Abstract
Many pneumococcal capsular polysaccharides (PSs) are similar in structure, and a pneumococcal antibody often binds to all of the PSs with a similar structure. Yet, these cross-reactive antibodies may bind to the structurally related pneumococcal capsular PSs with an avidity too low to be effective. If memory B cells producing such weakly cross-reactive antibodies are elicited with pneumococcal conjugate vaccines, the memory cells for low-avidity antibodies could compromise the subsequent immune responses to the cross-reactive PS (original antigenic sin). To investigate these issues, we produced 14 hybridomas secreting monoclonal antibodies (MAbs) to the capsular PS of Streptococcus pneumoniae serotype 6B by immunizing BALB/c mice with antigens containing 6B PS and studied their epitope, avidity, in vitro opsonizing capacity, in vivo protective capacity, and “antigen binding titer” by enzyme-linked immunosorbent assay (ELISA) of 6A and 6B capsular PSs. Six MAbs bound to the non-cross-reactive 6B-specific epitope, and seven MAbs bound to the cross-reactive epitope present in both 6A and 6B PSs One MAb (Hyp6BM6) revealed a novel epitope. This epitope was found on 6A PS in solution, but not on 6A PS adsorbed onto the plastic surface of the ELISA plates. The avidity of the MAb for 6A or 6B PS ranged from 7.8 × 106 M−1 to 4.1 × 1011 M−1. No MAbs were weakly cross-reactive, since none of the cross-reactive MAbs showed any tendency toward having less avidity to 6A PS (the cross-reactive PS) than to 6B PS. Avidity influenced the results of several antibody assays. When all of the hybridomas were examined, avidity strongly correlated with the titer of a unit amount of MAb to bind antigen-coated ELISA plates (r = 0.91) or to opsonize pneumococci in vitro (r = −0.85). Because both assay results are avidity dependent, the ELISA and the opsonization assay results were strongly correlated (r = 0.91), regardless of avidity. Avidity also correlated with the potency of a MAb to passively protect mice against pneumococcal infections. When only the immunoglobulin G hybridomas were examined, little increase in opsonizing capacity and in vivo protective potency was observed above 109 M−1. Taken together, an ELISA measuring antigen binding titer may be an adequate measure of the protective immunity induced with pneumococcal vaccines, and the absence of a partially cross-reactive MAb suggests that antigenic sin may not be significant in responses to vaccines against the S. pneumoniae 6B serotype.
Streptococcus pneumoniae is a well-known pathogen with a polysaccharide (PS) capsule. It is a major causative agent for pneumonia, sepsis, meningitis, and otitis media in infants under 2 years of age and the elderly (10). Pneumococcal sepsis is associated with very high rates of mortality, even with appropriate antibiotic treatments (10). Antibiotic treatments are becoming less effective due to a rapid increase in the prevalence of antibiotic-resistant S. pneumoniae in many parts of the world (4). Therefore, there is a need for pneumococcal vaccines that are effective in young children and the elderly.
Antibodies to capsular PS opsonize S. pneumoniae and provide protection against S. pneumoniae expressing the homologous (i.e., vaccine) or cross-reactive capsular serotypes. To provide protection against the majority of S. pneumoniae serotypes, currently available pneumococcal vaccines contain PSs of 23 different serotypes (29) chosen from 90 known serotypes (14). However PSs of many serotypes (e.g., 6B PS) in the 23-valent vaccine are not immunogenic in young children. When PS is conjugated to protein molecules, it often becomes immunogenic in young children. Recently a new pneumococcal conjugate vaccine containing seven serotypes (4, 6B, 9V, 14, 18C, 19F, and 23F) was approved for clinical use in the United States (11). While the heptavalent vaccine provides adequate coverage against invasive infections in the United States, it may not provide enough coverage in other countries (13). To improve the heptavalent conjugate vaccine, there is a large ongoing effort to produce conjugate pneumococcal vaccines, which will probably contain two to four more serotypes (34).
To facilitate the development of new pneumococcal vaccines, an accurate assessment of vaccine-induced immune response is important. However, the assessment of pneumococcal antibody response is not simple. Antibodies to PS antigens are often of low avidity and may not be so functional, yet assays for pneumococcal antibodies vary in their capacity to detect antibodies with very low avidity (1). Enzyme-linked immunosorbent assays (ELISAs) for pneumococcal antibodies may not be specific and may also detect antibodies to contaminants found in various preparations of “purified” capsular PS (7, 88). An individual expresses only a very few clones of anti-PS antibodies, and this situation may exaggerate the variability observed for pneumococcal antibody function and cross-reactivity among individuals (24). In addition, vaccine-induced immunity should be tested against the homologous serotypes included in the vaccine as well as their cross-reactive serotypes. Thus, there is a need to investigate assays measuring vaccine-induced immune responses to homologous and cross-reactive serotypes.
An additional complication in measuring vaccine-induced immune responses may arise due to cross-reactions among antibodies. An exposure to an antigen can induce immune memory, which can interfere with the subsequent immune response to a cross-reactive antigen (8). This phenomenon, which is named “original antigenic sin” (9), may be applicable to pneumococcal conjugate vaccines that elicit immune memory, since antibodies to one pneumococcal PS often cross-react with a pneumococcal PS with similar structure. A previous study suggested that pneumococcal antibodies may bind the PS of the vaccine serotype much more avidly than that of the cross-reactive serotype (24). To investigate these issues in detail, we studied 14 monoclonal antibodies (MAbs) from an inbred mouse strain immunized with 6B PS and tested the MAbs for their avidity and functional capacities.
MATERIALS AND METHODS
Hybridoma production.
The production of 14 hybridomas from four fusions is described here briefly, since it will be described in detail elsewhere (unpublished data). BALB/c mice (Jackson Laboratory, Bar Harbor, Maine) were immunized with various pneumococcal antigens. Formalin-fixed S. pneumoniae serotype 6B (strain L82016) (5) cells (108 CFU) were intraperitoneally injected into the mouse that was used for fusion 1 on days 0, 7, 14, 21, 49, 57, and 74. For fusions 2 and 3, the mouse was subcutaneously immunized with 2.5 μg of 6B capsular PS conjugated to keyhole limpet hemocyanin (PS/protein ratio of 2.2 by weight) along with 5 to 10 μg of Quil A on days 0 and 34 and intraperitoneally on day 59. For fusion 4, 1.3 μg of 6B capsular PS conjugated to a diphtheria toxin mutant (CRM197) (PS/protein ratio of =0.47 by weight) with 10 μg of QS-21 was given to the mouse subcutaneously on days 0 and 14 and intraperitoneally on day 28. The mouse spleens were harvested 5 to 7 days after the last immunization, and the splenocytes were fused with SP2/0-Ag14 cells as described previously (19). Hybridomas producing antibodies binding 6A or 6B PS, without binding C-polysaccharide (C-PS) were selected.
Sandwich-type ELISAs.
A standard sandwich-type ELISA (21) was used with minor variations as described below. To determine the antigen binding titers of antibodies, microtiter plates were coated with type 6A (a gift of G. Schiffman, Brooklyn, N.Y.) or 6B (American Type Culture Collection, Manassas, Va.) pneumococcal PS (38). After blocking the plates with the blocking solution (phosphate-buffered saline [PBS] with 1% skim milk and 0.05% Tween 20), samples (or standards) diluted in the blocking solution were added to microtiter wells. Following a 3-h incubation at room temperature, antimouse immunoglobulin (Ig) conjugated with alkaline phosphatase was added. After a 2-h incubation at room temperature, the wells were washed thoroughly and were filled with 150 μl of p-nitrophenyl phosphate in diethanolamine buffer (pH 9.8). Optical densities at 405 nm were converted into concentrations by comparing the optical densities to a standard. Specific preparations of Hyp6BM1 and Hyp6BM9 were used as standards with 10 U/ml for the anti-6B and anti-6A antibody assays, respectively. Antigen binding titers may reflect antibody avidity. To determine the concentration of Ig in the samples independent of avidity, the antibody concentrations were determined by capturing the antibody to the ELISA plates by the Fc region. To achieve this, the ELISA plates were coated with rabbit antibodies specific for all mouse Igs. Depending on the isotype of the samples, alkaline phosphatase-conjugated antimouse IgG or IgM antibody was used as the secondary antibody.
Avidity measurement.
A Farr assay (22) was used to determine avidity as described below. Radiolabeled 6A and 6B PSs were prepared by conjugating tyraminated 6A and 6B PSs with 125I. The radiolabel had a specific activity of about 50,000 cpm/ng and could be precipitated up to 75% with appropriate antibodies. The radioactive PSs were typically diluted to 1 × 10−12 M to 3 × 10−12 M based on the assumed molecular mass of 9.3 × 105 Da (3). The assay was performed by mixing 75 μl of radiolabeled PS with 75 μl of serially diluted antibody. After a 2-h incubation, 150 μl of saturated ammonium sulfate was added, and the antibody-bound radioactive PS was precipitated by centrifugation. After the supernatant solution containing unbound PS was removed, the radioactivity of the precipitate was determined.
Avidity (K) was calculated by using the equation K = Fb/[tAb(1 − Fb)] (12, 20), where tAb is total antibody concentration (in molar units) and Fb is the fraction of PS bound to antibody. Fb was determined by the equation [(CPM precipitated with test antibody − CPM precipitated without antibody)/(maximally precipitable CPM with control antibody − CPM precipitated without antibody)]. Molecular masses of antibodies were assumed to be 150 kDa for lgG and 900 kDa for IgM. To minimize the error in estimating the avidity values and to satisfy conditions for the avidity equation, the data used for the avidity calculation had Fb values between 0.2 and 0.9 and [tAb] ≥ 5× [total PS concentration].
Opsonization assays.
Opsonization assays were performed as follows. Pneumococci were grown in Todd-Hewitt broth with 0.5% yeast extract (THY medium), aliquoted in THY medium containing 15% glycerol, and kept frozen at −70°C until usè. The strains of bacteria used were DS2212 for 6B and 2727-97 for 6A, which were obtained, respectively, from G. Carlone and R. Facklam at the Centers for Disease Control and Prevention (Atlanta, Ga.). HL-60 cells were differentiated into granulocytic cells by culturing them in RPMI 1640 with 10% fetal calf serum and 0.8% dimethylformamide (Fisher Scientific, Pittsburgh, Pa.) for 5 to 6 days. After the differentiation, HL-60 cells were diluted to 107 cells/ml in dilution buffer (Hanks' buffer supplemented with 0.1% gelatin and 10% fetal calf serum). The dilution buffer was used also to perform serial dilutions of the test samples (e.g., serum). Ten microliters of pneumococcus solution containing 1,000 CFU and 20 μl of diluted test sample were placed in a well of 96-well microtiter plate. After a 15-min incubation at 37°C, 40 μl of HL-60 suspension and 10 μl of rabbit complement (Pelfreeze, Browndeer Wis.) were added to the well. The mixture was incubated for 1 h at 37°C with shaking. Ten microliters of the reaction mixture was plated in a THY agar plate. The plates were incubated in a candle jar at 37°C overnight, and bacterial colonies in the plates were counted. The opsonization titer of a serum is defined as the dilution of the serum that results in half as many colonies as are seen with complement controls.
Infection with S. pneumoniae serotype 6B.
S. pneumoniae serotype 6B strain BG9163 (5) was grown in 10 ml of THY medium until the optical density at 405 nm was 0.5 to 0.6. Bacteria were spun down and resuspended in 3 ml of normal saline, and then a portion was diluted 1:600 in normal saline containing 15% glycerol. The diluted bacteria were aliquoted and stored at −70°C. Frozen aliquots from the same batch of bacteria were used in all studies. At the beginning of the experiments, 200 μl of the normal saline containing various amounts of a MAb was subcutaneously injected into the nape of each CBA/N mouse (Jackson Laboratory). One day later, the mice were infected by injecting 8 × 104 to 12 × 104 CFU of bacteria in 100 μl of normal saline into the peritoneum of each mouse. The number of surviving mice was monitored for 35 days.
RESULTS
Cross-reactivity of anti-6B PS antibodies to 6A PS.
The cross-reactivity of the hybridomas was assessed by determining the avidity and opsonophagocytic killing capacity for 6B or 6A PS with a set of hybridoma supernatants. In order to avoid any influence of antibody avidity on the measurements of antibody concentration, the concentration of these antibodies was determined by detecting the Fc region of antibodies, not the Fab region. The avidity of antibodies was determined by a well-established method described by Griswold et al. (12) and was found to range from slightly less than 107 to 5 × 1011 M−1 (Table 1 and Fig. 1, left panel). IgM hybridomas tended to have higher avidity than IgG hybridomas. Only one of eight IgM samples displayed avidity less than 1010 M−1 for the 6B serotype, but only one out of six IgG hybridomas displayed avidity greater than 1010 M−1. This is likely due to the fact that IgM displays more binding sites than IgG. MAbs specific for 6B PS tended to have lower avidity than the cross-reactive antibodies. For instance, three IgG cross-reactive MAbs have higher avidity than the other three non-cross-reactive MAbs.
TABLE 1.
Summary of the data obtained in this study
| Fusion no. | Sample | Isotype | Avidity (M−1)
|
Concn of antibody for killing 50% (μg/ml)
|
Antigen binding titer/antibody concn (100)a
|
|||
|---|---|---|---|---|---|---|---|---|
| 6B | 6A | 6B | 6A | 6B | 6A | |||
| 1 | Hyp6BG2 | IgG | 7.82 × 106 | <8.79 × 105 | 0.246 | >6.675 | 0.41 | <0.04 |
| Hyp6BG3 | IgG | 2.84 × 107 | <3.95 × 105 | 0.544 | >12.25 | 0.61 | <0.02 | |
| Hyp6BG5 | IgG | 2.35 × 107 | <3.19 × 106 | 0.203 | >9 | 0.56 | <0.03 | |
| Hyp6BM1 | IgM | 3.83 × 1010 | <6.98 × 106 | 0.039 | >4.155 | 30.05 | <0.06 | |
| Hyp6BM3 | IgM | 1.02 × 1010 | <4.90 × 106 | 0.126 | >6.163 | 1.62 | <0.04 | |
| 2 | Hyp6BM6 | IgM | 1.69 × 1011 | 2.39 × 1011 | 0.056 | 0.04 | 66.42 | 60.39b |
| Hyp6BM7 | IgM | 5.49 × 108 | <4.25 × 106 | 0.022 | >3.585 | 13.1 | <0.07 | |
| 3 | Hyp6BM8 | IgM | 2.44 × 1011 | 6.41 × 1010 | 0.016 | 0.018 | 78.21 | 134.14 |
| Hyp6BM9 | IgM | 1.23 × 1011 | 5.55 × 1010 | 0.032 | 0.033 | 60.13 | 80.64 | |
| Hyp6BM10 | IgM | 4.11 × 1011 | 3.70 × 1011 | 0.011 | 0.022 | 79.63 | 203.52 | |
| 4 | Hyp6BM17 | IgM | 3.49 × 1011 | 2.38 × 1011 | 0.007 | 0.013 | 43.04 | 132.69 |
| Hyp6BG6 | IgG | 3.79 × 108 | 7.38 × 109 | 0.077 | 0.011 | 0.52 | 173.91 | |
| Hyp6BG7 | IgG | 1.86 × 109 | 3.65 × 1010 | 0.015 | 0.019 | 9.56 | 52.94 | |
| Hyp6BG8 | IgG | 1.51 × 1010 | 3.72 × 1010 | 0.014 | 0.022 | 78.57 | 73.57 | |
Antigen binding titer was measured by an ELISA utilizing ELISA plates coated with PS antigen. Antibody concentrations were determined with an ELISA detecting the Fc region of antibody.
After repeated freezing and thawing, Hyp6BM6 had a value of <1.
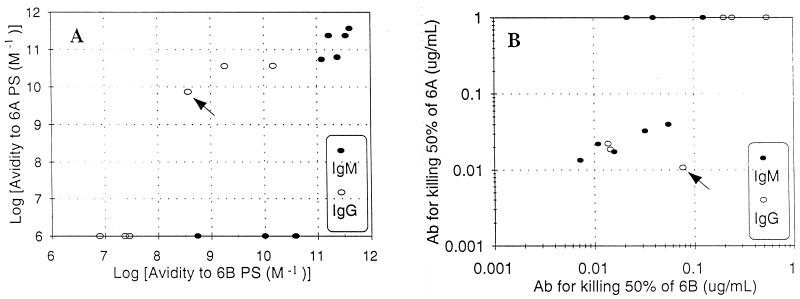
FIG. 1.
Comparison of binding of antibodies to 6B PS (x axis) versus 6A PS (y axis) measured by avidity (left panel) or opsonophagocytosis (right panel). For the left panel, the detection limit is 5 × 106 M−1. For the right panel, the amount of antibody (A6) necessary to kill 50% of the bacteria is plotted in the y axis. The data points along the top showed no detectable opsonic activity at more than 4 μg/ml.  , IgM;
, IgM;  , IgG. Arrows indicate Hyp6BG6.
, IgG. Arrows indicate Hyp6BG6.
The hybridomas were made by immunizing mice with 6B PS, and six hybridomas unambiguously bound only 6B PS and did not bind 6A PS in both assays for avidity and opsonophagocytic capacity (Fig. 1). These 6B-specific MAbs had undetectable avidity for 6A PS (Fig. 1, left panel) and did not display any opsonic capacity even at greater than 1 μg/ml (Fig. 1, right panel). Among the eight cross-reacting hybridomas, we initially expected that they would bind 6A PS less strongly than 6B PS, because 6A is the cross-reactive antigen of 6B PS. Unexpectedly, however, most cross-reacting MAbs reacted with 6A as well as 6B PS, as shown by the fact that the data points of cross-reacting MAbs lie along an approximate 45° angle in both panels of Fig. 1. For instance, the MAb required about 10 to 100 ng/ml to kill half of the pneumococci for both the 6A and 6B serotypes (Fig. 1, right panel). In some cases, there was a tendency for the MAb to bind 6A PS better than 6B PS. An IgG MAb named “Hyp6BG6” had about 10-fold higher avidity for 6A PS than 6B PS and had correspondingly more capacity to opsonize the 6A serotype than the 6B serotype. Thus, antibodies induced with 6B PS tend to cross-react fully with 6A PS or not cross-react with 6A PS at all.
Opsonophagocytic capacity of an antibody strongly correlates with its avidity.
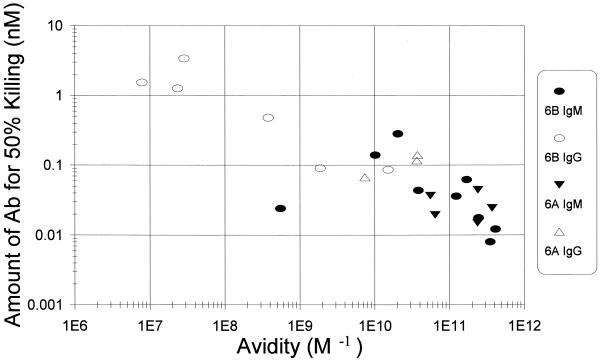
To assess the effect of avidity on the protective capacities of hybridomas, we compared the amount of antibody necessary for killing half of the bacteria against avidity (Fig. 2). While there was a MAb (Hyp6BM7) that was reproducibly more efficient in opsonization than expected by its avidity, most MAbs displayed an opsonic capacity strongly correlated (r = −0.85) with their avidity. When only IgG antibodies were examined, 109 appears to be the threshold, and an additional increase in avidity did not result in much increase in opsonizing potency. When all of the data from IgM and IgG hybridomas were examined, however, there was no evidence for low-and high-avidity thresholds, because the functional capacity of antibodies continued to increase or decrease following the changes in avidity, even when the hybridomas have very high (>1011 M−1) or low (108 M−1) avidity.
FIG. 2.
Comparison of the concentration of antibody needed for killing 50% of bacteria by opsonophagocytosis (y axis) to avidity (x axis). Solid symbols represent IgM, and open symbols represent IgG. Molecular mass was assumed to be 1.6 × 105 for IgG and 9 × 105 for IgM. Target bacteria were either the 6B serotype ( ● and ○) or the 6A serotype (▾ and ▵). The best fit line is y = −0.42x − 3.1. The correlation coefficient is −0.85.
The potency of in vivo protective IgG antibodies depends on their avidity.
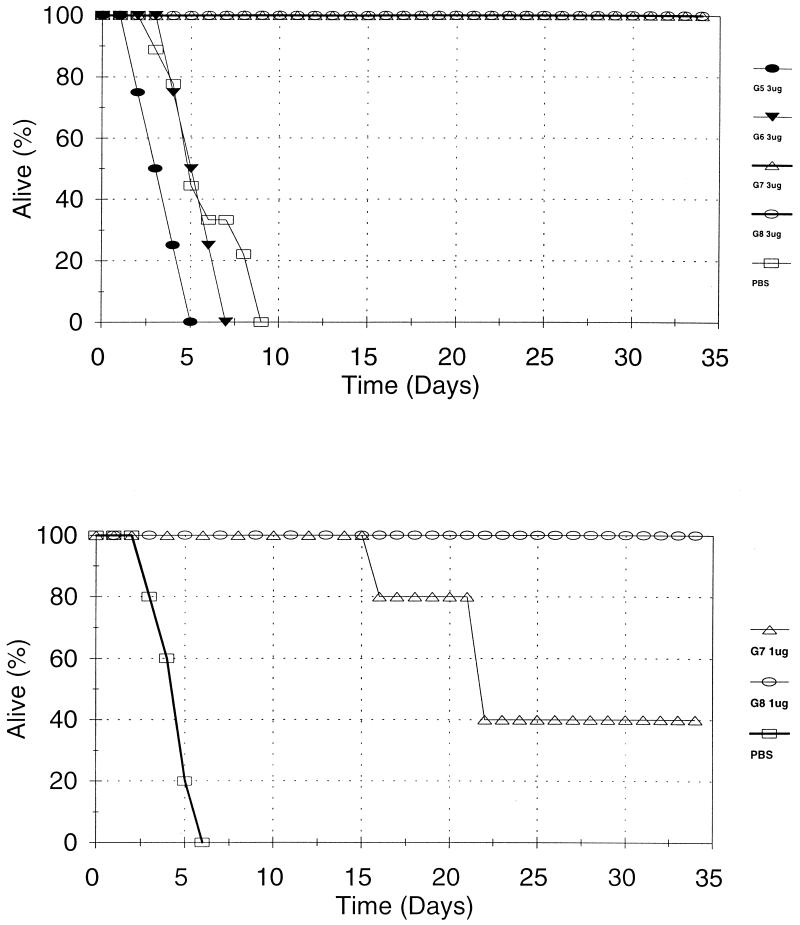
The above data suggested that 109 may be the functional threshold for IgG antibodies against pneumococcal capsular PS. Also, another study reported that antibodies may have equivalent in vivo protective potency as long as their avidity is above a minimal threshold of 107 to 108 M−1 (2). We therefore further investigated this issue with four IgG hybridomas expressing widely disparate avidities (Hyp6BG5, Hyp6BG6, Hyp6BG7, and Hyp6BG8) (Fig. 3 and Table 2). The avidities of these MAbs ranged from 2.4 × 107 to 1.5 × 1010 M−1 with an increment of 10-fold difference among the MAbs. As shown in Fig. 3 and Table 2, 3 or 30 μg of the two lgG hybridomas (Hyp6BG5 and Hyp6BG6), respectively, expressing an avidity of 107 or 3.8 × 108 M−1, did not protect the mice from infection, and they died as fast as the mice receiving PBS. More than 30 μg could not be evaluated for technical reasons. In contrast, 3 μg of Hyp6BG7 and Hyp6BG8, which had a higher avidity, provided mice with 100% protection from infection (P = 0.029 by day 8; Fisher's exact test). When less antibody (0.3 or 1 μg) was used, Hyp6BG7 appeared to be less protective than Hyp6BG8, but the differences were minimal even after multiple experiments (Table 2). These findings therefore provide independent support that the functional potential of lgG antibodies is less dependent on the antibody avidity above 109 M−1. Below 109 M−1, however, in vivo protective function and in vitro opsonophagocytic capacity of lgG antibodies correlate with avidity.
FIG. 3.
Survival of mice (y axis) versus number of days after infection (x axis). The amounts of antibody used for protection were 3 μg/mouse (top panel) and 1 μg/mouse (bottom panel). 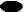 , Hyp6BG5 group; ▾, Hyp6BG6 group; ▵, Hyp6BG7 group;
, Hyp6BG5 group; ▾, Hyp6BG6 group; ▵, Hyp6BG7 group;  , Hyp6BG8 group;
, Hyp6BG8 group;  , PBS control. Four to five mice were in each group.
, PBS control. Four to five mice were in each group.
TABLE 2.
Ability of IgG antibodies to protect mice in vivo
| Hybridoma | Isotype | Avidity (M−1) | No. surviving/total at antibody dose (μg/mouse) ofa:
|
||||
|---|---|---|---|---|---|---|---|
| 0.3 | 1 | 3 | 10 | 30 | |||
| Hyp6BG5 | IgG2a | 2.35 × 107 | NDb | ND | 0/4 | 0/5 | 0/5 |
| Hyp6BG6 | IgG2b | 3.79 × 108 | ND | ND | 0/4 | 0/5 | 0/5 |
| Hyp6BG7 | IgG1 | 1.86 × 109 | 1/5 | 2/5, 3/5c | 4/4 | ND | ND |
| Hyp6BG8 | IgG1 | 1.51 × 1010 | 1/5 | 5/5, 5/5 | 4/4 | ND | ND |
Ratios of the number of mice surviving on day 35 over the total number of mice used for the experiment are shown.
ND, not done.
Two experiments were performed with Hyp6BG7 and Hyp6BG8 at 1 μg/mouse. In the first experiment, the results were 2 of 5 versus 5 of 5 for Hyp6BG7 and Hyp6BG8. In the second experiment, the results were 3 of 5 versus 5 of 5. By Fisher's exact test, P = 0.17 for 2 of 5 versus 5 of 5 and 0.033 for 5 of 10 versus 10 of 10.
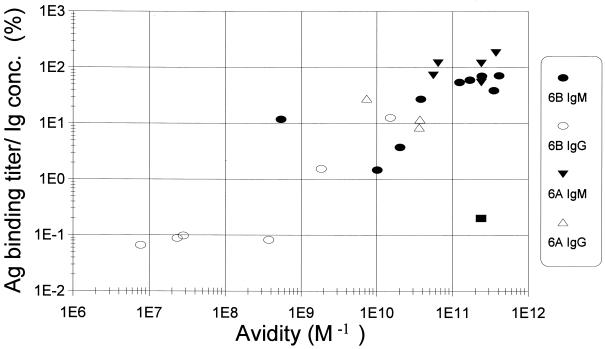
Antigen binding titer of an antibody depends on its avidity.
For this study, we determined the antibody concentrations with an assay detecting the constant region in order to avoid the influence of antibody avidity. In many other studies, however, antibody concentrations are estimated by measuring the amount of antibody bound to the antigen immobilized on the plastic wells by their V(variable) regions (27, 38). These V region-dependent assay results are referred as “antigen binding titers” here. To examine the effect of antibody avidity on the antigen binding titer, we plotted the antigen binding titer per unit amount of MAb versus avidity (Fig. 4). Few MAbs reproducibly behaved as outliers of the group. Hyp6BM7, the lgM hybridoma with the lowest avidity for 6B PS, had a higher antigen binding titer than expected (Fig. 4). While many preparations of Hyp6BM6 bound well to 6A PS immobilized on the ELISA plates (Table 1), some preparations of Hyp6BM6 (shown as a solid rectangle in Fig. 4) had strong avidity, but did not bind well to 6A PS immobilized on the ELISA plates. Despite these exceptional points, the antigen binding titer per unit amount of antibody was strongly dependent on antibody avidity (r = 0.91), and the antigen binding titer increased about 30-fold for every 100-fold increase in avidity. Reflecting the fact that both the antigen binding titer and the opsonophagocytic capacity are avidity dependent, the antigen binding titer and the opsonophagocytosis titer are strongly correlated (r = 0.91) (data not shown).
FIG. 4.
Comparison of antigen binding titer per unit antibody molecule (y axis) to avidity (x axis). Immunoglobulin concentration was expressed as nanomolar based on the molecular masses of 1.6 × 105 for IgG and 9 × 105 for IgM. ● and ○, binding to 6B PS; ▾ and ▵, binding to 6A PS. Solid symbols represent IgM, and open symbols represent IgG.  , preparation of Hyp6BM6 slightly denatured by repeated thawing and freezing. The best fit line for the data points (excluding the square) is y = 0.71x + 6.3, and their correlation coefficient is 0.91.
, preparation of Hyp6BM6 slightly denatured by repeated thawing and freezing. The best fit line for the data points (excluding the square) is y = 0.71x + 6.3, and their correlation coefficient is 0.91.
Hyp6BM6 hybridoma recognizes a novel epitope of 6A PS that disappears after immobilization.
The unusual binding behavior of Hyp6BM6 noted above was observed only with certain preparations. Therefore, we considered that denaturation of Hyp6BM6 might be responsible for this phenomenon. When an aliquot of Hyp6BM6 was frozen and thawed for several cycles, its ability to bind 6B PS did not diminish, but its ability to bind 6A PS drastically decreased (data not shown). Thus, the 6A binding capacity of Hyp6BM6 was dependent on the preservation conditions. Since all of the aliquots of Hyp6BM6 could efficiently opsonize the S. pneumoniae 6A serotype, we considered that the denatured Hyp6BM6 may bind 6A PS in solution or on the bacteria, but not 6A PS immobilized on the plastic. To directly test this possibility, the poorly binding aliquot of Hyp6BM6 was tested for binding 6A and 6B PS by various assays (Fig. 5). The binding capacity of Hyp6BM6 in this aliquot was 4-to 10-fold greater than that of an aliquot of Hyp6BM8 when binding to either 6B or 6A PS was determined in solution (Fig. 5) right panels Consistent with this observation, Hyp6BM6 and Hyp6BM8 appeared to contain equivalent binding capacities when the ELISA plates coated with 6B PS were used (Fig. 5, left upper panel). However, with ELISA plates coated with 6A PS, the antigen binding titer of this specific aliquot of Hyp6BM6 had much less than that of Hyp6BM8 (Fig. 5, left lower panel). These data indicate that Hyp6BM6 binds a unique epitope on 6A PS that disappears upon immobilization on the plastic plate. The data also show that the antigen binding titer may not always predict the capacity of an antibody to opsonize bacteria.
FIG. 5.
ELISA (left panels) and Farr assay (right panels) results obtained with 6B PS (top panels) and with 6A PS (bottom panels). The left panels show the optical density (y axis) in milliabsorbance versus the dilution of hybridoma supernatant (x axis). The right panels show the radioactivity of the antibody-bound PS antigen (y axis) versus the dilution of hybridoma supernatant (x axis). The hybridoma antibodies used for this study were Hyp6BM1 (▴), Hyp6BM6 (●), and Hyp6BM8 (■).
DISCUSSION
The original antigenic sin phenomenon was discovered when influenza virus vaccines were found to elicit memory B cells producing ineffective antibodies to cross-reactive serotypes and these B cells interfered with the subsequent immune responses to the influenza virus vaccination by cross-reactive serotype (9). This phenomenon has recently been proposed as a mechanism for ineffective cytotoxic T-cell immunity for some viruses (17). We considered that this phenomenon may also complicate the immune response to pneumococcal conjugate vaccines based on the studies of polyclonal antisera (24). However, our studies of the hybridomas produced with 6B PS (or conjugate vaccines) show that antibodies bound 6A PS either very strongly (avidity greater than 109 M−1) or not at all (avidity less than 107 M−1). No MAb displayed an intermediate avidity for 6A PS or ineffectively opsonized 6A serotype pneumococci. In view of there being no evidence for the B-cell clones making weakly cross-reactive antibodies, we speculate that pneumococcal vaccines may not induce memory B cells producing ineffective antibodies to the cross-reactive serotypes.
With this panel of MAbs displaying a wide range of avidity (107 to 1012 M−1), we examined the effect of avidity on in vitro opsonophagocytic killing capacity, a commonly used measure of antibody function. The vitro opsonophagocytic potency of all of the antibodies was tightly correlatable to their avidity (r = −0.85) without any obvious thresholds in either extreme of avidity. All data points formed one cluster (instead of two separate clusters) in Fig. 2 and 4, although our MAbs differed in their constant (lgM versus lgG) or variable (cross-reactive versus non-cross-reactive) regions. This finding suggests that vaccines containing the cross-reactive epitope of 6B PS should be as effective as those containing non-cross-reactive epitopes. Complement components provide the major opsonization signals to phagocytes, since opsonization is hardly demonstrable in the absence of complement (unpublished observation), and lgM can fix complement more efficiently than lgG in vitro (6). Consequently, we anticipated that lgM would have more opsonophagocytic potency than lgG, even when they have similar avidities. It was therefore surprising to observe that lgM and lgG antibody molecules, once their avidity is accounted for, have similar opsonizing capacities. A possible explanation may be that lgG (but not lgM) provides additional opsonization signals to phagocytes, perhaps through the Fcγ receptor. This possibility is consistent with the observation that in vitro opsonization assay results are dependent on the Fcγ receptor allotypes (16). Whatever the explanation may be, in vitro opsonophagocytic killing of pneumococci by anticapsular antibodies appears to be determined by the number of antibodies bound to the capsule of pneumococci.
We also examined the influence of avidity on antibody protection in vivo, since it is unclear whether antibody avidity is relevant for its in vivo function. For instance, Bachmann et al. (2) found that in vivo (but not in vitro) protection against a viral infection by mouse MAb does not depends on antibody avidity, once the avidity exceeds a maximal avidity threshold (about 107 to 108 M−1). In contrast to this finding, Usinger and Lucas found that, in vivo, protective potency of polyclonal pneumococcal antibody is associated with its avidity among antibodies expressing avidities ranging from 109 to 1010 M−1 (35). There are a number of differences between the two studies. First, different pathogens were used in the two studies, and neutralizing virus infectivity may be less dependent on antibody avidity than opsonizing bacteria. Second, viral antibodies were mouse MAbs, but pneumococcal antibodies were polyclonal human antisera. Third, for the calculation of the avidity of pneumococcal antibodies, the molecular weight of polysaccharide was assumed to be 10-fold smaller than what the literature suggests (3), and the low molecular weight could have rendered their estimation of the avidity less directly comparable. Our data based on mouse MAbs confirm and extend the observation by Usinger and Lucas. In vivo protection appears to depend on avidity when the avidity is less than 109, but the potency of lgG antibodies did not increase much once the avidity was greater than 109 to 1010 M−1.
While avidity accounts for most of the functional differences observed for our hybridomas, there appear to be additional factors influencing the function of anticapsular PS antibody, because there are reproducibly outlier data points. For instance, Hyp6BM7 has a relatively low avidity, yet it has 10-fold more opsonic activity than expected on the basis of its avidity. Several explanations are possible. It may be a hexameric IgM, which has been shown to be very efficient in fixing complement (28). Alternatively, Hyp6BM7 may recognize an epitope that is abundant on the surface of bacteria or that is efficient for opsonization. A MAb to group B streptococci was found to be very potent due to its recognition of a very densely expressed epitope (25). A recent study suggests that antibodies to the cryptococcal capsule recognize different epitopes and differ in their protective capacity (18). Even though only two epitopes have been defined for 6B PS so far (14), there may be new epitopes for 6B PS, and Hyp6BM7 may recognize a new epitope. Additional studies of Hyp6BM7 are in progress.
The presence of new epitopes for 6B PS is more strongly suggested with another outlier, Hyp6BM6. When it is slightly denatured (and only then), it recognizes an epitope of 6A PS that was found to disappear upon its adsorption to the plastic surface. Although new epitopes were shown to appear after adsorption of protein antigens to a plastic surface (36), our finding was unexpected, because a large repeating polymer molecule such as 6A PS should express each epitope multiple times, and it seemed unlikely that all of the epitopes disappeared upon adsorption to plastic. Also, as far as we are aware, this is the first report describing the importance of antibody denaturation in its antigen recognition. Our finding a new epitope is consistent with the growing body of evidence that a simple PS may express a large number of epitopes. For instance, a linear homopolymer of sialic acid expresses at least three epitopes (32). Because many PSs are conjugated to protein molecules for new vaccines, one must consider that some of the epitopes may be extinguished or produced during the conjugation step.
Antibody avidity is relevant not only for assessing the functionality of MAbs, but also for assessing protective immunity induced with pneumococcal vaccines in humans for several reasons listed below. Since pneumococcal vaccines elicit only a few antibody clones in a given individual (24), sera from immune individuals may vary significantly in avidity, and some individuals may have only expressed antibody clones with low avidity and functional capacity. In addition, the target populations for the pneumococcal vaccination are young children and older adults, who appear to produce antibodies with low avidity (15, 23, 31, 33). In a preliminary study, pneumococcal antibodies in a pool of sera from young adults immunized with a PS vaccine have an avidity of about 1010 M−1, and the avidity of the antibodies in the serum pools of 7-month-old children who received conjugate vaccines is severalfold lower than this (unpublished studies). Finally, pneumococci also often cause infections in areas of the body (e.g., the central nervous system) in which antibody concentrations are very low, and avidity could be especially important for antibody function in these areas.
If the protective capacities of pneumococcal antibodies depend on their avidity as well as their concentration, a simple measurement of antibody concentration would be inadequate for assessing pneumococcal vaccine-induced protective immunity. Yet, the protective immunity induced with pneumococcal vaccines is most commonly assessed by the antibody concentrations induced with the vaccines, and a standardized ELISA protocol using antigen-coated plates has been developed for the purpose of vaccine evaluations (26). Although good correlations between the standardized ELISA results and opsonophagocytosis titers have been reported (21, 30, 37), the standardized ELISA was found to have unsatisfactory specificity (7, 38), and its usefulness is controversial. Our experience with Hyp6BM6 shows that the standardized ELISA would never be a perfect tool for measuring protective immunity. However, our data also suggest that the results of the standardized pneumococcal ELISA reflect antibody avidity as well as antibody concentration, perhaps because the amount of the antigen immobilized on the ELISA plates is limiting (12, 20). Thus, once the pneumococcal antibody ELISA is made to be specific, a pneumococcal antibody ELISA would be a relatively good measure of protective immunity induced with pneumococcal vaccines, and functional assays such as the in vitro opsonophagocytosis assay may be used for unusual situations, such as that observed here with Hyp6BM6.
ACKNOWLEDGMENTS
This work was supported by a grant from the National Institutes of Health (AI-31473). M.H.N. is partially supported by NIAID contract NO1 AI-45248.
We thank J. M. Purkerson and C. E. Frasch for critical reading of the manuscript and E. Henderson for secretarial support.
REFERENCES
- 1.Anttila M, Eskola J, Ahman H, Kayhty H. Avidity of IgG for Streptococcus pneumoniae type 6B and 23F polysaccharides in infants primed with pneumococcal conjugates and boosted with polysaccharide or conjugate vaccines. J Infect Dis. 1998;177:1614–1621. doi: 10.1086/515298. [DOI] [PubMed] [Google Scholar]
- 2.Bachmann M F, Kalinke U, Althage A, Freer G, Burkhart C, Roost H-P, Aguet M, Hengartner H, Zinkernagel R M. The role of antibody concentration and avidity in antiviral protection. Science. 1997;276:2024–2027. doi: 10.1126/science.276.5321.2024. [DOI] [PubMed] [Google Scholar]
- 3.Bednar B, Hennessey J P., Jr Molecular size analysis of capsular polysaccharide preparations from Streptococcus pneumoniae. Carbohydr Res. 1994;243:115–130. doi: 10.1016/0008-6215(93)84085-k. [DOI] [PubMed] [Google Scholar]
- 4.Breiman R F, Butler J C, Tenover F C, Elliott J A, Facklam R R. Emergence of drug-resistant pneumococcal infections in the United States. JAMA. 1994;271:1831–1835. [PubMed] [Google Scholar]
- 5.Briles D E, Crain M J, Gray B M, Forman C, Yother J. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect Immun. 1992;60:111–116. doi: 10.1128/iai.60.1.111-116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper N R. The classical complement pathway: activation and regulation of the first complement component. Adv Immunol. 1985;37:151–216. doi: 10.1016/s0065-2776(08)60340-5. [DOI] [PubMed] [Google Scholar]
- 7.Coughlin R T, White A C, Anderson C A, Carlone G M, Klein D L, Treanor J. Characterization of pneumococcal specific antibodies in healthy unvaccinated adults. Vaccine. 1998;16:1761–1767. doi: 10.1016/s0264-410x(98)00139-x. [DOI] [PubMed] [Google Scholar]
- 8.Eisen H N, Little J R, Steiner L A, Simms E S, Gray W. Degeneracy in the secondary immune response: stimulation of antibody formation by cross-reacting antigens. Isr J Med Sci. 1969;5:338–350. [PubMed] [Google Scholar]
- 9.Fazekas de St. Groth B, Webster R G. Disquisition on original antigenic sin. I. Evidence in man. J Exp Med. 1966;124:331–345. doi: 10.1084/jem.124.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fedson D S, Musher D M. Pneumococcal vaccine. In: Plotkin S A, Mortimer E A, editors. Vaccines. Philadelphia, Pa: The W. B. Saunders Co.; 1994. pp. 517–564. [Google Scholar]
- 11.Food and Drug Administration. Infant pneumococcal vaccine. JAMA. 2000;283:2228. [Google Scholar]
- 12.Griswold W R, Lucas A H, Bastian J F, Garcia G. Functional affinity of antibody to the Haemophilus influenzae type b polysaccharide. J Infect Dis. 1989;159:1083–1087. doi: 10.1093/infdis/159.6.1083. [DOI] [PubMed] [Google Scholar]
- 13.Hausdorff W P, Bryant J, Paradiso P R, Siber G R. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin Infect Dis. 2000;30:100–121. doi: 10.1086/313608. [DOI] [PubMed] [Google Scholar]
- 14.Henrichsen J. Six newly recognized types of Streptococcus pneumoniae. J Clin Microbiol. 1995;33:2759–2762. doi: 10.1128/jcm.33.10.2759-2762.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hetherington S V, Rutkowski A F. Antibody affinity in infants after immunization with conjugated capsular polysaccharide from Haemophilus influenzae type b. J Infect Dis. 1990;162:1185–1188. doi: 10.1093/infdis/162.5.1185. [DOI] [PubMed] [Google Scholar]
- 16.Jansen W T M, Breukels M A, Snippe H, Sanders L A M, Verheul A F M, Rijkers G T. Fcγ-receptor polymorphisms determine the magnitude of in vitro phagocytosis of Streptococcus pneumoniae mediated by pneumococcal conjugate sera. J Infect Dis. 1999;180:888–891. doi: 10.1086/314920. [DOI] [PubMed] [Google Scholar]
- 17.Klenerman P, Zinkernagel R M. Original antigenic sin impairs cytotoxic T lymphocyte responses to viruses bearing variant epitopes. Nature. 1998;394:482–485. doi: 10.1038/28860. [DOI] [PubMed] [Google Scholar]
- 18.MacGill T C, MacGill R S, Casadevall A, Kozel T R. Biological correlates of capsular (Quellung) reactions of Cryptococcus neoformans. J Immunol. 2000;164:4835–4842. doi: 10.4049/jimmunol.164.9.4835. [DOI] [PubMed] [Google Scholar]
- 19.Nahm M H, Clevinger B L, Davie J M. Monoclonal antibodies to streptococcal group A carbohydrate. I. A dominant idiotypic determinant is located on Vk. J Immunol. 1982;129:1513–1518. [PubMed] [Google Scholar]
- 20.Nahm M H, Herzenberg L A, Little K, Little J R. A new method of applying the Sips equation. J Immunol. 1977;119:301–305. [PubMed] [Google Scholar]
- 21.Nahm M H, Olander J V, Magyarlaki M. Identification of cross-reactive antibodies with low opsonophagocytic activity for Streptococcus pneumoniae. J Infect Dis. 1997;176:698–703. doi: 10.1086/514093. [DOI] [PubMed] [Google Scholar]
- 22.Nahm M H, Siber G, Olander J V. A modified Farr assay is more specific than ELISA for measuring antibodies to S. pneumoniae capsular polysaccharides. J Infect Dis. 1996;173:113–118. doi: 10.1093/infdis/173.1.113. [DOI] [PubMed] [Google Scholar]
- 23.Nicoletti C, Yang X, Cerny J. Repertoire diversity of antibody response to bacterial antigens in aged mice III. Phosphorylcholine antibody from young and aged mice differ in structure and protective activity against infection with Streptococcus pneumoniae. J Immunol. 1993;150:543–549. [PubMed] [Google Scholar]
- 24.Park M K, Sun Y, Olander J V, Hoffmann J W, Nahm M H. The repertoire of human antibodies to the carbohydrate capsule of Streptococcus pneumoniae 6B. J Infect Dis. 1996;174:75–82. doi: 10.1093/infdis/174.1.75. [DOI] [PubMed] [Google Scholar]
- 25.Pincus S H, Shigeoka A O, Moe A A, Ewing L P, Hill H R. Protective efficacy of IgM monoclonal antibodies in experimental group B streptococcal infection is a function of antibody avidity. J Immunol. 1988;140:2779–2785. [PubMed] [Google Scholar]
- 26.Plikaytis B D, Goldblatt D, Frasch C E, Blondeau C, Bybel M J, Giebink G S, Jonsdottir I, Käyhty H, Konradsen H B, Madore D V, Nahm M H, Schulman C A, Holder P F, Lezhava T, Elie C M, Carlone G M. An analytical model applied to a multicenter pneumococcal enzyme-linked immunosorbent assay study. J Clin Microbiol. 2000;38:2043–2050. doi: 10.1128/jcm.38.6.2043-2050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quataert S A, Kirch C S, Quackenbush Wiedl L J, Phipps D C, Strohmeyer S, Cimino C O, Skuse J, Madore D V. Assignment of weight-based antibody units to a human antipneumococcal standard reference serum, lot 89-S. Clin Diagn Lab Immunol. 1995;2:590–597. doi: 10.1128/cdli.2.5.590-597.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reddy P S, Corley R B. The contribution of ER quality control to the biologic functions of secretory IgM. Immunol Today. 1999;20:582–588. doi: 10.1016/s0167-5699(99)01542-x. [DOI] [PubMed] [Google Scholar]
- 29.Robbins J B, Austrian R, Lee C J, Rastogi S C, Schiffman G, Henrichsen J, Makela P H, Broome C V, Facklam R R, Tiesjema R H, Parke J C., Jr Considerations for formulating the second-generation pneumococcal capsular polysaccharide vaccine with emphasis on the cross-reactive types within groups. J Infect Dis. 1983;148:1136–1159. doi: 10.1093/infdis/148.6.1136. [DOI] [PubMed] [Google Scholar]
- 30.Romero-Steiner S, Libutti D, Pais L B, Dykes J, Anderson P, Whitin J C, Keyserling H L, Carlone G M. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin Diagn Lab Immunol. 1997;4:415–422. doi: 10.1128/cdli.4.4.415-422.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romero-Steiner S, Musher D M, Cetron M S, Pais L B, Groover J E, Fiore A F, Plikaytis B D, Carlone G M. Reduction in functional antibody activity against Streptococcus pneumoniae in vaccinated elderly individuals highly correlates with decreased IgG antibody avidity. Clin Infect Dis. 1999;29:281–288. doi: 10.1086/520200. [DOI] [PubMed] [Google Scholar]
- 32.Rubinstein L J, Stein K E. Murine immune response to the Neisseria meningitidis group C capsular polysaccharide. II Specificity J Immunol. 1988;141:4357–4362. [PubMed] [Google Scholar]
- 33.Schlesinger Y, Granoff D M. Avidity and bactericidal activity of antibody elicited by different Haemophilus influenzae type b conjugate vaccines. JAMA. 1992;267:1489–1494. [PubMed] [Google Scholar]
- 34.Siber G R. Pneumococcal disease: prospects for a new generation of vaccines. Science. 1994;265:1385–1387. doi: 10.1126/science.8073278. [DOI] [PubMed] [Google Scholar]
- 35.Usinger W R, Lucas A H. Avidity as a determinant of the protective efficacy of human antibodies to pneumococcal capsular polysaccharides. Infect Immun. 1999;67:2366–2370. doi: 10.1128/iai.67.5.2366-2370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaidya H C, Dietzler D N, Ladenson J H. Inadequacy of traditional ELISA for screening hybridoma supernatants for murine monoclonal antibodies. Hybridoma. 1985;4:271–276. doi: 10.1089/hyb.1985.4.271. [DOI] [PubMed] [Google Scholar]
- 37.Vitharsson G, Jonsdottir I, Jonsson S, Valdimarsson H. Opsonization and antibodies to capsular and cell wall polysaccharides of Streptococcus pneumoniae. J Infect Dis. 1994;170:592–599. doi: 10.1093/infdis/170.3.592. [DOI] [PubMed] [Google Scholar]
- 38.Yu X, Sun Y, Frasch C, Concepcion N, Nahm M H. Pneumococcal capsular polysaccharide preparations may contain non-C-polysaccharide contaminants that are immunogenic. Clin Diagn Lab Immunol. 1999;6:519–524. doi: 10.1128/cdli.6.4.519-524.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]