Abstract
Amino acids are not only the building blocks of proteins, an indispensable component of cells, but also play versatile roles in regulating cell metabolism, proliferation, differentiation and growth by themselves or through their derivatives. At the whole body level, the bioavailability and metabolism of amino acids, interacting with other macronutrients, is critical for the physiological processes of reproduction including gametogenesis, fertilization, implantation, placentation, fetal growth and development. In fertilization and early pregnancy, histotroph in oviductal and uterine secretions provides nutrients and microenvironment for conceptus (embryo and extraembryonic membranes) development. These nutrients include select amino acids in histotroph (arginine, leucine and glutamine of particular interest) that stimulate conceptus growth and development, as well as interactions between maternal uterus and the conceptus, thus impacting maintenance of pregnancy, placental growth, development and functions, fetal growth and development, and consequential pregnancy outcomes. Gestational protein undernutrition causes fetal growth restriction and predisposes cardiovascular, metabolic diseases and others in offspring via multiple mechanisms, whereas the supplementation of glycine, leucine and taurine during pregnancy partially rescues growth restriction and beneficially modulates fetal programming. Thus, amino acids are essential for the fertility of humans and all animals.
Keywords: amino acid, nutrition, reproduction, metabolism, uterus, pregnancy, mechanistic target of rapamycin
Introduction
Nutrition is critical for any organism’s growth and reproduction because nutrients not only provide building blocks for the growth of any cell, organ and whole body, but also regulate the development of reproductive organs, onset of reproduction and fetal growth and development (Wallace et al. 2006; Wang et al. 2012; Bloomfield et al. 2013). Among nutrients, amino acids not only function as the building block of proteins, one of the major components of cells, tissues, organs or organisms, but also plays versatile roles in reproduction, development and production (Wu et al. 2004; Wu 2009; Wu et al. 2014). Here we review the essential functions of amino acids, particularly derived from diet, in reproductive processes including gametogenesis, conceptus development and the long term impacts of their nutritional status during pregnancy on the health and diseases of adult offspring. Because published studies that address the role of amino acid in reproduction focus on amino acid transport and metabolism, the related main findings will be the focus of this review. Mammals (e.g., humans, pigs, sheep and cattle) will be included, but knowledge from other vertebrates such as rodents is also discussed if appropriate.
2. Amino acids in gametogenesis
2.1. Oogenesis
In mammals, oogenesis is a long and complicated process, which requires the hormonal and nutritional interactions between oocyte and surrounding granulosa and/or cumulus cells, dependent on the stage of oogenesis. This event is also affected by the environment (Wu et al. 2019). To date, our understanding of the metabolic control of oocyte development primarily comes from the oocyte’s in vitro maturation, while little is known about its metabolism in the early stage of oogenesis (Gu et al. 2015). Dietary deficiency of essential amino acids rapidly induces cessation of the rat estrous cycle (Narita et al. 2011), and preovulatory exposure to a protein-restricted diet disrupts amino acid kinetics and alters mitochondrial structure and function in the rat oocyte (Schutt et al. 2019). Therefore, amino acid metabolism is indispensable for oocyte development. Decreased amino acid transport proteins levels and increased glucose/lipid content in oocytes have been implicated in meiotic defects, organelle dysfunction and epigenetic alteration (Gu et al. 2015). To date, amino acid transport and metabolism in mammalian oocyte growth and development remains largely unknown.
The expression of amino acid transporter(s) in oocytes and follicular cells demonstrates a developmental stage-dependent manner. In mice, amino acid transport systems b0,+, L, and asc/ASC are active throughout oocyte growth and maturation; amino acid transport systems XAG−, B0,+, A, and CAT/y+ are not active in growing or meiotically maturing oocytes; amino Acid transport systems GLY, β, and xc− are activated in oocytes during meiotic maturation (Pelland et al. 2009). In particular, glycine transport was mainly via system GLY and cysteine/glutamate transport was via system X−c in immature oocytes, while system β, L, GLY, X−c, and b0,+ were detected in matured oocytes(Haghighat and Van Winkle 1990; Van Winkle et al. 1990; Van Winkle et al. 1992). Similarly, follicular cells (granulosa and cumulus cells) also demonstrated development stage-dependent expression of amino acid transporters and ability to promote amino acid transport into oocyte (Eppig et al. 2005). The extent to which follicular cells enhance uptake of a particular amino acid into oocytes depends on at least three physiologically important variables, the stage of follicular development, the presence of other amino acids in the environment, and gap junctional communication (Haghighat and Van Winkle 1990). It is noteworthy that cumulus and granulosa cells have distinct pattern of expression of amino acid transporters, for instance, Slc38a3 (a transcript encoding a sodium-coupled neutral amino acid transporter that has high substrate preference for alanine) was abundantly expressed in cumulus cells (Eppig et al. 2005), while granulosa cells promote transport of glycine, alanine, taurine and lysine into oocytes (Colonna et al. 1983; Haghighat and Van Winkle 1990; Pelland et al. 2009).
The prolife of amino acids in follicular fluids in antral follicles changes with the progression of follicular development. In pigs, regardless of follicle size, Gly, Gly, Ala, Gln, and Pro were the most abundant amino acids in pig follicular fluid (Hong and Lee 2007). As follicle size increased in antral follicles, the concentration of Asn significantly increased, but the concentrations of other amino acids, except Arg and Trp, significantly decreased (Hong and Lee 2007).
Oocytes and follicular cells have different metabolic requirements. While growing oocytes preferentially metabolize pyruvate over glucose, the somatic compartment of ovarian follicles is more glycolytic. Accumulating evidence supports other nutrients, amino acids including Gln, Arg and Leu, and fatty acids, play an important role in the maturation of oocytes (Collado-Fernandez et al. 2012). Thus, the unusually high abundance of amino acids such as glutamine may complement the metabolism of glucose and fatty acids via transamination and participation of the TCA cycle (Cetica et al. 2003), and serve as the energy substrate to support oocyte development (Collado-Fernandez et al. 2012). During oocyte maturation, the metabolism rate is increased and the oxidative metabolism is supported by an increase in glucose oxidation via the Krebs cycle. This cycle is facilitated by pyruvate, glutamine and glycine metabolism (Rieger and Loskutoff 1994). The utilization of amino acids also represents the developmental competence of oocyte in early embryonic development (Thompson et al. 2007).
2.2. Spermatogenesis
Similar to oogenesis, spermatogenesis is an intricate and complex process, which occurs in the two specialized compartments in seminiferous tubules, basal and Sertoli cell formed compartments. To date, less has been known about the metabolism of germ cells in the basal compartment where spermatogonia use glucose as a fuel for ATP production. More developed germ cells, such as spermatids, are unable to use glucose, despite of expressing all enzymes for glycolysis, and utilize lactate for ATP production (Boussouar and Benahmed 2004). The metabolism of sperm cells closely interacts with that of Sertoli cells when sperm germ cells enter meiosis. In general, Sertoli cells are involved in the regulation of spermatogenesis, providing nutritional support for germ cells. The characteristic of Sertoli cells in metabolism is its potent capacity of glycolysis, and low capacity of oxidative metabolism (Robinson and Fritz 1981; Grootegoed et al. 1986). Lactate is primarily derived from glycolysis in Sertoli cells (Robinson and Fritz 1981). The export of lactate from Sertoli cells by specific monocarboxylate transporters is responsible for improved lactate supply to germ cells and Sertoli cells preferentially use lipids as an energy source primarily via beta oxidation of fatty acids (Xiong et al. 2009). Recent studies suggest that despite being an energy substrate, glucose is not the main metabolite used for ATP synthesis in Sertoli cells (Riera et al. 2009), because Sertoli cells possesses a strong capacity of metabolizing fatty acids via the mitochondrial β-oxidation pathway (Xiong et al. 2009) and of the oxidation of amino acids [primarily Gln and Leu and also other amino acids, such as Ala and Val (Kaiser et al. 2005)]. Macronutrients have regulatory roles in Sertoli cells. First, glucose metabolism could modulate the oxidation of Ala and Val by competing with acetyl-CoA. Second, glucose metabolism stimulates the conversion of Val into lipids. Third, glutamine inhibits the oxidation of Leu, Val, and Ala, but does not alter the conversion of these amino acids into lipids. Fourth, Gln also inhibits the incorporation of alanine into proteins (Kaiser et al. 2005). Fifth, Ala is the main glucogenic amino acid, since it can be converted into pyruvate that can be used as a substrate by Sertoli cells for several biochemical pathways, including the TCA cycle and possibly gluconeogenesis (Kaiser et al. 2005; Rato et al. 2012).
3. Amino acids in conceptus development
In most mammalian species, fertilization and early mitotic divisions of the zygotes occur in the oviduct, followed by hatching from zona pellucid, implantation, placentation and fetal development in the uterus. Thus, oocytes containing nutrients, the oviductal fluid, uterine fluid and maternal circulation serve as the nutrient sources for conceptus development.
3.1. Fertilization stage
Fertilization occurs in the oviduct for most mammals. The oviduct provides the minienvironment for fertilization and the subsequent development of zygotes and early embryo. In bovine oviduct fluid, concentrations of amino acids were not affected by day of cycle (Days 0, 2, 3, 4 and 6). Asp, Glu, Ser, Gly, Ala, Tyr, Phe and Lys in oviductal fluid were present in higher concentrations than in plasma. Gly was the most abundant amino acid, and the concentration of many amino acids in oviduct fluid are higher than their plasma levels, indicating their transport into the oviduct (Hugentobler et al. 2007). To date, how these amino acids are transported from maternal circulation to the oviductal fluid remains unknown; however, recent studies support the view that the presence of multiple embryos in the oviduct could induce many differentiated gene expressions in the oviductal epithelial cells (Maillo et al. 2015).
Amino acids in the oviductal fluid may affect the process of fertilization including sperm penetration and pronuclear formation. In pigs, sperm penetration was not altered by amino acid treatment during oocyte in vitro maturation (IVM), but monospermic fertilization was increased by Gln, Asp, and Val. All amino acids except Asp and Asn stimulated male pronuclear formation after IVF. Arg and Ala treatment during IVM improved blastocyst formation (Hong and Lee 2007). Interestingly, gonadotrophins (FSH and LH) plus 11 amino acids interacted with cysteamine (a mercaptoethylamine compound that is endogenously derived from the CoA degradation) to improve oocyte maturation, while enhancing the decondensation of spermatozoa and maternal pronuclear formation. However, the addition of 10% serum or gonadotrophins with or without amino acids did not support male pronuclear formation without cysteamine. This suggests the importance of cysteamine in the formation of male pronuclear formation. In contrast, female pronuclear formation was apparently similar between controls and IVM oocytes (Kito and Bavister 1997).
3.2. Peri-implantation stage
Embryonic loss represents a major constraint in both human reproduction and the livestock industry. There is a high rate of loss of early pregnancies in humans and 75 percent of this loss represents a failure of implantation (Wilcox et al. 1988). Much pregnancy wastage is caused by abnormal embryos (Roberts and Lowe 1975); however, it has been estimated that 27 percent of normal embryos are lost at the time of or soon after implantation (Clark 2003). Similarly, the incidence of embryonic loss in sheep is 20 to 30 percent, of which two-thirds occurs during the peri-implantation period between Days 12 and 18 of gestation (Nancarrow 1994), when rapid trophectoderm growth and conceptus elongation occur (Guillomot M 1993). In addition, up to 40% of cattle embryos die within 3 weeks of fertilization (Hugentobler et al. 2007). Therefore, peri-implantation stage is critical for improving pregnancy outcomes.
In most mammals, an embryo at the morula stage enters uterine lumen and further develops in the minienvironment provided by uterine endometrium and uterine secretions termed histotroph. The latter includes secretions of uterine epithelia and molecules transported into the uterine lumen, a complex mixture of enzymes, growth factors, cytokines, lymphokines, hormones, transport proteins and nutrients. Histotroph is critical for growth and development of ovine conceptuses as they undergo morphological transitions from spherical to tubular to filamentous forms, as well as differentiation between Days 13 and 16 of pregnancy and immediately prior to implantation (Bazer 1975; Spencer and Bazer 2004). In ewes, survival and elongation of the conceptus as well as growth of trophectoderm are dependent on uterine secretions (Heyman et al. 1984; Flechon et al. 1986), as conceptuses fail to elongate beyond the tubular stage of development in ewes lacking uterine glands (Gray et al. 2001). Histotroph supports conceptus survival and development during the critical peri-implantation period of pregnancy in mammals (Bazer 1975; Spencer and Bazer 2004).
3.2.1. Amino acid profile in uterine secretions
Among the components in histotroph, amino acids play critical roles in embryonic development, especially the trophectoderm development during peri-implantation stage. In reality, amino acid turnover has been identified as an indicator of embryonic viability in humans and cattle (Houghton et al. 2002; Sturmey et al. 2010). To date, the amino acid profile in the uterine secretions has been measured in sheep(Gao et al. 2009d), pigs (Bazer et al. 2015a), cattle (Forde et al. 2014), and humans (Kermack et al. 2015).
In sheep, total recoverable glucose, Arg, Gln, Leu, Asp, Glu, Asn, His, beta-Ala, Tyr, Trp, Met, Val, Phe, Ile, Lys, Cys, Pro, glutathione, calcium, and sodium were greater in the uterine fluid of pregnant compared with cyclic ewes between Days 10 and 16. In cyclic ewes, only modest changes in the total amounts of glucose, Asn, Cit, Tyr, Trp, Met, Val, Cys, glutathione, calcium, and potassium were detected between Days 3 and 16. However, in pregnant ewes, amounts of glucose, Arg, Gln, Glu, Gly, Cys, Leu, Pro, glutathione, calcium, and potassium in uterine fluids increased 3- to 23-fold between Days 10 and 14 and remained high to Day 16. Of particular interest were increases in glucose, Arg, Leu, and Gln in uterine flushings of pregnant ewes between Days 10 and 16 of pregnancy (Fig 1). Total amounts of His, ornithine, Lys, Ser, Thr, Ile, Phe, Trp, Met, and Cit in uterine fluids also increased, but to a lesser extent during early pregnancy. These novel results indicate activation of pregnancy-associated mechanisms for transport of nutrients into the uterine lumen, and they provide a framework for future studies of nutrients, including glucose, amino acids, and glutathione, required to activate nutrient-sensing cell signaling pathways for growth, development, and survival of conceptuses, as well as for optimization of culture media for in vitro studies of conceptus development (Gao et al. 2009d). The detailed amino acid profile in the ovine uterine flushings from cyclic and early pregnant ewes are described in our original publication (Gao et al. 2009d). Similarly, there are significant changes in amino acids in uterine flushing of pigs during the estrous cycle (Days 5–15) and pregnancy (Days 9–15). Among all amino acid investigated, concentrations of Arg, Gln, Leu, His, Orn, Lys, Asp, Ser and Cys were affected by the status of pregnancy or cyclicity; concentrations of Arg, His, Asn, Gly, Ala, Cys, Glutathione, Tyr, Trp and Pro were changed with the days investigated; concentrations of Gln, Glu, Ser, Cys and Trp were affected by the interaction of status of pregnancy and Days (Bazer et al. 2015a). Amino acid profiles in bovine uterine secretions are also changed with the status of the estrus cycle and/or pregnancy. The concentrations of multiple amino acids (Asp, Arg, Gln, His, Lys, Ile, Leu, Phe, tTyr, Glu, Asn and Val) in bovine uterine luminal fluid were increased during peri-implantation (Days 7–19 of pregnancy investigated) (Forde et al. 2014).
FIG. 1.
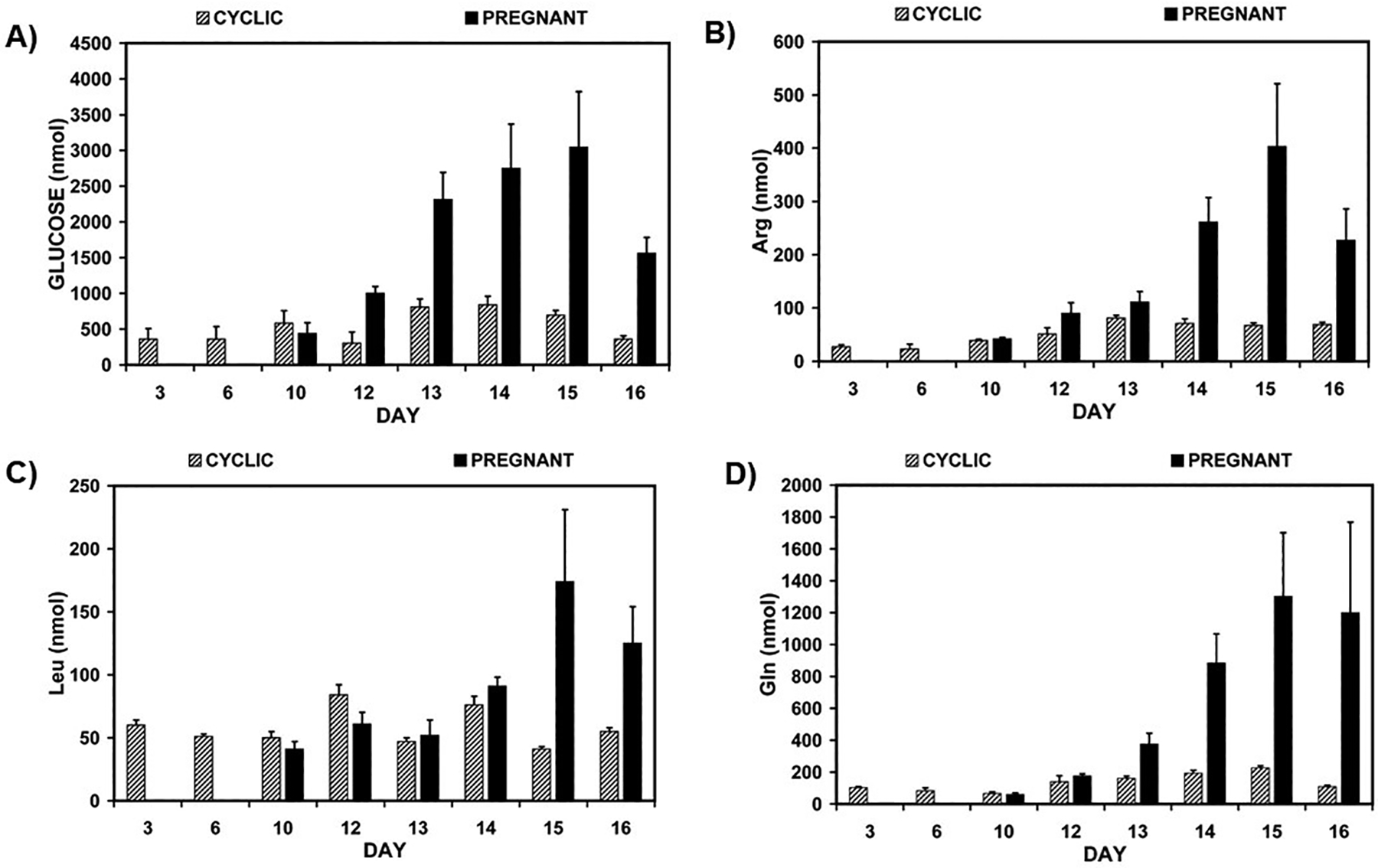
Total recoverable Glucose (A), arginine (B), leucine (C) and glutamine (D) (nmol) in uterine flushings from cyclic and pregnant ewes. Effects of day, pregnancy status and day by pregnancy status were significant (P < 0.05).
In literature, there is only one study of amino acid profile in human uterine secretions. In non-pregnant subjects, concentrations of 18 amino acids were not significantly altered by age, BMI, cycle phase or the presence of specific benign gynecological pathologies, although concentrations of several amino acids in the uterine fluid were increased by Western diet, including Asn, His, Ser, Gln, Val, Phe, Ile and Leu. In addition, there were no significant correlations between serum amino acid concentrations and those in the uterine fluid (Kermack et al. 2015).
3.2.2. Nutrient sensing: Mechanistic Target of Rapamycin (MTOR) signaling
MTOR, a highly conserved serine-threonine protein kinase, senses and responds to changes in amino acid levels and energy sufficiency, as well as select agents, such as hormones and mitogens (Dennis et al. 1996; Gingras et al. 1999; Gingras et al. 2001), thus integrating both extracellular and intracellular factors to control growth and development of cells and tissues. MTOR and associated proteins comprise two structurally and functionally distinct complexes, mTORC1 and mTORC2 (Guertin et al. 2006; Wullschleger et al. 2006; Liao et al. 2008). The MTOR-associated proteins appear to determine specificity of the two different cell signaling pathways (Schmelzle and Hall 2000; Gao et al. 2002; Zhang et al. 2003; Sarbassov et al. 2005) mediated by mTOR complexes, associated regulators and effectors.
MTORC1 and MTORC2 mediated cell signaling pathways may be critical for growth and development of the conceptus, as well as implantation. First, in mice, disruption of the MTOR gene leads to post-implantation lethality due to impaired cell proliferation and hypertrophy in both the embryonic disc and trophoblast (Gangloff et al. 2004; Murakami et al. 2004) and dysfunction of MTOR complex 1 and 2 leads to fetal lethality occurring at different stages of development (Guertin et al. 2006; Jacinto et al. 2006; Shiota et al. 2006). Second, MTOR signaling regulates translation of proteins in the uterus, including insulin-like growth factor-2 (IGF2), ornithine decarboxylase (ODC1) and nitric oxide synthases (NOS) (Nielsen et al. 1995; Kimball et al. 1999; Martin and Sutherland 2001), which play important roles in trophoblast cell proliferation, differentiation and migration in varied species including sheep and pigs (Kim et al. 2008; Kim et al. 2011; Kong et al. 2012; Kim et al. 2013; Kong et al. 2014; Wang et al. 2014a; Wang et al. 2014b; Wang et al. 2015; Wang et al. 2016; Lenis et al. 2018). Third, MTOR promotes translation of the “polypyrimidine tract” mRNA family (Jefferies et al. 1994) that is critical to fetal and placental development (Ohlsson et al. 1989; Zhou and Bondy 1992; Wathes et al. 1998), as well as trophoblast development, differentiation, and motility (Martin and Sutherland 2001; Martin et al. 2003; Wu et al. 2004). Fourth, emerging evidence indicates that the MTOR cell signaling pathways regulate expression of the glucose transporters SLC2A1 (Buller et al. 2008; Zhou et al. 2008) and SLC2A12 (Schmid et al. 2008), and amino acid transporters SLC1A5 (neutral amino acid transporter) (Fuchs and Bode 2005; Fuchs et al. 2007) and SLC7A5 (L-type amino acid transporter 1) (Liu et al. 2004; Fuchs and Bode 2005), which together with other amino acid transporters mediate the transport of glucose or amino acids from blood circulation into histotroph including oviductal and uterine secretions, critical microenvironments for fertilization and early conceptus development.
Our previous study demonstrated, for the first time, that MTOR and its complexes are present in both ovine uterine endometrium and conceptus during peri-implantation stage (Gao et al. 2009c). The mRNAs for MTOR, LST8, MAPKAP1, RAPTOR, RICTOR, TSC1, TSC2, RHEB, and EIF4EBP1 were localized to luminal, superficial glandular, and glandular epithelia and stromal cells of uteri from cyclic and pregnant ewes, as well as trophectoderm and endoderm of conceptuses between Days 13 and 18 of pregnancy. In endometria of pregnant ewes, increases in abundance of mRNAs for RICTOR, RHEB, and EIF4EBP1, as well as RHEB protein, correlated with rapid conceptus growth and development during the peri-implantation period. In addition, P4 and IFNT stimulated expression of RHEB and EIF4EBP1 in uterine endometria. These results suggest that the MTOR cell signaling pathway mediates interactions between the maternal uterus and peri-implantation conceptuses and that P4 and IFNT affect this pathway by regulating expression of RHEB and EIF4EBP1. To support this notion, further in vivo and ex vivo studies have demonstrated the critical role of MTOR signaling in early conceptus development in response to IGF2, SPP1 (osteopontin), select nutrients (glucose, Arg, Leu and Gln) and manipulation of production of NO and polyamine (Mehrotra et al. 1998; Wu and Morris 1998; Martin and Sutherland 2001; Martin et al. 2003; Kwon et al. 2004; Wu et al. 2005).
3.2.3. Amino acid transport
Amino acid transporters are required for amino acid transport from blood circulation into tissue and cells where amino acids are utilized. In human placentae, amino acid transport requires active amino acid transporters which belong to different families and systems (Regnault and Hay 2006; Grillo et al. 2008), but little is known about their expression in the uterine endometrium and conceptuses in livestock. Our studies, taking the advantage of prolonged period of peri-implantation in ovine conceptus development, we first revealed the expression of multiple amino acid transporters in ovine uterine endometrium and conceptus (Gao et al. 2009a,b).
Cationic amino acids including Arg are primarily transported by SLC7A1–3. SLC7A1 mRNA was most abundant in endometrial luminal (LE) and superficial glandular (sGE) epithelia in both cyclic and early pregnant ewes, SLC7A2 mRNA was most abundant in LE and mid to deep glandular (GE) epithelia on Days 14–20 of gestation and SLC7A3 is expressed ubiquitously in uterine endometrial cells. SLC7A1, SLC7A2, and SLC7A3 mRNAs were expressed in trophectoderm and endoderm of conceptuses. More importantly, the expression of SLC7A2 in uterine endometrium is induced by P4 and further stimulated by IFNT(Gao et al. 2009a), which may be responsible for the marked increase of Arg in uterine secretions during early pregnancy (Gao et al. 2009d). The detailed mRNA expression of neutral and acidic amino acids transporters in ovine uterine tissue and conceptus are summarized in Table 1. Briefly, SLC1A2, SLC1A3, SLC3A1, SLC6A14, SLC6A19, SLC7A6, SLC38A3, and SLC38A6 mRNAs were weakly expressed in the ovine endometrium. However, SLC1A4, SLC1A5, SLC7A8, and SLC43A2 mRNAs were detectable in uterine luminal epithelia (LE), superficial glandular epithelia (sGE), and/or glandular epithelia (GE). SLC1A1 and SLC7A5 mRNAs were most abundant in LE/sGE and GE. SLC1A3 and SLC38A4 mRNAs were most abundant in uterine stroma. SLC38A6 mRNA was detected only in cells with a stromal distribution suggesting immune lineage. SLC1A5 mRNA was expressed primarily in LE/sGE and stromal cells, and it was more abundant in uteri of pregnant ewes. Furthermore, P4 induced and IFNT further stimulated SLC1A5 expression in LE/sGE. Endometrial SLC1A1, SLC7A5, and SLC43A2 mRNAs demonstrated both temporal and cell SLC specific changes. Several mRNAs were detectable in trophectoderm (SLC6A19, SLC7A5, SLC7A6, and SLC43A2), while others were more abundant in endoderm (SLC1A4, SLC1A5, SLC6A19, SLC7A5, SLC7A6, SLC7A8, and SLC43A2) of conceptuses. These results document coordinate changes in expression of transporters that are likely responsible for increases in amounts of neutral and acidic amino acids in the uterine lumen to support conceptus growth, development, and survival (Gao et al. 2009b).
TABLE 1.
Gene name and function of members of Solute Carrier Families 1, 3, 7 and 43
| Gene Abbreviation | Full Name of Gene in HGNC and function |
|---|---|
| SLC1A1 a | solute carrier family 1 (neuronal/epithelial high affinity glutamate transporter, system XAG), member 1 |
| SLC1A2 * | solute carrier family 1 (glial high affinity glutamate transporter), member 2 |
| SLC1A3 *b | solute carrier family 1 (glial high affinity glutamate transporter), member 3 |
| SLC1A4 a,d | solute carrier family 1 (glutamate/neutral amino acid transporter), member 4 |
| SLC1A5 a,b,d | solute carrier family 1 (neutral amino acid transporter), member 5 |
| SLC3A1 * | solute carrier family 3 (cystine, dibasic and neutral amino acid transporters, activator of cystine, dibasic and neutral amino acid transport), member 1 |
| SLC6A14 * | solute carrier family 6 (amino acid transporter), member 14 |
| SLC6A19 *d | solute carrier family 6 (neutral amino acid transporter), member 19 |
| SLC7A5 b,d | solute carrier family 7 (cationic amino acid transporter, y+ system), member 5; transport large neutral amino acid |
| SLC7A6 *d | solute carrier family 7 (cationic amino acid transporter, y+ system), member 6; transport cationic and neutral amino acids |
| SLC7A8 a,d | solute carrier family 7 (cationic amino acid transporter, y+ system), member 8; transport large and small neutral amino acids |
| SLC38A3 * | solute carrier family 38, member 3; transport glutamine, histidine, and asparagine |
| SLC38A4 *b | solute carrier family 38, member 4; transport alanine, histidine, glutamine etc. |
| SLC38A6 c | solute carrier family 38, member 6; transport neutral amino acids |
| SLC43A2 a,d | solute carrier family 43, member 4; transport large neutral amino acids |
Weak to undetectable in uterine endometrial epithelia;
Detectable in endometrial luminal, superficial glandular and/or glandular epithelia;
Expression most abundant in endometrial stroma;
Detected in unidentified cells in endometrial stroma that appeared to be macrophages;
Detected in conceptus trophectoderm and endoderm.
Similar to sheep, temporal changes in the expression of amino acid transporters in the endometrium and conceptus occurs during early pregnancy in cattle (Days7-19 of pregnancy) and SLC1A1, −1A4, −1A5, −38A2, −38A4, −38A7, −43A2, −6A14, −7A1, −7A5 and −7A7 in the endometrium, some of which are modified by P4 (Bazer et al. 2015a). Temporal changes in expression of the cationic AA transporters SLC7A1, SLC7A4 and SLC7A6 occurred in the endometrium during the estrous cycle/early pregnancy coordinate with changes in conceptus expression of SLC7A4, SLC7A2 and SLC7A1. Only one acidic AA transporter (SLC1A5) increased in the endometrium while conceptus expression of SLC1A4 increased. The neutral AA transporters SLC38A2 and SLC7A5 increased in the endometrium in a temporal manner while conceptus expression of SLC38A7, SLC43A2, SLC38A11 and SLC7A8 also increased.
It is noteworthy that due to the lack of specific inhibitor for certain amino acid transporters and the substrate sharing among different amino acid transporters, it is difficult to analyze the activity of single amino acid transporter in vivo or ex vivo. More advanced techniques are highly demanded in the study of amino acid transport in both uterine endometrium and conceptus during peri-implantation and thereafter. Overexpression of genes or specific knockout of genes/proteins can provide a powerful tool to study a role of amino acid transporters in conceptus survival, growth and development.
3.2.4. Select amino acid metabolism in conceptus during peri-implantation stage
3.2.4.1. Arginine
Arg is a conditionally essential amino acid for conceptus survival, growth and development (Wu et al. 2013; 2017; 2018). In general, Arg exerts its functions primarily through its metabolites and is a precursor for important biological molecules including urea, creatinine, Orn, Pro, nitric oxide, agmatine (Agm) and polyamines (Wu and Morris 1998). Arginine metabolism via nitric oxide and polyamines play critical roles in conceptus development (Mehrotra et al. 1998; Wu and Morris 1998; Martin and Sutherland 2001; Martin et al. 2003; Kwon et al. 2004; Wu et al. 2005). In addition, Arg activates the mTOR cell signaling pathway to induce proliferation, migration and adhesion of ovine and pig trophectoderm cells required for implantation, survival and growth of blastocysts, as well as survival, growth, and health of mammalian conceptuses (Kong et al. 2012; Kong et al. 2014; Wang et al. 2014a; Bazer et al. 2015a; Wang et al. 2016).
In the ovine conceptus, mRNA expressions of SLC7A1–3 are present during peri-implantation stage. In vivo morpholino antisense oligonucleotide (MAO)-mediated knockdown of SLC7A1 mRNA in ovine trophectoderm report that SLC7A1 accounts for 73% of arginine uptake, thus being the key transporter of arginine by conceptus trophectoderm (Wang et al. 2014a). Interestingly, MAO knockdown of SLC7A1 also reduced the abundance of ornithine decarboxylase, and nitric oxide synthase (NOS3) proteins, arginine-related amino acids [Cit (76%) and Orn (40%)] and polyamines, which likely accounts for their retarded development (Wang et al. 2014a).
In ovine trophectoderm cells, arginine is used for biosynthesis of NO and polyamines (putrescine, spermidine and spermine) (Wang et al. 2014b; Wang et al. 2015; Lenis et al. 2018), which are critical to the morphological transition of conceptuses from the spherical to filamentous forms and signaling for pregnancy recognition by IFNT from conceptus trophectoderm. The NOS3 is rate-limiting enzymes in the production of NO in ovine conceptus, while ODC1, is the rate limiting enzyme in the production of polyamines from ornithine which is derivatives of arginine catalyzed by arginase I/II. In addition, Arg is also converted to agmatine by ADC and agmatine is converted to putrescine by AGAMAT in the uterus and conceptus trophectoderm to generate polyamines or agmatine may have direct effects on the uterus or conceptus (Wang et al. 2014b). Both putrescine and NO stimulate cell proliferation via activation of the TSC2-MTOR signaling cascade, whereas only putrescine increased IFNT production (Wang et al. 2015). (MAO-mediated knockdown ODC1 and ADC mRNAs was most detrimental to conceptus development and their production of interferon tau (IFNT). Agm, polyamines, amino acids, and adequate secretion of IFNT are critical for establishment and maintenance of pregnancy during the peri-implantation period of gestation in sheep (Lenis et al. 2018).
Arg also stimulates cell migration of ovine trophoblast cells and demonstrates synergistic effects on adhesion medicated by SPP1 in ovine trophectoderm cells. Those cooperative effects of arginine and SPP1 were mediated by focal adhesion assembly-MTORC2-cytoskeletal reorganization and MAPK pathways, which may play an important role in rapid elongation of ovine conceptuses during the peri-implantation period of pregnancy (Wang et al. 2016).
The acute stimulation of L-arginine on MTOR signaling has been reported in ovine and porcine trophoblast cells. In ovine trophoblast cells, Arg activates mTOR cell signaling and phosphorylation of RPS6, V-AKT murine thymoma viral oncogene homolog 1 (AKT1), glycogen synthase kinase 3-beta (GSK3B), mTOR and RPS6 kinase (RPS6K) proteins (Kim et al. 2010). Similarly, arginine activates MTOR signaling dose-dependently in porcine trophectoderm cell line (Kong et al. 2012) via the production of putrescine (Kong et al. 2014).
3.2.4.2. Leucine
Among all amino acids investigated before, the role of Leu in regulating activities of EIF4EBP1 and RPS6KB1 via MTOR signaling is well established (Proud 2002; Ban et al. 2004). Leu is the most effective single amino acid as other amino acids have little or no effect on phosphorylation or dephosphorylation of EIF4EBP1. Leu has a potent capability of regulating kinase activity of RPS6KB1 through phosphorylation (Ban et al. 2004). It has been proposed that Leu modulates MTOR function, in part, by regulating mitochondrial function and AMPK because it may serve both as a mitochondrial fuel through oxidative carboxylation and as an allosteric activator of glutamate dehydrogenase (Tokunaga et al. 2004). In ovine trophectoderm cells, leucine stimulates MTOR-RPS6K-RPS6 cell signaling pathways to stimulate hypertrophy, hyperplasia, and migration (Kim et al. 2011). Similarly, in porcine trophectoderm cells, physiological levels of Leu stimulate activities of mTOR and ribosomal protein S6 (RPS6) kinase, and proliferation of trophectoderm cells (Kim et al. 2013). More importantly, dietary supplementation of Leu during pregnancy may promote fetal growth and pregnancy outcome (Teodoro et al. 2012; Liu et al. 2018); therefore, Leu has the potential to be applied in the livestock industry and human clinic to overcome fetal growth restriction.
3.2.4.3. Glutamine
To date, little is known about the glutamine metabolism during early conceptus development in livestock. In ovine fetuses, however, Gln may be synthesized from branched-chain amino acids (BCAA) including Ile, Leu, and Val by BCAA transaminase in the placenta (Goodwin et al. 1987). Glutamine metabolism provides reducing equivalents for energy production in ovine (Wales and Du 1994) and bovine (Rieger et al. 1992) conceptuses, possibly to compensate for glucose metabolism (Gardner et al. 1993). In addition, Gln is essential for the synthesis of nucleotides, NAD(P)+, and aminosugars (glucosamine-6-phosphate, UDP-N-acetylgalactosamine, and UDP-N-acetylglucosamine), and a precursor for synthesis of all macromolecules containing amino sugars (Wu 2013; Flynn et al. 2002). Glutamine can be converted into citrulline, the precursor of Arg, in ovine placentae (Kwon et al. 2003a) and inhibit NO production from Arg (Wu et al. 2001); therefore, Gln and Arg are closely linked in conceptus metabolism. There is evidence that dietary supplementation with glutamine to swine during late gestation enhances pregnancy outcomes (Wu et al. 2011).
3.2.4.4. Proline
Little is known about proline metabolism and function in the conceptuses; however, ovine placentae have a high capacity for Pro catabolism and polyamine production as Pro is synthesized from pyrroline-5-carboxylate (P5C) by cytosolic NAD(P)H)-dependent P5C reductase, and P5C is formed mainly from Orn, Gln and Glu (Wu and Morris 1998; Wu et al. 2008b). Arg can also be converted into Pro in ovine placentae via the arginase pathway (Kwon et al. 2003b). Pro is a major substrate for polyamine synthesis via Pro oxidase, Orn aminotransferase and Orn decarboxylase in both ovine and porcine placentae. Pro can be converted into ornithine, which is subsequently converted into putrescine, spermidine and spermine via ornithine decarboxylase (ODC), spermidine synthase and spermine synthase, respectively in the cytosol (Wu et al. 2005). In addition, allantoic and amniotic fluids contain enzymes to convert Pro into Orn for delivery into the circulation of the conceptus (Kwon et al. 2003b). The important role of Pro in fetal development is supported by a positive association between fetal growth and Pro availability during pregnancy. Reduced placental and reduced fetal growth are associated with reductions in placental Pro transport, Pro oxidase activity, and concentrations of polyamines in gestating dams with either naturally occurring or malnutrition-induced growth retardation, while increasing Pro availability in maternal plasma through nutritional or pharmacological modulation in pigs and sheep enhances concentrations of Pro and polyamines in placentae and fetal fluids, as well as fetal growth (Wu et al. 2008a). In support of an important role of Pro in mammalian pregnancy, Liu et al. (2019a) recently reported that dietary supplementation with Pro to gestating mice enhanced the number of live-born pups. Interestingly, the F1 generation female offspring from Pro-supplemented dams had higher concentrations of Glu and Tau in plasma; of putrescine and spermidine in placental tissues; and of Gly, Tau, and spermidine in amniotic fluid at E12.5, as compared with F1 generation female offspring from dams without Pro supplementation (Liu et al. 2019b).
3.2.4.5. Other amino acids
Other amino acids play important roles in conceptus development. For example, Ala, and Ser, together with Gln, are major glucogenic precursors in humans (Wu 2013) and ewes (Clark et al. 1976). Ser also plays an important role in one-carbon unit metabolism essential for 2’-deoxythymidylate synthesis and methylation (Snell and Fell 1990). Ser participates in the synthesis of phosphatidylserine and ceramide (signaling molecules). These events are critical for DNA synthesis and consequently, cell proliferation. Gly and Ser are interconvertible via serine hydroxymethyltransferase, which also contributes to one-carbon unit metabolism essential for synthesis of purine and pyrimidine nucleotides in DNA synthesis and cell proliferation. Interestingly, Gly is the most abundant amino acid in ovine uterine arterial plasma (Kwon et al. 2003a), and uterine fluids from cyclic cows (Hugentobler et al. 2007).
3.3. Placentation stage and thereafter
In contrast to the extensive studies during peri-implantation stage, little is known about the amino acid transport and metabolism in placentation stage in most species. Placentation refers to the formation and arrangement of placenta, leading to a fully functional placenta, which maintains pregnancy, nurtures the fetus and modulates the bidirectional interactions between the mother and fetus. Like the formation of any organs, the development of placenta undergoes cell proliferation, death and differentiation in two major types of cells, trophoblast and endothelial cells, which execute two primary functions of the placenta, nutrient transport from the maternal circulation to fetal capillaries and hormone synthesis and release.
Studies of amino acids in the placenta can be primarily divided into two field of research, metabolic pathways and transport systems (Vonnahme et al. 2015). Previous research has shown net uteroplacental consumption of Ile, Leu, and Val, while Met was the only essential amino acid showing a net uteroplacental release (Chung et al. 1998). The ovine placenta has the capability of Gln synthesis due to the activity of Gln synthetase, which catalyzes the transamination of Glu, derived from α-ketoglutarate (Wu et al. 2015), a metabolite of branch-chained amino acids in the placenta (Chung et al. 1998). BCAA, especially Leu can activate mTOR signaling, a nutritional sensor, which stimulates cell growth and protein synthesis via increased rates of mRNA translation through the phosphorylation of eukaryotic initiation factor 4E-binding protein 1 (4E-BP1) and the ribosomal protein S6 kinase 1, and also cell proliferation, differentiation and migration. The regulatory mechanisms of amino acids on mTOR signaling has been described above. Supplementation of Leu to dams on a protein deficient diet can restore fetal growth and minimize the decreases in fetal organ mass and carcass fat, which is associated with increased mTOR signaling in the fetus (Teodoro et al. 2012). On the other hand, mTOR signaling regulates several type of amino acid transporters in trophoblast cells or cell lines at transcriptional and posttranslational levels (Edinger and Thompson 2002). In human primary trophoblast cell cultures, rapamycin, the inhibitor of mTOR complex 1, reduces the activity of system A, system L, and taurine amino acid transporters, but not protein expression, thus, amino acid transporter activation may be independent of protein synthesis (Edinger and Thompson 2002).
3.4. Beneficial Effects of Dietary Arginine Supplementation on Embryonic/Fetal Survival and Growth in Mammals
Dietary supplementation of Arg has been studied extensively in pregnant pigs the past decade, with the dose and timing of supplementation being determined to maximize the benefits of Arg in reproductive performance of pregnancy dams. To date, the effect of dietary supplementation with 0.4, 0.5, 0.83, 1 and 1.7% have been supplemented to pregnancy sows at the early, mid and late gestation. Most of these studies demonstrated the beneficial effect of Arg on pregnancy outcomes including increased live litter size and birthweight (Wu et al. 2017, 2018). An increase in the number of live-born pigs markedly increases the profit margin associated with reproduction and lactation performance in dams and reduced low-birth-weight piglets greatly improves the management of neonatal pigs and maximize pre-weaning survival and growth (Wu et al. 2010). However, dietary Arg supplementation immediately post-mating at the dose of 0.83% may have deleterious effects on reproductive performance of pigs (ovulation, luteinization and fetal development) (Li et al. 2010), and in stark contrast, dietary Arg supplementation starting 14 post-mating increased reproductive performance (Berard and Bee 2010; Li et al. 2014). In addition, Arg supplementation from early to late gestation increases pregnancy outcomes (increased live litter size and birth weight), but whether Arg supplementation at late gestation is beneficial is controversial (Bass et al. 2017; Nuntapaitoon et al. 2018). Thus, maternal dietary Arg supplementation holds great promise in improving reproductive efficiency in livestock industry.
Besides pregnant pigs, other species also benefit from the supplementation of Arg during pregnancy, including sheep, rats and humans. Intravenous administration of arginine prevents IUGR in underfed ewes (Lassala et al. 2010) and diet-induced obese ewes (Carey Satterfield et al. 2012). Dietary supplementation of pregnant rats with arginine increases the numbers of implantation sites and litter size by approximately three (Zeng et al. 2008; Zeng et al. 2013). More interestingly, intravenous Arg supplementation also improves fetal-placental growth and prevent IUGR in pregnant women by reducing placental apoptosis and improving fetal growth and development (Shen and Hua 2011), increasing birth weight at term (Xiao and Li 2005; Singh et al. 2015), reducing diastolic blood pressure and prolonging pregnancy in patients with gestational hypertension with or without proteinuria (Gui et al. 2014). Thus, Arg supplementation could be potentially used to prevent or treat IUGR and pregnancy related disorders.
4. Amino acids in developmental origins of health and diseases
4.1. Protein restriction during maternal gestation and associated fetal growth restriction and fetal programming of adult diseases
Evidence from numerous human studies and animal experiments supports the Barker Hypothesis, which propose that the in utero environment especially nutrition affects fetal growth and development and has long term effects on offspring health and diseases in adulthood (Fleming et al. 2015; Daniels 2016; Sferruzzi-Perri and Camm 2016). Pregnant rats with dietary protein insufficiency have been widely used as an animal models in the study of fetal programming of adult diseases, or developmental origin of adult health and diseases and other experimental models include mice (Gheorghe et al. 2009; Mortensen et al. 2010; Gonzalez et al. 2016) and non-human primate (Roberts et al. 2018). One of important findings in the study of gestational protein insufficiency is the association between fetal growth restriction and predisposition of adult diseases and gestational protein insufficiency programmed diseases include cardiovascular diseases (hypertension, cardiac and arterial disorders), metabolic diseases (obesity and diabetes) and endocrine disorders (Sferruzzi-Perri and Camm 2016). To date, although many proposed mechanisms have been proposed, the placenta is emerging as a critical player in fetal programming (Burton et al. 2016; Sferruzzi-Perri and Camm 2016; Myatt and Thornburg 2018). Placentas of dams with gestational protein insufficiency demonstrate impaired growth and placental efficiency (the ratio of fetal to placental weight) (Gao et al. 2012a), which is associated with reduced expression and/or activities of amino acid transporters and MTOR signaling (Jansson et al. 2006; Rosario et al. 2011; Sferruzzi-Perri and Camm 2016).
4.2. Amino acids in maternal and fetal plasma in response to a low protein diet during pregnancy
One may assume that the abundance of amino acids in maternal plasm will be decreased if pregnancy dams are fed a low protein diet. In striking contrast to this assumption, in pregnant rats fed the low protein diet during mid and late pregnancy, the total concentration of amino acids in maternal plasma is not reduced by protein insufficiency, resulting from increased levels of so-called “non-essential amino acids” and reduced levels of essential amino acids (Gao et al. 2012a) and altered maternal metabolism related to diet intake (Gao et al. 2015a; Gao et al. 2015b) and insulin secretion (Gao et al. 2017), which may represents a successful adaptation to maternal nutritional stresses. Similarly, protein-deficient gilts maintain maternal plasma concentrations of amino acids by mobilizing maternal protein stores and decreasing oxidation of amino acids during the first half of gestation (Wu et al. 1998). The “non-essential amino acids” likely play an important role in the adaptation of the conceptus to a nutritional insult for survival.
To date, few studies measured fetal plasma amino acids in response to maternal dietary protein insufficiency due to the technical limits in fetal plasma sampling in most experimental animals. In rats with gestational protein insufficiency, among all the measured amino acids, Thr is the only amino acid whose fetal plasma levels are remarkably reduced in late pregnancy (Rees et al. 1999). However, the dietary supplementation of Thr failed to rescue the fetal growth restriction and programmed adult diseases in offspring (Rees et al. 2006). In contrast, in pigs, reduced concentration of multiple amino acids are seen in both fetal plasma and allantoic fluid during mid-pregnancy (fetal plasma: Ala, Arg, BCAAs, Gln, Gly, Lys, Orn, Pro, Tau, Thr and urea; allantoic fluid: Ala, Arg, BCAA, Cit, Cys, Gly, His, Met, Pro, Ser, Tau, Thr and Tyr) (Wu et al. 1998). This discrepancy in fetal amino acid profile in response to gestational protein insufficiency may result from species differences in the metabolism, stage of pregnancy as well as different diet components.
4.3. Mechanisms of fetal growth restriction
To date, emerging evidence suggests the following mechanisms to be responsible for fetal growth restriction. Reduced mTOR signaling in the placenta. Gestational protein insufficiency in rats leads to reduced mTOR signaling, and decreased expressions and activities of several sodium-dependent neutral amino acid transporters such as system A in the placenta (Jansson et al. 2006), possibly due to reduced plasma BCAAs (Gao et al. 2012a) and insulin (Gao et al. 2017) which are known stimulators of MTOR signaling.
Activation of the amino acid response (AAR) pathway in the placenta and IGFBP-1 activity. The mammalian AAR pathway (activating transcription factor-3 and 4) in the placenta are upregulated by a maternal low-protein diet (Strakovsky et al. 2010) and the activation of AAR stimulates both IGFBP-1 secretion and hyperphosphorylation (pSer101/pSer119/pSer169), decreasing IGF-1 bioavailability and its activity as potent regulator of fetal growth (Karl 1995).
Altered renin-angiotensin system (RAS) in maternal, uterine and placental compartments. RAS plays a critical role in regulating blood flow, including the blood flow in maternal-utero-fetal units. The low protein diet alters the expression of RAS in maternal and utero-placental units, including enhanced angiotensin II production in maternal lung (Gao et al. 2012d; Gao et al. 2016), increased expression of angiotensin receptor type I in uterine artery (Gao et al. 2012d), reduced expression of angiotensin converting enzyme II (ACE2) in placental labyrinth zone (Gao et al. 2012c). All these alterations contribute to the reduced utero-fetal blood flow, a determinant of fetal growth (Lang et al. 2003), and local accumulation of angiotensin II in placental labyrinth zone inhibits amino acid transport in trophoblast cells (Shibata et al. 2006).
Increased testosterone. The LP diet enhances the plasma levels of testosterone in pregnant rats (Zambrano et al. 2005; Gao et al. 2012b). Increased testosterone inhibits the expression and activity of neutral amino acid transporters (Sathishkumar et al. 2011), thus resulting in IUGR as well as associated hypertension and other disorders in adult offspring (Chinnathambi et al. 2012; Chinnathambi et al. 2013).
Impaired mitochondrial function. Gestational protein insufficiency causes the mitochondrial abnormality with increased oxygen uptake and impaired oxidative phosphorylation (Rebelato et al. 2013), negatively affecting placental functions including amino acid transport which requires continuous energy supply. Improving anti-oxidative responses and reducing obesity likely play an important role in mitigating mitochondrial dysfunction (Ji et al. 2017).
4.4. Prevention of fetal programming in response to maternal gestational protein restriction
Supplementations of Gly (Jackson et al. 2002), taurine (Mortensen et al. 2010) and Leu (Teodoro et al. 2012) during pregnancy have been reported to benefit fetal growth and long-term health in response to maternal protein insufficiency, although the underlying mechanisms remain unclear. Dietary supplementation of Gly throughout pregnancy normalized the predisposed hypertension in offspring from pregnant rats fed a low protein diet (Jackson et al. 2002), possibly by reversing vascular dysfunction in mesenteric artery and improving NO release in maternal circulation. The supplementation of taurine in maternal gestational protein restriction partly rescues fetal growth retardation, restores fatty acid metabolism in the liver and oxidative phosphorylation and TCA cycle in skeletal muscle (Mortensen et al. 2010), normalizes proliferation and vascularization and cytokine sensitivity in pancreatic islets in offspring (Boujendar et al. 2002; Merezak et al. 2004). The supplementation with Leu also reversed this growth deficit, minimizing the difference or restoring the mass of organs and carcass fat, the liver and muscle protein, and the RNA concentrations in offspring of rats with gestational protein restriction, possibly by the activation of the mTOR signaling pathway (Teodoro et al. 2012). Dietary supplementation of Leu to premating SD rats improves the within-litter birth weight uniformity, antioxidative capability, and immune function (Liu et al. 2018). However, a recent study with a large cohort of human subjects suggested that the dietary amino acid pattern, rich in branched-chain, aromatic, and aliphatic amino acids, and proline could increase the risk of hypertension (Teymoori et al. 2017). The controversy on the benefits of dietary amino acids in pregnancy and non-pregnancy may result from the differences in the metabolic patterns of amino acids (including ammonia production) and metabolic adaptations during pregnancy (Herring et al. 2018).
5. Summary
Amino acids are critical for animal production and human reproduction because they modulate major processes of reproduction, including gametogenesis, fertilization, implantation, placentation, and fetal growth and development. Peri-implantation stage of pregnancy is associated with significant embryonic loss in both humans and livestock, and the remarkable increases of amino acids in the uterine secretions, hormonally regulated expression of amino acid transporters in the uterine endometrium, and the activation of MTOR signaling pathway provide an intricate regulatory system in conceptus growth and development, implantation and maintenance of pregnancy. Select amino acids in uterine secretions, particularly Arg, Leu and Gln, together with glucose, stimulates trophectoderm or trophoblast cell proliferation, differentiation and growth through the MTOR cell signaling. Arg exerts its stimulatory effects on placental and fetal growth primarily through its derivatives, NO and polyamines, whose production in the trophectoderm/trophoblast cells are regulated by key enzymes NOS3, ODC1, ADC, AGMAT, and proline oxidase. Dietary or intravenous Arg supplementation improves reproductive performances of domestic animals and humans and could serve as a potential means to prevent or treat fetal growth restriction. Dietary supplementation with Gln during late gestation can also improve fetal growth. Extreme nutritional conditions, including gestational protein undernutrition, causes fetal growth restriction and long-term effects on the health and disease in offspring via impaired MTOR and amino acid response signaling in the placenta, altered RAS in maternal, placental and fetal compartments, and elevated testosterone. Dietary supplementation of Gly, Leu and taurine to dams with a severe protein deficiency can partially rescue fetal growth restriction and fetal programming of adult diseases.
FIG. 2.
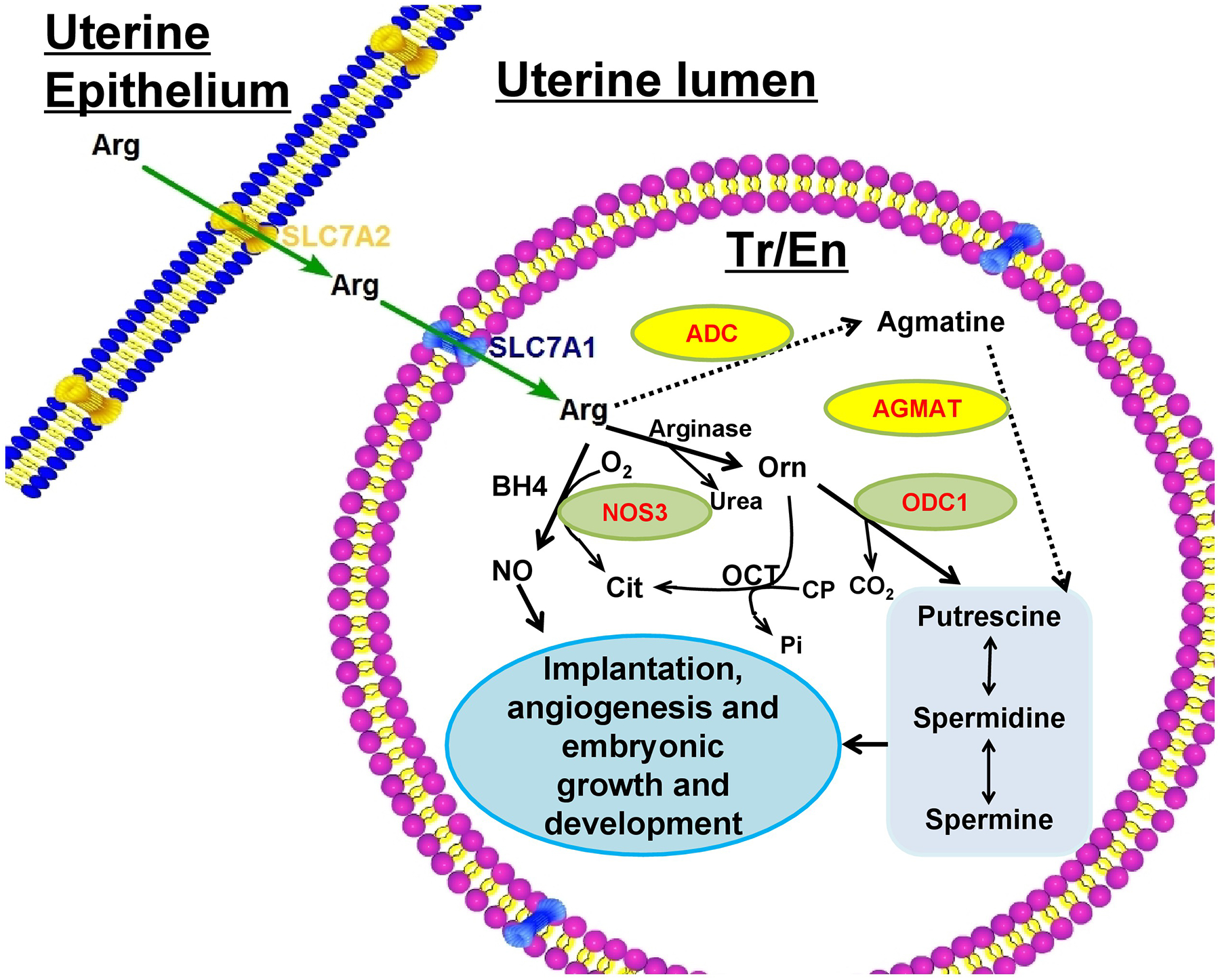
Biosynthesis of nitric oxide and polyamines from L-arginine in ovine trophectoderm cells. Arginine in uterine lumen, primarily transported by solute carrier family 7 member 2 (SLC7A2) in uterine endometrial epithelia, is transported into trophectoderm (Tr) and/or endoderm (En) cells by the solute carrier family 7 member 1 (SLC7A1) where it can be converted to nitric oxide (NO) by nitric oxide synthase 3 (NOS3) or arginine can be converted to ornithine by arginase and then ornithine is converted to putrescine by ornithine decarboxylase (ODC1). However, the sheep conceptus can also convert arginine to agmatine via arginine decarboxylase and agmatine can be converted to putrescine by agmatinase. ADC: arginine decarboxylase; BH4: tetrahydrobiopterin; OCT: optimal cutting temperature; AGMAT: agmatinase (Gao et al. 2009a; Bazer et al. 2015b).
References
- Ban H, Shigemitsu K, Yamatsuji T, Haisa M, Nakajo T, Takaoka M, Nobuhisa T, Gunduz M, Tanaka N, Naomoto Y. Arginine and Leucine regulate p70 S6 kinase and 4E-BP1 in intestinal epithelial cells. Int J Mol Med. 2004;13:537–43. [PubMed] [Google Scholar]
- Bass BE, Bradley CL, Johnson ZB, Zier-Rush CE, Boyd RD, Usry JL, Maxwell CV, Frank JW. Influence of dietary L-arginine supplementation of sows during late pregnancy on piglet birth weight and sow and litter performance during lactation. J Anim Sci. 2017;95:248–56. [DOI] [PubMed] [Google Scholar]
- Bazer FW. Uterine protein secretions: Relationship to development of the conceptus. J Anim Sci. 1975;41:1376–82. [DOI] [PubMed] [Google Scholar]
- Bazer FW, Johnson GA, Wu G. Amino acids and conceptus development during the peri-implantation period of pregnancy. Adv Exp Med Biol. 2015a;843:23–52. [DOI] [PubMed] [Google Scholar]
- Bazer FW, Wang X, Johnson GA, Wu G. Select nutrients and their effects on conceptus development in mammals. Anim Nutr. 2015b;1:85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berard J, Bee G. Effects of dietary l-arginine supplementation to gilts during early gestation on foetal survival, growth and myofiber formation. Animal. 2010;4:1680–7. [DOI] [PubMed] [Google Scholar]
- Bloomfield FH, Jaquiery AL, Oliver MH. Nutritional regulation of fetal growth. Nestle Nutr Inst Workshop Ser. 2013;74:79–89. [DOI] [PubMed] [Google Scholar]
- Boujendar S, Reusens B, Merezak S, Ahn MT, Arany E, Hill D, Remacle C. Taurine supplementation to a low protein diet during foetal and early postnatal life restores a normal proliferation and apoptosis of rat pancreatic islets. Diabetologia. 2002;45:856–66. [DOI] [PubMed] [Google Scholar]
- Boussouar F, Benahmed M. Lactate and energy metabolism in male germ cells. Trends Endocrinol Metab. 2004;15:345–50. [DOI] [PubMed] [Google Scholar]
- Buller CL, Loberg RD, Fan MH, Zhu Q, Park JL, Vesely E, Inoki K, Guan KL, Brosius FC III. A GSK-3/TSC2/mTOR pathway regulates glucose uptake and GLUT1 glucose transporter expression. Am J Physiol Cell Physiol. 2008;295:C836–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton GJ, Fowden AL, Thornburg KL. Placental Origins of Chronic Disease. Physiol Rev. 2016;96:1509–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey Satterfield M, Dunlap KA, Keisler DH, Bazer FW, Wu G. Arginine nutrition and fetal brown adipose tissue development in diet-induced obese sheep. Amino Acids. 2012;43:1593–603. [DOI] [PubMed] [Google Scholar]
- Cetica P, Pintos L, Dalvit G, Beconi M. Involvement of enzymes of amino acid metabolism and tricarboxylic acid cycle in bovine oocyte maturation in vitro. Reproduction. 2003;126:753–63. [PubMed] [Google Scholar]
- Chinnathambi V, Balakrishnan M, Ramadoss J, Yallampalli C, Sathishkumar K. Testosterone alters maternal vascular adaptations: role of the endothelial NO system. Hypertension. 2013;61:647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnathambi V, Balakrishnan M, Yallampalli C, Sathishkumar K. Prenatal testosterone exposure leads to hypertension that is gonadal hormone-dependent in adult rat male and female offspring. Biol Reprod. 2012;86:137, 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung M, Teng C, Timmerman M, Meschia G, Battaglia FC. Production and utilization of amino acids by ovine placenta in vivo. Am J Physiol. 1998;274:E13–22. [DOI] [PubMed] [Google Scholar]
- Clark DA. Is there any evidence for immunologically mediated or immunologically modifiable early pregnancy failure? J Assist Reprod Genet. 2003;20:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MG, Filsell OH, Jarrett IG. Gluconeogenesis in isolated intact lamb liver cells. Effects of glucagon and butyrate. Biochem J. 1976;156:671–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado-Fernandez E, Picton HM, Dumollard R. Metabolism throughout follicle and oocyte development in mammals. Int J Dev Biol. 2012;56:799–808. [DOI] [PubMed] [Google Scholar]
- Colonna R, Cecconi S, Buccione R, Mangia F. Amino acid transport systems in growing mouse oocytes. Cell Biol Int Rep. 1983;7:1007–15. [DOI] [PubMed] [Google Scholar]
- Daniels SR. The Barker hypothesis revisited. J Pediatr. 2016;173:1–3. [DOI] [PubMed] [Google Scholar]
- Dennis PB, Pullen N, Kozma SC, Thomas G. The principal rapamycin-sensitive p70(s6k) phosphorylation sites, T-229 and T-389, are differentially regulated by rapamycin-insensitive kinase kinases. Mol Cell Biol. 1996;16: 6242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger AL, Thompson CB. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol Biol Cell. 2002;13:2276–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppig JJ, Pendola FL, Wigglesworth K, Pendola JK. Mouse oocytes regulate metabolic cooperativity between granulosa cells and oocytes: amino acid transport. Biol Reprod. 2005;73:351–7. [DOI] [PubMed] [Google Scholar]
- Flechon JE, Guillomot M, Charlier M, Flechon B, Martal J. Experimental studies on the elongation of the ewe blastocyst. Reprod Nutr Dev. 1986;26:1017–24. [DOI] [PubMed] [Google Scholar]
- Fleming TP, Velazquez MA, Eckert JJ. Embryos, DOHaD and David Barker. J Dev Orig Health Dis. 2015;6:377–83. [DOI] [PubMed] [Google Scholar]
- Flynn NE, Meininger CJ, Haynes TE, and Wu G. The metabolic basis of arginine nutrition and pharmacotherapy. Biomed Pharmacother. 2002;56:427–38. [DOI] [PubMed] [Google Scholar]
- Forde N, Simintiras CA, Sturmey R, Mamo S, Kelly AK, Spencer TE, Bazer FW, and Lonergan P. Amino acids in the uterine luminal fluid reflects the temporal changes in transporter expression in the endometrium and conceptus during early pregnancy in cattle. PLoS One. 2014;9:e100010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs BC, and Bode BP. Amino acid transporters ASCT2 and LAT1 in cancer: partners in crime? Semin Cancer Biol. 2005;15:254–66. [DOI] [PubMed] [Google Scholar]
- Fuchs BC, Finger RE, Onan MC, Bode BP. ASCT2 silencing regulates mammalian target-of-rapamycin growth and survival signaling in human hepatoma cells. Am J Physiol. 2007;293:C55–63. [DOI] [PubMed] [Google Scholar]
- Gangloff YG, Mueller M, Dann SG, Svoboda P, Sticker M, Spetz JF, Um SH, Brown EJ, Cereghini S, Thomas G, Kozma SC. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol Cell Biol. 2004;24:9508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Ho E, Balakrishnan M, Yechoor V, Yallampalli C. Decreased insulin secretion in pregnant rats fed a low protein diet. Biol Reprod. 2017;97:627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Sathishkumar KR, Yallampalli U, Balakrishnan M, Li X, Wu G, Yallampalli C. Maternal protein restriction regulates IGF2 system in placental labyrinth. Front Biosci (Elite Ed). 2012a;4:1434–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Sisley S, Yallampalli C. Blunted hypothalamic ghrelin signaling reduces diet intake in rats fed a low-protein diet in late pregnancy. Physiol Rep. 2015a;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Tanchico DT, Yallampalli U, Balakrishnan MP, Yallampalli C. Appetite regulation is independent of the changes in ghrelin levels in pregnant rats fed low-protein diet. Physiol Rep. 2015b;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Tanchico DT, Yallampalli U, Yallampalli C. A Low-Protein Diet Enhances Angiotensin II Production in the Lung of Pregnant Rats but not Nonpregnant Rats. J Pregnancy. 2016;2016:4293431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Wu G, Spencer TE, Johnson GA, Bazer FW. Select nutrients in the ovine uterine lumen. III. Cationic amino acid transporters in the ovine uterus and peri-implantation conceptuses. Biol Reprod. 2009a;80:602–9. [DOI] [PubMed] [Google Scholar]
- Gao H, Wu G, Spencer TE, Johnson GA, Bazer FW. Select nutrients in the ovine uterine lumen. IV. Expression of neutral and acidic amino acid transporters in ovine uteri and peri-implantation conceptuses. Biol Reprod. 2009b;80:1196–208. [DOI] [PubMed] [Google Scholar]
- Gao H, Wu G, Spencer TE, Johnson GA, Bazer FW. Select nutrients in the ovine uterine lumen. VI. Expression of FK506-binding protein 12-rapamycin complex-associated protein 1 (FRAP1) and regulators and effectors of mTORC1 and mTORC2 complexes in ovine uteri and conceptuses. Biol Reprod. 2009c;81:87–100. [DOI] [PubMed] [Google Scholar]
- Gao H, Wu G, Spencer TE, Johnson GA, Li X, Bazer FW. Select nutrients in the ovine uterine lumen. I. Amino acids, glucose, and ions in uterine lumenal flushings of cyclic and pregnant ewes. Biol Reprod. 2009d;80:86–93. [DOI] [PubMed] [Google Scholar]
- Gao H, Yallampalli U, Yallampalli C. Gestational protein restriction reduces expression of Hsd17b2 in rat placental labyrinth. Biol Reprod. 2012b;87:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Yallampalli U, Yallampalli C. Maternal protein restriction reduces expression of angiotensin I-converting enzyme 2 in rat placental labyrinth zone in late pregnancy. Biol Reprod. 2012c;86:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Yallampalli U, Yallampalli C. Protein restriction to pregnant rats increases the plasma levels of angiotensin II and expression of angiotensin II receptors in uterine arteries. Biol Reprod. 2012d;86:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao K, Jiang Z, Lin Y, Zheng C, Zhou G, Chen F, Yang L, Wu G. Dietary L-arginine supplementation enhances placental growth and reproductive performance in sows. Amino Acids. 2012e;42:2207–14. [DOI] [PubMed] [Google Scholar]
- Gao X, Zhang Y, Arrazola P, Hino O, Kobayashi T, Yeung RS, Ru B, Pan D. Tsc tumour suppressor proteins antagonize amino-acid-TOR signalling. Nat Cell Biol. 2002;4:699–704. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Lane M, Batt P. Uptake and metabolism of pyruvate and glucose by individual sheep preattachment embryos developed in vivo. Mol Reprod Dev. 1993;36:313–9. [DOI] [PubMed] [Google Scholar]
- Gheorghe CP, Goyal R, Holweger JD, Longo LD. Placental gene expression responses to maternal protein restriction in the mouse. Placenta. 2009;30:411–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras AC, Gygi SP, Raught B, Polakiewicz RD, Abraham RT, Hoekstra MF, Aebersold R, Sonenberg N. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev. 1999;13:1422–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Gygi SP, Niedzwiecka A, Miron M, Burley SK, Polakiewicz RD, Wyslouch-Cieszynska A, Aebersold R, Sonenberg N. Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev. 2001;15:2852–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez PN, Gasperowicz M, Barbeito-Andres J, Klenin N, Cross JC, Hallgrimsson B. Chronic Protein Restriction in Mice Impacts Placental Function and Maternal Body Weight before Fetal Growth. PLoS One. 2016;11: e0152227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin GW, Gibboney W, Paxton R, Harris RA, Lemons JA. Activities of branched-chain amino acid aminotransferase and branched-chain 2-oxo acid dehydrogenase complex in tissues of maternal and fetal sheep. Biochem J. 1987;242:305–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray CA, Taylor KM, Ramsey WS, Hill JR, Bazer FW, Bartol FF, Spencer TE. Endometrial glands are required for preimplantation conceptus elongation and survival. Biol Reprod. 2001;64:1608–13. [DOI] [PubMed] [Google Scholar]
- Grillo MA, Lanza A, Colombatto S. Transport of amino acids through the placenta and their role. Amino Acids. 2008; 34:517–23. [DOI] [PubMed] [Google Scholar]
- Grootegoed JA, Oonk RB, Jansen R, van der Molen HJ. Metabolism of radiolabelled energy-yielding substrates by rat Sertoli cells. J Reprod Fertil. 1986;77:109–18. [DOI] [PubMed] [Google Scholar]
- Gu L, Liu H, Gu X, Boots C, Moley KH, Wang Q. Metabolic control of oocyte development: linking maternal nutrition and reproductive outcomes. Cell Mol Life Sci. 2015;72:251–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11:859–71. [DOI] [PubMed] [Google Scholar]
- Gui S, Jia J, Niu X, Bai Y, Zou H, Deng J, Zhou R. Arginine supplementation for improving maternal and neonatal outcomes in hypertensive disorder of pregnancy: a systematic review. J Renin Angiotensin Aldosterone Syst. 2014;15:88–96. [DOI] [PubMed] [Google Scholar]
- Guillomot MFJ, Leroy F. Blastocyst development and implantation. Eds. Thibault C, Levasseur MC & Hunter RHF. 1993;387–411. [Google Scholar]
- Haghighat N, Van Winkle LJ. Developmental change in follicular cell-enhanced amino acid uptake into mouse oocytes that depends on intact gap junctions and transport system Gly. J Exp Zool. 1990;253:71–82. [DOI] [PubMed] [Google Scholar]
- Herring CM, Bazer FW, Johnson GA, Wu G. Impacts of maternal dietary protein intake on fetal survival, growth and development. Exp Biol Med. 2018;243:525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman Y, Camous S, Fevre J, Meziou W, Martal J. Maintenance of the corpus luteum after uterine transfer of trophoblastic vesicles to cyclic cows and ewes. J Reprod Fertil. 1984;70:533–40. [DOI] [PubMed] [Google Scholar]
- Hong J, Lee E. Intrafollicular amino acid concentration and the effect of amino acids in a defined maturation medium on porcine oocyte maturation, fertilization, and preimplantation development. Theriogenology. 2007;68:728–35. [DOI] [PubMed] [Google Scholar]
- Houghton FD, Hawkhead JA, Humpherson PG, Hogg JE, Balen AH, Rutherford AJ, Leese HJ. Non-invasive amino acid turnover predicts human embryo developmental capacity. Hum Reprod. 2002;17:999–1005. [DOI] [PubMed] [Google Scholar]
- Hugentobler SA, Diskin MG, Leese HJ, Humpherson PG, Watson T, Sreenan JM, Morris DG. Amino acids in oviduct and uterine fluid and blood plasma during the estrous cycle in the bovine. Mol Reprod Dev. 2007;74:445–54. [DOI] [PubMed] [Google Scholar]
- Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–37. [DOI] [PubMed] [Google Scholar]
- Jackson AA, Dunn RL, Marchand MC, Langley-Evans SC. Increased systolic blood pressure in rats induced by a maternal low-protein diet is reversed by dietary supplementation with glycine. Clin Sci (Lond). 2002;103:633–9. [DOI] [PubMed] [Google Scholar]
- Jansson N, Pettersson J, Haafiz A, Ericsson A, Palmberg I, Tranberg M, Ganapathy V, Powell TL, Jansson T. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J Physiol. 2006;576:935–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Wu ZL, Dai ZL, Wang XL, Li J, Wang BG, Wu G. Fetal and neonatal programming of postnatal growth and feed efficiency in swine. J Anim Sci Biotechnol. 2017;8:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies HB, Reinhard C, Kozma SC, Thomas G. Rapamycin selectively represses translation of the “polypyrimidine tract” mRNA family. Proc Natl Acad Sci U S A. 1994;91:4441–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser GR, Monteiro SC, Gelain DP, Souza LF, Perry ML, Bernard EA. Metabolism of amino acids by cultured rat Sertoli cells. Metabolism. 2005;54:515–21. [DOI] [PubMed] [Google Scholar]
- Karl PI. Insulin-like growth factor-1 stimulates amino acid uptake by the cultured human placental trophoblast. J Cell Physiol. 1995;165:83–8. [DOI] [PubMed] [Google Scholar]
- Kermack AJ, Finn-Sell S, Cheong YC, Brook N, Eckert JJ, Macklon NS, Houghton FD. Amino acid composition of human uterine fluid: association with age, lifestyle and gynaecological pathology. Hum Reprod. 2015;30: 917–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Erikson DW, Burghardt RC, Spencer TE, Wu G, Bayless KJ, Johnson GA, Bazer FW. Secreted phosphoprotein 1 binds integrins to initiate multiple cell signaling pathways, including FRAP1/mTOR, to support attachment and force-generated migration of trophectoderm cells. Matrix Biol. 2010;29:369–82. [DOI] [PubMed] [Google Scholar]
- Kim J, Song G, Gao H, Farmer JL, Satterfield MC, Burghardt RC, Wu G, Johnson GA, Spencer TE, Bazer FW. Insulin-like growth factor II activates phosphatidylinositol 3-kinase-protooncogenic protein kinase 1 and mitogen-activated protein kinase cell Signaling pathways, and stimulates migration of ovine trophectoderm cells. Endocrinology. 2008;149:3085–94. [DOI] [PubMed] [Google Scholar]
- Kim J, Song G, Wu G, Gao H, Johnson GA, Bazer FW. Arginine, leucine, and glutamine stimulate proliferation of porcine trophectoderm cells through the MTOR-RPS6K-RPS6-EIF4EBP1 signal transduction pathway. Biol Reprod. 2013;88:113. [DOI] [PubMed] [Google Scholar]
- Kim JY, Burghardt RC, Wu G, Johnson GA, Spencer TE, Bazer FW. Select nutrients in the ovine uterine lumen. VII. Effects of arginine, leucine, glutamine, and glucose on trophectoderm cell signaling, proliferation, and migration. Biol Reprod. 2011;84:62–9. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Shantz LM, Horetsky RL, Jefferson LS. Leucine regulates translation of specific mRNAs in L6 myoblasts through mTOR-mediated changes in availability of eIF4E and phosphorylation of ribosomal protein S6. J Biol Chem. 1999;274:11647–52. [DOI] [PubMed] [Google Scholar]
- Kito S, Bavister BD. Male pronuclear formation and early embryonic development of hamster oocytes matured in vitro with gonadotrophins, amino acids and cysteamine. J Reprod Fertil. 1997;110: 35–46. [DOI] [PubMed] [Google Scholar]
- Kong X, Tan B, Yin Y, Gao H, Li X, Jaeger LA, Bazer FW, Wu G. L-Arginine stimulates the mTOR signaling pathway and protein synthesis in porcine trophectoderm cells. J Nutr Biochem. 2012;23:1178–83. [DOI] [PubMed] [Google Scholar]
- Kong X, Wang X, Yin Y, Li X, Gao H, Bazer FW, Wu G. Putrescine stimulates the mTOR signaling pathway and protein synthesis in porcine trophectoderm cells. Biol Reprod. 2014;91:106. [DOI] [PubMed] [Google Scholar]
- Kwon H, Spencer TE, Bazer FW, Wu G. Developmental changes of amino acids in ovine fetal fluids. Biol Reprod. 2003a;68:1813–20. [DOI] [PubMed] [Google Scholar]
- Kwon H, Wu G, Bazer FW, Spencer TE. Developmental changes in polyamine levels and synthesis in the ovine conceptus. Biol Reprod. 2003b;69:1626–34. [DOI] [PubMed] [Google Scholar]
- Kwon H, Wu G, Meininger CJ, Bazer FW, Spencer TE. Developmental changes in nitric oxide synthesis in the ovine placenta. Biol Reprod. 2004;70:679–86. [DOI] [PubMed] [Google Scholar]
- Lang U, Baker RS, Braems G, Zygmunt M, Kunzel W, Clark KE. Uterine blood flow--a determinant of fetal growth. Eur J Obstet Gynecol Reprod Biol. 2003;110 (Suppl 1):S55–61. [DOI] [PubMed] [Google Scholar]
- Lassala A, Bazer FW, Cudd TA, Datta S, Keisler DH, Satterfield MC, Spencer TE, Wu G. Parenteral administration of L-arginine prevents fetal growth restriction in undernourished ewes. J Nutr. 2010;140:1242–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenis YY, Johnson GA, Wang X, Tang WW, Dunlap KA, Satterfield MC, Wu G, Hansen TR, Bazer FW. Functional roles of ornithine decarboxylase and arginine decarboxylase during the peri-implantation period of pregnancy in sheep. J Anim Sci Biotechnol. 2018;9:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Xia H, Yao W, Wang T, Li J, Piao X, Thacker P, Wu G, Wang F. Effects of arginine supplementation during early gestation (day 1 to 30) on litter size and plasma metabolites in gilts and sows. J Anim Sci. 2015;93:5291–303. [DOI] [PubMed] [Google Scholar]
- Li X, Bazer FW, Johnson GA, Burghardt RC, Erikson DW, Frank JW, Spencer TE, Shinzato I, Wu G. Dietary supplementation with 0.8% L-arginine between days 0 and 25 of gestation reduces litter size in gilts. J Nutr. 2010;140:1111–6. [DOI] [PubMed] [Google Scholar]
- Li X, Bazer FW, Johnson GA, Burghardt RC, Frank JW, Dai Z, Wang J, Wu Z, Shinzato I, Wu G. Dietary supplementation with L-arginine between days 14 and 25 of gestation enhances embryonic development and survival in gilts. Amino Acids. 2014;46:375–84. [DOI] [PubMed] [Google Scholar]
- Liao XH, Majithia A, Huang X, Kimmel AR. Growth control via TOR kinase signaling, an intracellular sensor of amino acid and energy availability, with crosstalk potential to proline metabolism. Amino Acids. 2008;35:761–70. [DOI] [PubMed] [Google Scholar]
- Liu T, Zuo B, Wang W, Wang S, Wang J. Dietary Supplementation of Leucine in Premating Diet Improves the Within-Litter Birth Weight Uniformity, Antioxidative Capability, and Immune Function of Primiparous SD Rats. Biomed Res Int. 2018;2018:1523147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XM, Reyna SV, Ensenat D, Peyton KJ, Wang H, Schafer AI, Durante W. Platelet-derived growth factor stimulates LAT1 gene expression in vascular smooth muscle: role in cell growth. FASEB J. 2004;18:768–70. [DOI] [PubMed] [Google Scholar]
- Liu N, Dai ZL, Zhang YC, Chen JQ, Yang Y, Wu G, Tso P, Wu ZL. Maternal L-proline supplementation enhances fetal survival and placental nutrient transport in mice. Biol. Reprod 2019a;100:1073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Dai ZL, Jia H, Zhang YC, Chen JQ, Sun SQ, Wu G, Wu ZL. Maternal L-proline supplementation during gestation alters amino acid and polyamine metabolism in the first generation female offspring of C57BL/6J mice. Amino Acids. 2019b;51:805–11. [DOI] [PubMed] [Google Scholar]
- Maillo V, Gaora PO, Forde N, Besenfelder U, Havlicek V, Burns GW, Spencer TE, Gutierrez-Adan A, Lonergan P, Rizos D. Oviduct-Embryo Interactions in Cattle: Two-Way Traffic or a One-Way Street? Biol Reprod. 2015;92:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PM, Sutherland AE. Exogenous amino acids regulate trophectoderm differentiation in the mouse blastocyst through an mTOR-dependent pathway. Dev Biol. 2001;240:182–93. [DOI] [PubMed] [Google Scholar]
- Martin PM, Sutherland AE, Van Winkle LJ. Amino acid transport regulates blastocyst implantation. Biol Reprod. 2003;69:1101–8. [DOI] [PubMed] [Google Scholar]
- Mateo RD, Wu G, Bazer FW, Park JC, Shinzato I, Kim SW. Dietary L-arginine supplementation enhances the reproductive performance of gilts. J Nutr. 2007;137:652–6. [DOI] [PubMed] [Google Scholar]
- Mehrotra PK, Kitchlu S, Farheen S. Effect of inhibitors of enzymes involved in polyamine biosynthesis pathway on pregnancy in mouse and hamster. Contraception. 1998;57:55–60. [DOI] [PubMed] [Google Scholar]
- Merezak S, Reusens B, Renard A, Goosse K, Kalbe L, Ahn MT, Tamarit-Rodriguez J, Remacle C. Effect of maternal low-protein diet and taurine on the vulnerability of adult Wistar rat islets to cytokines. Diabetologia. 2004; 47:669–75. [DOI] [PubMed] [Google Scholar]
- Mortensen OH, Olsen HL, Frandsen L, Nielsen PE, Nielsen FC, Grunnet N, Quistorff B. A maternal low protein diet has pronounced effects on mitochondrial gene expression in offspring liver and skeletal muscle; protective effect of taurine. J Biomed Sci. 2010;17 (Suppl 1):S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Ichisaka T, Maeda M, Oshiro N, Hara K, Edenhofer F, Kiyama H, Yonezawa K, Yamanaka S. mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Mol Cell Biol. 2004;24:6710–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myatt L, Thornburg KL. Effects of prenatal nutrition and the role of the placenta in health and disease. Methods Mol Biol. 2018;1735:19–46. [DOI] [PubMed] [Google Scholar]
- Nancarrow CD. Embryonic mortality in the ewe and doe. In: Embryonic Mortality in Domestic Species, edited by Zavy MT and Geisert RD. CRC Press, Boca Raton, Florida. pp. 79–97, 1994. [Google Scholar]
- Narita K, Nagao K, Bannai M, Ichimaru T, Nakano S, Murata T, Higuchi T, Takahashi M. Dietary deficiency of essential amino acids rapidly induces cessation of the rat estrous cycle. PLoS One. 2011;6:e28136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen FC, Ostergaard L, Nielsen J, Christiansen J. Growth-dependent translation of IGF-II mRNA by a rapamycin-sensitive pathway. Nature. 1995;377:358–62. [DOI] [PubMed] [Google Scholar]
- Nuntapaitoon M, Muns R, Theil PK, Tummaruk P. l-arginine supplementation in sow diet during late gestation decrease stillborn piglet, increase piglet birth weight and increase immunoglobulin G concentration in colostrum. Theriogenology. 2018;121:27–34. [DOI] [PubMed] [Google Scholar]
- Ohlsson R, Larsson E, Nilsson O, Wahlstrom T, Sundstrom P. Blastocyst implantation precedes induction of insulin-like growth factor II gene expression in human trophoblasts. Development. 1989;106:555–9. [DOI] [PubMed] [Google Scholar]
- Pelland AM, Corbett HE, and Baltz JM. Amino Acid transport mechanisms in mouse oocytes during growth and meiotic maturation. Biol Reprod. 2009;81:1041–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proud CG. Regulation of mammalian translation factors by nutrients. Eur J Biochem. 2002;269:5338–49. [DOI] [PubMed] [Google Scholar]
- Rato L, Alves MG, Socorro S, Duarte AI, Cavaco JE, Oliveira PF. Metabolic regulation is important for spermatogenesis. Nat Rev Urol. 2012;9:330–8. [DOI] [PubMed] [Google Scholar]
- Rebelato HJ, Esquisatto MA, Moraes C, Amaral ME, Catisti R. Gestational protein restriction induces alterations in placental morphology and mitochondrial function in rats during late pregnancy. J Mol Histol. 2013;44:629–37. [DOI] [PubMed] [Google Scholar]
- Rees WD, Hay SM, Antipatis C. The effect of dietary protein on the amino acid supply and threonine metabolism in the pregnant rat. Reprod Nutr Dev. 2006;46:227–39. [DOI] [PubMed] [Google Scholar]
- Rees WD, Hay SM, Buchan V, Antipatis C, Palmer RM. The effects of maternal protein restriction on the growth of the rat fetus and its amino acid supply. Br J Nutr. 1999;81:243–50. [PubMed] [Google Scholar]
- Regnault TR, Hay WW Jr. In vivo techniques for studying fetoplacental nutrient uptake, metabolism, and transport. Methods Mol Med. 2006;122:207–24. [DOI] [PubMed] [Google Scholar]
- Rieger D, Loskutoff NM. Changes in the metabolism of glucose, pyruvate, glutamine and glycine during maturation of cattle oocytes in vitro. J Reprod Fertil. 1994; 100: 257–262. [DOI] [PubMed] [Google Scholar]
- Rieger D, Loskutoff NM, Betteridge KJ. Developmentally related changes in the uptake and metabolism of glucose, glutamine and pyruvate by cattle embryos produced in vitro. Reprod Fertil Dev. 1992;4:547–57. [DOI] [PubMed] [Google Scholar]
- Riera MF, Galardo MN, Pellizzari EH, Meroni SB, Cigorraga SB. Molecular mechanisms involved in Sertoli cell adaptation to glucose deprivation. Am J Physiol Endocrinol Metab. 2009;297:E907–14. [DOI] [PubMed] [Google Scholar]
- Roberts CJ, Lowe CR. Where have all the conceptions gone?. Lancet 1975;1:636–7. [PubMed] [Google Scholar]
- Roberts VHJ, Lo JO, Lewandowski KS, Blundell P, Grove KL, Kroenke CD, Sullivan EL, Roberts CT Jr, Frias AE. Adverse placental perfusion and pregnancy outcomes in a new nonhuman primate model of gestational protein restriction. Reprod Sci. 2018;25:110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson R, Fritz IB. Metabolism of glucose by Sertoli cells in culture. Biol Reprod. 1981;24:1032–41. [DOI] [PubMed] [Google Scholar]
- Rosario FJ, Jansson N, Kanai Y, Prasad PD, Powell TL, Jansson T. Maternal protein restriction in the rat inhibits placental insulin, mTOR, and STAT3 signaling and down-regulates placental amino acid transporters. Endocrinology. 2011;152:1119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. [DOI] [PubMed] [Google Scholar]
- Sathishkumar K, Elkins R, Chinnathambi V, Gao H, Hankins GD, Yallampalli C. Prenatal testosterone-induced fetal growth restriction is associated with down-regulation of rat placental amino acid transport. Reprod Biol Endocrinol. 2011;9:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000;103:253–62. [DOI] [PubMed] [Google Scholar]
- Schmid H, Bertoluci M, Coimbra TM. Glucose transporter 12 and mammalian target of rapamycin complex 1 signaling: a new target for diabetes-induced renal injury? Endocrinology. 2008;149:913–16. [DOI] [PubMed] [Google Scholar]
- Schutt AK, Blesson CS, Hsu JW, Valdes CT, Gibbons WE, Jahoor F, Yallampalli C. Preovulatory exposure to a protein-restricted diet disrupts amino acid kinetics and alters mitochondrial structure and function in the rat oocyte and is partially rescued by folic acid. Reprod Biol Endocrinol. 2019;17:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sferruzzi-Perri AN, Camm EJ. The programming power of the placenta. Front Physiol. 2016;7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen SF, Hua CH. Effect of L-arginine on the expression of Bcl-2 and Bax in the placenta of fetal growth restriction. J Matern Fetal Neonatal Med. 2011;24:822–6. [DOI] [PubMed] [Google Scholar]
- Shibata E, Powers RW, Rajakumar A, von Versen-Hoynck F, Gallaher MJ, Lykins DL, Roberts JM, Hubel CA. Angiotensin II decreases system A amino acid transporter activity in human placental villous fragments through AT1 receptor activation. Am J Physiol Endocrinol Metab. 2006;291:E1009–16. [DOI] [PubMed] [Google Scholar]
- Shiota C, Woo JT, Lindner J, Shelton KD, Magnuson MA. Multiallelic disruption of the rictor gene in mice reveals that mTOR complex 2 is essential for fetal growth and viability. Dev Cell. 2006; 11:583–9. [DOI] [PubMed] [Google Scholar]
- Singh S, Singh A, Sharma D, Singh A, Narula MK, Bhattacharjee J. Effect of L-arginine on nitric oxide levels in intrauterine growth restriction and its correlation with fetal outcome. Indian J Clin Biochem. 2015;30:298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell K, Fell DA. Metabolic control analysis of mammalian serine metabolism. Adv Enzyme Regul. 1990;30:13–32. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Bazer FW. Uterine and placental factors regulating conceptus growth in domestic animals. J Anim Sci. 2004;82 (E-Suppl):E4–13. [DOI] [PubMed] [Google Scholar]
- Strakovsky RS, Zhou D, Pan YX. A low-protein diet during gestation in rats activates the placental mammalian amino acid response pathway and programs the growth capacity of offspring. J Nutr. 2010;140:2116–20. [DOI] [PubMed] [Google Scholar]
- Sturmey RG, Bermejo-Alvarez P, Gutierrez-Adan A, Rizos D, Leese HJ, Lonergan P. Amino acid metabolism of bovine blastocysts: a biomarker of sex and viability. Mol Reprod Dev. 2010;77:285–296. [DOI] [PubMed] [Google Scholar]
- Teodoro GF, Vianna D, Torres-Leal FL, Pantaleao LC, Matos-Neto EM, Donato J Jr, Tirapegui J. Leucine is essential for attenuating fetal growth restriction caused by a protein-restricted diet in rats. J Nutr. 2012;142:924–30. [DOI] [PubMed] [Google Scholar]
- Teymoori F, Asghari G, Mirmiran P, Azizi F. Dietary amino acids and incidence of hypertension: A principle component analysis approach. Sci Rep. 2017;7:16838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JG, Lane M, Gilchrist RB. Metabolism of the bovine cumulus-oocyte complex and influence on subsequent developmental competence. Soc Reprod Fertil Suppl. 2007;64:179–90. [DOI] [PubMed] [Google Scholar]
- Tokunaga C, Yoshino K, Yonezawa K. mTOR integrates amino acid- and energy-sensing pathways. Biochem Biophys Res Commun. 2004;313:443–6. [DOI] [PubMed] [Google Scholar]
- Van Winkle LJ, Campione AL, Gorman JM, Weimer BD. Changes in the activities of amino acid transport systems b0,+ and L during development of preimplantation mouse conceptuses. Biochim Biophys Acta. 1990;1021: 77–84. [DOI] [PubMed] [Google Scholar]
- Van Winkle LJ, Mann DF, Wasserlauf HG, Patel M. Mediated Na+-independent transport of L-glutamate and L-cystine in 1- and 2-cell mouse conceptuses. Biochim Biophys Acta. 1992;1107:299–304. [DOI] [PubMed] [Google Scholar]
- Vonnahme KA, Lemley CO, Caton JS, Meyer AM. Impacts of maternal nutrition on vascularity of nutrient transferring Tissues during gestation and lactation. Nutrients. 2015;7:3497–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wales RG, Du ZF. The metabolism of glutamine by the preimplantation sheep conceptus and its interaction with glucose. Reprod Fertil Dev. 1994;6:659–67. [DOI] [PubMed] [Google Scholar]
- Wallace JM, Luther JS, Milne JS, Aitken RP, Redmer DA, Reynolds LP, Hay WW Jr. Nutritional modulation of adolescent pregnancy outcome -- a review. Placenta. 2006;27 (Suppl A): S61–68. [DOI] [PubMed] [Google Scholar]
- Wang J, Wu Z, Li D, Li N, Dindot SV, Satterfield MC, Bazer FW, Wu G. Nutrition, epigenetics, and metabolic syndrome. Antioxid Redox Signal. 2012;17:282–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Burghardt RC, Romero JJ, Hansen TR, Wu G, Bazer FW. Functional roles of arginine during the peri-implantation period of pregnancy. III. Arginine stimulates proliferation and interferon tau production by ovine trophectoderm cells via nitric oxide and polyamine-TSC2-MTOR signaling pathways. Biol Reprod. 2015;92:75. [DOI] [PubMed] [Google Scholar]
- Wang X, Frank JW, Little DR, Dunlap KA, Satterfield MC, Burghardt RC, Hansen TR, Wu G, Bazer FW. Functional role of arginine during the peri-implantation period of pregnancy. I. Consequences of loss of function of arginine transporter SLC7A1 mRNA in ovine conceptus trophectoderm. FASEB J. 2014a;28:2852–63. [DOI] [PubMed] [Google Scholar]
- Wang X, Johnson GA, Burghardt RC, Wu G, Bazer FW. Uterine Histotroph and Conceptus Development. II. Arginine and secreted phosphoprotein 1 cooperatively stimulate migration and adhesion of ovine trophectoderm cells via focal adhesion-MTORC2 mediated cytoskeleton reorganization. Biol Reprod. 2016;95:71. [DOI] [PubMed] [Google Scholar]
- Wang X, Ying W, Dunlap KA, Lin G, Satterfield MC, Burghardt RC, Wu G, Bazer FW. Arginine decarboxylase and agmatinase: an alternative pathway for de novo biosynthesis of polyamines for development of mammalian conceptuses. Biol Reprod. 2014b;90:84. [DOI] [PubMed] [Google Scholar]
- Wathes DC, Reynolds TS, Robinson RS, Stevenson KR. Role of the insulin-like growth factor system in uterine function and placental development in ruminants. J Dairy Sci. 1998;81:1778–89. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Weinberg CR, O’Connor JF, Baird DD, Schlatterer JP, Canfield RE, Armstrong EG, Nisula BC. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189–94. [DOI] [PubMed] [Google Scholar]
- Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37:1–17. [DOI] [PubMed] [Google Scholar]
- Wu G. Amino Acids: Biochemistry and Nutrition. CRC Press, Boca Raton, Florida, 2013. [Google Scholar]
- Wu G, Bazer FW, Burghardt RC, Johnson GA, Kim SW, Li XL, Satterfield MC, Spencer TE. Impacts of amino acid nutrition on pregnancy outcome in pigs: mechanisms and implications for swine production. J Anim Sci. 2010;88:E195–204. [DOI] [PubMed] [Google Scholar]
- Wu G, Bazer FW, Johnson GA, Knabe DA, Burghardt RC, Spencer TE, Li XL, Wang JJ. Important roles for L-glutamine in swine nutrition and production. J Anim Sci. 2011;89:2017–30. [DOI] [PubMed] [Google Scholar]
- Wu G, Bazer FW, Cudd TA, Meininger CJ, Spencer TE. Maternal nutrition and fetal development. J Nutr. 2004;134: 2169–72. [DOI] [PubMed] [Google Scholar]
- Wu G, Bazer FW, Dai Z, Li D, Wang J, Wu Z. Amino acid nutrition in animals: protein synthesis and beyond. Annu Rev Anim Biosci. 2014;2:387–417. [DOI] [PubMed] [Google Scholar]
- Wu G, Bazer FW, Datta S, Johnson GA, Li P, Satterfield MC, Spencer TE. Proline metabolism in the conceptus: implications for fetal growth and development. Amino Acids. 2008a;35:691–702. [DOI] [PubMed] [Google Scholar]
- Wu G, Bazer FW, Datta S, Johnson GA, Li P, Satterfield MC, Spencer TE. Proline metabolism in the conceptus: implications for fetal growth and development. Amino Acids. 2008b;35:691–702. [DOI] [PubMed] [Google Scholar]
- Wu G, Bazer FW, Hu J, Johnson GA, Spencer TE. Polyamine synthesis from proline in the developing porcine placenta. Biol Reprod. 2005;72:842–50. [DOI] [PubMed] [Google Scholar]
- Wu G, Bazer FW, Satterfield MC, Li X, Wang X, Johnson GA, Burghardt RC, Dai Z, Wang J, Wu Z. Impacts of arginine nutrition on embryonic and fetal development in mammals. Amino Acids. 2013;45:241–56. [DOI] [PubMed] [Google Scholar]
- Wu G, Bazer FW, Johnson GA, Herring C, Seo H, Dai ZL, Wang JJ, Wu ZL, Wang XL. Functional amino acids in the development of the pig placenta. Mol Reprod Dev. 2017;84:879–82. [DOI] [PubMed] [Google Scholar]
- Wu G, Bazer FW, Johnson GA, Hou YQ. Arginine nutrition and metabolism in growing, gestating and lactating swine. J Anim Sci. 2018;96:5035–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Haynes TE, Li H, Yan W, Meininger CJ. Glutamine metabolism to glucosamine is necessary for glutamine inhibition of endothelial nitric oxide synthesis. Biochem J. 2001;353:245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, and Morris SM Jr. Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Pond WG, Ott T, and Bazer FW. Maternal dietary protein deficiency decreases amino acid concentrations in fetal plasma and allantoic fluid of pigs. J Nutr. 1998;128:894–902. [DOI] [PubMed] [Google Scholar]
- Wu G, Brown J, Zamora ML, Miller A, Satterfield MC, Meininger CJ, Steinhauser CB, Johnson GA, Burghardt RC et al. Adverse organogenesis and predisposed long-term metabolic syndrome from prenatal exposure to fine particulate matter. Proc Natl Acad Sci USA. 2019;116:11590–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Xie C, Zhang Y, Fan Z, Yin Y, Blachier F. Glutamate-glutamine cycle and exchange in the placenta-fetus unit during late pregnancy. Amino Acids. 2015;47:45–53. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. [DOI] [PubMed] [Google Scholar]
- Xiao XM, Li LP. L-Arginine treatment for asymmetric fetal growth restriction. Int J Gynaecol Obstet. 2005;88:15–8. [DOI] [PubMed] [Google Scholar]
- Xiong W, Wang H, Wu H, Chen Y, Han D. Apoptotic spermatogenic cells can be energy sources for Sertoli cells. Reproduction. 2009;137:469–79. [DOI] [PubMed] [Google Scholar]
- Zambrano E, Martinez-Samayoa PM, Bautista CJ, Deas M, Guillen L, Rodriguez-Gonzalez GL, Guzman C, Larrea F, Nathanielsz PW. Sex differences in transgenerational alterations of growth and metabolism in progeny (F2) of female offspring (F1) of rats fed a low protein diet during pregnancy and lactation. J Physiol. 2005;566:225–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Mao X, Huang Z, Wang F, Wu G, Qiao S. Arginine enhances embryo implantation in rats through PI3K/PKB/mTOR/NO signaling pathway during early pregnancy. Reproduction. 2013;145:1–7. [DOI] [PubMed] [Google Scholar]
- Zeng X, Wang F, Fan X, Yang W, Zhou B, Li P, Yin Y, Wu G, Wang J. Dietary arginine supplementation during early pregnancy enhances embryonic survival in rats. J Nutr. 2008;138:1421–5. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gao X, Saucedo LJ, Ru B, Edgar BA, Pan D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol. 2003;5:578–81. [DOI] [PubMed] [Google Scholar]
- Zhou J, Bondy C. Insulin-like growth factor-II and its binding proteins in placental development. Endocrinology. 1992;131:1230–40. [DOI] [PubMed] [Google Scholar]
- Zhou QL, Jiang ZY, Holik J, Chawla A, Hagan GN, Leszyk J, Czech MP. Akt substrate TBC1D1 regulates GLUT1 expression through the mTOR pathway in 3T3-L1 adipocytes. Biochem J. 2008;411:647–55. [DOI] [PMC free article] [PubMed] [Google Scholar]


