Abstract
Objectives
The cytokine oncostatin M (OSM) is implicated in the pathology of SSc. Inhibiting OSM signalling using GSK2330811 (an anti-OSM monoclonal antibody) in patients with SSc has the potential to slow or stop the disease process.
Methods
This multicentre, randomized, double-blind, placebo-controlled study enrolled participants ≥18 years of age with active dcSSc. Participants were randomized 3:1 (GSK2330811:placebo) in one of two sequential cohorts to receive GSK2330811 (cohort 1: 100 mg; cohort 2: 300 mg) or placebo s.c. every other week for 12 weeks. The primary endpoint was safety; blood and skin biopsy samples were collected to explore mechanistic effects on inflammation and fibrosis. Clinical efficacy was an exploratory endpoint.
Results
Thirty-five participants were randomized to placebo (n = 8), GSK2330811 100 mg (n = 3) or GSK2330811 300 mg (n = 24). Proof of mechanism, measured by coordinate effects on biomarkers of inflammation or fibrosis, was not demonstrated following GSK2330811 treatment. There were no meaningful differences between GSK2330811 and placebo for any efficacy endpoints. The safety and tolerability of GSK2330811 were not favourable in the 300 mg group, with on-target, dose-dependent adverse events related to decreases in haemoglobin and platelet count that were not observed in the 100 mg or placebo groups.
Conclusion
Despite a robust and novel experimental medicine approach and evidence of target engagement, anticipated SSc-related biologic effects of GSK2330811 were not different from placebo and safety was unfavourable, suggesting OSM inhibition may not be a useful therapeutic strategy in SSc.
Trial registration number
ClinicalTrials.gov, NCT03041025; EudraCT, 2016-003417-95.
Keywords: biomarkers, fibrosis, GSK2330811, inflammation, monoclonal antibody, placebo-controlled trial, safety
Rheumatology key messages.
Preclinical evidence suggests that OSM is a cytokine implicated in the pathogenesis of SSc.
OSM inhibition by GSK2330811 did not modulate biomarkers of inflammation or fibrosis in SSc patients.
This study advances understanding of OSM biology and provides valuable insights into SSc trial design.
Introduction
Diffuse cutaneous SSc (dcSSc) is a systemic autoimmune rheumatic disorder characterized by dysregulated immune response and progressive fibrosis of the skin and internal organs [1, 2]. Nintedanib [3] and tocilizumab [4] are licenced for the treatment of SSc-associated interstitial lung disease (ILD) and haematopoietic stem cell transplantation can improve skin thickening, progression-free survival and long-term survival [5, 6]. However, effective disease-modifying therapies are needed [7].
Oncostatin M (OSM) is a member of the IL-6 cytokine family with a potentially important role in the pathogenesis of SSc. OSM levels are elevated in the serum of patients with SSc [8] and OSM receptor expression in the skin of patients with SSc is associated with more rapid progression of skin disease [9]. OSM induces a profibrotic response in human skin organoid and murine models [10–14] that may be driven in part by an increase in profibrotic M2-like macrophages [11].
GSK2330811, a humanized IgG1 monoclonal antibody, prevents OSM receptor binding. It was well tolerated in healthy volunteers following single s.c. doses of 0.1–6 mg/kg [15]. This is the first study of GSK2330811 in participants with SSc and investigated the safety, tolerability, pharmacokinetics (PK) and target engagement following repeat dosing. To explore mechanistic efficacy, we used multivariate modelling of biomarkers that reflect the anticipated mechanism of action of GSK2330811, representing a rigorous statistical framework for investigation of experimental medicine in complex immune-mediated disease.
Materials and methods
Additional methods are provided in the Supplementary Methods, available at Rheumatology online. Full study details are available in the protocol (Supplementary File S1, available at Rheumatology online).
Study design
This was a phase 2a, multicentre, randomized, double-blind (sponsor open), placebo-controlled, proof of mechanism study in adults with dcSSc conducted at 14 centres across four countries (Canada, the Netherlands, UK and USA) (Supplementary Table S1, available at Rheumatology online).
The study comprised a ≤6 week screening period, a 12 week treatment period and a 16 week post-treatment follow-up. Eligible participants were randomized (3:1) to six doses of s.c. GSK2330811 or placebo, administered every other week in one of two sequential cohorts, each with a block size of four: GSK2330811 100 mg or placebo (cohort 1) or GSK2330811 300 mg or placebo (cohort 2, stratified according to mycophenolate use).
Study population
Eligible participants were ≥18 years of age with active dcSSc and a modified Rodnan skin score (mRSS) ≥10 and ≤35 at screening and ≤5 years from onset of the first non-RP manifestation. Active SSc was defined as meeting at least one of the following criteria during screening: CRP ≥6 mg/l, disease duration ≤18 months (from the first non-RP manifestation), or any of the following within the previous 6 months: increase of ≥3 mRSS units, involvement of one new body area and an increase of ≥2 mRSS units, or involvement of two new body areas. Participants taking mycophenolate were eligible if they had been on a stable dosage for ≥3 months prior to the first dosing day and were willing to continue this dosage until the day 85 visit.
Endpoints
The primary endpoint was safety and tolerability. Secondary endpoints were PK, target engagement in blood and anti-drug antibodies (ADA). Exploratory endpoints included biomarkers of fibrosis, inflammation and vasculopathy in the blood and skin and clinical endpoints.
PK and target engagement
Blood samples were collected on days 1, 15, 29, 57, 85, 113, 155 and 197. A skin suction blister was optional for participants in cohort 2 and performed on day 57–85 as previously described [15]. The GSK2330811 concentration and free and total OSM in blood and skin blister fluid samples were analysed using validated assays [15]. Individual dosing information and parameters from a minimal physiologically based PK (mPBPK) and target engagement model [15] were used to simulate exposure and total and free OSM and derive the percentage target engagement [(1−free OSM/baseline OSM)*100].
Biomarkers
Four key biomarkers of inflammation and fibrosis were prespecified for analysis to determine proof of mechanism: alpha smooth muscle actin (αSMA) [16–19] measured by skin histology, 2-gene skin score (2GSSC) [19–21] derived from measurement of THBS1 and MS4A4A mRNA in the skin; procollagen type III N-terminal peptide (PIIINP) [17, 22–28] measured in serum and C-C motif chemokine ligand 2 (CCL2) [20, 21, 29] measured by mRNA in skin. These markers were selected based on the strength of evidence for modulation by OSM, differentiation between SSc and healthy populations, correlation with clinical endpoints and response to other therapies within 6 months [16, 19, 20, 28]. Serum IL-6, CRP and CCL2 levels and suppressor of cytokine signalling-3 (SOCS3) mRNA in skin were used as pharmacodynamic biomarkers of OSM signalling. Serum, plasma and skin punch biopsies were collected for biomarker assessment.
Clinical endpoints
Among others, mRSS and forced vital capacity (FVC) measured by respiratory laboratory tests (FVClab) and home spirometry (FVChome) were exploratory endpoints.
Statistical analyses
Sample size was based on feasibility, with the intention of randomizing 24–36 participants to cohort 2. No formal analyses were performed for safety. As the study was not designed for formal hypothesis testing, an estimation approach was taken. Bayesian analyses used non-informative priors, adjusting for mycophenolate use at day 1 and including terms for baseline. Longitudinal analysis was adjusted for visit, visit by treatment and visit by baseline interactions. Change from baseline to day 85 data for the four prespecified biomarkers (αSMA, 2GSSC, PIIINP and CCL2) were analysed using a multivariate model, adjusted for the corresponding baseline value and treatment. The study would be considered negative for proof of mechanism if the joint probability of a change from baseline for all combinations of three biomarkers was <50%. Spearman correlation coefficients were calculated for the baseline:change from baseline ratio by treatment group. No missing data were imputed.
Ethics approval and patient involvement
The study protocol was approved by each study site’s ethics committee or institutional review board, in accordance with the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), Good Clinical Practice (GCP) and applicable country-specific requirements.
Results
Participant disposition and baseline characteristics
The first participant enrolled on 22 June 2017 and the last participant completed the study on 7 July 2020. Of the 47 participants screened, 35 were eligible (Supplementary Fig. S1, available at Rheumatology online).
Of the 35 participants, 26 (74%) were women and the median disease duration from first non-RP symptom was 16 months (range 4–60). Most participants were taking mycophenolate at entry [n = 29 (83%)], with balanced use between the placebo and GSK2330811 300 mg groups. Study groups were similar at baseline, except the placebo group comprised fewer patients with ILD and had a higher mean FVC than the GSK2330811 300 mg group (Table 1; Supplementary Table S2, available at Rheumatology online).
Table 1.
Participant demographics and clinical characteristics at baseline (safety population, unless stated otherwise)
| Characteristics | Placebo (n = 8) | GSK2330811 100 mg (n = 3)a | GSK2330811 300 mg (n = 24) | Total (N = 35) |
|---|---|---|---|---|
| Age, years, mean (s.d.) | 50.8 (15.4) | 66.7 (NA) | 52.6 (12.6) | 53.4 (13.2) |
| Female, n (%) | 6 (75) | 1 (33) | 19 (79) | 26 (74) |
| Race, n (%) | ||||
| White | 7 (88) | 3 (100) | 20 (83) | 30 (86) |
| Black or African American | 0 | 0 | 3 (13) | 3 (9) |
| Asian | 1 (13) | 0 | 0 | 1 (3) |
| American Indian or Alaska Native | 0 | 0 | 1 (4) | 1 (3) |
| BMI, kg/m2, mean (s.d.) | 23.9 (4.1) | 27.9 (NA) | 29.4 (6.7) | 28.0 (6.4) |
| Time since onset of first non-Raynaud manifestation of disease, months, median (range) | 12.0 (7–21) | 14.0 (11–21) | 17.5 (4–60) | 16.0 (4–60) |
| Pulmonary fibrosisc, n (%) | 1 (13) | 1 (33) | 8 (33) | 10 (29) |
| Mycophenolate use at day 1, n (%) | 7 (88) | 2 (67) | 20 (83)a | 29 (83)a |
| Baseline mRSS | ||||
| Mean (s.d.) | 20.5 (4.5) | 32.0 (NA) | 20.5 (6.6) | 21.5 |
| Median (range) | 19 (16–29) | 31 (29–36) | 19 (11–32) | 19 (11–36) |
| Baseline FVC (% predicted)a | ||||
| Mean (s.d.) | 105 (23.1) | 104 (NA) | 87.7 (18.6) | – |
| Median (range) | 104 (79–155) | 105 (103–105) | 87 (54–129) | – |
| Baseline DLCO (% haemoglobin)a, corrected | ||||
| Mean (s.d.) | 78.8 (22.7) | 89.0 (NA) | 72.6 (18.7) | – |
| Median (range) | 72 (55–126) | 88 (80–99) | 69 (44–123) | – |
| Baseline CRP, mg/L,a median (range) | 2.0 (0.1–11.6) | 2.1 (0.7–3.3) | 1.7 (0.2–82.5) | – |
| Baseline PhGA,a median (range) | 3.5 (2–7) | 2.0 (2–6) | 4.0 (0–7) | – |
| Baseline PtGA,a,d median (range) | 2.0 (0–5) | 0 | 5.0 (1–8) | – |
| Baseline autoantibody profile, n (%) | ||||
| ANA | 8 (100) | 3 (100) | 21 (88) | 32 (91) |
| Anti-RNA polymerase III | 5 (63) | 2 (67) | 8 (33) | 15 (43) |
| Anti-Scl-70 | 2 (25) | 1 (33) | 4 (17) | 7 (20) |
| Anti-centromere | 1 (13) | 0 | 1 (4) | 2 (6) |
Data from the per-protocol population where one participant in the GSK2330811 300 mg group was excluded (n = 23).
s.d. not calculated in the GSK2330811 100 mg group, as there were only three participants.
Confirmed by high-resolution CT.
Data missing from one participant in the placebo group (n = 7) and two participants in the GSK2330811 100 mg group (n = 1).
DLCO: diffusing capacity of the lungs for carbon monoxide; NA: not applicable; PhGA: Physician’s Global Assessment; PtGA: Patient’s Global Assessment.
PK, target engagement and anti-drug antibodies
GSK2330811 plasma concentrations are shown in Supplementary Table S3, available at Rheumatology online. GSK2330811 concentrations in the two available skin blister fluid samples in the 300 mg group were 3293 ng/ml and 11 154 ng/ml.
Model parameter values from the mPBPK model are shown in Supplementary Table S4, available at Rheumatology online. The value of the model-predicted baseline OSM parameter was updated to reflect serum baseline OSM measured in this study (geometric mean 0.000957 nM; geometric coefficient of variation 59%). During the on-treatment phase, free OSM levels in serum were below the lower limit of quantitation (LLQ) in the 300 mg group, therefore the model-predicted free OSM concentration was used to derive target engagement. Mean serum total OSM levels increased after GSK2330811 dosing, suggesting GSK2330811–OSM binding and target engagement [15] (Supplementary Fig. S2A, available at Rheumatology online). Total:baseline OSM ratios for the 300 mg group are shown in Supplementary Table S5, available at Rheumatology online. The median (80% interval) model-derived target engagement in serum for this group was estimated at 91% (range 81–94%) on day 77. In the skin blister fluid, free OSM from two participants in the 300 mg group were below the LLQ; their total OSM values were 249.13 and 199.28 pg/ml.
Model-derived target engagement was predicted to be 87% (range 77–92) in well-perfused tissues and 67% (range 51–78) in poorly perfused tissues, but too few skin blister fluid samples were collected to estimate this directly for fibrotic skin.
In the GSK2330811 300 mg group, two (9%) participants were positive for ADA at any time post-baseline and all were negative for ADA at their final study visit. In the placebo and 100 mg groups, no participants were ADA positive any time post-baseline.
Biomarkers
Despite evidence of target engagement in serum following administration of GSK2330811 300 mg, there were no changes from baseline in SOCS3 in the skin (Supplementary Fig. S2B, available at Rheumatology online) or IL-6, CRP or CCL2 in serum (Supplementary Fig. S2C–E, available at Rheumatology online). No changes were observed for other disease and OSM-related genes analysed in the skin (Supplementary Table S6, available at Rheumatology online).
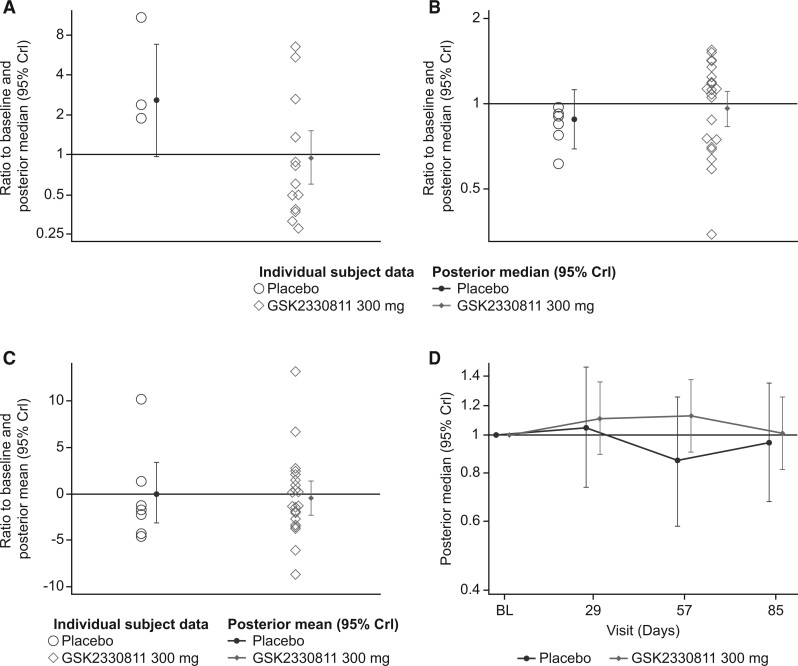
No reduction of potential markers of inflammation or fibrosis (αSMA, CCL2, 2GSSC and PIIINP) was observed from baseline to day 85 for the GSK2330811 300 mg group (Fig. 1). The jointly modelled probability that, based on the observed data, the reduction from baseline in at least three of four of these biomarkers was non-zero was 37%, which did not meet the prespecified 50% threshold. Although a difference from placebo was observed for αSMA, only three participants in the placebo group had sufficient skin biopsy tissue. Changes in key individual biomarkers following GSK2330811 300 mg were not well correlated (correlation coefficient <0.4; Supplementary Table S7, available at Rheumatology online); results from the univariate and multivariate models were similar for the fitted estimates (Supplementary Table S8, available at Rheumatology online) and the probability of a change in the biomarkers (Supplementary Table S9, available at Rheumatology online).
Fig. 1.
(A–C) Ratio to baseline at day 85 and (D) including visits up to day 85a
(A–C) Posterior medians are based on a Bayesian analysis at day 85 only. (D) Posterior medians are based on a Bayesian longitudinal analysis. aPer protocol population. αSMA: alpha smooth muscle actin; CCL2: C-C motif chemokine ligand 2; PIIINP: procollagen type III N-terminal peptide;
2GSSC: 2-gene skin score; BL: baseline; CrI: credible interval.
Of 22 biomarkers analysed in blood, only 3 (tissue inhibitor of metalloproteinases 1, soluble vascular cell adhesion molecule-1, vascular endothelial growth factor) showed a marginal change (≤1.3-fold) following GSK2330811 300 mg dosing that was not seen in the placebo group (Supplementary Fig. S3, available at Rheumatology online). GSK2330811 showed no effect on M1 and M2 macrophage and fibrosis gene signatures in skin (Supplementary Fig. S4 and Table S10, available at Rheumatology online).
Clinical endpoints
The mean change from baseline mRSS at day 85 showed no difference between the GSK2330811 300 mg and placebo groups (Table 2). No meaningful differences between the GSK2330811 300 mg and placebo groups were reported for any other clinical endpoint, including FVChome (Supplementary Fig. S5, available at Rheumatology online) or measures of participants’ health (Table 2; Supplementary Table S11, available at Rheumatology online). A post hoc analysis indicated that FVChome and FVClab measurements were highly correlated (correlation coefficients 0.8 at baseline and 0.9 at day 85) (Supplementary Fig. S6, available at Rheumatology online). Despite the small sample, an absence of outliers at week 12 suggests improvement in the home FVC technique over time.
Table 2.
Mean change from baseline to day 85 in exploratory efficacy endpoints (per protocol populationa)
| Efficacy endpoint | Placebo (n = 8) | GSK2330811 300 mg (n = 23) | Difference from placebo |
|---|---|---|---|
| mRSS | |||
| n | 8 | 21 | – |
| Change from baseline, posterior mean (95% CrI) | −3.13 (−6.20, −0.07) | −2.01 (−3.82, −0.20) | 1.12 (−2.43, 4.72) |
| Probability <0, % | 98 | 98 | 27 |
| FVClab | |||
| n | 8 | 20 | – |
| Change from baseline, posterior mean (95% CrI) | 0.04 (−0.20, 0.28) | 0.06 (−0.09, 0.21) | 0.02 (−0.27, 0.30) |
| Probability >0, % | 64 | 80 | 56 |
| ACR CRISS | |||
| n | 6 | 19 | – |
| Predicted probability of improvement from baseline, median (IQR) | 0.11 (0.00, 0.60) | 0.01 (0.00, 0.06) | – |
| HAQ-DI | |||
| n | 8 | 22 | – |
| Change from baseline, posterior mean (95% CrI) | 0.01 (−0.22, 0.25) | 0.05 (−0.10, 0.19) | 0.03 (−0.24, 0.31) |
| Probability <0, % | 45 | 26 | 41 |
| PhGA | |||
| n | 8 | 22 | – |
| Mean change from baseline (s.d.) | −0.8 (1.67) | 0.3 (2.45) | – |
| PtGA | |||
| n | 6 | 22 | – |
| Mean change from baseline (s.d.) | 1.0 (2.10) | 0.6 (2.04) | – |
Data for 100 mg group not shown.
CRISS: combined response index for systemic sclerosis; CrI: credible interval; HAQ-DI: HAQ-Disability Index; PhGA: Physician’s Global Assessment; PtGA: Patient’s Global Assessment.
Safety
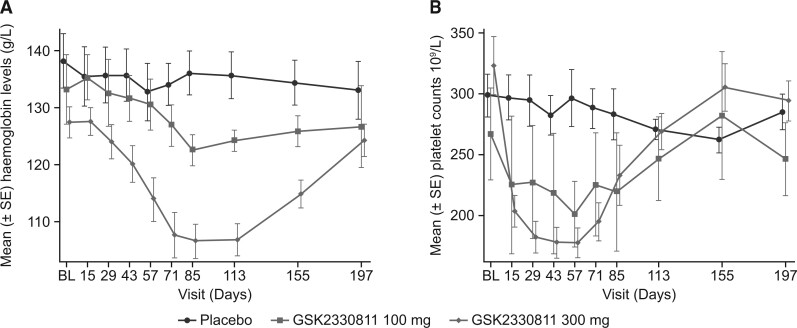
All participants reported at least one adverse event (AE) during the on-treatment phase (Supplementary Table S12, available at Rheumatology online). In the 300 mg group, nine (39%) participants experienced AEs of decreased haemoglobin (5 participants) or anaemia (4 participants). Laboratory values showed dose-dependent reductions in haemoglobin that worsened over time during treatment with GSK2330811 100 mg and 300 mg, with a median time to nadir in the 300 mg group at day 84 (range 16–154) and recovery off-treatment to near baseline by the end of follow-up (Fig. 2A). Two (25%) participants receiving placebo and 20 (87%) participants receiving 300 mg had an increase (worsening) of at least one grade for anaemia based on the Common Terminology Criteria for AEs (CTCAE). Of these, two (9%) participants receiving 300 mg worsened to grade 3 anaemia (haemoglobin <80 g/l), necessitating discontinuation of treatment as per protocol. However, the CTCAE anaemia grade was imbalanced at baseline, with nine (38%) participants in the 300 mg group having grade 1 anaemia pre-dose [haemoglobin <lower limit of normal (LLN) − 100 g/l] compared with none in the other groups.
Fig. 2.
Mean (A) blood haemoglobin levels and (B) platelet counts (safety population)
BL: baseline.
Decrease in mean platelet count was dose dependent. Six (26%) participants experienced AEs of either a platelet count decrease (13%) or thrombocytopenia (13%). In the 300 mg group, nine (39%) participants had an increase (worsening) in thrombocytopenia to grade 1 (platelet count <LLN − 75 × 109/l), with a median time to nadir at day 42 (range 16–84). Platelet counts recovered to baseline values by the end of follow-up (Fig. 2B). Changes in reticulocyte counts, erythropoietin and thrombopoietin were also treatment group dependent (Supplementary Fig. S7, available at Rheumatology online).
The mean total neutrophil counts decreased in the 300 mg group compared with placebo (Supplementary Fig. S7D, available at Rheumatology online); three participants (13%) worsened to grade 1 neutropenia (<LLN − 1.5 × 109/l), one participant (4%) worsened to grade 3 neutropenia (<1 − 0.5 × 109/l) and one participant (13%) in the placebo group worsened to grade 2 neutropenia.
Four (17%) participants in the 300 mg group permanently discontinued study treatment or withdrew from the study due to an AE, compared with none in the placebo or 100 mg group (Supplementary Table S13, available at Rheumatology online).
During the on-treatment phase, one participant in the 300 mg group experienced serious AEs (SAEs) of atrial fibrillation, mild hypotension and severe pericarditis. During follow-up, one participant in the placebo group experienced an infected digital ulcer. Two participants in the 300 mg group reported SAEs after completing participation in the study: one was diagnosed with urothelial cell cancer 236 days after the last dose and one was diagnosed with renal cell carcinoma 533 days after the last dose of GSK2330811. All SAEs were considered by the investigator to be unrelated or unlikely related to GSK2330811. No participant died during the study.
Discussion
As well as being the first repeat-dosing study of the anti-OSM monoclonal antibody GSK2330811 in participants with SSc, this study incorporated innovative study design elements to address well-highlighted challenges of conducting randomized controlled trials in SSc [30–32]. The multivariate modelling of predefined, carefully selected biomarkers to assess proof of mechanism avoids an inflated type I error, which can result when considering endpoints independently, and exemplifies the potential of an experimental medicine approach combined with a rigorous statistical framework in complex immune-mediated disease. Assessment of target engagement of GSK2330811 to OSM in the skin compartment using a skin suction blister model [15] and home FVC measurement are additional contributions towards innovation in SSc trial design.
Exposure of GSK2330811 in plasma was consistent with modelled expectations and confirmed target engagement in serum [15]. Skin blister fluid data were limited, but demonstrated that GSK2330811 reached the skin. Despite this, proof of mechanism, as measured by a coordinated effect on biomarkers of inflammation and fibrosis in skin and serum, was not demonstrated following treatment with GSK2330811. A correlation between OSM and CRP is well described [33], and SOCS3 (a signalling molecule downstream of OSM and IL-6) was downregulated by the IL-6 receptor blocker tocilizumab in the faSScinate study [20]. The lack of anticipated effect of GSK2330811 on CRP and SOCS3 implies possible redundancy in OSM signalling (e.g. IL-6). While OSM pathways are known to be activated in fibrosis and SSc [10, 13, 34], OSM blockade alone may be inadequate to ameliorate dcSSc disease pathology.
High background use of mycophenolate, which was excluded in faSScinate, may have dampened the effects of GSK2330811. Other hypotheses include the potential for lower GSK2330811 levels in fibrotic skin, leading to insufficient skin target engagement, and differential effects of OSM inhibition on signalling through its two receptors. If the low-affinity leukaemia inhibitory factor receptor (LIFR) mediates haemoglobin and platelet effects, and the high-affinity OSM receptor mediates targeted skin effects, increased OSM inhibition may elicit LIFR-mediated AEs before achieving potentially advantageous inhibition of OSM receptor signalling [35].
The safety of GSK2330811 was not favourable at 300 mg based on the observed degree of anaemia, thrombocytopenia and mild neutropenia. Anaemia and thrombocytopenia are consistent with a known role of OSM in bone marrow [36–38] and were reported in the phase 1 study of GSK2330811 in healthy participants [15]. However, anaemia was more pronounced than anticipated. Elevation of thrombopoietin and erythropoietin and the time course of reticulocytosis provide mechanistic insights into potential compensatory mechanisms following GSK2330811 treatment.
The study was limited by the small number of samples available for αSMA analysis; insufficient blister fluid samples for measurement of target engagement; unequal randomization of underlying ILD, FVC, autoantibodies and anaemia at baseline; and high background mycophenolate usage. Most participants were positive for ANA and anti-RNA polymerase III was the most common SSc-specific reactivity, reflecting recruitment of patients with early severe dcSSc in the USA and UK. Generalizability to other ANA subgroups may be limited but is likely irrelevant for interpretation of a negative trial. While 12 weeks was likely too short a treatment period to observe changes in clinical endpoints, in the absence of effects on inflammation and fibrosis biomarkers it is unlikely that a longer study would show clinical efficacy.
Although these data do not support further clinical development of GSK2330811 in SSc, this study advances our understanding of OSM biology and introduces innovations in early phase clinical study design in SSc.
Supplementary Material
Acknowledgements
The authors would like to thank the study participants as well as the study investigators, study coordinators, the study site teams (Supplementary Table S1, available at Rheumatology online) and the GlaxoSmithKline study teams. Editorial support (in the form of writing assistance, including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating authors’ comments for each draft, assembling tables and figures, grammatical editing and referencing) was provided by Hayley Butler, at Fishawack Indicia, UK, part of Fishawack Health, and was funded by GlaxoSmithKline.
Funding: This study (201247) was funded by GlaxoSmithKline (NCT03041025; Proof of Mechanism Study of GSK2330811 in Diffuse Cutaneous Systemic Sclerosis).
Disclosure statement: C.D. reports grants and personal fees from GlaxoSmithKline and CSL Behring; grants from Inventiva, Servier and Arxx Therapeutics; and personal fees from Bayer, Sanofi, Boehringer Ingelheim, Roche, Bristol-Myers Squibb, Corbus, Acceleron and Horizon. D.E.F. reports grants and research fees from Actelion, Amgen, Bristol-Myers Squibb, Corbus, Galapagos GlaxoSmithKline, National Institutes of Health (NIH), Novartis, Pfizer, Sanofi and Roche/Genentech and consultancy fees from Actelion, Amgen, Bristol-Myers Squibb, Corbus, Galapagos Novartis and Pfizer. D.K. reports personal fees from Acceleron, Actelion, Amgen, Bayer, Boehringer Ingelheim, CSL Behring, Corbus, Genentech/Roche, Horizon and Mitsubishi Tanabe Pharma and is Chief Medical Officer of Eicos Sciences, a subsidiary of CiviBioPharma, and has stock options. F.D.G. received research grant support and consultancy fees from AbbVie, AstraZeneca, Boehringer Ingelheim, Capella Biosciences, Chemomab, Kymab, Mitsubishi-Tanabe and Janssen. J.V. reports research grants from the NIH, Rheumatology Research Foundation, TeneoBio, Sun Pharma and Boehringer Ingelheim and consultancy fees from Emerald Therapeutics, Boehringer Ingelheim, TeneoBio, Arxx Therapeutics and Ribon Pharma. L.C. has received consulting fees from Boehringer Ingelheim, Bristol-Myers Squibb, Eicos, Genentech and Mitsubishi-Tanabe and served on the data and safety monitoring board for Reata. S.R.J. reports grants from Bayer, Boehringer Ingelheim and Corbus and consulting fees from Boehringer Ingelheim and Eicos. M.C.V. reports grants and personal fees from Boehringer Ingelheim, Bristol-Myers Squibb, Corbus, GlaxoSmithKline, Janssen, MSD, Novartis and Roche and grants from Boehringer Ingelheim, Ferrer, Galapagos and Janssen. J.T., C.Z., E.C., A.L., N.W., S.M.F. and J.R. are employees of GlaxoSmithKline and hold stock/shares in the company. The study protocol (File S1) was approved by each study site’s ethics committee or institutional review board, in accordance with the ICH, GCP and applicable country-specific requirements.
Contributor Information
Christopher P Denton, Centre for Rheumatology and Connective Tissue Diseases, Division of Medicine, University College London, London.
Francesco del Galdo, Institute of Rheumatic and Musculoskeletal Medicine, and Biomedical Research Centre, University of Leeds, Leeds, UK.
Dinesh Khanna, Scleroderma Program, University of Michigan, Ann Arbor, MI, USA.
Madelon C Vonk, Department of Rheumatology, Radboud University Medical Center, Nijmegen, The Netherlands.
Lorinda Chung, Stanford University School of Medicine and Palo Alto VA Health Care System, Palo Alto, CA, USA.
Sindhu R Johnson, Toronto Scleroderma Program, Toronto Western Hospital; Mount Sinai Hospital, Institute of Health Policy, Management and Evaluation, University of Toronto, Toronto, ON, Canada.
John Varga, Scleroderma Program, University of Michigan, Ann Arbor, MI, USA; Department of Medicine, Feinberg School of Medicine, Northwestern University, Chicago, IL.
Daniel E Furst, University of California, Los Angeles, Los Angeles, CA; University of Washington, Seattle, WA, USA; University of Florence, Florence, Italy.
Jane Temple, GlaxoSmithKline, Uxbridge.
Chiara Zecchin, GlaxoSmithKline, Stevenage, UK.
Eszter Csomor, GlaxoSmithKline, Stevenage, UK.
Amy Lee, GlaxoSmithKline, Mississauga, Canada.
Nicolas Wisniacki, GlaxoSmithKline, Stevenage, UK.
Shaun M Flint, GlaxoSmithKline, Stevenage, UK.
Juliet Reid, GlaxoSmithKline, Stevenage, UK.
Data availability statement
Anonymised individual participant data and additional study documents can be requested for further research from www.clinicalstudydatarequest.com.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Denton CP, Khanna D.. Systemic sclerosis. Lancet 2017;390:1685–99. [DOI] [PubMed] [Google Scholar]
- 2. Stern EP, Denton CP.. The pathogenesis of systemic sclerosis. Rheum Dis Clin North Am 2015;41:367–82. [DOI] [PubMed] [Google Scholar]
- 3. Electronic Medicines Compendium. Ofev 100 mg soft capsules. 2021. https://www.medicines.org.uk/emc/product/1786/smpc (14 July 2021, date last accessed).
- 4. US Food and Drug Administration. ACTEMRA prescribing information. 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125276s131lbl.pdf (27 July 2021, date last accessed).
- 5. Sullivan KM, Goldmuntz EA, Keyes-Elstein L. et al. Myeloablative autologous stem-cell transplantation for severe scleroderma. N Engl J Med 2018;378:35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Laar JM, Farge D, Sont JK. et al. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical trial. JAMA 2014;311:2490–8. [DOI] [PubMed] [Google Scholar]
- 7. Johnson SR, Furst DE.. Current therapeutic approaches in scleroderma: clinical models of effective antifibrotic therapies. Curr Treatm Opt Rheumatol 2020;6:382–93. [Google Scholar]
- 8. Feeney M, Syed F, Khan K. et al. Oncostatin M as a potential molecular target in systemic sclerosis. Arthritis Rheumatol 2015;67: 2297–8. [Google Scholar]
- 9. Stifano G, Sornasse T, Rice LM. et al. Skin gene expression is prognostic for the trajectory of skin disease in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheumatol 2018;70:912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marden G, Wan Q, Wilks J. et al. The role of the oncostatin M/OSM receptor beta axis in activating dermal microvascular endothelial cells in systemic sclerosis. Arthritis Res Ther 2020;22:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ayaub EA, Dubey A, Imani J. et al. Overexpression of OSM and IL-6 impacts the polarization of pro-fibrotic macrophages and the development of bleomycin-induced lung fibrosis. Sci Rep 2017;7:13281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Botelho FM, Rodrigues R, Guerette J. et al. Extracellular matrix and fibrocyte accumulation in BALB/c mouse lung upon transient overexpression of oncostatin M. Cells 2019;8:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mozaffarian A, Brewer AW, Trueblood ES. et al. Mechanisms of oncostatin M-induced pulmonary inflammation and fibrosis. J Immunol 2008;181:7243–53. [DOI] [PubMed] [Google Scholar]
- 14. Matsuda M, Tsurusaki S, Miyata N. et al. Oncostatin M causes liver fibrosis by regulating cooperation between hepatic stellate cells and macrophages in mice. Hepatology 2018;67:296–312. [DOI] [PubMed] [Google Scholar]
- 15. Reid J, Zamuner S, Edwards K. et al. In vivo affinity and target engagement in skin and blood in a first-time-in-human study of an anti-oncostatin M monoclonal antibody. Br J Clin Pharmacol 2018;84:2280–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Allanore Y, Distler O, Jagerschmidt A. et al. Lysophosphatidic acid receptor 1 antagonist SAR100842 for patients with diffuse cutaneous systemic sclerosis: a double-blind, randomized, eight-week placebo-controlled study followed by a sixteen-week open-label extension study. Arthritis Rheumatol 2018;70:1634–43. [DOI] [PubMed] [Google Scholar]
- 17. Denton CP, Ong VH, Xu S. et al. Therapeutic interleukin-6 blockade reverses transforming growth factor-beta pathway activation in dermal fibroblasts: insights from the faSScinate clinical trial in systemic sclerosis. Ann Rheum Dis 2018;77:1362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nagahama KY, Togo S, Holz O. et al. Oncostatin M modulates fibroblast function via signal transducers and activators of transcription proteins-3. Am J Respir Cell Mol Biol 2013;49:582–91. [DOI] [PubMed] [Google Scholar]
- 19. Rice LM, Padilla CM, McLaughlin SR. et al. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J Clin Invest 2015;125:2795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khanna D, Denton CP, Jahreis A. et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): a phase 2, randomised, controlled trial. Lancet 2016;387:2630–40. [DOI] [PubMed] [Google Scholar]
- 21. Rice LM, Ziemek J, Stratton EA. et al. A longitudinal biomarker for the extent of skin disease in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheumatol 2015;67:3004–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abignano G, Blagojevic J, Bissell LA. et al. European multicentre study validates enhanced liver fibrosis test as biomarker of fibrosis in systemic sclerosis. Rheumatology 2019;58:254–9. [DOI] [PubMed] [Google Scholar]
- 23. Abignano G, Cuomo G, Buch MH. et al. The enhanced liver fibrosis test: a clinical grade, validated serum test, biomarker of overall fibrosis in systemic sclerosis. Ann Rheum Dis 2014;73:420–7. [DOI] [PubMed] [Google Scholar]
- 24. Akesson A, Scheja A, Lundin A, Wollheim FA.. Improved pulmonary function in systemic sclerosis after treatment with cyclophosphamide. Arthritis Rheum 1994;37:729–35. [DOI] [PubMed] [Google Scholar]
- 25. Duncan MR, Hasan A, Berman B.. Oncostatin M stimulates collagen and glycosaminoglycan production by cultured normal dermal fibroblasts: insensitivity of sclerodermal and keloidal fibroblasts. J Invest Dermatol 1995;104:128–33. [DOI] [PubMed] [Google Scholar]
- 26. Guo X, Higgs BW, Bay-Jensen AC. et al. Suppression of T cell activation and collagen accumulation by an anti-IFNAR1 mAb, anifrolumab, in adult patients with systemic sclerosis. J Invest Dermatol 2015;135:2402–9. [DOI] [PubMed] [Google Scholar]
- 27. Lee YJ, Shin KC, Kang SW. et al. Type III procollagen N-terminal propeptide, soluble interleukin-2 receptor, and von Willebrand factor in systemic sclerosis. Clin Exp Rheumatol 2001;19:69–74. [PubMed] [Google Scholar]
- 28. Quillinan NP, McIntosh D, Vernes J, Haq S, Denton CP.. Treatment of diffuse systemic sclerosis with hyperimmune caprine serum (AIMSPRO): a phase II double-blind placebo-controlled trial. Ann Rheum Dis 2014;73:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Assassi S, Wu M, Tan FK. et al. Skin gene expression correlates of severity of interstitial lung disease in systemic sclerosis. Arthritis Rheum 2013;65:2917–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Johnson SR, Hinchcliff M, Asano Y.. Controversies: molecular vs. clinical systemic sclerosis classification. J Scleroderma Relat Disord 2016;1:277–85. [Google Scholar]
- 31. Johnson SR, Khanna D, Allanore Y, Matucci-Cerinic M, Furst DE.. Systemic sclerosis trial design moving forward. J Scleroderma Relat Disord 2016;1:177–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Johnson SR, Tomlinson GA, Granton JT, Hawker GA, Feldman BM.. Applied Bayesian methods in the rheumatic diseases. Rheum Dis Clin North Am 2018;44:361–70. [DOI] [PubMed] [Google Scholar]
- 33. Akarsu M, Hurşitoğlu M, Toprak Z. et al. Relationships among oncostatin M, insulin resistance, and chronic inflammation: a pilot study. Arch Endocrinol Metab 2020;64:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stawski L, Trojanowska M.. Oncostatin M and its role in fibrosis. Connect Tissue Res 2019;60:40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gearing DP, Bruce AG.. Oncostatin M binds the high-affinity leukemia inhibitory factor receptor. New Biol 1992;4:61–5. [PubMed] [Google Scholar]
- 36. Miyajima A, Kinoshita T, Tanaka M. et al. Role of oncostatin M in hematopoiesis and liver development. Cytokine Growth Factor Rev 2000;11:177–83. [DOI] [PubMed] [Google Scholar]
- 37. Tanaka M, Hirabayashi Y, Sekiguchi T. et al. Targeted disruption of oncostatin M receptor results in altered hematopoiesis. Blood 2003;102:3154–62. [DOI] [PubMed] [Google Scholar]
- 38. Wallace PM, Macmaster JF, Rillema JR. et al. In vivo properties of oncostatin M. Ann N Y Acad Sci 1995;762:42–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymised individual participant data and additional study documents can be requested for further research from www.clinicalstudydatarequest.com.