Abstract
Objectives
Routinely used performance status scales, assessing patients' suitability for cancer treatment, have limited ability to account for multimorbidity, frailty and cognition. The Clinical Frailty Scale (CFS) is a suggested alternative, but research detailing its use in oncology is limited. This study aims to evaluate if CFS is associated with prognosis and care needs on discharge in oncology inpatients.
Methods
We evaluated a large, single‐centre cohort study in this research. CFS was recorded for adult inpatients at a Regional Cancer Centre. The associations between CFS, age, tumour type, discharge destination and care requirements and survival were evaluated.
Results and Conclusions
A total of 676 patients were included in the study. Levels of frailty were high (Median CFS 6, 81.8% scored ≥5) and CFS correlated with performance status (R = 0.13: P = 0.047). Patients who were frail (CFS ≥ 5) were less likely to be discharged home (62.9%) compared with those who were not classed as frail (86.1%) (OR 3.6 [95%CI 2.1 to 6.3]: P < 0.001). Higher CFS was significantly associated with poorer prognosis in all ages. Solid organ malignancy (hazard ratio [HR] 2.60 [95%CI 2.05–3.32]) and CFS (HR 1.43 [95%CI 1.29–1.59]; P < 0.001) were independently associated with poorer survival. This study demonstrated that CFS may help predict prognosis in adult oncology inpatients of any age. This may aid informed shared decision‐making in this setting. Future work should establish if routine CFS measurement can aid the appropriate prescription of systemic therapy and enable early conversations about discharge planning.
Keywords: allied health personnel, cancer, frailty, palliative care, patient discharge, prognosis
What is already known about the topic
The Eastern Cooperative Oncology Group Performance Status Scale is a 40‐year‐old adaption of the Karnofsky Performance Status Scale, produced to assess oncology patients' suitability for treatments and clinical trials.
The Eastern Cooperative Oncology Group Performance Status Scale has limited ability to account for multimorbidity, frailty and cognition and reflect changes in patients' condition. Patients may therefore inappropriately receive or not receive systemic therapy.
Patient safety initiatives have recommended the use of the Rockwood CFS; however, there is limited evidence for its use in an oncology setting.
What this paper adds
Increasing frailty measured using the CFS is associated with poor prognosis in adult oncology and haemato‐oncology inpatients at any age.
Implications for practice, theory or policy
This paper supports use of the CFS in the oncology setting in patients of all age groups.
Further evaluation is required to establish if measuring frailty can enable early conversations about discharge planning.
1. INTRODUCTION
In oncology, functional ability is used to assess suitability for treatment. The primary tool for evaluation is the Eastern Co‐operative Oncology Group Performance Status Scale (ECOG PS). However, this scale has been criticised as subjective and prone to bias particularly in the older patient population (Simcock & Wright, 2020) with limited ability to dynamically respond to changes in a patient's condition. Frailty screening using the Clinical Frailty Scale (CFS) has been suggested as an alternative measure (Shah et al., 2022; Simcock & Wright, 2020).
Frailty can be defined as a heightened state of vulnerability and lack of resilience to cope with both physiological and psychological stressors that often, but not exclusively, correlate with age (Xue, 2011). It is a syndrome that reflects underlying defects in multiple physiological systems and represents a continuum along a spectrum rather than a binomial concept.
It is estimated that over 43% of patients with a diagnosis of cancer are frail although prevalence varies widely (range 7% to 63%) depending on the population studied and the scales used (Handforth et al., 2015). Both cancer symptoms and the impact of cancer therapy can precipitate functional decline and the development of frailty (Kirkhus, Harneshaug, et al., 2019). Frail patients with cancer have a poorer quality of life and a steeper deterioration in function (Kirkhus, Šaltytė, et al., 2019). Frailty is associated with increased complications from surgery and chemotherapy and consequently poorer prognosis (Cohen et al., 2016; Klingenschmid et al., 2022; O'Mahony et al., 2020). The importance of screening for frailty is increasingly recognised in the oncology and haemato‐oncology inpatient population. This objective assessment could facilitate discussions on the benefits and risks of anticancer treatments, prognosis and advance care planning.
Numerous measures of frailty are in use, some of which have been validated or designed for use in patients with cancer but there remains no international standard (Ethun et al., 2017). The CGA is recommended by the International Society of Geriatric Oncology as systematic assessment of geriatric domains better identifies frail oncology patients with a poor prognosis than subjective assessment by oncologists (Kirkhus et al., 2017). However, the CGA can be time and resource intensive (Dent et al., 2016).
The Rockwood CFS is a simple score designed to screen for frailty in subjects aged 65 years and over (Rockwood et al., 2005). It can be performed by all members of the multidisciplinary team with minimal training and is easily integrated into a standard consultation (Dent et al., 2016). Originally designed as a 7‐point scale, it was updated to a 9‐point scale in 2007. A score of 1 is very fit, with 8 being very severely frail and 9 being “terminally ill or with a life expectancy of less than 6 months”. The use of category 9 may be contentious in oncology and palliative care and omission of this point and use of an 8‐point scale has been suggested in these settings (Church et al., 2020; Shah et al., 2022).
In older patients without cancer, the CFS has been shown to predict in‐hospital mortality, new nursing home placement and length of hospital stay (Basic & Shanley, 2015). A recent review has demonstrated widespread use of the CFS in geriatric medicine, with some use in cardiology, intensive care, general medicine, emergency medicine, surgery and dialysis (Church et al., 2020). However, information from other medical specialties is lacking. Whether CFS has utility in an inpatient oncology setting, including in those patients aged under 65 is not yet known.
2. AIM
This study aims to evaluate if the CFS could be used to indicate prognosis or discharge in oncology and haemato‐oncology inpatients.
3. METHODS
3.1. Design
We evaluated a large, single‐centre cohort study of adult oncology and haemato‐oncology inpatients at a regional cancer centre over an 18‐month period.
3.2. Outcomes
The associations between CFS, age, tumour type, discharge destination and care requirements and survival were evaluated. The primary outcome measure was time from frailty screening to death. Secondary outcome measures were whether the patient was discharged or died during hospital admission and for the former discharge destination and care requirements on discharge.
3.3. Inclusion criteria
Patients aged >18 years were included if they were an inpatient at the Northern Centre for Cancer Care, Newcastle upon Tyne, UK, between 15 April 2020 and 31 October 2021, had a diagnosis of either a solid organ or haematological malignancy and had involvement from occupational therapy and/or physiotherapy.
3.4. Screening for frailty and measurement of other parameters
The 8‐point CFS was used to screen for frailty (Rockwood et al., 2005) Figure S1. A CFS eLearning package was used to increase confidence and accuracy of scoring. Individual scores were recorded; however, a CFS of 4 (vulnerable) was used as the threshold for frailty; those with a CFS of 4 or below were recorded as ‘not frail’, whereas a CFS of 5 or above were recorded as ‘frail’. Screening was performed by a physiotherapist (KH) or occupational therapist (JW/RR) on days 2 to 4 of the hospital admission. Exact timing was at the discretion of the screener. Measurement aimed to reflect a true picture of background frailty level, and therefore, a variable period of time from admission to screening was allowed to enable acute medical conditions to stabilise. Age, diagnosis, treatment intent and number of medications were also recorded. The most recent treatment received (within 3 months) was recorded. Patients not on any systemic treatment and not receiving curative intent radiotherapy were defined as being on active surveillance or best supportive care. As palliative radiotherapy may be given alongside systemic therapy and best supportive care, this was recorded separately.
3.5. Discharge and follow‐up
Outcome of admission was recorded as died or discharged and discharge destination was categorised as:
Fast‐track discharge (defined as ‘immediate provision of NHS continuing healthcare due to a rapidly deteriorating condition that may be entering a terminal phase’ (Department of Health and Social Care, 2018). In most cases, this care is delivered up to four times per day in the patient's home)
Transferred to a hospice
Repatriated to a local or community hospital
Discharged home with equipment
Discharged home with equipment and additional care
Discharged home independently or with support from family
Other.
The cohort was followed until death or the end of the study (31 October 2021).
3.6. Statistics
As would be expected in an oncology inpatient setting, age was not normally distributed (Kolmogorov–Smirnov test (P < 0.0001) and CFS and ECOG PS are ordinal scales; therefore, nonparametric statistics were used throughout. Age, PS and CFS are reported as median (range) with the correlation between PS and CFS assessed with the Spearman rank test. Rates of death versus discharge to the home environment were compared with a Fisher's exact test.
Median survival and hazard ratios (HR) for frailty was estimated using Kaplan Meier curves with CFS 4 used as the reference group. Univariate cox regression models assessing the impact of gender (using female as a reference), age, ECOG PS whether the patient had a haematological or solid organ malignancy (using haematological diagnosis as a reference), number of medications and CFS on survival were performed. Age was assessed both as a continuous variable and comparing patients younger than 65 years to those 65 years and older: This threshold was chosen as this is the age group in which CFS was validated. Significant factors in univariate analysis were subsequently included in the multivariate analysis. All statistics were performed using SPSS (version 27; IBM, New York, USA) and GraphPad Prism (version 8 GraphPad, California, USA) with P < 0.05 interpreted as significant throughout.
3.7. Approvals
This study was performed as part of a service evaluation within the NHS Elect Acute Frailty Network and was approved by the Newcastle Hospitals NHS Foundation Trust Clinical Governance Board. Patients verbally consented to CFS assessment as part of their clinical evaluation by an Allied Health Professional.
4. RESULTS
Over the 18 month study period, there were 4758 admissions (including re‐admissions) to the Northern Centre for Cancer Care. A total of 676 patients fit the inclusion criteria, and 672 had CFS recorded. One patient was imminently dying at the time of admission and therefore was not screened for frailty. The three remaining patients had no reason for lack of CFS screening recorded. The age of patients ranged from 18 to 99 with a median age of 66; 299 patients were under the age of 65 years.
4.1. Details of disease, diagnosis and treatment
The most common diagnoses were lymphomas (17.3% of patients), thoracic malignancies (14.3% of patients) and urological malignancies (11.1% of patients). Rates of utilisation of systemic treatment and radiotherapy varied by tumour site of origin (Table 1). Rates of polypharmacy were high with a median of 12 prescribed medications (range 1 to 40; interquartile range [IQR] 9–16).
TABLE 1.
Demographics of patients included in study including clinical frailty scale (CFS), performance status (PS) and most recent treatment received
| Age (median and range) | CFS (median and range) | PS (median and range) | Most recent treatment a | ||||
|---|---|---|---|---|---|---|---|
| Chemotherapy | Radiotherapy | Active surveillance/best supportive care | Other b | ||||
| Haematology | |||||||
| Acute lymphoblastic leukaemia (n = 21) | 36 (22–74) | 4.5 (3–7) | 0.5 (0–2) | 75% | ‐ | 25% | ‐ |
| Acute myeloid leukaemia (n = 52) | 65 (38–88) | 5 (2–8) | 1 (0–3) | 65% | 13% | 35% | ‐ |
| Chronic leukaemia (lymphocytic and myeloid: n = 17) | 64 (24 to 84) | 4 (2–7) | 1 (0–3) | 47% | 11.7% | 30% | 23% |
| Lymphoma (n = 117) | 66 (23–89) | 6 (2–8) | 1 (0–3) | 60% | 20% | 40% | ‐ |
| Myeloma (n = 48) | 73 (44–88) | 6 (3–8) | 1 (0–2) | 40% | 33% | 40% | 20% |
| Other (nonmalignant n = 24 c ) | 56 (23–92) | 5 (2–7) | 1 (0–1) | 17% | ‐ | 83% | ‐ |
| Solid tumour | |||||||
| Breast (n = 33) | 55 (48–88) | 6 (4–8) | 1 (1–2) | 64% | 12% | 18% | 18% |
| Colorectal (n = 34) | 61 (41–85) | 6 (4–8) | 1 (0–2) | 74% | 32% | 24% | ‐ |
| Oesophagogastric (n = 22) | 72.5 (55–88) | 5 (4–7) | 1 (1–3) | 45% | 27% | 45% | ‐ |
| Head and neck (n = 24) | 64 (36–92) | 6 (3–7) | 1 (0–2) | 46% | 92% | 8% | ‐ |
| Hepatobiliary malignancies (n = 31) | 65 (51 to 86) | 6 (3–8) | 1 (0–4) | 23% | 19% | 68% | 19% |
| Gynaecological cancers (n = 24 d ) | 67 (25–82) | 6 (3–8) | 2 (1–4) | 67% | 25% | 8% | ‐ |
| Sarcoma (n = 13) | 49 (22–73) | 6 (3–7) | 1 (1–3) | 85% | 15% | 0% | ‐ |
| Skin (n = 17) | 62 (29–83) | 6 (2–7) | 2 (1–4) | 18% | 65% | 24% | 24% |
| Thoracic malignancies (n = 97) | 70 (53–89) | 6 (3–8) | 1 (0–4) | 48% | 21% | 37% | 15% |
| Urology (n = 78 e ) | 67 (51–85) | 6 (4–8) | 1 (0–4) | 31% | 52% | 14% | 65% |
| Other (n = 24 f ) | 68 (21–84) | 6 (4–8) | 1 (0–4) | 25% | 42% | 50% | 4% |
May add up to more than 100% as more than one treatment modality may be used and palliative radiotherapy may be part of best supportive care.
Includes hormonal therapies, tyrosine kinase inhibitors and checkpoint inhibitors.
Includes myelodysplatic syndrome, histiocytosis and aplastic anaemia.
Ovarian cancer = 21: cervical cancer = 3.
Prostate cancer = 60, bladder cancer 8, renal cancer 10.
Central nervous system = 11: Cancer Unknown Primary = 7: Thyroid = 2: Other = 4.
4.1.1. Overall frailty levels
Levels of frailty were high with a median CFS of 6; however, patients with solid tumours had higher frailty levels (Median 6: IQR 5–7) than those with haematological diagnoses (Median 6: IQR 4 to 6: Mann–Witney: P < 0.0001). CFS by individual tumour type are outlined in Table 1 and demonstrated in the supporting information Figure S2. About 81.8% of patients had a CFS of 5 or above and therefore were deemed to be ‘frail’. High levels of frailty were demonstrated under the age of 65 (Median 6: IQR 4 to 6) although these were lower than in patients aged 65 and over (Median 6: IQR 5–7 Mann–Witney: P < 0.0001). Frailty correlated with ECOG PS but poorly (R = 0.13: P = 0.047).
4.1.2. Outcome of admission
Patients who were frail (CFS ≥ 5) were less likely to be discharged home (62.9%) compared with those who were not classed as frail (86.1%) (OR 3.6 [95%CI 2.1 to 6.3]: P < 0.001). The breakdown of discharge destination by CFS is shown in Figure 1.
FIGURE 1.
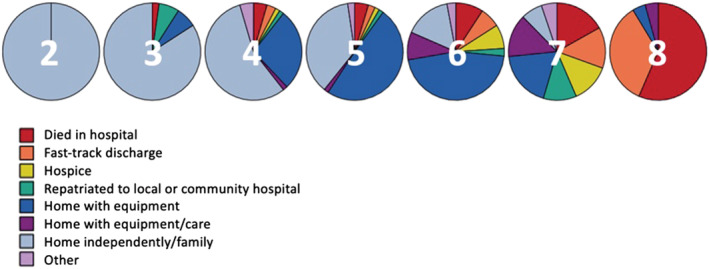
Pie chats demonstrating discharge destination by Clinical Frailty Scale (CFS) in total population
4.1.3. Association with death
A total of 317 patients had died at the time of analysis, with a median follow‐up in patients who have not died of 172 days. CFS significantly predicted time to death (as demonstrated in Figure 2a (P < 0.001) with significantly poorer survival in patients with CFS ≥ 6 compared with the reference group of patients with CFS 4 (Table 2). The higher the CFS, the higher the chance of death during the hospital admission. About 56.5% of patients with a CFS of 8 died while in hospital in comparison with only 4.5% of patients with a CFS of 4 (Fisher's exact test: P < 0.001). There was a similar association between CFS and survival when restricting to the age group of patients under 65 years old (log‐rank P < 0.001; Figure 2b).
FIGURE 2.
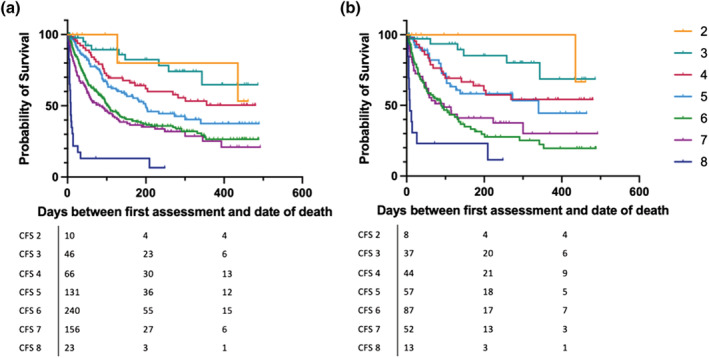
Overall survival from time of assessment by Clinical Frailty Scale (CFS) in (a) total population and (b) patients <65 years old
TABLE 2.
Survival median (days) per CFS
| CFS | Number of patients | Estimated median survival | 95% CI | Hazard ratio for death (reference group CFS 4) | P value |
|---|---|---|---|---|---|
| 2 | 10 | Not reached | ‐ | 1.04 (0.4–2.75) | 0.93 |
| 3 | 46 | Not reached | ‐ | 0.56 (0.29–1.08) | 0.09 |
| 4 | 66 | Not reached | ‐ | ‐ | ‐ |
| 5 | 131 | 200 | 139.3–260.7 | 1.42 (0.92–2.18) | 0.11 |
| 6 | 240 | 105 | 80.8–129.2 | 1.85 (1.32–2.59) | 0.0003 |
| 7 | 156 | 82 | 45.1–118.9 | 2.18 (1.51–3.15) | <0.0001 |
| 8 | 23 | 8 | 5.7–10.3 | 6.76 (2.66–17.19) | <0.0001 |
| Overall | 672 | 138 | 101.4–174.6 |
Abbreviation: CFS, Clinical Frailty Scale.
Despite the fact that having a solid organ malignancy, as opposed to a haematological malignancy, significantly predicted subsequent mortality (HR 2.9 [95%CI 2.3 to 3.7]: P < 0.001), CFS was associated with mortality in both solid organ and haematological malignancies (Figure 3a,b: log‐rank P < 0.001).
FIGURE 3.
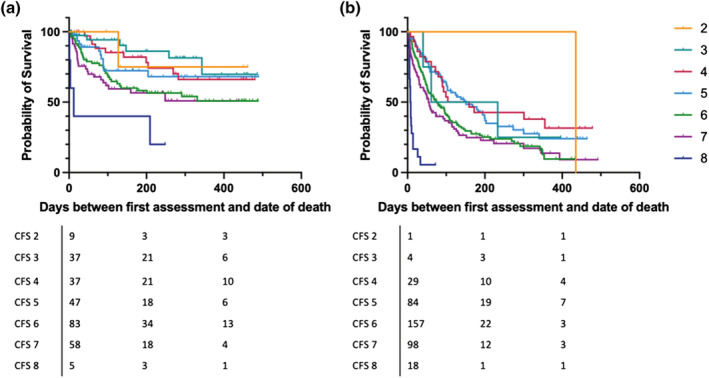
Overall survival from time of assessment by Clinical Frailty Scale (CFS) in (a) haematological malignancies and (b) solid organ malignancies
Univariable analysis and multivariable cox regression models assessing the impact of gender, age, whether the patient had a haematological or solid organ malignancy (using haematological malignancy as a reference), number of medications, CFS and performance status on survival were performed.
On univariate analysis performance status HR 1.28 (95%CI 1.05 to 1.56: P < 0.001) solid organ malignancy (HR 2.9 [95%CI 2.3 to 3.7]; P < 0.001), increasing age measured either as a continuous variable (HR 1.02 [95%CI 1.01 to 1.02]; P < 0.001) or comparing patients ≥65 with those <65 years old (HR 1.01 [95%CI 1.01 to 1.02]; P < 0.001) and CFS (HR 1.5 [95%CI 1.4 to 1.6]; P < 0.001) were all associated with poorer survival, while number of medications and gender were not (Table 3). In the multivariable analysis (using age as a continuous variable), having a solid organ malignancy and initial CFS continued to be associated with prognosis, while age and PS were no longer significant factors in the model (Table 3).
TABLE 3.
Hazard ratios from the cox regression model
| HR | 95% CI | P‐value | |
|---|---|---|---|
| Univariate analysis | |||
| Gender | 0.84 | 0.69–1.04 | 0.115 |
| Age (under 65 vs. 65 years and older) | 1.01 | 1.01–1.02 | <0.0001 |
| Age (continuous variable) | 1.02 | 1.01–1.02 | <0.0001 |
| Solid organ versus haematological malignancy | 2.9 | 2.3–3.7 | <0.0001 |
| Number of medications | 1.0 | 0.98–1.02 | 1.000 |
| CFS | 1.5 | 1.4–1.6 | <0.0001 |
| Performance status | 1.28 | 1.05–1.56 | 0.016 |
| Multivariate analysis | |||
| Age (continuous variable) | 1.00 | 1.00–1.01 | 0.21 |
| Solid organ versus haematological malignancy | 2.60 | 2.05–3.32 | <0.0001 |
| CFS | 1.43 | 1.29–1.59 | <0.0001 |
| Performance status | 1.15 | 0.95–1.35 | 0.31sss |
Note: Values in bold emphasis indicate that P < 0.05.
Abbreviations: CFS, Clinical Frailty Scale; HR, hazard ratio.
5. DISCUSSION
This study has demonstrated high levels of frailty amongst adult oncology and haemato‐oncology patients of all ages using CFS scoring. CFS may help predict prognosis in adult oncology inpatients of any age. Increasing frailty level is associated with lower levels of survival in all ages and diagnoses. Suffering from a solid organ malignancy and the presence of frailty were independently associated with poorer survival.
Frailty measured using the CFS has been found to correlate with prognosis across a range of patient populations including outpatients with hepatobiliary, central nervous system and haematological malignancies (Klingenschmid et al., 2022; Shah et al., 2022; Wall et al., 2021). However, this is the first study focussing specifically on its use in oncology and haemato‐oncology inpatients (Church et al., 2020). Unlike other oncology studies, we found a relatively weak correlation between CFS and PS, and PS did not independently predict survival (Klingenschmid et al., 2022; Shah et al., 2022). This highlights the challenge of assessing PS in the inpatient setting, where patients may have limited opportunities for mobilisation and self‐care, and rapid changes in physiology and function can occur.
Our findings are similar to those in a large cohort of older inpatients (where only 6% had cancer), and frailty status was shown to be associated with a number of poor outcomes including increased length of stay and inpatient mortality (Ellis et al., 2020; Hubbard et al., 2017). Higher CFS scores have also been shown to be associated with reduced survival in intensive care units (Muscedere et al., 2017), where, as in this study, it has shown utility in patients aged under as well as over 65 years (Bagshaw et al., 2016).
Frailty is an increasingly commonly used construct in modern healthcare. Our study supports the use of the CFS in oncology and haematology (Klingenschmid et al., 2022; Shah et al., 2022; Wall et al., 2021). Recognising the implications of frailty may help healthcare professionals discuss the risks and benefits of potential treatments with patients and their families. We recognise the need for education of healthcare professionals and the general public to enable this. Currently, there are different meanings and interpretations of the term (Lawless et al., 2020). Frailty may be viewed as a negative concept only associated with very old age, the approach of end‐of‐life or the development of dependency and incapacity (Lawless et al., 2020). We suggest that it may aid particularly difficult conversations in the oncology setting particularly where the rate of deterioration is rapid, with marked changes in patient and family roles requiring significant psychological adjustment (Fletcher et al., 2012). Frailty is a term recognised by patients and families as opposed to performance status which is often a completely alien concept.
Frailty assessment may aid early decisions regarding disposition and treatment escalation and help maximise and prioritise valuable time for patients and families. We have shown that frailty level can indicate which patients are likely to be discharged to their home environment and which might need significant care. For the latter, early referral to palliative care and consideration of hospice placement is likely appropriate.
Various measures have been validated for assessing frailty, ranging from 1‐ to 90‐point scales (Dent et al., 2016). Although the Comprehensive Geriatric Assessment is recommended by the International Society of Geriatric Oncology (Wildiers et al., 2014), it can be time‐consuming, making it difficult to fit into routine clinical encounters, and as such, opportunities to identify frailty may be missed. In routine practice, it is likely that the best tool to screen for frailty is one that is quick, easy to use and interpret, requires minimal training and can be applied by a range of healthcare professionals. This pragmatic approach is likely to maximise the number of patients with frailty being identified, help inform the best treatment decisions early and allow referral for further specialist assessment if required. The Rockwood CFS is advantageous in this regard as well as being familiar to staff across disciplines due to widespread use internationally. It has also demonstrated good inter‐rater reliability and acceptability across a range of healthcare professionals (Rockwood et al., 2005).
6. STRENGTHS
To our knowledge, this is the first large, cohort study assessing frailty and its relationships to survival and care requirements in the inpatient oncology and haemato‐oncology population. No patients were lost to follow‐up. Our data reflect patients across a range of ages and tumour types and demonstrate that Rockwood CFS is feasible in inpatient haematology and oncology wards. Physiotherapists and occupational therapists are ideal CFS screeners as their roles require the routine evaluation of functional ability.
7. LIMITATIONS
Patients were only included in the study if they presented with functional needs and were assessed by a physiotherapist or occupational therapist. The sample of patients therefore likely excluded patients who were imminently dying and those who were fully independent. This has the potential to artificially decrease and increase the frailty prevalence, respectively.
This is only a single‐centre study of hospital inpatients. Further work is required before conclusions can be applied to other settings. In particular, results should not be extrapolated to the outpatient setting. Inpatients will likely have more rapid trajectories of disease progression, a higher symptom burden and a poorer prognosis than patients reviewed in outpatients. Work to evaluate the potential utility in guiding treatment decisions in outpatients is ongoing (Gomes et al., 2020).
The decision was taken to perform screening several days into the hospital admission rather than immediately. This gave the acute medical conditions time to stabilise and therefore reflect a true picture of background frailty level. However, scores were assigned at several different time‐points during various treatment pathways, and some patients received subsequent cancer therapy that may have impacted on diagnosis and prognosis. The aforementioned training package was used to reduce inter‐rater variability; however, risk of this was not evaluated.
While PS, and number of medications, was routinely captured, levels of comorbidity such as the Charlson Comorbidity Index (Charlson et al., 1987) were not routinely measured in the inpatient service. Future work should assess whether this gives extra information over routine frailty screening.
8. CONCLUSIONS
This study demonstrates that a rapid frailty screening tool can be used in the oncology/haematology inpatient setting. The association between frailty level, prognosis and admission outcome may indicate that frailty can guide decisions as to prognosis, the potential utility of anticancer therapy or escalation of treatment to high dependency/intensive care. Occupational therapists and physiotherapists appear to be well‐placed to assess, monitor and intervene when frailty is identified. Future work as to how best to communicate about frailty with patients and family members and programmes to maintain function in patients admitted to hospital is now required.
CONFLICT OF INTEREST
All authors report no conflicts of interest.
Supporting information
Figure S1. Supporting Information
Figure S2. Clinical Frailty Scale by Tumour Type
Welford, J. , Rafferty, R. , Hunt, K. , Short, D. , Duncan, L. , Ward, A. , Rushton, C. , Todd, A. , Nair, S. , Hoather, T. , Clarke, M. , Dawes, L. , Anderson, V. , Pelham, A. , Lowe, H. , Dewhurst, F. , & Greystoke, A. (2022). The Clinical Frailty Scale can indicate prognosis and care requirements on discharge in oncology and haemato‐oncology inpatients: A cohort study. European Journal of Cancer Care, 31(6), e13752. 10.1111/ecc.13752
Funding information JW received funding from the Sir Bobby Robson Foundation and The Newcastle upon Tyne Hospitals NHS Foundation Trust cancer charity. HL received funding from the NIHR Newcastle Biomedical Research Centre.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Bagshaw, S. M. , Majumdar, S. R. , & Rolfson, D. B. (2016). A prospective multicenter cohort study of frailty in younger critically ill patients. Critical Care, 20, 175. 10.1186/s13054-016-1338-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basic, D. , & Shanley, C. (2015). Frailty in an older inpatient population: Using the clinical frailty scale to predict patient outcomes. Journal of Aging and Health, 27(4), 670–685. 10.1177/0898264314558202 [DOI] [PubMed] [Google Scholar]
- Charlson, M. E. , Pompei, P. , Ales, K. L. , & MacKenzie, C. R. (1987). A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases, 40, 373–383. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- Church, S. , Rogers, E. , Rockwood, K. , & Theou, O. (2020). A scoping review of the clinical frailty scale. BMC Geriatrics, 20(1), 1, 393–18. 10.1186/s12877-020-01801-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, H. J. , Smith, D. , Sun, C. L. , Tew, W. , Mohile, S. G. , Owusu, C. , Klepin, H. D. , Gross, C. P. , Lichtman, S. M. , Gajra, A. , Filo, J. , Katheria, V. , & Hurria, A. (2016). Cancer and aging research group. Frailty as determined by a comprehensive geriatric assessment‐derived deficit‐accumulation index in older patients with cancer who receive chemotherapy. Cancer, 122(24), 3865–3872. 10.1002/cncr.30269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent, E. , Kowal, P. , & Hoogendijk, E. O. (2016). Frailty measurement in research and clinical practice: A review. European Journal of Internal Medicine, 31, 3–10. 10.1016/j.ejim.2016.03.007 [DOI] [PubMed] [Google Scholar]
- Department of Health and Social Care . (2018). NHS continuing healthcare fast‐track pathway tool. Retrieved from https://www.gov.uk/government/publications/nhs-continuing-healthcare-fast-track-pathway-tool (Accessed: 28 January 2022).
- Ellis, H. L. , Wan, B. , Yeung, M. , Rather, A. , Mannan, I. , Bond, C. , Harvey, C. , Raja, N. , Dutey‐Magni, P. , Rockwood, K. , Davis, D. , & Searle, S. D. (2020). Complementing chronic frailty assessment at hospital admission with an electronic frailty index (FI‐Laboratory) comprising routine blood test results. CMAJ, 192(1), E3–E8. 10.1503/cmaj.190952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethun, C. G. , Bilen, M. A. , Jani, A. B. , Maithel, S. K. , Ogan, K. , & Master, V. A. (2017). Frailty and cancer: Implications for oncology surgery, medical oncology, and radiation oncology. CA: A Cancer Journal for Clinicians, 67, 362–377. 10.3322/caac.21406 [DOI] [PubMed] [Google Scholar]
- Fletcher, B. S. , Miaskowski, C. , Given, B. , & Schumacher, K. (2012). The cancer family caregiving experience: An updated and expanded conceptual model. European Journal of Oncology Nursing, 16(4), 387–398. 10.1016/j.ejon.2011.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes, F. , Lewis, A. , & Morris, R. (2020). The care of older cancer patients in the United Kingdom. Ecancer, 14, 1101. 10.3332/ecancer.2020.1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handforth, C. , Clegg, A. , Young, C. , Simpkins, S. , Seymour, M. T. , Selby, P. J. , & Young, J. (2015). The prevalence and outcomes of frailty in older cancer patients: A systematic review. Annals of Oncology, 26(6), 1091–1101. 10.1093/annonc/mdu540 [DOI] [PubMed] [Google Scholar]
- Hubbard, R. E. , Peel, N. M. , Samanta, M. , Gray, L. C. , Mitnitski, A. , & Rockwood, K. (2017). Frailty status at admission to hospital predicts multiple adverse outcomes. Age and Ageing, 46(5), 801–806. 10.1093/ageing/afx081 [DOI] [PubMed] [Google Scholar]
- Kirkhus, L. , Harneshaug, M. , Šaltytė, B. J. , Grønberg, B. H. , Rostoft, S. , Bergh, S. , Hjermstad, M. J. , Selbæk, G. , Wyller, T. B. , Kirkevold, Ø. , Borza, T. , Saltvedt, I. , & Jordhøy, M. S. (2019). Modifiable factors affecting older patients' quality of life and physical function during cancer treatment. Journal of Geriatric Oncology, 10(6), 904–912. 10.1016/j.jgo.2019.08.001 [DOI] [PubMed] [Google Scholar]
- Kirkhus, L. , Šaltytė, B. J. , Grønberg, B. H. , Hjermstad, M. J. , Rostoft, S. , Harneshaug, M. , Selbæk, G. , Wyller, T. B. , & Jordhøy, M. S. (2019). Frailty identified by geriatric assessment is associated with poor functioning, high symptom burden and increased risk of physical decline in older cancer patients: Prospective observational study. Palliative Medicine, 33(3), 312–322. 10.1177/0269216319825972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkhus, L. , Šaltytė Benth, J. , Rostoft, S. , Grønberg, B. H. , Hjermstad, M. J. , Selbæk, G. , Wyller, T. B. , Harneshaug, M. , & Jordhøy, M. S. (2017). Geriatric assessment is superior to oncologists' clinical judgement in identifying frailty. British Journal of Cancer, 117(4), 470–477. 10.1038/bjc.2017.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenschmid, J. , Krigers, A. , Pinggera, D. , Kerschbaumer, J. , Thomé, C. , & Freyschlag, C. F. (2022). The clinical frailty scale as predictor of overall survival after resection of high‐grade glioma. Journal of Neuro‐Oncology, 158(1), 15–22. 10.1007/s11060-022-04001-y Epub 2022 Apr 25. PMID: 35467234; PMCID: PMC9166827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawless, M. T. , Archibald, M. M. , Ambagtsheer, R. C. , & Kitson, A. L. (2020). Factors influencing communication about frailty in primary care: A scoping review. Patient Education and Counseling, 103(3), 436–450. 10.1016/j.pec.2019.09.014 [DOI] [PubMed] [Google Scholar]
- Muscedere, J. , Waters, B. , & Varambally, A. (2017). The impact of frailty on intensive care unit outcomes: A systematic review and meta‐analysis. Intensive Care Medicine, 43, 1105–1122. 10.1007/s00134-017-4867-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony, M. , Mohammed, K. , & Kasivisvanathan, R. (2020). Cardiopulmonary exercise testing versus frailty, measured by the clinical frailty score, in predicting morbidity in patients undergoing major abdominal Cancer surgery. World Journal of Surgery Epub ahead of print, 45, 116–125. 10.1007/s00268-020-05779-6 [DOI] [PubMed] [Google Scholar]
- Rockwood, K. , Song, X. , MacKnight, C. , Bergman, H. , Hogan, D. B. , McDowell, I. , & Mitnitski, A. (2005). A global clinical measure of fitness and frailty in elderly people. CMAJ, 173(5), 489–495. 10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah, D. , Kapacee, Z. A. , Lamarca, A. , Hubner, R. A. , Valle, J. W. , & McNamara, M. G. (2022). Use of the Rockwood clinical frailty scale in patients with advanced hepatopancreaticobiliary malignancies. Expert Review of Anticancer Therapy, 22(9), 1009–1015. 10.1080/14737140.2022.2096594 Epub 2022 Jul 6. PMID: 35768183 [DOI] [PubMed] [Google Scholar]
- Simcock, R. , & Wright, J. (2020). Beyond Performance Status. Clinical Oncology (Royal College of Radiologists), 32(9), 553–561. 10.1016/j.clon.2020.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall, S. A. , Huang, Y. , Keiter, A. , Funderburg, A. , Kloock, C. , Yuhasz, N. , Gure, T. R. , Folefac, E. , Stevens, E. , Presley, C. J. , Williams, N. O. , Krok‐Schoen, J. L. , Naughton, M. J. , & Rosko, A. E. (2021). Integration of a geriatric assessment with intervention in the Care of Older Adults with Hematologic Malignancies. Frontiers in Oncology, 11, 775050. 10.3389/fonc.2021.775050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildiers, H. , Heeren, P. , Puts, M. , Topinkova, E. , Janssen‐Heijnen, M. L. , Extermann, M. , Falandry, C. , Artz, A. , Brain, E. , Colloca, G. , Flamaing, J. , Karnakis, T. , Kenis, C. , Audisio, R. A. , Mohile, S. , Repetto, L. , Van Leeuwen, B. , Milisen, K. , & Hurria, A. (2014). International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. Journal of Clinical Oncology, 32(24), 2595–2603. 10.1200/JCO.2013.54.8347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue, Q. L. (2011). The frailty syndrome: Definition and natural history. Clinics in Geriatric Medicine, 27(1), 1–15. 10.1016/j.cger.2010.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Supporting Information
Figure S2. Clinical Frailty Scale by Tumour Type
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


