Abstract
By directly suppressing the function of certain immune cell subsets and by stimulating other cell populations related to immunopathology, parasite-derived substances play an important role in the chronic establishment of parasitic disease. Our objective was twofold: (i) to investigate further the role of Echinococcus granulosus antigen B (AgB) in the human early inflammatory response by determining its effect on polymorphonuclear cell (PMN) random migration, chemotaxis, and oxidative metabolism and (ii) to determine its action in acquired immunity by evaluating AgB and sheep hydatid fluid (SHF)-driven Th1 (gamma interferon [IFN-γ] and interleukin 12 [IL-12]) and Th2 (IL-4 and IL-13) cytokine production by peripheral blood mononuclear cells (PBMC) from 40 patients who had cured or stable or progressive cystic echinococcosis. AgB significantly inhibited PMN recruitment but left their random migration and oxidative metabolism unchanged. Patients' PBMC stimulated with AgB produced IL-4 and IL-13 but did not produce IL-12. They also produced significantly lower IFN-γ concentrations than did PBMC stimulated with SHF (P = 10−5). AgB skewed the Th1/Th2 cytokine ratios towards a preferentially immunopathology-associated Th2 polarization, predominantly in patients with progressive disease. AgB-stimulated patients' PBMC also proliferated less than SHF-stimulated PBMC (P = 9 × 10−3). In vitro Th2 cytokine production was reflected in vivo by elevated specific immunoglobulin E (IgE) and IgG4 antibodies binding to AgB. These findings confirm that AgB plays a role in the escape from early immunity by inhibiting PMN chemotaxis. They also add new information on the host-parasite relationship, suggesting that AgB exploits the activation of T helper cells by eliciting a nonprotective Th2 cell response.
Cystic echinococcosis (CE) is a widely endemic helminthic disease caused by infection with metacestodes (larval stage) of the tapeworm Echinococcus granulosus. It affects humans and a wide range of livestock species (28). The disease is characterized by the growth in the host internal organs, mostly liver and lungs, of steadily growing fluid-filled, unilocular cysts surrounded by a two-layered hydatid cyst wall. The main feature of the host-parasite relationship is the coexistence of the chronic infection with detectable humoral and cellular responses against the parasite. Parasite survival in vivo depends on efficient evasion mechanisms starting to operate as the parasite develops toward a hydatid cyst. A fibrotic host capsule of variable thickness usually develops, thus forming together with the parasite-derived acellular laminated layer a formidable cystic structure. As a consequence, the actively dividing germinal layer within the cyst along with its associated brood capsule and enclosed protoscoleces are effectively sequestered from the host immune responses. In addition to this physical barrier, the hydatid cyst has probably evolved other strategies for immune evasion. Although older models suggested a more passive role for parasites in immune evasion—for example sequestration, antigenic masking by host proteins, and global immunosuppression—later studies suggest in human parasitoses the active deployment of strategies that manipulate and exploit the host response (12, 30).
The search for E. granulosus defense molecules has highlighted the importance of both the host-exposed structure and inner components of the cyst, such as the protoscoleces or hydatid fluid, in immune evasion by the parasite (14, 15). Hydatid fluid is a complex mixture of distinct antigens of host and parasite origin. The two most abundant antigens are E. granulosus antigen 5 and antigen B (AgB), whose concentration ratio is about 1:10 (25). Antigen B (AgB), which accounts for as much as 10% of the total content of hydatid fluid, is a 160-kDa thermostable lipoprotein that produces three subunits at 8 or 12, 16, and 20 or 24 kDa in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing and nonreducing conditions (21, 36). Immunohistological studies have shown that this antigen is located in the protoscolex tegument and germinal membrane of the metacestode and therefore secreted into the cyst fluid (29, 35). Observing that the 12-kDa subunit of AgB is a protease inhibitor with the ability to inhibit neutrophil recruitment Shepherd et al. first suggested the role of this antigen in escape mechanisms from the early natural immunity (37). In studying acquired immunity to E. granulosus we have previously reported that crude sheep hydatid fluid (SHF) elicits both Th1 and Th2 cell activation: the Th2 response benefits the parasite, whereas the Th1 response benefits the host (31, 32, 33, 34). Thus, the characterization of parasite-derived immunoregulatory molecules associated with Th1 or Th2 polarization is an important prerequisite for identifying the basis of resistance or susceptibility. Although AgB induces a cellular response in patients with CE the precise type of T-cell activation remains unclear (20, 26). In the immune response to infections, cytokines, produced by Th lymphocytes, have a role in regulating antibody isotype production, and in particular, Th2 cytokines regulate synthesis of immunoglobulin E (IgE) and IgG4 (19). Several lines of evidence indicate that sera from patients with CE contain all antibody isotypes specific for AgB (11). The predominant IgG4 binding to AgB suggests a link between AgB and Th2 cell activation (20, 41).
Our aim in this study was to provide further immunological evidence on the involvement of E. granulosus AgB in evasion strategies enacted by the parasite to permit the establishment of chronic CE. To extend current knowledge on the ability of AgB to interfere with the early inflammatory response, we evaluated the effects of AgB and SHF on random motility, chemotaxis, and oxidative metabolism of polymorphonuclear cells (PMN) from uninfected controls. To investigate the potential role of AgB in acquired immunity we assessed in vitro parasite antigen-driven Th1 (gamma interferon [IFN-γ] and interleukin 12 [IL-12]) and Th2 (IL-4 and IL-13) cytokine production by peripheral blood mononuclear cells (PBMC) from patients with CE who had cured or stable, or progressive disease and from uninfected controls. Finally, we wanted to relate cytokine production in vitro to specific IgE and IgG subclass production in vivo.
MATERIALS AND METHODS
Blood samples.
Blood samples were obtained from 40 patients with CE (32 had cysts in the liver, 2 had cysts in the lung, 2 had cysts in the pancreas, 1 had cysts in the pelvis, and 3 had cysts in multiple sites) and from 15 sex- and age-matched uninfected controls. CE was diagnosed on findings from imaging techniques (ultrasonographic scanning or nuclear magnetic resonance or both), serological assays, and surgery or ex adiuvantibus after medical treatment. None of the subjects studied had a history of atopic manifestations. All patients had normal total Ig counts (IgG, 800 to 1700 mg/dl; IgA, 89 to 450 mg/dl; IgM, 60 to 250 mg/dl). Thirteen patients had received a 3-month cycle of albendazole (10 to 12 mg/kg of body weight per day) more than 12 months before the study. Hydatid cysts were classified into seven types according to Caremani et al. (8): type I (simple cysts), type II (multiple cysts), type III (cysts with detachment of the wall), type IV (cysts with a mixed pattern), type V (heterogeneous cysts), type VI (hyperechoic cysts), and type VII (calcified cysts). Patients were divided into two groups according to the outcome of the disease determined by objective criteria mainly based on imaging methods: (i) patients with cured or stable disease, who had a stationary or regressive course of disease irrespective of surgical intervention or chemotherapy and (ii) patients with progressive disease, who had progression or relapse (Table 1). All procedures were approved by the local Ethical Committee, and all subjects gave their informed consent to the study.
TABLE 1.
Clinical features of the 40 patients with CE
| Patient group | Patient no. | Sexa | Age (yr) | Previous surgery | Cyst site | Cyst typeb | Pharmacological treatment |
|---|---|---|---|---|---|---|---|
| Cured or stable disease | 1 | M | 60 | No | Liver | VI-VII | No |
| 2 | F | 62 | No | Liver | V | Yes | |
| 3 | F | 45 | No | Liver | IV-V | No | |
| 4 | M | 60 | No | Multiple | VI | Yes | |
| 5 | F | 48 | No | Liver | VI-VII | No | |
| 6 | M | 45 | No | Liver | IV | No | |
| 7 | F | 48 | No | Multiple | IV | Yes | |
| 8 | M | 60 | No | Liver | VI-VII | No | |
| 9 | F | 40 | No | Liver | VII | No | |
| 10 | M | 55 | No | Liver | V-VI | No | |
| 11 | F | 68 | No | Liver | VI | Yes | |
| 12 | F | 65 | No | Liver | VII | No | |
| 13 | F | 60 | No | Pelvis | VII | No | |
| 14 | M | 55 | No | Liver | V-VI | No | |
| 15 | F | 60 | No | Liver | VI-VII | No | |
| 16 | M | 62 | No | Liver | VI-VII | No | |
| 17 | F | 45 | No | Liver | V | Yes | |
| 18 | F | 48 | No | Liver | V | No | |
| 19 | F | 68 | No | Liver | V | Yes | |
| 20 | F | 51 | No | Liver | VI-VII | No | |
| 21 | M | 50 | No | Liver | VI | Yes | |
| Progressive disease | 1 | M | 60 | Yes | Liver | I | Yes |
| 2 | F | 50 | No | Liver | II | Yes | |
| 3 | M | 26 | No | Liver | I | Yes | |
| 4 | M | 35 | No | Multiple | I | Yes | |
| 5 | M | 55 | No | Liver | I | No | |
| 6 | M | 60 | No | Liver | I | No | |
| 7 | M | 40 | No | Liver | II | No | |
| 8 | F | 60 | No | Liver | II | No | |
| 9 | F | 65 | No | Pancreas | I | No | |
| 10 | F | 55 | No | Liver | II | No | |
| 11 | F | 76 | Yes | Liver | II-VI-VII | No | |
| 12 | F | 45 | No | Liver | VI-VII | No | |
| 13 | F | 48 | No | Liver | II | No | |
| 14 | F | 50 | No | Pancreas | I | No | |
| 15 | M | 35 | No | Lung | I | No | |
| 16 | F | 46 | No | Liver | VI | No | |
| 17 | F | 60 | No | Liver | II | Yes | |
| 18 | M | 61 | No | Liver | II | Yes | |
| 19 | M | 49 | No | Liver | I-II | No |
M, male; F, female.
According to the sonographic classification of Caremani et al. (8).
Antigens.
SHF was collected in the Latium region of Italy from fertile cysts for subsequent use as specific parasite antigen. SHF was prepared by the method of Bombardieri et al. (4). In brief, SHF was clarified by centrifugation at 10,000 × g at 4°C for 60 min, dialyzed against phosphate-buffered saline (PBS), pH 7.2, 10-fold concentrated with a collodion bag ultrafiltration apparatus (Sartorius GmbH, Göttingen, Germany), and lyophilized until use; a purified AgB preparation was obtained by electroelution as described by Ioppolo et al. (20). In brief, a partially purified SHF was separated in a nonreducing SDS–12% PAGE gel, the part of the gel below the 21.5-kDa marker was removed, and protein was eluted from polyacrylamide strips with a model 422 Electro-Eluter module (Bio-Rad, Richmond, Calif.) at 10 mA/tube for 3 h at 4°C as recommended by the manufacturer. SDS was removed through a Sephadex G-10 column. All the antigenic preparations were filtered through a 0.45-μm-pore-size membrane filter (Millex-HA; Millipore S.A., Molsheim, France) for subsequent use in cellular cultures. The total protein content was determined by the Bio-Rad protein assay as indicated by the manufacturer. The electroeluted AgB showed three bands at 8, 16, and 20 kDa upon SDS–12% PAGE under reducing and nonreducing conditions.
PMN separation.
PBMC were separated from plasma by Lymphoprep (Nyegaard & Co., Oslo, Norway) density gradient centrifugation by the method of Boyum (5) and PMN were obtained as previously described (9). The cell viability, checked by trypan blue exclusion, was always greater than 99%. The cell suspension consisted of 97 to 98% neutrophils and only 2 to 3% eosinophils. To avoid up-regulation of the nonspecific surface receptors, all the isolation steps were performed at 4°C (18).
Chemotactic assay.
PMN from each of three uninfected controls were divided into three aliquots and diluted in PBS Dulbecco solution to a final cell concentration of 106/ml: two aliquots were cultured with AgB at a concentration of 1 or 2 μg/ml, and the third aliquot was cultured without antigen (blank control), and all were tested in triplicate. Chemotaxis and random migration were assayed by the modified Boyden method, as described elsewhere (1). In brief, PMN random migration and chemotaxis were assessed in Perspex chemotactic chambers (B.M. Strumentazione Biomedica, Milan, Italy); mixed-ester filters (diameter, 13 mm; pore size, 3 μm; Millipore Corporation, Bedford, Mass.) were used between the two compartments. A supernatant of Escherichia coli culture (10%) was used as the attractant to evaluate chemotaxis, and Dulbecco solution was used in the lower compartment to evaluate random migration. After incubation of the chambers for 60 min in a humid atmosphere with 5% CO2 at 37°C, the filters were removed, fixed in 95% ethyl alcohol, stained with Harris hematoxylin, treated with 0.05% HCl and bluing agent, dehydrated in 95% ethyl alcohol and absolute isopropyl alcohol, cleared in xylol, and mounted on slides. Chemotaxis and random migration were evaluated in a single session by the staff involved in the image analysis workstation, who were blinded to the antigens used.
Computer-assisted image analysis.
The method of computer-assisted image analysis, already described elsewhere (1), provides a completely new approach for evaluating cell migration through micropore filters. The software used immediately, within a few seconds, gives the following information: (i) the percentage values for each migration plane; (ii) the logarithms of the measuring sequences and the square value of depth; (iii) the linear regression between the two variables; (iv) the constant term, the gradient, and the correlation index (adjusted R2) of the line interpolating the values; (v) the intersection point with depth axis (corresponding to a decrease of two logarithmic units in the count axis) and the square root of this value (this point, expressed in micrometers, is the true “final plane” value [FP] reached by the cells); and (vi) the interpolating curve that describes the kinetics of cell propagation through the filter (2, 3).
Superoxide anion production by PMN.
Superoxide dismutase-inhibitable ferricytochrome c reduction was studied using the method previously described (6). Cells were preincubated for 5 min at 37°C with three different concentrations of AgB (0.5, 1, and 2 μg/ml) or with bovine serum albumin (BSA) in PBS, pH 7.2, at the same concentrations as AgB (controls). The stimulating agent was 1 μg of f-formyl methionyl leucyl phenylalanine (f-MLP) or phorbol 12-myristate 13-acetate (PMA) per ml.
Luminol-enhanced PMN chemiluminescence.
The effects of AgB on f-MLP- and PMA-stimulated PMN chemiluminescence (CL) were evaluated using luminol as a CL amplifier, according to the method previously described (10). PMN (105/vial) were incubated in PBS with different concentrations of AgB (0.5, 1, and 2 μg/ml) for 2 min. PBS-BSA was added to control vials. Luminol (10 μl) was added to obtain a final concentration of 1 μM in a volume of 1 ml. After incubation, PMN were stimulated with either f-MLP (final concentration, 1 μM) or PMA (final concentration, 1 μg/ml). Stimulation lasted 15 min with f-MLP and 30 min with PMA. Data were expressed as counts per minute and plotted on a system of coordinates: x axis (time) and y axis (counts per minute). Results were expressed as integral over the total measuring time (counts × 106/105 PMN/measuring time). Variations of more than 25% from counts in cells incubated with buffer alone were considered significant for stimulation, and those of less than 25% were considered significant for inhibition. The cutoff was established in accordance with results obtained with 30 different uninfected controls. For superoxide anion production tests the cutoff for inhibition was calculated as the mean − 2 standard deviations (SD) of the CL values obtained with cells from 30 different donors.
Cytokine assays.
To test cytokine production, PBMC were cultured as described by Riganò et al. (31). In brief, cells were cultured in RPMI medium supplemented with 5% human AB serum, antibiotics (1% penicillin-streptomycin), and 1% glutamine at 5 × 106 cells per ml. Cells were stimulated with SHF (100 μg/ml) or with AgB (2 μg/ml). Unstimulated PBMC from patients with CE and PBMC from uninfected controls were also tested as controls. According to preliminary kinetics studies of cytokine production, culture supernatants were harvested 120 h later. IFN-γ, IL-12, IL-4, and IL-13 production was quantified with commercially available kits for enzyme-linked immunosorbent assay (ELISA) (Quantikine human IFN-γ, QuantiGlo human IL-12, Quantikine HS human IL-4, and Quantikine human IL-13; R&D Systems, Minneapolis, Minn.) as recommended by the manufacturer. The ranges of detection by ELISA kits were 15.6 to 1,000 pg/ml for IFN-γ, 0.7 to 7,000 pg/ml for IL-12, 0.25 to 16 pg/ml for IL-4, and 62.5 to 4,000 pg/ml for IL-13. The QuantiGlo IL-12 immunoassay is a solid-phase CL ELISA, while the immunoassays for the other cytokines are colorimetric ELISA.
Proliferation assay.
Proliferation was assayed by the established procedure (38). In brief, triplicate cultures of PBMC were prepared in flat-bottomed microwell culture plates (Falcon 3040; Becton Dickinson, San Jose, Calif.) at 105 cells/well, by the addition of 180 μl of cell suspension and 20 μl of sterile antigen preparation (corresponding to 100 μg of SHF per ml or 2 μg of AgB per ml). In all experiments, cultures with phytohemagglutinin (2 μg/ml) and cultures without antigen were also set up as positive and negative controls. After 8 days of culture at 37°C in a humidified atmosphere containing 5% CO2 in air, the proliferative response was assessed by the addition of 20 μl containing 0.5 μCi of 3H-methyl-thymidine (specific activity, 1 mCi/mmol; Amersham Life Science, Little Chalfont Buckinghamshire, United Kingdom) to each well. After a further 16 h at 37°C, cells were harvested on glass fiber filter paper (Wallac EG&G Company, Turku, Finland), using an automatic cell harvester (Harvester 96, MACH III M; TOMTEC). The uptake of 3H-methyl-thymidine into the DNA of cells was evaluated by reading samples in a β counter (1450 Microbeta Plus; Wallac EG&G Company). Net counts per minute of triplicate cultures were measured, and the proliferative response was expressed as the stimulation index (ratio between counts per minute in stimulated cultures and counts per minute in unstimulated cultures). The mean of stimulation indices (SIs) in uninfected controls + 2 SD was taken as the threshold level for a positive proliferative response.
Serological assays.
Patients' sera were tested by ELISA for IgG isotypes and IgE as described by Riganò et al. (32). Optical densities (OD) at 490 nm were considered positive when higher than 0.3 for IgE, 0.06 for IgG1, 0.007 for IgG2, 0.01 for IgG3, and 0.003 for IgG4 when the antigen was SHF and higher than 0.3 for IgE, 0.03 for IgG1, 0.003 for IgG2, 0.02 for IgG3, and 0.004 for IgG4 when the antigen was AgB (mean + 2 SD of absorbance readings in uninfected controls).
Statistical analysis.
Results of the cytokine ELISA were expressed as geometric means and ranges; Wilcoxon and Mann-Whitney sum-rank tests were used to compare differences between means and between Th1 and Th2 cytokine ratios. Results of Ig ELISA were expressed as arithmetic means and ranges; Student's t test was used to compare differences between arithmetic means. Spearman's rank correlation test was used to evaluate all the correlations. Differences with a confidence interval of 95% or higher were considered statistically significant (P ≤ 0.05).
RESULTS
Effect of AgB on neutrophil random migration.
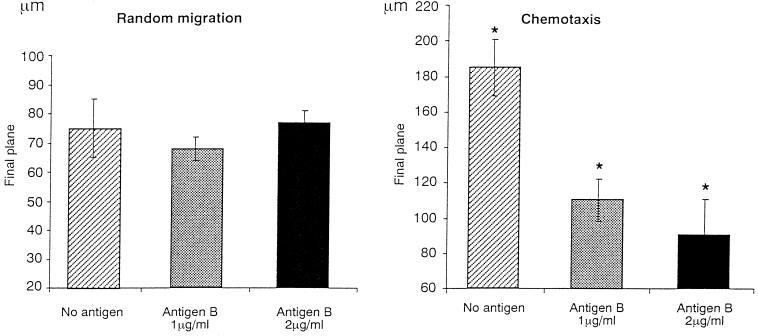
AgB left PMN random migration statistically unchanged, yielding an FP similar to that in control PMN (1 μg/ml, 68 ± 4; 2 μg/ml, 77 ± 5; and controls, 75 ± 10) (Fig. 1). Conversely, AgB strongly inhibited PMN chemotaxis, so that FPs differed significantly in AgB-stimulated and unstimulated PMN (1 μg/ml, 110 ± 12; 2 μg/ml, 91 ± 2; and controls, 185 ± 16; P = 3 × 10−3).
FIG. 1.
Effect of AgB on PMN random migration and chemotaxis. Data presented here are the means of triplicate experiments in three uninfected controls. Asterisks indicate statistically significant difference versus control (P < 0.05 by Student's t test). Error bars, SD.
Effect of AgB on PMN superoxide anion production and CL.
None of the AgB concentrations tested affected PMN superoxide anion production or CL in response to f-MLP or PMA, although AgB slightly yet not significantly reduced the CL response to f-MLP (data not shown).
Induction of Th1 and Th2 cytokine profiles by parasite antigens.
In unstimulated cultures, PBMC from patients and uninfected controls produced low amounts of IL-4, IFN-γ, and IL-13. Controls' PBMC produced significantly higher amounts of IL-12 than patients' PBMC (P = 10−2) (Table 2). PBMC from 95% of patients spontaneously produced IL-4 whereas only 10% produced IL-13. AgB increased IL-4 and IL-13 production exclusively in patients with CE, and mean IL-4 and IL-13 concentrations differed significantly in patients and uninfected controls (IL-4, P = 6 × 10−3; IL-13, P < 10−4). AgB induced low amounts of IFN-γ in both patients and controls (8.0 and 4.5 pg/ml, respectively). AgB failed to induce IL-12 production in either patients or controls, and concentrations of this cytokine were significantly lower in patients than in controls (P = 4 × 10−3). In patients, SHF stimulated production of all cytokines tested, and in uninfected controls it stimulated production of IFN-γ alone. Again, the mean IL-4 and IL-13 concentrations were significantly higher in patients than in controls (IL-4, P = 8 × 10−3; IL-13, P < 10−4). In patients with CE, SHF induced significantly higher concentrations of IFN-γ and IL-13 than did AgB (IFN-γ, P < 10−4; IL-13, P = 10−2).
TABLE 2.
Cytokine production in PBMC cultures from CE patients and uninfected controls
| Stimulus | Cytokine | Patients (n = 40)
|
Uninfected controls (n = 12)
|
P (patients vs controls) | ||
|---|---|---|---|---|---|---|
| No. testing positive (%) | Mean (range) concn (pg/ml)a | No. testing positive (%) | Mean (range) concn (pg/ml)a | |||
| None | IL-4 | 38 (95) | 0.3 (0–0.9) | 9 (75) | 0.2 (0–1.1) | |
| IL-13 | 4 (10) | 0.01 (0–164) | 5 (42) | 0.1 (0–124) | ||
| IFN-γ | 26 (65) | 0.2 (0–58) | 4 (33) | 0.3 (0–62) | ||
| IL-12 | 19 (48) | 0.01 (0–4.5) | 11 (92) | 0.6 (0.9–3.5) | 10−2 | |
| AgB | IL-4 | 40 (100) | 1.1 (0.3–4.9) | 11 (92) | 0.5 (0–1.12) | 6 × 10−3 |
| IL-13 | 35 (88) | 43.4* (0–2,415) | 5 (42) | 0.1 (0–136) | <10−4 | |
| IFN-γ | 35 (88) | 8.0† (0–523) | 11 (92) | 4.5 (0–94) | ||
| IL-12 | 20 (50) | 0.01 (0–3.8) | 11 (92) | 0.3 (0–3.0) | 4 × 10−3 | |
| SHF | IL-4 | 40 (100) | 1.2 (0.3–5.0) | 10 (83) | 0.4 (0–1.7) | 8 × 10−3 |
| IL-13 | 38 (95) | 182* (0–2,313) | 6 (50) | 0.2 (0–208) | <10−4 | |
| IFN-γ | 40 (100) | 231† (36–1,162) | 12 (100) | 194 (32–1,185) | ||
| IL-12 | 26 (65) | 0.1 (0–2.5) | 12 (100) | 0.5 (0.8–5.5) | ||
Statistically significant differences: *, P = 10−2; †, P < 10−4.
Relation between cytokine production and outcome of disease.
In unstimulated PBMC cultures cytokine production was similar in patients regardless of the outcome of the disease (Table 3). In response to AgB and to SHF, PBMC from CE patients with cured or stable disease expressed higher concentrations of all cytokines than PBMC from patients with progressive disease; the difference reached statistical significance for IL-12 in response to AgB (P = 3 × 10−2).
TABLE 3.
Cytokine production in PBMC cultures from CE patients divided into two groups according to clinical outcome of disease
| Stimulus | Cytokine | Patients with:
|
|||
|---|---|---|---|---|---|
| Cured or stable disease (n = 21)
|
Progressive disease (n = 19)
|
||||
| No. testing positive (%) | Mean (range) concn (pg/ml)a | No. testing positive (%) | Mean (range) concn (pg/ml)a | ||
| None | IL-4 | 21 (100) | 0.4 (0.02–0.9) | 17 (89) | 0.2 (0–0.8) |
| IL-13 | 2 (5) | 0.003 (0–27) | 2 (14) | 0.003 (0–164) | |
| IFN-γ | 15 (71) | 0.4 (0–58) | 11 (58) | 0.1 (0–31) | |
| IL-12 | 10 (48) | 0.02 (0–4.5) | 9 (47) | 0.01 (0–0.9) | |
| AgB | IL-4 | 21 (100) | 1.1 (0.35–4.9) | 19 (100) | 0.9 (0.2–3.1) |
| IL-13 | 18 (86) | 59.1 (0–2,415) | 17 (89) | 33.0 (0–1,144) | |
| IFN-γ | 17 (81) | 21.2 (0–285) | 18 (95) | 3.0 (0–523) | |
| IL-12 | 12 (57) | 0.048* (0–3.8) | 8 (42) | 0.004* (0–0.5) | |
| SHF | IL-4 | 21 (100) | 1.3 (0.4–5.0) | 19 (100) | 1.2 (0.2–3.9) |
| IL-13 | 20 (95) | 185.3 (0–2,313) | 18 (95) | 177.2 (0–2,193) | |
| IFN-γ | 21 (100) | 266.3 (9–1,000) | 19 (100) | 200.0 (32–1,162) | |
| IL-12 | 15 (71) | 0.1 (0–2.5) | 11 (58) | 0.03 (0–2.5) | |
Statistically significant difference: *, P = 3 × 10−2.
Th1/Th2 cytokine ratios.
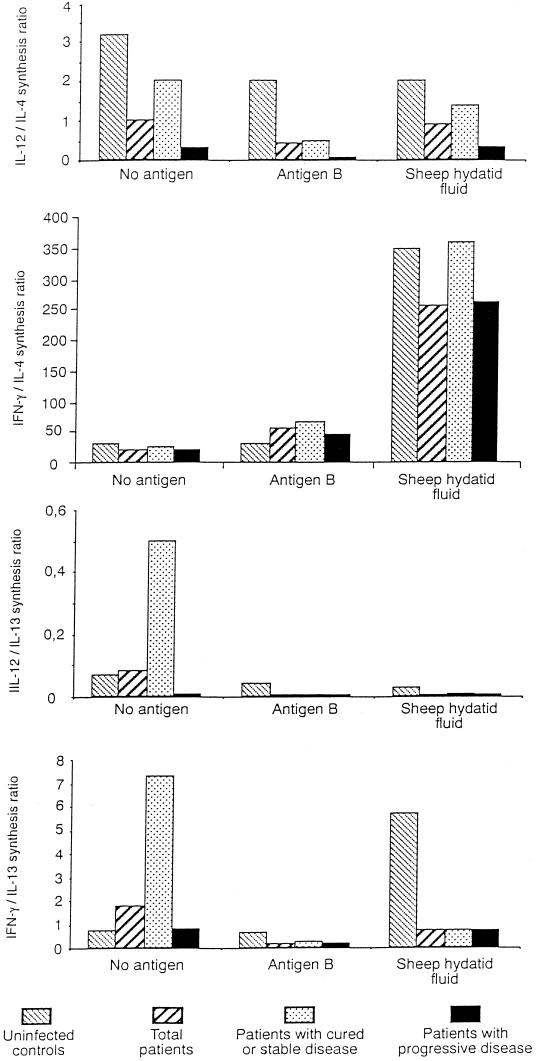
In unstimulated PBMC, the relationship between IL-12 and IL-4 concentrations in uninfected controls and patients with CE divided according to outcome of the disease showed a shift towards Th2 polarization in patients, especially in those with progressive disease (Fig. 2). In AgB-stimulated patients' PBMC, this polarization increased. The relationship between IFN-γ and IL-4 showed that SHF shifted the balance towards Th1 polarization, particularly in patients with cured or stable disease and uninfected controls. AgB only slightly increased the IFN-γ/IL-4 ratio in patients by inducing, as well as IL-4, small amounts of IFN-γ. The low IL-12/IL-13 and IFN-γ/IL-13 ratios further confirmed the preferential AgB stimulation of Th2 cells, especially in patients with progressive disease.
FIG. 2.
Ratio of unstimulated (no antigen), SHF-, AgB-stimulated Th1 to Th2 cytokine production in 40 patients with CE divided into two groups according to the outcome of the disease and in 12 uninfected controls.
Parasite antigen-driven T-cell proliferation.
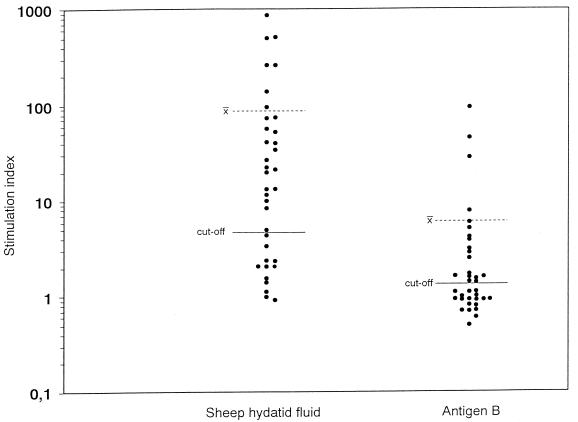
AgB induced a 13-fold significantly lower mean value of proliferation than SHF (SI, 6.3 ± 17 versus 87.5 ± 180; P = 9 × 10−3) (Fig. 3). A positive correlation was found between PBMC stimulation indices and the IFN-γ/IL-4 ratio in response to AgB (r = 0.8; P = 10−5) and SHF (r = 0.5; P = 9 × 10−3).
FIG. 3.
Proliferation of PBMC from patients with cystic echinococcosis in response to E. granulosus SHF and AgB. x̄, mean SIs in subjects tested.
Humoral response to SHF and AgB.
ELISAs determining the distribution of IgE and IgG subclass expression showed similar percentages of antibody responses to SHF and AgB in patients with CE and the highest number of positive responses for IgG1, IgE, and IgG4 (Table 4). Even though AgB and SHF yielded similar percentages of positive responses for all antibody isotypes, AgB elicited lower mean ELISA ODs for all subclasses except IgG2. Mean ODs differed significantly for IgG1 in total patients (P = 10−3) and in patients with cured or stable disease (P = 9 × 10−4) and differed for IgE in total patients and in the two groups of patients (P = 4 × 10−3; P = 4 × 10−2). In both antigen ELISAs, patients with progressive disease had higher mean OD values for IgE, whereas those with cured or stable disease had higher OD values for IgG4.
TABLE 4.
Ig isotype expression in 40 patients with CE
| Ag | Total patients
|
Patients with:
|
|||||
|---|---|---|---|---|---|---|---|
| Cured or stable disease
|
Progressive disease
|
||||||
| Ig isotype | No. (%) testing positive | Mean OD (range)a | No. (%) testing positive | Mean OD (range)a | No. (%) testing positive | Mean OD (range)a | |
| AgB | IgG1 | 38 (95) | 0.4* (0.0–2.6) | 21 (100) | 0.3† (0.04–1.3) | 17 (88) | 0.5 (0.0–2.6) |
| IgG2 | 25 (63) | 0.06 (0.0–0.7) | 13 (63) | 0.09 (0.0–0.7) | 12 (63) | 0.02 (0.0–0.2) | |
| IgG3 | 21 (53) | 0.03 (0.0–0.3) | 12 (58) | 0.03 (0.0–0.3) | 9 (50) | 0.02 (0.0–0.08) | |
| IgG4 | 33 (83) | 0.14 (0–1.5) | 18 (84) | 0.2 (0.0–1.5) | 15 (81) | 0.05 (0.0–0.5) | |
| IgE | 38 (95) | 0.3̂ (0.01–0.9) | 20 (95) | 0.2** (0.01–0.8) | 18 (94) | 0.4†† (0.04–0.95) | |
| SHF | IgG1 | 40 (100) | 0.9* (0.02–2.4) | 21 (100) | 0.9† (0.1–2.4) | 19 (100) | 0.9 (0.02–2.3) |
| IgG2 | 21 (53) | 0.06 (0–0.6) | 13 (63) | 0.07 (0.0–0.6) | 8 (44) | 0.04 (0.0–0.3) | |
| IgG3 | 23 (57) | 0.05 (0.0–0.5) | 11 (53) | 0.07 (0.002–0.5) | 12 (63) | 0.03 (0.0–0.2) | |
| IgG4 | 34 (85) | 0.3 (0–1.7) | 17 (79) | 0.4 (0.0–1.8) | 17 (88) | 0.13 (0.0–0.7) | |
| IgE | 38 (95) | 0.5̂ (0–1.12) | 20 (96) | 0.4** (0.0–0.9) | 18 (94) | 0.6†† (0.06–1.12) | |
Statistically significant differences by Student's t test: *, P = 10−3; †, P = 9 × 10−4; ^, P = 4 × 10−3; **, ††, P = 4 × 10−2.
DISCUSSION
Parasites have evolved a variety of adaptive strategies for evading or even exploiting their host's immune response. Some of these strategies are passive, whereas others involve active manipulations of the host's defensive responses. Clarifying the host immune response will make it easier to identify the critical measures taken by parasites for evasion and prolonged infestation. Increasing evidence shows that parasite-derived substances play an important role in initiating or maintaining the parasite's advantage, directly suppressing the function of certain subsets of immune cells as well as stimulating other cell populations related to immunopathology (7, 12, 23).
In this paper we show that E. granulosus AgB is an immunoregulatory molecule involved in the host-parasite interactions responsible for parasite survival. Our data here confirm previous evidence that AgB impairs the early inflammatory response (37). They also extend existing knowledge by showing that AgB influences the Th1-Th2 balance, thus permitting the lifelong coevolution of the host-parasite relationship. Our experiments specify that AgB inhibits PMN recruitment. But unlike other parasitic molecules, such as the 45-kDa glycoprotein derived from Trichinella spiralis (7), it alters neither PMN random migration nor oxidative metabolism.
Our CL test results showing that the AgB left cellular metabolism intact, suggest that AgB-induced inhibition of chemotaxis does not arise simply from the antigen's toxic effect. Neither does it arise from an impairment of the cytoskeleton, because AgB-stimulated PMN fully retained their ability to migrate spontaneously. Support for this conclusion comes also from experiments using SHF (data not shown). An important point is that instead of adding AgB to the bottom of the Boyden chamber, as others did (37), we added it to the top. In this way we directly evaluated the protein's action on cell motility, irrespective of its chemoattractant properties. The effects of AgB on circulating PMN from patients with CE might be interesting to evaluate, though we doubt whether the results would differ from those in cells from uninfected controls. Evaluating compartmentalized PMN (adjacent to cystic lesions) might yield different results.
In previous in vitro and in vivo studies we found high Th1 cytokine concentrations (IFN-γ) in patients who responded to chemotherapy and high Th2 cytokine concentrations (IL-4 and IL-10) in patients who did not (32, 33, 34). If Th1 responses begin to damage the parasite, then it would be to the parasite's advantage to create a Th2 bias through the release of antigens that induce Th2-promoting cytokines, such as IL-4 and IL-13. The results here confirm our previous data showing that SHF, a crude mixture that contains various antigens, can promote activation of Th1 and Th2 cells (31). They also provide new information on the productive immune response, indicating that AgB can skew the type 1-type 2 cytokine towards a preferentially Th2 polarization. In this study, AgB induced high IL-4 and IL-13, low IFN-γ, and no IL-12 production, yet Th1 and Th2 responses depend not only on the amount of cytokines produced but also on the Th1/Th2 ratio. The IFN-γ/IL-4 ratio is related to the lymphocyte phenotype. In our study the difference in IFN-γ/IL-4 ratios in response to both parasite antigens depended on IFN-γ production alone, because SHF and AgB induced similar amounts of IL-4. This ratio shows that AgB induces a more predominant Th2 cytokine response than SHF. Our finding that AgB induced this switch only in patients with CE further supports its important role in E. granulosus infection. This Th2 polarization is more evident in patients with progressive disease, in whom the stimulus with AgB increases the imbalance observed also in unstimulated cultures. Hence, we presume that AgB is the SHF component primarily responsible for inducing IL-4, whereas other antigenic subunits probably induce production of IFN-γ.
IL-13 is an abundantly secreted cytokine sharing many properties with IL-4, such as providing help for IgG4 and IgE production and acting as an anti-inflammatory cytokine via its effect on monocytes (27). Thus, IL-13 may initiate numerous, but not all IL-4-like effects. Although often considered a Th2 cytokine, IL-13 is a product of Th0, Th1, and Th2 cells (13). In this study we found no association between IL-13 and IL-4 production in either group of patients. Interestingly, although AgB and SHF induced equal amounts of IL-4, AgB induced lower amounts of IL-13. Previous investigations on E. granulosus infection have shown in patients' sera a strong positive correlation between IL-12 and IL-13 concentrations (16). In our study, we found no correlation between IL-12 and IL-13 in response to either of the parasite antigens tested, and the role of this cytokine in CE needs to be further investigated.
In a previous study investigating IL-12 gene expression in pharmacologically treated patients with CE, we suggested a role of this cytokine in resolving the disease, probably by promoting Th1 cell activation (IFN-γ) (34). In alveolar echinococcosis, in vivo treatment with recombinant IL-12 showed that this cytokine is of crucial importance in inhibiting larval growth (mainly through production of IFN-γ), suggesting its usefulness in therapy (17). In our study the only cytokine produced in significantly higher levels by unstimulated PBMC from controls was IL-12, suggesting a possible in vivo down-regulation of this Th1 cytokine in patients with CE. Consistent with our study, Dreweck et al. (16) recently found a lower IL-12 concentration in sera from patients with alveolar echinococcosis than in controls, but in contrast to us, they found higher IL-12 levels in sera from patients with progressive echinococcosis than in patients with stable disease. The discrepancies presumably depend on differences in the patients studied and on the samples used (serum or PBMC).
Our finding that AgB induces no IL-12 production in patients' PBMC strongly suggests that in vivo, it could down-modulate this cytokine, probably by selectively modulating macrophages themselves, the major IL-12 producers. Control over IL-12 production is the major factor driving the response towards the Th1 or Th2 phenotype. The most likely cause of Th2-cell polarization is the direct down-modulating action of AgB on Th1 cells. Future research should investigate the functional activity of AgB in the induction of other anti-parasite mechanisms, including nitric oxide production and chemokine expression by macrophages or the activation of cellular death machinery in host responding cells.
The effect of parasite antigens on Th-cell activation receives support from antigen-driven PBMC proliferation. Compared with SHF, AgB induced lower levels of IFN-γ and also less Th1-dependent PBMC proliferation.
In vitro Th2 cytokine production was reflected in vivo by elevated levels of AgB-specific IgE and, most strikingly, IgG4. Helminth parasites are particularly adept at stimulating IgE synthesis, and a number of parasite allergens have recently been cloned and characterized (22, 24). We cannot exclude the possibility that AgB, like an allergen, could act directly on the promotion of IL-4 synthesis, thus generating the early IL-4 milieu necessary for Th2 development. Our data here confirm recent observations that IgG4 and IgE antibodies strongly recognize in SHF the three bands of AgB at 8 or 12, 16, and 20 or 24 kDa (20, 39, 40, 41). The higher mean ODs of IgE ELISA for patients with progressive disease are consistent with the Th2 imbalance observed in these patients, predominantly in AgB-stimulated cultures. The opposite behavior of IgG4, higher in patients with stable or cured disease, conflicts with our previous observations of a parallel IgE and IgG4 regulation in patients under pharmacological treatment (32). One reason is that we grouped patients here according to cyst activity, regardless of the success of therapy.
In their study of patients with CE, Sterla et al. (40) found an enhanced production of low-avidity anticarbohydrate IgG2 specific for broad bands at 116, 55, and 24 kDa. Why we found IgG2 that binds AgB will be a matter of future research.
We conclude that AgB exploits T-cell activation by eliciting the nonprotective Th2 response. AgB may have a direct role in the escape from immunity, probably by inhibiting PMN chemotaxis. Further studies should focus on precisely when and how AgB modulates immune functions. They should also seek to identify pathologically relevant peptide sequences.
The Th1-Th2 model explaining how selective immune responses—including cell-mediated or humoral immunity—develop provides the rationale for new therapeutic strategies. The notion that a parasite antigen can exploit the host response to the parasite's advantage makes it worthwhile to continue to search for other antigenic components of SHF that favor protective immunity by inducing a preferential Th1 polarization.
ACKNOWLEDGMENTS
R.R. and E.P. contributed equally to this work.
This work was partially supported by (i) Italian Ministry of Health grants (“Surveillance project on emerging and re-emerging infectious disease” and “Allergic diseases: development of diagnostic and therapeutic tools and evaluation of their suitability for the management of the allergic patient”) from the Istituto Superiore di Sanità (art. 502/12) and (ii) MURST and the University of Pisa (“Analisi molecolare, immunologica e farmacologica delle interazioni tra parassiti, ospiti e vettori”) to F. Bruschi.
REFERENCES
- 1.Azzarà A, Chimenti M, Azzarelli L, Fantini E, Carulli G, Ambrogi F. An image processing workstation for automatic evaluation of human granulocyte motility. J Immunol Methods. 1992;148:29–40. doi: 10.1016/0022-1759(92)90155-m. [DOI] [PubMed] [Google Scholar]
- 2.Azzarà A, Chimenti M, Carulli G, Rizzuti-Gullaci A, Ambrogi F. An image processing procedure for the assessment of normality curves of motility of human granulocytes in micropore filters. Scand J Clin Lab Investig. 1995;55:399–406. doi: 10.3109/00365519509104979. [DOI] [PubMed] [Google Scholar]
- 3.Azzarà A, Carulli G, Rizzuti-Gullaci A, Minnucci S, Capochiani E, Ambrogi F. Motility of rhG-CSF-induced neutrophils in patients undergoing chemotherapy: evidence for inhibition detected by image analysis. Br J Haematol. 1996;92:161–168. doi: 10.1046/j.1365-2141.1996.00295.x. [DOI] [PubMed] [Google Scholar]
- 4.Bombardieri S, Giordano F, Ingrao F, Ioppolo S, Siracusano A, Vicari G. An evaluation of an agar gel diffusion test with crude and purified antigens in the diagnosis of hydatid disease. Bull WHO. 1974;51:525–530. [PMC free article] [PubMed] [Google Scholar]
- 5.Boyum A. Separation of leukocytes from blood and bone marrow. Scand J Clin Lab Investig. 1968;21:77–81. [PubMed] [Google Scholar]
- 6.Bruschi F, Carulli G, Azzarà A, Minnucci S. Inhibition of neutrophil oxidative metabolism by trichinellosis patient sera. Parasite origin or host induction? Parasite Immunol. 1995;17:253–260. doi: 10.1111/j.1365-3024.1995.tb01023.x. [DOI] [PubMed] [Google Scholar]
- 7.Bruschi F, Carulli G, Azzarà A, Homan W, Minnucci S, Rizzuti-Gullaci A, Sbrana S, Angiolini C. Inhibitory effects of human neutrophil functions by the 45 kD glycoprotein derived from the parasitic nematode Trichinella spiralis. Int Arch Allergy Immunol. 2000;122:58–65. doi: 10.1159/000024359. [DOI] [PubMed] [Google Scholar]
- 8.Caremani M, Benci A, Maestrini R, Rossi G, Menchetti D. Abdominal cystic hydatid disease (CHD): classification of sonographic appearance and response to treatment. J Clin Ultrasound. 1996;24:491–500. doi: 10.1002/(SICI)1097-0096(199611/12)24:9<491::AID-JCU1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 9.Carulli G, Gianfaldoni M, Azzarà A, Papineschi F, Vanacore R, Minnucci S, Testi R, Ambrogi F. FcRIII (CD 16) expression on neutrophils from chronic myeloid leukemia. A flow cytometric study. Leuk Res. 1992;16:1203–1209. doi: 10.1016/0145-2126(92)90120-v. [DOI] [PubMed] [Google Scholar]
- 10.Carulli G, Minnucci S, Gianfaldoni M L, Angiolini C, Azzarà A, Ambrogi F. Interactions between platelets and neutrophils in essential thrombocythaemia. Effects on neutrophil chemiluminescence and superoxide anion generation. Eur J Clin Investig. 1995;25:929–934. doi: 10.1111/j.1365-2362.1995.tb01969.x. [DOI] [PubMed] [Google Scholar]
- 11.Craig P S. Immunodiagnosis of Echinococcus granulosus and a comparison of techniques for diagnosis of canine echinococcosis. In: Andersen F L, Ouhelli H, Kachani M, editors. Compendium on cystic echinococcosis. Provo, Utah: Brigham Young University Print Services; 1997. pp. 85–118. [Google Scholar]
- 12.Damian R T. Parasite immune evasion and exploitation: reflections and projections. Parasitology. 1997;115:S169–S175. doi: 10.1017/s0031182097002357. [DOI] [PubMed] [Google Scholar]
- 13.de Waal Malefyt R, Abrams J S, Zurawski S M, Lecron J C, Mohan-Peterson S, Sanjanwala B, Bennett B, Silver J, de Vries J E, Yssel H. Differential regulation of IL-13 and IL-4 production by human CD8+ and CD4+ Th0, Th1 and Th2 T cell clones and EBV-transformed B cells. Int Immunol. 1995;7:1405–1416. doi: 10.1093/intimm/7.9.1405. [DOI] [PubMed] [Google Scholar]
- 14.Diaz A, Ferreira A, Sim R B. Complement evasion by Echinococcus granulosus. J Immunol. 1997;158:3779–3786. [PubMed] [Google Scholar]
- 15.Diaz A, Irigoin F, Ferreira F, Sim R B. Control of host complement activation by the Echinococcus granulosus hydatid cyst. Immunopharmacology. 1999;42:91–98. doi: 10.1016/s0162-3109(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 16.Dreweck C M, Soboslay P T, Schulz-Key H, Gottstein B, Kern P. Cytokine and chemokine secretion by human peripheral blood cells in response to viable Echinococcus multilocularis metacestode vesicles. Parasite Immunol. 1999;21:433–438. doi: 10.1046/j.1365-3024.1999.00243.x. [DOI] [PubMed] [Google Scholar]
- 17.Emery I, Leclerc C, Sengphommachanh K, Vuitton D A, Liance M. In vivo treatment with recombinant IL-12 protects C57BL/6J mice against secondary alveolar echinococcosis. Parasite Immunol. 1998;20:81–91. doi: 10.1046/j.1365-3024.1998.00131.x. [DOI] [PubMed] [Google Scholar]
- 18.Fearon D, Collins L A. Increased expression of C3b receptors on polymorphonuclear leukocytes induced by chemotactic factors and by purification procedures. J Immunol. 1983;130:370–375. [PubMed] [Google Scholar]
- 19.Finkelman F D, Holmes J, Katona I M, Urban J F, Beckmann M P, Park L S, Schooley K A, Coffman R L, Mosmann T R, Paul W E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 20.Ioppolo S, Notargiacomo S, Profumo E, Franchi C, Ortona E, Riganò R, Siracusano A. Immunological responses to antigen B from Echinococcus granulosus cyst fluid in hydatid patients. Parasite Immunol. 1996;18:571–578. doi: 10.1046/j.1365-3024.1996.d01-31.x. [DOI] [PubMed] [Google Scholar]
- 21.Maddison S E, Slemenda S B, Schantz P M, Fried J A, Wilson M, Tsang V C W. A specific diagnostic antigen of Echinococcus granulosus with an apparent molecular weight of 8kDa. Am J Trop Med Hyg. 1989;40:377–383. doi: 10.4269/ajtmh.1989.40.377. [DOI] [PubMed] [Google Scholar]
- 22.Margutti P, Ortona E, Vaccari S, Barca S, Riganò R, Teggi A, Muhschlegel F, Frosch M, Siracusano A. Cloning and expression of a cDNA encoding an elongation factor 1β/δ protein from Echinococcus granulosus with immunogenic activity. Parasite Immunol. 1999;21:485–492. doi: 10.1046/j.1365-3024.1999.00246.x. [DOI] [PubMed] [Google Scholar]
- 23.McKerrow J H. Cytokine induction and exploitation in schistosome infections. Parasitology. 1997;115:S107–S112. doi: 10.1017/s0031182097001765. [DOI] [PubMed] [Google Scholar]
- 24.McReynolds L A, Kennedy M W, Selkirk M E. The polyprotein allergens of nematodes. Parasitol Today. 1993;9:403–406. doi: 10.1016/0169-4758(93)90046-i. [DOI] [PubMed] [Google Scholar]
- 25.Musiani P, Piantelli M, Lauriola L, Arru E, Pozzuoli R. Echinococcus granulosus: specific quantification of the two most immunoreactive antigens in hydatid fluids. J Clin Pathol. 1978;31:475–478. doi: 10.1136/jcp.31.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Profumo E, Ortona E, Riganò R, Notargiacomo S, Ioppolo S, Teggi A, Siracusano A. Cellular and humoral responses to antigenic subunits of Echinococcus granulosus cyst fluid in hydatid patients. Parasite Immunol. 1994;16:393–398. doi: 10.1111/j.1365-3024.1994.tb00366.x. [DOI] [PubMed] [Google Scholar]
- 27.Punnonen J, Aversa G, Cocks B G, McKenzie A N J, Menon S, Zurawski G, De Waal Malefyt R, De Vries J E. Interleukin-13 induces IL-4 independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc Natl Acad Sci USA. 1993;90:3730–3734. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rausch R L. Echinococcus granulosus: biology and ecology. In: Andersen F L, Ouhelli H, Kachani M, editors. Compendium on cystic echinococcosis. Provo, Utah: Brigham Young University Print Services; 1997. pp. 18–53. [Google Scholar]
- 29.Rickard M D, Davies C, Bout D T, Smith J D. Immunohistological localization of two hydatid antigens (antigen 5 and antigen B) in the cyst wall, brood capsules and protoscoleces of Echinococcus granulosus (ovine and equine) and E. multilocularis using immunoperoxidase methods. J Helminthol. 1977;51:359–364. doi: 10.1017/s0022149x00007719. [DOI] [PubMed] [Google Scholar]
- 30.Rickard M D, Williams J F. Hydatidosis/cysticercosis: immune mechanisms and immunization against infection. Adv Parasitol. 1982;21:229–296. doi: 10.1016/s0065-308x(08)60277-8. [DOI] [PubMed] [Google Scholar]
- 31.Riganò R, Profumo E, Di Felice G, Ortona E, Teggi A, Siracusano A. In vitro production of cytokines by peripheral blood mononuclear cells from hydatid patients. Clin Exp Immunol. 1995;99:433–439. doi: 10.1111/j.1365-2249.1995.tb05569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riganò R, Profumo E, Ioppolo S, Notargiacomo S, Ortona E, Teggi A, Siracusano A. Immunological markers indicating the effectiveness of pharmacological treatment in human hydatid disease. Clin Exp Immunol. 1995;102:281–285. doi: 10.1111/j.1365-2249.1995.tb03778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riganò R, Profumo E, Ioppolo S, Notargiacomo S, Teggi A, Siracusano A. Serum cytokine detection in the clinical follow up of patients with cystic echinococcosis. Clin Exp Immunol. 1999;115:503–507. doi: 10.1046/j.1365-2249.1999.00843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riganò R, Profumo E, Buttari B, Teggi A, Siracusano A. Cytokine gene expression in peripheral blood mononuclear cells from patients with pharmacologically treated cystic echinococcosis. Clin Exp Immunol. 1999;118:95–101. doi: 10.1046/j.1365-2249.1999.01021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanchez F, March F, Mercader M, Coll P, Munoz C, Prats G. Immunochemical localization of major hydatid fluid antigens in protoscoleces and cysts of Echinococcus granulosus from human origin. Parasite Immunol. 1991;13:583–592. doi: 10.1111/j.1365-3024.1991.tb00554.x. [DOI] [PubMed] [Google Scholar]
- 36.Shepherd J C, McManus D P. Specific and cross-reactive antigens of Echinococcus granulosus cyst fluid. Mol Biochem Parasitol. 1987;25:143–154. doi: 10.1016/0166-6851(87)90003-x. [DOI] [PubMed] [Google Scholar]
- 37.Sheperd J C, Aitken A, McManus D P. A protein secreted in vivo by Echinococcus granulosus inhibits elastase activity and neutrophil chemotaxis. Mol Biochem Parasitol. 1991;44:81–90. doi: 10.1016/0166-6851(91)90223-s. [DOI] [PubMed] [Google Scholar]
- 38.Siracusano A, Teggi A, Quintieri F, Notargiacomo S, De Rosa F, Vicari G. Cellular immune responses of hydatid patients to Echinococcus granulosus antigens. Clin Exp Immunol. 1988;72:400–405. [PMC free article] [PubMed] [Google Scholar]
- 39.Siracusano A, Ioppolo S, Lastilla M, Notargiacomo S, Ortona E, Riganò R. Detection of IgE antibodies against antigen 5 and B of Echinococcus granulosus by immunoblotting. G Mal Infett Parassit. 1993;45:286–288. [Google Scholar]
- 40.Sterla S, Sato H, Nieto A. Echinococcus granulosus human infection stimulates low avidity anticarbohydrate IgG2 and high avidity antipeptide IgG4 antibodies. Parasite Immunol. 1999;21:27–34. doi: 10.1046/j.1365-3024.1999.00197.x. [DOI] [PubMed] [Google Scholar]
- 41.Wen H, Craig P S. Immunoglobulin G subclass responses in human cystic and alveolar echinococcosis. Am J Trop Med Hyg. 1994;51:741–748. doi: 10.4269/ajtmh.1994.51.741. [DOI] [PubMed] [Google Scholar]