Abstract
Objectives:
Ribavirin inhibits eukaryotic translation initiation factor 4E (eIF4E), thereby decreasing cap-dependent translation. In this two-part study, we assessed the pharmacodynamic effects and therapeutic potential of ribavirin in human papillomavirus (HPV)–related malignancies.
Methods:
In the pharmacodynamic study, ribavirin (400 mg BID for 14 days) was evaluated in 8 patients with HPV-positive localized oropharyngeal carcinoma with phosphorylated-eIF4E (p-eIF4E) ≥30%. In the therapeutic study, ribavirin (1400 mg BID in 28-day cycles, continuously dosed) was evaluated in 12 patients with recurrent and/or metastatic HPV-related cancer. Dose interruptions or reductions were allowed according to prespecified criteria. Toxicities were assessed in accordance with National Cancer Institute Common Terminology Criteria for Adverse Events version 4; response was assessed using Response Evaluation Criteria in Solid Tumors version 1.1. Patients remained on study until disease progression or unacceptable toxicity.
Results:
Six patients were evaluable in the pharmacodynamic study: 4 had decreased p-eIF4E after 14 days of ribavirin. In the therapeutic study, 12 patients were evaluable for toxicity, and 9 were evaluable for response. Among these, median follow-up was 3.5 months, and best overall response was stable disease in 5 patients and progression of disease in 4 patients. Median progression-free survival was 1.8 months. The most common treatment-related adverse events (grade >2) were anemia, dyspnea, and hyperbilirubinemia. All patients had anemia (grades 1–3), with 33% having at least 1 dose reduction.
Conclusion:
Oral ribavirin decreases p-eIF4E levels and is well-tolerated. However, a clear signal of efficacy in patients with recurrent and/or metastatic HPV-related cancers was not observed. (NCT02308241, NCT01268579)
Keywords: Ribavirin, human papillomavirus, HPV-related cancers, eIF4E, cap-dependent translation
INTRODUCTION
Human papillomavirus (HPV) is an oncovirus that has been implicated in the pathogenesis of nearly all cervical cancers and a substantial proportion of head and neck (HN), anal, penile, vaginal, and vulvar cancers. The number of HPV-related malignancies in the US remains above 30,000 per year, driven primarily by an increased incidence of HPV-related oropharyngeal squamous cell carcinomas (SCCs) despite decreased numbers of cervical cancers. While the prognosis of localized HPV-related cancers is generally favorable, recurrent and/or metastatic (R/M) disease carries a poor prognosis with a median overall survival of about 1 year [1–5]. Standard therapies developed for R/M HPV-related cancers improve clinical outcomes, but are not curative, leaving a persistent need to develop new therapies. For instance, the PD-1 checkpoint inhibitor pembrolizumab has a modest overall response rate of ~20% in R/M HNSCC and ~15% in advanced cervical cancer [5–7].
There is substantial interest in developing tissue agnostic therapies that target the biology of HPV-driven oncogenesis. HPV causes malignant transformation primarily through expression of E6 and E7 oncoproteins, which target p53 and pRb tumor suppressors for proteasomal degradation, respectively. E6 and E7 also cause the translation of mRNAs with 5’ 7-methyl-guanosine caps [8–12]. Activation of “cap-dependent translation” promotes cancer growth and survival through upregulation of cap-dependent proto-oncogene transcripts such as CCND1, VEGF, BCL2, MMP9, and c-MYC, as well as E6 and E7 [13–16].
The central regulator of cap-dependent translation is the eukaryotic translation initiation factor 4E (eIF4E), which is regulated by oncogenic PI3K (phosphatidylinositol 3-kinase)/AKT/mTOR signaling [17]. Stimulated by growth factors, cellular stress, or changes in nutrients, PI3K/AKT activates the mTORC1 kinase complex, which in turn phosphorylates the translational repressor protein eIF4E-binding protein–1 (4E-BP1). Hyperphosphorylated 4E-BP1 releases eIF4E to interact with the translation initiation complex to initiate cap-dependent translation [8]. Approximately 80% of HNSCCs possess increased PI3K/AKT/mTOR signaling, and phosphorylated-eIF4E (p-eIF4E) is detected in almost all HPV-related HNSCCs [18, 19]. Elevated levels of eIF4E and cap-dependent translation are also seen in cervical cancer, a disease almost entirely driven by HPV [20]. Inhibition of eIF4E suppresses the growth of HNSCC and cervical cancer cell lines in vitro[21, 22] and in mouse xenograft models[23], nominating eIF4E as a putative therapeutic target for HPV-related cancers.
Ribavirin is a guanosine ribonucleoside analog with activity against a wide range of viruses. Although its mechanism of action is not entirely understood[24], ribavirin mimics the 7-methyl-guanosine 5’ cap of mRNA, thereby blocking eIF4E binding and inhibiting translation of capped viral transcripts [25–27]. Ribavirin targets eIF4E and suppresses tumor growth in hypopharyngeal cancer cells, chemo-resistant nasopharyngeal cell lines, and xenografts [27, 28]. In HPV-positive cervical cancer cell lines and xenografts, ribavirin induces cell cycle arrest and apoptosis [29]. Clinically, ribavirin has activity against laryngeal papillomatosis, a rare HPV-related disease of the upper airway [30, 31]. Ribavirin reduced eIF4E levels in patients with relapsed acute myelogenous leukemia (AML) [32, 33]. Taken together, these data suggest that ribavirin may possess activity in HPV-related malignancies by targeting eIF4E [34, 35].
Herein, we describe a two-part study of single-agent ribavirin. First, we conducted a 14-day pharmacodynamic study to evaluate if ribavirin can reduce p-eIF4E levels in patients with localized HPV-related HNSCC. We subsequently conducted a pilot therapeutic study of ribavirin in patients with R/M HPV-related malignancies.
PATIENTS AND METHODS
Both studies were single-institution phase I studies approved by the institutional review board at Memorial Sloan Kettering Cancer Center. All patients gave informed written consent.
Pharmacodynamic Study
Eligible patients had localized HNSCC and a biopsy of the primary tonsil and/or base of tongue or involved neck lymph nodes that showed SCC positive for p16 by immunohistochemistry, with or without HPV on in situ hybridization, and p-eIF4E ≥30% on immunohistochemistry. Other inclusion criteria were age 18–65; Karnofsky performance status ≥80; adequate hematologic, renal, and liver function; negative pregnancy test (if of childbearing potential); ability to swallow oral medications; and agreement to undergo biopsy after ribavirin treatment (if the patient did not undergo planned surgery). Exclusion criteria were prior chemotherapy or radiation for tonsil or base-of-tongue cancer, ≥10 pack-years of smoking, history of hemolytic anemia or thalassemia, active infection or other serious medical condition that would prevent protocol treatment, current or prior treatment with ribavirin, active hepatitis B or C infection, and clinically significant cardiovascular disease.
Ribavirin was self-administered at 400 mg PO BID (800-mg flat total daily dose) for 14 days (and no less than 10 days). The safety and efficacy of this dosing was delineated in hepatitis C clinical trials [36, 37]. Medication compliance was assessed by pill diaries and pill counts at each visit. Surgical resection or research biopsy was performed on Day 15 or at the discretion of the surgeon. Toxicities were assessed in accordance with National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4. All evaluable patients were able to be maintained on the 400-mg BID dose.
The primary objective of the study was to determine whether 2 weeks of ribavirin decreased tumor expression of p-eIF4E among patients with HPV-associated tonsil or base-of-tongue cancer. Formalin-fixed, paraffin-embedded tissue was processed using established methods. p-eIF4E staining was performed using standard methodology with a primary antibody against phospho-eIF4E-ser209 (Cell Signaling Technologies #9741). p-eIF4E was scored as the percentage of the total cells that stained positive. Positive p-eIF4E was defined as ≥30% of cells with cytoplasmic and/or nuclear staining [19, 21, 38, 39].
Therapeutic Study
Eligible patients had R/M HPV-associated SCC of the cervix, anus, vagina, vulva, penis, or oropharynx and had received at least 1 prior systemic therapy for R/M disease. HPV was confirmed by p16 immunohistochemistry, with or without HPV on in situ hybridization, except for cervical cancer, where SCC was presumed to be HPV related. Biopsies were not required, otherwise the other inclusion/exclusion criteria were similar to those for the pharmacodynamic study.
Ribavirin was self-administered at 1400 mg PO BID (2800-mg total daily dose) with food in 28-day cycles with continuous dosing. This dose was chosen because it was the recommended phase II dose (RP2D) based on the phase I trial of ribavirin in AML [33]. Medication compliance was assessed by pill diaries and pill counts at each visit. Dose interruptions or reductions were allowed according to prespecified criteria: dose level −1, 1000 mg PO BID; dose level −2, 800 mg PO BID; dose level −3, 600 mg PO BID. Toxicities were assessed according to CTCAE version 4. Cross-sectional imaging (CT or MRI) was obtained at baseline and after every 2 cycles. Response was assessed by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Patients remained on study until disease progression or unacceptable toxicity.
The primary objective was to evaluate RECIST v1.1 response with ribavirin in patients with R/M HPV-related malignancies. Ribavirin would be considered worthy of further study if response was observed in at least 2 of 12 subjects, with the probability of missing an effect (observing 0–1 responses if the true response rate is 30%) of 0.09. This cutoff for response rate was based on historical efficacy data of other drugs used in second-line or greater for R/M head and neck, cervical, and anal cancers, for which response rates are typically 15%−20% [2–5]. Secondary objectives were to (1) determine overall survival and progression-free survival, (2) determine the safety and tolerability of ribavirin in this patient population, and (3) perform tissue correlative studies for the purpose of hypothesis generation.
RESULTS
Pharmacodynamic Study
From April 2011 to September 2013, 8 patients were enrolled, of whom 6 were evaluable (Figure 1). One withdrew consent, and 1 had no viable cells in the posttreatment biopsy specimen for analysis. Of these 6 patients, 4 had a marked decreased in p-eIF4E staining after 14 days of ribavirin (Table 1, Figure 2). We took these results as evidence that oral ribavirin could reach its target and exert an effect on p-eIF4E in the tumors and deemed the drug worthy of further evaluation in our therapeutic pilot study.
Figure 1.
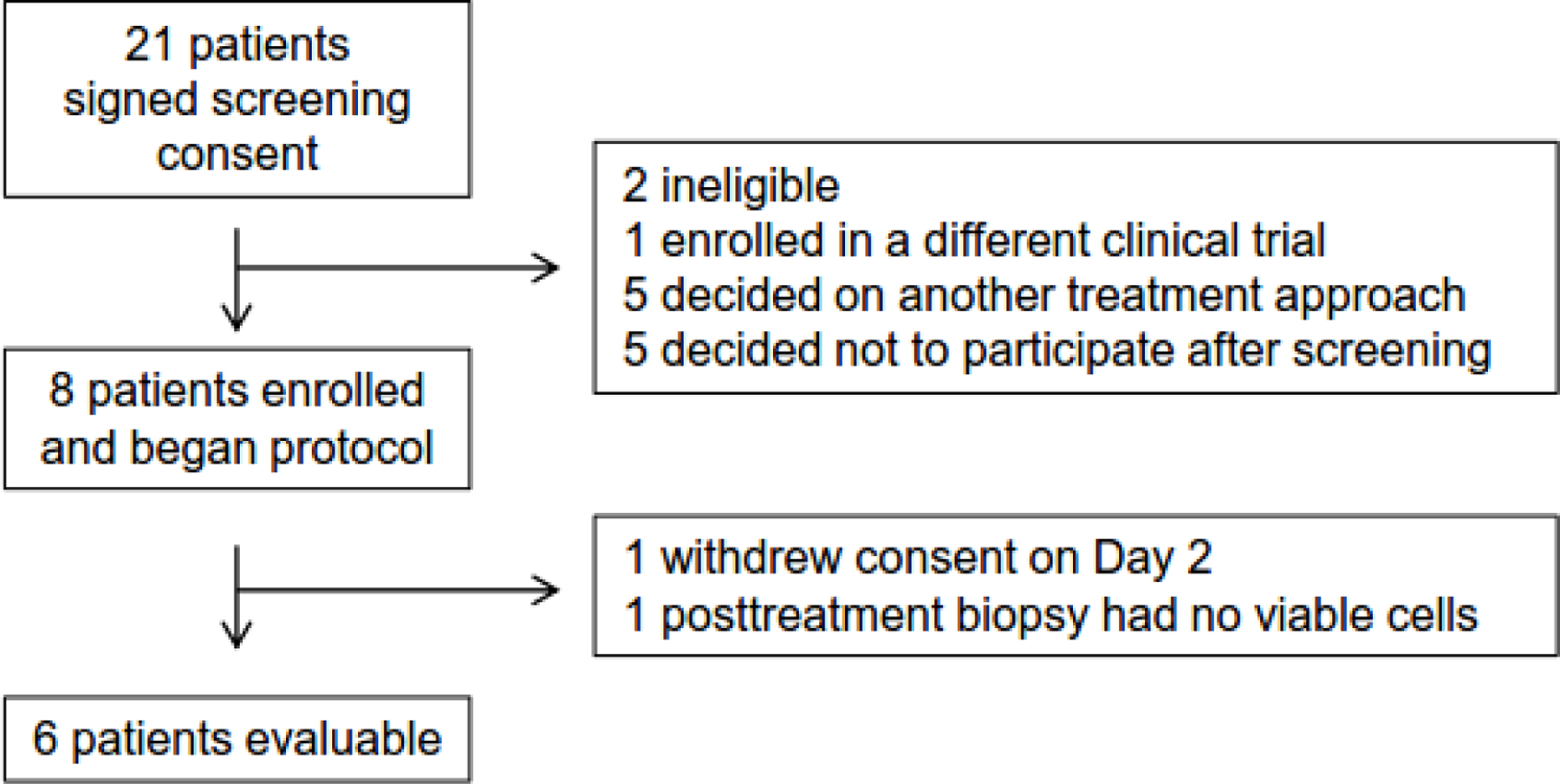
Enrollment schema of the pharmacodynamic study.
Table 1.
Summary of phospho-eIF4E staining in the 6 evaluable patients in the pharmacodynamic study
| Patient | Baseline phospho-eIF4E, % | Posttreatment phospho-eIF4E, % |
|---|---|---|
| 1 | 70 | 30 |
| 2 | 90 | 30 |
| 3 | 70–80 | 40–50 |
| 4 | 40 | 40 |
| 5 | 80 | 50 |
| 6 | 80 | 80 |
Results are scored as the percent of tumor cells that had positive nuclear and/or cytoplasmic phosho-eIF4E staining by immunohistochemistry. Samples from subjects 3 and 4 were surgical specimens; the remainder were fine-needle aspirates
Figure 2.
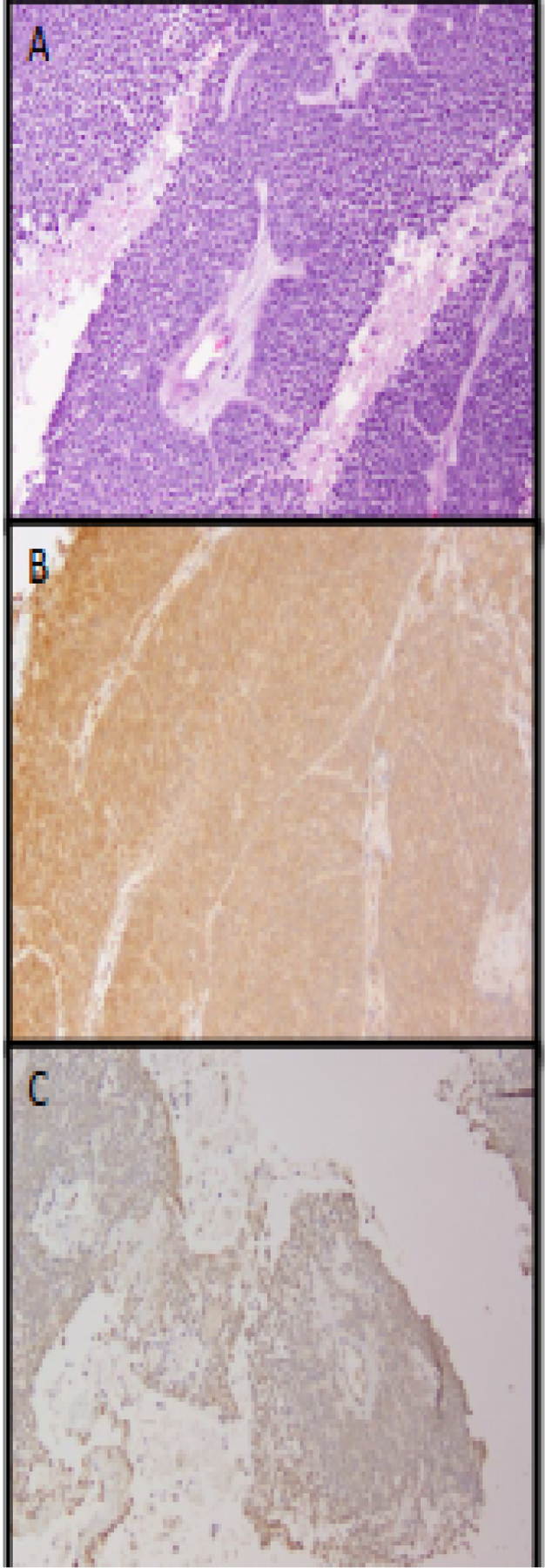
Reduction in phospho-eIF4E staining by immunohistochemistry in Patient 2. (A) Pretreatment biopsy specimen showing characteristics of nonkeratinizing squamous cell carcinoma. (B) Pretreatment biopsy specimen stained with phospho-eIF4E. (C) Posttreatment biopsy specimen stained with phospho-eIF4E.
No grade >2 toxicities were observed during the study. Anemia, the most common toxicity associated with ribavirin, was not observed in any patient; therefore, no dose reductions or interruptions were required for anemia. The most common laboratory abnormalities possibly related to treatment were a grade 1 increase in alanine aminotransferase in 3 patients and a grade 1 increase in bilirubin in 1 patient.
Of the 6 patients, 5 had paired pre- and post-treatment imaging available. Although clinical response was not formally addressed in the pharmacodynamic study, all 5 patients appeared to have stable disease. One patient had a decrease in FDG avidity on PET scan at the primary site (standardized uptake value, from 13.2 to 4.3).
Therapeutic Study
From January 2015 to April 2017, 12 patients were enrolled in this study (Figure 3). Median age was 59 years (range, 47–69). Five patients had anal canal cancer, 3 had cervical cancer, and 4 had oropharyngeal cancer. Patients had received a median of 3 lines of prior chemotherapy (range, 1–6), including chemotherapy given concurrently with radiation, and 11 had received prior radiation therapy. Baseline characteristics are presented in Supplementary Table 1.
Figure 3.
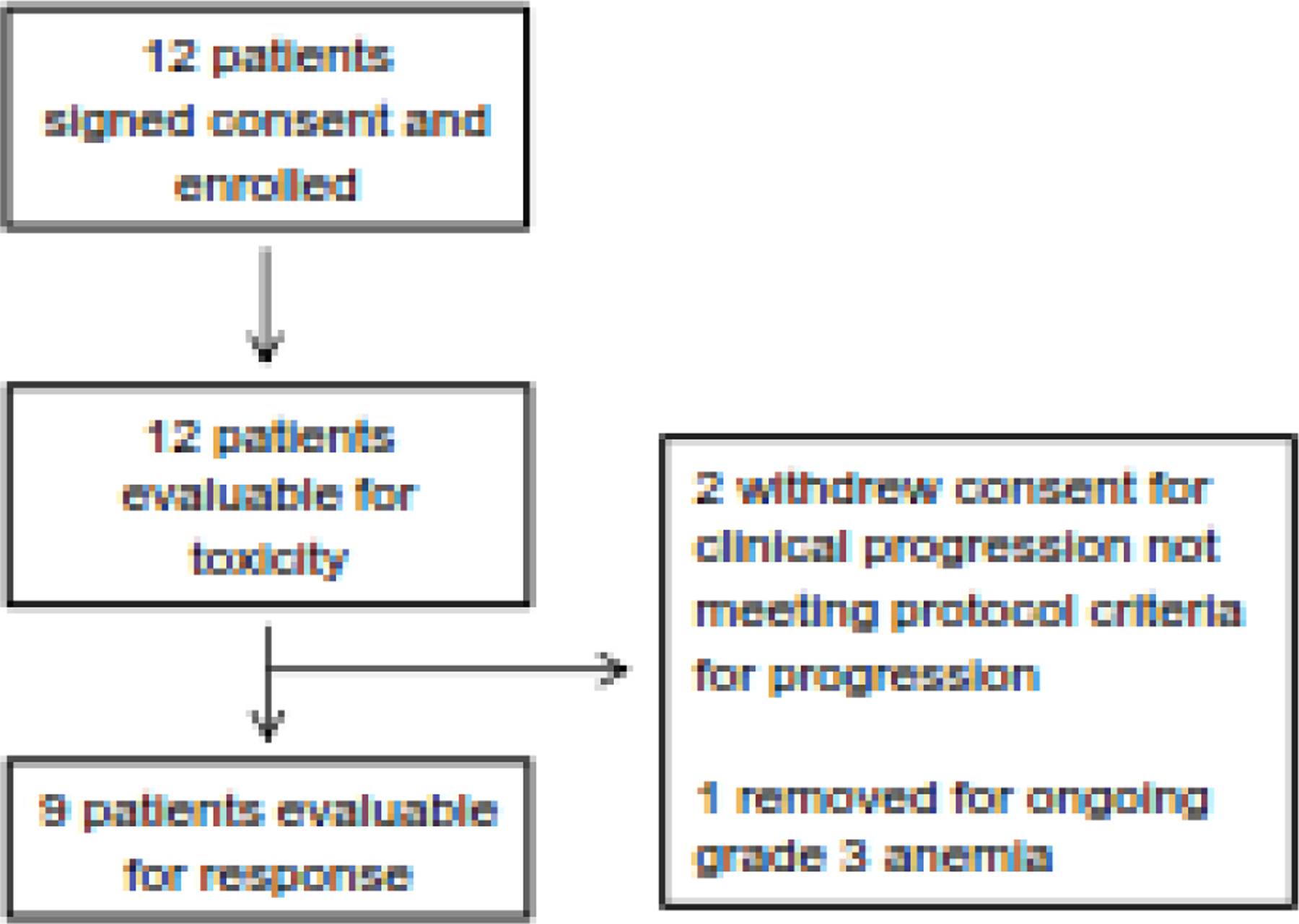
Enrollment schema of the therapeutic study.
All patients were evaluable for toxicity. The most common toxicity attributed to ribavirin was anemia: 25% grade 1, 50% grade 2, and 25% grade 3. One patient required discontinuation of ribavirin and removal from the study due to refractory grade 3 anemia that persisted after 2 dose reductions. One episode of grade 3 hyperbilirubinemia was attributed to the drug, which resolved after holding the drug and dose reduction. In total, 7 patients required dose reductions (4 for anemia and 3 for abnormal liver function tests), with 3 reaching dose level −1 (1000 mg BID) and 4 reaching dose level −2 (800 mg BID). Among the 12 evaluable patients, the median number of cycles completed was 2 (range, 1–8), and the median number of days of treatment was 34 (range, 19–222). There were no grade 4 or 5 toxicities in the study. No other grade 3 toxicities could clearly be attributed to the study drug. Treatment-related toxicities that occurred in ≥2 patients are listed in the Table 2.
Table 2.
Treatment-related toxicities occurring in ≥2 patients in therapeutic study
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 or 5 | Any grade |
|---|---|---|---|---|---|
| Anemia | 2 (17) | 3 (25) | 1 (8) | 6 (50) | |
| Dyspnea | 4 (33) | 1 (8) | 4 (42) | ||
| Blood bilirubin increase | 1 (8) | 1 (8) | 1 (8) | 3 (25) | |
| Fatigue | 1 (8) | 2 (17) | 3 (25) | ||
| Anorexia | 2 (17) | 2 (17) | |||
| Aspartate aminotransferase increase | 2 (17) | 2 (17) | |||
| Fever | 1 (8) | 1 (8) | 2 (17) | ||
| Gastroesophageal reflux | 2 (17) | 2 (17) | |||
| Weight loss | 2 (17) | 2 (17) | |||
| Alanine aminotransferase increase | 1 (8) | 2 (17) | |||
| Alkaline phosphatase increase | 1 (8) | 2 (17) |
Data are no. (%).
Nine patients were evaluable for response. One patient with anal cancer withdrew consent after 1 cycle owing to rectal bleeding believed to be due to clinical progression, without radiographic evidence of progression. Another patient with anal cancer withdrew consent after 1 cycle after imaging showed tumor growth. One patient with oropharynx cancer withdrew consent after 1 cycle owing to overall clinical decline. At the time of analysis, all patients had been removed from the study drug, with a median follow-up of 3.5 months (range, 1.4–10.4). Among these 9 patients, the best overall response was stable disease in 5 patients and progression of disease in 4 patients (Table 3). None of the patients had radiographic response that met the criteria for partial response (the largest RECIST response was −6%); thus, we did not meet our prespecified cutoff of 2 responses to warrant further study of ribavirin for this indication. Median progression-free survival was 1.8 months. At the time of analysis, 8 patients had died, and 4 had been censored, with a median survival of 8.6 months (95% confidence interval, 4.3–19.4) (Figure 4).
Table 3.
Summary of patient characteristics, best overall response, duration of response, reason for discontinuation, and molecular testing among patients in the therapeutic study.
| Patient | Tumor type | Prior lines ofchemotherapy | Best overall response | Best responseby RECIST, % | Treatmentduration, days | Reason for discontinuing study | Molecular testing |
|---|---|---|---|---|---|---|---|
| 1 | Oropharynx | 5 | SD | −6 | 222 | Disease progression | ASXL1; DNMT3B; ATR; EP300; MLL3; PIK3R1 |
| 2 | Anal | 1 | SD | 6 | 59 | Withdrawal of consenta | Not performed |
| 3 | Cervix | 3 | SD | 8 | 39 | Toxicity(anemia) | PIK3CA; SOX2; EIF4A2; TP63; BLM; FBXW7; FGFR1; GRIN2A; MGA; MLL2; NUP93; PAK7; RAD51C; RPTOR; SMO; SUFU; TSC2 |
| 4 | Anal | 2 | POD | 39 | 27 | Disease progression | TP63; CTNNB1 |
| 5 | Anal | 2 | SD | −1 | 55 | Progression (nontarget lesion) | PIK3CA; B2M; BRAF; CRKL; EP300; ERG; KDM6A; LATS2; MLL3; PIK3C3; RYBP; STAG2; TETI |
| 6b | Oropharynx | 3 | – | – | 27 | Clinical decline (pain) | ERBB2; TP63; DICER1; MDC1; NOTCH1; ATR |
| 7b | Anal | 6 | SD | 1 | 27 | Withdrawal of consenta | PIK3CA |
| 8 | Anal | 2 | SD | 3 | 173 | Disease progression | PIK3CA, GNA11; TGFBR2; ANKRD11; DICER1; DIS3; SPEN; KDM6A |
| 9 | Cervix | 3 | POD | 0 | 19 | Progression (nontarget lesion) | Not performed |
| 10 | Oropharynx | 4 | POD | 5 | 29 | Disease progression | GSK3B |
| 11b | Oropharynx | 5 | – | – | 19 | Withdrawal of consenta | PIK3CA; FBXW7; FOXA1; PTEN |
| 12 | Cervix | 2 | POD | −1 | 41 | Progression (nontarget lesion) | Not performed |
POD, progression of disease; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease.
Reasons for consent withdrawal: Patient 2: rectal bleeding; Patient 7: radiographic progression not meeting RECIST criteria; Patient 11: clinical decline.
Inevaluable for response: Patients 6 and 11: no interval imaging was performed; Patient 7: radiographic progression not meeting RECIST criteria, incomplete cycle.
Figure 4.
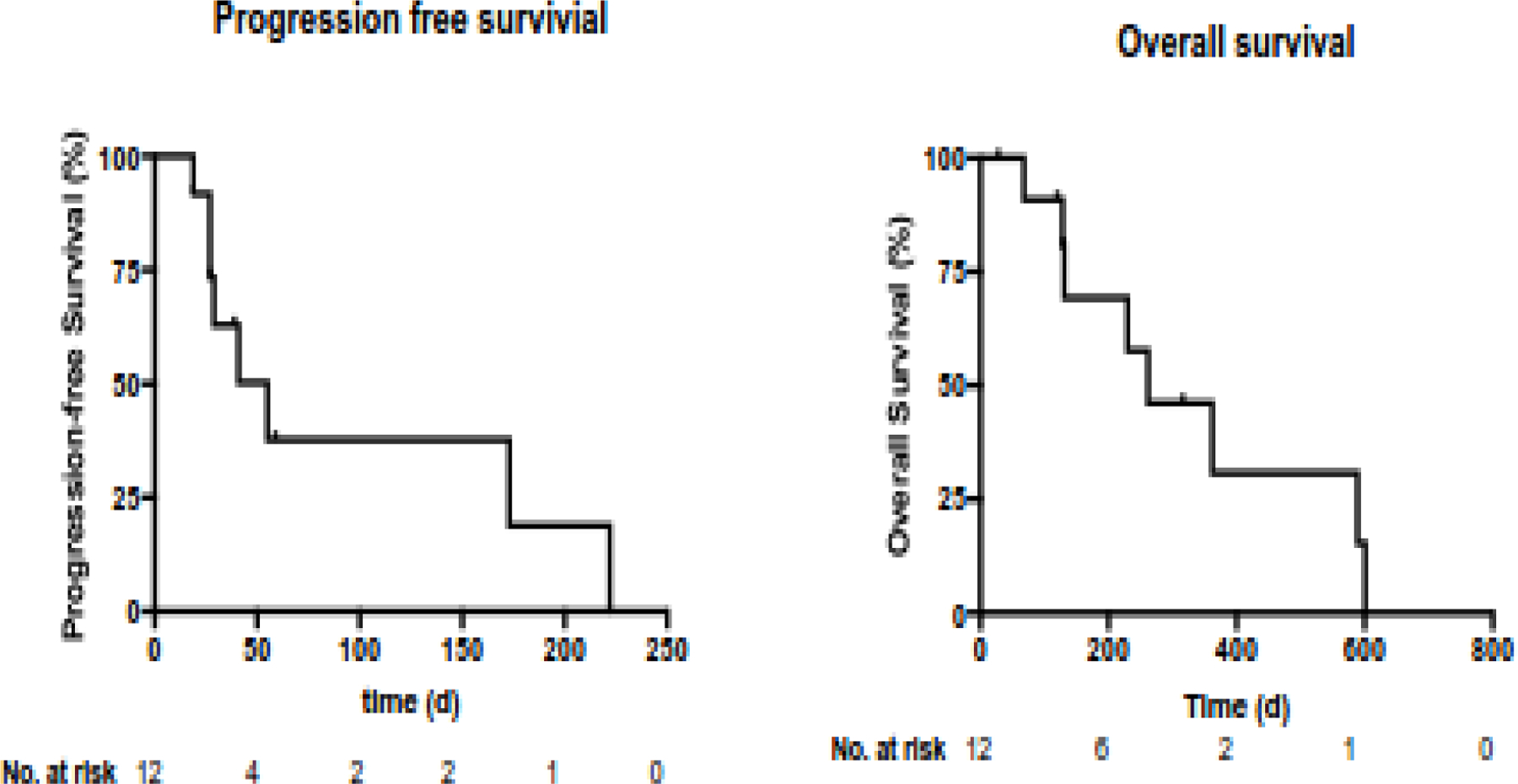
Progression-free survival and overall survival curves for patients in the therapeutic study.
All patients came off study within the first 2 cycles (8 for progression of disease, 1 for toxicity), except for 2 patients: 1 with oropharynx cancer that progressed after 8 cycles (222 days) and 1 with anal cancer that progressed after 6 cycles (173 days), although the clinical benefit of ribavirin in these cases is difficult to ascertain. The patient with stable disease for 222 days appeared to have an indolent course leading up to the trial; this patient had been treated with 5 lines of therapy over the course of 2 years for metastatic head and neck cancer and most recently had stable disease on single-agent paclitaxel for >7 months. The patient with stable disease for 173 days was treated relatively early in the course of his metastatic anal cancer, and thus the overall trajectory of his disease was not clear.
Nine of the 12 patients had molecular testing performed on their tumor (MSK-IMPACT; paired normal and tumor DNA). Typical for HPV-driven cancers, 5 tumors had mutations in PIK3CA (3 with E545K and 2 with E542K), and 1 had mutations in PIK3R1 (R755Fs), suggesting that response to ribavirin was not associated with tumors driven by activating mutations in the PI3K pathway (Table 3).
DISCUSSION
This two-part study demonstrated that oral ribavirin reduces p-eIF4E in early-stage HPV-related HNSCCs; however, it is unclear whether single-agent ribavirin suppresses tumor growth or leads to improved clinical outcomes in patients with R/M HPV-related cancers. Intriguingly, a ribavirin dose as low as 800 mg/day reduced tumor levels of p-eIF4E, indicating on-target effects. We chose a higher dose of 2800 mg/day for our therapeutic study based on the RP2D from a phase I trial of ribavirin in AML [33]. Although we observed no adverse events grade >3, all patients had some degree of anemia, with grade 3 anemia occurring in 25% of patients. Anemia, a known toxicity of ribavirin, caused 33% of patients to require at least 1 dose reduction, including 1 patient who developed refractory anemia and withdrew from the study. Of note, the AML study may have reached a higher RP2D because the protocol was more tolerant of anemia and ribavirin was combined with cytarabine, which can reduce its plasma levels [33].
The pharmacodynamic window study builds on our recently published report that HPV-positive oropharynx cancers universally express p-eIF4E, as well as on preclinical studies demonstrating that HPV E6 increases eIF4E-mediated cap-dependent translation through PI3K/AKT/mTOR signaling. Consistent with other studies, our data confirms that ribavirin significantly decreases p-eIF4E and cap-mediated translation. However, there are some limitations to our pharmacodynamic study. It is possible that the levels of p-eIF4E in tumors is inherently dynamic and an untreated control group was not analyzed to verify the biomarker decrease was specifically related to ribavirin treatment. Notably, the 2 patients without reduction had stable p-eIF4E staining between the two biopsies. Although 1 patient did have a decrease in PET signal of the primary tumor during the window study, the short treatment interval was not adequate to evaluate for significant clinical responses.
In our therapeutic pilot study, significant clinical activity was not detected. Two patients had atypically long intervals of stable disease (173 and 222 days), though the contribution of ribavirin to this clinical outcome is unclear. Limitations of this therapeutic evaluation were the small sample size and toxicities of high-dose ribavirin. The lack of activity may reflect that targeting eIF4E alone is insufficient to induce major tumor regressions. Alternatively, primary and acquired resistance to ribavirin can be mediated by increased expression of sonic hedgehog transcription factor glioma-associated protein 1 (GLI1), which drives UDP-glucuronosyltransferase–dependent glucuronidation of ribavirin [32, 33, 40, 41]. In phase I studies of ribavirin in AML, elevated GLI1 expression at baseline was associated with a lack of initial response, and in patients who did have an initial response, elevation in GLI1 was associated with acquired resistance [33, 40]. Interestingly, the hedgehog pathway inhibitor vismodegib restored sensitivity to ribavirin in vitro by downregulating GLI1[40], suggesting a combination of ribavirin and vismodegib could have therapeutic potential.
Beyond ribavirin, new potent and selective agents targeting eIF4E or cap-dependent translation are being developed for clinical testing and they may offer a better therapeutic window to elicit efficacy against HPV-related cancers [42]. Preclinical data demonstrate that inhibition of eIF4A, another subunit of the translation initiation complex, downregulates proteins associated with AKT and MAPK signaling; a phase I/II trial of a small molecule inhibitor is underway [43]. In addition, inhibitors of MAPK interacting kinase 1/2, such as tomivosertib, which blocks eIF4E phosphorylation, represent an area of active investigation with multiple compounds in clinical development [44]. However, all of these are still in early development, and studies in HPV-related diseases are needed.
Targeting the PI3K/AKT/mTOR pathway and downstream effectors remains an attractive therapeutic strategy for the treatment of HPV-positive and HPV-negative solid-tumor malignancies that rely on hyperactivation of this pathway. In our therapeutic study, 5 of 9 tumors with genomic profiling had activating PI3K mutations. To overcome feedback-based resistance mechanisms to traditional mTOR inhibitors, recent efforts to target the pathway have focused on targeting activating mutations of PI3K isoforms [45]. A phase I basket study of taselisib, a selective inhibitor of class I PI3K α, γ, and δ isoforms, showed an acceptable safety profile and a clinical response rate of 8.9% in 146 patients [46]. Interestingly, higher response rates were seen in patients with head and neck cancers (19.0%) and cervical cancer (10.5%) who may have HPV-positive tumors (subgroup analysis pending). However, in a subgroup analysis of buparlisib (BKM120), another oral pan-PI3K inhibitor, plus paclitaxel in patients with platinum-pretreated HNSCCs, objective response rate was slightly lower in patients with HPV-positive tumors (35.3%) than in patients with HPV-negative tumors (39.6%), although this difference was not statistically significant [47]. A trial of buparlisib combined with cisplatin and radiation therapy is underway and enrolling patients with HPV-positive and HPV-negative HNSCCs (NCT02113878). Whether these selective PI3K inhibitors inhibit eIF4E and cap-dependent translation remains to be determined.
Combination therapies using ribavirin and other agents with nonoverlapping toxicity profiles may lead to durable responses by increasing potency and overcoming resistance mechanisms. We recently reported the results of a phase Ib trial of induction chemotherapy with afatinib, ribavirin, and weekly carboplatin and paclitaxel for stage IVA/IVB HPV-positive oropharyngeal SCC in which patients received ribavirin daily for 3 21-day cycles before definitive treatment [48]. The best overall response was unconfirmed partial response in 6 patients (67%), stable disease in 2 patients (22%), and progression of disease in 1 patient (11%). Importantly, the regimen was safe and well tolerated, with no grade 3 anemia events. The utility of combining ribavirin with immune checkpoint inhibitors remains unclear. Two recent animal model studies revealed that inhibiting cap-dependent translation by targeting either eIF4F or MAPK interacting kinase 1/2 decreases expression of PD-L1, leading to tumor regressions and CD8+ T cell activation [49, 50]. However, increased eIF4F activation was associated with response to anti–PD-1 in patient melanoma samples [50]. In HPV-related cancers, targeting cap-dependent translation may decrease expression of E6 and E7, a source of unique viral antigens that is increasingly targeted by emerging cellular therapies and vaccines. Therefore, studies that combine cap-mediated translation inhibitors and immunotherapy for R/M HPV-positive cancers should be carefully considered.
CONCLUSION
In conclusion, ribavirin reduces levels of p-eIF4E, a downstream effector of PI3K/AKT/mTOR signaling and central regulator of cap-dependent translation. Although single-agent ribavirin downregulates a critical biomarker linked to HPV oncogenesis, it may be insufficient to control tumor growth in patients with R/M HPV-related cancers. More potent and selective inhibitors of PI3K and eIF4 isoforms, or combinations of ribavirin and other agents, may overcome resistance mechanisms and are currently being studied in clinical trials.
Supplementary Material
Highlights.
Therapies targeting the viral etiology of HPV-related cancers are lacking
HPV E6 and E7 oncogenesis is dependent on eIF4E-mediated cap-dependent translation
Ribavirin effectively reduces eIF4E levels in patients with HPV-related cancers
Ribavirin monotherapy likely has limited clinical efficacy in HPV-related cancers
Rational combinations with ribavirin or other eIF4E inhibitors should be considered
ACKNOWLEDGMENTS
We thank the Memorial Sloan Kettering Cancer Center research staff who coordinated execution of these clinical trials.
Funding:
The research was funded by the Garban Fund and NIH/NCI grant P30 CA008748. Bharat Burman is supported by the Conquer Cancer Foundation Young Investigator Award and NIH/NCI grant T32CA009207. The funders played no role in any aspect of the study.
Abbreviations:
- 4E-BP1
eIF4E-binding protein–1
- AML
acute myelogenous leukemia
- CTCAE
Common Terminology Criteria for Adverse Events
- eIF4E
eukaryotic translation initiation factor 4E
- GLI1
glioma-associated protein 1
- HN
head and neck
- HPV
human papillomavirus
- p-eIF4E
phospho-eIF4E
- POD
progression of disease
- RECIST
Response Evaluation Criteria in Solid Tumors
- R/M
recurrent and/or metastatic
- RP2D
recommended phase II dose
- SCCs
squamous cell carcinomas
- SD
stable disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
Bharat Burman, Nora Katabi, and Richard Wong: none declared. Scott Drutman is an employee and stockholder at Volastra Therapeutics. Matthew Fury is an employee and stockholders at Regeneron Pharmaceuticals. Alan Ho: advisory board/consulting at Eisai Pharmaceuticals, Sanofi Genzyme (Aventis), Novartis, AstraZeneca, Bristol-Myers Squibb, Genentech/Roche, Sun Pharmaceuticals, Ayala Pharmaceuticals, Regeneron, TRM Oncology, CureVac, Kura Oncology, Exelixis, Rgenta, Inxmed, Prelude Therapeutics, Merck, Ignyta, and Klus Pharma; data safety and monitoring committee for Affyimmune. David Pfister: advisory board/consulting at Boehringer Ingelheim and Incyte; clinical trial support from Merck and Hookipa Pharma.
REFERENCES
- [1].Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008;359:1116–27. [DOI] [PubMed] [Google Scholar]
- [2].Ghosn M, Kourie HR, Abdayem P, Antoun J, Nasr D. Anal cancer treatment: current status and future perspectives. World J Gastroenterol 2015;21:2294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pfaendler KS, Tewari KS. Changing paradigms in the systemic treatment of advanced cervical cancer. Am J Obstet Gynecol 2016;214:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Scatchard K, Forrest JL, Flubacher M, Cornes P, Williams C. Chemotherapy for metastatic and recurrent cervical cancer. Cochrane Database Syst Rev 2012;10:CD006469. [DOI] [PMC free article] [PubMed]
- [5].Burtness B, Harrington KJ, Greil R, Soulieres D, Tahara M, de Castro G, Jr., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 2019;394:1915–28. [DOI] [PubMed] [Google Scholar]
- [6].Frenel JS, Le Tourneau C, O’Neil B, Ott PA, Piha-Paul SA, Gomez-Roca C, et al. Safety and Efficacy of Pembrolizumab in Advanced, Programmed Death Ligand 1-Positive Cervical Cancer: Results From the Phase Ib KEYNOTE-028 Trial. J Clin Oncol 2017;35:4035–41. [DOI] [PubMed] [Google Scholar]
- [7].Chung HC, Ros W, Delord JP, Perets R, Italiano A, Shapira-Frommer R, et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol 2019;37:1470–8. [DOI] [PubMed] [Google Scholar]
- [8].Jackson RJ, Hellen CU, Pestova TV. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol 2010;11:113–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mantovani F, Banks L. The human papillomavirus E6 protein and its contribution to malignant progression. Oncogene 2001;20:7874–87. [DOI] [PubMed] [Google Scholar]
- [10].Spangle JM, Ghosh-Choudhury N, Munger K. Activation of cap-dependent translation by mucosal human papillomavirus E6 proteins is dependent on the integrity of the LXXLL binding motif. J Virol 2012;86:7466–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Spangle JM, Munger K. The human papillomavirus type 16 E6 oncoprotein activates mTORC1 signaling and increases protein synthesis. J Virol 2010;84:9398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang S, Pang T, Gao M, Kang H, Ding W, Sun X, et al. HPV E6 induces eIF4E transcription to promote the proliferation and migration of cervical cancer. FEBS Lett 2013;587:690–7. [DOI] [PubMed] [Google Scholar]
- [13].De Benedetti A, Graff JR. eIF-4E expression and its role in malignancies and metastases. Oncogene 2004;23:3189–99. [DOI] [PubMed] [Google Scholar]
- [14].Larsson O, Li S, Issaenko OA, Avdulov S, Peterson M, Smith K, et al. Eukaryotic translation initiation factor 4E induced progression of primary human mammary epithelial cells along the cancer pathway is associated with targeted translational deregulation of oncogenic drivers and inhibitors. Cancer Res 2007;67:6814–24. [DOI] [PubMed] [Google Scholar]
- [15].Fan S, Ramalingam SS, Kauh J, Xu Z, Khuri FR, Sun SY. Phosphorylated eukaryotic translation initiation factor 4 (eIF4E) is elevated in human cancer tissues. Cancer Biol Ther 2009;8:1463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Stacey SN, Jordan D, Williamson AJ, Brown M, Coote JH, Arrand JR. Leaky scanning is the predominant mechanism for translation of human papillomavirus type 16 E7 oncoprotein from E6/E7 bicistronic mRNA. J Virol 2000;74:7284–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, et al. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature 2004;428:332–7. [DOI] [PubMed] [Google Scholar]
- [18].Iglesias-Bartolome R, Martin D, Gutkind JS. Exploiting the head and neck cancer oncogenome: widespread PI3K-mTOR pathway alterations and novel molecular targets. Cancer Discov 2013;3:722–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fury MG, Drobnjak M, Sima CS, Asher M, Shah J, Lee N, et al. Tissue microarray evidence of association between p16 and phosphorylated eIF4E in tonsillar squamous cell carcinoma. Head Neck 2011;33:1340–5. [DOI] [PubMed] [Google Scholar]
- [20].Matthews-Greer J, Caldito G, de Benedetti A, Herrera GA, Dominguez-Malagon H, Chanona-Vilchis J, et al. eIF4E as a marker for cervical neoplasia. Appl Immunohistochem Mol Morphol 2005;13:367–70. [DOI] [PubMed] [Google Scholar]
- [21].DeFatta RJ, Nathan CO, De Benedetti A. Antisense RNA to eIF4E suppresses oncogenic properties of a head and neck squamous cell carcinoma cell line. Laryngoscope 2000;110:928–33. [DOI] [PubMed] [Google Scholar]
- [22].Oridate N, Kim HJ, Xu X, Lotan R. Growth inhibition of head and neck squamous carcinoma cells by small interfering RNAs targeting eIF4E or cyclin D1 alone or combined with cisplatin. Cancer Biol Ther 2005;4:318–23. [DOI] [PubMed] [Google Scholar]
- [23].Siegele B, Cefalu C, Holm N, Sun G, Tubbs J, Meschonat C, et al. eIF4E-targeted suicide gene therapy in a minimal residual mouse model for metastatic soft-tissue head and neck squamous cell carcinoma improves disease-free survival. J Surg Res 2008;148:83–9. [DOI] [PubMed] [Google Scholar]
- [24].Graci JD, Cameron CE. Mechanisms of action of ribavirin against distinct viruses. Rev Med Virol 2006;16:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Goswami BB, Borek E, Sharma OK, Fujitaki J, Smith RA. The broad spectrum antiviral agent ribavirin inhibits capping of mRNA. Biochem Biophys Res Commun 1979;89:830–6. [DOI] [PubMed] [Google Scholar]
- [26].Bougie I, Bisaillon M. The broad spectrum antiviral nucleoside ribavirin as a substrate for a viral RNA capping enzyme. J Biol Chem 2004;279:22124–30. [DOI] [PubMed] [Google Scholar]
- [27].Kentsis A, Topisirovic I, Culjkovic B, Shao L, Borden KL. Ribavirin suppresses eIF4E-mediated oncogenic transformation by physical mimicry of the 7-methyl guanosine mRNA cap. Proc Natl Acad Sci U S A 2004;101:18105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hu Z, Zhen L, Li Q, Han Q, Hua Q. Ribavirin sensitizes nasopharyngeal carcinoma to 5-fluorouracil through suppressing 5-fluorouracil-induced ERK-dependent-eIF4E activation. Biochem Biophys Res Commun 2019;513:862–8. [DOI] [PubMed] [Google Scholar]
- [29].Sharma S, Baksi R, Agarwal M. Repositioning of anti-viral drugs as therapy for cervical cancer. Pharmacol Rep 2016;68:983–9. [DOI] [PubMed] [Google Scholar]
- [30].McGlennen RC, Adams GL, Lewis CM, Faras AJ, Ostrow RS. Pilot trial of ribavirin for the treatment of laryngeal papillomatosis. Head Neck 1993;15:504–12; discussion 12–3. [DOI] [PubMed] [Google Scholar]
- [31].Goon P, Sonnex C, Jani P, Stanley M, Sudhoff H. Recurrent respiratory papillomatosis: an overview of current thinking and treatment. Eur Arch Otorhinolaryngol 2008;265:147–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Assouline S, Culjkovic B, Cocolakis E, Rousseau C, Beslu N, Amri A, et al. Molecular targeting of the oncogene eIF4E in acute myeloid leukemia (AML): a proof-of-principle clinical trial with ribavirin. Blood 2009;114:257–60. [DOI] [PubMed] [Google Scholar]
- [33].Assouline S, Culjkovic-Kraljacic B, Bergeron J, Caplan S, Cocolakis E, Lambert C, et al. A phase I trial of ribavirin and low-dose cytarabine for the treatment of relapsed and refractory acute myeloid leukemia with elevated eIF4E. Haematologica 2015;100:e7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Borden KL, Culjkovic-Kraljacic B. Ribavirin as an anti-cancer therapy: acute myeloid leukemia and beyond? Leuk Lymphoma 2010;51:1805–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Coleman LJ, Peter MB, Teall TJ, Brannan RA, Hanby AM, Honarpisheh H, et al. Combined analysis of eIF4E and 4E-binding protein expression predicts breast cancer survival and estimates eIF4E activity. Br J Cancer 2009;100:1393–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr., et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002;347:975–82. [DOI] [PubMed] [Google Scholar]
- [37].Jacobson IM, Brown RS Jr., Freilich B, Afdhal N, Kwo PY, Santoro J, et al. Peginterferon alfa-2b and weight-based or flat-dose ribavirin in chronic hepatitis C patients: a randomized trial. Hepatology 2007;46:971–81. [DOI] [PubMed] [Google Scholar]
- [38].Wendel HG, Silva RL, Malina A, Mills JR, Zhu H, Ueda T, et al. Dissecting eIF4E action in tumorigenesis. Genes Dev 2007;21:3232–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hernando E, Charytonowicz E, Dudas ME, Menendez S, Matushansky I, Mills J, et al. The AKT-mTOR pathway plays a critical role in the development of leiomyosarcomas. Nat Med 2007;13:748–53. [DOI] [PubMed] [Google Scholar]
- [40].Zahreddine HA, Culjkovic-Kraljacic B, Assouline S, Gendron P, Romeo AA, Morris SJ, et al. The sonic hedgehog factor GLI1 imparts drug resistance through inducible glucuronidation. Nature 2014;511:90–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zahreddine HA, Culjkovic-Kraljacic B, Borden KL. Sonic Hedgehog factor Gli1: As good as resistant. Mol Cell Oncol 2015;2:e961827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lu C, Makala L, Wu D, Cai Y. Targeting translation: eIF4E as an emerging anticancer drug target. Expert Rev Mol Med 2016;18:e2. [DOI] [PubMed] [Google Scholar]
- [43].Wolfe AL, Singh K, Zhong Y, Drewe P, Rajasekhar VK, Sanghvi VR, et al. RNA G-quadruplexes cause eIF4A-dependent oncogene translation in cancer. Nature 2014;513:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Dreas A, Mikulski M, Milik M, Fabritius CH, Brzozka K, Rzymski T. Mitogen-activated Protein Kinase (MAPK) Interacting Kinases 1 and 2 (MNK1 and MNK2) as Targets for Cancer Therapy: Recent Progress in the Development of MNK Inhibitors. Curr Med Chem 2017;24:3025–53. [DOI] [PubMed] [Google Scholar]
- [45].Thorpe LM, Yuzugullu H, Zhao JJ. PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nat Rev Cancer 2015;15:7–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Jhaveri K, Chang MT, Juric D, Saura C, Gambardella V, Melnyk A, et al. Phase I Basket Study of Taselisib, an Isoform-Selective PI3K Inhibitor, in Patients with PIK3CA-Mutant Cancers. Clin Cancer Res 2021;27:447–59. [DOI] [PubMed] [Google Scholar]
- [47].Soulieres D, Licitra L, Mesia R, Remenar E, Li SH, Karpenko A, et al. Molecular Alterations and Buparlisib Efficacy in Patients with Squamous Cell Carcinoma of the Head and Neck: Biomarker Analysis from BERIL-1. Clin Cancer Res 2018;24:2505–16. [DOI] [PubMed] [Google Scholar]
- [48].Dunn LA, Fury MG, Sherman EJ, Ho AA, Katabi N, Haque SS, et al. Phase I study of induction chemotherapy with afatinib, ribavirin, and weekly carboplatin and paclitaxel for stage IVA/IVB human papillomavirus-associated oropharyngeal squamous cell cancer. Head Neck 2018;40:233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Xu Y, Liao S, Wang L, Wang Y, Wei W, Su K, et al. Galeterone sensitizes breast cancer to chemotherapy via targeting MNK/eIF4E and beta-catenin. Cancer Chemother Pharmacol 2021;87:85–93. [DOI] [PubMed] [Google Scholar]
- [50].Cerezo M, Guemiri R, Druillennec S, Girault I, Malka-Mahieu H, Shen S, et al. Translational control of tumor immune escape via the eIF4F-STAT1-PD-L1 axis in melanoma. Nat Med 2018;24:1877–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


