PURPOSE
Heart failure (HF) is a potentially life-threatening complication of treatment for childhood cancer. We evaluated the risk and risk factors for HF in a large European study of long-term survivors. Little is known of the effects of low doses of treatment, which is needed to improve current treatment protocols and surveillance guidelines.
METHODS
This study includes the PanCareSurFup and ProCardio cohort of ≥ 5-year childhood cancer survivors diagnosed between 1940 and 2009 in seven European countries (N = 42,361). We calculated the cumulative incidence of HF and conducted a nested case-control study to evaluate detailed treatment-related risk factors.
RESULTS
The cumulative incidence of HF was 2% (95% CI, 1.7 to 2.2) by age 50 years. The case-control study (n = 1,000) showed that survivors who received a mean heart radiation therapy (RT) dose of 5 to < 15 Gy have an increased risk of HF (odds ratio, 5.5; 95% CI, 2.5 to 12.3), when compared with no heart RT. The risk associated with doses 5 to < 15 Gy increased with exposure of a larger heart volume. In addition, the HF risk increased in a linear fashion with higher mean heart RT doses. Regarding total cumulative anthracycline dose, survivors who received ≥ 100 mg/m2 had a substantially increased risk of HF and survivors treated with a lower dose showed no significantly increased risk of HF. The dose-response relationship appeared quadratic with higher anthracycline doses.
CONCLUSION
Survivors who received a mean heart RT dose of ≥ 5 Gy have an increased risk of HF. The risk associated with RT increases with larger volumes exposed. Survivors treated with < 100 mg/m2 total cumulative anthracycline dose have no significantly increased risk of HF. These new findings might have consequences for new treatment protocols for children with cancer and for cardiomyopathy surveillance guidelines.
BACKGROUND
Developments in the treatment for children with cancer have improved survival considerably over recent decades.1 However, long-term survivors are at risk of adverse effects induced by cancer and its treatment. One of the most severe effects is cardiotoxicity. This may occur as asymptomatic myocardial dysfunction and can progress to symptomatic heart failure (HF), which is related to increased morbidity and mortality.2-7
CONTEXT
Key Objective
Cardiotoxic cancer treatment is an important risk factor for heart failure (HF) in childhood cancer survivors. This Pan-European cohort (N = 42,361) and case-control study examined cumulative incidence of HF and whether low doses of anthracyclines and radiotherapy involving the heart (heart RT), estimated by dosimetry, are associated with HF. Our study provides accurate dose-response evidence because of its unprecedented number of cases (n = 500).
Knowledge Generated
The multivariable regression analyses demonstrated that a mean heart RT dose of 5 to < 15 Gy is associated with a serious risk of HF. An anthracycline dose of < 100 mg/m2 was not a significant risk factor of HF.
Relevance
Previous guidelines could not make cardiomyopathy surveillance recommendations for survivors treated with prescribed chest RT < 15 Gy because of lack of evidence. We propose to recommend follow-up for survivors exposed to a mean heart RT dose ≥ 5 Gy and reconsider surveillance of survivors treated with a total cumulative anthracycline dose < 100 mg/m2.
Previous studies among childhood cancer survivors (hereafter survivors) identified treatment-related risk factors for HF, including anthracyclines, mitoxantrone, and radiation therapy (RT) where the heart was in the radiation field.4,8-12 Anthracycline analogs that have been linked to cardiotoxicity comprise doxorubicin, daunorubicin, epirubicin, and idarubicin. Mitoxantrone is an anthraquinone and structurally comparable with doxorubicin.13 Of these chemotherapeutic agents, mitoxantrone has the greatest cardiotoxic potential, which may be related to differences in underlying pathophysiology.14,15 Other potential risk factors for HF are cyclophosphamide, sex, age at cancer diagnosis, and presence of traditional cardiovascular risk factors.2,4,7,16-18
Surveillance of myocardial function after cardiotoxic treatment is of great importance to detect treatable abnormalities at an early stage.19 The International Guideline Harmonization Group (IGHG) formulated cardiomyopathy surveillance recommendations in 2015 for survivors treated with anthracyclines (all doses) and survivors treated with radiotherapy involving the heart region of ≥ 15 Gy. Furthermore, this group highlighted future directions for research including the risk of symptomatic HF in survivors treated with < 15 Gy chest RT as little was known about the effects.19 New evidence for low doses of cardiotoxic treatments is needed to guide both updates of cardiomyopathy surveillance strategies and designs of treatment regimes.
Pooling data from two EU-funded consortia, the PanCareSurFup (PCSF) cardiac study20 and ProCardio,21 created a large cohort of survivors (N = 42,361) to investigate low treatment doses of anthracyclines and cardiac RT and the nature of dose responses, by using phantom-based radiation dosimetry including dose-volume histogram indicators. The latter technique calculates the estimated dose received by the organ at risk.
METHODS
In 2011, collaborative efforts initiated the PCSF cardiac study22 and ProCardio and designed them to be complementary with a view of pooling data. We conducted a cohort study and a nested case-control study using these data. We described the exact process below and show it by a flowchart in the Data Supplement (online only).
Study Population
We included ≥ 5-year survivors in whom cancer was diagnosed at age < 20 years between 1940 and 2009. The PCSF cardiac study comprised eight European subcohorts from France, Hungary, Italy (two subcohorts), the Netherlands, Slovenia, Switzerland, and the United Kingdom. The ProCardio project comprised survivors from France and the United Kingdom. The inclusion criteria, which are listed in the Data Supplement, differed slightly between the subcohorts. The study was performed after approval by a local Human Investigations Committee. Depending on the regulations in each country, informed consent was obtained from all individual participants included in the corresponding subcohort or the data were collected under national law.
Identification of Survivors With Heart Failure
We identified survivors with HF (hereafter case) as a first event by using multiple strategies, for example, linkage to population-based databases and patient-based questionnaires. A case was defined as having symptomatic HF graded according to the Common Terminology and Criteria for Adverse Events23 as grades 3, 4, and 5 (Data Supplement). The exact methods are described by Feijen et al.22
Case-Control Study: Control Selection
We randomly selected controls by density sampling and matched them with cases with HF (ratio 1:1) on subcohort, sex, age at first cancer diagnosis (±1 year), and calendar year of first cancer diagnosis (±3 year). The length of follow-up after first cancer diagnosis of controls was at least as long as the interval between cancer diagnosis and HF in the matched case, but controls had to be HF-free. When no suitable control could be found, the calendar period criterion was relaxed (maximum 10 years). If still no eligible control was available, then age at cancer diagnosis was relaxed (maximum 3 years).
Data Collection
For the cohort study, we collected baseline characteristics for all survivors included in the analysis. These data included sex, month and year of birth, month and year of first cancer diagnosis, morphology code, type of treatment, and the month and year of the start of treatment.22 For the case-control study, we collected details of treatment for all cases and controls from medical records by using a standardized extraction form. We collected data for each cycle of each cytotoxic agent to enable calculation of cumulative dose (or equivalent14,24,25). We performed radiation dosimetry for the whole body including seven parts of the heart for all cases and controls who received RT, as previously described.26-28 With dosimetry, we calculated the estimated average of the maximum dose that was given to different parts of the whole heart, and this measure is reflected by mean heart RT dose. In addition, we created dose volume variables by calculating the percentage of heart volume that received at least 5 (V5), 10 (V10), 15 (V15), 20 (V20), or 30 (V30) Gy. The variable V5-15 reflects the percentage of the cardiac volume that received a maximum dose of 5-15 Gy, and the variable V15 reflects the percentage of the cardiac volume that received at least 15 Gy. We collected all treatment data until the date of the cardiac event for cases and for the same period of follow-up from childhood cancer diagnosis for the matched controls.
Statistical Analysis
For the cohort study, the main outcome of interest was the first occurrence of symptomatic HF. Time at risk started 5 years after the first primary cancer diagnosis. Cardiac follow-up ended at the first occurrence of HF, death for deceased individuals, or at last date of exit from cardiac follow-up. To limit follow-up bias, we fixed the final end of follow-up date separately for each subcohort as the last date on which cardiac follow-up was available for ≥ 80% of subcohort members (Data Supplement). We calculated the cumulative incidence of symptomatic HF with attained age as the time scale and taking death into account as a competing risk.29 We analyzed cumulative incidence for the overall cohort, by subcohort and by treatment period until the number at risk was < 100. We performed Gray's test to test for unadjusted significant differences between the cumulative incidences.30
In the case-control study, we included all cases identified in the cohort study (100% of all subcohort members) and used a conditional logistic regression model to estimate odds ratios (ORs). The model included treatment-related exposures on the basis of the literature and clinical knowledge.7,31-33 See the Data Supplement for the complete list of chemotherapy agents that were tested. We started with a baseline model including total cumulative anthracycline dose and mean heart RT dose since these are well-established risk factors for HF.2,7,12 Thereafter, we expanded the baseline model by adding each potential covariate to the model and compared it with the baseline model with a likelihood ratio test. For the final model, we evaluated evidence of interaction between treatment variables and age at diagnosis. In addition, we analyzed heart RT dose-volume variables by including them instead of mean dose. We used R-studio (version 6.1.1) to analyze noncontinuous treatment exposures, and we used Epicure software34 to evaluate continuous exposures by fitting a linear model for the excess odds ratio (EOR) and to evaluate departures from linearity. For all analyses, we defined statistical significance as a two-sided P value of < .05.
RESULTS
The characteristics of survivors included in the cohort study are presented in the Data Supplement. The cohort included a total of 36,205 survivors (45% were female). The UK subcohort contributed 46%. The median age of the survivors was 5.8 years at the time of diagnosis and was 29.7 years at the end of follow-up. The most frequent cancer diagnoses were leukemia (27%), lymphoma (15%), central nervous system tumors (18%), and sarcoma (12%).
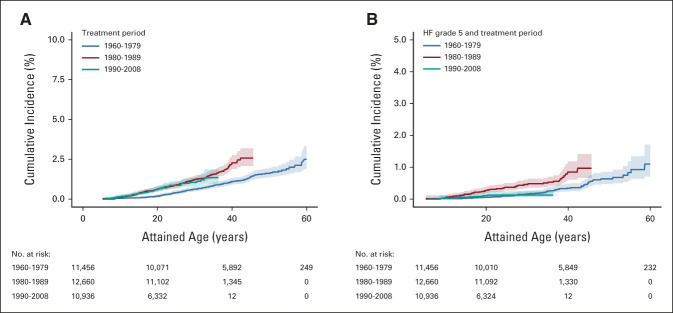
Figure 1 shows the cumulative incidence of HF by attained age. By 50 years of attained age, the cumulative incidence of HF was 2.0% (95% CI, 1.7 to 2.2). The Data Supplement illustrates the variation in cumulative incidence of HF between the subcohorts. The latest time point where we could analyze all different subcohorts was age 30 years at which the cumulative incidence ranged between 0.2% and 2.6%. For France, the United Kingdom, and the Netherlands, the risk by age 50 years was available and the cumulative incidence ranged from 1.0% to 5.2%. See the Data Supplement for the characteristic of the survivors by subcohort. The cumulative incidence of HF was greater among those with cancer diagnosed from 1980 onward than among those diagnosed before (Figure 2A). Figure 2B shows that the cumulative incidence of cardiac mortality because of HF was lower in the treatment period 1990-2008 compared with that in 1980-1990.
FIG 1.

Cumulative incidence of heart failure for all survivors (including all types of treatment) with attained age as the time scale. Shaded: 95% CI.
FIG 2.
(A) Cumulative incidence of HF for three different treatment periods: 1960-1979 (No. = 11,456 cohort members and No. = 150 cases), 1980-1989 (No. = 12,660 cohort members and No. = 169 cases), and 1990-2008 (No. = 10,936 cohort members and No. = 66 cases). Pairwise comparisons showed these degrees of significance: 1960-1979 versus 1980-1989, P = .0004; 1960-1979 versus 1990-2008, P = .00008; 1980-1989 versus 1990-2008, P = .3917. (B) Cumulative incidence of cardiac mortality because of HF for three different treatment periods: 1960-1979 (No. = 11,456 cohort members and No. = 56 cases), 1980-1989 (No. = 12,660 cohort members and No. = 62 cases), and 1990-2008 (No. = 10,936 cohort members and No. = 9 cases). Pairwise comparisons showed these degrees of significance: 1960-1979 versus 1980-1989, P = .0001; 1960-1979 versus 1990-2008, P = .73; and 1980-1989 versus 1990-2008, P = .0005. HF, heart failure.
The case-control study included 500 cases and 500 controls, and their characteristics are demonstrated in Table 1. Of all survivors, 366 had not received any RT and the RT exposure was unknown for one case and two controls. Among the 631 survivors who received RT, dosimetry was impossible for seven (1.1%) cases and five (0.8%) controls. The median of the mean heart RT dose in cases was 18.1 Gy, compared with 16.5 Gy in controls. The median cumulative anthracycline dose (including mitoxantrone) was 362 mg/m2 in cases and 218 mg/m2 in controls. Analyzing mitoxantrone as separate exposure would have led to underpowered results because only 29 survivors (nine of them with missing dose) received this agent. Dexrazoxane treatment was equal between cases (n = 4) and controls (n = 4).
TABLE 1.
Characteristics of the Survivors Included in the Case-Control Study
The final model included cumulative anthracycline dose and mean heart RT dose (Table 2). See the Data Supplement for the likelihood ratio tests for all analyzed covariates. The ORs of HF significantly increased with both the total cumulative anthracycline dose (Ptrend = < .0001) and mean heart RT dose (Ptrend = < .0001). When compared with survivors who did not receive anthracyclines, the OR associated with total cumulative anthracycline doses < 100 mg/m2 did not reach statistical significance (2.3; 95% CI, 0.7 to 7.1), the OR for 100 to < 250 mg/m2 was 5.8 (95% CI, 2.9 to 11.3), and the OR for ≥ 250 mg/m2 was 21.2 (95% CI, 11.4 to 39.2). When compared with survivors with a mean heart RT dose of 0 Gy, a mean heart dose of < 5 Gy was not associated with HF risk (1.3; 95% CI, 0.8 to 2.0), the OR for 5 to < 15 Gy was 5.5 (95% CI, 2.5 to 12.3), the OR for 15 to < 35 Gy was 9.0 (95% CI, 4.6 to 17.6), and the OR for ≥ 35 Gy was 22.6 (95% CI, 4.9 to 102.8). We also evaluated the noncontinuous dose-response relationship in more detail (Data Supplement). Further analyses provided no evidence of an effect modification by age at diagnosis regarding the roles of anthracyclines or heart RT in the risk of HF (Data Supplement). We refer the reader to the Data Supplement for the characteristics of the cases and controls who have only been exposed to heart RT and not to anthracyclines and for the characteristics of the cases and controls who have been exposed to a mean heart RT dose of 5 to < 15 Gy. In addition, we evaluated dose-volume RT variables instead of mean heart RT dose adjusted for total cumulative anthracycline dose. In survivors who received a maximum heart RT dose of 5 to < 15 Gy, the OR of HF was significantly increased if ≥ 50% of the volume was exposed (OR, 5.6; 95% CI, 1.5 to 20.6). In survivors who received ≥ 15 Gy, the risk was already significantly increased if < 50% of the heart was exposed (Table 3). The Data Supplement demonstrates the results of the remaining dose-volume variables.
TABLE 2.
Multivariable Conditional Logistic Regression Model of Grade 3-5 Heart Failure by Cancer Treatment Variables
TABLE 3.
Multivariable Conditional Logistic Regression Modelsa of Grade 3-5 Heart Failure by Volume of the Heart Exposed to the Individual Patients' Maximum Heart RT Dose
When fitting the continuous total cumulative anthracycline dose as a linear term (adjusted for heart RT), there was a significant departure from linearity (Data Supplement). The EOR per 100 mg/m2 total cumulative anthracycline dose is expressed by the following equation: EOR = –0.3 (dose/100) + 1.6 (dose/100)2 (Fig 3A). For mean heart RT dose, the dose-response relationship (adjusted for anthracyclines) was linear and yielded an EOR of 5.1 per 10 Gy (Fig 3B).
FIG 3.
(A) The ORs and corresponding 95% CIs (red dots and bars) of developing heart failure by the received total cumulative anthracycline dose and the fitted linear EOR and corresponding 95% CIs per 100 mg/m2 anthracyclines (solid blue and gray line), both of which were adjusted for mean heart RT dose. ORs were calculated relative to survivors treated without anthracyclines and are plotted at the mean cumulative anthracyclines dose of the controls within each relevant dose category. (B) The ORs and corresponding 95% CIs (red dots and bars) of developing heart failure by the received mean heart RT dose and the fitted linear EOR and corresponding 95% CIs per 10 Gy mean heart RT (solid blue and gray line), both of which were adjusted for cumulative anthracycline dose. ORs were calculated relative to survivors treated without heart RT and are plotted at the mean cumulative radiation dose of the controls within each relevant dose category. EOR, excess odds ratio; OR, odds ratio; RT, radiation therapy.
DISCUSSION
Insight into the risk factors for HF in survivors of childhood cancer is relevant for both the treatment of new children with cancer and cardiac surveillance in survivors after cardiotoxic treatment. This large pan-European nested case-control study shows important new findings. We show that survivors who received a comparatively low mean heart RT dose of 5 to < 15 Gy had a five times higher risk of HF compared with survivors who did not receive RT in the heart region, especially when more than half of the heart was exposed to low RT doses. Furthermore, we did not identify a significantly increased risk of HF for survivors treated with < 100 mg/m2 total cumulative anthracycline dose.
As emphasized by the IGHG cardiomyopathy surveillance guideline,19 little was known about the risk of HF for survivors exposed to lower doses of RT. Consequently, no recommendations could be made for survivors treated with chest RT < 15 Gy and a moderate recommendation (on the basis of weak quality evidence) could be made for 15-35 Gy.19 Previous studies in childhood cancer survivors have not found evidence that heart RT doses < 15 Gy calculated with dosimetry were associated with HF.2,12,16,35,36 This could be the result of insufficient statistical power. Recently, Bates et al11 demonstrated that phantom-based mean heart RT doses of 10-20 Gy are associated with a higher risk for HF in 24,214 survivors from the Childhood Cancer Survivor Study (CCSS; n = 371 HF events); however, they could not demonstrate a dose-volume relationship in this dose range. Within our large case-control study derived from an underlying cohort exceeding 50,000 survivors, we found that survivors treated with a mean heart RT dose 5-< 15 Gy are at risk of HF. Our results could be of great clinical importance because, on the basis of our data, a part of the survivors who are at risk will be labeled as low risk by current cardiomyopathy surveillance strategies19 (see the Data Supplement). We recognize that this concerns a small absolute number of cases; however, the proportion of survivors exposed to low mean heart RT doses is likely growing as a result of developments in radiotherapy techniques.37
Mean heart RT dose will be more and more available as it is part of current treatment planning in many institutions. Therefore, we propose to include this measure in the current cardiomyopathy surveillance guideline and recommend echocardiographic follow-up for survivors treated with a mean heart RT dose of ≥ 5 Gy. However, mean heart RT dose is not available for survivors who received radiotherapy before the introduction of advanced RT planning systems.38 For these survivors, the prescribed chest RT dose can be used as a surrogate for the maximum heart RT dose (calculated by dosimetry) in our dose-volume analysis. This analysis showed that in survivors treated with a maximum heart RT dose of 5 to < 15 Gy, the risk increased when larger cardiac volumes were exposed (≥ 50% of the total volume). Accordingly, one could consider monitoring survivors who were exposed to a prescribed chest RT dose of 5 to < 15 Gy when an experienced member of the pediatric radiotherapy planning team estimates that at least 50% of the heart was included in the original treatment field.
Regarding mean heart RT dose and the risk of HF, we show a linear dose-response relationship when adjusted for anthracyclines. By contrast, a case-control study by van Nimwegen et al,39 who included 369 adolescent or adult 5-year survivors of Hodgkin Lymphoma, demonstrated a nonlinear dose-response relationship. However, this was not adjusted for anthracycline dose, and the HF cases were older and exposed to higher doses of mean heart RT, which might have influenced their results.
Currently, the IGHG cardiomyopathy surveillance guideline includes a moderate (on the basis of weak-quality evidence) recommendation for cardiac surveillance for survivors treated with < 100 mg/m2 anthracyclines.19 Our study did not identify a significantly increased risk of HF for survivors treated with < 100 mg/m2 total cumulative anthracycline dose, in line with previous studies.16,36,40 Nevertheless, there were some cases with HF in this treatment group; possible reasons for this include the presence of cardiovascular risk factors and genetic susceptibility to anthracycline-induced cardiomyopathy.36,40 We calculated the total cumulative anthracycline dose on the basis of the results in the study by Feijen et al and included the mitoxantrone dose.14,24 Previous studies used different doxorubicin equivalent ratios, so a comparison with our study can be limited.16,36,40 Previous literature suggested that the dose response of HF and cardiac events more generally might increase substantially with higher anthracycline doses,5,40 which is confirmed by our study. On the basis of our data, the dose-response relationship appeared quadratic and Figure 3 reflects that the risk of HF increases exponentially with higher cumulative anthracycline doses. The results of our study and the cost-effectiveness study by Ehrhardt et al41 strengthen the need to reconsider the current recommendation for cardiac screening of low-risk survivors.19
In the cohort study, we evaluated the trend in cumulative incidence of both HF (grade ≥ 3) and HF-related mortality (grade 5). An important finding is that the cumulative incidence of HF increases more steeply with attained age in survivors treated ≥ 1980. In contrast to our results, in the CCSS, the cumulative incidence of HF was lower in the 1990s compared with earlier decades.2 Although detailed treatment information is not available for our cohort, we postulate that the difference in the degree of changes in treatment intensity2,7 and the difference in era grouping could play a role. In addition, the introduction of survivorship care in the 1990s potentially resulted in more survivors being monitored and being aware of cardiac diseases, and thus, they are more likely to visit the GP or late effects clinic in Europe. This could have led to more HF diagnoses after 1990. Furthermore, we showed that the cumulative incidence of HF-related mortality is lower for survivors who are diagnosed ≥ 1990 when compared with 1980-1989. As in the general population,42 this may be related to improvement in early diagnosis and treatment. As demonstrated in the Data Supplement, the cumulative incidence of HF varies between the subcohorts. This is most likely caused by different proportions of survivors exposed to cardiotoxic treatment as a result of the subcohort-specific inclusion criteria. Also, the differences in health care systems might have influenced the detection of grade 3 HF.
Beside the strengths of our study where we were able to provide precise estimates of HF risk in the low doses for heart RT and anthracycline, some limitations need to be considered. A potential limitation of the case-control study is that traditional cardiovascular risk factors could not be analyzed because data on, for example, hypertension, diabetes mellitus, and smoking status were missing for > 50% of cases and controls. However, such risk factors are unlikely to be strong confounding factors in the relationship between the investigated treatment factors and risk of HF. In Tables 8 and 9 of the Data Supplement, the actual risk might be underestimated for some cases as a result of missing anthracycline dose. Furthermore, the cumulative incidence of HF may be underestimated because of the methods of HF ascertainment in the cohort study. Despite the advantages of linkage, it is possible that some cases were missed.43 In the United Kingdom, most of the period at risk was covered by a questionnaire completed by the survivor followed by medical record validation; only a minority of follow-up was covered by linkage alone.
In conclusion, this study provides new evidence that survivors who received a mean heart RT dose of 5 to < 15 Gy have an increased risk for HF, especially when more than half of the heart was exposed to RT. Furthermore, this study did not identify a significantly increased risk of HF for survivors treated with < 100 mg/m2 total cumulative anthracycline dose. These new findings might have consequences for new treatment protocols for children with cancer and for cardiomyopathy surveillance guidelines.
ACKNOWLEDGMENT
We acknowledge the unique contribution of Stanislaw Garwicz (1935-2018) to the PanCareSurFup consortium.
Lars Hjorth
Stock and Other Ownership Interests: BioInvent, Camurus, Cantargia AB, SOBI
Honoraria: Roche, Bayer
Speakers' Bureau: Bayer
No other potential conflicts of interest were reported.
SUPPORT
Supported by European Union's Seventh Framework Programme for research, technological development, and demonstration (257505); The Dutch Heart Foundation (CVON2015-21); Dutch Cancer Society; Swiss Paediatric Oncology Group; Swiss Cancer Research (KFS-2783-02-2011, KFS-4722-02-2019, KFS-5027-02-2020, and KFS-5302-02-2021); the Swiss Cancer League (KLS/KFS-4825-01-2019); Slovenian Research Agency; the French Society of Childhood Cancer (SFCE); ARC foundation with the Pop-HaRC and CHART projects; the French National Cancer Institute (INCA) with Programme Hospitalier de Recherche Clinique; the Pfizer Foundation for childhood and adolescent health; the Ligue Nationale Contre le Cancer (LNCC); the Institut de Recherche en Santé Publique (IRESP); the French Agence Nationale Pour la Recherche Scientifique (Hope-EpiProject); AIRC Foundation for Cancer Research; the Compagnia San Paolo; Children with Cancer UK (17-247 and PGTaSFA-100033); and The Brain Tumor Charity (GN-000624).
AUTHOR CONTRIBUTIONS
Conception and design: Elizabeth A.M. Feijen, Julianne Byrne, Claudia E. Kuehni, Gill Levitt, Helena J.H. van der Pal, Carlotta Sacerdote, Nadia Haddy, Florent de Vathaire, Michael M. Hawkins, Leontien C.M. Kremer
Provision of study materials or patients: Elizabeth A.M. Feijen, Rodrigue S. Allodji, Desiree Grabow, Claudia E. Kuehni, Damien Llanas, Lorna Z. Zaletel, Lucia Miligi, Carlotta Sacerdote, Zsuzsanna Jakab, Cristina Veres, David L. Winter, Florent de Vathaire
Administrative support: Elizabeth A.M. Feijen
Collection and assembly of data: Esmée C. de Baat, Elizabeth A.M. Feijen, Raoul C. Reulen, Rodrigue S. Allodji, Francesca Bagnasco, Edit Bardi, Fabiën N. Belle, Julianne Byrne, Ghazi Debiche, Desiree Grabow, Momcilo Jankovic, Claudia E Kuehni, Damien Llanas, Jacqueline Loonen, Lorna Z. Zaletel, Milena M. Maule, Lucia Miligi, Helena J.H. van der Pal, Carlotta Sacerdote, Zsuzsanna Jakab, Cristina Veres, Nadia Haddy, David L. Winter, Florent de Vathaire, Michael M. Hawkins, Leontien C.M. Kremer
Data analysis and interpretation: Esmée C. de Baat, Elizabeth A.M. Feijen, Raoul C. Reulen, Rodrigue S. Allodji, Edit Bardi, Julianne Byrne, Elvira C. van Dalen, Lars Hjorth, Claudia E. Kuehni, Gill Levitt, Helena J.H. van der Pal, Cécile M. Ronckers, Roderick Skinner, Zsuzsanna Jakab, Cristina Veres, David L. Winter, Florent de Vathaire, Michael M. Hawkins, Leontien C.M. Kremer
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Risk Factors for Heart Failure Among Pan-European Childhood Cancer Survivors: A PanCareSurFup and ProCardio Cohort and Nested Case-Control Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Lars Hjorth
Stock and Other Ownership Interests: BioInvent, Camurus, Cantargia AB, SOBI
Honoraria: Roche, Bayer
Speakers' Bureau: Bayer
No other potential conflicts of interest were reported.
REFERENCES
- 1.Gatta G, Botta L, Rossi S, et al. : Childhood cancer survival in Europe 1999-2007: Results of EUROCARE-5—A population-based study. Lancet Oncol 15:35-47, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Mulrooney DA, Hyun G, Ness KK, et al. : Major cardiac events for adult survivors of childhood cancer diagnosed between 1970 and 1999: Report from the Childhood Cancer Survivor Study cohort. BMJ 368:l6794, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fidler MM, Reulen RC, Henson K, et al. : Population-based long-term cardiac-specific mortality among 34 489 five-year survivors of childhood cancer in Great Britain. Circulation 135:951-963, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong GT, Oeffinger KC, Chen Y, et al. : Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol 31:3673-3680, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Pal HJ, van Dalen EC, van Delden E, et al. : High risk of symptomatic cardiac events in childhood cancer survivors. J Clin Oncol 30:1429-1437, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Mertens AC, Yasui Y, Neglia JP, et al. : Late mortality experience in five-year survivors of childhood and adolescent cancer: The Childhood Cancer Survivor Study. J Clin Oncol 19:3163-3172, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Feijen EAML, Font-Gonzalez A, Van der Pal HJH, et al. : Risk and temporal changes of heart failure among 5-year childhood cancer survivors: A DCOG-LATER study. J Am Heart Assoc 8:e009122, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoff DD, Rozencweig M, Layard M, et al. : Daunomycine-induced cardiotoxicity in children and adults: A review of 110 cases. Am J Med 62:200-208, 1977 [DOI] [PubMed] [Google Scholar]
- 9.Hoff DD, Layard M, Basa P, et al. : Risk factors for doxorubicin-induced congestive heart failure. Arch Intern Med 91:710-717, 1979 [DOI] [PubMed] [Google Scholar]
- 10.Kremer LC, van der Pal HJ, Offringa M, et al. : Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: A systematic review. Ann Oncol 13:819-829, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Bates JE, Howell RM, Liu Q, et al. : Therapy-related cardiac risk in childhood cancer survivors: An analysis of the Childhood Cancer Survivor Study. J Clin Oncol 37:1090-1101, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mansouri I, Allodji RS, Hill C, et al. : The role of irradiated heart and left ventricular volumes in heart failure occurrence after childhood cancer. Eur J Heart Fail 21:509-518, 2019 [DOI] [PubMed] [Google Scholar]
- 13.Shenkenberg TD, Von Hoff DD: Mitoxantrone: A new anticancer drug with significant clinical activity. Ann Intern Med 105:67-81, 1986 [DOI] [PubMed] [Google Scholar]
- 14.Feijen EAM, Leisenring WM, Stratton KL, et al. : Derivation of anthracycline and anthraquinone equivalence ratios to doxorubicin for late-onset cardiotoxicity. JAMA Oncol 5:864-871, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damiani RM, Moura DJ, Viau CM, et al. : Pathways of cardiac toxicity: Comparison between chemotherapeutic drugs doxorubicin and mitoxantrone. Arch Toxicol 90:2063-2076, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Chow EJ, Chen Y, Kremer LC, et al. : Individual prediction of heart failure among childhood cancer survivors. J Clin Oncol 33:394-402, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chellapandian D, Pole JD, Nathan PC, et al. : Congestive heart failure among children with acute leukemia: A population-based matched cohort study. Leuk Lymphoma 60:385-394, 2019 [DOI] [PubMed] [Google Scholar]
- 18.Mulrooney DA, Armstrong GT, Huang S, et al. : Cardiac outcomes in adult survivors of childhood cancer exposed to cardiotoxic therapy. Ann Intern Med 164:93-101, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armenian SH, Hudson MM, Mulder RL, et al. : Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol 16:e123-e136, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grabow D, Kaiser M, Hjorth L, et al. : The PanCareSurFup cohort of 83,333 five-year survivors of childhood cancer: A cohort from 12 European countries. Eur J Epidemiol 33:335-349, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CORDIS : Final Report Summary - PROCARDIO (Cardiovascular risk from exposure to low-dose and low-dose-rate ionizing radiation. https://cordis.europa.eu/project/id/295823/reporting [Google Scholar]
- 22.Feijen EA, Font-Gonzalez A, van Dalen EC, et al. : Late cardiac events after childhood cancer: Methodological aspects of the Pan-European Study PanCareSurFup. PLoS One 11:e0162778, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NCI : Common terminology criteria for adverse events (CTCAE) V4.03. 2010. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm
- 24.Feijen EA, Leisenring WM, Stratton KL, et al. : Equivalence ratio for daunorubicin to doxorubicin in relation to late heart failure in survivors of childhood cancer. J Clin Oncol 33:3774-3780, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green DM, Nolan VG, Goodman PJ, et al. : The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: A report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer 61:53-67, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Badouna AN, Veres C, Haddy N, et al. : Total heart volume as a function of clinical and anthropometric parameters in a population of external beam radiation therapy patients. Phys Med Biol 57:473-484, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Veres C, Allodji RS, Llanas D, et al. : Retrospective reconstructions of active bone marrow dose-volume histograms. Int J Radiat Oncol Biol Phys 90:1216-1224, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Isambert A, Beaudre A, Ferreira I, et al. : Quality assurance of a virtual simulation software: Application to IMAgo and SIMAgo (ISOgray). Cancer Radiother 11:178-187, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Geskus RB: Data Analysis with Competing Risks and Intermediate States. London, UK, Taylor & Francis, 2016 [Google Scholar]
- 30.Gray RJ: A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16:1141-1154, 1988 [Google Scholar]
- 31.Pai VB, Nahata MC: Cardiotoxicity of chemotherapeutic agents: Incidence, treatment and prevention. Drug Saf 22:263-302, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Yeh ET, Bickford CL: Cardiovascular complications of cancer therapy: Incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol 53:2231-2247, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Chang HM, Moudgil R, Scarabelli T, et al. : Cardiovascular complications of cancer therapy: Best practices in diagnosis, Prevention, and management: Part 1. J Am Coll Cardiol 70:2536-2551, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Risk Sciences International: EPICURE user manual and command summary. http://epicurehelp.risksciences.com/
- 35.Mulrooney DA, Yeazel MW, Kawashima T, et al. : Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: Retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ 339:b4606, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y, Chow EJ, Oeffinger KC, et al. : Traditional cardiovascular risk factors and individual prediction of cardiovascular events in childhood cancer survivors. J Natl Cancer Inst 112:256-265, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bergom C, Bradley JA, Ng AK, et al. : Past, present, and future of radiation-induced cardiotoxicity: Refinements in targeting, surveillance, and risk stratification. JACC CardioOncol 3:343-359, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalapurakal JA, Gopalakrishnan M, Walterhouse DO, et al. : Cardiac-sparing whole lung IMRT in patients with pediatric tumors and lung metastasis: Final report of a prospective multicenter clinical trial. Int J Radiat Oncol Biol Phys 103:28-37, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Nimwegen FA, Ntentas G, Darby SC, et al. : Risk of heart failure in survivors of Hodgkin lymphoma: Effects of cardiac exposure to radiation and anthracyclines. Blood 129:2257-2265, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blanco JG, Sun CL, Landier W, et al. : Anthracycline-related cardiomyopathy after childhood cancer: Role of polymorphisms in carbonyl reductase genes—A report from the Children's Oncology Group. J Clin Oncol 30:1415-1421, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ehrhardt MJ, Ward ZJ, Liu Q, et al. : Cost-effectiveness of the International Late Effects of Childhood Cancer Guideline Harmonization Group screening guidelines to prevent heart failure in survivors of childhood cancer. J Clin Oncol 38:3851-3862, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones NR, Roalfe AK, Adoki I, et al. : Survival of patients with chronic heart failure in the community: A systematic review and meta-analysis. Eur J Heart Fail 21:1306-1325, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herrett E, Shah AD, Boggon R, et al. : Completeness and diagnostic validity of recording acute myocardial infarction events in primary care, hospital care, disease registry, and national mortality records: Cohort study. BMJ 346:f2350, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]