Abstract
Objectives
Patients with SLE have increased mortality compared with age- and sex-matched controls. LN is a severe manifestation of SLE and an important cause of death. We carried out a retrospective survival analysis to investigate factors that could influence the risk of mortality and LN in a large multi-ethnic cohort of patients with SLE.
Methods
By careful review of medical records, we identified 496 patients with SLE for whom we had complete information regarding the period of observation and occurrence of death and nephritis. Patients were stratified into groups according to sex, ethnicity, age at start of follow-up and time period of diagnosis. Kaplan–Meier analysis was used to investigate differences between the groups.
Results
Of the 496 patients in the study, 91 (18.3%) died, 165 (33.3%) developed LN and 33 (6.7%) developed end-stage renal failure. There was no difference between men and women in either mortality or development of LN. Caucasian patients were significantly less likely to develop LN than other ethnic groups (P < 0.0001) but not less likely to die. Patients diagnosed before the median age of 28 years were significantly more likely to develop LN (P < 0.0001) but significantly less likely to die (P = 0.0039) during the period of observation. There has been a significant improvement in survival in patients diagnosed between 1978 and 1989 and those diagnosed between 2006 and 2011 (P = 0.019).
Conclusion
In our cohort, non-Caucasian ethnicity and younger age at diagnosis are associated with the risk of developing LN. There is evidence of improvement in survival of patients with SLE over time.
Keywords: systemic lupus erythematosus, mortality, lupus nephritis, ethnicity, survival analysis
Rheumatology key messages.
In this British cohort of patients with SLE, sex and ethnicity did not affect survival.
Patients diagnosed younger were more likely to develop lupus nephritis but less likely to die. Non-Caucasians were more likely to develop nephritis than Caucasians.
Patients diagnosed between 2006 and 2011 had significantly improved survival compared with earlier cohorts.
Introduction
SLE is an autoimmune rheumatic disease with a prevalence of 97/100 000 in the UK [1]. Mortality from SLE has improved significantly over the last 50 years but is still higher than for age- and sex-matched people in the general population [2]. It has been argued that the improvement in mortality was greatest prior to 2000 [3] and has since levelled off and that it is not the same in countries with different levels of prosperity [3]. Numerous studies in different countries have estimated the standardized mortality ratio (SMR) for SLE at between 2.5 and 5, with a higher SMR in younger age groups [2, 4–15].
In calculating the SMR, the mortality rate for patients with SLE is compared with that for the same age and sex groups in the area from which the SLE cohort was drawn. This approach has advantages. For example, Bernatsky et al. [4] pointed out that it allows for the fact that men in general have higher mortality than women and thus avoids overestimating mortality risk in men with SLE compared with women with SLE. This method can be applied to very large groups such as the 9547 patients from multiple centres of the SLICC group studied by Bernatsky et al. [4].
The SMR method is not ideal for longitudinal analysis of survival in a group of patients that varies in terms of time of follow-up. Survival analysis using the Kaplan–Meier method is better from this point of view [16]. However, when comparing survival curves in different subgroups of patients with SLE it is necessary to have sufficiently long follow-up, enough individuals and enough events to analyse in each subgroup. This can be a problem when looking at sex and ethnicity. For example, a Korean study included only female subjects because only one male patient with lupus had died [10]. Many studies have been carried out in populations with little ethnic diversity, e.g. Denmark [12], Sweden [9], Greece [13], Korea [10] and China [14, 15, 17].
It is important to define whether some groups of patients with SLE have a higher mortality risk than others. Four characteristics of particular interest are sex, ethnicity, age at diagnosis and the time period when the patients were diagnosed. The impact of these factors on mortality will be investigated in this article using Kaplan–Meier survival analysis.
Although some groups have claimed higher mortality in men than women with SLE [11, 13, 17, 18], many others have found that this is not the case [4, 7, 8, 12, 15, 19, 20]. A systematic review of 12 studies including 27 210 subjects also found no difference in the SMR between men and women with SLE [2].
Articles from the USA have shown that African American patients have higher mortality than white patients [18, 20–22] and this was also shown in the USA subgroup of the SLICC multicentre study [4]. However, this relationship has not been demonstrated elsewhere and most SLE cohorts are not ethnically diverse enough to analyse the question. The UK has an ethnically diverse population, but previous articles from Birmingham [23] and London [24] have not provided a clear answer to the question of association between mortality and ethnicity.
Patients diagnosed at a younger age have been reported by some groups to have more aggressive forms of disease and higher SMRs [4–6, 8, 10, 25]. On the other hand, not all reports agree [9, 12, 15], and because these younger patients are further from the end of a natural lifespan, survival analysis by the Kaplan–Meier method has shown that their survival over time is actually better on average than that of patients diagnosed later in life [17, 26].
Some groups have reported that the SMR for patients with SLE improved considerably over time from 1970 to 2000 [4, 27]. However, in a meta-analysis of 171 studies, Tektonidou et al. [3] concluded that the improvement in mortality of patients with SLE seen up to 2000 has now slowed considerably. Most of those 171 articles, however, did not include data from later than 2005 and thus do not reflect improvements due to the introduction of, for example, biologic agents [3]. Singh and Yen [20] reviewed national census data from the USA collected between 1968 and 2013 and concluded that mortality from SLE only started to decrease in 1999 and decreased steadily after that.
LN is one of the most severe forms of SLE and a major cause of death in patients with this disease [28]. A large study in the SLICC inception cohort showed that LN occurred in 700 of 1827 patients [29] and was associated with a 3-fold increase in the risk of death. Risk factors for developing LN included male sex, non-white ethnicity and younger age [29]. Other groups have reported similar findings [30, 31]. We therefore expanded our analysis of the effects of ethnicity, sex and age of onset to include development of LN as well as mortality.
Patients and methods
Patients
We reviewed the medical records of patients treated at the Lupus Clinic at University College London Hospital (UCLH) since 1979. All met the ACR revised criteria for SLE [32] or previous criteria extant at the time of entry into the cohort. Causes of death in 725 patients treated at this unit have been described in a previous article [24]. However, that article did not include survival analysis, because clear information about the start and end of follow-up for each patient was not available.
In this article we have included 496 patients for whom we have established accurately the duration of follow-up, occurrence and year of death, occurrence and year of diagnosis of LN and date of censoring where appropriate. Any patient for whom all of these data points could not be established (e.g. because they had last been seen many decades ago) was excluded from the analysis.
Whenever a patient fulfils classification criteria for SLE and is enrolled into this UCLH cohort, a specific paper copy research folder is started in which data including the BILAG 2004 activity score and medications are recorded at each clinic visit. In addition, a baseline serum sample is taken and stored. To establish an accurate start date for follow-up, one author (W.L.) reviewed all available research folders to find the earliest assessment and another (A.R.) reviewed the dates of the earliest sample in storage. Whichever was the earliest of these dates was taken as the start date for follow-up. The age at diagnosis was obtained by subtracting the birth year from the year of starting follow-up. We reviewed the data for all patients up to December 2019.
We categorized the patients into four groups by the year of starting follow-up. These were group 1 (1978–89, n = 89), group 2 (1990–99, n = 155), group 3 (2000–2005, n = 112) and group 4 (2006–2011, n = 140). These groups were chosen so that no group covered too long a time frame, so that the cut points between groups were at the end of a calendar year (and for groups 3 and 4) to coincide with introduction of new treatments. We began to use mycophenolate more extensively in 2000 and rituximab more widely in the mid-2000s.
To establish the end date for follow-up, AR reviewed the medical records and research database to establish which patients remained under follow-up in 2019, which had died or been lost to follow-up and when these events had occurred.
Ethnicity and gender for each patient were entered by W.L. A data quality check was carried out by A.R. double-checking a random 25% of the entries and there was agreement in 99.5% of datapoints. Ethnicity was categorized as Caucasian, Afro-Caribbean, South Asian (Indian, Pakistani, Bangladeshi, Sri Lankan), East Asian (Chinese, Japanese, Korean, Filipino) and other (including mixed ethnicity).
F.F. and D.A.I. collected information for each patient with LN. Of the patients included, 95% had biopsy-proven LN [33]. For the patients that either declined or had a contraindication for renal biopsy, LN was assumed in the presence of two or more of the following—proteinuria >0.5 g/24 h, oedema requiring diuretic therapy, hypertension, creatinine clearance <60 ml/min or elevated serum creatinine—in the absence of another explanation for these findings [34].
This is an observational retrospective study of medical records collected over a period of >30 years. All data were derived from normal clinical management and no patients underwent extra questionnaires or research procedures. No individualized or identifiable data are presented in this study, therefore ethical approval and informed consent were not required. This is standard practice at our institution.
Statistical analysis
Kaplan–Meier survival curves were compared using the logrank test and hazard ratios (HRs) of mortality and incident LN were obtained through multivariate Cox regression analysis. All statistical tests were two-sided, conducted at a significance level of 0.05 and reported using P-values and/or 95% CIs. All statistical analyses were performed using Stata version 16.1 (StataCorp, College Station, TX, USA).
Results
The characteristics of patients enrolled in the study are shown in Table 1. Overall, there were 454 women and 42 men. The mean follow-up was 15.8 years (s.d. 8.75) with a maximum of 40 years. The median age at the start of follow-up was 28.0 years (IQR 21–37).
Table 1.
Demographic and clinical characteristic of patients enrolled
| Variables | Patients, n | Deaths, n | Patients with LN, n |
|---|---|---|---|
| Total | 496 | 91 | 165 |
| Gender, n (%) | |||
| Female | 454 (91.5) | 84 (92.3) | 152 (92.1) |
| Male | 42 (8.47) | 7 (7.69) | 13 (7.88) |
| Age at onset, n (%) | |||
| Above median (>28 years) | 246 (50.4) | 60 (65.9) | 58 (35.2 |
| Below median (<28 years) | 250 (49.6) | 31 (34.1) | 107 (64.9%) |
| Ethnicity, n (%) | |||
| Caucasian | 307 (61.9) | 59 (64.8) | 77 (46.7) |
| South Asian | 55 (11.1) | 12 (13.2) | 24 (14.6) |
| African Caribbean | 98 (19.8) | 15 (16.5) | 44 (26.7) |
| East Asian | 24 (4.8) | 2 (2.2) | 13 (7.88) |
| Mixed race | 12 (2.42) | 3 (3.3) | 7 (4.24) |
| Time of diagnosis, n (%) | |||
| 1978–89 | 89 (17.9) | 39 (42.9) | 36 (21.8) |
| 1990–99 | 155 (31.3) | 33 (36.4) | 38 (23.0) |
| 2000–5 | 112 (22.6) | 15 (16.5) | 55 (33.0) |
| 2006–11 | 140 (28.2) | 4 (4.4) | 36 (21.8) |
There were 91 (18.3% of cohort) deaths and 165 (33.3%) patients developed LN. There were 35 (7.1%) patients who were in both these groups (i.e. they developed LN and subsequently died), although only four patients died from LN itself. Of the 165 patients with LN, 33 eventually developed end-stage renal disease (6.7% of cohort).
Cause of death
The main causes of death were cancer, cardiovascular/thrombosis, infection and renal disease. The causes of death in each group of patients are shown in Table 2.
Table 2.
Causes of death
| Characteristics | Infection | Cardiovascular/thrombosis | Cancer | Renal | Other | Total |
|---|---|---|---|---|---|---|
| Total, n | 25 | 18 | 25 | 4 | 19 | 91 |
| By sex, n (%) | ||||||
| Male | 0 | 3 (17) | 3 (12) | 0 | 1 (5) | 7 (8) |
| Female | 25 (100) | 15 (83) | 22 (88 | 4 (100%) | 18 (95) | 84 (92) |
| By ethnicity, n (%) | ||||||
| Caucasian | 13 (52) | 14 (78) | 22 (88) | 1 (25) | 9 (47) | 59 (65) |
| Afro-Caribbean | 5 (20) | 1 (5.5) | 1 (4) | 3 (75) | 5 (26) | 15 (16) |
| South Asian | 4 (16) | 2 (11) | 2 (8) | 0 | 4 (21) | 12 (13) |
| East Asian | 0 | 1 (5.5) | 0 | 0 | 1 (5) | 2 (2) |
| Mixed race | 3 (12) | 0 | 0 | 0 | 0 | 3 (3) |
| By age of onset, n (%) | ||||||
| Above median | 16 (64) | 15 (83) | 16 (64) | 2 (50) | 12 (63) | 61 (67) |
| Below median | 9 (36) | 3 (17) | 9 (36) | 2 (50) | 7 (37) | 30 (33) |
| By time of diagnosis, n (%) | ||||||
| Group 1 | 10 (40) | 7 (39) | 10 (40) | 3 (75) | 9 (47) | 39 (43) |
| Group 2 | 8 (32) | 5 (28) | 13 (52) | 1 (25) | 7 (37) | 34 (37) |
| Group 3 | 7 (28) | 5 (28) | 1 (4) | 0 | 1 (5) | 14(15) |
| Group 4 | 0 | 1 (5.5) | 1 (4) | 0 | 2 (10) | 4 (4) |
Other causes of death included suicide (n = 2), road traffic accident (n = 1), old age (n = 1), liver failure (n = 2), unknown cause (n = 5), alcoholism (n = 1), thrombotic thrombocytopenic purpura (n = 1), haemorrhage (n = 3), haemolytic anaemia (n = 1), acute respiratory distress syndrome (n = 1) and chronic obstructive pulmonary disease (n = 1).
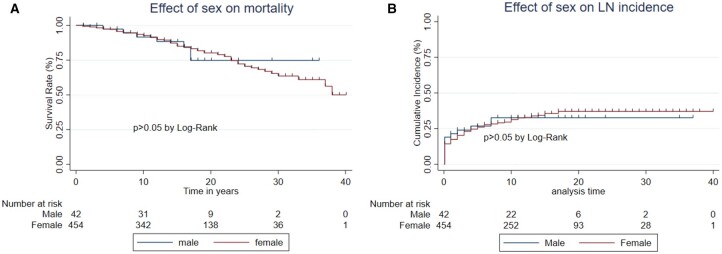
Effect of sex
Death during the observation period occurred in 84/454 (18.5%) women and 7/42 (16.7%) men. Fig. 1A shows that survival was not affected by gender. There were too few male deaths to reach conclusions about different causes of death between the sexes, although no men died of either infection or renal disease.
Fig. 1.
Effect of sex on mortality and LN
Comparison of (A) mortality and (B) incidence of LN between male and female patients.
LN occurred in 152/454 (33.5%) women and 13/42 (31.0%) men. Fig. 1B shows that there was no difference between the groups in the development of LN.
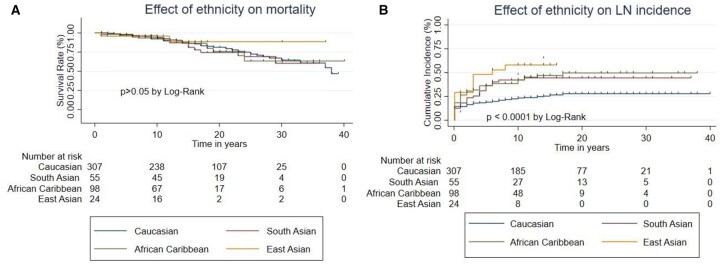
Effect of ethnicity
Death occurred in 59/301 (19.9%) Caucasian patients, 15/98 (15.3%) African/Caribbean patients, 12/55 (21.8%) South Asian patients and 2/24 (8.3%) East Asian patients. Fig. 2A shows that the survival curves for the different ethnic groups are very similar, with possible better survival in East Asians, although the curve only diverges from the other groups at a point where only two East Asians remained under follow-up, making it hard to reach a firm conclusion. Statistical analysis showed no significant difference between the groups. Three of the four renal deaths occurred in Afro-Caribbean patients.
Fig. 2.
Effect of ethnicity on mortality and LN
Comparison of (A) mortality and (B) incidence of LN between patients in different ethnic groups: Caucasian, Afro-Caribbean, South Asian and East Asian.
LN occurred in 77/307 (25.1%) Caucasian patients, 44/98 (44.9%) African/Caribbean patients, 24/55 (43.6%) South Asian patients and 13/24 (54.2%) East Asian patients. Fig. 2B shows that Caucasian patients had significantly lower risk of developing LN than any of the other ethnic groups.
By Cox proportional hazards analysis, Afro-Caribbean patients had a significantly increased risk of developing LN compared with Caucasians [HR 2.03 (95% CI 1.40, 2.94), P < 0.0001] and this was also true for South Asians [HR 1.85 (95% CI 1.17, 2.93), P = 0.08] and East Asians [HR 2.79 (95% CI 1.55, 5.02), P = 0.01].
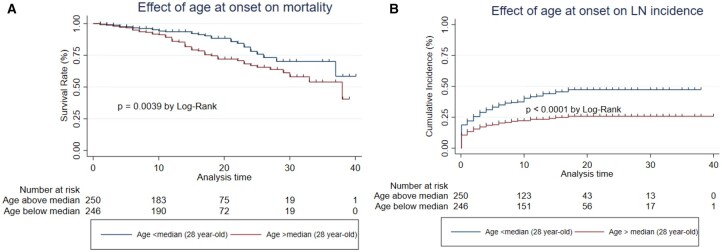
Effect of age at the start of follow-up
Death occurred in 60/246 (24.3%) patients diagnosed at greater than the median age of 28 years and 31/250 (12.4%) of patients diagnosed at ≤28 years. Fig. 3A shows a significant difference between the groups (P = 0.0039). Apart from renal disease, all causes of death were overrepresented in the older group, with a particularly large difference for cardiovascular/thrombosis.
Fig. 3.
Effect of age at onset of SLE on mortality and LN
Comparison of (A) mortality and (B) incidence of LN between patients diagnosed with SLE above and below the median age (28 years).
Whereas death was more likely to occur in the group diagnosed at an older age, the reverse was true for LN. LN developed in 107/250 (42.8%) of those diagnosed below the median age compared with 58/246 (23.6%) of those diagnosed above that age. Fig. 3B shows that this difference was significant (P < 0.0001).
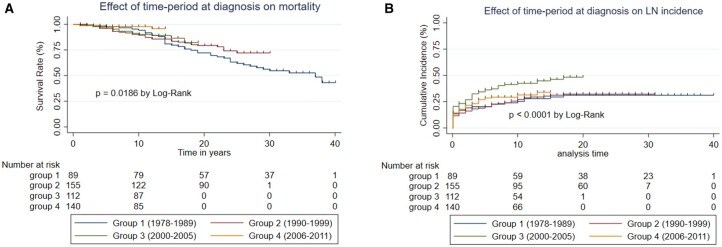
Effect of time of diagnosis
After stratification of the patients into four groups according to the date of starting follow-up, 39/89 (38.2%) of group 1, 34/155 (21.9%) of group 2, 14/112 (12.5%) of group 3 and 4/140 (2.9%) of group 4 had died during the period of observation. Fig. 4A shows an improvement in survival going from group 1 to group 4 that was statistically significant (P = 0.019).
Fig. 4.
Effect of time period of diagnosis on mortality and LN
Comparison of (A) mortality and (B) incidence of LN between patients diagnosed with SLE during the following time periods: group 1 (1978–89), group 2 (1990–99), group 3 (2000–5), group 4 (2006–11). A colour version is available online
LN occurred in 36/89 (40.4%) of group 1, 38/155 (24.5%) of group 2, 55/112 (49.1%) of group 3 and 36/140 (25.7%) of group 4. Fig. 4B shows a statistically significant increase in the diagnosis of LN in group 3 compared with the other groups (P < 0.05).
Using Cox proportional hazards analysis, there was a significantly lower risk of mortality for group 4 compared with group 1 [HR 0.22 (95% CI 0.07, 0.63), P = 0.005]. Significant differences were not seen for the other groups [group 2 vs group 1: HR 0.69 (95% CI 0.42, 1.11), group 3 vs group 1: HR 0.63 (95% CI 0.43, 1.18)].
Discussion
This is the largest survival analysis study ever undertaken in a British SLE cohort. By retrospective analysis of data from 496 patients, with representation of four ethnic groups, we have obtained new insights into the effects of sex, ethnicity, age at onset and time period of diagnosis.
Our results concur with studies from different units showing no difference in mortality between men and women with SLE [4, 7, 8, 12, 15, 19, 20]. A limitation is that there may have been too few men in our study to detect an increase in mortality or development of LN. Studies showing higher mortality in men with SLE [11, 13, 17, 18] have been from single centres (like our study), so the effect may be centre dependent. Wu et al. [17] considered the possibility that the apparent increased male mortality could be due to worse renal function and older age at onset in men in their cohort. In a large study (1979 patients, 157 men), Tan et al. [18] reported that 11.5% of men but 6.2% of women died. However, both Bultink et al. [19] in Britain and Singh and Yen [20] in the USA, reviewing census data of SLE deaths from national databases, concluded that women had higher mortality than men (though in the latter case this was not statistically significant). Unlike other studies [12, 18, 29], we did not find increased LN in men, but the number of men with LN in those studies was much higher than in ours. Feldman et al. [35] focussed on patients with incident LN identified from the Medicaid Analytic Extract from 29 American states. In this large study, 2467 women and 283 men were identified and 5 year cumulative mortality was 9.4% in men and 9.8% in women with no difference in mortality or end-stage renal disease between the sexes.
We found that non-Caucasian patients were not more likely to die than Caucasians. In fact, the only data showing that black patients with SLE, in particular, have a higher risk of mortality come from the USA [18, 21, 22]. The population studied by Lim et al. [21] in Georgia was notable for a very high proportion of black patients (76%) and a high death rate (401/1353), especially among black patients. In Baltimore, Tan et al. [18] studied 1979 patients (131 deaths, 77in black people and 54 in Caucasians) and concluded that black patients of both sexes had an increased risk of death. In the USA, however, associations between ethnicity, poverty and access to healthcare may mean that relationships between ethnicity and health outcomes may not be representative of other countries [36]. Guo et al. [22] pointed out that all-cause mortality is also significantly higher for black than white American women. Singh and Yen [20] found that relationships between ethnicity and mortality in SLE depended on geographical location within the USA and, in particular, that higher mortality occurred in areas with higher poverty rates. Only one study outside the USA has looked at the effect of ethnicity on mortality in patients with SLE. Hendler et al. [26] studied 600 patients in Brazil, 54 of whom died. However, ethnicity was only described in terms of European descent and was not associated with mortality in multivariate analysis. In the UK, although there are socio-economic differences between ethnic groups, healthcare is free at the point of delivery. Based on our findings, we therefore suggest that socio-economic factors and access to healthcare are likely to have been more important than genetic factors in the reports of higher mortality in black patients with SLE in the USA. We recognize, however, that the genetic background of African Americans is different from that of Afro-Caribbean patients in the UK.
We confirmed findings from other groups that LN is more common in non-Caucasian patients [18, 29]. The reasons for this difference are not fully understood. The fact that it was not reflected in increased deaths in non-Caucasians may be due to improved treatment of LN. Only four patients within our cohort died from renal lupus, although 35/91 patients who died from any cause had a previous history of LN [24].
Patients diagnosed with SLE earlier in life, particularly those with juvenile onset [5, 37], tend to have more active disease and a higher prevalence of major organ involvement, such as LN. The situation with regard to mortality depends on the method of analysis used. Articles that cite SMR almost invariably show higher SMRs in younger age groups [4–7, 10, 12, 25], where the death rate among the non-lupus population is very low. Articles that do not use SMR and/or describe cumulative deaths by means of survival analysis, however, show that patients diagnosed later in life are more likely to die within a given time period [17, 26]. Our results support these previous findings. Patients diagnosed with SLE below the median age were more likely to develop LN, but less likely to die during the period of observation. However, it may be that our follow-up is still not long enough to reach firm conclusions about this point. For example, damage due to early aggressive treatment of SLE may take many decades to impact on mortality.
The concept that mortality from SLE has improved over time has been supported by numerous studies in which authors subdivided cohorts of patients with SLE according to the time period of diagnosis and reported lower SMRs in the patients diagnosed more recently. Bernatsky et al. [4], studying 9547 patients, reported an SMR of 4.9 in those diagnosed between 1970 and 1979 but 2.0 in those diagnosed between 1990 and 2001. In 442 patients from Hong Kong, Mok et al. [6] reported a decrease in SMR from 7.88 to 2.17 between 2000 and 2006. In 1241 Canadian patients, Urowitz et al. [11] showed that the SMR was 12.6 for those diagnosed between 1970 and 1978 and 3.46 for those diagnosed between 1997 and 2005. Conversely, Ingvarsson et al. [9] found no improvement in mortality over time in 175 Swedish patients followed from 1981 to 2014 and a review of 125 studies of adult SLE patients suggested that improvement might have plateaued after the mid-1990s [3].
Our study has the advantages of follow-up until 2019 and the generation of survival curves for the different time groups. Fig. 4B clearly shows an improvement over time, which is statistically significant comparing group 4 with group 1. Reasons for this improvement, as discussed by other authors, could include diagnosis of milder cases and improvements in therapy [3]. The latter explanation would suggest that deaths from lupus itself (rather than infection, cancer or cardiovascular disease) should be less frequent in later cohorts, which has been shown by some authors [4, 7]. In our study, however, very few deaths were due directly to SLE, so we can make no such conclusion.
We were surprised by the finding that cases of LN were significantly higher in group 3 than the other groups. On further examination of the data, it seems likely that this is a coincidental finding due to the fact that significantly more African/Caribbean patients entered the cohort during the period 2000–2005 than at other times. Thus the proportions of African/Caribbean patients were 14/89 (15.7%) in group 1, 21/155 (13.5%) in group 2, 26/112 (32.1%) in group 3 and 27/140 (19.3%) in group 4.
Limitations of the study include the fact that it is from a single centre and, since the data were gathered over a period of many years, the results may have been affected by changes in referral practice, physicians and standards of care. Since this is a rheumatology department, patients with LN and advanced kidney failure may not be referred. This is the group with the highest mortality. Very few of our patients died of LN.
In conclusion, this very large, long-duration survival analysis study in an ethnically diverse British cohort of patients with SLE has shown that mortality does seem to be improving in patients diagnosed more recently. Greater mortality in African American patients with SLE may be a specific USA finding related to socio-economic factors.
Acknowledgements
F.F. was supported by Fundação para a Ciência e a Tecnologia (IP grant DFA/BD/7131/2020) and LUPUS UK (grant 1543411). W.L., F.F., D.A.I. and A.R. collected data, contributed to critical analysis of the manuscript and approved the final version. W.L., F.F. and A.R. carried out statistical analysis. A.R. wrote the final manuscript.
Funding: This work was carried out at a centre supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre.
Disclosure statement: The authors have declared no conflicts of interest.
Contributor Information
Weike Luo, Centre for Rheumatology Research, Division of Medicine, University College London, London, UK.
Filipa Farinha, Centre for Rheumatology Research, Division of Medicine, University College London, London, UK.
David A Isenberg, Centre for Rheumatology Research, Division of Medicine, University College London, London, UK.
Anisur Rahman, Centre for Rheumatology Research, Division of Medicine, University College London, London, UK.
Data availability statement
Data are available upon request to the authors.
References
- 1. Rees F, Doherty M, Grainge M. et al. The incidence and prevalence of systemic lupus erythematosus in the UK, 1999-2012. Ann Rheum Dis 2016;75:136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yurkovich M, Vostretsova K, Chen W, Avina-Zubieta JA.. Overall and cause-specific mortality in patients with systemic lupus erythematosus: a meta-analysis of observational studies. Arthritis Care Res 2014;66:608–16. [DOI] [PubMed] [Google Scholar]
- 3. Tektonidou MG, Lewandowski LB, Hu J, Dasgupta A, Ward MM.. Survival in adults and children with systemic lupus erythematosus: a systematic review and Bayesian meta-analysis of studies from 1950 to 2016. Ann Rheum Dis 2017;76:2009–16. [DOI] [PubMed] [Google Scholar]
- 4. Bernatsky S, Boivin JF, Joseph L. et al. Mortality in systemic lupus erythematosus. Arthritis Rheum 2006;54:2550–7. [DOI] [PubMed] [Google Scholar]
- 5. Hersh AO, Trupin L, Yazdany J. et al. Childhood-onset disease as a predictor of mortality in an adult cohort of patients with systemic lupus erythematosus. Arthritis Care Res 2010;62:1152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mok CC, To CH, Ho LY, Yu KL.. Incidence and mortality of systemic lupus erythematosus in a southern Chinese population, 2000–2006. J Rheumatol 2008;35:1978–82. [PubMed] [Google Scholar]
- 7. Bjornadal L, Yin L, Granath F, Klareskog L, Ekbom A.. Cardiovascular disease a hazard despite improved prognosis in patients with systemic lupus erythematosus: results from a Swedish population based study 1964–95. J Rheumatol 2004;31:713–9. [PubMed] [Google Scholar]
- 8. Bernatsky S, Clarke A, Gladman DD. et al. Mortality related to cerebrovascular disease in systemic lupus erythematosus. Lupus 2006;15:835–9. [DOI] [PubMed] [Google Scholar]
- 9. Ingvarsson RF, Landgren AJ, Bengtsson AA, Jonsen A.. Good survival rates in systemic lupus erythematosus in southern Sweden, while the mortality rate remains increased compared with the population. Lupus 2019;28:1488–94. [DOI] [PubMed] [Google Scholar]
- 10. Chun BC, Bae SC.. Mortality and cancer incidence in Korean patients with systemic lupus erythematosus: results from the Hanyang lupus cohort in Seoul, Korea. Lupus 2005;14:635–8. [DOI] [PubMed] [Google Scholar]
- 11. Urowitz MB, Gladman DD, Tom BDM, Ibañez D, Farewell VT.. Changing patterns in mortality and disease outcomes for patients with systemic lupus erythematosus. J Rheumatol 2008;35:2152–8. [DOI] [PubMed] [Google Scholar]
- 12. Jacobsen S, Petersen J, Ullman S. et al. A multicentre study of 513 Danish patients with systemic lupus erythematosus. II. Disease mortality and clinical factors of prognostic value. Clin Rheumatol 1998;17:478–84. [DOI] [PubMed] [Google Scholar]
- 13. Alamanos Y, Voulgari PV, Papassava M. et al. Survival and mortality rates of systemic lupus erythematosus patients in northwest Greece. Study of a 21-year incidence cohort. Rheumatology (Oxford) 2003;42:1122–3. [DOI] [PubMed] [Google Scholar]
- 14. Mok CC, Kwok CL, Ho LY, Chan PT, Yip SF.. Life expectancy, standardized mortality ratios, and causes of death in six rheumatic diseases in Hong Kong, China. Arthritis Rheum 2011;63:1182–9. [DOI] [PubMed] [Google Scholar]
- 15. Mu L, Hao Y, Fan Y. et al. Mortality and prognostic factors in Chinese patients with systemic lupus erythematosus. Lupus 2018;27:1742–52. [DOI] [PubMed] [Google Scholar]
- 16. Altman DG. Practical statistics for medical research. London: Chapman & Hall, 1991. [Google Scholar]
- 17. Wu G, Jia X, Gao D, Zhao Z.. Survival rates and risk factors for mortality in systemic lupus erythematosus patients in a Chinese center. Clin Rheumatol 2014;33:947–53. [DOI] [PubMed] [Google Scholar]
- 18. Tan TC, Fang H, Magder LS, Petri MA.. Differences between male and female systemic lupus erythematosus in a multiethnic population. J Rheumatol 2012;39:759–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bultink IEM, de Vries F, van Vollenhoven RF, Lalmohamed A.. Mortality, causes of death and influence of medication use in patients with systemic lupus erythematosus vs matched controls. Rheumatology (Oxford) 2021;60:207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singh RR, Yen EY.. SLE mortality remains disproportionately high, despite improvements over the last decade. Lupus 2018;27:1577–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lim SS, Helmick CG, Bao G. et al. Racial disparities in mortality associated with systemic lupus erythematosus – Fulton and Dekalb counties, Georgia, 2002–2016. MMWR Morb Mortal Wkly Rep 2019;68:419–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guo Q, Liang M, Duan J. et al. Age differences in secular trends in black-white disparities in mortality from systemic lupus erythematosus among women in the United States from 1988 to 2017. Lupus 2021;30:715–24. [DOI] [PubMed] [Google Scholar]
- 23. Yee CS, Su L, Toescu V. et al. Birmingham SLE cohort: outcomes of a large inception cohort followed for up to 21 years. Rheumatology (Oxford) 2015;54:836–43. [DOI] [PubMed] [Google Scholar]
- 24. Lorenzo-Vizcaya A, Isenberg D.. Analysis of trends and causes of death in SLE patients over a 40-years period in a cohort of patients in the United Kingdom. Lupus 2021;30:702–6. [DOI] [PubMed] [Google Scholar]
- 25. To CH, Mok CC, Tang SS. et al. Prognostically distinct clinical patterns of systemic lupus erythematosus identified by cluster analysis. Lupus 2009;18:1267–75. [DOI] [PubMed] [Google Scholar]
- 26. Hendler JV, de Souza L, de Freitas Zernow DC. et al. Survival analysis of patients with systemic lupus erythematosus in a tertiary hospital in southern Brazil. Clin Rheumatol 2017;36:2005–10. [DOI] [PubMed] [Google Scholar]
- 27. Urowitz MB, Gladman DD, Abu-Shakra M, Farewell VT.. Mortality studies in systemic lupus erythematosus. Results from a single center. III. Improved survival over 24 years. J Rheumatol 1997;24:1061–5. [PubMed] [Google Scholar]
- 28. Yap DY, Tang CS, Ma MK, Lam MF, Chan TM.. Survival analysis and causes of mortality in patients with lupus nephritis. Nephrol Dial Transplant 2012;27:3248–54. [DOI] [PubMed] [Google Scholar]
- 29. Hanly JG, O’Keeffe AG, Su L. et al. The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatology (Oxford) 2016;55:252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lim SS, Bayakly AR, Helmick CG. et al. The incidence and prevalence of systemic lupus erythematosus, 2002–2004: the Georgia Lupus Registry. Arthritis Rheumatol 2014;66:357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Somers EC, Marder W, Cagnoli P. et al. Population-based incidence and prevalence of systemic lupus erythematosus: the Michigan Lupus Epidemiology and Surveillance program. Arthritis Rheumatol 2014;66:369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 33. Weening JJ, D’Agati VD, Schwartz MM. et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol 2004;15:241–50. [DOI] [PubMed] [Google Scholar]
- 34. Gisca E, Duarte L, Farinha F, Isenberg DA.. Assessing outcomes in a lupus nephritis cohort over a 40-year period. Rheumatology (Oxford) 2021;60:1814–22. [DOI] [PubMed] [Google Scholar]
- 35. Feldman CH, Broder A, Guan H, Yazdany J, Costenbader KH.. Sex differences in health care utilization, end-stage renal disease, and mortality among Medicaid beneficiaries with incident lupus nephritis. Arthritis Rheumatol 2018;70:417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Duran S, Apte M, Alarcon GS, Lumina Study Group. Poverty, not ethnicity, accounts for the differential mortality rates among lupus patients of various ethnic groups. J Natl Med Assoc 2007;99:1196–8. [PMC free article] [PubMed] [Google Scholar]
- 37. Ambrose N, Morgan TA, Galloway J. et al. Differences in disease phenotype and severity in SLE across age groups. Lupus 2016;25:1542–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request to the authors.