways (i.e., MAPK and Akt) involved in cell cycle arrest and apoptosis induction and, therefore, associated with cancer development[ 4 , 66 , 67 , 68 ] (Table S2, Supporting Information). Other anticarcinogenic mechanisms contribute to understanding how Uro‐A and physiologically relevant mixtures mimicking UM‐A exert beneficial effects on colon cancer cells. A mechanism recently described involves induction of senescence‐associated with β‐galactosidase activity upon long‐term exposure via up‐regulation of tumor‐suppressor proteins expressions such as p21Cip1/Waf1 and p53.[ 69 , 70 ] The anticancer activity of Uro‐A is also associated with its capacity to induce autophagy and inhibition of metastasis involving the upregulation of LC3, a central protein in the autophagy pathway.[ 71 ]
The studies of Uros against cancer performed during the last decade have two common essential points to highlight: i) among all colonic Uros tested, Uro‐A exerts the highest anticarcinogenic effect and; ii) the circulating phase‐II conjugated Uros exert lower (or lack of) anticancer activities compared to their free forms.[ 68 , 72 , 73 ] In this regard, the evidence of the in vitro anticancer activity of Uros in systemic tissues such as prostate, breast, or liver is still limited and contradictory compared with those reported against intestinal cancer cell models. As stated before, while new in vitro studies using free colonic Uros with doubtful physiological relevance indicate a clear anticancer effect through different mechanisms of action, to date, there are still few in vitro studies mimicking the in vivo conditions of Uros at the systemic level (regarding metabolic form and concentrations) (Table S2, Supporting Information). Indeed, these few relevant studies have reported lower or absence of antiproliferative effect in phase‐II metabolites, unlike their free forms against different models of prostate,[ 74 ] and breast[ 46 , 73 ] cancer. In addition, contrasting with Uro‐A, its phase‐II conjugates failed to exert senescence on colon cancer cell lines upon long‐term effects.[ 69 ] However, it cannot be ruled out a possible effect on senescence induction against systemic cancer cells similar to those of other phenolics phase‐II conjugates such as resveratrol against breast cancer cells.[ 75 ] Therefore, it is still mandatory to design in vitro studies considering molecular forms physiologically relevant, concentrations achieved in vivo, representative mixtures, etc., to unequivocally identify the possible anticancer effects against systemic cancer cells and the molecule(s) responsible for the effects observed.
On the other hand, although the increasing number of in vitro studies indicate the potential anticancer effects of Uros, mainly Uro‐A, the in vivo evidence on the anticancer activity of Uros is still limited (Table 3). In this regard, preliminary animal studies (rat models) performed in 2010 reported that oral administration or in situ colon perfusion of Uro‐A modulated the gene expression in the colonic mucosa of several markers (p53, Rb1, Akt, CYP1A1, etc.) associated with colon cancer development.[ 57 , 58 ] Since then, two recent remarkable animal studies have explored the attractive Uros' anticancer effect observed in vitro. In 2018, Dahiya et al. reported that the oral administration of Uro‐A (50 mg kg−1) for 4–5 weeks decreased the tumor size and the expression of the proliferation marker Ki‐67 in prostate cancer cells xenografts.[ 76 ] One year later, following a pharmacological approach, Lv et al. observed the reduction in the average tumor volume and weight, and the Ki‐67 levels, of the hepatocellular tumor in a xenograft mouse model after intraperitoneal or subcutaneous administration of Uro‐B (40 mg kg−1 every 2 days, for 30 days).[ 77 ]
Overall, despite these promising results, more relevant preclinical studies, both in vitro and in animal models, are needed to provide more significant evidence and new insights to elucidate the potential anticancer effects of Uros.
1.5. Neuroprotective Effects and Improvement of Cognitive Function
In the past few years, several studies have begun to indicate that Uros may be partly responsible for the benefits against neurodegenerative diseases such as Alzheimer's or Parkinson's diseases attributed to ET‐rich foods consumption.[ 78 ] Hence, evidence is increasing supported by animal models of neuronal injury and(or) neurodegenerative diseases. These investigations indicate that administration (oral and intraperitoneal) of Uro‐A exerts an improvement in cognitive impairment, brain aging, and a reduction in the accumulation of amyloid plaques[ 79 , 80 , 81 , 82 ] (Table 3). In this regard, animal studies claim that the free form of Uro‐A can cross the blood‐brain barrier (BBB) and reach the brain in rats fed pomegranate juice (alone and combined with rotenone) after whole‐body perfusion to avoid overlapping of metabolites from those of the residual blood,[ 42 , 43 ] confirming previous results obtained for Uro‐A and Uro‐B by computational models[ 83 ] and animal models upon intravenous administration but in non‐perfused brains.[ 49 ] However, these enthusiastic results should be cautiously considered since the brain samples underwent enzymatic hydrolysis, and thus, the presence of free Uro‐A in the brain cannot be unequivocally confirmed. The concentrations detected (at the range of ng g−1 wet tissue) came from a single point after the intake. Thus, as commented before, whether conjugated glucuronides alone or in combination with their free forms might be able to cross BBB, as well as the physiological concentrations that reach the brain tissue remain unknown.[ 42 , 43 ]
Therefore, despite the neuroprotective effect observed against a wide range of neuronal cell models, mainly due to the (neuro)inflammation and antioxidant activities exerted by Uro‐A, and to a lesser extent Uro‐B, evidence is still weak. The main reason is the lack of physiologically relevant studies using the circulating conjugated Uros that might reach brain tissues (Table S3, Supporting Information). In this regard, to date, only a few studies follow this approach reporting a slight attenuation on the H2O2‐induced cytotoxicity in neuroblastoma SH‐SY5Y cells after pre‐treatment with conjugated glucuronides of Uro‐A and isoUro‐A, but not for Uro‐B glur,[ 84 ] as well as for methyl‐Uro‐A and methyl‐Uro‐B at similar concentrations in LPS‐stimulated BV‐2 microglia cells.[ 85 ] However, Xu et al. reported no effect of methylated Uros (never detected in circulation in humans), unlike their de‐methylated derivatives, under the similar (neuro)inflammation model in BV‐2 cells.[ 86 ]
Overall, there is still a need for well‐designed in vivo studies to identify if phase‐II conjugated Uros can cross the BBB, interact with neuronal cells, and delve into underlying molecular mechanisms associated with their neuroprotective effects.
1.6. Cardioprotective Effects
At present, many studies associate the consumption of ET‐rich foods with protection against cardiometabolic diseases such as hypertension, hyperlipidemia, obesity, and diabetes.[ 78 , 87 ] The question of what molecule(s) could be responsible for these beneficial effects has launched numerous studies that again placed Uros under the spotlight. Considering this, recent in vivo studies using a range of different rodent models mimicking cardiometabolic disorders (i.e., diabetes, obesity, cardiac dysfunction after ischemia‐reperfusion) described positive effects after oral and intraperitoneal administration of Uro‐A and, to a lesser extent, Uro‐B. These benefits encompassed improvement of the cardiac dysfunction systemic atherosclerotic lesions, a decrease of cardiometabolic risk markers (blood lipids, insulin sensitivity, fasting glucose, lipid peroxidation, body weight, and visceral adipose tissue mass), anti‐obesity effects,[ 38 , 88 , 89 , 90 ] and reduction of lipid plaques deposition and oxidized LDLc (oxLDLc) uptake in Uro‐B‐administered animals[ 91 ] (Table 3).
However, from a dietary point of view, whether Uros exert the cardioprotective effects by themselves or indirectly remains elusive. Thus, to date, the number of in vitro studies that investigate the impact of free and conjugated colonic Uros using the concentrations achieved at the systemic level is scant[ 4 ] (Table S4, Supporting Information). Among the physiologically relevant in vitro studies (that consider concentrations and metabolic form achieved in vivo), two studies reported a possible anti‐atherosclerosis effect for Uro‐A and Uro‐B glucuronides, partly due to their slight anti‐inflammatory activity on TNF‐α‐stimulated human endothelial cells,[ 92 ] as well as for Uro‐B sulfate that was able to induce a reverse cholesterol efflux in oxLDLc stimulated THP‐1 macrophages.[ 91 ] Additional benefits described for Uro‐B glur comprise attenuation of the direct detrimental effects of TMAO and the diabetic cardiomyopathy in rats‐isolated cardiomyocytes.[ 62 , 93 ] Regarding the anti‐obesity effect, Uros play an important role in reducing triglycerides accumulation, increasing fatty acid oxidation in adipocytes and hepatocytes,[ 94 , 95 ] and decreasing adipocyte formation.[ 96 ] However, there are no studies conducted with conjugated Uros to gain further insights into these possible effects against metabolic‐associated disorders such as obesity and related complications (Table S4, Supporting Information).
Therefore, to date, the evidence regarding the cardioprotective activities of Uros behind the health effects associated with ET‐rich foods consumption is somewhat limited by the in vitro to in vivo extrapolation and requires further research.
1.7. Miscellaneous Effects
The possible role of Uros on other health effects and how they can interact and(or) modulate receptors, proteins, signaling pathways, or microorganisms that may explain the beneficial health effects related to ET‐rich foods consumption, has also been investigated (Table 3 and Table S5, Supporting Information). Among these effects, one of the most studied is the role of Uros as molecules (partially) responsible for the antioxidant properties attributed to the consumption of ETs‐rich foods. In this regard, the evidence provided by in vitro and in vivo preclinical studies remains contradictory[ 4 , 97 ] (Table 3 and Table S5, Supporting Information). Thus, despite the relative protection against oxidative stress reported for the free forms of Uros in some cell models, the antioxidant capacity of the conjugated metabolites is unclear. In addition, animal models reporting an unequivocally antioxidant activity or protection against radical damage are still scarce and contradictory. For instance, the positive effect of Uro‐A administration in carrageenan‐induced paw edema mice model[ 98 ] and against cisplatin‐induced oxidative damage[ 99 ] contrasts with the absence of antioxidant effects observed in plasma and colon mucosa in a DSS‐intestinal inflammation animal model.[ 58 ]
The ability of Uros to act as phytoestrogens, due to their structural parallelism with estradiol, is another activity widely explored. Cellular and in silico models confirmed that free colonic Uros (Uro‐A, isoUro‐A, and Uro‐B) could interact with the estradiol receptors exhibiting estrogenic and antiestrogenic effects.[ 100 , 101 , 102 ] However, a recent in vitro study reported that the circulating conjugated Uros failed to exert in vitro estrogenic and anti‐estrogenic activities.[ 73 ] To the best of our knowledge, there is no in vivo study to corroborate these estrogenic and(or) anti‐estrogenic effects.
Moreover, studies in rodent models have characterized the effect of the oral administration of Uro‐A on gut microbiota modulation (i.e., by increasing potentially beneficial bacterial groups for health)[ 58 ] and on improving the intestinal barrier function by the induction tight junction proteins expression in epithelial cells.[ 59 ] Similarly, a recent study has also described that the intraperitoneal administration of Uro‐A and Uro‐B for 4 weeks in rats fed a high‐fat diet exerted changes in the gut microbiota composition, promoting the reduction of gut microbes related to body weight, dysfunctional lipid metabolism, and inflammation.[ 90 ] However, more in vivo studies are mandatory to confirm or reverse these promising effects.
Finally, Uro‐A also showed the capacity to stimulate mitophagy and improve muscle health in preclinical aging models and Duchenne muscular dystrophy (DMD) models in mice fed an Uro‐A‐enriched diet, in primary human myofibroblasts isolated from patients with DMD, and in Caenorhabditis elegans exposed to Uro‐A.[ 103 ] Besides, in the studies conducted in worms, Uro‐A extended their lifespan,[ 37 , 103 ] although more studies are needed to confirm these promising protection against aging and age‐related conditions.
2. Human Trials: Stratification by Urolithin Metabotypes
There is substantial human interindividual variability in response to (poly)phenols consumption. A complex combination of variables contributes to this interindividual variability, including age, sex, lifestyle, physiological status, gut microbiota, genetic makeup, dietary patterns, food matrix, and processing.[ 52 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 ]
The two‐way interaction between (poly)phenols and gut microbiota is crucial in the (poly)phenols and health puzzle.[ 52 , 111 , 112 ] Increasing evidence has recognized the phenolic‐derived microbial metabolites as possible major actors that explain the bioactivity of the ingested (poly)phenols.[ 50 , 52 , 111 ]
However, besides the potential activity of the microbial derivatives, each individual's gut microbiota, including the microbial ecology involved in (poly)phenols metabolism, is also a relevant driver in the (poly)phenols effects.[ 111 ] In this regard, some individuals have characteristic metabolic responses to dietary or pharmacological interventions. These metabolic phenotypes are known as “metabotypes” and are characterized by the subjects' (epi)genetics, physiology, and(or) gut microbiota composition and functionality. Therefore, metabotype is a broad term that can involve the gut microbial and host metabolism and subsequent biological activity (synthesis, transport, interaction with receptors, etc.) of either endogenous or exogenous compounds (cholesterol, glucose, dietary and environmental compounds, and drugs).[ 52 , 111 , 113 , 114 , 115 ] In the specific context of (poly)phenols and health, a metabotype associated with the metabolism of (poly)phenols by the gut microbiota refers to a metabolic phenotype characterized by specific metabolites produced from the catabolism of specific (poly)phenol precursors by a particular gut microbial ecology.[ 52 , 111 , 116 ] A qualitative criterion (i.e., producer vs non‐producer of specific metabolites) defines a metabotype associated with the gut microbiota. On the other hand, a quantitative criterion (i.e., high producer vs low producers) should not be used to establish a possible metabotype. The establishment of the cut‐off between high and low producers is arbitrary. It depends on many variables, including the gastrointestinal motility, sample collection time, food matrix, the lag period between the last intake of (poly)phenolic precursor and the sample analysis, and the sensitivity of the analytical procedure.[ 52 , 108 , 111 ]
To date, in the context of (poly)phenols, the metabotypes identified unambiguously are those involved in the metabolism of isoflavones (equol producers vs non‐producers) and EA (Uro metabotypes, UMs). The present review will not address all the characteristics of these metabotypes systematically. Previous publications provide a comprehensive description of these metabotypes and their possible association with human health.[ 52 , 111 ] However, in the context of the present review, it is worth highlighting the main characteristics of UMs because the essential question is to elucidate whether Uros drive the effect on human health as bioactive compounds and(or) by the gut microbial ecology associated with their UM.[ 52 , 111 ]
Briefly, in the case of UMs, three metabotypes have been described (UM‐A, UM‐B, and UM‐0): UM‐A individuals are those producers of only Uro‐A as final Uro, UM‐B subjects also produce Uro‐A, and distinctively, isoUro‐A, and Uro‐B. Finally, UM‐0 individuals cannot produce Uros (only the pentahydroxy‐Uro precursor Uro‐M5 has been detected). Furthermore, the distribution of UM‐A and UM‐B is critically affected by aging. The percentage of UM‐0 was reported to be approximately constant (10%) in the range from 5 to 90 years of age in a Caucasian cohort (n = 839). In contrast, UM‐A is more prevalent at an early age, decreasing from 85% up to 55% in adulthood, parallel to an increase of UM‐B from 15% up to 45%. From 35 to 40 years of age, the proportion of UM‐A and UM‐B (55%, and 45%, respectively) remains approximately unaltered.[ 52 , 117 ] The human gut microbial ecologies associated with UMs, and their associations with cardiometabolic markers, have been described in healthy normoweight, overweight, and obese individuals (n = 249), showing lower richness and diversity in UM‐0 individuals compared to UM‐A and UM‐B.[ 118 ]
Recently, Cortés‐Martín et al.[ 119 ] reported that overweight‐obesity prevalence in a Caucasian group of children and adolescents (n = 415) was related to being a boy (9−12 years old) with either UM‐B or UM‐0, low adherence to the Mediterranean diet, and high contribution of a specific consortium of 24 single‐nucleotide polymorphisms (SNPs) from a total of 53 SNPs related to obesity and cardiometabolic diseases. These results also highlighted the multifactorial etiology of obesity since every single variable (sex, diet, UMs, SNPs, age, physical activity) was not independently associated with overweight‐obesity.[ 119 ] The same authors also described that UMs distribution in polymedicated metabolic syndrome patients was almost similar to that described for healthy young adults.[ 120 ] The authors confirmed that the gut microbiota of these patients was in dysbiosis, mainly in hypertensive patients with an overabundance of LPS‐producing members of the Enterobacteriaceae family.[ 121 ] Overall, polymedication affected the microbial ecology involved in Uros production and also determined the prebiotic effect of a pomegranate extract in these patients.[ 120 ]
The current evidence, although still low, suggests that the gut microbial ecology of UM‐A could be “protective,” while UM‐B could be potentially prone to dysbiosis and cardiometabolic risk.[ 52 , 111 , 118 , 122 ] In this regard, for the first time, González‐Sarrías et al.[ 123 ] described the improvement of cardiometabolic risk biomarkers in UM‐B individuals after consuming an ET‐rich pomegranate extract. In contrast, no statistically significant effects were observed in UM‐A individuals or the whole group before clustering individuals according to their UMs. In general, the relatively low number of volunteers with UM‐0 in different human studies has prevented drawing any statistically significant conclusion regarding the possible impact of this non‐producer UM in the outcomes of the studies.[ 120 , 122 , 123 , 124 ]
Therefore, the stratification of individuals according to their gut microbiota (poly)phenol metabotypes could help understand individuals’ response to dietary (poly)phenols, something essential in the context of personalized nutrition.[ 52 , 111 , 123 ]
3. Safety and Bioactivity of Urolithins in Humans
To date, Uros are well‐known biomarkers of ETs consumption.[ 50 , 125 ] However, the evidence supporting their role as bioactive metabolites with differential impact on human health is very scarce so far because it has been primarily addressed by correlating the presence of free and conjugated Uros in blood, urine, and(or) feces with different health biomarkers.
Several associations between basal cardiometabolic risk factors and the urine Uro metabolomics signature were reported in healthy overweight‐obese individuals and subjects with metabolic syndrome traits after clustering by their UMs.[ 126 ] In healthy overweight‐obese individuals, urinary Phase‐II Uro‐A conjugates (primarily in UM‐A) were positively associated with apolipoprotein A‐I and intermediate‐HDLc. At the same time, Uro‐B and isoUro‐A (characteristic from UM‐B) were positively correlated with total cholesterol, LDLc, apolipoprotein B, VLDLc, IDLc, oxLDL, and the apolipoprotein B/apolipoprotein A‐I ratio.[ 126 ] Only Uro‐A‐derived metabolites correlated inversely with glucose levels in volunteers with metabolic syndrome risk factors.[ 126 ] Another study reported that Uro‐A glur was inversely associated with basal abdominal adiposity, fasting insulin, and HOMA‐IR after 12‐weeks consumption of mixed nuts (30 g day−1) by volunteers with metabolic syndrome risk factors.[ 127 ] In healthy overweight‐obese individuals, urinary excreted phase‐II Uro conjugates were significantly correlated with improving several cardiometabolic risk markers after consuming a high dose (640 mg phenolics day−1) of a pomegranate extract for 3 weeks.[ 123 ] In this study, urinary Uro‐A metabolites were associated with the decrease of total cholesterol, non‐HDLc, and LDLc, while changes of LDLc correlated with both Uro‐A and isoUro‐A + Uro‐B conjugates. However, these associations were only observed in UM‐B individuals. In contrast, no significant correlations were found in UM‐A individuals, where cardiovascular risk markers did not change.[ 123 ] The presence of nanomolar concentrations of Uro‐A conjugates was also associated with improved flow‐mediated dilation in healthy subjects (n = 10) after consuming red raspberries.[ 128 ] However, statistically significant associations between excreted or circulating Uros in all the above studies do not ineludibly involve causality or clinical relevance.[ 52 , 111 ] In this regard, other studies failed to find a significant correlation between Uros occurrence in plasma, urine, feces, or colonic tissues and cancer‐related markers and metabolic endotoxemia in colorectal cancer patients (n = 45),[ 124 , 129 ] metabolic endotoxemia in overweight‐obese individuals (n = 49),[ 130 ] and the blood lipid profile (n = 32),[ 33 ] and antioxidant markers (n = 31)[ 131 ] in healthy subjects.
Overall, the possible associations between excreted or circulating metabolites and health‐related biomarkers detected in a specific moment might be casual rather than causal, which is somewhat logical due to the highly variable turnover of these metabolites in the bloodstream and other reservoirs.[ 52 , 111 ]
As in the case of other microbial‐derived metabolites, an Uro production gradient within UM‐A and UM‐B individuals defines tentatively “high” and “low” producers of Uros. However, as explained above, the criterion for establishing the boundary between high and low Uro producers (also extrapolated to other microbial‐derived metabolites such as equol, enterolignans, and hydroxyvalerolactones) is arbitrary.[ 111 ] This means that the possible associations between “high” or “low” Uros production and health issues may not be reproducible in all circumstances or conditions.
Therefore, whether a specific microbial‐derived metabolite exerts biological activity, dependently or not of the associated microbiota, requires evaluating the effects upon direct administration of the metabolite. In this regard, oral Uro‐A administration was safe in both preclinical[ 39 , 58 , 59 ] and human studies,[ 34 ] and it was recognized as GRAS (generally recognized as safe) for its use as an ingredient in several food products by the Food and Drug Administration (FDA). These facts predict an increase in human trials to evaluate the specific health effects of Uro‐A in the coming years. Two human studies, funded by a private company, have been conducted with a commercial oral synthetic Uro‐A supplement. The first study reported the safety, tolerability, and improved mitochondrial and cellular health by regulating gene expression associated with cellular and mitochondrial function in healthy elderly subjects after consuming 500 and 1000 mg Uro‐A in a single and multiple dose‐escalation phase 1 study.[ 34 ] The second human study claimed that consuming 500 mg of Uro‐A was more efficient than the single intake of a specific pomegranate juice to achieve more plasma Uro‐A‐derived conjugates after 6 and 24 h.[ 35 ] However, i) the use of snapshot plasma levels of microbial circulating metabolites is questionable for this comparison (the use of 24h‐volume urine is a more appropriate quantitative approach), ii) the overall bioavailability and plasma pharmacokinetics of Uro‐A and derived metabolites after oral Uro‐A administration have been reported to be low,[ 43 , 132 ] iii) the approach used for clustering “Uro‐A producers” and “Uro‐A non‐producers” was arbitrary and did not take into account many interfering variables, and iv) there may be a bias in the results depending on the ETs concentration and food matrix chosen (e.g., walnuts intake could have yielded a lower number of Uro‐A non‐producers and promoted much more Uro‐A production than that specific pomegranate juice).[ 133 ]
4. New Insights into Microorganisms Involved in Urolithin Production
Uros occur naturally in the gut by the action of the gut microbiota on dietary ETs, but EA needs to be produced first. EA can be obtained from EA glycosides or ETs possessing a hexa‐hydroxydiphenic acid (HHDP) moiety by chemical or biological methods such as acidic or enzymatic hydrolyses. β‐Glucosidase has been associated with EA glycoside hydrolysis.[ 134 ] In addition, microbial enzymes known as tannases (tannin acyl hydrolase) have been related to tannin hydrolysis. Tannases hydrolyze the ester bonds present in gallotannins and ETs, although they do not affect the C‐C bonds.[ 4 , 135 , 136 ] Therefore, they have no activity on condensed tannins. The ability to degrade tannins is different between bacteria, fungi, and yeasts. Bacteria and fungi can hydrolyze high molecular weight compounds such as ETs and gallotannins. In contrast, yeasts only hydrolyze gallotannins easily.[ 136 ] The tannase activity of bacteria isolated from different sources, mainly from the intestines of animals and humans and from fermented food and fruit wastes has been described. Many of them are lactic acid bacteria such as Lactobacillus plantarum, L. paraplantarum and L. pentosus.[ 136 ] This can explain why (poly)phenols and particularly hydrolyzable tannins have consistent “prebiotic‐like” effects and promote the growth of intestinal lactobacilli.[ 5 , 137 ] Homologous genes to the known L. plantarum tannase gene have been described in other gut bacteria genera with potential tannase activity such as Roseburia, Fusobacterium, Streptococcus, and Slackia.[ 138 ] These authors suggested that gut tannin‐degrading bacteria that selectively colonize tumorous tissues but not adjacent non‐malignant tissues might be involved in preventing colon tumor progression. Some Bacillus species isolated recently from traditional fermented tea leaves have been identified as extracellular tannase producers and suggested as potential probiotics.[ 139 ] However, there are few studies on the biodegradation of ETs specifically. Tannases involved in ETs hydrolysis are known as ETs acyl hydrolases or ellagitannases.[ 140 ] Preliminary evidence of the fungal biodegradation pathway of ETs has revealed the existence of a fungal ellagitannase, which hydrolyzes pomegranate ETs (punicalagin) where punicalin and gallagic acid would serve as intermediates.[ 141 ] Punicalagin and punicalin from pomegranate husk have been reported as an optimal carbon source for EA production by Aspergillus niger in a solid‐state culture using polyurethane foam as support and pomegranate ETs as substrate.[ 142 ] Human gut microbiota also contain bacteria with ellagitannase activity against punicalagin. Still, also other ETs holding an HHDP moiety, such as pedunculagin, potentillin, casuarictin, coriariin B, vescalagin, castalagin, casuarinin, stenophyllinin A, and stenophyllanin A, resulted in Uro production as shown in a fecal fermentation study in vitro.[ 143 ] However, the individual microbiota composition, the food matrix, and the type of ingested ETs could determine the rate of Uro production.[ 133 , 143 ] After hydrolysis of ellagitannin‐O‐glycosides by ellagitannase, the released HHDP, a non‐stable form, suffers a double lactonization to move on to the stable EA.[ 144 ]
EA can be dehydroxylated by the gut microbiota to render EA metabolites called nasutins in which two hydroxyls from the two catechols have been removed. This has been described in beavers and pigs, producing nasutins and Uros in their intestine.[ 9 ] The gut microbiota of other animals, such as Australian termites, can only dehydroxylate the hydroxyl groups of EA, leading to nasutins. The production of nasutins by the human microbiota has not been described so far, although it cannot be ruled out. In contrast, the conversion of EA to Uros by the gut microbiota of humans and other mammals has been demonstrated.[ 4 , 112 , 144 ]
Microorganisms able to produce nasutins are still unknown. On the contrary, in the last decade, the isolation, identification, and characterization of the microorganisms responsible for Uro production and their health implications have been actively investigated.[ 118 , 145 ] The genus Gordonibacter from the Eggerthellaceae family accommodates bacterial species that are part of the human gut microbiota and can metabolize EA into Uro‐M5, Uro‐M6, and Uro‐C.[ 146 , 147 ] Cells of this genus are Gram‐positive, obligatory anaerobic, motile, and non‐spore‐forming short‐rod‐shaped. The first described species of the genus Gordonibacter, i.e., G. pamelaeae, was isolated from the sigmoid region of the colon of a patient suffering acute Crohn's disease.[ 148 ] The other three described species G. urolithinfaciens, G. faecihominis and “G. massiliensis,” were isolated from human feces of healthy volunteers.[ 149 , 150 , 151 ] However, G. faecihominis has recently been recognized as a later heterotypic synonym of G. urolithinfaciens when its genome was compared with that of G. urolithinfaciens.[ 149 , 150 , 151 , 152 ] On the other hand, the name of “G. massiliensis” has been published but not yet validated according to the International Code of Nomenclature of Bacteria (Bacteriological Code). A specific and suitable real‐time qPCR system was developed to detect and quantify the Gordonibacter genus. Gordonibacter is present in human fecal samples (104–1010 cells g−1) and positively correlates with Uro‐A production whereas inversely correlates with isoUro‐A and(or) Uro‐B.[ 133 ] The distribution of Gordonibacter throughout the digestive tract (small intestine, ascending colon, transverse colon, and descending colon) was also explored with a gastrointestinal simulation model TWIN‐SHIME that showed the highest prevalence in the descending colon.[ 5 ] Furthermore, a direct relationship between Gordonibacter and Uro‐A production along the digestive tract was confirmed. The associations between basal cardiometabolic risk factors and Gordonibacter were studied in humans.[ 126 ] Uro‐A production was positively related to fecal Gordonibacter, and, as commented before, positively correlated with HDL‐cholesterol, while Uro‐B and isoUro‐A, which are inversely correlated with Gordonibacter, were positively correlated with total‐cholesterol, LDLc, and oxLDLc.[ 126 ] Further use of cultivation‐independent 16S rRNA gene‐based methods on an HiSeq‐Illumina platform shows that Gordonibacter is reduced in smokers with Crohn´s disease (CD) compared with nonsmokers with CD.[ 153 ] The authors of this study suggested that intestinal microbes could mediate the adverse effects of smoking in CD. In contrast, consumption of ETs rich foods such as pomegranate or walnuts can increase Gordonibacter levels in the human gut.[ 123 , 154 ] Other studies that sequenced fecal DNA on a MiSeq‐Illumina platform demonstrated in normoweight–overweight‐obese adults,[ 118 ] healthy women during the first year of postpartum,[ 122 ] and metabolic syndrome (MetS) patients.[ 120 ] that Gordonibacter and two other genera of the Eggerthellaceae family (Eggerthella and Adlercreutzia) predominate in UM‐A (characterized by Uro‐A production) versus UM‐B individuals (characterized by the production of Uro‐B and isoUro‐A in addition to Uro‐A). Gordonibacter colonization in infants occurs throughout their first year of life, and neither breastfeeding nor vaginal delivery seems to be an essential condition for this. However, the kinetics of Gordonibacter colonization in babies is conditioned by their mother's UM.[ 7 ]
Ellagibacter, another genus from Eggerthellaceae family, can also convert EA into Uros (Uro‐M5, Uro‐M6, Uro‐C, and isoUro‐A).[ 155 ] The only currently described species of the genus Ellagibacter, i.e., E. isourolithinifaciens, was isolated from the feces of a healthy volunteer.[ 156 ] Cells are Gram‐positive, non‐motile, obligatory anaerobic, non‐spore‐forming short‐rods. Further use of cultivation‐independent 16S rRNA gene‐based methods in different cohorts of Caucasian adults (n = 249),[ 118 ] in healthy women during the first year of postpartum,[ 122 ] and in metabolic syndrome (MetS) patients,[ 120 ] demonstrated that Ellagibacter predominates in donors from UM‐B versus those from UM‐A. Ellagibacter was also detected in fecal samples of healthy elderly Korean women but only after consuming a synbiotic drink composed of probiotics (Bifidobacterium animalis spp. lactis HY8002, Lactobacillus casei HY2782, and L. plantarum HY2782), dietary fiber, FOS, xylooligosaccharides, and isomalto‐oligosaccharides.[ 157 ]
G. urolithinfaciens and E. isourolithinifaciens can also transform other phenolics, including caffeic acid (dehydroxylation and reduction), dihydrocaffeic acid (dehydroxylation), and their ester conjugates rosmarinic acid (reduction), and chlorogenic acid (quinic acid degradation, reduction, and dehydroxylation). E. isourolithinifaciens is more efficient than G. urolithinfaciens in the catechol‐dehydroxylase activity, while the reductase activity is more relevant in G. urolithinfaciens.[ 158 ] The enzymes producing these dehydroxylations to form Uros are catechol dehydroxylases, where NADPH and FAD are involved in the electron transportation systems.[ 159 , 160 ] Microorganisms containing 3‐dehydroxylase activity to render R urolithins and those responsible for 9‐dehydroxylase and 8‐dehydroxylase activities to render the final Uros (Uro‐A and Uro‐B) of the route are unknown, but they are under study. Strains of the closest neighbors of Gordonibacter (Paraeggerthella and Eggerthella) and Ellagibacter (Senegalimassilia and Adlercreutzia) based on 16S rRNA gene sequence phylogeny were also tested for their ability to catabolize EA, but they did not produce Uros.[ 146 , 156 ] Further studies on bacterial species capable of producing these Uros are guaranteed.
The human gut microbial ecology associated with UMs and the divergence between the enterotypes and UMs have been identified. More than 30% of the discriminating genera between UM‐A (Gordonibacter, Paraeggerthella, and Eggerthella) and UM‐B (Ellagibacter, Senegalimassilia, Slackia, and Adlercreutzia) belongs to the Eggerthellaceae family.[ 118 ] Therefore, variations in the gut microbiota ecology among individuals can affect (poly)phenol metabolism capacity. Still, at the same time, (poly)phenol gut metabotypes can indirectly reflect the individual's gut microbiome (biomarkers of gut microbiota) and health status (biomarkers of health status).[ 52 , 111 , 145 ] Further research should be performed to elucidate the potential health benefits of Gordonibacter and Ellagibacter and other species from the Eggerthellaceae family due to their ability to produce bioactive metabolites from several dietary (poly)phenols.
5. Future Directions
Various crucial issues need further research despite the significant evolution of urolithin research. Overall, whether the in vivo biological activity endorsed to Uros is due to each specific metabolite and(or) the physiological circulating mixture of metabolites and(or) the gut microbial ecology associated with their production is still poorly understood.
The ability of Uros to cross the blood‐brain barrier and the nature of the metabolites and concentrations reached in the brain tissues need to be clarified.
The specific in vivo activity for each free and conjugated Uro metabolite is unknown. Therefore, studies on the different Uro metabolites and their phase‐II conjugates are needed to understand their role in human health.
The evidence on the safety and impact of Uros on human health is still scarce and only partially available for Uro‐A.
More human studies are needed to unravel the role of UMs in human health, with a particular focus on UM‐0 (Uro non‐producers).
It is unknown whether there are potential common links between the gut microbial ecologies of the two unambiguously described metabotypes so far, i.e., equol (isoflavones) and Uros (ellagitannins).
Studying the precise differential transcriptomics between UMs and their comparison with other metabolic processes could help unravel the complex enzymatic machinery of these gut microbial ecologies and their correlation to human health.
The gut microbes responsible for producing the different Uros still need to be better identified and characterized and the biochemical pathways and enzymes involved.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
SUPPLEMENTARY INFORMATION
Acknowledgements
This research was supported by the Project PID2019‐103914RB‐I00 from the Ministry of Science and Innovation (MICINN, Spain) and the Projects 20880/PI/18 and 19900/GERM/15 (Fundación Séneca de la Región de Murcia, Spain). J.A.G.‐B. was supported by a Standard European Marie Curie Fellowship from the European Commission. This project has received funding from the European Union's Horizon 2020 research and innovation program under the Marie Sklodowska‐Curie grant agreement no. 838991.
Biographies
Rocío García‐Villalba: Dr. Rocío García‐Villalba is chemical engineer and completed her Ph.D. at the Department of Analytical Chemistry at the University of Granada (2006–2010). Since then, she has worked as a postdoctoral researcher at the Food Science and Technology Department of CEBAS‐CSIC in Murcia. Her main research line deals with polyphenols and health, specifically in analyzing secondary metabolites, mainly polyphenols, in plant extracts and biological samples, studying their metabolism, bioavailability, pharmacokinetics, and interactions with the gut microbiota.

Juan Carlos Espín: Prof. J.C. Espín (CEBAS‐CSIC, Murcia, Spain) has been a highly‐cited researcher since 2018. His research lines deal with polyphenols and health, integrating clinical trials, animal and cell models, and multi‐omics approaches. The main pillars on which his research focuses are to investigate i) the in vivo traceability of polyphenols to understand the drivers of polyphenol effects and ii) the high interindividual variability in the metabolism and activity observed after consumption of polyphenols, with emphasis on the gut microbiota. His approach suggests considering the health effects more individually in healthy people and patients, highlighting the concepts "personalized nutrition and precision medicine".

Antonio González‐Sarrías: Antonio González‐Sarrías is a Tenured Scientist at CEBAS‐CSIC (Murcia, Spain). He is a biologist and obtained his Ph.D. with honors in 2009. His scientific career has been focused on evaluating the anti‐carcinogenic, anti‐inflammatory, and cardioprotective activities of different dietary polyphenols and derived in vivo metabolites in preclinical (cell and animal models) and clinical studies. Remarkably, the scientific impact of his research has led to being a highly‐cited researcher since 2020 (Clarivate, Web of Science).

Mena P., Crozier A., García‐Villalba R., Giménez‐Bastida J. A., Cortés‐Martín A., Ávila‐Gálvez M. Á., Tomás‐Barberán F. A., Selma M. V., Espín J. C., González‐Sarrías A., Urolithins: a Comprehensive Update on their Metabolism, Bioactivity, and Associated Gut Microbiota. Mol. Nutr. Food Res. 2022, 66, 2101019. 10.1002/mnfr.202101019
Data Availability Statement
Data available on request from the authors.
References
- 1. Cerdá B., Llorach R., Cerón J. J., Espín J. C., Tomás‐Barberán F. A., Eur. J. Nutr. 2003, 42, 18. [DOI] [PubMed] [Google Scholar]
- 2. Cerdá B., Cerón J. J., Tomás‐Barberán F. A., Espín J. C., J. Agric. Food Chem. 2003, 31, 3493. [DOI] [PubMed] [Google Scholar]
- 3. Cerdá B., Espín J. C., Parra S., Martínez P., Tomás‐Barberán F. A., Eur. J. Nutr. 2004, 43, 205. [DOI] [PubMed] [Google Scholar]
- 4. Tomás‐Barberán F. A., González‐Sarrías A., García‐Villalba R., Nuñez‐Sánchez M. A., Selma M. V., García‐Conesa M. T., Mol. Nutr. Food Res. 2017, 1, 1500901. [DOI] [PubMed] [Google Scholar]
- 5. García‐Villalba R., Vissenaekens H., Pitart J., Romo‐Vaquero M., Espín J. C., Grootaert C., Selma M. V., Raes K., Smagghe G., Possemiers S., Van Camp J., Tomás‐Barberán F. A., J. Agric. Food Chem. 2017, 65, 5480. [DOI] [PubMed] [Google Scholar]
- 6. García‐Villalba R., Selma M. V., Espín J. C., Tomás‐Barberán F. A., J. Agric. Food Chem. 2019, 67, 11099. [DOI] [PubMed] [Google Scholar]
- 7. Cortés‐Martín A., García‐Villalba R., García‐Mantrana I., Rodríguez‐Varela A., Romo‐Vaquero M., Collado M. C., Tomás‐Barberán F. A., Espín J. C., Selma M. V., J. Agric. Food Chem. 2020, 68, 12606. [DOI] [PubMed] [Google Scholar]
- 8. Cerdá B., Periago P., Espín J. C., Tomás‐Barberán F. A., J. Agric. Food Chem. 2005, 53, 5571. [DOI] [PubMed] [Google Scholar]
- 9. González‐Barrio R., Truchado P., Ito H., Espín J. C., Tomás‐Barberán F. A., J. Agric. Food Chem. 2011, 59, 1152. [DOI] [PubMed] [Google Scholar]
- 10. Kosmala M., Jurgonski A., Juskiewicz J., Karlinska E., Macierzynski J., Roj E., Zdunczyk Z., J. Agric. Food Chem. 2017, 65, 5470. [DOI] [PubMed] [Google Scholar]
- 11. Milala J., Kosmala M., Karlinska E., Juskiewicz J., Zdunczyk Z., Fotschki B., J. Agric. Food Chem. 2017, 65, 10738. [DOI] [PubMed] [Google Scholar]
- 12. Jurgonski A., Juskiewicz J., Fotschki B., Kolodziejczyk K., Milala J., Kosmala M., Grzelak‐Blaszczyk K., Markiewicz L., Eur. J. Nutr. 2017, 56, 853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zary‐Sikorska E., Kosmala M., Milala J., Fotschki B., Ognik K., Juskiewicz J., Pol. J. Food Nutr. Sci. 2019, 69, 379. [Google Scholar]
- 14. Zary‐Sikorska E., Fotschki B., Jurgonski A., Kosmala M., Milala J., Kolodziejczyk K., Majewski M., Ognik K., Juskiewicz J., Molecules 2020, 25, 5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Piwowarski J. P., Stanislawska I., Granica S., Stefanska J., Kiss A. K., Drug Metab. Dispos. 2017, 45, 657. [DOI] [PubMed] [Google Scholar]
- 16. Dacrema M., Sommella E., Santarcangelo C., Bruno B., Marano M. G., Insolia V., Saviano A., Capiglia P., Stornaiuolo M., Daglia M., Biomed. Pharmacother. 2020, 131, 110670. [DOI] [PubMed] [Google Scholar]
- 17. Teixeira L. L., Costa G. R., Dörr F. A., Ong T. P., Pinto E., Lajolo F. M., Hassimotto N. M. A., Food Fuct. 2017, 8, 2266. [DOI] [PubMed] [Google Scholar]
- 18. Inada K. O. P., Tomás‐Barberán F. A., Perrone D., Monteiro M., J. Funct. Foods 2019, 57, 299. [Google Scholar]
- 19. de Araujo R. L., Tomás‐Barberán F. A., Ferreira dos Santos R., Martínez‐Blázquez J. A., Genovese M. I., Food Res. Int. 2021, 142, 110209. [DOI] [PubMed] [Google Scholar]
- 20. Quatrin A., Rampelotto C., Pauletto R., Maurer L. H., Nichelle S. M., Klein B., Rodrigues R. F., Junior M. R. M., Fonseca B. d. S., Ragagnin de Menezes C., Mello R. d. O., Rodrigues E., Bochi V. C., Emanuelli T., J. Funct. Foods 2020, 65, 103714. [Google Scholar]
- 21. Yang X., Tomás‐Barberán F. A., J. Agric. Food Chem. 2019, 67, 5394. [DOI] [PubMed] [Google Scholar]
- 22. Meir A. Y., Tuohy K., Von Bergen M., Krajmalnik‐Brown R., Heining U., Zelicha H., Tsaban G., Rinott E., Kaplan A., Aharoni A., Zeibich L., Chang D., Dirks B., Diotallevi C., Arapitsas P., Vrhovsek U., Ceglarek U., Haange S. B., Rolle‐Kampczyk U., Engelmann B., Lapidot M., Colt M., Sun Q., Shai I., Nutrients 2021, 13, 1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dadi T. H., Vahjen W., Zentek J., Melzing M. F., Granica S., Piwowarski J. P., J. Ethnopharmacol. 2020, 261, 113073. [DOI] [PubMed] [Google Scholar]
- 24. Volpp L., Ferianec V., Jezovicova M., Durackova Z., Scherf‐Clavel O., Högger P., Front. Pharmacol. 2020, 11, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kresty L. A., Fromkes J. J., Frankel W. L., Hammond C. D., Seeram N. P., Baird M., Stoner G. D., Oncotarget 2018, 9, 35356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sandhu A. K., Miller M. G., Thangthaeng N., Scott T. M., Shukitt‐Hale B., Edirisinghe I., Burton‐Freeman B., Food Funct. 2018, 9, 96. [DOI] [PubMed] [Google Scholar]
- 27. Puupponen‐Pimiä R., Seppänen‐Laakso T., Kankainen M., Maukonen J., Törrönen R., Kolehmaninen M., Leppänen T., Moilanen E., Nohynek L., Aura A. M., Poutanen K., Tomás‐Barberán F. A., Espín J. C., Oksman‐Caldentey K. M., Mol. Nutr. Food. Res. 2013, 57, 2258. [DOI] [PubMed] [Google Scholar]
- 28. Jarrard D., Filon M., Huang W., Havighurst T., DeShong K., Kim K., Konety B. R., Saltzstein D., Mukhtar H., Wollmer B., Suen C., House M. G., Parnes H. L., Bailey H. H., The Prostate 2021, 81, 41. [DOI] [PubMed] [Google Scholar]
- 29. Roberts K. M., Grainger E. M., Thomas‐Ahner J. M., Hinton A., Gu J., Riedl K. M., Vodovotz Y., Abaza R., Schwartz S. J., Clinton S. K., Mol. Nutr. Food Res. 2017, 61, 1600224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roberts K. M., Grainger E. M., Thomas‐Ahner J. M., Hinton A., Gu J., Riedl K. M., Vodovotz Y., Abaza R., Schwartz S. J., Clinton S. K., Mol. Nutr. Food Res. 2020, 64, 1900800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang X., Zhao A., Sandhu A. K., Edirisinghe I., Burton‐Freeman B. M., Nutrients 2020, 12, 3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang X., Fan J., Xiao D., Edirisinghe I., Burton‐Freeman B. M., Sandhu A. K., J. Agric. Food Chem. 2021, 69, 9238. [DOI] [PubMed] [Google Scholar]
- 33. Zhang X., Sandhu A., Edirisinghe I., Burton‐Freeman B. M., Molecules 2020, 25, 4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Andreux P. A., Blanco‐Bose W., Ryu D., Burdet F., Ibberson M., Aebischer P., Auwerx J., Singh A., Rinsch C., Nat. Metabol. 2019, 1, 595. [DOI] [PubMed] [Google Scholar]
- 35. Singh A., Dámico D., Andreux P. A., Dunngalvin G., Kern T., Blanco‐Bose W., Auwerx J., Aebischer P., Rinsch C., Eur. J. Clin. Nutr. 2021, 10.1038/s41430-021-00950-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zou D., Ganugula R., Arora M., Nabity M. B., Sheikh‐Hamad D., Ravi Kumar M. N. V., Am. J. Physiol. Renal. Physiol. 2019, 317, F1255. [DOI] [PubMed] [Google Scholar]
- 37. Ryu D., Mouchiroud L., Andreux P. A., Katsyuba E., Moullan N., Nicolet‐Dit‐Félix A. A., Williams E. G., Jha P., Lo Sasso G., Huzard D., Aebischer P., Sandi C., Rinsch C., Auwerx J., Nat. Med. 2016, 22, 879. [DOI] [PubMed] [Google Scholar]
- 38. Toney A. M., Fan R., Xian Y., Chaidez V., Ramer‐Tait A. E., Chung S., Obesity 2019, 27, 612. [DOI] [PubMed] [Google Scholar]
- 39. Savi M., Bocchi L., Mena P., Dall'Asta M., Crozier A., Brighenti F., Stilli D., Del Rio D., Cardiovasc. Diabetol. 2017, 16, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Heilman J., Andreux P., Tran N., Rinsch C., Blanco‐Bose W., Food Chem. Toxicol. 2017, 108, 289. [DOI] [PubMed] [Google Scholar]
- 41. Ávila‐Gálvez M. A., Giménez‐Bastida J. A., González‐Sarrías A., Espín J. C., Food Funct. 2019, 10, 3135. [DOI] [PubMed] [Google Scholar]
- 42. Kujawska M., Jourdes M., Kurpik M., Szulc M., Szaefer H., Chmielarz P., Kreiner G., Krajka‐Kuźniak V., Mikołajczak P. L., Teissedre P. Ł., Jodynis‐Liebert J., Int. J. Mol. Sci. 2019, 21, 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kujawska M., Jourdes M., Witucki L., Karaźniewicz‐Łada M., Szulc M., Górska A., Mikołajczak P. L., Teissedre P. Ł., Jodynis‐Liebert J., Brain Sci 2021, 11, 1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gu J., Thomas‐Ahner J. M., Riedl K. M., Bailey M. T., Vodovotz Y., Schwartz S. J., Clinton S. K., Mol. Nutr. Food Res. 2019, 63, 1800636. [DOI] [PubMed] [Google Scholar]
- 45. Ávila‐Gálvez M. A., Romo‐Vaquero M., González‐Sarrías A., Espín J. C., J. Funct. Foods 2019, 61, 103516. [Google Scholar]
- 46. Ávila‐Gálvez M. A., García‐Villalba R., Martínez‐Díaz F., Ocaña‐Castillo B., Monedero‐Saiz T., Torrecillas‐Sánchez A., Abellán B., González‐Sarrías A., Espín J. C., Mol. Nutr. Food Res. 2019, 63, 1801239. [DOI] [PubMed] [Google Scholar]
- 47. Espín J. C., González‐Barrio R., Cerdá B., López‐Bote C., Rey A. I., Tomás‐Barberán F. A., J. Agric. Food Chem 2007, 55, 10476. [DOI] [PubMed] [Google Scholar]
- 48. Seeram N. P., Aronson W. J., Zhang Y., Henning S. M., Moro A., Lee R. P., Sartippour M., Harris D. M., Rettig M., Suchard M. A., Pantuck A. J., Belldegrun A., Heber D., D. J. Agric. Food Chem. 2007, 55, 7732. [DOI] [PubMed] [Google Scholar]
- 49. Gasperotti M., Passamonti S., Tramer F., Masuero D., Guella G., Mattivi F., Vrhovsek U., ACS Chem. Neurosci. 2015, 6, 1341. [DOI] [PubMed] [Google Scholar]
- 50. Cerdá B., Tomás‐Barberán F. A., Espín J. C., J. Agric. Food Chem. 2005, 53, 227. [DOI] [PubMed] [Google Scholar]
- 51. González‐Sarrías A., Espín J. C., Tomás‐Barberán F. A., Trends Food Sci. Technol. 2017, 69, 281. [Google Scholar]
- 52. Cortés‐Martín A., Selma M. V., Tomás‐Barberán F. A., González‐Sarrías A., Espín J. C., Mol. Nutr. Food Res. 2020, 64, 1900952. [DOI] [PubMed] [Google Scholar]
- 53. García‐Villalba R., Giménez‐Bastida J. A., Ávila‐Gálvez M. A., Tomás‐Barberán F. A., Espín J. C., González‐Sarrías A., in Diet. Polyphenols, John Wiley & Sons, Ltd, 2020, pp. 319. [Google Scholar]
- 54. Ávila‐Gálvez M. Á., González‐Sarrías A., Espín J. C., J. Agric. Food Chem. 2018, 66, 7857. [DOI] [PubMed] [Google Scholar]
- 55. Mena P., Del Rio D., J. Agric. Food Chem. 2018, 66, 8221. [DOI] [PubMed] [Google Scholar]
- 56. Giménez‐Bastida J. A., Larrosa M., González‐Sarrías A., Tomás‐Barberán F., Espín J. C., García‐Conesa M. T., J. Agric. Food Chem. 2012, 60, 8866. [DOI] [PubMed] [Google Scholar]
- 57. González‐Sarrías A., Larrosa M., Tomás‐Barberán F. A., Dolara P., Espín J. C., Br. J. Nutr. 2010, 104, 503. [DOI] [PubMed] [Google Scholar]
- 58. Larrosa M., González‐Sarrías A., Yáñez‐Gascón M. J., Selma M. V., Azorín‐Ortuño M., Toti S., Tomás‐Barberán F., Dolara P., Espín J. C., J. Nutr. Biochem. 2010, 21, 717. [DOI] [PubMed] [Google Scholar]
- 59. Singh R., Chandrashekharappa S., Bodduluri S. R., Baby B. V., Hegde B., Kotla N. G., Hiwale A. A., Saiyed T., Patel P., Vijay‐Kumar M., Langille M. G. I., Douglas G. M., Cheng X., Rouchka E. C., Waigel S. J., Dryden G. W., Alatassi H., Zhang H.‐G., Haribabu B., Vemula P. K., Jala V. R., Nat. Commun. 2019, 10, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hering N. A., Luettig J., Jebautzke B., Schulzke J. D., Rosenthal R., Front. Pharmacol. 2021, 12, 610164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Giménez‐Bastida J. A., González‐Sarrías A., Espín J. C., Schneider C., Mol. Nutr. Food Res. 2020, 2000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sala R., Mena P., Savi M., Brighenti F., Crozier A., Miragoli M., Stilli D., Del Rio D., J. Funct. Foods 2015, 15, 97. [Google Scholar]
- 63. Rønning S. B., Voldvik V., Bergum S. K., Aaby K., Borge G. I. A., Food Funct. 2020, 11, 7946. [DOI] [PubMed] [Google Scholar]
- 64. Bobowska A., Granica S., Filipek A., Melzig M. F., Moeslinger T., Zentek J., Kruk A., Piwowarski J. P., Eur. J. Nutr. 2021, 60, 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. González‐Sarrías A., Espín J.‐C., Tomás‐Barberán F. A., García‐Conesa M.‐T., Mol. Nutr. Food Res. 2009, 53, 686. [DOI] [PubMed] [Google Scholar]
- 66. Al‐Harbi S. A., Abdulrahman A. O., Zamzami M. A., Khan M. I., Front. Nutr. 2021, 8, 647582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. González‐Sarrías A., Núñez‐Sánchez M. Á., Tomé‐Carneiro R. J., Tomás‐Barberán F. A., García‐Conesa M. T., Espín J. C., Mol. Nutr. Food Res. 2016, 60, 701. [DOI] [PubMed] [Google Scholar]
- 68. González‐Sarrías A., Núñez‐Sánchez M. Á., García‐Villalba R., Tomás‐Barberán F. A., Espín J. C., Eur. J. Nutr. 2017, 56, 831. [DOI] [PubMed] [Google Scholar]
- 69. Giménez‐Bastida J. A., Ávila‐Gálvez M. Á., Espín J. C., González‐Sarrías A., Food Chem. Toxicol. 2020, 139, 111260. [DOI] [PubMed] [Google Scholar]
- 70. Norden E., Heiss E. H., Carcinogenesis 2019, 40, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhao W., Shi F., Guo Z., Zhao J., Song X., Yang H., Mol. Carcinog. 2018, 57, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. González‐Sarrías A., Giménez‐Bastida J. A., Núñez‐Sánchez M. Á., Larrosa M., García‐Conesa M. T., Tomás‐Barberán F. A., Espín J. C., Eur. J. Nutr. 2014, 53, 853. [DOI] [PubMed] [Google Scholar]
- 73. Ávila‐Gálvez M. Á., Espín J. C., González‐Sarrías A., J. Agric. Food Chem. 2018, 66, 8547. [DOI] [PubMed] [Google Scholar]
- 74. Giorgio C., Mena P., Del Rio D., Brighenti F., Barocelli E., Hassan‐Mohamed I., Callegari D., Lodola A., Tognolini M., Mol. Nutr. Food Res. 2015, 59, 2155. [DOI] [PubMed] [Google Scholar]
- 75. Giménez‐Bastida J. A., Ávila‐Gálvez M. Á., Espín J. C., González‐Sarrías A., Mol. Nutr. Food Res. 2019, 63, 1900629. [DOI] [PubMed] [Google Scholar]
- 76. Dahiya N. R., Chandrasekaran B., Kolluru V., Ankem M., Damodaran C., Vadhanam M. V., Mol. Carcinog. 2018, 57, 1332. [DOI] [PubMed] [Google Scholar]
- 77. Lv M.‐Y., Shi C.‐J., Pan F.‐F., Shao J., Feng L., Chen G., Ou C., Zhang J.‐F., Fu W.‐M., J. Cell. Biochem. 2019, 120, 17273. [DOI] [PubMed] [Google Scholar]
- 78. Giménez‐Bastida J. A., Ávila‐Gálvez M. Á., Espín J. C., González‐Sarrías A., Trends Food Sci. Technol. 2021, 114, 410. [Google Scholar]
- 79. Gong Z., Huang J., Xu B., Ou Z., Zhang L., Lin X., Ye X., Kong X., Long D., Sun X., He X., Xu L., Li Q., Xuan A., J. Neuroinflammation 2019, 16, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ahsan A., Zheng Y.‐R., Wu X.‐L., Tang W.‐D., Liu M.‐R., Ma S.‐J., Jiang L., Hu W.‐W., Zhang X.‐N., Chen Z., CNS Neurosci. Ther. 2019, 25, 976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chen P., Chen F., Lei J., Li Q., Zhou B., Neurother. J. Am. Soc. Exp. Neurother. 2019, 16, 1269. [Google Scholar]
- 82. Lee H. J., Jung Y. H., Choi G. E., Kim J. S., Chae C. W., Lim J. R., Kim S. Y., Yoon J. H., Cho J. H., Lee S.‐J., Han H. J., Cell Death Differ. 2021, 28, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yuan T., Ma H., Liu W., Niesen D. B., Shah N., Crews R., Rose K. N., Vattem D. A., Seeram N. P., ACS Chem. Neurosci. 2016, 7, 26. [DOI] [PubMed] [Google Scholar]
- 84. González‐Sarrías A., Núñez‐Sánchez M. Á., Tomás‐Barberán F. A., Espín J. C., J. Agric. Food Chem. 2017, 65, 752. [DOI] [PubMed] [Google Scholar]
- 85. DaSilva N. A., Nahar P. P., Ma H., Eid A., Wei Z., Meschwitz S., Zawia N. H., Slitt A. L., Seeram N. P., Nutr. Neurosci. 2019, 22, 185. [DOI] [PubMed] [Google Scholar]
- 86. Xu J., Yuan C., Wang G., Luo J., Ma H., Xu L., Mu Y., Li Y., Seeram N. P., Huang X., Li L., J. Agric. Food Chem. 2018, 66, 571. [DOI] [PubMed] [Google Scholar]
- 87. García‐Conesa M. T., Chambers K., Combet E., Pinto P., Garcia‐Aloy M., Andrés‐Lacueva C., de Pascual‐Teresa S., Mena P., Konic Ristic A., Hollands W. J., Kroon P. A., Rodríguez‐Mateos A., Istas G., Kontogiorgis C. A., Rai D. K., Gibney E. R., Morand C., Espín J. C., González‐Sarrías A., Int. J. Mol. Sci. 2018, 19, 694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Cui G. H., Chen W. Q., Shen Z. Y., Pharmacol. Rep. 2018, 70, 519. [DOI] [PubMed] [Google Scholar]
- 89. Abdulrahman A. O., Kuerban A., Alshehri Z. A., Abdulaal W. H., Khan J. A., Khan M. I., Diabetes Metab. Syndr. Obes. 2020, 13, 3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Abdulrahman A. O., Alzubaidi M. Y., Nadeem M. S., Khan J. A., Rather I. A., Khan M. I., Int. J. Food Sci. Nutr. 2021, 72, 923. [DOI] [PubMed] [Google Scholar]
- 91. Zhao W., Wang L., Haller V., Ritsch A., Mol. Nutr. Food Res. 2019, 63, 1800887. [DOI] [PubMed] [Google Scholar]
- 92. Giménez‐Bastida J. A., González‐Sarrías A., Larrosa M., Tomás‐Barberán F., Espín J. C., García‐Conesa M. T., Mol. Nutr. Food Res. 2012, 56, 784. [DOI] [PubMed] [Google Scholar]
- 93. Savi M., Bocchi L., Bresciani L., Falco A., Quaini F., Mena P., Brighenti F., Crozier A., Stilli D., Del Rio D., Mol. Basel Switz 2018, 23, E549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Cisneros‐Zevallos L., Bang W. Y., Delgadillo‐Puga C., Int. J. Mol. Sci. 2020, 21, 2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kang I., Kim Y., Tomás‐Barberán F. A., Espín J. C., Chung S., Mol. Nutr. Food Res. 2016, 60, 1129. [DOI] [PubMed] [Google Scholar]
- 96. Les F., Arbonés‐Mainar J. M., Valero M. S., López V., J. Ethnopharmacol. 2018, 220, 67. [DOI] [PubMed] [Google Scholar]
- 97. Djedjibegovic J., Marjanovic A., Panieri E., Saso L., Oxid. Med. Cell. Longev. 2020, 5194508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ishimoto H., Shibata M., Myojin Y., Ito H., Sugimoto Y., Tai A., Hatano T., Bioorg. Med. Chem. Lett. 2011, 21, 5901. [DOI] [PubMed] [Google Scholar]
- 99. Jing T., Liao J., Shen K., Chen X., Xu Z., Tian W., Wang Y., Jin B., Pan H., Food Chem. Toxicol. 2019, 129, 108. [DOI] [PubMed] [Google Scholar]
- 100. Larrosa M., González‐Sarrías A., García‐Conesa M. T., Tomás‐Barberán F. A., Espín J. C., J. Agric. Food Chem. 2006, 54, 1611. [DOI] [PubMed] [Google Scholar]
- 101. Dellafiora L., Mena P., Cozzini P., Brighenti F., Del Rio D., Food Funct. 2013, 4, 1442. [DOI] [PubMed] [Google Scholar]
- 102. Zhang W., Chen J.‐H., Aguilera‐Barrantes I., Shiau C.‐W., Sheng X., Wang L.‐S., Stoner G. D., Huang Y.‐W., Mol. Nutr. Food Res. 2016, 60, 2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Luan P., D'Amico D., Andreux P. A., Laurila P.‐P., Wohlwend M., Li H., Imamura de Lima T., Place N., Rinsch C., Zanou N., Auwerx J., Sci. Transl. Med. 2021, 13, eabb0319. [DOI] [PubMed] [Google Scholar]
- 104. Milenkovic D., Morand C., Aedin C., Konic‐Ristic A., Tomás‐Barberán F. A., Ordovas J. M., Kroon P., De Caterina R., Rodríguez‐Mateos A., Adv. Nutr. 2017, 8, 558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Cassidy A., Minihane A. M., Am. J. Clin. Nutr. 2017, 105, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Scholl A., Lepper A., Lehr T., Hanke N., Schneider K. L., Brockmöller J., Seufferlein T., Stingl J. C., PLoS One 2018, 13, e0193074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Gibney E. R., Milenkovic D., Combet E., Ruskovska T., Greyling A., González‐Sarrías A., de Roos B., Tomás‐Barberán F. A., Morand C., Rodríguez‐Mateos A., Eur. J. Nutr. 2019, 58, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cortés‐Martín A., Selma M. V., Espín J. C., GarcíaVillalba R., Mol. Nutr. Food Res. 2019, 63, 1800819. [DOI] [PubMed] [Google Scholar]
- 109. Tomás‐Barberán F. A., Espín J. C., Annu. Rev. Food Sci. Technol. 2019, 10, 221. [DOI] [PubMed] [Google Scholar]
- 110. Zhao C., Wang Y., Zhang B., Yue Y., Zhang J., Sci. Rep. 2020, 10, 9521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Iglesias‐Aguirre C. E., Cortés‐Martín A., Ávila‐Gálvez M. A., Giménez‐Bastida J. A., Selma M. V., González‐Sarrías A., Espín J. C., Food Funct. 2021, 12, 10324. [DOI] [PubMed] [Google Scholar]
- 112. Selma M. V., Espín J. C., Tomás‐Barberán F. A., J. Agric. Food Chem. 2009, 57, 6485. [DOI] [PubMed] [Google Scholar]
- 113. Camps S. G., Koh H. R., Wang N. X., Henry C. J., Nutrition 2020, 78, 110799. [DOI] [PubMed] [Google Scholar]
- 114. Palmnäs M., Brunius C., Shi L., Rostgaard‐Hansen A., Torres N. E., González‐Domínguez R., Zamora‐Ros R., Ye Y. L., Halkjær J., Tjønneland A., Riccardi G., Giacco R., Costabile G., Vetrani C., Nielsen J., Andres‐Lacueva C., Landberg R., Adv. Nutr. 2020, 11, 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wawro N., Pestoni G., Riedl A., Breuninger T. A., Peters A., Rathmann W., Koenig W., Huth C., Meisinger C., Rohrmann S., Linseisen J., Nutrients 2020, 12, 1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Espín J. C., González‐Sarrías A., Tomás‐Barberán F. A., Biochem. Pharmacol. 2017, 139, 82. [DOI] [PubMed] [Google Scholar]
- 117. Cortés‐Martín A., García‐Villalba R., González‐Sarrías A., Romo‐Vaquero M., Loria‐Kohen V., Ramírez‐de‐Molina A., Tomás‐Barberán F. A., Selma M. V., Espín J. C., Food Funct. 2018, 9, 4100. [DOI] [PubMed] [Google Scholar]
- 118. Romo‐Vaquero M., Cortés‐Martín A., Loria‐Kohen V., Ramírez‐de‐Molina A., García‐Mantrana I., Collado M. C., Espín J. C., Selma M. V., Mol. Nutr. Food Res. 2019, 63, 1800958. [DOI] [PubMed] [Google Scholar]
- 119. Cortés‐Martín A., Colmenarejo G., Selma M. V., Espín J. C., Sci. Rep. 2020, 10, 7850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Cortés‐Martín A., Iglesias‐Aguirre C. E., Meoro A., Selma M. V., Espín J. C., Mol. Nutr. Food Res. 2021, 65, 2001048. [DOI] [PubMed] [Google Scholar]
- 121. Cortés‐Martín A., Iglesias‐Aguirre C. E., Meoro A., Selma M. V., Espín J. C., Microorganisms 2020, 8, 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Cortés‐Martín A., Romo‐Vaquero M., García‐Mantrana I., Rodríguez‐Varela A., Collado M. C., Espín J. C., Selma M. V., Nutrients 2019, 11, 2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. González‐Sarrías A., García‐Villalba R., Romo‐Vaquero M., Alasalvar C., Örem A., Zafrilla P., Tomás‐Barberán F. A., Selma M. V., Espín J. C., Mol. Nutr. Food Res. 2017, 61, 1600830. [DOI] [PubMed] [Google Scholar]
- 124. Núñez‐Sánchez M. Á., González‐Sarrías A., García‐Villalba R., Monedero‐Saiz T., García‐Talavera N. V., Gómez‐Sánchez M. B., Sánchez‐Álvarez C., García‐Albert A. M., Rodríguez‐Gil F. J., Ruiz‐Marín M., Pastor‐Quirante F. A., Martínez‐Díaz F., Tomás‐Barberán F. A., Espín J. C., García‐Conesa M. T., J. Nutr. Biochem. 2017, 42, 126. [DOI] [PubMed] [Google Scholar]
- 125. Tomas‐Barberan F. A., Selma M. V., Espín J. C., J. Agric. Food Chem. 2018, 66, 3593. [DOI] [PubMed] [Google Scholar]
- 126. Selma M. V., González‐Sarrías A., Salas‐Salvadó J., Andrés‐Lacueva C., Alasalvar C., Örem A., Tomás‐Barberán F. A., Espín J. C., Clin. Nutr. 2018, 37, 897. [DOI] [PubMed] [Google Scholar]
- 127. Mora‐Cubillos X., Tulipani S., Garcia‐Aloy M., Bulló M., Tinahones F. J., Andres‐Lacueva C., Mol. Nutr. Food Res. 2015, 59, 2480. [DOI] [PubMed] [Google Scholar]
- 128. Istas G., Feliciano R. P., Weber T., García‐Villalba R., Tomás‐Barberán F. A., Heiss C., Rodríguez‐Mateos A., Arch. Biochem. Biophys. 2018, 651, 43. [DOI] [PubMed] [Google Scholar]
- 129. González‐Sarrías A., Núñez‐Sánchez M. A., Ávila‐Gálvez M. A., Monedero‐Saiz T., Rodríguez‐Gil F. J., Martínez‐Díaz F., Selma M. V., Espín J. C., Food Funct. 2018, 9, 2617. [DOI] [PubMed] [Google Scholar]
- 130. González‐Sarrías A., Romo‐Vaquero M., García‐Villalba R., Cortés‐Martín A., Selma M. V., Espín J. C., Mol. Nutr. Food Res. 2018, 62, 1800160. [DOI] [PubMed] [Google Scholar]
- 131. Bialasiewicz P., Prymont‐Przyminska A., Zwolinska A., Sarniak A., Wlodarczyk A., Krol M., Glusac J., Nowak P., Markowski J., Rutkowski K. P., Nowak D., J. Am. Coll. Nutr. 2014, 33, 274. [DOI] [PubMed] [Google Scholar]
- 132. González‐Sarrías A., Azorín‐Ortuño M., Yáñez‐Gascón M. J., Tomás‐Barberán F. A., García‐Conesa M. T., Espín J. C., J. Agric. Food. Chem. 2009, 57, 5623. [DOI] [PubMed] [Google Scholar]
- 133. Romo‐Vaquero M., García‐Villalba R., González‐Sarrías A., Beltrán D., Tomás‐Barberán F. A., Espín J. C., Selma M. V., J. Funct. Foods 2015, 17, 785. [Google Scholar]
- 134. Lee J. H., Talcott S. T., J. Agric. Food Chem. 2004, 52, 361. [DOI] [PubMed] [Google Scholar]
- 135. Jimenez N., Esteban‐Torres M., Mancheno J. M., de las Rivas B., Munoz R., Appl. Environ. Microbiol. 2014, 80, 2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Aguilar‐Zarate P., Cruz‐Hernandez M. A., Montañez J. C., Belmares‐Cerda R. E., Aguilar C. N., Rev. Mex. Ing. Quim. 2014, 13, 63. [Google Scholar]
- 137. Tomás‐Barberán F. A., Selma M. V., Espín J. C., I Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 471. [DOI] [PubMed] [Google Scholar]
- 138. de Felipe F. L., de las Rivas B., Muñoz R., Front. Microbiol. 2014, 5, 684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Unban K., Kochasee P., Shetty K., Khanongnuch, Foods 2020, 9, 490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Evtyugin D. D., Magina S., Evtuguin D. V., Molecules 2020, 25, 2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ascacio‐Valdés J. A., Buenrostro J. J., De la Cruz R., Sepúlveda L., Aguilera A. F., Prado A., Contreras J. C., Rodríguez R., Aguilar C. N., J. Basic Microbiol. 2014, 54, 28. [DOI] [PubMed] [Google Scholar]
- 142. Ascacio‐Valdés J. A., Aguilera‐Carbó A. F., Buenrostro J. J., Prado‐Barragán A., Rodríguez‐Herrera R., Aguilar C. N., J. Basic Microbiol. 2016, 56, 329. [DOI] [PubMed] [Google Scholar]
- 143. Piwowarski J. P., Granica S., Stefańska J., Kiss A. K., J. Nat. Prod. 2016, 79, 3022. [DOI] [PubMed] [Google Scholar]
- 144. García‐Villalba R., Beltrán D., Espín J. C., Selma M. V., Tomás‐Barberán F. A., J. Agric. Food Chem. 2013, 61, 8797. [DOI] [PubMed] [Google Scholar]
- 145. Selma M. V., Tomás‐Barberán F. A., Romo‐Vaquero M., Cortés‐Martín A., Espín J. C., In Dietary Polyphenols: Metabolism and Health Effects, Wiley, Oxford. 2021, 13, pp. 497–532. [Google Scholar]
- 146. Selma M. V., Beltrán D., García‐Villalba R., Espín J. C., Tomás‐Barberán F. A., Food Funct. 2014, 5, 1779. [DOI] [PubMed] [Google Scholar]
- 147. Selma M. V., Tomás‐Barberán F. A., Beltrán D., García‐Villalba R., Espín J. C., Int. J. Syst. Evol. Microbiol. 2014, 64, 2346. [DOI] [PubMed] [Google Scholar]
- 148. Würdemann D., Tindall B. J., Pukall R., Lünsdorf H., Strömpl C., Namuth T., Nahrstedt H., Wos‐Oxley M., Ott S., Schreiber S., Timmis K. N., Oxley A. P. A., Int. J. Syst. Evol. Microbiol. 2009, 59, 1405. [DOI] [PubMed] [Google Scholar]
- 149. Martínez‐Blanch J. F., Ramón D., Beltrán D., Romo‐Vaquero M., García‐Villalba R., Espín J. C., Tomás‐Barberán F. A., Codoñer F. M., Selma M. V., G. Announc. 2017, 5, e01120‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Jin J. S., Lee K. C., Park I. S., Kim K. K., Ahn J. S., Benno Y., Hattori M., Lee J.‐S., Antonie Van Leeuwenhoek 2014, 106, 439. [DOI] [PubMed] [Google Scholar]
- 151. Ngom I. I., Hasni I., Lo C. I., Traore S. I., Fontanini A., Raoult D., Fenollar F., New Microbe New Infect 2020, 33, 100624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Danylec N., Stoll D. A., Huch M., Int. J. Syst. Evol. Microbiol. 2019, 69, 2527. [DOI] [PubMed] [Google Scholar]
- 153. Opstelten J. L., Plassais J., van Mil S. W., Achouri E., Pichaud M., Siersema P. D., Oldenburg B., Cervino A. C., Inflamm Bowel Dis 2016, 22, 2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. García‐Mantrana I., Calatayud M., Romo‐Vaquero M., Espín J. C., Selma M. V., Collado M. C., Nutrients 2019, 11, 2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Selma M. V., Beltrán D., Luna M. C., Romo‐Vaquero M., García‐Villalba R., Mira A., Espín J. C., Tomás‐Barberán F. A., Front. Microbiol. 2017, 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Beltrán D., Romo‐Vaquero M., Espín J. C., Tomás‐Barberán F. A., Selma M. V., Int. J. Syst. Evol. Microbiol. 2018, 68, 1707. [DOI] [PubMed] [Google Scholar]
- 157. Lee S. H., You H. S., Kang H. G., Kang S. S., Hyun S. H., Nutrients 2020, 12, 3112.33053824 [Google Scholar]
- 158. García‐Villalba R., Beltrán D., Frutos M. D., Selma M. V., Tomás‐Barberán F. A., Espín J. C., Food Funct. 2020, 11, 7012. [DOI] [PubMed] [Google Scholar]
- 159. Rekdal V. M., Bess E. N., Bisanz J. E., Turnbaugh P. J., Balskus E. P., Science (80‐.). 2019, 364, aau6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Watanabe H., Kishino S., Kudoh M., Yamamoto H., Ogawa J., J. Biosci. Bioeng. 2020, 129, 552. [DOI] [PubMed] [Google Scholar]
- 161. Tang L., Mo Y., Li Y., Zhong Y., He S., Zhang Y., Tang Y., Fu S., Wang X., Chen A., Biochem. Biophys. Res. Commun. 2017, 486, 774. [DOI] [PubMed] [Google Scholar]
- 162. Guada M., Ganugula R., Vadhana M., Ravi Kumar M. N. V., J. Pharmacol. Exp. Ther. 2017, 363, 58. [DOI] [PubMed] [Google Scholar]
- 163. Liu H., Kang H., Song C., Lei Z., Li L., Guo J., Xu Y., Guan H., Fang Z., Li F., Front. Pharmacol. 2018, 9, 1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.a) Wang Y., Huang H., Jin Y., Shen K., Chen X., Xu Z., Jin B., Pan H., Food Chem. Toxicol. 2019, 131, 110591. [DOI] [PubMed] [Google Scholar]; b) Chen P., Chen F., Lei J., Li Q., Zhou B., Neurotherapeutics 2019, 16, 1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Fu X., Gong L. F., Wu Y. F., Lin Z., Jiang B. J., Wu L., Yu K. H., Food Funct. 2019, 10, 6135. [DOI] [PubMed] [Google Scholar]
- 166. Zheng D., Liu Z., Zhou Y., Hou N., Yan W., Qin Y., Ye Q., Cheng X., Xiao Q., Bao Y., Luo J., Wu X., Pharmacol. Res. 2020, 153, 104655. [DOI] [PubMed] [Google Scholar]
- 167. Mousavi S., Weschka D., Bereswill S., Heimesaat M. N., Pathogens 2020, 10, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Yang J., Guo Y., Henning S. M., Chan B., Long J., Zhong J., Acin‐Perez R., Petcherski A., Shirihai O., Heber D., Li Z., Mol. Nutr. Food Res. 2020, 64, 2000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Lin X. H., Ye X. J., Li Q. F., Gong Z., Cao X., Li J. H., Zhao S. T., Sun X. D., He X. S., Xuan A. G., Neuroscience 2020, 448, 94. [DOI] [PubMed] [Google Scholar]
- 170. Ghosh N., Das A., Biswas N., Gnyawali S., Singh K., Gorain M., Polcyn C., Khanna S., Roy S., Sen C. K., Sci. Rep. 2020, 10, 20184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Xia B., Shi X. C., Xie B. C., Zhu M. Q., Chen Y., Chu X. Y., Cai G. H., Liu M., Yang S. Z., Mitchell G. A., Pang W. J., Wu J. W., PLoS Biol. 2020, 18, e3000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Adachi S. I., Sasaki K., Kondo S., Komatsu W., Yoshizawa F., Isoda H., Yagasaki K., Molecules 2020, 25, 5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Tuohetaerbaike B., Zhang Y., Tian Y., Zhang N. N., Kang J., Mao X., Zhang Y., Li X., J. Ethnopharmacol. 2020, 250, 112479. [DOI] [PubMed] [Google Scholar]
- 174. Shen P. X., Li X., Deng S. Y., Zhao L., Zhang Y. Y., Deng X., Han B., Yu J., Li Y., Wang Z. Z., Zhang Y., EBioMedicine 2021, 64, 103227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Wang Y., Jasper H., Toan S., Muid D., Chang X., Zhou H., Redox Biol. 2021, 45, 102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTARY INFORMATION
Data Availability Statement
Data available on request from the authors.


