Abstract
Objective
The aim of this study was to describe possible remodeling (i.e., dilatation and elongation) of papillary capillaries induced by increased oxygen demand for the repair process following a skin wound.
Methods
Computer‐assisted video microscopy was used to examine 10 healthy volunteers before (baseline) and after (≈1 h and ≈24 h) an incision (5 mm long and 1 mm deep) on the forearm, 0–1 mm and 30 mm (control site) from the incision. We defined categories from 0 (low) to 3 (high) to grade dilatation and elongation of the nutritive papillary capillaries, as well as the visibility of the superficial vascular plexus. Approximately 10 000 capillaries from 200 films were scored.
Results
The nutritive papillary capillaries were dilated and elongated (p < 0.01) after ≈24 h; that is, elongation (score 1.9 ± 0.9) vs baseline (score 0.9 ± 0.6), p < 0.01 and dilatation (score 2.2 ± 0.7) vs baseline (score 0.3 ± 0.3), p < 0.01. Superficial plexus visibility increased (p < 0.01) after ≈1 h (score 2.0 ± 0.7) and ≈24 h (score 2.7 ± 0.3) vs baseline (score 0.8 ± 0.4).
Conclusion
The superficial vascular skin plexus showed enhanced visibility already ≈1 h after the skin trauma. Morphological remodeling in the nutritive papillary capillaries—dilatation and elongation after ≈24 h—facilitate increased O2 supply.
Keywords: computer‐assisted video microscopy, skin microcirculation, skin trauma response
Abbreviations
- CAVM
computer‐assisted video microscopy
- CF
circulatory failure
- CFV
capillary flow velocities
- FCD
functional capillary density
- ROI
region of interest
- ICC
intraclass correlation coefficient
- LDPM
laser Doppler perfusion measurements
- SaO2
arterial oxygen saturation
- SmvO2
microvascular oxygen saturation
- VEGF
vascular endothelial growth factor
1. INTRODUCTION
Remodeling of tissue is a way of maintaining homeostasis in response to physiological and pathological stimuli such as aging, disease, and injury. 1
Microscopy (electron and light) of skin biopsies has been used to visualize human microvascular anatomy, 2 and microvascular remodeling has been studied in animal models and computer simulation. 3 To capture dynamic processes, other methods are needed—for example, in vivo microscopy.
Healing of a skin trauma leads to an increase in local metabolic rate for proliferation of cells and production of extracellular matrix. Because of poor diffusion capacity of O2 in human tissues, the maximal O2 diffusion distance from a perfused capillary before chronic hypoxia develops has been estimated to be in the range of 0.04−0.14 mm. 4 Consequently, O2 supply for proliferation of cells at the epidermal basement membrane is delivered by the non‐innervated nutritive papillary skin capillaries arising from a subpapillary superficial plexus. 2 These capillaries can be studied in vivo by non‐invasive computer‐assisted video microscopy—CAVM. Analyses of frames and films can be used to quantify functional capillary densities (FCD) and capillary flow velocities (CFV), 5 , 6 , 7 , 8 while diffuse reflectance spectroscopy (DRS) can be used to measure microvascular oxygen saturation (SmvO2) and quantification of O2 extraction (arterial oxygen saturation (SaO2) minus SmvO2) in the sub‐epidermal vessels.
In a model of human skin microtrauma—presented in two previous trauma studies (T1 9 and T2 10 )—we used CAVM and DRS to quantify trauma responses in subepithelial vessels after ≈1 h and ≈24 h. After ≈1 h, SmvO2 had increased from ≈50% to ≈70%, while FCD and CFV 9 were unchanged; after ≈24 h, SmvO2 remained at ≈70%, while FCD and CFV had increased. 10 Perfusion in the deep thermoregulatory plexus (regulated by autonomous nerves), assessed with laser Doppler perfusion measurements, LDPM, showed a ≈5‐fold increase after ≈1 h and a near 10‐fold increase after ≈24 h. 9 , 10 Our interpretation is that the ≈1 h increase in the LDPM signal is compatible with activation of the axon reflex; that is, a reflex that increases perfusion in the deep skin thermoregulatory plexus which in turn increases perfusion in the superficial plexus (and SmvO2 which is included in the DRS measuring volume). At ≈1 h post trauma, the regulatory mechanisms for papillary nutritive capillary perfusion have not yet been activated. 9 , 10 The changes in FCD, CFV, and SmvO2 at ≈24 h, all imply increased O2 delivery.
The O2 diffusion capacity is also proportional to the area of the membrane. In the present study, we reanalyzed the films from the same healthy individuals as in the T2 study, 10 with the aim to quantify morphological changes which increase the capillary membrane surface (i.e., dilatation and elongation) of the nutritive papillary capillaries, ≈24 h after a skin injury. With the CAVM technique, the superficial plexus vessels are usually not clearly seen. During conditions with increased perfusion, the vessels may become easier to visualize. Therefore, we also wanted to investigate how this parameter changed following the skin trauma.
2. MATERIAL AND METHODS
We investigated the same 10 subjects as in the T2 study. 10 CAVM was used to capture information about morphology after a standardized incision (5 × 1 mm) on the forearm, at two distances (0.1 mm and 30 mm) from the incision after 30−60 min (subsequently referred to as ≈1 h) and 24 h±1 h (subsequently referred to as ≈24 h).
All participants gave informed written consent, and the study was approved by the Norwegian regional committee for medical and health research ethics (2014/1767).
2.1. Selection and preparation of subjects, induction of trauma
The inclusion criteria were healthy non‐pregnant volunteers; 18–50 years of age; Caucasian skin type; no regular use of medication, nicotine, or alcohol; no physical exercise on the day of the examination; no tea and coffee for 5 h before the examination. The exclusion criteria were blood pressure, heart rate, or arterial oxygen saturation outside reference levels, and a change in skin temperature during the experiment of more than ±1˚C.
We selected a skin area on the volar surface of the left forearm, without hair or skin damage for measurements. 9 Before baseline measures and trauma induction, subjects were prepared as in the T1 study; that is, we measured blood pressure, arterial oxygen saturation, and heart rate after an acclimatization procedure of 10 min, and continuously monitored skin temperature 10 cm from the region of interest (ROI). 9 The same preparations were done before the measurements after 24 h (±1 h).
The standardized disposable mechanical device (Surgicutt®, Technidyne Corporation) was used to create a skin trauma of 5 mm length and 1 mm depth, parallel to the elbow crease. 9
2.2. Techniques, data capture, and scoring
To capture data, we used a mobile microvascular laboratory (mLab, ODI Medical AS) containing the CAVM microscope (Mediscope, D1, Optilia) with a lens with 300 x magnification, field of view of 1.3 × 0.7 mm, image format 1600 × 1200 pixels, and a frame rate of 7 frames/s.
To measure distances from the incision, we used the incision and the ≈1 mm field of view of the microscope as references. 10
2.3. Changes in capillaries and the superficial plexus after trauma
Changes in the superficial plexus were defined as degree of increased visibility.
Changes in the nutritive papillary capillaries were defined as degrees of elongation and dilatation (Table 1).
TABLE 1.
Scoring of observed changes—definitions per film sequence
| Score | Superficial plexus visibility a | Nutritive papillary capillaries | |
|---|---|---|---|
| Dilatation | Elongation b | ||
| 0 | Not visible | No visible capillaries with increased diameter (all capillaries <10 μm) | No visible elongated capillaries in relation to baseline controls |
| 1 | One blurry red vessel or more pink background color | One or up to 10% of capillaries with increased diameter (>10 μm) | Some, but less than <10% of capillaries have more than double length in relation to baseline controls |
| 2 | One clearly visible blood vessel or 2 blurry red vessels | 11%–50% of capillaries with diameter >10 μm | 11%–50% of capillaries have more than double length |
| 3 | >2 clearly visible blood vessels or several blurry red vessels | More than 50% of capillaries with increased diameter >10 μm | >50% of capillaries are elongated |
The blood vessels counted as part of this plexus were defined as having diameters of 20–100 μm.
Elongation was defined as the change in the length of visible papillary capillary loops in relation to baseline.
2.4. Scoring
In the skin of the volar aspect of the forearm, around half of the capillaries are organized perpendicular to the skin surface, and half parallel to the skin surface. The horizontal capillaries were used to evaluate elongation. To compare the capillary lengths, 5 random baseline films were used for comparison. The resolution of the films was sufficient to visualize individual erythrocytes within capillaries. The diameter of a single erythrocyte is ≈8–9 μm, and was used as a reference to define a subjective scale from 0 (low) to 3 (high) degree of capillary dilatation, and for scoring of superficial plexus visibility (Table 1).
2.5. Data analyses
The 200 CAVM files were numbered with a computer‐generated code consisting of 12 random letters and numbers and then analyzed off‐line on an analyzing platform (cLab research, ODI). Results are presented as mean ± SD. To compare results, the two‐sided t‐test for paired data was used. The level of significance was set to 5%.
To investigate the inter‐ and intra‐observer reliability, a random selection of 20 recoded films containing an even distribution of films from baseline, ≈1 and ≈24 h post trauma and the control site was analyzed again by the first observer (observer 1), as well as by a second independent examiner (observer 2) and compared to observer 1. Test–retest reliability was determined by intraclass correlation coefficient (ICC). SPSS Version 26 (SPSS Inc.) was used for statistical analysis.
3. RESULTS
3.1. Computer‐assisted video microscopy
In total 200 film sequences with more than 10 000 individual capillaries were analyzed.
The superficial vascular skin plexus showed enhanced visibility already ≈1 h after the skin trauma, Figure 1B. After ≈24 h, the papillary nutritive capillaries were elongated and dilated, Figure 1C. Examples of these findings are shown in Figure 2.
FIGURE 1.
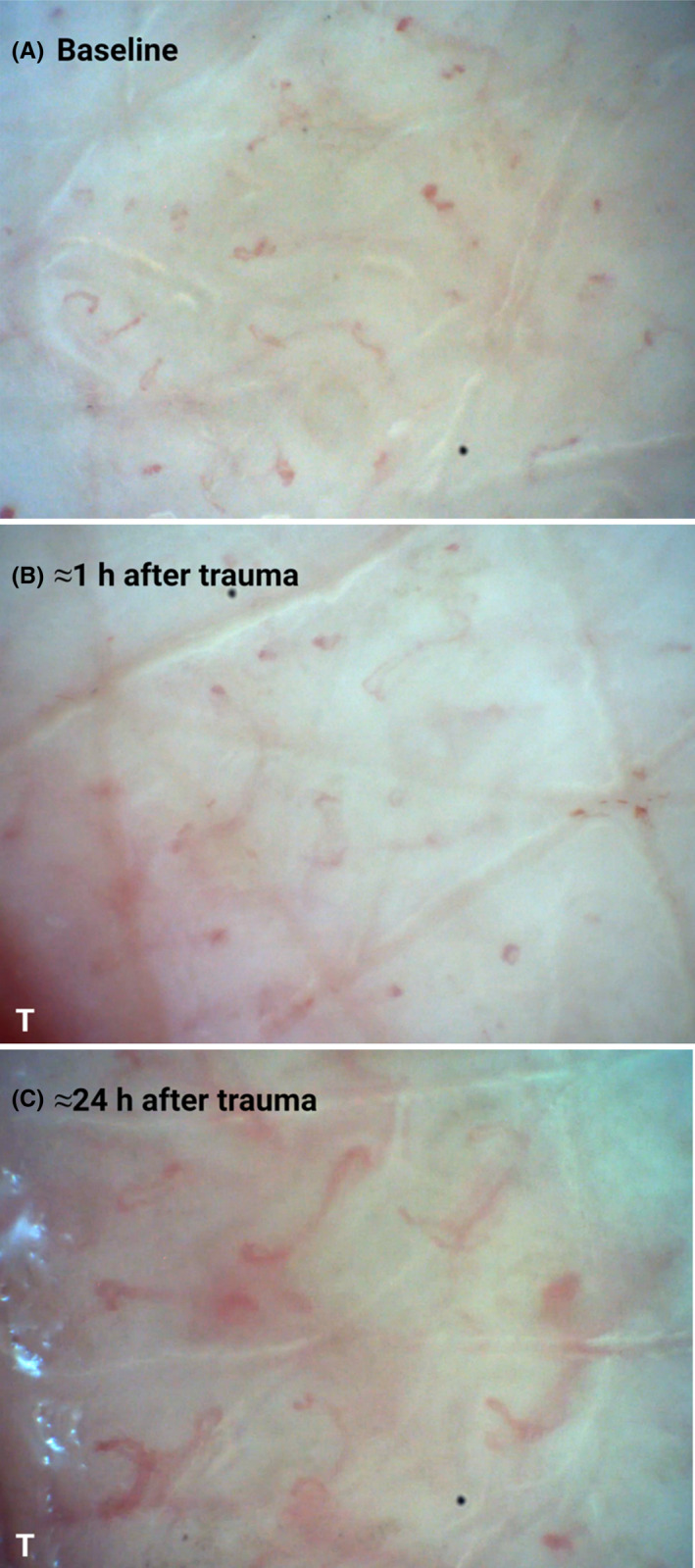
(A–C). Photographs from a CAVM film with magnification 300x, at (A) baseline, (B) ≈1 h after the trauma at 0–1 mm distance from the trauma line (T), with unchanged capillaries, but increased visibility of the superficial plexus (the pink background color), and (C) ≈24 h after the trauma (T), with dilated and elongated capillaries in addition to increased visibility of the superficial plexus. Created with BioRender.com
FIGURE 2.
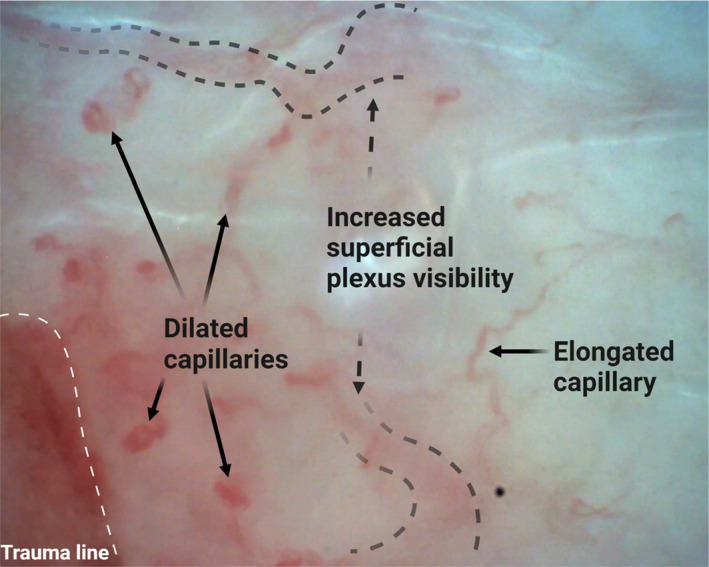
Picture from CAVM film with magnification 300× from the volar aspect of the forearm at 0–1 mm distance from the trauma line ≈24 h after the trauma. Created with BioRender.com
3.2. Papillary nutritive capillary dilatation and elongation
There were no changes in papillary nutritive capillary dilatation or elongation ≈1 h after the trauma, Table 2, Figures 3 and 4. After ≈24 h, the dilatation increased from score 0.3 ± 0.3 at baseline to score 2.2 ± 0.7 (p < 0.01), Table 2 and Figure 3. The elongation score increased from 0.9 ± 0.6 at baseline to 1.9 ± 0.9 (p < 0.01) after ≈24 h, Table 2 and Figure 4.
TABLE 2.
CAVM results before and after trauma, n = 10
| Morphological parameter | Score by time after trauma and distance from trauma line, mean ± SD | |||
|---|---|---|---|---|
| Baseline | 0−1 mm | 30 mm (control site) | ||
| 30–60 min | 24±1 h | 24±1 h | ||
| Capillary dilatation a | 0.31 ± 0.3 | 0.44 ± 0.3 | 2.2 ± 0.7* | 0.36 ± 0.3 |
| Capillary elongation a | 0.91 ± 0.6 | 0.67 ± 0.7 | 1.94 ± 0.9* | 0.68 ± 0.5 |
| Superficial plexus visibility a | 0.77 ± 0.4 | 2.0 ± 0.7* | 2.7 ± 0.3* |
0.74 ± 0.4 |
Average of five 20 s recordings per individual.
p < 0.01.
FIGURE 3.
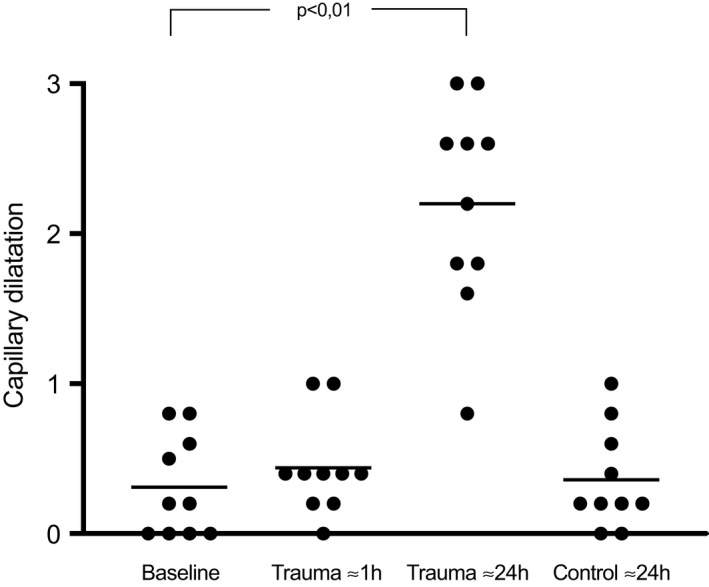
A scatter plot of study subjects showing capillary dilatation at baseline, ≈1 h, and ≈24 h after the trauma, at 0–1 mm distance as well as the control site at 30 mm from the trauma line. Each dot represents the average of 5 recordings of each study subject. The horizontal lines are the mean values
FIGURE 4.
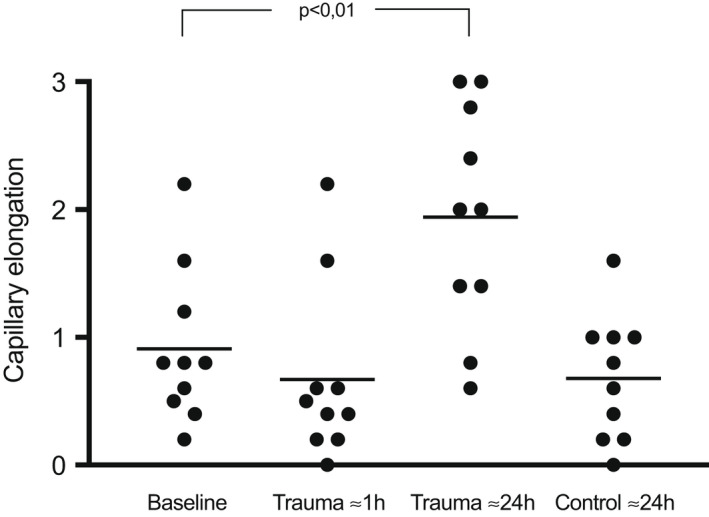
Capillary elongation at baseline, ≈1 h, and ≈24 h after the trauma, at 0–1 mm distance as well as the control site at 30 mm from the trauma line. Each dot represents the average of 5 recordings of each study subject. The horizontal lines are the mean values
3.3. Superficial plexus visibility
The superficial plexus visibility increased from score 0.8 ± 0.4 at baseline to 2.0 ± 0.7 after ≈1 h (p < 0.01), and the increase remained at ≈24 h with a score of 2.7 ± 0.3, Table 2 and Figure 5.
FIGURE 5.
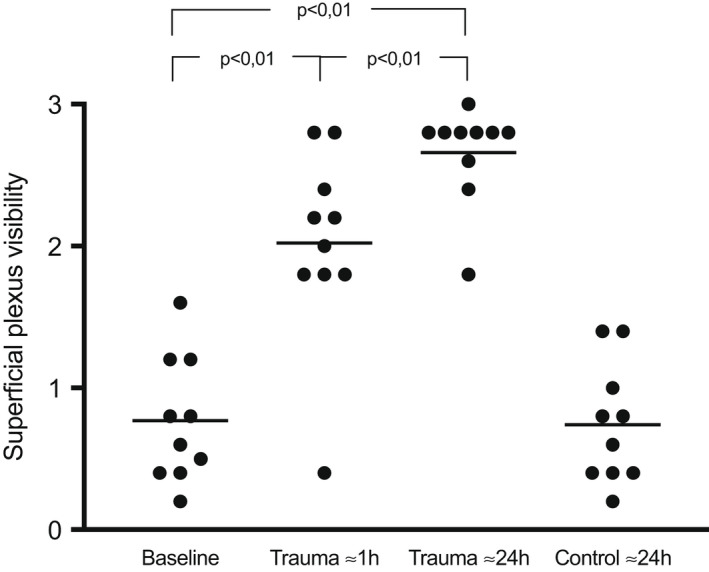
Score of the visibility of the superficial plexus at baseline, ≈1 h, and ≈24 h after the trauma, at 0–1 mm distance as well as the control site at 30 mm from the trauma line. Each dot represents a study subject and an average of 5 recordings. The horizontal lines are the mean values
3.4. Test–retest reliability
Intra‐observer reliability between the first and second analysis by observer 1 was good for elongation and superficial plexus visibility with an ICC of 0.88 and 0.86, and excellent for dilatation with an ICC of 0.96.
Inter‐observer reliability was also investigated, and the same 20 random films were analyzed by observer 2 and compared to observer 1. Both observers found similar increases in score of all three parameters in these films ≈24 h after trauma vs baseline, Table 3.
TABLE 3.
CAVM results of observer 1 and observer 2 by morphological parameter
| Observer 1 | Observer 2 | |||||
|---|---|---|---|---|---|---|
| Baseline | Trauma ≈1 h | Trauma ≈24 h | Baseline | Trauma ≈1 h | Trauma ≈24 h | |
| Capillary dilatation | 0.2 | 0.2 | 2.6 | 0.25 | 0.8 | 1.8 |
| Capillary elongation | 0.4 | 0.6 | 2.4 | 0.8 | 0.8 | 2.2 |
| Superficial plexus visibility | 0.4 | 1.6 | 2.2 | 0.2 | 1.2 | 1.8 |
Average score (0–3) from observer 1 and 2 at baseline and after trauma of each morphological parameter in 20 random of totally 200 CAVM films.
The ICC score was moderate for elongation (0.69) and dilatation (0.67), but poor for superficial plexus visibility (0.43) with the CAVM technique. The percentage where observer 2 scored within score ±1 of observer 1 was 90% of the films for the parameters capillary dilatation and elongation, and 80% for the parameter visibility of the superficial plexus.
4. DISCUSSION
In this digital microscopy study of healthy humans, we demonstrated that ≈24 h after a skin trauma, papillary nutritive capillaries are elongated and dilated, and the superficial vascular plexus vessels are more visible.
A tissue's metabolic rate and O2 need depend on several factors. In the skin, a large part of the energy consumption under physiological conditions is used for stem cells production of new epidermal cells. During unphysiological conditions (e.g., hypo‐ or hyperthermia) as well as pathological conditions (e.g., inflammation, tumor growth, or tissue repair), O2 demand may vary considerably, requiring a flexible and well‐regulated microcirculatory function.
We have previously described a technique for creating a mini trauma in skin of healthy volunteers and found changes in FCD, CFV, and SmvO2 in sub‐epidermal vessels ≈24 h after the trauma, compatible with increased O2 delivery for the healing process. 9 , 10
According to Fick's first law, a gas diffuses passively across a membrane from high to low concentration, with a magnitude that is proportional to the concentration gradient. The diffusion capacity is also proportional to the area of the membrane. Both the dilatation and elongation of nutritive papillary capillaries found in this study increase the capillary membrane surface area.
The degree of dilatation was evaluated according to the percentage of capillaries that had a diameter >10 μm (a single erythrocyte is ≈8–9 μm), and a significant increase in the number of dilated capillaries after ≈24 h was found, (Figure 3). Since the capillary surface area can be expressed as 2 x capillary length (r = radius), the capillary dilatation facilitates increased O2 delivery.
Angiogenesis, the growth of new capillaries from pre‐existing vasculature, is a highly dynamic process in wound healing, involving complex coordination of multiple cell types. This process of structural adaptation occurs on a longer time scale than 24 h. However, vascular remodeling is a rapidly emerging field of research, and there is now evidence that quick remodeling of the microcirculation can occur in hours to a few days. 11 The mechanisms behind our findings are uncertain and not the focus of this paper, but it is fascinating to show the plasticity of capillary structures to adapt to physiological needs.
In the T1 study, SmvO2 increased following the trauma, in spite of unchanged FCD and CFV in nutritive capillaries. The finding was compatible with a measuring volume of the DRS technique including the superficial vascular plexus and that perfusion in this plexus increased in parallel with increased perfusion in the deep thermoregulatory plexus caused by the axon reflex. 9 In the present study, the superficial plexus vessels visibility increased after one hour, with further increase after ≈24 h. The changes in visibility from baseline, to ≈1 h and ≈24 h paralleled the increase in laser Doppler perfusion signal found in T1 and T2 study, and the increased visibility is probably caused by increased perfusion in the superficial plexus vessels.
4.1. Perspectives
The dilatation and elongation of the nutritive papillary capillaries demonstrated in this study ≈24 h after a small skin trauma show the plasticity of the microcirculatory system and a quick remodeling process increasing the capacity for O2 supply for the repair processes. Skin digital in vivo microscopy can be used to study microvascular plasticity.
4.2. Limitations
O2 diffusion is temperature‐dependent, that is, increased at higher temperatures and reduced at lower temperatures. As we found no changes in skin temperature, we do not believe the results in this study are affected by temperature changes.
In this study, we defined criteria for scoring of morphology parameters, but the scoring is subjective. The intra‐observer reliability of capillary dilatation and elongation were good and excellent, and the inter‐observer reliability was moderate. Both observers found similar increases in these two parameters after the trauma. The most difficult parameter to assess was the degree of superficial plexus visibility, with good intra‐observer reliability and poor inter‐observer reliability, but also for this parameter the two observers found a similar increase after the trauma. The percentage where observer 2 scored within score ±1 of observer 1 was 80%–90% of the films for all parameters.
Capillary elongation was found to be near to 1 for baseline and control films, Figure 4. According to the definitions in Table 1, this value should have been near to zero. For analyses, the films were compared to five random reference films used to determine “normal” capillary length. This result indicates that the five reference films had less than average capillary length. Nevertheless, capillary length had increased significantly after ≈24 h.
4.3. Implications
Circulatory failure can be defined as the respiratory/circulatory systems’ insufficient capacity to deliver sufficient O2 to all the ≈1014 cells of our body. Transport of O2 is dependent on heart function and flow in arteries and veins, while delivery mainly takes place from the capillaries. ICU treatment is hampered by lack of coherence between patient outcomes and routine clinical assessments of the transport function, that is, blood pressures and heart function, and the lack of techniques for assessing microvascular function.
It has been debated whether assessments of skin circulation can be used as a surrogate biomarker for systemic microvascular dysfunction. Lack of understanding of function and regulation of skin perfusion, of knowledge of measuring depths of technologies used for assessments of skin perfusion, lack of consensus of clinical protocols for data acquisition and standardized parameters for expression of microvascular function, has hindered development of clinically applicable microvascular diagnostic concepts.
In the present study, as well as in the T1 and T2 studies, we used a mini‐trauma model in skin of healthy volunteers. The microvascular trauma response was examined using the ODIN concept (oxygen delivery index) including standardized technologies and clinical protocols for data acquisition (ODI Medical AS). In T1 and T2, an analyzing platform with proprietary software was used, and after ≈24 h both FCD and CFV increased in papillary capillaries. All these findings in addition to the changes in morphology showed in this paper, are in accordance with increased microvascular O2 delivery for the healing process. The studies show that the ODIN concept has sufficient sensitivity to demonstrate local increased O2 delivery as a response to increased metabolic demand.
Testing of skin ODIN examinations during reduction in systemic metabolic demands was performed in a study of asphyxiated newborns, treated with systemic hypothermia where the infant's mean core temperature was reduced to 33.5 C, causing a reduction in metabolic rate of approximately 20%–30%. 5 The results showed that microvascular downregulation of O2 delivery was achieved by reduction of capillary flow velocities (i.e., number of erythrocytes passing through each capillary per time unit), accompanied by increase in functional capillary density (O2 is reduced at lower temperatures) and increased microvascular O2 extraction (the erythrocyte transit time increased giving more time for pO2 equilibration across the capillary membrane).
In another study, increased mean value and heterogeneity of skin nutritive functional capillary densities and capillary flow velocities have been shown to be significantly changed in patients dying during treatment with extracorporeal membrane oxygenation (ECMO) for acute heart failure, as compared to healthy controls and ECMO survivors. 7
CONFLICTS OF INTEREST
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Research funding: The Medical Student Research Program, University of Oslo funded the experimental expenses at Oslo University Hospital. Østfold hospital trust is funding the PhD‐fellowship of Liv Wikslund. The funding contributors have not had any involvement in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. Kvernebo, K is founder, shareholder, and currently, CMO of ODI Medical AS. ODI Medical AS has provided the analysis algorithms and analysis platform used in this study.
AUTHOR CONTRIBUTIONS
KK developed the theoretical framework. LKW and KK conceived and designed the study, and VSA contributed to the experimental setup. LKW and VSA did the practical work, supervised by KK. MLK reanalyzed the CAVM data. LKW designed the figures and wrote the manuscript, with support from MLK and KK, and input from all the other authors.
ACKNOWLEDGMENT
Professor Leiv Sandvik has given statistical advice.
Wikslund LK, Kaljusto M‐L, Amundsen VS, Kvernebo K. Microvascular remodeling following skin injury. Microcirculation. 2022;29:e12755. doi: 10.1111/micc.12755
REFERENCES
- 1. Ambrosi DBAM, Cyron CJ, DeSimone A, Goriely A, Humphrey JD, Kuhl E. Growth and remodelling of living tissues: perspectives, challenges and opportunities. J R Soc Interface. 2019;16(157):20190233. Epub 2019 Aug 21. PMID: 31431183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Braverman IM. The cutaneous microcirculation: ultrastructure and microanatomical organization. Microcirculation. 1997;4(3):329‐340. [DOI] [PubMed] [Google Scholar]
- 3. Santamaría R, González‐Álvarez M, Delgado R, Esteban S, Arroyo AG. Remodeling of the microvasculature: may the blood flow be with you. Front Physiol. 2020;15(11). PMID: 33178049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Forster JCH‐PW, Douglass MJJ, Bezak E. A review of the development of tumor vasculature and its effects on the tumor microenvironment. Hypoxia (Auckl). 2017;5:21‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fredly S, Fugelseth D, Nygaard CS, Salerud EG, Stiris T, Kvernebo K. Noninvasive assessments of oxygen delivery from the microcirculation to skin in hypothermia‐treated asphyxiated newborn infants. Pediatr Res. 2016;79(6):902‐906. [DOI] [PubMed] [Google Scholar]
- 6. Fredly S, Fugelseth D, Wester T, Haggblad E, Kvernebo K. Skin microcirculation in healthy term newborn infants–assessment of morphology, perfusion and oxygenation. Clin Hemorheol Microcirc. 2015;59(4):309‐322. [DOI] [PubMed] [Google Scholar]
- 7. Wester T, Awan ZA, Kvernebo TS, Salerud G, Kvernebo K. Skin microvascular morphology and hemodynamics during treatment with veno‐arterial extra‐corporeal membrane oxygenation. Clin Hemorheol Microcirc. 2014;56(2):119‐131. [DOI] [PubMed] [Google Scholar]
- 8. Wester T, Haggblad E, Awan ZA, et al. Assessments of skin and tongue microcirculation reveals major changes in porcine sepsis. Clin Physiol Funct Imaging. 2011;31(2):151‐158. [DOI] [PubMed] [Google Scholar]
- 9. Sundheim LK, Sporastoyl AH, Wester T, Salerud G, Kvernebo K. Acute skin trauma induces hyperemia, but superficial papillary nutritive perfusion remains unchanged. Microcirculation. 2017;24(7)):e12389. [DOI] [PubMed] [Google Scholar]
- 10. Wikslund LK, Amundsen VS, KverneboAK S‐V, Kvernebo K. Skin trauma rapidly inducesthermoregulatory plexus hyperemia, while an increasednutritive papillary capillary function can be detected after 24 h. Microcirculation. 2022;29:e12735. doi: 10.1111/micc.12735 [DOI] [PubMed] [Google Scholar]
- 11. Ricard N, Simons M. When it is better to regress: dynamics of vascular pruning. PLoS Biol. 2015;13(5):e1002148. [DOI] [PMC free article] [PubMed] [Google Scholar]


