To the Editor:
The incidence of kidney failure in African Americans is 2-3 times higher than that in White Americans. A portion of the increased kidney risk appears to be because of polymorphisms in the gene encoding apolipoprotein L1 (APOL1). The precise mechanism of how APOL1 renal-risk variants accelerate kidney disease progression is a matter of intense research, and currently there are no proven treatments for APOL1-related kidney disease. Currently, there are large National Institutes of Health-funded national studies (APOL1 Long-term Kidney Transplantation Outcomes Network [“APOLLO”] and Living Donor Extended Time Study [“LETO”]) underway to help define the role of APOL1 genotyping in the context of kidney donation and transplantation.1 However, the role of APOL1 genotyping in chronic kidney disease (CKD) care remains unclear.2,3
To date, few studies have examined patient perspectives and attitudes about testing for APOL1.4, 5, 6, 7, 8 APOL1 renal-risk variants have only been detected among individuals of recent African ancestry, and given the impacts of racism on African Americans as well as the mistrust and underrepresentation of African Americans in clinical trials, it is essential to engage and incorporate patient perspectives and attitudes about APOL1 genotyping in clinical decision making and formulation of best practices.
In this pilot study, we offered APOL1 genetic testing and counseling and assessed the attitudes and concerns related to APOL1 testing and kidney risk management among self-identified African Americans seen in the Hypertension and Nephrology Clinics at a Midwestern academic medical center. In the first phase of this ongoing project, we recruited 128 participants who self-identified as African American. Baseline surveys to assess patient attitudes and concerns about APOL1 genetic testing and kidney risk management were completed before blood samples were drawn and sent to a Clinical Laboratory Improvement Amendments-approved laboratory for APOL1 genotyping (Item S1, Item S2, Table S1).
Among the cohort, 71 (55%) were women, and the mean age was 57 years. Nearly 40% of participants (n = 51; 39%) reported an annual family income of <$15,000. Obesity was present in 93 (73%) participants. The median CKD Epidemiology Collaboration 2021 equation estimated glomerular filtration rate (eGFR) was 42 mL/min/1.73 m2 and median urinary albumin-creatinine ratio was 93 mg/g. A mean of 3 antihypertensive medications were used to achieve a mean systolic blood pressure of 146 mm Hg, and 81 (63%) participants were receiving angiotensin-converting enzyme inhibitor or angiotensin receptor blocker therapy (Table 1).
Table 1.
Participant Characteristics, by CKD Stage.
| Characteristics | Overall | Stages 1-2 (eGFR ≥60) | Stage 3 (eGFR 30-59) | Stages 4-5 (eGFR <30) |
|---|---|---|---|---|
| African American race, n | 128 | 38 | 55 | 35 |
| Age, y, mean (SD) | 57 (13) | 50 (11) | 60 (11) | 61 (13) |
| Sex, n (%) | ||||
| Women | 71 (55) | 19 (50) | 30 (55) | 22 (63) |
| Men | 57 (44) | 19 (50) | 25 (45) | 13 (37) |
| Educational attainment, n (%) | ||||
| High school graduate or greater | 101 (79) | 30 (79) | 43 (78) | 28 (80) |
| Less than high school | 24 (19) | 6 (16) | 12 (22) | 6 (17) |
| Not reported | 3 (2) | 2 (5) | 0 | 1 (3) |
| Annual family income, n (%) | ||||
| Less than $15,000 | 51 (40) | 21 (55) | 18 (33) | 12 (34) |
| 15,000-$30,000 | 18 (14) | 4 (11) | 11 (20) | 3 (9) |
| $30,000-$45.000 | 15 (12) | 3 (8) | 9 (16) | 3 (9) |
| ≥ $45,000 | 22 (17) | 6 (16) | 8 (15) | 8 (23) |
| Not reported | 22 (17) | 4 (11) | 9 (16) | 9 (26) |
| Self-reported overall health, n (%) | ||||
| Good to excellent | 48 (38) | 18 (47) | 19 (35) | 11 (31) |
| Fair | 59 (46) | 15 (39) | 25 (45) | 19 (54) |
| Poor to very poor | 21 (16) | 5 (13) | 11 (20) | 5 (14) |
| Blood pressure, mm Hg, mean (SD) | ||||
| Systolic | 146 (22) | 150 (26) | 142 (17) | 148 (24) |
| Diastolic | 84 (14) | 89 (14) | 82 (14) | 82 (15) |
| Serum creatinine level, mg/dL, median (IQR) | 1.5 (1.2-2.2) | 1.0 (0.8-1.2) | 1.6 (1.4-1.9) | 2.9 (2.3-4.4) |
| eGFR, mL/min/1.73 m2, median (IQR) | 42 (29-63) | 76 (66-90) | 42 (37-50) | 20 (13-26) |
| Urinary albumin-to-creatinine ratio, mg/g, median (IQR)a | 93 (11-634) | 52 (8-403) | 31 (8-163) | 644 (125-1240) |
| Serum lipid levels, mg/dL, mean (SD) | ||||
| Total cholesterolb | 166 (50) | 170 (57) | 170 (52) | 156 (42) |
| High density lipoprotein cholesterolb | 49 (16) | 47 (13) | 49 (19) | 48 (14) |
| Low density lipoprotein cholesterolc | 99 (33) | 103 (35) | 103 (37) | 89 (26) |
| Triglyceridesc | 135 (94) | 141 (78) | 130 (113) | 137 (79) |
| Fasting blood glucose, mg/dL, mean (SD) | 113 (55) | 109 (49) | 118 (56) | 108 (60) |
| Body mass index level, n (%) | ||||
| Underweight (<18.5 kg/m2) | 1 (1) | 0 | 0 | 1 (3) |
| Normal (18.5 to <25.0 kg/m2) | 17 (13) | 6 (16) | 6 (11) | 5 (14) |
| Overweight (25.0 to <30.0 kg/m2) | 17 (13) | 5 (13) | 7 (13) | 5 (14) |
| Obese (≥30.0 kg/m2) | 93 (73) | 27 (71) | 42 (76) | 24 (69) |
| Antihypertensive agent use, n (%) | ||||
| ACEi/ARBs | 81 (63) | 24 (63) | 35 (64) | 22 (63) |
| β-blockers | 65 (51) | 13 (34) | 33 (60) | 19 (54) |
| Calcium channel blockers | 84 (66) | 21 (55) | 38 (69) | 25 (71) |
| Diuretics | 65 (51) | 17 (45) | 29 (53) | 19 (54) |
| Vasodilators | 23 (18) | 4 (11) | 10 (18) | 9 (26) |
| Number of antihypertensive agents, mean (SD) | 3 (1) | 3 (1) | 3 (1) | 3 (1) |
Note: Percentages reflect column percentages.
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; IQR, interquartile range; SD, standard deviation.
40% unavailable
28% unavailable
30% unavailable
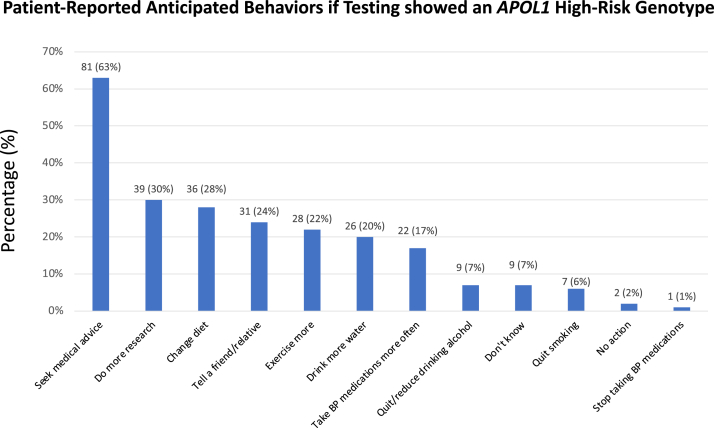
Overall, nearly all participants (120 [94%]) reported being concerned about kidney disease. When stratified by CKD stages, 36 (94%), 50 (91%), and 34 (97%) participants with eGFR rates of ≥60, 30-59, and <30 mL/min/1.73 m2, respectively, reported being concerned about kidney disease (Table S2). Most of the participants thought it was a good idea to be tested for genes that may impact kidney disease (120 [94%]) and would want APOL1 testing for their children (104 [81%]). Only a small portion (21 [16%]) reported that they would be very upset if genetic results showed that they had a high-risk APOL1 genotype. Survey responses did not differ appreciably when stratified by CKD stages (Table S2). Participants reported that knowledge of a high-risk APOL1 genotype would lead to positive changes in health-related behaviors, including seeking medical advice and dietary and lifestyle modification (Fig 1). Few individuals (n = 2; <2%) reported that they would take no action and only 1 (<1%) would stop taking blood pressure medications if they were found to have a high-risk APOL1 genotype. Among the participants genotyped to date, 50 (39%) had 0, 56 (44%) had 1, and 22 (17%) had 2 APOL1 renal-risk variants (high-risk genotypes).
Figure 1.
Patient-reported anticipated behaviors if testing showed APOL1 high-risk genotype. Abbreviation: BP, blood pressure.
To our knowledge, our study is one of the first and largest to directly solicit attitudes about APOL1 genotyping from individuals with CKD. In contrast, prior studies have solicited attitudes about APOL1 from African Americans in hypothetical nonclinical settings,4,5 among prior kidney transplant donors,6,7 and from African Americans without CKD.8 Our findings of support for APOL1 testing are largely consistent with these prior reports4, 5, 6, 7 but extend to a clinical population receiving general nephrology and hypertension care, in which the impact of APOL1 is also relevant.
Patients’ strong support for APOL1 testing was first reported in a smaller (n = 26) study that conducted in-depth interviews on related beliefs and attitudes.9 In that study, participants endorsed that knowledge of APOL1 risk would not inspire fatalism or decision regret but motivate health-promoting behaviors9—a theme that emerged in a recent clinical trial8 and in our study. A recent report of 76 interviews, including researchers, clinicians, and African American patients, family, and community members supported the concept of offering genetic testing to patients, although concerns included risks, such as psychological burdens, misunderstanding, and potential stigma and discrimination.10 These collective observations suggest that even though there are no proven therapies for APOL1-related kidney disease to date, incorporating APOL1 testing may provide benefits in educating patients about kidney risk and encouraging positive health changes.
Two different expert panels have offered recommendations about APOL1 testing in clinical settings.2,3 However, these panels disagree in their recommendations about APOL1 testing in nontransplant settings, with one earlier panel “recommending against the routine offer of APOL1 testing in clinical care”2 and a more recent panel recommending that APOL1 genotyping “should be considered in all patients with kidney disease who have African ancestry, particularly when there is a family history of CKD.”3 Our findings suggest that APOL1 testing is well received by patients, which lends support to consideration in clinical practice.
Although we did not assess health care providers’ attitudes about the APOL1 testing, identifying APOL1 risk status in patients with CKD may guide clinicians in monitoring and managing patients at risk of rapid CKD progression. Strict blood pressure control may have a mortality benefit in CKD patients with high-risk APOL1 genotypes,11 and a recent randomized clinical trial showed that disclosing APOL1 results to clinicians and patients was associated with improved blood pressure control and kidney disease screening.8 In addition, knowledge of APOL1 status can motivate clinicians to refer patients for ongoing APOL1 clinical trials.12
Our study has limitations. Findings from 1 center may not generalize to other populations and practice settings. Patients who agreed to participate may differ from the broader population (e.g., they may be more interested in their own health), which may contribute to selection bias. The proportion of high-risk genotypes (17%) was slightly higher than prior general population estimates of ∼13%, which likely reflects sampling from nephrology clinics. In addition, although we found that participants may be motivated to engage in health-promoting behaviors after genotyping, the current design did not measure the impact of APOL1 testing on patient outcomes. However, a recent trial demonstrated improved blood pressure control,8 and we intend to create a prospective cohort to provide insights on this topic over time.
In conclusion, we report that African American patients at an urban Midwestern medical center were receptive toward APOL1 genetic testing and believed that testing would motivate changes in health-related behavior. Further research is necessary to determine the optimal patient-centered use of this emerging risk-assessment tool.
Article Information
Authors’ Contributions
Research concept and study design: KLL, ANM, YC; participant recruitment: KLL, YC, KKL, JCE, AAM, KNM, AKM, TMV, MDP; data acquisition: KLL, KKL; genotyping: BIF, AC; statistical analysis: KLL, ANM, CYH; data interpretation: KLL, ANM, YC, KKL, JCE, AAM, KNM, AKM, TMV, BIF, AC, CYH, MDP. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This work was supported by a grant from the Mid-America Transplant Foundation. KLL is supported by the Mid-America Transplant/Jane A. Beckman Endowed Chair in Transplantation. ANM is supported by University of California, San Francisco, Dean’s Diversity award (Watson Scholar), R01DK114014 diversity supplement, and K23DK119562. CYH is supported by K24DK92291. KLL, ANM, BIF, and CYH receive research funding related to APOL1 in transplantation from the National Institutes of Health (NIH: U01DK116042 and R01DK120551). JCE receives NIH research funding related to APOL1 biology (R01DK120651).
Financial Disclosure
Dr Lentine receives consulting fees from CareDx and speaker honoraria from Sanofi. Drs Edwards and Freedman received research funding from Vifor Pharma. Dr Freedman and Wake Forest University Health Sciences have rights to a US patent involving APOL1 genetic testing. Dr Freedman also receives research funding from AstraZeneca and RenalytixAI and consulting fees from AstraZeneca, RenalytixAI, and XinThera. The remaining authors declare that they have no relevant financial interests.
Acknowledgements
We thank study participants, clinicians and staff at Saint Louis University Hospital and Clinic for sharing about the study, research assistants Addie Wisniewski and Kennan Maher for assistance with data management, and new coordinator joining the team, Heather Kuenz, RN. We are grateful to the entire Mid-America Transplant Foundation Board for their support of community-based kidney research, including leadership Kevin Lee, MA, and Diane Brockmeier, BSN, MA, and former chair Will Ross, MD, MPH, for advocacy related to launching this project, along with recent past chair Nesa Joseph, EdD, MHA, and current chair Ellen Barnidge, PhD, for support of the ongoing project.
Prior Presentation
An abstract describing portions of this work was presented at the National Kidney Foundation virtual Spring Clinical Meeting, March 2021.
Peer Review
Received April 21, 2022, as a submission to the expedited consideration track with 3 external peer reviews. Direct editorial input from the Statistical Editor and the Editor-in-Chief. Accepted in revised form August 9, 2022.
Footnotes
Item S1: Additional References
Item S2: Detailed Methods
Table S1: Selected Survey Items
Table S2: Survey Responses Stratified by CKD Stage
Supplementary Material
Item S1, S2; Table S1, S2.
References
- 1.Lentine K.L., Mannon R.B. Apolipoprotein L1: role in the evaluation of kidney transplant donors. Curr Opin Nephrol Hypertens. 2020;29(6):645–655. doi: 10.1097/MNH.0000000000000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young B.A., Blacksher E., Cavanaugh K.L., et al. Apolipoprotein L1 testing in African Americans: involving the community in policy discussions. Am J Nephrol. 2019;50(4):303–311. doi: 10.1159/000502675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freedman B.I., Burke W., Divers J., et al. Diagnosis, education, and care of patients with APOL1-associated nephropathy: a Delphi consensus and systematic review. J Am Soc Nephrol. 2021;32(7):1765–1778. doi: 10.1681/ASN.2020101399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Umeukeje E.M., Young B.A., Fullerton S.M., et al. You are just now telling us about this? African American perspectives of testing for genetic susceptibility to kidney disease. J Am Soc Nephrol. 2019;30(4):526–530. doi: 10.1681/ASN.2018111091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berrigan M., Austrie J., Fleishman A., et al. Opinions of African American adults about the use of apolipoprotein L1 (ApoL1) genetic testing in living kidney donation and transplantation. Am J Transplant. 2021;21(3):1197–1205. doi: 10.1111/ajt.16206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon E.J., Amόrtegui D., Blancas I., Wicklund C., Friedewald J., Sharp R.R. African American living donors’ attitudes about APOL1 genetic testing: a mixed methods study. Am J Kidney Dis. 2018;72(6):819–833. doi: 10.1053/j.ajkd.2018.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon E.J., Amόrtegui D., Blancas I., Wicklund C., Friedewald J., Sharp R.R. A focus group study on African American living donors’ treatment preferences, sociocultural factors, and health beliefs about apolipoprotein L1 genetic testing. Prog Transplant. 2019;29(3):239–247. doi: 10.1177/1526924819854485. [DOI] [PubMed] [Google Scholar]
- 8.Nadkarni G.N., Fei K., Ramos M.A., et al. Effects of testing and disclosing ancestry-specific genetic risk for kidney failure on patients and health care professionals: a randomized clinical trial. JAMA Netw Open. 2022;5(3) doi: 10.1001/jamanetworkopen.2022.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horowitz C.R., Ferryman K., Negron R., et al. Race, genomics and chronic disease: what patients with African ancestry have to say. J Health Care Poor Underserved. 2017;28(1):248–260. doi: 10.1353/hpu.2017.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.West K.M., Cavanaugh K.L., Blacksher E., et al. Stakeholder perspectives on returning nonactionable apolipoprotein L1 (APOL1) genetic results to African American research participants. J Empir Res Hum Res Ethics. 2022;17(1-2):4–14. doi: 10.1177/15562646211063267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ku E., Lipkowitz M.S., Appel L.J., et al. Strict blood pressure control associates with decreased mortality risk by APOL1 genotype. Kidney Int. 2017;91(2):443–450. doi: 10.1016/j.kint.2016.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daehn I.S., Duffield J.S. The glomerular filtration barrier: a structural target for novel kidney therapies. Nat Rev Drug Discov. 2021;20(10):770–788. doi: 10.1038/s41573-021-00242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Item S1, S2; Table S1, S2.