ABSTRACT
Chimeric antigen receptor (CAR)-T cells have demonstrated significant improvements in the treatment of refractory B-cell malignancies that previously showed limited survival. In contrast, early-phase clinical studies targeting solid tumors have been disappointing. This may be due to both a lack of specific and homogeneously expressed targets at the surface of tumor cells, as well as intrinsic properties of the solid tumor microenvironment that limit homing and activation of adoptive T cells. Faced with these antagonistic conditions, radiotherapy (RT) has the potential to change the overall tumor landscape, from depleting tumor cells to reshaping the tumor microenvironment. In this article, we describe the current landscape and discuss how RT may play a pivotal role for enhancing the efficacy of adoptive T-cell therapies in solid tumors. Indeed, by improving homing, expansion and activation of infused T cells while reducing tumor volume and heterogeneity, the use of RT could help the implementation of engineered T cells in the treatment of solid tumors.
KEYWORDS: Radiotherapy, CAR-T cell, engineered T-cell, adoptive T-cell, solid tumors, immunotherapy, combination treatment
1. Introduction
Despite great advances in B-cell malignancies including acute lymphoblastic leukemia (B-ALL),1,2 chronic lymphocytic leukemia,3,4 mantle cell lymphoma,5 large B-cell lymphoma (LBCL),6–8 and multiple myeloma,9 the majority of clinical investigations involving CAR-T cells in solid tumors provided poor outcomes.10 To date, CAR-T cell therapies have been experimented in approximately 250 clinical trials as a treatment for a large variety of solid tumors, from primitive brain tumors to pelvic malignancies.11 Except for the slight effects observed in gastric and pancreatic cancers treated with anti-claudin 18.2 CAR-T cells12 and encouraging results with local administration of anti-mesothelin CAR-T cells in malignant pleural disease,13 results obtained in these trials were disappointing. Of note, even when targeting relevant antigens, such as PSMA in prostate cancer, the efficacy of CAR-T cells remains low and their engraftment is uncertain. This underscores the fact that, in solid tumors treated with CAR-T cells, targeting the right antigen is necessary but not sufficient. Moreover, the use of CAR-T cells in solid tumors often resulted in on-target/off-cancer toxicities.14 Indeed, compared to hematological malignancies, the specificities of the tumor microenvironment (TME) in solid tumors are of critical importance in thwarting the action of adoptive cells. Herein, we aim to describe how TME limits both homing and activation of infused T cells, preventing them from reaching tumor cells and exerting their antitumor effects. Finally, we will provide a speculative point of view on how RT could be involved in combating these deleterious conditions.
2. Optimizing the efficacy of T-cell therapies in solid tumors using radiotherapy: rationale
As demonstrated notably in esophageal squamous cell carcinoma,15,16 primitive brain tumors17 including glioblastoma,18 head and neck cancers19 and lung tumors,20 a key issue for the development of solid tumors and their escape to immune surveillance is the polarization of the immune microenvironment into an immunosuppressive and tolerant phenotype.21 This can be done through various pathways, of which the most known are the secretion of immunosuppressive chemokines such as interleukin (IL)-10 or transforming growth-factor (TGF)-β,22 or the upregulation of several druggable inhibitory immune checkpoints which will be described later in this review. This results in a progressive change from “hot” tumors (T-cell inflamed) to “cold” tumors, either showing no immune cell infiltration (called “immune deserts”) or with effector cells located at the periphery of tumor and not infiltrating tumor core (called “immune excluded”). In addition to limiting the infiltration of CAR-T cells into their core, cold tumors also promote the recruitment of regulatory T cells (Tregs), leading to the exhaustion of patient’s effector cells. Tregs also dampen the efficacy of infused T cells, the reason why they have been used to prevent graft versus host disease after hematopoietic stem cell transplantation.23 Radiotherapy has interesting properties prone to combat the antagonistic immune landscape of advanced solid tumors and by this way improve the efficacy of CAR-T cells in this setting.
2.1. RT-induced tumor debulking may increase antitumor efficacy while decreasing toxicity of CAR-T cells
As shown in B-ALL and LBCL,24,25 a lower initial tumor burden is a major predictor of response to CAR-T cell treatment. Indeed, a strong correlation was observed between durable response and the peak CAR-T cell levels in blood normalized to pretreatment tumor burden in LBCL. These observations highlight a minimum threshold of specific T cells relative to the number of tumor cells for the treatment to be efficient, which refers to the classical effector to tumor cell ratio in immunology.26 Furthermore, decreasing tumor burden and tumor heterogeneity before CAR-T cell infusion may reduce the risk of inducing tumor resistance due to the selection of refractory clones characterized by loss or decrease of the antigen targeted by CAR-T cells.27,28 Moreover, a high marrow tumor burden has been identified as an independent predictor of cytokine release syndrome (CRS) in 133 patients enrolled in a prospective phase I/II trial receiving anti-CD19 CAR-T cells.29 Therefore, by debulking tumors, RT aims to increase the efficiency of CAR-T cells by depleting some resistant clones while limiting the rate of CRS by decreasing tumor burden.
2.2. Promoting the homing of CAR-T cells into solid tumors
Solid tumors include a large number of stromal cells of various types. These cells, by forming a natural barrier, restrict the possibility for immune cells to penetrate TME.30 Radiotherapy (RT) has shown its ability to permeabilize the peritumoral stroma on various levels:
Remodeling vascularization
The dysfunctional vascularization often encountered in solid tumors hampers the homing of effector T cells within the tumor microenvironment, leading to tumor immune escape.31 Therefore, normalizing vascularization and reducing hypoxia are required for the efficacy of immunotherapies, including adoptive T cells.32 Mondini et al. first demonstrated by using a murine orthotopic model of head and neck squamous cell carcinoma (HNSCC) expressing HPV16 that a combination of single-dose of 7.5 Gy and anti-HPV vaccine increased both pericyte coverage and the expression of ICAM-1 on tumor vessels. This led to enhanced intra-tumor vascular permeability, an increased number of intra-tumor CD8+ T cells, and decreased tumor-growth rate and improved survival that were not observed when using vaccine or RT alone.33 Moreover, human CAR-T cells show both reduced expansion and cytokine secretion when cultured in hypoxic conditions.34 From this point of view, hypofractionated ablative RT has shown its ability to normalize tumor vasculature in murine lung tumors,35 as well as in non-small cell lung cancer patients’ derived xenografts.36 Moreover, it induced both an intense infiltration by CD8+ T cells and a loss of myeloid-derived suppressor cells (MDSC) in two murine colon carcinoma models.37 A similar pattern of vasculature normalization was assessed using functional imaging in murine pancreatic carcinoma models receiving 35 to 45 Gy in 4 to 5 fractions.38,39 Therefore, using RT may provide an option for better homing of adoptive T-cell therapies. RT also promotes the growth of new blood vessels through an increase in endothelial nitric oxide synthase (NOS) abundance, contributing to revascularization.40 Finally, irradiated endothelium is prone to show a more adhesive phenotype, thus facilitating the transmigration of immune cells.41,42 Therefore, since radiotherapy has showed its effects on both revascularization and re-oxygenation of solid tumors, with benefits observable with a large array of doses ranging from low doses of 1–2 Gy to dozens of grays,43–45 further experimentations should consider it as a prime partner in CAR-T cell-based approaches, with the aim to improve the homing of infused cells.
Effects of RT on chemotaxis
In some aspects, RT has the ability to polarize the tumor microenvironment into an inflammatory phenotype prone to exert an efficient antitumor action and to show a synergistic action with immunotherapy.46 This polarization is associated with type I and II interferon responses47,48 with cGAS-STING pathway activation and with the secretion of multiple chemokines within tumor microenvironment,49 such as CXCL9,50 CXCL10,51 CXCL1152 or CXCL16,53 leading to chemoattraction of either endogenous or adoptive lymphocytes to the tumor site. Moreover, immunogenic cell death induced by RT acts as a trigger for harnessing the host’s immune system to attack remaining tumor cells.54 All these features contribute to an inflammatory immune environment, which could foster the homing of CAR-T cells. However, RT also promotes immunosuppressive mechanisms, by notably increasing the infiltration of regulatory T cells (Tregs),55 myeloid-derived suppressor cells (MDSCs)56 and developing tolerant anti-inflammatory macrophages.57,58 Faced with this observation, whether RT will promote or limit the attraction and homing of infused T cells will depend on a balance between subsequent activating and inhibiting signals. In this regard, the scheme of RT more prone to potentiate immune antitumor signaling is still debatable, with scarce preclinical elements indicating a deleterious profile mediated by the expression of exonuclease TREX1 following high doses per fraction beyond 12 Gy.59 Therefore, the translation of these findings requires further clarification among preclinical models used, as well as considerations about radiation timing and dose.
Cancer-associated fibroblasts (CAF)
Lastly, tumor stroma and CAFs are known to play a crucial role in immune escape.60 In a mouse model of intrarectal orthotopic tumor, Nicolas et al. demonstrated RT’s ability to modulate the phenotype of inflammatory CAF (iCAF), shown as predictors of poor response to neoadjuvant therapy in rectal cancer patients,61 depending on IL-1. In another intrarectal orthotopic model, RT-activated CAFs promoted survival of tumor cells through the activation of IGF1 receptor (IGF1R), and RT followed by the neutralization of IGF1R reduced the number of mice with organ metastases.62 These studies demonstrate the relevance of associations using RT and agents targeting tumor stroma.
2.3. To enhancing activation of adoptive T-cells in the solid tumor core
TCR engineered T cells (TCR-T cells), in contrast to CAR-T cells, bind to their corresponding antigen after the antigen has been processed by major histocompatibility complex class I (MHC I). This process is represented in Figure 1. This makes TCR-T cells able to recognize mostly intracellular antigens, whereas CAR-T cells only bind cell surface antigens. RT is known for its ability to enhance the presence of MHC I at the surface of tumor cells. This was first-assessed in the early 2000ʹs by Garnett et al. in preclinical work in which eight human carcinoma cell lines (five colon, three lung) showed increased expression of MHC I 72 hours after non-lethal irradiation of 10 to 20 Gy, with subsequently enhanced susceptibility of tumor cells to T-cell mediated attack.63 Two years later, Reits et al. confirmed these findings in human cutaneous melanoma cells for doses beyond 7 Gy, as well as in MC38 mouse colon carcinoma cells. In addition, in MC38 subcutaneous tumors, the combination of 10 Gy irradiation and adoptive T-cells led to marked inhibition of tumor growth,64 thus creating a solid view for the future of such an association in clinical trials. Finally, since RT is able to enhance the expression of MHC class I at the surface of tumor cells, it may also limit immune escape which is related to the loss of MHC class I expression in multiple cancers.65–67 This could enhance the objective response rate of TCR-T cell therapies and limit further tumor immune escape in the setting of combinatorial approaches.
Figure 1.
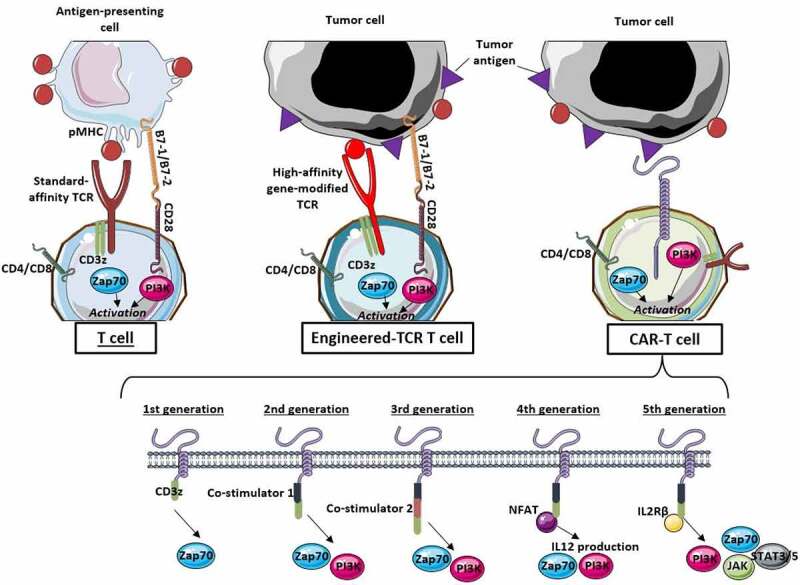
Patterns of activation of conventional T cells, gene-modified TCR-T cells and CAR-T cells in the tumor immune microenvironment.
(pMHC: peptide presented by major histocompatibility complex; TCR: T-cell receptor; PI3K: phosphoinositide-3 kinase; JAK: janus kinase; STAT: signal transducer and activator of transcription)
In regard to CAR-T cells, particular attention should be given to those targeting cell surface antigens for which high expression levels are associated with poor response to RT, i.e. the ErbB family including epidermal growth factor receptor EGFR (ErbB1)68,69 and ErbB2 (HER2).70,71 Thus, targeting these pathways using CAR-T cells could result in limited survival of radio-resistant clones prone to drive tumor progression or relapse. Although, even if more than 40 clinical trials experimenting HER2 (NCT03182816; NCT05341492; NCT02873390; NCT03198052; NCT0368167) or EGFR (NCT03696030; NCT04650451; NCT04995003; NCT0092044) targeted CAR-T cells in solid tumors are ongoing, only two trials experiment associations between CAR-T cells targeting EGFR and radiotherapy in glioblastoma. These trials are included in Table 1. In the other trials, conditioning regimens, when described in protocols, use chemotherapy alone and do not include radiotherapy as a possible option.
Table 1.
Brief summary of current clinical trials involving adoptive T-cell therapies and radiotherapy in solid tumors.
| Clinicaltrials.gov identifier | Study phase | Conditions | Interventions | Locations | Status |
|---|---|---|---|---|---|
| NCT03412526 | Phase II | Ovarian Cancer | Total body irradiation 2GyIL-2 Tumor-infiltrating lymphocytes (TIL) | Ramat Gan, Israel | Recruiting |
| NCT03396575 | Phase I | Pediatric glioma | Radiotherapy Temozolomide Ex-vivo expanded TIL | Gainesville, USA | Recruiting |
| NCT02584829 | Phase I | Merkel cell carcinoma | Radiotherapy Avelumab | Seattle, USA | Active, not recruiting |
| Phase II | Autologous T-CD8+ cells Interferon beta | Washington, USA | |||
| NCT03347097 | Early Phase I | Glioblastoma | Chemoradiotherapy TIL expressing PD1 | Shanghai, China | Active, not recruiting |
| NCT01758458 | Phase I | Merkel cell carcinoma | Radiotherapy Recombinant human IL-2 | Seattle, USA | Terminated |
| Phase II | Intralesional injection of interferon beta | Washington, USA | |||
| NCT00512889 | Phase I | Melanoma | Radiotherapy GM-SF Autologous activated lymphocytes | Boston, USA | Completed |
| NCT03344250 | Phase I | Glioblastoma | Anti-EGFR TCR-T cells Chemoradiotherapy | Charlottesville, USA | Active, not recruiting |
| NCT05022849 | Phase I | Castration-resistant prostate cancer | KLK2 CAR-T cells Radiotherapy (option) Chemotherapy (option) | Multiple centers, USA | Recruiting |
| NCT03132922 | Phase I | Multiple solid tumors | Low-dose radiotherapy 1.4 Gy Gene-modified TCR-T cells | Multiple centers, USA | Active, not recruiting |
| NCT02664363 | Phase I | Gliblastoma | Chemoradiotherapy EGFRvIII CAR-T cells | Durham, USA | Terminated |
Another issue to consider for the activation of adoptive T cells in solid tumors is the up-regulation of immune suppressive checkpoints within the tumor microenvironment likely to thwart the action of both endogenous and adoptive T cells and drive tumor immune escape. This challenge is of critical importance since high doses of RT are likely to enhance the expression of several of these checkpoints, including programmed-cell death ligand 1 (PD-L1),72–74 cytotoxic T-lymphocyte associated protein 4 (CTLA-4),75,76 lymphocyte-activation gene 3 (LAG-3)77 and T cell immunoreceptor with Ig and ITIM domains (TIGIT).78 However, these findings were the substrate for breakthrough trials experimenting combinatorial approaches associating RT and immune-checkpoint blockades, leading to practice changes and improvements in patients’ survival.79,80 Therefore, this negative feature does not impair the potential benefits of combining RT with adoptive T-cell therapies in solid tumors. Indeed, several options may be used to counteract these effects. These options may include the association of immune-checkpoint blockades to adoptive T cells and RT, or the use of newly engineered CAR-T cells either showing dominant negative TGF β,81 or switch receptors PD1/CD28,82 or even producing immune checkpoint blockades (ICB) targeting PD1.83 Moreover, since RT may up-regulate these inhibitory checkpoints in a dose- and fractionation-dependent manner,78 research must be conducted on the best RT schedule to both maximize activating and minimize inhibiting features.
2.4. Radiation-induced lymphopenia as support for proper expansion of engineered T cells
Radiation-induced lymphopenia (RIL) is a common adverse event in radiotherapy treatments, often reported as jeopardizing the efficacy of either concurrent or subsequent immunotherapy administration.84–86 This frequent condition is largely due to the high radiosensitivity of lymphocytes, assessed in-vitro with near 90% of lethality in a population of human lymphocytes irradiated with a single dose of 2 Gy.87 This observation was the basis for further studies highlighting the impact of RT on the reduction of circulating lymphocytes following conventional fractionation RT for the treatment of high-grade gliomas.88 Of note, even before the era of intensity-modulated RT and radioimmunotherapy combinations, the effects of RT on reducing lymphocyte counts were well established.89–91 Therefore, even when practiced in non-myeloablative conditions, e.g. as a local ablative treatment, RT can induce lymphopenia in the patient and therefore may favor the expansion of infused T cells in the setting of CAR-T cell transplantation. In this setting, the sequence between ablative RT and CAR-T cell infusion is crucial, since RT should be delivered before the infusion of T cells.
Interestingly, the ability of RT to deplete patient’s lymphocytes led to consider total-body irradiation (TBI) as a valuable conditioning option before hematopoietic cell transplantation (HCT) in combination with chemotherapy.92 In this setting, two trials have demonstrated the equivalence of chemoradiotherapy (CRT) and chemotherapy (CT)-based regimens in chronic myeloid leukemia. Moreover, in acute myeloid leukemia,93,94 one trial has showed both clinical and biological superiority of the CRT-based approach over CT alone.95 Although a strategy of lymphocyte depletion may be controversial in case of a metastatic patient, it remains crucial before CAR-T cell infusion to allow their proper expansion, whether it uses CT, RT or both. From this point of view, adding a large-volume and low-dose irradiation to CT during the conditioning phase would combine an efficient lymphocyte depletion to the biological benefits of low-dose RT in solid tumors relying upon mobilizing both innate and adaptive immunity. These benefits are detailed later in this article and notably include the secretion of IFN γ,96 the recruitment of Th1 CD4+ and activated dendritic cells,97 the polarization of macrophages, the decrease of TGF β secretion and the infiltration of NK cells.98 All these features are prone to enhance the action of subsequently infused T cells.
However, the duration and depth of lymphopenia after conditioning are crucial issues. Indeed, patients have to recover an appropriate lymphocyte count fast enough not to compromise long-term adaptive response against solid tumors. Moreover, lymphocyte depletion must not lead to the progression of patients before T-cell infusion. From this perspective, experiments remain to be done about the best CT and RT doses and timing to achieve positive and safe outcomes before HCT. A recent case series of patients with relapsed or refractory large B-cell lymphoma has demonstrated the safety and efficacy of a CRT-based conditioning option.99 In this retrospective review, 12 patients received bridging RT on target lesions before the infusion of Axicabtagene Ciloleucel. RT was delivered on a large volume, ranging from one hip to the whole abdomen. The dose of RT was flexible, ranging from 6 Gy in 3 fractions to 36.5 Gy in 14 fractions, according to irradiated volume. Four patients out of 12 did not receive any concurrent bridging systemic therapy. No significant toxicity was observed during bridging RT. At 30 days, the objective response rate was 81.8%, with complete response in 27% of evaluable patients. These outcomes were observed despite a slight decrease in lymphocyte counts in 10 patients.
Therefore, RT in a large volume with a controlled dose may represent an interesting conditioning option with CT instead of myeloablative TBI questionable in a patient with metastatic solid tumor. A comparison of the various available regimens of CRT in the setting of CAR-T cells according to their respective immunomodulatory effects is needed.
2.5. Promoting a durable response despite radiation-induced lymphopenia: an impossible challenge?
The currently scarce use of CRT-based conditioning regimens before CAR-T cell infusion may be explained by the apprehension of inducing excessive lymphopenia and therefore compromising the generation of a durable antitumor adaptive immunity driven by endogenous memory T cells. Indeed, because the presence of infused T cells is time-limited, especially in the case of allogeneic T cells eliminated by the host immune system, the generation of an adaptive immunity is necessary to achieve a long event-free survival. By using an oncolytic vaccine and a preclinical model of methylcholanthrene-induced fibrosarcoma, Walsh et al. demonstrated the contribution of both transferred and endogenous T cells in providing long-term survival after adoptive T-cell therapy.100 Thus, transferred T-cells destroyed tumor masses, while endogenous T-cells prevented immune escape by limiting the emergence of new tumor-associated antigens (TAA). Another study by Alizadeh et al. illustrated in a syngeneic model of murine glioblastoma the critical role played by adoptive T cells in the induction of endogenous T-cell memory response through activation of the IFNγ pathway and activation of intratumoral macrophages.101
Moreover, beyond the issue of radiation-induced lymphopenia, high doses of focal RT have shown their ability to release great amounts of tumor-specific antigens that further act like danger-associated molecular patterns (DAMPs) for the activation of endogenous T-cells mediated by antigen-presenting cells (APC).102,103 This process leads to the generation of endogenous memory T-cells acting as an antitumor in-situ vaccine represented in Figure 2.
Figure 2.
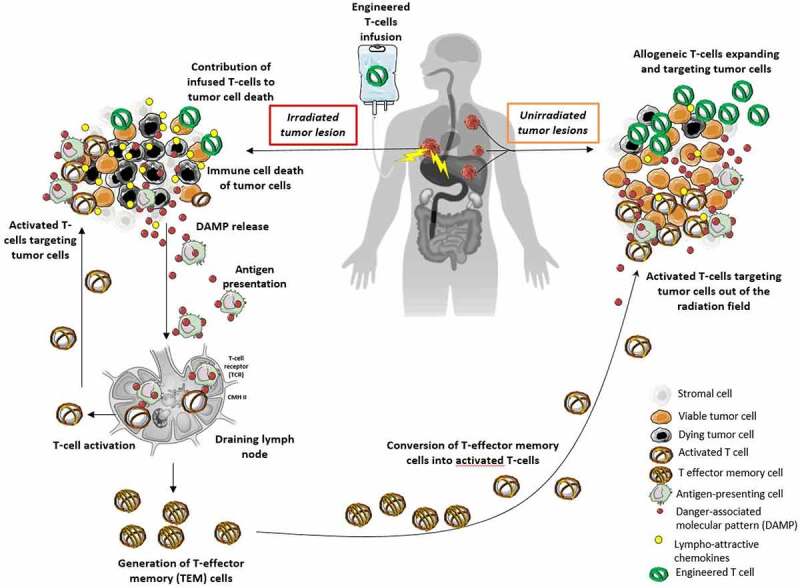
Radiotherapy as an in-situ vaccine in combination with adoptive T-cell therapies.
Therefore, facing these data, solutions must be found to limit lymphopenia during conditioning phase in the setting of combinatorial approaches associating adoptive T cells with RT. This is a highly challenging condition, since lymphopenia must be present to ensure an appropriate expansion of adoptive cells, but must not be too high, as to hamper further antitumor adaptive immunity. The following options may be explored to reach this purpose.
Choosing an appropriate conditioning regimen
The question of the best conditioning regimen before HCT remains controversial. Moreover, RT is inconsistently used with CT in this setting. Nissani et al. made a comparison between different non-myeloablative conditioning regimens in patients with metastatic melanoma treated using an infusion of tumor-infiltrating lymphocytes (TILs).104 They demonstrated that a combination of low-dose TBI of 2 Gy and 75 mg/m2 fludarabine was less efficient in depleting lymphocytes compared to chemotherapy regimens with cytarabine and fludarabine. The authors negatively concluded about the association between TBI and fludarabine, arguing its use resulted in insufficient bone marrow suppression. However, this treatment provided at least 2 days of severe lymphopenia in a large proportion of patients by using a low dose of RT and therefore showed a good safety profile. Based on the assumption that excessive lymphopenia would compromise long-term immune response, these results should reconsider the option of CRT-based conditioning regimens, with the possibility for clinicians to make TBI dose vary according to the desired depth of lymphopenia. Alternatives to TBI may include high-dose focal irradiation of target lesions, with surrounding areas irradiated with scattered low-doses. A brief overview of clinical trials associating CAR-T or TCR-T cells and RT is exposed in Table 1. Of note, only two trials are currently reported as experimenting a combination of chemotherapy and low-dose irradiation followed by TCR-T or CAR-T cell infusion in solid tumors.
Challenging the conditions of irradiation
Beyond the question of conditioning regimen, radiation oncologists must pay careful attention to dosimetry, notably within areas surrounding high-dose fields. Indeed, these volumes are often significant and receive scattered low-doses likely to impact lymphocyte survival. From this point of view, a comprehensive understanding of the expected occurrence, duration and depth of lymphopenia caused by his treatment is a difficult but crucial challenge to the radiation oncologist. It may require usual analysis of dose-volume histograms105–107 as well as a composite set of criteria108 or use recent machine learning solutions.109
Moreover, in the setting of metastatic patients, internal radiotherapy delivered using α particles may be a promising option, as it deposits high energy over a short path length. In hematological malignancies, this approach is experimented in acute myeloid leukemia (AML) with the anti-CD33 antibody lintuzumab conjugated with actinium-225. The results of a recent phase I trial showed a maximum tolerated dose of 111 kBq/kg.110 Up to that dose, no event of myelosuppression longer than 35 days was reported. The prevalence of treatment-related lymphopenia was difficult to evaluate due to coexisting baseline suppression of hematopoiesis by active AML present in 11 patients out of 18.
Menager et al. tested the combination of α-radiotherapy with adoptive T cell transfer in a murine model of myeloma expressing the tumor antigen CD138 and ovalbumin (OVA).111 By using an anti-CD138 antibody coupled to bismuth-213 and followed by adoptive transfer of OVA-specific CD8+ T cells, they observed a significant tumor growth control and an improved survival in the animals treated with multimodal treatment. Interestingly, at the end of the experiment, infused T-cells were still present in tumor and lymph nodes, assessing the persistence of infused lymphocytes despite the concurrent presence of α emitters.
In the setting of solid tumors, α-radiotherapy is a source of major advances in castration-resistant prostate cancer. In a phase III trial, the use of lutetium-177-prostate specific membrane antigen (PSMA) led to improvements in progression-free survival (PFS) and overall survival (OS) compared to standard of care.112 Moreover, according to a recent study of biodistribution and safety, lutetium-177-PSMA lost 90% of its initial activity within the first 70 hours following administration.113 This resulted in only 2 patients out of 51 presenting leukocytopenia of grade 3 or more, which is congruent with the 2.5% rate of leukopenia grade 3 or more presented in the phase III trial previously mentioned. Therefore, in prostate cancer, α-radiotherapy should not impair the efficacy of a subsequent adoptive T-cell transfer due to cytotoxicity against infused T cells. A similar approach of combined treatments using α-radiotherapy may be experimented in a wide variety of solid tumors, as the field of available radiopharmaceuticals is rapidly growing.114
Finally, an appropriate solution to improving homing and activation of infused T cells and allow their proper expansion by depleting endogenous lymphocytes without compromising further immunity would be to combine both high-dose and low-dose irradiation. In this way, treating different volumes with different levels of dose could help to treat a high number of lesions in a patient. Indeed, in patients with advanced and refractory diseases, it may be difficult to treat a significant number of lesions with conventional or high-dose RT because of unacceptable toxicity. In such a setting, the complementary use of low-dose RT could make these patients benefit from the radiobiological properties of both high-dose and low-dose RT in combination with CAR-T cells. From such a perspective, an overview of the features of RT within the tumor microenvironment according to fractionation is presented in Figure 3. The issues of combining low-dose RT, high-dose RT and adoptive T-cell therapies are described later in this article.
Figure 3.
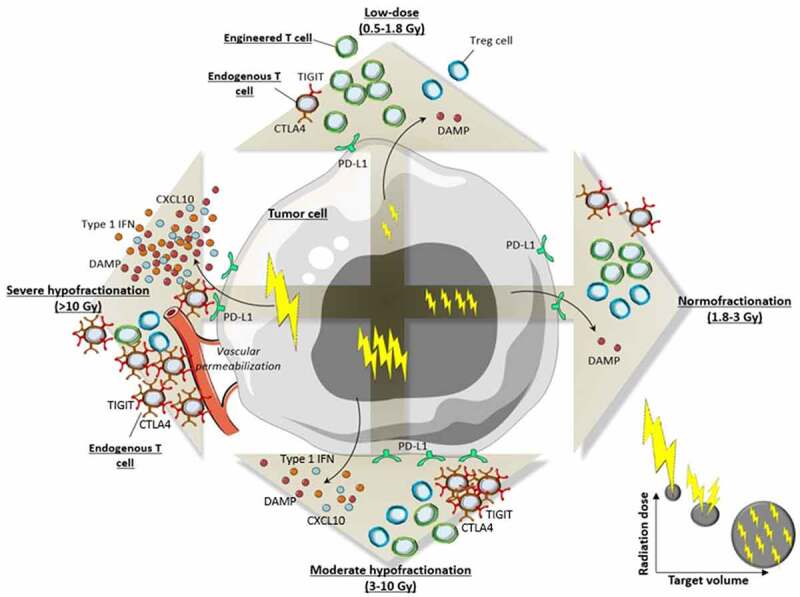
Comparative biological effects of RT within tumor microenvironment according to fractionation.
(DAMP: danger-associated molecular pattern; CTLA4: cytotoxic T-lymphocyte-associated protein 4; PD-L1: programmed-death ligand 1; TIGIT: T cell immunoreceptor with Ig and ITIM domains; IFN: interferon; Gy: Gray)
2.6. CAR-NK cells better than CAR-T cells in combination with RT? The latest arrival in the field of engineered-cell therapies
Natural killer (NK) cells constitute an important component of innate immunity, accounting for 5–15% of peripheral blood leucocytes in human. Conversely to T cells, NK cells can generate tumor cell death through a large variety of mechanisms that do not require the presence of antigen-specific TCR. Their activation is mostly based on a balance between germline-encoded activating and inhibiting signals, with thus a natural trend towards killing tumor cells known for their ability to down-regulate the expression of HLA.115 In a study by Weiss et al., a CAR-T cell based on the activating NK signal NKG2D has demonstrated prolonged survival in mice bearing glioblastoma, and even cured a fraction of them. Moreover, the combination of NKG2D-based CAR-T cells with RT resulted in a synergistic activity in two different mouse glioma models.116
After the large success of engineered T-cell therapies in the treatment of refractory hematological malignancies, researchers have shifted their attention towards the creation of modified NK cells targeting antigens of interest, including chimeric antigen receptor NK cells, or CAR NK-cells. The early-phase experimentation of these new agents in CD-19 positive tumors showed low rates of cytokine release syndrome and neurotoxicity with encouraging signs of efficacy.117 This resulted in widening the scope of CAR-NK-based treatments by using new NK-based constructs targeting HER2 in breast cancer,118 EGFR in glioblastoma119 or mesothelin in ovarian cancer.120 However, the activity of CAR-NK cells can be thwarted by the up-regulation of inhibitory ligands at the surface of tumor cells, e.g. HLA-G in Ewing sarcoma.121,122
From this perspective, high doses of RT beyond 10 Gy are prone to up-regulate activating ligands at the surface of tumor cells, notably those recognized by NKG2D, as it has been demonstrated in-vitro.123–125 Furthermore, in an in-vivo model of canine sarcoma, a single-fraction of 2 Gy followed by injection of a plasmid encoding recombinant human interleukin 15 (rhIL-15) enhanced both intra-tumor homing and cytotoxicity of subsequently infused NK cells.126 Finally, RT in combination with blocking of PD-L1/PD-1 pathway increased antitumor cytotoxicity of NK cells in human nasopharyngeal carcinoma.127
Considering these encouraging data, RT, either used alone or in association with chemotherapy or immune-checkpoint blockades, should be considered in further early-phase clinical trials in association with CAR-NK cells. Such a combination may challenge the line between their inhibiting and activating signals and thus polarizing them into an inflammatory and antitumor phenotype. For this purpose, targeting the inhibitory checkpoint NKG2A128 or the heterogeneous KIR family129 at the surface of host NK cells may also constitute an interesting option in association with RT.
3. Optimizing the efficacy of T-cell therapies in solid tumors using radiotherapy: how?
The outstanding technical improvements in RT practices over the last decade have greatly broadened the indications and modalities of RT in the treatment of solid tumors, with more than 60% of cancer patients requiring RT.130 Therefore, at the era of widespread use of immunotherapy, an important concern remains, regarding the scheme of RT more prone to enhance the action of immunotherapy in a combinatorial setting.131 While this issue is the field for a large body of research regarding the sequences of immune checkpoint blockades and RT, a similar reflection should be engaged as part of the optimization in further combinatorial strategies associating T-cell therapies and RT.132,133 Different settings regarding the volume to irradiate and the dose to deliver can be compared one with another with the aim to improve the efficacy of adoptive T-cell therapies using RT.
3.1. Option 1: a small volume irradiated with high doses of RT, improving the homing and efficacy of CAR-T cells
The implementation of stereotactic radiosurgery (SRS) has improved local control in most solid tumor types.134 By delivering high doses of radiation per fraction on a small volume, i.e. macroscopic tumor with limited margins, SRS is able to increase the biologically equivalent dose (BED) delivered, leading to higher local control rate.135–137
Congruently with its direct antitumor effects, SRS is also able to reshape the tumor microenvironment (TME) in a way that could synergize with adoptive T-cell therapies to various levels. First, high doses of RT are able to induce immunogenic cell death (ICD), a critical feature for dendritic cell (DC) activation and effector T-cell priming.138,139 This condition can be enhanced by the concomitant use of radiosensitizers, as demonstrated with hafnium oxide nanoparticles NBTXR3. In a preclinical model of murine colorectal tumor, NBTXR3 injected intratumorally and then activated by 12 Gy in 3 fractions increased CD4+, CD8+ and CD68+ cell infiltrates compared to radiotherapy alone. Moreover, significant modifications in TCR repertoire diversity were found in radiotherapy-activated NBTXR3 group, not only in tumors treated with combination but also in distant untreated tumors.140 Other alternatives of radiosensitizers prone to increase ICD in combination with high-dose RT include gold nanoparticles141 or repurposing anti-alcoholism drug disulfiram.142
Furthermore, the ability of high-dose RT to activate immune microenvironment in solid tumors depends on the dose and fractionation.143 As an example, Vanpouille-Box et al. demonstrated 24 Gy delivered in 3 fractions of 8 Gy increased CD8+ and Th1 cells within tumor microenvironment in TSA murine tumors through the secretion of activating cytokines and cGAS-STING pathway. However, in three different murine cell lines, when the single-fraction dose exceeded 12 Gy, the activation of DNA exonuclease TREX1 impaired the activation of cGAS-STING pathway by decreasing the amount of double-stranded DNA within the cytoplasm of tumor cells.59 The overexpression of several immune checkpoints may also participate to this relative immunosuppression induced by high doses, as demonstrated with PD-L1, Galectin-9 and herpes virus entry mediator (HVEM) in B16-F10 cells.144 This supports the association of immune checkpoint blockades to high-dose RT described later in this section.
Similarly, high doses of RT above 5 Gy increase the expression of cell-adhesion molecules such as intracellular adhesion molecule (ICAM-1) and vascular-cell adhesion molecule (VCAM-1),145 enhancing the adhesion of lymphocytes to endothelial cells and then their extravasation to TME. Moreover, the high level of lethality observed in tumor cells following SRS may lead to the depletion of clones likely to develop mechanisms of resistance to adoptive T-cells and therefore limit further immune escape.
On the other hand, high doses per fraction can also promote the recruitment within TME of regulatory T-cells (Tregs), myeloid-derived suppressor cells (MDSCs), and anti-inflammatory tumor-associated macrophages, known for their pro-tumor/immunosuppressive properties.131 Such findings force the development of strategies prone to maintain a positive antitumor immune balance within TME and hence ensure the action of both endogenous and infused T cells. Facing this challenge, recent improvements in functional imaging and radiomics are prone to estimate in a non-invasive manner the amount of effector cells146–148 as well as the presence of hypoxic areas149 within tumors. This could help to select lesions more likely to benefit from RT while increasing the dose in the immunological “no man’s land” constituted by hypoxic areas.150
Several other options can also be experimented, including the simultaneous use of ICB targeting PD-1, CTLA-4, PD-L1 or TIGIT that are up-regulated by RT in a dose- and fractionation-dependent manner as mentioned earlier in this section. Newly engineered CAR-T cells harboring switch receptors or producing ICB may also be a solution and are currently challenged in early-phase experiments. Other options may include administrations of cyclophosphamide, known for efficiently depleting Tregs, as demonstrated in animals as in humans with good tolerance.151–153 Therefore, the association of such treatments to associations between CAR-T cells and RT should deserve more consideration from further experiments, since it is likely to enhance the efficacy of CAR-T cells by combating immunosuppressive pathways within tumor microenvironment.
3.2. Option 2: a large volume irradiated with low doses of RT, facilitating the expansion of CAR-T cells and making the most of the surprising properties of low-dose RT
3.2.1. The definition of low-dose RT
From a technical point of view, there is no clear consensus on the definition of a low dose of RT. In preclinical settings, a recent review analyzed 37 studies challenging combinations between RT and immunotherapy in various animal models from 1996 to 2019.154 Of the 36 studies delivering local irradiation, 24 delivered a total dose of at least 10 Gy and 17 delivered at least 15 Gy. Moreover, RT was delivered in a single fraction in 15 studies, and in 1 to 4 fractions in 20 studies, with such hypofractionation increasing biologically effective dose (BED). As a comparison, most of preclinical studies experimenting low-dose RT and presented later in this section use total irradiation doses ranged from 0.1 to 2 Gy. Therefore, even if no clear definition exists about low-dose RT, such a level of dose may legitimately be evaluated as low. Furthermore, some authors differentiate a low dose of RT defined as up to 0.1 Gy from a moderate dose ranging from 0.1 to 2 Gy.96 Obviously, even a dose of 2 Gy is far different from the usual preclinical radiation schemes described earlier and therefore may be considered as a low dose.
In a clinical setting, in contrast to preclinical experiments, there are clear recommendations on the prescription of dose according to the histopathology and staging of tumors. This dose usually varies between 50 and 80 Gy in 25 to 40 fractions for tumors treated with curative intent and may be over 100 Gy BED in case of hypofractionated stereotactic radiosurgery (SRS). Therefore, in this setting, as experimented in publications mentioned later in this review, 1 to 4 Gy delivered in a few fractions may legitimately be regarded as low-dose RT.
3.2.2. The interesting properties of low doses and applications
Historically, incidental RT has been presented as increasing the risk of cancer. This observation is largely due to the dose–response curves made on the basis of Hiroshima and Nagasaki bomb survivors.155 However, these conclusions are unclear for doses inferior to 1 Gy and for low-dose rate irradiation.156 In addition, the incidence of cancer in patients after radiation exposure varies according to their age when exposed, with a relative decrease in older patients. Moreover, in this study, the dose received by survivors was retrospectively estimated according to their location and shielding at the time of bombing, and radiation consisted in gamma rays and neutrons which differ from X-rays in their radiobiological properties. For these reasons, low doses received by these cohorts should not be systematically extrapolated to medical workers or patients exposed to a low dose from a known source of x-rays. From that perspective, an epidemiologic study demonstrated male radiologists registered after 1955 in England have a negative standard mortality ratio (SMR) for death from cancer compared to all male medical practitioners (SMR 0.68).157 This trend was similar in 145,915 radiologic technologists registered between 1926 and 1982 in the United States.158,159 Finally, in patients, a recent retrospective work showed that, after RT for breast cancer, radiation-induced sarcomas mostly occur, not within areas irradiated with low doses but within those irradiated with doses superior to 30 Gy.160
Low-dose RT has been an expanding field of research over recent years. In contrast to high-dose conventional RT, it is able to treat large targets without compromising surrounding healthy tissues. Beyond depleting endogenous lymphocytes to allow a proper expansion of subsequently infused T cells, low-dose RT has also interesting radiobiological properties to exploit in solid tumors. When low doses of RT are delivered to non-transformed cells surrounding tumor cells, they are prone to induce apoptosis in cancer cells through reactive-oxygen species (ROS) signaling.161,162 The unexpected properties of low doses translate into multiple animal studies showing tumor reduction and changes in host immune response after low-dose irradiation163–172
According to clinical practice, several applications of low-dose RT have emerged in recent years. By using a pre-clinical model of lung adenocarcinoma, Barsoumian et al. showed the synergistic effect of the association between a single low-dose of 2 Gy and a combination of both anti-PD1 and anti-CTLA498. This result was explained by the ability of low-dose RT to reshape tumoral stroma into a proactive, antitumor phenotype. Moreover, in a pre-clinical model of advanced ovarian carcinoma, Herrera et al. used an association of low-dose RT with three different immunotherapies and cyclophosphamide to obtain a significant tumor growth delay through the activation of various pathways.97 This resulted in early-phase experimentations of low-dose RT and immunotherapy in patients harboring advanced and metastatic ovarian carcinomas (NCT03728179), with expected results to come.
Regarding some other aspects of the immunosuppressive landscape in solid tumors, low-dose RT can play a key role, notably by reshaping the immune phenotype of the myeloid compartment within tumor microenvironment, with subsequent benefits on T cell migration and activity.173–175 Moreover, a large amount of preclinical reports on various tumor models assessed the immune benefits of a low dose delivered in a large volume (whole-body irradiation), including the secretion of various pro-inflammatory cytokines as IFN .163,166,167,176,177
Therefore, even in the absence of preclinical data and on-going trial associating low-dose RT and adoptive T-cell therapy, it makes sense to consider that low-dose RT may not be limited to the simple function of depleting patients’ lymphocytes in order to ensure an optimal expansion of infused cells. The unique radiobiological effects provided by low doses could be helpful for the immune activation of solid tumors and thus could participate in the efficiency of infused T cells, in a different but complementary way to conventional/high-dose RT.
3.3. Final option: combining options 1 and 2, associating the properties of both low-dose and high-dose RT
Considering the data mentioned above and the different but complementary effects shown by high-dose and low-dose RT, a promising idea would be to associate both of them. This appears to be technically possible thanks to the ability of RT being spatially adjustable, which allows combining different doses delivered on different volumes in a same treatment. Such combinations between high-dose and low-dose irradiation may not require any specific process, since focal high-dose ablative RT often scatters large fields of low-dose within surrounding areas. As an example, this is illustrated in the dosimetry of a real patient treated with high-dose ablative RT, 60 Gy in 8 fractions delivered on 2 lung metastases, presented in Figure 4. In a setting of CAR-T cell therapy, this would result in the combination of positive effects underlying both high-dose and low-dose radiation. Such combinations are displayed in Figure 5, with examples of clinical outcomes already existing in literature. Menon et al. worked on a cohort of patients with various histological types receiving SRS associated with immune-checkpoint blockade.178 Interestingly, they demonstrated that not only lesions irradiated with high-dose SRS showed good response but also lesions receiving 5 to 10 Gy (versus 50 Gy for high-dose RT) experienced a surprisingly high objective response rate, with more than half of them (58%) meeting the PR/CR criteria for RECIST. Of note, these lesions received either an intentional low-dose RT or low doses scattered from the volume treated with high-dose RT. This observation paved the way for further perspectives of associations between high-dose and low-dose RT in patients with solid metastatic tumors in association with immunotherapy, with promising results.179–181
Figure 4.
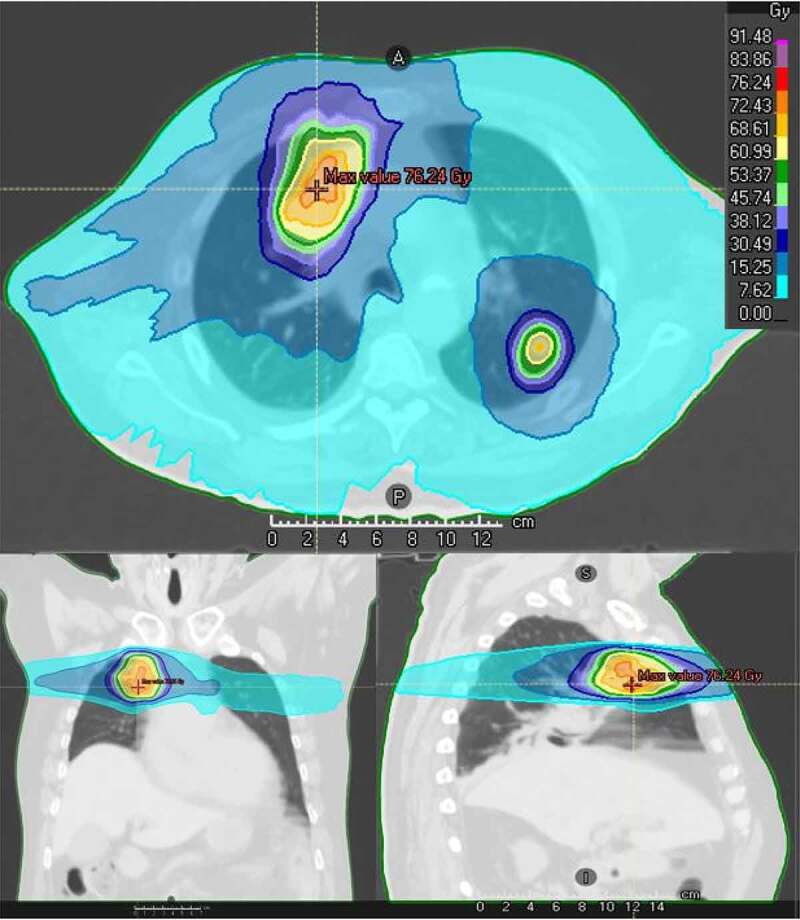
An example of patient treated with high-dose focal RT, 60 Gy in 8 fractions on 2 pulmonary lesions, illustrating large incidental low-dose radiation surrounding treated volumes.
Figure 5.
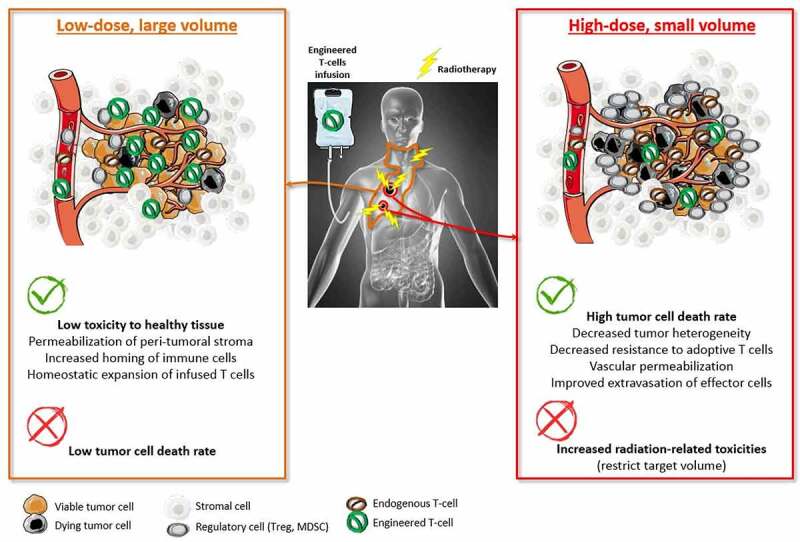
Complementary features between low-dose and high-dose RT in the setting of combinatorial approaches using engineered T-cell therapies.
Therefore, with regard to CAR-T cell therapies and similarly to observations in radioimmunotherapy combinatorial approaches, combining a low-dose of radiation delivered in a large volume with a high dose delivered in a limited volume would result in improving both the expansion, the homing and the activation of infused T cells. This hypothesis should be the starting point for the initiation of future clinical trials centered on spatially modulated bridging RT as a prime conditioning option, with the help of concurrent chemotherapy.
However, in this setting, there is no consensus regarding the optimal volume to irradiate with low or high dose. A suitable option may be to treat a limited volume of macroscopic tumor with a high-dose of RT in order to maximize homing and activation of infused T cells while preventing the patient from toxicity. For this purpose, partial irradiation could be a promising option, since it is likely to reshape the tumor microenvironment in a similar manner to conventional whole-tumor RT, leading to in-situ abscopal effect as demonstrated in two mice models.182
Finally, in the frequent setting of lung metastases, an interesting option may be the combination of low-dose total-lung irradiation (TLI) associated with focal high-dose RT on macroscopic tumors. In this setting, whole-lung irradiation may ensure the occurrence of radiation-induced lymphopenia while treating microscopic tumor involvement. In addition, according to safety profiles, it would provide a limited rate of radiation-induced lung injury for doses up to 5 Gy.183
4. New approaches in RT: paving the way for further preclinical and clinical experiments in the field of radioimmunotherapy combinations
As described in this review, defining the best conditions for the clinical implementation of combinatorial strategies using RT and CAR-T cells is challenging. Therefore, scientists and physicians must make the most of innovations designed in recent years.
4.1. Preclinical innovations in RT: treating mice as humans
The use of appropriate animal models remains crucial to increase the reproducibility of results and enable a good « bench to bedside » approach. Recent improvements include the use of next-generation devices able to deliver RT in similar conditions in mice than in patients. Thanks to their on-board imaging system, these devices deliver precise and controlled irradiation in mice due to visualization of the target.184,185 In the near future, preclinical research will not be limited to TBI, subcutaneous tumors or in-vitro studies.
Moreover, to facilitate the transition between preclinical and clinical research, preclinical models should be as similar as possible to their clinical counterpart. In such a goal, humanized mice should be used more often. After myeloablative treatment and then transfusion with human hematopoietic precursor cells, they may represent an appropriate model to experiment new radioimmunotherapy combinations using patient-derived xenografts (PDX).186 Of note, studies using humanized mice in the field of radioimmunotherapy combinations are currently scarce. Moreover, current preclinical associations between CAR-T cells and RT involve PDX of pancreatic187 or prostate cancer188 in immune deficient mice treated with fractionated low-dose. The use of a syngeneic model of murine glioblastoma in immunocompetent mice is also reported.189
4.2. Technological innovations in RT: FLASH ultra-high dose rate RT, proton beams, minibeams
Significant technological advances over the past decade in RT have translated into great opportunities and major changes in the landscape of cancer management, with the continuous aim to spare normal tissue as efficiently as possible. From this perspective, ultra-high dose rate RT, also called as FLASH-RT, has demonstrated its ability to enhance the differential effect of RT between tumors and normal tissues.190 However, the biological mechanisms underlying this effect are unclear, and a high variability remains depending on the type of tissue irradiated (acute or late-responding tissue), the particle used (photon or proton), or differences in dose and dose-rate.191
Another breakthrough innovation is spatially-fractionated RT (SFRT) with the use of highly spatially modulated beams, such as minibeams192 and microbeams.193 Notably, proton minibeam RT markedly increased the tolerance of normal brain tissue in rats compared to conventional homogeneous irradiation.194
Therefore, using FLASH or SFRT in immunocompetent animal models and analyzing their respective impacts on lymphocyte survival may provide informative content to researchers in the field of radioimmunotherapy.
5. Conclusion
As previously mentioned, clinical trials involving engineered T cells, in particular CAR-T cells, for the treatment of solid tumors have provided disappointing results, in contrast with their increasingly wide use in refractory hematological malignancies. The heterogeneity of solid tumors as well as the variety of oncogenic drivers could result in such a failure. However, the modalities and sequence of conditioning treatments play a crucial role in the efficacy of engineered T cells. From this perspective, radiotherapy, inconsistently used in conditioning phase, and currently underused in ongoing trials experimenting CAR-T cells in solid tumors may enhance the efficacy of these latter in solid tumors. Far beyond the old example of TBI, current RT is a flexible tool, spatially adjustable, with the ability to sculpt dose-delivering and thus deliver different doses in different volumes of interest in a same treatment. Considering the differential effects of high doses versus low doses of RT and the high radiosensitivity of resident T cells depleted during the conditioning phase, radiotherapy should deserve further consideration from clinical research in this field. Notably, local RT could be widely used as part of the conditioning phase in combination with chemotherapy as it provides crucial benefits for the action of adoptive T cells:
i) Deep remodeling of the tumor microenvironment by changes in tumor vasculature and cytokine secretion, thus increasing the attraction, homing and activation of engineered T cells in solid tumors.
ii) Fighting the immune no-go zone represented by hypoxic areas within the tumor.
iii) Depleting clones likely to further escape adoptive T cell therapy.
Whether these effects will be observed only in some selected radiosensitive settings or in a wide area of cancers will depend on ongoing and future experiments involving relevant preclinical models as well as early-phase clinical trials.
For its part, CT would consolidate the depletion of host T-cells in addition to significant antitumor effects.
A major challenge for RT in the future of combinatorial approaches associating CAR-T cells will be to find the optimal dose and volumes of irradiation to both maximize the benefits mentioned above and prevent excessive radiation-induced lymphopenia to improve long-term adaptive immunity. In addition to the options mentioned earlier in this review, particular emphasis should be placed on the implementation of functional imaging and radiomics in patients. Indeed, by using these non-invasive tools, we would be able to make iterative evaluations of the tumor immune landscape either before or after infusion of engineered T cells and selectively deliver on-demand RT to cold tumors, with the objective of strengthening the action of infused cells in real time.
Therefore, in the field of engineered T cells, a fine multidisciplinary management involving hematologists, medical and radiation oncologists, radiologists, biologists and medical physicists is the path towards overcoming the resistance of solid malignancies to adoptive T-cell therapies. Thus, after recent disappointments in this field, we may revive the phoenix from its ashes.
Funding Statement
The author(s) reported that there is no funding associated with the work featured in this article.
Disclosure statement
E.D. reports grants and personal fees from Roche Genentech; grants from Servier; grants from AstraZeneca; grants and personal fees from Merck-Serono; grants from BMS; and grants from MSD outside the submitted work. S.D. is the founder of ErVaccine Technologies. The remaining authors declare that no conflict of interest exists.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, et al. Tisagenlecleucel in children and young adults with B-Cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–18. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, Wolters P, Martin S, Delbrook C, Yates B, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24(1):20–28. doi: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gill SI, Vides V, Frey NV, Metzger S, O’Brien M, Hexner E, Mato AR, Lacey SF, Melenhorst JJ, Pequignot E, et al. Prospective clinical trial of anti-CD19 CAR T cells in combination with ibrutinib for the treatment of chronic lymphocytic leukemia shows a high response rate. Blood. 2018;132(Supplement 1):298–298. doi: 10.1182/blood-2018-99-115418. [DOI] [Google Scholar]
- 4.Siddiqi T, Soumerai JD, Dorritie KA, Stephens DM, Riedell PA, Arnason JE, Kipps TJ, Gillenwater HH, Gong L, Yang L, et al. Phase 1 TRANSCEND CLL 004 study of lisocabtagene maraleucel in patients with relapsed/refractory CLL or SLL. Blood. 2021 Oct 26, blood.2021011895. doi: 10.1182/blood.2021011895. [DOI] [PubMed] [Google Scholar]
- 5.Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, Timmerman JM, Holmes H, Jaglowski S, Flinn IW, et al. KTE-X19 CAR T-Cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382(14):1331–1342. doi: 10.1056/NEJMoa1914347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, et al. Axicabtagene ciloleucel CAR T-Cell therapy in refractory large B-Cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, Lin Y, Braunschweig I, Hill BT, Timmerman JM, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20(1):31–42. doi: 10.1016/S1470-2045(18)30864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, Jäger U, Jaglowski S, Andreadis C, Westin JR, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-Cell lymphoma. N Engl J Med. 2019;380(1):45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 9.Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, Liedtke M, Rosenblatt J, Maus MV, Turka A, et al. Anti-BCMA CAR T-Cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380(18):1726–1737. doi: 10.1056/NEJMoa1817226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner J, Wickman E, DeRenzo C, Gottschalk S.. CAR T cell therapy for solid tumors: bright future or dark reality? Molecular Therapy. 2020;28(11):2320–2339. doi: 10.1016/j.ymthe.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaft N. The landscape of CAR-T cell clinical trials against solid tumors—A comprehensive overview. Cancers. 2020;12(9):2567. doi: 10.3390/cancers12092567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhan X, Wang B, Li Z, Li J, Wang H, Chen L, Jiang H, Wu M, Xiao J, Peng X, et al. Phase I trial of Claudin 18.2-specific chimeric antigen receptor T cells for advanced gastric and pancreatic adenocarcinoma. JCO. 2019;37(15_suppl):2509–2509. doi: 10.1200/JCO.2019.37.15_suppl.2509. [DOI] [Google Scholar]
- 13.Adusumilli PS, Zauderer MG, Rivière I, Solomon SB, Rusch VW, O’Cearbhaill RE, Zhu A, Cheema W, Chintala NK, Halton E, et al. A phase I trial of regional mesothelin-targeted CAR T-cell therapy in patients with malignant pleural disease, in combination with the anti–PD-1 agent Pembrolizumab. Cancer Discov. 2021;11(11):2748–2763. doi: 10.1158/2159-8290.CD-21-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359(6382):1361–1365. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 15.Zheng Y, Chen Z, Han Y, Han L, Zou X, Zhou B, Hu R, Hao J, Bai S, Xiao H, et al. Immune suppressive landscape in the human esophageal squamous cell carcinoma microenvironment. Nat Commun. 2020;11(1):6268. doi: 10.1038/s41467-020-20019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang T-X, Fu L. The immune landscape of esophageal cancer. Cancer Commun. 2019;39(1):79. doi: 10.1186/s40880-019-0427-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quail DF, Joyce JA. The Microenvironmental Landscape of Brain Tumors. Cancer Cell. 2017;31(3):326–341. doi: 10.1016/j.ccell.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daubon T, Hemadou A, Romero Garmendia I, Saleh M. Glioblastoma immune landscape and the potential of new immunotherapies. Front. Immunol. 2020;11:585616. doi: 10.3389/fimmu.2020.585616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandal R, Şenbabaoğlu Y, Desrichard A, Havel JJ, Dalin MG, Riaz N, Lee K-W, Ganly I, Hakimi AA, Chan TA, et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight. 2016;1(17). doi: 10.1172/jci.insight.89829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lavin Y, Kobayashi S, Leader A, Amir ED, Elefant N, Bigenwald C, Remark R, Sweeney R, Becker CD, Levine JH, et al. Innate immune landscape in early lung adenocarcinoma by paired single-cell analyses. Cell. 2017;169(4):750–765.e17. doi: 10.1016/j.cell.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39(4):782–795. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Fleming V, Hu X, Weber R, Nagibin V, Groth C, Altevogt P, Utikal J, Umansky V. Targeting myeloid-derived suppressor cells to bypass tumor-induced immunosuppression. Front. Immunol. 2018;9:398. doi: 10.3389/fimmu.2018.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elias S, Rudensky AY. Therapeutic use of regulatory T cells for graft‐versus‐host disease. Br J Haematol. 2019;187(1):25–38. doi: 10.1111/bjh.16157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li M, Xue S-L, Tang X, Xu J, Chen S, Han Y, Qiu H, Miao M, Xu N, Tan J, et al. The differential effects of tumor burdens on predicting the net benefits of ssCART-19 cell treatment on r/r B-ALL patients. Sci Rep. 2022;12(1):378. doi: 10.1038/s41598-021-04296-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Locke FL, Rossi JM, Neelapu SS, Jacobson CA, Miklos DB, Ghobadi A, Oluwole OO, Reagan PM, Lekakis LJ, Lin Y, et al. Tumor burden, inflammation, and product attributes determine outcomes of axicabtagene ciloleucel in large B-cell lymphoma. Blood Advances. 2020;4(19):4898–4911. doi: 10.1182/bloodadvances.2020002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mallet M, Boulos RE, Alcazer V, Bonaventura P, Estornes Y, Chuvin N, Depil S. Tumour burden and antigen-specific T cell magnitude represent major parameters for clinical response to cancer vaccine and TCR-engineered T cell therapy. Eur J Cancer. 2022;171:96–105. doi: 10.1016/j.ejca.2022.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Vasan N, Baselga J, Hyman DM. A view on drug resistance in cancer. Nature. 2019;575(7782):299–309. doi: 10.1038/s41586-019-1730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lemoine J, Ruella M, Houot R. Born to survive: how cancer cells resist CAR T cell therapy. J Hematol Oncol. 2021;14(1):199. doi: 10.1186/s13045-021-01209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hay KA, Hanafi L-A, Li D, Gust J, Liles WC, Wurfel MM, López JA, Chen J, Chung D, Harju-Baker S, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor–modified T-cell therapy. Blood. 2017;130(21):2295–2306. doi: 10.1182/blood-2017-06-793141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsumura Y. Cancer stromal targeting (CAST) therapy. Adv Drug Deliv Rev. 2012;64(8):710–719. doi: 10.1016/j.addr.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 31.Dirkx AEM, Oude EMGA, Castermans K, Schaft DWJ, Thijssen VLJL, Dings RPM, Kwee L, Mayo KH, Wagstaff J, Steege JCAB, et al. Anti‐angiogenesis therapy can overcome endothelial cell anergy and promote leukocyte‐endothelium interactions and infiltration in tumors. FASEB J. 2006;20(6):621–630. doi: 10.1096/fj.05-4493com. [DOI] [PubMed] [Google Scholar]
- 32.Huang Y, Kim BYS, Chan CK, Hahn SM, Weissman IL, Jiang W. Improving immune–vascular crosstalk for cancer immunotherapy. Nat Rev Immunol. 2018;18(3):195–203. doi: 10.1038/nri.2017.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mondini M, Nizard M, Tran T, Mauge L, Loi M, Clémenson C, Dugue D, Maroun P, Louvet E, Adam J, et al. Synergy of radiotherapy and a cancer vaccine for the treatment of HPV-associated head and neck cancer. Mol Cancer Ther. 2015;14(6):1336–1345. doi: 10.1158/1535-7163.MCT-14-1015. [DOI] [PubMed] [Google Scholar]
- 34.Berahovich R, Liu X, Zhou H, Tsadik E, Xu S, Golubovskaya V, Wu L. Hypoxia selectively impairs CAR-T cells in vitro. Cancers. 2019;11(5):602. doi: 10.3390/cancers11050602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lan J, Wan X-L, Deng L, Xue J-X, Wang L-S, Meng M-B, Ling H, Zhang X, Mo X-M, Lu Y. Ablative hypofractionated radiotherapy normalizes tumor vasculature in lewis lung carcinoma mice model. Radiat Res. 2013;179(4):458–464. doi: 10.1667/RR3116.1. [DOI] [PubMed] [Google Scholar]
- 36.Tong F, Xiong C, Wei C, Wang Y, Liang Z, Lu H, Pan H, Dong J, Zheng X, Wu G, et al. Hypo-fractionation radiotherapy normalizes tumor vasculature in non-small cell lung cancer xenografts through the p-STAT3/HIF-1 alpha signaling pathway. Ther Adv Med Oncol. 2020;12:175883592096585. doi: 10.1177/1758835920965853. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Filatenkov A, Baker J, Mueller AMS, Kenkel J, Ahn G-O, Dutt S, Zhang N, Kohrt H, Jensen K, Dejbakhsh-Jones S, et al. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin Cancer Res. 2015;21(16):3727–3739. doi: 10.1158/1078-0432.CCR-14-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor E, Zhou J, Lindsay P, Foltz W, Cheung M, Siddiqui I, Hosni A, Amir AE, Kim J, Hill RP, et al. Quantifying reoxygenation in pancreatic cancer during stereotactic body radiotherapy. Sci Rep. 2020;10(1):1638. doi: 10.1038/s41598-019-57364-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee Y-H, C-F Y, Yang Y-C, Hong J-H, Chiang C-S. Ablative radiotherapy reprograms the tumor microenvironment of a pancreatic tumor in favoring the immune checkpoint blockade therapy. IJMS. 2021;22(4):2091. doi: 10.3390/ijms22042091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sonveaux P, Brouet A, Havaux X, Grégoire V, Dessy C, Balligand J-L, Feron O. Irradiation-induced angiogenesis through the up-regulation of the nitric oxide pathway: implications for tumor radiotherapy. Cancer Res. 2003;63:1012–1019. [PubMed] [Google Scholar]
- 41.Gabryś D, Greco O, Patel G, Prise KM, Tozer GM, Kanthou C. Radiation effects on the cytoskeleton of endothelial cells and endothelial monolayer permeability. International Journal of Radiation Oncology*Biology*Physics. 2007;69(5):1553–1562. doi: 10.1016/j.ijrobp.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 42.Rousseau M, Gaugler M-H, Rodallec A, Bonnaud S, Paris F, Corre I. RhoA GTPase regulates radiation-induced alterations in endothelial cell adhesion and migration. Biochem Biophys Res Commun. 2011;414(4):750–755. doi: 10.1016/j.bbrc.2011.09.150. [DOI] [PubMed] [Google Scholar]
- 43.Fowler JF. The rationale of dose fractionation. In: Vaeth JM, editor. Frontiers of radiation therapy and oncology Vol. 3. Basel: S. Karger AG: 1968. p. 6–23 doi: 10.1159/000386971. [DOI] [Google Scholar]
- 44.Kallman RF. The Phenomenon of Reoxygenation and Its Implications for Fractionated Radiotherapy. Radiology. 1972;105(1):135–142. doi: 10.1148/105.1.135. [DOI] [PubMed] [Google Scholar]
- 45.Withers HR. The four R’s of radiotherapy. Advances in radiation biology. Vol. 5. ed. John T. Lett, Howard Adler. 1975. 241–271. doi: 10.1016/B978-0-12-035405-4.50012-8. Amsterdam: Elsevier. [DOI] [Google Scholar]
- 46.Deutsch E, Chargari C, Galluzzi L, Kroemer G. Optimising efficacy and reducing toxicity of anticancer radioimmunotherapy. Lancet Oncol. 2019;20(8):e452–e463. doi: 10.1016/S1470-2045(19)30171-8. [DOI] [PubMed] [Google Scholar]
- 47.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, X-D L, Mauceri H, Beckett M, Darga T, et al. STING-Dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41(5):843–852. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM. Radiation-Induced IFN-γ production within the tumor microenvironment influences antitumor immunity. The Journal of Immunology. 2008;180(5):3132–3139. doi: 10.4049/jimmunol.180.5.3132. [DOI] [PubMed] [Google Scholar]
- 49.Storozynsky Q, Hitt MM. The impact of radiation-induced DNA damage on cGAS-STING-mediated immune responses to cancer. IJMS. 2020;21(22):8877. doi: 10.3390/ijms21228877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng -C-C, Chang Y-F, A-S H, Sie Z-L, Chang J-S, Peng C-L, Chang -C-C. Irradiation mediates IFNα and CXCL9 expression in Non-small cell lung cancer to stimulate CD8+ T cells activity and migration toward tumors. Biomedicines. 2021;9(10):1349. doi: 10.3390/biomedicines9101349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seyedin SN, Hasibuzzaman MM, Pham V, Petronek MS, Callaghan C, Kalen AL, Mapuskar KA, Mott SL, Spitz DR, Allen BG, et al. Combination therapy with radiation and PARP inhibition enhances responsiveness to anti-PD-1 therapy in colorectal tumor models. International Journal of Radiation Oncology*Biology*Physics. 2020;108(1):81–92. doi: 10.1016/j.ijrobp.2020.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lai J-Z, Zhu -Y-Y, Ruan M, Chen L, Zhang Q-Y. Local irradiation sensitized tumors to adoptive T cell therapy via enhancing the cross-priming, homing, and cytotoxicity of antigen-specific CD8 T cells. Front. Immunol. 2019;10:2857. doi: 10.3389/fimmu.2019.02857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, Babb JS, Schneider RJ, Formenti SC, Dustin ML, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181(5):3099–3107. doi: 10.4049/jimmunol.181.5.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Golden EB, Apetoh L. Radiotherapy and Immunogenic Cell Death. Semin Radiat Oncol. 2015;25(1):11–17. doi: 10.1016/j.semradonc.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 55.Mondini M, Loyher P-L, Hamon P, Gerbé de Thoré M, Laviron M, Berthelot K, Clémenson C, Salomon BL, Combadière C, Deutsch E, et al. CCR2-dependent recruitment of tregs and monocytes following radiotherapy is associated with TNFα-mediated resistance. Cancer Immunol Res. 2019;7(3):376–387. doi: 10.1158/2326-6066.CIR-18-0633. [DOI] [PubMed] [Google Scholar]
- 56.Xu J, Escamilla J, Mok S, David J, Priceman S, West B, Bollag G, McBride W, Wu L. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013;73(9):2782–2794. doi: 10.1158/0008-5472.CAN-12-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsai C-S, Chen F-H, Wang -C-C, Huang H-L, Jung S-M, C-J W, Lee -C-C, McBride WH, Chiang C-S, Hong J-H. Macrophages from irradiated tumors express higher levels of iNOS, Arginase-I and COX-2, and promote tumor growth. International Journal of Radiation Oncology*Biology*Physics. 2007;68(2):499–507. doi: 10.1016/j.ijrobp.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 58.Chiang C-S, Fu SY, Wang S-C, C-F Y, Chen F-H, Lin C-M, Hong J-H, Randall RL. Irradiation promotes an M2 macrophage phenotype in tumor hypoxia. Front. Oncol. 2012;2:2. doi: 10.3389/fonc.2012.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, Inghirami G, Coleman CN, Formenti SC, Demaria S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8(1):15618. doi: 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov. 2019;18(2):99–115. doi: 10.1038/s41573-018-0004-1. [DOI] [PubMed] [Google Scholar]
- 61.Nicolas AM, Pesic M, Engel E, Ziegler PK, Diefenhardt M, Kennel KB, Buettner F, Conche C, Petrocelli V, Elwakeel E, et al. Inflammatory fibroblasts mediate resistance to neoadjuvant therapy in rectal cancer. Cancer Cell. 2022;40(2):168–184.e13. doi: 10.1016/j.ccell.2022.01.004. [DOI] [PubMed] [Google Scholar]
- 62.Tommelein J, De Vlieghere E, Verset L, Melsens E, Leenders J, Descamps B, Debucquoy A, Vanhove C, Pauwels P, Gespach CP, et al. Radiotherapy-Activated cancer-associated fibroblasts promote tumor progression through paracrine IGF1R activation. Cancer Res. 2018;78(3):659–670. doi: 10.1158/0008-5472.CAN-17-0524. [DOI] [PubMed] [Google Scholar]
- 63.Garnett CT, Palena C, Chakarborty M, Tsang K-Y, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64(21):7985–7994. doi: 10.1158/0008-5472.CAN-04-1525. [DOI] [PubMed] [Google Scholar]
- 64.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, K.Wansley E, Camphausen K, Luiten RM, de RAH, Neijssen J, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203(5):1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamamoto K, Venida A, Yano J, Biancur DE, Kakiuchi M, Gupta S, Sohn ASW, Mukhopadhyay S, Lin EY, Parker SJ, et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature. 2020;581(7806):100–105. doi: 10.1038/s41586-020-2229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watson NFS, Ramage JM, Madjd Z, Spendlove I, Ellis IO, Scholefield JH, Durrant LG. Immunosurveillance is active in colorectal cancer as downregulation but not complete loss of MHC class I expression correlates with a poor prognosis. Int J Cancer. 2006;118(1):6–10. doi: 10.1002/ijc.21303. [DOI] [PubMed] [Google Scholar]
- 67.Garrido F. MHC/HLA class I loss in cancer cells. In: MHC class-I loss and cancer immune escape. Vol. 1151, ed. Garrido F. Cham (Switzerland): Springer International Publishing; 2019. p. 15–78. doi: 10.1007/978-3-030-17864-2_2. Advances in Experimental Medicine and Biology. [DOI] [Google Scholar]
- 68.Zhang M, Han N, Jiang Y, Wang J, Li G, Lv X, Li G, Qiao Q. EGFR confers radioresistance in human oropharyngeal carcinoma by activating endoplasmic reticulum stress signaling PERK-eIF2α-GRP94 and IRE1α-XBP1-GRP78. Cancer Med. 2018;7(12):6234–6246. doi: 10.1002/cam4.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jedlinski A, Ansell A, Johansson A-C, Roberg K. EGFR status and EGFR ligand expression influence the treatment response of head and neck cancer cell lines: EGFR status influences treatment response. Journal of Oral Pathology & Medicine. 2013;42(1):26–36. doi: 10.1111/j.1600-0714.2012.01177.x. [DOI] [PubMed] [Google Scholar]
- 70.Duru N, Fan M, Candas D, Menaa C, Liu H-C, Nantajit D, Wen Y, Xiao K, Eldridge A, Chromy BA, et al. HER2-associated radioresistance of breast cancer stem cells isolated from HER2-negative breast cancer cells. Clin Cancer Res. 2012;18(24):6634–6647. doi: 10.1158/1078-0432.CCR-12-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Candas-Green D, Xie B, Huang J, Fan M, Wang A, Menaa C, Zhang Y, Zhang L, Jing D, Azghadi S, et al. Dual blockade of CD47 and HER2 eliminates radioresistant breast cancer cells. Nat Commun. 2020;11(1):4591. doi: 10.1038/s41467-020-18245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, Stratford IJ, Poon E, Morrow M, Stewart R, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74(19):5458–5468. doi: 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]
- 73.Chen M-F, Chen P-T, Chen W-C, M-S L, Lin P-Y, Lee K-D. The role of PD-L1 in the radiation response and prognosis for esophageal squamous cell carcinoma related to IL-6 and T-cell immunosuppression. Oncotarget. 2016;7(7):7913–7924. doi: 10.18632/oncotarget.6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ehlerding EB, Lee HJ, Barnhart TE, Jiang D, Kang L, McNeel DG, Engle JW, Cai W. Noninvasive imaging and quantification of radiotherapy-Induced PD-L1 upregulation with 89 Zr–Df–Atezolizumab. Bioconjugate Chem. 2019;30(5):1434–1441. doi: 10.1021/acs.bioconjchem.9b00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vanpouille-Box C, Pilones KA, Wennerberg E, Formenti SC, Demaria S. In situ vaccination by radiotherapy to improve responses to anti-CTLA-4 treatment. Vaccine. 2015;33(51):7415–7422. doi: 10.1016/j.vaccine.2015.05.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Formenti SC, Rudqvist N-P, Golden E, Cooper B, Wennerberg E, Lhuillier C, Vanpouille-Box C, Friedman K, Ferrari de Andrade L, Wucherpfennig KW, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med. 2018;24(12):1845–1851. doi: 10.1038/s41591-018-0232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wagener-Ryczek S, Schoemmel M, Kraemer M, Bruns C, Schroeder W, Zander T, Gebauer F, Alakus H, Merkelbach-Bruse S, Buettner R, et al. Immune profile and immunosurveillance in treatment-naive and neoadjuvantly treated esophageal adenocarcinoma. Cancer Immunol Immunother. 2020;69(4):523–533. doi: 10.1007/s00262-019-02475-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grapin M, Richard C, Limagne E, Boidot R, Morgand V, Bertaut A, Derangere V, Laurent P-A, Thibaudin M, Fumet JD, et al. Optimized fractionated radiotherapy with anti-PD-L1 and anti-TIGIT: a promising new combination. J. Immunotherapy Cancer. 2019;7(1):160. doi: 10.1186/s40425-019-0634-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, et al. Overall survival with durvalumab after chemoradiotherapy in Stage III NSCLC. N Engl J Med. 2018;379(24):2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 80.Mondini M, Levy A, Meziani L, Milliat F, Deutsch E. Radiotherapy–immunotherapy combinations – perspectives and challenges. Mol Oncol. 2020;14(7):1529–1537. doi: 10.1002/1878-0261.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hou AJ, Chang ZL, Lorenzini MH, Zah E, Chen YY. TGF-β-responsive CAR-T cells promote anti-tumor immune function. Bioengineering & Translational Medicine. 2018;3(2):75–86. doi: 10.1002/btm2.10097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen C, Y-M G, Zhang F, Zhang Z-C, Zhang Y-T, Y-D H, Wang L, Zhou N, Tang F-T, Liu H-J, et al. Construction of PD1/CD28 chimeric-switch receptor enhances anti-tumor ability of c-Met CAR-T in gastric cancer. OncoImmunology. 2021;10(1):1901434. doi: 10.1080/2162402X.2021.1901434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ping Y, Li F, Nan S, Zhang D, Shi X, Shan J, Zhang Y. Augmenting the effectiveness of CAR-T cells by enhanced self-delivery of PD-1-Neutralizing scFv. Front. Cell Dev. Biol. 2020;8:803. doi: 10.3389/fcell.2020.00803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Venkatesulu BP, Mallick S, Lin SH, Krishnan S. A systematic review of the influence of radiation-induced lymphopenia on survival outcomes in solid tumors. Crit Rev Oncol Hematol. 2018;123:42–51. doi: 10.1016/j.critrevonc.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 85.Wang X, Wang P, Zhao Z, Mao Q, Yu J, Li M. A review of radiation-induced lymphopenia in patients with esophageal cancer: an immunological perspective for radiotherapy. Ther Adv Med Oncol. 2020;12:175883592092682. doi: 10.1177/1758835920926822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Holub K, Vargas A, Biete A. Radiation-induced lymphopenia: the main aspects to consider in immunotherapy trials for endometrial and cervical cancer patients. Clin Transl Oncol. 2020;22(11):2040–2048. doi: 10.1007/s12094-020-02345-3. [DOI] [PubMed] [Google Scholar]
- 87.Nakamura N, Kusunoki Y, Akiyama M. Radiosensitivity of CD4 or CD8 positive human T-Lymphocytes by an in vitro colony formation assay. Radiat Res. 1990;123(2):224. doi: 10.2307/3577549. [DOI] [PubMed] [Google Scholar]
- 88.Yovino S, Kleinberg L, Grossman SA, Narayanan M, Ford E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling Radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. 2013;31(2):140–144. doi: 10.3109/07357907.2012.762780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meyer KK. Radiation-Induced lymphocyte-immune deficiency: a factor in the increased visceral metastases and decreased hormonal responsiveness of breast cancer. Arch Surg. 1970;101(2):114. doi: 10.1001/archsurg.1970.01340260018003. [DOI] [PubMed] [Google Scholar]
- 90.Newman GH, Rees GJG, Jones RSJ, Grove EA, Preece AW. Changes in helper and suppressor T lymphocytes following radiotherapy for breast cancer. Clin Radiol. 1987;38(2):191–193. doi: 10.1016/S0009-9260(87)80032-6. [DOI] [PubMed] [Google Scholar]
- 91.Petrini B, Wasserman J, Blomgren H, Baral E. Blood lymphocyte subpopulations in breast cancer patients following radiotherapy. Clin. Exp. Immunol. 1977;29:36–42. [PMC free article] [PubMed] [Google Scholar]
- 92.Gyurkocza B, Sandmaier BM. Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood. 2014;124(3):344–353. doi: 10.1182/blood-2014-02-514778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Clift R, Buckner C, Thomas E, Bensinger W, Bowden R, Bryant E, Deeg H, Doney K, Fisher L, Hansen J. Marrow transplantation for chronic myeloid leukemia: a randomized study comparing cyclophosphamide and total body irradiation with busulfan and cyclophosphamide. Blood. 1994;84(6):2036–2043. doi: 10.1182/blood.V84.6.2036.2036. [DOI] [PubMed] [Google Scholar]
- 94.Devergie A, Blaise D, Attal M, Tigaud J, Jouet J, Vernant J, Bordigoni P, Ifrah N, Dauriac C, Cahn J. Allogeneic bone marrow transplantation for chronic myeloid leukemia in first chronic phase: a randomized trial of busulfan-cytoxan versus cytoxan-total body irradiation as preparative regimen: a report from the French Society of Bone Marrow Graft (SFGM). Blood. 1995;85(8):2263–2268. doi: 10.1182/blood.V85.8.2263.bloodjournal8582263. [DOI] [PubMed] [Google Scholar]
- 95.Blaise D, Maraninchi D, Archimbaud E, Reiffers J, Devergie A, Jouet J, Milpied N, Attal M, Michallet M, Ifrah N. Allogeneic bone marrow transplantation for acute myeloid leukemia in first remission: a randomized trial of a busulfan-Cytoxan versus Cytoxan-total body irradiation as preparative regimen: a report from the Group d’Etudes de la Greffe de Moelle Osseuse [see comments]. Blood. 1992;79(10):2578–2582. doi: 10.1182/blood.V79.10.2578.2578. [DOI] [PubMed] [Google Scholar]
- 96.Janiak MK, Wincenciak M, Cheda A, Nowosielska EM, Calabrese EJ. Cancer immunotherapy: how low-level ionizing radiation can play a key role. Cancer Immunol Immunother. 2017;66(7):819–832. doi: 10.1007/s00262-017-1993-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Herrera FG, Ronet C, Ochoa de Olza M, Barras D, Crespo I, Andreatta M, Corria-Osorio J, Spill A, Benedetti F, Genolet R, et al. Low dose radiotherapy reverses tumor immune desertification and resistance to immunotherapy. Cancer Discov. 2021 Sep 3, candisc.0003.2021. doi: 10.1158/2159-8290.CD-21-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barsoumian HB, Ramapriyan R, Younes AI, Caetano MS, Menon H, Comeaux NI, Cushman TR, Schoenhals JE, Cadena AP, Reilly TP, et al. Low-dose radiation treatment enhances systemic antitumor immune responses by overcoming the inhibitory stroma. J Immunother Cancer. 2020;8(2):e000537. doi: 10.1136/jitc-2020-000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sim AJ, Jain MD, Figura NB, Chavez JC, Shah BD, Khimani F, Lazaryan A, Krivenko G, Davila ML, Liu HD, et al. Radiation therapy as a bridging strategy for CAR T cell therapy with axicabtagene ciloleucel in diffuse large B-Cell lymphoma. International Journal of Radiation Oncology*Biology*Physics. 2019;105(5):1012–1021. doi: 10.1016/j.ijrobp.2019.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Walsh SR, Simovic B, Chen L, Bastin D, Nguyen A, Stephenson K, Mandur TS, Bramson JL, Lichty BD, Wan Y. Endogenous T cells prevent tumor immune escape following adoptive T cell therapy. J Clin Invest. 2019;129(12):5400–5410. doi: 10.1172/JCI126199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alizadeh D, Wong RA, Gholamin S, Maker M, Aftabizadeh M, Yang X, Pecoraro JR, Jeppson JD, Wang D, Aguilar B, et al. IFNγ is critical for CAR T Cell–Mediated myeloid activation and induction of endogenous immunity. Cancer Discov. 2021;11(9):2248–2265. doi: 10.1158/2159-8290.CD-20-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Formenti SC, Demaria S. Radiation therapy to convert the tumor into an in situ vaccine. International Journal of Radiation Oncology*Biology*Physics. 2012;84(4):879–880. doi: 10.1016/j.ijrobp.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Demaria S, Golden EB, Formenti SC. Role of Local Radiation Therapy in Cancer Immunotherapy. JAMA Oncol. 2015;1(9):1325. doi: 10.1001/jamaoncol.2015.2756. [DOI] [PubMed] [Google Scholar]
- 104.Nissani A, Lev-Ari S, Meirson T, Jacoby E, Asher N, Ben-Betzalel G, Itzhaki O, Shapira-Frommer R, Schachter J, Markel G, et al. Comparison of non-myeloablative lymphodepleting preconditioning regimens in patients undergoing adoptive T cell therapy. J Immunother Cancer. 2021;9(5):e001743. doi: 10.1136/jitc-2020-001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chadha AS, Liu G, Chen H-C, Das P, Minsky BD, Mahmood U, Delclos ME, Suh Y, Sawakuchi GO, Beddar S, et al. Does unintentional splenic radiation predict outcomes after pancreatic cancer radiation therapy? International Journal of Radiation Oncology*Biology*Physics. 2017;97(2):323–332. doi: 10.1016/j.ijrobp.2016.10.046. [DOI] [PubMed] [Google Scholar]
- 106.Ellsworth SG. Field size effects on the risk and severity of treatment-induced lymphopenia in patients undergoing radiation therapy for solid tumors. Advances in Radiation Oncology. 2018;3(4):512–519. doi: 10.1016/j.adro.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang Q, Qiu Q, Zhang Z, Zhang J, Yang G, Liu C, Li B. Bone marrow dosimetric analysis of lymphopenia in patients with esophageal squamous cell carcinoma treated with chemoradiotherapy. Cancer Med. 2021;10(17):5847–5858. doi: 10.1002/cam4.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van Rossum PSN, Deng W, Routman DM, Liu AY, Xu C, Shiraishi Y, Peters M, Merrell KW, Hallemeier CL, Mohan R, et al. Prediction of severe lymphopenia during chemoradiation therapy for esophageal cancer: development and validation of a pretreatment nomogram. Pract Radiat Oncol. 2020;10(1):e16–e26. doi: 10.1016/j.prro.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhu C, Lin SH, Jiang X, Xiang Y, Belal Z, Jun G, Mohan R. A novel deep learning model using dosimetric and clinical information for grade 4 radiotherapy-induced lymphopenia prediction. Phys. Med. Biol. 2019 Dec 18. doi: 10.1088/1361-6560/ab63b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rosenblat TL, McDevitt MR, Carrasquillo JA, Pandit-Taskar N, Frattini MG, Maslak PG, Park JH, Douer D, Cicic D, Larson SM, et al. Treatment of patients with acute myeloid leukemia with the targeted alpha-particle nanogenerator actinium-225-lintuzumab. Clinical Cancer Research. 2022;28(10):2030–2037. doi: 10.1158/1078-0432.CCR-21-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ménager J, Gorin J-B, Maurel C, Drujont L, Gouard S, Louvet C, Chérel M, Faivre-Chauvet A, Morgenstern A, Bruchertseifer F, et al. Combining α-radioimmunotherapy and adoptive t cell therapy to potentiate tumor destruction Shiku H, editor. PLoS ONE. 2015;10(6):e0130249. doi: 10.1371/journal.pone.0130249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, Tagawa ST, Nordquist LT, Vaishampayan N, El-Haddad G, et al. Lutetium-177–PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385(12):1091–1103. doi: 10.1056/NEJMoa2107322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schuchardt C, Zhang J, Kulkarni HR, Chen X, Müller D, Baum RP. Prostate-specific membrane antigen radioligand therapy using 177 Lu-PSMA I&T and 177 Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer: comparison of safety, biodistribution, and dosimetry. J Nucl Med. 2022;63(8):1199–1207. doi: 10.2967/jnumed.121.262713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sgouros G, Bodei L, McDevitt MR, Nedrow JR. Radiopharmaceutical therapy in cancer: clinical advances and challenges. Nat Rev Drug Discov. 2020;19(9):589–608. doi: 10.1038/s41573-020-0073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xie G, Dong H, Liang Y, Ham JD, Rizwan R, Chen J. CAR-NK cells: a promising cellular immunotherapy for cancer. EBioMedicine. 2020;59:102975. doi: 10.1016/j.ebiom.2020.102975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Weiss T, Weller M, Guckenberger M, Sentman CL, Roth P. NKG2D-Based CAR T cells and radiotherapy exert synergistic efficacy in glioblastoma. Cancer Res. 2018;78(4):1031–1043. doi: 10.1158/0008-5472.CAN-17-1788. [DOI] [PubMed] [Google Scholar]
- 117.Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, Nassif Kerbauy L, Overman B, Thall P, Kaplan M, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med. 2020;382(6):545–553. doi: 10.1056/NEJMoa1910607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kruschinski A, Moosmann A, Poschke I, Norell H, Chmielewski M, Seliger B, Kiessling R, Blankenstein T, Abken H, Charo J. Engineering antigen-specific primary human NK cells against HER-2 positive carcinomas. Proceedings of the National Academy of Sciences. 2008;105(45):17481–17486. doi: 10.1073/pnas.0804788105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Han J, Chu J, Keung Chan W, Zhang J, Wang Y, Cohen JB, Victor A, Meisen WH, Kim S, Grandi P, et al. CAR-engineered NK cells targeting wild-type EGFR and EGFRvIII enhance killing of glioblastoma and patient-derived glioblastoma stem cells. Sci Rep. 2015;5(1):11483. doi: 10.1038/srep11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li Y, Hermanson DL, Moriarity BS, Kaufman DS. Human iPSC-Derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell. 2018;23(2):181–192.e5. doi: 10.1016/j.stem.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kailayangiri S, Altvater B, Spurny C, Jamitzky S, Schelhaas S, Jacobs AH, Wiek C, Roellecke K, Hanenberg H, Hartmann W, et al. Targeting Ewing sarcoma with activated and GD2-specific chimeric antigen receptor-engineered human NK cells induces upregulation of immune-inhibitory HLA-G. OncoImmunology. 2017;6(1):e1250050. doi: 10.1080/2162402X.2016.1250050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Spurny C, Kailayangiri S, Altvater B, Jamitzky S, Hartmann W, Wardelmann E, Ranft A, Dirksen U, Amler S, Hardes J, et al. T cell infiltration into Ewing sarcomas is associated with local expression of immune-inhibitory HLA-G. Oncotarget. 2018;9(5):6536–6549. doi: 10.18632/oncotarget.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim J-Y, Son Y-O, Park S-W, Bae J-H, Chung JS, Kim HH, Chung B-S, Kim S-H, Kang C-D. Increase of NKG2D ligands and sensitivity to NK cell-mediated cytotoxicity of tumor cells by heat shock and ionizing radiation. Exp Mol Med. 2006;38(5):474–484. doi: 10.1038/emm.2006.56. [DOI] [PubMed] [Google Scholar]
- 124.Butler JE, Moore MB, Presnell SR, Chan H-W, Chalupny NJ, Lutz CT. Proteasome regulation of ULBP1 transcription. J Immunol. 2009;182(10):6600–6609. doi: 10.4049/jimmunol.0801214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bedel R, Thiery-Vuillemin A, Grandclement C, Balland J, Remy-Martin J-P, Kantelip B, Pallandre J-R, Pivot X, Ferrand C, Tiberghien P, et al. Novel role for STAT3 in transcriptional regulation of NK immune cell targeting receptor MICA on cancer cells. Cancer Res. 2011;71(5):1615–1626. doi: 10.1158/0008-5472.CAN-09-4540. [DOI] [PubMed] [Google Scholar]
- 126.Canter RJ, Grossenbacher SK, Foltz JA, Sturgill IR, Park JS, Luna JI, Kent MS, Culp WTN, Chen M, Modiano JF, et al. Radiotherapy enhances natural killer cell cytotoxicity and localization in pre-clinical canine sarcomas and first-in-dog clinical trial. J. Immunotherapy Cancer. 2017;5(1):98. doi: 10.1186/s40425-017-0305-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Makowska A, Lelabi N, Nothbaum C, Shen L, Busson P, Tran TTB, Eble M, Kontny U. Radiotherapy combined with PD-1 inhibition increases NK cell cytotoxicity towards nasopharyngeal carcinoma cells. Cells. 2021;10(9):2458. doi: 10.3390/cells10092458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.André P, Denis C, Soulas C, Bourbon-Caillet C, Lopez J, Arnoux T, Bléry M, Bonnafous C, Gauthier L, Morel A, et al. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell. 2018;175(7):1731–1743.e13. doi: 10.1016/j.cell.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhao X-Y, X-X Y, Z-L X, Cao X-H, Huo M-R, Zhao X-S, Chang Y-J, Wang Y, Zhang X-H, L-P X, et al. Donor and host coexpressing KIR ligands promote NK education after allogeneic hematopoietic stem cell transplantation. Blood Advances. 2019;3(24):4312–4325. doi: 10.1182/bloodadvances.2019000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Orth M, Lauber K, Niyazi M, Friedl AA, Li M, Maihöfer C, Schüttrumpf L, Ernst A, Niemöller OM, Belka C. Current concepts in clinical radiation oncology. Radiat Environ Biophys. 2014;53(1):1–29. doi: 10.1007/s00411-013-0497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Boustani G, Laurent A, Mirjolet. The 6th R of Radiobiology: reactivation of Anti-Tumor Immune Response. Cancers. 2019;11(6):860. doi: 10.3390/cancers11060860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Minn I, Rowe SP, Pomper MG. Enhancing CAR T-cell therapy through cellular imaging and radiotherapy. Lancet Oncol. 2019;20(8):e443–e451. doi: 10.1016/S1470-2045(19)30461-9. [DOI] [PubMed] [Google Scholar]
- 133.Hauth F, Ho AY, Ferrone S, Duda DG. Radiotherapy to enhance chimeric antigen receptor T-cell therapeutic efficacy in solid tumors: a narrative review. JAMA Oncol. 2021 Apr 22;7(7):1051. doi: 10.1001/jamaoncol.2021.0168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, Mulroy L, Lock M, Rodrigues GB, Yaremko BP, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. The Lancet. 2019;393(10185):2051–2058. doi: 10.1016/S0140-6736(18)32487-5. [DOI] [PubMed] [Google Scholar]
- 135.Hiraoka M, Matsuo Y, Nagata Y. Stereotactic body radiation therapy (SBRT) for early-stage lung cancer. Cancer/Radiothérapie. 2007;11(1–2):32–35. doi: 10.1016/j.canrad.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 136.Mahadevan A, Blanck O, Lanciano R, Peddada A, Sundararaman S, D’Ambrosio D, Sharma S, Perry D, Kolker J, Davis J. Stereotactic Body Radiotherapy (SBRT) for liver metastasis – clinical outcomes from the international multi-institutional RSSearch® patient registry. Radiat Oncol. 2018;13(1):26. doi: 10.1186/s13014-018-0969-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Viani GA, Arruda CV, Hamamura AC, Faustino AC, Freitas Bendo Danelichen A, Guimarães FS. Stereotactic body radiotherapy for oligometastatic prostate cancer recurrence: a meta-analysis. Am J Clin Oncol. 2020;43(2):73–81. doi: 10.1097/COC.0000000000000635. [DOI] [PubMed] [Google Scholar]
- 138.Golden EB, Pellicciotta I, Demaria S, Barcellos-Hoff MH, Formenti SC. The convergence of radiation and immunogenic cell death signaling pathways. Front. Oncol. 2012;2:2. doi: 10.3389/fonc.2012.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Golden EB, Frances D, Pellicciotta I, Demaria S, Helen Barcellos-Hoff M, Formenti SC. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. OncoImmunology. 2014;3(4):e28518. doi: 10.4161/onci.28518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Darmon A, Zhang P, Marill J, Mohamed Anesary N, Da Silva J, Paris S. Radiotherapy-activated NBTXR3 nanoparticles modulate cancer cell immunogenicity and TCR repertoire. Cancer Cell Int. 2022;22(1):208. doi: 10.1186/s12935-022-02615-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Qin X, Yang C, Xu H, Zhang R, Zhang D, Tu J, Guo Y, Niu B, Kong L, Zhang Z. Cell‐Derived biogenetic gold nanoparticles for sensitizing radiotherapy and boosting immune response against cancer. Small. 2021;17(50):2103984. doi: 10.1002/smll.202103984. [DOI] [PubMed] [Google Scholar]
- 142.Sun T, Yang W, Toprani SM, Guo W, He L, DeLeo AB, Ferrone S, Zhang G, Wang E, Lin Z, et al. Induction of immunogenic cell death in radiation-resistant breast cancer stem cells by repurposing anti-alcoholism drug disulfiram. Cell Commun Signal. 2020;18(1):36. doi: 10.1186/s12964-019-0507-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Demaria S, Formenti SC, Chaturvedi A, Schiffman JD, Jones KB, Lessnick SL, Beckerle M, Randall RL. Radiation as an immunological adjuvant: current evidence on dose and fractionation. Front. Oncol. 2012;2:2. doi: 10.3389/fonc.2012.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rückert M, Deloch L, Frey B, Schlücker E, Fietkau R, Gaipl US. Combinations of radiotherapy with vaccination and immune checkpoint inhibition differently affect primary and abscopal tumor growth and the tumor microenvironment. Cancers. 2021;13(4):714. doi: 10.3390/cancers13040714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hallahan D, Kuchibhotla J, Wyble C. Cell adhesion molecules mediate radiation-induced leukocyte adhesion to the vascular endothelium’. Cancer Res. 1996;56:5150–5155. [PubMed] [Google Scholar]
- 146.Sun R, Limkin EJ, Vakalopoulou M, Dercle L, Champiat S, Han SR, Verlingue L, Brandao D, Lancia A, Ammari S, et al. A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: an imaging biomarker, retrospective multicohort study. Lancet Oncol. 2018;19(9):1180–1191. doi: 10.1016/S1470-2045(18)30413-3. [DOI] [PubMed] [Google Scholar]
- 147.Sun R, Sundahl N, Hecht M, Putz F, Lancia A, Rouyar A, Milic M, Carré A, Battistella E, Alvarez Andres E, et al. Radiomics to predict outcomes and abscopal response of patients with cancer treated with immunotherapy combined with radiotherapy using a validated signature of CD8 cells. J Immunother Cancer. 2020;8(2):e001429. doi: 10.1136/jitc-2020-001429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sun R, Henry T, Laville A, Carré A, Hamaoui A, Bockel S, Chaffai I, Levy A, Chargari C, Robert C, et al. Imaging approaches and radiomics: toward a new era of ultraprecision radioimmunotherapy? J Immunother Cancer. 2022;10(7):e004848. doi: 10.1136/jitc-2022-004848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kakkad S, Krishnamachary B, Jacob D, Pacheco-Torres J, Goggins E, Bharti SK, Penet M-F, Bhujwalla ZM. Molecular and functional imaging insights into the role of hypoxia in cancer aggression. Cancer Metastasis Rev. 2019;38(1–2):51–64. doi: 10.1007/s10555-019-09788-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tubin S, Khan MK, Salerno G, Mourad WF, Yan W, Jeremic B. Mono-institutional phase 2 study of innovative Stereotactic Body RADIOTHERAPY targeting PArtial Tumor HYpoxic (SBRT-PATHY) clonogenic cells in unresectable bulky non-small cell lung cancer: profound non-targeted effects by sparing peri-tumoral immune microenvironment. Radiat Oncol. 2019;14(1):212. doi: 10.1186/s13014-019-1410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lutsiak MEC, Semnani RT, De Pascalis R, Kashmiri SVS, Schlom J, Sabzevari H. Inhibition of CD4+25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105(7):2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 152.Traverso I, Fenoglio D, Negrini S, Parodi A, Battaglia F, Kalli F, Conteduca G, Tardito S, Traverso P, Indiveri F, et al. Cyclophosphamide inhibits the generation and function of CD8+ regulatory T cells. Hum Immunol. 2012;73(3):207–213. doi: 10.1016/j.humimm.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 153.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, Solary E, Le Cesne A, Zitvogel L, Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56(5):641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Vanneste BGL, Van Limbergen EJ, Dubois L, Samarska IV, Wieten L, Aarts MJB, Marcelissen T, De Ruysscher D. Immunotherapy as sensitizer for local radiotherapy. OncoImmunology. 2020;9(1):1832760. doi: 10.1080/2162402X.2020.1832760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ozasa K, Shimizu Y, Suyama A, Kasagi F, Soda M, Grant EJ, Sakata R, Sugiyama H, Kodama K. Studies of the mortality of atomic bomb survivors, report 14, 1950–2003: an overview of cancer and noncancer diseases. Radiat Res. 2012;177(3):229–243. doi: 10.1667/RR2629.1. [DOI] [PubMed] [Google Scholar]
- 156.Kamiya K, Ozasa K, Akiba S, Niwa O, Kodama K, Takamura N, Zaharieva EK, Kimura Y, Wakeford R. Long-term effects of radiation exposure on health. The Lancet. 2015;386(9992):469–478. doi: 10.1016/S0140-6736(15)61167-9. [DOI] [PubMed] [Google Scholar]
- 157.Berrington A, Darby SC, Weiss HA, Doll R. 100 years of observation on British radiologists: mortality from cancer and other causes 1897–1997. BJR. 2001;74(882):507–519. doi: 10.1259/bjr.74.882.740507. [DOI] [PubMed] [Google Scholar]
- 158.Mohan A, Hauptmann M, Doody M, Freedman D, Alexander B, Boice J, Mandel J, Correa-Villasenor A, Matanoski G, Linet M. Mortality among radiologic technologists in the United States (1926–1997). Ann Epidemiol. 2000;10(7):480. doi: 10.1016/S1047-2797(00)00142-3. [DOI] [PubMed] [Google Scholar]
- 159.Mohan AK, Hauptmann M, Freedman DM, Ron E, Matanoski GM, Lubin JH, Alexander BH, Boice JD, Morin Doody M, Linet MS. Cancer and other causes of mortality among radiologic technologists in the United States. Int J Cancer. 2003;103(2):259–267. doi: 10.1002/ijc.10811. [DOI] [PubMed] [Google Scholar]
- 160.Mirjolet C, Diallo I, Bertaut A, Veres C, Sargos P, Helfre S, Sunyach M-P, Truc G, Le Pechoux C, Paumier A, et al. Treatment related factors associated with the risk of breast radio-induced-sarcoma. Radiotherapy and Oncology. 2022;171:14–21. doi: 10.1016/j.radonc.2022.04.004. [DOI] [PubMed] [Google Scholar]
- 161.Portess DI, Bauer G, Hill MA, O’Neill P. Low-Dose irradiation of nontransformed cells stimulates the selective removal of precancerous cells via intercellular induction of apoptosis. Cancer Res. 2007;67(3):1246–1253. doi: 10.1158/0008-5472.CAN-06-2985. [DOI] [PubMed] [Google Scholar]
- 162.Bauer G. Low dose radiation and intercellular induction of apoptosis: potential implications for the control of oncogenesis. Int J Radiat Biol. 2007;83(11–12):873–888. doi: 10.1080/09553000701727523. [DOI] [PubMed] [Google Scholar]
- 163.Hashimoto S, Shirato H, Hosokawa M, Nishioka T, Kuramitsu Y, Matushita K, Kobayashi M, Miyasaka K. The suppression of metastases and the change in host immune response after low-dose total-body irradiation in tumor-bearing rats. Radiat Res. 1999;151(6):717–724. doi: 10.2307/3580211. [DOI] [PubMed] [Google Scholar]
- 164.Mitchel REJ, Jackson JS, Morrison DP, Carlisle SM. Low doses of radiation increase the latency of spontaneous lymphomas and spinal osteosarcomas in cancer-prone, radiation-sensitive Trp53 heterozygous mice. Radiat Res. 2003;159(3):320–327. doi: 10.1667/0033-7587(2003)159[0320:LDORIT2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 165.H-S Y, Liu Z-M, X-Y Y, Song A-Q, Liu N, Wang H. Low-dose Radiation Induces Antitumor Effects and Erythrocyte System Hormesis. Asian Pacific Journal of Cancer Prevention. 2013;14(7):4121–4126. doi: 10.7314/APJCP.2013.14.7.4121. [DOI] [PubMed] [Google Scholar]
- 166.Jin SZ, Pan XN, Wu N, Jin GH, Liu SZ. Whole-Body low dose irradiation promotes the efficacy of conventional radiotherapy for cancer and possible mechanisms. Dose-Response. 2007;5(4). doi: 10.2203/dose-response.07-020.Jin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wu N, Jin S-Z, Pan X-N, Liu S-Z. Increase in efficacy of cancer radiotherapy by combination with whole-body low dose irradiation. Int J Radiat Biol. 2008;84(3):201–210. doi: 10.1080/09553000801902133. [DOI] [PubMed] [Google Scholar]
- 168.Nowosielska EM, Cheda A, Wrembel-Wargocka J, Janiak MK. Immunological mechanism of the low-dose radiation-induced suppression of cancer metastases in a mouse model. Dose-Response. 2010;8(2). doi: 10.2203/dose-response.09-016.Nowosielska. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nowosielska EM, Cheda A, Wrembel-Wargocka J, Janiak MK. Anti-neoplastic and immunostimulatory effects of low-dose X-ray fractions in mice. Int J Radiat Biol. 2011;87(2):202–212. doi: 10.3109/09553002.2010.519422. [DOI] [PubMed] [Google Scholar]
- 170.Nowosielska EM, Cheda A, Wrembel-Wargocka J, Janiak MK. Effect of low doses of low-let radiation on the innate anti-tumor reactions in radioresistant and radiosensitive mice. Dose-Response. 2012;10(4). doi: 10.2203/dose-response.12-018.Nowosielska. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cheda A, Wrembel-Wargocka J, Lisiak E, Nowosielska EM, Marciniak M, Janiak MK. Single low doses of X rays inhibit the development of experimental tumor metastases and trigger the activities of NK cells in mice. Radiat Res. 2004;161(3):335–340. doi: 10.1667/rr3123. [DOI] [PubMed] [Google Scholar]
- 172.Takahashi M, Kojima S. Suppression of atopic dermatitis and tumor metastasis in mice by small amounts of radon. Radiat Res. 2006;165(3):337–342. doi: 10.1667/RR3501.1. [DOI] [PubMed] [Google Scholar]
- 173.Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, Pfirschke C, Voss RH, Timke C, Umansky L, et al. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24(5):589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 174.Nadella V, Singh S, Jain A, Jain M, Vasquez KM, Sharma A, Tanwar P, Rath GK, Prakash H. Low dose radiation primed iNOS + M1macrophages modulate angiogenic programming of tumor derived endothelium. Mol Carcinog. 2018;57(11):1664–1671. doi: 10.1002/mc.22879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Deloch L, Fuchs J, Rückert M, Fietkau R, Frey B, Gaipl US. Low-dose irradiation differentially impacts macrophage phenotype in dependence of fibroblast-like synoviocytes and radiation dose. Journal of Immunology Research. 2019;2019:1–11. doi: 10.1155/2019/3161750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pandey R, Shankar BS, Sharma D, Sainis KB. Low dose radiation induced immunomodulation: effect on macrophages and CD8+ T cells. Int J Radiat Biol. 2005;81(11):801–812. doi: 10.1080/09553000500531886. [DOI] [PubMed] [Google Scholar]
- 177.Liu J, Zhou J, Wu M, Hu C, Yang J, Li D, Wu P, Chen Y, Chen P, Lin S, et al. Low-dose total body irradiation can enhance systemic immune related response induced by hypo-fractionated radiation. Front. Immunol. 2019;10:317. doi: 10.3389/fimmu.2019.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Menon H, Chen D, Ramapriyan R, Verma V, Barsoumian HB, Cushman TR, Younes AI, Cortez MA, Erasmus JJ, de Groot P, et al. Influence of low-dose radiation on abscopal responses in patients receiving high-dose radiation and immunotherapy. Journal for ImmunoTherapy of Cancer. 2019;7(1). doi: 10.1186/s40425-019-0718-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sezen D, Patel RR, Tang C, Onstad M, Nagarajan P, Patel SP, Welsh JW, Lin LL. Immunotherapy combined with high- and low-dose radiation to all sites leads to complete clearance of disease in a patient with metastatic vaginal melanoma. Gynecol Oncol. 2021;161(3):645–652. doi: 10.1016/j.ygyno.2021.03.017. [DOI] [PubMed] [Google Scholar]
- 180.Patel RR, Verma V, Barsoumian HB, Ning MS, Chun SG, Tang C, Chang JY, Lee PP, Gandhi S, Balter P, et al. Use of multi-site radiation therapy for systemic disease control. International Journal of Radiation Oncology*Biology*Physics. 2021;109(2):352–364. doi: 10.1016/j.ijrobp.2020.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Patel RR, He K, Barsoumian HB, Chang JY, Tang C, Verma V, Comeaux N, Chun SG, Gandhi S, Truong MT, et al. High-dose irradiation in combination with non-ablative low-dose radiation to treat metastatic disease after progression on immunotherapy: results of a phase II trial. Radiotherapy and Oncology. 2021;162:60–67. doi: 10.1016/j.radonc.2021.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Markovsky E, Budhu S, Samstein RM, Li H, Russell J, Zhang Z, Drill E, Bodden C, Chen Q, Powell SN, et al. An antitumor immune response is evoked by partial-volume single-dose radiation in 2 murine models. International Journal of Radiation Oncology*Biology*Physics. 2019;103(3):697–708. doi: 10.1016/j.ijrobp.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hanania AN, Mainwaring W, Ghebre YT, Hanania NA, Ludwig M. Radiation-induced lung injury. Chest. 2019;156(1):150–162. doi: 10.1016/j.chest.2019.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Matinfar M, Iordachita I, Ford E, Wong J, Kazanzides P. Precision radiotherapy for small animal research. Metaxas D, Axel L, Fichtinger G, Székely G. editors. Medical Image Computing and Computer-Assisted Intervention – MICCAI 2008. Vol. 5242. Springer Berlin Heidelberg;Berlin, Heidelberg. 2008. Lecture Notes in Computer Science 619–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wong J, Armour E, Kazanzides P, Iordachita I, Tryggestad E, Deng H, Matinfar M, Kennedy C, Liu Z, Chan T, et al. High-resolution, small animal radiation research platform with x-ray tomographic guidance capabilities. International Journal of Radiation Oncology*Biology*Physics. 2008;71(5):1591–1599. doi: 10.1016/j.ijrobp.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cogels MM, Rouas R, Ghanem GE, Martinive P, Awada A, Van Gestel D, Krayem M. Humanized mice as a valuable pre-clinical model for cancer immunotherapy research. Front. Oncol. 2021;11:784947. doi: 10.3389/fonc.2021.784947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.DeSelm C, Palomba ML, Yahalom J, Hamieh M, Eyquem J, Rajasekhar VK, Sadelain M. Low-dose radiation conditioning enables CAR T cells to mitigate antigen escape. Mol Ther. 2018;26(11):2542–2552. doi: 10.1016/j.ymthe.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang Y, He L, Sadagopan A, Ma T, Dotti G, Wang Y, Zheng H, Gao X, Wang D, DeLeo AB, et al. Targeting radiation-resistant prostate cancer stem cells by B7-H3 CAR T cells. Mol Cancer Ther. 2021;20(3):577–588. doi: 10.1158/1535-7163.MCT-20-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Murty S, Haile ST, Beinat C, Aalipour A, Alam IS, Murty T, Shaffer TM, Patel CB, Graves EE, Mackall CL, et al. Intravital imaging reveals synergistic effect of CAR T-cells and radiation therapy in a preclinical immunocompetent glioblastoma model. OncoImmunology. 2020;9(1). doi: 10.1080/2162402X.2020.1757360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bourhis J, Sozzi WJ, Jorge PG, Gaide O, Bailat C, Duclos F, Patin D, Ozsahin M, Bochud F, Germond J-F, et al. Treatment of a first patient with FLASH-radiotherapy. Radiotherapy and Oncology. 2019;139:18–22. doi: 10.1016/j.radonc.2019.06.019. [DOI] [PubMed] [Google Scholar]
- 191.Vozenin M-C, Bourhis J, Durante M. Towards clinical translation of FLASH radiotherapy. Nat Rev Clin Oncol. 2022 Oct 27;19(12):791–803. doi: 10.1038/s41571-022-00697-z [DOI] [PubMed] [Google Scholar]
- 192.Meyer J, Eley J, Schmid TE, Combs SE, Dendale R, Prezado Y. Spatially fractionated proton minibeams. BJR. 2019;92(1095):20180466. doi: 10.1259/bjr.20180466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Prise KM, Schettino G. Microbeams in radiation biology: review and critical comparison. Radiat Prot Dosimetry. 2011;143(2–4):335–339. doi: 10.1093/rpd/ncq388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Prezado Y, Jouvion G, Hardy D, Patriarca A, Nauraye C, Bergs J, González W, Guardiola C, Juchaux M, Labiod D, et al. Proton minibeam radiation therapy spares normal rat brain: long-term clinical, radiological and histopathological analysis. Sci Rep. 2017;7(1):14403. doi: 10.1038/s41598-017-14786-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


