Abstract
Objective
Salivary glands of primary SS (pSS) patients characteristically harbour periductal infiltrates, in which lymphoepithelial lesions (LELs) can develop. LELs are composed of hyperplastic ductal epithelium with infiltrating lymphocytes and may assist in the challenging diagnostic process of pSS. As manual identification of LELs remains difficult, we aimed to identify LELs by using an objective digital image analysis (DIA) algorithm that detects intraepithelial lymphocytes.
Methods
A virtual triple-staining technique developed for this study was used to count intraepithelial lymphocytes in consecutive slides stained for CD3 (T-lymphocytes), high-molecular-weight cytokeratin (hmwCK) (striated ducts) and CD20 (B-lymphocytes) in labial and parotid gland biopsies in a diagnostic cohort of 109 sicca patients. Patients were classified as having pSS or non-SS according to the ACR-EULAR classification criteria.
Results
T-lymphocytes were detected in almost all analysed ducts of pSS and non-SS sicca patients, whereas intraepithelial B-lymphocytes were present in 59–68% of labial and parotid gland biopsies of pSS patients, against only 2–3% of patients classified as non-SS. Intraepithelial B-lymphocytes were found in almost all striated ducts with hyperplasia (LELs). Remarkably, ∼25% of analysed striated ducts without hyperplasia of pSS patients also contained B-lymphocytes (precursor-LELs). Furthermore, presence of intraepithelial B-lymphocytes was associated with clinical parameters of pSS (i.e. serology).
Conclusion
The presence of intraepithelial B-lymphocytes in salivary gland biopsies of sicca patients is a clear indicator of pSS and can be used as an objective alternative to LEL scoring. Therefore, identification of B-lymphocyte–containing ducts should be added to the diagnostic histopathological work-up of patients suspected of pSS.
Keywords: SS, histopathology, lymphoepithelial lesions, salivary gland, parotid gland, labial gland
Rheumatology key messages.
The presence of intraepithelial B-lymphocytes in salivary glands of sicca patients is a clear indicator of primary SS.
Digital image analysis of intraepithelial B-lymphocytes reveals lymphoepithelial lesions and precursor lymphoepithelial lesions.
Presence of B-lymphocyte–containing ducts is associated with clinical parameters of primary SS.
Introduction
Primary SS (pSS) is a systemic autoimmune disease characterized by sicca symptoms and lymphocytic infiltration of the lacrimal and salivary glands [1]. The infiltrates mainly consist of B- and T-lymphocytes and are located around striated ducts [2]. The number of periductal foci (cluster of >50 lymphocytes) can be calculated into the focus score (FS), where ≥1 focus per 4 mm2 of glandular parenchyma is used for classification of pSS [3]. Although FS is the leading histopathological parameter for the diagnosis and classification of pSS [4, 5], it has important limitations. Calculation of FS is difficult when other salivary gland conditions, such as acinar atrophy, non-specific chronic sialadenitis, or sclerosing chronic sialadenitis are present. Furthermore, FS does not include the extent of lymphocytic infiltration, as it does not take the size of foci into account [6].
Another histopathological feature that may assist in the diagnosis of pSS is the presence of lymphoepithelial lesions (LELs) within salivary gland biopsies. The presence of LELs has been suggested to be more indicative of pSS than a positive FS alone [7–10]. Initially, LELs were described as hyperplasia of myoepithelial cells and named myoepithelial lesions or myoepithelial islands [11, 12]. More recent studies have shown, however, that basal ductal cells are responsible for the ductal hyperplasia [13–15]. LELs are currently defined as striated ducts composed of hyperplastic ductal epithelium with intraepithelial lymphocytes. The hypothesis is that LELs develop after an influx of lymphocytes into the epithelium, leading to partial hyperplasia and ultimately to complete obstruction of ducts (Fig. 1) [8, 16]. Despite consensus about the definition of LELs and the development of a grading system on haematoxylin and eosin (H&E) staining [16], evaluation of LELs is still a subjective process and can be challenging. Tangentially sectioned striated ducts may resemble ductal hyperplasia, and distinction between fully obstructed ducts and germinal centres can be difficult, as both can appear as lighter-staining areas containing lymphocytes on H&E staining [17, 18].
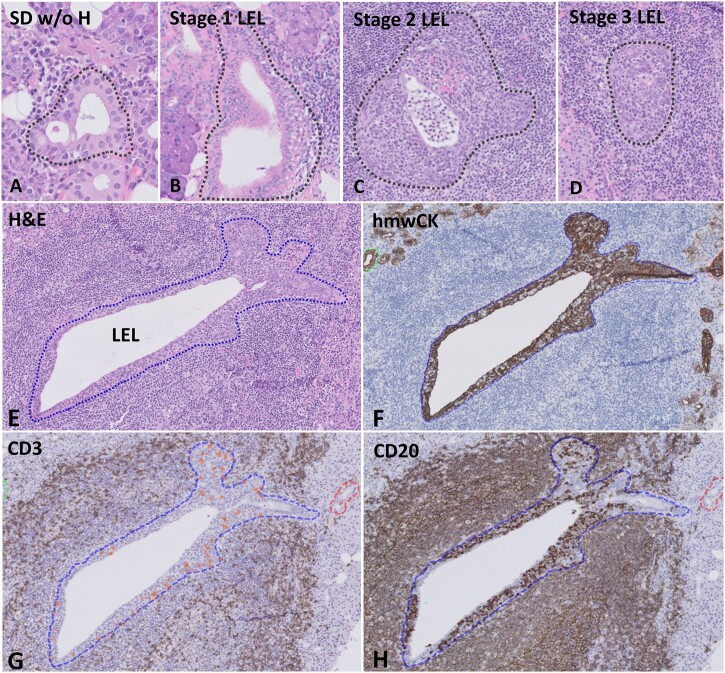
Fig. 1.
Histological analysis of striated ducts on H&E staining and digital image analysis of intraepithelial lymphocytes
(A–D) Grading of striated ducts on H&E-stained sections. (A) SD w/o H: striated duct with intraepithelial lymphocytes, without ductal hyperplasia. (B) Stage 1 lymphoepithelial lesion (LEL): lymphocytic ductal infiltration and ductal hyperplasia affecting <50% of the epithelium. (C) Stage 2 LEL: lymphocytic ductal infiltration and hyperplasia affecting ≥50% of the epithelium. (D) Stage 3 LEL: lymphocytic ductal infiltration and fully circumferentially hyperplastic epithelium without lumen. (E–H) Digital image analysis by using a virtual triple-staining technique. (E) H&E staining of striated duct with ductal hyperplasia and infiltrating lymphocytes (stage 2 LEL). (F) Detection of ductal epithelium by using staining for hmwCK. (G) Detection of intraepithelial CD3+ T-lymphocytes within the selected striated duct and (H) detection of CD20+ B-lymphocytes. H&E: haematoxylin and eosin.
A more objective histological parameter that could assist in identification of LELs is the presence of intraepithelial lymphocytes within striated ducts. We have previously shown that both B- and T-lymphocytes are present within LELs, and that there is an association between intraepithelial B-lymphocytes and hyperplasia of the ductal epithelium [16]. These results, however, are based on a relatively small cohort and warrant validation in a larger cohort. Furthermore, associations between the clinical parameters of pSS patients and the presence of intraepithelial B-lymphocytes and LEL stage are not yet known.
Therefore, the objectives of this study were (1) to investigate whether objective detection of intraepithelial lymphocytes using digital image analysis (DIA) can be used as an alternative to conventional methods for the assessment of LELs in salivary gland biopsies of patients suspected of having pSS and (2) to correlate the presence of intraepithelial lymphocytes and the grade of LELs to clinical parameters and disease activity of pSS patients.
Materials and methods
Patients
The study population consisted of consecutive patients with sicca symptoms who underwent a complete diagnostic work-up for pSS, including a simultaneously taken labial and parotid biopsy. Incisional parotid gland biopsies were performed under local anaesthesia, as previously described [19]. Exclusion criteria were age <18 years, diagnosis of an associated autoimmune disease, presence of a salivary gland mucosa–associated lymphoid tissue lymphoma, or insufficient biopsy material. The diagnostic work-up included all items of the ACR-EULAR classification criteria [5], serological parameters and the EULAR SS Disease Activity Index (ESSDAI) scores. As the ACR-EULAR criteria, which were developed from labial gland biopsy results, also have excellent accuracy when using parotid gland biopsy results [20], patients were classified as having pSS or non-SS based on the ACR-EULAR criteria, either including the labial gland biopsy focus score (ACR-EULAR labial), or the parotid gland biopsy focus score (ACR-EULAR parotid).
Histochemical and immunohistochemical staining
Formalin-fixed, paraffin-embedded labial and parotid salivary gland tissue samples were serially sectioned at 3 µm thickness and deparaffinized. Automatic staining was performed with H&E, and tissue samples were manually stained for CD3 (clone 2GV6, Roche), high-molecular-weight cytokeratin (hmwCK, clone 34βE12, Roche) and CD20 (clone L-26, Roche). Antigen retrieval was performed for 15 min in EDTA buffer (98°C, pH 8.0), and endogenous peroxidase was blocked. Pre-fixed dilutions of primary antibodies (1% BSA-PBS, Roche) were applied for 75 min. Primary antibodies were visualized by using 3,3′-diaminobenzidine (DAB) after incubation with a poly-horseradish peroxidase-labelled secondary antibody (Thermo Scientific). Tonsillar tissue was used as both a positive and negative control, as the tonsillar epithelium expresses hmwCK and does not express CD3 or CD20, and the tonsillar lymphoid tissue expresses CD3 and CD20 but does not express hmwCK.
Histological analysis
FS was calculated on H&E-stained sections. For analysis of ductal hyperplasia (LELs) and presence of intraepithelial lymphocytes, both the H&E staining and sections stained for hmwCK were used. To be able to compare manual LEL scoring and the results of DIA, regions of interest comprising striated ducts were manually selected on H&E-stained sections, with a maximum of 10 regions of interest per biopsy. The 10 striated ducts with intraepithelial lymphocytes that contained the most pronounced ductal hyperplasia (if present) were selected. Consecutive sections stained for hmwCK were used to correctly identify the ductal epithelium and to detect occluded LELs without lumina. In cases where fewer than 10 striated ducts with hyperplasia (LELs) were present, striated ducts with infiltrating lymphocytes without hyperplasia, preferably within infiltrated areas, were selected on H&E staining and included to make the number up to 10 ducts. In cases where no striated ducts with hyperplasia (LELs) were present, a maximum of 5 striated ducts without hyperplasia with infiltrating lymphocytes was selected. Striated ducts with hyperplasia (LELs) were scored in three stages of severity, based on H&E staining (Fig. 1B–D).
Digital image analysis
All stained slides were digitized using a Philips UFS slide scanner (Philips, Best, The Netherlands). Digital slides were stored on a central image server, and slides stained for CD3, hmwCK and CD20 were loaded into the DIA platform Visiopharm Integrator System (VIS) (Visiopharm, Hørsholm, Denmark). A virtual triple-staining technique (VTS) was developed for this study to align consecutive sections stained for CD3, hmwCK and CD20 (Fig. 1). Visual verification of the alignment was performed for all cases, and alignment was optimized manually if needed. Biopsies were excluded from further analyses if alignment of all three slides failed. In case of inability to align either the CD3 or CD20 slide with the hmwCK slide, only this particular CD3 or CD20 slide was excluded. After alignment, an algorithm developed for this study was used to detect and count intraepithelial CD3+ T-lymphocytes and CD20+ B-lymphocytes within the previously selected hmwCK-positive striated ducts. Intraepithelial B/T ratios were calculated. In cases where no intraepithelial lymphocytes were detected in the serial sections stained for CD3 and CD20, B/T-lymphocyte ratios were defined as 0. In cases where intraepithelial B-lymphocytes were detected in the absence of intraepithelial T-lymphocytes, B/T ratios were defined as 1. To validate the DIA algorithm, data for the numbers of intraepithelial lymphocytes obtained by DIA were compared with data from manual counting of a random sample of the total cohort (15 pSS and 15 non-SS sicca patients). Manual counting analysis was performed as previously described [16].
Statistical analysis
Descriptive statistics were described as number (%), mean (s.d.) or median (IQR). Mann–Whitney U tests and Fisher’s exact tests were used to compare differences in FS and in the presence of striated ducts with hyperplasia (LELs) between pSS patients and non-SS sicca patients. The Wilcoxon signed rank test was used to compare differences in the mean stage of LELs per biopsy comparing labial and parotid gland biopsies of pSS patients. Spearman correlation was used to explore interobserver agreement between manual and digital analysis of intraepithelial lymphocytes, and to analyse the association between FS and B/T-lymphocyte ratios, and between B/T ratios within labial and parotid gland biopsies. In addition, Bland–Altman plots were created to evaluate systematic differences and to calculate 95% limits of agreements. Generalized estimating equations (GEEs) were used to analyse B/T-lymphocyte ratios within striated ducts of patients with pSS over the different stages of severity. Fisher’s exact tests and Mann–Whitney U tests were used to test differences in clinical parameters between pSS patients with and without intraepithelial B-lymphocytes within the salivary gland biopsies. For comparing differences in clinical parameters between three or more groups based on LEL stages, Kruskal–Wallis tests were used, followed by Mann–Whitney U tests. Statistical analyses were performed in IBM SPSS Statistics 23.
Ethics
The study was performed in accordance with the Declaration of Helsinki. All participants gave written informed consent, and the study was approved by the Medical Ethics Committee of the University Medical Center Groningen (METc 2013.066).
Results
A flow chart illustrating the process of inclusion and exclusion of patients and their biopsies is shown in Fig. 2. When the labial gland biopsy was used for classification, 43 patients (42%) were classified as having pSS according to the criteria (ACR-EULAR labial). When the parotid gland was taken into account, 32 patients (32%) were classified as having pSS according to the criteria (ACR-EULAR parotid). Characteristics of the patients classified as having pSS or non-SS are presented in Table 1.
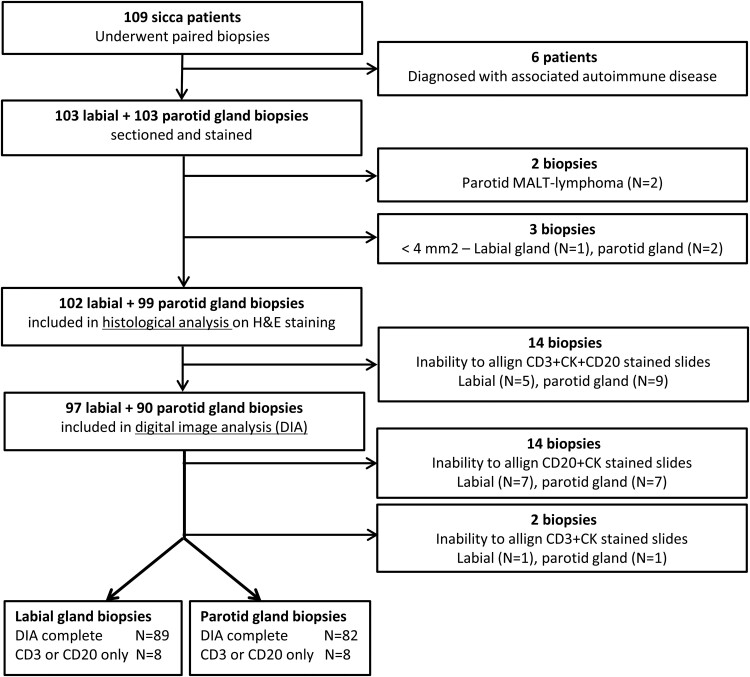
Fig. 2.
Flowchart of the process of inclusion and exclusion of patients and salivary gland biopsies
MALT: Mucosa-Associated Lymphoid Tissue; H&E: haematoxylin and eosin; DIA: digital image analysis.
Table 1.
Characteristics of pSS and non-SS sicca patients included in the diagnostic cohort
| (A) ACR-EULAR (labial) |
(B) ACR-EULAR (parotid) |
|||||
|---|---|---|---|---|---|---|
|
N = 102 patients |
N = 99 patients |
|||||
| pSS | Non-SS | pSS | Non-SS | |||
| N = 43 | N = 59 | N = 32 | N = 67 | |||
| Age | 53.4 (14.3) | 48.5 (12.8) | 49.7 (14.0) | 50.0 (12.9) | ||
| Female | 42 (98) | 50 (85) | 31 (97) | 58 (87) | ||
| ACR-EULAR items | ||||||
| Anti-SSA positivity | 33 (77) | 2 (3) | 30 (94) | 2 (3) | ||
| Anti-SSB positivity | 18 (42) | 0 0 | 15 (47) | 0 0 | ||
| FS ≥1 | 30 (70) | 2 (3) | 19 (60) | 2 (3) | ||
| UWS ≤0.1 ml/min | 25 (58) | 24 (41) | 20 (63) | 26 (39) | ||
| OSS ≥5 | 19 (45) | 6 (10) | 16 (52) | 6 (9) | ||
| Schirmer’s test ≤5 mm | 35 (81) | 33 (56) | 26 (81) | 40 (60) | ||
|
| ||||||
| Histological analysis | Labial gland | P-value | Parotid gland | P-value | ||
|
| ||||||
| FS | 1.6 (0.8–2.8) | 0.0 (0.0–0.6) | <0.001 | 1.3 (0.5–1.8) | 0.0 (0.0–0.2) | <0.001 |
| LELs | 18 (42) | 1 (2) | <0.001 | 18 (56) | 0 0 | <0.001 |
| Mean stage of LELs (stage 1–3) | 1.0 (1.0–1.25) | NA | NA | 1.7 (1.0-2.0) | NA | NA |
Data are presented as mean (s.d.), number (%) or median (IQR). Patients were classified as primary SS (pSS) or non-SS by using the 2016 ACR-EULAR criteria, based on either (A) only the labial gland result or (B) only the parotid gland result. FS: focus score; UWS: unstimulated whole saliva; OSS: ocular staining score; LELs: lymphoepithelial lesions.
Histological analysis
In total, 562 and 516 regions of interests containing striated ducts were selected within the included labial and parotid gland biopsies, respectively. Evaluation of ductal hyperplasia on H&E staining showed that striated ducts with hyperplasia (LELs) were exclusively found in labial and parotid gland biopsies of pSS patients, except for one labial gland biopsy (FS > 1.0) of a patient classified as non-SS, in which one LEL was found (Table 1). More severe LEL stages (≥ stage 2) were more often found in parotid gland biopsies compared with labial gland biopsies (Fig. 3). In the subgroup of pSS patients with presence of LELs in both the labial and the parotid gland biopsy (N = 13), the mean stage of LELs was significantly higher in the parotid gland biopsy compared with the labial gland biopsy (P = 0.025). FS was higher in biopsies of pSS patients with LELs compared with biopsies of pSS patients without LELs (P = 0.024 for the labial and P < 0.001 for the parotid gland).
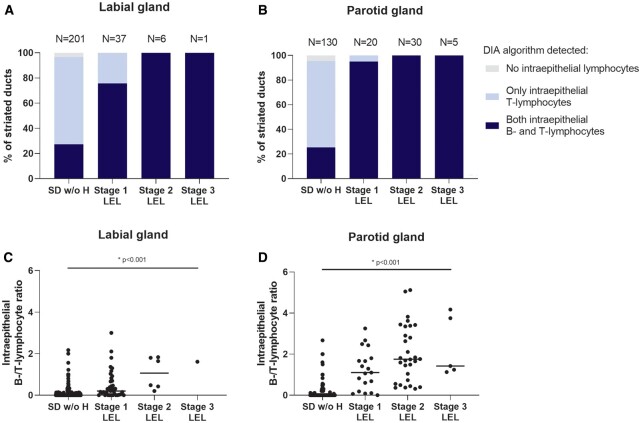
Fig. 3.
Presence of intraepithelial lymphocytes within analysed striated ducts with and without hyperplasia of pSS patients
(A–B) Percentages of analysed striated ducts with presence of intraepithelial B- and T-lymphocytes or presence of T-lymphocytes only. Results are shown for striated ducts without hyperplasia (SD w/o H) and striated ducts with hyperplasia (lymphoepithelial lesions—LELs) in both (A) labial and (B) parotid gland biopsies pSS patients. LELs were scored in three stages of severity based on the percentage of ductal hyperplasia (see Fig. 1). Numbers of ducts included in the DIA are shown above the bars. (C–D) B/T-lymphocyte ratios within SD w/o H and striated ducts with hyperplasia (LELs) of pSS patients in both (C) labial and (D) parotid gland biopsies. Horizontal lines show medians. Generalized estimating equation (GEE) analyses were used to test differences in B/T-lymphocyte ratios over the stages of LELs within both biopsies. DIA: digital image analysis
Correlation of digital image analysis and manual counting
DIA images are shown in Fig. 1F–H. Interobserver agreement between manual counting and digital analysis was very high: ρ = 0.93 for numbers of intraepithelial B-lymphocytes and ρ = 0.94 for numbers of intraepithelial T-lymphocytes (Supplementary Fig. S1, available at Rheumatology online). Bland–Altman plots show that mean differences for B-lymphocytes were not far from zero (95% CI of the mean: +0.99, –1.74), indicating that there was no systematic bias. For T-lymphocytes, there was a pattern for DIA results to be higher compared with manual results, which was shown by the positive mean difference (Supplementary Fig. S1, available at Rheumatology online). Outliers in the Bland–Altman plots are only present in the higher scoring ranges for both intraepithelial B- and T-lymphocytes, as the differences between both methods become larger with increasing averages.
Digital image analysis
Within salivary gland biopsies of pSS patients, the DIA algorithm detected T-lymphocytes in all striated ducts with hyperplasia (LELs) and in nearly all (95% of) analysed striated ducts without hyperplasia. Intraepithelial B-lymphocytes were found in almost all (90% of) striated ducts with hyperplasia (LELs) and in 27% and 25% of analysed striated ducts without hyperplasia in labial and parotid gland biopsies of pSS patients (Fig. 3A, B). In total, 59% of analysed labial gland and 68% of analysed parotid gland biopsies of pSS patients contained striated ducts with intraepithelial B-lymphocytes (Table 2). Within striated ducts with hyperplasia, the intraepithelial B-lymphocytes were predominantly located in the area of ductal hyperplasia. Although both B- and T-lymphocytes were almost always present within striated ducts with hyperplasia (LELs), the B/T-lymphocyte ratio became higher and shifted to B-lymphocytes when the severity of LELs increased (Fig. 3C, D). The B/T ratio was associated with the focus score in both labial (ρ = 0.42) and parotid (ρ = 0.61) gland biopsies of pSS patients. In the subgroup of pSS patients for whom we analysed both biopsies (N = 33), intraepithelial B-lymphocytes were found in 20 patients, and of these 15 patients (75%) had B-lymphocyte–containing ducts in both the labial and the parotid gland biopsy. Furthermore, there was a good association between the B/T-lymphocyte ratios within the labial and the parotid gland of these pSS patients (ρ = 0.74).
Table 2.
Clinical parameters of pSS patients with and without the presence of B-lymphocyte–containing striated ducts
| (A) Labial gland |
(B) Parotid gland |
|||||
|---|---|---|---|---|---|---|
| ACR-EULAR+ (labial) |
ACR-EULAR+ (parotid) |
|||||
|
N = 39 pSS patientsa |
N = 28 pSS patientsa |
|||||
| Intraepithelial B-lymphocytes absent | Intraepithelial B-lymphocytes present | P-value | Intraepithelial B-lymphocytes absent | Intraepithelial B-lymphocytes present | P-value | |
| (N = 16 patients) | (N = 23 patients) | (N = 9 patients) | (N = 19 patients) | |||
| Focus score | 1.33 (0.65–1.98) | 1.91 (0.79–3.69) | 0.098 | 0.15 (0.00–1.30) | 1.52 (0.95–2.89) | 0.006 |
| Oral and ocular function tests | ||||||
| UWS (ml/min) | 0.18 (0.02–0.30) | 0.05 (0.01–0.11) | 0.089 | 0.09 (0.01–0.28) | 0.08 (0.04–0.18) | 0.658 |
| SWS (ml/min) | 0.32 (0.22–0.81) | 0.18 (0.02–0.32) | 0.011 | 0.30 (0.18–1.12) | 0.21 (0.05–0.42) | 0.190 |
| OSS | 2.5 (1.0–4.8) | 5.5 (1.8–7.3) | 0.048 | 1.0 (0.0–6.5) | 5.0 (1.75–7.0) | 0.169 |
| Schirmer’s test | 4.0 (2.3–8.8) | 2.0 (0.0–5.0) | 0.071 | 4.0 (0.0–16.5) | 2.0 (0.0–5.0) | 0.401 |
| Clinical examination | ||||||
| ESSDAI total score | 3.0 (1.0–8.5) | 5.0 (2.0–16) | 0.144 | 2.0 (1.5–6.5) | 4.0 (1.0–13.0) | 0.311 |
| Serological parameters | ||||||
| Anti-SSA positivity | 9 (56) | 21 (91) | 0.015 | 8 (89) | 18 (95) | 0.548 |
| Anti-SSB positivity | 2 (13) | 14 (61) | 0.003 | 2 (22) | 12 (63) | 0.052 |
| RF positivity (>5 IU/ml) | 3 (19) | 20 (87) | <0.001 | 2 (22) | 17 (90) | 0.001 |
| RF levels (IU/ml) | 1.3 (0.0–3.5) | 38.5 (9.6–82.0) | <0.001 | 1.40 (0.38–4.65) | 51.0 (20.0–80.0) | 0.001 |
| IgG levels (g/l) | 11.5 (9.7–16.6) | 18.8 (13.9–20.2) | 0.004 | 11.5 (9.15–14.8) | 17.2 (13.9–20.1) | 0.029 |
| ESR | 9.5 (6.0–39.5) | 33.0 (18.0–47.0) | 0.026 | 9.0 (5.5–31.5) | 33.0 (18.0–50.0) | 0.019 |
Data are presented as median (IQR) or number (%).
Data for four pSS patients is missing because of an inability to align the slides stained for CD20. UWS: unstimulated whole saliva; SWS: stimulated whole saliva; OSS: ocular staining score; ESSDAI: EULAR EULAR SS Disease Activity Index.
Within the salivary gland biopsies of patients classified as non-SS, all analysed striated ducts were without hyperplasia, except for one labial gland (FS > 1) that harboured one duct with hyperplasia (LEL). The DIA algorithm detected T-lymphocytes in nearly all (94% of) analysed striated ducts without hyperplasia of non-SS patients. Intraepithelial B-lymphocytes, on the other hand, were only detected in four striated ducts without hyperplasia (0.8%), within one labial and two parotid gland biopsies of three patients of the non-SS group (2–3%). Two of these patients classified as non-SS were diagnosed as having pSS by the treating physician, based on the combination of sicca symptoms, abnormal findings by sialochemistry and ophthalmological evaluation, and FS of the biopsies. The third patient with intraepithelial B-lymphocytes was not diagnosed as having pSS by the treating physician, but was clinically suspected of developing a different systemic autoimmune disease.
Together, presence of intraepithelial B-lymphocytes in labial or parotid salivary gland biopsies seems to be a clear indicator of pSS. Intraepithelial B-lymphocytes were not only present within striated ducts with hyperplasia (LELs), but also within around 25% of the analysed striated ducts without hyperplasia in biopsies of pSS patients. This finding strongly supports the hypothesis that presence of B-lymphocytes precedes the hyperplastic reaction of the ductal epithelium forming LELs in pSS patients [16]. Therefore, striated ducts without hyperplasia, but with intraepithelial B-lymphocytes, will from now on be referred to as precursor LELs (pre-LELs).
Associations with clinical parameters
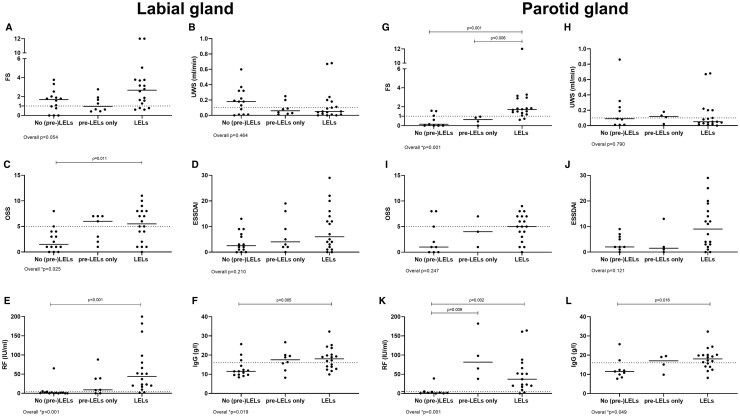
In parotid gland biopsies, FS was significantly higher when ducts with intraepithelial B-lymphocytes were present (Table 2). Furthermore, differences were found in oral and ocular function tests (stimulated whole saliva; ocular staining score) between pSS patients with and without ducts with intraepithelial B-lymphocytes (Table 2). Although ESSDAI scores were higher in patients with ducts containing intraepithelial B-lymphocytes, differences were not significant. However, when intraepithelial B-lymphocytes were not taken into account, ESSDAI scores were significantly higher in patients with striated ducts with hyperplasia (LELs), compared with pSS patients without LELs in the parotid biopsy (P = 0.038). Serological parameters were consistently higher in pSS patients with presence of intraepithelial B-lymphocytes within striated ducts, irrespective whether the labial or parotid gland was taken into account (Table 2). In Fig. 4, pSS patients without the presence of striated ducts with hyperplasia (LELs), but with the presence of pre-LELs, are presented as a separate group (pre-LELs only). For most clinical parameters, the median values of the pre-LEL only group were in between the median values of the groups with and without LELs. For IgG and RF levels, the median values of patients with pre-LELs and patients with LELs were above the clinical cut-off values, in contrast to patients without (pre-)LELs (Fig. 4). Although FS increased over the stages of LELs, the severest LEL stage per biopsy was not associated with other clinical parameters (Supplementary Fig. S2, available at Rheumatology online).
Fig. 4.
Clinical parameters in pSS patients with different (pre)LEL stages in labial and parotid gland biopsies
Clinical parameters in three groups of pSS patients: (1) patients without LELs and without pre-LELs, (2) patients with pre-LELs only, and (3) patients with full-blown LELs within labial (A–F) and parotid (G–L) salivary gland biopsies. (A, G) focus score (FS); (B, H) unstimulated whole saliva (UWS); (C, I) ocular staining scores (OSS); (D, J) Eular Sjögren’s syndrome disease activity index (ESSDAI) scores; (E, K) RF levels; (F, L) IgG levels. Horizontal bars represent medians. Overall differences between groups were analysed by Kruskal–Wallis tests, overall P-values are presented below the graphs. In cases where overall P-values were <0.05, Mann–Whitney U tests were used to test between-group differences. Dotted lines represent cut-off values of clinical parameters: (B, K) UWS = 0.10 ml/min; (C, I) OSS = 5; (E, K) RF = 5 IU/ml; (F, L) IgG = 16 g/l. LEL: lymphoepithelial lesion, pre-LEL: striated duct without hyperplasia with infiltrating B-lymphocytes.
Discussion
In this study, we developed a DIA algorithm that objectively detected B- and T-lymphocytes within striated ducts in labial and parotid salivary glands of patients suspected of having pSS. We showed that presence of intraepithelial B-lymphocytes, and not intraepithelial T-lymphocytes, was a key finding in salivary gland biopsies of pSS patients, in contrast to biopsies of non-SS patients. In total, 59% of labial and 68% of parotid gland biopsies of pSS patients contained intraepithelial B-lymphocytes. These B-lymphocytes were detected within (almost) all striated ducts with hyperplasia (LELs), but also in ∼25% of analysed striated ducts without hyperplasia. Importantly, the presence of ducts with intraepithelial B-lymphocytes was associated with clinical parameters of pSS, such as oral and ocular function tests and serological parameters.
The DIA algorithm presented in this study is an accurate and objective method for detecting intraepithelial lymphocytes within striated ducts in salivary gland tissue. In the Bland–Altman plots, mean differences in numbers of intraepithelial B-lymphocytes between DIA and manual counting were not far from zero (Supplementary Fig. S1, available at Rheumatology online). Outliers in these plots were only seen in the ducts with the highest numbers of lymphocytes, which can be explained by difficulties in manual identification of the borders of largely infiltrated striated ducts. Therefore, using a DIA algorithm to precisely identify ductal borders makes DIA results more reliable compared with manual analyses. Although interobserver agreement between manual and digital analysis of T-lymphocytes was high, there was a positive mean difference in T-lymphocyte counts between DIA and manual counting, indicating a pattern that DIA counted more T-lymphocytes. However, based on these results, evaluation of the number of intraepithelial CD3+ T-lymphocytes will not assist in the detection of LELs, as we have shown that intraepithelial T-lymphocytes are found in almost all analysed striated ducts of both non-SS and pSS patients. Although there is no added value of detecting intraepithelial CD3+ T-lymphocytes for LEL evaluation, T-lymphocytes may contribute (directly or indirectly) to the development of LELs. Therefore, it would be interesting to analyse the phenotype of intraepithelial T-lymphocytes in pSS more thoroughly in future studies. One of the drawbacks of the VTS technique used in this study is the need to align three slides stained by immunohistochemistry, which places high demands on serial sectioning of the slides. As we have shown that intraepithelial T-lymphocytes do not assist in LEL detection, a dual- (CK and CD20) rather than triple-staining technique could be used in future studies and clinical practice. This would reduce alignment difficulties. Furthermore, deep learning–based algorithms that allow identification of striated ducts on the slides stained for CD20 are currently under investigation. This approach will avoid virtual dual staining altogether. Another option would be the use of multiplexed tissue imaging technologies [21]. However, while digital and computational pathology algorithms are upcoming in routine clinical pathology practice, multiplexing techniques are far from clinical implementation.
In contrast to intraepithelial T-lymphocytes, ductal intraepithelial B-lymphocytes were largely restricted to salivary gland tissue of pSS patients. Furthermore, B-lymphocytes were not only found in the hyperplastic area of striated ducts with hyperplasia (LELs), but also in a proportion of analysed striated ducts without hyperplasia of pSS patients, which we called precursor LELs (pre-LELs). These results are in line with our previous hypothesis that ductal hyperplasia leading to LEL formation is driven by infiltrating B-lymphocytes [16, 22]. However, the DIA algorithm did not detect B-lymphocytes in 10 ducts with hyperplasia scored as stage 1 LELs (10%) (Fig. 3). In 5 of these 10 ducts, manual revision showed that the DIA algorithm had failed to detect intraepithelial B-lymphocytes. The effect of the missed B-lymphocytes was, however, negligible on the biopsy levels, as intraepithelial B-lymphocytes were detected in other ducts within the same biopsy in 4 of these 5 cases. An explanation for the apparent absence of B-lymphocytes in the 5 remaining stage 1 LELs is that intraepithelial B-lymphocytes are present at a higher or lower section level and not in the section that was analysed. Another explanation is that these 5 ducts without B-lymphocytes were erroneously scored as stage 1 LELs on H&E staining. For all 5 ducts, a consensus meeting was needed, as it remains difficult to distinguish a tangentially sectioned duct from a small area of ductal hyperplasia on H&E staining. Previously, multiple studies showed that DIA algorithms can overcome manual immunohistochemistry scoring issues by improving reliability and reproducibility [23]. For analysis of salivary glands specifically, Lucchesi et al. recently showed that using DIA to assess FS in salivary gland biopsies improved inter-rater agreement [24]. The DIA algorithm presented in the present study assists in objective detection of intraepithelial B-lymphocytes on slides stained for CD20 and hmwCK, thereby improving the subjective manual evaluation of LELs. The algorithm that was used in this study not only enables objective detection of LELs, but also reveals pre-LELs, which is not possible with manual scoring of H&E-stained slides.
Although the incidence of more severe LEL stages is higher in the parotid gland compared with the labial gland, DIA results were comparable between the two biopsy types. Most patients with B-lymphocyte–containing ducts in at least one of their biopsies had intraepithelial B-lymphocytes (with or without hyperplasia) in both the labial and the parotid gland (75%). Furthermore, there was a strong association between B/T-lymphocyte ratios in the two glands (ρ = 0.74). Together, these results show that the algorithm that was used in this study can be used in both types of glands and will produce similar results.
Around 60–70% of biopsies taken from pSS patients contained intraepithelial B-lymphocytes within striated ducts, against only three patients classified as non-SS (2–3%). Interestingly, two of these three patients were diagnosed as having pSS by the treating physician. As the ACR-EULAR criteria are developed to select a homogeneous group of patients for clinical trials and are not designed for clinical diagnosis at individual patient level, misclassification remains possible [5, 25, 26]. Taking this into account, we can conclude that all patients with intraepithelial B-lymphocytes in striated ducts in this cohort were diagnosed with pSS, except for one patient who is clinically at risk of developing pSS or another autoimmune disease in the future. Thus, the presence of B-lymphocyte–containing ducts within salivary gland biopsies of sicca patients is a strong indicator of pSS, and these B-lymphocyte–containing ducts can be assessed by an objective DIA algorithm using slides stained for CD20 and hmwCK.
In this diagnostic cohort, the presence of intraepithelial B-lymphocytes within striated ducts of salivary gland biopsies was associated with differences in ocular and oral function test results and serological parameters, such as the presence of autoantibodies and levels of RF, IgG and ESR (Table 2). Interestingly, no significant differences were found in unstimulated whole saliva (UWS) levels between pSS patients with and without intraepithelial B-lymphocytes within either the labial or parotid gland biopsy. Apparently, lower UWS levels in pSS patients are not directly related to presence of LELs or pre-LELs, which could be explained by the fact that totally occluded stage 3 LELs that can block salivary flow are not frequently found in salivary glands of pSS patients. Furthermore, other histological parameters, such as FS, percentage of inflammatory infiltrate, and presence of germinal centres, do not correlate with salivary flow either [27–29]. This indicates that other mechanisms than the formation of periductal infiltrates and LELs, such as defects in acinar cells, may play a dominant role in decreased saliva production in pSS patients [22]. However, the presence of LELs may contribute to the altered electrolyte composition of the saliva of pSS patients, in particular sodium concentration [30]. Although not significant, pSS patients with striated ducts with hyperplasia (LELs) seem to have higher disease activity in terms of ESSDAI, serological parameters, and ocular and oral function test results, compared with patients with pre-LELs only (Fig. 4). Stage of LEL severity (stage 1–3), on the other hand, was not associated with the clinical parameters of pSS (Supplementary Fig. S2, available at Rheumatology online). Further longitudinal studies should provide evidence regarding whether the presence of ductal intraepithelial B-lymphocytes in glandular biopsies of pSS patients can be predictive of developing higher disease activity.
In conclusion, the presence of intraepithelial B-lymphocytes, and not intraepithelial T-lymphocytes, is a key finding in salivary gland biopsies of pSS patients and is strongly suggestive of pSS. The objective DIA algorithm used in this study detected B-lymphocytes within almost all striated ducts with hyperplasia (LELs), and also in one-quarter of the analysed striated ducts without hyperplasia of pSS patients. These B-lymphocyte–containing ducts without hyperplasia likely represent a precursor stage of LELs (pre-LELs). Thus, the detection of intraepithelial B-lymphocytes by using DIA is not only an objective alternative for the subjective evaluation of LELs on H&E staining, but also allows detection of pre-LELs, which is not possible on H&E staining. Furthermore, the presence of intraepithelial B-lymphocytes was associated with clinical parameters of pSS, such as oral and ocular function test results and serology. Together, the data indicate that identification of B-lymphocyte containing ducts in salivary gland biopsies should be used as an objective marker in the diagnostic work-up of sicca patients suspected of having pSS.
Funding: This study was financially supported by grants from the NIH (USA; RFA-DE-06–004) and the Jan-Kornelis de Cock Foundation (Groningen, the Netherlands). There was no involvement of these funding sources in the study design, data collection, analysis and interpretation of data, or writing of this report.
Disclosure statement: S.H. is an employee of Visiopharm. B.v.d.V. is on the scientific advisory board of Visiopharm, for which UMCG is compensated. The other authors have declared no conflicts of interest.
Supplementary Material
Contributor Information
Martha S van Ginkel, Department of Rheumatology and Clinical Immunology.
Tineke van der Sluis, Department of Pathology and Medical Biology, University of Groningen and University Medical Center Groningen, Groningen, The Netherlands.
Marian L C Bulthuis, Department of Pathology and Medical Biology, University of Groningen and University Medical Center Groningen, Groningen, The Netherlands.
Henk J Buikema, Department of Pathology and Medical Biology, University of Groningen and University Medical Center Groningen, Groningen, The Netherlands.
Erlin A Haacke, Department of Rheumatology and Clinical Immunology; Department of Pathology and Medical Biology, University of Groningen and University Medical Center Groningen, Groningen, The Netherlands.
Suzanne Arends, Department of Rheumatology and Clinical Immunology.
Stine Harder, Visiopharm A/S, Hørsholm, Denmark.
Fred K L Spijkervet, Department of Oral and Maxillofacial Surgery, University of Groningen and University Medical Center Groningen, Groningen, The Netherlands.
Hendrika Bootsma, Department of Rheumatology and Clinical Immunology.
Arjan Vissink, Department of Oral and Maxillofacial Surgery, University of Groningen and University Medical Center Groningen, Groningen, The Netherlands.
Frans G M Kroese, Department of Rheumatology and Clinical Immunology.
Bert van der Vegt, Department of Pathology and Medical Biology, University of Groningen and University Medical Center Groningen, Groningen, The Netherlands.
Data availability statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Brito-Zerón P, Baldini C, Bootsma H. et al. Sjögren syndrome. Nat Rev Dis Primers 2016;2:16047. [DOI] [PubMed] [Google Scholar]
- 2. Voulgarelis M, Tzioufas AG.. Pathogenetic mechanisms in the initiation and perpetuation of Sjögren’s syndrome. Nat Rev Rheumatol 2010;6:529–37. [DOI] [PubMed] [Google Scholar]
- 3. Greenspan JS, Daniels TE, Talal N, Sylvester RA.. The histopathology of Sjögren’s syndrome in labial salivary gland biopsies. Oral Surg Oral Med Oral Pathol 1974;37:217–29. [DOI] [PubMed] [Google Scholar]
- 4. Daniels TE. Labial salivary gland biopsy in Sjögren’s syndrome. Assessment as a diagnostic criterion in 362 suspected cases. Arthritis Rheum 1984;27:147–56. [DOI] [PubMed] [Google Scholar]
- 5. Shiboski CH, Shiboski SC, Seror R. et al. ; International Sjögren's Syndrome Criteria Working Group. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis 2017;76:9–16. [DOI] [PubMed] [Google Scholar]
- 6. Kroese FGM, Haacke EA, Bombardieri M.. The role of salivary gland histopathology in primary Sjögren’s syndrome: promises and pitfalls. Clin Exp Rheumatol 2018;36:S222–33. [PubMed] [Google Scholar]
- 7. Leroy JP, Pennec YL, Letoux G, Youinou P.. Lymphocytic infiltration of salivary ducts: a histopathologic lesion specific for primary Sjögren’s syndrome? Arthritis Rheum 1992;35:481–2. [DOI] [PubMed] [Google Scholar]
- 8. Ihrler S, Zietz C, Sendelhofert A, Riederer A, Löhrs U.. Lymphoepithelial duct lesions in Sjogren-type sialadenitis. Virchows Archiv 1999;434:315–23. [DOI] [PubMed] [Google Scholar]
- 9. LeRoy JP, Pennec YL, Jouquan J, Lelong A, Youinou P.. Relations of the histopathology of sublingual and labial salivary glands to the clinical presentation in primary Sjögren’s syndrome. Clin Exp Rheumatol 1989;7:171–4. [PubMed] [Google Scholar]
- 10. Leroy JP, Pennec YL, Soulier C. et al. Follow up study of labial salivary gland lesions in primary Sjogren’s syndrome. Ann Rheum Dis 1992;51:777–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morgan WS, Castleman B.. A clinicopathologic study of Mikulicz’s disease. Am J Pathol 1953;29:471–503. [PMC free article] [PubMed] [Google Scholar]
- 12. Dardick I, Van Nostrand AWP, Rippstein P. et al. Characterization of epimyoepithelial islands in benign lymphoepithelial lesions of major salivary gland: an immunohistochemical and ultrastructural study. Head Neck Surg 1988;10:168–78. [DOI] [PubMed] [Google Scholar]
- 13. Pammer J, Horvat R, Weninger W, Ulrich W.. Expression of bcl-2 in salivary glands and salivary gland adenomas. A contribution to the reserve cell theory. Pathol Res Pract 1995;191:35–41. [DOI] [PubMed] [Google Scholar]
- 14. Yoshihara T, Morita M, Ishii T.. Ultrastructure and three-dimensional imaging of epimyoepithelial islands in benign lymphoepithelial lesions. Oto-Rhino-Laryngol 1995;252:106–11. [DOI] [PubMed] [Google Scholar]
- 15. Pijpe J, Meijer JM, Bootsma H, van der Wal JE. et al. Clinical and histologic evidence of salivary gland restoration supports the efficacy of rituximab treatment in Sjögren’s syndrome. Arthritis Rheum 2009;60:3251–6. [DOI] [PubMed] [Google Scholar]
- 16. van Ginkel MS, Haacke EA, Bootsma H. et al. Presence of intraepithelial B-lymphocytes is associated with the formation of lymphoepithelial lesions in salivary glands of primary Sjögren’s syndrome patients. Clin Exp Rheumatol 2019;37:S42–8. [PubMed] [Google Scholar]
- 17. Nakshbandi U, Haacke EA, Bootsma H. et al. Bcl6 for identification of germinal centres in salivary gland biopsies in primary Sjögren’s syndrome. Oral Diseases 2020;26:707–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Delli K, Haacke EA, Ihrler S. et al. Need for consensus guidelines to standardise the assessment of germinal centres and other histopathological parameters in salivary gland tissue of patients with primary Sjögren’s syndrome. Ann Rheum Dis 2016;75:e32. [DOI] [PubMed] [Google Scholar]
- 19. Pijpe J, Kalk WWI, van der WAL JE. et al. Parotid gland biopsy compared with labial biopsy in the diagnosis of patients with primary Sjogren’s syndrome. Rheumatology (Oxford) 2007;46:335–41. [DOI] [PubMed] [Google Scholar]
- 20. van Nimwegen JF, van Ginkel MS, Arends S. et al. Validation of the ACR-EULAR criteria for primary Sjögren’s syndrome in a Dutch prospective diagnostic cohort. Rheumatology (Oxford) 2018;57:818–25. [DOI] [PubMed] [Google Scholar]
- 21. Tan WCC, Nerurkar SN, Cai HY. et al. Overview of multiplex immunohistochemistry/immunofluorescence techniques in the era of cancer immunotherapy. Cancer Commun (Lond) 2020;40:135–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Verstappen GM, Pringle S, Bootsma H, Kroese FGM.. Epithelial–immune cell interplay in primary Sjögren syndrome salivary gland pathogenesis. Nat Rev Rheumatol 2021;17:333–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hamilton PW, Bankhead P, Wang Y, Hutchinson R. et al. Digital pathology and image analysis in tissue biomarker research. Methods 2014;70:59–73. [DOI] [PubMed] [Google Scholar]
- 24. Lucchesi D, Pontarini E, Donati V. et al. The use of digital image analysis in the histological assessment of Sjögren’s syndrome salivary glands improves inter-rater agreement and facilitates multicentre data harmonisation. Clin Exp Rheumatol 2020;38:180–8. [PubMed] [Google Scholar]
- 25. Jonsson R, Brokstad KA, Jonsson MV, Delaleu N, Skarstein K.. Current concepts on Sjögren’s syndrome – classification criteria and biomarkers. Eur J Oral Sci 2018;126:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baldini C, Ferro F, Bombardieri S.. Classification criteria in Sjögren’s syndrome. Ann Transl Med 2017;5:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mossel E, van Ginkel MS, Haacke EA. et al. Histopathology, salivary flow and ultrasonography of the parotid gland: three complementary measurements in primary Sjögren’s syndrome. Rheumatology 2022;61:2472--82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bookman AAM, Shen H, Cook RJ. et al. Whole stimulated salivary flow: correlation with the pathology of inflammation and damage in minor salivary gland biopsy specimens from patients with primary Sjögren’s syndrome but not patients with sicca. Arthritis Rheum 2011;63:2014–20. [DOI] [PubMed] [Google Scholar]
- 29. Risselada AP, Looije MF, Kruize AA, Bijlsma JWJ, van Roon JAG.. The role of ectopic germinal centers in the immunopathology of primary Sjögren’s Syndrome: a systematic review. Semin Arthritis Rheum 2013;42:368–76. [DOI] [PubMed] [Google Scholar]
- 30. Pringle SA, Berkhof B, van Ginkel M. et al. Parotid salivary sodium levels of pSS patients suggest B-cell mediated epithelial sodium channel dysruption. Clin Exp Rheumatol 2021;39:30–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.