Abstract
Objective
To assess the in vitro efficacy on antioxidant potential, protection against global oxidative stress, and effect on collagen neosynthesis of minimalist formula (Peptide‐C ampoules product) containing 10% natural vitamin C, rice and lupin bio‐peptides, hyaluronic acid, and Vichy volcanic mineralizing water (active mix).
Methods
In‐tube quantitative tests (“in‐tube screening”) assessed global antioxidant properties, anti‐lipid peroxidation, anti‐protein glycosylation, and metalloproteinase inhibition (anti‐collagenase, anti‐elastase, and anti‐hyaluronidase activity) properties of the formula. Protection against oxidative stress was evaluated on human keratinocyte monolayer cultures, and collagen neosynthesis was quantified on fibroblast monolayer cultures treated with supernatants from product‐treated reconstructed human epidermis.
Results
Product (5% concentration) showed high antioxidant ability (blocking 99.0% oxidation), protection against oxidative stress damage (51.8% lipid peroxidation and 37.8% protein glycosylation decreases), and inhibition of hyaluronidase (21.9%), elastase (47.1%), and collagenase (61.8%). The protective effect was validated on human keratinocyte monolayer cultures in the presence of active mix (0.025%). Oxidative stress (ROS) was reduced by 99.0%, while global oxidative stress (RMS) induced by pollution, UVA radiation, and a combination of both factors was reduced by 48.94%, 8.7%, and 96.28%, respectively. The product increased collagen neosynthesis (11.21%) by cellular dialogue in fibroblasts incubated with product/mix‐treated‐RHE supernatants.
Conclusion
The combination of ingredients in the product showed high global antioxidant capacity, as well as a protective effect against oxidative stress induced by UVA, pollution, or both combined factors and an ability to stimulate collagen neosynthesis in in vitro studies, which support the clinical efficacy of this product.
Keywords: anti‐aging, cell culture, oxidative stress, peptides, skin barrier, skin physiology/structure
Abbreviations
- AGEs
advanced glycosylation end products
- DNA
Deoxyribonucleic acid
- DMEM
Dulbecco’s Modified Eagle Medium
- ECM
extracellular matrix
- EDTA
ethylenediaminetetraacetic acid
- EM
emission
- EX
excitation
- HA
hyaluronic acid
- H 2 DCF‐DA
2’,7’ dichlorodihydrofluorescein diacetate fluorescent probe
- VVMW
Vichy volcanic mineralizing water
- MMPs
metalloproteinases
- MTT
3‐(4,5‐dimethyl‐2‐thiazolyl)‐2,5‐diphenyl‐2H‐tetrazolium bromide
- IR
infrared (solar radiation)
- PMSF
phenylmethylsulfonyl fluoride
- PS
pollution
- RCS
reactive carbon species
- RHE
reconstructed human epidermis
- RMS
reactive molecular species
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- UV
ultraviolet
1. INTRODUCTION
Since Christopher Wild coined the term exposome in 2005, proposing a more holistic approach to the factors that affect an individual, there has been a gradual integration of the concept into the field of cosmetics. In recent years, the term “skin aging exposome” has been used to define external and internal factors and their interactions, which affect a human individual from conception to death, as well as the body's response to these factors, which lead to clinical and biological signs of skin aging. 1
Exposome factors such as external factors (ultraviolet [UV], visible light and Infrared [IR] solar radiation, environmental pollution, tobacco smoke, and temperature) and internal factors (food, alcohol consumption, stress, and lack of sleep) may induce oxidative stress through the generation of free radicals, including reactive oxygen species (ROS), reactive nitrogen species (RNS), and reactive carbonyl species (RCS). 2 , 3 Although ROS, RNS, and RCS are all different molecular entities, they are interrelated highly reactive molecules that generate other molecules, and thus, the damage produced is exponentially increased. 4
Oxidative stress is responsible for damage to cell membranes such as lipid peroxidation (especially in mitochondria), changes in the structure and function of proteins (protein glycosylation and/or activation of enzymes that degrade the proteins of the extracellular matrix [ECM]), alterations in DNA, and/or modifications in gene expression. 5
The initial cause of structural alterations in skin is oxidative stress from an increase in ROS. Polyunsaturated lipids of membranes and lipoproteins are the main targets of ROS. ROS‐mediated lipid peroxidation generates RCS, which amplifies the damage caused by ROS altering other structures. Protection against lipid peroxidation prevents indiscriminate ROS toxicity. 6 In this way, oxidative stress causes the alteration of the protein structure and its function, for example, in protein glycosylation with the formation of advanced glycosylation end products (AGEs). 5
High levels of ROS also activate matrix metalloproteinases (MMPs), which degrade the molecules that constitute the ECM in the dermis, such as collagen, hyaluronic acid, and elastin. The increased degradation and reduced de novo synthesis of these proteins, together with a decreased rate of skin cell renewal, are the main processes involved in the loss of elasticity and the appearance of wrinkles. 5
Finally, in an attempt to mitigate oxidative stress, inflammation and melanin synthesis are induced in the skin, which results in the appearance of dark spots and hyperpigmentation.
The use of cosmetics may help to protect the skin against exposome factors and maintain adequate physiological conditions to prevent cell damage. Therefore, research is essential for the development of effective products proven to protect against oxidation, maintain the natural balance and the functions of the skin, and ultimately improve skin aging signs.
The formula of Peptide‐C ampoules described here has been developed at low pH (pH2.85) and contains pure vitamin C (10%), peptides (rice and lupin), hyaluronic acid (HA), and Vichy volcanic mineralizing water (VVMW). Vitamin C has well‐known anti‐aging effects on skin and has been shown to prevent epidermal damage from pollution and ultraviolet radiation. 7 , 8 Peptides are increasingly used as dermoscometic ingredients for anti‐aging effects, and rice di‐ and tripeptides have shown an anti‐wrinkle effect. 9 HA has been reported to have anti‐inflammatory properties and antioxidative properties. 10 Finally, VVMW is a highly mineralized mineral water shown to increase catalase activity, which is an important antioxidant enzyme. 11 Furthermore, the formula has demonstrated effectiveness and high subject satisfaction for wrinkle reduction and skin rejuvenation in three clinical studies. 12
To further assess the product, in vitro screening was carried out to test anti‐oxidation effects and efficacy against oxidation‐derived processes, including inhibition of ECM metalloproteases.
2. MATERIALS AND METHODS
2.1. Test sample
Two versions of the sample were used in this study: final formula and mix of actives. The product (final formula) includes vehicles and a minimalist formula of only 10 ingredients (mix of actives: 10% natural vitamin C, rice and lupin bio‐peptides, hyaluronic acid, and Vichy volcanic mineralizing water), with no preservatives, in amber glass ampoules (LiftActiv Specialist Peptide‐C ampoules; Vichy, Paris, France [Peptide‐C ampoules]). The mix of actives only was used in cellular cultures in order to avoid cytotoxicity.
2.2. In‐tube tests
The evaluation of the final formula in blister format was carried out using the INVITOOLS commercial kits (Invitrotecnia).
The reagents and samples were prepared using the diluent included in the corresponding kit, following the manufacturer's instructions. Measurements were taken using a Biotek Synergy HT (Winooski, VT) Microplate reader.
2.2.1. Anti‐general oxidant ability
150 μl of the substrate and 50 μl of the positive control (vitamin E analogue at final concentration of 31.25 μg.mL−1) and intermediate dilutions of the sample/s were dispensed in triplicate. 50 μl of diluent was used as negative control. After incubation for 30 min at 25°C, activity was measured by spectrophotometry at 410 nm.
2.2.2. Anti‐lipid peroxidation ability
25 μl of the substrate, 12.5 μl of the oxidant agent, and 12.5 μl of the positive control (vitamin E analogue at final concentration of 250 μg.mL−1) and intermediate dilutions of the sample/s were dispensed in triplicate. For the negative control, 12.5 μl of Diluent was used. After incubation for 2 h at 37°C, fluorometer readings were performed (EX: 500 nm; EM: 530 nm).
2.2.3. Anti‐protein glycosylation ability
187.5 μl of the substrate and 62.5 μl of the positive control (aminoguanidine at a final concentration of 690 μg.mL−1) and intermediate dilutions of the sample/s were dispensed in triplicate. For the negative control, 62.5 μl of diluent was served. It was incubated for72 h at 37°C. After adding the developer solution, it was incubated for 15 min at 95°C and read by fluorimetry (EX: 370 nm; EM: 440 nm).
2.2.4. Inhibition of Collagenase
5 μl of enzyme and 5 μl of the positive control (Ethylenediaminetetraacetic acid, EDTA at final concentration of 5 mg.mL−1) and intermediate dilutions of the sample/s were dispensed in triplicate. For the negative control, 5 μl of reaction buffer was used. It was incubated 15 min at 37°C. Finally, 10 µl of substrate was added before incubation for 45 min at 37°C. After adding the development solutions, spectrophotometry was performed at 570 nm.
2.2.5. Inhibition of Elastase
50 μl of enzyme and 50 μl of the positive control (phenylmethylsulfonyl fluoride, PMSF at final concentration of 130 μg.mL−1) and intermediate dilutions of the sample/s were dispensed in triplicate. For the negative control, 50 μl of reaction buffer was used. It was incubated 15 min at 25°C. Finally, 50 µl of substrate was added before incubation for 45 min at 25°C. Spectrophotometry readings were performed at 410 nm.
2.2.6. Inhibition of hyaluronidase
10 μl of enzyme and 10 μl of the positive control (Ethylenediaminetetraacetic acid, EDTA at final concentration of 1.25 mg.mL−1) and intermediate dilutions of the sample/s were dispensed in triplicate and incubated for 15 min at 37°C. For the negative control, 10 μl of reaction buffer was used. Finally, 20 µl of substrate was added followed by incubation for 45 min at 37°C. After adding the development solutions, spectrophotometry readings were performed at 540 nm.
2.3. Cellular assays
2.3.1. Cultures
To evaluate the protective effect against reactive molecular species (RMS), human keratinocytes (spontaneously immortalized cell line HaCaT, CLS GmbH) were grown until confluence was reached, and 2 × 106 cells/plate were seeded in a 96‐well plate (Sarstedt).
To perform the collagen quantification, human fibroblasts (derived and adapted by the R&D department of Invitrotecnia) were grown until they reached confluence. Afterward, 5·105 cells/plate were seeded in a 24‐well plate (Sarstedt).
Both types of cell lines were cultured in Dulbecco's Modified Eagle Medium (DMEM; Gibco) and supplemented with 10% fetal bovine serum (FBS; Sigma‐Aldrich) and 1% penicillin‐streptomycin (Gibco). Once the cultures were established (after 24 h incubation), the treatments were applied.
2.3.2. Tissues
Reconstructed human epidermis (RHE; SkinEthic, France) was used. Tissues were maintained with 1 ml of growth medium (SkinEthics, France) overnight, following the manufacturer's instructions. Once the model was established, product or mix of actives was applied.
2.4. Treatments
2.4.1. Oxidative stress conditions
The keratinocyte cultures in a confluent monolayer (100% confluence) were washed and incubated in absence (standard culture conditions ‐negative control‐ and damage control) and presence of active Mix for 24 h, in the following cases:
To evaluate global ROS damage, human keratinocyte cultures at confluence were treated with hydrogen peroxide (H2O2, Sigma‐Aldrich) at a concentration of 0.0017% for 2 h. After the treatment, cells were washed twice with PBS1X and the oxidative stress was quantified. Finally, plates were washed with PBS1X and cellular viability was evaluated.
To induce oxidative stress by pollution, human keratinocyte cultures at confluence were treated with diesel particles (Urban dust, NIST® SRM®; Sigma‐Aldrich) at a concentration of 700µM for 24 h. Cells were washed twice with PBS1X, and the oxidative stress was quantified.
Afterward, plates were washed with PBS1X and cellular viability was evaluated.
To induce oxidative stress by UVA radiation, human keratinocyte cultures at confluence were irradiated at 3 J.cm−2 in Bio‐Link Crosslinker BLX‐365 (Vilber Lourmat). One plate not irradiated was used as control of production of ROS and cellular viability. Cells were washed twice with PBS1X, and the oxidative stress was quantified.
Afterward, plates were washed with PBS1X and cellular viability was evaluated.
2.4.2. Deep product effect
The RHE tissues were moistened to facilitate the sample application, were treated with the product (simulating normal use conditions). After 24 h, supernatants of the treated‐RHE tissues (SkinEthic) were transferred to adapted‐human fibroblast cultures and incubated for 24 h. The process was repeated until the adapted‐fibroblast culture reached confluence. Sodium ascorbate was used as a positive control (0.01%, Sigma‐Aldrich). Collagen was then quantified by the INVITOOLS commercial kit (Invitrotecnia), following the manufacturer's instructions.
2.5. Quantification methods
2.5.1. Oxidative stress
The quantification was performed with 2’,7’ dichlorodihydrofluorescein diacetate (H 2 DCF‐DA) fluorescent probe (Molecular Probes; Invitrogen, Europe).
The H 2 DCF‐DA fluorescent probe was added to the whole plate. After 30 min, the fluorescent signal was recorded.
2.5.2. Cell viability
The MTT assay was performed with 0.5 mg.mL−1 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide in culture medium. The optical density was measured at 570 nm in Microplate reader SPECTROstar Omega (BMG LabTech GmbH).
2.5.3. Total amount of collagen
Total collagen was quantified using the INVITOOLS commercial kits (Invitrotecnia, Spain), following the manufacturer's instructions. After fixation of the cells, 500 μL of the staining solution were added. After 1 h, 100 μl of the extraction solution was added, and then, spectrophotometry was performed at 540 nm.
2.6. Statistical analysis
The paired Student t test was used to compare the means of two groups, which were usually the negative or positive control (depending on the group considered as the reference) and the sample tested (product or active mix‐treatments).
To test antioxidant capacity and efficacy on derived effects, the results obtained for the sample (5% concentration) for each test (general anti‐oxidation, lipid anti‐peroxidation, protein glycosylation and inhibition of hyaluronidase, and elastase and collagenase activities) were compared with the activity of negative control.
To test protective effect against damage by global oxidative stress, results obtained from cellular viability and level of ROS were compared with the positive control, for each condition (treatments with H2O2, pollution, and UVA radiation) in the cultures treated with or without 0.025% of mix.
In addition, ANOVA tests were performed where necessary to compare different quantitative variables in more than two groups (positive control, final formula, and actives mix) with one outcome variable to evaluate the statistically significant difference between the samples and positive control.
In all the statistical tests, a p value of p < 0.05 was taken as significant.
3. RESULTS
3.1. Very high direct antioxidant capacity and efficacy on derived effects: lipid peroxidation, protein glycosylation, inhibition of hyaluronidase, and elastase and collagenase activities
The product was evaluated at concentrations of 1, 5, 10, 20, and 25% for all in‐tube tests. Global oxidation and derived processes were tested (lipid peroxidation and protein glycosylation) as well as MMP inhibition capacity, assessed by the inhibition of hyaluronidase, elastase, and collagenase activity.
An effect was observed at 5% concentration of the product in all the assays (Figure 1). Effectiveness in blocking oxidation, reduction in lipid peroxidation, and reduction in protein glycosylation were 99.0% (p < 0.001 compared to the negative control), 51.8% (p < 0.01 compared to the negative control), and 37.7% (p < 0.05 compared to the negative control), respectively; inhibition of hyaluronidase activity, elastase activity, and collagenase was 21.9%, 47.1% (p < 0.01 compared to the negative control), and 61.8% (p < 0.01 compared to the negative control), respectively.
FIGURE 1.
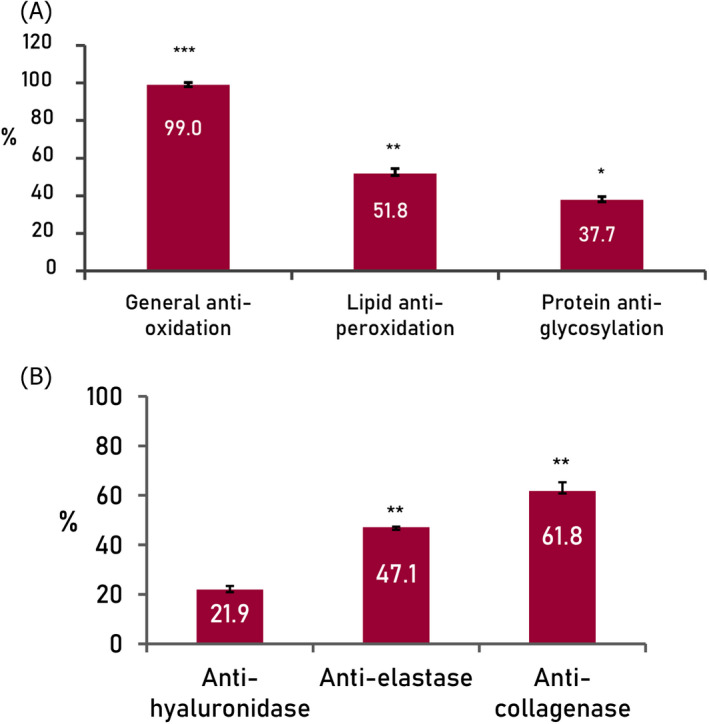
Results from in‐tube assays showing general anti‐oxidation, lipid anti‐peroxidation, and protein anti‐glycosylation effectiveness of 5% product (A), and inhibition of hyaluronidase, elastase, and collagenase activities by 5% final formula (B). Negative control adjusted to 0% of inhibition/100% of activity. ***p < 0.001, **p < 0.01
3.2. Protective effect against damage by global oxidative stress from reactive molecular species
For human cell in vitro assays, the mix of actives of the product was used to avoid cytotoxic effects from non‐active ingredients of the product. Working concentrations were determined by cytotoxicity curves. Values of cellular viability around 85% were acceptable (non‐cytotoxic).
3.2.1. Protective effect of the mix of actives against ROS‐induced damage
The exposure of the keratinocyte culture to hydrogen peroxide (Figure 2), under controlled conditions (0.0017%, 2 h), induced a decrease in cellular viability of 30.2% and a statistically significant increase in free radical levels (228.2%), compared with standard culture conditions (100%).
FIGURE 2.
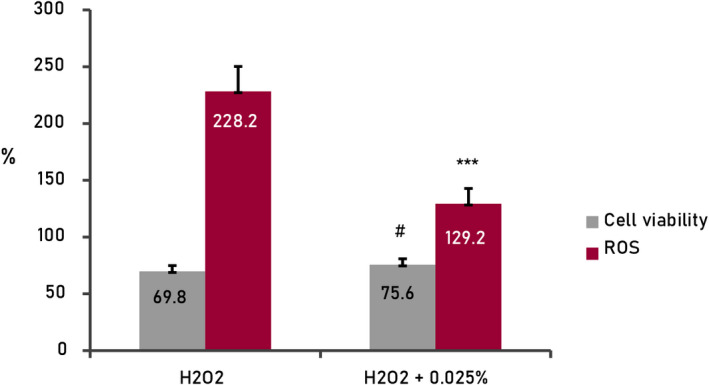
Results from cellular assays (human keratinocytes) treated with active mix (0.025%). Oxidative stress by ROS (H2O2, hydrogen peroxide 150µM, 2 h); 0.025%+H2O2, cells treated with active mix and H2O2 150 µM, 2 h. Cellular viability and basal levels of ROS adjusted at 100% in standard conditions of the culture (negative control, non‐treated cells). ***p < 0.001, # p < 0.05
In presence of the mix of actives (0.025%), the cellular viability increased by 5.9%, statistically significant (p < 0.05), and the levels of free radicals decreased to 98.98%, statistically significant (p < 0.001). These results are indicative of the strong antioxidant potential of the product against global oxidative stress and its derived effects increasing the cellular viability.
3.2.2. Protective effect of the actives mix against pollution‐induced damage
The exposure of the keratinocyte culture to diesel particles (Figure 3), under controlled conditions (700 μM, for 24 h), caused an increase in free radical levels (188.1%) and a decrease in cell viability (75.2%), statistically significant compared with standard culture conditions (100%).
FIGURE 3.
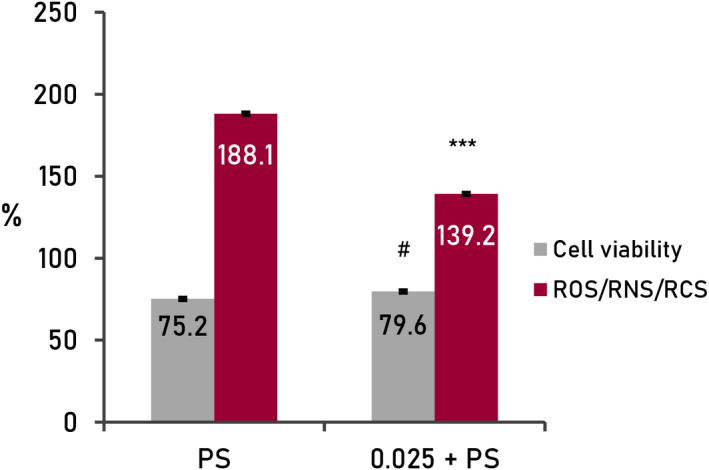
Results from cellular assays (human keratinocytes) treated with active mix (0.025%). Oxidative stress (RMS) by pollution (cells treated with diesel particles 700 µM, 24 h; 0.025%+pollution, cells treated with formulation and diesel particles 700 µM, 24 h). Cellular viability and basal levels of ROS adjusted at 100% in standard conditions of the culture (negative control, non‐treated cells). ***p < 0.001, #p < 0.05
In the presence of the mix of actives (0.025%), there was a decrease in free radical levels (−48.9%) and an increase in cell viability (+4.9%), both statistically significant (p < 0.001 and p < 0.05, respectively). These results are indicative of a wide protective effect against damage caused by the increase in specific free radicals of contamination and its derived effects increasing the cellular viability.
3.2.3. Protective effect of the actives mix against UVA‐induced damage
The exposure of the keratinocyte culture to UVA radiation (Figure 4), under controlled conditions (3 J.cm−2), caused an increase in free radical levels (139.8%) and a decrease in cell viability (84.3%), statistically significant compared with standard culture conditions (100%).
FIGURE 4.
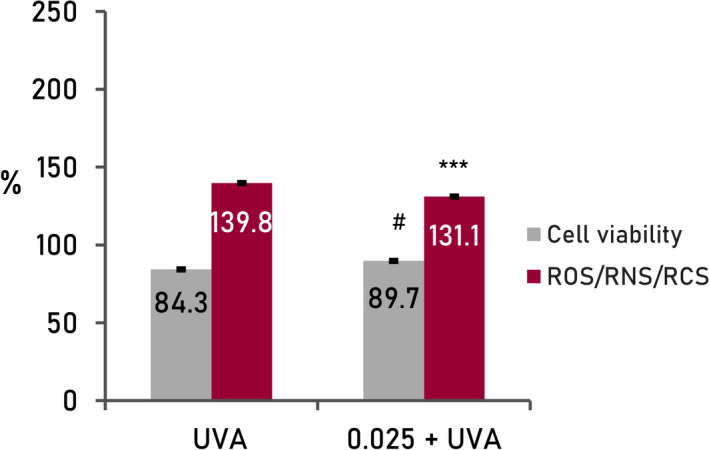
Results from cellular assays with human keratinocytes treated with active mix (0.025%). Reactive molecular species oxidative stress by UVA radiation (cells treated with UVA radiation, 3 J/cm2); 0.025%+UVA, cells treated with UVA radiation, 3 J/cm2). Cellular viability and basal levels of ROS adjusted at 100% in standard conditions of the culture (negative control, non‐treated cells). ***p < 0.001, #p < 0.05
In the presence of the actives of the formula (0.025%), there was a decrease in free radical levels (−8.8%) and an increase in cell viability (+5.5%), both statistically significant (p < 0.001, p < 0.05, respectively). These results are indicative of a clear protective effect against damage caused by the increase in free radicals generated by radiation.
3.2.4. Protective effect of the actives mix against damage caused by pollution and UVA combined
In the case of the combination of pollution and UVA radiation (Figure 5), exposure to combined factors causes an increase in free radical levels (278.7%) and a decrease in cell viability (66.95%) greater than the factors separately for both parameters, statistically significant compared with the culture conditions standard (100%).
FIGURE 5.
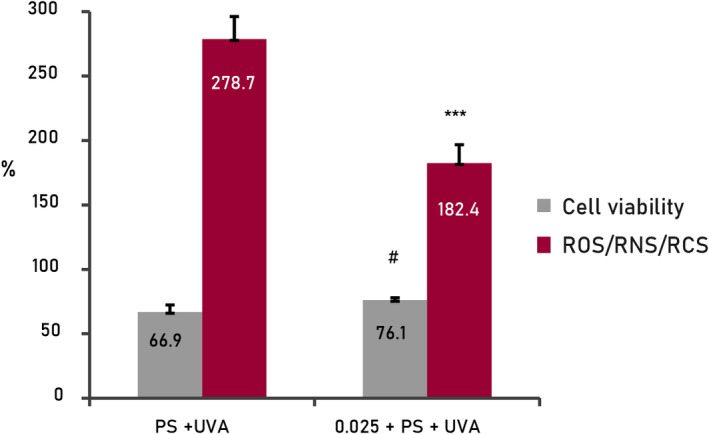
Results from cellular assays with human keratinocytes treated with active mix (0.025%). Reactive molecular species oxidative stress by combined pollution and UVA. Cellular viability and basal levels of ROS adjusted at 100% in standard conditions of the culture (negative control, non‐treated cells). PS, pollution, ***p < 0.001, #p < 0.05
In the presence of the mix of actives (0.025%), free radical levels decreased (−96.3%) and cell viability increased (+9.19%), both statistically significant (p < 0.01 and p < 0.05 respectively). This is indicative of a clear protective effect against damage caused by global oxidative stress, induced by the combination of external environmental factors.
3.3. Collagen neosynthesis by RHE treated with peptide C formulation
To assess the effectiveness of the product to stimulate (new) collagen synthesis, reconstituted human epidermis (RHE, SkinEthic) was treated with the final formula and with the active mix (0.01%).
As shown in Figure 6, fibroblasts treated with supernatants from epidermis treated with the mix of actives or treated with supernatants from epidermis treated with the product increased collagen synthesis by 11.9% and 11.2%, respectively, compared with the negative control (p < 0.01 and p < 0.05, respectively), similar to values of positive control (sodium ascorbate 0.01%) which is known to be a powerful antioxidant.
FIGURE 6.
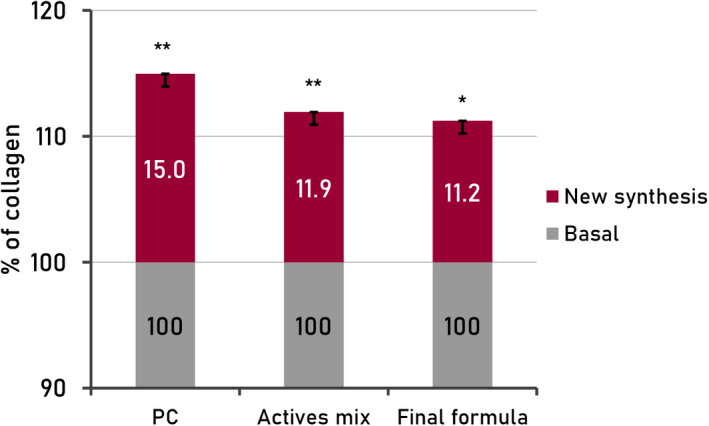
Quantification (%) of total collagen on human fibroblasts treated with reconstituted human epidermis supernatants (RHE; SkinEthic) treated with active mix and final formula. PC, sodium ascorbate (0.01%); SD, standard deviation
This result is indicative of cellular dialogue between keratinocytes and fibroblast that stimulate the synthesis of new collagen from the cells in the deeper layers of the skin without penetration.
4. DISCUSSION
Skin aging results in a thinner and less well‐protected skin. 13 , 14 It is caused by a combination of genetic factors and exposome factors (environmental and biological factors) on top of chronological aging. 15 Due to modern urban life, the population is exposed on a daily basis to solar radiation and other external factors including air pollution, tobacco smoke, stress, or solar radiation. 16 Oxidative stress has been described as the most important biological consequence of these exposome factors, resulting in the formation of RMS (ROS, RNS, and RCS), which can play a major role in the alteration of the homeostasis of the skin and premature aging by degradation of components of the ECM and reduction in antioxidant capabilities in the skin. 13 , 17
Antioxidants can provide protection against this release of radicals and are becoming more widely used, in the form of vitamins or plant extracts, in cosmetic formulations. 8 , 15 , 17 Vitamin C is the most abundant antioxidant, that can interact with harmful free radicals and donate its electron. This strong effect is especially relevant in photoaged skin, quenching ROS induced by UV‐radiation, and in protection of epidermal morphology, blocking phototoxicity of polycyclic aromatic hydrocarbons in pollution combined with sunlight. 8 , 15 In addition, recent studies have proven that HA has antioxidant properties (in vivo and vitro), inhibiting lipid peroxidation and reducing the activity of ROS by scavenging ROS free radicals. 18
In‐tube screening of the product, containing pure vitamin C (10% L‐ascorbic acid, at pH = 2.8), revealed a strong antioxidant potential and a high efficacy against lipid peroxidation and protein glycosylation. The protective effect of the formula against RMS‐induced damage was validated in human keratinocyte cultures. The monolayers showed a significant decrease in free radical levels and an increase in cell viability when they were incubated with the combination of actives at 0.025%, and after, they were exposed to global oxidative stress, generated by peroxide, UVA, and pollution (diesel particles). High protection against exposure to combined factors, that caused a greater increase in free radical levels and a decrease in cell viability than the factors separately, was especially relevant.
A direct consequence of antioxidant effectiveness of the product is the protection of the integrity of proteins in the ECM. Metalloproteases are activated by oxidative stress and degradation of the structural proteins of the ECM of the dermis occurs. The main consequences of these processes are the formation of wrinkles. 5 This study demonstrates, by in‐tube screening, that the product has the capacity to inhibit collagenase, elastase, and hyaluronidase metalloproteinases, showing the ability of the final formula to protect the deeper layers of the skin. The other main process involved in the formation of wrinkles is the decrease in the neosynthesis of matrix proteins. The formula contains bio‐peptides, polypeptides, or oligopeptides has been used in cosmetic products as effective anti‐aging ingredients. 19 , 20 They are composed of amino acids, and they can imitate peptide sequences or act as messenger molecules stimulating the production of collagen, elastin, and lumican rebuilding the dermal matrix, reducing the appearance of wrinkles and to increase firmness. 21 , 22 , 23 , 24 Collagen (COL) is the major ECM molecule in the dermis, along with other components that confer properties on the skin. 14 The main ones are I, III, VI, and XIV (dermis), IV (basal membranes), VII and XVII (epidermis‐dermis junctions), XIII (epidermis), and XV and XVI (fibroblasts). 25
The application of the product or the mix of active ingredients on reconstructed human epidermis demonstrated the positive effect on collagen neosynthesis, in both cases, which could be the result of a keratinocyte‐fibroblast dialogue.
5. CONCLUSION
The results of these in vitro studies revealed high antioxidant capacity of the product, as well as a protective effect against oxidative stress‐derived processes, such as lipid peroxidation and protein glycosylation. Moreover, broad protective effect against global oxidative stress and RMS from UVA, pollution, and both combined exposome factors was demonstrated.
In addition, the inhibition of collagenase, elastase, and hyaluronidase and ability to stimulate collagen neosynthesis demonstrate the potential of the product to protect the ECM and provide anti‐aging efficacy.
CONFLICT OF INTEREST
The study was funded by L’Oreal España S.A. Madrid, Spain.
AUTHORS CONTRIBUTIONS
M. Matabuena‐Yzaguirre, M. Herranz‐López, and E. Barrajón‐Catalán involved in conception and study design. A. Martín‐Martínez, N. Sánchez‐Marzo, and D. Martínez‐Casanova involved in acquisition of data. A. Martín‐Martínez, N. Sánchez‐Marzo, D. Martínez‐Casanova, M. Abarquero‐Cerezo, M. Herranz‐López, E. Barrajón‐Catalán, and M. Matabuena‐Yzaguirre involved in the analysis and/or interpretation of data, revised the manuscript critically for important intellectual content, and approved the version of the manuscript to be published. A. Martín‐Martínez, M. Abarquero‐Cerezo, and M. Matabuena‐Yzaguirre involved in drafting the manuscript.
6. ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to. No ethical approval was required as this is an article with data obtained from in vitro assays, no human or animal experimentation.
ACKNOWLEDGMENT
These studies were financially supported by Laboratories Vichy (L'Oréal), and M. Abarquero‐Cerezo is an employee of Laboratories Vichy (L'Oréal).
Martín‐Martínez A, Sánchez‐Marzo N, Martínez‐Casanova D, et al. High global antioxidant protection and stimulation of the collagen synthesis of new anti‐aging product containing an optimized active mix. J Cosmet Dermatol. 2022;21:3993–4000. doi: 10.1111/jocd.14703
Almudena Martín‐Martínez and Noelia Sánchez‐Marzo should be considered joint first author.
This study was presented at the International Master Course on Aging Science Annual World Congress January 30 ‐February 1, 2020; Paris, France.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Krutmann J, Bouloc A, Sore G, Bernard BA, Passeron T. The skin aging exposome. J Dermatol Sci. 2017;85(3):152‐161. [DOI] [PubMed] [Google Scholar]
- 2. Dunn JH, Koo J. Psychological Stress and skin aging: a review of possible mechanisms and potential therapies. Dermatol Online J. 2013;19(6):18561. [PubMed] [Google Scholar]
- 3. Dupont E, Gomez J, Bilodeau D. Beyond UV radiation: a skin under challenge. Int J Cosmet Sci. 2013;35(3):224‐232. [DOI] [PubMed] [Google Scholar]
- 4. Bild W, Ciobica A, Padurariu M, Bild V. The interdependence of the reactive species of oxygen, nitrogen, and carbon. J Physiol Biochem. 2013;69(1):147‐154. [DOI] [PubMed] [Google Scholar]
- 5. Rittié L, Fisher GJ. UV‐light‐induced signal cascades and skin aging. Ageing Res Rev. 2002;1(4):705‐720. [DOI] [PubMed] [Google Scholar]
- 6. Singh M, Kapoor A, Bhatnagar A. Oxidative and reductive metabolism of lipid‐peroxidation derived carbonyls. Chem Biol Interact. 2015;234:261‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pullar JM, Carr AC, Vissers MCM. The roles of vitamin C in skin health. Nutrients. 2017;9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dimitrov A, Zanini M, Zucchi H, et al. Vitamin C prevents epidermal damage induced by PM‐associated pollutants and UVA1 combined exposure. Exp Dermatol. 2021;30(11):1693‐1698. 10.1111/exd.14315. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 9. Jouandeaud M. Di‐ and tripeptides: a new approach to skin nutrition. Personal Care Magazine. 2002;2002:1‐4. [Google Scholar]
- 10. Litwiniuk M, Krejner A, Speyrer MS, Gauto AR, Grzela T. Hyaluronic acid in inflammation and tissue regeneration. Wounds. 2016;28(3):78‐88. [PubMed] [Google Scholar]
- 11. Bruneau F, Bernard D, Ragueneau N, Montastier C. Effect of Vichy water on catalase activity in the stratum corneum. Int J Cosmet Sci. 1996;18(6):269‐277. [DOI] [PubMed] [Google Scholar]
- 12. Escobar S, Valois A, Nielsen M, Closs B, Kerob D. Effectiveness of a formulation containing peptides and vitamin C in treating signs of facial ageing: three clinical studies. Int J Cosmet Sci. 2021;43(2):131‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garre A, Narda M, Valderas‐Martinez P, Piquero J, Granger C. Antiaging effects of a novel facial serum containing L‐Ascorbic acid, proteoglycans, and proteoglycan‐stimulating tripeptide: ex vivo skin explant studies and in vivo clinical studies in women. Clin Cosmet Investig Dermatol. 2018;11:253‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barba C, Alonso C, Sanchez I, Suner E, Saez‐Martin LC, Coderch L. Soybean‐fragmented proteoglycans against skin aging. J Cosmet Laser Ther. 2017;19(4):237‐244. [DOI] [PubMed] [Google Scholar]
- 15. Rinnerthaler M, Bischof J, Streubel MK, Trost A, Richter K. Oxidative stress in aging human skin. Biomolecules. 2015;5(2):545‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Puizina‐Ivić N. Skin aging. Acta Dermatovenerol Alp Pannonica Adriat. 2008;17(2):47‐54. [PubMed] [Google Scholar]
- 17. Wölfle U, Seelinger G, Bauer G, Meinke MC, Lademann J, Schempp CM. Reactive molecule species and antioxidative mechanisms in normal skin and skin aging. Skin Pharmacol Physiol. 2014;27(6):316‐332. [DOI] [PubMed] [Google Scholar]
- 18. Ke C, Sun L, Qiao D, Wang D, Zeng X. Antioxidant acitivity of low molecular weight hyaluronic acid. Food Chem Toxicol. 2011;49(10):2670‐2675. [DOI] [PubMed] [Google Scholar]
- 19. Gorouhi F, Maibach HI. Role of topical peptides in preventing or treating aged skin. Int J Cosmet Sci. 2009;31(5):327‐345. [DOI] [PubMed] [Google Scholar]
- 20. Schagen SK. Topical peptide treatments with effective anti‐aging results. Cosmetics. 2017;4:1‐14. [Google Scholar]
- 21.Ant 23‐ Lintner K, Peschard O. Biologically active peptides: from a laboratory bench curiosity to a functional skin care product. Int J Cosmet Sci. 2000;22(3):207‐218. [DOI] [PubMed] [Google Scholar]
- 22. Katayama K, Armendariz‐Borunda J, Raghow R, Kang AH, Seyer JM. A pentapeptide from type I procollagen promotes extracellular matrix production. J Biol Chem. 1993;268(14):9941‐9944. [PubMed] [Google Scholar]
- 23. Lintner K. Promoting production in the extracellular matrix without compromising barrier. Cutis. 2002;70(6 Suppl):13‐16. discussion 21–3. [PubMed] [Google Scholar]
- 24. Pauly G, Contet‐Audonneau J‐L, Moussou P, et al. Small proteoglycans in the skin: new targets in the fight against skin aging. Int J Cosmet Sci. 2009;31(2):154. [Google Scholar]
- 25. Kliment CR, Englert JM, Crum LP, Oury TD. A novel method for accurate collagen and biochemical assessment of pulmonary tissue utilizing one animal. Int J Clin Exp Pathol. 2011;4(4):349‐355. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


