Abstract
Granuloma formation around schistosomal eggs is induced by soluble egg antigens (SEA) and mediated by the activity of CD4+ Th lymphocytes and their cytokines. Regulation of the inflammatory Th cell response during infection is still insufficiently understood. The hypothesis of this study was that activation-induced cell death (AICD) of CD4+ T cells is involved in the immune inflammatory response. This study investigated the dynamics of splenic and granuloma CD4+ Th cell apoptosis and Fas ligand (FasL) expression during the acute and chronic stages of murine schistosomal infection. Enhanced apoptosis of freshly isolated CD4+ Th lymphocytes commenced after egg deposition and persisted during the peak and modulated phases of granuloma formation. After oviposition, CD4+, CD8+, and CD19+ splenocytes and granuloma cells expressed elevated levels of FasL but FasL expression declined during the downmodulated stage of infection. In culture, SEA induced splenic and granuloma CD4+ T-cell apoptosis and stimulated expression of FasL on splenic but not granuloma CD4+ T cells, CD8+ T cells, and CD19+ B cells. SEA-stimulated splenocytes and granuloma cells preferentially lysed a Fas-transfected target cell line. Depletion of B cells from SEA-stimulated splenic cultures decreased CD4+ T cell apoptosis. Coculture of purified splenic B cells with CD4+ T cells and adoptive transfer of purified B cells indicated that antigen-stimulated B cells can kill CD4+ Th cells. However, CD4+ T cells were the dominant mediators of apoptosis in the granuloma. This study indicates that AICD is involved in the apoptosis of CD4+ T cells during schistosomal infection.
The host granulomatous inflammatory response to deposited worm eggs leads to hepatic and intestinal fibrosis, the major pathological consequences of infection with the parasitic helminth Schistosoma mansoni (3). Previous studies in the murine model have demonstrated that granuloma formation was induced by soluble egg antigens (SEA) released from schistosomal eggs (6) and granulomatous inflammation was dependent on the activation of CD4+ T helper lymphocytes (26). SEA has been used extensively in vitro to stimulate proliferation and cytokine production by spleen and granuloma cells from infected mice (6, 11, 24). Two important regulatory events in the granuloma have been identified: (i) acute-stage CD4+ Th1-Th2 switching (5, 24, 31) and (ii) chronic-stage downmodulation of the inflammatory response (7, 11). The early CD4+ Th cell response before oviposition and during initial granuloma formation is dominated by the release of Th1-type cytokines (24, 31), whereas after egg deposition with the full development of the granulomatous response, cytokine production is switched to a Th2-type profile. This Th1-Th2 switch of cytokine release results in enhanced granulomatous inflammation and increased fibrosis. Following the peak of granuloma formation, a spontaneous downmodulation of the inflammatory response occurs with diminished Th2-type cytokine production, decreased granuloma formation, and cumulative fibrosis (4). The factors involved in regulation of the CD4+ Th cell response at the acute and chronic stages of infection are still being investigated.
Downregulation of peripheral T helper cell function is important in limiting tissue damage and other side effects caused by sustained inflammation (22). A major mechanism of peripheral T cell regulation is activation-induced cell death (AICD), which is mediated through upregulated expression of death effector molecules such as Fas ligand (FasL), tumor necrosis factor, and perforin-granzyme B (1, 2, 19, 28). Inducible expression of FasL has generally been studied on T lymphocytes following activation by mitogens or through the T cell receptor complex (21). However, several recent reports indicate that activated B cells can express functional FasL (8, 16, 30, 34). Susceptibility to FasL-mediated apoptosis is determined by the expression of the death receptor, Fas (CD95, Apo1), and by the activation state of the target cell (29).
All of the previous studies of apoptosis in schistosomiasis have been focused on the acute stage of the infection. In the first study, splenocytes from infected mice were sensitive to mitogen-induced apoptosis that was ameliorated by neutralized interleukin-10 activity and apoptosis was detected in histological spleen and granuloma sections (12). Another study demonstrated that splenic Th1 cells were more susceptible to apoptosis than their Th2 counterparts (13). The third study determined a high level of lymphocyte apoptosis in granulomas but not in splenic cells of infected mice (33). These studies did not examine the dynamics of CD4+ Th cell apoptosis during the chronic stage of infection, SEA-induced AICD of CD4+ Th lymphocytes, or the role of FasL-bearing effector cell populations in mediating CD4+ Th cell apoptosis.
The hypothesis of this study was that the previously observed decrease in the relative number of splenic T cells at the early chronic stage of the infection (10) was the result of SEA-induced AICD. This study investigated the dynamics of apoptosis of freshly isolated spleen and granuloma CD4+ T cells to gain an insight into the in vivo apoptotic events during the infection. The ex vivo expression of FasL as a marker of AICD during both the acute and chronic stages of infection was detected. Culture of splenocytes and granuloma lymphocytes with SEA induced CD4+ Th cell apoptosis and increased functional FasL display on the surfaces of CD4+ and CD8+ T lymphocytes and CD19+ B lymphocytes. Remarkably, SEA-stimulated splenic B cells were shown to function as effector cells in CD4+ T cell apoptosis.
MATERIALS AND METHODS
Mice, infection, and cell preparation.
Six- to eight-week-old female CBA/Jk mice (Jackson Laboratory, Bar Harbor, Maine) were injected subcutaneously with 25 cercariae of the Puerto Rican strain of S. mansoni. Infected mice received standard mouse chow and acidified water. Mice were sacrificed at the indicated times of infection, and spleens and livers were removed aseptically. The erythrocytes from dispersed splenocytes were removed by hypotonic shock, and the remaining cells were washed in culture medium consisting of RPMI 1640 medium (Sigma, St. Louis, Mo.), 10% fetal calf serum (Gibco BRL, Gaithersburg, Md.), 2 mM pyruvate, 0.05 mM 2-mercaptoethanol, 2 mM l-glutamine, penicillin at 100 U/ml, and streptomycin at 0.1 mg/ml. Granuloma cells were prepared as previously described (32). Isolated granuloma cells were resuspended in culture medium, and adherent cells were removed by incubation on plastic petri dishes (Becton Dickinson, Franklin Lakes, N.J.) for 90 min at 37°C in 5% CO2. The viability of isolated cells was determined, by trypan blue exclusion, to be >95% for splenocytes and >80% for granuloma cell preparations.
Antibodies, reagents, and cell lines.
Conjugated monoclonal antibodies against murine lymphocyte surface markers (CD4-fluorescein isothiocyanate [FITC], CD8-FITC, CD3-phycoerythrin [PE], and FasL-PE), as well as FITC-conjugated annexin V reagent and FcBlock, were purchased from Pharmingen (San Diego, Calif.). Anti-CD4–PE and anti-CD19–FITC were purchased from Caltag (Burlingame, Calif.), and isotype-matched murine immunoglobulin G2bκ-PE (control for FasL-PE), and propidium iodide (PI) were purchased from Sigma. The Fas-transfected L1210 lymphoblastic leukemia line and untransfected L1210 cells were the generous gift of Chris Bleackley (University of Alberta, Edmonton, Alberta, Canada) with the kind permission of Pierre Golstein (Université Marseille-Luminy, Marseille, France). SEA was prepared as previously described (6).
Detection of Th apoptosis by annexin V-based, three-color flow cytometry.
Freshly isolated spleen and granuloma cells (5 × 105 cells/tube) were washed in labeling buffer (1× phosphate-buffered saline [PBS], 0.2% bovine serum albumin [BSA], 0.1% Na azide). Cell pellets were incubated with 0.5 μg of FcBlock for 10 min at 4°C before incubation with 0.1 μg of CD4-PE antibody for 30 min at 4°C. Labeled cells were washed once in 1× PBS and once in annexin V labeling buffer (10 mM HEPES, 140 mM NaCl, 2.5 mM CaCl2), and then 3 μl of annexin V-FITC and 0.5 μg of PI were added to the resuspended cell pellet and the combination was incubated for 10 min at room temperature. Labeling was terminated by the addition of 0.3 ml of annexin V labeling buffer, and data were acquired immediately on a FACScan instrument (Becton Dickinson, San Jose, Calif.). Viable lymphocytes were gated using forward scatter versus side scatter characteristics, and analysis of Th cell apoptosis was performed by gating of the CD4+ PI− population followed by analysis of annexin V-FITC labeling. Data were plotted as the percentage of viable T cells that were positive for annexin V. For in vitro SEA-stimulated Th cell apoptosis, splenocytes and nonadherent granuloma cells (105/well) were cultured in round-bottom 96-well plates in the presence of medium alone or medium containing SEA at 10 μg/ml for 60 h and then collected and stained as described above. For CD8+ T cell and B cell depletion, splenocytes and granuloma cells were labeled with manufacturer-suggested concentrations of MACS murine anti-CD8 and/or anti-CD19 magnetic microbeads (Miltenyi Biotech, Auburn, Calif.) in degassed PBS–2 mM EDTA–0.5% BSA for 10 min at 6°C, washed, and passed through a depletion column (Miltenyi). Two-color flow cytometry confirmed that <3% B cells or CD8+ T cells remained after the depletions. Depleted cell preparations were resuspended in culture medium with or without SEA, cultured, and stained as described above.
Detection of FasL surface expression on T and B cells.
Freshly isolated splenocytes were washed three times in PBS, resuspended in 1% paraformaldehyde, incubated for 1 h at 4°C, and washed three times with PBS and then once with labeling buffer. Cells were then incubated with FcBlock for 10 min at 4°C and then incubated with either 0.5 μg of anti-CD4–FITC, 0.5 μg of anti-CD8–FITC, or 0.2 μg of anti-CD19–FITC and either 0.4 μg of anti-FasL–PE or 0.4 μg of immunoglobulin G2bκ-PE (control antibody) for 30 min at 4°C. After labeling, the cells were washed twice in PBS and data were acquired on the FACScan. Viable cells were gated by forward scatter-side scatter, and then FITC-positive cells were gated and analyzed for display of FasL-PE compared with the isotype-matched control antibody. For analysis of SEA-stimulated surface FasL expression, splenocytes or granuloma cells were cultured for 36 or 48 h, respectively, in medium with or without SEA at 10 μg/ml prior to fixation and staining. Trypan blue (Gibco) was added to stained granuloma cells at a final concentration of 0.03% prior to flow cytometry to quench the autofluorescence of eosinophils (27).
Chromium release assay.
FasL-transfected and nontransfected L1210 lymphoblastoid leukemia cells (2 × 106) were labeled with 0.2 mCi of 51Cr for 2 h at 37°C with frequent mixing. Labeled cells were washed three times with culture medium and plated in 96-well round-bottom plates at 1.25 × 104 cells/well with various effector cell populations. Splenocytes preincubated with or without SEA at 10 μg/ml for 36 h at 37°C were washed, counted, and mixed with target cells at the indicated effector-to-target cell (E:T) ratios. SEA-stimulated granuloma cells were spun through Ficoll or labeled with magnetic microbeads and passed through a depletion column before culture with labeled target cells. Cytotoxicity was determined on a gamma counter by measuring the counts per minute of released 51Cr in 150 μl of culture medium after 16 h of incubation. Percent specific lysis was determined using the following formula, in which spont. cpm refers to the release by target cells cultured in medium alone and total cpm refers to release by target cells cultured in medium containing 1% Triton X-100: [(sample cpm − spont. cpm)/(total cpm − spont cpm)] × 100.
CD4+ T cell apoptosis induced by purified B cells.
Splenocytes from mice infected 8 weeks previously were isolated as described above. Target CD4+ T cells were prepared by magnetic microbead depletion of CD8+ T cells and CD19+ B cells, followed by culture in medium for 36 h. Effector CD19+ B cells were prepared by culture of splenocytes in the presence or absence of SEA at 10 μg/ml for 36 h, followed by magnetic microbead purification of B cells. Target CD4+ T cells and effector CD19+ B cells were greater than 85% and 95% pure, respectively. Target cells were plated at 5 × 104 cells/well in 96-well round-bottom plates, effector cells were added at the indicated E:T ratios, and the combination was incubated for an additional 24 h. Cells from two wells were pooled and stained for CD4+ T cell apoptosis as described above. Error bars indicate the standard deviation of six replicates from a representative of three independent experiments.
Adoptive transfer of purified B cells.
Splenocytes from mice infected 8 weeks previously were cultured in the presence or absence of SEA at 10 μg/ml for 36 h. Cultured cells were washed in RPMI 1640 medium–0.3% BSA–20 mM HEPES and incubated with anti-Thy1.2 and anti-asialo GM1 antibodies (4 μg/10 million cells) for 1 h at 4°C, and then low-toxicity rabbit complement (Accurate Chemical, Westbury, N.Y.) was added and the mixture was incubated for 1 h at 37°C. After several washes, 20 million T- and NK-depleted splenocytes (>70% B cells, <8, 3, and 2% CD4+, CD8+, and NK cells, respectively) were injected into the tail veins of recipient mice infected 7 weeks previously (three mice per group). Recipient mice were sacrificed 24 h postinjection, splenocytes were prepared separately for each mouse, and CD4+ T cell apoptosis was measured in 12 replicates for each condition shown in Fig. 1B.
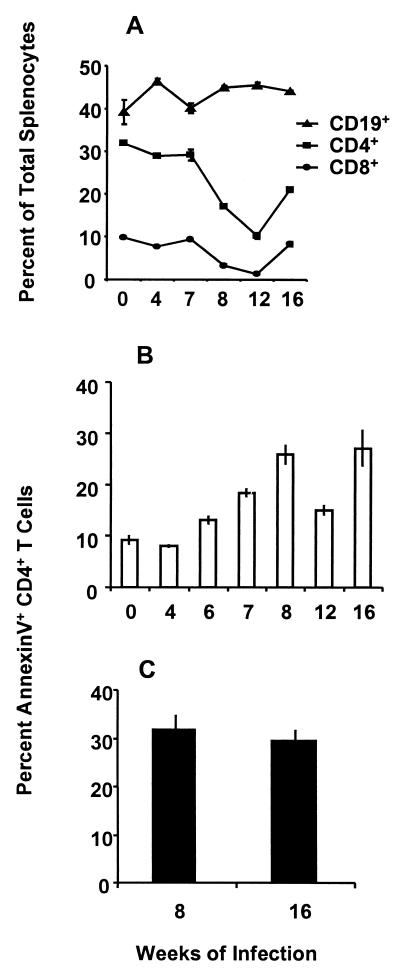
FIG. 1.
Dynamics of CD4+ T lymphocyte apoptosis during the course of infection. (A) Two-color flow cytometric analysis of T and B cell populations was performed on freshly isolated splenocytes at the indicated times of infection. Data are the ratio of the given population of cells versus the total splenocyte population. (B) At specified intervals of infection, spleens were removed and the dispersed cells were immediately stained with anti-CD4-PE, annexin V-FITC, and PI as described in Materials and Methods. CD4-positive, PI-negative cells were analyzed and plotted as percent annexin-positive cells within the total CD4+ T cell population. Cells collected at the indicated times of infection were stained on the same day to minimize interassay variability. (C) Freshly isolated granuloma CD4+ T cells from 8 and 16 weeks of infection were analyzed for apoptosis as described for panel B. Error bars indicate the standard deviation of quadruplicate samples. Similar results were obtained in at least three separate experiments for each time point, representing a total of 15 to 20 mice per time point.
SEA-stimulated proliferation.
Splenocytes from mice infected 8 and 16 weeks previously were depleted of CD19+ B cells and/or CD8+ T cells by magnetic microbead separation prior to culture in the presence of SEA at 10 μg/ml. The proliferation of 105 cells/well was determined by addition of [3H]thymidine at 104 h of culture and harvest at 120 h. Tritium uptake was determined using a scintillation counter. Mean counts per minute and standard deviations were determined for six replicate samples.
Statistical analysis.
Mean percentages and standard deviations were determined by normalization of flow cytometric and chromium release data by arcsine transformation. The statistical significance of the data was determined using analysis of variance and the paired Student t test.
RESULTS
CD4+ T cell apoptosis during the course of S. mansoni infection.
Reduced percentages of T cells in the spleens, granulomas, and circulation of mice following the acute stage of infection with S. mansoni have been previously reported by our laboratory (10). Flow cytometric analysis of splenic CD19+ B cells and CD4+ and CD8+ T cells extended that study and revealed that relative to the total splenocyte population, both the CD4+ and CD8+ T cell subsets are reduced (Fig. 1A). These reduced percentages were not due to loss of CD4 or CD8 expression, since no increase in CD3+ double-negative (CD4− CD8−) T cells was observed. Because B cell expansion and splenomegaly are hallmarks of this infection, it was unknown whether some of the reduced percentage of CD4+ T cells was attributable to apoptotic cell death. To assess the putative role of apoptosis in CD4+ T lymphocyte regulation during schistosomal infection, freshly isolated spleen and granuloma cells, without further stimulus, were examined for binding of the early apoptosis marker annexin V (25, 35). The results in Fig. 1B demonstrate that apoptosis of freshly isolated splenic CD4+ Th cells did not increase above the background during larval maturation (4 weeks) but significantly increased (P < 0.001) by 6 weeks of infection, correlating with the initiation of egg deposition in the liver. Splenic CD4+ Th cell apoptosis peaked at the acute stage (8 weeks), decreased by 12 weeks, and then increased at the chronic stage (16 weeks) of infection. As shown in Fig. 1C, 30% of granuloma CD4+ Th cells were apoptotic during both the acute and chronic, downmodulated stages of granulomatous inflammation.
SEA-induced apoptosis in cultured CD4+ Th cells.
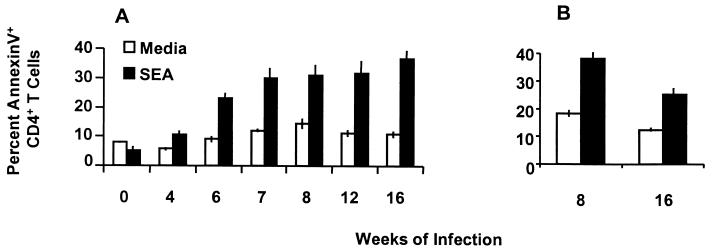
The finding that CD4+ Th cell apoptosis rose at 6 weeks of infection and increased further thereafter suggested that antigenic stimulation by worm eggs could be involved in the induction of apoptosis. To test this hypothesis, SEA was used to stimulate splenic and granuloma CD4+ T cell apoptosis in vitro (Fig. 2A and B). Cells cultured in medium alone for 60 h exhibited a reduction in apoptosis compared with freshly isolated cells, as shown in Fig. 1B and C. SEA had little effect on splenic cells derived from uninfected mice or mice infected 4 weeks before but was a potent stimulator of apoptosis in cells from 6 to 16 weeks of infection (all P < 0.001), the period that encompasses early, peak, and downmodulated granuloma formation (Fig. 2A). Compared with the 4-week pre-egg deposition time point, SEA stimulated nearly threefold increases (P < 0.001) in splenic CD4+ Th cell apoptosis after egg deposition commenced. As shown in Fig. 2B, granuloma CD4+ Th cells analyzed at the peak (8 weeks) and downmodulated (16 weeks) stages of the infection were also stimulated by SEA to undergo a twofold increase in apoptosis (both P < 0.001). However, SEA-stimulated CD4+ Th cell apoptosis was higher in granuloma cells isolated from the acute stage (8 weeks) of infection compared to granuloma cells from the downmodulated (16 weeks) stage (P < 0.001).
FIG. 2.
SEA-stimulated apoptosis of CD4+ T helper lymphocytes. Splenocytes and nonadherent granuloma cells collected at the indicated times of infection were cultured with or without SEA at 10 μg/ml for 60 h and then subjected to annexin V binding analysis as described in the legend to Fig. 1B. (A) Spleen data were obtained from a single experiment and are representative of at least three experiments with a total of 10 to 20 mice per time point. (B) Data on SEA-stimulated granuloma CD4+ Th cell apoptosis at 8 and 16 weeks of infection are representative of at least three experiments with a total of 20 to 30 mice per time point. Error bars indicate the standard deviations of quadruplicate samples analyzed by arcsine transformation of percentage data.
Detection of FasL expression on T and B lymphocyte populations between 0 and 16 weeks of infection.
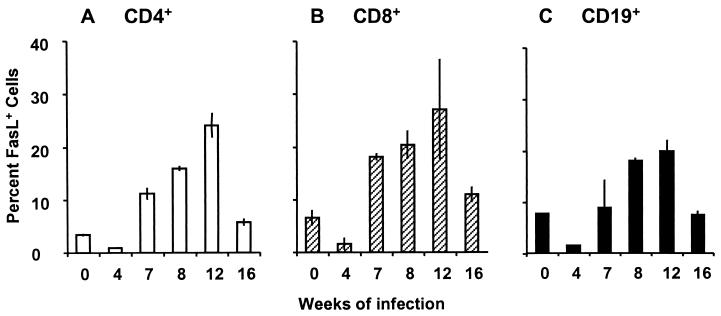
The observed antigen-stimulated increase in CD4+ Th cell apoptosis indicated that AICD was a potential contributing factor in Th cell regulation. Because the interaction of Fas (CD95, Apo-1) with its ligand (FasL) is a major component of AICD for T helper lymphocytes (28), we assessed FasL expression during infection. Flow cytometric assays for surface FasL expression on freshly isolated, unstimulated spleen cells indicated that both CD4+ and CD8+ T cells expressed FasL during infection. Interestingly, a large splenic population that expressed surface FasL was identified as CD19+ B cells. A comparison of FasL expression by CD4+, CD8+, and CD19+ cell populations is presented in Fig. 3. For each cell type, the level of FasL expression was lowest at the pre-egg deposition stage (4 weeks) of infection, rose to a peak between 8 and 12 weeks, coincident with maximal granuloma development, and then decreased at the downmodulated, chronic (16 weeks) stage of infection.
FIG. 3.
FasL expression on freshly isolated spleen T and B cells. Expression of FasL by spleen lymphocytes freshly isolated at the indicated times of infection was analyzed by two-color flow cytometry. The percentage of cells within each population that stained with anti-FasL–PE compared with an isotype-matched control antibody was plotted. (A) CD4+ T lymphocytes. (B) CD8+ T lymphocytes. (C) CD19+ B lymphocytes. Data were obtained from a single assay performed on the same day and are representative of at least three experiments with a total of 10 to 15 mice for each time point. Error bars indicate the standard deviation of duplicate samples analyzed by arcsine transformation of percentage data.
SEA-stimulated FasL expression on splenic and granuloma T and B cells.
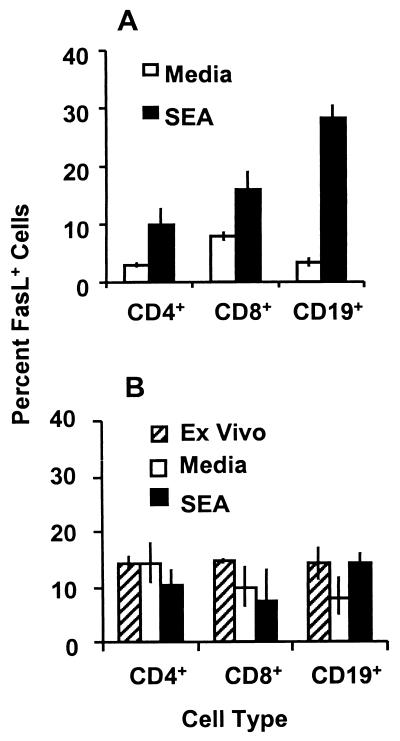
Because SEA could drive Th cell apoptosis in vitro and the dynamics of FasL expression paralleled that of apoptosis in freshly isolated spleen CD4+ Th cells, the effect of the SEA stimulus on the expression of FasL was assessed in splenocyte and granuloma cell cultures from mice infected 8 weeks before. All splenocytes showed reduced surface expression of FasL after 24 h of culture, regardless of the presence or absence of SEA (data not shown). FasL expression was partially regained on CD4+ and CD8+ T cells (P < 0.05 compared to medium-treated controls) and completely regained on CD19+ B cells (P < 0.001) after 36 h of culture with SEA (Fig. 4A). Trypan blue exclusion analysis revealed no evidence of preferential death in the medium-treated cultures or proliferation in the SEA-stimulated cultures to explain the differences in FasL expression. Reverse transcription-PCR analysis revealed that FasL mRNA expression is lost by 12 h of culture with or without SEA and is regained at 21 h of culture in SEA but not in medium-treated controls. SEA did not stimulate FasL mRNA or surface expression on splenocytes from uninfected mice and mice infected 4 weeks before (data not shown). Fifteen percent of granuloma CD4+, CD8+, and CD19+ lymphocytes from mice infected 8 weeks before expressed FasL ex vivo (Fig. 4B). FasL expression on granuloma lymphocytes was not significantly reduced by culture in medium alone and was not further enhanced by culture with SEA. It is noteworthy that unstimulated granuloma CD4+ T cells cultured in medium showed significantly higher expression of FasL than splenic CD4+ T cells under the same conditions (P < 0.01).
FIG. 4.
SEA-stimulated expression of FasL by cultured T and B lymphocytes. Splenocytes (A) and nonadherent granuloma cells (B) from mice infected for 8 weeks were cultured with or without SEA at 10 μg/ml for 36 and 48 h, respectively, and analyzed for surface expression of FasL by two-color flow cytometry. Ex vivo surface FasL expression on freshly isolated granuloma T and B cells from mice infected for 8 weeks was also analyzed (striped bars). Error bars indicate the standard deviation of triplicate samples normalized by arcsine transformation. The data shown are representative of three repeated experiments utilizing a total of 10 to 15 mice per time point.
SEA-stimulated lymphocytes induce lysis of Fas-bearing targets.
To confirm that SEA-induced FasL was functional at inducing apoptosis, chromium release assays were performed utilizing an indicator lymphoblastic leukemia cell line transfected with Fas, L1210-Fas, which was sensitive to anti-Fas monoclonal antibody-induced lysis. SEA-stimulated splenocytes from mice infected 8 and 16 week before were able to preferentially lyse L1210-Fas target cells in a dose-dependent manner (Fig. 5A and B). The lytic ability of splenocytes collected at 16 weeks of infection was half that of cells collected at 8 weeks of infection (P < 0.01). Lysis of the nontransfected L1210 parent cell line by splenic effectors from mice infected 8 weeks before indicated that target lysis was not mediated solely by FasL. This did not seem to be the case with splenocytes collected at 16 weeks of infection. SEA-stimulated splenocytes from uninfected mice and mice infected 4 weeks before lysed less than 5% of L1210-Fas cells at all of the E:T ratios tested, similar to splenocytes from all of the infection time points tested cultured without SEA (data not shown). Granuloma cells collected at 8 weeks of infection and stimulated with SEA were able to preferentially lyse the Fas-sensitive target cells (Fig. 5C) but were much less effective than splenocytes. Lack of lysis of the nontransfected L1210 parent cell line indicated no alternate lytic mechanism expressed by granuloma cells.
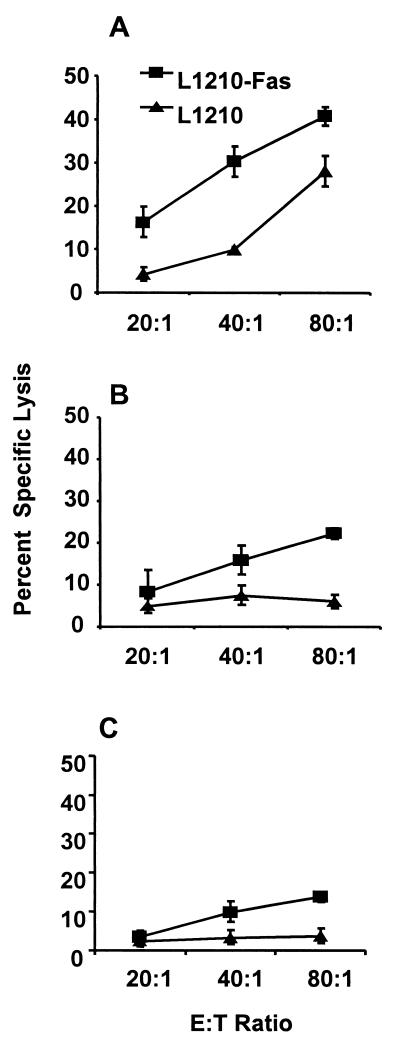
FIG. 5.
Lysis of Fas-sensitive and Fas-resistant target cells by SEA-stimulated effector cells. Splenocytes from animals infected for 8 (A) or 16 (B) weeks were cultured with or without SEA at 10 μg/ml for 36 h prior to use as effector cells. Target cells were 51Cr-labeled L1210-Fas (squares) and control L1210 (triangles) lymphoblastoid leukemia cells. Effector and target cells were cultured together at the indicated E:T ratios for an additional 16 h. Error bars represent the standard deviation of quadruplicate samples normalized by arcsine transformation. (C) Granuloma cells collected from mice infected for 8 weeks and stimulated for 36 h with SEA were spun through Ficoll to remove dead cells and debris and then cultured with 51Cr-labeled L1210-Fas and control L1210 parent cells for 16 h. Data were analyzed as described above. Lysis of both target cell lines by spleen and granuloma effector cells cultured in medium without SEA was less than 5% at all of the E:T ratios used.
Determination of the predominant death effector cell populations in the spleen and granulomas.
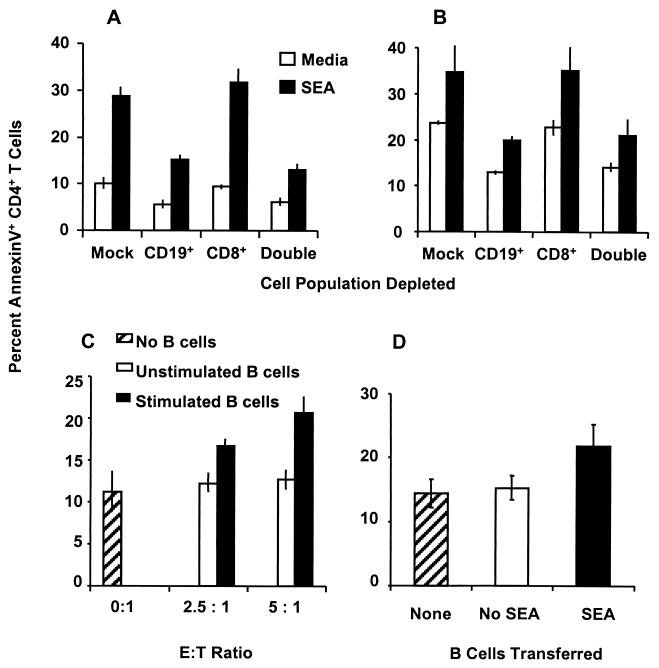
To assess the relative importance of CD8+ and CD19+ cells in inducing CD4+ Th cell apoptosis, the respective cell populations were selectively removed from spleen and granuloma cell preparations by magnetic microbead separation. As shown in Fig. 6A, compared with unseparated splenocytes (mock depletion), which showed about 30% SEA-induced apoptosis, removal of CD8+ T cells from splenocytes of mice infected for 8 weeks did not decrease SEA-stimulated CD4+ Th cell apoptosis. In contrast, removal of CD19+ B cells led to a marked reduction in CD4+ Th cell apoptosis (P < 0.001). This was similar to the reduced level of CD4+ T cell apoptosis observed when both CD8+ T cells and CD19+ B cells were removed. However, a residual cell death of CD4+ T cells was still observed in cultures depleted of CD8+ and CD19+ lymphocytes. These data indicated a dominant role of splenic B cell effectors compared to CD8+ T cells in the induction of CD4+ T cell apoptosis. Depletion analysis of splenocytes from mice infected for 16 weeks (Fig. 6B) showed essentially the same results and confirmed that not CD8+ T cells but rather CD19+ B cells had the dominant effector function at the chronic stage of infection (P < 0.001). To ensure that the reduction in CD4+ T cell apoptosis was not due to decreased activation, proliferation assays were performed in parallel. As shown in Table 1, no reduction in SEA-stimulated proliferation was observed following depletion of CD8+ T cells or B cells. To confirm that SEA-stimulated B cells could directly mediate apoptosis of CD4+ T cells, coculture experiments with purified, activated effector B cells and target CD4+ T cells were performed. SEA-stimulated B cells induced dose-dependent apoptosis of CD4+ T cells (P < 0.001 compared to unstimulated B cells at both E:T ratios) at fairly low E:T ratios (Fig. 6C).
FIG. 6.
Analysis of splenic B cell-mediated CD4+ Th cell apoptosis following depletion, coculture, or adoptive transfer. Splenocytes from mice infected for 8 (A) or 16 (B) weeks were depleted of CD8+ T cells and/or B cells by magnetic microbead separation before culture with or without SEA (10 μg/ml) for 60 h and then analyzed for CD4+ T cell apoptosis. (C) Effector CD19+ B cells were purified (>95% purity) from splenocytes of mice infected for 8 weeks by positive selection with magnetic microbeads after culture in the presence or absence of SEA for 36 h. Target CD4+ T cells (>85% purity) were prepared by depletion of CD8+ T cells and CD19+ B cells from splenocytes of mice infected for 8 weeks and then cultured in medium without SEA for 36 h. Effector and target cells were then mixed and incubated for an additional 24 h at the indicated E:T ratios prior to analysis of CD4+ T cell apoptosis. (D) Splenocytes from donor mice infected for 8 weeks were cultured with or without SEA, depleted of T and NK cells, and then injected into recipient mice infected for 7 weeks (three mice per group). Recipients were sacrificed at 24 h postinjection, and splenic CD4+ T cell apoptosis was determined as described in the legend to Fig. 1B. All data are from a representative of three experiments, and error bars indicate the standard deviation of 6 (A, B, and C) or 12 (D) replicates.
TABLE 1.
Proliferation and CD4+ T cell apoptosis following cell depletions
| Cells depleted | 8-wk infection
|
16-wk infection
|
||
|---|---|---|---|---|
| Proliferationa (cpm, 10−4) | Apoptosisb (% annexin V+) | Proliferation (cpm, 10−4) | Apoptosis (% annexin V+) | |
| None (mock depletion) | 2.9 ± 0.1 | 29.2 ± 2.2 | 1.9 ± 0.1 | 33.6 ± 6.4 |
| CD19 | 2.9 ± 0.2 | 15.1 ± 1.1 | 2.1 ± 0.9 | 19.9 ± 0.8 |
| CD8 | 2.8 ± 0.2 | 31.8 ± 2.8 | 1.7 ± 0.1 | 35.2 ± 4.9 |
| CD8, CD19 | 3.5 ± 0.1 | 13.1 ± 1.4 | 2.3 ± 0.3 | 21.2 ± 3.5 |
Uptake of [3H]thymidine by splenocytes at 120 h of culture in the presence of SEA (10 μg/ml). The data are counts per minute ± the standard deviation of six replicate samples.
Annexin V binding of CD4+ PI− cells measured at 60 h of culture in the presence of SEA (10 μg/ml). The data are mean percent annexin V+ cells among viable CD4+ T cells ± the standard deviation of six replicates.
In order to determine whether SEA-stimulated, FasL bearing B cells could induce CD4+ T cell apoptosis in vivo, adoptive-transfer experiments were performed. The intravenous transfer of 20 million enriched, SEA-stimulated B cells resulted in a statistically significant (P < 0.001) increase in splenic CD4+ T cell apoptosis at 24 h postinjection compared to mice that received unstimulated B cells or RPMI 1640 medium (Fig. 6D).
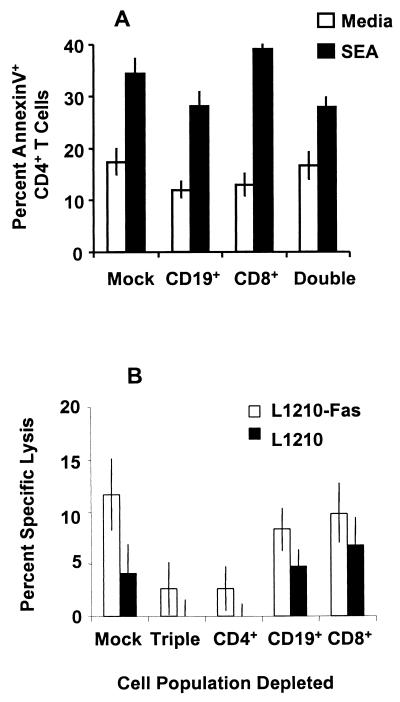
In contrast to the effects mediated by splenic B cells, depletion of CD19+ B cells from granuloma lymphocytes led to only a small, although significant (P < 0.01), reduction in CD4+ T cell apoptosis compared with mock depletion (Fig. 7A). As was observed for splenocytes, depletion of CD8+ T cells from the granuloma lymphocytes did not reduce CD4+ T cell apoptosis. These data suggested that CD4+ T cells might be the dominant effectors in the granuloma. To support this contention, cytotoxicity assays of the FasL-transfected cell line were performed. The low level of granuloma cell-induced lysis of L1210-Fas cells was completely abrogated by magnetic microbead-mediated depletion of CD4+ T cells (P < 0.001) but was not affected by CD8+ T cell or CD19+ B cell depletion from the effector population (Fig. 7B).
FIG. 7.
Analysis of the dominant effector cells within the granuloma. (A) Nonadherent granuloma cells were depleted of CD19+ B cells and CD8+ T cells using magnetic microbeads prior to culture in the presence or absence of SEA for 60 h. CD4+ Th cell apoptosis was assessed as described in the legend to Fig. 1C on six replicate samples. The data presented are from a representative of three experiments which utilized a total of 25 to 30 mice. Error bars indicate the standard deviation of arcsine-transformed percentages. (B) Nonadherent granuloma cells from mice infected for 8 weeks were cultured in the presence or absence of SEA for 36 h prior to depletion of the indicated cell populations using magnetic microbead separations. The depleted granuloma cells were then mixed with 51Cr-labeled L1210-Fas or parent L1210 lymphoblastoid leukemia cells at an E:T ratio of 32:1 and incubated for an additional 16 h. Specific lysis was determined and is presented as described in the legend to Fig. 5. Error bars indicate the standard deviation of six replicates in this representative of three experiments.
DISCUSSION
The data presented here indicate that the egg-induced granulomatous inflammation that occurs during S. mansoni infection in mice is accompanied by CD4+ Th cell apoptosis. CD4+ Th cell apoptosis commenced after egg deposition (5 weeks), was found at the early and florid granulomatous stages (6 to 10 weeks), and persisted throughout the chronic downmodulated stage (16 weeks) of the infection. The percentage of annexin V-binding CD4+ Th cells isolated ex vivo from the spleen and granulomas was higher than the expected percentage of antigen-specific Th cells, indicating that apoptosis was an active, ongoing mechanism that could influence antigen-specific, as well as bystander, Th cell turnover in vivo. A sharp drop in the percentage of splenic CD4+ T cells between 7 and 12 weeks of infection paralleled the increase in CD4+ T cell apoptosis, suggesting that apoptosis plays a role in the control of Th cell expansion during schistosomal infection. SEA was able to further stimulate apoptosis of egg-primed splenic and granuloma CD4+ T cells in vitro. The higher percentage of CD4+ T cell apoptosis in granulomas at 8 weeks versus 16 weeks of infection correlates with the increased activity of acute-stage granuloma Th cells. Because pronounced CD4+ Th cell apoptosis was observed in ex vivo, as well as SEA-stimulated, spleen and granuloma cell cultures only after oviposition, it was concluded that CD4+ T cell apoptosis was due to SEA-induced AICD.
The interaction of the Fas death receptor and its ligand is one of the major mechanisms of AICD and plays an important role in peripheral deletion of mature circulating Th lymphocytes (22, 28). The present study indicates that the expression of FasL on splenocytes was upregulated during the course of the granulomatous response and correlated fairly well with increased CD4+ Th lymphocyte apoptosis. Exceptions to this correlation were found at the 12- and 16-week stages of the infection. At 12 weeks, FasL expression was high but the observed CD4+ T cell apoptosis was low. This could be due to the sharp drop in the CD4+ T cell percentage observed at 12 weeks of infection and to the resistance of the residual splenic CD4+ T cells to AICD. The discrepancy in the high level of splenic CD4+ T cell apoptosis and low FasL expression at 16 weeks remains unclear.
It is noteworthy that during infection and SEA stimulation, splenic CD4+ and CD8+ T cells, as well as CD19+ B cells, displayed increased expression of FasL which paralleled cell activation by egg antigens. On a percentage basis, FasL expression peaked with maximal granulomatous inflammation (8 to 12 weeks) in all three cell populations and declined during the downmodulated infection stage (16 weeks). This decline may be attributable to the lower level of cellular activation during downmodulation as a consequence of decreased cytokine production (5, 36). The high percentage of FasL-expressing cells in each population relative to the expected low percentage of antigen-specific B and T cells suggested that both antigen-specific and bystander cells were stimulated to express FasL and may participate in killer-effector function.
The ex vivo expression of FasL on splenocytes was found to be transient. FasL expression on splenocytes cultured in medium alone or with SEA was diminished at 24 h of culture but was regained at 36 h of culture with the antigen. This stimulated expression was due to de novo FasL gene transcription (data not shown). SEA-induced FasL expression was most pronounced on the CD19+ B cell population. In contrast, ex vivo FasL expression by granuloma cells at 8 weeks of infection did not diminish in culture with medium and the SEA stimulus did not enhance granuloma lymphocyte FasL expression. This data suggested that the granuloma cells had received maximal stimulation in vivo and could not be further stimulated to express FasL.
The preferential lysis of L1210-Fas cells, compared with the parent cell line, by SEA-stimulated spleen and granuloma cells confirmed that FasL+ cells were functional in mediating apoptosis. The reduced cytotoxicity exhibited by splenocytes at 16 weeks of infection and granuloma cells at 8 weeks of infection corresponded to the reduced level of FasL expression by these cells. As expected, splenocytes from uninfected mice and mice infected for 4 weeks, which did not express FasL after SEA stimulation, did not lyse either target cell line. Lysis of the parent cell line by splenocytes at 8 weeks of infection indicated that other non-FasL-mediated mechanisms of cell death were stimulated by culture in the presence of SEA.
The removal of CD8+ T cells from SEA-stimulated cultures did not lead to reductions in CD4+ T cell apoptosis, suggesting that CD8+ T cells are not critical to SEA-induced AICD. This result may correlate with previous findings that granuloma downmodulation is not dependent on CD8+ T cells (37) and could be attributable to the relatively low number of CD8+ T cells compared to CD4+ T cells and CD19+ B cells in the granuloma and spleen, respectively.
In contrast, the killer-effector role of SEA-stimulated B cells was indicated by several lines of evidence. The removal of B cells from acute- and chronic-stage splenocyte cultures led to a dramatic reduction in CD4+ T cell apoptosis. Reduced Th cell death could not be attributed to the loss of antigen-presenting cells because proliferation assays indicated that antigen presentation was not impaired by the removal of B cells from these cultures. Moreover, enhanced CD4+ T cell apoptosis in vitro caused by coculture with antigen-stimulated, purified B cells was observed. Significantly, an in vivo role for B cell-mediated apoptosis during infection was suggested by the increased CD4+ T cell death following adoptive transfer of SEA-stimulated, FasL-bearing B cells. Previous studies have shown that mitogen-activated B cells expressed surface FasL (16) and that FasL expression was detectable on B cells isolated from human tonsil and bone marrow (20, 30). Recent studies have demonstrated Epstein-Barr virus- and lectin-inducible FasL expression on B cells (8, 34). To our knowledge, the current study is the first to demonstrate that activated B cells can directly mediate CD4+ T cell apoptosis.
The possibility that splenic B cells can mediate apoptosis of CD4+ Th cells is particularly intriguing in the context of schistosomal infection. Our observations are consistent with previous studies that indicated the importance of splenic B cells in the regulation of granuloma formation. In splenectomized, B cell-depleted and B cell-deficient infected mice, granuloma downmodulation was impaired (9, 14, 17, 18). It is noteworthy that these previous findings are consistent with the current data which showed that B cells were active in mediating CD4+ Th cell apoptosis in vivo. Additional studies are under way to examine FasL expression on B cells and to correlate B cell-mediated apoptosis of CD4+ T cells and regulation of the granulomatous response. Preliminary experiments using CD4+ T cell depletion and culture of purified B cells with SEA-stimulated splenocyte supernatants indicate that FasL expression on B cells is dependent on a soluble factor from CD4+ T cells (data not shown). This may explain why the percentages of FasL+ T and B cells are higher than the expected percentages of antigen-specific cells (15, 23).
Unlike the dominant role of splenic B cells in CD4+ T cell apoptosis, granuloma B cells at 8 weeks of infection, that comprised less than 4% of the total granuloma cells, appeared to play a minor role in mediating granuloma CD4+ Th cell death. A relatively high sustained level of FasL expression was observed on granuloma CD4+ T cells, which are the largest lymphocytic population within the lesions. Although analysis of CD4+ Th cell apoptosis by depletion of CD4+ Th cells was not feasible, the low level of granuloma cell-mediated L1210-Fas cell cytotoxicity was eliminated by removal of CD4+ but not CD8+ T cells or B cells from the effector population. These data suggest that CD4+ T cells are the major mediators of apoptosis within the granuloma.
A previous study has indicated that splenocytes of S. mansoni-infected mice are susceptible to mitogen-induced apoptosis (12). The present study demonstrated that SEA is also a potent stimulator of CD4+ Th cell apoptosis. A second study showed higher lymphocyte apoptosis in granuloma than in splenocyte populations at the acute stage of infection (33). We did not find substantial differences in apoptosis between splenic and granuloma cells in the isolated CD4+ Th cell population. This discrepancy may be attributable to the difference between the assays that were used to analyze total-lymphocyte instead of CD4+ T cell apoptosis. Another study indicated that Th1-type cells of schistosome-infected mice are more susceptible than Th2-type cells to mitogen-induced apoptosis (13). Our current study, although it did not address AICD in Th cell subpopulations, indicated that CD4+ Th cell apoptosis commenced at 6 weeks of infection, before the Th1- to Th2-type response switch, and peaked at 8 weeks, coincident with the fully developed Th2-type granulomatous response. Thus, the SEA-induced and AICD-mediated regulation of CD4+ T cells was active throughout the early and florid stages of granuloma formation and was not limited to apoptosis of Th1 cells.
In conclusion, we propose that the SEA stimulus induces almost simultaneous CD4+ T cell activation and activation-induced cell death. During the early stages of granuloma development, cell activation-increased cytokine production and cell proliferation are dominant, resulting in the observed increases in granuloma size and intense inflammation. The results of the current study suggest that as the infection continues, the proapoptotic machinery expands to a point at which the balance is shifted toward cell death. In the face of continued egg production and antigenic stimuli, AICD of activated lymphocytes that mediate the inflammatory response seems to be an important regulatory mechanism which diminishes unchecked lymphocyte proliferation, inflammatory cytokine production, and granulomatous inflammation. Further studies of the role of CD4+ T cell apoptosis in the downmodulation of the inflammatory response should lead to a better understanding of pathogenesis during schistosomal infection.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant AI-12913 from the National Institute of Allergy and Infectious Diseases. Schistosome life stages and materials for this work were supplied through NIH-NIAID contract N01-AI55270.
We thank Joel Whitfield and Eric VanBuren for excellent technical assistance and Chris Bleackley for providing the L1210-Fas and L1210 parent cell lines.
REFERENCES
- 1.Alderson M R, Tough T W, Davis-Smith T, Braddy S, Falk B, Schooley K A, Goodwin R G, Smith C A, Ramsdell F, Lynch D A. Fas ligand mediates activation-induced cell death in human T lymphocytes. J Exp Med. 1995;181:71–77. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berke G. Killing mechanisms of cytotoxic lymphocytes. Curr Opin Hematol. 1997;4:32–40. doi: 10.1097/00062752-199704010-00006. [DOI] [PubMed] [Google Scholar]
- 3.Boros D L. Immunopathology of Schistosoma mansoni infection. Clin Microbiol Rev. 1989;2:250–269. doi: 10.1128/cmr.2.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boros D L. The role of cytokines in the formation of the schistosome egg granuloma. Immunobiology. 1994;191:441–450. doi: 10.1016/S0171-2985(11)80450-X. [DOI] [PubMed] [Google Scholar]
- 5.Boros D L. T helper cell populations, cytokine dynamics, and pathology of the schistosome egg granuloma. Microbes Infect. 1999;1:511–516. doi: 10.1016/s1286-4579(99)80090-2. [DOI] [PubMed] [Google Scholar]
- 6.Boros D L, Warren K S. Delayed hypersensitivity-type granuloma formation and dermal reaction induced and elicited by a soluble factor isolated from Schistosoma mansoni eggs. J Exp Med. 1970;132:488–507. doi: 10.1084/jem.132.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boros D L, Pelley R P, Warren K S. Spontaneous modulation of granulomatous hypersensitivity in schistosomiasis mansoni. J Immunol. 1975;114:1437–1441. [PubMed] [Google Scholar]
- 8.Bussing A, Stein G M, Pfuller U, Schietzel M. Induction of Fas ligand by the toxic mistletoe lectins in human lymphocytes. Anticancer Res. 1999;19:1785–1790. [PubMed] [Google Scholar]
- 9.Cheever A W, Byram J E, Hieny S, von Lichtenberg F, Lunde M N, Sher A. Immunopathology of Schistosoma japonicum and S. mansoni infection in B cell depleted mice. Parasite Immunol. 1985;7:387–398. doi: 10.1111/j.1365-3024.1985.tb00086.x. [DOI] [PubMed] [Google Scholar]
- 10.Chensue S W, Boros D L. Population dynamics of T and B lymphocytes in the lymphoid organs, circulation, and granulomas of mice infected with Schistosoma mansoni. Am J Trop Med Hyg. 1979;28:291–299. doi: 10.4269/ajtmh.1979.28.291. [DOI] [PubMed] [Google Scholar]
- 11.Colley D G. Immune responses to a soluble schistosomal egg antigen preparation during chronic primary infection with Schistosoma mansoni. J Immunol. 1975;115:150–156. [PubMed] [Google Scholar]
- 12.Estaquier J, Marguerite M, Sahuc F, Bessis N, Auriault C, Ameisen J C. Interleukin 10-mediated T cell apoptosis during the T helper type 2 cytokine response in murine Schistosoma mansoni parasite infection. Eur Cytokine Netw. 1997;8:153–160. [PubMed] [Google Scholar]
- 13.Fallon P G, Smith P, Dunne D W. Type 1 and type 2 cytokine producing mouse CD4+ and CD8+ T cells in acute Schistosoma mansoni infection. Eur J Immunol. 1998;28:1408–1416. doi: 10.1002/(SICI)1521-4141(199804)28:04<1408::AID-IMMU1408>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 14.Ferru I, Roye O, Delacre M, Auriault C, Wolowczuk I. Infection of B-cell-deficient mice by the parasite Schistosoma mansoni: demonstration of the participation of B cells in granuloma modulation. Scand J Immunol. 1998;48:233–240. doi: 10.1046/j.1365-3083.1998.00376.x. [DOI] [PubMed] [Google Scholar]
- 15.Fischer E, Camus D, Santoro F, Capron A. Schistosoma mansoni: autoantibodies and polyclonal B cell activation in infected mice. Clin Exp Immunol. 1981;46:89–97. [PMC free article] [PubMed] [Google Scholar]
- 16.Hahne M, Renno T, Schroeter M, Irmler M, French L, Bornand T, MacDonald H R, Tschopp J. Activated B cells express functional Fas ligand. Eur J Immunol. 1996;26:721–724. doi: 10.1002/eji.1830260332. [DOI] [PubMed] [Google Scholar]
- 17.Hood A T, Boros D L. The effect of splenectomy on the pathophysiology and egg-specific immune response of Schistosoma mansoni-infected mice. Am J Trop Med Hyg. 1980;29:586–591. doi: 10.4269/ajtmh.1980.29.586. [DOI] [PubMed] [Google Scholar]
- 18.Jankovic D, Cheever A W, Kullberg M C, Wynn T A, Yap G, Caspar P, Lewis F A, Clynes R, Ravetch J V, Sher A. CD4+ T cell-mediated granulomatous pathology in schistosomiasis is downregulated by a B cell-dependent mechanism requiring Fc receptor signaling. J Exp Med. 1998;187:619–629. doi: 10.1084/jem.187.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ju S T, Panka D J, Cui H, Ettinger R, El-Khatib M, Sherr D H, Stanger B Z, Marshak-Rothstein A. Fas (CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 20.Kondo E, Yoshino T, Nishiuchi R, Sakuma I, Nishizaki K, Yayagaki N, Yagita H, Akagi T. Expression of Fas ligand mRNA in germinal centers of the human tonsil. J Pathol. 1997;183:75–79. doi: 10.1002/(SICI)1096-9896(199709)183:1<75::AID-PATH1084>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Latinis K M, Carr L L, Peterson E J, Norian L A, Eliason S L, Koretsky G A. Regulation of CD95 (Fas) ligand expression by TCR-mediated signaling events. J Immunol. 1997;158:4602–4611. [PubMed] [Google Scholar]
- 22.Lenardo M F, Chan K M, Hornung F, McFarland H, Siegel R, Wang J, Zheng L. Mature lymphocyte apoptosis-immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol. 1999;17:221–253. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 23.Lopes L M, Pereira M A, Gerken S E, Vaz N. Polyclonal activation of B lymphocytes during experimental infection with Schistosoma mansoni. Parasitology. 1990;100:83–91. doi: 10.1017/s0031182000060145. [DOI] [PubMed] [Google Scholar]
- 24.Lukacs N W, Boros D L. Utilization of fractionated soluble egg antigens reveals selectively modulated granulomatous and lymphokine responses during murine schistosomiasis mansoni. Infect Immun. 1992;60:3209–3216. doi: 10.1128/iai.60.8.3209-3216.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin S J, Reutelingsperger C P M, McGahon A J, Rader J A, VanSchie R C A A, LaFace D M, Green D R. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathew R C, Boros D L. Anti-L3T4 antibody treatment suppresses hepatic granuloma formation and abrogates antigen-induced interleukin-2 production in Schistosoma mansoni infection. Infect Immun. 1986;54:820–826. doi: 10.1128/iai.54.3.820-826.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mosiman V L, Patterson B K, Canterero L, Goolsby C L. Reducing cellular autofluorescence in flow cytometry: an in situ method. Cytometry. 1997;30:151–156. [PubMed] [Google Scholar]
- 28.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 29.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1455. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 30.Nilsson N, Ingvarsson S, Borrebaeck C A K. Immature B cells in bone marrow express Fas/FasL. Scand J Immunol. 2000;51:279–284. doi: 10.1046/j.1365-3083.2000.00701.x. [DOI] [PubMed] [Google Scholar]
- 31.Pearce E J, Caspar P, Grzych J M, Lewis F A, Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991;173:159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ragheb S, Mathew R C, Boros D L. Establishment and characterization of an antigen-specific T-cell line from liver granulomas of Schistosoma mansoni-infected mice. Infect Immun. 1987;55:2625–2630. doi: 10.1128/iai.55.11.2625-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rumbley C A, Zekavat S A, Sugaya H, Perrin P J, Ramadan M A, Phillips S M. The schistosome granuloma: characterization of lymphocyte migration, activation, and cytokine production. J Immunol. 1998;161:4129–4137. [PubMed] [Google Scholar]
- 34.Tanner J E, Alfieri C. Epstein-Barr virus induces fas (CD95) in T cells and fas ligand in B cells leading to T-cell apoptosis. Blood. 1999;94:3439–3447. [PubMed] [Google Scholar]
- 35.VanEngeland M, Nieland L J W, Ramaekers F C S, Schutte B, Reutelingsperger C P M. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31:1–9. doi: 10.1002/(sici)1097-0320(19980101)31:1<1::aid-cyto1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 36.Wynn T A, Cheever A W. Cytokine regulation of granuloma formation in schistosomiasis. Curr Opin Immunol. 1995;7:505–511. doi: 10.1016/0952-7915(95)80095-6. [DOI] [PubMed] [Google Scholar]
- 37.Yap G, Cheever A W, Caspar P, Jankovic D, Sher A. Unimpaired down-modulation of the hepatic granulomatous response in CD8 T-cell and gamma interferon-deficient mice chronically infected with Schistosoma mansoni. Infect Immun. 1997;65:2583–2586. doi: 10.1128/iai.65.7.2583-2586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]