Abstract
Objective
Mucosal initiated immune responses may be involved in the pathophysiology of RA. The most abundant immunoglobulin at mucosal surfaces is IgA, of which two subclasses exist: IgA1 and IgA2. IgA2 is mainly present at mucosal sites and has been ascribed pro-inflammatory properties. As IgA subclasses might provide insights into mucosal involvement and pro-inflammatory mechanisms, we investigated IgA responses in sera of RA patients.
Methods
In two cohorts of RA patients, the EAC and IMPROVED, total IgA1 and IgA2 were measured by ELISA. Furthermore, IgA subclass levels of RF and anti-citrullinated protein antibodies (anti-CCP2) were determined. The association of these IgA subclass levels with CRP and smoking was investigated.
Results
Total IgA1 and IgA2 were increased in RA patients compared with healthy donors in both cohorts. This increase was more pronounced in seropositive RA vs seronegative RA. For RF and anti-CCP2, both IgA1 and IgA2 could be detected. No strong associations were found between IgA subclasses (total, RF and anti-CCP2) and CRP. In smoking RA patients, a trend towards a selective increase in total IgA2 and RF IgA1 and IgA2 was observed.
Conclusion
RA patients have raised IgA1 and IgA2 levels. No shift towards IgA2 was observed, indicating that the increase in total IgA is not due to translocation of mucosal IgA into the bloodstream. However, mucosal inflammation might play a role, given the association between smoking and total IgA2 levels. Despite its pro-inflammatory properties, IgA2 does not associate strongly with pro-inflammatory markers in RA patients.
Keywords: RA, IgA subclasses, ACPA, rheumatoid factor, mucosal immunity
Rheumatology key messages.
Both total IgA1 and total IgA2 levels are raised in seropositive RA patients.
The increase in serum total IgA levels doesn’t seem to be due to translocation of mucosal IgA.
Smoking RA patients tend to have increased total IgA2 and RF IgA subclass levels.
Introduction
IgA is the most abundant class of immunoglobulin at mucosal sites and it has an important function in maintaining intestinal homeostasis. Recently, mucosal immune responses have gained increasing attention for their potential role in the pathophysiology of (seropositive) rheumatoid arthritis [1]. Therefore, it is worthwhile to study the IgA response in more detail in RA patients.
Several findings suggest mucosal involvement in the disease mechanisms of seropositive RA. Smoking is a well-known risk factor for the development of RA [1], implying that processes occurring at the pulmonary mucosa could play a role in disease pathogenesis. Furthermore, ACPA have been detected in sputum and saliva of seropositive RA patients, suggesting local production of autoantibodies [2, 3]. The gut, another vast mucosal site, might also be important and dysbiosis of the gut microbiome has been described in RA [4]. It has been hypothesized that dysbiosis of the microbiome could lead to local inflammation, loss of barrier function and possibly even bacterial translocation. Given that IgA plays an important role in mucosal immune responses, the characteristics of the IgA response might provide more insight into local inflammatory processes and thus possible mucosal origins of RA.
Humans harbour two IgA subclasses: IgA1 and IgA2 [5]. The biggest differences between IgA1 and IgA2 are the structure of the hinge region and their distribution at different sites. In serum, over 90% of total IgA is IgA1, whereas the IgA subclasses are more balanced at mucosal sites, with exact ratios depending on the location [5]. Furthermore, a recent report described a pro-inflammatory effect of IgA2 on neutrophils and macrophages, while this was not found for IgA1 [6]. Thus IgA1 and IgA2 not only differ in structure and localization, but may also recruit different effector functions. Moreover, the same study reported total IgA subclass levels to be lower in RA patients compared with healthy controls, and most strikingly, that ACPA IgA in serum is more often of the IgA2 subclass (up to 80% of all ACPA IgA) compared with total IgA. A higher proportion of ACPA IgA2 was also positively correlated with DAS [6] and ACPA IgA2 levels were weakly correlated with the severity of flares during DMARD tapering [7]. These findings suggest that ACPA IgA may have a mucosal origin, compatible with the high amount of IgA2, and that it might translocate from the mucosa into the bloodstream, leading to an elevated ACPA IgA2 percentage in serum. Furthermore, the pro-inflammatory properties of ACPA IgA2 might contribute to disease processes in RA.
Thus, studying IgA subclasses might provide more insight into both the origin as well as the potential pro-inflammatory pathophysiological mechanisms in RA. We hypothesized that mucosal inflammation in RA patients might result in elevated IgA2 levels, which could contribute to the ongoing pro-inflammatory processes in RA. To investigate these hypotheses, we set out to examine IgA subclass levels of total and autoantibody-specific IgA in sera of RA, as well as their link with inflammation and smoking, as a proxy for mucosal inflammation.
Patients and methods
Stored sera from two cohorts of early RA patients were used, the IMPROVED study and the Leiden Early Arthritis Clinic (EAC), of which details are described elsewhere [8, 9]. All patients fulfilled either the 2010 (IMPROVED) or the 1987 (EAC) ACR criteria for RA and gave written informed consent. Samples were selected based on previous autoantibody measurements [8, 9]. In this study, seropositivity was defined as positivity for anti-CCP2 IgG and/or RF IgM. Of the IMPROVED study, baseline samples from 125 seropositive RA patients were included, most of whom previously tested positive for anti-CCP2 IgA and RF IgA, as well as baseline sera of 68 seropositive RA patients who were negative for RF IgA and anti-CCP2 IgA, and 56 RA patients who were seronegative for both anti-CCP2 IgG and RF IgM. To investigate the generalizability of the findings, samples of a second cohort, the EAC, were used. This included sera of 95 seropositive, mostly anti-CCP2 IgA positive, and 64 seronegative RA patients collected at the one-year visit. Sera of 60 healthy donors, not matched for age or sex, were taken along as control.
Total, RF and anti-CCP2 IgA subclasses were measured with in-house ELISA (Supplementary Data S1 for details, available at Rheumatology online). To test whether RF IgA could influence the readout of the anti-CCP2 IgA subclass ELISA’s by binding to anti-CCP2 IgG, sera of anti-CCP2 IgG-positive/IgA-negative, RF IgA-negative patients were mixed 1:1 with sera of anti-CCP2 IgG-negative/IgA-negative, RF IgA-positive patients or with control sera before addition to the plate. Moreover, part of the samples were tested side-by-side before and after IgG depletion on anti-CCP2 IgA2 ELISA (Supplementary Data S1, available at Rheumatology online). As RF was measured in arbitrary units and not in exact amounts, no percentage RF IgA2 of total RF could be calculated. Instead the ratio RF IgA1/RF IgA2 was used.
Mann–Whitney U tests were used to compare IgA-levels between groups. Multivariate linear regression was performed to analyse total IgA subclass levels in RA patients vs healthy controls corrected for known confounders age and gender [10], using the IgA subclass levels (10log transformed due to skewness of the data) as the dependent variable. To assess the relationship between IgA subclass levels and CRP, DAS, HAQ score (HAQ) and BMI, Spearman's rank correlation coefficients were calculated, due to the presence of outliers. Patients with missing CRP values (IMPROVED n = 5, EAC n = 20) or smoking status (IMPROVED n = 1) were excluded from analyses involving CRP and smoking, respectively. For smoking analysis, a multivariate linear regression model including the possible confounders age, gender and CRP was used, with the log-transformed IgA subclass levels as the dependent variable. For analysis regarding CRP, similar models were performed for each IgA subclass separately (included as independent variable), together with age, gender and smoking as confounders, using the log-transformed CRP levels as the dependent variable. The associations between RF IgA subclass levels and smoking or CRP were analysed within patients who tested positive for both RF IgA1 and IgA2. Analyses regarding anti-CCP2 IgA1 levels were performed within the anti-CCP2 IgA1-positive group only. The Holm-Bonferroni method was applied to correct for multiple testing.
Ethics approval and consent to participate
Both the IMPROVED study and the EAC were conducted with the approval of the regional ethics committee at Leiden University Medical Center. The IMPROVED study was approved by the Medical Ethics Committees of all participating hospitals: the Groene Hart Hospital, Franciscus Hospital, Lievensberg Hospital, Admiral de Ruyter Hospital, ZorgSaam Hospital and the Medical Ethical Committee of South Holland for Haaglanden Medical Center, Haga Hospital, Bronovo Hospital and Reinier de Graaf Hospital. Written informed consent was obtained from all participants.
Results
Total IgA subclasses in RA
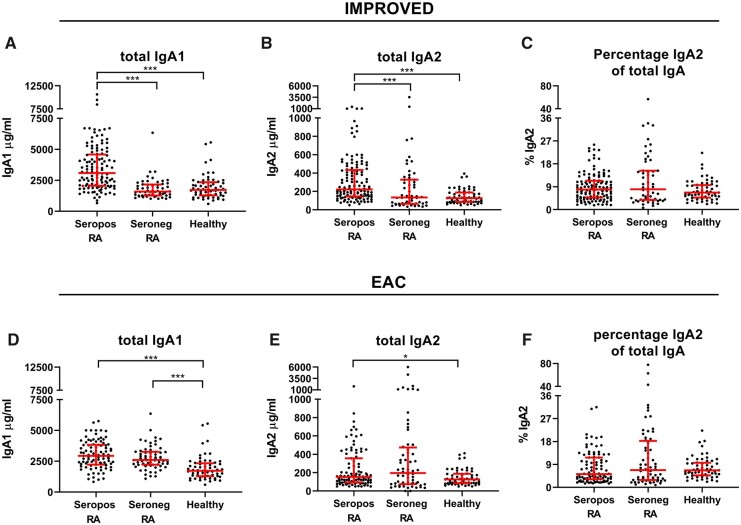
In seropositive RA IMPROVED patients, both total IgA1 (tIgA1, P < 0.001) and total IgA2 (tIgA2, P < 0.001) levels were strikingly elevated compared with healthy donors (Fig. 1A and B). Similar results were found using linear regression including age and gender as potential confounders (Supplementary Table S1, available at Rheumatology online). Both IgA subclasses were raised to the same extent, as the percentage of IgA2 (%tIgA2, P = 0.18) did not differ between seropositive patients and healthy donors (Fig. 1C). In seronegative IMPROVED patients, total IgA1, total IgA2 and the %tIgA2 were not raised compared with healthy controls (Fig. 1A and B, Supplementary Table S1, available at Rheumatology online). To investigate if the raise in total IgA levels in seropositive RA was due to the presence of IgA autoantibodies, seropositive RA patients who were negative for RF IgA and anti-CCP2 IgA were tested. In those patients, total IgA1 (P < 0.001) and IgA2 levels (tIgA2 P < 0.001) were also elevated (Supplementary Fig. S1, available at Rheumatology online). This indicates that the elevation in total IgA in seropositive RA is not solely caused by the presence of IgA autoantibodies.
Fig. 1.
Total IgA subclasses
Total IgA1, total IgA2 and percentage IgA2 of total IgA in seropositive and seronegative RA patients compared to healthy controls. Mann–Whitney U tests to compare IgA levels between seropositive or seronegative RA patients and healthy donors. Red bars: median and interquartile range. *P < 0.05, **P < 0.01, ***P < 0.001, no brackets shown when P > 0.05. Not all samples were measured in the same experiment. Colour version available online.
To investigate the generalizability of these findings, we also performed these measurements on sera of patients included in the EAC. While IMPROVED-sera were collected at baseline, these EAC-sera were collected at the patients’ one-year visit. A complete overview of difference between the cohorts can be found in Table 1. Also in the EAC, total IgA1 and IgA2 levels were raised in seropositive RA patients compared with healthy donors, although total IgA2 was just not significant after multiple testing correction (Fig. 1D and E) (tIgA1 P < 0.001, tIgA2 P = 0.02). Increased total IgA1 levels were now also observed in seronegative RA patients compared with healthy donors (tIgA1 P < 0.001, tIgA2 P = 0.06). Linear regression with correction for age and gender yielded similar results (Supplementary Table S1, available at Rheumatology online). IgA subclass levels did not differ between seropositive and seronegative EAC patients (Fig. 1D and E) (tIgA1 P = 0.13, tIgA2 P = 0.70). Thus, both total IgA1 and IgA2 levels are raised in seropositive RA compared with healthy controls and might also be higher in seronegative RA patients with longer disease duration.
Table 1.
Characteristics of the different patient subsets
| IMPROVED seropositivea RA | IMPROVED seronegative RA | IMPROVED seropositive, RF IgA-/CCP2 IgA- RA | EAC seropositivea RA | EAC seronegative RA | Healthy donors | |
|---|---|---|---|---|---|---|
| Number | 125 | 56 | 68 | 95 | 64 | 60 |
| Time sampling | Baseline | Baseline | Baseline | 1 -year visit | 1-year visit | NA |
| Age, mean (s.d.), years | 53.3 (12.4) | 54.3 (15.1) | 47.1 (14.7) | 59.1 (15.3) | 59.1 (16.8) | 44.7 (14.4) |
| Female, n (%) | 80 (64) | 39 (70) | 48 (71) | 64 (67)b | 41 (64)b | 35 (58) |
| Ever smoking, n (%) | 76 (61) | 23 (42) | 18 (27) | 50 (53)b | 22 (35)b | Unknown |
| CRP, mg/L, mean (s.d.) | 24.5 (34.1) (n = 120) | 22.2 (32.3) (n = 55) | 25.3 (33.0) (n = 64) | 26.3 (37.7) (n = 75) |
11.0 (21.8) (n = 55) |
NA |
| DAS, mean (s.d.) | 3.29 (0.91) | 3.70 (0.85) | 3.20 (0.83) | Unknown | Unknown | NA |
| RF IgM positivity, n (%) | 112 (92) | 0 0 | 56 (85) | 75 (79)b | 0 0b | NA |
| RF IgA positivity, n (%) | 106 (85) | NA | 0 0 | Unknown | NA | NA |
| Anti-CCP2 IgG positivity, n (%) | 118 (95) | 0 0 | 38 (57) | 95 (100)b | 0 0b | NA |
| Anti-CCP2 IgA positivity, n (%) | 102 (82) | NA | 0 0 | 63 (67)b | NA | NA |
| Total IgA1, μg/ml, median (IQR) | 3082 (2057–4579) | 1581 (1273–2155) | 2913 (1968–3912) | 2936 (2229–3828) | 2605 (2215–3259) | 1720 (1280–2338) |
| Total IgA2, μg/ml, median (IQR) | 221 (145–433) | 134 (65–328) | 197 (139–400) | 153 (95–356) | 196 (74–473) | 127 (86–188) |
| % IgA2 total IgA, median (IQR) | 7.9 (4.7–11.4) | 8.0 (3.8–15.3) | 6.0 (4.5–9.2) | 5.2 (3.2–11.7) | 6.7 (2.9–18.3) | 6.7 (4.8–9.6) |
Enriched for ACPA IgA positivity.
Information collected at baseline.
RF and anti-CCP2 IgA1 and IgA2
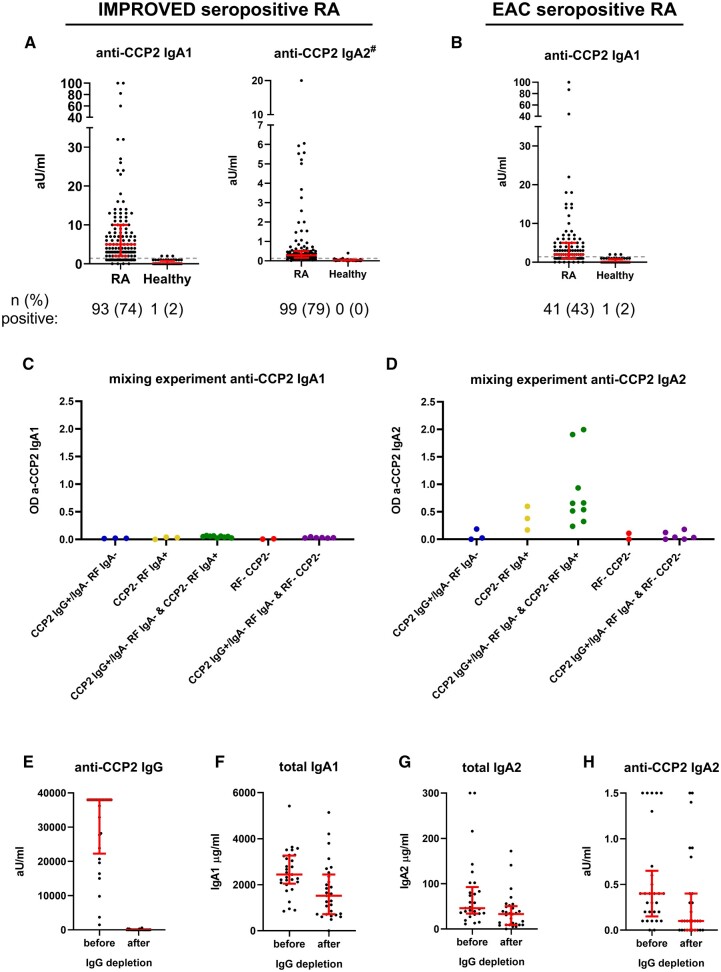
Next, we established assays to measure RF and anti-CCP2 IgA subclasses. For RF, both IgA subclasses could be readily detected in the seropositive IMPROVED RA patients with 77% positivity for RF IgA1 and 70% for RF IgA2 (Fig. 2A and B). Replication in the EAC showed 85% positivity for RF IgA1 and 60% RF IgA2 in seropositive RA (Fig. 2C and D). Anti-CCP2 IgA1 was present in 74% of seropositive patients in the IMPROVED (Fig. 3A). In the EAC, 43% of seropositive RA patients tested positive for anti-CCP2 IgA1 (Fig. 3B), which is partly due to the lower amount of anti-CCP2 IgA-positive patients within the seropositive group in the EAC compared with IMPROVED. Where anti-CCP2 IgA1 could be readily detected, the detection of anti-CCP2 IgA2 proved challenging. The ELISA protocol needed to be modified extensively to obtain decent signals for anti-CCP2 IgA2 (Supplementary Data S1, available at Rheumatology online). However, with these adaptations, including the use of less diluted serum and incubation of serum overnight, we eventually managed to obtain sufficient readouts (Fig. 3A). During quality controls of this new protocol, mixing experiments showed no effect of RF on the outcome of the anti-CCP2 IgA1 ELISA (Fig. 3C). However, using the anti-CCP2 IgA2 protocol, RF IgA2 could bind to anti-CCP2 IgG and give a false-positive anti-CCP2 IgA2 signal (Fig. 3D). Therefore, we concluded that anti-CCP2 IgA2 could not be reliably detected in sera containing RF IgA2. To further assess the impact of this RF IgA interference on anti-CCP2 IgA2, IgG depletion was performed in a subset of patients and anti-CCP2 IgA2 measurements were compared before and after IgG depletion. As expected, anti-CCP2 IgG was undetectable after IgG depletion (Fig. 3E), whereas only a slight non-specific IgA loss was observed after the procedure (Fig. 3F and G). Anti-CCP2 IgA2 could still be detected after IgG depletion in part of the seropositive RA samples (Fig. 3H). Moreover, anti-CCP2 IgA2 could also be detected in some RF-negative patients, indicating that not the entire signal could be attributed to RF. In conclusion, anti-CCP2 IgA2 can be present in part of seropositive RA patients, but technical difficulties posed by RF IgA interference prohibited precise determination of anti-CCP2 IgA2 levels. Therefore, no further analyses using anti-CCP2 IgA2 levels were performed.
Fig. 2.
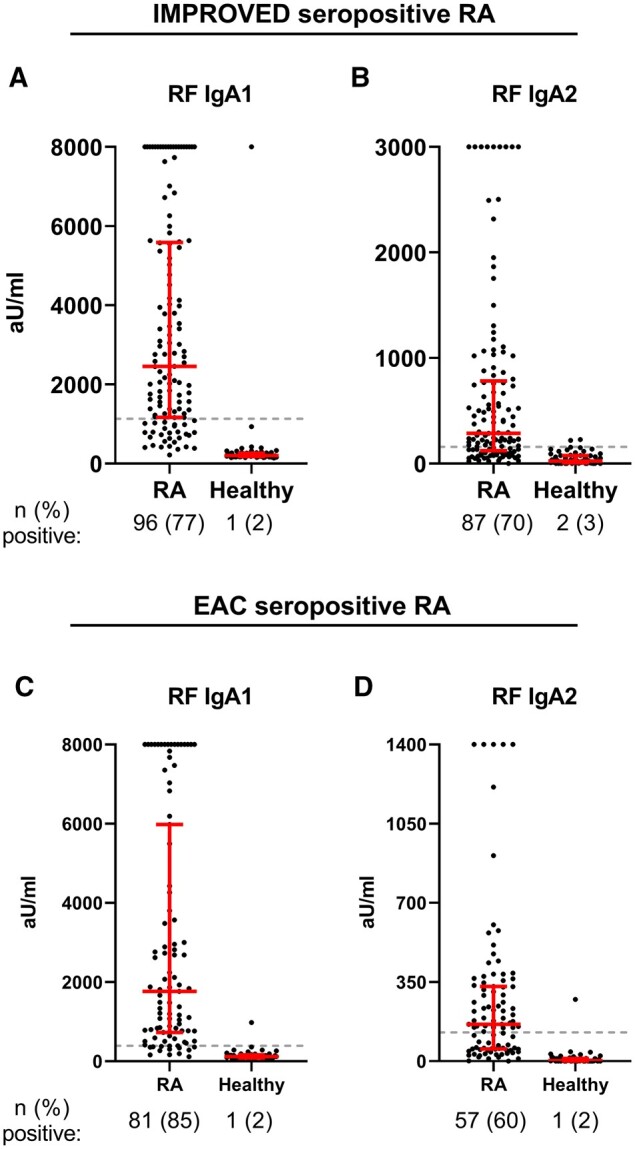
RF IgA subclasses
(A) RF IgA1 levels and (B) RF IgA2 levels in IMPROVED seropositive RA patients, (C) RF IgA1 levels and (D) RF IgA2 levels in EAC seropositive RA patients. Gray dashed line represents cut-off. Red bars: median and interquartile range. Arbitrary units cannot be directly compared between subclasses. Colour version is available online.
Fig. 3.
Anti-CCP2 IgA subclasses
(A) Anti-CCP2 IgA1 and IgA2 subclass measurements in seropositive IMPROVED RA patients and healthy controls. Number and percentage of patients above the cut-off is indicated. Gray dashed line represents cut-off. Red bars: median and interquartile range. #Results might be influenced by RF IgA2 binding. (B) Anti-CCP2 IgA1 in seropositive EAC RA patients. (C–D) Mixing experiment for anti-CCP2 IgA1 and IgA2 ELISA to investigate whether RF IgA binding could influence the read-out. For anti-CCP2 IgA1, no RF interference is observed, for the anti-CCP2 IgA2 ELISA results could be influenced by RF IgA2 binding, as the combination of anti-CCP2 IgG+ IgA- RF IgA- serum with anti-CCP2- RF IgA+ serum can give high OD values. (E–H) Measurements before and after IgG depletion in a selection of IMRPOVED seropositive RA patients. After IgG depletion, the anti-CCP2 IgA2 signal remains clearly visible in part of the samples, while all anti-CCP2 IgG is depleted. The procedure led to some specific loss of total IgA1 and total IgA2. Colour version is available online
IgA subclasses and inflammation
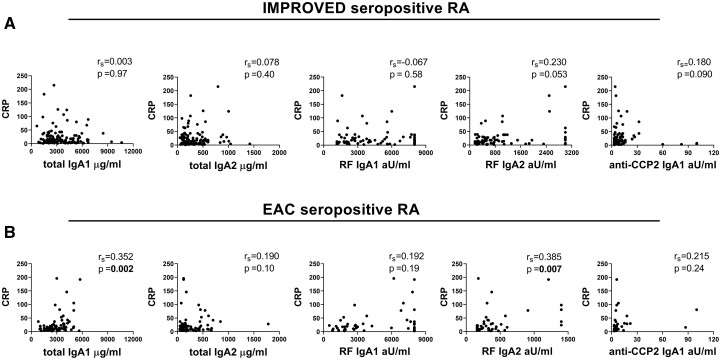
To investigate whether total and antigen-specific IgA subclass levels in seropositive RA are associated with inflammation, correlations with CRP were examined. In the IMPROVED, no association was observed for either total or antigen-specific IgA1 or IgA2 and CRP in univariate analysis (Fig. 4A). Also, no correlations were seen for %tIgA2 and RF IgA subclass ratio (Supplementary Fig. S2A, available at Rheumatology online). To correct for confounders, analyses were adjusted for age, gender and smoking. After this correction, a small difference was seen for RF IgA2 (P = 0.007), but this did not remain significant after multiple testing correction (Supplementary Table S2, available at Rheumatology online). In seropositive EAC patients, Spearman correlation yielded significant results for CRP and total IgA1 (rs = 0.352, P = 0.002) and RF IgA2 (rs = 0.385, P = 0.007) (Fig. 4B, Supplementary Fig. S2B, available at Rheumatology online). However, no clear pattern was visible in the scatterplots and the strength of the correlation was limited. Similarly, in multivariate analyses, small, but significant associations were found between CRP and total IgA1 and RF IgA2 (Supplementary Table S2, available at Rheumatology online). In the IMPROVED cohort, similar analyses with DAS, HAQ, BMI and the presence of erosions were performed. No significant associations with total or antigen-specific IgA subclasses was observed for these parameters, after correction for multiple testing (Supplementary Fig. S3, available at Rheumatology online). In conclusion, although there were some correlations between CRP and IgA antibodies of both subclasses, especially regarding RF IgA2-levels, these associations were not very strong in both cohorts.
Fig. 4.
Correlation between IgA subclasses and CRP in seropositive RA patients
Correlation between IgA subclass levels and CRP levels in (A) IMPROVED seropositive RA patients and in (B) EAC seropositive patients, calculated using Spearman’s rank correlation coefficient (rs). In RF IgA subclass analyses, only patients positive for both RF IgA1 and RF IgA2 are included. For anti-CCP2 IgA1 analysis, only anti-CCP2 IgA1 positive patients are included. Of note, RF and anti-CCP2 IgA subclass levels were not titrated.
IgA subclasses and smoking
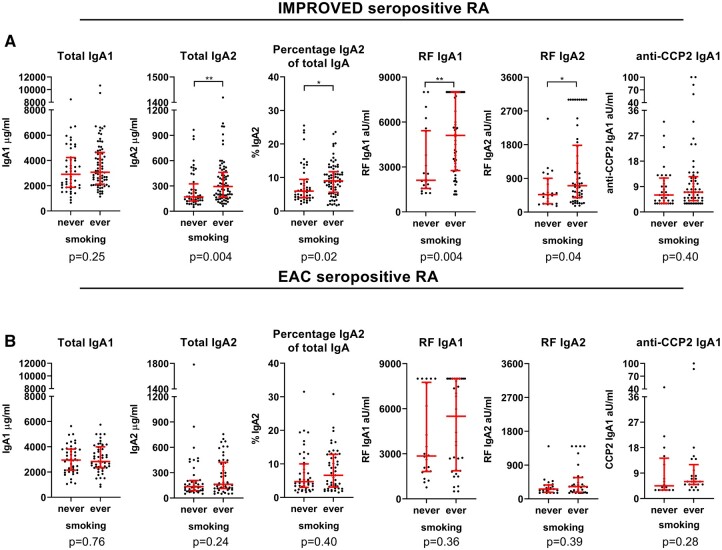
As IgA is the dominant antibody at mucosal surfaces, mucosal inflammation – for example, caused by long-term smoking – might lead to a more prominent IgA response and higher IgA serum levels. To investigate this hypothesis, levels of total, RF and anti-CCP2 IgA subclasses were compared between smoking and non-smoking RA patients. In seropositive IMPROVED patients, smokers had significantly increased serum levels of total IgA2 (P = 0.004), as well as higher RF IgA1 levels (P = 0.004) (Fig. 5A). Percentage IgA2 of total IgA (P = 0.02) and RF IgA2 (P = 0.04) were not significant after multiple testing correction. However, the ratio RF IgA1/RF IgA2 was similar in smokers vs non-smokers (Supplementary Fig. S4A, available at Rheumatology online), indicating that both RF subclasses are elevated in smokers, even though this was not significant for RF IgA2-levels. Interestingly, levels of total IgA1 and anti-CCP2 IgA1 were not elevated in smokers. Similar results were found in multivariate analyses corrected for age, gender and CRP (Supplementary Table S3, available at Rheumatology online). In the EAC seropositive samples, RF IgA1 levels appeared to be higher in smokers, but this was not statistically significant (P = 0.36) (Fig. 5B). Smoking was also not associated with other total or antigen-specific subclass levels (Fig. 5B, Supplementary Fig. S4B and Table S3, available at Rheumatology online). Similar to the pattern observed in seropositive RA, in seronegative IMPROVED patients, total IgA2 levels were also selectively increased in smokers (tIgA2 P = 0.004, %tIgA2 P = 0.002) (Supplementary Fig. S4C, Table S3, available at Rheumatology online), while no difference was observed between smoking vs non-smoking seronegative EAC patients after 1 year of treatment (Supplementary Fig. S4D, Table S3, available at Rheumatology online). In conclusion, smoking, a proxy for mucosal inflammation, might lead to a selective increase in serum total IgA2 levels and RF IgA levels at disease onset, but does not seem to affect serum total IgA1 and anti-CCP2 IgA1 in RA patients.
Fig. 5.
Association between IgA subclasses and smoking in seropositive RA patients
IgA subclass levels in ever- versus never-smoking seropositive RA patients in (A) IMPROVED and (B) EAC, analysed using Mann–Whitney U tests. Red bars: median and interquartile range. *P < 0.05, **P < 0.01, ***P < 0.001, #not significant after correction for multiple testing. In RF IgA subclass analyses, only patients positive for both RF IgA1 and RF IgA2 are included. For anti-CCP2 IgA1 analysis, only anti-CCP2 IgA1 positive patients are included. Of note, RF and anti-CCP2 IgA subclass levels were not titrated. Colour version available online.
Discussion
Because studying IgA subclasses can provide insight in both mucosal involvement as well as potential pro-inflammatory pathophysiological processes, we explored the IgA subclass distribution of total IgA as well as RF- and anti-CCP2-specific IgA in RA. Strikingly, total IgA1 and IgA2 levels were increased in seropositive RA patients in both the IMPROVED and EAC cohort. This was much less pronounced for seronegative patients, in whom only total IgA1 in the EAC was significant. Furthermore, both RF and anti-CCP2 IgA1 and IgA2 were detectable in a subset of seropositive RA patients. However, technical difficulties posed by RF IgA interference prohibited precise determination of anti-CCP2 IgA2 levels.
Several observations, for example the detection of RF and ACPA in sputum and saliva of seropositive RA patients, indicate that the mucosal immune system might be involved in the pathophysiology of RA [1–3, 11]. Both IgA subclasses have an important function in mucosal immune responses, but the relative amount of IgA2 is increased at mucosal sites [12]. A previous study suggested that also in serum of RA patients the relative amount of IgA2 is increased [6]. Based on these findings, one might hypothesize that in RA patients IgA2 can translocate from (inflamed) mucosal sites, where it is highly abundant, into the circulation, leading to an elevated percentage of IgA2 in serum. However, we found that both IgA1 and IgA2 levels were elevated in seropositive RA patients. The percentage of IgA2 in serum was not increased in RA patients when compared with healthy donors. Thus, these data do not support the notion that direct translocation of mucosal IgA [2] is one of the main mechanisms leading to the elevated IgA subclass levels in RA.
Nonetheless, chronic mucosal inflammation might still be involved in the hyperproduction of IgA subclasses in RA patients. The link between mucosal immune responses and serum immunoglobulins is currently not completely understood. Research in celiac disease showed that mucosal and serum IgA are related, but produced by different plasma cells [13]. Therefore, one might hypothesize that it is possible that the initial mucosal response is predominantly of the IgA2 subclass, while the related serum response is predominantly IgA1. This means the elevated IgA1 and IgA2 serum levels in RA could still be the result of increased mucosal IgA responses, most likely not by direct translocation of mucosal IgA, but potentially via the generation of specific plasma cell populations that contribute to the serum antibody pool. On the other hand, various studies have described that IgG and IgM can also be elevated in RA [14–17]. This suggests the elevated IgA subclass levels could also be part of a general immunoglobulin (Ig) hyperproduction in RA patients, for example due to a specific B-cell hyperreactivity in the context of systemic inflammation. Another possibility is that the hyperglobulinemia reflects intrinsic B-cell alterations in RA patients, which could be in line with the important role that B cells play in the pathophysiology of RA [18–20].
To investigate whether chronic mucosal inflammation might play a role in the elevated IgA subclass levels in RA patients, we used smoking status as a proxy for mucosal inflammation, as smoking is known to cause chronic pulmonary inflammation [21]. Intriguingly, smoking was associated with a selective increase in total IgA2 levels in serum of RA patients in the IMPROVED. Also RF IgA subclass levels tended to be increased in smoking RA patients in IMPROVED, whereas anti-CCP2 IgA1 levels were not. This is interesting, as both RF and anti-CCP2 total IgA have been detected in sputum of RA patients, suggesting they are produced locally in the lungs [2]. However, our findings suggest that smoking might have a larger influence on the production of RF IgA than on anti-CCP2 IgA. This is in line with the observations that smoking is associated with RF IgM positivity rather than the presence of ACPA IgG [22, 23]. Of note, we could not replicate these associations with smoking in the EAC, where autoantibody levels were overall lower than in the IMPROVED, possibly due to the immunosuppressive treatment that most patients had received [9]. In conclusion, our data suggest that chronic mucosal inflammation may be one of the mechanisms playing a role in the elevated RF IgA and total IgA2 levels in RA, although smoking status does not explain the full extent of the increase in total IgA subclasses in RA patients.
Although the presence of IgA2 in humans was described decades ago, novel findings regarding pro-inflammatory effector functions of IgA2 were recently described [6]. However, in RA patients we did not observe an association between total IgA2 levels and two important markers of inflammation, CRP and DAS. On the other hand, a significant correlation between total IgA1 levels and CRP was seen in the EAC. Furthermore, significant associations between CRP and RF IgA2-levels were found, although the effect was small and not significant after correction for multiple testing correction in the IMPROVED. Higher anti-CCP2 IgA1 levels were not associated with lower inflammation in our study, in contrast to a weak correlation between low anti-CCP2 IgA1 and high DAS described before [6]. A recent study also describes that anti-CCP2 IgA2 levels decline in ongoing remission, although, based on our data regarding RF interference, this effect might be mediated by a decline in RF IgA levels [7, 9]. Taken together, our findings do not appear to support an essential role for IgA2 in the ongoing pro-inflammatory processes in RA.
One of the limitations of our study is the use of in-house ELISAs. Technical difficulties posed by RF IgA interference prohibited precise determination of anti-CCP2 IgA2 levels. To obtain decent signals in the ELISA, serum was diluted less and incubated overnight instead of 1 h, which might have provided RF IgA with the chance to bind anti-CCP2 IgG. As a result, the anti-CCP2 IgA1/IgA2 ratio could not be calculated. This precluded our attempts to replicate the findings that the ACPA IgA response is shifted towards IgA2 [6]. It is unclear whether possible interference of RF IgA2 was investigated in other studies. However, based on the fact that anti-CCP2 IgA1 was readily detectable whereas anti-CCP2 IgA2 was not, it seemed unlikely that a majority of total ACPA IgA was of the IgA2 subclass. On the contrary, both RF IgA1 and IgA2 were readily detectable, in line with previous studies [11].
Furthermore, we repeated the measurements on an independent cohort to investigate the generalizability of our findings. The results of the in-house IgA subclass ELISAs were largely reproducible between the two different cohorts. Another strength is that many anti-CCP2 IgA positive patients were included and detailed information regarding smoking status and inflammatory markers was available. Although our findings are in contrast with a previous study on IgA subclasses in RA (which found both IgA1 and IgA2 to be lower in RA patients) [6], multiple other studies have described raised total IgA levels in RA [14–16, 24–27].
In conclusion, seropositive RA patients have raised total IgA1 and IgA2 levels and can also harbour RF and ACPA IgA subclasses. Because no shift towards the IgA2 subclass was observed, the increase in total IgA levels appears not to be due to translocation of mucosal IgA over mucosal barriers into the bloodstream. However, chronic mucosal inflammation might be one of the mechanisms involved in the raise in IgA [2] levels in RA, given the association between smoking and total IgA2 levels. Despite the pro-inflammatory properties of IgA2, our data does not seem to support a large role of IgA2 in chronic inflammatory processes in RA patients.
Funding: This work is supported by a FOREUM career grant [to D.vdW.], the Target to B consortium [to D.vdW. and R.E.M.T.] and the Dutch Arthritis Foundation [to R.E.M.T. and A.H.M.vdH.-VM.].
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary Material
Contributor Information
Veerle F A M Derksen, Department of Rheumatology, Leiden University Medical Center, Leiden.
Cornelia F Allaart, Department of Rheumatology, Leiden University Medical Center, Leiden.
Annette H M Van der Helm-Van Mil, Department of Rheumatology, Leiden University Medical Center, Leiden; Department of Rheumatology, Erasmus Medical Center, Rotterdam, The Netherlands.
Tom W J Huizinga, Department of Rheumatology, Leiden University Medical Center, Leiden.
René E M Toes, Department of Rheumatology, Leiden University Medical Center, Leiden.
Diane van der Woude, Department of Rheumatology, Leiden University Medical Center, Leiden.
Data availability statement
The data underlying this article will be shared on reasonable request.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Holers VM, Demoruelle MK, Kuhn KA. et al. Rheumatoid arthritis and the mucosal origins hypothesis: protection turns to destruction. Nat Rev Rheumatol 2018;14:542–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Willis VC, Demoruelle MK, Derber LA. et al. Sputum autoantibodies in patients with established rheumatoid arthritis and subjects at risk of future clinically apparent disease. Arthritis Rheum 2013;65:2545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Svard A, Kastbom A, Sommarin Y, Skogh T.. Salivary IgA antibodies to cyclic citrullinated peptides (CCP) in rheumatoid arthritis. Immunobiology 2013;218:232–7. [DOI] [PubMed] [Google Scholar]
- 4. Zhang X, Zhang D, Jia H. et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med 2015;21:895–905. [DOI] [PubMed] [Google Scholar]
- 5. Woof JM, Russell MW.. Structure and function relationships in IgA. Mucosal Immunol 2011;4:590–7. [DOI] [PubMed] [Google Scholar]
- 6. Steffen U, Koeleman CA, Sokolova MV. et al. IgA subclasses have different effector functions associated with distinct glycosylation profiles. Nat Commun 2020;11:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sokolova MV, Hagen M, Bang H. et al. IgA anti-citrullinated protein antibodies (IgA ACPA) are associated with flares during DMARD tapering in rheumatoid arthritis. Rheumatology 2021;61:2124--31. [DOI] [PubMed] [Google Scholar]
- 8. Verpoort KN, Jol-van der Zijde CM, Papendrecht-van der Voort EA. et al. Isotype distribution of anti-cyclic citrullinated peptide antibodies in undifferentiated arthritis and rheumatoid arthritis reflects an ongoing immune response. Arthritis Rheum 2006;54:3799–808. [DOI] [PubMed] [Google Scholar]
- 9. de Moel EC, Derksen V, Trouw LA. et al. In rheumatoid arthritis, changes in autoantibody levels reflect intensity of immunosuppression, not subsequent treatment response. Arthritis Res Ther 2019;21:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Berth M, Delanghe J, Langlois M, De Buyzere M.. Reference values of serum IgA subclasses in caucasian adults by immunonephelometry. Clin Chem 1999;45:309–10. [PubMed] [Google Scholar]
- 11. Ottèn HG, Daha MR, van Laar JM, de Rooy HH, Breedveld FC.. Subclass distribution and size of human IgA rheumatoid factor at mucosal and nonmucosal sites. Arthritis Rheumatol 1991;34:831–9. [DOI] [PubMed] [Google Scholar]
- 12. Brandtzaeg P, Johansen FE.. Mucosal B cells: phenotypic characteristics, transcriptional regulation, and homing properties. Immunolog Rev 2005;206:32–63. [DOI] [PubMed] [Google Scholar]
- 13. Iversen R, Snir O, Stensland M. et al. Strong clonal relatedness between serum and gut IgA despite different plasma cell origins. Cell Rep 2017;20:2357–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Lloyd KA, Melas I. et al. Rheumatoid arthritis patients display B-cell dysregulation already in the naïve repertoire consistent with defects in B-cell tolerance. Sci Rep 2019;9:19995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gravina GE, Bossios A, Ekerljung L, Malmhäll C. et al. Low serum levels of immunoglobulin D recognize autoantibody production in rheumatoid arthritis. J Mol Sci 2018;2:5. [Google Scholar]
- 16. Ayyappan P, Harms RZ, Seifert JA. et al. Heightened levels of antimicrobial response factors in patients with rheumatoid arthritis. Front Immunol 2020;11:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin G, Li J.. Elevation of serum IgG subclass concentration in patients with rheumatoid arthritis. Rheumatol Int 2010;30:837–40. [DOI] [PubMed] [Google Scholar]
- 18. Moura RA, Fonseca JE.. JAK inhibitors and modulation of B cell immune responses in rheumatoid arthritis. Front Med (Lausanne) 2021;7:607725. [DOI] [PMC free article] [PubMed]
- 19. Wu F, Gao J, Kang J. et al. B cells in rheumatoid arthritis pathogenic mechanisms and treatment prospects. Front immunol 2021;12:750753. [DOI] [PMC free article] [PubMed]
- 20. Volkov MA-O, van Schie KA-O, van der Woude DA-O.. Autoantibodies and B Cells: the ABC of rheumatoid arthritis pathophysiology. Immunol Rev 2020;294:148–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gonçalves RB, Coletta RD, Silvério KG. et al. Impact of smoking on inflammation: overview of molecular mechanisms. Inflamm Res 2011;60:409–24. [DOI] [PubMed] [Google Scholar]
- 22. van Wesemael TJ, Ajeganova S, Humphreys J. et al. Smoking is associated with the concurrent presence of multiple autoantibodies in rheumatoid arthritis rather than with anti-citrullinated protein antibodies per se: a multicenter cohort study. Arthritis Res Ther 2016;18:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Regueiro C, Rodriguez-Rodriguez L, Lopez-Mejias R. et al. A predominant involvement of the triple seropositive patients and others with rheumatoid factor in the association of smoking with rheumatoid arthritis. Sci Rep 2020;10:3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Badcock LJ, Clarke S, Jones PW, Dawes PT, Mattey DL.. Abnormal IgA levels in patients with rheumatoid arthritis. Ann Rheum Dis 2003;62:83–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jorgensen C, Anaya JM, Cognot C, Sany J.. Rheumatoid arthritis associated with high levels of immunoglobulin a: clinical and biological characteristics. Clin Exp Rheumatol 1992;10:571–5. [PubMed] [Google Scholar]
- 26. Kanerud L, Engström GN, Tarkowski A.. Evidence for differential effects of sulphasalazine on systemic and mucosal immunity in rheumatoid arthritis. Ann Rheum Dis 1995;54:256–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Delft MAM, van der Woude D, Toes REM, Trouw LA.. Secretory form of rheumatoid arthritis-associated autoantibodies in serum are mainly of the IgM isotype, suggesting a continuous reactivation of autoantibody responses at mucosal surfaces. Ann Rheum Dis 2019;78:146–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request.