Abstract
Solid tumors have a unique tumor microenvironment (TME), which includes hypoxia, low acidity, and high hydrogen peroxide and glutathione (GSH) levels, among others. These unique factors, which offer favourable microenvironments and nourishment for tumor development and spread, also serve as a gateway for specific and successful cancer therapies. A good example is metal peroxide structures which have been synthesized and utilized to enhance oxygen supply and they have shown great promise in the alleviation of hypoxia. In a hypoxic environment, certain oxygen-dependent treatments such as photodynamic therapy and radiotherapy fail to respond and therefore modulating the hypoxic tumor microenvironment has been found to enhance the antitumor impact of certain drugs. Under acidic environments, the hydrogen peroxide produced by the reaction of metal peroxides with water not only induces oxidative stress but also produces additional oxygen. This is achieved since hydrogen peroxide acts as a reactive substrate for molecules such as catalyse enzymes, alleviating tumor hypoxia observed in the tumor microenvironment. Metal ions released in the process can also offer distinct bioactivity in their own right. Metal peroxides used in anticancer therapy are a rapidly evolving field, and there is good evidence that they are a good option for regulating the tumor microenvironment in cancer therapy. In this regard, the synthesis and mechanisms behind the successful application of metal peroxides to specifically target the tumor microenvironment are highlighted in this review. Various characteristics of TME such as angiogenesis, inflammation, hypoxia, acidity levels, and metal ion homeostasis are addressed in this regard, together with certain forms of synergistic combination treatments.
1. Introduction
Cancer has risen to become one of the major threats to human health, and it is reported to have caused approximately 10 million deaths globally in 2020 alone, according to data available on the WHO website (https://www.who.int/news-room/fact-sheets/detail/cancer). This accounts for nearly one in six deaths, making cancer the biggest cause of mortality globally, with breast, lung, colon, rectum, and prostate cancers being the most prevalent types of cancer.
According to the American Cancer Society, in the United States of America alone, an estimated 609,000 people died of cancer in the year 2021 with lung and colon cancers being the most prevalent among both men and women. In the same period, 1,898,160 new cases of cancer were reported (Figure 1) [1].
Figure 1.
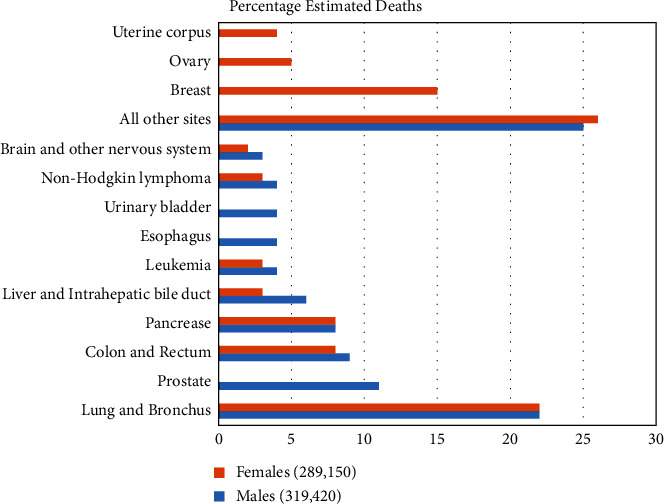
Estimated percentage of deaths from various cancers in the US in 2021.
From such grim statistics, the advancement and development of new approaches to combat this disease are critical. With the advancement of technology in scientific research, a number of unique cancer treatment approaches have been developed. These include photodynamic therapy (PDT), chemodynamic therapy (CDT), photothermal therapy, and nanoparticles, among others, which have been developed to complement the standard treatments which utilize chemotherapy and radiation [2].
Many technologies are now being investigated in clinical trials, and some have even been adopted in clinical practice. Targeted delivery of active drugs, delivery by nanoparticles, and targeting overexpressed proteins and antigens on tumor cell surfaces are a few of these methods [3]. The ability to treat cancer has advanced enormously over the past 70 years, from cytotoxic medications that shrink tumors but have serious systemic side effects, to targeted therapies that may kill cancer cells while sparing healthy organs. This article discusses some of the new approaches that target the tumor microenvironment (TME) by the use of metal peroxides to alter the oxygen levels in tumors [4, 5].
Many issues still need to be studied in order to further understand cancer therapy. Research is making significant efforts to find novel and efficient treatments that can lessen side effects. This has been the focus over the past ten years, where several research studies have concentrated on developing alternative therapies to reduce the negative effects of conventional medications [6, 7].
The departure from the cisplatin operation model, which employs the metal as the principal active centre of the therapy is a developing trend, even though the traditional chemotherapy approach using DNA as the set target continues to yield significant results. Most of the available and effective anticancer medications currently on the market are met with high toxicity levels [8, 9]. Cancer research may be considered as an area with significant unmet needs since cancers also acquire immunity against most medications [10–12].
Metal-based drug platforms for cancer therapy have been used for a long time and have been shown to perform well in the detection and treatment of illnesses, and they are critical in the early stages of therapeutic development. Chief among these are platinum-containing medications which are the most extensively studied and used as antitumor chemotherapy treatments [13–15]. They constitute one of the main triumphs in the field of application of medical bioinorganic chemistry. These drugs which include cisplatin, carboplatin, and oxaliplatin, all in the class of cell cycle nonspecific treatments, are being employed in disorders such as gynaecological and digestive system cancers [16, 17].
Their mechanism of action involves penetrating the nucleus of a cell and reacting with DNA molecules to produce platinum-DNA complexes, which distort DNA structure and prevent replication and transcription [18, 19]. Antitumor medicines containing platinum, on the other hand, have drawbacks such as limited bioavailability, high systemic toxicity, resistance, and limited selectivity for cancerous cells [20, 21].
In particular, when platinum medications and proteins are combined in the blood, the reaction inactivates most of these drugs before they reach the desired targets [22]. This results in severe side effects as well as decreased bioavailability, thereby reducing the efficacy of these treatments [21]. Furthermore, typical platinum medications primarily target the genome, which in some cases is easily repaired by cancer cells. This raises the susceptibility of these cells to platinum drug resistance [11, 23].
In the physiological environment, metal ions are known to play key roles in a variety of important cellular metabolic pathways including material movement into and out of the cell across the cell membrane, energy production, and transmission of information, among others [24, 25]. When these ions are not properly distributed or absorbed in cells, this can obstruct the aforementioned processes, resulting in permanent cellular injury, or the activation of metabolic responses which may lead to apoptosis [26, 27].
Metal peroxides have attracted particular research interest in biology and medicine due to their peculiar chemical reactions, associated reaction products, and particular biochemical effects of the released metal ions [28, 29]. Recently, versatile metal peroxide nanoparticles including CuO2, CaO2, MgO2, ZnO2, BaO2, and TiOx have been developed for therapeutic applications [30–32]. These include areas of cancer treatment, bacterial infection prevention, and tissue regeneration, where they have received substantial research attention [33, 34].
For many years, the logical design of drug targeting techniques has been explored, and flexible targeting protocols have been suggested to increase targeting effectiveness [35, 36]. However, targeting strategies are still far from ideal. The investigation of disease-specific therapy by inducing chemical reactions in situ has sparked intense study interest. Numerous nanoparticles that can initiate favourable chemical processes for the treatment of diseases are now being developed, either as nanocatalysts or nanoreactants [37, 38]. Since these nanomedicines are designed to respond to specific disease microenvironments, they are expected to improve drug targeting and therefore efficacy, at the same time reducing undesirable side effects.
Consisting primarily of metal ions and peroxo groups, metal peroxides may combine with water to form hydrogen peroxide and release metal ions in the process [34]. Numerous biological applications can benefit from the postgenerated hydrogen peroxide. For instance, in catalytic medicine, hydrogen peroxide can function as the reactant in a Fenton-like enzymatic reaction to produce large amounts of extremely harmful hydroxyl radicals [39]. The therapeutic effectiveness of procedures that involve oxygen, such as photodynamic treatment (PDT) and radiotherapy, can also be increased by the self-decomposition of hydrogen peroxide to create oxygen [40, 41].
The metal-ion component of metal peroxides takes part in a variety of biological processes, such as biochemical reactions and the process of tissue regeneration [42, 43]. On this basis, metal peroxide-based nanoparticles serve as nascent nanosystem with distinct intrinsic physicochemical characteristics, reactive aspects, and bioactivities for fulfilling diverse requirements of biological applications. Copper peroxide (CuO2), calcium peroxide (CaO2), magnesium peroxide (MgO2), zinc peroxide (ZnO2), barium peroxide (BaO2), and titanium peroxide (TiOx) are some of the metal peroxide nanosystems which have attracted interest in this area. As shown in Figure 2, they have been extensively investigated in several biomedical fields, including catalytic nanomedicine, based on their reactivity for hydrogen peroxide and oxygen generation and metal ion-based bioactivity [44–46].
Figure 2.
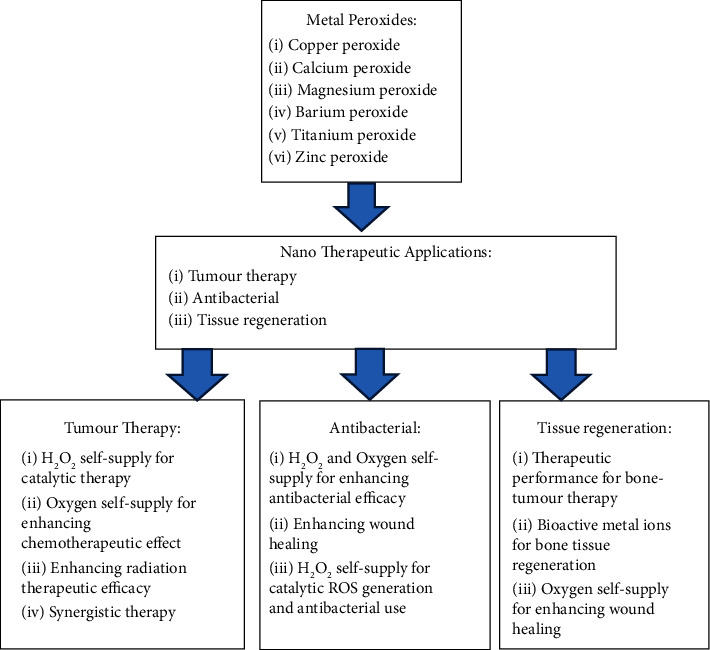
Applications for various metal peroxide nanotherapeutics.
2. Targeting the Tumor Microenvironment
Without a crucial interaction between cancerous cells and their immediate environment, the malignant characteristics of cancer cells cannot appear. Cancer growth is actively aided by the tumor infiltrate, which is made up of immune cells, angiogenic vasculature, lymphatic cells, and cancer-associated fibroblastic cells [47]. The capacity to alter these conditions is a crucial trait that allows tumor cells to develop some of the characteristic abilities required for tumor development and metastatic spread. Therefore, it has become essential in the area of cancer therapy to target the tumor microenvironment as a viable frontier in cancer treatment.
The notion of a complex tumor environment that promotes tumor growth and metastatic dispersion has replaced the tumor cell-centered perspective of cancer development as a result of the realization of the TME's crucial role in the genesis and progression of cancer [48]. As a result, new TME targets have been found that may assist, guide, and enhance the effects of numerous cancer medicines. The functioning of the tumor microenvironment (TME) dictates its fundamental and essential role in tumor morphology and physiology [49].
Numerous immune and nonimmune cell types may be detected inside the TME infrastructure, and together with the numerous substances they emit, these cells help to create an intratumoral milieu that is chronically inflammatory, immunosuppressive, and proangiogenic [50]. In these conditions, cancer cells can adapt and develop with a considerably lower chance of being found and eliminated by host immune surveillance. The number of biological molecules and mechanistic pathways that might be targeted for cancer treatment grows as our understanding of the TME expands. Here, a few of these particular microenvironments shown in Figure 3 are discussed.
Figure 3.
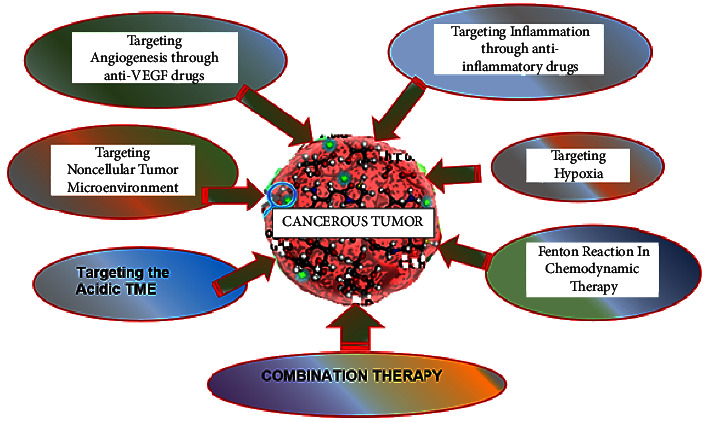
Approaches for targeting the tumor microenvironment.
2.1. Targeting Angiogenesis through Anti-VEGF Drugs
Proangiogenic and antiangiogenic factors generated by both malignant and nonmalignant cells tightly regulate the complicated process of vascularization in tumors through a number of signalling channels [51]. When proangiogenic factors are more prevalent, angiogenesis, sometimes referred to as the “angiogenic switch,” is activated [52]. The main proangiogenic factor in endothelial cell activation is the vascular endothelial growth factor-A (VEGF-A), although numerous other growth factors, including fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), and endothelial growth factor (EGF), are also proangiogenic [53]. The tumor vasculature with a deficit in pericytes and perivascular cells, as well as an increased permeability, leads to a leaky vascular system [54, 55]. This is in contrast to normal vasculature, which is characterized by an organized formation of mature endothelial cells covered with pericytes [56].
In vasculogenesis, the newly created blood vessels' ability to supply oxygen and nutrients contributes to tumor growth and proliferation [52]. Therefore, targeting angiogenesis is a potential option for therapeutic intervention in cancer treatment. Antiangiogenic medications which cause leaky vasculature have now been the subject of numerous clinical studies globally [57]. When used in conjunction with traditional chemotherapy treatments, the anti-VEGF antibody bevacizumab improves overall survival in patients with metastatic colorectal cancer, nonsmall cell lung cancer, and breast cancer [58, 59].
2.2. Targeting Inflammation through Anti-Inflammatory Drugs
Studies recognise persistent inflammation as a key player in the development of cancer [60]. Laboratory studies suggest that the presence of active chronic innate immune cell types, such as neutrophils, macrophages, and mast cells (MCs) promote tumorigenesis [61]. This is performed through tissue remodelling, instigation of angiogenesis, and uncontrolled cell proliferation, leading to the growth and advancement of malignant cells into ectopic tissue [61]. In this regard, a number of anti-inflammatory medications, including cyclooxygenase 2 inhibitors have been tested for colorectal and chemotherapy-resistant breast cancer [62, 63]. Nonsteroidal anti-inflammatory drugs are reported for breast, colorectal, and prostate cancer treatment [64]. Anti-inflammatory steroid drugs such as dexamethasone used for the treatment of brain tumors, have been found to lower tumor incidence and slow down tumor progression and lower overall mortality rates [65].
2.3. Targeting the Noncellular Tumor Microenvironment
In addition to extracellular matrix (ECM) molecules, the noncellular environment also consists of physical and chemical elements including pH, oxygen tension, interstitial pressure, and fluid flow [66]. Therefore, any alterations in ECM in the context of the tumor environment will have an impact on cancer cell activity. The ECM is increasingly understood to be a dynamic component of the tumor microenvironment rather than a static structure that only preserves tissue shape. Cell proliferation, migration, angiogenesis, and cancer metastasis are all known to be regulated by ECM components and their metabolites [67, 68].
The ability of the tumor microenvironment to support cancer cell proliferation, migration, and invasion, as well as to affect inflammatory responses and lymphangiogenesis, can be significantly impacted by changes in ECM degradation such as density and stiffness [69]. A deeper comprehension of this complex ecosystem will be necessary to enhance cancer therapy due to the complexity of tumor cell-host cell interactions and cell-ECM interactions inside a tumor. It seems improbable that focusing on a specific molecular pathway or kind of cell would result in effective anticancer treatments and prevent the development of drug resistance. To achieve long-term effectiveness, it is necessary to combine conventional “cell-centred” chemotherapies and radiation therapies with strategies that target the no-cellular tumor microenvironment.
2.4. Targeting Hypoxia
One of the main characteristics that distinguish cancer cells from normal cells is their uncontrolled proliferative behaviour, which is partly caused by abnormal vasculature [70]. The oxygen level in places with solid tumors decreases as a result of the cancer cells' fast oxygen consumption. The fast proliferating cells result in a significant diffusion distance for oxygen, from the network of blood vessels, and the cancer cells. The result is a highly hypoxic scenario as a result of the tumor's lack of oxygenated blood, which encourages the growth of cells that turns tumorous [71]. Additionally, hypoxic circumstances encourage cancer cells to switch from oxidative phosphorylation to anaerobic glycolysis, which naturally causes lactic acid to accumulate and lower extracellular pH in the tumor microenvironment [72, 73].
Additionally, tumor-associated and/or therapy-induced anaemia reduces the blood's ability to carry oxygen, which results in hypoxia in tumor areas [74]. Poor prognosis, radiotherapy/chemotherapy resistance, and tumor metastasis are all linked to hypoxia [75]. Tumor cells can adapt to hypoxic conditions by producing erythropoietin (EPO), switching from aerobic to anaerobic metabolism, downregulating DNA repair pathways, enlisting the assistance of stromal cells, and upregulating protooncogenes as well as hypoxia-inducible factor (HIF) 1 and HIF 2 [76]. Therapeutic medicines are frequently created as low-toxicity prodrugs in normoxic environments and are then selectively activated in hypoxic tumor regions to address hypoxia in TME [77].
The hypoxia-activated prodrug AQ4N ((1,4-bis((2-(dimethylamino-N-oxide)ethyl)amino)5,8-dihydroxy-anthracene-9,10-dione), also known as banoxantrone, is transformed into AQ4, a strong inhibitor of topoisomerase II, in hypoxic environments, and treat solid malignancies including bronchoalveolar lung carcinoma and ovarian cancer [78]. Hemeproteins transform AQ4 from the aliphatic N-oxide prodrug by two successive 2e reductions. Under hypoxic circumstances, hypoxia-activated prodrugs called enamine N-oxides can release small molecules through selective bioreductive two-electron bioreduction processes [79]. The resultant iminium ion, which is unsaturated, is easily reactive with biological nucleophiles [80].
The reaction scheme in Figure 4 shows the mechanism of action for the AQ4N and enamine N-oxide prodrugs.
Figure 4.
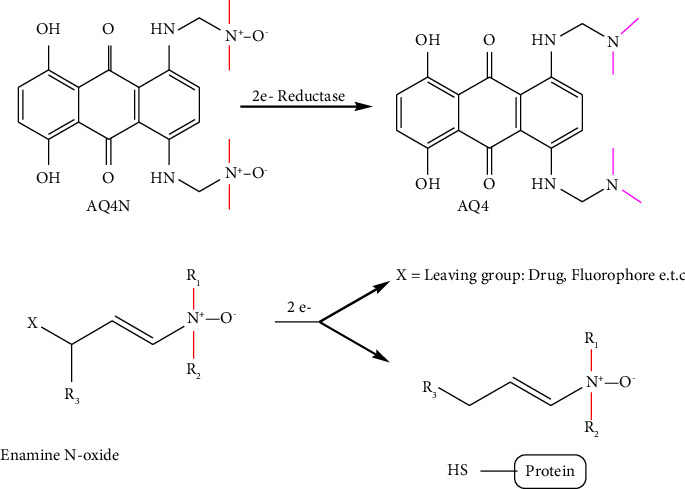
Mechanism of hypoxia-activated AQ4N and enamine N-oxide prodrugs.
Tirapazamine (TPZ), another hypoxia cell toxin, selectively shows cytotoxic effects under an hypoxic environment [81]. Its mode of action is based on the process where several intracellular reductases catalyse TPZ to generate a radical by adding an electron [82]. In an hypoxic environment, this highly reactive TPZ radical can result in DNA single- or double-strand breaks [83]. The TPZ radical's cytotoxicity is quickly reduced when it is oxidized back to its harmless parent under aerobic circumstances [84]. Under hypoxic conditions, the metabolism of TPZ leads to the loss of a water molecule to generate the benzotriazinyl (BTZ) radical, which also leads to DNA damage as illustrated by the reaction mechanism in Figure 5.
Figure 5.
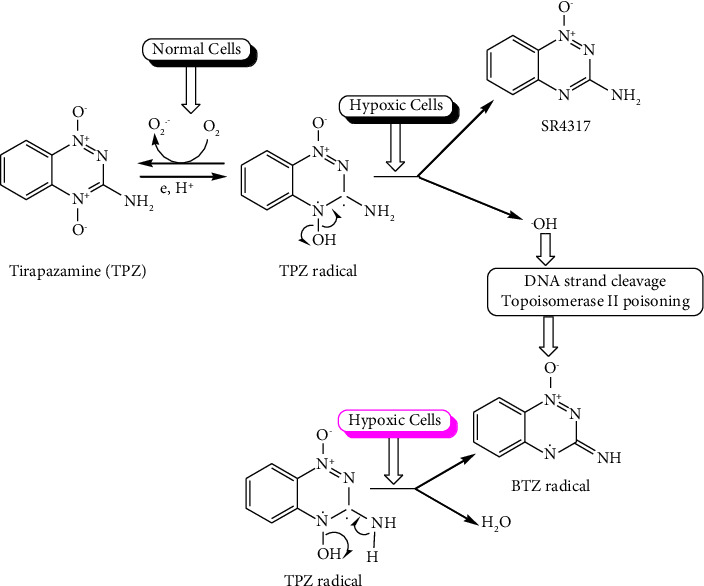
Summary mechanism of tripazamine under normal and hypoxic cells.
2.5. Targeting the Acidic TME
Normal tissues have an extracellular pH of 7.4, but the pH in TME is substantially lower (6.7–7.1). There are several processes through which tumors develop an acidic pH. As discussed earlier, tumor cells in a hypoxic environment primarily employ aerobic glycolysis as an energy metabolism process [73]. This results in increased production of lactic acid and H+, which are then released in the tumor microenvironment (TME) by passive diffusion and active membrane-based ion transport [85]. Tumor cells have greatly elevated levels of the H + -ATPases, Na+-H+ exchanger NHE1, and monocarboxylate-H+ efflux cotransporters MCT1 and MCT4, and these factors all contribute to H+ efflux [86]. Additionally, the preservation of low pH in TME is also aided by carbonic anhydrase 9 (CA9), which is overexpressed in several cancer types [87].
To address acidity in TME, several researchers have reported that proton pump inhibitors may slow the growth of hepatoblastoma and oesophageal adenocarcinoma [88–90]. Proton pump inhibitors, such as omeprazole, esomeprazole, rabeprazole, pantoprazole, or lansoprazole, significantly slow the growth and development of neoplasms in individuals with Barrett's oesophagus [91]. Bafilomycin A1, a proton pump inhibitor of the vacuolar type, has demonstrated viability to induce apoptosis in hepatoblastoma cells but not in healthy cells, suggesting that it may be used as a cancer therapy [92].
3. Fenton-Reaction Approach
With a high level of tumor selectivity, Fenton reaction-based catalytic nanoparticles have become a unique tumor-therapeutic technique [93, 94]. Typically, Fenton compounds are used in oxidative treatment to cause a disproportionate reaction and transform tumors with overexpressed hydrogen peroxide into toxic hydroxyl radicals [95–97]. However, the therapeutic effectiveness of these catalytic-process-based nanotherapeutics is significantly constrained by the low intratumoral hydrogen peroxide level of around 100 μm [98, 99]. The ability of metal peroxides to produce hydrogen peroxide opens up the idea of creating Fenton nanoagents for catalytic nanotherapeutics. Copper chloride, hydrogen peroxide, and sodium hydroxide have been used in an aqueous reaction system to easily create multifunctional copper peroxide (CuO2) nanodots [100, 101].
This procedure included polyvinylpyrrolidone (PVP) which not only regulates nanodot particle diameter but also supplies the surface functionalization necessary to ensure the excellent stability of nanodots under a physiological environment [102, 103]. Their particle size of about 5 nm allowed for effective accumulation in tumors [102, 103]. By reacting with water, the created CuO2 nanodots sparked a chemical change that produced hydrogen peroxide, and the presence of Cu2+ as catalysts sparked a Fenton-like process that produced the very reactive hydroxyl radicals with hydrogen peroxide acting as the reactant on its own [104]. By causing lysosomal lipid peroxidation, the generated hydroxyl radicals caused cancer cells to undergo cell death by lysosomal membrane permeabilization [105, 106].
CaO2 nanoparticles should be combined with other Fenton compounds in order to achieve therapeutic goals since the chemically inert Calcium portion cannot cause chemical reactions [107]. With the help of hyaluronate acid, CaO2 nanoparticles were combined with widely studied and highly biocompatible Fe3O4 Fenton nanoagents to create CaO2-Fe3O4/HA hybrid nanostructure, which led to hydrogen peroxide self-supply and Fenton-based tumor killing process [100].
Chemodynamic therapy is a new nanotheranostic method that uses a meticulously synthesized Fenton nanocatalyst to accelerate the conversion of hydrogen peroxide to OH [108, 109]. The impact of chemodynamic therapy is generally inadequate because it is restricted by the quantity of endogenous hydrogen peroxide in the tumor [106, 110]. Since metal peroxides can produce hydrogen peroxide in the mildly acidic TME, it can be used to improve chemodynamic therapy effectiveness. Furthermore, the metal ions that make up metal peroxides, such as Cu2+, Co2+, and Mn2+, have strong Fenton catalytic performance, rendering metal peroxide a prospective hydrogen peroxide self-supply chemodynamic therapy agent [111].
Several researchers have reported Fenton-type copper peroxide (CP) nanodots that were attached by PVP using hydroxide ions [100, 112–115]. As discussed in the abovementioned paragraph, in an acidic condition, the produced CP nanodots may reversibly degrade into Cu2+ and hydrogen peroxide, allowing the hydrogen peroxide self-supplying chemodynamic therapy to be produced. The pH-sensitive CP nanodots were absorbed by tumors due to improved permeation and retainment properties, and they produce huge quantities of OH in the acidic endo/lysosomal compartments via a Fenton-like process, which can cause lysosomal membrane permeabilization-mediated tumor cell death via lysosomal lipid peroxidation [37, 106, 113]. Finally, researchers used inductively coupled plasma optical emission spectrometry to examine the physiological dispersion of CP nanopods in U87MG tumor-bearing mice, finding that tumor absorption of CP nano pods was 5.96 0.79 percent, with outstanding chemodynamic therapy antitumor efficacy and minimal loss in weight [113].
The Fenton reaction has been widely employed in water treatment studies since it was first described [116]. In general, the interaction between Fe2+ and H2O2 might result in •OH, which could then destroy the water contaminants [117]. For the Fenton reaction to function properly in industrial settings, a number of parameters, such as the high demand for H2O2 and maintaining a small optimal pH window, are crucial. Researchers have shown that the Fenton reaction can cause oxidative damage to the cancer cells' DNA, proteins, or lipids, which can be targeted for treatment [118]. The right conditions are created for the Fenton reaction (Fe2+ + H2O2 ⟶ Fe3+ + •OH + OH−) to take place by the overexpression of H2O2 (100 M) and moderate acidity of TME [119].
Designing Fe-based nanosystems for targeted intracellular Fenton reaction with noninvasive therapy effectiveness makes sense given the extremely short half-life of •OH (109 s) [120]. Other transition metal ions, such as Mo4+, Ti3+, Cu+, Mn2+, Ag+, and V2+ have been included in the construction of many nanosystems and nanozymes to help further ease the small window of acidic pH required for effective cancer CDT [46]. Cu+ may, for instance, carry out Fenton-like reactions up to 160 times more quickly than Fe2+ and is said to be more effective in producing toxic •OH in TME (pH 6.5–6.9) [121]. These Fenton-like reactions caused by transition metals have a number of benefits, including excellent performance in nearly neutral environments and a large natural abundance of structurally diverse oxide products [122].
Fe-based nanocatalysts require low pH levels and large catalyst dosages, but in contrast to other species, they have the best activity at low H2O2 concentrations and low activation energies [123]. Before precisely designing a Fenton/Fenton-like reaction-based nanomedicine, it is important to take into account the feasibility of active redox cycles in the pH state, catalyst loading, and stability of oxidation products. The full potential of chemodynamic cancer therapy is frequently limited by the intricacy of TME and the preparation of an “all-in-one” chemodynamic drug [124]. Designing appropriate Fenton nanosystems and modifying TME in favour of CDT is therefore of utmost importance.
According to certain theories, ROS trigger intracellular lipid peroxidation, which results in ferroptosis [125] as illustrated in Figure 6. However, treatment is challenging due to the tiny levels of produced OH in cells [126]. There have been reports of several nanoparticles that improve the efficacy of Fenton reactions for medicinal applications [95].
Figure 6.
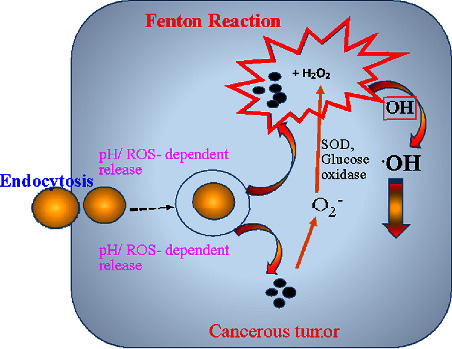
Mechanism of ROS generation by ferroptosis.
Typically made primarily of metal ions and peroxo groups, metal peroxides may combine with water to form hydrogen peroxide (H2O2). There are several biological uses for the postgenerated H2O2. For instance, in catalytic medicine, H2O2 can function as the reactant in a Fenton-like catalytic reaction to produce enormous amounts of hydroxyl radicals (•OH) [127]. Additionally, H2O2 has the ability to self-decompose to create oxygen (O2), which may be used to increase the therapeutic effectiveness of other O2-involved modalities including radiation treatment and photodynamic therapy (PDT). In order to produce O2 and H2O2, metal peroxide can therefore serve as a solid precursor [33]. With a high level of tumor selectivity, Fenton reaction-based catalytic nanotherapeutics have become a unique tumor-therapeutic technique [128].
The development of MO2 as a self-supplying source of O2 and H2O2 (Figures 7 and 8) has made it a very promising therapeutic treatment for tumors [129]. Under acidic circumstances, the produced H2O2 from MO2 reacting with H2O not only causes oxidative stress but also generates additional O2 by serving as a reaction substrate for molecules like CAT or MnO2 to reduce tumor hypoxia and reverse TME [130].
Figure 7.
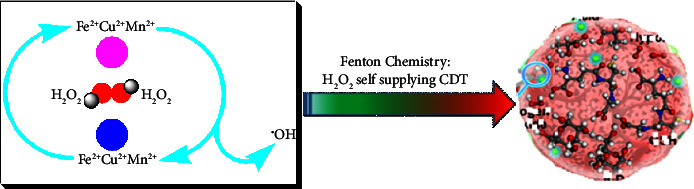
Fenton chemistry of MO2 as a self-supplying source of O2 and H2O2.
Figure 8.
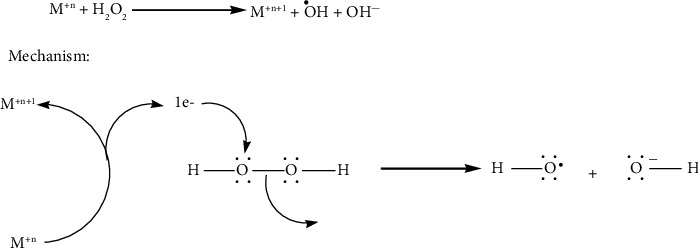
Mechanism of catalytic chemistry of Fenton nanocatalysts for versatile radical nanotherapeutics.
3.1. Reactive Oxygen Species in Apoptosis
Superoxide radicals (O2−), singlet oxygen (1O2), hydrogen peroxide, and hydroxyl radicals are examples of reactive oxygen species (ROS) that may damage lipids, proteins, and DNA, causing cell death and apoptosis. Oxidative stress occurs when ROS levels surpass the antioxidant capability of cells, resulting in cell death [131, 132]. Metal peroxides are excellent in inducing oxidative stress in cells, and it has been widely employed in anticancer therapy in recent times. The mildly acidic tumor microenvironment is expected to break down MO2 into M2+ and hydrogen peroxide.
Several researchers have reported the synthesis of transferrin-modified MgO2 nanosheets (TMNSs), which have a similar reaction to the neutral pH and low CAT activity of the tumor microenvironment [133, 134]. MgO2 interacts with H+ to produce hydrogen peroxide quickly, damaging the morphology of transferrin on the nanosheets' surface [135]. The trapped Fe3+ is then released by transferrin, which causes the Fenton reaction to produce cytotoxic Hydroxyl radicals [98, 136].
Figure 9 is a Jablonski diagram showing the mechanism of photodynamic cancer therapy mediated by photosensitizers. Photodynamic therapy uses photosensitizers (PS) to transform local molecular oxygen into cytotoxic reactive oxygen species (ROS), which can destroy biomolecules and cause cell death [137, 138]. However, because photodynamic therapy's efficacy is highly dependent on oxygen levels, solid tumor hypoxia reduces its efficacy, and increased oxygen consumption by photodynamic therapy would exacerbate the tumor's hypoxia, creating a vicious cycle [139]. Metal peroxides act as an oxygen self-sufficient compound which improves the effect of the aforesaid challenges of photodynamic therapy.
Figure 9.
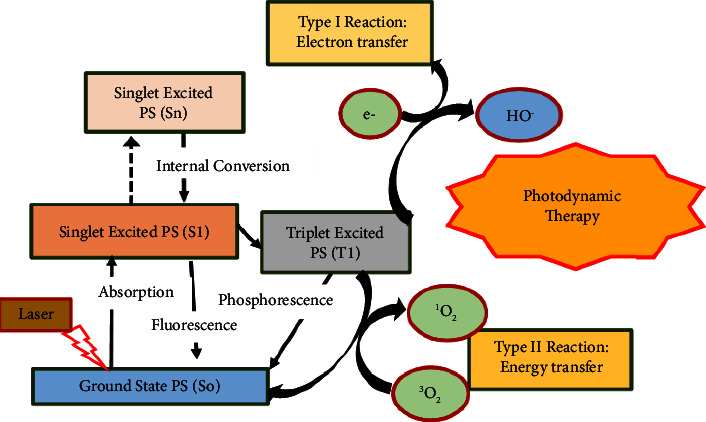
Mechanism of photosensitizer-mediated photodynamic cancer therapy.
Zhang et al., for example, created a double light-driven photodynamic therapy using a liposome-based nanosystem [140]. The hydrophilic PS (methylene blue, MB) and CaO2 NPs were enclosed in the aqueous cavity and the hydrophobic layer, respectively. When LipoMB/CaO2 reaches the tumor tissue, the CaO2 inside the liposomes reacts with water to produce oxygen in the mildly acidic TME, alleviating tumor hypoxia [141].
During the first phase, brief irradiation is used to rupture the liposome by oxidizing the phospholipid bilayer and to activate singlet oxygen (1O2) [141, 142]. CaO2 is then exposed to water and generates additional oxygen. Finally, after irradiation is supplied, the photodynamic therapy impact will be much enhanced in the oxygen-adequate TME. This well-conceived two-stage irradiation method based on CaO2 maximizes CaO2's oxygen supply capability.
Self-supplying oxygen photodynamic therapy treatment using CaO2 and hydrophilic ammonium bicarbonate (NH4HCO3) encapsulated in PEG-shelled liposomes has also been described using aza Boron-dipyrromethene (BODIPY) dye as photosensitizer (Figure 10) [143]. NH4HCO3 is used as a thermoresponsive compound in this experiment. Aza BODIPY dye raises the temperature of the liposome system when it is treated with near-infrared (NIR) [143]. When the temperature reaches 40 degree Celsius, NH4CO3 thermally decomposes to form CO2, which expands and destroys the liposomes, enabling CaO2 and CO2 to completely react to release oxygen, and enhancing the photodynamic therapy effectiveness [143].
Figure 10.
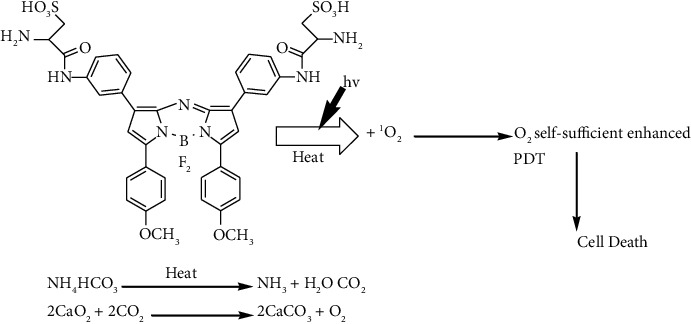
Two-stage mechanism of CaO2 and boron aza BODIPY photodynamic therapy.
Photodynamic therapy using rose bengal as PS was developed with the aid of CaO2 NPs and it achieved the best results in these tests [144, 145].
3.2. Improved Chemodynamic Therapy
Chemodynamic therapy (CDT) is a developing, minimally invasive technique, which disproportionates endogenous H2O2 via Fenton or Fenton-like processes into the highly toxic hydroxyl radical (•OH) [95]. By destroying DNA, inactivating proteins, and inducing phospholipid membrane peroxidation, •OH can induce a significant extent of cell death in cancerous cells [146]. CDT is selective since it works well under increased production of hydrogen peroxide in tumors relative to normal tissue. This minimizes the harm to normal tissue. Therefore, compared to conventional treatment options, CDT has a number of benefits, including low invasiveness, excellent selectivity, and fewer adverse effects.
The endogenous concentration of H2O2 is 10–50 μM [147]. However, this level is inadequate to produce enough hydroxyl radicals to ensure that CDT works effectively. Therefore, the creation of new techniques that will raise the level of H2O2 in the tumor will raise the level of hydroxyl radical produced by Fenton or processes that are similar to Fenton, which will boost the effects of CDT [148].
The utilization of biochemical processes is one such method to raise the endogenous levels of H2O2 in tumors [149]. Enzyme catalysis is the major method used for this [150]. Two biological processes have been employed recently to produce H2O2 in tumors. First, glucose oxidase (GOx) is used to accelerate the reaction of water, oxygen, and glucose to form gluconic acid and H2O2 [151]. The alternative process uses superoxide dismutase (SOD) to catalyse the production of H2O2 from superoxide anion radicals [150]. Both processes create H2O2 by catalytic reactions using chemicals found in the tumor, which can effectively raise the H2O2 content.
Metal peroxide can create oxygen or function as a reaction substrate to counteract tumor hypoxia and provide more oxygen for chemo-drugs to deliver improved chemotherapeutic treatment [100]. Due to the limited negative effects, they have on normal tissues in vivo, metal peroxides which are broken down by the acidic microenvironment of the tumor to produce metal ions and H2O2 are a credible alternative source of H2O2 [129]. In response to the acidic tumor microenvironment, the transferrin-modified MgO2 nanosheets rapidly generate a substantial amount of H2O2 and then undergo a Fenton reaction with metal released from transferrin, which substantially enhanced the production of toxic •OH for the effective cancer therapy [152].
MnO2 nanoparticles have been investigated as a smart chemodynamic approach to improve CDT in cancer therapy. After being taken up by the cell, MnO2 can interact with intracellular GSH to form GSSG and Mn2+, which has good Fenton-like activity when it comes to producing highly reactive hydroxyl radicals from endogenous H2O2 in the presence of physiological HCO3− ions. Figure 11 shows a scheme of how GSH depletion impairs the antioxidant defence system (ADS), making cancer cells more susceptible to OH radicals produced in the Mn2+-mediated Fenton-like process, permitting increased CDT leading to cell death [153].
Figure 11.
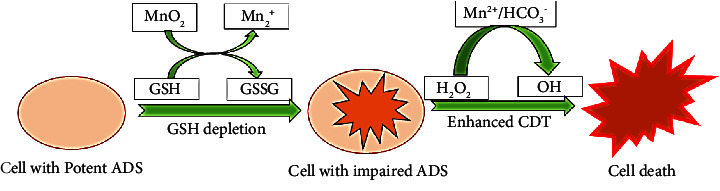
Cancer treatment through nanoparticle-facilitated Fenton process.
4. Targeting Metal Ion Homeostasis
So far, only a few forms of metal peroxide have been described for tumor treatment, with the majority of the studies focusing on CaO2-based nanostructures [154, 155]. Other metal peroxide-based nanoparticles, such as MgO2, BaO2, ZnO2, and CuO2-based materials, have yet to be fully realized, and their physiological uses are equally restricted [44]. Improving them by correctly altering them or coupling them with other chemotherapeutic drugs might be a potential research trend [156]. CaO2 has the highest clinical translation value in the metal peroxide indicated above, in our opinion. CaO2 has strong biocompatibility because Ca2+ is extensively dispersed in the body [157].
Furthermore, because Ca2+ is dispersed throughout cancer cells, therapy tactics such as calcium stress are universal, and Ca2+ has the function of speeding osteogenesis, which might be beneficial in the management of bone cancers such as osteosarcoma [158]. However, the synthesis and preservation of CaO2 and metal peroxide face difficulties due to their instability; the shape, size, and dispersion of metal peroxide are hard to accurately regulate, making mass synthesis challenging [34, 159].
Calcium excess is triggered by a malfunction of the calcium balancing system and a problem of calcium transport, which results in an excessive rise in intracellular calcium levels [160, 161]. Calcium excess can disrupt the mitochondrial oxidative phosphorylation pathway, reduce mitochondrial membrane potential, and activate phospholipases and proteases in the cytoplasm, resulting in permanent cell damage [162]. Internal calcification is commonly detected in some cancers following radiation or chemotherapy in clinical treatment, therefore calcification is typically thought of as a byproduct of tumor treatment, and it has been discovered that calcified tumors often respond better to treatment [163, 164].
Given the significance of Ca2+ in cell growth, respiration and mortality, the overload mechanism might be destructive to cancerous cells, providing a drug-free approach to cancer treatment [164]. Signal transmission in cells is a fundamental and crucial aspect of life. Ca2+ is a broadly distributed intracellular messenger where it regulates nearly all cellular functions in cells, including muscular movement, neurotransmission from neurons and astrocytes, tissue repair, and respiratory functions in the liver and pancreas, together with cellular mitosis, maturity, and death, among others. Ca2+ regulates the growth of cancerous cells, tumor progression, invasion, and spread, among other things [164].
Under typical conditions, cells have a very stringent Ca2+ level regulation system. However, in an oxidatively stressed environment, cells struggle to maintain Ca2+ balance due to aberrant intracellular Ca2+ channel activity, culminating in calcium excess-induced cell death [165]. As a result, one of the probable approaches for antitumor treatment is the disruption of tumor physiological Ca2+ balance by calcium overload [166].
An oxidatively stressed environment will alter the protein functions and prevent the proper relay of the calcium signal in CAT-downregulated cancerous cells, resulting in unrestrained Ca2+ build-up and cell death [167, 168]. Similarly, nanosystems which used CaO2 as an oxygen source and hematoporphyrin monomethyl as a photosensitizer have been synthesized and reported [169, 170]. This approach effectively coupled photodynamic therapy with calcium overload.
Cancer cells can also be destroyed by disrupting intracellular Zn2+ homeostasis, where ZnO, ZnO2, and other Zn-based nanoparticles that may release Zn2+ at tumor locations have been studied for tumor treatment [45, 171]. Excess Zn2+ can cause apoptotic cell death and lactate dehydrogenase release by depolarizing mitochondrial membrane potential, activating caspase-3, and causing cell death [172]. Simultaneously, by blocking the mitochondrial electron transport chain, Zn2+ can boost the production of endogenous ROS [173, 174]. As a result, for Ca2+ or Zn2+ ion antitumor treatment, the design and synthesis of degradable nanoparticles containing these ions hold great promise.
The produced hydrogen peroxide combines with Fenton or Fenton-like compounds (such as Fe2+, Mn2+, Cu+, and Co2+) to form hydroxyl radicals and achieve chemodynamic therapy in an acidic environment [127, 175]. The hydrogen peroxide generated can be degraded by CAT or MnO2 to generate oxygen, enhancing the efficiency of oxygen-dependent cancer treatments such as photodynamic therapy and radiation treatment [176, 177].
The metal ions released after the degradation are observed to have some significant ramifications, such as excess calcium caused by Ca2+ ions released from CaO2 which is thought to cause mitochondrial damage [166, 178, 179]. The released Ba2+ ions produced by the degradation reaction of BaO2 are known to act as a potassium ion pump suppressor, inhibiting tumor progression [180, 181].
5. Combination Therapies
Even though it is currently a highly popular treatment option for many different types of cancer, monotherapy is usually thought to be less efficient than combination therapy. Traditional monotherapy approaches nonselectively target cells that are actively multiplying, which eventually results in the death of both malignant and healthy cells.
For the majority of cancers, the treatment's effectiveness with monotherapy is insufficient and therefore essential to combine two or even more treatment approaches [182, 183]. Each therapeutic drug has antitumor action, and by combining them, they can provide the effects of combination therapy. Additionally, the “1 + 1 > 2” synergistic therapeutic outcomes can be achieved if the tumor-killing mechanisms of each therapeutic drug can complement one another [184].
More significantly, the properties of metal peroxides may be precisely paired with photosensitizers, enzymes, metal nanoparticles, Fenton reagents, or chemotherapeutic medications, among other things, to help and encourage different therapies including photodynamic therapy, chemodynamic therapy, and chemotherapy [170, 185, 186]. When several therapies are coupled, metal peroxide-based coadministration achieves much better antiproliferative results [187].
Because metal peroxides are unstable, some surface modification using molecules such as polyvinyl pyrrolidone (PVP) and hyaluronic acid (HA) is required for improved biological applications in physiological media [188, 189]. Surface modification enhances not only the stability of metal peroxide but also the dispersibility of nanoparticles (NPs), making tumor targeting feasible [190, 191].
A key component of cancer therapy is combination therapy, a mode of care that combines two or more therapeutic drugs. The combination of anticancer medications improves efficacy in comparison to monotherapy because it targets important pathways in a manner that is often additive or synergistic [192]. In addition to therapeutic anticancer effects including reducing tumor growth and metastatic potential, this strategy may also diminish drug resistance [79]. Being able to target several pathways effectively reduces drug resistance because cancer cells typically cannot adapt to the concurrent harmful effects of two therapeutic drugs. The process of creating a new anticancer medicine is expensive and time-consuming. New tactics are thus being proposed that focus on survival routes that deliver efficient and effective outcomes at a reasonable cost.
Combination therapy with drugs originally prescribed for the management of conditions other than cancer is one such strategy. In the end, this has a synergistic or cumulative effect, necessitating a smaller therapeutic dosage of each drug, because they enable the use of individual medications in lower dosages while maintaining therapeutic efficiency. These combination drug regimens lessen the overall toxicity of the treatment.
This strategy works best when an FDA-approved medication targets pathways that are comparable to those seen in cancer [193]. The overall cost of combination treatment research is decreased because one of the medications utilized in it is already FDA-approved [194]. The various outcomes from monotherapies and combination therapies are summarized in Figure 12.
Figure 12.
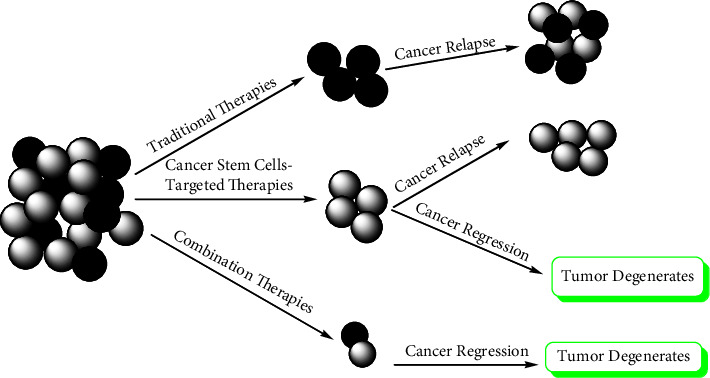
Comparison of the effectiveness of traditional monotherapies and combination therapies.
Traditional cancer treatments generally only destroy differentiated cancer cells and miss the cancer stem cells (CSC). Thus, CSC is capable of surviving and may cause relapses. CSC-targeted medicines either eradicate CSC or cause differentiation in cancer cells, which may then lead to apoptosis-mediated cell death. However, combined therapy may be the most successful method of removing tumors.
6. Synthetic Procedures for Metal Peroxides
The most extensively used process for preparing metal peroxides is hydrolyzation-precipitation [100, 195]. Metal chloride, metal acetate, or metal carbonate are commonly utilized as precursors in this process, which involves adding hydrogen peroxide to an alkaline aqueous medium of metal salt to precipitate the water-insoluble metal peroxide particles [196]. The procedure is relatively simple and is carried out under mild conditions making the process to be cheap, and the size of NPs may be controlled to several nanometres.
For example, in the synthesis of CaO2, the CaO2 hydrate was formed using equation (1) and the process was subsequently aided in precipitating the metal peroxide by adding ammonia to neutralize the HCl, as shown [196, 197]:
| (1) |
It is important to note that, in addition to regulating the size of the particles, PVP also works as a stabilizer in hydrolyzed precipitation [35, 198]. The synthesis of BaO2 by hydrolysis and precipitation process described the use of PEG as a stabilization agent to change the outer layer of metal peroxide [199].
In a nutshell, sodium formate and BaCl2 aqueous solution were ultrasonically combined before being added to dry methanol. After aggressively swirling, hydrogen peroxide is added to the mixture, and the BaO2 NPs were precipitated using an aqueous solution of choline hydroxide [200].
They noted that the organic ligands used had a significant impact on the growth rate and orientation of BaO2 nanocrystals, resulting in a variety of sizes of particles and morphologies. When a ligand with a specified coordination capacity with Ba2+ is added to the process, the crystal development is successfully regulated, resulting in nanosized BaO2 particles [200]. Nanoparticles with varied particular surface areas and functionalities have varied shape controllability, which is an important link in the design of nanoparticle theranostic platforms. To limit the toxicity of free Ba2+ to healthy tissue, the scientists combined BaO2 with a biodegradable potent chelating ligand L-glutamic acid (N-diacetic acid) in the abovementioned illustration [200]. Effective surface modification not only enhances the stability of metal peroxide but also the dispersion of NPs, making tumor targeting possible.
The Leidenfrost dynamic chemistry approach is another synthetic option. Here in this method, the formation and development of NPs are split into the following two sections: initially, nanochemistry happens in the heated zone, and the generated NPs create nanoclusters; secondly, these nanoclusters move into cooler regions, where they will continue to grow [200, 201]. This propensity might be used to control the size of NPs in the future. Zinc acetate solution was combined with hydrogen peroxide and put in a Petri dish, which was then rapidly exposed to a superheated plate (300 C), causing the solution to change colour from colourless to milky white, resulting in the formation of ZnO2 NPs [200, 201]. Overall, by adjusting the concentration of zinc acetate, the size of ZnO2 NPs produced by this approach could be controlled.
Literature reports on the production of MgO2 nanosheets have been published via a microemulsion system, where cyclohexane and CO-520 were added to an MgCl2 solution [200, 202–204]. After 30 minutes of stirring, ammonium hydroxide was quickly introduced to generate Mg(OH)2 and stirred for another 30 minutes. To generate MgO2 nanosheets, hydrogen peroxide was introduced to regulate the reaction process, and anhydrous ethanol was used to break the reverse microemulsion system [200]. Similarly, a reverse microemulsion approach to synthesize CaO2 nanoparticles by simultaneously incorporating cisplatin, and capping with negatively charged phospholipid has been reported [205, 206].
The sizes of NPs may be controlled in a microemulsion process by altering the moisture content and pH of the micelles [207, 208]. In the microemulsion process, the organic solvent layer and surfactant layer efficiently separated the precipitated particles and increased particle dispersibility [209, 210]. Some chemotherapy medications can be introduced directly to the microemulsion system to create NPs while also achieving drug loading [205].
The ideal approach for the research study on the biomimetic production of calcium carbonate (CaCO3) minerals is the gas diffusion technique, which has the benefits of ease of operation and monitoring [94, 211]. Deng and coworkers devised a new CaO2 production method based on CaCO3 gas diffusion chemistry. A beaker with ethanol solution with CaCl2 and hydrogen peroxide is typically covered with parafilm with some holes, and then another separate beaker holding ammonia is then introduced in the same desiccator. The CaO2 synthesis is completed after a 2-hour gas diffusion process at 35°C [212].
ZnO2 nanoparticles have been produced via an innovative sonochemical method [213, 214]. The method is a straightforward reaction where ZnSO4H2O was dissolved in distilled water and NaOH was added dropwise to adjust the pH up to 8.0 [213]. After that, hydrogen peroxide was added, and the mixture was sonicated with ultrasound for half an hour, yielding ZnO2 nanoparticles with very uniform size ranges and a spherical shape [213]. However, nanoparticle aggregation was detected. The best way to modify size distribution and optimize NP distribution has never been straightforward [215]. Metal peroxide has been used as an oxygen-generating compound in the production of potential tumor theranostics nanoplatforms in the past few years, which may modulate the tumor microenvironment to generate a new working environment for therapies whose effectiveness is restricted by the underlying tumor microenvironment [44, 46, 213, 215, 216].
7. Conclusion
Even if there are still many issues to be resolved, metal peroxides have introduced innovative methods for treating tumors, and their use in biology merits further research and development. Metal peroxide nanostructures have been produced and applied as a supply of oxygen and hydrogen peroxide in the cancer tumor microenvironment with promising results [100]. Under acidic environments, the hydrogen peroxide produced by the reaction of metal peroxide with water has a dual role of inducing oxidative stress and producing surplus oxygen from the reaction with molecules such as catalyse enzymes. These alleviate tumor hypoxia thereby reversing the low oxygen levels observed in the tumor microenvironment [29, 216, 217]. In addition, the properties of metal peroxides may be precisely paired with other molecules such as photosensitizers, enzymes, metal nanoparticles, Fenton reagents, or chemotherapeutic medications, to achieve combination therapies including photodynamic therapy, chemodynamic therapy, and chemotherapy [170, 185–187]. Metal peroxide-based coadministration with other therapies has been shown to achieve much better antiproliferative results [187].
Though not exhaustive, this review visited the most widely studied metal peroxide nanosystems that have been applied in cancer studies. It covers common synthesis procedures for these nanomaterials and a comprehensive overview of applications in the noncellular cancer tumor microenvironment.
Data Availability
All data used to support this study are included within the article.
Disclosure
This study was conducted as part of the employment of the author at Kisii University.
Conflicts of Interest
The author declares no conflicts of interest.
References
- 1.Baldari S., Di Rocco G., Toietta G. Current biomedical use of copper chelation therapy. International Journal of Molecular Sciences . 2020;21(3):p. 1069. doi: 10.3390/ijms21031069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vines J. B., Lim D.-J., Park H. Contemporary polymer-based nanoparticle systems for photothermal therapy. Polymers . 2018;10(12):p. 1357. doi: 10.3390/polym10121357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aghebati‐Maleki A., Dolati S., Ahmadi M., et al. Nanoparticles and cancer therapy: Perspectives for application of nanoparticles in the treatment of cancers. Journal of Cellular Physiology . 2020;235(3):1962–1972. doi: 10.1002/jcp.29126. [DOI] [PubMed] [Google Scholar]
- 4.Bahrami B., Hojjat-Farsangi M., Mohammadi H., et al. Nanoparticles and targeted drug delivery in cancer therapy. Immunology Letters . 2017;190:64–83. doi: 10.1016/j.imlet.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Kang C., Sun Y., Zhu J., et al. Delivery of nanoparticles for treatment of brain tumor. Current Drug Metabolism . 2016;17(8):745–754. doi: 10.2174/1389200217666160728152939. [DOI] [PubMed] [Google Scholar]
- 6.Liang Y., Xie L., Liu K., et al. Bergapten: a review of its pharmacology, pharmacokinetics, and toxicity. Phytotherapy Research . 2021;35(11):6131–6147. doi: 10.1002/ptr.7221. [DOI] [PubMed] [Google Scholar]
- 7.Shah K. K., Bennett B., Lenny A., et al. Adapting preference-based utility measures to capture the impact of cancer treatment-related symptoms. The European Journal of Health Economics . 2021;22(8):1301–1309. doi: 10.1007/s10198-021-01337-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Francia R., Crisci S., De Monaco A., et al. Response and toxicity to cytarabine therapy in leukemia and lymphoma: from dose puzzle to pharmacogenomic biomarkers. Cancers . 2021;13(5):p. 966. doi: 10.3390/cancers13050966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang S., Shim M. K., Kim W. J., et al. Cancer-activated doxorubicin prodrug nanoparticles induce preferential immune response with minimal doxorubicin-related toxicity. Biomaterials . 2021;272 doi: 10.1016/j.biomaterials.2021.120791.120791 [DOI] [PubMed] [Google Scholar]
- 10.Kaemmerer E., Loessner D., Avery V. M. Addressing the tumor microenvironment in early drug discovery: a strategy to overcome drug resistance and identify novel targets for cancer therapy. Drug Discovery Today . 2021;26(3):663–676. doi: 10.1016/j.drudis.2020.11.030. [DOI] [PubMed] [Google Scholar]
- 11.Nussinov R., Tsai C.-J., Jang H. Anticancer drug resistance: an update and perspective. Drug Resistance Updates . 2021;59 doi: 10.1016/j.drup.2021.100796.100796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao X., Wang K., Zong Q., Tu Y., Dong Y., Yuan Y. Polyprodrug with glutathione depletion and cascade drug activation for multi-drug resistance reversal. Biomaterials . 2021;270 doi: 10.1016/j.biomaterials.2020.120649.120649 [DOI] [PubMed] [Google Scholar]
- 13.Gigante E., Hobeika C., Le Bail B., et al. Systemic treatments with tyrosine kinase inhibitor and platinum-based chemotherapy in patients with unresectable or metastatic hepatocholangiocarcinoma. Liver Cancer . 2022;11 doi: 10.1159/000525488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hajipour F., Mahdavinia M., Fereidoonnezhad M. Half-lantern Cyclometalated platinum (II) complexes as anticancer agents: molecular Docking, apoptosis, cell cycle analysis, and cytotoxic activity Evaluations. Anti-Cancer Agents in Medicinal Chemistry . 2022;22(6):1149–1158. doi: 10.2174/1871520621666210713112105. [DOI] [PubMed] [Google Scholar]
- 15.Li B. T., Smit E. F., Goto Y., et al. Trastuzumab deruxtecan in HER2-mutant non–small-cell lung cancer. New England Journal of Medicine . 2022;386(3):241–251. doi: 10.1056/nejmoa2112431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan C. y., Li H., Wu M. f., et al. A dose-finding trial for hyperthermic intraperitoneal cisplatin in gynecological cancer patients receiving hyperthermic intraperitoneal chemotherapy. Frontiers in Oncology . 2021;11 doi: 10.3389/fonc.2021.616264.616264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wazqar D. Y., Thabet H. A., Safwat A. M. A Quasi-experimental study of the effect of Ginger Tea on preventing Nausea and Vomiting in patients with gynecological cancers receiving cisplatin-based regimens. Cancer Nursing . 2021;44(6):E513–E519. doi: 10.1097/ncc.0000000000000939. [DOI] [PubMed] [Google Scholar]
- 18.Lu Y., Ma X., Chang X., et al. Recent development of gold (I) and gold (III) complexes as therapeutic agents for cancer diseases. Chemical Society Reviews . 2022;51(13):5518–5556. doi: 10.1039/d1cs00933h. [DOI] [PubMed] [Google Scholar]
- 19.Xiong X., Liu L.-Y., Mao Z.-W., Zou T. Approaches towards understanding the mechanism-of-action of metallodrugs. Coordination Chemistry Reviews . 2022;453 doi: 10.1016/j.ccr.2021.214311.214311 [DOI] [Google Scholar]
- 20.Fronik P., Gutmann M., Vician P., et al. A platinum (IV) prodrug strategy to overcome glutathione-based oxaliplatin resistance. Communications Chemistry . 2022;5(1):46–13. doi: 10.1038/s42004-022-00661-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang C., Xu C., Gao X., Yao Q. Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics . 2022;12(5):2115–2132. doi: 10.7150/thno.69424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding Y., Tong Z., Jin L., et al. An NIR Discrete Metallacycle constructed from Perylene Bisimide and Tetraphenylethylene Fluorophores for imaging‐guided cancer Radio‐chemotherapy. Advanced Materials . 2022b;34(7) doi: 10.1002/adma.202106388.2106388 [DOI] [PubMed] [Google Scholar]
- 23.Ulldemolins A., Seras-Franzoso J., Andrade F., et al. Perspectives of nano-carrier drug delivery systems to overcome cancer drug resistance in the clinics. Cancer Drug Resistance . 2021;4(1):44–68. doi: 10.20517/cdr.2020.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song X., Liu J., Geng N., et al. Multi-omics analysis to reveal disorders of cell metabolism and integrin signaling pathways induced by PM2. 5. Journal of Hazardous Materials . 2022;424 doi: 10.1016/j.jhazmat.2021.127573.127573 [DOI] [PubMed] [Google Scholar]
- 25.Tian Y., Tian Y., Yuan Z., et al. Iron metabolism in aging and Age-related diseases. International Journal of Molecular Sciences . 2022;23(7):p. 3612. doi: 10.3390/ijms23073612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alsagaby S. A. Transcriptomics-based investigation of molecular mechanisms underlying apoptosis induced by ZnO nanoparticles in human Diffuse large B-cell lymphoma. International Journal of Nanomedicine . 2022;17:2261–2281. doi: 10.2147/ijn.s355408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao J., Chen J., Zhu X., et al. Multichannel sensor array of carbon dots-metal ion pairs for accurate biological thiols analysis and cancer cell discrimination. Sensors and Actuators B: Chemical . 2022;353 doi: 10.1016/j.snb.2021.131119.131119 [DOI] [Google Scholar]
- 28.Liu Q., Wu B., Li M., Huang Y., Li L. Heterostructures made of upconversion nanoparticles and metal–organic frameworks for biomedical applications. Advanced Science . 2022;9(3) doi: 10.1002/advs.202103911.2103911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun X., Zhang G., Ding X., et al. A DNA functionalized metal–organic framework combined with magnesium peroxide nanoparticles: targeted and enhanced photodynamic therapy. Materials Chemistry Frontiers . 2022;6(7):956–965. doi: 10.1039/d1qm01475g. [DOI] [Google Scholar]
- 30.Hao H., Yu M., Yi Y., et al. Mesoporous calcium peroxide-ignited no generation for amplifying photothermal immunotherapy of breast cancer. Chemical Engineering Journal . 2022;437 doi: 10.1016/j.cej.2022.135371.135371 [DOI] [Google Scholar]
- 31.Meng Q., Yang K., Zhao K., et al. Mechanistic revelation into the degradation of organic pollutants by calcium peroxide nanoparticles@ polydopamine in Fe (III)-based catalytic systems. Separation and Purification Technology . 2022;296 doi: 10.1016/j.seppur.2022.121412.121412 [DOI] [Google Scholar]
- 32.Zhou M., Li B., Li N., Li M., Xing C. Regulation of Ca2+ for cancer cell apoptosis through photothermal conjugated nanoparticles. ACS Applied Bio Materials . 2022;5(6):2834–2842. doi: 10.1021/acsabm.2c00236. [DOI] [PubMed] [Google Scholar]
- 33.Bi X., Bai Q., Liang M., et al. Silver peroxide nanoparticles for combined antibacterial sonodynamic and photothermal therapy. Small . 2022;18(2) doi: 10.1002/smll.202104160.2104160 [DOI] [PubMed] [Google Scholar]
- 34.Rastinfard A., Dalisson B., Barralet J. Aqueous decomposition behavior of solid peroxides: effect of pH and buffer composition on oxygen and hydrogen peroxide formation. Acta Biomaterialia . 2022;145:390–402. doi: 10.1016/j.actbio.2022.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Fu D.-Y., Liu X., Zheng X., et al. Polymer-metal-organic framework hybrids for bioimaging and cancer therapy. Coordination Chemistry Reviews . 2022;456 doi: 10.1016/j.ccr.2021.214393.214393 [DOI] [Google Scholar]
- 36.Gera A. K., Burra R. K. The rise of polymeric Microneedles: recent developments, advances, challenges, and applications with regard to Transdermal drug delivery. Journal of Functional Biomaterials . 2022;13(2):p. 81. doi: 10.3390/jfb13020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tian H., Zhao S., Nice E. C., et al. A cascaded copper-based nanocatalyst by modulating glutathione and cyclooxygenase-2 for hepatocellular carcinoma therapy. Journal of Colloid and Interface Science . 2022;607:1516–1526. doi: 10.1016/j.jcis.2021.09.049. [DOI] [PubMed] [Google Scholar]
- 38.Yang B., Yao H., Yang J., Chen C., Shi J. Construction of a two-dimensional artificial antioxidase for nanocatalytic rheumatoid arthritis treatment. Nature Communications . 2022;13(1):1988–2016. doi: 10.1038/s41467-022-29735-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu H., Huang Z., Liu C. Development of a horseradish peroxidase-Fenton-like system for the degradation of sulfamethazine under weak acid condition. Environmental Science and Pollution Research . 2022;29(8):12065–12074. doi: 10.1007/s11356-021-16681-6. [DOI] [PubMed] [Google Scholar]
- 40.Shih C.-Y., Wang P.-T., Su W.-C., Teng H., Huang W.-L. Nanomedicine-based strategies assisting photodynamic therapy for hypoxic tumors: state-of-the-Art approaches and Emerging trends. Biomedicines . 2021;9(2):p. 137. doi: 10.3390/biomedicines9020137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu X., Xu N., Zhang L., Wang D., Zhang P. Novel design of multifunctional nanozymes based on tumor microenvironment for diagnosis and therapy. European Journal of Medicinal Chemistry . 2022;238 doi: 10.1016/j.ejmech.2022.114456.114456 [DOI] [PubMed] [Google Scholar]
- 42.Wong C.-C., Lu C.-X., Cho E.-C., et al. Calcium peroxide aids tyramine-alginate gel to crosslink with tyrosinase for efficient cartilage repair. International Journal of Biological Macromolecules . 2022;208:299–313. doi: 10.1016/j.ijbiomac.2022.03.044. [DOI] [PubMed] [Google Scholar]
- 43.Zu Y., Wang Y., Yao H., Yan L., Yin W., Gu Z. A copper peroxide fenton Nanoagent-hydrogel as an in situ pH-responsive Wound Dressing for effectively trapping and eliminating Bacteria. ACS Applied Bio Materials . 2022;5(4):1779–1793. doi: 10.1021/acsabm.2c00138. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y., Gao F., Li X., et al. Tumor microenvironment-responsive fenton nanocatalysts for intensified anticancer treatment. Journal of Nanobiotechnology . 2022;20(1):1–33. doi: 10.1186/s12951-022-01278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu S., Zhang K., Liang Y., et al. Nano‐enabled tumor Systematic energy exhaustion via zinc (II) Interference mediated glycolysis inhibition and specific GLUT1 depletion. Advanced Science . 2022;9(7) doi: 10.1002/advs.202103534.2103534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhuang Y., Han S., Fang Y., Huang H., Wu J. Multidimensional transitional metal-actuated nanoplatforms for cancer chemodynamic modulation. Coordination Chemistry Reviews . 2022;455 doi: 10.1016/j.ccr.2021.214360.214360 [DOI] [Google Scholar]
- 47.Kumari S., Advani D., Sharma S., Ambasta R. K., Kumar P. Combinatorial therapy in tumor microenvironment: where do we stand? Biochimica et Biophysica Acta, Reviews on Cancer . 2021;1876(2) doi: 10.1016/j.bbcan.2021.188585.188585 [DOI] [PubMed] [Google Scholar]
- 48.Barnestein R., Galland L., Kalfeist L., Ghiringhelli F., Ladoire S., Limagne E. Immunosuppressive tumor microenvironment modulation by chemotherapies and targeted therapies to enhance immunotherapy effectiveness. OncoImmunology . 2022;11(1) doi: 10.1080/2162402x.2022.2120676.2120676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Christofides A., Strauss L., Yeo A., Cao C., Charest A., Boussiotis V. A. The complex role of tumor-infiltrating macrophages. Nature Immunology . 2022;23(8):1148–1156. doi: 10.1038/s41590-022-01267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tatarova Z., Blumberg D. C., Korkola J. E., et al. A multiplex implantable microdevice assay identifies synergistic combinations of cancer immunotherapies and conventional drugs. Nature Biotechnology . 2022;40:1–11. doi: 10.1038/s41587-022-01379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manuelli V., Pecorari C., Filomeni G., Zito E. Regulation of redox signaling in HIF‐1‐dependent tumor angiogenesis. FEBS Journal . 2022;289(18):5413–5425. doi: 10.1111/febs.16110. [DOI] [PubMed] [Google Scholar]
- 52.Bergers G., Benjamin L. E. Tumorigenesis and the angiogenic switch. Nature Reviews Cancer . 2003;3(6):401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 53.Markman M. Antiangiogenic drugs in ovarian cancer. Expert Opinion on Pharmacotherapy . 2009;10(14):2269–2277. doi: 10.1517/14656560903120907. [DOI] [PubMed] [Google Scholar]
- 54.Huang M., Liu M., Huang D., et al. Tumor perivascular cell-derived extracellular vesicles promote angiogenesis via the Gas6/Axl pathway. Cancer Letters . 2022;524:131–143. doi: 10.1016/j.canlet.2021.10.023. [DOI] [PubMed] [Google Scholar]
- 55.Xian X., Håkansson J., Ståhlberg A., et al. Pericytes limit tumor cell metastasis. Journal of Clinical Investigation . 2006;116(3):642–651. doi: 10.1172/jci25705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meijer E. M., van Dijk C. G. M., Kramann R., Verhaar M. C., Cheng C. Implementation of pericytes in vascular regeneration strategies. Tissue Engineering Part B Reviews . 2022;28(1):1–21. doi: 10.1089/ten.teb.2020.0229. [DOI] [PubMed] [Google Scholar]
- 57.Augustin H. G., Koh G. Y. Antiangiogenesis: Vessel Regression, vessel Normalization, or both? Cancer Research . 2022;82(1):15–17. doi: 10.1158/0008-5472.can-21-3515. [DOI] [PubMed] [Google Scholar]
- 58.Browder T., Butterfield C. E., Kräling B. M., et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Research . 2000;60(7):1878–1886. [PubMed] [Google Scholar]
- 59.Kamba T., McDonald D. M. Mechanisms of adverse effects of anti-VEGF therapy for cancer. British Journal of Cancer . 2007;96(12):1788–1795. doi: 10.1038/sj.bjc.6603813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Balkwill F., Coussens L. M. An inflammatory link. Nature . 2004;431(7007):405–406. doi: 10.1038/431405a. [DOI] [PubMed] [Google Scholar]
- 61.Lampiasi N. Interactions between macrophages and mast cells in the Female Reproductive system. International Journal of Molecular Sciences . 2022;23(10):p. 5414. doi: 10.3390/ijms23105414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ahmed M., Hussain A. R., Siraj A. K., et al. Co-targeting of Cyclooxygenase-2 and FoxM1 is a viable strategy in inducing anticancer effects in colorectal cancer cells. Molecular Cancer . 2015;14(1):131–214. doi: 10.1186/s12943-015-0406-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakanishi C., Toi M. Nuclear factor-κB inhibitors as sensitizers to anticancer drugs. Nature Reviews Cancer . 2005;5(4):297–309. doi: 10.1038/nrc1588. [DOI] [PubMed] [Google Scholar]
- 64.O’Malley D. M., Blair C. K., Greenbaum A., et al. Colorectal cancer Survivors’ Receptivity toward genomic testing and targeted Use of non-steroidal anti-inflammatory drugs to prevent cancer Recurrence. Journal of Community Genetics . 2022;13(2):201–214. doi: 10.1007/s12687-021-00574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Upadhyayula P. S., Higgins D. M., Argenziano M. G., et al. The sledgehammer in precision medicine: dexamethasone and immunotherapeutic treatment of glioma. Cancer Investigation . 2022;40(6):554–566. doi: 10.1080/07357907.2021.1944178. [DOI] [PubMed] [Google Scholar]
- 66.Kharaishvili G., Simkova D., Bouchalova K., Gachechiladze M., Narsia N., Bouchal J. The role of cancer-associated fibroblasts, solid stress and other microenvironmental factors in tumor progression and therapy resistance. Cancer Cell International . 2014;14(1):41–48. doi: 10.1186/1475-2867-14-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Natua S., Dhamdhere S. G., Mutnuru S. A., Shukla S. Interplay within tumor microenvironment orchestrates neoplastic RNA metabolism and transcriptome diversity. Wiley interdisciplinary reviews. RNA . 2022;13(2) doi: 10.1002/wrna.1676.e1676 [DOI] [PubMed] [Google Scholar]
- 68.Prasad S., Saha P., Chatterjee B., Chaudhary A. A., Lall R., Srivastava A. K. Complexity of tumor microenvironment: therapeutic role of Curcumin and its metabolites. Nutrition and Cancer . 2022;1–13:1–13. doi: 10.1080/01635581.2022.2096909. [DOI] [PubMed] [Google Scholar]
- 69.Jiang Y., Zhang H., Wang J., Liu Y., Luo T., Hua H. Targeting extracellular matrix stiffness and mechanotransducers to improve cancer therapy. Journal of Hematology & Oncology . 2022;15(1):34–15. doi: 10.1186/s13045-022-01252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wesley U. V., Sutton I., Clark P. A., et al. Enhanced expression of pentraxin-3 in glioblastoma cells correlates with increased invasion and IL8-VEGF signaling axis. Brain Research . 2022;1776 doi: 10.1016/j.brainres.2021.147752.147752 [DOI] [PubMed] [Google Scholar]
- 71.Seledtsov V. I., von Delwig A. A. Oxygen therapy in traditional and immunotherapeutic treatment protocols of cancer patients: current reality and future prospects. Expert Review of Anticancer Therapy . 2022;22(6):575–581. doi: 10.1080/14737140.2022.2070153. [DOI] [PubMed] [Google Scholar]
- 72.Apostolova P., Pearce E. L. Lactic acid and lactate: revisiting the physiological roles in the tumor microenvironment. Trends in Immunology . 2022;43(12):969–977. doi: 10.1016/j.it.2022.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miao P., Sheng S., Sun X., Liu J., Huang G. Lactate dehydrogenase A in cancer: a promising target for diagnosis and therapy. IUBMB Life . 2013;65(11):904–910. doi: 10.1002/iub.1216. [DOI] [PubMed] [Google Scholar]
- 74.Schiavo F., Kjellsson Lindblom E., Toma-Dasu I. Towards the virtual tumor for optimizing radiotherapy treatments of hypoxic tumors: a novel model of heterogeneous tissue vasculature and oxygenation. Journal of Theoretical Biology . 2022;547 doi: 10.1016/j.jtbi.2022.111175.111175 [DOI] [PubMed] [Google Scholar]
- 75.Giatromanolaki A., Gkegka A. G., Pouliliou S., Biziota E., Kakolyris S., Koukourakis M. Hypoxia and anaerobic metabolism relate with immunologically cold breast cancer and poor prognosis. Breast Cancer Research and Treatment . 2022;194:13–23. doi: 10.1007/s10549-022-06609-0. [DOI] [PubMed] [Google Scholar]
- 76.Harris A. L. Hypoxia—a key regulatory factor in tumor growth. Nature Reviews Cancer . 2002;2(1):38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 77.Li J., Shang W., Li Y., Fu S., Tian J., Lu L. Advanced nanomaterials targeting hypoxia to enhance radiotherapy. International Journal of Nanomedicine . 2018;13:5925–5936. doi: 10.2147/ijn.s173914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Papadopoulos K. P., Goel S., Beeram M., et al. A phase 1 open-label, accelerated dose-escalation study of the hypoxia-activated prodrug AQ4N in patients with advanced malignancies. Clinical Cancer Research . 2008;14(21):7110–7115. doi: 10.1158/1078-0432.ccr-08-0483. [DOI] [PubMed] [Google Scholar]
- 79.Anduran E., Dubois L. J., Lambin P., Winum J.-Y. Hypoxia-activated prodrug derivatives of anti-cancer drugs: a patent review 2006–2021. Expert Opinion on Therapeutic Patents . 2022;32(1):1–12. doi: 10.1080/13543776.2021.1954617. [DOI] [PubMed] [Google Scholar]
- 80.Kang D., Lee S., Kim J. Bioorthogonal click and release: a general, rapid, chemically revertible bioconjugation strategy employing enamine N-oxides. Chem . 2022;8(8):2260–2277. doi: 10.1016/j.chempr.2022.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ajnai G., Cheng C.-C., Kan T.-C., et al. Improving Tirapazamine (TPZ) to target and eradicate hypoxia tumors by gold nanoparticle Carriers. Pharmaceutics . 2022;14(4):p. 847. doi: 10.3390/pharmaceutics14040847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang X., Cheng L., Lu Y., et al. A mxene-based bionic cascaded-enzyme nanoreactor for tumor phototherapy/enzyme dynamic therapy and hypoxia-activated chemotherapy. Nano-Micro Letters . 2022;14(1):1–21. doi: 10.1007/s40820-021-00761-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang B., Xue R., Lyu J., Gao A., Sun C. Tumor acidity/redox hierarchical-activable nanoparticles for precise combination of X-ray-induced photodynamic therapy and hypoxia-activated chemotherapy. Journal of Materials Chemistry B . 2022;10(20):3849–3860. doi: 10.1039/d2tb00303a. [DOI] [PubMed] [Google Scholar]
- 84.Brown M. The remarkable yin and yang of tumor hypoxia. International Journal of Radiation Biology . 2010;86(11):907–917. doi: 10.3109/09553002.2010.492492. [DOI] [PubMed] [Google Scholar]
- 85.Worsley C. M., Veale R. B., Mayne E. S. The acidic tumor microenvironment: Manipulating the immune response to elicit escape. Human Immunology . 2022;83 doi: 10.1016/j.humimm.2022.01.014. [DOI] [PubMed] [Google Scholar]
- 86.Lardner A. The effects of extracellular pH on immune function. Journal of Leukocyte Biology . 2001;69(4):522–530. doi: 10.1189/jlb.69.4.522. [DOI] [PubMed] [Google Scholar]
- 87.Queen A., Bhutto H. N., Yousuf M., Syed M. A., Hassan M. I. Carbonic anhydrase IX: a tumor acidification switch in heterogeneity and chemokine regulation. Seminars in Cancer Biology . 2022;86:899–913. doi: 10.1016/j.semcancer.2022.01.001. [DOI] [PubMed] [Google Scholar]
- 88.Hebert K. A., Bonnen M. D., Ghebre Y. T. Proton pump inhibitors and sensitization of cancer cells to radiation therapy. Frontiers in Oncology . 2022;12 doi: 10.3389/fonc.2022.937166.937166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lindner K., Borchardt C., Schöpp M., et al. Proton pump inhibitors (PPIs) impact on tumor cell survival, metastatic potential and chemotherapy resistance, and affect expression of resistance-relevant miRNAs in esophageal cancer. Journal of Experimental & Clinical Cancer Research . 2014;33(1):73–12. doi: 10.1186/s13046-014-0073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Poly T. N., Lin M.-C., Syed-Abdul S., Huang C.-W., Yang H.-C., Li Y. C. J. Proton pump inhibitor Use and Risk of gastric cancer: current evidence from Epidemiological studies and critical Appraisal. Cancers . 2022;14(13):p. 3052. doi: 10.3390/cancers14133052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Richter J. E., Penagini R., Pohl D., et al. Barrett’s esophagus: proton pump inhibitors and chemoprevention II. Annals of the New York Academy of Sciences . 2011;1232(1):114–139. doi: 10.1111/j.1749-6632.2011.06048.x. [DOI] [PubMed] [Google Scholar]
- 92.Yeo M., Kim D.-K., Kim Y.-B., et al. Selective induction of apoptosis with proton pump inhibitor in gastric cancer cells. Clinical Cancer Research . 2004;10(24):8687–8696. doi: 10.1158/1078-0432.ccr-04-1065. [DOI] [PubMed] [Google Scholar]
- 93.Xu L., Wang J., Wang J., et al. Polypyrrole-iron phosphate-glucose oxidase-based nanocomposite with cascade catalytic capacity for tumor synergistic apoptosis-ferroptosis therapy. Chemical Engineering Journal . 2022a;427 doi: 10.1016/j.cej.2021.131671.131671 [DOI] [Google Scholar]
- 94.Xu M., Zhang J., Mu Y., Foda M. F., Han H. Activation of TRPV1 by capsaicin-loaded CaCO3 nanoparticle for tumor-specific therapy. Biomaterials . 2022;284 doi: 10.1016/j.biomaterials.2022.121520.121520 [DOI] [PubMed] [Google Scholar]
- 95.Sun Q., Wang Z., Liu B., et al. Recent advances on endogenous/exogenous stimuli-triggered nanoplatforms for enhanced chemodynamic therapy. Coordination Chemistry Reviews . 2022;451 doi: 10.1016/j.ccr.2021.214267.214267 [DOI] [Google Scholar]
- 96.Xie W., Zhang G., Guo Z., et al. Ultra‐sensitive iron‐doped Palladium nanocrystals with enhanced hydroxyl radical generation for chemo‐/chemodynamic Nanotherapy. Advanced Functional Materials . 2022;32(12) doi: 10.1002/adfm.202107518.2107518 [DOI] [Google Scholar]
- 97.Zhang S., Jin L., Liu J., et al. Novel FeF 2/Fe 1– x S nanoreactor‐mediated mitochondrial Dysfunction via oxidative stress and Fluoride ions overloaded for synergistic chemodynamic therapy and photothermal therapy. Advanced Functional Materials . 2022;32(23) doi: 10.1002/adfm.202113397.2113397 [DOI] [Google Scholar]
- 98.Yang J., Yao H., Guo Y., Yang B., Shi J. Enhancing tumor catalytic therapy by Co‐catalysis. Angewandte Chemie . 2022b;61(17) doi: 10.1002/anie.202200480.e202200480 [DOI] [PubMed] [Google Scholar]
- 99.Zhao Y., Xiao X., Jiang F., et al. Bioinspired nanocatalysts as hydrogen peroxide homeostasis regulators for tumor-specific synergistic therapy. Biomaterials Science . 2022;10(5):1364–1372. doi: 10.1039/d1bm02000e. [DOI] [PubMed] [Google Scholar]
- 100.Jia C., Guo Y., Wu F. Chemodynamic therapy via fenton and fenton‐like nanomaterials: strategies and recent advances. Small . 2022;18(6) doi: 10.1002/smll.202103868.2103868 [DOI] [PubMed] [Google Scholar]
- 101.Zhang Lu, Li C., Wan S., Zhang X. Nanocatalyst‐mediated chemodynamic tumor therapy. Advanced Healthcare Materials . 2022;11(2) doi: 10.1002/adhm.202101971.2101971 [DOI] [PubMed] [Google Scholar]
- 102.Abbas F., Kumar S., Pal S. K., Panda D. Carbon nanodot doped in polymer film: Plasmophore enhancement, catalytic amination and white-light generation. Journal of Molecular Liquids . 2022;347 doi: 10.1016/j.molliq.2021.118001.118001 [DOI] [Google Scholar]
- 103.Yang L., Jia P., Song S., et al. On-demand triggered chemodynamic therapy by NIR-II light on oxidation-Prevented Bismuth nanodots. ACS Applied Materials & Interfaces . 2022;14(19):21787–21799. doi: 10.1021/acsami.1c22631. [DOI] [PubMed] [Google Scholar]
- 104.Yang N., Li H., Cao C., et al. Tumor microenvironment-activated theranostic nanoreactor for NIR-II Photoacoustic imaging-guided tumor-specific photothermal therapy. Fundamental Research . 2022 doi: 10.1016/j.fmre.2022.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cao X., Li S., Chen W., et al. Multifunctional hybrid hydrogel system enhanced the therapeutic efficacy of treatments for Postoperative glioma. ACS Applied Materials & Interfaces . 2022;14(24) doi: 10.1021/acsami.2c05147.27623 [DOI] [PubMed] [Google Scholar]
- 106.Deng H., Yang Z., Pang X., et al. Self-sufficient copper peroxide loaded pKa-tunable nanoparticles for lysosome-mediated chemodynamic therapy. Nano Today . 2022;42 doi: 10.1016/j.nantod.2021.101337.101337 [DOI] [Google Scholar]
- 107.Nielsen M. M., Pedersen C. M. Vessel effects in organic chemical reactions; a century-old, overlooked phenomenon. Chemical Science . 2022;13(21):6181–6196. doi: 10.1039/d2sc01125e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yu Z., Hu Y., Sun Y., Sun T. Chemodynamic therapy combined with multifunctional nanomaterials and their applications in tumor treatment. Chemistry--A European Journal . 2021;27(56) doi: 10.1002/chem.202101514.13953 [DOI] [PubMed] [Google Scholar]
- 109.Zhou J., Lei M., Peng X.-L., Wei D.-X., Yan L.-K. Fenton reaction induced by Fe-based nanoparticles for tumor therapy. Journal of Biomedical Nanotechnology . 2021;17(8):1510–1524. doi: 10.1166/jbn.2021.3130. [DOI] [PubMed] [Google Scholar]
- 110.Hou S., Gao Y.-E., Ma X., et al. Tumor microenvironment responsive biomimetic copper peroxide nanoreactors for drug delivery and enhanced chemodynamic therapy. Chemical Engineering Journal . 2021;416 doi: 10.1016/j.cej.2021.129037.129037 [DOI] [Google Scholar]
- 111.Sun Y., Zhou J., Liu D., Li X., Liang H. Enhanced catalytic performance of Cu-doped MnFe2O4 magnetic ferrites: Tetracycline hydrochloride attacked by superoxide radicals efficiently in a strong alkaline environment. Chemosphere . 2022;297 doi: 10.1016/j.chemosphere.2022.134154.134154 [DOI] [PubMed] [Google Scholar]
- 112.Lin L.-S., Huang T., Song J., et al. Synthesis of copper peroxide nanodots for H2O2 self-supplying chemodynamic therapy. Journal of the American Chemical Society . 2019;141(25):9937–9945. doi: 10.1021/jacs.9b03457. [DOI] [PubMed] [Google Scholar]
- 113.Liu Y., Bhattarai P., Dai Z., Chen X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chemical Society Reviews . 2019;48(7):2053–2108. doi: 10.1039/c8cs00618k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tang Z., Zhao P., Wang H., Liu Y., Bu W. Biomedicine meets Fenton chemistry. Chemical Reviews . 2021;121(4):1981–2019. doi: 10.1021/acs.chemrev.0c00977. [DOI] [PubMed] [Google Scholar]
- 115.Wu W., Pu Y., Lu X., Lin H., Shi J. Transitional metal‐based Noncatalytic medicine for tumor therapy. Advanced Healthcare Materials . 2021;10(11) doi: 10.1002/adhm.202001819.2001819 [DOI] [PubMed] [Google Scholar]
- 116.Lin S. H., Lin c. M., Leu H. G. Operating characteristics and kinetic studies of surfactant wastewater treatment by Fenton oxidation. Water Research . 1999;33(7):1735–1741. doi: 10.1016/s0043-1354(98)00403-5. [DOI] [Google Scholar]
- 117.Li D., Yu J., Jia J., et al. Coupling electrode aeration and hydroxylamine for the enhanced Electro-Fenton degradation of organic contaminant: Improving H2O2 generation, Fe3+/Fe2+ cycle and N2 selectivity. Water Research . 2022;214 doi: 10.1016/j.watres.2022.118167.118167 [DOI] [PubMed] [Google Scholar]
- 118.Wang K., Li J., Yi Y., et al. Polyamine-activated carbonyl stress strategy for oxidative damage therapy. Nano Today . 2022;42 doi: 10.1016/j.nantod.2021.101355.101355 [DOI] [Google Scholar]
- 119.Lu Z., Bai S., Jiang Y., et al. Porphyrin‐based Covalent organic framework for imaging‐guided cancer Combinatorial Immuno‐sonodynamic therapy. Advanced Functional Materials . 2022;32(45) doi: 10.1002/adfm.202207749.2207749 [DOI] [Google Scholar]
- 120.Lin L., Chen H., Zhao R., Zhu M., Nie G. Nanomedicine targets iron metabolism for cancer therapy. Cancer Science . 2022;113(3):828–837. doi: 10.1111/cas.15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang L., Xu Y., Liu C., et al. Copper-doped MOF-based nanocomposite for GSH depleted chemo/photothermal/chemodynamic combination therapy. Chemical Engineering Journal . 2022;438 doi: 10.1016/j.cej.2022.135567.135567 [DOI] [Google Scholar]
- 122.Zhang H., Ma W., Wang Z., et al. Self-supply oxygen ROS reactor via fenton-like reaction and modulating glutathione for Amplified cancer therapy effect. Nanomaterials . 2022;12(14):p. 2509. doi: 10.3390/nano12142509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li D., Yang T., Liu Z., et al. Green synthesis of graphite-based photo-Fenton nanocatalyst from waste tar via a self-reduction and solvent-free strategy. Science of the Total Environment . 2022;824 doi: 10.1016/j.scitotenv.2022.153772.153772 [DOI] [PubMed] [Google Scholar]
- 124.Zhang Li, Forgham H., Shen A., Qiao R., Guo B. Recent advances in single Fe-based Nanoagents for photothermal–chemodynamic cancer therapy. Biosensors . 2022;12(2):p. 86. doi: 10.3390/bios12020086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Han N., Li L.-G., Peng X.-C., et al. Ferroptosis triggered by dihydroartemisinin facilitates chlorin e6 induced photodynamic therapy against lung cancer through inhibiting GPX4 and enhancing ROS. European Journal of Pharmacology . 2022;919 doi: 10.1016/j.ejphar.2022.174797.174797 [DOI] [PubMed] [Google Scholar]
- 126.Qi Y.-L., Wang H.-R., Chen L.-L., Duan Y.-T., Yang S.-Y., Zhu H.-L. Recent advances in small-molecule fluorescent probes for studying ferroptosis. Chemical Society Reviews . 2022;51(18):7752–7778. doi: 10.1039/d1cs01167g. [DOI] [PubMed] [Google Scholar]
- 127.Cao C., Wang X., Yang N., Song X., Dong X. Recent advances of cancer chemodynamic therapy based on Fenton/Fenton-like chemistry. Chemical Science . 2022;13(4):863–889. doi: 10.1039/d1sc05482a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tan X., Liao D., Rao C., et al. Recent advances in nano-architectonics of metal-organic frameworks for chemodynamic therapy. Journal of Solid State Chemistry . 2022;314 doi: 10.1016/j.jssc.2022.123352.123352 [DOI] [Google Scholar]
- 129.Liu F., He T., Gong S., et al. A tumor pH-responsive autocatalytic nanoreactor as a H2O2 and O2 self-supplying depot for enhanced ROS-based chemo/photodynamic therapy. Acta Biomaterialia . 2022;154:510–522. doi: 10.1016/j.actbio.2022.10.002. [DOI] [PubMed] [Google Scholar]
- 130.Khorshidi S., Younesi S., Karkhaneh A. Peroxide mediated oxygen delivery in cancer therapy. Colloids and Surfaces B: Biointerfaces . 2022;219 doi: 10.1016/j.colsurfb.2022.112832.112832 [DOI] [PubMed] [Google Scholar]
- 131.Albert-Garay J. S., Riesgo-Escovar J. R., Salceda R. High glucose concentrations induce oxidative stress by inhibiting Nrf2 expression in rat Müller retinal cells in vitro. Scientific Reports . 2022;12(1):1261–1312. doi: 10.1038/s41598-022-05284-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang H., Zhao F., Gai X., et al. Astilbin attenuates apoptosis induced by cadmium through oxidative stress in carp (Cyprinus carpio L.) head kidney lymphocyte. Fish & Shellfish Immunology . 2022;125 doi: 10.1016/j.fsi.2022.05.021. [DOI] [PubMed] [Google Scholar]
- 133.He C., Zhang X., Xiang G. Nanoparticle facilitated delivery of peroxides for effective cancer treatments. Biomaterials Science . 2020;8(20):5574–5582. doi: 10.1039/d0bm01265c. [DOI] [PubMed] [Google Scholar]
- 134.Tang Z., Liu Y., Ni D., et al. Biodegradable nanoprodrugs:“delivering” ROS to cancer cells for molecular dynamic therapy. Advanced Materials . 2020;32(4) doi: 10.1002/adma.201904011.1904011 [DOI] [PubMed] [Google Scholar]
- 135.He Y., Ingudam S., Reed S., Gehring A., Strobaugh T. P., Irwin P. Study on the mechanism of antibacterial action of magnesium oxide nanoparticles against foodborne pathogens. Journal of Nanobiotechnology . 2016;14(1):54–59. doi: 10.1186/s12951-016-0202-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Geng Y.-K., Zhou Y. Reduction of refractory Maillard reaction products by Fe3+ during thermal hydrolysis pretreatment and enhanced sludge biodegradability. Journal of Hazardous Materials . 2022;430 doi: 10.1016/j.jhazmat.2022.128400.128400 [DOI] [PubMed] [Google Scholar]
- 137.Kubrak T., Karakuła M., Czop M., Kawczyk-Krupka A., Aebisher D. Advances in management of Bladder cancer—the role of photodynamic therapy. Molecules . 2022;27(3):p. 731. doi: 10.3390/molecules27030731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li J., Zhuang Z., Zhao Z., Tang B. Z. Type I AIE photosensitizers: mechanism and application. View . 2022;3(2) doi: 10.1002/viw.20200121.20200121 [DOI] [Google Scholar]
- 139.Lee H., Dey D. K., Kim K., et al. Hypoxia-responsive nanomedicine to overcome tumor microenvironment-mediated resistance to chemo-photodynamic therapy. Materials Today Advances . 2022;14 doi: 10.1016/j.mtadv.2022.100218.100218 [DOI] [Google Scholar]
- 140.Zhang R., Song X., Liang C., et al. Catalase-loaded cisplatin-prodrug-constructed liposomes to overcome tumor hypoxia for enhanced chemo-radiotherapy of cancer. Biomaterials . 2017;138:13–21. doi: 10.1016/j.biomaterials.2017.05.025. [DOI] [PubMed] [Google Scholar]
- 141.Liu L. H., Zhang Y. H., Qiu W. X., et al. Dual-stage light Amplified photodynamic therapy against hypoxic tumor based on an O2 self-sufficient Nanoplatform. Small . 2017;13(37) doi: 10.1002/smll.201701621.1701621 [DOI] [PubMed] [Google Scholar]
- 142.Widomska J., Welc R., Gruszecki W. I. The effect of carotenoids on the concentration of singlet oxygen in lipid membranes. Biochimica et Biophysica Acta (BBA) - Biomembranes . 2019;1861(4):845–851. doi: 10.1016/j.bbamem.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 143.Yu Q., Huang T., Liu C., et al. Oxygen self-sufficient NIR-activatable liposomes for tumor hypoxia regulation and photodynamic therapy. Chemical Science . 2019;10(39):9091–9098. doi: 10.1039/c9sc03161h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Accioni F., Rassu G., Begines B., et al. Novel utilization of therapeutic coatings based on infiltrated encapsulated Rose Bengal microspheres in porous titanium for Implant Applications. Pharmaceutics . 2022;14(6):p. 1244. doi: 10.3390/pharmaceutics14061244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Borodziuk A., Kowalik P., Duda M., et al. Unmodified Rose Bengal photosensitizer conjugated with NaYF4: Yb, Er upconverting nanoparticles for efficient photodynamic therapy. Nanotechnology . 2020;31(46) doi: 10.1088/1361-6528/aba975.465101 [DOI] [PubMed] [Google Scholar]
- 146.Ding M., Fan Y., Lv Y., et al. A prodrug hydrogel with tumor microenvironment and near-infrared light dual-responsive action for synergistic cancer immunotherapy. Acta Biomaterialia . 2022;149:334–346. doi: 10.1016/j.actbio.2022.06.041. [DOI] [PubMed] [Google Scholar]
- 147.Avdonin P. V., Nadeev A. D., Mironova G. Y., Zharkikh I. L., Avdonin P. P., Goncharov N. V. Enhancement by hydrogen peroxide of calcium signals in endothelial cells induced by 5-HT1B and 5-HT2B receptor agonists. Oxidative Medicine and Cellular Longevity . 2019;2019 doi: 10.1155/2019/1701478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tang Z., Liu Y., He M., Bu W. Chemodynamic therapy: tumor microenvironment‐mediated Fenton and Fenton‐like reactions. Angewandte Chemie . 2019;131(4):958–968. doi: 10.1002/ange.201805664. [DOI] [PubMed] [Google Scholar]
- 149.Huang H., Wang Z., Chen L., Yu H., Chen Y. Catalytic biomaterials and nanomedicines with exogenous and endogenous activations. Advanced Healthcare Materials . 2022 doi: 10.1002/adhm.202201607.2201607 [DOI] [PubMed] [Google Scholar]
- 150.Islam M. N., Rauf A., Fahad F. I., et al. Superoxide dismutase: an updated review on its health benefits and industrial applications. Critical Reviews in Food Science and Nutrition . 2022;62(26):7282–7300. doi: 10.1080/10408398.2021.1913400. [DOI] [PubMed] [Google Scholar]
- 151.Hong Y., Tao Q., Liu Y.-Y., Wang Z., Wang H., Sun L. Copper peroxide coated upconversion nanoparticle modified with glucose oxidase for H2O2 self-supplying starvation-enhanced chemodynamic therapy in vitro. Dalton Transactions . 2022;51(30) doi: 10.1039/d2dt00163b.11325 [DOI] [PubMed] [Google Scholar]
- 152.Wu Y., Li Y., Lv G., Bu W. Redox dyshomeostasis strategy for tumor therapy based on nanomaterials chemistry. Chemical Science . 2022;13(8):2202–2217. doi: 10.1039/d1sc06315d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Alizadeh N., Salimi A. Multienzymes activity of metals and metal oxide nanomaterials: applications from biotechnology to medicine and environmental engineering. Journal of Nanobiotechnology . 2021;19(1):26–31. doi: 10.1186/s12951-021-00771-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rastinfard A. Montréal, Canada: McGill University; 2022. Aqueous decomposition behaviour of oxygen releasing peroxy-compounds and assessment of their therapeutic potential. Department of Experimental Surgery. [Google Scholar]
- 155.Vellingiri K., Choudhary V., Kumar S., Philip L. Sorptive removal versus catalytic degradation of aqueous BTEX: a comprehensive review in the perspective of life-cycle assessment. Environmental Sciences: Water Research & Technology . 2022;8 doi: 10.1039/D1EW00918D. [DOI] [Google Scholar]
- 156.Ahmad T., Iqbal A., Halim S. A., et al. Recent advances in Electrochemical sensing of hydrogen peroxide (H2O2) released from cancer cells. Nanomaterials . 2022;12(9):p. 1475. doi: 10.3390/nano12091475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang C., Li Z., Bai J. Bubble-assisted HIFU ablation enabled by calcium peroxide. Journal of Materials Chemistry B . 2022;10(23):4442–4451. doi: 10.1039/d2tb00587e. [DOI] [PubMed] [Google Scholar]
- 158.Ahmed A. S. I., Sheng M. H. C., Lau K.-H. W., et al. Calcium released by osteoclastic resorption stimulates autocrine/paracrine activities in local osteogenic cells to promote coupled bone formation. American Journal of Physiology - Cell Physiology . 2022;322(5):C977–C990. doi: 10.1152/ajpcell.00413.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jeevanandam J., Kiew S. F., Boakye-Ansah S., et al. Green approaches for the synthesis of metal and metal oxide nanoparticles using microbial and plant extracts. Nanoscale . 2022;14(7):2534–2571. doi: 10.1039/d1nr08144f. [DOI] [PubMed] [Google Scholar]
- 160.Morciano G., Rimessi A., Patergnani S., et al. Calcium dysregulation in heart diseases: targeting calcium channels to achieve a correct calcium homeostasis. Pharmacological Research . 2022;177 doi: 10.1016/j.phrs.2022.106119.106119 [DOI] [PubMed] [Google Scholar]
- 161.Pu F., Chen N., Xue S. Calcium intake, calcium homeostasis and health. Food Science and Human Wellness . 2016;5(1):8–16. doi: 10.1016/j.fshw.2016.01.001. [DOI] [Google Scholar]
- 162.Glancy B., Willis W. T., Chess D. J., Balaban R. S. Effect of calcium on the oxidative phosphorylation cascade in skeletal muscle mitochondria. Biochemistry . 2013;52(16):2793–2809. doi: 10.1021/bi3015983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mercado C., Chhor C., Scheel J. R. MRI in the setting of neoadjuvant treatment of breast cancer. Journal of Breast Imaging . 2022;4(3):320–330. doi: 10.1093/jbi/wbab059. [DOI] [PubMed] [Google Scholar]
- 164.Zhou Y.-W., Long Y.-X., Liu X., Liu J.-Y., Qiu M. Tumor calcification is associated with better survival in metastatic colorectal cancer patients treated with bevacizumab plus chemotherapy. Future Oncology . 2022;18(22):2453–2464. doi: 10.2217/fon-2021-1422. [DOI] [PubMed] [Google Scholar]
- 165.Hahn V. S., Zhang K. W., Sun L., Narayan V., Lenihan D. J., Ky B. Heart failure with targeted cancer therapies: mechanisms and cardioprotection. Circulation Research . 2021;128(10):1576–1593. doi: 10.1161/circresaha.121.318223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zheng P., Ding J. Calcium ion nanomodulators for mitochondria-targeted multimodal cancer therapy. Asian Journal of Pharmaceutical Sciences . 2022;17(1):1–3. doi: 10.1016/j.ajps.2021.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang X., Li C., Jin H., et al. Mutual promotion of oxidative stress amplification and calcium overload by degradable spatially selective self-cascade catalyst for synergistic tumor therapy. Chemical Engineering Journal . 2022;432 doi: 10.1016/j.cej.2021.134438.134438 [DOI] [Google Scholar]
- 168.Yao J., Peng H., Qiu Y., et al. Nanoplatform-mediated calcium overload for cancer therapy. Journal of Materials Chemistry B . 2022;10(10):1508–1519. doi: 10.1039/d1tb02721b. [DOI] [PubMed] [Google Scholar]
- 169.Kalyane D., Choudhary D., Polaka S., et al. Reactive oxygen nano-generators for cancer therapy. Progress in Materials Science . 2022;130 doi: 10.1016/j.pmatsci.2022.100974.100974 [DOI] [Google Scholar]
- 170.Patel M., Prabhu A. Smart nanocomposite assemblies for multimodal cancer theranostics. International Journal of Pharmaceutics . 2022;618 doi: 10.1016/j.ijpharm.2022.121697.121697 [DOI] [PubMed] [Google Scholar]
- 171.Matsui C., Takatani‐Nakase T., Hatano Y., Kawahara S., Nakase I., Takahashi K. Zinc and its transporter ZIP6 are key mediators of breast cancer cell survival under high glucose conditions. FEBS Letters . 2017;591(20):3348–3359. doi: 10.1002/1873-3468.12797. [DOI] [PubMed] [Google Scholar]
- 172.Rozenberg J. M., Kamynina M., Sorokin M., et al. The role of the metabolism of zinc and Manganese ions in human Cancerogenesis. Biomedicines . 2022;10(5):p. 1072. doi: 10.3390/biomedicines10051072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chen G.-H., Song C.-C., Zhao T., et al. Mitochondria-dependent oxidative stress mediates ZnO nanoparticle (ZnO NP)-induced mitophagy and lipotoxicity in freshwater teleost fish. Environmental Science & Technology . 2022;56(4):2407–2420. doi: 10.1021/acs.est.1c07198. [DOI] [PubMed] [Google Scholar]
- 174.Dabravolski S. A., Sadykhov N. K., Kartuesov A. G., Borisov E. E., Sukhorukov V. N., Orekhov A. N. Interplay between Zn2+ homeostasis and mitochondrial functions in Cardiovascular diseases and heart ageing. International Journal of Molecular Sciences . 2022;23(13):p. 6890. doi: 10.3390/ijms23136890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Xie W., Ye J., Guo Z., et al. TME-responded Full-biodegradable nanocatalyst for mitochondrial calcium Overload-induced hydroxyl radical bursting cancer treatment. Chemical Engineering Journal . 2022;438 doi: 10.1016/j.cej.2022.135372.135372 [DOI] [Google Scholar]
- 176.Chang M., Hou Z., Wang M., Li C., Al Kheraif A. A., Lin J. Tumor microenvironment responsive single-Atom nanozymes for enhanced antitumor therapy. Chemistry (Rajkot, India) . 2022;28(15) doi: 10.1002/chem.202104081.e202104081 [DOI] [PubMed] [Google Scholar]
- 177.Yang N., Gong F., Cheng L. Recent advances in upconversion nanoparticle-based nanocomposites for gas therapy. Chemical Science . 2022;13(7):1883–1898. doi: 10.1039/d1sc04413c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chen J., Qiu M., Zhang S., et al. A calcium phosphate drug carrier loading with 5-fluorouracil achieving a synergistic effect for pancreatic cancer therapy. Journal of Colloid and Interface Science . 2022;605:263–273. doi: 10.1016/j.jcis.2021.07.080. [DOI] [PubMed] [Google Scholar]
- 179.Zhou Y., Jing S., Liu S., et al. Double-activation of mitochondrial permeability transition pore opening via calcium overload and reactive oxygen species for cancer therapy. Journal of Nanobiotechnology . 2022;20(1):188–214. doi: 10.1186/s12951-022-01392-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bo W., Silkunas M., Mangalanathan U., et al. Probing Nanoelectroporation and Resealing of the cell membrane by the Entry of Ca2+ and Ba2+ ions. International Journal of Molecular Sciences . 2020;21(9):p. 3386. doi: 10.3390/ijms21093386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Payne S. L., Ram P., Srinivasan D. H., Le T. T., Levin M., Oudin M. J. Potassium channel-driven bioelectric signalling regulates metastasis in triple-negative breast cancer. EBioMedicine . 2022;75 doi: 10.1016/j.ebiom.2021.103767.103767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Boone C. E., Wang L., Gautam A., Newton I. G., Steinmetz N. F. Combining nanomedicine and immune checkpoint therapy for cancer immunotherapy. Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology . 2022;14(1):p. e1739. doi: 10.1002/wnan.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Feng S., De Carvalho D. D. Clinical advances in targeting epigenetics for cancer therapy. FEBS Journal . 2022;289(5):1214–1239. doi: 10.1111/febs.15750. [DOI] [PubMed] [Google Scholar]
- 184.Feng S., Lu J., Wang K., et al. Advances in smart mesoporous carbon nanoplatforms for photothermal–enhanced synergistic cancer therapy. Chemical Engineering Journal . 2022;435 doi: 10.1016/j.cej.2022.134886.134886 [DOI] [Google Scholar]
- 185.Karges J. Clinical development of metal complexes as photosensitizers for photodynamic therapy of cancer. Angewandte Chemie . 2022;61(5) doi: 10.1002/anie.202112236.e202112236 [DOI] [PubMed] [Google Scholar]
- 186.Shi X., Zhang X., Zhang X., Guo H., Wang S. The Integration of reactive oxygen species generation and prodrug activation for cancer therapy. BIO Integration . 2022;3(1):32–40. [Google Scholar]
- 187.Wang Q., Gao Z., Zhao K., et al. Co-delivery of enzymes and photosensitizers via metal-phenolic network capsules for enhanced photodynamic therapy. Chinese Chemical Letters . 2022;33(4):1917–1922. doi: 10.1016/j.cclet.2021.11.040. [DOI] [Google Scholar]
- 188.Baker A., Khan M. S. Handbook of Oxidative Stress in Cancer: Therapeutic Aspects . New York, NY, USA: Springer; 2022. Biogenic nanomaterials derived ROS for cancer therapy; pp. 1–14. [Google Scholar]
- 189.Sanati M., Afshari A. R., Kesharwani P., Sukhorukov V. N., Sahebkar A. Recent trends in the application of nanoparticles in cancer therapy: the involvement of oxidative stress. Journal of Controlled Release . 2022;348:287–304. doi: 10.1016/j.jconrel.2022.05.035. [DOI] [PubMed] [Google Scholar]
- 190.Ansari A. A., Parchur A. K., Chen G. Surface modified lanthanide upconversion nanoparticles for drug delivery, cellular uptake mechanism, and current challenges in NIR-driven therapies. Coordination Chemistry Reviews . 2022;457 doi: 10.1016/j.ccr.2022.214423.214423 [DOI] [Google Scholar]
- 191.Kohestanian M., Pourjavadi A., Keshavarzi N. Facile and tunable method for polymeric surface modification of magnetic nanoparticles via RAFT polymerization: preparation, characterization, and drug release properties. European Polymer Journal . 2022;167 doi: 10.1016/j.eurpolymj.2022.111067.111067 [DOI] [Google Scholar]
- 192.Siddharth S., Kuppusamy P., Wu Q., Nagalingam A., Saxena N. K., Sharma D. Metformin enhances the anti-cancer efficacy of Sorafenib via suppressing MAPK/ERK/Stat3 Axis in hepatocellular carcinoma. International Journal of Molecular Sciences . 2022;23(15):p. 8083. doi: 10.3390/ijms23158083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Islam S., Wang S., Bowden N., Martin J., Head R. Repurposing existing therapeutics, its importance in oncology drug development: Kinases as a potential target. British Journal of Clinical Pharmacology . 2022;88(1):64–74. doi: 10.1111/bcp.14964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.El Zarif T., Yibirin M., De Oliveira-Gomes D., et al. Overcoming therapy resistance in colon cancer by drug repurposing. Cancers . 2022;14(9):p. 2105. doi: 10.3390/cancers14092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Liu C., Cao Y., Cheng Y., et al. An open source and reduce expenditure ROS generation strategy for chemodynamic/photodynamic synergistic therapy. Nature Communications . 2020;11(1):1735–1739. doi: 10.1038/s41467-020-15591-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sharma A., Saini A. K., Kumar N., et al. Methods of preparation of metal-doped and hybrid tungsten oxide nanoparticles for anticancer, antibacterial, and biosensing applications. Surfaces and Interfaces . 2022;28 doi: 10.1016/j.surfin.2021.101641.101641 [DOI] [Google Scholar]
- 197.Zhong Z., Liu C., Xu Y., et al. γ‐Fe2O3 loading Mitoxantrone and glucose oxidase for pH‐responsive chemo/chemodynamic/photothermal synergistic cancer therapy. Advanced Healthcare Materials . 2022;11(11) doi: 10.1002/adhm.202102632.2102632 [DOI] [PubMed] [Google Scholar]
- 198.Pan Q., Xie L., Liu R., et al. Two birds with one stone: copper metal-organic framework as a carrier of disulfiram prodrug for cancer therapy. International Journal of Pharmaceutics . 2022;612 doi: 10.1016/j.ijpharm.2021.121351.121351 [DOI] [PubMed] [Google Scholar]
- 199.Anastasiadis S. H., Chrissopoulou K., Stratakis E., Kavatzikidou P., Kaklamani G., Ranella A. How the physicochemical properties of Manufactured nanomaterials affect their performance in dispersion and their applications in biomedicine: a review. Nanomaterials . 2022;12(3):p. 552. doi: 10.3390/nano12030552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.He J., Fu L.-H., Qi C., Lin J., Huang P. Metal peroxides for cancer treatment. Bioactive Materials . 2021;6(9):2698–2710. doi: 10.1016/j.bioactmat.2021.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Elbahri M., Abdelaziz R., Disci-Zayed D., et al. Underwater Leidenfrost nanochemistry for creation of size-tailored zinc peroxide cancer nano therapeutics. Nature Communications . 2017;8(1) doi: 10.1038/ncomms15319.15319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Li S., Zhou B., Ren B., et al. Preparation of MgO nanomaterials by microemulsion-based oil/water interface precipitation. Materials Letters . 2016;171:204–207. doi: 10.1016/j.matlet.2016.02.048. [DOI] [Google Scholar]
- 203.Zardi P., Carofiglio T., Maggini M. Mild microfluidic approaches to Oxide nanoparticles synthesis. Chemistry (Rajkot, India) . 2022;28(9) doi: 10.1002/chem.202103132.e202103132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhang C., Belwal T., Luo Z., Su B., Lin X. Application of nanomaterials in isothermal nucleic acid amplification. Small . 2022;18(6) doi: 10.1002/smll.202102711.2102711 [DOI] [PubMed] [Google Scholar]
- 205.He C., Zhang X., Yan R., et al. Enhancement of cisplatin efficacy by lipid–CaO2 nanocarrier-mediated comprehensive modulation of the tumor microenvironment. Biomaterials Science . 2019;7(10):4260–4272. doi: 10.1039/c9bm00797k. [DOI] [PubMed] [Google Scholar]
- 206.Ullah A., Chen G., Yibang Z., et al. A new approach based on CXCR4-targeted combination liposomes for the treatment of liver fibrosis. Biomaterials Science . 2022;10(10):2650–2664. doi: 10.1039/d2bm00242f. [DOI] [PubMed] [Google Scholar]
- 207.Eastoe J., Tabor R. F. Colloidal Foundations of Nanoscience . Amsterdam, Netherlands: Elsevier; 2022. Surfactants and nanoscience; pp. 153–182. [Google Scholar]
- 208.Jimaja S., Varlas S., Foster J. C., Taton D., Dove A. P., O’Reilly R. K. Stimuli-responsive and core cross-linked micelles developed by NiCCo-PISA of helical poly (aryl isocyanide) s. Polymer Chemistry . 2022;13(27):4047–4053. doi: 10.1039/d2py00397j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Narita A., Naka K., Chujo Y. Facile control of silica shell layer thickness on hydrophilic iron oxide nanoparticles via reverse micelle method. Colloids and Surfaces A: Physicochemical and Engineering Aspects . 2009;336(1–3):46–56. doi: 10.1016/j.colsurfa.2008.11.013. [DOI] [Google Scholar]
- 210.Wu X., Xu N., Cheng C., et al. Encapsulation of hydrophobic capsaicin within the aqueous phase of water-in-oil high internal phase emulsions: controlled release, reduced irritation, and enhanced bioaccessibility. Food Hydrocolloids . 2022;123 doi: 10.1016/j.foodhyd.2021.107184.107184 [DOI] [Google Scholar]
- 211.Zheng A., Zhu S., Zhou J., Wang H. Dopamine-and citrate-mediated, rapid synthesis of hollow calcium carbonate nanoparticles: their formation, metastability and transformation. Colloids and Surfaces A: Physicochemical and Engineering Aspects . 2022;634 doi: 10.1016/j.colsurfa.2021.128056.128056 [DOI] [Google Scholar]
- 212.Deng S. G., Cao J. M., Feng J., et al. A bio-inspired approach to the synthesis of CaCO3 spherical assemblies in a soluble ternary-additive system. The Journal of Physical Chemistry B . 2005;109(23) doi: 10.1021/jp050290b.11473 [DOI] [PubMed] [Google Scholar]
- 213.Ghosh S., Sarkar B., Kaushik A., Mostafavi E. Nanobiotechnological prospects of probiotic microflora: synthesis, mechanism, and applications. Science of the Total Environment . 2022;838 doi: 10.1016/j.scitotenv.2022.156212.156212 [DOI] [PubMed] [Google Scholar]
- 214.Mahdavi R., Ashraf Talesh S. S. Effect of modified ZnO nanoparticles synthesized via the sonochemical method on the self-cleaning behavior of a waterborne acrylic coating. Journal of Dispersion Science and Technology . 2022;43(2):188–198. doi: 10.1080/01932691.2020.1847663. [DOI] [Google Scholar]
- 215.Malburet C., Leclercq L., Cotte J.-F., et al. Size and Charge characterization of lipid nanoparticles for mRNA Vaccines. Analytical Chemistry . 2022;94(11):4677–4685. doi: 10.1021/acs.analchem.1c04778. [DOI] [PubMed] [Google Scholar]
- 216.Koo S., Park O. K., Kim J., et al. Enhanced chemodynamic therapy by Cu–Fe peroxide nanoparticles: tumor microenvironment-mediated synergistic fenton reaction. ACS Nano . 2022;16(2):2535–2545. doi: 10.1021/acsnano.1c09171. [DOI] [PubMed] [Google Scholar]
- 217.Chen X., Yin X., Zhan L., et al. Organelle‐specific Anchored delivery system Stretching a reversal of tumor hypoxia microenvironment to a combinational chemo‐photothermal therapy. Advanced Functional Materials . 2022;32(15) doi: 10.1002/adfm.202108603.2108603 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used to support this study are included within the article.


