Abstract
Purpose:
Multiple sclerosis (MS) is a multifocal demyelinating disease that affects the central nervous system (CNS) and commonly leads to neurogenic lower urinary tract dysfunction (NLUTD). Proper storage and release of urine relies on synchronized activity of the LUT, which is meticulously regulated by supraspinal circuits, making it vulnerable to diseases such as MS. NLUTD, characterized by voiding dysfunction (VD), storage issues, or a combination of both is a common occurrence in MS. Unfortunately, there are limited treatment options for NLUTD, making the search for alternative treatments such as transcranial rotating permanent magnet stimulation (TRPMS) of utmost importance. To assess effectiveness of treatment we also need to understand underlying factors that may affect outcomes, which we addressed here.
Methods:
Ten MS subjects with VD and median age of 54.5 years received daily TRPMS sessions for two weeks. Five pre-determined regions of interest (ROIs) known to be involved in the micturition cycle were modulated (stimulated or inhibited) using TRPMS. Clinical data (non-instrumented uroflow and urodynamics parameters, PVR, bladder symptom questionnaires) and neuro-imaging data were collected at baseline and following TRPMS via 7-Tesla Siemens MAGNETOM Terra magnetic resonance imaging (MRI) scanner. Each participant underwent functional MRI (fMRI) concurrently with a repeated urodynamic study (UDS). Baseline data of each arm was evaluated to determine any indicators of successful response to treatment.
Keywords: Multiple sclerosis, Voiding dysfunction, Magnetic resonance imaging, Neuromodulation
1. Introduction
Multiple sclerosis (MS) is a chronic auto-immune disease of the central nervous system (CNS) which leads to inflammation-induced demyelination, axonal degeneration, and neuronal injury [1]. Over time, MS lesions (plaques) spread within the CNS leading to various focal neurologic symptoms such as visual, sensory or motor impairments, or even difficulty with bowel or bladder control [2,3].
Voiding dysfunction (VD) symptoms include difficulty with bladder emptying, urinary hesitancy, straining, and even urinary retention. Although the prevalence of VD amongst MS individuals varies from one study to the other (12%–40%) [4,5], a considerable number of patients with MS suffer from VD which can decrease quality of life significantly. Unfortunately, effective treatment options for VD are limited. Intermittent self-catheterization is the preferred treatment for patients who cannot empty their bladder; however, neurologic impairments due to MS can make catheterization extremely challenging or impossible [6]. Even for those who can follow the regimen, the morbidity – hematuria, infection, pain, and trauma – as well as the cost associated with catheterization can be a substantial burden [7].
A previous study incorporating a brain-bladder functional magnetic resonance imaging (fMRI) protocol was conducted on brain activation during the micturition cycle (including both storage and voiding phases) in MS patients with neurogenic lower urinary tract dysfunction (NLUTD) [3]. Results showed significantly different activation patterns compared to MS patients without VD and healthy controls (HC) [3]. This prompted us to look for therapy options such as neuromodulation to augment the excitability of brain regions that are possibly associated with bladder filling and voiding [7].
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive therapeutic tool that uses a sequence of consecutive magnetic field pulses to induce electrical activity in focal brain regions. High/low frequency trains of rTMS can increase/inhibit cortical excitability, respectively. Utilizing the efficacy and safe profile of rTMS [8–10], an innovative transcranial rotating permanent magnetic stimulator (TRPMS) was developed by the Neurophysiology and Neuromodulation Laboratory at our institution. Compared to conventional TMS devices, TRPMS is portable and capable of modulating multiple cortical foci simultaneously with potential benefits including improved uniformity and consistency in targeting areas with anticipated anatomical variabilities [7,11]. Using TRPMS, our group has shown, for the first time, the effect of TRPMS in improving VD parameters in 10 women with MS through both neuroimaging (brain activation during voiding initiation) and clinical data (non-instrumented uroflow and validated questionnaires) [7].
With the promising results from our pilot trial, we seek to enhance our current understanding of TRPMS treatment in women with MS and VD by identifying baseline factors that could serve as predictors for responses to multi-focal rTMS. We hypothesize there are baseline clinical characteristics or baseline supraspinal activation patterns of patients that contribute to a successful TRPMS response.
2. Methods and materials
2.1. Subjects
Our initial intention was to recruit individuals with neurogenic bladder from various etiologies such as spinal cord injury, multiple sclerosis, Parkinson’s disease, cerebrovascular accident, and major spine surgery. However to provide a more homogeneous cohort for this pilot trial, we decided to revise the protocol to only include women with MS largely due to our extensive experience with MS individuals. Men were excluded because of potential confounding factors of voiding dysfunction by bladder outlet obstruction secondary to benign prostatic hyperplasia and pathology (which are common in this age group). In addition, posture during urination (lying flat in the scanner versus sitting or standing) appears to affect voiding, more noticeably in men than women, especially when neurogenic lower urinary tract symptoms are present. Moreover, Gender distribution with respect to MS prevalence in the community is 14:4 female to male ratio. Based on the inclusion and exclusion criteria (Table 1), ten adult women (≥18 years) with clinically stable MS (no new clinical or radiological activity) for ≥ 6 months with Expanded Disability Status Score (EDSS) ≤ 6.5 and neurogenic VD were recruited. Neurogenic VD was defined as either having %post-void residual/bladder capacity (%PVR/BC) ≥ 40%, being ≤10th percentile of the Liverpool nomogram or performing self-catheterization. Fig. 1 contains a CONSORT flow diagram detailing the process of participant enrollment in our study and Table 2 details demographic information about study participants.
Table 1.
Inclusion and exclusion criteria.
| Inclusion | Exclusion |
|---|---|
|
|
Fig. 1.
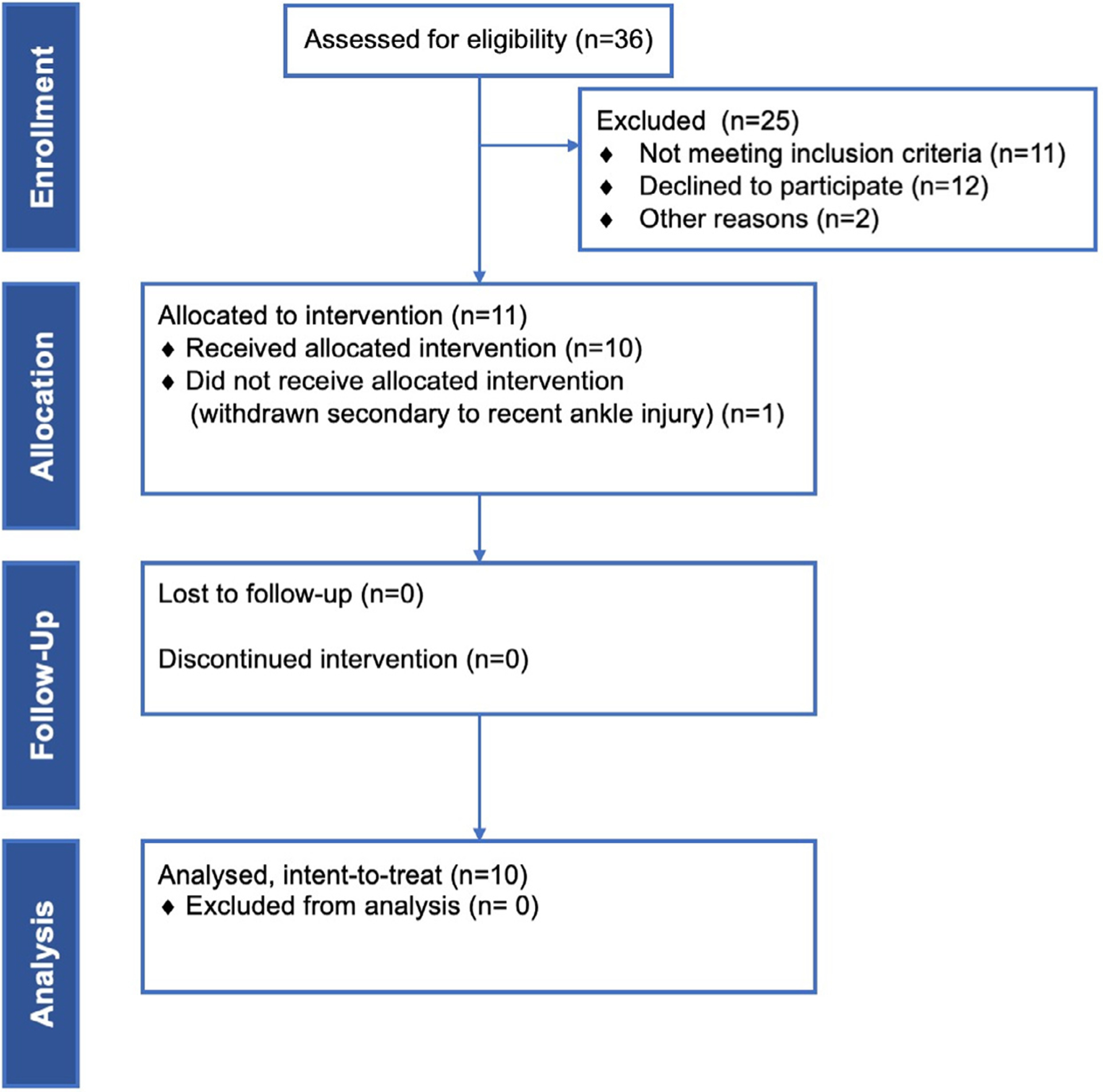
CONSORT flow diagram.
Table 2.
Patient demographics.
| Mean | Range | |
|---|---|---|
| Age (year) | 53.40 | 35–77 |
| Body mass index (kg/m2) | 26.83 | 19.9–41.8 |
| Number of deliveries | 1.00 | 0–3 |
| Multiple sclerosis duration (year) | 16.30 | 1–44 |
| Expanded Disability Status Scale | 3.80 | 1.5–6.5 |
| Baseline %PVR/BC | 54.04 | 32.05–81.59 |
| Baseline Liverpool nomogram percentile | 34.00 | 5–96 |
| Number of voided during baseline fMRI/ total subjects | 4/10 | N/A |
| Number of neurogenic detrusor overactivity during baseline fMRI/ total subjects | 4/10 | N/A |
| Post-treatment %PVR/BC | 29.46 | 0.76–64.54 |
| Post-treatment Liverpool nomogram percentile | 62.80 | 3–99 |
| Number of voided during post-treatment fMRI/ total subjects | 6/10 | N/A |
| Number of neurogenic detrusor overactivity during post-treatment fMRI/ total subjects | 4/10 | N/A |
2.2. Data acquisition
Prior to treatment, baseline clinical characteristics (age, body mass index, parity, etc.), non-instrumented uroflow and urodynamics parameters, PVR, and relevant validated questionnaires were obtained for each subject. The uroflow parameters collected included void volume, PVR, bladder volume, %PVR/BV, maximum and mean urine flow rate, voiding time, and Liverpool nomogram percentile. The collected urodynamic parameters were volume at first sensation of bladder filling, volume at first desire to void, volume at strong desire to void, presence of stress urinary incontinence, occurrence of neurogenic detrusor overactivity, straining during voiding, abdominal pressure at maximum urinary flow rate, detrusor sphincter dyssynergia, detrusor or bladder leak point pressure, maximum detrusor pressure (during storage and at maximum urinary flow rate), corrected end-filling pressure, corrected maximum cystometric capacity (MCC), volume leaked, PVR, %PVR/MCC, and compliance. The questionnaires included Urogenital Distress Inventory-6 (UDI-6), Incontinence Impact Questionnaire-7 (IIQ-7), American Urological Association Symptom Score (AUASS), Hamilton Rating Scale for Anxiety (HAM-A), Hamilton Depression Rating Scale (HAM-D), Hospital Anxiety and Depression Scale (HADS), and Neurogenic Bladder Symptom Score (NBSS).
2.3. Concurrent fMRI/UDS and diffusion tensor imaging (DTI) examination
Anatomical and functional MRI images were collected per our protocol using a 7-Tesla Siemens MAGNETOM Terra MRI scanner with a 32-channel single transmit head coil [7,12]. Structural images were obtained via the T1-weighted magnetization-prepared rapid gradient-echo (MPRAGE) sequence through the sagittal plane (repetition time (TR)=2200 ms, echo time (TE)=2.95 ms, voxel size=0.35 mm × 0.35 mm × 0.70 mm). To capture changes in the brain activation at “strong desire to void” and “voiding initiation”, both at baseline and post-treatment, a concurrent fMRI/UDS examination was performed using echo-planar T2*-weighted blood-oxygen-level-dependent (BOLD) sequence (axial, TR=2500 ms, TE=24 ms, 1.4 mm in-plane resolution, and 1.4 mm slice thickness). MRI-compatible double-lumen 7 French urodynamic study (UDS) vesical and rectal catheters were used for this concurrent fMRI/UDS setup. Inside the MRI scanner, the subject’s bladder was gradually filled with sterile saline at 75 mL/min until the subject signaled a “strong desire to void”. The subject was then instructed to hold for 30 s, after permission to void was given. If subjects were unable to void, the bladder was manually aspirated. This cycle was repeated up to four times. UDS was performed concurrently during fMRI to monitor the entire filling and voiding cycles. The collected UDS parameters during this concurrent setup include PVR, occurrence of neurogenic detrusor overactivity, capability of voiding inside the MRI scanner. A reduced number of UDS parameters during scanning was collected in order to minimize artifacts during the fMRI session. In addition, the UDS catheters were mainly used to fill the bladder and assess its pressure during storage and voiding.
Standard BOLD fMRI data preprocessing was completed using open-source software named Analysis of Functional NeuroImages (AFNI, https://afni.nimh.nih.gov). These steps included slice time correction, motion correction, spatial normalization, and spatial smoothing. Individual fMRI activation maps at voiding initiation were created using a generalized linear model. Group analysis of the brain activation difference (responders vs. non-responders) was then created and voxels with significant differences were identified using Student’s t-test (uncorrected p<0.05).
Diffusion tensor imaging (DTI) (158 × 158 matrix, 1.4 mm slice thickness, 220 × 220 cm2 Field-of-View, 64 directions and b-value=1000 s/mm2) data were also collected. DTI is a technique used to characterize the architecture of white matter tracts in the CNS by analyzing the directionality and magnitude of water molecules. DTI allows the assessment of fractional anisotropy (FA) and mean diffusivity (MD), which measure the overall directionality of water diffusion and the rotationally invariant magnitude of water diffusion within the CNS respectively [13]. Structural connectivity of the brain was assessed by extracting FA and MD from fifty white matter tracts and aligning them onto the International Consortium of Brain Mapping (ICBM) DTI-81 atlas.
2.4. TRPMS treatment
TRPMS is a wearable and portable cortical neuromodulation device with multifocal stimulation capability [11]. TRPMS consists of assemblies of axially magnetized cylindrical magnets attached to battery-operated direct-current motors, called microstimulators, that can generate an oscillating magnetic field to depolarize the cell membrane of cortical neurons, resulting in net cortical excitation or inhibition depending on the stimulus parameters [11]. For this pilot trial, the total duration of stimulation was 40 min (480 stimuli) for excitation and ten minutes (120 stimuli) for inhibition. Each stimulus was 100 ms and delivered at the rate of 0.2 Hz [7]. During this study we delivered excitatory stimulation to regions known to be involved in voiding initiation and inhibitory stimulation to regions activated during pelvic floor (PF) contraction to promote relaxation of the urethral sphincter (PF complex) facilitating urine passage. We refined the list of regions to target based on literature (Table 3) with five regions modulated (stimulated/inhibited) for this pilot trial. Before treatment started, each subject received a TRPMS treatment cap-fitting session where their head measurement was obtained to aid the microstimulator placement process (five corresponding to each target region), allowing the treatment to be personalized based on their brain anatomy. Every participant received ten sessions of TRPMS treatment in a two-week period.
Table 3.
Cortical regions to modulate and their corresponding tasks. BPS = bladder pain syndrome.
| Cortical regions | Task | Stimulate/Inhibit? |
|---|---|---|
| Right inferior frontal gyrus (IFG) | Voiding initiation [14,15] | Stimulate (40 mins) |
| Left dorsolateral prefrontal cortex (dlPFC) | Decision to void [16] | Stimulate (40 mins) |
| Bilateral supplementary motor area (SMA) | Pelvic floor contraction [17] | Inhibit (10 mins) |
| Right middle frontal gyrus (MFG) | Pelvic floor contraction [15] | Inhibit (10 mins) |
| Right dorsolateral prefrontal cortex (dlPFC) | Anxiety, Pain/BPS [18] | Inhibit (10 mins) |
2.5. Criteria for TRPMS treatment responders
Response to TRPMS treatment was defined as accomplishing at least one of the following: (1) %PVR/BC ≤ 20% post-treatment if ≥ 40% at baseline, (2) %PVR/BC decrease by half its baseline value or more, or (3) Liverpool nomogram percentile ≥ 25% post-treatment if < 10% at baseline. According to the criteria, each subject was assigned to a responder or non-responder group. The baseline data of each group was evaluated for any statistically significant association with successful response to the treatment. Student’s t-test was used for BOLD and DTI signals. Fisher’s exact test was used for categorical variables. Welch’s T-test or Wilcoxon signed-rank test were used for continuous variables, depending on the data’s normality.
2.6. Feasibility outcome analysis
Additionally, we calculated feasibility outcome measures as part of the proposed analysis in our trial protocol [7] since follow-up data collection had concluded. We calculated recruitment rate by dividing the number of enrolled participants by the number of those screened for eligibility. Retention rate was calculated as the percentage of participants that completed the study in its entirety. Lastly, we determined effect size using patients’ %PVR/BC at baseline and post-treatment.
3. Results
Clinical data analysis: All MS women enrolled in the study completed the two-week neuromodulation and six of them showed improvement after TRPMS treatment, using the response criteria. At baseline, the responder group (n=6) showed significantly lower values in the number of deliveries (p = 0.048), first sensation of filling (FSF) during clinic UDS (p = 0.011), and consequences domain in the NBSS questionnaire (p = 0.019) compared to non-responders (n=4), as seen in Fig. 2. There was no significant difference in other characteristics such as PVR, presence of DSD, or EDSS between the two groups.
Fig. 2.
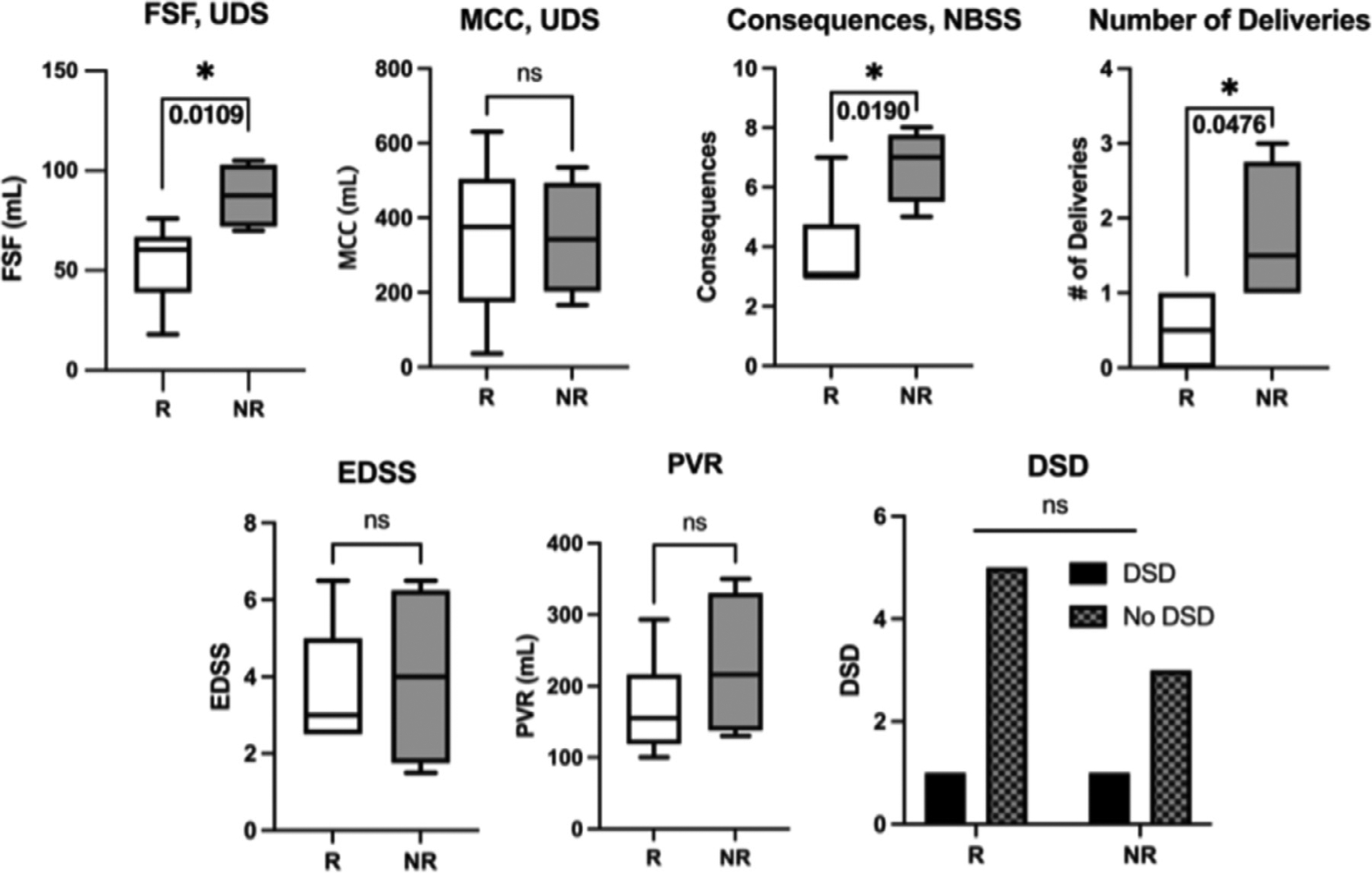
Results of statistical analysis between responders (R) and non-responders (NR) group. Responders showed significantly lower values in first sensation of filling (FSF), consequences domain in NBSS, and number of deliveries as compared to non-responders. Numerical values from the statistical analysis are listed in Table 4. UDS: urodynamic study, MCC: maximum cystometric capacity, PVR: post-void residual, AUASS: American Urological Association Symptom Score, NBSS: Neurogenic Bladder Symptom Score, EDSS: Expanded Disability Status Scale.
Functional MRI data analysis at (or attempt of) voiding initiation during baseline scan: whole brain BOLD group analysis at voiding (attempt of) initiation revealed multiple cortical areas of the brain with higher activation in the responders compared to non-responders (Fig. 3). Among the modulated cortical regions chosen, the right inferior frontal gyrus (t = 3.36) and middle frontal gyrus (t = 3.46) yielded higher BOLD activations in the responder group (p<0.05). There was no difference in other modulated regions of interest (ROIs) between the two groups. Overall, at voiding initiation, MS patients who responded to TRPMS treatment demonstrated a unique supraspinal activation pattern compared to MS patients who did not. No significant difference in white matter integrity between the two groups was noted based on the DTI analysis results.
Fig. 3.
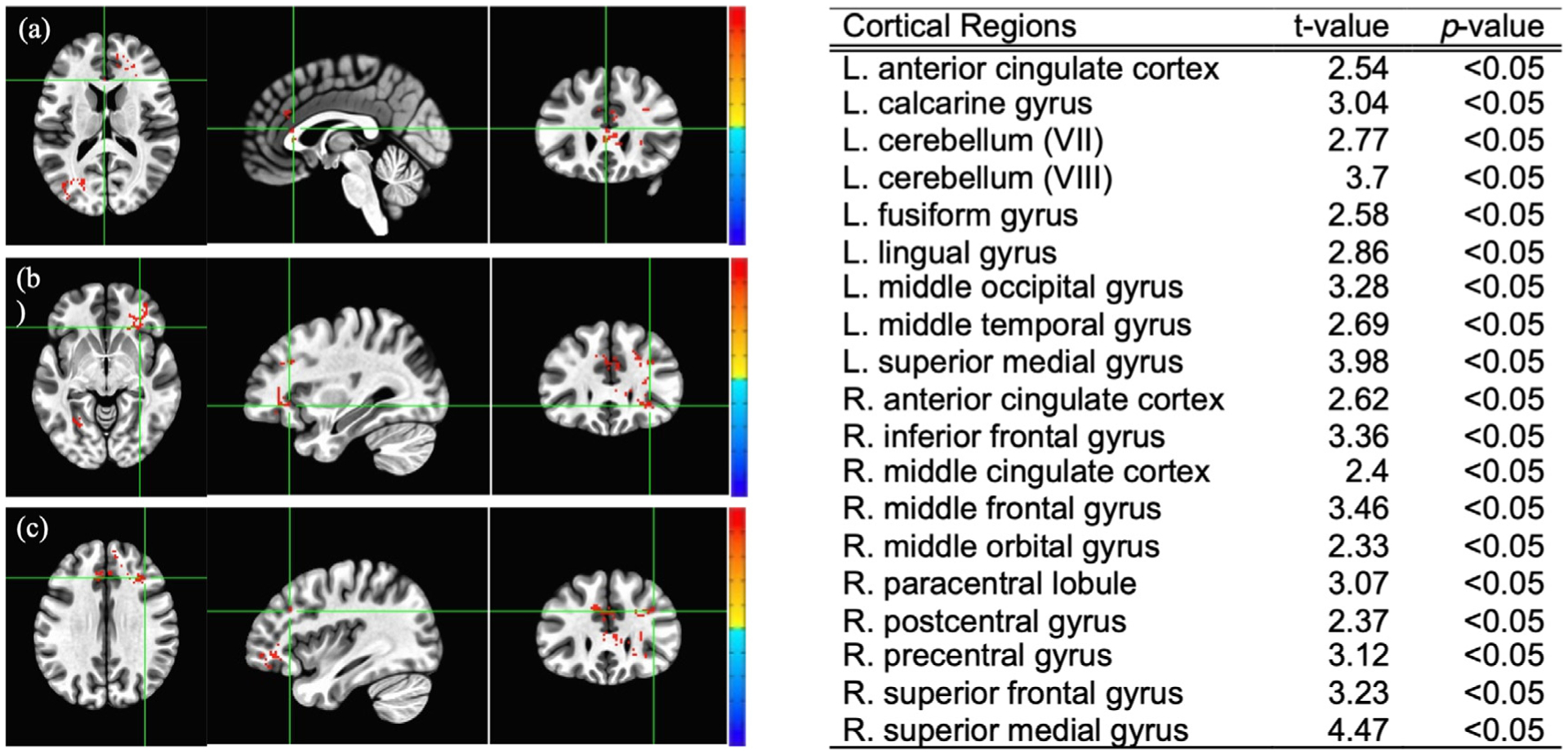
Blood-oxygen-level-dependent (BOLD) signal group analysis at baseline between the responder and non-responder group (Student’s t-test values listed on the right). Red indicates significantly higher cortical activation in the responder group at voiding initiation with a positive t-value; blue indicates significantly lower activation with a negative t-value. Some examples of cortical regions with significantly higher activation in the responder group are: (a) Left anterior cingulate cortex, (b) Right inferior frontal gyrus, (c) Right middle frontal gyrus.
Additionally, feasibility outcome analysis yielded a recruitment rate of 27.8%, a retention rate of 100% as no patients were lost during the study or follow-up, and effect size of 1.23.
4. Discussion
The purpose of this study was to enhance our understanding of rTMS treatment in MS individuals with VD and identify baseline factors that could potentially serve as predictors of response to neuromodulation treatment. By identifying factors that could signal potential response to treatment we aim to optimize future clinical trials and effectively treat those who could potentially benefit without subjecting others to unnecessary interventions.
4.1. Baseline clinical and urodynamics parameters
Interestingly, there were no significant differences in all questionnaires at baseline between responders and non-responses such as the total NBSS and AUASS. The consequences domain of the validated NBSS deals with pain associated with urination or catheter use, UTI, renal/bladder stones, and bladder medication reliance [19]. We found lower scores in the consequences domain at baseline to be significantly different between the two groups, possibly reflecting less severe NLUTD in the responder group prior to receiving TRPMS treatment.
Fewer numbers of deliveries in the responder group could be partially explained by the burden of childbirths on obstetrical neuromuscular injury. There is a strong association between vaginal childbirths and development of pelvic floor disorders later in life [20]. Although, this is a small pilot trial to infer any conclusions on this finding.
A statistically lower volume at FSF of the bladder was observed in the responder group. Since there was no significant difference in maximum cystometric capacity (MCC) between the groups, the FSF finding cannot be due to smaller bladders, instead it may suggest that responders had heightened bladder sensitivity to stored urine volume compared to non-responders. Contrary to storage phase findings, no significant difference was observed in PVR between the groups. This suggests storage phase parameters may better predict response to TRPMS than voiding phase parameters.
The Expanded Disability Status Scale (EDSS) used to quantify disability in MS, yielded no significant difference between the two groups [21]. Leading us to believe the disability burden of MS alone is not a predictor of successful response to TRPMS. However, with the subjective and objective data suggesting an underlying association between preserved local peripheral neural network/musculature involved in micturition and successful response, we cannot dismiss the possibility that non-response to therapy was due to peripheral damage to the urinary system. This argument can be further strengthened by the lack of significant differences between both groups in individuals with Detrusor Sphincter Dyssynergia (DSD). DSD is a common occurrence in patients with MS and can only occur in the presences of a pathology affecting the CNS [22]. One would expect individuals with DSD to be solely or primarily found in the non-responders group if they have more damaged supraspinal aspects of the micturition circuit; however, no significant difference in DSD EDSS between groups could point to other etiologies such as local neuromuscular injury. Moreover, the lack of significant differences in white matter integrity between the two groups further strengthens this argument.
4.2. Brain activation pattern
MS is typically thought to affect white matter; however, it has been shown that gray matter is also affected [23]. BOLD fMRI analysis was used to evaluate cortical activation patterns. The BOLD group analysis demonstrated stronger brain activations in the responder group within various cortical regions at baseline during initiation (or attempt of) voiding.
Specifically, the responding group from our study displayed stronger activations in cortical regions, including the superior frontal gyrus, middle frontal gyrus, inferior frontal gyrus of the prefrontal cortex, and the precentral gyrus and paracentral lobule of the precentral cortex in the right hemisphere. Another notable finding was stronger activation at the left anterior cingulate gyrus in the responder group, a cortical region directly associated with initiation and coordination of micturition [24]. Significantly stronger BOLD activations in these cortical regions represent increased cerebral blood oxygen levels generally associated with increased neuronal activity [25]. It is possible that preserved integrity of potential functional connectivity among some or all of the cortical regions (especially, the prefrontal and precentral cortices and left anterior cingulate gyrus) with stronger BOLD activations led to a successful response to TRPMS treatment. It should be noted that there was no significant difference in structural connectivity (white matter integrity) between the two groups.
5. Limitations
Despite our cohort being homogeneous via stringent inclusion/exclusion criteria, it consists of only ten patients with an inherently heterogeneous disease. Not having a placebo control is a major limitation of this study. However, this pilot trial served to enhance power calculations, ROI refinement and stimulation protocols for future randomized clinical trials. Also, it would have been meaningful to assess age-related changes in brain activation patterns by including subjects with VD absent of MS. It would not only reveal a unique pattern specific for MS patients with VD but also point us to more effective neuromodulation targets. Lastly, urinating in a supine position for our fMRI/UDS protocol may have resulted in additional voiding difficulty for our subjects.
6. Conclusion
TRPMS is a novel technology for non-invasive and individualized modulation of multiple cortical regions simultaneously. Based on the observed therapeutic efficacy in more than half of our cohort, TRPMS seems to be a promising therapeutic option. Our study revealed baseline clinical/UDS parameters and distinctive supraspinal activation patterns that may predict successful response to TRPMS treatment. While we are still trying to unveil the association between supraspinal changes and voiding dysfunction, we hope our predictors study will help phenotype MS women with VD for future rTMS studies.
Table 4.
Results of statistical analysis between responders (R) and non-responders (NR) group.
| R | NR | Mean difference | SE of mean | 95% Confidence Interval | t-value | df | p | ||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| FSF (mL), UDS | 54.17 | 87.50 | 33.33 | 11.56 | 6.38 | 60.28 | 2.88 | 7.54 | 0.011* |
| MCC (mL), UDS | 349.8 | 346.3 | −3.58 | 113.2 | −265.7 | 258.5 | 0.03 | 7.80 | 0.976 |
| PVR (mL) | 169.8 | 228.0 | 58.17 | 57.97 | −92.18 | 208.5 | 1.00 | 4.86 | 0.363 |
| Total, AUASS | 22.83 | 23.50 | 0.67 | 3.97 | −8.72 | 10.05 | 0.17 | 7.03 | 0.871 |
| Total, NBSS | 33.17 | 36.50 | 3.33 | 4.78 | −8.22 | 14.89 | 0.70 | 6.34 | 0.511 |
| Consequences, NBSS | 3.0 | 7.0 | NA | NA | NA | NA | NA | NA | 0.019* |
| Number of Deliveries | 0.5 | 1.5 | NA | NA | NA | NA | NA | NA | 0.048* |
| EDSS | 3.67 | 4.00 | 0.33 | 1.35 | −3.20 | 3.86 | 0.25 | 4.75 | 0.816 |
Acknowledgments
We would like to thank Hamida Rajab for her effort during the recruitment and performance of the clinical trial. Additionally, we want to show our gratitude to Ms. Carlina Acosta for her assistance with UDS.
Funding
This investigator-initiated study by Dr. Rose Khavari was supported in part through an award by the National Institute of Diabetes and Digestive and Kidney Diseases (1K23DK118209-01) and Houston Methodist Clinician Scientist Program.
Research Involving Human Participants:
This study was performed in line with the principles of the Declaration of Helsinki. Ethical approval was granted by the Institutional Review Board at Houston Methodist Hospital (PRO00019329) prior to conducting the research.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Yongchang Jang: Data collection, Data analysis, Manuscript writing. Khue Tran: Data collection, Data analysis. Zhaoyue Shi: Data analysis and interpretation, Manuscript editing. Karmonik Christof: Extensive manuscript editing. Darshil Choksi: Data analysis, Manuscript editing. Betsy H. Salazar: Manuscript writing and editing. John A. Lincoln: Manuscript writing and editing. Rose Khavari: Project development, Data Collection, Data interpretation, Manuscript writing and editing.
Informed Consent: All participants signed an informed consent form to participate in the study and publish the results of the study.
References
- [1].Zurawski J, Stankiewicz J, Multiple sclerosis re-examined: Essential and emerging clinical concepts, Am. J. Med 131 (5) (2018) 464–472, 10.1016/j.amjmed.2017.11.044. [DOI] [PubMed] [Google Scholar]
- [2].Jacob L, Tanislav C, Kostev K, Multiple sclerosis and incidence of urinary and fecal incontinence in almost 9,000 patients followed up for up to 10 years in Germany, Neuroepidemiology 55 (2) (2021) 92–99, 10.1159/000513234. [DOI] [PubMed] [Google Scholar]
- [3].Khavari R, Chen J, Boone T, Karmonik C, Brain activation patterns of female multiple sclerosis patients with voiding dysfunction, Neurourol. Urodyn 39 (3) (2020) 969–977, 10.1002/nau.24304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Aharony SM, Lam O, Corcos J, Evaluation of lower urinary tract symptoms in multiple sclerosis patients: Review of the literature and current guidelines, Can. Urol. Assoc. J 11 (1–2) (2017) 61–64, 10.5489/cuaj.4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mahajan ST, Patel PB, Marrie RA, Under treatment of overactive bladder symptoms in patients with multiple sclerosis: An ancillary analysis of the NARCOMS patient registry, J. Urol 183 (4) (2010) 1432–1437, 10.1016/j.juro.2009.12.029. [DOI] [PubMed] [Google Scholar]
- [6].Groen J, Pannek J, Castro Diaz D, Del Popolo G, Gross T, Hamid R, Karsenty G, Kessler TM, Schneider M, t Hoen L, Blok B, Summary of European association of urology (EAU) guidelines on neuro-urology, Eur. Urol 69 (2) (2016) 324–333, 10.1016/j.eururo.2015.07.071. [DOI] [PubMed] [Google Scholar]
- [7].Tran K, Shi Z, Karmonik C, John B, Rajab H, Helekar SA, Boone T, Khavari R, Therapeutic effects of non-invasive, individualized, transcranial neuromodulation treatment for voiding dysfunction in multiple sclerosis patients: Study protocol for a pilot clinical trial, Pilot Feasibility Stud. 7 (1) (2021) 83, 10.1186/s40814-021-00825-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Brusa L, Finazzi Agro E, Petta F, Sciobica F, Torriero S, Lo Gerfo E, Iani C, Stanzione P, Koch G, Effects of inhibitory rTMS on bladder function in Parkinson’s disease patients, Mov. Disord 24 (3) (2009) 445–448, 10.1002/mds.22434. [DOI] [PubMed] [Google Scholar]
- [9].Centonze D, Petta F, Versace V, Rossi S, Torelli F, Prosperetti C, Rossi S, Marfia GA, Bernardi G, Koch G, Miano R, Boffa L, Finazzi-Agro E, Effects of motor cortex rTMS on lower urinary tract dysfunction in multiple sclerosis, Mult. Scler 13 (2) (2007) 269–271, 10.1177/1352458506070729. [DOI] [PubMed] [Google Scholar]
- [10].Cervigni M, Onesti E, Ceccanti M, Gori MC, Tartaglia G, Campagna G, Panico G, Vacca L, Cambieri C, Libonati L, Inghilleri M, Repetitive transcranial magnetic stimulation for chronic neuropathic pain in patients with bladder pain syndrome/interstitial cystitis, Neurourol. Urodyn 37 (8) (2018) 2678–2687, 10.1002/nau.23718. [DOI] [PubMed] [Google Scholar]
- [11].Helekar SA, Convento S, Nguyen L, John BS, Patel A, Yau JM, Voss HU, The strength and spread of the electric field induced by transcranial rotating permanent magnet stimulation in comparison with conventional transcranial magnetic stimulation, J. Neurosci. Methods 309 (2018) 153–160, 10.1016/j.jneumeth.2018.09.002. [DOI] [PubMed] [Google Scholar]
- [12].Khavari R, Tran K, Helekar SA, Shi Z, Karmonik C, Rajab H, John B, Jalali A, Boone T, Noninvasive, individualized cortical modulation using transcranial rotating permanent magnet stimulator for voiding dysfunction in women with multiple sclerosis: A pilot trial, J. Urol 207 (3) (2022) 657–668, 10.1097/JU.0000000000002297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Clark KA, Nuechterlein KH, Asarnow RF, Hamilton LS, Phillips OR, Hageman NS, Woods RP, Alger JR, Toga AW, Narr KL, Mean diffusivity and fractional anisotropy as indicators of disease and genetic liability to schizophrenia, J. Psychiatr. Res 45 (7) (2011) 980–988, 10.1016/j.jpsychires.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shy M, Fung S, Boone TB, Karmonik C, Fletcher SG, Khavari R, Functional magnetic resonance imaging during urodynamic testing identifies brain structures initiating micturition, J. Urol 192 (4) (2014) 1149–1154, 10.1016/j.juro.2014.04.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Khavari R, Karmonik C, Shy M, Fletcher S, Boone T, Functional magnetic resonance imaging with concurrent urodynamic testing identifies brain structures involved in micturition cycle in patients with multiple sclerosis, J. Urol 197 (2) (2017) 438–444, 10.1016/j.juro.2016.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ketai LH, Komesu YM, Dodd AB, Rogers RG, Ling JM, Mayer AR, Urgency urinary incontinence and the interoceptive network: A functional magnetic resonance imaging study, Am. J. Obstet. Gynecol 215 (4) (2016) 449 e1–449 e17, 10.1016/j.ajog.2016.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schrum A, Wolff S, van der Horst C, Kuhtz-Buschbeck JP, Motor cortical representation of the pelvic floor muscles, J. Urol 186 (1) (2011) 185–190, 10.1016/j.juro.2011.03.001. [DOI] [PubMed] [Google Scholar]
- [18].Nizard J, Esnault J, Bouche B, Suarez Moreno A, Lefaucheur JP, Nguyen JP, Long-term relief of painful bladder syndrome by high-intensity, low-frequency repetitive transcranial magnetic stimulation of the right and left dorsolateral prefrontal cortices, Front. Neurosci 12 (2018) 925, 10.3389/fnins.2018.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Welk B, Lenherr S, Elliott S, Stoffel J, Gomes CM, de Bessa J, Cintra LKL, Myers JB, G. Neurogenic Bladder Research, The creation and validation of a short form of the neurogenic bladder symptom score, Neurourol. Urodyn 39 (4) (2020) 1162–1169, 10.1002/nau.24336. [DOI] [PubMed] [Google Scholar]
- [20].Hallock JL, Handa VL, The epidemiology of pelvic floor disorders and childbirth: An update, Obstet. Gynecol. Clin. North Am 43 (1) (2016) 1–13, 10.1016/j.ogc.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cao H, Peyrodie L, Agnani O, Cavillon F, Hautecoeur P, Donze C, Evaluation of an expanded disability status scale (EDSS) modeling strategy in multiple sclerosis, Med. Biol. Eng. Comput 53 (11) (2015) 1141–1151, 10.1007/s11517-015-1383-7. [DOI] [PubMed] [Google Scholar]
- [22].Stoffel JT, Detrusor sphincter dyssynergia: A review of physiology, diagnosis, and treatment strategies, Transl. Androl. Urol 5 (1) (2016) 127–135, 10.3978/j.issn.2223-4683.2016.01.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Calabrese M, Reynolds R, Magliozzi R, Castellaro M, Morra A, Scalfari A, Farina G, Romualdi C, Gajofatto A, Pitteri M, Benedetti MD, Monaco S, Regional distribution and evolution of gray matter damage in different populations of multiple sclerosis patients, PLoS One 10 (8) (2015) e0135428, 10.1371/journal.pone.0135428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nour S, Svarer C, Kristensen JK, Paulson OB, Law I, Cerebral activation during micturition in normal men, Brain 123 (Pt 4) (2000) 781–789, 10.1093/brain/123.4.781. [DOI] [PubMed] [Google Scholar]
- [25].Shmuel A, Yacoub E, Pfeuffer J, Van de Moortele PF, Adriany G, Hu X, Ugurbil K, Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain, Neuron 36 (6) (2002) 1195–1210, 10.1016/s0896-6273(02)01061-9. [DOI] [PubMed] [Google Scholar]


