Abstract
Objective
To explore the longitudinal association of quantitative infrapatellar fat pad (IPFP) signal intensity alteration with OA-related biomarkers.
Methods
Eighteen OA-related biochemical biomarkers of 600 knee OA participants in the Foundation for the National Institutes of Health OA Biomarkers Consortium (FNIH) study were extracted. The quantitative IPFP signal intensity measures were acquired based on magnetic resonance imaging, including mean value [Mean (IPFP)] and standard deviation [sDev (IPFP)] of the whole IPFP signal intensity, median value [Median (H)] and upper quartile value [UQ (H)] of high signal intensity, the ratio of volume of high signal intensity to volume of whole IPFP signal intensity [Percentage (H)] and Clustering factor (H). The linear mixed-effect model was applied to determine the longitudinal associations between IPFP signal intensity alteration and biochemical biomarkers over 2 years.
Results
All IPFP measures except for Clustering factor (H) were positively associated with urine collagenase-cleaved type II collagen neoepitope (uC2C), urine C-terminal cross-linked telopeptide of type II collagen (uCTX-II), urine C-terminal cross-linked telopeptide of type I collagen-α (uCTX-Iα) and urine N-terminal cross-linked telopeptide of type I collagen (uNTX-I). Mean (IPFP), Median (H) and Percentage (H) were positively associated with the nitrated form of an epitope located in the triple helix of type II collagen (Coll2-1 NO2). Mean (IPFP), Median (H) and UQ (H) were positively associated with sCTX-I and uCTX-Iβ. Positive associations between sDev (IPFP), Percentage (H) and serum hyaluronic acid (sHA) were found.
Conclusion
Our results suggest a role of IPFP signal intensity alteration in joint tissue remodelling on a molecular level.
Keywords: OA, infrapatellar fat pad, MRI, FNIH, biomarker
Rheumatology key messages.
The longitudinal associations between IPFP signal alteration and systemic OA-related biochemical biomarkers were evaluated.
Multiple IPFP signal measures were significantly associated with biomarkers of cartilage and bone turnover.
We demonstrated the role of IPFP signal alteration in OA on a molecular level.
Introduction
OA is a highly prevalent arthritis associated with tremendous individual and socioeconomic burden. While almost all joints could be involved, the joints most commonly affected by OA are the hip, knee, hand, foot and spine [1]. It has been increasingly appreciated that OA is an abnormal process of joint tissue remodelling, and is now widely viewed as a disease affecting the whole joint, including cartilage, bone, synovium, meniscus, ligaments, muscles and the joint capsule [2].
Recently, the infrapatellar fat pad (IPFP), also known as Hoffa's fat pad, has been regarded as an additional active tissue implicated in the pathogenesis of knee OA [3, 4]. When the knee is flexed, the IPFP could protect surrounding tissues and absorb shock through the joint, thus playing a protective role in the development of knee OA [5]. However, novel emerging evidence indicates that an abnormal IPFP might play a detrimental role in knee OA through releasing a wide range of pro-inflammatory mediators, including cytokines, adipokines, chemokines and inflammatory lipid mediators [3, 4]. Abnormality of IPFP could be evaluated via non-contrast-enhanced fat-suppressed MRI, and IPFP high signal alteration graded using a semi-quantitative scoring system, Hoffa’s synovitis has long been used as a surrogate for synovitis [6], which has been shown to be linked to knee osteoarthritic structural abnormalities in multiple studies [7–11]. To overcome the drawbacks of the semi-quantitative approach, such as outputs insensitive to change, time-intensive analysis and low reproducibility, we have recently developed an efficient and reproducible method to measure the IPFP signal intensity alteration quantitatively [12]. Based on this method, we have previously reported that quantitative IPFP signal intensity alteration at baseline could predict the incidence of radiographic knee OA [13], knee osteoarthritic structural progression [14] and total knee replacement (TKR) [15]. Collectively, all these studies focusing on semi-quantitative or quantitative IPFP signal intensity alteration in knee OA examined its role in morphological knee structural changes or TKR, which occurs in the late stage of the disease course of knee OA.
Molecular alterations of joint tissues in OA can occur prior to the morphological structural changes such as anatomical and/or physiological changes, including cartilage degradation, bone remodelling and osteophyte formation [16]. Of note, we previously demonstrated that serum levels of several pro-inflammatory mediators such as interleukin-17 (IL-17) and resistin, which could originate from IPFP and were linked to IPFP signal intensity alteration [17, 18], were significantly associated with knee osteoarthritic structural abnormalities [18–20]. Since the pro-inflammatory mediators released by IPFP have been strongly implicated in the remodelling of knee joint tissues [21], there is face validity to the hypothesis that IPFP signal intensity alteration might be associated with systemic biochemical biomarkers reflective of joint tissues turnover. Nevertheless, there is no study examining the relationships between IPFP signal intensity alteration and biochemical biomarkers reflecting knee joint tissue turnover in knee OA, particularly in longitudinal studies. The sample and data publicly available from the Foundation for the National Institute of Health (FNIH) OA Biomarkers Consortium study provided us with a unique opportunity to perform the present exploratory study to test this hypothesis, and the included biomarkers represent the best-qualified OA-related biochemical biomarkers to date [22].
Methods
Study design and participants
All 600 participants (one index knee per participant) selected for the FNIH OA Biomarkers Consortium study, a nested case–control study within the OA Initiative (OAI), were included in the present study. The goal of the OAI, a multicentre prospective cohort study of knee OA, was to study the natural history of, and risk factors for, the onset and progression of knee OA, and the validity of imaging, biochemical and genetic measures as biomarkers and surrogate endpoints for knee OA [23]. The OAI study has received ethics board approval by the institutional review board at the University of California, San Francisco (OAI Coordinating Center; Approval Number: 10-00532), and written informed consent was obtained from all participants. The FNIH study was conducted based on the subsets of OAI, and the detailed study design has been published previously [22]. In brief, the FNIH study comprises four mutually exclusive groups of participants according to the radiographic and/or pain progression status in an index knee. Eligible participants were those with at least one knee with a Kellgren/Lawrence (K/L) grade of 1–3 at baseline from central reading and availability at baseline and 24 months of knee radiographs, knee MRI, stored biological specimens and clinical data. Radiographic progression was defined as a decrease of ≥0.7 mm in minimum joint space width (minJSW) of the medial femorotibial compartment from baseline to 24, 36 or 48 months. Pain progression was defined as a Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain increase of ≥9 points, on a 0–100 normalized score, at two or more time points from the 24–60 months. The final sample include about one-quarter OA progressors with both symptomatic and radiographic progression, about one-quarter non-progressors, and about one-half either symptomatic only or radiographic only progressors.
Measurements of IPFP signal intensity
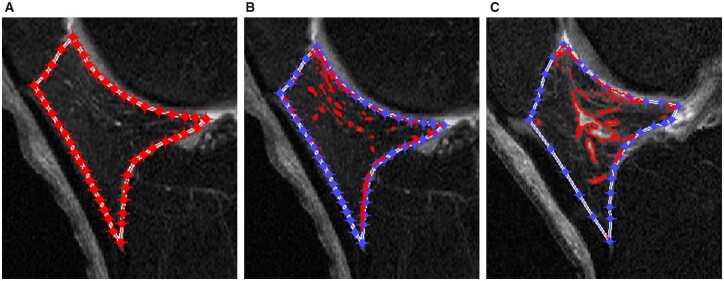
Based on our semi-automatic method, the IPFP signal intensity alteration was quantitatively measured using sagittal planes of fat-saturated T2-weighted images acquired from 3.0-T MRI on three time points (baseline, 12 months and 24 months), and all the measurements were performed blinded to the knee OA progression status. The details of this method were documented previously [12]. Concisely, an initial lasso consisted of a set of points was manually created around the outer contour of IPFP and then automatically contracted inward to approximate the actual outline of IPFP (Fig. 1A). The high signal intensity regions were obtained automatically (Fig. 1B). Finally, the measures of IPFP signal intensity alteration were calculated and output automatically. Since the IPFP signal intensity alteration was not uniform in different individuals, the mean value [Mean (IPFP)] and standard deviation [sDev (IPFP)] of the whole IPFP signal intensity were introduced to reflect the average level and variation of the whole IPFP signal intensity alteration. The median value [Median (H)] and upper quartile value [UQ (H)] of the high signal intensity were applied as the measures of high signal intensity. The volume of high signal intensity [Volume (H)] was calculated based on the slice thickness and the area on each slice, and the ratio of Volume (H) to volume of the whole IPFP signal intensity [Percentage (H)] was employed to denote the adjusted quantity of high signal intensity regions. The clustering regions with high signal intensity differed in participants, and Clustering factor (H) was utilized to reflect this clustering effect. The bigger the Clustering factor (H), the greater the clustering effect (Fig. 1C). Collectively, these six measures could be classified into four categories: signal intensity alteration of the whole IPFP, high signal intensity alteration of IPFP, adjusted quantity and clustering effect of high signal intensity. The intraclass correlation coefficients (ICCs) and inter-observer correlation coefficients were high (>0.90) for all measurements [12].
Fig. 1.
Segmentation and measurements of IPFP signal intensity alteration using MATLAB.
The segmentation and measurements of infrapatellar fat pad (IPFP) signal intensity alteration based on sagittal planes of fat-saturated T2-weighted images using MATLAB (The MathWorks, Natick, MA, USA). (A) The IPFP was segmented semi-automatically first. An initial lasso consisting of a set of points was manually created around the outer contour of IPFP and then automatically contracted inward to approximate the actual outline of IPFP. (B) The high signal intensity regions (red areas) were obtained automatically after outlining the boundary of IPFP. (C) The clustering effect of high signal intensity was greater than that in (B).
Serum (s) and urine (u) biomarkers
Morning blood and second morning void urine specimens were collected after an overnight fast using a uniform protocol at three time points (baseline, 12 months, 24 months); the biochemical biomarkers analysed are summarized in Table 1. The biomarker assays were conducted by LabCorp Clinical Trials, a Clinical Laboratory Improvement Amendments (CLIA) and College of American Pathologists (CAP) certified division within LabCorp (San Leandro, USA), with the exception of the urine nitrated form of an epitope located in the triple helix of type II collagen (uColl2-1 NO2) assay, which was performed by Artialis, Inc. (Liège, Belgium). Creatinine-adjusted values for urine biomarkers were calculated by dividing the urine assay values by the corresponding creatinine result. For the results below the lowest reportable value, two different methods were employed. For the first method, results below the lowest reportable value were replaced with a pre-specified value just below the lowest reportable value. For the second method, results below the lowest reportable value were interpolated by the manufacturer’s software by extending the standard curve below the lowest positive standard as a function of the assay standard curve fitting specified by the assay manufacturer, and this method is mainly used in FNIH study. For the uColl2-1 NO2 data provided by Artialis, the first method was applied and a value of 80% of the lower limit of quantification (LLOQ) (0.048nM) was used for the alternative numeric value for samples below the lowest reportable result. The details of biochemical biomarker assays can be found in the published primary FNIH analyses [24]. We classified these biochemical biomarkers into three groups based on the fact that they are derived from mainly implicated tissues and inflammatory response: systemic biomarkers of cartilage turnover [collagenase-cleaved type II collagen as well as type I collagen neoepitope (C1, 2C), collagenase-cleaved type II collagen neoepitope (C2C), Coll2-1 NO2, cartilage oligomeric matrix protein (COMP), C-propeptide of type II procollagen (CPII), chondroitin sulphate 846 (CS846), C-terminal cross-linked telopeptide of type II (CTX-II), type IIA procollagen amino terminal propeptide (PIIANP)], bone turnover [C-terminal cross-linked telopeptide of type I collagen (CTX-I) and N-terminal cross-linked telopeptide of type I collagen (NTX-I)] and inflammation [hyaluronic acid (HA) and matrix metalloproteinase 3 (MMP3)].
Table 1.
Serum and urine biomarkers included in the present study
| Biomarkers | Manufacturer | Biological process |
|---|---|---|
| Serum/urine C1, 2C | IBEX | Types I and II collagen degradation |
| Serum/urine C2C | IBEX | Type II collagen degradation |
| Serum/urine Coll2-1 NO2 | Artialis | Type II collagen degradation |
| Serum COMP | BioVendor | Cartilage degeneration |
| Serum CPII | IBEX | Type II collagen synthesis |
| Urine CTX-II | BioVendor | Type II collagen degradation |
| Serum CS846 | IBEX | Cartilage aggrecan synthesis/turnover |
| Serum PIIANP | Merck Group/Millipore | Type II collagen synthesis |
| Serum CTX-I | IDS | Bone resorption |
| Urine CTX-Iα | IDS | Turnover of newly formed bone |
| Urine CTX-1β | IDS | Bone resorption |
| Serum/urine NTX-I | ALERE-Osteomark | Bone resorption |
| Serum HA | Corgenix | Osteophyte burden, synovitis |
| Serum MMP3 | Invitrogen | Total (active and inactive) metalloprotease involved with joint tissue degradation |
ALERE-Osteomark: Princeton, New Jersey, USA; Artialis: Liège, Belgium; BioVendor: Brno, Czech Republic; Corgenix: Broomfield, Colorado, USA; IBEX Pharmaceuticals: Montréal, Québec, Canada; IDS: Baldon, UK; Invitrogen: Camarillo, California, USA; Merck Group/Millipore: St. Charles, Missouri, USA; Thermo Fisher Scientific: Waltham, MA, USA. C1, 2C: collagenase-cleaved type II collagen as well as type I collagen neoepitope; C2C: collagenase-cleaved type II collagen neoepitope; Coll2-1 NO2: nitrated form of an epitope located in the triple helix of type II collagen; COMP: cartilage oligomeric matrix protein; CPII: C-propeptide of type II procollagen; CS846: chondroitin sulphate 846; CTX-I: C-terminal cross-linked telopeptide of type I collagen; CTX-II: C-terminal cross-linked telopeptide of type II collagen; HA: hyaluronic acid; NTX-I: N-terminal cross-linked telopeptide of type I collagen; MMP3: matrix metalloproteinase 3; PIIANP: type IIA procollagen amino terminal propeptide.
Statistical analysis
The continuous and categorical variables were expressed as means (s.d.) and percentages, respectively. A linear mixed-effects model is appropriate for analysing longitudinal data of repeated measures, including baseline and follow-up data, and the correlation between repeated measures could be taken into account [25]. Thus, we utilized the linear mixed-effects model to evaluate the longitudinal associations between IPFP signal intensity measures and biochemical biomarkers after adjustment for baseline age, sex, body mass index (BMI), race, KL grade, WOMAC pain score, minJSW, pain medication use and use of medications with effect on bone (parathyroid hormone in the past 6 months and bisphosphonate in the past 12 months). The temporal relationship between IPFP signal intensity measures and biochemical biomarkers was further explored by adding the interaction term between IPFP signal intensity measures and time in the model. The IPFP signal intensity measures and biochemical biomarker concentrations were transposed to Z-scores prior to the data analysis, and β-coefficients and 95% CIs denoted the change in S.D. of biochemical biomarkers associated with per 1 s.d. increase in IPFP signal intensity measures. All statistical analysis were performed using Stata version 15.0 for Windows (StataCorp LLC, College Station, TX, USA), and a two-tailed P-value <0.05 was considered statistically significant.
Results
As shown in Table 2, the participants were on average 61.55 (8.88) years old with a mean BMI of 30.72 (4.78) kg/m2, and 353 were female (58.83%). The majority of included participants were white with knee K/L grade 2. Overall, 600, 582 and 600 MRI images with good quality were suitable for measuring IPFP signal intensity alteration at baseline, 12 months and 24 months, respectively.
Table 2.
Baseline characteristics of participants
| Variable | Value (n = 600) |
|---|---|
| Age, mean (s.d.), years | 61.55 (8.88) |
| Female, n (%) | 353 (58.83) |
| BMI, mean (s.d.), kg/m2 | 30.72 (4.78) |
| Race, White, n (%) | 475 (79.17) |
| K/L grade 1/2/3, n (%) | 75 (12.50)/306 (51.00)/219 (36.50) |
| WOMAC pain score, mean (s.d.) | 12.08 (15.61) |
| Pain medication use, n (%) | 177 (29.50) |
| Minimum medial radiographic JSW, mean (s.d.), mm | 3.83 (1.18) |
| Mean (IPFP), mean (s.d.) | 0.19 (0.04) |
| sDev (IPFP), mean (s.d.) | 0.09 (0.02) |
| Median (H), mean (s.d.) | 0.34 (0.06) |
| UQ (H), mean (s.d.) | 0.40 (0.07) |
| Percentage (H), mean (s.d.) | 0.13 (0.01) |
| Clustering factor (H), mean (s.d.) | 0.73 (0.05) |
IPFP: infrapatellar fat pad; JSW: joint space width; KL: Kellgren–Lawrence; Mean (IPFP): mean value of the whole IPFP signal intensity; Median (H): median value of high signal intensity; Percentage (H): ratio of volume of high signal intensity to volume of whole IPFP signal intensity; sDev (IPFP): standard deviation of the whole IPFP signal intensity; UQ (H): upper quartile value of high signal intensity; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index.
Results based on mixed-effect linear regression for longitudinal associations of IPFP signal intensity measures with systemic biomarkers of cartilage turnover over 2 years are shown in Table 3. In multivariable analysis with adjustment for baseline age, sex, BMI, race, KL grade, WOMAC pain score, minJSW and pain medication use, all IPFP signal intensity measures except for Clustering factor (H) were positively associated with uC2C (β varying from 0.11 to 0.16) and uCTX-II (β varying from 0.07 to 0.13). Furthermore, there were significant positive associations of Mean (IPFP) with Coll2-1 NO2 in serum and urine, namely sColl2-1 NO2 (β = 0.05, 95% CI: 0.01, 0.09) and uColl2-1 NO2 (β = 0.08, 95% CI: 0.03, 0.14), as well as of Median (H) (β = 0.06, 95% CI: 0.00, 0.11) and Percentage (H) (β = 0.09, 95% CI: 0.04, 0.14) with uColl2-1 NO2. However, no significant interaction between time and corresponding IPFP signal intensity measures was found in the aforementioned significant associations.
Table 3.
Longitudinal associations of IPFP signal measures with systemic biomarkers of cartilage turnover over 2 years
| IPFP SI measure | sC1, 2C β (95% CI) | sC2C β (95% CI) | sColl2-1 NO2 β (95% CI) | sCOMP β (95% CI) | sCPII β (95% CI) | sCS846 β (95% CI) | sPIIANP β (95% CI) | uC1, 2C β (95% CI) | uC2C β (95% CI) | uColl2-1 NO2 β (95% CI) | uCTX-II β (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (IPFP) | 0.04 (−0.01, 0.09) | −0.03 (−0.08, 0.03) | 0.05 (0.01, 0.09) | −0.05 (−0.10, 0.01) | 0.02 (−0.02, 0.07) | 0.04 (−0.01, 0.09) | 0.01 (−0.05, 0.06) | −0.01 (−0.06, 0.05) | 0.15 (0.09, 0.20) | 0.08 (0.03, 0.14) | 0.11 (0.05, 0.17) |
| sDev (IPFP) | 0.02 (−0.03, 0.06) | −0.02 (−0.06, 0.03) | 0.01 (−0.03, 0.04) | −0.02 (−0.07, 0.03) | −0.01 (−0.05, 0.03) | 0.01 (−0.03, 0.05) | 0.01 (−0.04, 0.06) | 0.02 (−0.03, 0.07) | 0.15 (0.10, 0.20) | 0.03 (−0.02, 0.08) | 0.12 (0.07, 0.17) |
| Median (H) | 0.03 (−0.02, 0.08) | −0.02 (−0.07, 0.03) | 0.03 (−0.01, 0.07) | −0.03 (−0.09, 0.02) | 0.01 (−0.03, 0.05) | 0.03 (−0.02, 0.07) | 0.01 (−0.04, 0.06) | 0.01 (−0.05, 0.06) | 0.16 (0.11, 0.21) | 0.06 (0.00, 0.11) | 0.13 (0.07, 0.18) |
| UQ (H) | 0.03 (−0.02, 0.07) | −0.02 (−0.07, 0.03) | 0.02 (−0.02, 0.06) | −0.03 (−0.08, 0.02) | 0.00 (−0.04, 0.04) | 0.02 (−0.02, 0.07) | 0.01 (−0.04, 0.06) | 0.00 (−0.05, 0.06) | 0.16 (0.10, 0.21) | 0.04 (−0.01, 0.09) | 0.12 (0.07, 0.18) |
| Percentage (H) | 0.01 (−0.04, 0.05) | 0.01 (−0.04, 0.06) | 0.01 (−0.03, 0.05) | 0.00 (−0.06, 0.05) | 0.01 (−0.03, 0.06) | −0.01 (−0.05, 0.04) | −0.02 (−0.07, 0.03) | 0.04 (−0.01, 0.10) | 0.11 (0.06, 0.17) | 0.09 (0.04, 0.14) | 0.07 (0.02, 0.13) |
| Clustering factor (H) | −0.02 (−0.06, 0.02) | 0.04 (−0.00, 0.09) | 0.01 (−0.02, 0.04) | 0.01 (−0.04, 0.06) | 0.01 (−0.03, 0.05) | −0.01 (−0.05, 0.03) | 0.00 (−0.05, 0.04) | 0.01 (−0.04, 0.06) | 0.05 (−0.00, 0.10) | 0.03 (−0.02, 0.08) | 0.02 (−0.03, 0.07) |
With adjustment for baseline age, sex, body mass index, race, Kellgren–Lawrence grade, Western Ontario and McMaster Universities Osteoarthritis Index pain score, minimum joint space width and pain medication use. Bold values represent statistically significant associations, P < 0.05. C1, 2C: collagenase-cleaved type II collagen as well as type I collagen neoepitope; C2C: collagenase-cleaved type II collagen neoepitope; Coll2-1 NO2: nitrated form of an epitope located in the triple helix of type II collagen; COMP: cartilage oligomeric matrix protein; CPII: C-propeptide of type II procollagen; CS846: chondroitin sulphate 846; CTX-II: C-terminal cross-linked telopeptide of type II collagen; IPFP: infrapatellar fat pad; Mean (IPFP): mean value of the whole IPFP signal intensity; Median (H): median value of high signal intensity; Percentage (H): ratio of volume of high signal intensity to volume of whole IPFP signal intensity; PIIANP: type IIA procollagen amino terminal propeptide; s: serum; sDev (IPFP): standard deviation of the whole IPFP signal intensity; u: urine; UQ (H): upper quartile value of high signal intensity.
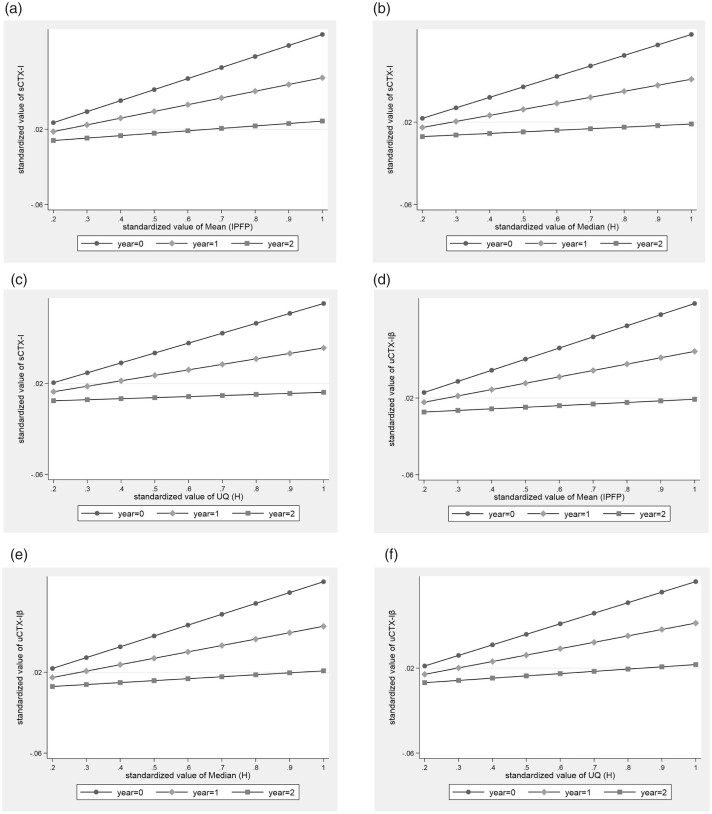
Results based on mixed-effect linear regression for longitudinal associations of IPFP signal intensity measures with systemic biomarkers of bone turnover over 2 years are shown in Table 4. After adjustment for baseline age, sex, BMI, race, KL grade, WOMAC pain score, minJSW, pain medication use, use of medications with effect on bone (parathyroid hormone in the past 6 months and bisphosphonate in the past 12 months), all IPFP signal intensity measures with the exception of Clustering factor (H) were positively associated with uCTX-Iα (β varying from 0.06 to 0.07) and uNTX-I (β varying from 0.06 to 0.13). Mean (IPFP), Median (H) and UQ (H) were positively associated with sCTX-I [Mean (IPFP): β = 0.12, 95% CI: 0.05, 0.18; Median (H): β = 0.10, 95% CI: 0.04, 0.16; UQ (H): β = 0.09, 95% CI: 0.03, 0.15] and uCTX-Iβ [Mean (IPFP): β = 0.12, 95% CI: 0.05, 0.18; Median (H): β = 0.11, 95% CI: 0.04, 0.17; UQ (H): β = 0.10, 95% CI: 0.03, 0.16]. In addition, Median (H) was positively associated with sNTX-I (β = 0.05, 95% CI: 0.00, 0.09). Notably, significant interactions between Mean (IPFP), Median (H) and UQ (H) and time were found for sCTX-I and uCTX-Iβ (Fig. 2). Moreover, there was a significant interaction between Mean (IPFP) and time on uNTX-I. Based on the coefficients of interaction terms (Supplementary Table S1, available at Rheumatology online), these results indicated that the strength of association between the aforementioned IPFP signal intensity measures and bone-related biochemical biomarkers decreased over time.
Table 4.
Longitudinal associations of IPFP signal measures with systemic biomarkers of bone turnover over 2 years
| IPFP SI measure | sCTX-I β (95% CI) | sNTX-I β (95% CI) | uCTX-Iα β (95% CI) | uCTX-1β β (95% CI) | uNTX-I β (95% CI) |
|---|---|---|---|---|---|
| Mean (IPFP) | 0.12 (0.05, 0.18)a | 0.05 (−0.00, 0.10) | 0.07 (0.01, 0.12) | 0.12 (0.05, 0.18)a | 0.13 (0.05, 0.20)a |
| sDev (IPFP) | 0.03 (−0.02, 0.08) | 0.03 (−0.01, 0.07) | 0.07 (0.01, 0.11) | 0.05 (−0.00, 0.10) | 0.07 (0.02, 0.12) |
| Median (H) | 0.10 (0.04, 0.16)a | 0.05 (0.00, 0.09) | 0.06 (0.01, 0.11) | 0.11 (0.04, 0.17)a | 0.07 (0.02, 0.12) |
| UQ (H) | 0.09 (0.03, 0.15)a | 0.04 (−0.00, 0.09) | 0.06 (0.01, 0.11) | 0.10 (0.03, 0.16)a | 0.06 (0.01, 0.12) |
| Percentage (H) | 0.04 (−0.01, 0.09) | 0.02 (−0.02, 0.07) | 0.06 (0.01, 0.12) | 0.03 (−0.03, 0.08) | 0.06 (0.01, 0.11) |
| Clustering factor (H) | 0.01 (−0.04, 0.06) | 0.01 (−0.03, 0.05) | −0.03 (−0.08, 0.02) | 0.00 (−0.05, 0.05) | −0.03 (−0.08, 0.02) |
With adjustment for baseline age, sex, body mass index, race, Kellgren/Lawrence grade, Western Ontario and McMaster Universities Osteoarthritis Index pain score, minimum joint space width, pain medication use, use of medications with effect on bone (parathyroid hormone in the past 6 months and bisphosphonate in the past 12 months).
There is an interaction between IPFP signal intensity measure and time. Bold values represent statistically significant associations, P < 0.05. CTX-I: C-terminal cross-linked telopeptide of type I collagen; IPFP: infrapatellar fat pad; Mean (IPFP): mean value of the whole IPFP signal intensity; Median (H): median value of high signal intensity; NTX-I: N-terminal cross-linked telopeptide of type I collagen; Percentage (H): ratio of volume of high signal intensity to volume of whole IPFP signal intensity; s: serum; sDev (IPFP): standard deviation of the whole IPFP signal intensity; u: urine; UQ (H): upper quartile value of high signal intensity.
Fig. 2.
Interaction between IPFP signal measures and time on circulating sCTX-I, uCTX-Iβ adjusted for potential covariates
Significant interaction between quantitative IPFP signal intensity measures and time on circulating sCTX-I and uCTX-Iβ with adjustment for potential covariates. (A) Significant interaction between Mean (IPFP) and time on sCTX-I. (B) Significant interaction between Median (H) and time on sCTX-I. (C) Significant interaction between UQ (H) and time on sCTX-I. (D) Significant interaction between Mean (IPFP) and time on uCTX-Iβ. (E) Significant interaction between Median (H) and time on uCTX-Iβ. (F) significant interaction between UQ (H) and time on uCTX-Iβ. CTX-I: C-terminal cross-linked telopeptide of type I collagen; IPFP: infrapatellar fat pad; Mean (IPFP): mean value of the whole IPFP signal intensity; Median (H): median value of high signal intensity; s: serum; u: urine; UQ (H): upper quartile value of high signal intensity.
Results based on mixed-effect linear regression for longitudinal associations of IPFP signal intensity measures with systemic biomarkers of inflammation over 2 years are shown in Table 5. In multivariable analysis with adjustment for baseline age, sex, BMI, race, KL grade, WOMAC pain score, minJSW and pain medication use, positive associations were found between sDev (IPFP) (β = 0.05, 95% CI: 0.00, 0.10), Percentage (H) (β = 0.05, 95% CI: 0.00, 0.10) and sHA, whereas there was no significant interaction between time and sDev (IPFP) or Percentage (H).
Table 5.
Longitudinal associations of IPFP signal measures with systemic biomarkers of inflammation over 2 years
| IPFP SI measure | sHA β (95% CI) | sMMP3 β (95% CI) |
|---|---|---|
| Mean (IPFP) | 0.02 (−0.03, 0.07) | −0.01 (−0.06, 0.04) |
| sDev (IPFP) | 0.05 (0.00, 0.10) | 0.02 (−0.03, 0.06) |
| Median (H) | 0.04 (−0.01, 0.09) | 0.00 (−0.04, 0.05) |
| UQ (H) | 0.04 (−0.01, 0.08) | 0.02 (−0.03, 0.07) |
| Percentage (H) | 0.05 (0.00, 0.10) | 0.00 (−0.05, 0.05) |
| Clustering factor (H) | 0.01 (−0.03, 0.06) | 0.04 (0.00, 0.08) |
With adjustment for baseline age, sex, body mass index, race, Kellgren–Lawrence grade, Western Ontario and McMaster Universities Osteoarthritis Index pain score, minimum joint space width and pain medication use. Bold values represent statistically significant associations, P < 0.05. HA: hyaluronic acid; IPFP: infrapatellar fat pad; Mean (IPFP): mean value of the whole IPFP signal intensity; Median (H): median value of high signal intensity; MMP3: matrix metalloproteinase 3; Percentage (H): ratio of volume of high signal intensity to volume of whole IPFP signal intensity; s: serum; sDev (IPFP): standard deviation of the whole IPFP signal intensity; u: urine; UQ (H): upper quartile value of high signal intensity.
Discussion
To the best of our knowledge, this is the first exploratory study to comprehensively examine the in vivo longitudinal associations of quantitative IPFP signal intensity measures with systemic OA-related biomarkers. After adjusting for potential confounders, all IPFP signal intensity measures except for Clustering factor (H) were positively associated with cartilage degradation biomarkers (uC2C and uCTX-II), with Mean (IPFP), Median (H) and Percentage (H) additionally found to be positively associated with another cartilage degradation biomarker (Coll2-1 NO2). Remarkably, the majority of quantitative IPFP signal intensity measures were positively associated with at least one of the assayed systemic bone turnover biomarkers. Specifically, all IPFP signal intensity measures except for Clustering factor (H) were positively associated with bone turnover biomarkers (uCTX-Iα and uNTX-I), with Mean (IPFP), Median (H) and UQ (H) additionally shown to be positively associated with another two bone resorption biomarkers (sCTX-I and uCTX-Iβ). Besides, Median (H) was positively associated with sNTX-I, a biomarker of bone resorption. Last, sDev (IPFP) and Percentage (H) were positively associated with a systemic biomarker of inflammation (sHA).
There is a large body of in vitro evidence suggesting that adipokines, such as leptin, resistin and visfatin [26], and pro-inflammatory cytokines, such as IL-6 and TNF-α [27], produced by IPFP could act alone or synergistically to induce the production of matrix-degrading enzymes such as collagenases and aggrecanases, which in turn degrade the extracellular matrix components of cartilage. In addition, the results derived from several studies using conditioned medium and co-culture models provided direct evidence for the deleterious role of IPFP in the catabolism of cartilage [28–30]. In contrast, two other studies have reported a protective role of IPFP-conditioned medium in the metabolism of cartilage [31, 32], and the differences in species and particularly the conditions of examined tissues (diseased or healthy) might explain the discrepancy. Our previous studies demonstrated that the baseline IPFP high signal intensity alteration, semi-quantitatively as well as quantitatively measured, was positively associated with cartilage defects cross-sectionally and increases in tibiofemoral cartilage defects longitudinally [14, 33], indicating that the IPFP pathology might play a pivotal role in the morphological changes of cartilage. This is supported by our prior findings that the serum levels of IL-17 and resistin, which could be released from IPFP, were positively associated with IPFP signal intensity alteration [17, 18] and tibiofemoral cartilage defect [18, 20]. Our present findings of positive relationships between quantitative IPFP signal intensity measures and several systemic biomarkers indicative of degradation of cartilage, specifically type II collagen, are consistent with our previous findings, and the pro-catabolic effect of pro-inflammatory mediators produced by IPFP might account for the potential link. However, the interaction term between time and IPFP signal intensity measures was non-significant, indicating that the temporal relationship between IPFP signal intensity alteration and systemic biomarkers of cartilage was unclear.
Compared with cartilage, our current knowledge about the interaction between IPFP and subchondral bone is relatively limited. However, there is increasing evidence suggesting that the pro-inflammatory cytokines, such as IL-6 and TNF-α, [27] and chemokines, such as IL-8 and monocyte chemoattractant protein 1, produced by IPFP are strongly implicated in bone resorption [34]. Similar to cartilage defects, we previously reported significant positive associations of semi-quantitative as well as quantitative IPFP signal intensity alteration with bone marrow lesions (BMLs), one of the most commonly used MRI-assessed subchondral bone abnormalities, cross-sectionally and longitudinally [14, 33]. Apart from revealing positive associations of serum levels of resistin and IL-17 with BMLs [18, 20], we also elucidated that the baseline levels of serum IL-6 were positively associated with an increase in BMLs over 2 years [19]. Consistent with these studies, the most striking findings in our present study are the significant associations between almost all IPFP signal intensity measures and systemic biomarkers indicative of bone resorption. Presumably, the pro-inflammatory cytokines and chemokines produced by the abundant immune cells infiltrated in IPFP might be responsible for the augmented osteoclastic bone resorption. Of great interest, significant interactions between time and IPFP signal intensity measures were also found, indicating that there is a temporal relationship between IPFP signal intensity alteration and bone resorption.
In addition to showing similar inflammatory cell composition, phenotype [35, 36] and histopathological changes [37] between IPFP and synovium from knee OA patients, emerging evidence also elucidated that knee osteoarthritic IPFP could substantially contribute to synovitis based on the findings of pro-inflammatory effects of IPFP-conditioned medium on fibroblast-like synoviocytes (FLS) [29, 38]. Gross et al. reported that the IPFP-conditioned medium collected from OA patients induced the expression of cyclooxygenase-2 and microsomal prostaglandin E synthase and the synthesis of prostaglandin E2 in FLS [29]. Similarly, in another study performed by Eymard et al. [38], the response of FLS to autologous IPFP from patients with severe knee OA was examined, and the IPFP-conditioned medium was found to remarkably induce the gene expression and release of inflammatory and prodegradative mediators by FLS. Collectively, these findings lend strong support to the potential role of osteoarthritic IPFP in the induction of synovitis, and this might account for the positive associations of IPFP signal intensity alteration measures with sHA, a biomarker indicative of synovitis, observed in our present study.
The main strength of our present study lies in the availabilities of multiple measurements of IPFP signal intensity alteration and systemic biomarkers for longitudinal association analysis. However, several limitations of our present study should be acknowledged. First, the levels of systemic biochemical biomarkers measured in serum or urine may represent the disease burden and activity of all joints, and thus the synovial fluid levels of these biomarkers, unavailable in this study, could be more suitable. Next, multiple testing corrections were not performed given the exploratory nature of this study. Following Bender and Lang, it is not a standard practice to adjust for multiple comparisons in exploratory studies [39]. Nonetheless, our results require further validation. Last, the results of our current study are derived from analysis of a selected nested case–control based on the data of the FNIH OA Biomarkers Consortium study, consisting of four groups of knee OA participants selected from the OAI on the basis of radiographic and/or pain progression status. Thus, whether our findings could be generalized to other non-selected OA cohorts needs further validation.
In conclusion, our results suggest a role of IPFP signal intensity alteration in joint tissue remodelling on a molecular level.
Supplementary Material
Acknowledgements
We thank the Osteoarthritis Research Society International (OARSI) for their leadership and expertise on the FNIH project. Data provided from the FNIH OA Biomarkers Consortium Project made possible through grants and direct or in-kind contributions by: AbbVie, Amgen, Arthritis Foundation, Artialis, Bioiberica, BioVendor, DePuy, Flexion Therapeutics, GSK, IBEX, IDS, Merck Serono, Quidel, Rottapharm | Madaus, Sanofi, Stryker, the Pivotal OAI MRI Analyses (POMA) study (NIH HHSN2682010000 21C) and the Osteoarthritis Research Society International. The OAI is a public–private partnership comprising five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health. Funding partners include Merck Research Laboratories, Novartis Pharmaceuticals Corporation, GlaxoSmithKline and Pfizer, Inc. Private sector funding for the Consortium and OAI is managed by the Foundation for the National Institutes of Health.
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. C.D. (changhai.ding@utas.edu.au) had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Conception and design: C.D., D.H. and H.C.; acquisition of data: H.C., Q.Y., W.H., T.M. and Z.C.; analysis and interpretation of data: H.C., Q.Y., D.H. and CD.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: DH provides consulting advice on scientific advisory boards for Pfizer, Lilly, TLCBio, Novartis, Tissuegene, Biobone. All other authors declare no other competing interests.
Contributor Information
Han Cen, Institute of Geriatrics, the Affiliated Hospital of Medical School; Department of Preventive Medicine, School of Medicine, Ningbo University, Ningbo, China; Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia.
Qingran Yan, Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia; Department of Rheumatology, Renji Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai.
Weiyu Han, Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia; Clinical Research Centre, Zhujiang Hospital, Southern Medical University, Guangzhou.
Tao Meng, Department of Rheumatology and Immunology, The Second Hospital of Anhui Medical University, Hefei.
Zhongshan Chen, Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia; School of Mathematics and Information Science, Nanjing Normal University of Special Education, Nanjing.
Guangfeng Ruan, Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia; Clinical Research Centre, Zhujiang Hospital, Southern Medical University, Guangzhou.
Tian Wang, Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia; Department of Rheumatology and Clinical Immunology, Beijing An Zhen Hospital, Capital Medical University, Beijing.
Feng Pan, Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia.
Di Chen, Faculty of Pharmaceutical Sciences, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China.
Virginia Byers Kraus, Duke Molecular Physiology Institute and Division of Rheumatology, Department of Medicine, Duke University School of Medicine, Durham, NC, USA.
David J Hunter, Clinical Research Centre, Zhujiang Hospital, Southern Medical University, Guangzhou; Department of Rheumatology, Royal North Shore Hospital and Institute of Bone and Joint Research, Kolling Institute, University of Sydney, Sydney, Australia.
Changhai Ding, Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia; Clinical Research Centre, Zhujiang Hospital, Southern Medical University, Guangzhou.
Data availability statement
Data are available from the corresponding author upon reasonable request.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Kloppenburg M, Berenbaum F.. Osteoarthritis year in review 2019: epidemiology and therapy. Osteoarthritis Cartilage 2020;28:242–8. [DOI] [PubMed] [Google Scholar]
- 2. Loeser RF, Goldring SR, Scanzello CR, Goldring MB.. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum 2012;64:1697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chang J, Liao Z, Lu M. et al. Systemic and local adipose tissue in knee osteoarthritis. Osteoarthritis Cartilage 2018;26:864–71. [DOI] [PubMed] [Google Scholar]
- 4. Zeng N, Yan ZP, Chen XY, Ni GX.. Infrapatellar fat pad and knee osteoarthritis. Aging Dis 2020;11:1317–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gallagher J, Tierney P, Murray P, O’Brien M.. The infrapatellar fat pad: anatomy and clinical correlations. Knee Surg Sports Traumatol Arthrosc 2005;13:268–72. [DOI] [PubMed] [Google Scholar]
- 6. Hunter DJ, Guermazi A, Lo GH. et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthritis Cartilage 2011;19:990–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roemer FW, Kwoh CK, Hannon MJ. et al. What comes first? Multitissue involvement leading to radiographic osteoarthritis: magnetic resonance imaging-based trajectory analysis over four years in the osteoarthritis initiative. Arthritis Rheumatol 2015;67:2085–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roemer FW, Kwoh CK, Hannon MJ. et al. Can structural joint damage measured with MR imaging be used to predict knee replacement in the following year? Radiology 2015;274:810–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Atukorala I, Kwoh CK, Guermazi A. et al. Synovitis in knee osteoarthritis: a precursor of disease? Ann Rheum Dis 2016;75:390–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harkey MS, Davis JE, Lu B. et al. Early pre-radiographic structural pathology precedes the onset of accelerated knee osteoarthritis. BMC Musculoskelet Disord 2019;20:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davis JE, Ward RJ, MacKay JW. et al. Effusion-synovitis and infrapatellar fat pad signal intensity alteration differentiate accelerated knee osteoarthritis. Rheumatology (Oxford) 2019;58:418–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lu M, Chen Z, Han W. et al. A novel method for assessing signal intensity within infrapatellar fat pad on MR images in patients with knee osteoarthritis. Osteoarthritis Cartilage 2016;24:1883–9. [DOI] [PubMed] [Google Scholar]
- 13. Wang K, Ding C, Hannon MJ. et al. Quantitative signal intensity alteration in infrapatellar fat pad predicts incident radiographic osteoarthritis: the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2019;71:30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Han W, Aitken D, Zheng S. et al. Association between quantitatively measured infrapatellar fat pad high signal-intensity alteration and magnetic resonance imaging-assessed progression of knee osteoarthritis. Arthritis Care Res (Hoboken) 2019;71:638–46. [DOI] [PubMed] [Google Scholar]
- 15. Wang K, Ding C, Hannon MJ. et al. Signal intensity alteration within infrapatellar fat pad predicts knee replacement within 5 years: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 2018;26:1345–50. [DOI] [PubMed] [Google Scholar]
- 16. Kraus VB, Blanco FJ, Englund M, Karsdal MA, Lohmander LS.. Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthritis Cartilage 2015;23:1233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang K, Xu J, Cai J. et al. Serum levels of interleukin-17 and adiponectin are associated with infrapatellar fat pad volume and signal intensity alteration in patients with knee osteoarthritis. Arthritis Res Ther 2016;18:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Han W, Aitken D, Zheng S. et al. Higher serum levels of resistin are associated with knee synovitis and structural abnormalities in patients with symptomatic knee osteoarthritis. J Am Med Dir Assoc 2019;20:1242–6. [DOI] [PubMed] [Google Scholar]
- 19. Zhu Z, Otahal P, Wang B. et al. Cross-sectional and longitudinal associations between serum inflammatory cytokines and knee bone marrow lesions in patients with knee osteoarthritis. Osteoarthritis Cartilage 2017;25:499–505. [DOI] [PubMed] [Google Scholar]
- 20. Wang K, Xu J, Cai J. et al. Serum levels of resistin and interleukin-17 are associated with increased cartilage defects and bone marrow lesions in patients with knee osteoarthritis. Mod Rheumatol 2017;27:339–44. [DOI] [PubMed] [Google Scholar]
- 21. Robinson WH, Lepus CM, Wang Q. et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol 2016;12:580–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hunter DJ, Nevitt M, Losina E, Kraus V.. Biomarkers for osteoarthritis: current position and steps towards further validation. Best Pract Res Clin Rheumatol 2014;28:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eckstein F, Wirth W, Nevitt MC.. Recent advances in osteoarthritis imaging—the osteoarthritis initiative. Nat Rev Rheumatol 2012;8:622–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kraus VB, Collins JE, Hargrove D. et al. Predictive validity of biochemical biomarkers in knee osteoarthritis: data from the FNIH OA Biomarkers Consortium. Ann Rheum Dis 2017;76:186–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Finucane MM, Samet JH, Horton NJ.. Translational methods in biostatistics: linear mixed effect regression models of alcohol consumption and HIV disease progression over time. Epidemiol Perspect Innov 2007;4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tu C, He J, Wu B, Wang W, Li Z.. An extensive review regarding the adipokines in the pathogenesis and progression of osteoarthritis. Cytokine 2019;113:1–12. [DOI] [PubMed] [Google Scholar]
- 27. Wang T, He C.. Pro-inflammatory cytokines: the link between obesity and osteoarthritis. Cytokine Growth Factor Rev 2018;44:38–50. [DOI] [PubMed] [Google Scholar]
- 28. Hui W, Litherland GJ, Elias MS. et al. Leptin produced by joint white adipose tissue induces cartilage degradation via upregulation and activation of matrix metalloproteinases. Ann Rheum Dis 2012;71:455–62. [DOI] [PubMed] [Google Scholar]
- 29. Gross JB, Guillaume C, Gegout-Pottie P. et al. The infrapatellar fat pad induces inflammatory and degradative effects in articular cells but not through leptin or adiponectin. Clin Exp Rheumatol 2017;35:53–60. [PubMed] [Google Scholar]
- 30. He J, Jiang Y, Alexander PG. et al. Infrapatellar fat pad aggravates degeneration of acute traumatized cartilage: a possible role for interleukin-6. Osteoarthritis Cartilage 2017;25:138–45. [DOI] [PubMed] [Google Scholar]
- 31. Bastiaansen-Jenniskens YM, Clockaerts S, Feijt C. et al. Infrapatellar fat pad of patients with end-stage osteoarthritis inhibits catabolic mediators in cartilage. Ann Rheum Dis 2012;71:288–94. [DOI] [PubMed] [Google Scholar]
- 32. Nishimuta JF, Bendernagel MF, Levenston ME.. Co-culture with infrapatellar fat pad differentially stimulates proteoglycan synthesis and accumulation in cartilage and meniscus tissues. Connect Tissue Res 2017;58:447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Han W, Aitken D, Zhu Z. et al. Signal intensity alteration in the infrapatellar fat pad at baseline for the prediction of knee symptoms and structure in older adults: a cohort study. Ann Rheum Dis 2016;75:1783–8. [DOI] [PubMed] [Google Scholar]
- 34. Brylka LJ, Schinke T.. Chemokines in physiological and pathological bone remodeling. Front Immunol 2019;10:2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Apinun J, Sengprasert P, Yuktanandana P. et al. Immune mediators in osteoarthritis: infrapatellar fat pad-infiltrating CD8+ T cells are increased in osteoarthritic patients with higher clinical radiographic grading. Int J Rheumatol 2016;2016:9525724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Klein-Wieringa IR, de Lange-Brokaar BJ, Yusuf E. et al. Inflammatory cells in patients with endstage knee osteoarthritis: a comparison between the synovium and the infrapatellar fat pad. J Rheumatol 2016;43:771–8. [DOI] [PubMed] [Google Scholar]
- 37. Favero M, El-Hadi H, Belluzzi E. et al. Infrapatellar fat pad features in osteoarthritis: a histopathological and molecular study. Rheumatology (Oxford) 2017;56:1784–93. [DOI] [PubMed] [Google Scholar]
- 38. Eymard F, Pigenet A, Citadelle D. et al. Induction of an inflammatory and prodegradative phenotype in autologous fibroblast-like synoviocytes by the infrapatellar fat pad from patients with knee osteoarthritis. Arthritis Rheumatol 2014;66:2165–74. [DOI] [PubMed] [Google Scholar]
- 39. Bender R, Lange S.. Adjusting for multiple testing—when and how? J Clin Epidemiol 2001;54:343–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the corresponding author upon reasonable request.