ABSTRACT
OBJECTIVE
To explore the effect of topical cannabidiol (CBD) in treating digital ulcers in patients with systemic sclerosis (SSc).
METHODS
In total, 45 patients with SSc who had digital ulcers were consecutively enrolled between January 2019 and December 2019. Of the participants, 25 were treated with CBD during surgical debridement and 20 were treated with standard local therapy. A numeric rating scale for pain and Health Assessment Questionnaire Disability Index were administered at the baseline and at the end of treatment.
RESULTS
Local treatment with CBD was significantly associated with lower pain scores, higher health assessment scores, and an increase in participants’ total hours of sleep. Patients in the control group more frequently required additional analgesic therapy.
CONCLUSIONS
Topical CBD may be a valuable tool to treat pain related to digital ulcers in patients with SSc.
KEYWORDS: cannabidiol, digital ulcers, Health Assessment Questionnaire Disability Index, pain management, quality of life, systemic sclerosis
INTRODUCTION
Systemic sclerosis digital ulcers (SSc-DUs) are extremely painful, hard to heal, and frequently responsible for an unsatisfying quality of life (QoL).1 The treatment of skin ulcers should be multidisciplinary and individualized for patients according to the DU features. One aspect of wound-related pain in SSc is volitional incident pain (ie, pain triggered not only by voluntary actions, such as dressing changes and cleaning, but also by specific procedures, especially surgical debridement). However, following the wound bed preparation framework, debridement is fundamental for DU healing.2 A study of patients with SSc-DUs found that 90% have volitional incident pain with a median score of 4.5 on a visual analog scale, indicating poorly controlled wound-related pain. Such wound-related pain may affect patients’ adherence to treatment and QoL and can delay healing.3
The pain relief provided by standard therapy (eg, nonsteroidal anti-inflammatory drugs, tramadol, opioids) is often inadequate, or the dose is limited by adverse effects. A wide range of topically applied agents have been studied for use with SSc-DUs;4 however, their efficacy remains questionable, particularly that of morphine-based drugs. There are also emerging concerns regarding opioid-induced inhibition of wound healing.5
Three main molecular families are expressed by species of the Cannabis plant, namely, cannabinoids, terpenes, and flavonoids. Cannabidiol (CBD) has been applied to wounds for thousands of years and has received renewed interest in the treatment of numerous pathologic conditions.6 In this article, the authors report their recent experience with the use of topical CBD for the management of SSc-DUs along with a review of the literature on this topic.
METHODS
The researchers retrospectively evaluated 45 patients with SSc (40 women; mean age, 53.0 [SD, 14.6] years; mean disease duration, 10.68 [SD, 5.2] years; cutaneous subsets, 35 limited/10 diffuse) who were followed up at the university-based scleroderma unit of Policlinico of Modena in 2019. Patients were included if they had been diagnosed with SSc, had one or more DUs, and were 18 years or older. Patients with bone or tendon exposure were excluded. All patients satisfied the American College of Rheumatology classification criteria for SSc.7
The enrolled patients all had long-standing, painful DUs that were resistant to opioid therapy at the maximum tolerated dose. All patients carried out both systemic and local therapies: all patients underwent periodic iloprost infusion every 30 to 40 days; calcium-channel blockers, phosphodiesterase type 5 inhibitors (sildenafil), and/or endothelin receptor antagonists (bosentan or macitentan) were administered according to clinical picture, ulcers, tolerance, and/or the presence of comorbidities (eg, pulmonary arterial hypertension). Surgical debridement was regularly performed on all participants following the wound bed preparation procedures, and advanced dressings (alginate, hydrocolloid, hydrofiber, hydrogel, and polyurethane foam or film) were applied.1
Among the 45 included patients, 25 were randomized to the CBD group, and 20 were randomized to the control group. In the CBD-treated group, the wound bed and periwound/perilesional skin were medicated with Cannabis sativa seed oil, a good manufacturing practice preparation produced in pharmaceutical laboratories. Cannabidiol was extracted through cold pressing C sativa seeds, obtaining a preparation of 10% CBD oil in acidic form and 90% hemp oil, free from tetrahydrocannabinol, according to the data sheet. Four drops of CBD oil were applied over the clean wound bed and covered with nonadhesive gauze. Oil-soaked dressings were changed every 24 hours. The CBD was locally administered daily for a 2-month follow-up period.
Wound-related pain was classified as severe in all participants according to World Health Organization guidelines.8 At the beginning of the study, a clinical assessment of DU characteristics was performed by a clinician and dedicated nurse (dimension; depth; presence of exudate, smell, and/or other signs of infection; perilesional skin; and wound bed). In addition, both at baseline and during medical procedures, researchers performed a clinical assessment of subjective symptoms due to background pain and determined volitional incident pain (during local management of DUs) according to clinical practice and guidelines.
The researchers gave each patient a diary and asked them to record the following symptoms daily: self-evaluation of wound-related pain (recorded at the same time every evening using a numeric rating scale [NRS] score), use of other analgesics, and any adverse effects. In addition, the Health Assessment Questionnaire Disability Index (HAQ-DI), a self-reported validated questionnaire, was administered at baseline and at the end of follow-up to assess patients’ QoL.9 Researchers evaluated CBD safety through patients’ diary records of adverse effects. Clinical parameter variations of DUs were monitored on a weekly basis during medication; researchers defined DU closure as when the wound bed was 100% epithelialized.
The study was approved by the local ethical committee (protocol no. 282/15). At the enrollment phase, each patient received exhaustive information about the use and the mode of application of CBD. All participants provided written informed consent.
Literature Review
The researchers performed a thorough search in PubMed, EMBASE, Scopus, Web of Science, Asian Science Citation Index, IranMedex, Scientific Information Database, PaKMediNet, IndMed, and Index Medicus for the World Health Organization Eastern Mediterranean Region for studies about SSc skin ulcers treated with CBD using the key words scleroderma, systemic sclerosis, topical CBD, cannabidiol, and cannabis.
Statistical Analysis
Continuous variables were expressed by mean and SD, whereas categorical variables were expressed as participant numbers and percentages. Continuous variables were checked for normal distribution using the Shapiro-Wilk test. Continuous variables following a Gaussian distribution were compared with a one-way analysis of variance followed by the Tukey-Kramer post hoc comparison test; otherwise, the Kruskal-Wallis rank sum test was used. P < .05 was considered significant. All statistical calculations were made with SPSS version 20 software (IBM Corporation).
RESULTS
Comparable clinical features of DUs were observed in both the CBD treatment group and the control group (Table 1).
Table 1.
CHARACTERISTICS OF PATIENTS WITH SYSTEMIC SCLEROSIS SKIN ULCERS AND THEIR RESPONSE TO CBD COMPARED WITH A CONTROL GROUP
| Characteristics | CBD Group | Control Group |
|---|---|---|
| Patients | ||
| Sex, n (%) | ||
| Female | 22 (88) | 18 (90) |
| Male | 3 (12) | 2 (10) |
| Age, mean (SD), y | 53.0 (14.6) | 57.3 (13.2) |
| Disease duration, mean (SD), y | 10.6 (5.2) | 14.3 (8.6) |
| Cutaneous subset, n | ||
| Limited | 18 | 17 |
| Diffuse | 7 | 3 |
| Treatment, n (%) | ||
| Systemic standard therapies | 25 (100) | 20 (100) |
| Local standard therapies | 25 (100) | 20 (100) |
| Local CBD treatments | 25 (100) | 0 (0) |
| CBD oral administration oil | 25 (100) | 0 (0) |
| Pain VAS (0–100), mean (SD) | ||
| Baseline | 8.40 (0.8) | 8.44 (0.77) |
| End of follow-up (T1)a | 6.00 (0.82) | 7.88 (0.73) |
| Hours of sleep, mean (SD) | ||
| Baseline | 2.56 (1.28) | 2.65 (1.16) |
| End of follow-up (T1)a | 5.67 (0.85) | 3.25 (1.34) |
| HAQ-DI (0–;3), mean (SD) | ||
| Baseline | 2.19 (0.67) | 2.24 (0.26) |
| End of follow-up (T1) | 1.61 (0.41) | 2.19 (0.62) |
| Additional analgesic therapy, n (%) | ||
| Baseline | ||
| End of follow-up (T1) | 12 (48) | 20 (100) |
| Skin ulcer evolution, n (%) | ||
| Healing | 18 (72) | 6 (30) |
| Adherence to CBD protocol, n (%) | 25 (100) | N/A |
| Local adverse effects of CBD, n (%) | ||
| Severe adverse events | 0 (0) | N/A |
| Mild adverse effects (itch, perilesional erythema) | 7 (28) | N/A |
aP = .0001
Abbreviations: CBD, cannabidiol; HAQ-DI, Health Assessment Questionnaire Disability Index; N/A, not applicable; VAS, visual analog scale.
CBD Group
For the 25 patients in the CBD group, CBD was administered locally for 1.20 (SD, 0.50) months. Basal wound-related pain NRS scores decreased from 8.4 (SD, 0.8) at the baseline (T0) to 6.0 (SD, 0.82) after 1 month of CBD treatment (T1; P < .0001). Across the same time period, volitional incident pain NRS scores decreased from 9.32 (SD, 0.75; T0) to 6.8 (SD, 1.12; T1; P < .0001; Table 2). In addition, mean total hours of sleep per night increased from 2.56 (SD, 1.28) to 5.67 (SD, 0.85) hours (P < .0001). Additional analgesic therapy was necessary in 12 of the 25 CBD group patients: four used acetaminophen, two used acetaminophen plus codeine, four used oxycodone, and two used morphine. Patients’ HAQ-DI scores decreased from 2.19 (SD, 0.67) at the baseline evaluation (T0) to 0.79 (SD, 0.46) at the final evaluation.
Table 2.
BASAL AND VOLITIONAL INCIDENT PAIN NRS SCORE AMONG PATIENTS TREATED WITH CBD AND CONTROLS
| Group | T0 | T1 | P |
|---|---|---|---|
| Basal NRS score | |||
| CBD, mean (SD) | 8.4 (0.8) | 6 (0.82) | <.0001 |
| Control, mean (SD) | 8.44 (0.77) | 7.88 (0.73) | .0327 |
| Comparison at T0 and T1 | P = .8580 | P < .0001 | — |
| Volitional incident pain NRS score | |||
| CBD, mean (SD) | 9.32 (0.75) | 6.8 (1.12) | <.0001 |
| Control, mean (SD) | 9.25 (0.91) | 9.3 (0.57) | .8363 |
| Comparison at T0 and T1 | P = .7784 | P < .0001 | — |
Abbreviations: CBD, cannabidiol; NRS, numeric rating scale.
Control Group
Control group patients reported less satisfactory outcomes. Their baseline mean wound-related pain NRS score was 8.44 (SD, 0.77; T0); after 1 month, their background wound-related pain NRS score decreased to 7.88 (SD, 0.73; T1; P = .0327). The volitional incident pain NRS score remained stable during the overall observational period (Table 2). The HAQ-DI score and mean total hours of sleep per night also did not show significant variations throughout the follow-up period. Further, all 20 patients in the control group needed additional analgesic therapy: all 20 patients used acetaminophen, 17 patients added oxycodone, and two used morphine.
Between-Group Comparisons
Although mean wound-related pain NRS scores did not differ between CBD-treated patients and control patients at baseline (P = .8688), their mean scores differed significantly after 1 month (P < .0001). Volitional incident pain NRS score also showed statistically significant differences (P < .0001) from T0 to T1 between groups (Table 2).
In terms of DU healing, 18 of the 25 patients in the CBD-treated group (72%) experienced complete healing by the end of the study (Figure). In contrast, complete healing was observed in 6 of the 20 control group participants (30%).
Figure.
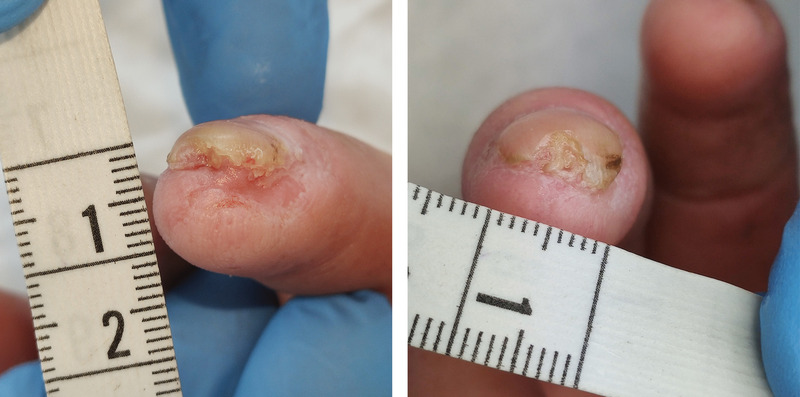
DIGITAL ULCER BEFORE AND AFTER TOPICAL TREATMENT WITH CANNABIDIOL OIL
A, Before treatment; B, after treatment.
Throughout the entire follow-up period, no significant adverse effects were recorded, either systemically or locally. No patient experienced severe adverse events related to CBD treatment. In particular, no alteration of the perilesional skin was noticed on physical examination. Seven of the 25 CBD-group participants (28%) recorded mild adverse effects, namely, itch and perilesional erythema, and none of the patients discontinued the CBD treatment. No DU infection was observed in the CBD group, but six control group patients required antibiotic therapy to treat DU infections that occurred during the period of observation.
DISCUSSION
Although wound-related pain is a common symptom in patients with SSc, particularly those with SSc-DUs, pain management is poorly investigated.1 Standardized analgesic strategies are not yet available for this condition, and little is known about volitional incident pain treatment. Studies have investigated the usefulness of topical analgesic drugs, especially opioids, for painful skin lesions including scleroderma.10–12 However, a recent randomized controlled trial did not demonstrate any significant effect of topical morphine in chronic skin lesions.13 Wound-related pain remains an area of significant and unmet need within scleroderma management.
The clinical utility of CBD in the treatment of SSc skin ulcers may be related to its anti-inflammatory and antifibrotic effects on modulating the associated chemokines/cytokines and receptor-mediated pathways. Cannabidiol acts via the endocannabinoid system (ECS), a chemical endogen signaling system that influences a wide variety of physiologic processes, including immunomodulation and analgesia, and is potentially capable of promoting wound healing.2 The ECS is composed of endocannabinoid ligands (eCBs) and eCBs-responsive receptors: two distinct extracellular receptors (CB1 and CB2) and other extracellular receptors involved in different pain pathways, including ionotropic, novel, and noncannabinoid target receptors. Cannabidiol receptors are present throughout the human body, in particular in the skin. Depending on their concentration, eCBs can modulate intracellular pathways acting on nuclear targets, such as the peroxisome proliferator-activated receptor family and nuclear factor κ-light-chain enhancer of activated B cells.14 The complex interactions between the ECS members in the skin, even if not yet fully clarified, seem to play a key function in controlling local immune responses.15 Interestingly, the ECS seems to play a role in epigenetic modification, resulting in tissue proliferation and modulation of wound-related pain.16 Moreover, the ECS could regulate pathways involved in keratinocyte differentiation, skin development, and epidermal cell differentiation.17
Recent research on fibrotic models in mice and the expression of ECS components in the skin of patients with scleroderma indicates that activation of CB2 and/or peroxisome proliferator-activated receptor γ as well as antagonism of CB1 and/or A2A adenosine receptors may become potent tools in the management of SSc and perhaps other fibrotic diseases as well, because of the inhibitory effect on fibrotic and inflammatory process.14
Despite an extensive literature search, the authors did not find any research investigating the use of CBD in SSc-DU treatment. Only seven published reports have investigated CBD treatment of skin ulcers (Table 3):6,18–23 a single case report,6 two case series,19,20 and one prospective open-label trial with different reports from the same study.18,21–23 Generally, the studies reported small patient series (between 3 and 33 patients) with varied dosages and modalities of CBD administration. The indications for CBD treatment were skin ulcers, pain, and wound healing.
Table 3.
PUBLISHED STUDIES ON CBM
| Article | Study type | Patient Population | Treatment with CBM | Follow-up, wk | Main Indications | Results | Analgesics | Adverse Events |
|---|---|---|---|---|---|---|---|---|
| Chelliah et al (2018)16 | Case series | 3 | Topical CBD | ND | Epidermolysis bullosa | Significant reduction of blistering and pain | Morphine; naproxene and gabapentin | NR |
| Maida and Corban (2017)17 | Case series | 3 | TCBM | 33; 9; 21 | Pyoderma gangrenosum | Significant pain reduction; significant reduction of opioid requirement | Opioid; acetaminophen | NR |
| Maida (2017)6 | Case report | 1 | Medical cannabis (vaporized topical oil) | 4 | Skin squamous cell cancer | Modest regression of malignant wound; reduction in pain and analgesic requirement | Hydromorphone, pregabalin | NR |
| Maida et al (2021)15 | Reports from prospective open-label serial case series | 1 | TCBM | 14 | Sickle cell disease leg ulcer | Faster wound healing than expected | Hydromorphone | NR |
| Maida et al (2020)18 | Reports from prospective open-label serial case series | 2 | TCBM | 11 | Non-uremic calciphylaxis leg ulcers | Faster wound healing than expected; reduction in analgesic requirement | Codeine and acetaminophen; morphine sulfate | NR |
| Maida et al (2021)19 | Reports from prospective open-label serial case series | 14 (3 lost to follow-up) | TCBM | ND | Venous leg ulcers | Faster wound healing than expected; complete wound closure within a median of 34 d | ND | NR |
Abbreviations: CBM, cannabis-based medicine; ND, not defined; NR, not referred; TCBM, topical cannabis-based medicine (composed of mixtures of cannabinoids, terpenes, and flavonoids).
In a case series of three children affected by epidermolysis bullosa, Chelliah et al19 reported the application of cannabis oil containing only CBD; they found that cannabis oil demonstrated analgesia, an opioid-sparing effect, and a trend toward wound healing. In a case report of a patient with a malignant skin wound, Maida6 reported a clinically significant relief of wound-related pain and reduction of opioid requirement using medical cannabis (vaporized and topical oil).
Similar results were also observed in a case series of patients with pyoderma gangrenosum who were treated with topical cannabis-based medicine (TCBM), a mixture of cannabinoids, terpenes, and flavonoids.20 In the prospective open-label trial (ISRCTN 16488940) performed by Maida et al21 in 2020, 33 patients, 31 with cutaneous membrane wounds and 2 with mucous membrane wounds, were treated using TCBM. The treatment promoted wound closure in up to 90% of cases.21 From the same trial, Maida et al18–22 reported the case of two patients affected by nonuremic calciphylaxis leg ulcer and a case of a sickle cell disease leg ulcer: treatment with TCBM resulted in faster-than-expected wound healing and reduced analgesic use. The largest cohort of patients investigated from the trial was composed of 14 patients with 16 recalcitrant leg ulcers, which were treated with TCBM in conjunction with compression bandaging. The results showed rapid wound closure of the previously nonhealing venous leg ulcers. This evidence suggests that TCBM may be an effective adjuvant in conjunction with compression therapy.23 No randomized controlled trials have been carried out though, and further research is needed.
To the authors’ knowledge, the present study is the first to report the effectiveness of local CBD treatment in the management of SSc-DUs. The results of this preliminary report encourage further exploration of CBD oil as a potentially helpful topical treatment in the management of SSc-DUs, having no major adverse effects and few minor adverse effects.
Favorable results were primarily observed for background and volitional incident pain. In particular, the improvement of mean chronic wound-related pain NRS scores was clearly quantifiable in the majority of patients treated with CBD. In addition, 72% of patients in the CBD group experienced clear-cut improvement in DU healing with complete re-epithelialization of the wound bed. As highlighted by follow-up HAQ-DI scores, CBD use was also associated with improved QoL. The above findings are comparable with those emerging from the literature on the use of cannabis derivatives in local treatment.
The rapid and significant reduction of wound-related pain in the 25 patients with SSc who received CBD therapy is supported by the results of a recent systematic review and meta-analysis of cannabinoids for medical use.24 Unlike intact skin, which is polar and hydrophilic, skin ulcers lack epithelial coverage and are nonpolar and lipophilic. Therefore, lipophilic compounds such as CBD could be readily absorbed through all classes of cutaneous wounds, including scleroderma skin ulcers, contributing to the significant reduction of volitional incident pain in the T1 CBD group. The improvement of DU healing may be related to better patient adherence to the medication. However, a direct action of CBD in the CB1-expressing cells, mononuclear cells (fibroblastic cells and monocytes) related to inflammation, and immune response that are present in the skin lesions cannot be excluded.21 The suggested effects of CBD on the release of interleukins, vascular endothelial growth factor, and metalloproteinase-9 could be related, even with the observed lack of DU infections in the CBD-treated group of patients.24
CONCLUSIONS
Topical administration of CBD is a safe, effective, noninvasive tool that is associated with improved wound-related pain, DU healing, and QoL of patients with SSc. Larger, randomized controlled, multicenter trials should be conducted to determine the potential clinical applications of topical CBD in the management of SSc-DUs.
Footnotes
The authors have disclosed no financial relationships related to this article.
REFERENCES
- 1.Giuggioli D, Manfredi A, Lumetti F, Colaci M, Ferri C. Scleroderma skin ulcers definition, classification and treatment strategies our experience and review of the literature. Autoimmunity Rev 2018;17(2):155–64. [DOI] [PubMed] [Google Scholar]
- 2.Maida V, Biasi SA. “Less pain with more gain”—Managing wound-related pain with cannabis-based medicines. Wound Repair Regen 2021;29(2):338–41. [DOI] [PubMed] [Google Scholar]
- 3.Giuggioli D, Manfredi A, Vacchi C, Sebastiani M, Spinella A, Ferri C. Procedural pain management in the treatment of scleroderma digital ulcers. Clin Exp Rheumatol 2015;33(1):5–10. [PubMed] [Google Scholar]
- 4.Lebedoff N French TM Shanmugam VK, et al. Review of local wound management for scleroderma-associated digital ulcers. J Scleroderma Relat Disord 2018;3:66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shanmugam VK, Couch KS, McNish S, Amdur RL. Relationship between opioid treatment and rate of healing in chronic wounds. Wound Repair Regen 2017;25(1):120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maida V. Medical cannabis in the palliation of malignant wounds—a case report. J Pain Symptom Manage 2017;53(1):e4–6. [DOI] [PubMed] [Google Scholar]
- 7.van den Hoogen F Khanna D Fransen J, et al. Classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum 2013;65(11):2737–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nugraha B Gutenbrunner C Barke A, et al. The IASP classification of chronic pain for ICD-11: functioning properties of chronic pain. Pain 2019;160(1):88–94. [DOI] [PubMed] [Google Scholar]
- 9.Pope J. Measures of systemic sclerosis (scleroderma): Health Assessment Questionnaire (HAQ) and Scleroderma HAQ (SHAQ), physician- and patient-rated global assessments, Symptom Burden Index (SBI), University of California, Los Angeles, Scleroderma Clinical Trials Consortium Gastrointestinal Scale (UCLA SCTC GIT) 2.0, Baseline Dyspnea Index (BDI) and Transition Dyspnea Index (TDI) (Mahler’s index), Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR), and Raynaud’s Condition Score (RCS). Arthritis Care Res 2011;63(Suppl 11):S98–111. [DOI] [PubMed] [Google Scholar]
- 10.van Ingen IL, Jansen MM, Barrera P. Topical opioids for painful ulcers in systemic sclerosis. Ann Rheum Dis 2008;67(3):427. [DOI] [PubMed] [Google Scholar]
- 11.Giuggioli D, Manfredi A, Colaci M, Ferri C. Oxycodone in the long-term treatment of chronic pain related to scleroderma skin ulcers. Pain Med 2010;11(10):1500–3. [DOI] [PubMed] [Google Scholar]
- 12.Ughi N, Crotti C, Ingegnoli F. Effectiveness and safety of oxycodone/naloxone in the management of chronic pain in patients with systemic sclerosis with recurrent digital ulcers: two case reports. Clin Interv Aging 2016;14;11:307–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaslansky R, Schramm C, Stein C, Güthoff C, Schmidt-Westhausen AM. Topical application of morphine for wound healing and analgesia in patients with oral lichen planus: a randomized, double-blind, placebo-controlled study. Clin Oral Investig 2018;22(1):305–11. [DOI] [PubMed] [Google Scholar]
- 14.Oddi S, Maccarrone M. Endocannabinoids and skin barrier function: molecular pathways and therapeutic opportunities. In: Wondrak GT, ed. Skin Stress Response Pathways. Cham, Switzerland: Springer; 2016: 301–23. [Google Scholar]
- 15.Toth KF, Adam D, Biro T, Olah A. Cannabinoid signaling in the skin: therapeutic potential of the “c(ut)annabinoid” system. Molecules 2019;24(5):918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis CJ, Mardaryev AN, Sharov AA, Fessing MY, Botchkarev VA. The epigenetic regulation of wound healing. Adv Wound Care 2014;3(7):468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruhl T, Kim BS, Beier JP. Cannabidiol restores differentiation capacity of LPS exposed adipose tissue mesenchymal stromal cells. Exp Cell Res 2018;370(2):653–62. [DOI] [PubMed] [Google Scholar]
- 18.Maida V, Shi RB, Fazzari FGT, Zomparelli L-M. A new treatment paradigm for sickle cell disease leg ulcers: topical cannabis-based medicines. Exp Dermatol 2021;30(2):291–3. [DOI] [PubMed] [Google Scholar]
- 19.Chelliah MP, Zinn Z, Khuu P, Teng JMC. Self-initiated use of topical cannabidiol oil for epidermolysis bullosa. Pediatr Dermatol 2018;35(4):e224–7. [DOI] [PubMed] [Google Scholar]
- 20.Maida V, Corban J. Topical medical cannabis: a new treatment for wound pain—three cases of pyoderma gangrenosum. J Pain Symptom Manage 2017;54(5):732–6. [DOI] [PubMed] [Google Scholar]
- 21.Maida V, Shi RB, Fazzari FGT, Zomparelli L. Promoting wound healing of uremic calciphylaxis leg ulcers using topical cannabis-based medicines. Dermatol Ther 2020;33(6):e14419. [DOI] [PubMed] [Google Scholar]
- 22.Maida V, Shi RB, Fazzari FGT, Zomparelli L. Topical cannabis-based medicines—a novel paradigm and treatment for non-uremic calciphylaxis leg ulcers: an open label trial. Int Wound J 2020;17(5):1508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maida V, Shi RB, Fazzari FGT, Zomparelli L. Topical cannabis-based medicines—a novel adjuvant treatment for venous leg ulcers: an open-label trial. Exp Dermatol 2021;30(9):1258–67. [DOI] [PubMed] [Google Scholar]
- 24.Baswan SM Klosner AE Glynn K, et al. Therapeutic potential of cannabidiol (CBD) for skin health and disorders. Clin Cosmet Investig Dermatol 2020;13:927–42. [DOI] [PMC free article] [PubMed] [Google Scholar]


