Abstract
Purpose of review
As the biology of metastatic renal cell carcinoma (mRCC) continues to be elucidated, novel treatments focused around immunotherapies and targeted therapies will continue to emerge. In this review, we will highlight recent treatment advances and their implications for surgical and systemic therapy.
Recent findings
Several new treatments, including the tyrosine kinase inhibitor cabozantinib, the combination of a PD-1 antibody (nivolumab) with a CTLA-4 antibody (ipilimumab), and the combination of axitinib with pembrolizumab or avelumab have been approved by the FDA as first-line therapy for the treatment of mRCC. Although promising survival benefits have been seen with these new therapies, careful patient selection is still critical.
Summary
The introduction of novel therapies and the investigation of combinatorial therapies have shifted the treatment paradigm for advanced RCC. Current trials have provided promising data that could lead to further therapeutic advances.
Keywords: Immunotherapy, renal cell carcinoma, resistance, targeted therapy, cytoreductive nephrectomy
Introduction
In the United States, an estimated 73,750 new cases and 14,830 deaths due to kidney and renal pelvis cancer are projected to occur in 2020 (1). Renal cell carcinoma (RCC) accounts for 90% of the kidney cancer cases, with the clear cell subtype as the most common form. Importantly, about 30% of patients initially present with metastatic RCC (mRCC) (2). Furthermore, one-third of RCC patients who undergo surgical resection for the primary tumor have cancer recurrence with distant metastases (3). Conventional chemotherapy and radiation therapy are often ineffective in treating mRCC. Therefore, developing novel treatment options has remained an important priority in the management of mRCC. In fact, the last decade has seen an extraordinary increase in the approved therapies to treat advanced RCC, which initially focused on mammalian target of rapamycin (mTOR) pathway inhibition, vascular endothelial growth factor (VEGF) pathway inhibition, and most recently, on immunotherapy (alone and in combination with other agents) (Table 1). In this review, we will highlight recent treatment advances and their implications for surgical and systemic therapy.
Table 1:
Current treatments for metastatic clear cell RCC by date of FDA approval
| Therapy | FDA Approval | Mechanism of Action | Treatment Line | Risk Groups | Comparison Arm | Endpoint | Route |
|---|---|---|---|---|---|---|---|
| Interleukin-2 | May 1992 | Cytokine immunotherapy | First-line | Favorable | Phase II - None | ORR | IV |
| Sorafenib | Dec 2005 | VEGFR, PDGFR, KIT inhibitor | Cytokine failure | Favorable Intermediate |
Placebo | OS | PO |
| Sunitinib | Jan 2006 | VEGFR, PDGFR inhibitor | First-line | Favorable Intermediate |
IFN-α | PFS | PO |
| Temsirolimus | May 2007 | mTOR inhibitor | First-line | Intermediate Poor |
IFN-α | OS | IV |
| Everolimus | Mar 2009 | mTOR inhibitor | VEGFR failure | All groups | Placebo | PFS | PO |
| Bevacizumab + IFN-α | Jul 2009 | Anti-VEGF monoclonal antibody | First-line | Favorable Intermediate |
IFN-α ± Placebo | OS | IV + SC |
| Pazopanib | Oct 2009 | VEGFR, PDGFR, KIT inhibitor | First-line or Cytokine failure | Favorable Intermediate |
Placebo | PFS | PO |
| Axitinib | Jan 2012 | VEGFR inhibitor | Second-line | Sorafenib | PFS | PO | |
| Nivolumab | Nov 2015 | Anti-PD1 monoclonal antibody | Second-line | All groups | Everolimus | OS | IV |
| Cabozantinib | Apr 2016 | VEGFR, MET, AXL inhibitor | Second-line | All groups | Everolimus | PFS | PO |
| Lenvantinib + Everolimus | May 2016 | VEGFR, PDGFR, KIT, FGFR, RET inhibitor mTOR inhibitor |
Second-line | All groups | Everolimus or Levantinib | PFS | PO |
| Cabozantinib | Dec 2017 | VEGFR, MET, AXL inhibitor | First-line | Intermediate Poor |
Sunitinib | PFS | PO |
| Nivolumab + Ipilimumab | Apr 2018 | Anti-PD1 monoclonal antibody Anti-CTLA-4 monoclonal antibody |
First-line | Intermediate Poor |
Sunitinib | OS ORR PFS |
IV + IV |
| Pembrolizumab + Axitinib | Apr 2019 | Anti-PD1 monoclonal antibody VEGFR inhibitor |
First-line | All groups | Sunitinib | OS PFS |
IV + PO |
| Avelumab + Axitinib | May 2019 | Anti-PD-L1 monoclonal antibody VEGFR inhibitor |
First-line | All groups PD-L1+ tumor |
Sunitinib | OS PFS |
IV + PO |
ORR: objective response rate; OS: overall survival; PFS: progression-free survival; IV: intravenous; PO: per os; SC: subcutaneous
Surgical Therapy
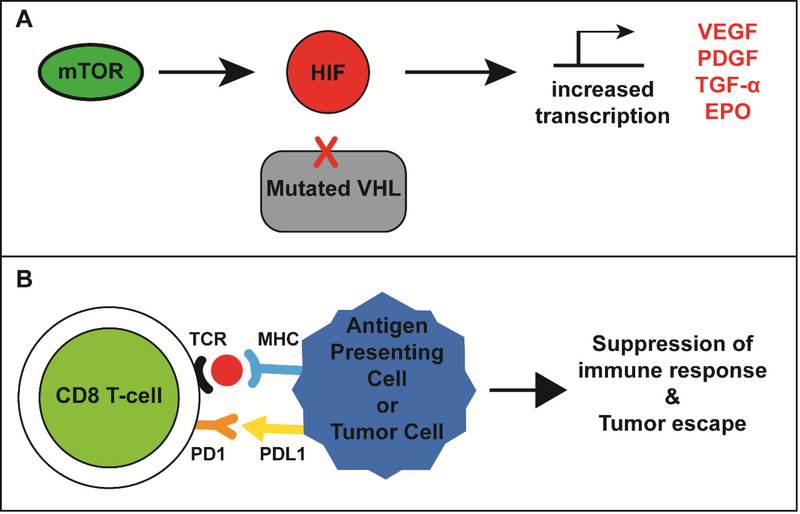
Approximately 75% of RCC cases are clear cell RCC (ccRCC). Interestingly, 90% of the sporadic ccRCC cases contain a mutation or epigenetic silencing that leads to von Hippel-Lindau (VHL) protein inactivation (4). This fundamental discovery has provided key insights into targeting the downstream consequences of VHL inactivation, which results in dysregulated expression of several tumorigenic and pro-angiogenic factors including vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and transforming growth factor-α (TGF-α) mediated by hypoxia-inducible factor (HIF) activation (Figure 1A). The discovery of HIF-1 and VHL pathway and their link to oxygen-sensing has expanded our understanding of RCC and it is no surprise that this discovery was recognized in the 2019 Nobel Prize in Physiology and Medicine (5–8). More recently, immunoregulatory pathways that promote T-cell inactivation and inhibit the anti-tumor response were found to be upregulated in RCC (Figure 1B). Activation of immune checkpoints, such as CTLA-4, PD-1, LAG-3, and TIM-3, expressed on T cells are known to be necessary for a robust antitumor response (9). More recently, modulation of this immunoregulatory pathway has proven to be critical for treating a multitude of malignancies in addition to RCC. The discovery of the CTLA-4 pathway by Dr. James P. Allison and PD-1 pathway by Dr. Tasuku Honjo was awarded the 2018 Nobel Prize in Physiology and Medicine (10, 11). Given the multitude of pathways that promote tumor survival, evolving strategies for treating mRCC have focused on modulating these pathways of tumor proliferation and tumor survival.
Figure 1:
VHL and PD1 pathway in RCC. (A) Mutated von Hippel-Lindau (VHL) protein cannot ubiquitinate hypoxia-inducible factor (HIF), which then translocates to the nucleus. Mammalian target of rapamycin (mTOR) complex 1 also positively regulates HIF activation. Together, this leads to increased transcription of growth factors, such as VEGF, that promote RCC growth and development. (B) T-cell receptors (TCR) on CD8 T cells engage with the major histocompatibility complex 1 (MHC1) on tumor cells and antigen-presenting cells. When programmed death ligand-1 (PDL-1) binds to programmed cell death protein 1 (PD-1) on CD8 T cells, this induces pathways that lead to tumor tolerance and suppression of tumor-specific immune response.
Many patients are found to have metastatic disease at the time of diagnosis. Cytoreductive nephrectomy has historically been a viable initial, non-pharmacological treatment option for managing mRCC, based on the findings that tumor debulking was associated with improved survival (12–14). While often utilized in the era of cytokine therapy, this approach in combination with the use of targeted systemic therapies has been questioned. To demonstrate the utility of this approach in patients receiving VEGFR-targeted therapy, a randomized phase III trial comparing cytoreductive nephrectomy followed by sunitinib versus sunitinib alone in the first-line setting (CARMENA) evaluated 450 patients with previously untreated metastatic clear cell RCC (15). All patients had intermediate- or poor-risk disease with a median follow-up of 51 months. Overall survival (OS) in the sunitinib alone group was non-inferior to the nephrectomy-sunitinib group (stratified HR for death, 0.89, 95% CI 0.71–1.1). Furthermore, the objective response (OR) and disease control rates were not significantly different between the two groups. While the CARMENA trial was not without its limitations, which are discussed later in this review, the results raise the possibility that systemic therapy alone can provide similar outcomes to cytoreductive nephrectomy followed by systemic therapy.
When systemic targeted therapy is given in combination with surgery for mRCC, the optimal sequencing of treatments is unclear. The SURTIME phase III clinical trial investigated this by comparing 99 patients with metastatic ccRCC who were randomized to receive either immediate cytoreductive nephrectomy followed by sunitinib or 3 cycles of sunitinib followed by cytoreductive nephrectomy (16). Due to poor accrual, progression-free survival (PFS) could not be assessed as a primary endpoint, so 28-week progression-free rate (PFR) for the intention-to-treat cohort was used instead. The PFR was similar (42% immediate nephrectomy vs 43% deferred nephrectomy) between the two arms with a median follow-up of 3.3 years. Of the 46 patients who underwent immediate nephrectomy, 40 received sunitinib, of whom 34 had to discontinue treatment due to disease progression. In the deferred nephrectomy arm, 48 patients received neoadjuvant sunitinib, of whom 38 patients underwent deferred nephrectomy and 26 patients then received postoperative sunitinib. Importantly, median OS was improved with deferred nephrectomy compared to immediate nephrectomy (32.4 months vs. 15 months; HR 0.57, p =0.03). Taken together, Bex et al. suggest that deferred nephrectomy allows more patients to receive sunitinib and may contribute to increased OS, although the trial findings should be interpreted with caution given the sample size and substituted PFR endpoint. Additional studies are needed now that we have entered the immunotherapy (IO) + tyrosine kinase inhibitor (TKI) combination era.
Monotherapy
Several strategies aimed at improving outcomes associated with front line systemic therapies in advanced RCC have been evaluated recently. The CABOSUN trial compared cabozantinib, a newer targeted therapy that inhibits multiple pathways (MET; VEGFR-1,2,3; AXL; RET; ROS1; TYRO3; MER; KIT; TRKB; FLT-3; and TIE-2), to sunitinib in 157 randomly assigned patients with previously untreated metastatic clear cell RCC with poor or intermediate risk features. Findings from this study were recently updated and demonstrated that progression-free survival (PFS) was significantly prolonged with cabozantinib (8.6 vs. 5.3 months, HR 0.48, 95% CI 0.31–0.74), but OS was not significantly increased with cabozantinib compared to sunitinib (26.6 vs. 21.2 months, HR 0.80, 95% CI 0.53–1.21) (17, 18). Based on the results of the phase II CABOSUN trial, cabozantinib was granted FDA-approval as a first-line therapy for mRCC (19).
Cabozantinib has also been studied in patients who have progressed after previously being treated with at least one VEGFR tyrosine kinase inhibitor (TKI) in the METEOR trial (20). Of note, patients who had disease progression on bevacizumab (anti-VEGF antibody), interleukin-2 (cytokine), interferon-alpha (cytokine), and nivolumab (checkpoint inhibitor) were also included in this study. In this phase III trial, 658 patients with metastatic ccRCC were assigned to cabozantinib or the mTOR inhibitor everolimus. Patients treated with cabozantinib had significantly longer PFS compared with everolimus (7.4 vs. 3.9 months, HR 0.51, 95% CI 0.41–0.62) as well as better OS (21.4 vs. 17.1 months, HR 0.70, 95% CI 0.58–0.85) (21). A sub-group analysis within this larger study was also done to look at outcomes for 142 patients with bone metastases, a subset of patients known to have poor prognosis (22–25). When analyzed by independent radiology committee, cabozantinib had a higher ORR (17% vs. 0%, 95% CI 9–27), increased PFS (7.4 vs. 2.7 months, HR 0.33, 95% CI 0.21–0.51) and increased OS (20.1 vs. 12.1 months, HR 0.54, 95% CI 0.34–0.84) among patients with bone metastases compared to everolimus, suggesting its utility in this challenging population (26).
Recent trials have also focused on immune checkpoint inhibitors such as pembrolizumab, a highly selective anti-PD-1 humanized monoclonal antibody, which was studied in the first line setting in the phase II Keynote 427 trial. In this trial, a cohort of 110 previously untreated patients with histologically confirmed metastatic ccRCC were treated with pembrolizumab, with data available after a median follow-up of 12 months (27). An independent radiology committee (IRC) blinded to treatment arm confirmed that the objective response rate (ORR) was 38.2%, including 3 patients with complete responses and 39 patients with partial responses. Patients with IMDC favorable risk and intermediate/poor risk mRCC had ORR of 31.7% (95% CI 18.1–48.1) and 42.0% (95% CI 30.2–54.5), respectively. ORR for patients also differed by PD-L1 expression. Patients with PD-L1 expression had ORR of 50% (95% 34.9–65.1) and without PD-L1 expression 26.4% (95% CI 15.3–40.3). The activity of pembrolizumab monotherapy as a first line treatment for metastatic ccRCC in this single arm study appears promising across risk groups, with particularly encouraging activity in intermediate/poor risk groups and patients with increased PD-L1 expression.
Combination Therapy
Immunotherapy + Immunotherapy (IO+IO)
Checkpoint inhibitor-based combination therapy was evaluated in the phase III Checkmate 214 trial, which compared combined therapy with nivolumab-ipilimumab to sunitinib monotherapy in previously untreated patients with mRCC (28). Nivolumab is an antibody against the immune checkpoint PD-1 and ipilimumab is an antibody against cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4). A cohort of 1096 patients were randomly assigned to receive either nivolumab plus ipilimumab every 3 weeks for four doses, followed by nivolumab every 2 weeks or sunitinib once daily for 4 weeks (with 2 weeks off). The coprimary end points were OS, PFS, and ORR among intermediate or poor risk patients. For the entire study population, OS was significantly increased with nivolumab-ipilimumab (median not reached vs 32.9 months, HR 0.68, 99.8% CI 0.49–0.95). Of note, nivolumab-ipilimumab and sunitinib groups contained 425 and 422 patients, respectively, with intermediate or poor risk mRCC. In these risk groups, at a median follow up of 25.2 months there was a significant improvement in OS with nivolumab-ipilimumab versus sunitinib, with median not reached versus 26 months, respectively (HR 0.63, 95% CI 0.44–0.82), but no significant improvement in median PFS (11.6 vs 8.4 months; HR 0.82, 95% CI 0.64–1.05, p=0.03). The ORR was 42% with combined immune therapy compared to 27% with sunitinib. Remarkably, complete response was seen in 9% of patients receiving nivolumab-ipilimumab compared to 1% of patients receiving sunitinib within the intermediate/poor-risk group. Given these results, the US Food and Drug Administration (FDA) granted approval to nivolumab-ipilimumab in combination for the treatment of intermediate or poor-risk, previously untreated metastatic ccRCC.
Given the mechanism of action of nivolumab, patients were further stratified by PD-L1 expression to see if the response rate differed. Among patients with intermediate/poor-risk disease and ≥ 1% PD-L1 expression, patients receiving nivolumab-ipilimumab had an improved ORR (58% vs 25%, HR 0.48, 95% CI 0.28–0.82) and median PFS (22.8 vs 5.9 months) compared to sunitinib. Interestingly, patients with intermediate/poor-risk disease and ≤ 1% PD-L1 expression receiving nivolumab-ipilimumab benefited in terms of increased OS (median not reached for either group, HR 0.73, 95% CI 0.56–0.96) without significant differences in ORR or PFS when compared to sunitinib. While PD-L1 expression may be a useful marker, it cannot provide either predictive or prognostic data with regards to response or OS benefits.
Although efficacious, combination therapy also introduces the potential for additional adverse events. The safety profiles for both nivolumab and ipilimumab have been characterized when used as monotherapy (29–31), and have demonstrated generally manageable toxicity such as fatigue, nausea, diarrhea, rash and musculoskeletal pain. In combination, 46% of patients experienced Grade 3 or 4 adverse events compared to 63% for the sunitinib treatment group. Notably, a large proportion of patients (22% vs 12%) discontinued nivolumab-ipilimumab compared to sunitinib due to treatment-related adverse events, although the reason for this discrepancy in discontinuation rates is unclear. Importantly, there were 8 and 4 treatment-related deaths in the nivolumab-ipilimumab and sunitinib groups, respectively. Among the patients treated with nivolumab-ipilimumab and experienced adverse events, 35% received high-dose steroids. These percentages likely underestimate treatment-related adverse events as the CheckMate 214 trial used ≥ 15% prevalence rate as a threshold to be considered a treatment-related adverse event. While not directly comparable, adverse events occurred at a higher rate within the combination therapy arm relative to monotherapy with either nivolumab or ipilimumab alone. The role of single agent PD1 inhibitors in this setting remains unclear; head to head comparisons with sunitinib and/or nivolumab-ipilimumab have not been performed. An approach utilizing single agent nivolumab followed by addition of ipilimumab in non-responding patients is being evaluated to determine if the toxicity associated with combination therapy can be avoided in some patients without compromising efficacy (32).
Immunotherapy (IO) + Tyrosine Kinase Inhibitor (TKI)
Additional encouraging data comes from the recently published phase III KEYNOTE-426 trial comparing the combination of axitinib (VEGF TKI) and pembrolizumab (anti-PD-1) to sunitinib in the first-line setting in 861 patients with advanced ccRCC (33). The primary endpoints were OS and PFS in the intention-to-treat population and secondary endpoint was ORR. At 1 year, OS was 89.9% in the pembrolizumab-axitinib group and 78.3% in the sunitinib group (p<0.0001). Pembrolizumab-axitinib improved PFS (15.1 months vs 11.1 months, HR 0.69, p<0.0001) compared to sunitinib. Subgroup analysis showed that PFS and OS were improved among intermediate/poor risk groups and PD-L1 positive patients when treated with combination therapy compared to sunitinib. Pembrolizumab-axitinib combination had an improved complete response rate (5.8% vs 1.9%) compared to sunitinib. Furthermore, partial response rate was also improved (53.5% vs 33.8%) with the combination over sunitinib. Adverse events occurred in 62.9% of patients receiving pembrolizumab-axitinib compared to 58.1% receiving sunitinib. Among patients receiving combination therapy, adverse events led to discontinuation of one drug in 30.5% of patients, discontinuation of both drugs in 10.7%, interruption of either drug in 69.9%, and dose reduction of axitinib in 20.3%. Sunitinib was discontinued by 13.9% of patients, interrupted in 49.9%, and dose reduced in 30.1%. Treatment-related deaths were similar between pembrolizumab-axitinib and sunitinib (0.9% vs 1.6%). In April 2019, the FDA approved the use of pembrolizumab and axitinib for the first-line treatment of patients with advanced RCC.
Similarly, the phase III JAVELIN Renal 101 trial compared treatment with sunitinib or the combination of avelumab (anti-PD-L1 antibody) and axitinib (VEGF TKI) in the first-line setting for advanced ccRCC (34, 35). The primary endpoints were PFS and OS among patients with PD-L1 positive tumors and secondary endpoint was PFS in the overall population. Compared to sunitinib, median PFS was significantly improved in the PD-L1+ group (13.8 vs 7.2 months; p<0.0001) with the combination arm. In the overall population, median PFS was also improved in the combination arm (13.8 vs 8.4 months; p<0.001). ORR among PD-L1 positive patients was 55.2% for avelumab-axitinib compared to 25.5% with sunitinib. Furthermore, subgroup analysis demonstrated that the risk of disease progression or death was lower with avelumab-axitinib across all IMDC risk groups compared to the sunitinib. Avelumab-axitinib combination showed higher complete response rates in the PD-L1 positive patients (4.4% vs 2.1%) and the overall population (3.4% vs 1.8%). Majority of patients with PD-L1 positive tumors (50.7%) and within the overall population (48%) demonstrated partial response with the combination, whereas the majority of patients receiving sunitinib were seen to have stable disease after treatment. Overall, the therapies were well tolerated. While adverse events of any grade were seen in 99.5% of patients receiving the combination therapy and 99.3% of patients receiving sunitinib, 7.6% of patients receiving avelumab-axitinib and 13.4% of patients receiving sunitinib had to discontinue treatment due to adverse events. Additionally, deaths due to either therapy were negligible. Of the patients receiving the combination therapy, 38.2% were deemed to have immune-related adverse events, of which 48 (11.1%) patients received high-dose steroids. Given this data, the FDA approved the use of avelumab in combination with axitinib in May 2019 as a first-line treatment for patients with advanced RCC.
Patient and Treatment Selection
Cytoreductive Surgery + TKI
As the treatment options for mRCC evolve, it is apparent that not every therapy is suitable for each patient. Given the complexity of the disease, no single therapy applies to patients in all risk categories. Thus, initiating patients on the “correct” therapy needs to be a judicious decision and should consider patient comorbidities, risk factors that have been utilized for prognostic models, as well as other relevant factors such as agent toxicity profile (Table 2). For example, the results from the CARMENA trial highlight the importance of patient selection for cytoreductive nephrectomy. While Méjean et al. suggest sunitinib is not inferior to nephrectomy for patients with intermediate- or poor-risk mRCC, cytoreductive nephrectomy is considered an integral part of the management of patients with favorable risk disease or those with low metastatic burden by many urologic oncologists. In fact, the earliest study demonstrating the utility of cytoreductive nephrectomy after treatment with IFNα−2b showed that among patients who underwent cytoreductive nephrectomy, patients with an ECOG performance status of 0 survived longer (17.4 vs 6.9 months) than those with performance status of 1 (13, 36).
Table 2:
Comparison of MSKCC and IMDC prognostic models for RCC
| Risk Factors | MSKCC Model (48) | IMDC Model (49, 50) |
|---|---|---|
| Time from diagnosis to treatment | < 1 year | < 1 year |
| Karnofsky performance status | < 80% | < 80% |
| Hemoglobin | < LLN | < LLN |
| Corrected calcium | > 10 mg/dL | > 10 mg/dL |
| LDH | > 1.5x ULN | - |
| Platelets | > ULN | |
| Absolute neutrophil count | > ULN |
Favorable: 0 factors; Intermediate: 1–2 factors; Poor: ≥ 3 factors
LLN: lower limit of normal; ULN: upper limit of normal; LDH: lactate dehydrogenase
Moreover, data from CARMENA should be interpreted in the context of prior reports. For instance, a retrospective analysis of data from Memorial Sloan Kettering Cancer Center and MD Anderson Cancer Center shows that cytoreductive nephrectomy should be avoided in patients with poor-risk disease, given that many of these patients will likely have a prolonged recovery prior to initiating systemic therapy (37). On the other hand, a retrospective analysis of over 15,000 patients with mRCC from 2006–2013 who were treated with cytoreductive nephrectomy and/or targeted therapy demonstrated that cytoreductive nephrectomy was associated with improved OS, although it is important to note that patient selection and other biases might influence these outcomes (38). Additionally, a meta-analysis comparing outcomes after cytoreductive nephrectomy or targeted therapy demonstrated similar results, suggesting that patient selection is a key feature influencing patient survival (39).
Approximately 40% of the patients in the CARMENA trial had poor-risk disease and a higher percentage of patients with advanced RCC with T3 or T4 tumors were offered nephrectomy compared to the sunitinib only group. It can be argued that the outcomes seen in this study were a function of patient selection and that the trial did not adequately address the role of cytoreductive nephrectomy in patients with better prognosis or those with lower metastatic burden, a setting where this approach is considered most effective. Additionally, OS in a per-protocol analysis where patients randomized to the nephrectomy arm but who did not undergo nephrectomy (7%) or did not receive sunitinib after nephrectomy (15%), and patients randomized to the sunitinib arm but did not get the sunitinib (approximately 5%) were excluded from the analysis, failed to show noninferiority (HR 0.98, 95% CI 0.77–1.25, upper limit of noninferiority ≤1.20), which further supports that sunitinib alone may not necessarily replace nephrectomy as an initial therapy for advanced RCC. Given the above considerations, it is our opinion that cytoreductive nephrectomy as a prelude to systemic therapy is still a reasonable option in well-selected patients.
Similarly, the SURTIME trial assessed the role of deferring cytoreductive nephrectomy in patients with intermediate risk or favorable surgical risk factors (16). The trial originally started as a phase 3 study to evaluate three months of upfront sunitinib followed by nephrectomy (deferred nephrectomy) versus immediate nephrectomy followed by sunitinib, with the primary endpoint being PFS. Due to poor accrual (n=99 patients accrued in 5.7 years) the trial was subsequently closed, and the primary endpoint was changed from PFS to progression free rate (PFR) at 28 weeks in the intent to treat (ITT) population. While the two arms were imbalanced in terms of proportion of patients receiving sunitinib or undergoing nephrectomy, no differences in PFR at week 28 were observed between the two arms. While the study highlights the potential advantage of deferring surgery, with only 99 study participants in the trial it is significantly underpowered to make an impactful conclusion. Moreover, the management of metastatic RCC has changed so dramatically since the start of SURTIME that sunitinib is no longer the optimal first-line treatment to use in this setting. Furthermore, deferring nephrectomy for systemic therapy raises the same concerns brought up with the CARMENA trial. However, Bex et al. suggest that initiating sunitinib prior to cytoreductive nephrectomy can provide insight into potential resistance to therapy and patients who progress on systemic therapy would likely not be good candidates for cytoreductive nephrectomy. While this may be true, patients with good performance status and minimal surgical risk factors may benefit from tumor debulking and the subsequent option of other systemic therapies (single agent or combination therapy).
Future studies will need to assess the benefit of different systemic therapies in the neoadjuvant/adjuvant nephrectomy population and to determine the clinical benefit of deferring nephrectomy. Appropriate biomarkers and surrogate endpoints are also necessary to better assess therapy efficacy. Furthermore, clinicians are currently in a conundrum: for patients who progress on first-line systemic therapy, should they be offered a cytoreductive nephrectomy prior to receiving second-line therapy if they are fit surgical candidates?
TKI Monotherapy
Patients with intermediate- or poor-risk advanced disease represent 70% to 80% of all patients with metastatic disease. For many years, sunitinib or pazopanib have remained the systemic first line therapies of choice for treating patients with advanced RCC, including primarily good to intermediate risk patients. Both CABOSUN and Checkmate 214 trials went head-to-head with sunitinib and demonstrated superior results with cabozantinib and nivolumab-ipilimumab, respectively, among patients with intermediate/poor risk disease (Table 1). Moreover, other ongoing trials in the first-line setting have shown promise and add to the arsenal of systemic therapies available for targeting advanced RCC (Table 3).
Table 3:
Ongoing Phase III combination studies for metastatic RCC
| Therapy | Study | Mechanism of Action | Population | End point |
|---|---|---|---|---|
| Bevacizumab + Atezolizumab | IMmotion151 NCT02420821 |
VEGF+PD-L1 | Untreated metastatic RCC with clear cell and sarcomatoid histology | PFS, PD in PD-L1+ OS in overall pop |
| Pembrolizumab + Lenvatinib | CLEAR NCT02811861 |
PD-1 + TKI | Untreated & treated metastatic ccRCC | PFS |
| Nivolumab + Cabozantinib ± Ipilimumab | CHECKMATE 9ER NCT03141177 |
PD-1 + TKI | Untreated metastatic or one prior non-VEGF/VEGFR neo/adjuvant treated metastatic ccRCC | PFS |
OS: overall survival; PFS: progression-free survival; PD: disease progression; TKI: tyrosine kinase inhibitor; PD-1: program death 1; ccRCC: clear cell renal cell carcinoma
The CABOSUN trial demonstrated that in previously untreated patients cabozantinib improved PFS and objective response rate compared with sunitinib. Furthermore, the METEOR trial demonstrated that cabozantinib was superior to everolimus in treating patients who progressed after initial VEGFR TKI treatment (40), demonstrating the versatility of cabozantinib in the first- and beyond settings. Subgroup analysis was also conducted in the Checkmate 214 trial, but did not demonstrate significant improvement in survival between nivolumab-ipilimumab and sunitinib in patients with bony metastases; however, nivolumab-ipilimumab did improve OS in patients with liver (HR 0.64, 95% CI 0.42–0.96) and lung metastases (HR 0.61, 95% CI 0.47–0.78) (28). Given the efficacy of cabozantinib in patients with bony metastases and nivolumab-ipilimumab in those with lung or liver metastases, they may be viable options as first-line therapies in these subgroups of patients.
IO Monotherapy and Combination Therapy
Immunotherapy regimens such as with single agent pembrolizumab or the nivolumab-ipilimumab doublet have shown promising results in previously untreated patients with intermediate-/poor-risk disease, although there is limited data available in the form of large scale phase 3 trials with single agent IOs, including nivolumab or ipilimumab monotherapy, in the first-line setting for mRCC (21). However, the Checkmate 214 trial provides compelling data that this combination is efficacious as a first-line therapy in patients with intermediate or poor-risk disease. Nevertheless, sunitinib had a higher ORR (52% vs 29%, p<0.001) and longer PFS (25.1 months vs 15.3 months) compared to nivolumab-ipilimumab combination in patients with favorable risk disease. This may suggest that distinct differences at the molecular level may be driving the response to sunitinib or nivolumab-ipilimumab. In fact, the IMmotion 150 phase II trial demonstrated that distinct subgroups of tumors characterized by distinct gene expression signatures for angiogenesis, T cell response, and inflammatory markers have differentially associated PFS when treated with atezolizumab+bevacizumab, atezolizumab alone, or sunitinib alone (41). The recently published phase III trials, JAVELIN 101 and KEYNOTE-426, have also expanded the options for treating advanced ccRCC. This, however, also raises questions on how to determine which immunotherapy combination will be most appropriate for patients and what subgroups may have the greatest benefit from these newer combination therapies (42).
Future Directions
The results of the Checkmate 214, JAVELIN 101, and KEYNOTE-426 trials have ushered in the era of combination therapy for mRCC, and additional studies are ongoing (Table 3). The IMmotion151 randomized phase III trial demonstrated that the combination of bevacizumab (anti-VEGF) and atezolizumab (PD-L1 inhibitor) delays PFS compared to sunitinib in PD-L1 positive individuals with mRCC, which was consistent across all risk groups in subgroup analysis (41, 43). Uniquely, this trial included both clear cell and sarcomatoid histology and demonstrated that patients with sarcomatoid histology benefited from the combination therapy compared to sunitinib (HR 0.46). While data from the IMmotion151 phase III trial has not matured, PD-L1+ patients benefited with improved PFS (11.2 vs 7.7 months, p=0.02) when receiving bevacizumab-atezolizumab compared to sunitinib after a median follow-up of 15 months (44, 45). Interim results from the CLEAR and Checkmate 9ER trials (Table 3) are still pending, but the data from these trials thus far are intriguing. However, the goal of therapeutic synergy with minimal or only additive toxicity has yet to be achieved.
Conclusions
The last decade has seen a rapid expansion in the number of therapies approved to treat metastatic clear cell RCC. Combination therapies offer great promise, as nivolumab-ipilimumab has the highest CR rate, especially in patients with intermediate/poor risk disease. As newer trial data expands the therapeutic horizon for treating advanced RCC, the benchmark for treating advanced RCC will continue to change. This is reminiscent of how TKIs supplanted high dose IL-2 because of their efficacy and side effect profile (46). Additional work into combination therapies using multiple immunotherapies, immunotherapy and TKI, and multiple immunotherapies with TKI will continue to change the mRCC landscape. The search for novel biomarkers will continue since currently we lack reliable diagnostic, therapeutic, and prognostic RCC biomarkers. These biomarkers will also be needed to help select the patient populations best served by cytoreductive surgery followed by systemic therapy (47). Similarly, elucidating the role of tumor resistance and heterogeneity will help inform first line and subsequent line treatment selection. Given the added toxicity of combination therapy, renewed attention to best supportive care/symptom management starting at the time of diagnosis is also critical.
Key Points.
As the therapeutic landscape for mRCC continues to evolve, identifying the “correct” patient for a given therapy is of utmost importance.
Cytoreductive surgery remains an important consideration for the carefully selected patient.
The evaluation of novel combination therapies provides a promising future for treating patients with mRCC.
Acknowledgments
Financial support and sponsorship
The research was funded by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research, Bethesda, Maryland, USA, and by a grant from the National Cancer Institute (P30CA072720).
Footnotes
Conflicts of interest
EAS receives research support from Astellas/Medivation.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Zisman A, Pantuck AJ, Wieder J, Chao DH, Dorey F, Said JW, et al. Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. J Clin Oncol. 2002;20(23):4559–66. [DOI] [PubMed] [Google Scholar]
- 3.Nerich V, Hugues M, Paillard MJ, Borowski L, Nai T, Stein U, et al. Clinical impact of targeted therapies in patients with metastatic clear-cell renal cell carcinoma. Onco Targets Ther. 2014;7:365–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore LE, Nickerson ML, Brennan P, Toro JR, Jaeger E, Rinsky J, et al. Von Hippel-Lindau (VHL) inactivation in sporadic clear cell renal cancer: associations with germline VHL polymorphisms and etiologic risk factors. PLoS Genet. 2011;7(10):e1002312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292(5516):464–8. [DOI] [PubMed] [Google Scholar]
- 6.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292(5516):468–72. [DOI] [PubMed] [Google Scholar]
- 7.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399(6733):271–5. [DOI] [PubMed] [Google Scholar]
- 8.Semenza GL, Nejfelt MK, Chi SM, Antonarakis SE. Hypoxia-inducible nuclear factors bind to an enhancer element located 3’ to the human erythropoietin gene. Proc Natl Acad Sci U S A. 1991;88(13):5680–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsieh JJ, Purdue MP, Signoretti S, Swanton C, Albiges L, Schmidinger M, et al. Renal cell carcinoma. Nat Rev Dis Primers. 2017;3:17009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11(11):3887–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–6. [DOI] [PubMed] [Google Scholar]
- 12.Flanigan RC, Mickisch G, Sylvester R, Tangen C, Van Poppel H, Crawford ED. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol. 2004;171(3):1071–6. [DOI] [PubMed] [Google Scholar]
- 13.Flanigan RC, Yonover PM. The role of radical nephrectomy in metastatic renal cell carcinoma. Semin Urol Oncol. 2001;19(2):98–102. [PubMed] [Google Scholar]
- 14.Mickisch GH, Garin A, van Poppel H, de Prijck L, Sylvester R, European Organisation for R, et al. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001;358(9286):966–70. [DOI] [PubMed] [Google Scholar]
- 15. Mejean A, Ravaud A, Thezenas S, Colas S, Beauval JB, Bensalah K, et al. Sunitinib Alone or after Nephrectomy in Metastatic Renal-Cell Carcinoma. N Engl J Med. 2018;379(5):417–27. *A study to evaluate the use of sunitinib alone versus sunitnib following cytoreductive nephrectomy for advanced RCC.
- 16. Bex A, Mulders P, Jewett M, Wagstaff J, van Thienen JV, Blank CU, et al. Comparison of Immediate vs Deferred Cytoreductive Nephrectomy in Patients with Synchronous Metastatic Renal Cell Carcinoma Receiving Sunitinib: The SURTIME Randomized Clinical Trial. JAMA Oncol. 2018. *Comparing the timing of cytoreductive nephrectomy in patients with metastatic RCC receiving sunitinib.
- 17.Choueiri TK, Halabi S, Sanford BL, Hahn O, Michaelson MD, Walsh MK, et al. Cabozantinib Versus Sunitinib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial. J Clin Oncol. 2017;35(6):591–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choueiri TK, Hessel C, Halabi S, Sanford B, Michaelson MD, Hahn O, et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): Progression-free survival by independent review and overall survival update. Eur J Cancer. 2018;94:115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Administration USFaD. FDA grants regular approval to Cabometyx for first-line treatment of advanced renal cell carcinoma 2017.
- 20.Choueiri TK, Escudier B, Powles T, Tannir NM, Mainwaring PN, Rini BI, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17(7):917–27. [DOI] [PubMed] [Google Scholar]
- 21.Motzer RJ, Escudier B, Powles T, Scheffold C, Choueiri TK. Long-term follow-up of overall survival for cabozantinib versus everolimus in advanced renal cell carcinoma. Br J Cancer. 2018;118(9):1176–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beuselinck B, Oudard S, Rixe O, Wolter P, Blesius A, Ayllon J, et al. Negative impact of bone metastasis on outcome in clear-cell renal cell carcinoma treated with sunitinib. Ann Oncol. 2011;22(4):794–800. [DOI] [PubMed] [Google Scholar]
- 23.McKay RR, Kroeger N, Xie W, Lee JL, Knox JJ, Bjarnason GA, et al. Impact of bone and liver metastases on patients with renal cell carcinoma treated with targeted therapy. Eur Urol. 2014;65(3):577–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKay RR, Lin X, Perkins JJ, Heng DY, Simantov R, Choueiri TK. Prognostic significance of bone metastases and bisphosphonate therapy in patients with renal cell carcinoma. Eur Urol. 2014;66(3):502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17(8):2530–40. [DOI] [PubMed] [Google Scholar]
- 26.Escudier B, Powles T, Motzer RJ, Olencki T, Aren Frontera O, Oudard S, et al. Cabozantinib, a New Standard of Care for Patients With Advanced Renal Cell Carcinoma and Bone Metastases? Subgroup Analysis of the METEOR Trial. J Clin Oncol. 2018;36(8):765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDermott DF, Lee J, Szczylik C, Donskov F, Malik J, Alekseev BY, et al. , editors. Pembrolizumab Monotherapy as First-Line Therapy in Advanced Clear Cell RCC: Results from Cohort A of KEYNOTE-427. ASCO; 2018; 2018; Chicago, IL. [Google Scholar]
- 28. Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. 2018;378(14):1277–90. **First study to demonstrate efficacy of combination immunotherapy in treating advanced RCC.
- 29.Choueiri TK, Fishman MN, Escudier B, McDermott DF, Drake CG, Kluger H, et al. Immunomodulatory Activity of Nivolumab in Metastatic Renal Cell Carcinoma. Clin Cancer Res. 2016;22(22):5461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.George S, Motzer RJ, Hammers HJ, Redman BG, Kuzel TM, Tykodi SS, et al. Safety and Efficacy of Nivolumab in Patients With Metastatic Renal Cell Carcinoma Treated Beyond Progression: A Subgroup Analysis of a Randomized Clinical Trial. JAMA Oncol. 2016;2(9):1179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.A Phase II Open-Label Study of Single Agent MDX-010 for the Treatment of IL-2 Refractory or IL-2 Ineligible Patients With Stage IV Renal Cancer [Internet]. 2003.
- 32.Phase II Sequential Treatment Trial of Single Agent Nivolumab, Then Combination Ipilimumab + Nivolumab in Metastatic or Unresectable Non-Clear Cell Renal Cell Carcinoma (ANZUP1602) (UNISoN) [Internet]. 2017.
- 33. Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. New England Journal of Medicine. 2019. **Efficacy of pembrolizumab and axinitib for treating advanced RCC.
- 34.Motzer RJ, editor JAVELIN Renal 101: a randomized, phase 3 study of avelumab + axitinib vs sunitinib as first-line treatment of advanced renal cell carcinoma (aRCC). European Society for Medical Oncology (ESMO) 2018 Congress; 2018. 10/21/2018; Munich, Germany: ESMO. [Google Scholar]
- 35. Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. New England Journal of Medicine. 2019. **Expands the therapies used to treat advanced RCC.
- 36.Flanigan RC, Salmon SE, Blumenstein BA, Bearman SI, Roy V, McGrath PC, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345(23):1655–9. [DOI] [PubMed] [Google Scholar]
- 37. Motzer RJ, Russo P. Cytoreductive Nephrectomy - Patient Selection Is Key. N Engl J Med. 2018;379(5):481–2. **Important commentary on the role of patient selection for cytoreductive nephrectomy for treating advanced RCC.
- 38.Bhindi B, Habermann EB, Mason RJ, Costello BA, Pagliaro LC, Thompson RH, et al. Comparative Survival following Initial Cytoreductive Nephrectomy versus Initial Targeted Therapy for Metastatic Renal Cell Carcinoma. J Urol. 2018;200(3):528–34. [DOI] [PubMed] [Google Scholar]
- 39.Bhindi B, Abel EJ, Albiges L, Bensalah K, Boorjian SA, Daneshmand S, et al. Systematic Review of the Role of Cytoreductive Nephrectomy in the Targeted Therapy Era and Beyond: An Individualized Approach to Metastatic Renal Cell Carcinoma. Eur Urol. 2018. [DOI] [PubMed] [Google Scholar]
- 40.Choueiri TK, Escudier B, Powles T, Mainwaring PN, Rini BI, Donskov F, et al. Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373(19):1814–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McDermott DF, Huseni MA, Atkins MB, Motzer RJ, Rini BI, Escudier B, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med. 2018;24(6):749–57. *Distinct gene signatures are differentially associated with PFS when treated with atezolizumab+bevacizumab, atezolizumab alone, or sunitinib alone.
- 42. Escudier B Combination therapy as first-line treatment in metastatic renal-cell carcinoma. New England Journal of Medicine. 2019. **Commentary providing comparison of recent combination therapies and limitations.
- 43.Escudier B, Motzer RJ, Rini BI, Powles T, McDermott DF, Suarez C, et al. Patient-reported outcomes (PROs) in IMmotion151: Atezolizumab (atezo) + bevacizumab (bev) vs sunitinib (sun) in treatment (tx) naive metastatic renal cell carcinoma (mRCC). Journal of Clinical Oncology. 2018;36(15_suppl):4511-. [Google Scholar]
- 44.Motzer RJ, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C, et al. IMmotion151: A Randomized Phase III Study of Atezolizumab Plus Bevacizumab vs Sunitinib in Untreated Metastatic Renal Cell Carcinoma (mRCC). Journal of Clinical Oncology. 2018;36(6_suppl):578-. [Google Scholar]
- 45. Rini BI, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet. 2019;393(10189):2404–15. **Shows efficacy of anti-VEGF and anti-PDL1 combination in treating mRCC in first-line setting
- 46.Allard CB, Gelpi-Hammerschmidt F, Harshman LC, Choueiri TK, Faiena I, Modi P, et al. Contemporary trends in high-dose interleukin-2 use for metastatic renal cell carcinoma in the United States. Urol Oncol. 2015;33(11):496 e11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shinder BM, Rhee K, Farrell D, Farber NJ, Stein MN, Jang TL, et al. Surgical Management of Advanced and Metastatic Renal Cell Carcinoma: A Multidisciplinary Approach. Front Oncol. 2017;7:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20(1):289–96. [DOI] [PubMed] [Google Scholar]
- 49.Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27(34):5794–9. [DOI] [PubMed] [Google Scholar]
- 50.Heng DY, Xie W, Regan MM, Harshman LC, Bjarnason GA, Vaishampayan UN, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol. 2013;14(2):141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]